A Low-Cost Hydrogel Electrode for Multifunctional Sensing: Strain, Temperature, and Electrophysiology
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
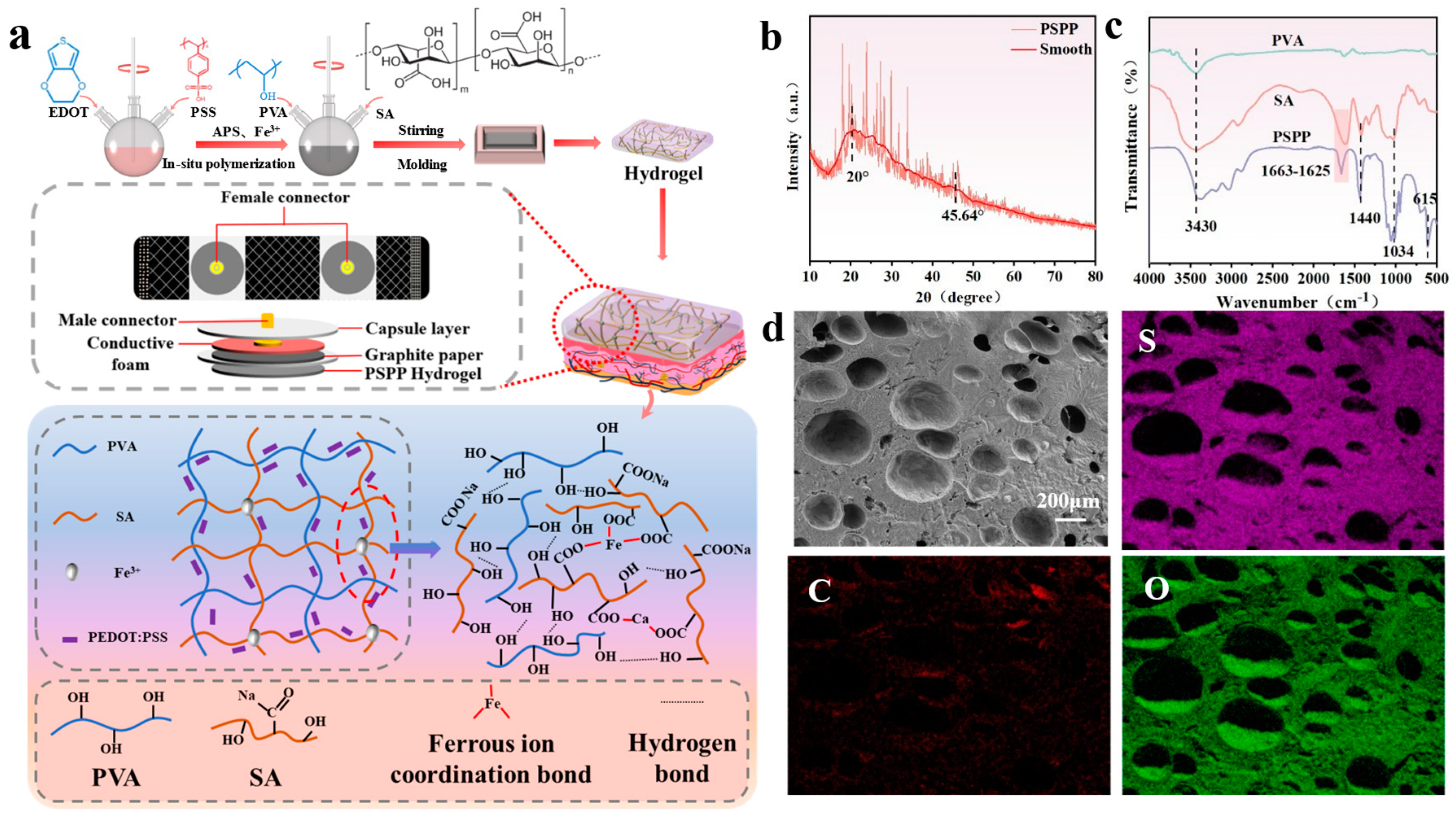
2.2. Preparation of PVA/SA/PEDOT:PSS Hydrogel
2.3. Characterization and Testing
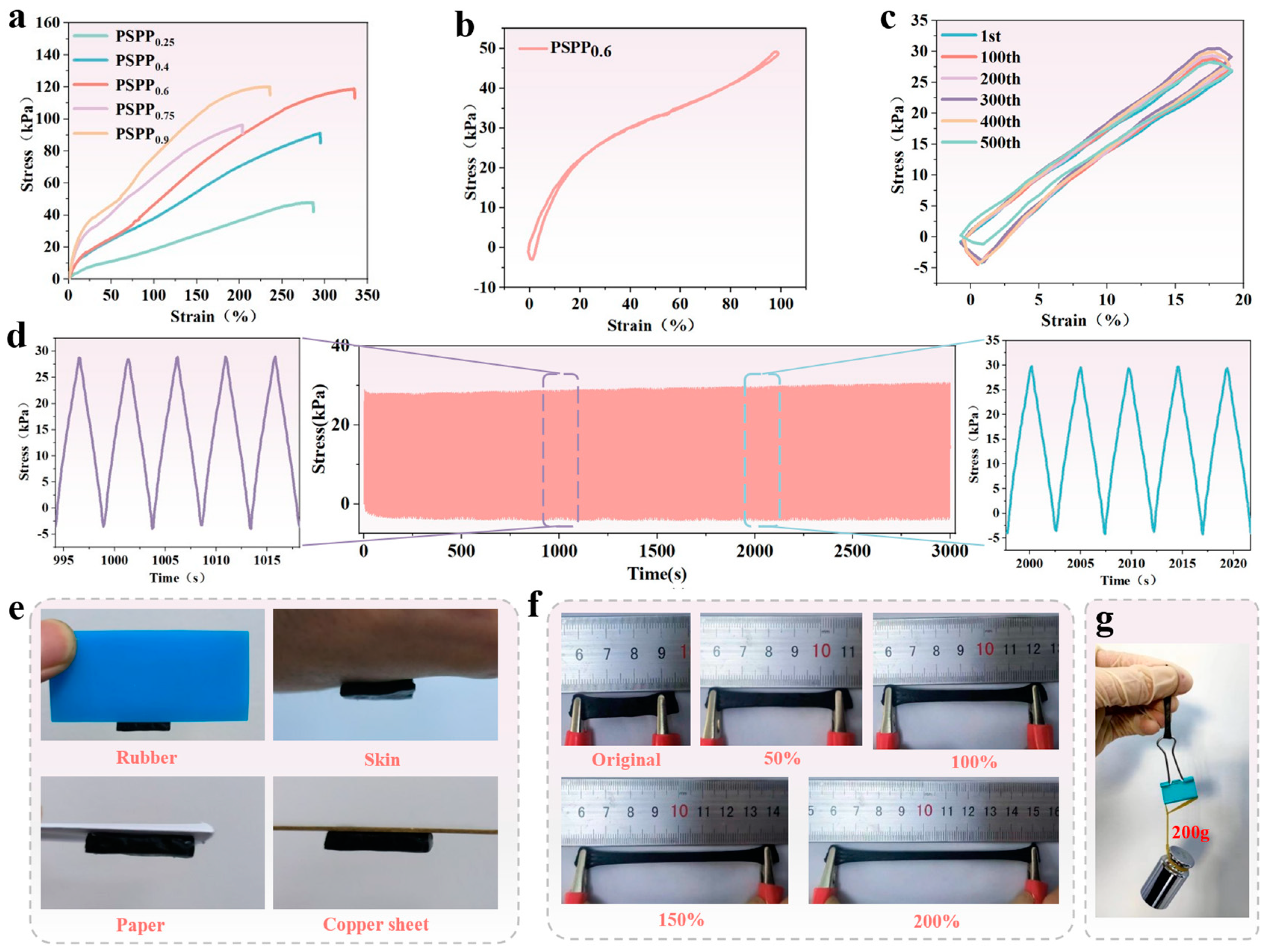
2.4. Mechanical Properties of PSPP Hydrogels
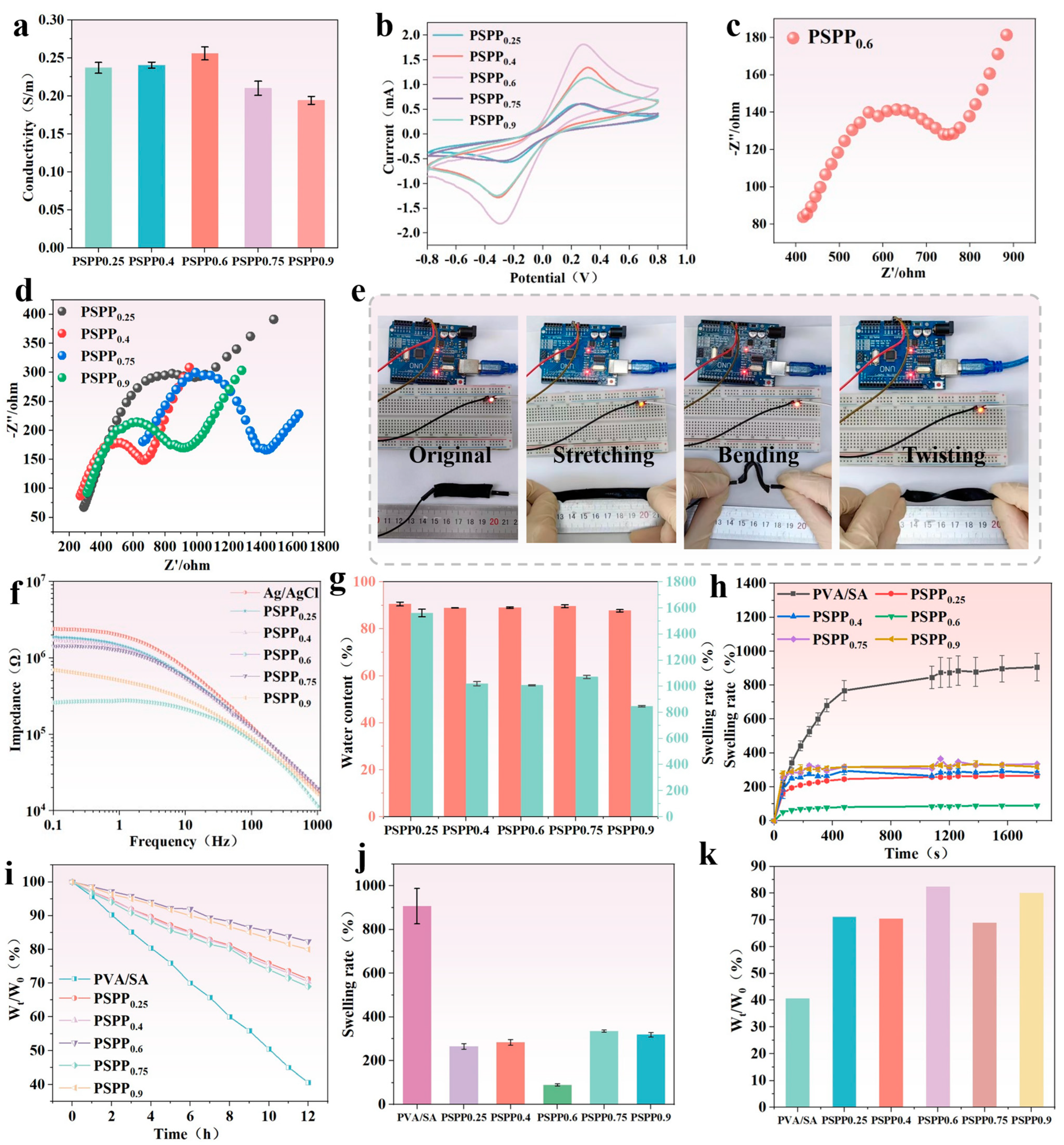
2.5. Electrochemical Properties of PSPP Hydrogels
2.6. Contact Impedance of PSPP Hydrogel with Skin
2.7. Swelling Ratio and Water Content of PSPP Hydrogel
2.8. Sensing Properties of PSPP Hydrogel
3. Results and Discussion
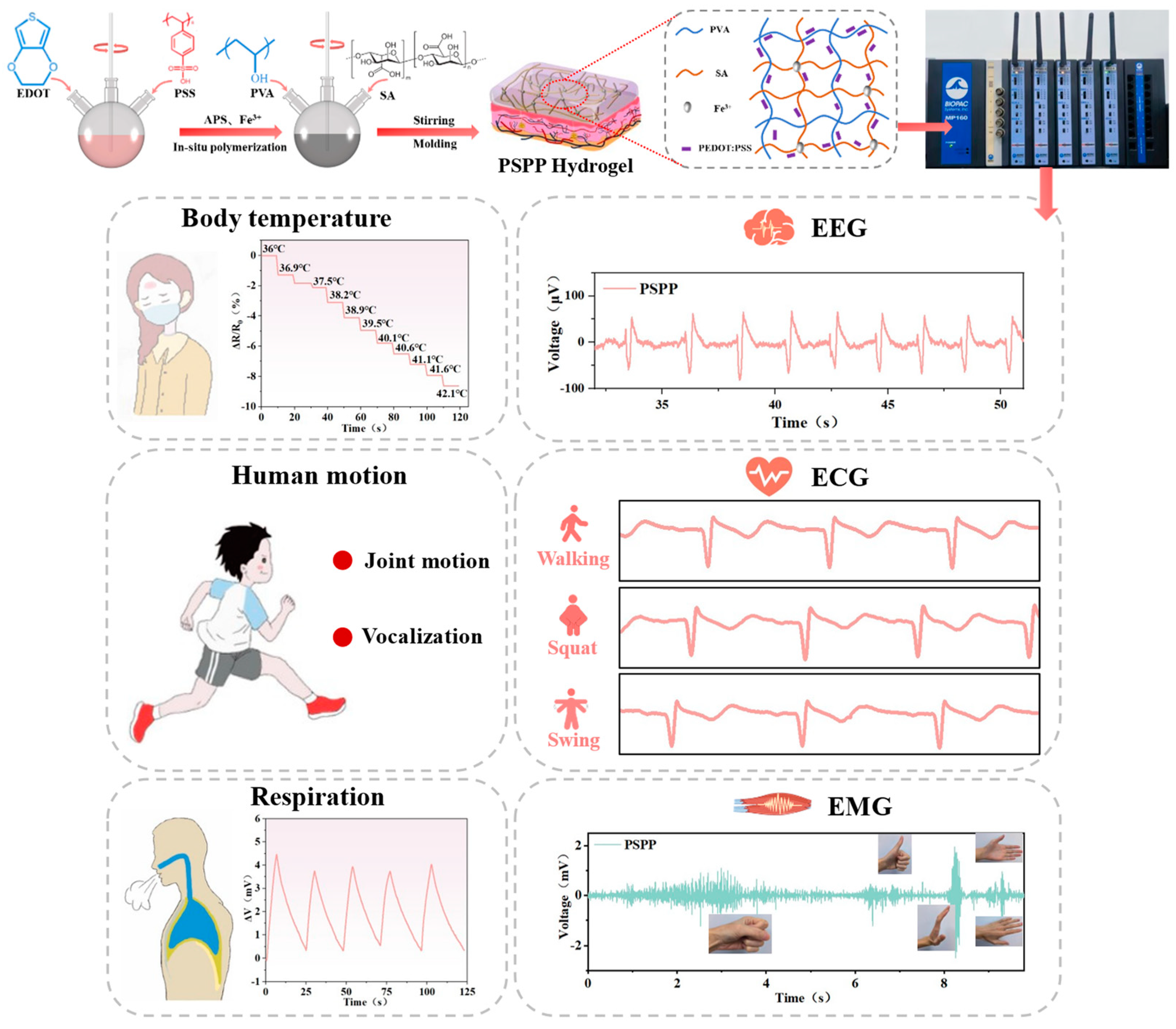
3.1. Structural Design of PSPP Hydrogel
3.2. Mechanical Properties of PSPP Hydrogel
3.3. Electrochemical Performance of PSPP Hydrogel
3.4. Swelling Behavior and Water Content of PSPP Hydrogel
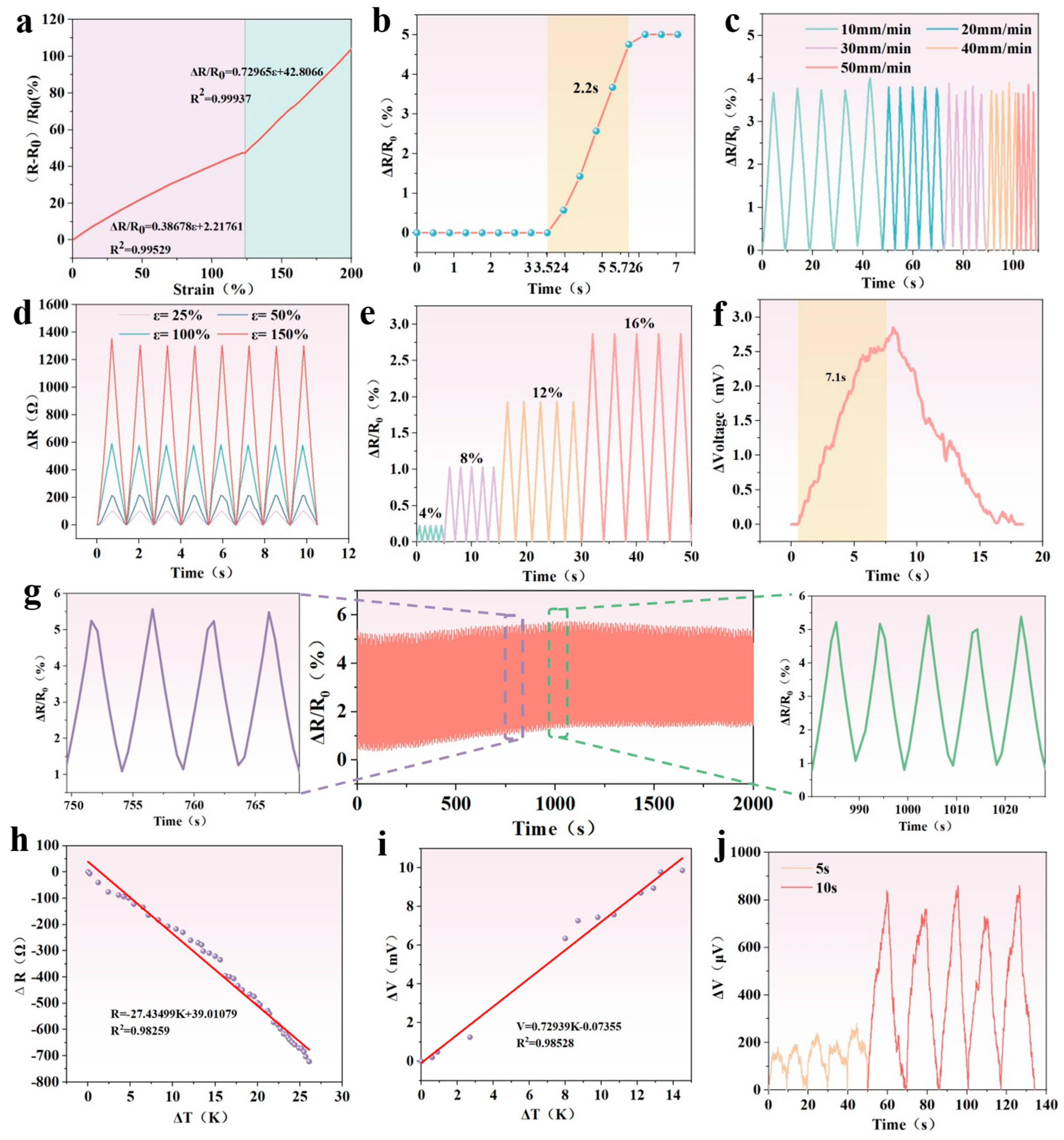
3.5. Strain Sensing of PSPP Hydrogel
3.6. Temperature Sensing of PSPP Hydrogel
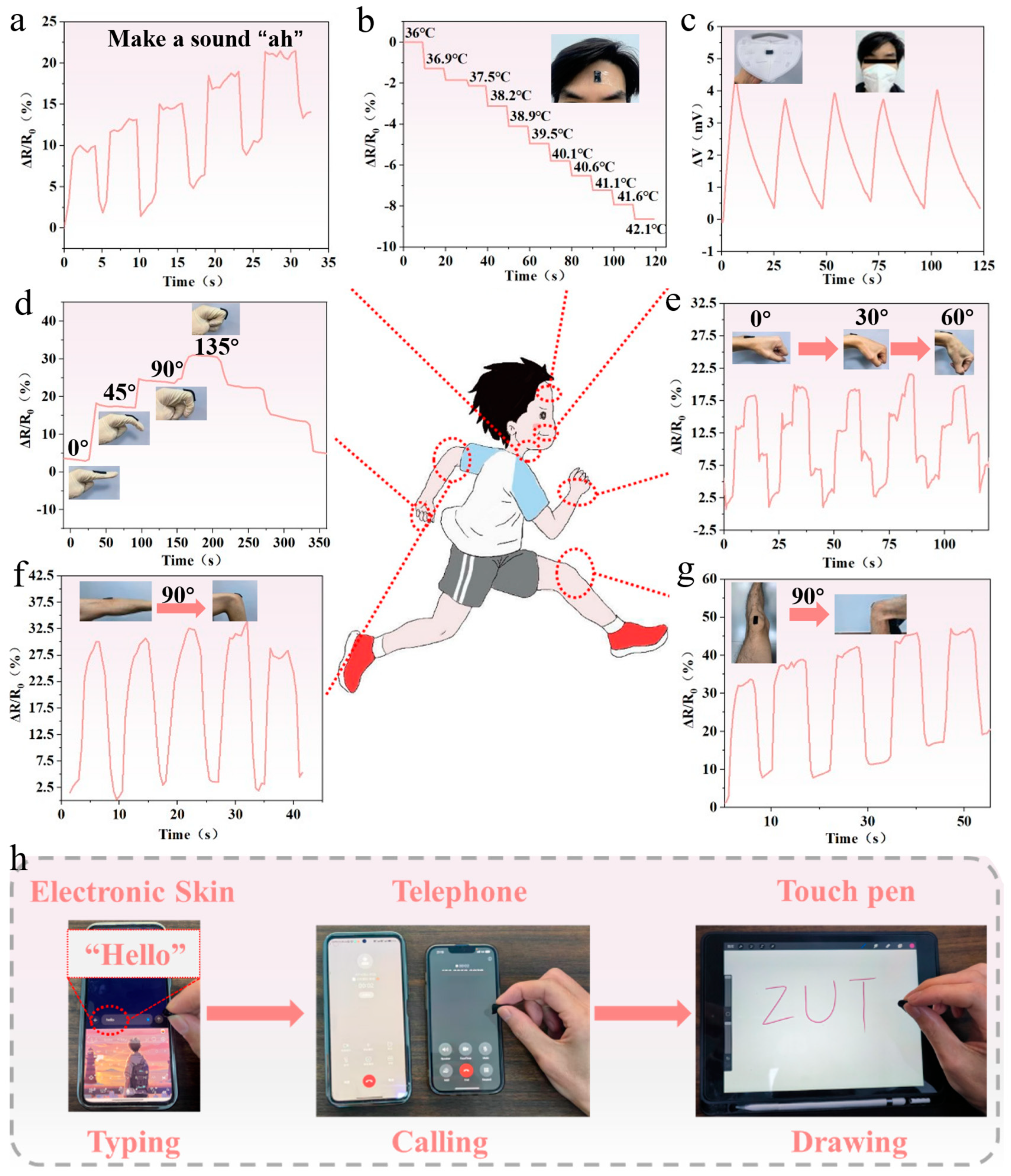
3.7. Monitoring Human Motion with PSPP Hydrogel
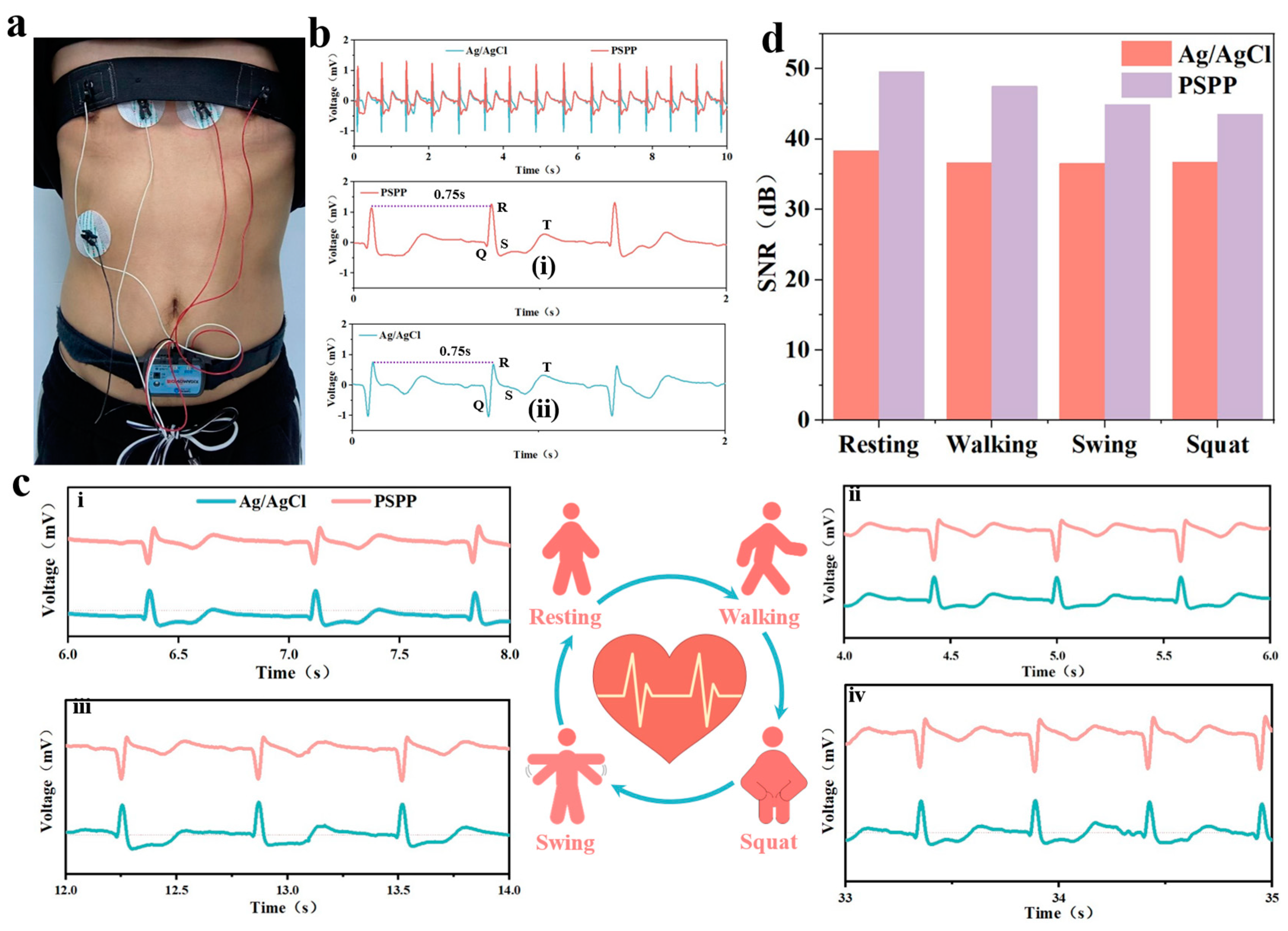
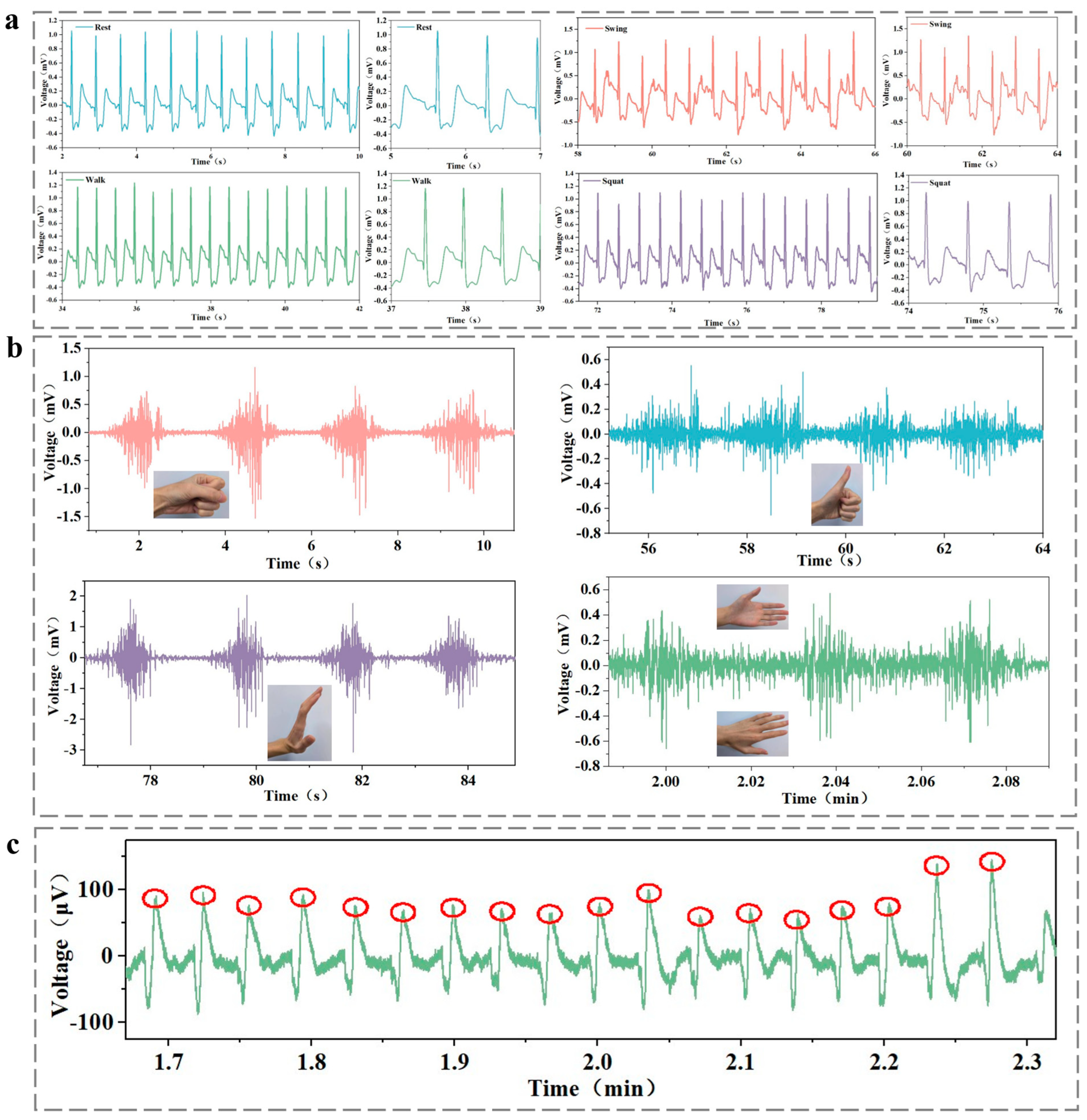
3.8. Monitoring Electrophysiological Signals with PSPP Hydrogel
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Fang, Z.; Wei, D.; Liu, Y. Flexible Pressure, Humidity, and Temperature Sensors for Human Health Monitoring. Adv. Healthc. Mater. 2024, 13, 2401532. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, B.S.; Mitra, R.; Manju, U. Titania nanoparticle-stimulated ultralow frequency detection and high-pass filter behavior of a flexible piezoelectric nanogenerator: A self-sustaining energy harvester for active motion tracking. ACS Appl. Mater. Interfaces 2023, 15, 45812–45822. [Google Scholar] [CrossRef] [PubMed]
