A Review of Readout Circuit Schemes Using Silicon Nanowire Ion-Sensitive Field-Effect Transistors for pH-Sensing Applications
Abstract
1. Introduction
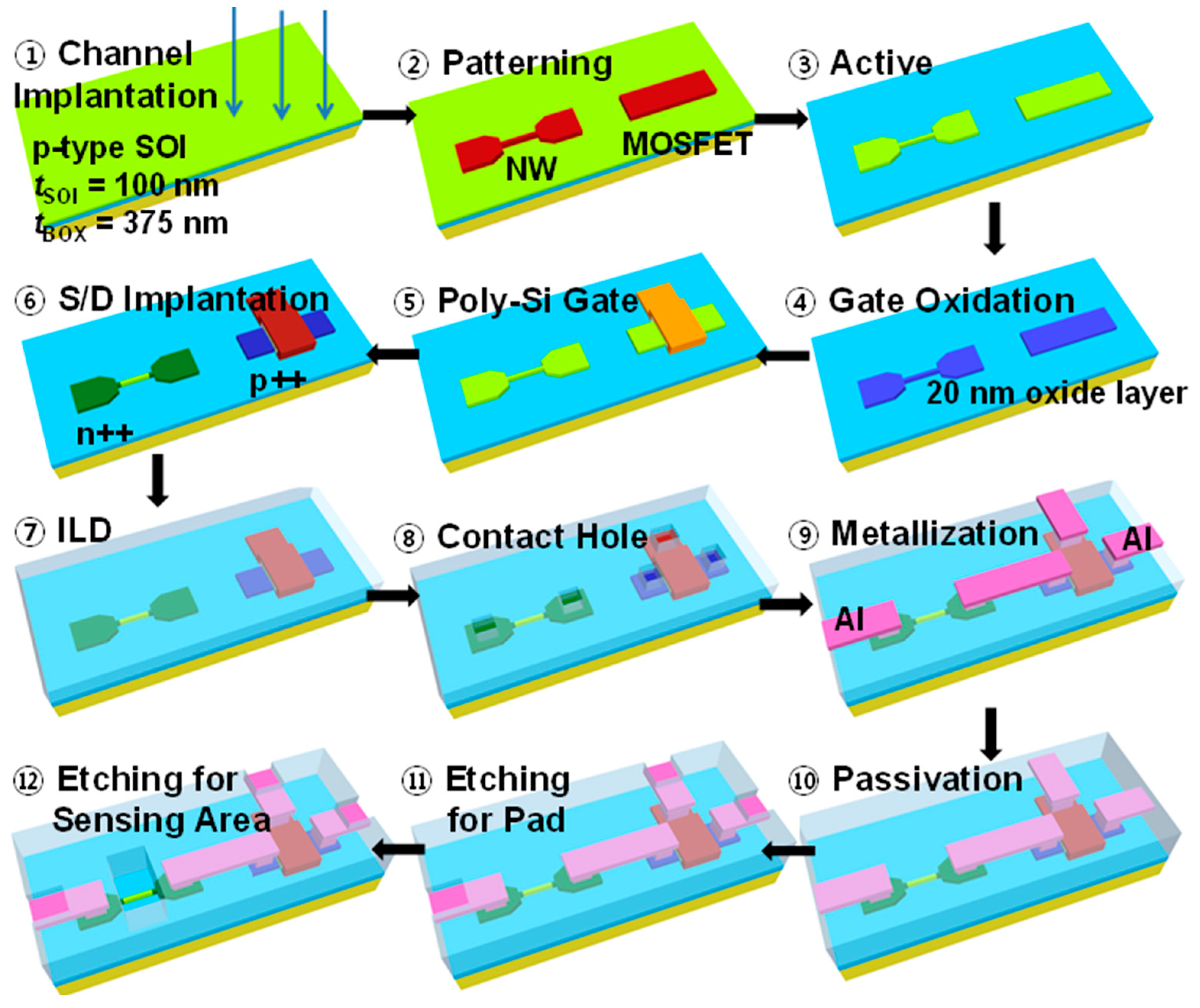
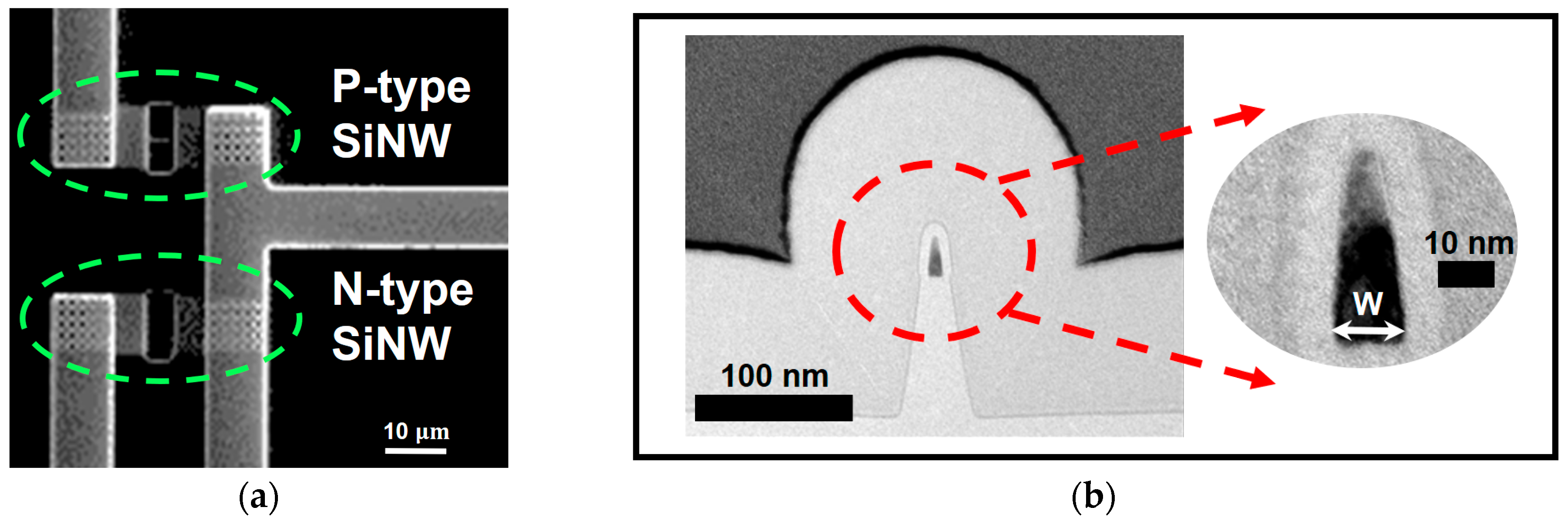
2. Fabrication Process of a SiNW ISFET
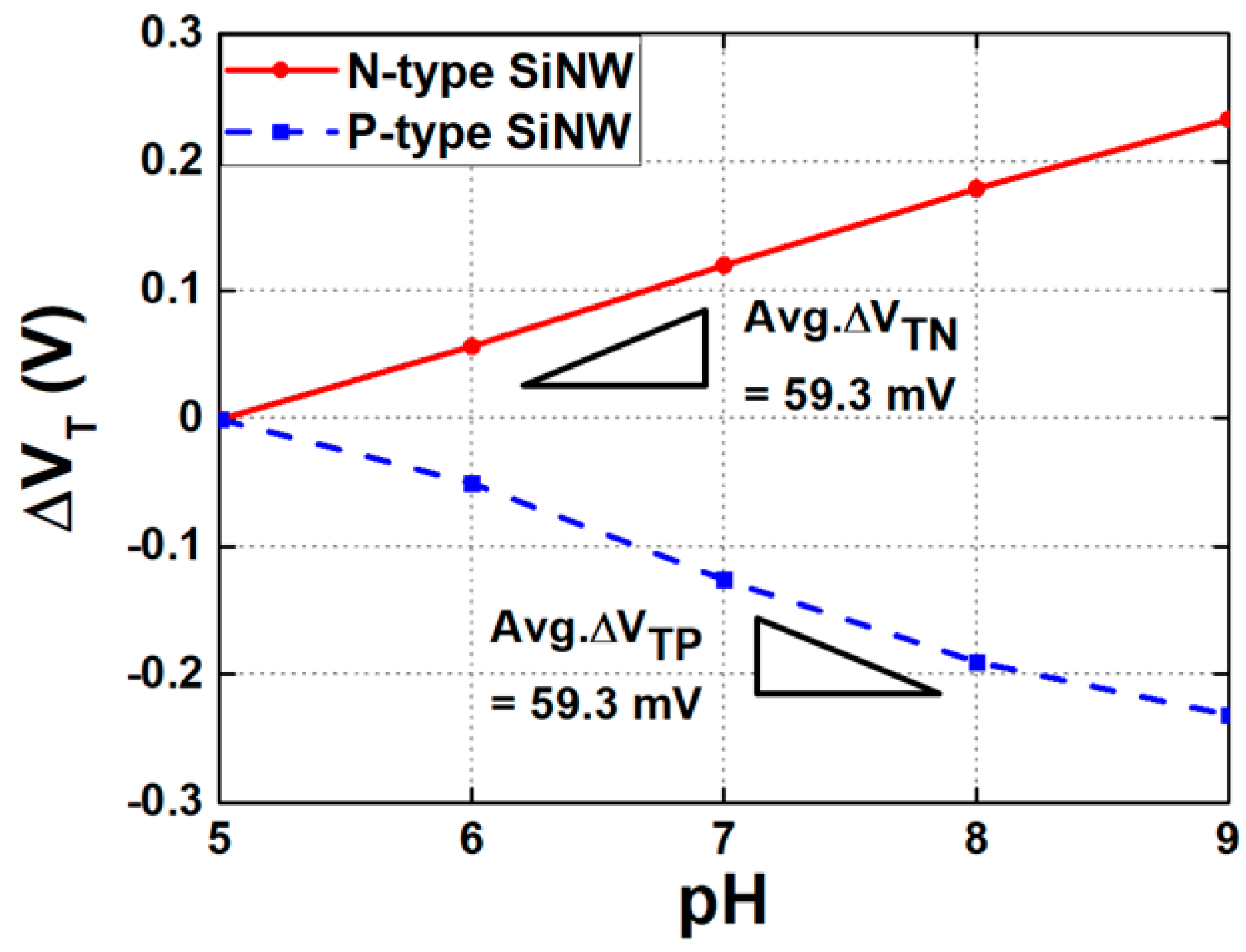
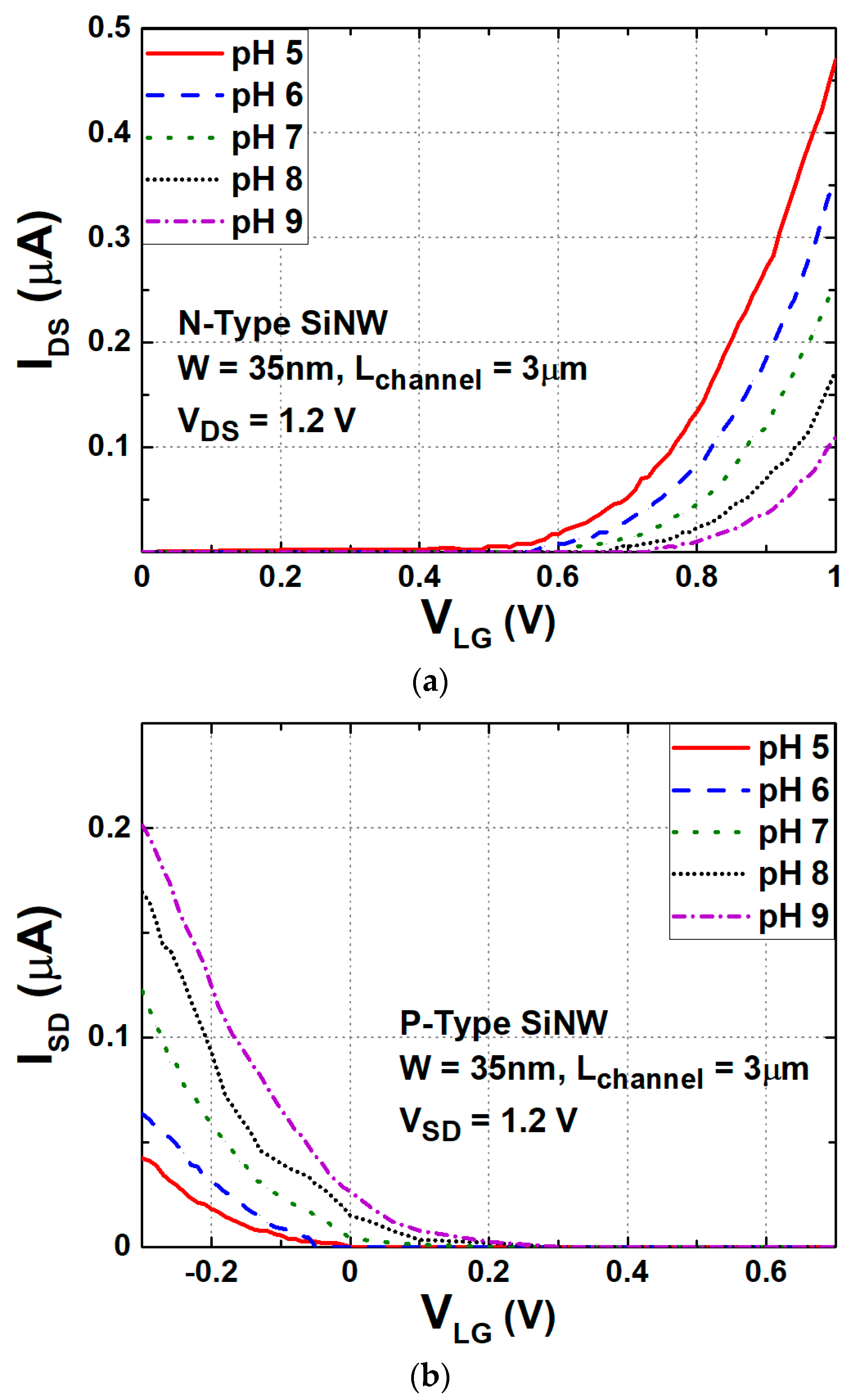
3. SiNW ISFET as a Hydrogen Ion Sensor
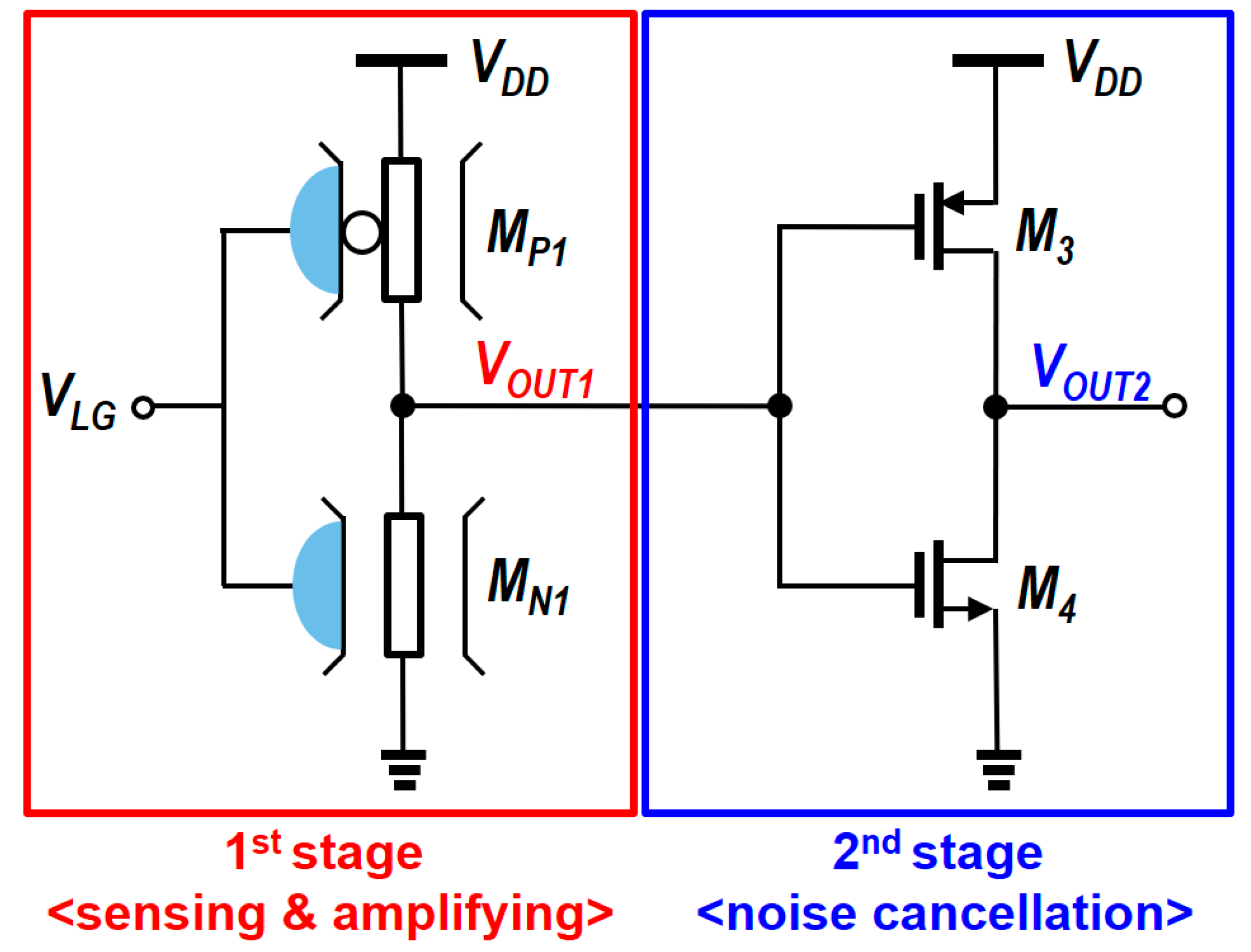
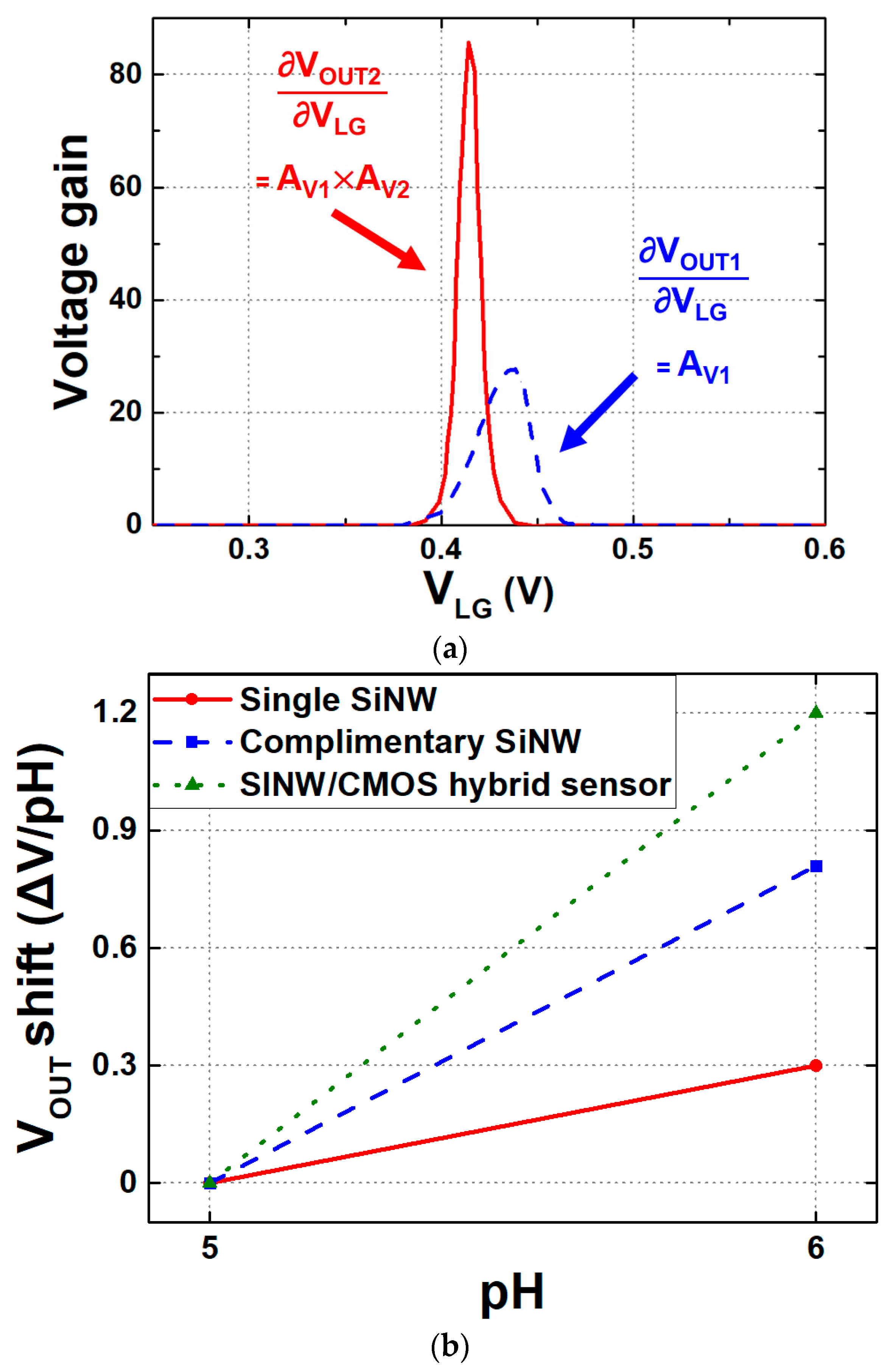
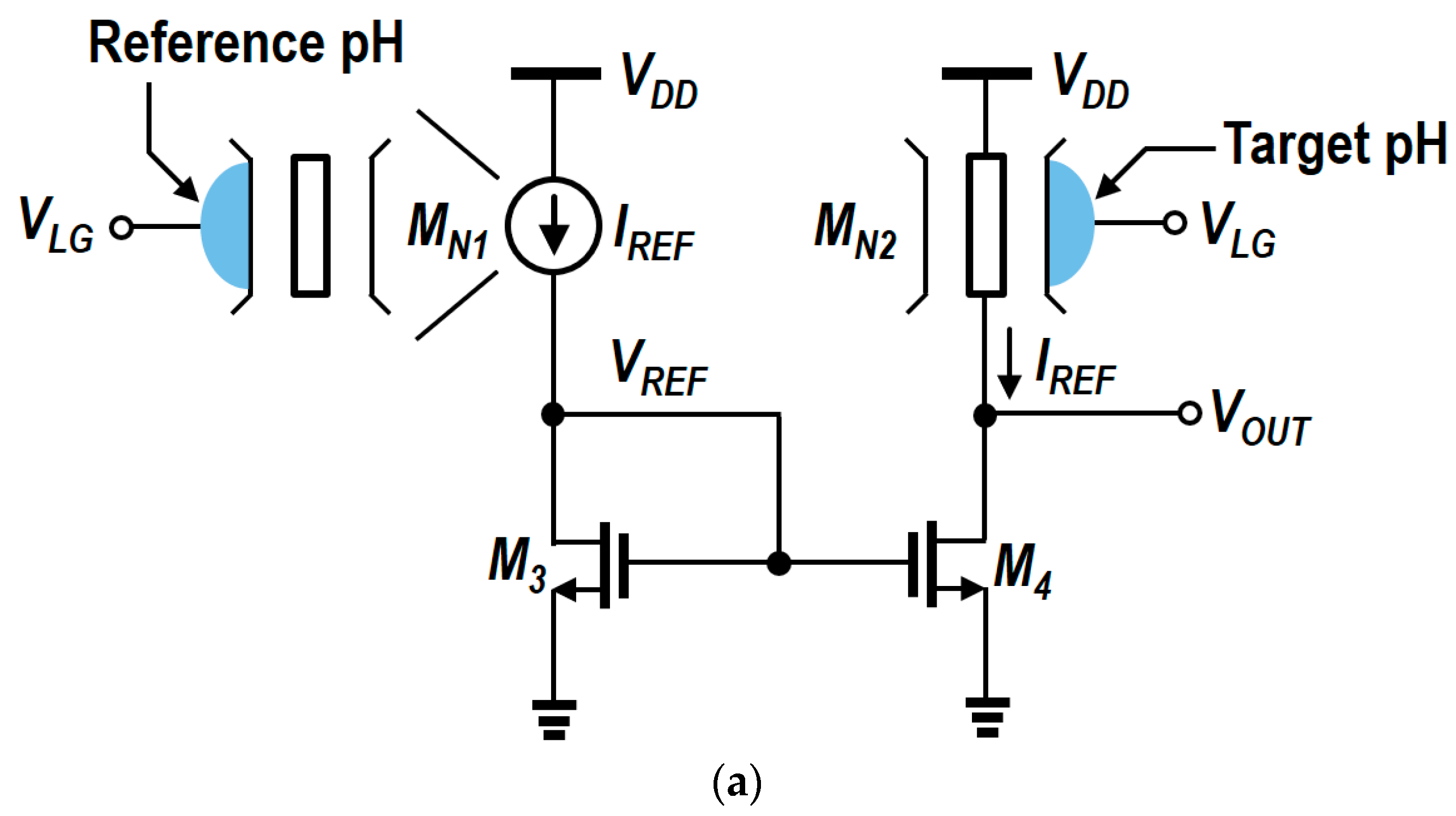
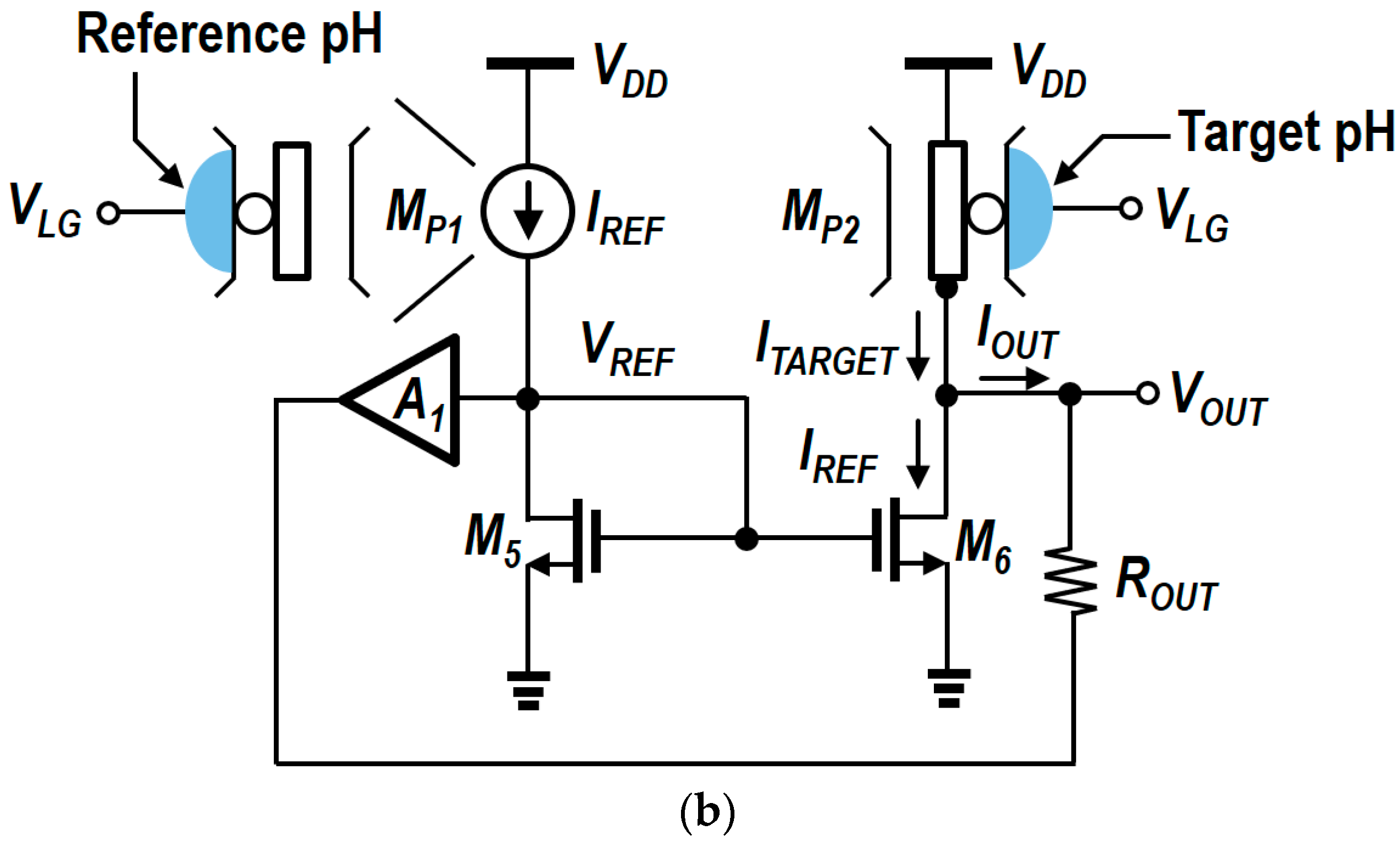
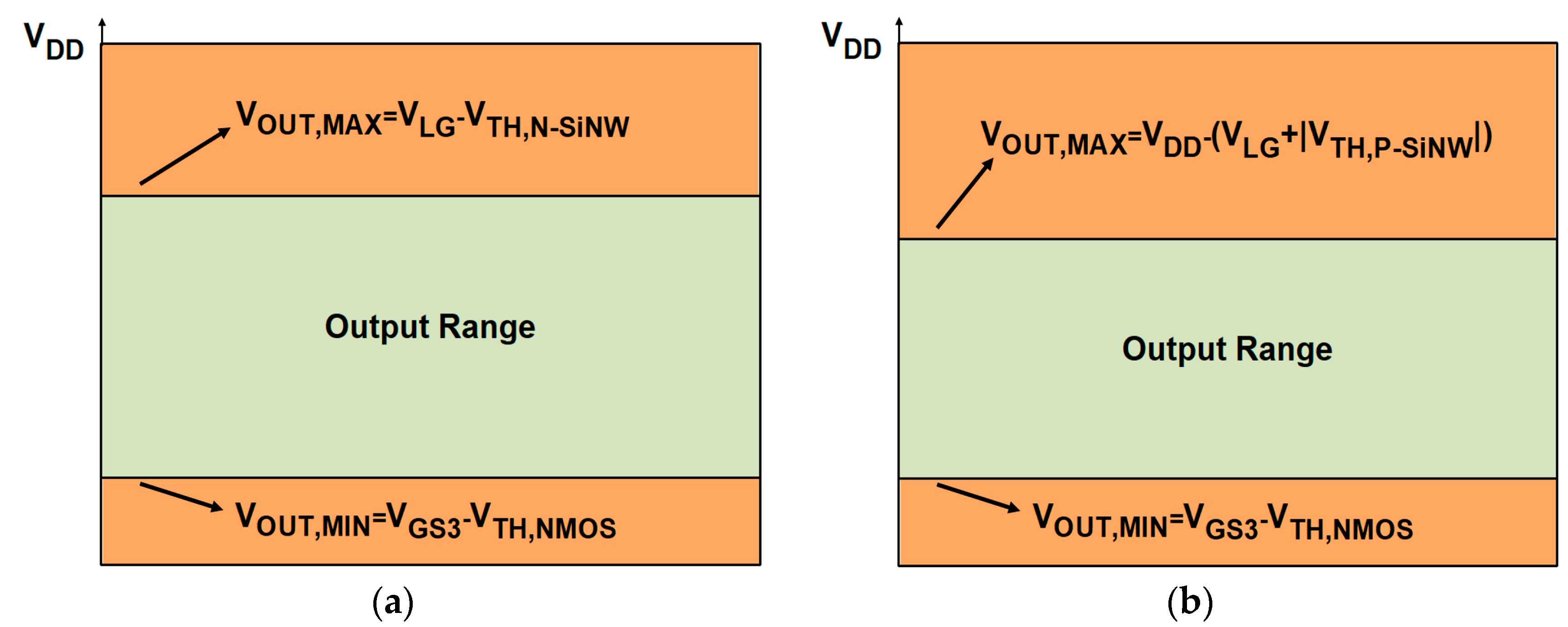
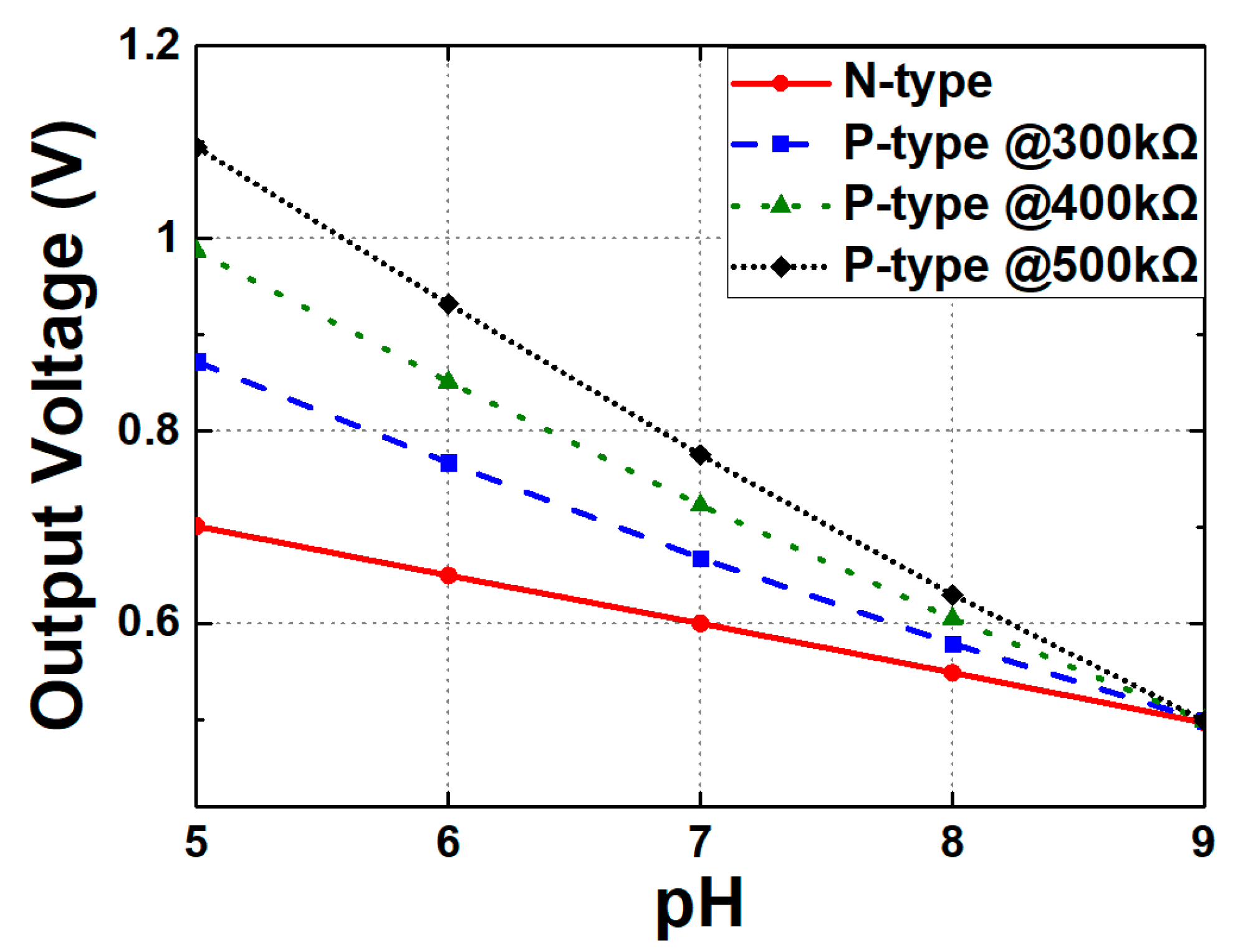
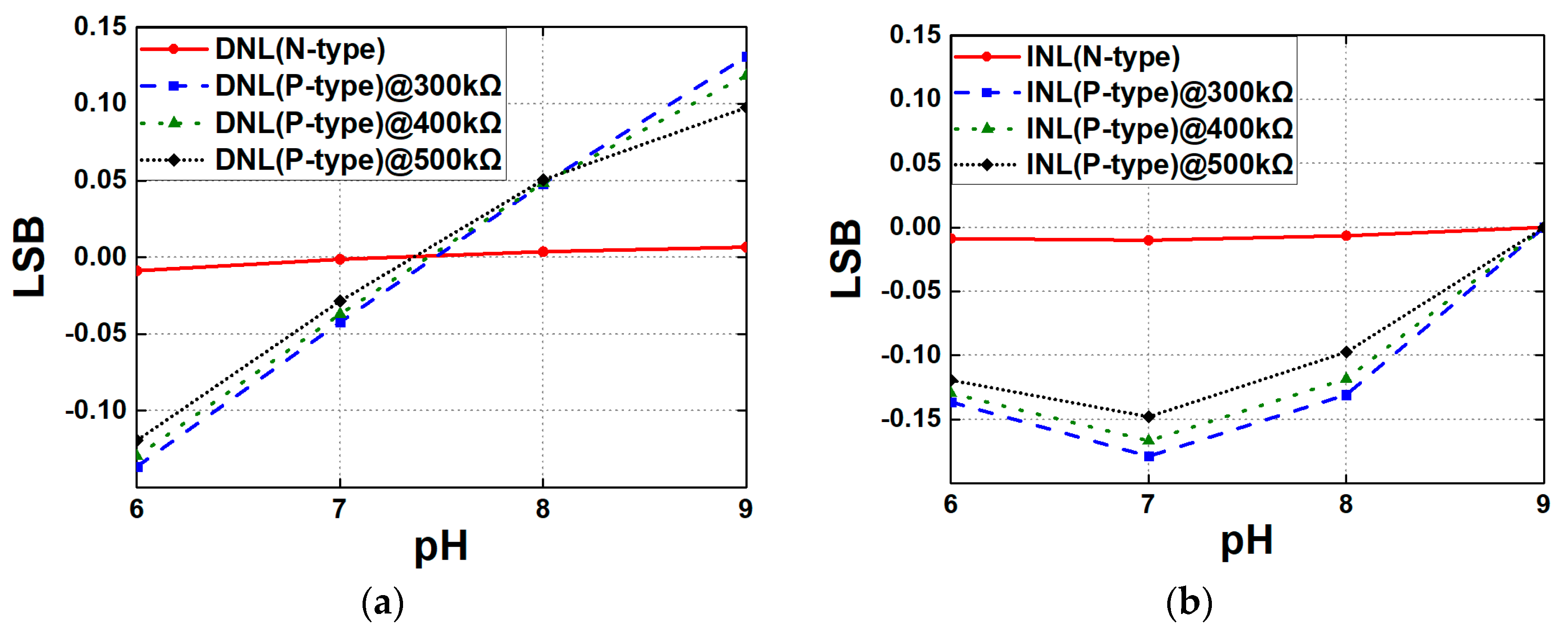
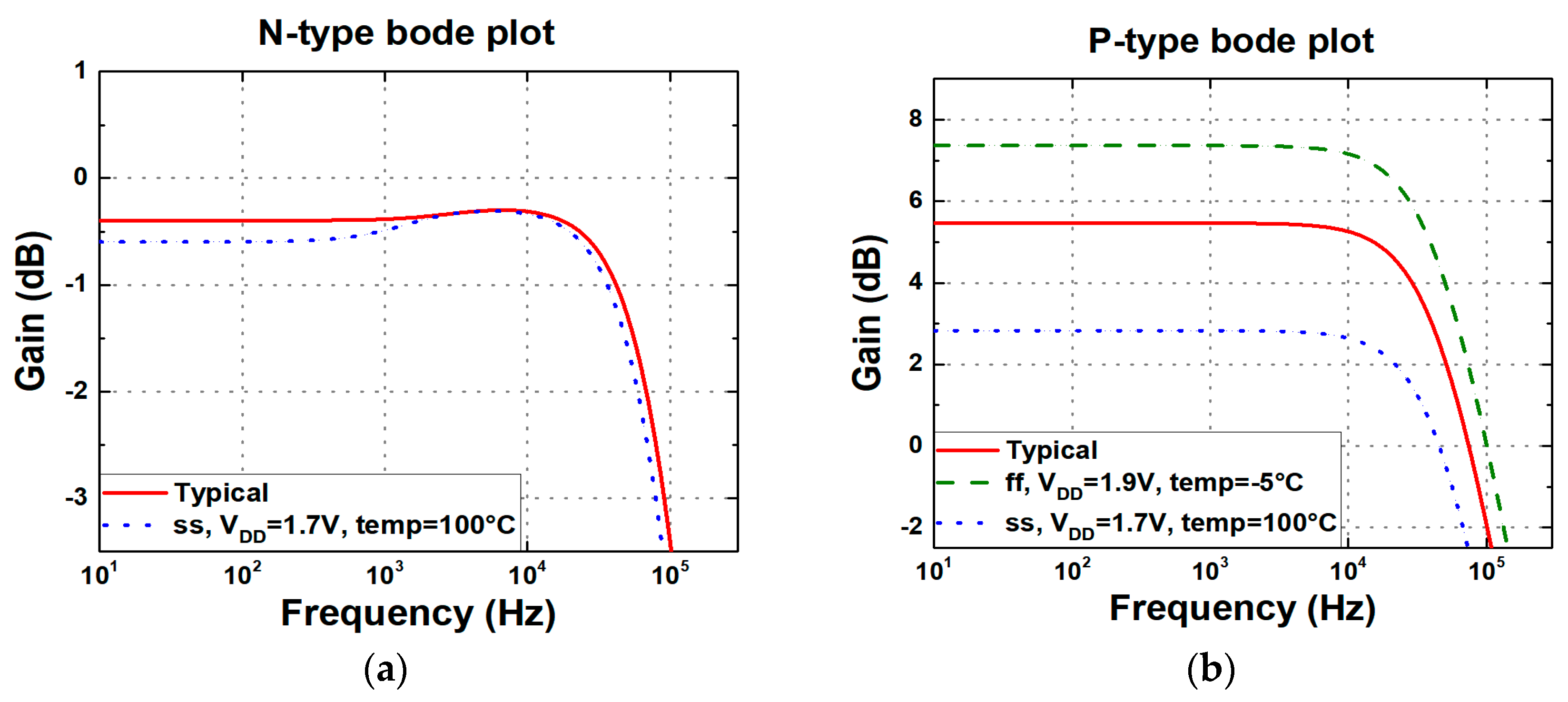
4. Circuit Design and Analysis of Readout Circuits
5. Modified N-Type Readout Scheme
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
Appendix B

References
- Shinwari, M.W.; Deen, M.J.; Landheer, D. Study of the electrolyte-insulator-semiconductor field-effect transistor (EISFET) with applications in biosensor design. Microelectron. Reliab. 2007, 47, 2025–2057. [Google Scholar]
- Suhito, I.R.; Koo, K.-M.; Kim, T.-H. Recent Advances in Electrochemical Sensors for the Detection of Biomolecules and Whole Cells. Biomedicines 2021, 9, 15. [Google Scholar]
- Tsai, H.-C.; Doong, R. A Simultaneous determination of pH, urea, acetylcholine and heavy metals using array-based enzymatic optical biosensor. Biosens. Bioelectron. 2005, 20, 1796–1804. [Google Scholar]
- Kumar, S.; Singh, R. Recent optical sensing technologies for the detection of various biomolecules: Review. Opt. Laser. Technol. 2021, 134, 106620. [Google Scholar]
- Nelson, R.W.; Nedelkov, D.; Tubbs, K.A. Biosensor chip mass spectrometry: A chip-based proteomics approach. Electrophoresis 2000, 21, 1155–1163. [Google Scholar] [CrossRef]
- Breuker, K.; Jin, M.; Han, X.; Jiang, H.; McLafferty, F.W. Top-Down Identification and Characterization of Biomolecules by Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2008, 19, 1045–1053.6. [Google Scholar]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef]
- Stern, E.; Klemic, J.F.; Routenberg, D.A.; Wyrembak, P.N.; Turner-Evans, D.B.; Hamilton, A.D.; LaVan, D.A.; Fahmy, T.M.; Reed, M.A. Label-free immunodetection with CMOS-compatible semiconducting nanowires. Nature 2007, 445, 519–522. [Google Scholar]
- Sharma, A.; AlGhamdi, W.S.; Faber, H.; Lin, Y.-H.; Liu, C.-H.; Hsu, E.-K.; Lin, W.-Z.; Naphade, D.; Mandal, S.; Heeney, M.; et al. Non-invasive, ultrasensitive detection of glucose in saliva using metal oxide transistors. Biosens. Bioelectron. 2023, 237, 115448. [Google Scholar]
- Ishikawa, F.N.; Chang, H.-K.; Curreli, M.; Liao, H.-I.; Olson, C.A.; Chen, P.-C.; Zhang, R.; Roberts, R.W.; Sun, R.; Cote, R.J.; et al. Label-free, electrical detection of the SARS virus N-protein with nanowire biosensors utilizing antibody mimics as capture probes. ACS Nano 2009, 3, 1219–1224. [Google Scholar]
- Liu, A. Towards development of chemosensors and biosensors with metaloxide-based nanowires or nanotubes. Biosens. Bioelectron. 2008, 24, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, J.; Yoon, J.; Chae, I.; Choi, S.-J.; Kim, D.M.; Mo, H.S.; Kim, D.H. The Module of Gain-controllable Amplifier Readout Circuits based on Si Nanowire ISFET for Biochips for Optimization of Dynamic Range, Linearity, and Resolution. In Proceedings of the ESSDERC 2019-49th European Solid-State Device Research Conference (ESSDERC), Cracow, Poland, 23–26 September 2019. [Google Scholar]
- Lei, K.-M.; Mak, P.-I.; Law, M.-K.; Martins, R.P. CMOS biosensors for in vitro diagnosis—Transducing mechanisms and applications. Lab. Chip 2016, 16, 3664–3681. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, D.; Chen, H.; Yue, X.; Fan, K.; Dong, L.; Wang, G. Application of Silicon Nanowire Field Effect Transistor (SiNW-FET) Biosensor with High Sensitivity. Sensors 2023, 23, 6808. [Google Scholar] [CrossRef] [PubMed]
