Sea Urchin-like Magnetic Microbeads-Based Electrochemical Biosensor for Highly Sensitive Detection of Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Fe3O4 Magnetic Microbeads (MMBs), Urchin-like Fe3O4@ZnO Magnetic Microbeads (SMMBs), and SMMB/Oxidase
2.3. Electrochemical Detection of Metabolites
3. Results
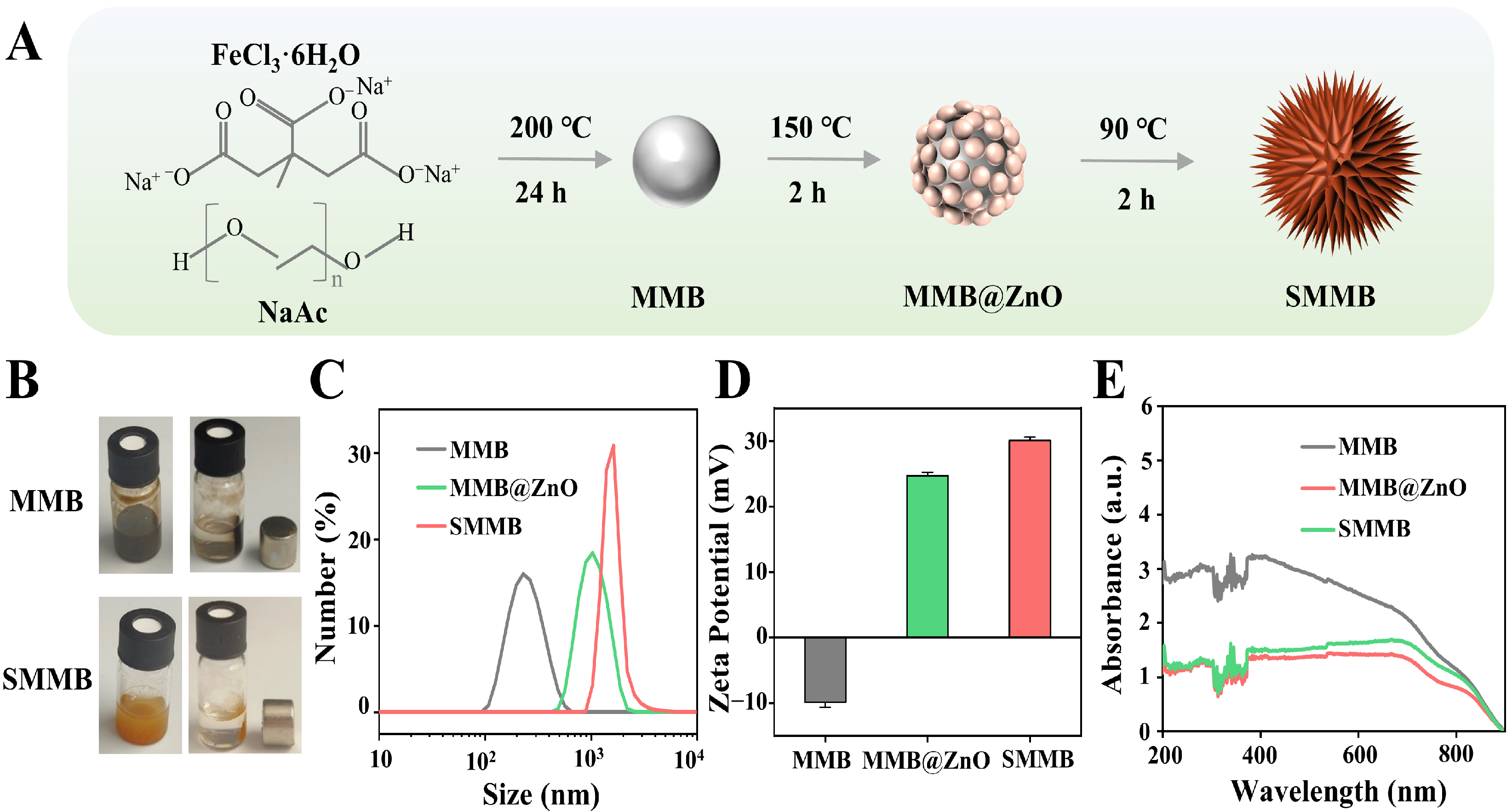
3.1. Preparation of SMMBs
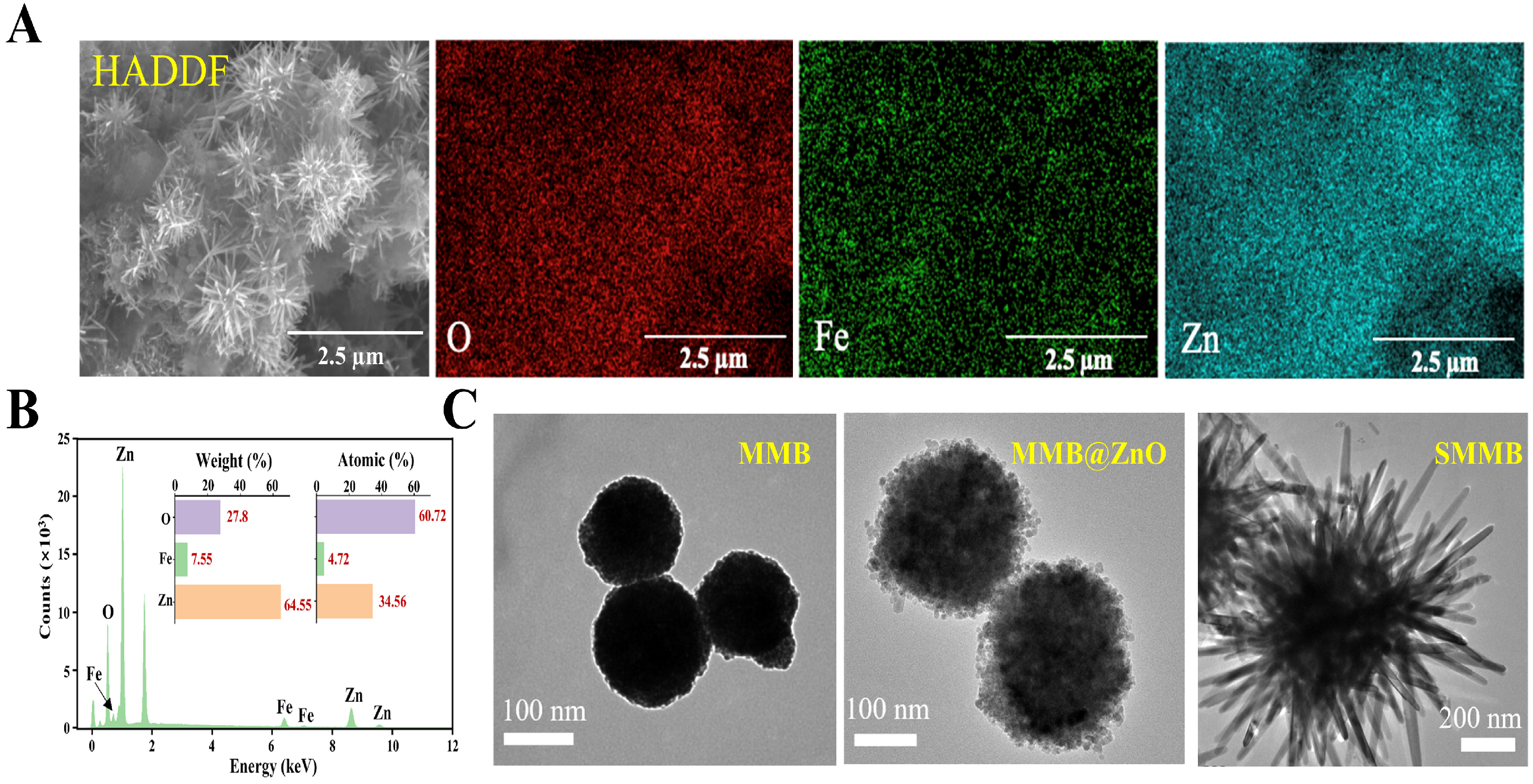
3.2. Characterization of SMMBs
3.3. Preparation of SMMB-Based Electrochemical Biosensor for Glucose Detection
3.4. Analyzing the Performance of the SMMB-Based Electrochemical Biosensor for Glucose Detection
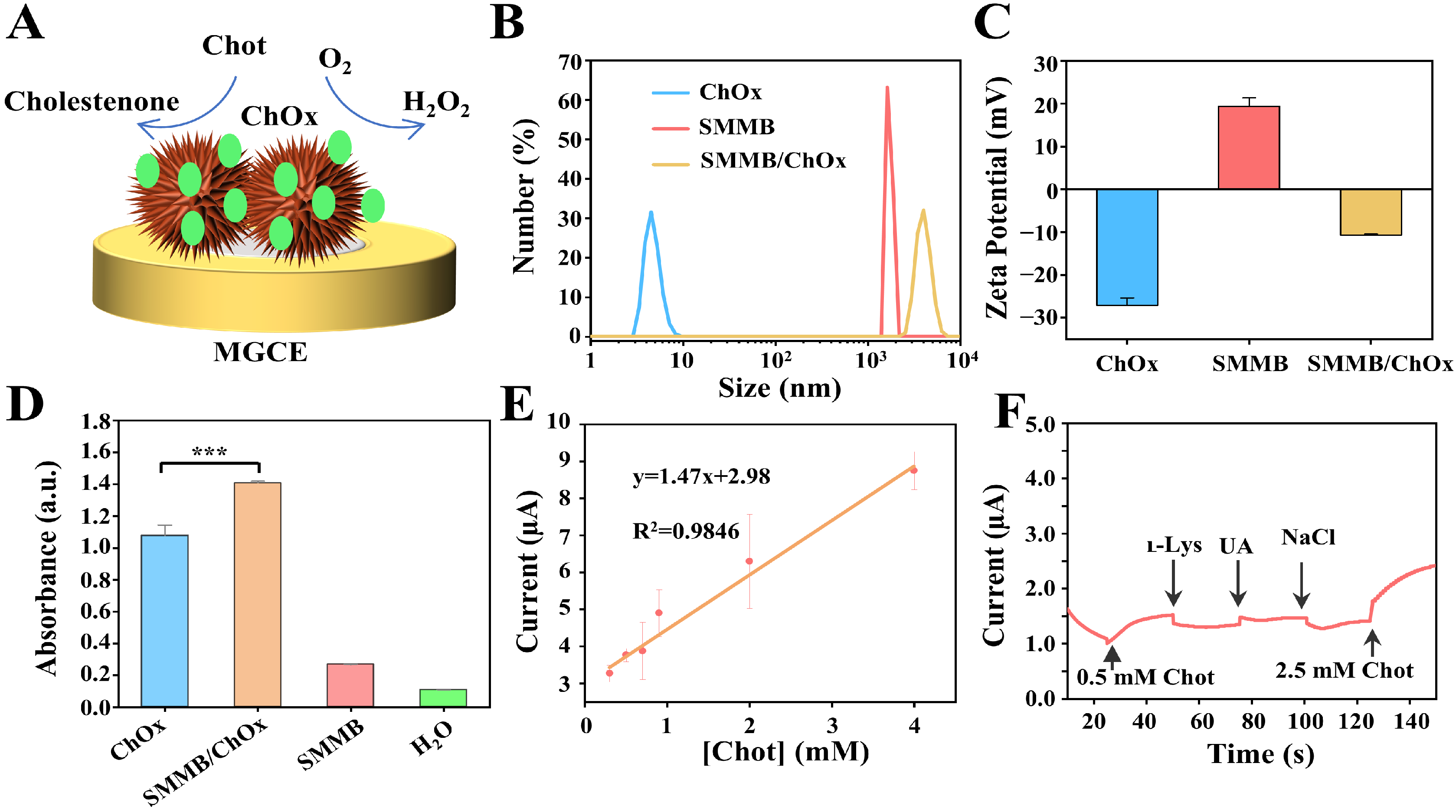
3.5. Analyzing the Performance of the SMMB-Based Electrochemical Biosensor for Cholesterol Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, D.; Xiong, Q.; Liu, W.; Liang, L.; Duan, H. Nanozymatic magnetic nanomixers for enzyme immobilization and multiplexed detection of metabolic disease biomarkers. Biosens. Bioelectron. 2023, 219, 114795. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Koklu, A.; Ohayon, D.; Wustoni, S.; Druet, V.; Saleh, A.; Inal, S. Organic bioelectronic devices for metabolite sensing. Chem. Rev. 2022, 122, 4581–4635. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, Y.; Li, S.; Yang, J.; Dong, J.; Lu, X. MXene/CNTs/Cu-MOF electrochemical probe for detecting tyrosine. Carbon 2022, 199, 110–118. [Google Scholar] [CrossRef]
- Jia, D.; Cui, M.; Ding, X. Visualizing DNA/RNA, proteins, and small molecule metabolites within live cells. Small 2024, 20, 2404482. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Wu, M.; Yu, W.; Wang, M.; Niu, M.; Meng, F.; Zhao, Y.; Osman, A.; Mousa, N.O.; et al. Plasmonic array assisted mass spectrometry for preferential metabolite detection. Chem. Eng. J. 2024, 486, 150224. [Google Scholar] [CrossRef]
