Doping Detection Based on the Nanoscale: Biosensing Mechanisms and Applications of Two-Dimensional Materials
Abstract
1. Introduction
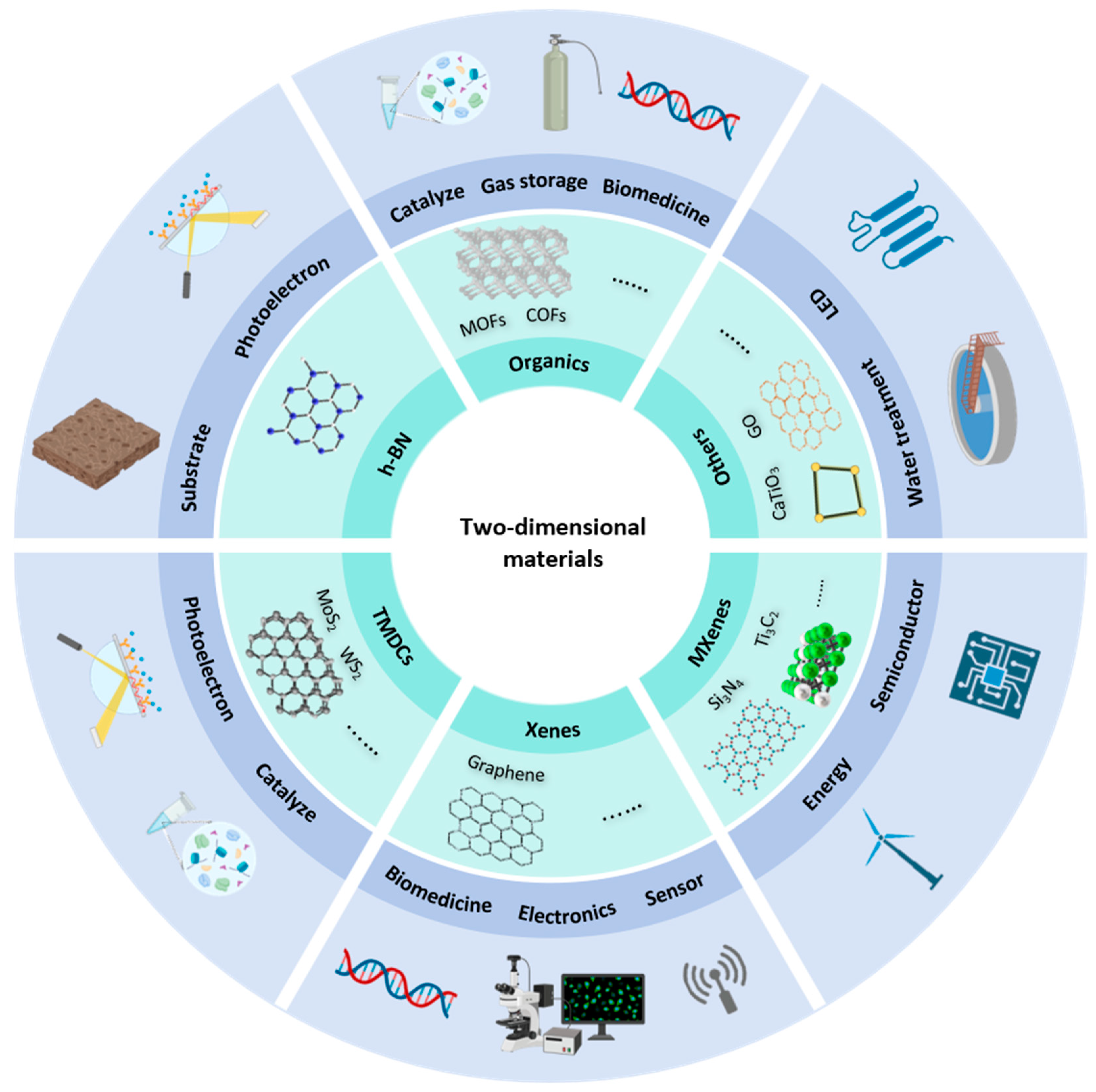
2. Characteristics of 2D Materials
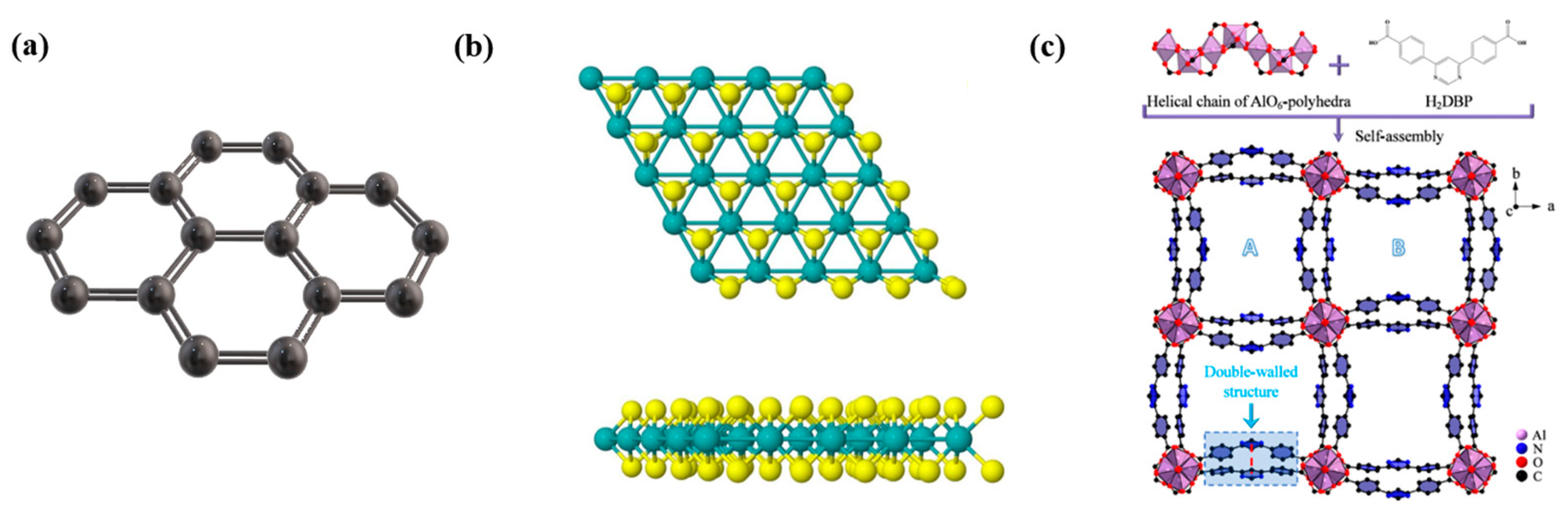
2.1. Structure and Properties
2.2. Advantages of Applications in Biosensing
3. Application of 2D Materials in Doping Detection
3.1. Detection of Anabolic Steroids
3.2. Detection of Stimulants
3.3. Detection of Peptide Hormones
4. Biosensing Mechanisms for Doping Detection
4.1. Principles of Molecular Recognition
4.2. Signal Transduction Mechanisms
4.3. Factors Affecting Sensitivity and Selectivity
5. Challenges and Prospects
Funding
Conflicts of Interest
Abbreviations
| LC-MS | Liquid chromatography–mass spectrometry |
| GC-MS | Gas chromatography–mass spectrometry |
| 2D | Two-dimensional |
| MOFs | Metal–organic frameworks |
| WADA | World Anti-Doping Agency |
| OLEDs | Organic light-emitting diodes |
| Mo | Molybdenum |
| S | Sulfur |
| ZnO | Zinc oxide |
| SERS | Surface-enhanced Raman spectroscopy |
| rGo | Reduced graphene oxide |
| BC-rGO | Biocarbon-derived porous reduced graphene oxide |
| NiO/NGO | Nickel oxide–nitrogen-doped graphene oxide |
| GCE | Glassy carbon electrode |
| IC-MOFs | Ion-conducting metal–organic frameworks |
| MPEA | N-methylphenethylamine |
| EPO | Erythropoietin |
| Hgh | Human growth hormone |
| N-rGO | Nitrogen-doped reduced graphene oxide |
| CuO | Copper oxide |
| Si3N4 | Silicon nitride |
| SiO2 | Silicon dioxide |
| COFs | Covalent organic frameworks |
| SPR | Surface plasmon resonance |
References
- Palmi, I.; Berretta, P.; Tini, A.; Ricci, G.; Marinelli, S. The unethicality of doping in sports. Clin. Ter. 2019, 170, e100–e101. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, J.; Henning, A.; Cohen, A.F.; Kayser, B. Dealing with doping. A plea for better science, governance and education. Br. J. Clin. Pharmacol. 2022, 88, 566–578. [Google Scholar] [CrossRef] [PubMed]
- Davoren, A.K.; Rulison, K.; Milroy, J.; Grist, P.; Fedoruk, M.; Lewis, L.; Wyrick, D. Doping Prevalence among U.S. Elite Athletes Subject to Drug Testing under the World Anti-Doping Code. Sports Med. Open 2024, 10, 57. [Google Scholar] [CrossRef]
- Gjelstad, A.; Herlofsen, T.M.; Bjerke, A.L.; Lauritzen, F.; Björnsdottir, I. Use of pharmaceuticals amongst athletes tested by Anti-Doping Norway in a five-year period. Front. Sports Act. Living 2023, 5, 1260806. [Google Scholar] [CrossRef]
- Christiansen, A.V.; Frenger, M.; Chirico, A.; Pitsch, W. Recreational Athletes’ Use of Performance-Enhancing Substances: Results from the First European Randomized Response Technique Survey. Sports Med. Open 2023, 9, 1. [Google Scholar] [CrossRef]
- Colpaert, T.; Risseeuw, M.; Deventer, K.; Van Eenoo, P. Investigating the detection of the novel doping-relevant peptide kisspeptin-10 in urine using liquid chromatography high-resolution mass spectrometry. Biomed. Chromatogr. BMC 2024, 38, e5946. [Google Scholar] [CrossRef]
- Narciso, J.; Luz, S.; Bettencourt da Silva, R. Assessment of the Quality of Doping Substances Identification in Urine by GC/MS/MS. Anal. Chem. 2019, 91, 6638–6644. [Google Scholar] [CrossRef]
- Klöppner, L.; Harps, L.C.; Parr, M.K. Sample Preparation Techniques for Growth-Promoting Agents in Various Mammalian Specimen Preceding MS-Analytics. Molecules 2024, 29, 330. [Google Scholar] [CrossRef]
- Zhao, X.; Yuan, Y.; Wei, H.; Fei, Q.; Luan, Z.; Wang, X.; Xu, Y.; Lu, J. Identification and characterization of higenamine metabolites in human urine by quadrupole-orbitrap LC-MS/MS for doping control. J. Pharm. Biomed. Anal. 2022, 214, 114732. [Google Scholar] [CrossRef]
- You, Y.; Proctor, R.M.; Haughan, J.; Missanelli, J.R.; Robinson, M.A. Use of Liquid Chromatography--Tandem Mass Spectrometry to Quantify and Confirm the Fentanyl Metabolite N-[1-(2-Phenethy-4-Piperidinyl)] Maloanilinic Acid in Equine Urine for Doping Control. J. Anal. Toxicol. 2023, 47, 393–402. [Google Scholar] [CrossRef]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Govindaraju, T.; Avinash, M.B. Two-dimensional nanoarchitectonics: Organic and hybrid materials. Nanoscale 2012, 4, 6102–6117. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wang, X.; Zhou, F.; Qu, J.; Song, J. 2D van der Waals Heterojunction Nanophotonic Devices: From Fabrication to Performance. Advenced Funct. Mater. 2021, 31, 2104260. [Google Scholar] [CrossRef]
- Chang, C.; Chen, W.; Chen, Y.; Chen, Y.; Chen, Y.; Ding, F.; Fan, C.; Fan, H.J.; Fan, Z.; Gong, C.; et al. Recent Progress on Two-Dimensional Materials. Acta Phys. Chim. Sin. 2021, 37, 2108017. [Google Scholar] [CrossRef]
- Park, H.; Oh, D.S.; Lee, K.J.; Jung, D.Y.; Lee, S.; Yoo, S.; Choi, S.Y. Flexible and Transparent Thin-Film Transistors Based on Two-Dimensional Materials for Active-Matrix Display. ACS Appl. Mater. Interfaces 2020, 12, 4749–4754. [Google Scholar] [CrossRef]
- Nirosha, R.; Agarwal, R. Gate dielectric based steady state & transient analysis of channel characteristics for organic thin-film transistors. J. Mater. Sci. Mater. Electron. 2023, 34, 2120. [Google Scholar] [CrossRef]
- Wang, P.; Chen, B.; Li, R.; Wang, S.; Li, Y.; Du, X.; Zhao, Y.; Zhang, X. 2D perovskite or organic material matter? Targeted growth for efficient perovskite solar cells with efficiency exceeding 24%. Nano Energy 2022, 94, 106914. [Google Scholar] [CrossRef]
- Yang, Y.; Yao, C.; Li, L.; Bo, M.; He, M.; Wang, J. Isomerization of two-dimensional non-fullerene electron acceptor materials for developing high-performance organic solar cells. J. Mater. Chem. C 2022, 10, 11286–11295. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, S.; Guo, X.; Wang, Y.; Lv, Y.; Zou, D.; Song, L.; Liu, X. Regulating the continuous and concentrated distribution of Quasi-2D perovskite phases to achieve Sky-Blue Light-Emitting diodes with efficiency approaching 17%. Chem. Eng. J. 2024, 482, 148875. [Google Scholar] [CrossRef]
- Maßmeyer, O.; Günkel, R.; Glowatzki, J.; Klement, P.; Ojaghi Dogahe, B.; Kachel, S.R.; Gruber, F.; Müller, M.; Fey, M.; Schörmann, J.; et al. Synthesis of 2D Gallium Sulfide with Ultraviolet Emission by MOCVD. Small 2024, 20, 2402155. [Google Scholar] [CrossRef]
- Hu, M.; Lyu, J.; Murrietta, N.; Fernández, S.; Michaels, W.; Zhou, Q.; Narayanan, P.; Congreve, D.N. 2D mixed halide perovskites for ultraviolet light-emitting diodes. Device 2024, 2, 100511. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, M.; Shang, S.; Gao, W.; Wang, X.; Hong, J.; Hua, C.; You, Z.; Liu, Y.; Chen, J. Recrystallization of 2D C-MOF Films for High-Performance Electrochemical Sensors. ACS Appl. Mater. Interfaces 2023, 15, 16991–16998. [Google Scholar] [CrossRef]
- Wang, S.; Xue, Y.; Yu, Z.; Huang, F.; Jin, Y. Layered 2D MOF nanosheets grown on CNTs substrates for efficient nitrite sensing. Mater. Today Chem. 2023, 30, 101490. [Google Scholar] [CrossRef]
