Pathway-like Activation of 3D Neuronal Constructs with an Optical Interface
Abstract
1. Introduction
2. Materials and Methods
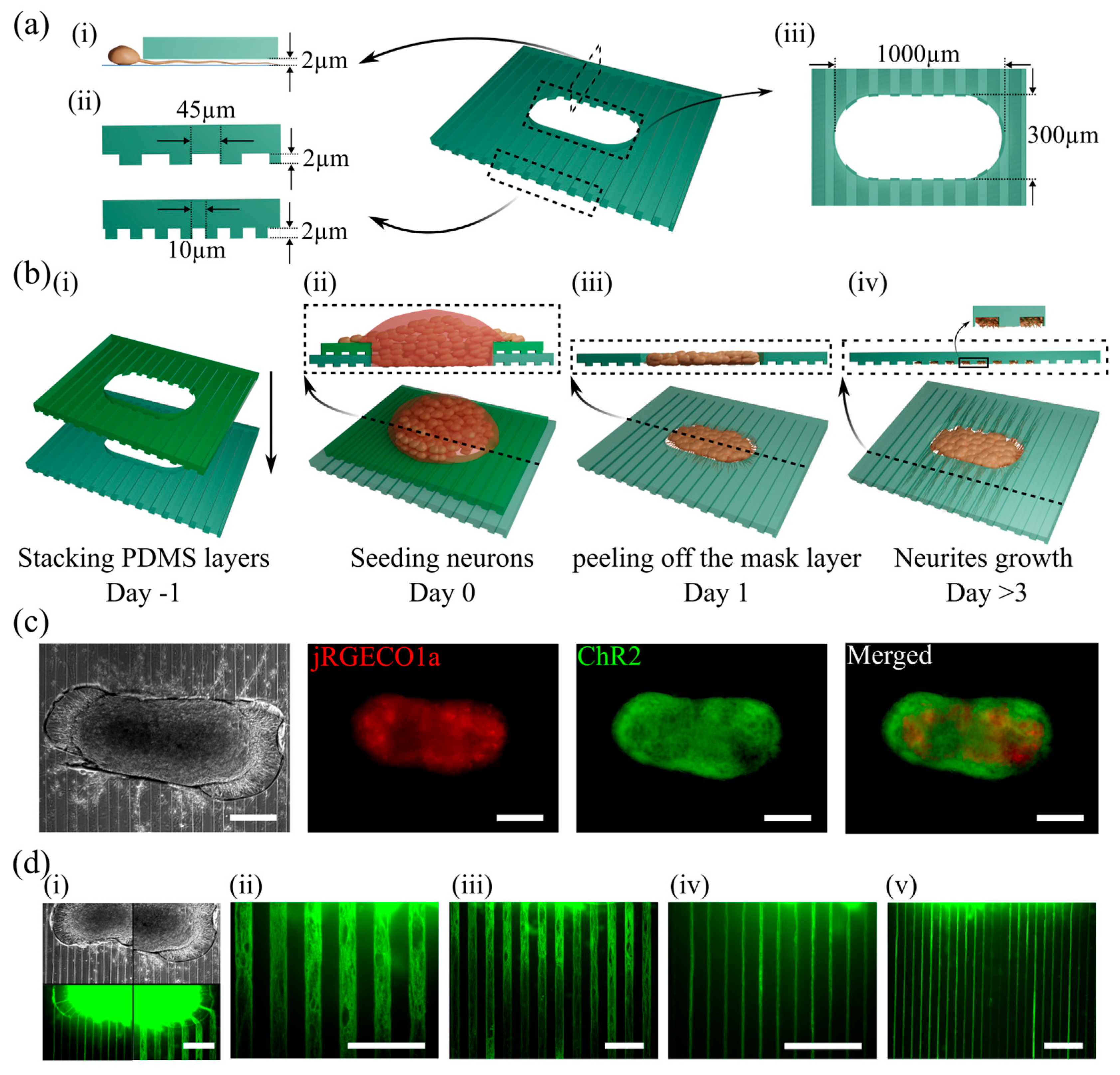
2.1. Fabrication of the PDMS Devices
2.2. Cell Culture
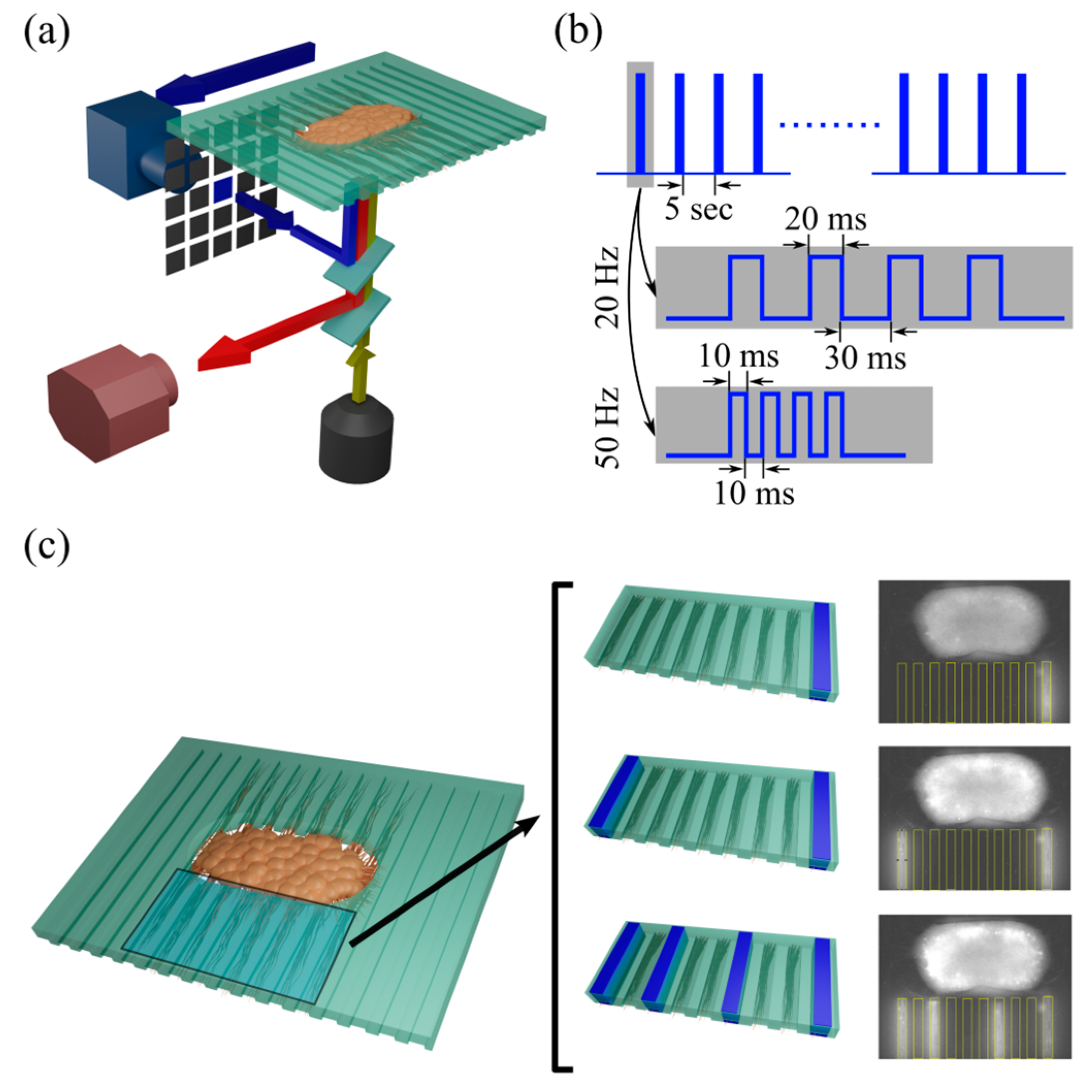
2.3. Optical Recording and Optogenetic Stimulation
2.4. Neural Activity Analysis
3. Results
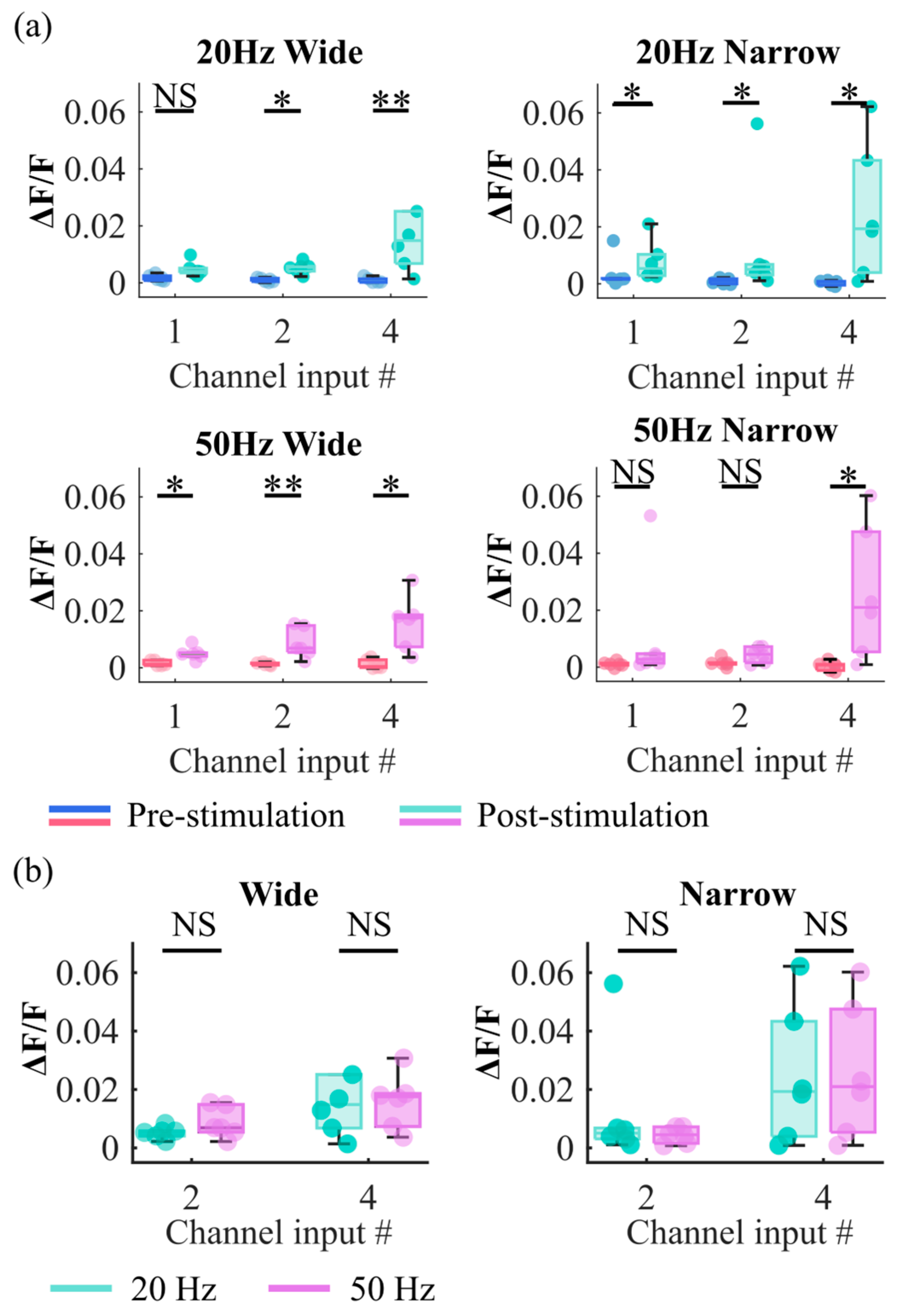
3.1. Stimulation of Axon Bundles in Microchannels Evoked Responses in the 3D Neural Culture
3.2. Responses Evoked by Different Stimulation Patterns Are Separable and Classifiable
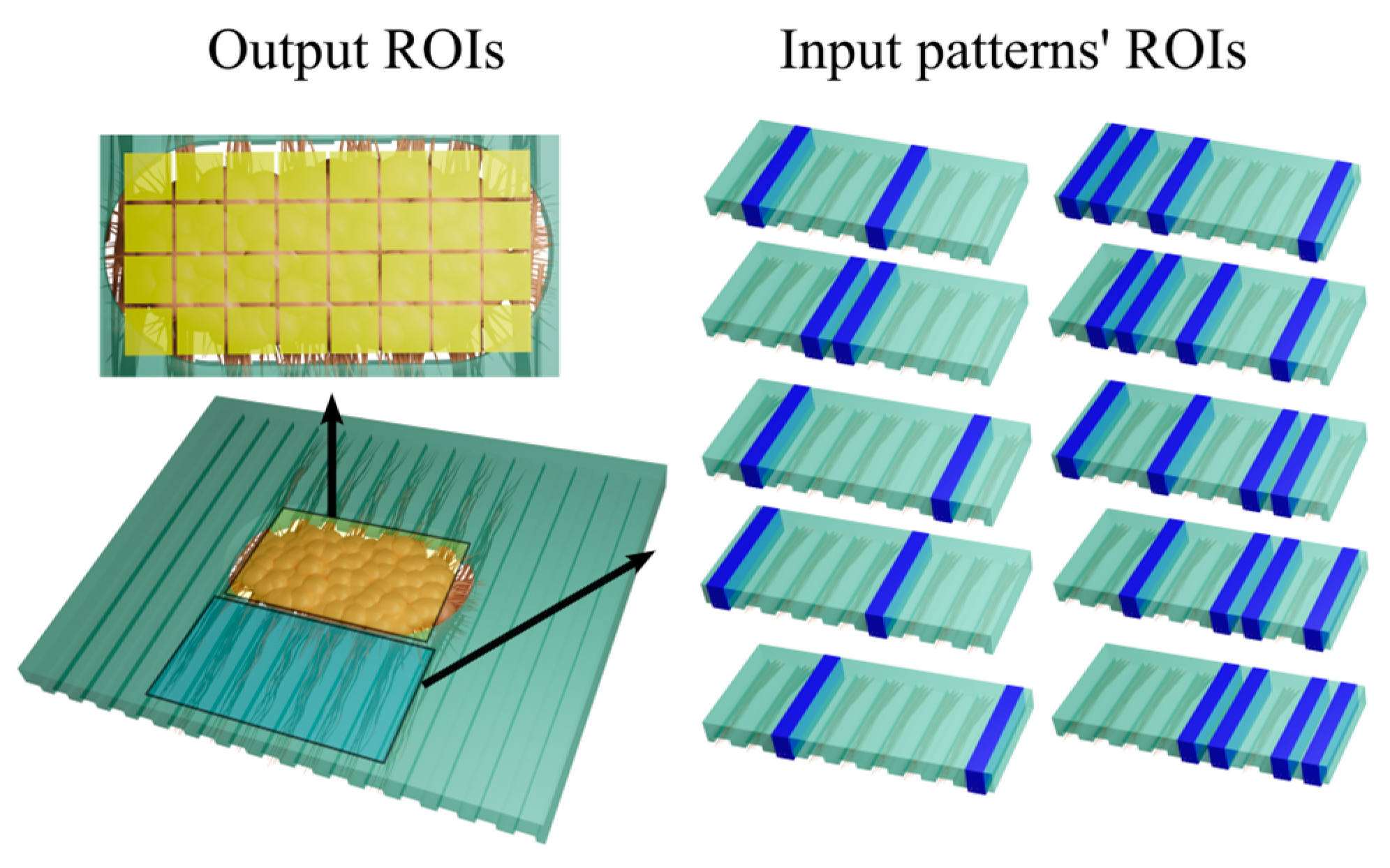
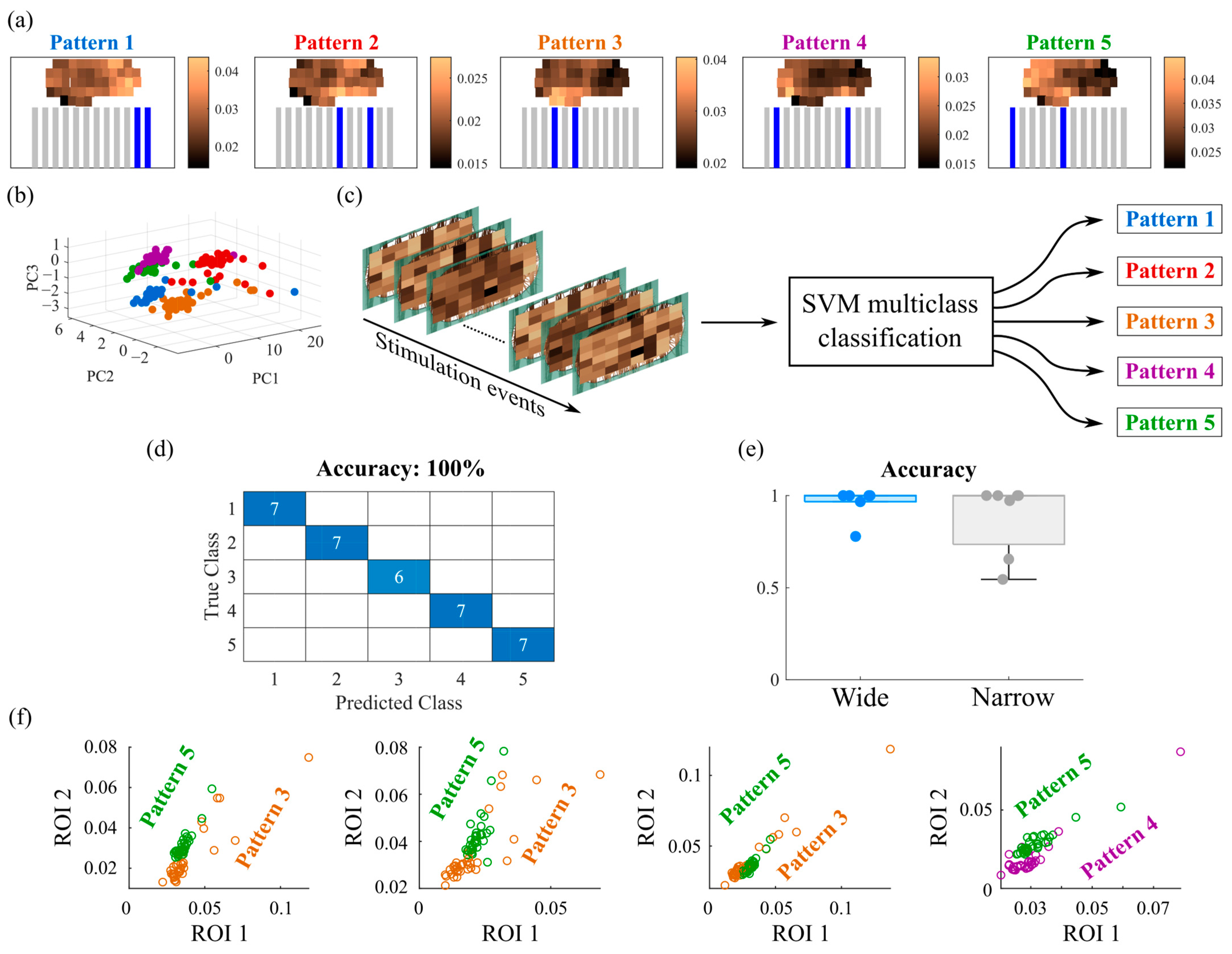
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- D’Antoni, C.; Mautone, L.; Sanchini, C.; Tondo, L.; Grassmann, G.; Cidonio, G.; Bezzi, P.; Cordella, F.; Di Angelantonio, S. Unlocking Neural Function with 3D In Vitro Models: A Technical Review of Self-Assembled, Guided, and Bioprinted Brain Organoids and Their Applications in the Study of Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2023, 24, 10762. [Google Scholar] [CrossRef]
- Zhuang, P.; Sun, A.X.; An, J.; Chua, C.K.; Chew, S.Y. 3D Neural Tissue Models: From Spheroids to Bioprinting. Biomaterials 2018, 154, 113–133. [Google Scholar] [CrossRef]
