Wearable Electrochemical Glucose Sensors for Fluid Monitoring: Advances and Challenges in Non-Invasive and Minimally Invasive Technologies
Abstract
1. Introduction
2. Overview of Wearable Electrochemical Glucose Sensors
2.1. Electrochemical Sensors
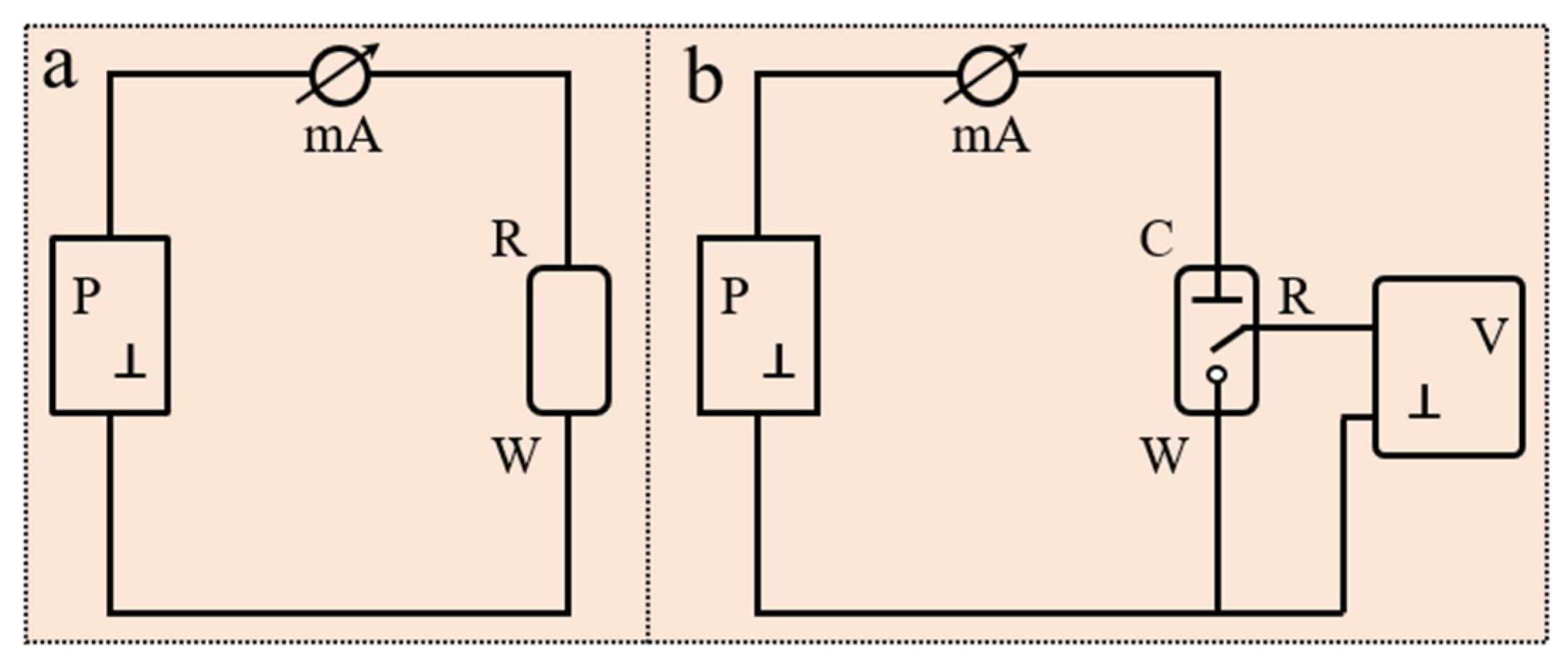
2.1.1. Basic Components of an Electrochemical Sensor
2.1.2. Electrochemical Detection Mechanism
2.2. Electrochemical Glucose Sensor
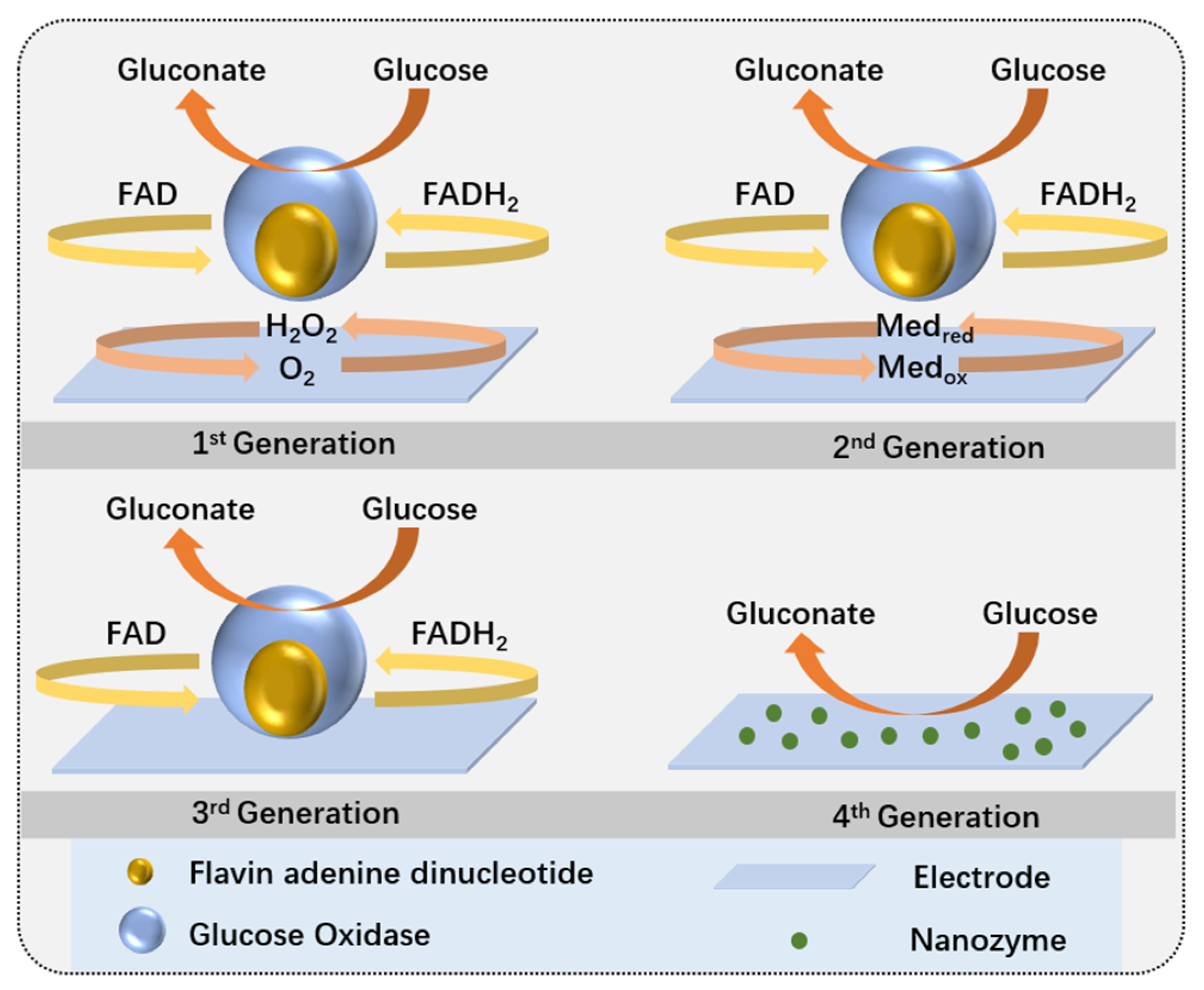
2.2.1. Enzymatic Glucose Sensors
2.2.2. Non-Enzymatic Glucose Sensors
2.3. Wearable Electrochemical Glucose Sensors
3. Advanced Microfluidic and Wearable Sensing Technologies
3.1. Microfluidics
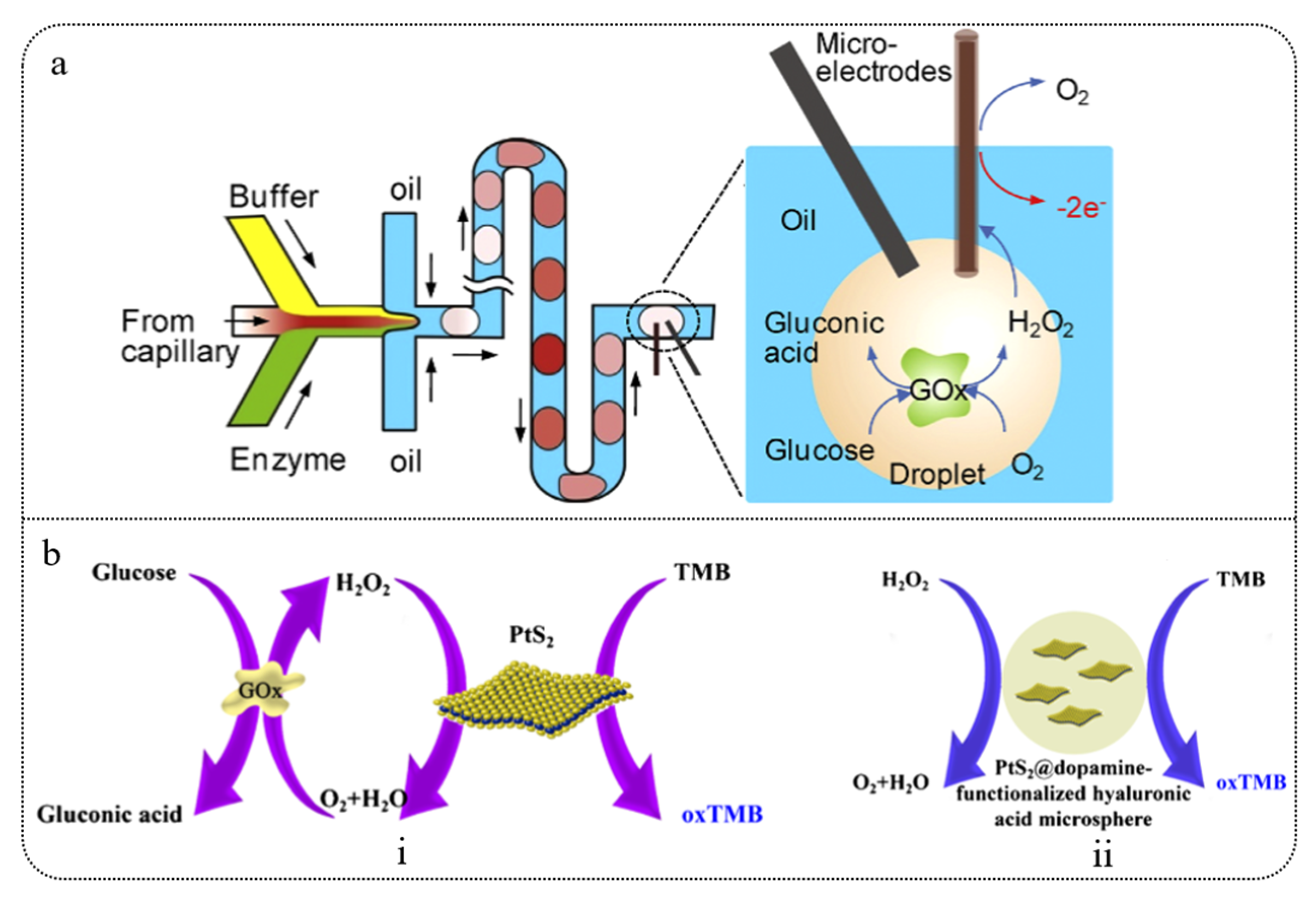
3.1.1. Droplet Microfluidics
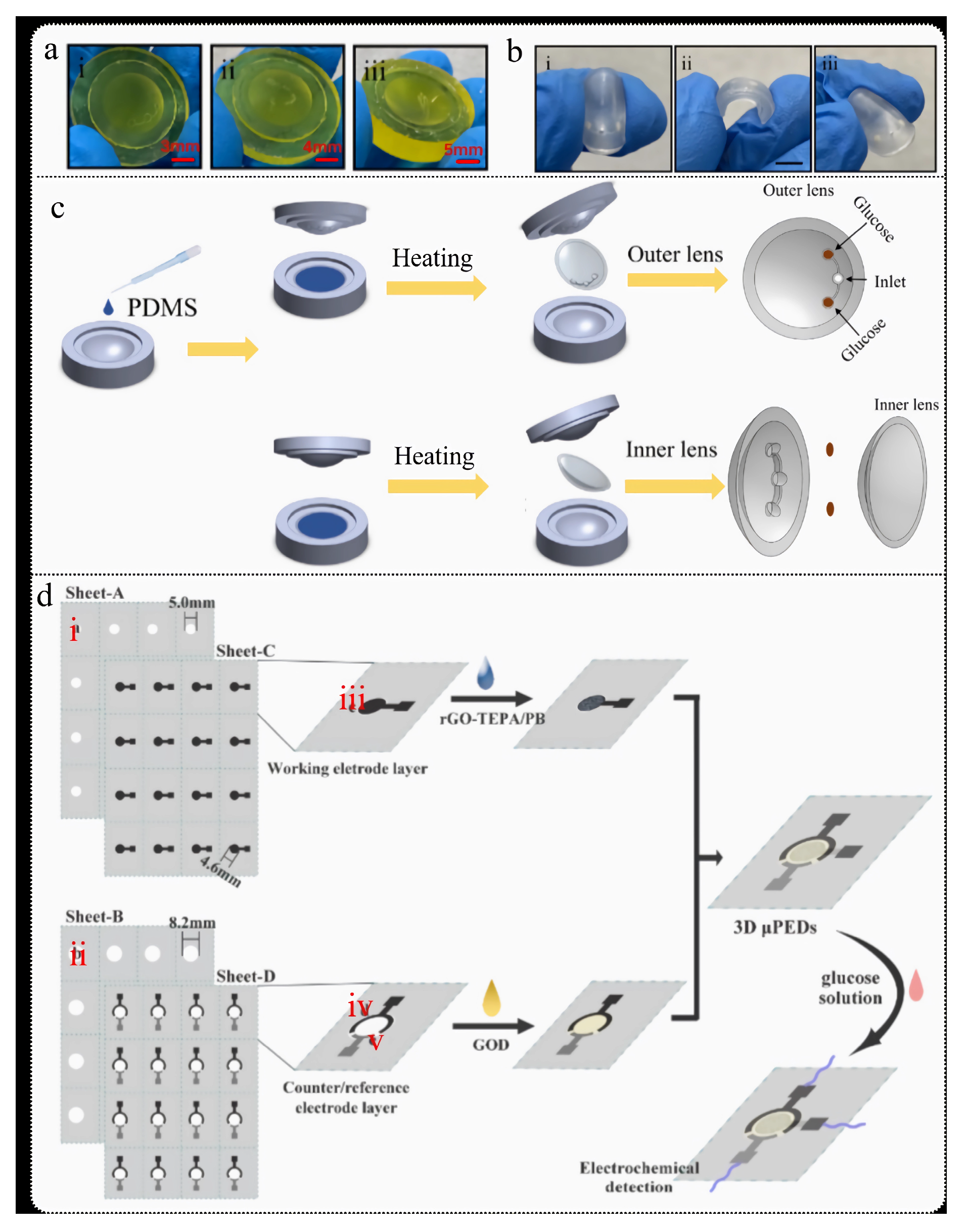
3.1.2. Paper-Based Microfluidics
3.1.3. 3D-Printed Microfluidics
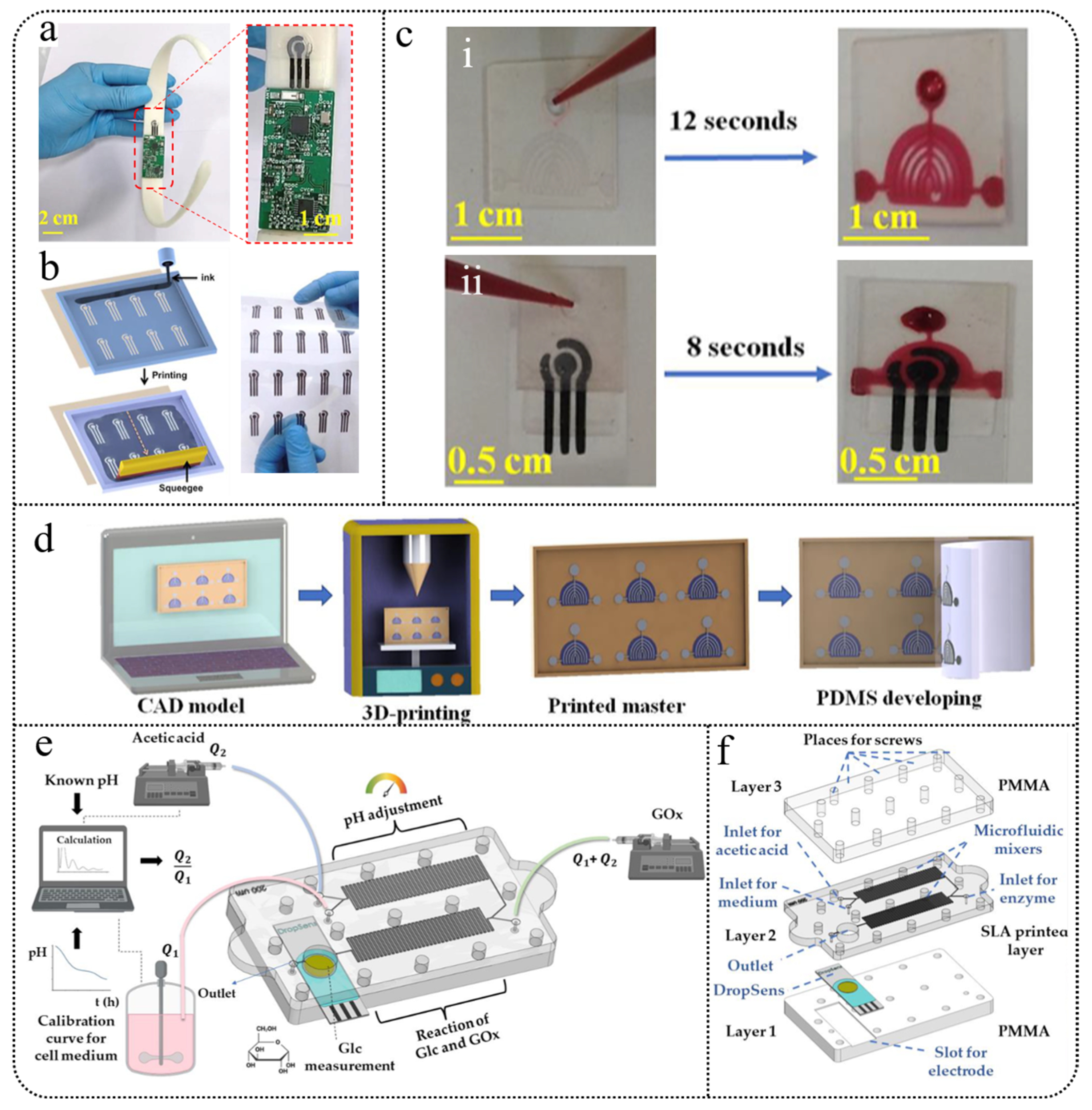
3.2. Wearable Fabric Sensors
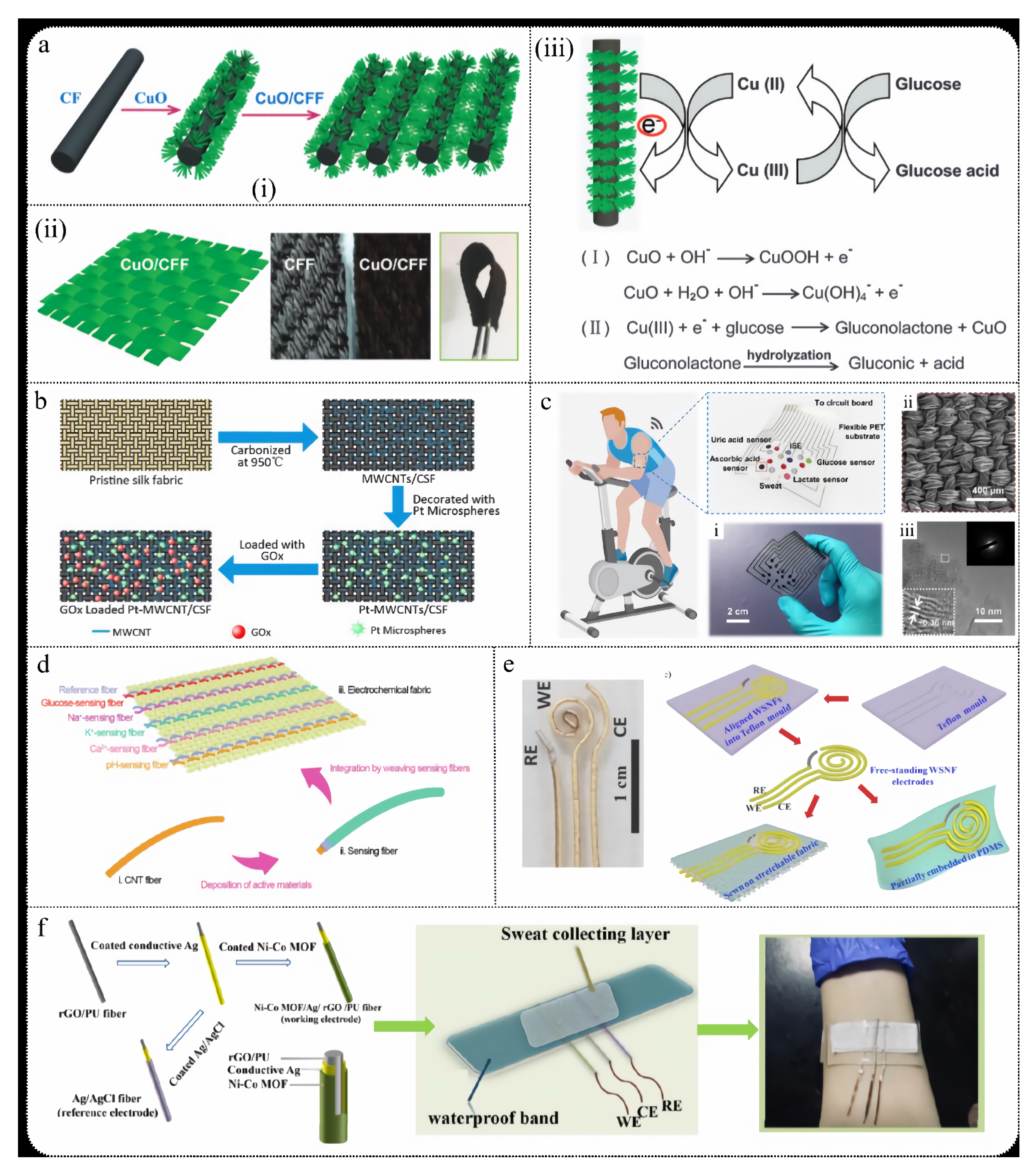
3.3. Self-Powered
3.3.1. TENG
3.3.2. TEG
3.3.3. BFC
3.3.4. FLIB