- Koechlin, A.; Boyle, P. Lung cancer risk, diabetes, and diabetes treatments. J. Clin. Oncol. 2016, 34, 1577. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Krinsley, J.S.; Faber, C.L.; Hirsch, I.B.; Brownlee, M. Brain glucose sensing and the problem of relative hypoglycemia. Diabetes Care 2023, 46, 237–244. [Google Scholar] [CrossRef]
- Ceriello, A.; Prattichizzo, F.; Phillip, M.; Hirsch, I.B.; Mathieu, C.; Battelino, T. Glycaemic management in diabetes: Old and new approaches. Lancet Diabetes Endocrinol. 2022, 10, 75–84. [Google Scholar] [CrossRef]
- Grom, L.C.; Coutinho, N.M.; Guimarães, J.T.; Balthazar, C.F.; Silva, R.; Rocha, R.S.; Freitas, M.Q.; Duarte, M.C.K.H.; Pimentel, T.C.; Esmerino, E.A.; et al. Probiotic dairy foods and postprandial glycemia: A mini-review. Trends Food Sci. Technol. 2020, 101, 165–171. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, L.J.; Wang, F.; Hong, T.; Liu, Z. Molecular imaging of diabetes and diabetic complications: Beyond pancreatic β-cell targeting. Adv. Drug Deliv. Rev. 2019, 139, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Yeon, S.Y.; Seo, M.; Kim, Y.; Hong, H.; Chung, T.D. Paper-based electrochromic glucose sensor with polyaniline on indium tin oxide nanoparticle layer as the optical readout. Biosens. Bioelectron. 2022, 203, 114002. [Google Scholar] [CrossRef] [PubMed]
- Teymourian, H.; Barfidokht, A.; Wang, J. Electrochemical glucose sensors in diabetes management: An updated review (2010–2020). Chem. Soc. Rev. 2020, 49, 7671–7709. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Zou, L.; Guo, X.; Ma, X.; Zhang, C.; Zou, Z.; Zhang, Y.; Hu, F.; Lu, Z.; Tang, K.; et al. Rising mesopores to realize Direct electrochemistry of glucose oxidase toward highly sensitive detection of glucose. Adv. Funct. Mater. 2019, 29, 1903026. [Google Scholar] [CrossRef]
