Aspects of Electrochemical Biosensors Using Affinity Assays
Abstract
1. Introduction
2. Electrodes
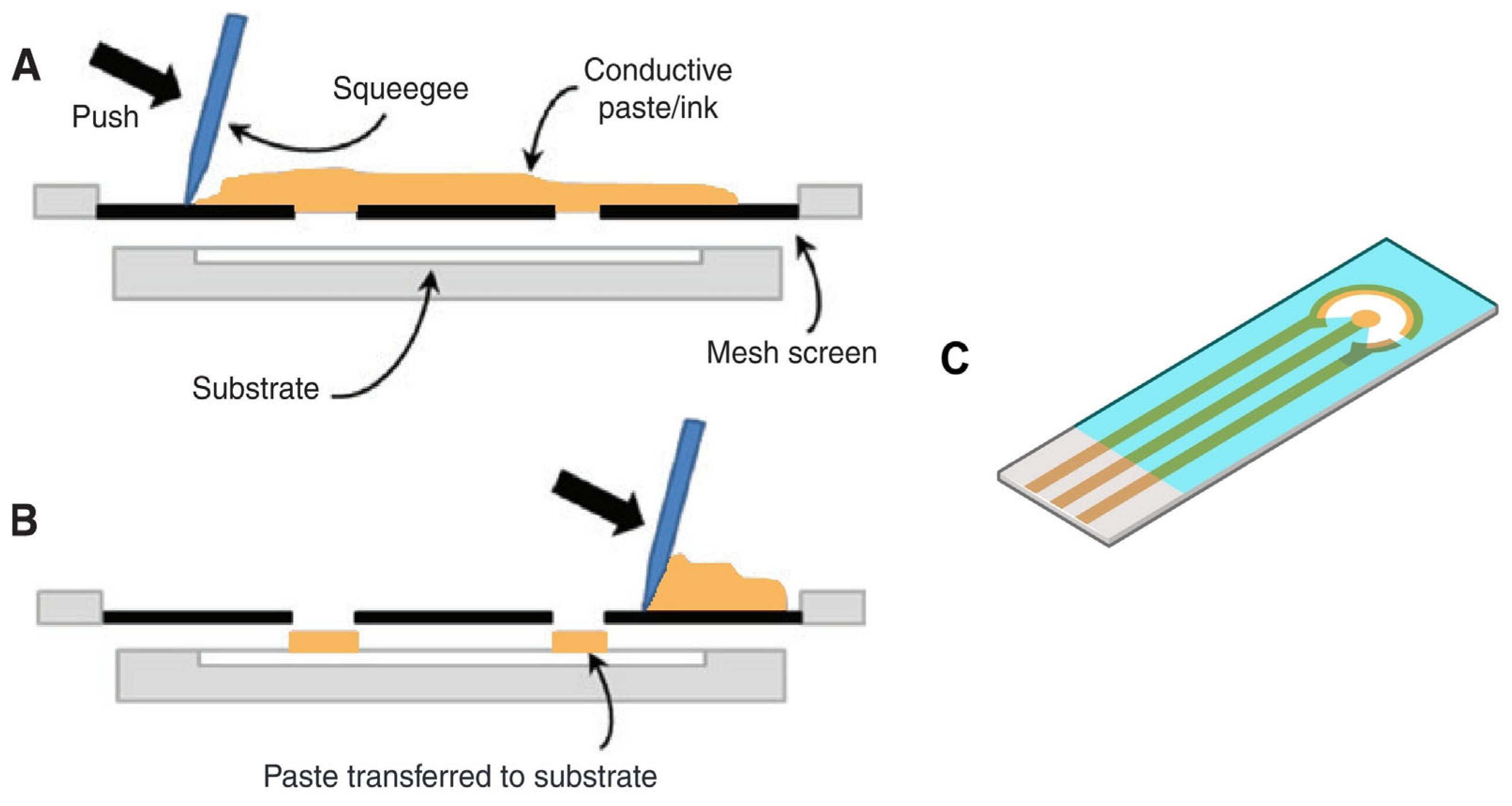
2.1. Electrode Types
| Screen-Printed Electrodes | |||
|---|---|---|---|
| Material | Produce/Supplier | Notes | Reference |
| Gold | DropSens | BT220, AT220, AT250 | [30,31,32,33,34,35,36,37,38,39,40] |
| BVT Technologies | BVT-AC1.W1.RS.Dw2 | [41] | |
| PalmSens | Italsens | [42,43] | |
| Zimmer and Peacock | A-AD-GG-101-N | [44] | |
| Zensor R&D | X | [45] | |
| Micrux | X | X | |
| Gamry | X | X | |
| Pine Research | X | X | |