4. Minimally Invasive Technology
4.1. Microneedles
4.2. Subcutaneous Sensor
4.3. Microdialysis
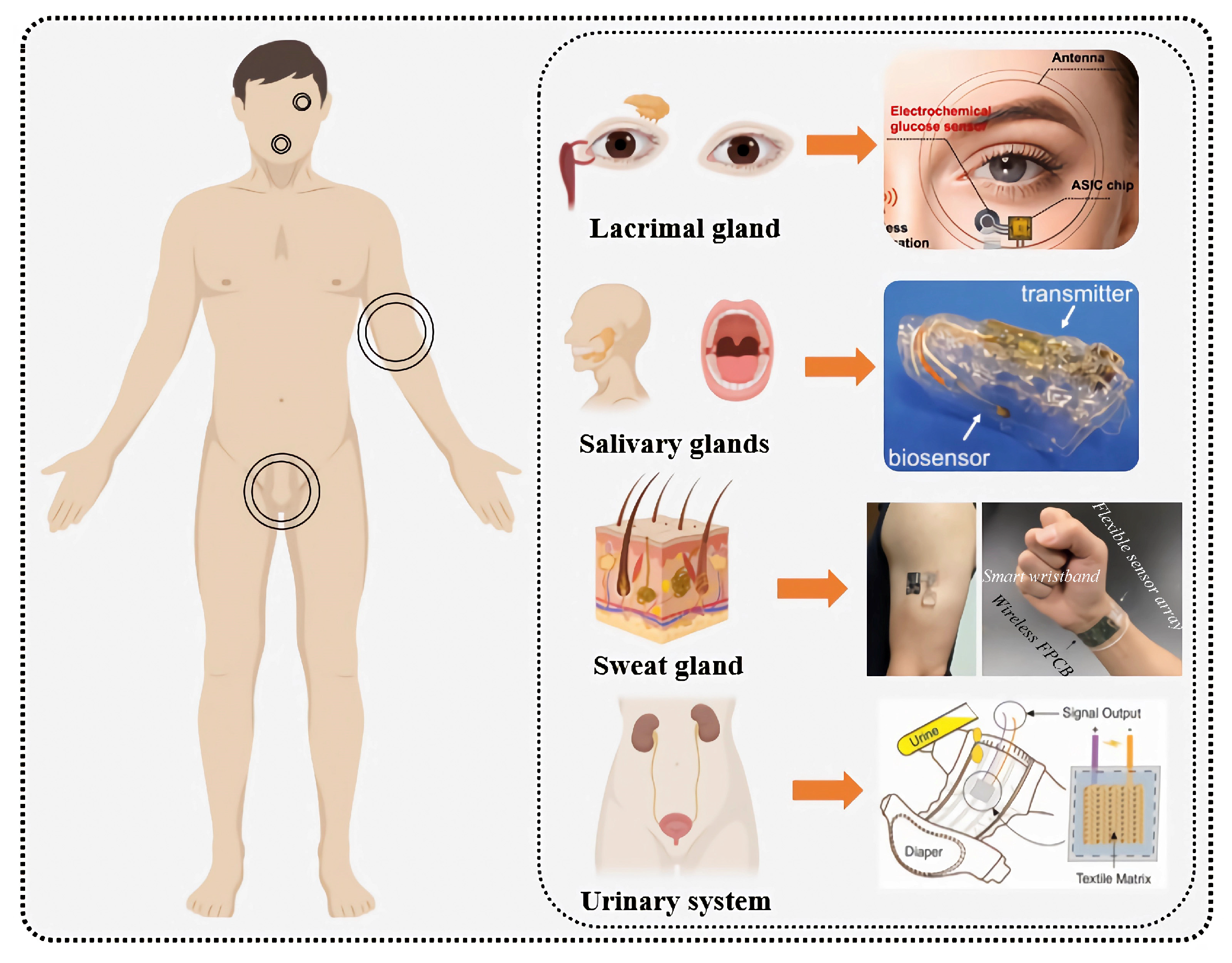
5. Glucose Monitoring Applications Based on Biological Fluids
5.1. Tear Sensors
5.1.1. Smart Contact Lenses (SCLs)
5.1.2. Ocular Disease Detection
5.2. Saliva Sensor

5.3. Sweat Sensor
5.3.1. Exercise-Induced Hypoglycemia
5.3.2. Glucose and ECG

5.4. Urine Sensor

5.5. ISF Sensor

6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tian, S.; Wu, J.; Liu, J.S.; Zou, B.S.; Kong, L.Q. Type 2 diabetes. Ann. Intern. Med. 2020, 172, 704–705. [Google Scholar] [CrossRef] [PubMed]
- Ben Guebila, M.; Thiele, I. Dynamic flux balance analysis of whole-body metabolism for type 1 diabetes. Nat. Comput. Sci. 2021, 1, 348–361. [Google Scholar] [CrossRef]
- Vijan, S. Type 2 diabetes. Ann. Intern. Med. 2010, 152, ITC3-1. [Google Scholar] [CrossRef]
- World Health Organization. Noncommunicable Diseases. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 5 May 2025).