- Chen, K.; Pan, J.; Yin, W.; Ma, C.; Wang, L. Flexible electronics based on one-dimensional inorganic semiconductor nanowires and two-dimensional transition metal dichalcogenides. Chin. Chem. Lett. 2023, 34, 108226. [Google Scholar] [CrossRef]
- Hoang, A.T.; Hu, L.; Katiyar, A.K.; Ahn, J.-H. Two-dimensional layered materials and heterostructures for flexible electronics. Matter 2022, 5, 4116–4132. [Google Scholar] [CrossRef]
- Liao, K.; Lei, P.; Tu, M.; Luo, S.; Jiang, T.; Jie, W.; Hao, J. Memristor Based on Inorganic and Organic Two-Dimensional Materials: Mechanisms, Performance, and Synaptic Applications. ACS Appl. Mater. Interfaces 2021, 13, 32606–32623. [Google Scholar] [CrossRef]
- Parreiras, S.O.; Martín-Fuentes, C.; Moreno, D.; Mathialagan, S.K.; Biswas, K.; Muñiz-Cano, B.; Lauwaet, K.; Valvidares, M.; Valbuena, M.A.; Urgel, J.I.; et al. 2D Co-Directed Metal-Organic Networks Featuring Strong Antiferromagnetism and Perpendicular Anisotropy. Small 2024, 20, e2309555. [Google Scholar] [CrossRef]
- An, J.D.; Wang, T.T.; Shi, Y.F.; Wu, X.X.; Liu, Y.Y.; Huo, J.Z.; Ding, B. A multi-responsive regenerable water-stable two-dimensional cadmium (II) fluorescent probe for highly selective, sensitive and real-time sensing of nitrofurazone and cupric ion. J. Mol. Struct. 2020, 1216, 128328. [Google Scholar] [CrossRef]
- Wang, F.; Wu, J.; Zhang, Y.; Yang, S.; Zhang, N.; Li, H.; Zhai, T. High-sensitivity shortwave infrared photodetectors of metal-organic frameworks integrated on 2D layered materials. Sci. China Mater. 2022, 65, 451–459. [Google Scholar] [CrossRef]
- Su, F.; Zhang, S.; Ji, H.; Zhao, H.; Tian, J.Y.; Liu, C.S.; Zhang, Z.; Fang, S.; Zhu, X.; Du, M. Two-Dimensional Zirconium-Based Metal-Organic Framework Nanosheet Composites Embedded with Au Nanoclusters: A Highly Sensitive Electrochemical Aptasensor toward Detecting Cocaine. ACS Sens. 2017, 2, 998–1005. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, D.; Yu, J.; Huang, Z.; Wang, L. Porous carbon nanosheets derived from two-dimensional Fe-MOF for simultaneous voltammetric sensing of dopamine and uric acid. Nanotechnology 2023, 34, 495102. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, D. Progress in electrochemical analysis of sports doping substances with two-dimensional materials. Int. J. Electrochem. Sci. 2024, 19, 100465. [Google Scholar] [CrossRef]
- Mari, E.; Duraisamy, M.; Eswaran, M.; Sellappan, S.; Won, K.; Chandra, P.; Tsai, P.-C.; Huang, P.-C.; Chen, Y.-H.; Lin, Y.-C.; et al. Highly electrochemically active Ti3C2Tx MXene/MWCNT nanocomposite for the simultaneous sensing of paracetamol, theophylline, and caffeine in human blood samples. Microchim. Acta 2024, 191, 212. [Google Scholar] [CrossRef]
- Lobo-Checa, J.; Hernández-López, L.; Otrokov, M.M.; Piquero-Zulaica, I.; Candia, A.E.; Gargiani, P.; Serrate, D.; Delgado, F.; Valvidares, M.; Cerdá, J.; et al. Ferromagnetism on an atom-thick & extended 2D metal-organic coordination network. Nat. Commun. 2024, 15, 1858. [Google Scholar] [CrossRef]
- Slathia, S.; Tripathi, M.; Tromer, R.; Gowda, C.C.; Pandey, P.; Galvao, D.S.; Dalton, A.; Tiwary, C.S. Thickness dependent nanoscale magnetism in two-dimensional manganese telluride (MnTe). Mater. Today Chem. 2024, 38, 102134. [Google Scholar] [CrossRef]
- Seki, S.; Paitandi, R.P.; Choi, W.; Ghosh, S.; Tanaka, T. Electron Transport over 2D Molecular Materials and Assemblies. Acc. Chem. Res. 2024, 57, 2665–2677. [Google Scholar] [CrossRef]
- Li, Z.; Tsuneyuki, T.; Paitandi, R.; Nakazato, T.; Odawara, M.; Tsutsui, Y.; Tanaka, T.; Miyake, Y.; Shinokubo, H.; Takagi, M.; et al. Ultrafine Spatial Modulation of Diazapyrene-Based Two-Dimensional Conjugated Covalent Organic Frameworks. J. Am. Chem. Soc. 2024, 146, 23497–23507. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Zhong, H.; Huang, X.; Li, W.; Hambsch, M.; Zhang, P.; Wang, Z.; St Petkov, P.; Heine, T.; et al. Surface-Modified Phthalocyanine-Based Two-Dimensional Conjugated Metal-Organic Framework Films for Polarity-Selective Chemiresistive Sensing. Angew. Chem. 2021, 60, 18666–18672. [Google Scholar] [CrossRef]
- Xiong, H.; Liu, H.; Feng, X.; Sun, Y.; Huang, Q.; Xiao, C. A review of two-dimensional porous graphene with in-plane pores: Pore construction and membrane applications. Carbon 2024, 229, 119547. [Google Scholar] [CrossRef]
- Li, X.; Li, B.-h.; He, Y.-b.; Kang, F.-y. A review of graphynes: Properties, applications and synthesis. New Carbon Mater. 2020, 35, 619–629. [Google Scholar] [CrossRef]
- Majidi, R. A review of structural and electronic properties of graphyne-based nanotubes. J. Comput. Electron. 2024, 23, 759–781. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Y.; Gao, Z.; Yuan, K.; Zhao, X. Theoretically modelling graphene-like carbon matryoshka with strong stability and particular three-center two-electron π bonds. Phys. Chem. Chem. Phys. 2021, 23, 11907–11916. [Google Scholar] [CrossRef] [PubMed]
- Jhaa, G.; Pancharatna, P.D.; Balakrishnarajan, M.M. Topological Impact of Delocalization on the Stability and Band Gap of Partially Oxidized Graphene. ACS Omega 2023, 8, 5124–5135. [Google Scholar] [CrossRef] [PubMed]
- Biswas, M.C.; Lubna, M.M.; Mohammed, Z.; Ul Iqbal, M.H.; Hoque, M.E. Graphene and Carbon Nanotube-Based Hybrid Nanocomposites: Preparation to Applications. In Graphene and Nanoparticles Hybrid Nanocomposites: From Preparation to Applications; Qaiss, A.e.K., Bouhfid, R., Jawaid, M., Eds.; Springer: Singapore, 2021; pp. 71–112. [Google Scholar]
- Lu, X.; Cui, M.; Pan, X.; Wang, P.; Sun, L. Investigation of the structural and electronic properties of pristine and Au-embedded MoS2/C60 and WSe2/C60 van der Waals heterostructures: A first-principles study. Appl. Surf. Sci. 2020, 503, 144328. [Google Scholar] [CrossRef]
- Hu, L.; Wu, W.; Hu, M.; Jiang, L.; Lin, D.; Wu, J.; Yang, K. Double-walled Al-based MOF with large microporous specific surface area for trace benzene adsorption. Nat. Commun. 2024, 15, 3204. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Stollenwerk, A.J.; Cunningham, C.; Sadler, C.; O’Leary, E.; Kidd, T.E.; Lukashev, P.V. Adsorption of Ag, Au, Cu, and Ni on MoS2: Theory and experiment. J. Phys. Condens. Matter An. Inst. Phys. J. 2024, 37, 015001. [Google Scholar] [CrossRef]
- Singh, E.; Singh, P.; Kim, K.S.; Yeom, G.Y.; Nalwa, H.S. Flexible Molybdenum Disulfide (MoS2) Atomic Layers for Wearable Electronics and Optoelectronics. ACS Appl. Mater. Interfaces 2019, 11, 11061–11105. [Google Scholar] [CrossRef]
- Su, H.-Y.; Calle-Vallejo, F.; Sun, K. A structure-sensitive descriptor for the design of active sites on MoS2 catalysts. Catal. Sci. Technol. 2023, 13, 5290–5300. [Google Scholar] [CrossRef]