- Manikandan, R.; Jang, H.-G.; Kim, C.-S.; Yoon, J.-H.; Lee, J.; Paik, H.-j.; Chang, S.-C. Nano-engineered paper-based electrochemical biosensors: Versatile diagnostic tools for biomarker detection. Coord. Chem. Rev. 2025, 523, 216261. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Topolnikova, Y.V.; Soldatkin, O.O. Advances in the biosensors for lactate and pyruvate detection for medical applications: A review. Trends Anal. Chem. 2019, 110, 160–172. [Google Scholar] [CrossRef]
- Lian, K.; Feng, H.; Liu, S.; Wang, K.; Liu, Q.; Deng, L.; Wang, G.; Chen, Y.; Liu, G. Insulin quantification towards early diagnosis of prediabetes/diabetes. Biosens. Bioelectron. 2022, 203, 114029. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Peng, Z.; Yang, J.; Li, Y. A novel photoelectrochemical microfluidic chip for multi-index determination of diabetes and its complications. Biosens. Bioelectron. 2022, 217, 114719. [Google Scholar] [CrossRef]
- Shang, J.; Zhao, M.; Qu, H.; Li, H.; Chen, S. Fabrication of CQDs/MoS2/Mo foil for the improved electrochemical detection. Anal. Chim. Acta 2019, 1079, 79–85. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Wang, S.; Xie, L.; Zhao, P.; Li, L.; Ge, S.; Yu, J. Rolling circle amplification assisted CRISPR/Cas12a dual-cleavage photoelectrochemical biosensor for highly sensitive detection of miRNA-21. Anal. Chim. Acta 2024, 1287, 342125. [Google Scholar] [CrossRef]
- Daniel, M.; Mathew, G.; Anpo, M.; Neppolian, B. MOF based electrochemical sensors for the detection of physiologically relevant biomolecules: An overview. Coord. Chem. Rev. 2022, 468, 214627. [Google Scholar] [CrossRef]
- Lu, N.; Zhang, M.; Ding, L.; Zheng, J.; Zeng, C.; Wen, Y.; Liu, G.; Aldalbahi, A.; Shi, J.; Song, S.; et al. Yolk–shell nanostructured Fe3O4@C magnetic nanoparticles with enhanced peroxidase-like activity for label-free colorimetric detection of H2O2 and glucose. Nanoscale 2017, 9, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Liu, C.; Zhang, L.; Liu, T.; Wang, Z.; Song, Z.; Cai, H.; Fang, Z.; Chen, J.; Wang, J.; et al. Wearable and flexible electrochemical sensors for sweat analysis: A review. Microsyst. Nanoeng. 