- Pari, B.; Gallucci, M.; Ghigo, A.; Brizzi, M.F. Insight on Infections in Diabetic Setting. Biomedicines 2023, 11, 971. [Google Scholar] [CrossRef]
- Ma, R.; Shao, R.; An, X.; Zhang, Q.; Sun, S. Recent advancements in noninvasive glucose monitoring and closed-loop management systems for diabetes. J. Mater. Chem. B 2022, 10, 5537–5555. [Google Scholar] [CrossRef] [PubMed]
- Sempionatto, J.R.; Lasalde-Ramírez, J.A.; Mahato, K.; Wang, J.; Gao, W. Wearable chemical sensors for biomarker discovery in the omics era. Nat. Rev. Chem. 2022, 6, 899–915. [Google Scholar] [CrossRef]
- IIqbal, S.M.; Mahgoub, I.; Du, E.; Leavitt, M.A.; Asghar, W. Advances in healthcare wearable devices. NPJ Flex. Electron. 2021, 5, 9–22. [Google Scholar] [CrossRef]
- Vaghasiya, J.V.; Mayorga-Martinez, C.C.; Pumera, M. Wearable sensors for telehealth based on emerging materials and nanoarchitectonics. NPJ Flex. Electron. 2023, 7, 26–39. [Google Scholar] [CrossRef]
- Park, H.; Park, W.; Lee, C.H. Electrochemically active materials and wearable biosensors for the in situ analysis of body fluids for human healthcare. NPG Asia Mater. 2021, 13, 23–36. [Google Scholar] [CrossRef]
- Zou, Y.; Chu, Z.; Guo, J.; Liu, S.; Ma, X.; Guo, J. Minimally invasive electrochemical continuous glucose monitoring sensors: Recent progress and perspective. Biosens. Bioelectron. 2023, 225, 115103–115122. [Google Scholar] [CrossRef]
- Bellido, V.; Freckman, G.; Pérez, A.; Galindo, R.J. Accuracy and Potential Interferences of Continuous Glucose Monitoring Sensors in the Hospital. Endocr. Pract. 2023, 29, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Voelker, R. Blood Glucose Readings Made Possible Via Smartphone. J. Am. Med. Assoc. 2018, 319, 1758. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Ma, N.; Yang, T.; Zhang, Y.; Miao, Q.; Tao, J. A multi-level hypoglycemia early alarm system based on sequence pattern mining. BMC Med. Inform. Decis. Mak. 2021, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, X.; Yang, J. A predictive model incorporating the change detection and Winsorization methods for alerting hypoglycemia and hyperglycemia. Med. Biol. Eng. Comput. 2021, 59, 2311–2324. [Google Scholar] [CrossRef]
- Rodbard, D. Continuous glucose monitoring: A review of successes, challenges, and opportunities. Diabetes Technol. Ther. 2016, 18, S2-3–S2-13. [Google Scholar] [CrossRef]
- Li, G.; Wen, D. Sensing nanomaterials of wearable glucose sensors. Chin. Chem. Lett. 2021, 32, 221–228. [Google Scholar] [CrossRef]
- Gupta, S.; Tai, N. Carbon nanomaterials and their composites for electrochemical glucose biosensors: A review on fabrication and sensing properties. J. Taiwan Inst. Chem. Eng. 2024, 154, 104957–104985. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Treacy, M.; Ebbesen, T.; Gibson, J. Exceptionally high Young’s modulus observed for individual carbon nanotubes. Nature 1996, 381, 678–680. [Google Scholar] [CrossRef]
- Wong, E.W.; Sheehan, P.E.; Lieber, C.M. Nanobeam Mechanics: Elasticity, Strength, and Toughness of Nanorods and Nanotubes. Science 1997, 277, 1971–1975. [Google Scholar] [CrossRef]
- Salvetat, J.P.; Bonard, J.M.; Thomson, N.H.; Kulik, A.J.; Forro, L.; Benoit, W.; Zuppiroli, L. Mechanical properties of carbon nanotubes. Appl. Phys. A 1999, 69, 255–260. [Google Scholar] [CrossRef]
- Colomer, J.F.; Stephan, C.; Lefrant, S.; Van Tendeloo, G.; Willems, I.; Konya, Z.; Nagy, J.B. Large-scale synthesis of single-wall carbon nanotubes by catalytic chemical vapor deposition (CCVD) method. Chem. Phys. Lett. 2000, 317, 83–89. [Google Scholar] [CrossRef]
- Piyush, K.; Narvdeshwar Pawan, K. Characteristics of Carbon Nanotubes and Their High-Performance Composite Materials. J. Compos. Mater. 2001, 18, 1–5. [Google Scholar]
- Yuwen, T.; Shu, D.; Zou, H.; Yang, X.; Wang, S.; Zhang, S.; Zang, G. Carbon nanotubes: A powerful bridge for conductivity and flexibility in electrochemical glucose sensors. J. Nanobiotechnology 2023, 21, 320–348. [Google Scholar] [CrossRef]
- Amara, U.; Mahmood, K.; Riaz, S.; Nasir, M.; Hayat, A.; Hanif, M.; Nawaz, M.H. Self-assembled perylene-tetracarboxylicacid/multi-walled carbon nanotube adducts based modification of screen-printed interface for efficient enzyme immobilization towards glucose biosensing. Microchem. J. 2021, 165, 106–109. [Google Scholar] [CrossRef]
- Tahir, M.A.; Bajwa, S.Z.; Mansoor, S.; Briddon, R.W.; Khan, W.S.; Scheffler, B.E.; Amin, I. Evaluation of carbon nanotube based copper nanoparticle composite for the efficient detection of agroviruses. J. Hazard. Mater. 2018, 346, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Gupta, V.K.; Huseinov, A.; Rahm, C.E.; Gazica, K.; Alvarez, N.T. Highly sensitive non-enzymatic glucose sensor based on carbon nanotube microelectrode set. Sens. Actuators B Chem. 2021, 348, 130688–130689. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.E.; Zhang, Y.; Dubonos, S.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Long, W.; Huang, R. The Chemical Mysteries and Research Progress of Graphene. J. Luoyang Inst. Technol. (Nat. Sci. Ed.) 2012, 22, 1–4+12. [Google Scholar]
- Zhang, C.; Zhang, Z.; Yang, Q.; Chen, W. Graphene-based Electrochemical Glucose Sensors: Fabrication and Sensing Properties. Electroanalysis 2018, 30, 2504–2524. [Google Scholar] [CrossRef]
- Al Faruque, M.A.; Syduzzaman, M.; Sarkar, J.; Bilisik, K.; Naebe, M. A review on the production methods and applications of graphene-based materials. Nanomaterials 2021, 11, 2414. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Paeng, K.; Kim, I.S. A review of doping modulation in graphene. Synth. Met. 2018, 244, 36–47. [Google Scholar] [CrossRef]
- Narang, G.; Bansal, D.; Joarder, S.; Singh, P.; Kumar, L.; Mishra, V.; Kumari, K. A Review on the Synthesis, Properties, and Applications of Graphynes. Chem. Flat Mater. 2023, 40, 100517–100540. [Google Scholar] [CrossRef]
- Kaushal, S.; Kaur, M.; Kaur, N.; Kumaria, V.; Singh, P.P. Heteroatom-doped graphene as sensing materials: A mini review. RSC Adv. 2020, 10, 28608–28629. [Google Scholar] [CrossRef]
- Reynoso-Soto, E.A.; Félix-Navarro, R.M.; Rivera-Lugo, Y.Y.; Lozano-Garcia, A.; Dominguez-Vargas, D.A.; Silva-Carrillo, C. Electrochemical Determination of Glucose Using Nitrogen-Doped Graphene. Top. Catal. 2022, 65, 1235–1243. [Google Scholar] [CrossRef]
- Chen, G.; Liu, Y.; Liu, Y.; Tian, Y.; Zhang, X. Nitrogen and sulfur dual-doped graphene for glucose biosensor application. J. Electroanal. Chem. 2015, 738, 100–107. [Google Scholar] [CrossRef]
- Razmi, H.; Mohammad-Rezaei, R. Graphene quantum dots as a new substrate for immobilization and direct electrochemistry of glucose oxidase: Application to sensitive glucose determination. Biosens. Bioelectron. 2013, 41, 498–504. [Google Scholar] [CrossRef]
- Ran, P.; Song, J.; Mo, F.; Wu, J.; Liu, P.; Fu, Y. Nitrogen-doped graphene quantum dots coated with gold nanoparticles for electrochemiluminescent glucose detection using enzymatically generated hydrogen peroxide as a quencher. Microchim. Acta 2019, 186, 3397–3406. [Google Scholar] [CrossRef]
- Jeong, J.; Yang, M.; Kim, D.S.; Lee, T.J.; Choi, B.G.; Kim, D.H. High performance electrochemical glucose sensor based on three-dimensional MoS2/graphene aerogel. J. Colloid Interface Sci. 2017, 506, 379–385. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Mohammadpour-Haratbar, S.; Zare, Y.; Rhee, K.Y.; Park, S.J. A Review on Non-Enzymatic Electrochemical Biosensors of Glucose Using Carbon Nanofiber Nanocomposites. Biosensors 2022, 12, 1004. [Google Scholar] [CrossRef] [PubMed]
- Yadav, D.; Amini, F.; Ehrmann, A. Recent advances in carbon nanofibers and their applications—A review. Eur. Polym. J. 2020, 138, 109963–109976. [Google Scholar] [CrossRef]
- Mohammadpour-Haratbar, A.; Mosallanejad, B.; Zare, Y.; Rhee, K.Y.; Park, S.J. Co3O4 nanoparticles embedded in electrospun carbon nanofibers as free-standing nanocomposite electrodes as highly sensitive enzyme-free glucose biosensors. Rev. Adv. Mater. Sci. 2022, 6, 744–755. [Google Scholar] [CrossRef]
- Van der Ham, M.P.; Hersbach, T.J.P.; Delgado, J.J.; Matson, B.D.; Lim, J.; Führer, M.; Bitter, J.H. Improved electrocatalytic activity of Pt on carbon nanofibers for glucose oxidation mediated by support oxygen groups in Pt perimeter. Appl. Catal. B Environ. 2023, 338, 123046. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Wu, T.; Wang, Q.; Wang, H.; Liang, J.; Chen, S. Flexible and conductive titanium carbide–carbon nanofibers for high-performance glucose biosensing. Electrochim. Acta 2018, 281, 517–524. [Google Scholar] [CrossRef]
- Huan, K.; Li, Y.; Deng, D.; Wang, H.; Wang, D.; Li, M.; Luo, L. Composite-controlled electrospinning of CuSn bimetallic nanoparticles/carbon nanofibers for electrochemical glucose sensor. Appl. Surf. Sci. 2022, 573, 151528. [Google Scholar] [CrossRef]
- Yu, S.; Liu, S.; Jiang, X.; Yang, N. Recent advances on electrochemistry of diamond related materials. Carbon 2022, 200, 517–542. [Google Scholar] [CrossRef]
- Lu, J.; Xu, D.; Huang, N.; Jiang, X.; Yang, B. One-dimensional diamond nanostructures: Fabrication, properties and applications. Carbon 2024, 223, 119020. [Google Scholar] [CrossRef]
- Lavini, F.; Rejhon, M.; Riedo, E. Two-dimensional diamonds from sp 2-to-sp 3 phase transitions. Nat. Rev. Mater. 2022, 7, 814–832. [Google Scholar] [CrossRef]
- Kumar, S.; Nehra, M.; Kedia, D.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.H. Nanodiamonds: Emerging face of future nanotechnology. Carbon 2019, 143, 678–699. [Google Scholar] [CrossRef]
- Yang, N.; Foord, J.S.; Jiang, X. Diamond electrochemistry at the nanoscale: A review. Carbon 2016, 99, 90–110. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical applications of nanodiamond. Nanotechnology 2017, 28, 252001. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.R.; Carvalho, J.H.; Stefano, J.S.; Oliveira, G.G.; Prakash, J.; Janegitz, B.C. Electrochemical sensors and biosensors based on nanodiamonds: A review. Mater. Today Commun. 2023, 35, 106142. [Google Scholar] [CrossRef]
- Luo, D.; Wu, L.; Zhi, J. Fabrication of boron-doped diamond nanorod forest electrodes and their application in nonenzymatic amperometric glucose biosensing. ACS Nano 2009, 3, 2121–2128. [Google Scholar] [CrossRef] [PubMed]