- Numnuam, A.; Thavarungkul, P.; Kanatharana, P. An amperometric uric acid biosensor based on chitosan-carbon nanotubes electrospun nanofiber on silver nanoparticles. Anal. Bioanal. Chem. 2014, 406, 3763–3772. [Google Scholar] [CrossRef] [PubMed]
- Thriveni, G.; Ghosh, K. Advancement and Challenges of Biosensing Using Field Effect Transistors. Biosensors 2022, 12, 647. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Li, L.; Mu, X.; Genov, R.; Mason, A.J. CMOS Electrochemical Instrumentation for Biosensor Microsystems: A Review. Sensors 2017, 17, 74. [Google Scholar] [CrossRef]
- Samuel, V.R.; Rao, K.J. A review on label free biosensors. Biosens. Bioelectron. X 2022, 11, 100216. [Google Scholar] [CrossRef]
- Nair, P.R.; Alam, M.A. Performance limits of nanobiosensors. Appl. Phys. Lett. 2006, 88, 233120. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Y.; Li, X.; Kamins, T.I.; Nauka, K.; Williams, R.S. Sequence-specific label-free DNA sensors based on Silicon Nanowires. Nano Lett. 2004, 4, 245–247. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Zhang, L.; Huang, M.J.; Luo, Z.H.H.; Tay, G.K.I.; Lim, E.-J.A.; Kang, T.G.; Chen, Y. Silicon nanowire biosensor for highly sensitive and rapid detection of Dengue virus. Sens. Actuators B Chem. 2010, 146, 138–144. [Google Scholar] [CrossRef]
- Presnova, G.; Presnov, D.; Krupenin, V.; Grigorenko, V.; Trifonov, A.; Andreeva, I.; Ignateko, O.; Egorov, A.; Rubtsova, M. Biosensor based on a silicon nanowire field-effect transistor functionalized by gold nanoparticles for the highly sensitive determination of prostate specific antigen. Biosens. Bioelectron. 2017, 88, 283–289. [Google Scholar] [PubMed]
- Curreli, M.; Zhang, R.; Ishikawa, F.N.; Chang, H.-K.; Cote, R.J.; Zhou, C.; Thompson, M.E. Real-time, label-free detection of biological entities using nanowire-based FET. IEEE Trans. Nanotechnol. 2008, 7, 651–667. [Google Scholar]
- Chen, W.; Yao, H.; Tzang, C.H.; Zhu, J.; Yang, M.; Lee, S.-T. Silicon nanowires for high-sensitivity glucose detection. Appl. Phys. Lett. 2006, 88, 213104. [Google Scholar] [CrossRef]
- Gao, Z.; Agarwal, A.; Trigg, A.D.; Singh, N.; Fang, C.; Tung, C.-H.; Fan, Y.; Buddharaju, K.D.; Kong, J. Silicon Nanowire Arrays for Label-Free Detection of DNA. Anal. Chem. 2007, 79, 3291–3297. [Google Scholar] [CrossRef]
- Jayakumar, G.; Asadollahi, A.; Hellstrӧm, P.-E.; Garidis, K.; Östling, M. Silicon nanowires integrated with CMOS circuits for biosensing application. Solid States Electron. 2014, 98, 26–31. [Google Scholar]
- Schwierz, F. Graphene Transistors: Status, Prospects, and Problems. Proc. IEEE 2013, 101, 1567–1584. [Google Scholar]
- Zhan, B.; Li, C.; Yang, J.; Jenkins, G.; Huang, W.; Dong, X. Graphene Field-Effect Transistor and Its Application for Electronic Sensing. Small 2014, 10, 4042–4065. [Google Scholar]
- Wang, J. Carbonnanotube based electrochemical biosensors: A review. Electroanalysis 2005, 17, 7–14. [Google Scholar]
- Sotiropoulou, S.; Chaniotakis, N.A. Carbon nanotube array-based biosensor. Anal. Bioanal. Chem. 2003, 375, 103–105. [Google Scholar] [CrossRef]
- Maehashi, K.; Katsura, T.; Kerman, K.; Takamura, Y.; Matsumoto, K.; Tamiya, E. Label-free protein biosensor based on aptamer-modified carbon nanotube fieldeffecttransistors. Anal. Chem. 2007, 79, 782–787. [Google Scholar]
- Lin, Y.; Lu, F.; Tu, Y.; Ren, Z. Glucose biosensors based on carbon nanotube nanoelectrode ensembles. Nano Lett. 2004, 4, 191–195. [Google Scholar]
- Liu, X.; Lin, P.; Yan, X.; Kang, Z.; Zhao, Y.; Lei, Y.; Li, C.; Du, H.; Zhang, Y. nzyme-coated single ZnO nanowire FET biosensor for detection of uric acid. Sens. Actuators B Chem. 2013, 176, 22–27. [Google Scholar]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-Based Materials for Biosensors: A Review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef] [PubMed]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, BME-17, 70–71. [Google Scholar]
