Insights to Resistive Pulse Sensing of Microparticle and Biological Cells on Microfluidic Chip
Abstract
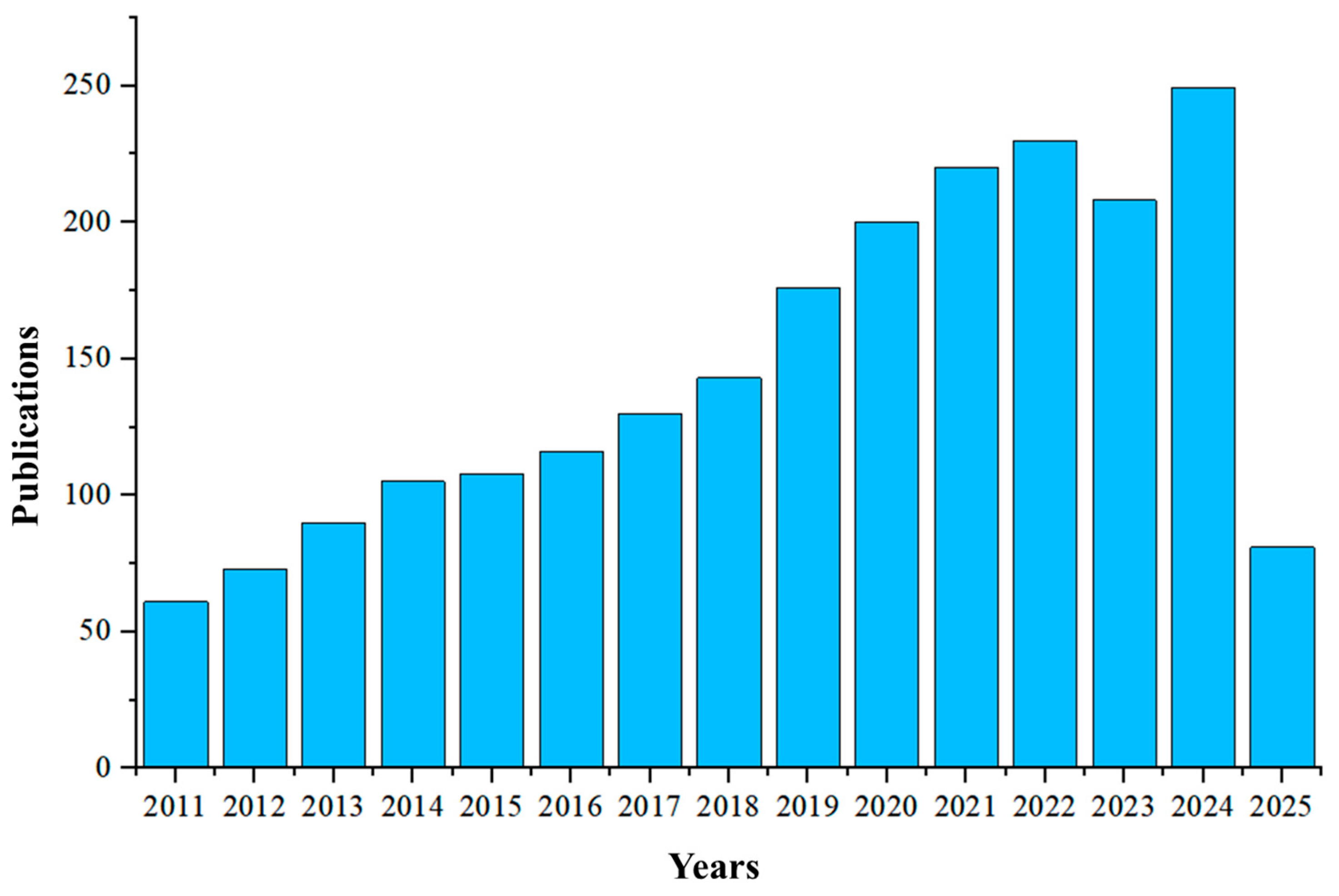
1. Introduction
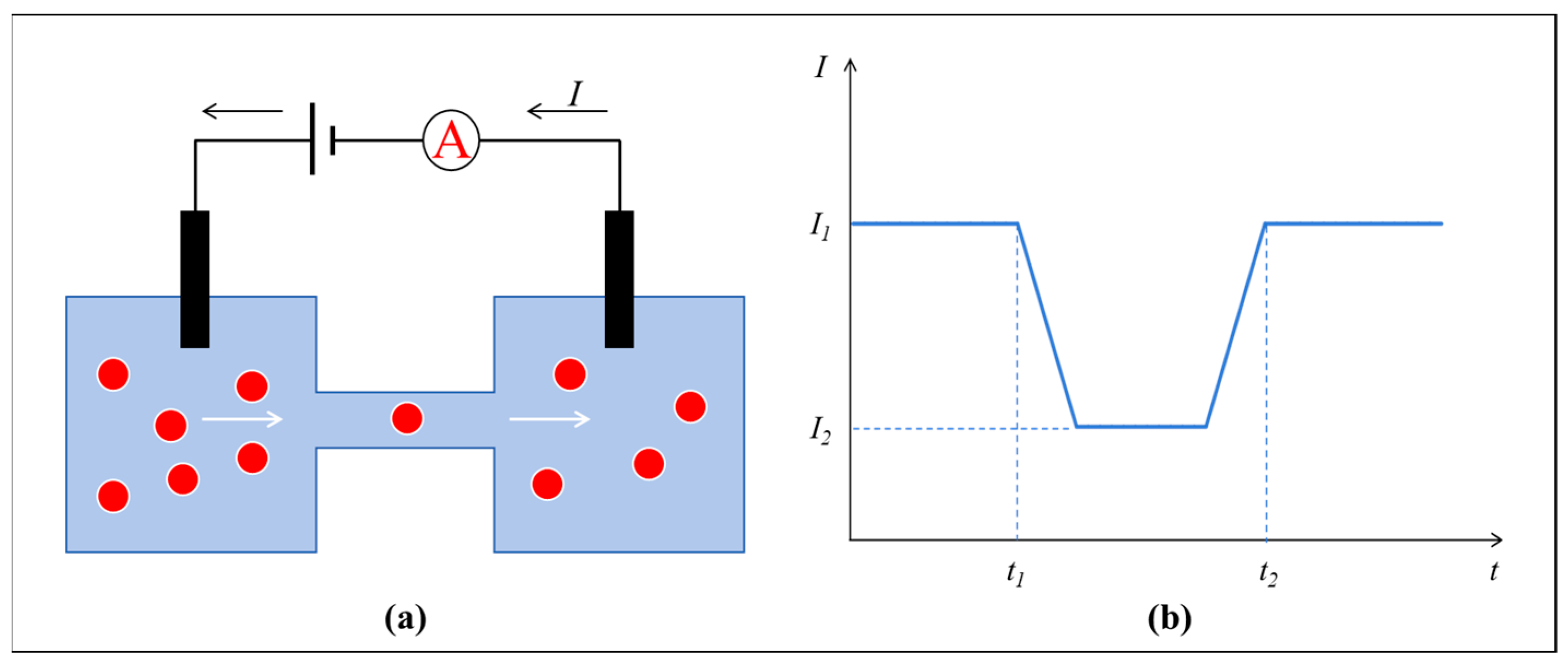
2. Theory of RPS
3. RPS Detection of Micro-Target
3.1. Biological Sensing Pores

| Serial No. | Micro/Nano-Target | Electric Field Signal | Sensing Pore Information | Ref. | ||
|---|---|---|---|---|---|---|
| Type | Material | Voltage | Frequency | Type | ||
| 1 | Molecules | Poly4 | 100 mV | 11 kHz | Wild-type aerolysin | [45] |
| 2 | Molecules | Cytolysin A | 200 mV | 10 kHz | Cytolysin A | [62] |
| 3 | Molecules | ssDNA | 200 mV | 10 kHz | Alpha-hederin | [51] |
| 4 | Molecules | granzyme B | 200 mV | 20 kHz | Perforin | [57] |
| 5 | Molecules | DNA/RNA | 120 mV | 50 kHz | Wild-type α-hemolysin | [63] |
| 6 | Proteins | Protein biomarkers | 400 mV | 100 kHz | Wild-type aerolysin | [64] |
| 7 | Molecules | Peptide | 90 mV | 500 kHz | Fragaceatoxin C | [56] |
| 8 | Molecules | DNA Sequence | 180 mV | 100 kHz | MspA | [53] |
| 9 | Molecules | polydeoxyadenines | 120 mV | 10 kHz | Aerolysin | [65] |
| 10 | Molecules | DNA hybrids | 120–160 mV | 100 kHz | DPhPC bilayer | [66] |
| 11 | Molecules | α-syn124–140 | 100 mV | 10 kHz/200 kHz | Toxin aerolysin | [67] |
| 12 | Cells | Ramos cells | 80–120 mV | 10 kHz | Aerolysin | [68] |
| 13 | Proteins | Plasma Proteins | 50/100 mV | 10 kHz | Pleurotolysin toxin | [46] |
| 14 | Proteins | nucleocapsid protein | 100–120 mV | 100 kHz | α-hemolysin | [69] |
| 15 | Proteins | Nanomolar proteins | 75 mV/150 mV | 50 kHz | YaxAB | [59] |
| 16 | Molecules | DNA Complexes | 100 mV/120 mV | 20 kHz | α-hemolysin | [47] |
| 17 | Proteins | Folded proteins | 150 mV | 20 kHz | Poly (C9) | [58] |
| 18 | Molecules | miRNA | 150 mV | 50 kHz | α-hemolysin | [70] |
| 19 | Proteins | Streptavidin | 35–100 mV | 0.1 Hz–1 MHz | Lipid bilayers | [71] |
| 20 | Molecules | prion protein | 100 mV | 10 kHz | DNA aptamer | [72] |
3.2. Solid-State Sensing Pores

| Serial No. | Micro/Nano-Target | Electric Field Signal | Sensing Pore Information | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Type | Size (Diameter/Length) | Material | Voltage | Frequency | Type | Size (Diameter/Width/Height) | ||
| 1 | Proteins | — | Carcinoembryonic antigen | 80–120 mV | 100 kHz | SiNx | 4 nm | [78] |
| 2 | Molecules | — | dsDNA. | 100 mV | 500 kHz | SiN | 3 nm | [81] |
| 3 | Molecules | — | DNA | 200 mV | 250 kHz | SiN | 8.5 nm | [90] |
| 4 | NPs | 80 nm/100 nm | PS | 100 mV | 30 kHz | SiN | 200 nm | [91] |
| 5 | Cells | — | human PANC-1/HPNE cells | 1–2 V | 3, 10 kHz | Quartz capillaries | 30 nm | [92] |
| 6 | Virus | — | HBV | 10 V | 1 kHz | Glass | 50 nm/50 nm | [93] |
| 7 | NPs | 160 nm | PMMA | 200 mV | 1 MHz | Borosilicate glass capillaries | 340 nm | [94] |
| 8 | NPs | 50 nm/ 100 nm | PS | 500 mV | 3.2–10 GHz | Si3N4 | 450 nm | [95] |
| 9 | NPs | 100 nm | PS/E. coli | 1.2 V | 10 kHz | Glass | 150 nm | [96] |
| 10 | Virus | — | Adeno-associated virus | 100–300 mV | 10 kHz | SiN | 66 nm/90 nm | [97] |
| 11 | NPs | 70 nm | Latex nanoparticles | 200 mV | 7.3 kHz | Quartz capillaries | — | [98] |
| 12 | Molecules | — | Enzyme molecules | 500 mV | 100 kHz | Pt | 250 nm | [99] |
| 13 | Bio-pellets | 60–160 nm | EV | 1 V | 100 kHz | D263 glass | 200 nm | [100] |
| 14 | Bio-pellets | 60 nm/90 nm | EV | 400 mV | 10 kHz | Gold | — | [101] |
| 15 | Virus | 30–35 nm | HBV | 0.5–2.5 V | 10 kHz | D263 glass | 60 nm/60 nm | [102] |
| 16 | Proteins | — | Aβ42 | 500 mV | 20 kHz | Quartz nanopipette | 34 nm | [103] |
| 17 | Bio-pellets | — | ADSC-EVs | 320 mV | 10 kHz | SiN | — | [104] |
| 18 | Molecules | — | miRNA | 1–5 V | 1 kHz | Gold | — | [105] |
3.3. Other Sensing Pores

| Serial No. | Micro/Nano-Target | Electric Field Signal | Sensing Pore Information | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Type | Size (Diameter/Length) | Material | Voltage | Frequency | Type | Size (Width/Height) | ||
| 1 | Bio-pellets | 30–100 nm | EV | 1–5 V | 1–5 kHz | PDMS | — | [121] |
| 2 | NPs | 100 nm/300 nm/350 nm | PS | 0.4–4 V | 5–50 kHz | PMMA | — | [122] |
| 3 | Molecules | — | λ-DNA | 1 V | — | PMMA-COC | 154 nm/203 nm | [123] |
| 4 | Molecules | — | ssDNA | 5–20 V | 10 kHz | PET | — | [124] |
| 5 | NPs | 100 nm | PS | 10 V | 500 kHz | PDMS | — | [125] |
| 6 | NPs | 160 nm | Nanoparticle | 200 mV | 10 kHz | — | 250 nm | [119] |
| 7 | Virus | — | SARS-CoV-2 | 0.1–1 V | 100 kHz | PDMS | — | [126] |
| 8 | Bio-pellets | 30–200 nm | EV | 10 V | 10 kHz | — | — | [127] |
| 9 | NPs | 15 μm/20 μm | PS | 1–5 V | 1–10 MHz | PDMS | 40 μm/35 μm | [128] |
| 10 | Molecules | — | 100 bp DNA | 600 mV | 5 kHz | PET | 21 nm/23 nm/27 nm/30 nm/42 nm | [129] |
| 11 | Virus | 68–77 nm | CNP | 500 mV | 1 GHz | SiN | — | [130] |