- Nantaphol, S.; Watanabe, T.; Nomura, N.; Siangproh, W.; Chailapakul, O.; Einaga, Y. Bimetallic Pt–Au nanocatalysts electrochemically deposited on boron-doped diamond electrodes for nonenzymatic glucose detection. Biosens. Bioelectron. 2017, 98, 76–82. [Google Scholar] [CrossRef]
- Gao, Q.; Zhang, W.; Zhao, C.; Yan, W.; Han, S.; Li, Y.; Liu, Q.; Li, X.; Liu, D. Laser-Engraved Boron-Doped Diamond for Copper Catalyzed Non-Enzymatic Glucose Sensing. Adv. Mater. Interfaces 2022, 9, 2200034. [Google Scholar] [CrossRef]
- Afreen, S.; Muthoosamy, K.; Manickam, S.; Hashim, U. Functionalized fullerene (C60) as a potential nanomediator in the fabrication of highly sensitive biosensors. Biosens. Bioelectron. 2015, 63, 354–364. [Google Scholar] [CrossRef]
- Chuang, C.W.; Shih, J.S. Preparation and application of immobilized C60-glucose oxidase enzyme in fullerene C60-coated piezoelectric quartz crystal glucose sensor. Sens. Actuators B Chem. 2001, 81, 1–8. [Google Scholar] [CrossRef]
- Gao, Y.F.; Yang, T.; Yang, X.L.; Zhang, Y.S.; Jiao, B.L.; Hong, J.; Moosavi-Movahedi, A.A. Direct electrochemistry of glucose oxidase and glucose biosensing on a hydroxyl fullerenes modified glassy carbon electrode. Biosens. Bioelectron. 2014, 60, 30–34. [Google Scholar] [CrossRef]
- Ma, X.X.; Li, Y.Y.; Song, X.Y.; Xu, K.X.; Chen, Y.J.; Meng, X.; Bao, L.X. Electrochemical Properties Analysis of Immobilized Glucose Oxidase on Multi-Walled Carbon Nanotubes and Hydroxy-Fullerene Nanocomposites. J. Electrochem. Soc. 2023, 170, 75504–77513. [Google Scholar] [CrossRef]
- Hassanvand, Z.; Jalali, F.; Nazari, M.; Parnianchi, F.; Santoro, C. Carbon nanodots in electrochemical sensors and biosensors: A review. ChemElectroChem 2021, 8, 15–35. [Google Scholar] [CrossRef]
- Liu, J.; Li, R.; Yang, B. Carbon dots: A new type of carbon-based nanomaterial with wide applications. ACS Cent. Sci. 2020, 6, 2179–2195. [Google Scholar] [CrossRef]
- Liu, H.; Zhong, X.; Pan, Q. Carbon dots: A new type of carbon-based nhesis strategy. Coord. Chem. Rev. 2024, 498, 215468–215484. [Google Scholar] [CrossRef]
- Guan, X.; Li, Z.; Geng, X.; Lei, Z.; Karakoti, A.; Wu, T.; Vinu, A. Emerging Trends of Carbon-Based Quantum Dots: Nanoarchitectonics and Applications. Small 2023, 19, 2207181–2207258. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon dots: Synthesis, properties and applications. Nanomaterials 2021, 11, 3419. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Wang, H.; Wang, B.; Zhou, Y.; Liu, Y.; Kang, Z. Chiral Control of Carbon Dots via Surface Modification for Tuning the Enzymatic Activity of Glucose Oxidase. ACS Appl. Mater. Interfaces 2021, 13, 5877–5886. [Google Scholar] [CrossRef] [PubMed]
- Sanjuan-Navarro, L.; Moliner-Martínez, Y.; Campíns-Falcó, P. The state of art of nanocarbon black as analyte in a variety of matrices: A review. TrAC Trends Anal. Chem. 2022, 157, 116769–116779. [Google Scholar] [CrossRef]
- Anwer, A.H.; Ahtesham, A.; Shoeb, M.; Mashkoor, F.; Ansari, M.Z.; Zhu, S.; Jeong, C. State-of-the-art advances in nanocomposite and bio-nanocomposite polymeric materials: A comprehensive review. Adv. Colloid Interface Sci. 2023, 318, 102955–102987. [Google Scholar] [CrossRef]
- Vicentini, F.C.; Silva, T.A.; Fatibello-Filho, O. Carbon black electrodes applied in electroanalysis. Curr. Opin. Electrochem. 2023, 43, 101415–101423. [Google Scholar] [CrossRef]
- Atanasov, P.; Kaisheva, A.; Iliev, I.; Razumas, V.; Kulys, J. Glucose biosensor based on carbon black strips. Biosens. Bioelectron. 1992, 7, 361–365. [Google Scholar] [CrossRef]
- Piñón-Balderrama, C.I.; Hernández-Escobar, C.A.; Reyes-López, S.Y.; Conejo-Dávila, A.S.; Estrada-Monje, A.; Zaragoza-Contreras, E.A. Non-Enzymatic Electrochemical Sensing of Glucose with a Carbon Black/Polyaniline/Silver Nanoparticle Composite. Chemosensors 2024, 12, 26. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Q.Y.; Jiang, M.; Li, S.S. Application of valence-variable transition-metal-oxide-based nanomaterials in electrochemical analysis: A review. Anal. Chim. Acta 2024, 1295, 342270–342285. [Google Scholar] [CrossRef]
- Niu, X.; Li, X.; Pan, J.; He, Y.; Qiu, F.; Yana, Y. Recent advances in non-enzymatic electrochemical glucose sensors based on non-precious transition metal materials: Opportunities and challenges. RSC Adv. 2016, 6, 84893–84905. [Google Scholar] [CrossRef]
- Xuan, C.T.; Oanh, V.T.; Viet, N.X.; Tu, L.M.; Loan, T.T.; Van Tuan, C.; Hoa, N.D. One-Step Method for the Direct Growth of NiO Nanoflowers on Pencil Graphite Electrode for Highly Sensitive Non-Enzymatic Glucose Sensing. J. Electrochem. Soc. 2024, 171, 17505–17513. [Google Scholar]
- Zhu, H.; Li, L.; Zhou, W.; Shao, Z.; Chen, X. Advances in non-enzymatic glucose sensors based on metal oxides. J. Mater. Chem. B 2016, 4, 7333–7349. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Azeem, M.; Wang, Z.; Ashraf, G.; Liu, H. A review on electrochemical biosensing platform based on layered double hydroxides for small molecule biomarkers determination. Adv. Colloid Interface Sci. 2018, 262, 21–38. [Google Scholar] [CrossRef]
- Jamal, F.; Rafique, A.; Moeen, S.; Haider, J.; Nabgan, W.; Haider, A.; Maqbool, M. (Review of Metal Sulfide Nanostructures and their Applications. ACS Appl. Nano Mater. 2023, 6, 7077–7106. [Google Scholar] [CrossRef]
- Arivazhagan, M.; Kannan, P.; Maduraiveeran, G. Nanostructured Transition Metal Sulfide-based Glucose and Lactic Acid Electrochemical Sensors for Clinical Applications. Curr. Top. Med. Chem. 2023, 23, 284–294. [Google Scholar]
- Chen, Y.; Wang, H.; Chen, H.; Song, J.; Deng, D.; Luo, L. Synthesis of Quaternary (Ni, Co, Cu) Se2 Nanosheet Arrays on Carbon Cloth for Non-Enzymatic Glucose Determination. Chemosensors 2023, 11, 530. [Google Scholar] [CrossRef]
- Khan, M.D.; Malik, M.A.; Revaprasadu, N. Progress in selenium based metal-organic precursors for main group and transition metal selenide thin films and nanomaterials. Coord. Chem. Rev. 2019, 388, 24–47. [Google Scholar] [CrossRef]
- Lakshmy, S.; Santhosh, S.; Kalarikkal, N.; Rout, C.S.; Chakraborthy, B. A review of electrochemical glucose sensing based on transition metal phosphides. J. Appl. Phys. 2023, 133, 070702–070746. [Google Scholar] [CrossRef]
- He, L.; Li, J.; Cao, J.; Li, X.; Feng, X.; Zhang, J.; Yang, Y. High performance of non-enzymatic glucose biosensors based on the design of microstructure of Ni2P/Cu3P nanocomposites. Appl. Surf. Sci. 2022, 593, 153395–153404. [Google Scholar] [CrossRef]
- Zhang, T.; Ran, J.; Ma, C.; Yang, B. A Universal Approach to Enhance Glucose Biosensor Performance by Building Blocks of Au Nanoparticles. Adv. Mater. Interfaces 2020, 7, 2000227–2000235. [Google Scholar] [CrossRef]
- Zhao, J.; Hu, X.; Huang, X.; Koh, K.; Chen, H. A facile gold nanoparticles embeded hydrogel for non-enzymatic sensing of glucose. Colloids Surf. B Biointerfaces 2019, 183, 110404–110427. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, M.; Yadav, P.; Dalal, S.; Kataria, S.K. A review on ameliorative green nanotechnological approaches in diabetes management. Biomed. Pharmacother. 2020, 127, 110198–110213. [Google Scholar] [CrossRef] [PubMed]
- Lipińska, W.; Grochowska, K.; Siuzdak, K. Enzyme Immobilization on Gold Nanoparticles for Electrochemical Glucose Biosensors. Nanomaterials 2021, 11, 1156. [Google Scholar] [CrossRef]
- Karra, S.; Wooten, M.; Griffith, W.; Gorski, W. Morphology of Gold Nanoparticles and Electrocatalysis of Glucose Oxidation. Electrochim. Acta 2016, 218, 8–14. [Google Scholar] [CrossRef]
- Kim, S.K.; Jeon, C.; Lee, G.H.; Koo, J.; Cho, S.H.; Han, S.; Hahn, S.K. Hyaluronate–Gold Nanoparticle/Glucose Oxidase Complex for Highly Sensitive Wireless Noninvasive Glucose Sensors. ACS Appl. Mater. Interfaces 2019, 11, 37347–37356. [Google Scholar] [CrossRef]
- Zhao, X.H.; Li, Q.; Ma, X.M.; Xiong, Z.; Quan, F.Y.; Xia, Y.Z. Alginate fibers embedded with silver nanoparticles as efficient catalysts for reduction of 4-nitrophenol. RSC Adv. 2015, 5, 49534–49540. [Google Scholar] [CrossRef]
- Setyorini, D.A.; Noviandri, I.; Amran, M.B.; Rizki, W.O.S.; Serunting, M.A. (A green-micro-synthesis of curcumin functionalized silver nanoparticles for bacteria inhibition and glucose sensor electrode modifier. Mater. Today Sustain. 2024, 25, 100648–100657. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, S.J.; Yun, S.J.; Jang, J.; Kang, H.; Kim, K.; Choi, I.; Park, S. Silver nanoparticles affect glucose metabolism in hepatoma cells through production of reactive oxygen species. Int. J. Nanomed. 2016, 11, 55–68. [Google Scholar]
- Guascito, M.R.; Chirizzi, D.; Picca, R.A.; Mazzotta, E.; Malitesta, C. Ag nanoparticles capped by a nontoxic polymer: Electrochemical and spectroscopic characterization of a novel nanomaterial for glucose detection. Mater. Sci. Eng. C 2011, 31, 606–611. [Google Scholar] [CrossRef]
- Setyorini, D.A.; Noviandri, I.; Amran, M.B.; Rizki, W.O.S.; Serunting, M.A. Surface functionalized halloysite nanotubes decorated with silver nanoparticles for enzyme immobilization and biosensing. J. Mater. Chem. B 2016, 4, 2553–2560. [Google Scholar]
- Shabbir, S.A.; Tariq, S.; Ashiq, M.G.B.; Khan, W.A. Non-enzymatic glucose sensor with electrodeposited silver/carbon nanotubes composite electrode. Biosci. Rep. 2019, 39, 20181983–20181999. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhang, J.; Yu, Q.; Guo, X.; Chen, S.; Sun, H.; Gao, D. Highly biocompatible zwitterionic dendrimer-encapsulated platinum nanoparticles for sensitive detection of glucose in complex medium. New J. Chem. 2019, 43, 9076–9083. [Google Scholar] [CrossRef]
- Wu, Y.S.; Wang, T.P.; Chen, P.Y.; Lee, C.L. Vacant graphene Nanosheet-Supported platinum nanoparticles as catalysts for neutral glucose oxidation reaction. Appl. Surf. Sci. 2022, 578, 152060–152069. [Google Scholar] [CrossRef]
- Rathod, D.; Dickinson, C.; Egan, D.; Dempsey, E. Platinum nanoparticle decoration of carbon materials with applications in non-enzymatic glucose sensing. Sens. Actuators B Chem. 2010, 143, 547–554. [Google Scholar] [CrossRef]
- Zhao, Q.; Yu, H.; Hu, D.; Li, L.; Jin, J.; Ai, M.; Wei, J.; Song, K. Recent advances in electrochemical sensors based on palladium nanoparticles. Chin. J. Anal. Chem. 2022, 50, 100144–100158. [Google Scholar] [CrossRef]