2023, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Jin, K.-Q.; Li, J.-D.; Sheng, K.-K.; Huang, W.-H.; Liu, Y.-L. Flexible and stretchable electrochemical sensors for biological monitoring. Adv. Mater. 2023, 2305917. [Google Scholar] [CrossRef]
- Yang, L.; Gu, X.; Liu, J.; Wu, L.; Qin, Y. Functionalized nanomaterials-based electrochemiluminescent biosensors and their application in cancer biomarkers detection. Talanta 2024, 267, 125237. [Google Scholar] [CrossRef]
- Myndrul, V.; Coy, E.; Babayevska, N.; Zahorodna, V.; Balitskyi, V.; Baginskiy, I.; Gogotsi, O.; Bechelany, M.; Giardi, M.T.; Iatsunskyi, I. MXene nanoflakes decorating ZnO tetrapods for enhanced performance of skin-attachable stretchable enzymatic electrochemical glucose sensor. Biosens. Bioelectron. 2022, 207, 114141. [Google Scholar] [CrossRef]
- Chavhan, M.P.; Khandelwal, M.; Arya, S.; Das, T.; Singh, A.; Ghodbane, O. A review of nanocomposites/hybrids made from biomass-derived carbons for electrochemical capacitors. Chem. Eng. J. 2024, 500, 157267. [Google Scholar] [CrossRef]
- Ahmad, R.; Lee, B.-I. Facile fabrication of palm trunk–like ZnO hierarchical nanostructure–based biosensor for wide-range glucose detection. Chem. Eng. J. 2024, 492, 152432. [Google Scholar] [CrossRef]
- Chavez-Urbiola, I.R.; Reséndiz-Jaramillo, A.Y.; Willars-Rodriguez, F.J.; Martinez-Saucedo, G.; Arriaga, L.G.; Alcantar-Peña, J.; Escalona-Villalpando, R.A.; Ledesma-García, J. Glucose biosensor based on a flexible Au/ZnO film to enhance the glucose oxidase catalytic response. J. Electroanal. Chem. 2022, 926, 116941. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X. Nanotechnology and nanomaterial-based no-wash electrochemical biosensors: From design to application. Nanoscale 2019, 11, 19105–19118. [Google Scholar] [CrossRef]