| 12 | Virus | 129–141 nm | VLP | 200 mV | — | NP200 | 220 nm | [131] |
| 13 | Proteins | 30–150 nm | miR-21-5p | — | — | NP100 | — | [132] |
| 14 | NPs | 700 nm/830 nm | PS | 1 V | 2 kHz | PDMS | — | [133] |
| 15 | NPs | 300 nm/1 μm | PS | 4 V | 100 kHz | PDMS | — | [134] |
| 16 | Proteins | 75 nm | PC3 CM | 5 V | 100 kHz | Ts-400 | 375 nm | [110] |
| 17 | NPs | 600 nm/1 μm | PS/ Yeast cells | 10 V | 400 kHz | PDMS | 2 μm | [135] |
| 18 | Bio-pellets | 252 nm/460 nm | EV | 10–20 V | 400 kHz | PDMS | 500 nm/600 nm | [136] |
| 19 | Bio-pellets | 50–330 nm | EV | 700 V | 100 kHz | — | 47 nm | [137] |
| 20 | Molecules | 200 nm | MDA-MB-231/PS | 10–25 V | 200 kHz | PDMS | 10 μm/10 μm | [138] |
4. Advantages, Challenges, and Future Scope
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, M.D. The Coulter Principle: A history. Cytom. Part A 2022, 101, 8–11. [Google Scholar] [CrossRef]
- Fan, X.; Batchelor-McAuley, C.; Yang, M.; Barton, S.; Rickaby, R.E.M.; Bouman, H.A.; Compton, R.G. Quantifying the Extent of Calcification of a Coccolithophore Using a Coulter Counter. Anal. Chem. 2022, 94, 12664–12672. [Google Scholar] [CrossRef]
- Peng, R.; Li, D. Detection and sizing of nanoparticles and DNA on PDMS nanofluidic chips based on differential resistive pulse sensing. Nanoscale 2017, 9, 5964–5974. [Google Scholar] [CrossRef]
- Anderson, W.; Lane, R.; Korbie, D.; Trau, M. Observations of Tunable Resistive Pulse Sensing for Exosome Analysis: Improving System Sensitivity and Stability. Langmuir 2015, 31, 6577–6587. [Google Scholar] [CrossRef]
- Tsutsui, M.; Yoshida, T.; Yokota, K.; Yasaki, H.; Yasui, T.; Arima, A.; Tonomura, W.; Nagashima, K.; Yanagida, T.; Kaji, N.; et al. Discriminating single-bacterial shape using low-aspect-ratio pores. Sci. Rep. 2017, 7, 17371. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, H.; Cai, M.; Song, J. A Multifunctional Flexible Tactile Sensor Based on Resistive Effect for Simultaneous Sensing of Pressure and Temperature. Adv. Sci. 2024, 11, e2307693. [Google Scholar] [CrossRef]
- Khuje, S.; Sheng, A.; Yu, J.; Ren, S. Flexible Copper Nanowire Electronics for Wireless Dynamic Pressure Sensing. ACS Appl. Electron. Mater. 2021, 3, 5468–5474. [Google Scholar] [CrossRef]
- Weatherall, E.; Willmott, G.R. Applications of tunable resistive pulse sensing. Analyst 2015, 140, 3318–3334. [Google Scholar] [CrossRef] [PubMed]
- Willmott, G.R. Tunable Resistive Pulse Sensing: Better Size and Charge Measurements for Submicrometer Colloids. Anal. Chem. 2018, 90, 2987–2995. [Google Scholar] [CrossRef] [PubMed]
- Harrer, S.; Kim, S.C.; Schieber, C.; Kannam, S.; Gunn, N.; Moore, S.; Scott, D.; Bathgate, R.; Skafidas, S.; Wagner, J.M. Label-free screening of single biomolecules through resistive pulse sensing technology for precision medicine applications. Nanotechnology 2015, 26, 182502. [Google Scholar] [CrossRef] [PubMed]
- Reitemeier, J.; Metro, J.; Fu, K.X. Nanopore sensing and beyond: Electrochemical systems for optically-coupled single-entity studies, stimulus-responsive gating applications, and point-of-care sensors. Sens. Actuators Rep. 2024, 8, 100225. [Google Scholar] [CrossRef]
- Camazzola, A.; Buglakova, E.; Perrin, L.W.; Rukes, V.; Cao, C. Eliminating the Interference of Neighboring Nucleobases in Aerolysin for Nanopore Sequencing. ACS Sens. 2025, 10, 4202–4208. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, J.; Li, D. Microfluidic and nanofluidic resistive pulse sensing: A review. Micromachines 2017, 8, 204. [Google Scholar] [CrossRef]
- Prakash, S.; Pinti, M.; Bhushan, B. Theory, fabrication and applications of microfluidic and nanofluidic biosensors. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 2269–2303. [Google Scholar] [CrossRef]
- Jarrah, A.M.A.; AlMahasneh, M.M. Using parallel plates capacitor as a volumetric flow rate sensor and direction detection for microfluidic/nanofluidic and extra smaller applications. Sens. Int. 2023, 4, 100247. [Google Scholar] [CrossRef]
- Shim, S.; Shim, J.; Taylor, W.R.; Kosari, F.; Vasmatzis, G.; Ahlquist, D.A.; Bashir, R. Magnetophoretic-based microfluidic device for DNA Concentration. Biomed. Microdevices 2016, 18, 28. [Google Scholar] [CrossRef]
- He, F.; Liao, Y.; Lin, J.; Song, J.; Qiao, L.; Cheng, Y.; He, F.; Sugioka, K. Femtosecond laser fabrication of monolithically integrated microfluidic sensors in glass. Sensors 2014, 14, 19402–19440. [Google Scholar] [CrossRef] [PubMed]
- Pezzuoli, D.; Angeli, E.; Repetto, D.; Ferrera, F.; Guida, P.; Firpo, G.; Repetto, L. Nanofluidic-based accumulation of antigens for miniaturized immunoassay. Sensors 2020, 20, 1615. [Google Scholar] [CrossRef]
- Hur, J.; Chung, A.J. Microfluidic and Nanofluidic Intracellular Delivery. Adv. Sci. 2021, 8, 2004595. [Google Scholar] [CrossRef]