- Zou, J.; Bai, H.; Zhang, L.; Shen, Y.; Yang, C.; Zhuang, W.; Hu, J.; Yao, Y.; Hu, W. Ion-sensitive field effect transistor biosensors for biomarker detection: Current progress and challenges. J. Mater. Chem. B 2024, 12, 8523–8542. [Google Scholar] [PubMed]
- Tabata, M.; Miyahara, Y. Control of interface functions in solid-state biosensors for stable detection of molecular recognition. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2024, 100, 32–56. [Google Scholar]
- Tans, S.J.; Verschueren, A.R.M.; Dekker, C. Room-temperature transistor based on a single carbon nanotube. Nature 1998, 393, 49–52. [Google Scholar]
- Collins, P.G.; Arnold, M.S.; Avouris, P. Engineering Carbon Nanotubes and Nanotube Circuits Using Electrical Breakdown. Science 2001, 292, 706–709. [Google Scholar]
- Cui, Y.; Duan, X.; Hu, J.; Lieber, C.M. Doping and Electrical Transport in Silicon Nanowires. J. Phys. Chem. B 2000, 104, 22. [Google Scholar]
- Cui, Y.; Lieber, C.M. Functional Nanoscale Electronic Devices Assembled Using Silicon Nanowire Building Blocks. Science 2001, 291, 851–853. [Google Scholar]
- Duan, X.; Huang, Y.; Cui, Y.; Wang, J.; Lieber, C.M. Indium phosphide nanowires as building blocks for nanoscale electronic and optoelectronic devices. Nature 2001, 409, 66–69. [Google Scholar] [PubMed]
- Mu, L.; Chang, Y.; Sawtelle, S.D.; Wipf, M.; Duan, X.; Reed, M.A. Silicon Nanowire Field-Effect Transistors—A Versatile Class of Potentiometric Nanobiosensors. IEEE Access 2015, 3, 287–302. [Google Scholar]
- Gooding, J.J. Nanoscale Biosensors: Significant Advantages over Larger Devices? Small 2006, 2, 313–315. [Google Scholar]
- Huang, Y.; Duan, X.; Wei, Q.; Lieber, C.M. Directed Assembly of One-Dimensional Nanostructures into Functional Networks. Science 2001, 291, 630–633. [Google Scholar]
- Yang, X.; Frensley, W.R.; Zhou, D.; Hu, W. Performance analysis of Si nanowire biosensor by numerical modeling for charge sensing. IEEE Trans. Nanotechnol. 2012, 11, 501–512. [Google Scholar]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar]
- Gao, A.; Lu, N.; Dai, P.; Li, T.; Pei, H.; Gao, X.; Gong, Y.; Wang, Y.; Fan, C. Silicon-nanowire-based CMOS-compatible field-effect transistor nanosensors for ultrasensitive electrical detection of nucleic acids. Nano Lett. 2011, 11, 3974–3978. [Google Scholar] [PubMed]
- Dorvel, B.R.; Reddy, B.; Go, J.; Duarte Guevara, C.; Salm, E.; Alam, M.A.; Bashir, R. Silicon Nanowires with High-k Hafnium Oxide Dielectrics for Sensitive Detection of Small Nucleic Acid Oligomers. ACS Nano 2012, 6, 6150–6164. [Google Scholar]
- Panteli, C.; Georgiou, P.; Fobelets, K. Performance improvement of commercial ISFET sensors using reactive ion etching. Microelectron. Eng. 2018, 192, 61–65. [Google Scholar]
- Gao, X.P.A.; Zheng, G.; Lieber, C.M. Subthreshold regime has the optimal sensitivity for nanowire FET biosensors. Nano Lett. 2010, 10, 547–552. [Google Scholar]
- Chen, Y.; Wang, X.; Erramilli, S.; Mohanty, P.; Kalinowski, A. Siliconbased nanoelectronic field-effect pH sensor with local gate control. Appl. Phys. Lett. 2006, 89, 223512. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Kim, J.-Y.; Seol, M.-L.; Baek, D.J.; Guo, Z.; Kim, C.-H.; Choi, S.-J.; Choi, Y.-K. A pH sensor with a double-gate silicon nanowire field-effect transistor. Appl. Phys. Lett. 2013, 102, 083701. [Google Scholar] [CrossRef]
- Hahm, J.-I.; Lieber, C.M. Direct ultrasensitive electrical detection of DNA and DNA sequence variations using nanowire nanosensors. Nano Lett. 2004, 4, 51–54. [Google Scholar]
- Li, Z.; Rajendran, B.; Kamins, T.I.; Li, X.; Chen, Y.; Williams, R.S. Silicon nanowires for sequence-specific DNA sensing: Device fabrication and simulation. Appl. Phys. A 2005, 80, 1257–1263. [Google Scholar]