- Ruan, D.; Chen, G.; Luo, X.; Chen, Y.; Wu, H.; Liu, A. Bionic octopus-like flexible three-dimensional force sensor for meticulous handwriting recogn Liu Y ition in human-computer interactions. Nano Energy 2024, 123, 109357. [Google Scholar] [CrossRef]
- Mo, F.; Zhou, P.; Lin, S.; Zhong, J.; Wang, Y. A Review of Conductive Hydrogel-Based Wearable Temperature Sensors. Adv. Healthc. Mater. 2024, 13, 2401503. [Google Scholar] [CrossRef]
- Chen, J.; Liu, F.; Abdiryim, T.; Liu, X. An overview of conductive composite hydrogels for flexible electronic devices. Adv. Compos. Hybrid Mater. 2024, 7, 35. [Google Scholar] [CrossRef]
- Du, T.; Zhu, Z.; Chen, M.; Yan, X.; Li, Y. Functional hydrogel strain sensors for smart electronic devices: Strategies and recent progress. ACS Appl. Electron. Mater. 2024, 6, 5402–5428. [Google Scholar] [CrossRef]
- Li, G.; Li, C.; Li, G.; Yu, D.; Song, Z.; Wang, H.; Liu, X.; Liu, H.; Liu, W. Development of conductive hydrogels for fabricating flexible strain sensors. Small 2022, 18, 2101518. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, Y.; Zhang, Y.; Zhu, P.; Mao, Y. Recent Advances of Stretchable Nanomaterial-Based Hydrogels for Wearable Sensors and Electrophysiological Signals Monitoring. Nanomaterials 2024, 14, 1398. [Google Scholar] [CrossRef]
- Ji, R.; Yan, S.; Zhu, Z.; Wang, Y.; He, D.; Wang, K.; Zhou, D.; Jia, q.; Wang, X.; Zhang, B. Ureido-Ionic Liquid Mediated Conductive Hydrogel: Superior Integrated Properties for Advanced Biosensing Applications. Adv. Sci. 2024, 11, 2401869. [Google Scholar] [CrossRef]