- Su, H.-Y.; Liao, W.; Sun, K. First-principles and microkinetic simulation studies of CO2 hydrogenation mechanism and active site on MoS2 catalyst. Appl. Surf. Sci. 2023, 635, 157721. [Google Scholar] [CrossRef]
- Mehdizadeh, A.; Zeynali, M.; Karimi, M. Engineering of MoS2 nanoribbons as high-performance materials for biosensing applications. Appl. Surf. Sci. 2021, 540, 148349. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, S.; Wang, B.; Jiang, Z.; Fang, T. Recent Progress in Phase Regulation, Functionalization, and Biosensing Applications of Polyphase MoS2. Nano Micro Small 2022, 18, 2202956. [Google Scholar] [CrossRef]
- Pariari, D.; Varma, R.M.; Nair, M.N.; Zeller, P.; Amati, M.; Gregoratti, L.; Nanda, K.K.; Sarma, D.D. On the origin of metallicity and stability of the metastable phase in chemically exfoliated MoS2. Appl. Mater. Today 2020, 19, 100544. [Google Scholar] [CrossRef]
- Chatterjee, S.; Singh, H.; Hudda, D.; Sweety; Kumar, D. A Novel Acetylcholinesterase-Based Electrochemical Biosensor Using g-C3N4@MoS2 Nanohybrid for the Detection of Trichlorfon. Appl. Organomet. Chem. 2024, 38, e7721. [Google Scholar] [CrossRef]
- Li, P.; Ye, Y.; Li, Y.; Xie, Z.; Ye, L.; Huang, J. A MoS2 nanosheet-based CRISPR/Cas12a biosensor for efficient miRNA quantification for acute myocardial infarction. Biosens. Bioelectron. 2024, 251, 116129. [Google Scholar] [CrossRef]
- Pham, H.T.B.; Choi, J.Y.; Fang, X.; Claman, A.; Huang, S.; Coates, S.; Wayment, L.; Zhang, W.; Park, J. Macrocyclic ligand-driven ion selectivity and high surface area in a 2D conductive MOF. Chem 2024, 10, 199–210. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, W.; Hu, L.; Xu, M.; Gu, W.; Sun, H.; Zhu, C. Tuning atomic-scale sites in metal–organic framework-based nanozymes for sensitive biosensing. Sens. Diagn. 2023, 2, 1376–1389. [Google Scholar] [CrossRef]
- Xu, D.; Yang, F.; Zheng, D.; Gao, L.; Zhao, G.; Muhammad, P.; Wu, Q. MOF-derived yolk-shell CoN/Co-NC@SiO2 nanozyme with oxidase mimetic activities for colorimetric detection of glutathione. Microchem. J. 2024, 201, 110671. [Google Scholar] [CrossRef]
- Wang, R.; Liu, C.; Wei, Y.; Ran, Z.; Jiang, T.; Liu, C.; Shi, C.; Ren, Z.; Wang, X.; Liu, Z.; et al. Fiber SPR biosensor sensitized by MOFs for MUC1 protein detection. Talanta 2023, 258, 124467. [Google Scholar] [CrossRef]
- Elgazar, A.; Sabouni, R.; Ghommem, M.; Majdalawieh, A.F. Sustainable synthesis of MOF for COVID-19 detection using a 23 factorial design. Colloids Surf. A Physicochem. Eng. Asp. 2025, 705, 135616. [Google Scholar] [CrossRef]
- Xu, J.; Cui, X.; Wang, L.; Chen, G.; Ji, S.; Zhao, S.; Wang, H.; Luo, Z.; Zeng, A.; Fu, Q. DNA-functionalized MOF fluorescent probes for the enzyme-free and pretreatment-free detection of MicroRNA in serum. Talanta 2024, 275, 126083. [Google Scholar] [CrossRef]
- Zhang, R.; Yang, J.; Cao, Y.; Zhang, Q.; Xie, C.; Xiong, W.; Luo, X.; He, Y. Efficient 2D MOFs nanozyme combining with magnetic SERS substrate for ultrasensitive detection of Hg2+. Spectrochim. Acta. Part A Mol. Biomol. Spectrosc. 2024, 312, 124062. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Liang, J.; Wang, D.; Wang, R.; Janiak, C. Host molecules inside metal–organic frameworks: Host@MOF and guest@host@MOF (Matrjoschka) materials. Chem. Soc. Rev. 2025, 54, 601–622. [Google Scholar] [CrossRef] [PubMed]
- Piguillem, S.V.; Gomez, G.E.; Tortella, G.R.; Seabra, A.B.; Regiart, M.D.; Messina, G.A.; Fernández-Baldo, M.A. Paper-Based Analytical Devices Based on Amino-MOFs (MIL-125, UiO-66, and MIL-101) as Platforms towards Fluorescence Biodetection Applications. Chemosensors 2024, 12, 208. [Google Scholar] [CrossRef]
- Pandey, S.; Demaske, B.; Ejegbavwo, O.A.; Berseneva, A.A.; Setyawan, W.; Shustova, N.; Phillpot, S.R. Electronic structures and magnetism of Zr-, Th-, and U-based metal-organic frameworks (MOFs) by density functional theory. Comput. Mater. Sci. 2020, 184, 109903. [Google Scholar] [CrossRef]
- Pal, T.K. Metal–organic framework (MOF)-based fluorescence “turn-on” sensors. Mater. Chem. Front. 2023, 7, 405–441. [Google Scholar] [CrossRef]
- Liao, S.; Gui, L.; Yang, Y.; Liu, Y.; Hu, X. Fluorescence/visual aptasensor based on Au/MOF nanocomposite for accurate and convenient aflatoxin B1 detection. Microchim. Acta 2024, 191, 497. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Singh, A.; Shishodia, M.S. Graphene vs. silica coated refractory nitrides based core-shell nanoparticles for nanoplasmonic sensing. Phys. E Low Dimens. Syst. Nanostructures 2020, 124, 114288. [Google Scholar] [CrossRef]
- Ribeiro, D.S.; Santos, J.C.C.; Grieger, S.; Campos, J.L.E.; Machado, L.R.P.; Pacheco, F.G.; Fernandes, T.F.D.; Haase, C.C.; Silva, D.L.; Guterres, M.; et al. Measuring the Surface Area Concentration and Specific Surface Area of Mass-Produced Graphene Nanoflakes via Fluorescence Quenching. ACS Appl. Nano Mater. 2023, 6, 11198–11210. [Google Scholar] [CrossRef]
- Xiao, Y.; Cui, D.; Zhong, Y.; Li, Z.; Zhang, J.; Yu, J. Theoretical Design of Near-Infrared Tunable Surface Plasmon Resonance Biosensors Based on Gate-Controlled Graphene Plasmons. Coatings 2024, 14, 56. [Google Scholar] [CrossRef]
- Narayanan, T.N.; Vusa, C.S.; Alwarappan, S. Selective and efficient electrochemical biosensing of ultrathin molybdenum disulfide sheets. Nanotechnology 2014, 25, 335702. [Google Scholar] [CrossRef]
- Sun, B.; Wang, P.; Liang, Z.; Li, Z.; Ma, Q. MoS(2)/MXene Van der Waals heterojunction-based electrochemiluminescence sensor for triple negative breast cancer detection. Talanta 2024, 277, 126343. [Google Scholar] [CrossRef]
- Khoddam, Z.; Pourmadadi, M.; Abdouss, M.; Mazinani, S.; Behzadmehr, R.; Rahdar, A.; Ali Aboudzadeh, M. Hybrid nanocarriers based on polyacrylic acid, polyvinyl pyrrolidone, and molybdenum disulfide for enhanced 5-fluorouracil delivery in lung cancer therapy. Inorg. Chem. Commun. 2024, 159, 111749. [Google Scholar] [CrossRef]
- Wu, J.; Chang, X.; Guo, Y.; Xia, N. Synthesis of a hybrid nanocomposite: Incorporating MoS2 within a ZIF-8 for fluorescence-based detection and adsorption of tetracyclines antibiotics. Dye. Pigment. 2024, 226, 112137. [Google Scholar] [CrossRef]
- Song, R.; Zhang, H.; Lv, J. NiO Nanoparticle-decorated graphene oxide nanosheets modified glassy carbon electrode for sensitive electrochemical detection of pethidine. Int. J. Electrochem. Sci. 2023, 18, 100221. [Google Scholar] [CrossRef]
- Xu, T.; Wang, R.; Gu, C.; Jiang, T. Recyclable detection of gefitinib in clinical sample mediated by multifunctional Ag-anchored g-C3N4/MoS2 composite substrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 299, 122801. [Google Scholar] [CrossRef]
- Elfiky, M.; Gaber, M.; Mousa, M.; Salahuddin, N. Synthesis and validation of ultrasensitive stripping voltammetric sensor based on polypyrrole@ZnO/Fe3O4 core–shell nanostructure for picomolar detection of artesunate and dopamine drugs. Anal. Methods 2022, 14, 3739–3750. [Google Scholar] [CrossRef]