- Bunimovich, Y.L.; Shin, Y.S.; Yeo, W.-S.; Amori, M.; Kwong, G.; Heath, J.R. Quantitative real-time measurements of DNA hybridization with alkylated nonoxidized silicon nanowires in electrolyte solution. J. Am. Chem. Soc. 2006, 128, 16323–16331. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Zhang, G.; Chua, J.H.; Chee, R.-E.; Wong, E.H.; Agarwal, A.; Buddharaju, K.D.; Singh, N.; Gao, Z.Q.; Balasubramanian, N. DNA sensing by silicon nanowire: Charge layer distance dependence. Nano Lett. 2008, 8, 1066–1070. [Google Scholar] [CrossRef]
- Cattani-Scholz, A.; Pedone, D.; Dubey, M.; Neppl, S.; Nickel, B.; Feulner, P.; Schwartz, J.; Abstreiter, G.; Tornow, M. Organophosphonate-based PNA-functionalization of silicon nanowires for label-free DNA detection. ACS Nano 2008, 2, 1653–1660. [Google Scholar]
- Zhang, G.-J.; Chua, J.H.; Chee, R.-E.; Agarwal, A.; Wong, S.M.; Buddharaju, K.D.; Balasubramanian, N. Highly sensitive measurements of PNA-DNA hybridization using oxide-etched silicon nanowire biosensors. Biosens. Bioelectron. 2008, 23, 1701–1707. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Chua, J.H.; Chee, R.-E.; Agarwal, A.; Wong, S.M. Label-free detection of MiRNAs with silicon nanowire biosensors. Biosens. Bioelectron. 2009, 24, 2504–2508. [Google Scholar]
- Nair, P.R.; Alam, M.A. Design considerations of silicon nanowire biosensors. IEEE Trans. Electron Devices 2007, 54, 3400–3408. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [PubMed]
- Kim, A.; Ah, C.S.; Yu, H.Y.; Yang, J.H.; Baek, I.-B.; Ahn, C.-G.; Park, C.W.; Jun, M.S.; Lee, S. Ultrasensitive, label-free, and real-time immunodetection using silicon field-effect transistors. Appl. Phys. Lett. 2007, 91, 103901. [Google Scholar]
- Chua, J.; Chee, R.-E.; Agarwal, A.; Wong, S.M.; Zhang, G.-J. Label-free electrical detection of cardiac biomarker with complementary metal-oxide semiconductor-compatible silicon nanowire sensor arrays. Anal. Chem. 2009, 81, 6266–6271. [Google Scholar]
- Tian, R.; Regonda, S.; Gao, J.; Liu, Y.; Hu, W. Ultrasensitive protein detection using lithographically defined Si multi-nanowire field effect transistors. Lab Chip 2011, 11, 1952–1961. [Google Scholar]
- Lin, S.-P.; Pan, C.-Y.; Tseng, K.-C.; Lin, M.-C.; Chen, C.-D.; Tsai, C.-C.; Yu, S.-H.; Sun, Y.-C.; Lin, T.-W.; Chen, Y.-T. A reversible surface functionalized nanowire transistor to study protein–protein interactions. Nano Today 2009, 4, 235–243. [Google Scholar] [CrossRef]
- Lin, T.-W.; Hsieh, P.-J.; Lin, C.-L.; Fang, Y.-Y.; Yang, J.-X.; Tsai, C.-C.; Chiang, P.-L.; Pan, C.-Y.; Chen, Y.-T. Label-free detection of protein-protein interactions using a calmodulin-modified nanowire transistor. Proc. Natl. Acad. Sci. USA 2010, 107, 1047–1052. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Luo, Z.H.H.; Huang, M.J.; Ang, J.A.J.; Kang, T.G.; Ji, H.M. An integrated chip for rapid, sensitive, and multiplexed detection of cardiac biomarkers from fingerprick blood. Biosens. Bioelectron. 2011, 28, 459–463. [Google Scholar]
- Patolsky, F.; Timko, B.P.; Yu, G.; Fang, Y.; Greytak, A.B.; Zheng, G.; Lieber, C.M. Detection, stimulation, and inhibition of neuronal signals with high-density nanowire transistor arrays. Science 2006, 313, 1100–1104. [Google Scholar] [CrossRef]
- Tian, J.; Ma, D.; Wei, D.; Xu, M.; Fan, J.; Zhu, X.; Guo, M.; Rong, B.; Ke, L.; Mei, X.; et al. Clinical research on thoraco-abdominal two-field lymph node dissection and regularity of metastasis in middle-third thoracic esophageal carcinoma. Science 2010, 329, 831–834. [Google Scholar]
- Park, I.; Li, Z.; Li, X.; Pisano, A.P.; Williams, R.S. Towards the silicon nanowire-based sensor for intracellular biochemical detection. Biosens. Bioelectron. 2007, 22, 2065–2070. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Huang, M.J.; Luo, Z.H.H.; Tay, G.K.I.; Lim, E.-J.A.; Liu, E.T.; Thomsen, J.S. Highly sensitive and reversible silicon nanowire biosensor to study nuclear hormone receptor protein and response element DNA interactions. Biosens. Bioelectron. 2010, 26, 365–370. [Google Scholar] [PubMed]