- Alsaafeen, N.B.; Bawazir, S.S.; Jena, K.K.; Seitak, A.; Fatma, B.; Pitsalidis, C.; Khandoker, A.; Pappa, A.M. One-Pot Synthesis of a Robust Crosslinker-Free Thermo-Reversible Conducting Hydrogel Electrode for Epidermal Electronics. ACS Appl. Mater. Interfaces 2024, 16, 61435–61445. [Google Scholar] [CrossRef]
- Yang, G.; Lan, Z.; Gong, H.; Wen, J.; Pang, B.; Qiu, Y.; Zhang, Y.; Guo, W.; Bu, T.; Xie, B. A Nepenthes-Inspired Hydrogel Hybrid System for Sweat-Wicking Electrophysiological Signal Recording during Exercises. Adv. Funct. Mater. 2024, 2417841. [Google Scholar] [CrossRef]
- Wu, Q.; Chen, A.; Xu, Y.; Han, S.; Zhang, J.; Chen, Y.; Hang, J.; Yang, X.; Guan, L. Multiple physical crosslinked highly adhesive and conductive hydrogels for human motion and electrophysiological signal monitoring. Soft Matter 2024, 20, 3666–3675. [Google Scholar] [CrossRef]
- He, M.L.; Chen, N.F.; Wang, P.X.; Chen, H.Y.; Lai, W.Y.; Liao, P.T.; Yu, J.; Wei, Y.; Chung, R.J.; Hsu, C.H.; et al. Mussel-inspired sticky self-healing conductive hydrogels composites for physiological electrical sensing. J. Mater. Chem. A 2024, 12, 22859–22866. [Google Scholar] [CrossRef]
- Su, H.; Mao, L.; Chen, X.; Liu, P.; Pu, J.; Mao, Z.; Fujiwara, T.; Ma, Y.; Mao, X.; Li, L. A Complementary Dual-Mode Ion-Electron Conductive Hydrogel Enables Sustained Conductivity for Prolonged Electroencephalogram Recording. Adv. Sci. 2024, 11, 2405273. [Google Scholar] [CrossRef] [PubMed]
- Hasan, N.; Bhuyan, M.M.; Jeong, J.H. Single/Multi-Network Conductive Hydrogels—A Review. Polymers 2024, 16, 2030. [Google Scholar] [CrossRef] [PubMed]