- Alanazi, S.; Rhouati, A.; Chrouda, A.; Cialla-May, D.; Popp, J.; Muthana, S.; Dasouki, M.; Zourob, M. Design of an innovative aptasensor for the detection of chemotherapeutic drug Fludarabine phosphate. Sci. Rep. 2024, 14, 26300. [Google Scholar] [CrossRef]
- Kokab, T.; Shah, A.; Khan, M.A.; Nisar, J.; Ashiq, M.N. Electrochemical sensing platform for the simultaneous femtomolar detection of amlodipine and atorvastatin drugs. RSC Adv. 2021, 11, 27135–27151. [Google Scholar] [CrossRef]
- Nair, A.J.S.; Saisree, S.; Sandhya, K.Y. Picomolar level electrochemical detection of hydroquinone, catechol and resorcinol simultaneously using a MoS2 nano-flower decorated graphene. Analyst 2022, 147, 2966–2979. [Google Scholar] [CrossRef]
- Wiwasuku, T.; Chuaephon, A.; Habarakada, U.; Boonmak, J.; Puangmali, T.; Kielar, F.; Harding, D.; Youngme, S. A Water-Stable Lanthanide-Based MOF as a Highly Sensitive Sensor for the Selective Detection of Paraquat in Agricultural Products. ACS Sustain. Chem. Eng. 2022, 10, 2761–2771. [Google Scholar] [CrossRef]
- Zhang, T.; Cao, R.; Tang, H.; Feng, W.; Zhang, Z. Ratiometric fluorescence turn-off probe for highly selective recognition of 2-pentanone based on a Eu3+- functionalized MOF. Inorganica Chim. Acta 2024, 565, 122003. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, M.; Lu, Y.; Liu, Q.; Niu, Q.; You, T. Metal-organic-framework-confined quantum dots enhance photocurrent signals: A molecularly imprinted photoelectrochemical cathodic sensor for rapid and sensitive tetracycline detection. Anal. Chim. Acta 2024, 1293, 342269. [Google Scholar] [CrossRef]
- Zuo, C.; Guo, Y.; Li, J.; Peng, Z.; Bai, S.; Yang, S.; Wang, D.; Chen, H.; Xie, G. A nanoprobe for fluorescent monitoring of microRNA and targeted delivery of drugs. RSC Adv. 2021, 11, 8871–8878. [Google Scholar] [CrossRef]
- Kulandaivel, S.; Lu, Y.-K.; Lin, C.-H.; Yeh, Y.-C. Dual-functional PCN-242 (Fe2Co) MOF for sensitive bacterial endotoxin detection. J. Mater. Chem. B 2025, 13, 151–159. [Google Scholar] [CrossRef]
- Khafaga, D.S.R.; El-Morsy, M.T.; Faried, H.; Diab, A.H.; Shehab, S.; Saleh, A.M.; Ali, G.A.M. Metal–organic frameworks in drug delivery: Engineering versatile platforms for therapeutic applications. RSC Adv. 2024, 14, 30201–30229. [Google Scholar] [CrossRef]
- Nikshitha, M.; Sudhakara, S.M.; Shetty, M.S. Physics. Organic-inorganic hybrids: A comprehensive review on synthesis and their potential applications. Mater. Chem. Phys. 2025, 331, 130181. [Google Scholar] [CrossRef]
- Gayathri, K.; Arumugam Madan, K.; Chinmai Sriamulya, Y.; Nagabhishek Sirpu, N.; Rajender, B.; Ramyakrishna, P. Combination of ZnO Nanoparticle with Marine Sponge Derived Dipeptide for Enhanced Anticancer Efficacy in Liver Cancer Cells and their Toxicity Evaluation on Embryonic Zebrafish. Curr. Anal. Chem. 2021, 17, 677–688. [Google Scholar] [CrossRef]
- Ouyang, Y.; Zhou, M.; Liu, Y.; Zhang, L.; Zhong, C.; Yang, Q.; Liu, M. Mg-doped ZnO nanoparticle as an effective nanocarrier in delivery of 5-Fluorouracil anti-gastric cancer drug. J. Mol. Struct. 2024, 1314, 138706. [Google Scholar] [CrossRef]
- Li, Y.; Chai, B.-L.; Xu, H.; Zheng, T.-F.; Chen, J.-L.; Liu, S.-J.; Wen, H.-R. Temperature- and solvent-induced reversible single-crystal-to-single-crystal transformations of TbIII-based MOFs with excellent stabilities and fluorescence sensing properties toward drug molecules. Inorg. Chem. Front. 2022, 9, 1504–1513. [Google Scholar] [CrossRef]
- Chen, X.-X.; Hou, M.-J.; Mao, G.-J.; Wang, W.-X.; Xu, F.; Li, Y.; Li, C.-Y. ATP-responsive near-infrared fluorescence MOF nanoprobe for the controlled release of anticancer drug. Microchim. Acta 2021, 188, 287. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yang, Q.; Wang, J.-S.; Xie, F.-Y.; Yu, H.-Y.; Li, Y.; Ma, Y.-X.; Ruan, W.-J. An anionic-ligand installed pyrene-based MOF for the fluorescence detection of paraquat. New J. Chem. 2021, 45, 4401–4407. [Google Scholar] [CrossRef]
- Yan, X.; Tan, D.; Yu, L.; Li, D.; Huang, W.; Huang, W.; Wu, H. A High-Throughput and Logarithm-Serial-Dilution Microfluidic Chip for Combinational Drug Screening on Tumor Organoids. ACS Pharmacol. Transl. Sci. 2024, 7, 4135–4143. [Google Scholar] [CrossRef]
- Hamimed, S.; Jabberi, M.; Chatti, A. Nanotechnology in drug and gene delivery. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2022, 395, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Merone, G.M.; Tartaglia, A.; Rossi, S.; Santavenere, F.; Bassotti, E.; D’Ovidio, C.; Bonelli, M.; Rosato, E.; de Grazia, U.; Locatelli, M.; et al. Fast Quantitative LC-MS/MS Determination of Illicit Substances in Solid and Liquid Unknown Seized Samples. Anal. Chem. 2021, 93, 16308–16313. [Google Scholar] [CrossRef]
- Xiao, J.; Ding, J.; Sun, C.; Liu, D.; Gao, H.; Liu, Y.; Lu, Y.; Gao, X. Simultaneous Detection of Clenbuterol and Higenamine in Urine Samples Using Interference-Free SERS Tags Combined with Magnetic Separation. ACS Sens. 2024, 9, 5394–5404. [Google Scholar] [CrossRef]
- Su, S.; Sun, Q.; Ma, J.; Zhu, D.; Wang, F.; Chao, J.; Fan, C.; Li, Q.; Wang, L. Ultrasensitive analysis of microRNAs with gold nanoparticle-decorated molybdenum disulfide nanohybrid-based multilayer nanoprobes. Chem. Commun. 2020, 56, 9012–9015. [Google Scholar] [CrossRef]
- Thevis, M.; Görgens, C.; Guddat, S.; Thomas, A.; Geyer, H. Mass spectrometry in sports drug testing-Analytical approaches and the athletes’ exposome. Scand. J. Med. Sci. Sports 2024, 34, e14228. [Google Scholar] [CrossRef]
- Thomas, A.; Thevis, M. Recent advances in mass spectrometry for the detection of doping. Expert Rev. Proteom. 2024, 21, 27–39. [Google Scholar] [CrossRef]
- Göktaş, E.F.; Kabil, E.; Arıöz, F. Quantification and validation of nine nonsteroidal anti-inflammatory drugs in equine urine using gas chromatography-mass spectrometry for doping control. Drug Test. Anal. 2020, 12, 1065–1077. [Google Scholar] [CrossRef]
- Duan, M.; Che, X.; Ma, S.; Xu, H.; Zhen, Q.; Zhang, C.; Pang, H. Efficient luteolin detection sensor achieved by CdS-MoS2 heterostructures. Inorg. Chem. Commun. 2024, 163, 112374. [Google Scholar] [CrossRef]
- Wubie, S.T. Analytical Detection Methods of Performance Enhancing Drugs in Athletes, Review Study. Glob. Acad. J. Pharm. Drug Res. 2021, 3, 29–43. [Google Scholar]
- Wen, X.; Cheng, H.; Zhang, W.; You, L.; Li, J. Multifunctional Ni(OH)2/Ag composites for ultrasensitive SERS detection and efficient photocatalytic degradation of ciprofloxacin and methylene blue. Talanta 2024, 266, 125140. [Google Scholar] [CrossRef] [PubMed]
- WADA. Prohibited List. 2025. Available online: https://www.wada-ama.org/en/resources/world-anti-doping-code-and-international-standards/prohibited-list (accessed on 1 January 2025).