- Chen, X.; Li, T.; Shen, J.; Hu, Z. Fractal design of microfluidics and nanofluidics—A review. Chemom. Intell. Lab. Syst. 2016, 155, 19–25. [Google Scholar] [CrossRef]
- Yarin, A.L. Novel nanofluidic and microfluidic devices and their applications. Curr. Opin. Chem. Eng. 2020, 29, 17–25. [Google Scholar] [CrossRef]
- Waghchoure, A.P.; Reddy, J.P.; Bhosale, R.S. Fluorescence Based Miniaturized Microfluidic and Nanofluidic Systems for Biomedical Applications, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2022; Volume 186, ISBN 9780323988995. [Google Scholar]
- Meng, L.; He, X.; Gao, J.; Li, J.; Wei, Y.; Yan, J. A Novel Nanofabrication Technique of Silicon-Based Nanostructures. Nanoscale Res. Lett. 2016, 11, 2004595. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, Z.; Zheng, J.; Shi, J.; Zeng, B.F.; Yang, Y.; Hong, W.; Tian, Z.Q. Application of Micro/Nanofabrication Techniques to On-Chip Molecular Electronics. Small Methods 2021, 5, 2001034. [Google Scholar] [CrossRef]
- Weatherall, E.; Hauer, P.; Vogel, R.; Willmott, G.R. Pulse Size Distributions in Tunable Resistive Pulse Sensing. Anal. Chem. 2016, 88, 8648–8656. [Google Scholar] [CrossRef]
- Villone, M.M.; Nunes, J.K.; Li, Y.; Stone, H.A.; Maffettone, P.L. Design of a microfluidic device for the measurement of the elastic modulus of deformable particles. Soft Matter 2019, 15, 880–889. [Google Scholar] [CrossRef]
- Jia, Q.; Ye, W.; Zhang, C.; Jia, Z.; Liu, J.; Wang, T.; Zhao, L.; Liu, Y.; Wang, C.; Sun, P.; et al. Wearable Multimodal Sensing System for Synchronously Health-Environmental Monitoring via Hybrid Neuroevolutionary Signal Decoupling. Nano Lett. 2025, 25, 9726–9733. [Google Scholar] [CrossRef] [PubMed]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Hu, R.; Tong, X.; Zhao, Q. Four Aspects about Solid-State Nanopores for Protein Sensing: Fabrication, Sensitivity, Selectivity, and Durability. Adv. Healthc. Mater. 2020, 9, e2000933. [Google Scholar] [CrossRef]
- Adela Booth, M.; Vogel, R.; Curran, J.M.; Harbison, S.A.; Travas-Sejdic, J. Detection of target-probe oligonucleotide hybridization using synthetic nanopore resistive pulse sensing. Biosens. Bioelectron. 2013, 45, 136–140. [Google Scholar] [CrossRef]
- Vogel, R.; Coumans, F.A.W.; Maltesen, R.G.; Böing, A.N.; Bonnington, K.E.; Broekman, M.L.; Broom, M.F.; Buzás, E.I.; Christiansen, G.; Hajji, N.; et al. A standardized method to determine the concentration of extracellular vesicles using tunable resistive pulse sensing. J. Extracell. Vesicles 2016, 5, 31242. [Google Scholar] [CrossRef]
- Misra, R.; Fung, G.; Sharma, S.; Hu, J.; Kirkitadze, M. Assessment of Tunable Resistive Pulse Sensing (TRPS) Technology for Particle Size Distribution in Vaccine Formulations—A Comparative Study with Dynamic Light Scattering. Pharm. Res. 2024, 41, 1021–1029. [Google Scholar] [CrossRef]
- Blundell, E.L.C.J.; Vogel, R.; Platt, M. Particle-by-Particle Charge Analysis of DNA-Modified Nanoparticles Using Tunable Resistive Pulse Sensing. Langmuir 2016, 32, 1082–1090. [Google Scholar] [CrossRef]
- Akpinar, F.; Yin, J. Characterization of vesicular stomatitis virus populations by tunable resistive pulse sensing. J. Virol. Methods 2015, 218, 71–76. [Google Scholar] [CrossRef]
- Vecchio, G.; Amaducci, S.; Cosentino, L.; Finocchiaro, P. Pulse identification and shape analysis by derivative-based peak detection using a Convolutional Neural Network. J. Instrum. 2022, 17, P09040. [Google Scholar] [CrossRef]
- Yilmaz, D.; Kaya, D.; Kececi, K.; Dinler, A. Role of Nanopore Geometry in Particle Resolution by Resistive-Pulse Sensing. ChemistrySelect 2021, 6, 59–67. [Google Scholar] [CrossRef]
- Pollard, M.; Hunsicker, E.; Platt, M. A Tunable Three-Dimensional Printed Microfluidic Resistive Pulse Sensor for the Characterization of Algae and Microplastics. ACS Sens. 2020, 5, 2578–2586. [Google Scholar] [CrossRef]
- Hsu, W.L.; Hwang, J.; Daiguji, H. Theory of Transport-Induced-Charge Electroosmotic Pumping toward Alternating Current Resistive Pulse Sensing. ACS Sens. 2018, 3, 2320–2326. [Google Scholar] [CrossRef]
- Vaclavek, T.; Prikryl, J.; Foret, F. Resistive pulse sensing as particle counting and sizing method in microfluidic systems: Designs and applications review. J. Sep. Sci. 2019, 42, 445–457. [Google Scholar] [CrossRef]
- Das, N.; Chakraborty, B.; RoyChaudhuri, C. A review on nanopores based protein sensing in complex analyte. Talanta 2022, 243, 123368. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, D. A method to improve the resistive pulse sensing by modifying surface charge of nanochannels. Sens. Actuators B Chem. 2021, 337, 129773. [Google Scholar] [CrossRef]