- Imani KB, C.; Dodda, J.M.; Yoon, J.; Torres, F.G.; Imran, A.B.; Deen, G.R.; Al-Ansari, R. Seamless Integration of Conducting Hydrogels in Daily Life: From Preparation to Wearable Application. Adv. Sci. 2024, 11, 2306784. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Lv, J.; Liu, B.; Li, N.; Zhang, H. Facile One-Pot Preparation of Polypyrrole-Incorporated Conductive Hydrogels for Human Motion Sensing. Sensors 2024, 24, 5814. [Google Scholar] [CrossRef]
- Niu, M.; Chen, K.; Li, W.; Hu, J.; Zhang, J.; Zhu, P.; Pan, Z.; Mao, Y. Ionic hydrogels-based electronic skins for electrophysiological monitoring. J. Mater. Res. 2024, 39, 188–211. [Google Scholar] [CrossRef]
- Luo, J.; Sun, C.; Chang, B.; Zhang, B.; Li, K.; Li, Y.; Zhang, Q.; Wang, H.; Hou, C. On-Skin Paintable Water-Resistant Biohydrogel for Wearable Bioelectronics. Adv. Funct. Mater. 2024, 34, 2400884. [Google Scholar] [CrossRef]
- Wu, J.; Xian, J.; He, C.; Lin, H.; Li, J.; Li, F. Asymmetric Wettability Hydrogel Surfaces for Enduring Electromyographic Monitoring. Adv. Mater. 2024, 36, 2405372. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, M.; Zhuang, L.; Li, L.; Zhao, S.; Guo, J.; Zhao, Y.; Peng, Z.; Lian, J.; Liu, B.; et al. MRI and CT compatible asymmetric bilayer hydrogel electrodes for EEG-based brain activity monitoring. Microsyst. Nanoeng. 2024, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Zhong, H.J.; Ding, H.; Yu, B.; Ma, X.; Liu, X.; Chong, C.M.; He, J. Polyvinyl alcohol (PVA)-based hydrogels: Recent progress in fabrication, properties, and multifunctional applications. Polymers 2024, 16, 2755. [Google Scholar] [CrossRef] [PubMed]