- Pașca, S.P.; Arlotta, P.; Bateup, H.S.; Camp, J.G.; Cappello, S.; Gage, F.H.; Knoblich, J.A.; Kriegstein, A.R.; Lancaster, M.A.; Ming, G.-L.; et al. A Nomenclature Consensus for Nervous System Organoids and Assembloids. Nature 2022, 609, 907–910. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.O.; Shin, S.-R.; Bañobre-López, M. Brain-on-a-Chip: An Emerging Platform for Studying the Nanotechnology-Biology Interface for Neurodegenerative Disorders. J. Nanobiotechnol. 2024, 22, 573. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, D.M.; Pereira, N.; Rombo, D.M.; Beltrão-Cavacas, C.; Sebastião, A.M.; Valente, C.A. Ex Vivo Model of Epilepsy in Organotypic Slices-a New Tool for Drug Screening. J. Neuroinflamm. 2018, 15, 203. [Google Scholar] [CrossRef]
- Villanueva, R. Advances in the Knowledge and Therapeutics of Schizophrenia, Major Depression Disorder, and Bipolar Disorder from Human Brain Organoid Research. Front. Psychiatry 2023, 14, 1178494. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, N.; Murugan, N.J.; Kaplan, D.L. Toward Studying Cognition in a Dish. Trends Cogn. Sci. 2021, 25, 294–304. [Google Scholar] [CrossRef]
- Cai, H.; Ao, Z.; Tian, C.; Wu, Z.; Liu, H.; Tchieu, J.; Gu, M.; Mackie, K.; Guo, F. Brain Organoid Reservoir Computing for Artificial Intelligence. Nat. Electron. 2023, 6, 1032–1039. [Google Scholar] [CrossRef]
- Morales Pantoja, I.E.; Smirnova, L.; Muotri, A.R.; Wahlin, K.J.; Kahn, J.; Boyd, J.L.; Gracias, D.H.; Harris, T.D.; Cohen-Karni, T.; Caffo, B.S.; et al. First Organoid Intelligence (OI) Workshop to Form an OI Community. Front. Artif. Intell. 2023, 6, 1116870. [Google Scholar] [CrossRef]