- Weatherall, E.; Willmott, G.R. Conductive and biphasic pulses in tunable resistive pulse sensing. J. Phys. Chem. B 2015, 119, 5328–5335. [Google Scholar] [CrossRef]
- Hu, Z.L.; Huo, M.Z.; Ying, Y.L.; Long, Y.T. Biological Nanopore Approach for Single-Molecule Protein Sequencing. Angew. Chem.—Int. Ed. 2021, 60, 14738–14749. [Google Scholar] [CrossRef]
- Halimeh, I.; Cao, C.; Baaken, G.; Long, Y.-T.; Behrends, J.C. Length- and Species-Selective Detection of Short Oligonucleotides using a Microelectrode Cavity Array of Biological Nanopores. Biophys. J. 2016, 110, 200a. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, L.; Wang, C.; Zhong, C.; Wei, N.; Ying, Y.; Long, Y.; Yan, F.; Zhang, L. A High-Bandwidth, Low-Noise Front-End Readout Integrated Chip for Nanopore. Int. J. Circuit Theory Appl. 2025, 1–13. [Google Scholar] [CrossRef]
- Huang, G.; Willems, K.; Bartelds, M.; Van Dorpe, P.; Soskine, M.; Maglia, G. Electro-osmotic vortices promote the capture of folded proteins by plyab nanopores. Nano Lett. 2020, 20, 3819–3827. [Google Scholar] [CrossRef]
- Celaya, G.; Perales-Calvo, J.; Muga, A.; Moro, F.; Rodriguez-Larrea, D. Label-Free, Multiplexed, Single-Molecule Analysis of Protein-DNA Complexes with Nanopores. ACS Nano 2017, 11, 5815–5825. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, S.; Zhang, P.; Zeng, Z.; Zhao, D.; Wang, J.; Chen, H.; Huang, S. Osmosis-Driven Motion-Type Modulation of Biological Nanopores for Parallel Optical Nucleic Acid Sensing. ACS Appl. Mater. Interfaces 2018, 10, 7788–7797. [Google Scholar] [CrossRef]
- Huang, S. Microscopic Imaging of Restriction Engineered Biological Nanopores for Highly Specific Spotting of Epigenetic Markers. Biophys. J. 2019, 116, 149a. [Google Scholar] [CrossRef]
- Zhang, Z.; Bloch, D.; Yu, L.; Chen, Y.; Kang, X.; Makhamreh, A.K.; Foster, J.C.; Maglia, G.; Chen, M.; Wanunu, M. Terminal tagging of full-length proteins for enhanced capturing by biological nanopores. Biophys. J. 2024, 123, 149a. [Google Scholar] [CrossRef]
- Jeong, K.B.; Luo, K.; Lee, H.; Lim, M.C.; Yu, J.; Choi, S.J.; Kim, K.B.; Jeon, T.J.; Kim, Y.R. Alpha-Hederin Nanopore for Single Nucleotide Discrimination. ACS Nano 2019, 13, 1719–1727. [Google Scholar] [CrossRef] [PubMed]
- Motone, K.; Cardozo, N.; Nivala, J. Herding cats: Label-based approaches in protein translocation through nanopore sensors for single-molecule protein sequence analysis. iScience 2021, 24, 103032. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Yoo, J.; Aksimentiev, A. Water Mediates Recognition of DNA Sequence via Ionic Current Blockade in a Biological Nanopore. ACS Nano 2016, 10, 4644–4651. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Chen, X.; Guan, X.; Wang, L. Analysis with biological nanopore: On-pore, off-pore strategies and application in biological fluids. Talanta 2021, 223, 121684. [Google Scholar] [CrossRef]
- Mohammadi, M.M.; Bavi, O. DNA sequencing: An overview of solid-state and biological nanopore-based methods. Biophys. Rev. 2022, 14, 99–110. [Google Scholar] [CrossRef]
- Restrepo-Pérez, L.; Huang, G.; Bohländer, P.R.; Worp, N.; Eelkema, R.; Maglia, G.; Joo, C.; Dekker, C. Resolving Chemical Modifications to a Single Amino Acid within a Peptide Using a Biological Nanopore. ACS Nano 2019, 13, 13668–13676. [Google Scholar] [CrossRef]
- Watanabe, H.; Gubbiotti, A.; Chinappi, M.; Takai, N.; Tanaka, K.; Tsumoto, K.; Kawano, R. Analysis of Pore Formation and Protein Translocation Using Large Biological Nanopores. Anal. Chem. 2017, 89, 11269–11277. [Google Scholar] [CrossRef] [PubMed]
- Chanakul, W.; Mukhopadhyay, A.; Awasthi, S.; Protopopova, A.D.; Ianiro, A.; Mayer, M. Large and Stable Nanopores Formed by Complement Component 9 for Characterizing Single Folded Proteins. ACS Nano 2025, 19, 5240–5252. [Google Scholar] [CrossRef] [PubMed]
- Straathof, S.; Di Muccio, G.; Maglia, G. Nanopores with an Engineered Selective Entropic Gate Detect Proteins at Nanomolar Concentration in Complex Biological Sample. J. Am. Chem. Soc. 2025, 147, 15050–15065. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Wang, J.; Tang, R.; Jiang, Y.; Xi, D. Nucleic Acid-Based Biological Nanopore Sensing Strategies for Tumor Marker Detection. Langmuir 2024, 40, 21327–21340. [Google Scholar] [CrossRef]
- Yin, Y.D.; Zhang, L.; Leng, X.Z.; Gu, Z.Y. Harnessing biological nanopore technology to track chemical changes. TrAC—Trends Anal. Chem. 2020, 133, 116091. [Google Scholar] [CrossRef]
- Willems, K.; Ruic, D.; Lucas, F.L.R.; Barman, U.; Verellen, N.; Hofkens, J.; Maglia, G.; Van Dorpe, P. Accurate modeling of a biological nanopore with an extended continuum framework. Nanoscale 2020, 12, 16775–16795. [Google Scholar] [CrossRef]