- Zhang, G.-J.; Huang, M.J.; Ang, J.A.J.; Liu, E.T.; Desai, K.V. Self-assembled monolayer-assisted silicon nanowire biosensor for detection of protein–DNA interactions in nuclear extracts from breast cancer cells. Biosens. Bioelectron. 2011, 26, 3233–3239. [Google Scholar] [PubMed]
- Lin, C.-H.; Hung, C.-H.; Hsiao, C.-Y.; Lin, H.-C.; Ko, F.-H.; Yang, Y.-S. Poly-silicon nanowire field-effect transistor for ultrasensitive and label-free detection of pathogenic avian influenza DNA. Biosens. Bioelectron. 2009, 24, 3019–3024. [Google Scholar] [PubMed]
- Zheng, G.; Lieber, C.M. Nanowire Biosensors for Label-Free, Real-Time, Ultrasensitive Protein Detection. Methods Mol Biol. 2011, 790, 223–237. [Google Scholar]
- Patolsky, F.; Zheng, G.; Hayden, O.; Lakadamyali, M.; Zhuang, X.; Lieber, C.M. Electrical detection of single viruses. Proc. Natl. Acad. Sci. USA 2004, 101, 14017–14022. [Google Scholar]
- Wasilewski, T.; Kamysz, W.; Gębicki, J. AI-Assisted Detection of Biomarkers by Sensors and Biosensors for Early Diagnosis and Monitoring. Biosensors 2024, 14, 356. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Jiang, N.; Yetisen, A.K. Wearable Artificial Intelligence Biosensor Networks. Biosens. Bioelectron. 2023, 219, 114825. [Google Scholar]
- Ayadi, N.; Lale, A.; Hajji, B.; Launay, J.; Temple-Boyer, P. Machine Learning-Based Modeling of pH-Sensitive Silicon Nanowire (SiNW) for Ion Sensitive Field Effect Transistor (ISFET). Sensors 2024, 24, 8091. [Google Scholar] [CrossRef]
- Lee, J.; Jang, J.; Choi, B.; Yoon, J.; Kim, J.-Y.; Choi, Y.-K.; Kim, D.M.; Kim, D.H.; Choi, S.-J. A Highly Responsive Silicon Nanowire/Amplifier MOSFET Hybrid Biosensor. Sci. Rep. 2015, 5, 12286. [Google Scholar]
- Lee, J.; Choi, B.; Hwang, S.; Lee, J.H.; Park, B.-G.; Park, T.J.; Kim, D.M.; Kim, D.H.; Choi, S.-J. Investigation of Sensor Performance in Accumulation- and Inversion-Mode Silicon Nanowire pH Sensors. IEEE Trans. Electron Devices 2014, 61, 1607–1610. [Google Scholar]
- Bergveld, P. Thirty years of ISFETOLOGY: What happened in the past 30 years and what may happen in the next 30 years. Sens. Actuators B Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef]
- Zhang, J.-R.; Rupakula, M.; Bellando, F.; Garcia Cordero, E.; Longo, J.; Wildhaber, F.; Herment, G.; Guérin, H.; Ionescu, A.M. All CMOS Integrated 3D-Extended Metal Gate ISFETs for pH and Multi-Ion (Na+,K+,Ca2+) sensing. In Proceedings of the 2018 IEEE International Electron Devices Meeting (IEDM), San Francisco, CA, USA, 1–5 December 2018. [Google Scholar]
- Himori, S.; Nishitani, S.; Sakata, T. Aptamer-based nanofilter interface for small-biomarker detection with potentiometric biosensor. Electrochim. Acta 2021, 368, 137631. [Google Scholar] [CrossRef]
- Nakatsuka, N.; Yang, K.-A.; Abendroth, J.M.; Cheung, K.M.; Xu, X.; Yang, H.; Zhao, C.; Zhu, B.; Rim, Y.S.; Yang, Y.; et al. Aptamer-field-effect transistors overcome Debye length limitations for small-molecule sensing. Science 2018, 362, 319–324. [Google Scholar] [CrossRef]
- Chung, W.-Y.; Yang, C.-H.; Pijanowska, D.G.; Grabiec, P.B.; Torbicz, W. ISFET performance enhancement by using the improved circuit techniques. Sens. Actuators B Chem. 2006, 113, 555–562. [Google Scholar] [CrossRef]
- Gaddour, A.; Dghais, W.; Hamdi, B.; Ben Ali, M. Temperature Compensation Circuit for ISFET Sensor. J. Low Power Electron. Appl. 2020, 10, 2. [Google Scholar] [CrossRef]
- Withey, G.D.; Lazareck, A.D.; Tzolov, M.B.; Yin, A.; Aich, P.; Yeh, J.I.; Xu, J.M. Ultra-High Redox Enzyme Signal Transduction Using Highly Ordered Carbon Nanotube Array Electrodes. Biosens. Bioelectron. 2006, 21, 1560–1565. [Google Scholar] [CrossRef]
- Bezuneh, T.T.; Fereja, T.H.; Kitte, S.A.; Li, H.; Jin, Y. Gold Nanoparticle-Based Signal Amplified Electrochemiluminescence for Biosensing Applications. Talanta 2022, 248, 123611. [Google Scholar] [CrossRef]
- Nishitani, S.; Sakata, T. Enhancement of Signal-to-Noise Ratio for Serotonin Detection with Well-Designed Nanofilter-Coated Potentiometric Electrochemical Biosensor. ACS Appl. Mater. Interfaces 2020, 12, 14761–14769. [Google Scholar] [CrossRef]