- Alaqarbeh, M.; Adil, S.F.; Ghrear, T.; Khan, M.; Bouachrine, M.; Al-Warthan, A. Recent Progress in the Application of Palladium Nanoparticles: A Review. Catalysts 2023, 13, 1343. [Google Scholar] [CrossRef]
- Zhao, P.; Sun, X.; Hao, S.; Zhang, Y.; Chen, J.; Zhang, H.; Dong, S. Glucose Oxidase-like Rhodium Single-Atom Nanozymes: A Mimic Platform for Biometabolism and Electrometabolism of Glucose Oxidation at Neutral pH. ACS Energy Lett. 2023, 8, 1697–1704. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Soleimani, F.; Bidgoli, N.S.S.; Nezafat, Z.; Orooji, Y.; Baran, T. Recent developments in polymer-supported ruthenium nanoparticles/complexes for oxidation reactions. J. Organomet. Chem. 2021, 933, 121658–121704. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X.; Wang, W.; Liang, J.; Orooji, Y.; Dai, C.; Zhu, J. Efficient Sorbitol Producing Process through Glucose Hydrogenation Catalyzed by Ru Supported Amino Poly (Styrene-co-Maleic) Polymer (ASMA) Encapsulated on γ-Al2O3. Catalysts 2020, 10, 1068. [Google Scholar] [CrossRef]
- Pavel, I.-A.; Lakard, S.; Lakard, B. Flexible Sensors Based on Conductive Polymers. Chemosensors 2022, 10, 97. [Google Scholar] [CrossRef]
- Bagdžiūnas, G.; Palinauskas, D. Poly(9H-carbazole) as a Organic Semiconductor for Enzymatic and Non-Enzymatic Glucose Sensors. Biosensors 2020, 10, 104. [Google Scholar] [CrossRef]
- Nayana, V.; Kandasubramanian, B. Polycarbazole and its derivatives: Progress, synthesis, and applications. J. Polym. Res. 2020, 27, 285–308. [Google Scholar] [CrossRef]
- Cosnier, S.; Marks, R.S.; Lellouche, J.P.; Perie, K.; Fologea, D.; Szunerits, S. Electrogenerated Poly(ChiralDicarbazole) Films for the Reagentless Grafting of Enzymes. Electroanalysis 2000, 12, 1107–1112. [Google Scholar] [CrossRef]
- Fan, S.N.; Liu, R.W.; Ma, R.S.; Yu, S.S.; Li, M.; Zheng, W.T.; Hu, S.X. Two-dimensional polyaniline nanosheets via liquid-phase exfoliation. Chin. Phys. B 2017, 26, 476–483. [Google Scholar] [CrossRef]
- Kazemi, F.; Naghib, S.M.; Mohammadpour, Z. Multifunctional micro-/nanoscaled structures based on polyaniline: An overview of modern emerging devices. Mater. Today Chem. 2020, 16, 100249–100272. [Google Scholar] [CrossRef]
- Lai, J.; Yi, Y.; Zhu, P.; Shen, J.; Wu, K.; Zhang, L.; Liu, J. Polyaniline-based glucose biosensor: A review. J. Electroanal. Chem. 2016, 782, 138–153. [Google Scholar] [CrossRef]
- Huang, M.R.; Peng, Q.Y.; Li, X.G. Rapid and effective adsorption of lead ions on fine poly (phenylenediamine) microparticles. Chem.–A Eur. J. 2006, 12, 4341–4350. [Google Scholar] [CrossRef]
- Zhang, L.; Chai, L.; Liu, J.; Wang, H.; Yu, W.; Sang, P. pH manipulation: A facile method for lowering oxidation state and keeping good yield of poly (m-phenylenediamine) and its powerful Ag+ adsorption ability. Langmuir 2011, 27, 13729–13738. [Google Scholar] [CrossRef]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, H.; Luo, S.; Chen, J.; Wei, W.; Kuang, Y. Preparation and bioelectrochemical responses of the poly(m-phenylenediamine) glucose oxidase electrode. Sens. Actuators B Chem. 2004, 101, 224–230. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Y.; Lv, H.; Wang, G.; Hu, X.; Wang, C. Construction of a non-enzymatic glucose sensor based on copper nanoparticles/poly(o-phenylenediamine) nanocomposites. J. Solid. State Electrochem. 2015, 19, 731–738. [Google Scholar] [CrossRef]
- Wang, J.; Wang, M.; Guan, J.; Wang, C.; Wang, G. Construction of a non-enzymatic sensor based on the poly(o-phenylenediamine)/Ag-NPs composites for detectingglucose in blood. Mater. Sci. Eng. C 2017, 71, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Garjonyte, R.; Malinauskas, A. Glucose biosensor based on glucose oxidase immobilized in electropolymerized polypyrrole and poly(o-phenylenediamine) films on a Prussian Blue-modified electrode. Sens. Actuators B Chem. 2000, 63, 122–128. [Google Scholar] [CrossRef]
- Vidal, J.C.; Méndez, S.; Castillo, J.R. Electropolymerization of pyrrole and phenylenediamine over an organic conducting salt based amperometric sensor of increased selectivity for glucose determination. Anal. Chim. Acta 1999, 385, 203–211. [Google Scholar] [CrossRef]
- Kuceková, Z.; Rejmontová, P.; Humpolíček, P.; Kašpárková, V.; Bober, P.; Sáha, P.; Stejskal, J. Cytotoxicity of poly(p-phenylenediamine). Chem. Pap. 2017, 71, 367–372. [Google Scholar] [CrossRef]
- Rothwell, S.A.; Killoran, S.J.; O’Neill, R.D. Enzyme immobilization strategies and electropolymerization conditions to control sensitivity and selectivity parameters of a polymer-enzyme composite glucose biosensor. Sensors 2010, 10, 6439–6462. [Google Scholar] [CrossRef]
- Ekinci, E.; Karagözler, A.A.; Karagözler, A.E. Electrochemical synthesis and sensor application of poly (1,4-diaminobenzene). Synth. Met. 1996, 79, 57–61. [Google Scholar] [CrossRef]
- Kaloni, T.P.; Giesbrecht, P.K.; Schreckenbach, G.; Freund, M.S. Polythiophene: From Fundamental Perspectives to Applications. Chem. Mater. 2017, 29, 10248–10283. [Google Scholar] [CrossRef]
- Khalid, H.; Yu, H.; Wang, L.; Amer, W.A.; Akram, M.; Abbasi, N.M.; Saleem, M. Synthesis of ferrocene-based polythiophenes and their applications. Polym. Chem. 2014, 5, 6879–6892. [Google Scholar] [CrossRef]
- Fusco, G.; Göbel, G.; Zanoni, R.; Bracciale, M.P.; Favero, G.; Mazzei, F.; Lisdat, F. Aqueous polythiophene electrosynthesis: A new route to an efficient electrode coupling of PQQ-dependent glucose dehydrogenase for sensing and bioenergetic applications. Biosens. Bioelectron. 2018, 112, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Hocevar, M.A.; Fabregat, G.; Armelin, E.; Ferreira, C.A.; Alemán, C. Nanometric polythiophene films with electrocatalytic activity for non-enzymatic detection of glucose. Eur. Polym. J. 2016, 79, 132–139. [Google Scholar] [CrossRef]
- Huynh, T.P.; Sharma, P.S.; Sosnowska, M.; D’Souza, F.; Kutner, W. Functionalized polythiophenes: Recognition materials for chemosensors and biosensors of superior sensitivity, selectivity, and detectability. Prog. Polym. Sci. 2015, 47, 1–25. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Mousavi, S.M.; Bahrani, S.; Ramakrishna, S. Polythiophene silver bromide nanostructure as ultra-sensitive non-enzymatic electrochemical glucose biosensor. Eur. Polym. J. 2020, 138, 109959–109972. [Google Scholar] [CrossRef]
- Bao, W.; Hai, W.; Bao, L.; Yang, F.; Liu, Y.; Goda, T.; Liu, J. Poly(3,4-ethylenedioxythiophene) bearing fluoro-containing phenylboronic acid for specific recognition of glucose. Mater. Chem. Front. 2021, 5, 7684. [Google Scholar] [CrossRef]
- Seiti, M.; Giuri, A.; Corcione, C.E.; Ferraris, E. Advancements in tailoring PEDOT: PSS properties for bioelectronic applications: A comprehensive review. Biomater. Adv. 2023, 154, 213655–213671. [Google Scholar] [CrossRef]
- Das, R.; Nag, S.; Banerjee, P. Electrochemical nanosensors for sensitization of sweat metabolites: From concept mapping to personalized health monitoring. Molecules 2023, 28, 1259. [Google Scholar] [CrossRef]
- Worsfold, P.; Townshend, A.; Poole, C.F.; Miró, M. Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2019; pp. 70–79. [Google Scholar]
- Nernst, W. Die elektromotorische wirksamkeit der jonen. Z. Für Phys. Chem. 1889, 4, 129–181. [Google Scholar] [CrossRef]
- Ševčík, A. Oscillographic polarography with periodical triangular voltage. Collect. Czechoslov. Chem. Commun. 1948, 13, 349–377. [Google Scholar] [CrossRef]
- Wang, G.; He, X.; Wang, L.; Gu, A.; Huang, Y.; Fang, B.; Zhang, X. Non-Enzymatic Electrochemical Sensing of Glucose. Microchim. Acta 2013, 180, 161–186. [Google Scholar] [CrossRef]
- Wilson, R.; Turner, A. Glucose Oxidase: An Ideal Enzyme. Biosens. Bioelectron. 1992, 7, 165–185. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J. A Novel Improved Design for the First-Generation Glucose Biosensor. Food Technol. Biotechnol. 2001, 39, 55–58. [Google Scholar]
- Toghill, K.E.; Compton, R.G. Electrochemical Non-Enzymatic Glucose Sensors: A Perspective and an Evaluation. Int. J. Electrochem. Sci. 2010, 5, 1246–1301. [Google Scholar] [CrossRef]
- Ates, H.C.; Nguyen, P.Q.; Gonzalez-Macia, L.; Morales-Narváez, E.; Güder, F.; Collins, J.J.; Dincer, C. End-to-end design of wearable sensors. Nat. Rev. Mater. 2022, 7, 887–907. [Google Scholar] [CrossRef]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Wang, J. Wearable sensors: Modalities, challenges, and prospects. Lab. A Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Gambhir, S.S.; Ge, T.J.; Vermesh, O.; Spitler, R.; Gold, G.E. Continuous health monitoring: An opportunity for precision health. Sci. Transl. Med. 2021, 13, 5383–5389. [Google Scholar] [CrossRef]
- Kim, J.; Campbell, A.S.; de Ávila, B.E.F.; Wang, J. Wearable biosensors for healthcare monitoring. Nat. Biotechnol. 2019, 37, 389–406. [Google Scholar] [CrossRef]
- Wang, L.; Lou, Z.; Jiang, K.; Shen, G. Bio-multifunctional smart wearable sensors for medical devices. Adv. Intell. Syst. 2019, 1, 1900040–1900056. [Google Scholar] [CrossRef]
- Yang, Y.; Song, Y.; Bo, X.; Min, J.; Pak, O.S.; Zhu, L.; Gao, W. A laser-engraved wearable sensor for sensitive detection of uric acid and tyrosine in sweat. Nat. Biotechnol. 2020, 38, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device integration of electrochemical biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef]
- Hajam, M.I.; Khan, M.M. Microfluidics: A concise review of the history, principles, design, applications, and future outlook. Biomater. Sci. 2024, 12, 218–251. [Google Scholar] [CrossRef]
- Chen, L.; Yang, C.; Yan, X.; Hu, L.; Eggersdorfer, M.; Ye, F. Millifluidics, microfluidics, and nanofluidics: Manipulating fluids at varying length scales. Mater. Today Nano 2021, 16, 100136–100147. [Google Scholar] [CrossRef]
- Niculescu, A.G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Fabrication and applications of microfluidic devices: A review. Int. J. Mol. Sci. 2021, 22, 2011. [Google Scholar] [CrossRef]
- Chen, S.; Qiao, Z.; Niu, Y.; Yeo, J.C.; Liu, Y.; Qi, J.; Lim, C.T. Wearable flexible microfluidic sensing technologies. Nat. Rev. Bioeng. 2023, 1, 950–971. [Google Scholar] [CrossRef]