- Adelnia, H.; Ensandoost, R.; Moonshi, S.S.; Gavgani, J.N.; Vasafi, E.I.; Ta, H.T. Freeze/thawed polyvinyl alcohol hydrogels: Present, past and future. Eur. Polym. J. 2022, 164, 110974. [Google Scholar] [CrossRef]
- Li, M.; Wang, Y.; Wei, Q.; Zhang, J.; Chen, X.; An, Y. A High-Stretching, Rapid-Self-Healing, and Printable Composite Hydrogel Based on Poly (Vinyl Alcohol), Nanocellulose, and Sodium Alginate. Gels 2024, 10, 258. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Hashem, A.H.; Khattab, T.A.; Kamel, S. New antibacterial hydrogels based on sodium alginate. Int. J. Biol. Macromol. 2023, 248, 125872. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, N.; Zhang, H.; Sun, Y.; Lin, Z.; Hou, L. Preparation and characterization of poly (vinyl alcohol)/sodium alginate hydrogel with high toughness and electric conductivity. Carbohydr. Polym. 2018, 186, 377–383. [Google Scholar] [CrossRef]
- Dong, Y.; Gao, Z.; Mi, Q.; Tian, Y.; Zou, F.; Pan, C.; Tang, D.; Yu, H.Y. Highly sensitive and structure stable polyvinyl alcohol hydrogel sensor with tailored free water fraction and multiple networks by reinforcement of conductive nanocellulose. Int. J. Biol. Macromol. 2024, 281, 136128. [Google Scholar] [CrossRef]
- Ding, H.; Gu, Y.; Ren, Y.; Hu, C.; Qiu, Q.; Wu, D.; Mou, J.; Wu, Z.; Zhou, H. The latest research progress of conductive hydrogels in the field of electrophysiological signal acquisition. J. Mater. Chem. C 2024, 12, 3030–3052. [Google Scholar] [CrossRef]