- Hagedorn, H.-W.; Zankl, H.; Grund, C.; Schulz, R. Excretion of the anabolic steroid boldenone by racing pigeons. Am. J. Vet. Res. 1997, 58, 224–227. [Google Scholar] [CrossRef]
- Li, C.; Han, D.; Liang, Z.; Han, F.; Fu, W.; Wang, W.; Han, D.; Wang, Y.; Niu, L. Novel electrochemical-surface plasmon resonance (EC-SPR) sensor for amphetamine-type stimulants detection based on molecularly imprinted strategy. Sens. Actuators B Chem. 2022, 369, 132258. [Google Scholar] [CrossRef]
- Jung, J.; Jeong, Y.; Xu, Y.; Yi, J.; Kim, M.; Jeong, H.-J.; Shin, S.H.; Yang, Y.-H.; Son, J.; Sung, C. Production and engineering of nanobody-based quenchbody sensors for detecting recombinant human growth hormone and its isoforms. Drug Test. Anal. 2023, 15, 1439–1448. [Google Scholar] [CrossRef]
- Evans, R.L.; Bryant, D.J.; Voliotis, A.; Hu, D.; Wu, H.; Syafira, S.A.; Oghama, O.E.; McFiggans, G.; Hamilton, J.F.; Rickard, A.R. A Semi-Quantitative Approach to Nontarget Compositional Analysis of Complex Samples. Anal. Chem. 2024, 96, 18349–18358. [Google Scholar] [CrossRef]
- Ishii, H.; Shibuya, M.; Kusano, K.; Sone, Y.; Kamiya, T.; Wakuno, A.; Ito, H.; Miyata, K.; Sato, F.; Kuroda, T.; et al. Generic approach for the discovery of drug metabolites in horses based on data-dependent acquisition by liquid chromatography high-resolution mass spectrometry and its applications to pharmacokinetic study of daprodustat. Anal. Bioanal. Chem. 2022, 414, 8125–8142. [Google Scholar] [CrossRef]
- Ye, M.; Tang, S. Construction of SERS chip based on silver nanoparticles and detection of sports doping β-agonists. Alex. Eng. J. 2023, 83, 134–139. [Google Scholar] [CrossRef]
- Skoupá, K.; Šťastný, K.; Sládek, Z. Anabolic Steroids in Fattening Food-Producing Animals-A Review. Anim. Open Access J. 2022, 12, 2115. [Google Scholar] [CrossRef]
- Peng, C.; Wang, C.; Li, Z.; Wang, Z. A novel approach to detecting doping agents in food using electrochemical sensor based on zinc oxide/graphene oxide nanocomposites. J. Food Meas. Charact. 2024, 18, 6770–6781. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, L.; Li, Y.; Liu, X.; Fan, D.; Wu, D.; Wei, Q. Porphyrin-based metal-organic frameworks enhanced electrochemiluminescence (ECL) by overcoming aggregation-caused quenching: A new ECL emitter for the detection of trenbolone. Anal. Chim. Acta 2023, 1276, 341616. [Google Scholar] [CrossRef] [PubMed]
- Oleksak, P.; Nepovimova, E.; Valko, M.; Alwasel, S.; Alomar, S.; Kuca, K. Comprehensive analysis of prohibited substances and methods in sports: Unveiling trends, pharmacokinetics, and WADA evolution. Environ. Toxicol. Pharmacol. 2024, 108, 104447. [Google Scholar] [CrossRef]
- Huang, S.; Feng, R. A new electrochemical biosensor based on graphene oxide for rapid detection of synthetic testosterone as performance-enhancing drugs in athletes. Alex. Eng. J. 2024, 98, 281–289. [Google Scholar] [CrossRef]
- Ram, G.D.; Kumar, S.P. Bio-carbon-derived porous reduced graphene oxide photo- and electrochemical sensor for ultra-sensitive detection of testosterone hormone. Chem. Pap. 2024, 78, 9543–9557. [Google Scholar] [CrossRef]
- Sun, J.-Y.; Huang, K.-J.; Wei, S.-Y.; Wu, Z.-W.; Ren, F.-P. A graphene-based electrochemical sensor for sensitive determination of caffeine. Colloids Surf. B Biointerfaces 2011, 84, 421–426. [Google Scholar] [CrossRef]
- Li, Y.; Liu, W. Electrochemical detection of blood doping in sports: A novel biosensor based on nickel oxide/nitrogen-doped graphene oxide nanocomposite. Alex. Eng. J. 2024, 96, 176–184. [Google Scholar] [CrossRef]
- Zhang, Y. A novel approach to detect salbutamol as a doping agent in athletes: A nanoparticle-modified electrode. Alex. Eng. J. 2024, 96, 185–194. [Google Scholar] [CrossRef]
- Zhao, Z.; Mu, S.; Zheng, J.; Gu, L.; Shen, G.; Shen, Y. A new modification method of a Cetyl Trimethyl Ammonium Bromide/Nano-ZnO and Multi-walled Carbon Nanotubes Electrode for Determination of Anti Doping in Urine. IOP Conf. Ser. Mater. Sci. Eng. 2017, 220, 012009. [Google Scholar] [CrossRef]
- Liu, X.; Jade, N.; Rouhi, O. Reduced graphene oxide-based sensor for triamcinolone acetonide detection: Advancements in doping agent surveillance for athletes. Inorg. Chem. Commun. 2024, 162, 112285. [Google Scholar] [CrossRef]
- Lu, C.; Xu, M.; Lu, Y.; Zhang, Z.; Han, W.; Mahdi, A.B. Manufacturing of a Novel Sensor Based CuO@Graphene Catalyst for Voltammetric Detection of Prednisolone as an Important Doping Agent in Sport. In Topics in Catalysis; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Hudari, F.F.; Zanoni, M.V.B. A glassy carbon electrode modified with reduced graphene oxide for sensitive determination of bumetanide in urine at levels required for doping analysis. Microchim. Acta 2017, 184, 4117–4124. [Google Scholar] [CrossRef]
- Adegoke, O.; Zolotovskaya, S.; Abdolvand, A.; Daeid, N.N. Biomimetic graphene oxide-cationic multi-shaped gold nanoparticle-hemin hybrid nanozyme: Tuning enhanced catalytic activity for the rapid colorimetric apta-biosensing of amphetamine-type stimulants. Talanta 2020, 216, 120990. [Google Scholar] [CrossRef]
- Tian, Y.; Cao, S.; Xi, C.; Su, H.; Chen, Z. Designing polydopamine nanohybrid based on template-mediated for effectively remove amphetamine-type stimulants in sewage: Performance and mechanism. J. Environ. Chem. Eng. 2021, 9, 105870. [Google Scholar] [CrossRef]
- Gupta, P.; Goyal, R.N. Amino Functionalized Graphene Oxide and Polymer Nanocomposite Based Electrochemical Platform for Sensitive Assay of Anti-Doping Drug Atenolol in Biological Fluids. J. Electrochem. Soc. 2016, 163, B601. [Google Scholar] [CrossRef]
- Anvari, L.; Ghoreishi, S.M.; Faridbod, F.; Ganjali, M.R. Electrochemical Determination of Methamphetamine in Human Plasma on a Nanoceria Nanoparticle Decorated Reduced Graphene Oxide (rGO) Glassy Carbon Electrode (GCE). Anal. Lett. 2021, 54, 2509–2522. [Google Scholar] [CrossRef]
- Zhao, L.; Song, X.; Li, Y.; Jia, H.; Zhang, N.; Wei, Q.; Wu, D.; Ju, H. Europium-based metal-organic framework with acid-base buffer structure as electrochemiluminescence luminophore for hyperstatic trenbolone trace monitoring under wide pH range. Biosens. Bioelectron. 2023, 221, 114925. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, M.; Song, X.; Liu, X.; Ju, H.; Ai, H.; Wei, Q.; Wu, D. Annihilation luminescent Eu-MOF as a near-infrared electrochemiluminescence probe for trace detection of trenbolone. Chem. Eng. J. 2022, 434, 134691. [Google Scholar] [CrossRef]
- Jalalvand, A.R.; Rashidi, Z.; Khajenoori, M. Sensitive and selective simultaneous biosensing of nandrolone and testosterone as two anabolic steroids by a novel biosensor assisted by second-order calibration. Steroids 2022, 189, 109138. [Google Scholar] [CrossRef]