- Kundu, S.; Boutin, M.E.; Strong, C.E.; Voss, T.; Ferrer, M. High Throughput 3D Gel-Based Neural Organotypic Model for Cellular Assays Using Fluorescence Biosensors. Commun. Biol. 2022, 5, 1236. [Google Scholar] [CrossRef]
- Ming, Y.; Hasan, M.F.; Tatic-Lucic, S.; Berdichevsky, Y. Micro Three-Dimensional Neuronal Cultures Generate Developing Cortex-Like Activity Patterns. Front. Neurosci. 2020, 14, 563905. [Google Scholar] [CrossRef]
- Wilson, D.M.; Cookson, M.R.; Van Den Bosch, L.; Zetterberg, H.; Holtzman, D.M.; Dewachter, I. Hallmarks of Neurodegenerative Diseases. Cell 2023, 186, 693–714. [Google Scholar] [CrossRef]
- Kahn, R.S.; Sommer, I.E.; Murray, R.M.; Meyer-Lindenberg, A.; Weinberger, D.R.; Cannon, T.D.; O’Donovan, M.; Correll, C.U.; Kane, J.M.; van Os, J.; et al. Schizophrenia. Nat. Rev. Dis. Primers 2015, 1, 15067. [Google Scholar] [CrossRef] [PubMed]
- Stöber, T.M.; Batulin, D.; Triesch, J.; Narayanan, R.; Jedlicka, P. Degeneracy in Epilepsy: Multiple Routes to Hyperexcitable Brain Circuits and Their Repair. Commun. Biol. 2023, 6, 479. [Google Scholar] [CrossRef]