- Nishitani, S.; Fukuma, T.; Himori, S.; Man, Y.; Shiratori, R.; Sakata, T. Densification of Diazonium-Based Organic Thin Film as Bioelectrical Interface. Langmuir 2021, 37, 14369–14379. [Google Scholar] [CrossRef]
- Cao, S.; Sun, P.; Xiao, G.; Tang, Q.; Sun, X.; Zhao, H.; Zhao, S.; Lu, H.; Yue, Z. ISFET-based sensors for (bio)chemical applications: A review. Electrochem. Sci. Adv. 2022, 3, 4. [Google Scholar] [CrossRef]
- Sinha, S.; Pal, T. A comprehensive review of FET-based pH sensors: Materials, fabrication technologies, and modeling. Electrochem. Sci. Adv. 2021, 2, 5. [Google Scholar]
- Baghini, M.S.; Vilouras, A.; Douthwaite, M.; Georgiou, P.; Dahiya, R. Ultra-thin ISFET-based sensing systems. Electrochem. Sci. Adv. 2021, 2, 6. [Google Scholar]
- Moser, M.; Lande, T.S.; Toumazou, C.; Georgiou, P. ISFETs in CMOS and Emergent Trends in Instrumentation: A Review. IEEE Sens. J. 2016, 16, 6496–6514. [Google Scholar]
- Knopfmacher, O.; Tarasov, A.; Fu, W.; Wipf, M.; Niesen, B.; Calame, M.; Schönenberger, C. Nernst Limit in Dual-Gated Si-Nanowire FET Sensors. Nano Lett. 2010, 10, 2268–2274. [Google Scholar]
- Lee, J.; Hwang, S.; Choi, B.; Lee, J.H.; Moon, D.-I.; Seol, M.-L.; Kim, C.-H.; Chung, I.-Y.; Park, B.-G.; Choi, Y.-K.; et al. A novel SiNW/CMOS hybrid biosensor for high sensitivity/low noise. In Proceedings of the 2013 IEEE International Electron Devices Meeting (IEDM), Washington, DC, USA, 9–11 December 2013. [Google Scholar]
- Kim, S. Current-Mirror Type Silicon Nanowire Field-Effect Transistor-Based Biosensors. Master’s Thesis, Kookmin University, Seoul, Republic of Korea, 2016. [Google Scholar]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nat. Protoc. 2006, 1, 1711–1724. [Google Scholar] [CrossRef]
- Shin, K.-S.; Lee, K.; Kang, J.Y.; Chui, C.O. Novel T-Channel Nanowire FET Built-in Signal Amplification for pH Sensing. In Proceedings of the 2009 IEEE International Electron Devices Meeting (IEDM), Baltimore, MD, USA, 7–9 December 2009. [Google Scholar]
- Hsiao, C.-Y.; Lin, C.-H.; Hung, C.-H.; Su, C.-J.; Lo, Y.-R.; Lee, C.-C.; Lin, H.-C.; Ko, F.-H.; Huang, T.-Y.; Yang, Y.-S. Novel poly-silicon nanowire field effect transistor for biosensing application. Biosens. Bioelectron. 2009, 24, 1223–1229. [Google Scholar] [CrossRef]
- Ginet, P.; Akiyama, S.; Takama, N.; Fujita, H.; Kim, B. CMOS-compatible fabrication of top-gated field-effect transistor silicon nanowire-based biosensors. J. Micromech. Microeng. 2011, 21, 065008. [Google Scholar]
- Park, I.; Li, Z.; Pisano, A.P.; Williams, R.S. Top-down fabricated silicon nanowire sensors for real-time chemical detection. Nanotechnology 2009, 21, 015501. [Google Scholar]
- Choi, C.L.; Alivisatos, A.P. From artificial atoms to nanocrystal molecules: Preparation and properties of more complex nanostructures. Annu. Rev. Phys. Chem. 2010, 61, 369–389. [Google Scholar]
- Lieber, C.M. Semiconductor nanowires: A platform for nanoscience and nanotechnology. MRS Bull. 2011, 36, 1052–1063. [Google Scholar]
- Yang, P.; Yan, R.; Fardy, M. Semiconductor nanowire: What’s next? Nano Lett. 2010, 10, 1529–1536. [Google Scholar] [PubMed]
- Hobbs, R.G.; Petkov, N.; Holmes, J.D. Semiconductor Nanowire Fabrication by Bottom-Up and Top-Down Paradigms. Chem. Mater. 2012, 24, 1975–1991. [Google Scholar]
- Pennelli, G. Top down fabrication of long silicon nanowire devices by means of lateral oxidation. Microelectron. Eng. 2009, 86, 2139–2143. [Google Scholar]
- Kim, K.; Lee, J.K.; Han, S.J.; Lee, S. A Novel Top-Down Fabrication Process for Vertically-Stacked Silicon-Nanowire Array. Appl. Sci. 2020, 10, 1146. [Google Scholar] [CrossRef]
- Mohd Azmi, M.A.; Tehrani, Z.; Lewis, R.P.; Walker, K.-A.D.; Jones, D.R.; Daniels, S.H.; Doak, S.H.; Guy, O.J. Highly sensitive covalently functionalised integrated silicon nanowire biosensor devices for detection of cancer risk biomarker. Biosens. Bioelectron. 2014, 52, 216–224. [Google Scholar]