- Wan, R.; Liu, S.; Li, Z.; Li, G.; Li, H.; Li, J.; Xu, J.; Liu, X. 3D printing of highly conductive and strongly adhesive PEDOT: PSS hydrogel-based bioelectronic interface for accurate electromyography monitoring. J. Colloid Interface Sci. 2025, 677, 198–207. [Google Scholar] [CrossRef]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure pedot: Pss hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y. Development of flexible and wearable temperature sensors based on PEDOT: PSS. IEEE Trans. Electron. Devices 2019, 66, 3129–3133. [Google Scholar] [CrossRef]
- Yin, Y.; Guo, C.; Li, W.; Liu, H.; Mu, Q. A super-elastic wearable strain sensor based on PU/CNTs yarns for human-motion detection. Compos. Commun. 2024, 50, 102017. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, Y.; Xu, J.; Qian, R.; Yu, Z.; Ye, C. Stretchable, adhesive and self-healing conductive hydrogels based on PEDOT: PSS-stabilized liquid metals for human motion detection. Chem. Eng. J. 2024, 494, 152971. [Google Scholar] [CrossRef]
- Chai, X.; Tang, J.; Li, Y.; Cao, Y.; Chen, X.; Chen, T.; Zhang, Z. Highly stretchable and stimulus-free self-healing hydrogels with multiple signal detection performance for self-powered wearable temperature sensors. ACS Appl. Mater. Interfaces 2023, 15, 18262–18271. [Google Scholar] [CrossRef]
- Zhu, Y.; Yao, D.; Gao, X. Recyclable Bimodal Polyvinyl Alcohol/PEDOT: PSS Hydrogel Sensors for Highly Sensitive Strain and Temperature Sensing. ACS Appl. Mater. Interfaces 2024, 16, 32466–32480. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Zhu, Y.; Wang, J.H.; Li, X.N.; Wu, X.G.; Qin, Y.X.; Chen, W.Y. Fe3+, NIR light and thermal responsive triple network composite hydrogel with multi-shape memory effect. Compos. Sci. Technol. 