- Huang, Q.; Tang, B.; Romero, J.C.; Yang, Y.; Elsayed, S.K.; Pahapale, G.; Lee, T.-J.; Morales Pantoja, I.E.; Han, F.; Berlinicke, C.; et al. Shell Microelectrode Arrays (MEAs) for Brain Organoids. Sci. Adv. 2022, 8, eabq5031. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Franz, C.K.; Ryu, H.; Luan, H.; Cotton, K.Y.; Kim, J.U.; Chung, T.S.; Zhao, S.; Vazquez-Guardado, A.; Yang, D.S.; et al. Three-Dimensional, Multifunctional Neural Interfaces for Cortical Spheroids and Engineered Assembloids. Sci. Adv. 2021, 7, eabf9153. [Google Scholar] [CrossRef]
- Shin, H.; Jeong, S.; Lee, J.-H.; Sun, W.; Choi, N.; Cho, I.-J. 3D High-Density Microelectrode Array with Optical Stimulation and Drug Delivery for Investigating Neural Circuit Dynamics. Nat. Commun. 2021, 12, 492. [Google Scholar] [CrossRef] [PubMed]
- Felleman, D.J.; Van Essen, D.C. Distributed Hierarchical Processing in the Primate Cerebral Cortex. Cereb. Cortex 1991, 1, 1–47. [Google Scholar] [CrossRef]
- Van Essen, D.C.; Anderson, C.H.; Felleman, D.J. Information Processing in the Primate Visual System: An Integrated Systems Perspective. Science 1992, 255, 419–423. [Google Scholar] [CrossRef]
- Priebe, N.J.; Ferster, D. Inhibition, Spike Threshold, and Stimulus Selectivity in Primary Visual Cortex. Neuron 2008, 57, 482–497. [Google Scholar] [CrossRef]
- Saxena, S.; Cunningham, J.P. Towards the Neural Population Doctrine. Curr. Opin. Neurobiol. 2019, 55, 103–111. [Google Scholar] [CrossRef]