- Lu, P.; Zhan, C.; Huang, C.; Miao, L.; Chen, R.; Zhao, Y.; Xianyu, Y.; Chen, X.; Chen, Y. A wash-free spheres-on-sphere strategy for on-site and multiplexed biosensing. ACS Nano 2024, 18, 8270–8282. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Han, F.; Zhou, H.; Wang, H.; Zhao, J.; Zhao, G.; Zhang, Y.; Zhang, N.; Wang, Y.; Luan, M.; et al. Construction of electrochemical immunosensors based on Au@MXene and Au@CuS nanocomposites for sensitive detection of carcinoembryonic antigen. Talanta 2025, 283, 127147. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; An, J.; Zhang, M.; Zhang, Q.; Si, Y.; Zhang, Y.; Chen, C.; Zhang, D.; Fang, Y. Nanomaterial-based electrochemical biosensors as tools for detecting the tumor biomarker miR-21. Talanta 2025, 283, 127183. [Google Scholar] [CrossRef]
- Maafa, I.M. Potential of zinc oxide nanostructures in biosensor application. Biosensors 2025, 15, 61. [Google Scholar] [CrossRef]
- Keles, G.; Sifa Ataman, E.; Taskin, S.B.; Polatoglu, İ.; Kurbanoglu, S. Nanostructured metal oxide-based electrochemical biosensors in medical diagnosis. Biosensors 2024, 14, 238. [Google Scholar] [CrossRef]
- Mei, H.; Liu, X.; Song, Y.; Teng, B.; Zhong, D.; Zhang, J. Piezoelectric ZnO nanoarrays for catalytic detection of H2O2 with ultra sensitivity. Appl. Phys. Lett. 2023, 122, 223701. [Google Scholar] [CrossRef]
- Feng, A.; Hu, Y.; Yang, X.; Lin, H.; Wang, Q.; Xu, J.; Liu, A.; Wu, G.; Li, Q. ZnO nanowire arrays decorated with Cu nanoparticles for high-efficiency nitrate to ammonia conversion. ACS Catal. 2024, 14, 5911–5923. [Google Scholar] [CrossRef]
- Heydari, M.; Ghoreishi, S.M.; Khoobi, A. Chemometrics-assisted determination of Sudan dyes using zinc oxide nanoparticle-based electrochemical sensor. Food Chem. 2019, 283, 68–72. [Google Scholar] [CrossRef]