- Shan, L. Electrochemical Determination of Methandrostenolone Using a Molecularly Imprinted Sensor. Int. J. Electrochem. Sci. 2020, 15, 12587–12598. [Google Scholar] [CrossRef]
- Xu, X.; Bai, G.; Song, L.; Zheng, Q.; Yao, Y.; Liu, S.; Yao, C. Fast steroid hormone metabolism assays with electrochemical liver microsomal bioreactor based on polydopamine encapsulated gold-graphene nanocomposite. Electrochim. Acta 2017, 258, 1365–1374. [Google Scholar] [CrossRef]
- Alimohammadi, S.; Kiani, M.A.; Imani, M.; Rafii-Tabar, H.; Sasanpour, P. Electrochemical Determination of Dexamethasone by Graphene Modified Electrode: Experimental and Theoretical Investigations. Sci. Rep. 2019, 9, 11775. [Google Scholar] [CrossRef]
- Khoshhesab, Z.M. Simultaneous electrochemical determination of acetaminophen, caffeine and ascorbic acid using a new electrochemical sensor based on CuO–graphene nanocomposite. RSC Adv. 2015, 5, 95140–95148. [Google Scholar] [CrossRef]
- Rocha, D.P.; Dornellas, R.M.; Nossol, E.; Richter, E.M.; Silva, S.G.; Santana, M.H.P.; Munoz, R.A.A. Electrochemically Reduced Graphene Oxide for Forensic Electrochemistry: Detection of Cocaine and its Adulterants Paracetamol, Caffeine and Levamisole. Electroanalysis 2017, 29, 2418–2422. [Google Scholar] [CrossRef]
- Maleki, F.; Rashidi, M.-R.; Razmi, H.; Ghorbani, M. Label-free electrochemical immunosensor for detection of insulin-like growth factor-1 (IGF-1) using a specific monoclonal receptor on electrospun Zein-based nanofibers/rGO-modified electrode. Talanta 2023, 265, 124844. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Chen, J.; Huang, S.; Yan, Y.; Niu, Y.; Zeng, J.; Huang, Q.; Jin, M.; Shui, L. Label-free biosensor for trace insulin-like growth factor-I assay based on rGO-SnS2 heterostructure nanocomposite. Sens. Actuators B Chem. 2022, 370, 132415. [Google Scholar] [CrossRef]
- González-Guerrero, A.B.; Maldonado, J.; Dante, S.; Grajales, D.; Lechuga, L.M. Direct and label-free detection of the human growth hormone in urine by an ultrasensitive bimodal waveguide biosensor. J. Biophotonics 2017, 10, 61–67. [Google Scholar] [CrossRef]
- Xiong, C.; Li, L.; Yang, M.; Chen, Y.; Wang, L.; Xiong, L.; Huang, J.; Zou, Y. Rationally Designed Ionically Conductive Metal-Organic Frameworks for Ultrasensitive Methamphetamine Analogs Sensor. Adv. Funct. Mater. 2024, 34, 2316511. [Google Scholar] [CrossRef]
- Tiwari, N.K.; Sathyanesan, M.; Kumar, V.; Newton, S.S. A Comparative Analysis of Erythropoietin and Carbamoylated Erythropoietin Proteome Profiles. Life 2021, 11, 359. [Google Scholar] [CrossRef]
- Bohlooli, S.; Kia, S.; Bohlooli, S.; Sariri, R. Development of molecularly imprinted polymer on ferric oxide nanoparticles modified electrode as electrochemical sensor for detection of human growth hormone. Monatshefte Für Chem. Chem. Mon. 2022, 153, 39–48. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, S. A bioanalytical tool for monitoring athlete health and detecting performance-enhancing drugs: An electrochemical biosensor based on graphene oxide. Alex. Eng. J. 2024, 102, 58–65. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Q.-H.; Zhou, Y.-R.; Wang, S.-L.; Fu, J.; Liu, J.-Y.; Zhang, G.-H.; Ma, L.; Tao, G.; Tao, G.-H.; et al. Hydrogen-bonding and π-π interaction promoted solution-processable covalent organic frameworks. Nat. Commun. 2023, 14, 8181. [Google Scholar] [CrossRef]
- Patnaik, A. Hydrogen Bond Directed 2D Materials at Modulated Interfaces. In Advanced Supramolecular Nanoarchitectonics; Ariga, K., Aono, M., Eds.; William Andrew Publishing: Norwich, NY, USA, 2019; Chapter 1.3; pp. 31–87. [Google Scholar]
- Lin, X.; Lu, J.C.; Shao, Y.; Zhang, Y.Y.; Wu, X.; Pan, J.B.; Gao, L.; Zhu, S.Y.; Qian, K.; Zhang, Y.F.; et al. Intrinsically patterned two-dimensional materials for selective adsorption of molecules and nanoclusters. Nat. Mater. 2017, 16, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, M.; Nozawa, T.; Matsumoto, T.; Yagihashi, F.; Kikuchi, T.; Sato, K. Parallel-stacked aromatic molecules in hydrogen-bonded inorganic frameworks. Nat. Commun. 2021, 12, 7025. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, M.; Liu, J. π–π Stacking Interaction: A Nondestructive and Facile Means in Material Engineering for Bioapplications. Cryst. Growth Des. 2018, 18, 2765–2783. [Google Scholar] [CrossRef]
- Lin, S.-P. (Invited) Development of Cell-Based Biosensor Platform for Monitoring the Effect of Functional Nanocarriers As Drug Delivery System on Breast Cancer Cells. ECS Meet. Abstr. 2023, 243, 1928. [Google Scholar] [CrossRef]
- Altalbawy, F.M.A.; Altameemi, K.K.A.; Ballal, S.; Monsi, M.; Walia, C.; Prasad, G.V.S.; Al-saray, M.J.; Alsaadi, S.B.; Al-Mashhadani, Z.I.; Alsayah, A.M. A comparative DFT study of drug delivery system based on Pt-doped and Au-modified MoS2 nanosheets for β-lapachone drug. In Structural Chemistry; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Wei, W.; Zhou, S.; Ma, D.-D.; Li, Q.; Ran, M.; Li, X.; Wu, X.-T.; Zhu, Q.-L. Ultrathin Conductive Bithiazole-Based Covalent Organic Framework Nanosheets for Highly Efficient Electrochemical Biosensing. Adv. Funct. Mater. 2023, 33, 2302917. [Google Scholar] [CrossRef]
- Arshad, M.; Arshad, S.; Majeed, M.K.; Frueh, J.; Chang, C.; Bilal, I.; Niaz, S.I.; Khan, M.S.; Tariq, M.A.; Yasir Mehboob, M. Transition Metal-Decorated Mg12O12 Nanoclusters as Biosensors and Efficient Drug Carriers for the Metformin Anticancer Drug. ACS Omega 2023, 8, 11318–11325. [Google Scholar] [CrossRef]
- Wong, K.-Y.; Liu, Y.; Phan, C.-M.; Jones, L.; Wong, M.-S.; Liu, J. Selection of DNA aptamers for sensing drugs treating eye disease: Atropine and timolol maleate. Sens. Diagn. 2024, 3, 1679–1688. [Google Scholar] [CrossRef]
- Chen, S.; Ju, Y.; Yang, Y.; Xiang, F.; Yao, Z.; Zhang, H.; Li, Y.; Zhang, Y.; Xiang, S.; Chen, B.; et al. Multistate structures in a hydrogen-bonded polycatenation non-covalent organic framework with diverse resistive switching behaviors. Nat. Commun. 2024, 15, 298. [Google Scholar] [CrossRef]
- Piao, H.; Choi, G.; Sanoj Rejinold, N.; Yu, J.; Choi, S.-J.; Choy, J.-H. Pemetrexed-layered double hydroxide with Eudragit® S100; 2D van der Waals nanohybrid with drug delivery function. J. Ind. Eng. Chem. 2024, 130, 572–579. [Google Scholar] [CrossRef]
- Sayed, Z.S.; Hieba, E.M.; Batakoushy, H.A.; Rashdan, H.R.M.; Ismail, E.; Elkatlawy, S.M.; Elzwawy, A. Cancer treatment approaches within the frame of hyperthermia, drug delivery systems, and biosensors: Concepts and future potentials. RSC Adv. 2024, 14, 39297–39324. [Google Scholar] [CrossRef] [PubMed]
- Dinda, S.; Mahato, B.; Maiti, A.; Ghoshal, D. Selective Detection of Primary Aromatic Amines through Enhanced Luminescence of a 2D + 2D Inclined Polycatenated Microporous Nitro-Functionalized Metal–Organic Framework. Inorg. Chem. 2024, 63, 5996–6004. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, Z.; Xu, C.; Zhan, M.; Koh, K.; Niu, J.; Chen, H. 2D MOF-enhanced SPR sensing platform: Facile and ultrasensitive detection of Sulfamethazine via supramolecular probe. J. Hazard. Mater. 2023, 456, 131642. [Google Scholar] [CrossRef]