- Chung, S.; Abbott, L.F. Neural Population Geometry: An Approach for Understanding Biological and Artificial Neural Networks. Curr. Opin. Neurobiol. 2021, 70, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Berdichevsky, Y.; Staley, K.J.; Yarmush, M.L. Building and Manipulating Neural Pathways with Microfluidics. Lab. Chip 2010, 10, 999–1004. [Google Scholar] [CrossRef]
- Peyrin, J.-M.; Deleglise, B.; Saias, L.; Vignes, M.; Gougis, P.; Magnifico, S.; Betuing, S.; Pietri, M.; Caboche, J.; Vanhoutte, P.; et al. Axon Diodes for the Reconstruction of Oriented Neuronal Networks in Microfluidic Chambers. Lab. Chip 2011, 11, 3663–3673. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, M.K.; Bakkum, D.J.; Rompani, S.B.; Hierlemann, A. Recording Large Extracellular Spikes in Microchannels along Many Axonal Sites from Individual Neurons. PLoS ONE 2015, 10, e0118514. [Google Scholar] [CrossRef]
- Girardin, S.; Clément, B.; Ihle, S.J.; Weaver, S.; Petr, J.B.; Mateus, J.C.; Duru, J.; Krubner, M.; Forró, C.; Ruff, T.; et al. Topologically Controlled Circuits of Human iPSC-Derived Neurons for Electrophysiology Recordings. Lab. Chip 2022, 22, 1386–1403. [Google Scholar] [CrossRef] [PubMed]
- Ming, Y.; Abedin, M.J.; Tatic-Lucic, S.; Berdichevsky, Y. Microdevice for Directional Axodendritic Connectivity between Micro 3D Neuronal Cultures. Microsyst. Nanoeng. 2021, 7, 67. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Yang, Y.; Zhang, K.; Liang, W.; Xu, Z.; Wu, Y.; Luo, J.; Zhuang, C.; Cai, X. Recent Progress and Perspectives on Neural Chip Platforms Integrating PDMS-Based Microfluidic Devices and Microelectrode Arrays. Micromachines 2023, 14, 709. [Google Scholar] [CrossRef]