2021, 206, 108653. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, G.; Cheng, X.; Deng, H.; Fu, Q. Stretchable and healable conductive elastomer based on PEDOT: PSS/natural rubber for self-powered temperature and strain sensing. ACS Appl. Mater. Interfaces 2021, 13, 14599–14611. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, Z.; Wang, W.; Yu, D. PVA/CMC/PEDOT: PSS mixture hydrogels with high response and low impedance electronic signals for ECG monitoring. Colloids Surf. B Biointerfaces 2021, 208, 112088. [Google Scholar] [CrossRef]
- Hou, S.; Lv, D.; Li, Y.; Li, Z.; Liu, M.; Yu, X.; Han, Y. Highly Stretchable Self-Adhesive PEDOT: PSS Dry Electrodes for Biopotential Monitoring. ACS Appl. Polym. Mater. 2024, 6, 7781–7793. [Google Scholar] [CrossRef]
- Niu, L.; Shen, Z.; Wang, Z.; Qi, L.; Niu, H.; Zhou, H.; Zhang, C.; Xu, J.; Fang, J. Low-Contact Impedance Textile Electrode for Real-Time Detection of ECG Signals. ACS Appl. Mater. Interfaces 2024, 16, 57860–57869. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, F.; Bai, Y. Flexible surface myoelectric electrodes based on fiber skeleton reinforcement. China Plast. 2024, 38, 68–73. [Google Scholar]
- Kim, K.H.; Bang, S.W.; Kim, S.R. Emotion recognition system using short-term monitoring of physiological signals. Med. Biol. Eng. Comput. 2004, 42, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Chen, S.; Wang, F.; Liu, M.; Li, J.; Liu, H.; Yang, Y.; Zhang, H.; Liu, D.; He, R.; et al. High-Conductivity, Self-Healing, and Adhesive Ionic Hydrogels for Health Monitoring and Human-Machine Interactions Under Extreme Cold Conditions. Adv. Sci. 2025, 2412726, Online ahead of print. [Google Scholar]