- Das, G.; Prakasam, T.; Alkhatib, N.; AbdulHalim, R.G.; Chandra, F.; Sharma, S.K.; Garai, B.; Varghese, S.; Addicoat, M.A.; Ravaux, F.; et al. Light-driven self-assembly of spiropyran-functionalized covalent organic framework. Nat. Commun. 2023, 14, 3765. [Google Scholar] [CrossRef]
- Chintala, K. 5-Graphene based materials for electrochemical sensing. In 2D Materials-Based Electrochemical Sensors; Rout, C.S., Ed.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 159–198. [Google Scholar]
- Uçar, A.; Aydoğdu Tığ, G.; Er, E. Recent advances in two dimensional nanomaterial-based electrochemical (bio)sensing platforms for trace-level detection of amino acids and pharmaceuticals. TrAC Trends Anal. Chem. 2023, 162, 117027. [Google Scholar] [CrossRef]
- Yang, T.; Jiang, X.; Huang, Y.; Tian, Q.; Zhang, L.; Dai, Z.; Zhu, H. Mechanical sensors based on two-dimensional materials: Sensing mechanisms, structural designs and wearable applications. iScience 2022, 25, 103728. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, D.; Chen, Z.; Liu, X.; Xu, Y.; Guo, L. Enhanced Polymeric Carbon Nitride Nanosheet-Based Fluorescence for Biosensing Applications. ACS Appl. Nano Mater. 2023, 6, 1441–1447. [Google Scholar] [CrossRef]
- Zhong, Y.; Guo, L.; Zou, Y.; Ge, J.; Chen, Y.; Lu, Z.; Wang, D. Cys-Functionalized MoS2 Quantum Dots and Calcium Ion for Ratiometric Fluorescence Probes for Doxycycline. ACS Appl. Nano Mater. 2023, 6, 22355–22362. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, R.; Liu, L.; Xing, X.; Cai, H.; Fu, Y.; Sun, J.; Ruan, W.; Chen, J.; Qiu, X.; et al. Study of cell and drug interactions based on dual-mode detection using SPR and fluorescence imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 314, 124170. [Google Scholar] [CrossRef]
- Yang, H.; Xu, X.; Shu, B. Surface plasmon resonance (EC-SPR) sensor based on molecularly imprinted strategy for the determination of stimulant modafinil in saliva samples. Int. J. Electrochem. Sci. 2024, 19, 100739. [Google Scholar] [CrossRef]
- Basova, T. Phthalocyanine and Porphyrin Derivatives and Their Hybrid Materials in Optical Sensors Based on the Phenomenon of Surface Plasmon Resonance. Chemosensors 2024, 12, 56. [Google Scholar] [CrossRef]
- Yesudasu, V.; Srivastava, R.; Pal, S.; Verma, A.; Prajapati, Y.K. Performance Enhancement of SPR Sensor for Dengue Virus Detection: Influence of Aluminum Nitride and 2D Materials. In Plasmonics; Springer: Singapore, 2024. [Google Scholar] [CrossRef]
- Feng, D.; Xu, S.-S.; Wen, B.-Y.; Murugavel, K.; Zhang, Y.-J.; Wang, A.; Zhang, F.-L.; Jin, S.; Li, J.-F. Ultrasensitive and rapid detection for multiple dopings in saliva and urine using surface-enhanced Raman spectroscopy. Spectrosc. Lett. 2023, 56, 249–262. [Google Scholar] [CrossRef]
- Piras, A.; Ehlert, C.; Gryn’ova, G. Sensing and sensitivity: Computational chemistry of graphene-based sensors. WIREs Comput. Mol. Sci. 2021, 11, e1526. [Google Scholar] [CrossRef]
- Li, H.; Brédas, J.-L. Impact of Structural Defects on the Elastic Properties of Two-Dimensional Covalent Organic Frameworks (2D COFs) under Tensile Stress. Chem. Mater. 2021, 33, 4529–4540. [Google Scholar] [CrossRef]
- Choi, P.G.; Fuchigami, T.; Kakimoto, K.-i.; Masuda, Y. Effect of Crystal Defect on Gas Sensing Properties of Co3O4 Nanoparticles. ACS Sens. 2020, 5, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Jamdade, S.; Yu, Z.; Boulfelfel, S.E.; Cai, X.; Thyagarajan, R.; Fang, H.; Sholl, D.S. Probing Structural Defects in MOFs Using Water Stability. J. Phys. Chem. C 2024, 128, 3975–3984. [Google Scholar] [CrossRef]
- He, M.; Zhang, W.; Zhang, H.; Lian, J.; Gao, Y.; Wang, J.; Lv, Y.; Wang, X.; Miao, R.; Wang, L. Controlling amine monomers via UiO-66-NH2 defect sites to enhance forward osmosis membrane performance for lithium recovery. Chem. Eng. J. 2024, 493, 152321. [Google Scholar] [CrossRef]
- Sen, D.; Lazenby, R.A. Selective Aptamer Modification of Au Surfaces in a Microelectrode Sensor Array for Simultaneous Detection of Multiple Analytes. Anal. Chem. 2023, 95, 6828–6835. [Google Scholar] [CrossRef]
- Rashed, M.A.; Ahmed, J.; Faisal, M.; Alsareii, S.A.; Jalalah, M.; Tirth, V.; Harraz, F.A. Surface modification of CuO nanoparticles with conducting polythiophene as a non-enzymatic amperometric sensor for sensitive and selective determination of hydrogen peroxide. Surf. Interfaces 2022, 31, 101998. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, N.; Casotto, A.; Sangaletti, L.; Comini, E. Tailoring the surface chemistry of ZnO nanowires via mixed self-assembly of organosilanes for selective acetone detection. Sens. Actuators B Chem. 2023, 384, 133653. [Google Scholar] [CrossRef]
- Scott, A.J.; Duever, T.A.; Penlidis, A. The role of pH, ionic strength and monomer concentration on the terpolymerization of 2-acrylamido-2-methylpropane sulfonic acid, acrylamide and acrylic acid. Polymer 2019, 177, 214–230. [Google Scholar] [CrossRef]
- Malakhovsky, P.; Reznikov, I.; Murausky, D.; Artemyev, M. Role of Particle Shape, Substrate Streaming Potential, and Ionic Strength in Kinetics and Spatial Distribution of Electrostatically Deposited Silver Nanoparticles. J. Phys. Chem. C 2023, 127, 20398–20410. [Google Scholar] [CrossRef]
- Luo, W.; Meng, J.; Li, X.; Xie, Q.; Yi, D.; Wang, Y.; Hong, X. Temperature effects on surface plasmon resonance sensor based on side-polished D-shaped photonic crystal fiber. Measurement 2021, 181, 109504. [Google Scholar] [CrossRef]
- Shao, W.; Zeng, Z.; Star, A. An Ultrasensitive Norfentanyl Sensor Based on a Carbon Nanotube-Based Field-Effect Transistor for the Detection of Fentanyl Exposure. ACS Appl. Mater. Interfaces 2023, 15, 37784–37793. [Google Scholar] [CrossRef] [PubMed]
- Guselnikova, O.; Lim, H.; Kim, H.-J.; Kim, S.H.; Gorbunova, A.; Eguchi, M.; Postnikov, P.; Nakanishi, T.; Asahi, T.; Na, J.; et al. New Trends in Nanoarchitectured SERS Substrates: Nanospaces, 2D Materials, and Organic Heterostructures. Small 2022, 18, 2107182. [Google Scholar] [CrossRef]
- Cannaert, A.; Vandeputte, M.; Wille, S.M.R.; Stove, C.P. Activity-based reporter assays for the screening of abused substances in biological matrices. Crit. Rev. Toxicol. 2019, 49, 95–109. [Google Scholar] [CrossRef]
- Garrido, M.; Naranjo, A.; Pérez, E.M. Characterization of emerging 2D materials after chemical functionalization. Chem. Sci. 2024, 15, 3428–3445. [Google Scholar] [CrossRef]
- Du, Z.; Qiao, F.; Tong, L.; Zhang, W.; Mou, X.; Zhao, X.; Maitz, M.F.; Wang, H.; Huang, N.; Yang, Z. Mimicking Mytilus edulis foot protein: A versatile strategy for robust biomedical coatings. Innovation 2024, 5, 100671. [Google Scholar] [CrossRef]