- Vu, X.T.; GhoshMoulick, R.; Eschermann, J.F.; Stockmann, R.; Offenhäusser, A.; Ingebrandt, S. Fabrication and application of silicon nanowire transistor arrays for biomolecular detection. Sens. Actuators B Chem. 2010, 144, 354–360. [Google Scholar]
- Kong, T.; Su, R.; Zhang, B.; Zhang, Q.; Cheng, G. CMOS-compatible, label-free silicon-nanowire biosensors to detect cardiac troponin I for acute myocardial infarction diagnosis. Biosens. Bioelectron. 2012, 34, 267–272. [Google Scholar]
- Juang, Y.-Z.; Lin, C.-F.; Tsai, H.-H.; Su, Y.-K.; Wang, R.-L.; Li, W.-L.; Kung, P.-C. A fully compatible CMOS-based hydrogen ion sensor using natural forming sensing membrane for urea detecting application. Procedia Chem. 2009, 1, 1067–1070. [Google Scholar]
- Chung, W.-Y.; Cruz, F.R.G.; Yang, C.-H.; He, F.S.; Liu, T.-T.; Pijanowska, D.G.; Torbicz, W.; Grabiec, P.B.; Jarosewicz, B. CMOS Readout Circuit Developments for Ion Sensitive Field Effect Transistor Based Sensor Applications. In Solid State Circuits Technologies; Intechopen: London, UK, 2010; pp. 421–444. ISBN 9789533070452. [Google Scholar]
- Naumova, O.V.; Fomin, B.I.; Nasimov, D.A.; Dudchenko, N.V.; Devyatova, S.F.; Zhanaev, E.D.; Popov, V.P.; Latyshev, A.V.; Aseev, A.L.; Ivanov, Y.D.; et al. SOI nanowires as sensors for charge Detection. Semicond. Sci. Technol. 2010, 25, 055004. [Google Scholar]
- Lee, J. Top-Down Fabricated Silicon Nanowire/CMOS Hybrid Biosensors. Ph.D. Dissertation, Kookmin University, Seoul, Republic of Korea, 2014. [Google Scholar]
- Malpartida-Cardenas, K.; Miscourides, N.; Rodriguez-Manzano, J.; Yu, L.S.; Moser, N.; Baum, J.; Georgiou, P. Quantitative and rapid Plasmodium falciparum malaria diagnosis and artemisinin-resistance detection using a CMOS Lab-on-Chip platform. Biosens. Bioelectron. 2019, 145, 111678. [Google Scholar]
- Nemati, S.S.; Dehghan, G.; Sheibani, N.; Abdi, Y. Ion-Sensitive Field-Effect Transistor-Based Biosensors: The Sources of Gates and Their Sensitive Layers—A Review. IEEE Sens. J. 2024, 24, 17324–17336. [Google Scholar]
- Analog Devices. Available online: https://www.analog.com/en/lp/002/tools/ltspice-simulator-kr.html (accessed on 17 August 2024).
- Cadence Design Systems. Available online: https://www.cadence.com/ko_KR/home/tools/custom-ic-analog-rf-design/virtuoso-studio.html (accessed on 17 August 2024).










| Paper | Main Research Focus | Limitations and Differences from This Study |
|---|---|---|
| Cao et al. [92] | ISFET sensors for biomolecule detection and commercialization | Existing reviews focus on ISFET applications, materials, and fabrication but lack a detailed circuit-level analysis, including design strategies, optimization techniques, and their impact on sensitivity, noise immunity, and linearity. |
| Sinha et al. [93] | Materials, fabrication, and modeling methods for FET-based pH sensors | |
| Baghini et al. [94] | Ultra-thin ISFET sensor systems, noise compensation, and flexible electronics | |
| Moser et al. [95] | CMOS ISFET instrumentation and front-end circuit design | |
| This Study | SiNW ISFET readout circuit analysis, performance evaluation, and proposal of a novel N-type circuit | Provides a comprehensive circuit-level analysis and optimization. Proposes a novel N-type readout circuit to enhance sensitivity while maintaining stability. |
| Abbreviation | Definition |
|---|---|
| ADC | Analog-to-Digital Converter |
| BOX | Buried Oxide |
| BSiNW | Conduction Parameter of SiNW |
| CMOS | Complementary Metal Oxide Semiconductor |
| CMP | Chemical Mechanical Planarization |
| Cmole | Concentration Range of Target Biomolecules |
| Cox | Unit Oxide Capacitance |
| DNL | Differential Nonlinearity |
| DR | Dynamic Range |
| FET | Field-Effect Transistor |
| gm,SiNW | Transconductance of the SiNW ISFET |
| GFET | Graphene Field-Effect Transistor |
| HDP-CVD | High-Density Plasma Chemical Vapor Deposition |
| HSQ | Hydrogen Silsesquioxane |