- Xu, H.; Hang, Y.; Wu, Z.; Lei, X.; Deng, J.; Yang, J. Capillary-driven microchip integrated with nickel phosphide hybrid-modified electrode for the electrochemical detection of glucose. Anal. Chim. Acta 2024, 1316, 342882. [Google Scholar] [CrossRef]
- Vellanki, P. Glucose and the brain: A new target in diabetes. Sci. Transl. Med. 2020, 12, eabb5677. [Google Scholar] [CrossRef]
- Pan, Y.; Zuo, J.; Hou, Z.; Huang, Y.; Huang, C. Preparation of electrochemical sensor based on zinc oxide nanoparticles for simultaneous determination of AA, DA, and UA. Front. Chem. 2020, 8, 592538. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Jiao, L.; Wu, N.; Xu, W.; Wu, Z.; Wu, Y.; Hu, P.; Gu, W.; Zhu, C. Defect engineering of PdMo metallene for sensitive electrochemical detection of dopamine. Chem. Eng. J. 2023, 466, 143075. [Google Scholar] [CrossRef]
- Patil, Y.N.; Nandibewoor, S.T. Development of novel Ce doped ZnO/graphene based sensor for electrochemical investigation of potassium-competitive acid blocker: Vonoprazan. Mater. Sci. Semicond. Process. 2024, 171, 108039. [Google Scholar] [CrossRef]
- Savari, R.; Fakhar, O.; Rouhi, J. An electrochemical sensor based on ZnO–CuO nanocomposites for vancomycin detection in food samples. Ceram. Int. 2025; in press. [Google Scholar] [CrossRef]
- Duan, C.; Chen, G.; Wang, Z.; Li, H.; Zhang, Z.; Liu, Y.; Lu, M. An ultra-sensitive electrochemical sensing platform based on nanoflower-like Au/ZnO array on carbon cloth for the rapid detection of the nitrite residues in food samples. Food Chem. 2024, 437, 137892. [Google Scholar] [CrossRef]
- Ji, H.; Zhou, F.; Gu, J.; Shu, C.; Xi, K.; Jia, X. Nitrogen-doped carbon dots as a new substrate for sensitive glucose determination. Sensors 2016, 16, 630. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Deng, J.; Liu, H.; Ma, Y.; Li, Z.; Zhang, T. Flexible electrochemical glucose sensor based on PtNPs modified MXene/SF membrane. IEEE Sens. J. 2023, 1. [Google Scholar] [CrossRef]