- Ohnuma, A.; Tozaki, T.; Kikuchi, M.; Ishige, T.; Kakoi, H.; Hirota, K.-i.; Takahashi, Y.; Nagata, S.-i. Multiplex Detection of Transgenes Using πCode Technology for Gene Doping Control. Anal. Chem. 2023, 95, 10149–10154. [Google Scholar] [CrossRef]
- Argyriou, A.V.; Tektonidis, N.; Alevizos, E.; Ferentinos, K.P.; Kourgialas, N.N.; Mathioudakis, M.M. Precision Farming Multimodal Technologies Using Optical Sensors for the Detection of Citrus Tristeza Virus Endemics. Sustainability 2024, 16, 5748. [Google Scholar] [CrossRef]
- Liu, Q.; You, J.; Xiong, Y.; Liu, W.; Song, M.; Ren, J.; Xue, Q.; Tian, J.; Zhang, H.; Wang, X. Synergistic effect of interstitial phosphorus doping and MoS2 modification over Zn0.3Cd0.7S for efficient photocatalytic H2 production. J. Colloid Interface Sci. 2024, 675, 772–782. [Google Scholar] [CrossRef] [PubMed]
- Ramachandran, R.; Chen, T.-W.; Chen, S.-M.; Baskar, T.; Kannan, R.; Elumalai, P.; Raja, P.; Jeyapragasam, T.; Dinakaran, K.; Gnana kumar, G.p. A review of the advanced developments of electrochemical sensors for the detection of toxic and bioactive molecules. Inorg. Chem. Front. 2019, 6, 3418–3439. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Chand, R.; Kumar, S.; Mittal, N.; Srinivasan, S.; Rajabzadeh, A.R. Recent biosensing advances in the rapid detection of illicit drugs. TrAC Trends Anal. Chem. 2020, 131, 116006. [Google Scholar] [CrossRef]
- Jayawardena, H.S.N.; Liyanage, S.H.; Rathnayake, K.; Patel, U.; Yan, M. Analytical Methods for Characterization of Nanomaterial Surfaces. Anal. Chem. 2021, 93, 1889–1911. [Google Scholar] [CrossRef]
- Bahru, T.B.; Ajebe, E.G. A Review on Nanotechnology: Analytical Techniques Use and Applications. Int. Res. J. Pure Appl. Chem. 2019, 19, 45545. [Google Scholar] [CrossRef]
- Lu, Y.; Yan, J.; Ou, G.; Fu, L. A Review of Recent Progress in Drug Doping and Gene Doping Control Analysis. Molecules 2023, 28, 5483. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Mathew, M.; Rout, C.S. Microfluidic sensors based on two-dimensional materials for chemical and biological assessments. Mater. Adv. 2022, 3, 1874–1904. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Phuong, N.T.T.; Trinh, T.N.D.; Tran, N.H.T.; Trinh, K.T.L. Synthesis and applications of MXene-based materials in biosensors for screening infectious pathogens. Sens. Actuators A Phys. 2024, 378, 115784. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, W.; Sun, X.; Chen, Q.; Niu, B. The Application of Machine Learning in Doping Detection. J. Chem. Inf. Model. 2024, 64, 8673–8683. [Google Scholar] [CrossRef]
- Wang, J.; Duan, Z.; Luo, D. Fiber Optic SPR POCT: A Novel Nucleic Acid Detection Biosensor for Environmental Viruses. Research 2024, 7, 0296. [Google Scholar] [CrossRef]
- Suan Ng, S.; Ling Lee, H.; Bothi Raja, P.; Doong, R.-a. Recent Advances in Nanomaterial-based Optical Biosensors as Potential Point-of-Care Testing (PoCT) Probes in Carcinoembryonic Antigen Detection. Chem. Asian J. 2022, 17, e202200287. [Google Scholar] [CrossRef] [PubMed]
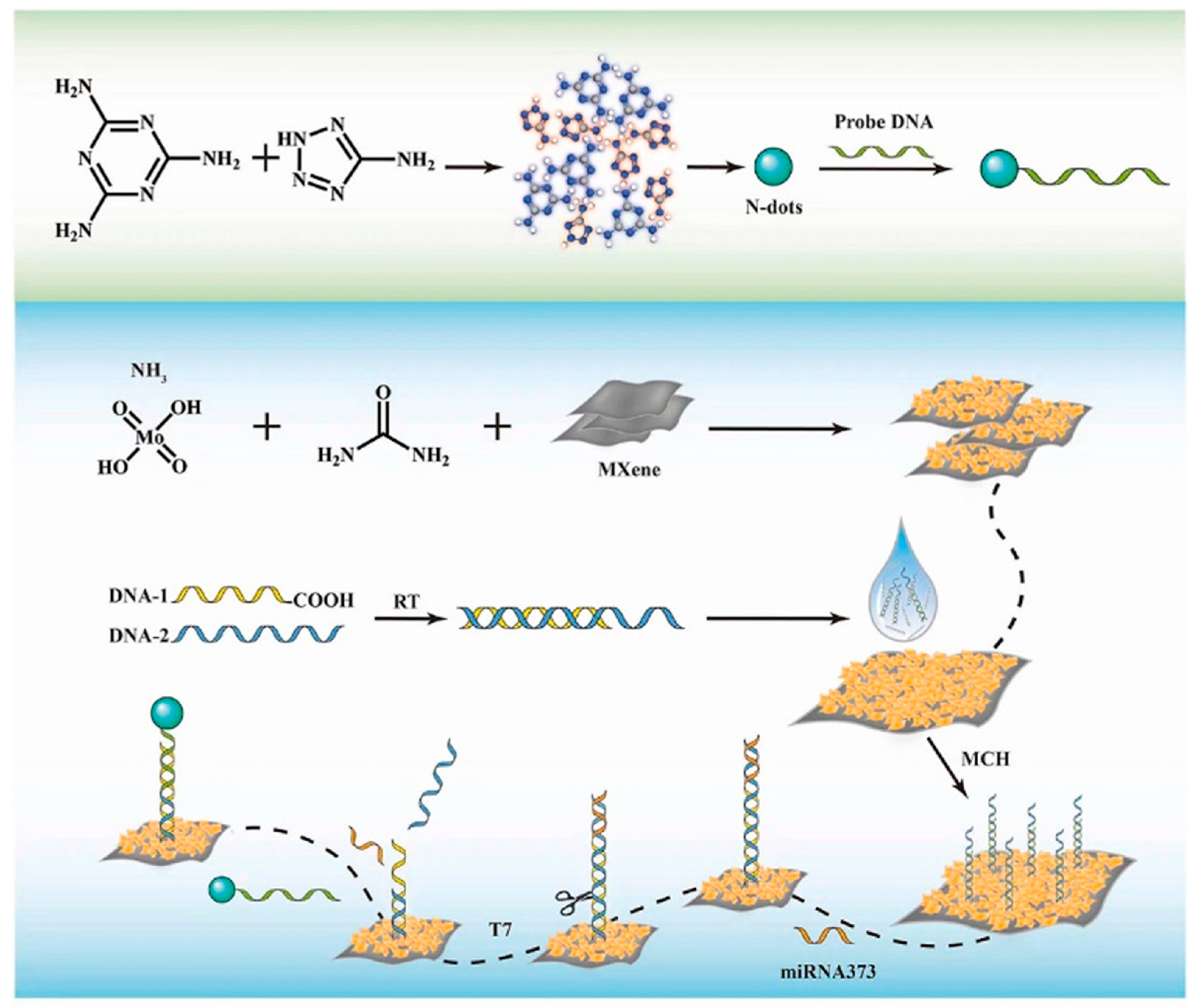






| Sensor | Doping | Doping Category | LOD | Linear Range | RSD | Recovery Rate | Reference |
|---|---|---|---|---|---|---|---|
| 2D AuNCs@521-MOF | Cocaine | S6 | 1.29 pM | 0.001–1.0 ng·mL | 0.02% | 101.2–112.7% | [30] |
| NiO–GO/GCE | Pethidine (PTD) | S7 | 3.8 ng/mL | 0–500 μg/mL | 5.41% | 90.00–99.66% | [76] |
| ZnO/GO/MOF | Nandrolone (NDL) | S1 | 0.09 µM | 0.5 µM–138 µM | 4.18% | 94.00–99.00% | [113] |
| Pt-NPs@MOFs/Au-ZnO | Trenbolone (TB) | S1 | 3.61f g/mL | 10 fg/mL–100 ng/mL | 2.3–4.5% | 96–107% | [114] |
| rGO/GCE | Testosterone | S1 | 0.1 nM | 2.0–210.0 nM | 1.97% | 98.1–104.2% | [116] |
| Nafion–Gr/GCE | Caffeine * | --- | 0.12 µM | 0.4–40 μM | 5.20% | 98.6–102.0% | [118] |
| NiO/NGO | Ephedrine (EPD) | S6 | 0.09 μM | 10–2580 μM | 3.591–4.21% | 98.80–99.40% | [119] |
| Tb4O7/RGO | Salbutamol (SBM) | S3 | 21 nM | 1 μM–710 μM | 4.17% | 96.50% | [120] |
| CTAB/ZnO | Carteolol Hydrochloride (CH) | P1 | 0.2 μg/mL | 1 × 10−3–2 × 10−1 mg/mL | 1.97–4.29% | 96.5–110.5% | [121] |
| Ag-rGOPE | Triamcinolone Acetonide (TAA) | S9 | 0.005 μM | 10–300 μM | 4.37% | 96.00% | [122] |
| Gr-CuO | Prednisolone | S9 | 0.008 µM | 0.01–25 µM | 3.40% | 97.1–103.5% | [123] |
| rGO/GCE | Bumetanide (BMT) | S5 | 75 nM | 0.255–50.0 μM | 3.40% | 99.3–101% | [124] |
| GO-CTAB-AuNP | Methamphetamine (MAMP) | S6 | 28.6 ng/mL | 0.5–100 μM | 4.70% | 74.6–84.0% | [125] |
| magGO/PDA | Amphetamine-type stimulants (ASTs) | S6 | 0.272 μg/L | 2–100 μg/L | 0.9–6.0% | 85.7–121.9% | [126] |
| GO/p-(AHNSA) | Atenolol (ATN) | P1 | 20 nM | 0.1–300 μM | 2.70% | 97% | [127] |
| CeO2/rGO | Methamphetamine (MAMP) | S6 | 8.7 µM | 25.0–166.6 µM | 4.70% | —— | [128] |
| Eu(H4BDPO)-MOF | Trenbolone (TB) | S1 | 2.36 fg/mL | 10 fg/mL–100 ng/mL | 3.20% | 96.7–103% | [129] |
| Eu-MOF (Eu2[Ru(dcbpy)3]3) | Trenbolone (TB) | S1 | 4.83 fg/mL | 5 fg/mL–100 ng/mL | 2.70% | 98.3–103.3% | [130] |
| C60/MWCNTs-Gr-IL/ITO | Nandrolone Decanoate (ND) Testosterone Decanoate (TS) | S1 | —— | 2.12–11.38 Μm 1.03–7.75 μM | 3.08% 3.33% | 97–106% 96.3–103% | [131] |
| PtNPs/AuNWs/IL/CrGO | Metandienone | S1 | 0.4 nM | 2 nM–9.6 μM | 7.70% | 95.70% | [132] |
| HLM/Au@RGO-CS/GCE | Testosterone | S1 | 4.2 mM | 25–200 mM | 3.13% | —— | [133] |
| GNP/GCE | Dexamethasone (DEX) | S9 | 15 nM | 0.1–50 μM | 0.40% | —— | [134] |
| CuO-Gr/CPE | Caffeine (CA) | --- | 0.01 μM | 0.025–5.3 μM | 3.70% | 96.5–98% | [135] |
| ERGO | Caffeine (CAF) | --- | 0.055 μM | 50–650 μM | 5.00% | 103.00% | [136] |
| PAN/Zein-rGO | IGF-1 | S2 | 55.72 fg/mL | 1 pg/mL–10 ng/mL | 0.03% | 95.81–101.54% | [137] |
| AuNPs@rGO-SnS | AgIGF-I | S2 | 0.12 pg/mL | 0.25–750.0 pg/mL | 3.12% | 96.86–123.55% | [138] |
| BiMW/Si3N4/SiO2 | hGH | S2 | 0.30 pg/mL | 3–10 pg/mL | —— | —— | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wang, Y.; Liu, B. Doping Detection Based on the Nanoscale: Biosensing Mechanisms and Applications of Two-Dimensional Materials. Biosensors 2025, 15, 227. https://doi.org/10.3390/bios15040227
Zhao J, Wang Y, Liu B. Doping Detection Based on the Nanoscale: Biosensing Mechanisms and Applications of Two-Dimensional Materials. Biosensors. 2025; 15(4):227. https://doi.org/10.3390/bios15040227
Chicago/Turabian StyleZhao, Jingjing, Yu Wang, and Bing Liu. 2025. "Doping Detection Based on the Nanoscale: Biosensing Mechanisms and Applications of Two-Dimensional Materials" Biosensors 15, no. 4: 227. https://doi.org/10.3390/bios15040227
APA StyleZhao, J., Wang, Y., & Liu, B. (2025). Doping Detection Based on the Nanoscale: Biosensing Mechanisms and Applications of Two-Dimensional Materials. Biosensors, 15(4), 227. https://doi.org/10.3390/bios15040227