- Perera, R.T.; Fleming, A.M.; Peterson, A.M.; Heemstra, J.M.; Burrows, C.J.; White, H.S. Unzipping of A-Form DNA-RNA, A-Form DNA-PNA, and B-Form DNA-DNA in the α-Hemolysin Nanopore. Biophys. J. 2016, 110, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Acharya, A.; Prajapati, J.D.; Kleinekathöfer, U. Atomistic Simulation of Molecules Interacting with Biological Nanopores: From Current Understanding to Future Directions. J. Phys. Chem. B 2022, 126, 3995–4008. [Google Scholar] [CrossRef]
- Cao, C.; Long, Y.T. Biological Nanopores: Confined Spaces for Electrochemical Single-Molecule Analysis. Acc. Chem. Res. 2018, 51, 331–341. [Google Scholar] [CrossRef]
- Fang, Z.; Liu, L.; Wang, Y.; Xi, D.; Zhang, S. Unambiguous Discrimination of Multiple Protein Biomarkers by Nanopore Sensing with Double-Stranded DNA-Based Probes. Anal. Chem. 2020, 92, 1730–1737. [Google Scholar] [CrossRef]
- Cao, C.; Magalhães, P.; Krapp, L.F.; Bada Juarez, J.F.; Mayer, S.F.; Rukes, V.; Chiki, A.; Lashuel, H.A.; Dal Peraro, M. Deep Learning-Assisted Single-Molecule Detection of Protein Post-translational Modifications with a Biological Nanopore. ACS Nano 2024, 18, 1504–1515. [Google Scholar] [CrossRef]
- Xi, D.; Li, Z.; Liu, L.; Ai, S.; Zhang, S. Ultrasensitive Detection of Cancer Cells Combining Enzymatic Signal Amplification with an Aerolysin Nanopore. Anal. Chem. 2018, 90, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Yin, B.; Tang, P.; Wang, L.; Xie, W.; Chen, X.; Wang, Y.; Weng, T.; Tian, R.; Zhou, S.; Wang, Z.; et al. An aptamer-assisted nanopore strategy with a salt gradient for direct protein sensing. J. Mater. Chem. B 2023, 11, 11064–11072. [Google Scholar] [CrossRef] [PubMed]
- Hiratani, M.; Kawano, R. DNA Logic Operation with Nanopore Decoding to Recognize MicroRNA Patterns in Small Cell Lung Cancer. Anal. Chem. 2018, 90, 8531–8537. [Google Scholar] [CrossRef]
- Skalová, Š.; Vyskočil, V.; Barek, J.; Navrátil, T. Model Biological Membranes and Possibilities of Application of Electrochemical Impedance Spectroscopy for their Characterization. Electroanalysis 2018, 30, 207–219. [Google Scholar] [CrossRef]
- Healey, M.J.; Sivakumaran, M.; Platt, M. Rapid quantification of prion proteins using resistive pulse sensing. Analyst 2020, 145, 2595–2601. [Google Scholar] [CrossRef]
- Yan, H.; Chen, T.; Hu, G.; Ma, J.; Wu, L.; Tu, J. A long-term stable solid-state nanopore for the dynamic monitoring of DNA synthesis. Anal. Chim. Acta 2025, 1344, 343710. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Liu, Y.; Fang, S.; Li, Y.; Weng, T.; Tian, R.; Yin, Y.; Zhou, D.; Yin, B.; Wang, Y.; et al. Solid-State nanopore DNA Sequencing: Advances, challenges and prospects. Coord. Chem. Rev. 2024, 510, 215816. [Google Scholar] [CrossRef]
- Muhammad Sajeer, P.; Simran; Nukala, P.; Manoj, M.V. TEM based applications in solid state nanopores: From fabrication to liquid in-situ bio-imaging. Micron 2022, 162, 103347. [Google Scholar] [CrossRef]
- Eggenberger, O.M.; Ying, C.; Mayer, M. Surface coatings for solid-state nanopores. Nanoscale 2019, 11, 19636–19657. [Google Scholar] [CrossRef]
- Zhang, Q.; Cheng, Y.; Cao, P.; Gu, Z. Solid-state nanopores for ion and small molecule analysis. Chin. Chem. Lett. 2019, 30, 1607–1617. [Google Scholar] [CrossRef]
- Tian, R.; Weng, T.; Chen, S.; Wu, J.; Yin, B.; Ma, W.; Liang, L.; Xie, W.; Wang, Y.; Zeng, X.; et al. DNA nanostructure-assisted detection of carcinoembryonic antigen with a solid-state nanopore. Bioelectrochemistry 2023, 149, 108284. [Google Scholar] [CrossRef]
- Raveendran, M.; Leach, A.R.; Hopes, T.; Aspden, J.L.; Actis, P. Ribosome Fingerprinting with a Solid-State Nanopore. ACS Sens. 2020, 5, 3533–3539. [Google Scholar] [CrossRef]
- Goto, Y.; Akahori, R.; Yanagi, I. Single Molecule and Single Cell Sequencing; Springer Nature: Singapore, 2019; Volume 1129, ISBN 978-981-13-6036-7. [Google Scholar]
- Beamish, E.; Tabard-Cossa, V.; Godin, M. Identifying Structure in Short DNA Scaffolds Using Solid-State Nanopores. ACS Sens. 2017, 2, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Shi, X.; Liu, S.C.; Ying, Y.L.; Li, Q.; Gao, R.; Fathi, F.; Long, Y.T.; Tian, H. Characterization of DNA duplex unzipping through a sub-2 nm solid-state nanopore. Chem. Commun. 2017, 53, 3539–3542. [Google Scholar] [CrossRef]
- Li, X.-N.; Li, Y.-X.; Yang, Y.; Wang, G.; Liu, H.-F.; Mohammad, M.A.; Qiu, H.-C.; Ren, T.-L. Fabrication of Hourglass-Shaped Solid-State Nanopores. ECS J. Solid State Sci. Technol. 2016, 5, P228–P232. [Google Scholar] [CrossRef]
- Roman, J.; Français, O.; Jarroux, N.; Patriarche, G.; Pelta, J.; Bacri, L.; Le Pioufle, B. Solid-State Nanopore Easy Chip Integration in a Cheap and Reusable Microfluidic Device for Ion Transport and Polymer Conformation Sensing. ACS Sens. 2018, 3, 2129–2137. [Google Scholar] [CrossRef]