| ICP | Inductively Coupled Plasma |
| ID | Drain Current |
| ILD | Inter-Layer Dielectric |
| INL | Integral Nonlinearity |
| IREF | Reference Current |
| ISFET | Ion-Sensitive Field-Effect Transistor |
| LPCVD | Low-Pressure Chemical Vapor Deposition |
| LSB | Least-Significant Bit |
| Op-Amp | Operational Amplifier |
| PR | Photoresist |
| PVT | Process, Voltage, and Temperature |
| ROUT | Output Resistance |
| SiNW | Silicon Nanowire |
| SNR | Signal-to-Noise Ratio |
| SOI | Silicon-on-Insulator |
| TEM | Transmission Electron Microscopy |
| TEOS | Tetraethyl Orthosilicate |
| VDD | Supply Voltage |
| VDS | Drain-Source Voltage |
| VLTH | Logic Threshold Voltage |
| VOUT | Output Voltage |
| VREF | Reference Voltage |
| VTH | Threshold Voltage |
| Mobility of Carriers | |
| ro,SiNW | Output Resistance of the SiNW ISFET |
| Top-Down Method | Bottom-Up Method | |
|---|---|---|
| Fabrication Process | SiNWs are synthesized from molecular precursors rather than bulk semiconductor wafers, enabling the fabrication of complex superlattice structures | SiNWs are produced from molecular precursors by using a metal nano-cluster mediated vapor-liquid-solid mechanism |
| Advantages | Characterized by high yield and optimized for large-scale production | Enables flexibility in selecting materials for nanowire synthesis |
| Provides reliable and consistent synthesis processes | Facilitates in situ doping with diverse dopants for tailored electronic properties | |
| Seamlessly integrates with CMOS technology and other systems | Supports the synthesis of SiNWs with diameters smaller than 10 nm | |
| Limitations | Requires extensive processing time | Achieving uniformity in SiNWs bridging source and drain electrodes is highly challenging. |
| Significant silicon waste during etching | Device assembly needs precise pre-alignment and placement of SiNWs, causing integration challenges | |
| Challenges in achieving sub-10 nm structures with lithography | Mass production of SiNW devices remains impractical |
| N-Type | P-Type | Improved N-Type | |
|---|---|---|---|
| Number of circuit components | 2 × NMOS/2 × SiNW | 2 × NMOS/2 × SiNW/ 1 × Buffer/1 × R | 2 × NMOS/2 × SiNW/ 1 × op-amp/2 × R |
| Linearity (DNL/INL) | 0.009 LSB/0.01 LSB | 0.13 LSB/0.18 LSB | 0.003 LSB/0.003 LSB |
| Sensitivity increase rate, max value (%, mV/pH) | 0, 51 | 60, 149 @ 500 kΩ | 98, 172 @ ratio = 3 |
| Output voltage range | VDD − (VLG − VTH,N-SiNW + VOV 1,NMOS) | VDD − (VOV,P-SiNW + VOV,NMOS) | VDD − (VLG − VTH,N-SiNW + VOV,NMOS) |
| Linearity (DNL/INL) | 0.009 LSB/0.01 LSB | 0.13 LSB/0.18 LSB | 0.003 LSB/0.003 LSB |
| Supply induced output variations (±10% on top of VDD, mV) | 1.2 | 343 | 5.1 |
| Gain (dB) | −0.4 | 5.5 | 10.3 |
| 3 dB bandwidth (kHz) | 112.2 | 46.3 | 92.5 |
| Power consumption (μW) | 4.13 | 4.32 + 1.8 (op-amp) | 4.30 + 1.8 (op-amp) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Joo, J.; Mo, H.; Kim, S.; Shin, S.; Song, I.; Kim, D.H. A Review of Readout Circuit Schemes Using Silicon Nanowire Ion-Sensitive Field-Effect Transistors for pH-Sensing Applications. Biosensors 2025, 15, 206. https://doi.org/10.3390/bios15040206
Joo J, Mo H, Kim S, Shin S, Song I, Kim DH. A Review of Readout Circuit Schemes Using Silicon Nanowire Ion-Sensitive Field-Effect Transistors for pH-Sensing Applications. Biosensors. 2025; 15(4):206. https://doi.org/10.3390/bios15040206
Chicago/Turabian StyleJoo, Jungho, Hyunsun Mo, Seungguk Kim, Seonho Shin, Ickhyun Song, and Dae Hwan Kim. 2025. "A Review of Readout Circuit Schemes Using Silicon Nanowire Ion-Sensitive Field-Effect Transistors for pH-Sensing Applications" Biosensors 15, no. 4: 206. https://doi.org/10.3390/bios15040206
APA StyleJoo, J., Mo, H., Kim, S., Shin, S., Song, I., & Kim, D. H. (2025). A Review of Readout Circuit Schemes Using Silicon Nanowire Ion-Sensitive Field-Effect Transistors for pH-Sensing Applications. Biosensors, 15(4), 206. https://doi.org/10.3390/bios15040206