- Zhang, S.; Staples, A.E. Microfluidic-based systems for the management of diabetes. Drug Deliv. Transl. Res. 2024, 14, 2989–3008. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, X.; Hou, J.; Huang, L.; Yao, Y.; Ding, Z.; Hao, N. Droplet Microfluidic Devices: Working Principles, Fabrication Methods, and Scale-Up Applications. Small Methods 2024, 8, 2301406–2301418. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.; Zhang, H.; Weitz, D.A.; Shum, H.C. Development and future of droplet microfluidics. Lab. A Chip 2024, 24, 1135–1153. [Google Scholar] [CrossRef]
- Shang, L.; Cheng, Y.; Zhao, Y. Emerging droplet microfluidics. Chem. Rev. 2017, 117, 7964–8040. [Google Scholar] [CrossRef]
- Gu, S.; Lu, Y.; Ding, Y.; Li, L.; Song, H.; Wang, J.; Wu, Q. A droplet-based microfluidic electrochemical sensor using platinum-black microelectrode and its application in high sensitive glucose sensing. Biosens. Bioelectron. 2014, 55, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, X.; Cui, T.; Li, S.; Qian, Y.; Yue, Y.; Yue, W. PtS2 nanosheets as a peroxidase-mimicking nanozyme for colorimetric determination of hydrogen peroxide and glucose. Microchim. Acta 2021, 188, 174–182. [Google Scholar] [CrossRef]
- Araujo, F.; Shrestha, N.; Gomes, M.J.; Herranz-Blanco, B.; Liu, D.; Hirvonen, J.J.; Sarmento, B. In vivo dual-delivery of glucagon like peptide-1 (GLP-1) and dipeptidyl peptidase-4 (DPP4) inhibitor through composites prepared by microfluidics for diabetes therapy. Nanoscale 2016, 8, 10706–10713. [Google Scholar] [CrossRef]
- Li, H.; He, W.; Feng, Q.; Chen, J.; Xu, X.; Lv, C.; Dong, H. Engineering superstable islets-laden chitosan microgels with carboxymethyl cellulose coating for long-term blood glucose regulation in vivo. Carbohydr. Polym. 2024, 323, 121425–121438. [Google Scholar] [CrossRef] [PubMed]
- Nishat, S.; Jafry, A.T.; Martinez, A.W.; Awan, F.R. Based microfluidics: Simplified fabrication and assay methods. Sens. Actuators B Chem. 2021, 336, 129681–129693. [Google Scholar] [CrossRef]
- Isgor, P.K.; Abbasiasl, T.; Das, R.; Istif, E.; Yener, U.C.; Beker, L. Paper integrated microfluidic contact lens for colorimetric glucose detection. Sens. Diagn. 2024, 3, 1743–1748. [Google Scholar] [CrossRef]
- Cao, L.; Han, G.C.; Xiao, H.; Chen, Z.; Fang, C. A novel 3D paper-based microfluidic electrochemical glucose biosensor based on rGO-TEPA/PB sensitive film. Anal. Chim. Acta 2020, 1096, 34–43. [Google Scholar] [CrossRef]
- Duarte, L.C.; Figueredo, F.; Chagas, C.L.; Cortón, E.; Coltro, W.K. A review of the recent achievements and future trends on 3D printed microfluidic devices for bioanalytical applications. Anal. Chim. Acta 2024, 1229, 342429–342439. [Google Scholar] [CrossRef]
- Gonzalez, G.; Roppolo, I.; Pirri, C.F.; Chiappone, A. Current and emerging trends in polymeric 3D printed microfluidic devices. Addit. Manuf. 2022, 55, 102867–102913. [Google Scholar] [CrossRef]
- Chen, C.; Fu, Y.; Sparks, S.S.; Lyu, Z.; Pradhan, A.; Ding, S.; Qiu, K. 3D-Printed Flexible Microfluidic Health Monitor for In Situ Sweat Analysis and Biomarker Detection. ACS Sens. 2024, 9, 3212–3223. [Google Scholar] [CrossRef]
- Mwaurah, M.M.; Vinoth, R.; Nakagawa, T.; Mathiyarasu, J.; Mohan, A.V. A Neckband-Integrated Soft Microfluidic Biosensor for Sweat Glucose Monitoring. ACS Appl. Nano Mater. 2024, 7, 17017–17028. [Google Scholar] [CrossRef]
- Podunavac, I.; Djocos, M.; Ve, M.; Birgermajer, S.; Pavlovic, Z.; Kojic, S.; Radonic, V. 3D-Printed Microfluidic Chip for Real-Time Glucose Monitoring in Liquid Analytes. Micromachines 2023, 14, 503. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Tao, X. Sensing mechanism of a carbon nanocomposite-printed fabric as a strain sensor. Compos. Part A Appl. Sci. Manuf. 2021, 144, 106350–106357. [Google Scholar] [CrossRef]
- Xu, W.; Dai, S.; Wang, X.; He, X.; Wang, M.Y.; Hu, C. Nanorod-aggregated flower-like CuO grown on a carbon fiber fabric for a super high sensitive non-enzymatic glucose sensor. J. Mater. Chem. B 2015, 3, 5777–5785. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ran, R.; Yang, Z.; Lv, R.; Shen, W.; Kang, F.; Huang, Z.H. An efficient flexible electrochemical glucose sensor based on carbon nanotubes/carbonized silk fabrics decorated with Pt microspheres. Sens. Actuators B Chem. 2018, 256, 63–70. [Google Scholar] [CrossRef]
- He, W.; Wang, C.; Wang, H.; Jian, M.; Lu, W.; Liang, X.; Zhang, Y. Integrated textile sensor patch for real-time and multiplex sweat analysis. Sci. Adv. 2019, 5, 649–656. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.; Zhang, Y.; Pan, J.; Li, S.; Sun, X.; Peng, H. Weaving sensing fibers into electrochemical fabric for real-time health monitoring. Adv. Funct. Mater. 2018, 28, 1804456–1804464. [Google Scholar] [CrossRef]
- Toi, P.T.; Trung, T.Q.; Dang, T.M.L.; Bae, C.W.; Lee, N.E. Highly electrocatalytic, durable, and stretchable nanohybrid fiber for on-body sweat glucose detection. ACS Appl. Mater. Interfaces 2019, 11, 10707–10717. [Google Scholar] [CrossRef]
- Shu, Y.; Su, T.; Lu, Q.; Shang, Z.; Xu, Q.; Hu, X. Highly stretchable wearable electrochemical sensor based on Ni-Co MOF nanosheet-decorated Ag/rGO/PU fiber for continuous sweat glucose detection. Anal. Chem. 2021, 93, 16222–16230. [Google Scholar] [CrossRef]
- Fan, F.; Tian, Z.; Wang, Z. Flexible triboelectric generator. Nano Energy 2012, 1, 328–334. [Google Scholar] [CrossRef]
- Cheng, T.; Shao, J.; Wang, Z. Triboelectric nanogenerator. Nat. Rev. Methods Primers 2023, 3, 39–51. [Google Scholar] [CrossRef]
- Yang, W.; Cai, X.; Guo, S.; Wen, L.; Sun, Z.; Shang, R.; Li, Z. A High Performance Triboelectric Nanogenerator Based on MXene/Graphene Oxide Electrode for Glucose Detection. Materials 2023, 16, 841. [Google Scholar] [CrossRef]
- Kanokpaka, P.; Chang, Y.H.; Chang, C.C.; Rinawati, M.; Wang, P.C.; Chang, L.Y.; Yeh, M.H. Enabling glucose adaptive self-healing hydrogel based triboelectric biosensor for tracking a human perspiration. Nano Energy 2023, 112, 108513–108549. [Google Scholar] [CrossRef]
- Yu, R.; Pan, C.; Chen, J.; Zhu, G.; Wang, Z.L. Enhanced Performance of a ZnO Nanowire-Based Self-Powered Glucose Sensor by Piezotronic Effect. Adv. Funct. Mater. 2013, 23, 5868–5874. [Google Scholar] [CrossRef]
- Zhao, T.; Fu, Y.; Sun, C.; Zhao, X.; Jiao, C.; Du, A.; Liu, B. Wearable biosensors for real-time sweat analysis and body motion capture based on stretchable fiber-based triboelectric nanogenerators. Biosens. Bioelectron. 2022, 205, 114115–114124. [Google Scholar] [CrossRef]
- Liu, Q.; Li, G.; Zhu, H.; Zhao, H. Micro thermoelectric devices: From principles to innovative applications. Chin. Phys. B 2022, 31, 47204–47223. [Google Scholar] [CrossRef]
- Siddique, A.R.M.; Mahmud, S.; Heyst, B.V. A review of the state of the science on wearable thermoelectric power generators (TEGs) and their existing challenges. Renew. Sustain. Energy Rev. 2017, 73, 730–744. [Google Scholar] [CrossRef]
- Kim, J.; Khan, S.; Kim, E.K.; Kil, H.J.; Kang, B.M.; Lee, H.G.; Kim, W. A true continuous healthcare system for type 1 diabetes. Nano Energy 2023, 113, 108553. [Google Scholar] [CrossRef]
- Mashayekhi Mazar, F.; Alijanianzadeh, M.; Jamshidy Nia, Z.; Molaei Rad, A. Introduction to biofuel cells: A biological source of energy. Energy Sources Part A Recovery Util. Environ. Eff. 2017, 39, 419–425. [Google Scholar] [CrossRef]
- Bandodkar, A.J.; Wang, J. Wearable biofuel cells: A review. Electroanalysis 2016, 28, 1188–1200. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, L.; Zhang, Z.; Guo, S.; Shang, H.; Li, Y.; Liu, J. Wearable biofuel cells based on the classification of enzyme for high power outputs and lifetimes. Biosens. Bioelectron. 2019, 124, 40–52. [Google Scholar] [CrossRef]
- Bandodkar, A.J. Wearable biofuel cells: Past, present and future. J. Electrochem. Soc. 2016, 164, 3007–3014. [Google Scholar] [CrossRef]
- Geetha, M.; Sadasivuni, K.K.; Al-Ejji, M.; Sivadas, N.; Baig, M.Z.; Promi, T.J.; Al-Shaibah, F.N. Review of Progress and Prospects in Research on Enzymatic and Non-Enzymatic Biofuel Cells; Specific Emphasis on 2D Nanomaterials. Curr. Biotechnol. 2022, 11, 212–229. [Google Scholar] [CrossRef]
- Jayapiriya, U.S.; Goel, S. Microfluidic non-enzymatic biofuel cell integrated with electrodeposited metallic catalysts on a paper based platform. J. Power Sources 2021, 510, 230405–230414. [Google Scholar]
- Bae, C.W.; Chinnamani, M.V.; Lee, E.H.; Lee, N.E. Stretchable non-enzymatic fuel cell-based sensor patch integrated with thread-embedded microfluidics for self-powered wearable glucose monitoring. Adv. Mater. Interfaces 2022, 9, 2200492–2200501. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Park, W.; Li, J.; Ma, J.; Yiu, C.K.; Yu, X. Intelligent soft sweat sensors for the simultaneous healthcare monitoring and safety warning. Adv. Healthc. Mater. 2023, 12, 2202846–2202855. [Google Scholar] [CrossRef] [PubMed]
- Safdar, M.; Jänis, J.; Sanchez, S. Microfluidic fuel cells for energy generation. Lab. A Chip 2016, 16, 2754–2758. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, T.; Tao, K.; Chang, H. Generating electricity on chips: Microfluidic biofuel cells in perspective. Ind. Eng. Chem. Res. 2018, 57, 2746–2758. [Google Scholar] [CrossRef]
- Frei, M.; Martin, J.; Kindler, S.; Cristiano, G.; Zengerle, R.; Kerzenmacher, S. Power supply for electronic contact lenses: Abiotic glucose fuel cells vs. Mg/air batteries. J. Power Sources 2018, 401, 403–414. [Google Scholar] [CrossRef]
- Yun, J.; Li, Z.; Miao, X.; Li, X.; Lee, J.Y.; Zhao, W.; Lee, S.W. A tear-based battery charged by biofuel for smart contact lenses. Nano Energy 2023, 110, 108344–108351. [Google Scholar] [CrossRef]
- Sankauskaite, A.; Pauliukaite, R.; Baltusnikaite-Guzaitiene, J.; Abraitiene, A. Smart Textile with Integrated Wearable Electrochemical Sensors. Curr. Opin. Electrochem. 2023, 42, 101410–101439. [Google Scholar] [CrossRef]
- Manjakkal, L.; Pullanchiyodan, A.; Yogeswaran, N.; Hosseini, E.S.; Dahiya, R. A wearable supercapacitor based on conductive PEDOT: PSS-coated cloth and a sweat electrolyte. Adv. Mater. 2020, 32, 1907254–1907263. [Google Scholar] [CrossRef] [PubMed]
- Du, K.; Lin, R.; Yin, L.; Ho, J.S.; Wang, J.; Lim, C.T. Electronic textiles for energy, sensing, and communication. Iscience 2022, 25, 104174–104193. [Google Scholar] [CrossRef] [PubMed]