- Shi, X.; Li, Q.; Gao, R.; Si, W.; Liu, S.C.; Aksimentiev, A.; Long, Y.T. Dynamics of a Molecular Plug Docked onto a Solid-State Nanopore. J. Phys. Chem. Lett. 2018, 9, 4686–4694. [Google Scholar] [CrossRef]
- Li, J.; Yu, D.; Zhao, Q. Solid-state nanopore-based DNA single molecule detection and sequencing. Microchim. Acta 2016, 183, 941–953. [Google Scholar] [CrossRef]
- Zhou, W.; Guo, Y.; Guo, W.; Qiu, H. High-Resolution and Low-Noise Single-Molecule Sensing with Bio-Inspired Solid-State Nanopores. J. Phys. Chem. Lett. 2024, 15, 5556–5563. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dai, Y.; Sun, J.; Shen, J.; Lin, M.; Xia, F. Solid-State Nanopore/Nanochannel Sensors with Enhanced Selectivity through Pore-in Modification. Anal. Chem. 2024, 96, 2277–2285. [Google Scholar] [CrossRef]
- Zahid, O.K.; Zhao, B.S.; He, C.; Hall, A.R. Quantifying mammalian genomic DNA hydroxymethylcytosine content using solid-state nanopores. Sci. Rep. 2016, 6, 29565. [Google Scholar] [CrossRef]
- Fujinami Tanimoto, I.M.; Zhang, J.; Cressiot, B.; Le Pioufle, B.; Bacri, L.; Pelta, J. Dynamics of DNA Through Solid-state Nanopores Fabricated by Controlled Dielectric Breakdown. Chem.—An Asian J. 2022, 17, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Peng, B.; Sabuncu, A.C.; Nam, S.; Ahn, C.W.; Kim, M.J.; Kim, M.J. Multiple consecutive recapture of rigid nanoparticles using a solid-state nanopore sensor. Electrophoresis 2018, 39, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, Z.Q.; Zheng, Y.W.; Song, J.; Zhao, W.W.; Xu, J.J. Bridging Ionic Current Rectification and Resistive-Pulse Sensing for Reliable Wide-Linearity Detection. Anal. Chem. 2024, 96, 6444–6449. [Google Scholar] [CrossRef]
- Young, T.W.; Kappler, M.P.; Call, E.D.; Brown, Q.J.; Jacobson, S.C. Integrated In-Plane Nanofluidic Devices for Resistive-Pulse Sensing. Annu. Rev. Anal. Chem. 2024, 17, 221–242. [Google Scholar] [CrossRef]
- Tognoni, E.; Adinolfi, B.; Ballestri, M.; Varchi, G.; Pellegrino, M. Effect of capillary action and gravitational force on resistive pulse sensing with nanopipettes. J. Electroanal. Chem. 2024, 975, 118764. [Google Scholar] [CrossRef]
- Secme, A.; Kucukoglu, B.; Pisheh, H.S.; Alatas, Y.C.; Tefek, U.; Uslu, H.D.; Kaynak, B.E.; Alhmoud, H.; Hanay, M.S. Dielectric Detection of Single Nanoparticles Using a Microwave Resonator Integrated with a Nanopore. ACS Omega 2023, 9, 7827–7834. [Google Scholar] [CrossRef]
- Schmeltzer, A.J.; Peterson, E.M.; Harris, J.M.; Lathrop, D.K.; German, S.R.; White, H.S. Simultaneous Multipass Resistive-Pulse Sensing and Fluorescence Imaging of Liposomes. ACS Nano 2024, 18, 7241–7252. [Google Scholar] [CrossRef]
- Tsutsui, M.; Wada, M.; Arima, A.; Tsunekawa, Y.; Sasaki, T.; Sakamoto, K.; Yokota, K.; Baba, Y.; Kawai, T.; Okada, T. Identifying Viral Vector Characteristics by Nanopore Sensing. ACS Nano 2024, 18, 15695–15704. [Google Scholar] [CrossRef] [PubMed]
- Terejánszky, P.; Papp, S.; Berényi, S.; Gyurcsányi, R.E. Resistive Pulse Sensing as a High-Resolution Nanoparticle Sizing Method: A Comparative Study. Part. Part. Syst. Charact. 2019, 36, 1800543. [Google Scholar] [CrossRef]
- Pan, R.; Wang, D.; Liu, K.; Chen, H.Y.; Jiang, D. Electrochemical Molecule Trap-Based Sensing of Low-Abundance Enzymes in One Living Cell. J. Am. Chem. Soc. 2022, 144, 17558–17566. [Google Scholar] [CrossRef] [PubMed]
- Young, T.W.; Kappler, M.P.; Hockaden, N.M.; Carpenter, R.L.; Jacobson, S.C. Characterization of Extracellular Vesicles by Resistive-Pulse Sensing on In-Plane Multipore Nanofluidic Devices. Anal. Chem. 2023, 95, 16710–16716. [Google Scholar] [CrossRef]
- Kurtjak, M.; Kereïche, S.; Klepac, D.; Križan, H.; Perčić, M.; Alić, V.K.; Lavrin, T.; Lenassi, M.; Wechtersbach, K.; Kojc, N.; et al. Unveiling the Native Morphology of Extracellular Vesicles from Human Cerebrospinal Fluid by Atomic Force and Cryogenic Electron Microscopy. Biomedicines 2022, 10, 1251. [Google Scholar] [CrossRef]
- Zhou, J.; Zlotnick, A.; Jacobson, S.C. Disassembly of Single Virus Capsids Monitored in Real Time with Multicycle Resistive-Pulse Sensing. Anal. Chem. 2022, 94, 985–992. [Google Scholar] [CrossRef]
- Meyer, N.; Bentin, J.; Janot, J.M.; Abrao-Nemeir, I.; Charles-Achille, S.; Pratlong, M.; Aquilina, A.; Trinquet, E.; Perrier, V.; Picaud, F.; et al. Ultrasensitive Detection of Aβ42 Seeds in Cerebrospinal Fluid with a Nanopipette-Based Real-Time Fast Amyloid Seeding and Translocation Assay. Anal. Chem. 2023, 95, 12623–12630. [Google Scholar] [CrossRef]
- Symonds, E.K.C.; Black, B.; Brown, A.; Meredith, I.; Currie, M.J.; Hally, K.E.; Danielson, K.M. Adipose derived stem cell extracellular vesicles modulate primary human macrophages to an anti-inflammatory phenotype in vitro. J. Extracell. Biol. 2023, 2, e104. [Google Scholar] [CrossRef]