- Pigareva, Y.; Gladkov, A.; Kolpakov, V.; Mukhina, I.; Bukatin, A.; Kazantsev, V.B.; Pimashkin, A. Experimental Platform to Study Spiking Pattern Propagation in Modular Networks In Vitro. Brain Sci. 2021, 11, 717. [Google Scholar] [CrossRef]
- Ihle, S.J.; Girardin, S.; Felder, T.; Ruff, T.; Hengsteler, J.; Duru, J.; Weaver, S.; Forró, C.; Vörös, J. An Experimental Paradigm to Investigate Stimulation Dependent Activity in Topologically Constrained Neuronal Networks. Biosens. Bioelectron. 2022, 201, 113896. [Google Scholar] [CrossRef]
- Moutaux, E.; Charlot, B.; Genoux, A.; Saudou, F.; Cazorla, M. An Integrated Microfluidic/Microelectrode Array for the Study of Activity-Dependent Intracellular Dynamics in Neuronal Networks. Lab. Chip 2018, 18, 3425–3435. [Google Scholar] [CrossRef]
- Poli, D.; Wheeler, B.C.; DeMarse, T.B.; Brewer, G.J. Pattern Separation and Completion of Distinct Axonal Inputs Transmitted via Micro-Tunnels between Co-Cultured Hippocampal Dentate, CA3, CA1 and Entorhinal Cortex Networks. J. Neural Eng. 2018, 15, 046009. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.F.; Berdichevsky, Y. Neuron and Astrocyte Aggregation and Sorting in Three-Dimensional Neuronal Constructs. Commun. Biol. 2021, 4, 587. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Torricelli, J.R.; Evege, E.K.; Price, P.J. Optimized Survival of Hippocampal Neurons in B27-Supplemented Neurobasal, a New Serum-Free Medium Combination. J. Neurosci. Res. 1993, 35, 567–576. [Google Scholar] [CrossRef]
- Eilers, P.H.; Boelens, H.F. Baseline Correction with Asymmetric Least Squares Smoothing; Leiden University Medical Centre: Leiden, The Netherlands, 2005. [Google Scholar]
- Fusi, S.; Miller, E.K.; Rigotti, M. Why Neurons Mix: High Dimensionality for Higher Cognition. Curr. Opin. Neurobiol. 2016, 37, 66–74. [Google Scholar] [CrossRef]
- Anastasiades, P.G.; Marques-Smith, A.; Butt, S.J.B. Studies of Cortical Connectivity Using Optical Circuit Mapping Methods. J. Physiol. 2018, 596, 145–162. [Google Scholar] [CrossRef]
- Smetters, D.; Majewska, A.; Yuste, R. Detecting Action Potentials in Neuronal Populations with Calcium Imaging. Methods 1999, 18, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Vogelstein, J.T.; Packer, A.M.; Machado, T.A.; Sippy, T.; Babadi, B.; Yuste, R.; Paninski, L. Fast Nonnegative Deconvolution for Spike Train Inference from Population Calcium Imaging. J. Neurophysiol. 2010, 104, 3691–3704. [Google Scholar] [CrossRef]
- Allen Brain Observatory technical Whitepaper: Stimulus Set and Response Analysis. Available online: https://community.brain-map.org/t/documentation-brain-observatory/3026 (accessed on 19 December 2024).