- Weng, W.; Chen, P.; He, S.; Sun, X.; Peng, H. Smart electronic textiles. Angew. Chem. Int. Ed. 2016, 55, 6140–6169. [Google Scholar] [CrossRef]
- Agcayazi, T.; Chatterjee, K.; Bozkurt, A.; Ghosh, T.K. Flexible interconnects for electronic textiles. Adv. Mater. Technol. 2018, 3, 1700277–1700308. [Google Scholar] [CrossRef]
- He, J.; Lu, C.; Jiang, H.; Han, F.; Shi, X.; Wu, J.; Peng, H. Scalable production of high-performing woven lithium-ion fibre batteries. Nature 2021, 597, 57–63. [Google Scholar] [CrossRef]
- Lu, C.; Jiang, H.; Cheng, X.; He, J.; Long, Y.; Chang, Y.; Peng, H. High-performance fibre battery with polymer gel electrolyte. Nature 2024, 629, 86–91. [Google Scholar] [CrossRef]
- Miller, P.R.; Skoog, S.A.; Edwards, T.L.; Wheeler, D.R.; Xiao, X.; Brozik, S.M.; Narayan, R.J. Hollow microneedle-based sensor for multiplexed transdermal electrochemical sensing. JoVE (J. Vis. Exp.) 2012, 64, 4067–4073. [Google Scholar]
- Donnelly, R.F.; Singh, T.R.R.; Garland, M.J.; Migalska, K.; Majithiya, R.; McCrudden, C.M.; Woolfson, A.D. Hydrogel-forming microneedle arrays for enhanced transdermal drug delivery. Adv. Funct. Mater. 2012, 22, 4879–4890. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.H.; Prausnitz, M.R. Dissolving microneedles for transdermal drug delivery. Biomaterials 2008, 29, 2113–2124. [Google Scholar] [CrossRef]
- Hu, Y.; Chatzilakou, E.; Pan, Z.; Traverso, G.; Yetisen, A.K. Microneedle Sensors for Point-of-Care Diagnostics. Adv. Sci. 2024, 11, 2306560–2306583. [Google Scholar] [CrossRef] [PubMed]
- El-Laboudi, A.; Oliver, N.S.; Cass, A.; Johnston, D. Use of microneedle array devices for continuous glucose monitoring: A review. Diabetes Technol. Ther. 2013, 15, 101–115. [Google Scholar] [CrossRef]
- Samant, P.P.; Prausnitz, M.R. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl. Acad. Sci. USA 2018, 115, 4583–4588. [Google Scholar] [CrossRef]
- Wang, P.M.; Cornwell, M.; Prausnitz, M.R. Minimally invasive extraction of dermal interstitial fluid for glucose monitoring using microneedles. Diabetes Technol. Ther. 2005, 7, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Samant, P.P.; Niedzwiecki, M.M.; Raviele, N.; Tran, V.; Mena-Lapaix, J.; Walker, D.I.; Prausnitz, M.R. Sampling interstitial fluid from human skin using a microneedle patch. Sci. Transl. Med. 2020, 12, 285–329. [Google Scholar] [CrossRef]
- Sharma, S.; Huang, Z.; Rogers, M.; Boutelle, M.; Cass, A.E. Evaluation of a minimally invasive glucose biosensor for continuous tissue monitoring. Anal. Bioanal. Chem. 2016, 408, 8427–8435. [Google Scholar] [CrossRef] [PubMed]
- Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Donnelly, R.F.; De Wael, K. Wearable hollow microneedle sensing patches for the transdermal electrochemical monitoring of glucose. Talanta 2022, 249, 123695–123724. [Google Scholar] [CrossRef]
- Henninger, N.; Woderer, S.; Kloetzer, H.M.; Staib, A.; Gillen, R.; Li, L.; Pill, J. Tissue response to subcutaneous implantation of glucose-oxidase-based glucose sensors in rats. Biosens. Bioelectron. 2007, 23, 26–34. [Google Scholar] [CrossRef]
- Huang, X.; Leduc, C.; Ravussin, Y.; Li, S.; Davis, E.; Song, B.; Lin, Q. A differential dielectric affinity glucose sensor. Lab. A Chip 2014, 14, 294–301. [Google Scholar] [CrossRef]
- Bolinder, J.; Hagström, E.; Ungerstedt, U.; Arner, P. Microdialysis of subcutaneous adipose tissue in vivo for continuous glucose monitoring in man. Scand. J. Clin. Lab. Investig. 1989, 49, 465–474. [Google Scholar] [CrossRef]
- Li, D.; Xu, Q.; Liu, Y.; Wang, R.; Xu, K.; Yu, H. A high-accuracy measurement method of glucose concentration in interstitial fluid based on microdialysis. Meas. Sci. Technol. 2017, 28, 115701–115717. [Google Scholar] [CrossRef]
- Najmi, A.; Saidi, M.S.; Shahrokhian, S.; Hosseini, H.; Hannani, S.K. Fabrication of a microdialysis-based nonenzymatic microfluidic sensor for regular glucose measurement. Sens. Actuators B Chem. 2021, 333, 129569–129578. [Google Scholar] [CrossRef]
- Hayashi, R.; Okubo, T.; Kudo, Y.; Ishikawa, Y.; Imaizumi, T.; Suzuki, K.; Nishida, K. Generation of 3D lacrimal gland organoids from human pluripotent stem cells. Nature 2022, 605, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, Y.; Shi, K.; Wu, H.; Ou, S. Advances in clinical examination of lacrimal gland. Front. Med. 2023, 10, 1257209–1257219. [Google Scholar] [CrossRef] [PubMed]
- Tóth-Molnár, E.; Ding, C. New insight into lacrimal gland function: Role of the duct epithelium in tear secretion. Ocul. Surf. 2020, 18, 595–603. [Google Scholar] [CrossRef]
- Pieczyński, J.; Szulc, U.; Harazna, J.; Szulc, A.; Kiewisz, J. Tear fluid collection methods: Review of current techniques. Eur. J. Ophthalmol. 2021, 31, 2245–2251. [Google Scholar] [CrossRef]
- Kagie, A.; Bishop, D.K.; Burdick, J.; La Belle, J.T.; Dymond, R.; Felder, R.; Wang, J. Flexible rolled thick-film miniaturized flow-cell for minimally invasive amperometric sensing. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2008, 20, 1610–1614. [Google Scholar] [CrossRef]
- Yao, H.; Liao, Y.; Lingley, A.R.; Afanasiev, A.; Lähdesmäki, I.; Otis, B.P.; Parviz, B.A. A contact lens with integrated telecommunication circuit and sensors for wireless and continuous tear glucose monitoring. J. Micromech. Microeng. 2012, 22, 075007. [Google Scholar] [CrossRef]
- Keum, D.H.; Kim, S.K.; Koo, J.; Lee, G.H.; Jeon, C.; Mok, J.W.; Hahn, S.K. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 2020, 6, 3252–3561. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Kim, S.Y.; Cheong, W.H.; Jang, J.; Park, Y.G.; Park, J.U. Soft, smart contact lenses with integrations of wireless circuits, glucose sensors, and displays. Sci. Adv. 2018, 4, 9841–9852. [Google Scholar] [CrossRef]
- Li, Z.; Yun, J.; Li, X.; Kim, M.; Li, J.; Lee, D.; Wu, A.; Lee, S.W. Power-Free Contact Lens for Glucose Sensing. Adv. Funct. Mater. 2023, 33, 2304647. [Google Scholar] [CrossRef]
- Lee, E.; Kang, S.; Shim, J.; Jeong, D.; Jeong, Y.; Ahn, J.; Seo, K. Quantification of tear glucose levels and their correlation with blood glucose levels in dogs. Vet. Med. Sci. 2022, 8, 1816–1824. [Google Scholar] [CrossRef]
- Park, W.; Seo, H.; Kim, J.; Hong, Y.M.; Song, H.; Joo, B.J.; Park, J.U. In-depth correlation analysis between tear glucose and blood glucose using a wireless smart contact lens. Nat. Commun. 2024, 15, 2828–2842. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.R.; Hung, C.C.; Chiu, H.Y.; Chang, P.H.; Li, B.R.; Cheng, S.J.; Chen, G.Y. Noninvasive glucose monitoring with a contact lens and smartphone. Sensors 2018, 18, 3208. [Google Scholar] [CrossRef]
- Zou, R.; Shan, S.; Huang, L.; Chen, Z.; Lawson, T.; Lin, M.; Liu, Y. High-performance intraocular biosensors from chitosan-functionalized nitrogen-containing graphene for the detection of glucose. ACS Biomater. Sci. Eng. 2019, 6, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Wang, M.; Zhao, C.; Wang, S.; Chen, K.; Li, X.; Ma, W. Flexible organic integrated electronics for self-powered multiplexed ocular monitoring. NPJ Flex. Electron. 2022, 6, 77–84. [Google Scholar] [CrossRef]
- Chong, D.D.; Das, N.; Singh, R.P. Diabetic retinopathy: Screening, prevention, and treatment. Nat. Rev. Dis. Primers 2016, 2, 16012–16019. [Google Scholar] [CrossRef]
- Obrosova, I.G.; Chung, S.S.M.; Kador, P.F. Diabetic cataracts: Mechanisms and management. Diabetes/Metab. Res. Rev. 2010, 26, 172–180. [Google Scholar] [CrossRef]
- Han, J.H.; Cho, Y.C.; Koh, W.G.; Choy, Y.B. Preocular sensor system for concurrent monitoring of glucose levels and dry eye syndrome using tear fluids. PLoS ONE 2020, 15, 239317–239332. [Google Scholar] [CrossRef]
- Nguyen-Khuong, T.; Everest-Dass, A.V.; Kautto, L.; Zhao, Z.; Willcox, M.D.; Packer, N.H. Glycomic characterization of basal tears and changes with diabetes and diabetic retinopathy. Glycobiology 2015, 25, 269–283. [Google Scholar] [CrossRef]
- Cullen, C.L.; Ihle, S.L.; Webb, A.A.; McCarville, C. Keratoconjunctival effects of diabetes mellitus in dogs. Vet. Ophthalmol. 2005, 8, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Hanaki, M.; Nakagaki, H.; Nakamura, H.; Kondo, K.; Weatherell, J.A.; Robinson, C. Glucose clearance from different surfaces of human central incisors and first molars. Arch. Oral Biol. 1993, 38, 479–482. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, T.; Kuroki, Y.; Nitta, H.; Chouhan, P.; Toma, K.; Sawada, S.I.; Mitsubayashi, K. Mouthguard biosensor with telemetry system for monitoring of saliva glucose: A novel cavitas sensor. Biosens. Bioelectron. 2016, 84, 106–111. [Google Scholar] [CrossRef]
- Mitsubayashi, K. Cavitas Biosensors: Noninvasive Approaches to Blood Glucose Monitoring for Diabetes Mellitus. Sens. Mater. 2018, 30, 2313–2320. [Google Scholar] [CrossRef]
- Arakawa, T.; Tomoto, K.; Nitta, H.; Toma, K.; Takeuchi, S.; Sekita, T.; Mitsubayashi, K. A wearable cellulose acetate-coated mouthguard biosensor for in vivo salivary glucose measurement. Anal. Chem. 2020, 92, 12201–12207. [Google Scholar] [CrossRef]
- Garcia-Carmona, L.; Martín, A.; Sempionatto, J.R.; Moreto, J.R.; Gonzalez, M.C.; Wang, J.; Escarpa, A. Pacifier Biosensor: Toward Noninvasive Saliva Biomarker Monitoring. Anal. Chem. 2019, 91, 13883–13891. [Google Scholar] [CrossRef]
- Liu, Y.; Yue, W.; Cui, Y. Development of an amperometric biosensor on a toothbrush for glucose. Sens. Actuators Rep. 2023, 5, 100133–100141. [Google Scholar] [CrossRef]
- Sha, P.; Luo, X.; Shi, W.; Liu, Y.; Cui, Y. A Smart Dental Floss for Biosensing of Glucose. Electroanalysis 2019, 31, 791–796. [Google Scholar] [CrossRef]
- Wiorek, A.; Parrilla, M.; Cuartero, M.; Crespo, G.A. Epidermal patch with glucose biosensor: pH and temperature correction toward more accurate sweat analysis during sport practice. Anal. Chem. 2020, 92, 10153–10161. [Google Scholar] [CrossRef]
- Fischer, C.; Fraiwan, A.; Choi, S. A 3D paper-based enzymatic fuel cell for self-powered, low-cost glucose monitoring. Biosens. Bioelectron. 2016, 79, 193–197. [Google Scholar] [CrossRef]