- Boateng, D.; Li, X.; Zhu, Y.; Zhang, H.; Wu, M.; Liu, J.; Kang, Y.; Zeng, H.; Han, L. Recent advances in flexible hydrogel sensors: Enhancing data processing and machine learning for intelligent perception. Biosens. Bioelectron. 2024, 261, 116499. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Margel, S. Fluorescent quantum dots-based hydrogels: Synthesis, Fabrication and multimodal biosensing. Talanta Open 2023, 8, 100243. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Zhou, J.; Zhao, Y.; Wang, C.; Fan, M.; Li, Y.; Yang, C.; Yang, H. A Low-Cost Hydrogel Electrode for Multifunctional Sensing: Strain, Temperature, and Electrophysiology. Biosensors 2025, 15, 177. https://doi.org/10.3390/bios15030177
Zheng J, Zhou J, Zhao Y, Wang C, Fan M, Li Y, Yang C, Yang H. A Low-Cost Hydrogel Electrode for Multifunctional Sensing: Strain, Temperature, and Electrophysiology. Biosensors. 2025; 15(3):177. https://doi.org/10.3390/bios15030177
Chicago/Turabian StyleZheng, Junjie, Jinli Zhou, Yixin Zhao, Chenxiao Wang, Mengzhao Fan, Yunfei Li, Chaoran Yang, and Hongying Yang. 2025. "A Low-Cost Hydrogel Electrode for Multifunctional Sensing: Strain, Temperature, and Electrophysiology" Biosensors 15, no. 3: 177. https://doi.org/10.3390/bios15030177
APA StyleZheng, J., Zhou, J., Zhao, Y., Wang, C., Fan, M., Li, Y., Yang, C., & Yang, H. (2025). A Low-Cost Hydrogel Electrode for Multifunctional Sensing: Strain, Temperature, and Electrophysiology. Biosensors, 15(3), 177. https://doi.org/10.3390/bios15030177