- Seršić, L.V.; Alić, V.K.; Biberić, M.; Zrna, S.; Jagoić, T.; Tarčuković, J.; Grabušić, K. Real-Time PCR Quantification of 87 miRNAs from Cerebrospinal Fluid: miRNA Dynamics and Association with Extracellular Vesicles after Severe Traumatic Brain Injury. Int. J. Mol. Sci. 2023, 24, 4751. [Google Scholar] [CrossRef]
- Zhang, M.; Harms, Z.D.; Greibe, T.; Starr, C.A.; Zlotnick, A.; Jacobson, S.C. In-Plane, In-Series Nanopores with Circular Cross Sections for Resistive-Pulse Sensing. ACS Nano 2022, 16, 7352–7360. [Google Scholar] [CrossRef]
- Hussein, E.A.; White, R.J. Silver Nanoneedle Probes Enable Sustained DC Current, Single-Channel Resistive Pulse Nanopore Sensing. Anal. Chem. 2021, 93, 11568–11575. [Google Scholar] [CrossRef]
- Vogel, R.; Pal, A.K.; Jambhrunkar, S.; Patel, P.; Thakur, S.S.; Reátegui, E.; Parekh, H.S.; Saá, P.; Stassinopoulos, A.; Broom, M.F. High-Resolution Single Particle Zeta Potential Characterisation of Biological Nanoparticles using Tunable Resistive Pulse Sensing. Sci. Rep. 2017, 7, 17479. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, Y.; Goda, T.; Matsumoto, A.; Takeuchi, H.; Yamaoka, S.; Miyahara, Y. Gold Nanoparticles with Ligand/Zwitterion Hybrid Layer for Individual Counting of Influenza A H1N1 Subtype Using Resistive Pulse Sensing. Langmuir 2019, 35, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Cimorelli, M.; Nieuwland, R.; Varga, Z.; van der Pol, E. Standardized procedure to measure the size distribution of extracellular vesicles together with other particles in biofluids with microfluidic resistive pulse sensing. PLoS ONE 2021, 16, e0249603. [Google Scholar] [CrossRef] [PubMed]
- Maugi, R.; Salkenova, Z.; Platt, M. Incorporating peptide aptamers into resistive pulse sensing. Med. Devices Sens. 2020, 3, e10059. [Google Scholar] [CrossRef]
- Chibuike, M.; Rathnayaka, C.; Shivanka, S.; Choi, J.; Verber, M.; Park, S.; Soper, S.A. Millisecond Label-Free Single Peptide Detection and Identification Using Nanoscale Electrochromatography and Resistive Pulse Sensing. Anal. Chem. 2024, 97, 427–435. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Shiri, F.; Verber, M.; McKinney, C.; Choi, J.; Park, S.; Surtan, J.; Prasannakumari, S.S.; Ritola, K.D.; Soper, S.A. Single-capsid identification of full and empty status of recombinant adeno-associated viruses via resistive pulse sensing. Sens. Actuators Rep. 2024, 8, 100242. [Google Scholar] [CrossRef]
- Horiguchi, Y.; Naono, N.; Sakamoto, O.; Takeuchi, H.; Yamaoka, S.; Miyahara, Y. Methodology to Detect Biological Particles Using a Biosensing Surface Integrated in Resistive Pulse Sensing. ACS Appl. Mater. Interfaces 2022, 14, 20168–20178. [Google Scholar] [CrossRef]
- Healey, M.J.; Rowe, W.; Siati, S.; Sivakumaran, M.; Platt, M. Rapid Assessment of Site Specific DNA Methylation through Resistive Pulse Sensing. ACS Sens. 2018, 3, 655–660. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, R.; Zhu, R.; Lu, W.; Wei, G.; Zhao, J.; Gu, Z.Y.; Zhao, Q. Metal–Organic Cage as Single-Molecule Carrier for Solid-State Nanopore Analysis. Small Methods 2022, 6, e2200743. [Google Scholar] [CrossRef]
- Balogun, Y.; Yang, R.; Wang, G. Resistive pulse sensing of pre-nucleation activities during single-entity lysozyme crystallization on single nanopipettes. Sens. Actuators Rep. 2025, 9, 100281. [Google Scholar] [CrossRef]
- Tsutsui, M.; Takaai, T.; Yokota, K.; Kawai, T.; Washio, T. Deep Learning-Enhanced Nanopore Sensing of Single-Nanoparticle Translocation Dynamics. Small Methods 2021, 5, 2100191. [Google Scholar] [CrossRef] [PubMed]
- Bakouei, M.; Abdorahimzadeh, S.; Taghipoor, M. Effects of cone angle and length of nanopores on the resistive pulse quality. Phys. Chem. Chem. Phys. 2020, 22, 25306–25314. [Google Scholar] [CrossRef] [PubMed]
- Shimada, T.; Fujino, K.; Yasui, T.; Kaji, N.; Ueda, Y.; Fujii, K.; Yukawa, H.; Baba, Y. Resistive Pulse Sensing on a Capillary-Assisted Microfluidic Platform for On-Site Single-Particle Analyses. Anal. Chem. 2023, 95, 18335–18343. [Google Scholar] [CrossRef]
- Maller, J.; Morgan, T.; Morita, M.; McCarthy, F.; Jung, Y.; Svensson, K.J.; Elias, J.E.; Macaubas, C.; Mellins, E. Extracellular vesicles in systemic juvenile idiopathic arthritis. J. Leukoc. Biol. 2023, 114, 387–403. [Google Scholar] [CrossRef]
- Salehirozveh, M.; Porro, A.; Thei, F. Large-scale production of polyimide micropore-based flow cells for detecting nano-sized particles in fluids. RSC Adv. 2023, 13, 873–880. [Google Scholar] [CrossRef]
- Jia, Z.; Choi, J.; Lee, S.; Soper, S.A.; Park, S. Modifying surface charge density of thermoplastic nanofluidic biosensors by multivalent cations within the slip plane of the electric double layer. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129147. [Google Scholar] [CrossRef]