- Cho, E.; Mohammadifar, M.; Choi, S. A single-use, self-powered, paper-based sensor patch for detection of exercise-induced hypoglycemia. Micromachines 2017, 8, 265. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Hou, W.; Ji, Z.; Sun, Z.; Li, M.; Lian, B. Wearable Electrochemical Sensor for Sweat-Based Potassium Ion and Glucose Detection in Exercise Health Monitoring. Chem. Open 2024, 13, 202300217–202300223. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Li, Y.; Li, Y.; Li, D.; Guo, T.; Deng, X.; Lu, X. Tuning water-resistant networks in mussel-inspired hydrogels for robust wet tissue and bioelectronic adhesion. ACS Nano 2023, 17, 2745–2760. [Google Scholar] [CrossRef]
- Hou, Z.; Gao, T.; Liu, X.; Guo, W.; Bai, L.; Wang, W.; Wei, D. Dual detection of human motion and glucose in sweat with polydopamine and glucose oxidase doped self-healing nanocomposite hydrogels. Int. J. Biol. Macromol. 2023, 252, 126473–126522. [Google Scholar] [CrossRef]
- Li, T.; Liang, B.; Ye, Z.; Zhang, L.; Xu, S.; Tu, T.; Ye, X. An integrated and conductive hydrogel-paper patch for simultaneous sensing of Chemical–Electrophysiological signals. Biosens. Bioelectron. 2022, 198, 113855–113865. [Google Scholar] [CrossRef]
- Nugba, B.E.; Mousa, N.O.; Osman, A.; El-Moneim, A.A. Non-enzymatic amperometric biosensor with anchored Ni nanoparticles for urinary glucose quantification. Diam. Relat. Mater. 2023, 137, 110171–110180. [Google Scholar] [CrossRef]
- Ouyang, Y.; O’Hagan, M.P.; Willner, I. Functional catalytic nanoparticles (nanozymes) for sensing. Biosens. Bioelectron. 2022, 218, 114768–114869. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, D.; Yang, Y.; Song, X. An electrochemical nonenzymatic microsensor modified by nickel cobaltate nanospheres for glucose sensing in urine. IEEE Sens. J. 2021, 21, 13074–13081. [Google Scholar] [CrossRef]
- Gomes, N.O.; Paschoalin, R.T.; Bilatto, S.; Sorigotti, A.R.; Farinas, C.S.; Mattoso, L.H.C.; Raymundo-Pereira, P.A. Flexible, bifunctional sensing platform made with biodegradable mats for detecting glucose in urine. ACS Sustain. Chem. Eng. 2023, 11, 2209–2218. [Google Scholar] [CrossRef]
- Fan, S.; Chang, W.; Fei, C.; Zhang, Z.; Hou, B.; Shi, Z.; Hui, Y. Stretchable and bendable textile matrix based on cellulose fibers for wearable self-powered glucose biosensors. Cellulose 2022, 29, 8919–8935. [Google Scholar] [CrossRef]
- Zhu, J.; Zhou, X.; Kim, H.J.; Qu, M.; Jiang, X.; Lee, K.; Khademhosseini, A. Gelatin methacryloyl microneedle patches for minimally invasive extraction of skin interstitial fluid. Small 2020, 16, 1905910–1905918. [Google Scholar] [CrossRef] [PubMed]
- Hakala, T.A.; Zschaechner, L.K.; Vänskä, R.T.; Nurminen, T.A.; Wardale, M.; Morina, J.; García Pérez, A. Pilot study in human healthy volunteers on the use of magnetohydrodynamics in needle-free continuous glucose monitoring. Sci. Rep. 2022, 12, 18318–18328. [Google Scholar] [CrossRef]
- Zhu, W.; Yu, H.; Pu, Z.; Guo, Z.; Zheng, H.; Li, C.; Li, D. Effect of interstitial fluid pH on transdermal glucose extraction by reverse iontophoresis. Biosens. Bioelectron. 2023, 235, 115406–115415. [Google Scholar] [CrossRef]
- He, Q.Y.; Zhao, J.H.; Du, S.M.; Li, D.G.; Luo, Z.W.; You, X.Q.; Liu, J. Reverse iontophoresis generated by porous microneedles produces an electroosmotic flow for glucose determination. Talanta 2024, 267, 125156–125165. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Sheng, C.; Dong, F.; Liu, S. An integrated wearable differential microneedle array for continuous glucose monitoring in interstitial fluids. Biosens. Bioelectron. 2024, 256, 116280–116288. [Google Scholar] [CrossRef]
- Pang, Y.; Li, Y.; Chen, K.; Wu, M.; Zhang, J.; Sun, Y.; Kong, D. Porous Microneedles Through Direct Ink Drawing with Nanocomposite Inks for Transdermal Collection of Interstitial Fluid. Small 2024, 20, 2305838–2305847. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, H.; Park, S.; Han, S.Y.; Hong, H.; Yang, D.S.; Ryu, W. Individually-addressable composite microneedle electrode array by mold-and-place method for glucose detection. Sens. Actuators B Chem. 2024, 401, 134884–134894. [Google Scholar] [CrossRef]
- Abbasiasl, T.; Mirlou, F.; Mirzajani, H.; Bathaei, M.J.; Istif, E.; Shomalizadeh, N.; Cebecioğlu, R.E.; Özkahraman, E.E.; Yener, U.C.; Beker, L. A Wearable Touch-Activated Device Integrated with Hollow Microneedles for Continuous Sampling and Sensing of Dermal Interstitial Fluid. Adv. Mater. 2024, 36, 2304704–2304720. [Google Scholar] [CrossRef]
- Shukla, S.; Machekposhti, S.A.; Joshi, N.; Joshi, P.; Narayan, R. Microneedle-Integrated Device for Transdermal Sampling and Analyses of Targeted Biomarkers. Small Sci. 2023, 3, 2200087–2200102. [Google Scholar] [CrossRef]
- Wu, T.; You, X.; Chen, Z. Hollow microneedles on a paper fabricated by standard photolithography for the screening test of prediabetes. Sensors 2022, 22, 4253. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Cho, S. Porous platinum black-coated minimally invasive microneedles for non-enzymatic continuous glucose monitoring in interstitial fluid. Nanomaterials 2020, 11, 37. [Google Scholar] [CrossRef]
- Wu, L.; Shrestha, P.; Iapichino, M.; Cai, Y.; Kim, B.; Stoeber, B. Characterization method for calculating diffusion coefficient of drug from polylactic acid (PLA) microneedles into the skin. J. Drug Deliv. Sci. Technol. 2021, 61, 102192–102202. [Google Scholar] [CrossRef]
- Yang, B.; Wang, H.; Kong, J.; Fang, X. Long-term monitoring of ultratrace nucleic acids using tetrahedral nanostructure-based NgAgo on wearable microneedles. Nat. Commun. 2024, 15, 1936–1950. [Google Scholar] [CrossRef]
- Najmi, A.; Saidi, M.S.; Hannani, S.K. Design of the micropump and mass-transfer compartment of a microfluidic system for regular nonenzymatic glucose measurement. Biotechnol. Rep. 2022, 34, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Heifler, O.; Borberg, E.; Harpak, N.; Zverzhinetsky, M.; Krivitsky, V.; Gabriel, I.; Patolsky, F. Clinic-on-a-needle array toward future minimally invasive wearable artificial pancreas applications. ACS Nano 2021, 15, 12019–12033. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Yu, Q.; Liu, Y.; Gai, W.; Ye, L.; Yang, L.; Cui, Y. Closed-loop diabetes minipatch based on a biosensor and an electroosmotic pump on hollow biodegradable microneedles. ACS Sens. 2022, 7, 1347–1360. [Google Scholar] [CrossRef]
- Parrilla, M.; Detamornrat, U.; Domínguez-Robles, J.; Tunca, S.; Donnelly, R.F.; De Wael, K. Wearable microneedle-based array patches for continuous electrochemical monitoring and drug delivery: Toward a closed-loop system for methotrexate treatment. ACS Sens. 2023, 8, 4161–4170. [Google Scholar] [CrossRef]
- Jiang, Y.; Trotsyuk, A.A.; Niu, S.; Henn, D.; Chen, K.; Shih, C.C.; Bao, Z. Wireless, closed-loop, smart bandage with integrated sensors and stimulators for advanced wound care and accelerated healing. Nat. Biotechnol. 2023, 41, 652–662. [Google Scholar] [CrossRef]

| Principle | Biofluid Interference | Underlying Cause |
|---|---|---|
| Amperometry | Reduced current sensitivity Signal drift | Low ionic strength→Higher solution resistance (Rs) [130] Protein adsorption→Electrode fouling [134] |
| Potentiometry | Nernstian slope deviation Reference electrode instability | Variable Na+/K+ levels→Ionic activity fluctuations [131] |
| Voltammetry | Peak broadening Redox potential shifts | Fluid viscosity→Altered diffusion coefficients [133] |
| Impedance | Non-linear Nyquist plots Rct-concentration correlation loss | Complex composition→Parasitic capacitances [6] |
| MNmaterials | Center-to-Center (μm) | Edge Width (μm) | Height (μm) | Ref. |
|---|---|---|---|---|
| Acupuncture needle | — | 80 | 3000 | [257] |
| Composite ink | — | <4 | 611 ± 22 | [258] |
| SWCNTs | — | — | 640 | [259] |
| PDMS | 1200 | 600 | 1500 | [260] |
| Polymer | 2500 | 800 | 900 | [261] |
| PEG-DA | 20 | 160 | 600 | [262] |
| Au | — | <300 | 100.16 | [263] |
| PLA | 100 | 200 | 500 | [264] |
| NgAgo | 300 ± 10 | — | 800 ± 10 | [265] |
| Glass and silicon | 300 | — | 150 | [266] |
| BF | M | D | E V(μL) | S (μA/mM/cm2) | LOD (μM) | LR(mM) | LT | Ref |
|---|---|---|---|---|---|---|---|---|
| Tear | GC-COOH | Amperometric method | — | 110.92 | 9.5 | 0–12 | >1 day | [227] |
| PBA/HEMA | — | 1500 | — | — | 0.1–0.6 | >10 h | [226] | |
| PET | — | 0.4 | — | — | — | — | [231] | |
| Saliva | CA/PDMS | — | — | — | — | 0.005–1 | >5 h | [237] |
| Ag/AgCl Oil ink | — | 0.5 | — | 40 | 100–1400 | >60 m | [238] | |
| Ag/AgCl | Voltammetric method | 5 | 0.1214 | 5 | 180–5220 | <3000 s | [239] | |
| Carbon graphite &Ag/AgCl ink | Voltammetric method | 10 | 0.0352 | — | 48–19,500 | — | [240] | |
| Sweat | Graphene/chitosan /PEDOT:PSS | Voltammetric method | 0.02–1.0 | 1.35 | — | — | — | [243] |
| EFC | — | 20 | 0.02 | — | 1–5 | — | [242] | |
| GOX/PEDOT:PSS | Amperometric method | 2 | — | 0.075 | 2–32 | — | [244] | |
| PAA/PAM/PDA | — | 4 | 1576 | 0.28 | 0–0.205 | — | [245] | |
| PEDOT:PSS | Cyclic voltammetry | — | 325.99 ± 0.8 | 10.3 | 0–12 | >10 days | [247] | |
| Urine | NiCo2O4 | — | — | 3449.14 | 0.376 | 1–100,000 | — | [250] |
| LIG/Ni | — | — | 5796.18 | 0.0152 | 12–1500 | — | [248] | |
| PLA/PEG | Amperometric method | 20 | — | 197 | 500–5500 | >60 days | [251] | |
| MCNT/RGO | — | 250 | — | 3.95 | 0–4000 | — | [252] | |
| ISF | MWCNTs/CSF | — | — | 288.86 | — | 0–5000 | — | [167] |
| — | Differential pulse voltammetry | — | 0.549 | 0.08 | 25–300 | >28 days | [168] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zheng, J.; Zhang, G.; Lu, S.; Zhou, J. Wearable Electrochemical Glucose Sensors for Fluid Monitoring: Advances and Challenges in Non-Invasive and Minimally Invasive Technologies. Biosensors 2025, 15, 309. https://doi.org/10.3390/bios15050309
Wang M, Zheng J, Zhang G, Lu S, Zhou J. Wearable Electrochemical Glucose Sensors for Fluid Monitoring: Advances and Challenges in Non-Invasive and Minimally Invasive Technologies. Biosensors. 2025; 15(5):309. https://doi.org/10.3390/bios15050309
Chicago/Turabian StyleWang, Ming, Junjie Zheng, Ge Zhang, Shiyan Lu, and Jinli Zhou. 2025. "Wearable Electrochemical Glucose Sensors for Fluid Monitoring: Advances and Challenges in Non-Invasive and Minimally Invasive Technologies" Biosensors 15, no. 5: 309. https://doi.org/10.3390/bios15050309
APA StyleWang, M., Zheng, J., Zhang, G., Lu, S., & Zhou, J. (2025). Wearable Electrochemical Glucose Sensors for Fluid Monitoring: Advances and Challenges in Non-Invasive and Minimally Invasive Technologies. Biosensors, 15(5), 309. https://doi.org/10.3390/bios15050309