- Lee, S.; Meyer, J.F.; Park, J.; Smirnakis, S.M. Visually Driven Neuropil Activity and Information Encoding in Mouse Primary Visual Cortex. Front. Neural Circuits 2017, 11, 50. [Google Scholar] [CrossRef]
- Trautmann, E.M.; O’Shea, D.J.; Sun, X.; Marshel, J.H.; Crow, A.; Hsueh, B.; Vesuna, S.; Cofer, L.; Bohner, G.; Allen, W.; et al. Dendritic Calcium Signals in Rhesus Macaque Motor Cortex Drive an Optical Brain-Computer Interface. Nat. Commun. 2021, 12, 3689. [Google Scholar] [CrossRef]
- Cunningham, J.P.; Yu, B.M. Dimensionality Reduction for Large-Scale Neural Recordings. Nat. Neurosci. 2014, 17, 1500–1509. [Google Scholar] [CrossRef]
- DiCarlo, J.J.; Zoccolan, D.; Rust, N.C. How Does the Brain Solve Visual Object Recognition? Neuron 2012, 73, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Ferster, D. The Synaptic Inputs to Simple Cells of the Cat Visual Cortex. Prog. Brain Res. 1992, 90, 423–441. [Google Scholar] [CrossRef] [PubMed]
- Sumi, T.; Yamamoto, H.; Katori, Y.; Ito, K.; Moriya, S.; Konno, T.; Sato, S.; Hirano-Iwata, A. Biological Neurons Act as Generalization Filters in Reservoir Computing. Proc. Natl. Acad. Sci. USA 2023, 120, e2217008120. [Google Scholar] [CrossRef] [PubMed]
- Ju, H.; Dranias, M.R.; Banumurthy, G.; VanDongen, A.M.J. Spatiotemporal Memory Is an Intrinsic Property of Networks of Dissociated Cortical Neurons. J. Neurosci. 2015, 35, 4040–4051. [Google Scholar] [CrossRef]
- Dockendorf, K.P.; Park, I.; He, P.; Príncipe, J.C.; DeMarse, T.B. Liquid State Machines and Cultured Cortical Networks: The Separation Property. Biosystems 2009, 95, 90–97. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omidi, S.; Berdichevsky, Y. Pathway-like Activation of 3D Neuronal Constructs with an Optical Interface. Biosensors 2025, 15, 179. https://doi.org/10.3390/bios15030179
Omidi S, Berdichevsky Y. Pathway-like Activation of 3D Neuronal Constructs with an Optical Interface. Biosensors. 2025; 15(3):179. https://doi.org/10.3390/bios15030179
Chicago/Turabian StyleOmidi, Saeed, and Yevgeny Berdichevsky. 2025. "Pathway-like Activation of 3D Neuronal Constructs with an Optical Interface" Biosensors 15, no. 3: 179. https://doi.org/10.3390/bios15030179
APA StyleOmidi, S., & Berdichevsky, Y. (2025). Pathway-like Activation of 3D Neuronal Constructs with an Optical Interface. Biosensors, 15(3), 179. https://doi.org/10.3390/bios15030179