- Kececi, K. A Comparable Study of Single Stranded DNA Sensing Using Track-Etched Nanopore Sensors. ChemistrySelect 2023, 8, e202302856. [Google Scholar] [CrossRef]
- Pan, W.; You, R.; Zhang, S.; Chang, Y.; Zhou, F.; Li, Q.; Chen, X.; Duan, X.; Han, Z. Tunable nanochannel resistive pulse sensing device using a novel multi-module self-assembly. Anal. Chim. Acta 2023, 1251, 341035. [Google Scholar] [CrossRef]
- Varga, Z.; Madai, M.; Kemenesi, G.; Beke-Somfai, T.; Jakab, F. Single-particle detection of native SARS-CoV-2 virions by microfluidic resistive pulse sensing. Colloids Surf. B Biointerfaces 2022, 218, 112716. [Google Scholar] [CrossRef]
- Ibrahim, P.; Denniston, R.; Mitsuhashi, H.; Yang, J.; Fiori, L.M.; Żurawek, D.; Mechawar, N.; Nagy, C.; Turecki, G. Profiling Small RNA From Brain Extracellular Vesicles in Individuals With Depression. Int. J. Neuropsychopharmacol. 2024, 27, pyae013. [Google Scholar] [CrossRef]
- Xu, R.; Ouyang, L.; Shaik, R.; Chen, H.; Zhang, G.; Zhe, J. Rapid Detection of Microparticles Using a Microfluidic Resistive Pulse Sensor Based on Bipolar Pulse-Width Multiplexing. Biosensors 2023, 13, 721. [Google Scholar] [CrossRef]
- Kececi, K.; Kaya, D.; Martin, C.R. Resistive-pulse Sensing of DNA with a Polymeric Nanopore Sensor and Characterization of DNA Translocation. ChemNanoMat 2022, 8, e202100424. [Google Scholar] [CrossRef]
- Van Bavel, N.; Issler, T.; Pang, L.; Anikovskiy, M.; Prenner, E.J. A Simple Method for Synthesis of Chitosan Nanoparticles with Ionic Gelation and Homogenization. Molecules 2023, 28, 4328. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.B.; Silva, R.J.S.; Sousa, M.F.Q.; Peixoto, C.; Roldão, A.; Carrondo, M.J.T.; Alves, P.M. Bioanalytics for Influenza Virus-Like Particle Characterization and Process Monitoring. Front. Bioeng. Biotechnol. 2022, 10, 805176. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Xu, X.; Zhang, M.; Song, X. Human urine-derived stem cell-derived exosomal miR-21-5p promotes neurogenesis to attenuate Rett syndrome via the EPha4/TEK axis. Lab. Investig. 2021, 101, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Nakatsuka, R.; Tsuji, T.; Doi, K.; Kawano, S. Synchronized resistive-pulse analysis with flow visualization for single micro- and nanoscale objects driven by optical vortex in double orifice. Sci. Rep. 2021, 11, 9323. [Google Scholar] [CrossRef]
- Han, Z.; Liu, J.; Liu, Z.; Pan, W.; Yang, Y.; Chen, X.; Gao, Y.; Duan, X. Resistive pulse sensing device with embedded nanochannel (nanochannel-RPS) for label-free biomolecule and bionanoparticle analysis. Nanotechnology 2021, 32, 295507. [Google Scholar] [CrossRef]
- Ahmadi, H.; Hossein Asli Ardebili, A.; Taghipoor, M. Fabrication of low aspect ratio solid-state pores from sub-micron to microscales utilizing crossing blades. Sens. Actuators A Phys. 2024, 377, 115682. [Google Scholar] [CrossRef]
- Calado, M.R.C.; Lage, T.C.; André, D.A.M.; Calaza, C.; Marques, C.; Herrero, C.; Piteira, J.; Montelius, L.; Petrovykh, D.Y.; Diéguez, L.; et al. Nanofluidic resistive pulse sensing for characterization of extracellular vesicles. Lab. Chip 2024, 24, 4028–4038. [Google Scholar] [CrossRef] [PubMed]
- Yarana, C.; Maneechote, C.; Khuanjing, T.; Ongnok, B.; Prathumsap, N.; Thanasrisuk, S.; Pattanapanyasat, K.; Chattipakorn, S.C.; Chattipakorn, N. Potential roles of 4HNE-adducted protein in serum extracellular vesicles as an early indicator of oxidative response against doxorubicin-induced cardiomyopathy in rats. Curr. Res. Toxicol. 2023, 5, 100134. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Kwon, S.Y.; Lee, J.Y.; Kim, S.D.; Kim, D.Y.; Kim, H.; Jang, N.; Wang, J.; Han, M.; Kong, S.H. High-throughput multi-gate microfluidic resistive pulse sensing for biological nanoparticle detection. Lab Chip 2023, 23, 1945–1953. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Zhao, K.; Jia, H.; Wei, Z.; Huo, Y.; Zhang, Y.; Zhang, K. Insights to Resistive Pulse Sensing of Microparticle and Biological Cells on Microfluidic Chip. Biosensors 2025, 15, 496. https://doi.org/10.3390/bios15080496
Yao Y, Zhao K, Jia H, Wei Z, Huo Y, Zhang Y, Zhang K. Insights to Resistive Pulse Sensing of Microparticle and Biological Cells on Microfluidic Chip. Biosensors. 2025; 15(8):496. https://doi.org/10.3390/bios15080496
Chicago/Turabian StyleYao, Yiming, Kai Zhao, Haoxin Jia, Zhengxing Wei, Yiyang Huo, Yi Zhang, and Kaihuan Zhang. 2025. "Insights to Resistive Pulse Sensing of Microparticle and Biological Cells on Microfluidic Chip" Biosensors 15, no. 8: 496. https://doi.org/10.3390/bios15080496
APA StyleYao, Y., Zhao, K., Jia, H., Wei, Z., Huo, Y., Zhang, Y., & Zhang, K. (2025). Insights to Resistive Pulse Sensing of Microparticle and Biological Cells on Microfluidic Chip. Biosensors, 15(8), 496. https://doi.org/10.3390/bios15080496