- Chen, D.; Wang, X.; Zhang, K.; Cao, Y.; Tu, J.; Xiao, D.; Wu, Q. Glucose photoelectrochemical enzyme sensor based on competitive reaction of ascorbic acid. Biosens. Bioelectron. 2020, 166, 112466. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, H.; Wang, Y.; Laurindo, M.B.J.; Zhao, J.; Hasebe, Y.; Zhang, Z. Crab gill–derived nanorod-like carbons as bifunctional electrochemical sensors for detection of hydrogen peroxide and glucose. Ionics 2024, 30, 3541–3552. [Google Scholar] [CrossRef]
- Xu, J.; Xu, K.; Han, Y.; Wang, D.; Li, X.; Hu, T.; Yi, H.; Ni, Z. A 3D porous graphene aerogel@GOx based microfluidic biosensor for electrochemical glucose detection. Analyst 2020, 145, 5141–5147. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Yuan, X.; Tian, E.; Tan, Y.; Li, L.; Huang, R. Sea Urchin-like Magnetic Microbeads-Based Electrochemical Biosensor for Highly Sensitive Detection of Metabolites. Biosensors 2025, 15, 225. https://doi.org/10.3390/bios15040225
Chen B, Yuan X, Tian E, Tan Y, Li L, Huang R. Sea Urchin-like Magnetic Microbeads-Based Electrochemical Biosensor for Highly Sensitive Detection of Metabolites. Biosensors. 2025; 15(4):225. https://doi.org/10.3390/bios15040225
Chicago/Turabian StyleChen, Bin, Xiaosu Yuan, Enze Tian, Yunjie Tan, Le Li, and Ru Huang. 2025. "Sea Urchin-like Magnetic Microbeads-Based Electrochemical Biosensor for Highly Sensitive Detection of Metabolites" Biosensors 15, no. 4: 225. https://doi.org/10.3390/bios15040225
APA StyleChen, B., Yuan, X., Tian, E., Tan, Y., Li, L., & Huang, R. (2025). Sea Urchin-like Magnetic Microbeads-Based Electrochemical Biosensor for Highly Sensitive Detection of Metabolites. Biosensors, 15(4), 225. https://doi.org/10.3390/bios15040225