| Carbon/graphite/graphene | DropSens | 110, C110, 110CNT | [34,36,46,47,48,49] |
| BVT Technologies | AC3.W4 | [50] | |
| Micrux | S1PE | [51,52,53] | |
| PalmSens | Italsens | [54] | |
| Zimmer and Peacock | X | [55,56] | |
| Zensor R&D | TE100 | [57,58,59,60] | |
| iGii | Gii-Sens (3D foam) | [61,62] | |
| Custom | X | [16,17,63,64,65] | |
| Conductive Technologies | X | X | |
| Gamry | X | X | |
| Pine Research | X | X | |
| Thin-Film Electrodes | |||
| Material | Producer/Supplier | Notes | Reference |
| Gold | DropSens | Interdigitated electrode | [66] |
| Micrux | Interdigitated electrode and SE1-AuPT | [67,68,69,70] | |
| Custom-made | X | [71,72,73,74,75] | |
| Macias Sensors | PCB electrodes | X | |
| Conductive Technologies | X | X | |
| Zimmer and Peacock | X | X | |
| Carbon/graphite/graphene | Custom | X | [76,77] |
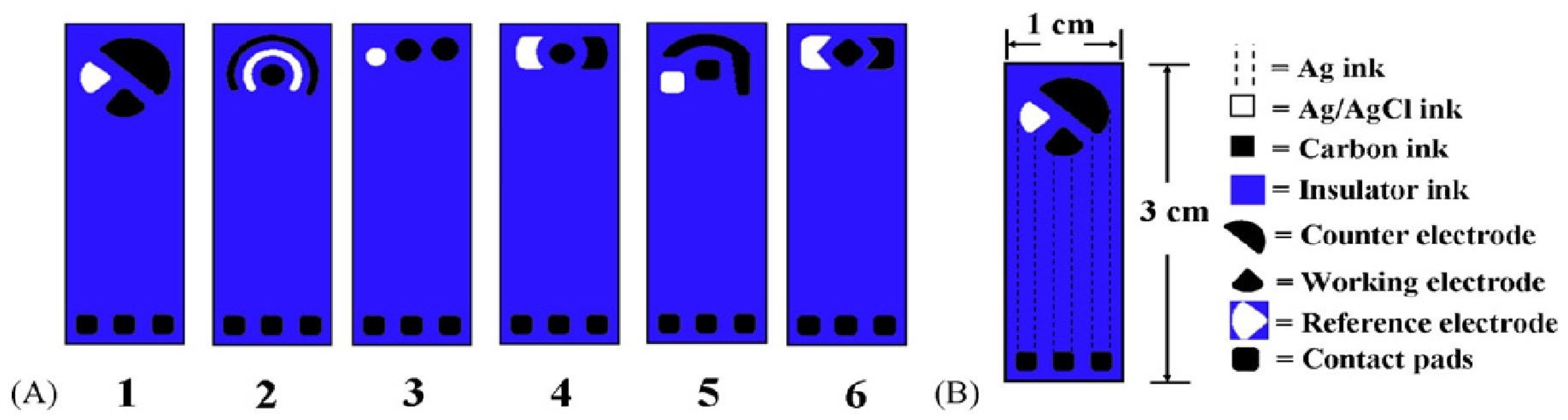
2.2. Electrode Geometries
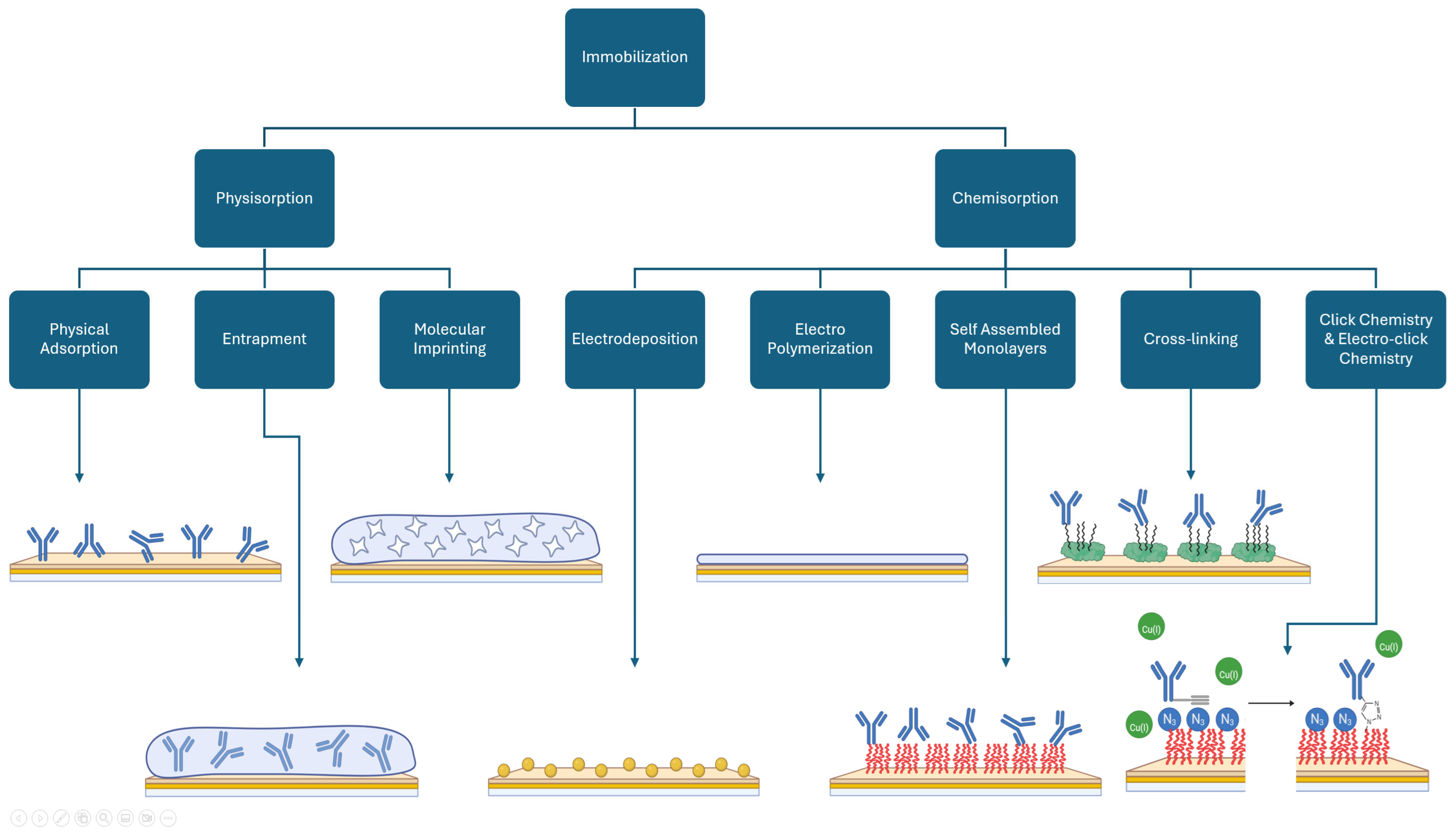
3. Immobilization
3.1. Physisorption
3.1.1. Physical/Passive Adsorption
3.1.2. Entrapment
3.1.3. Molecular Imprinting
3.2. Chemisorption
3.2.1. Electrodeposition
3.2.2. Electropolymerization
3.2.3. Self Assembled Monolayers
3.2.4. Cross-Linking
3.2.5. Click and Electro-Click Chemistry
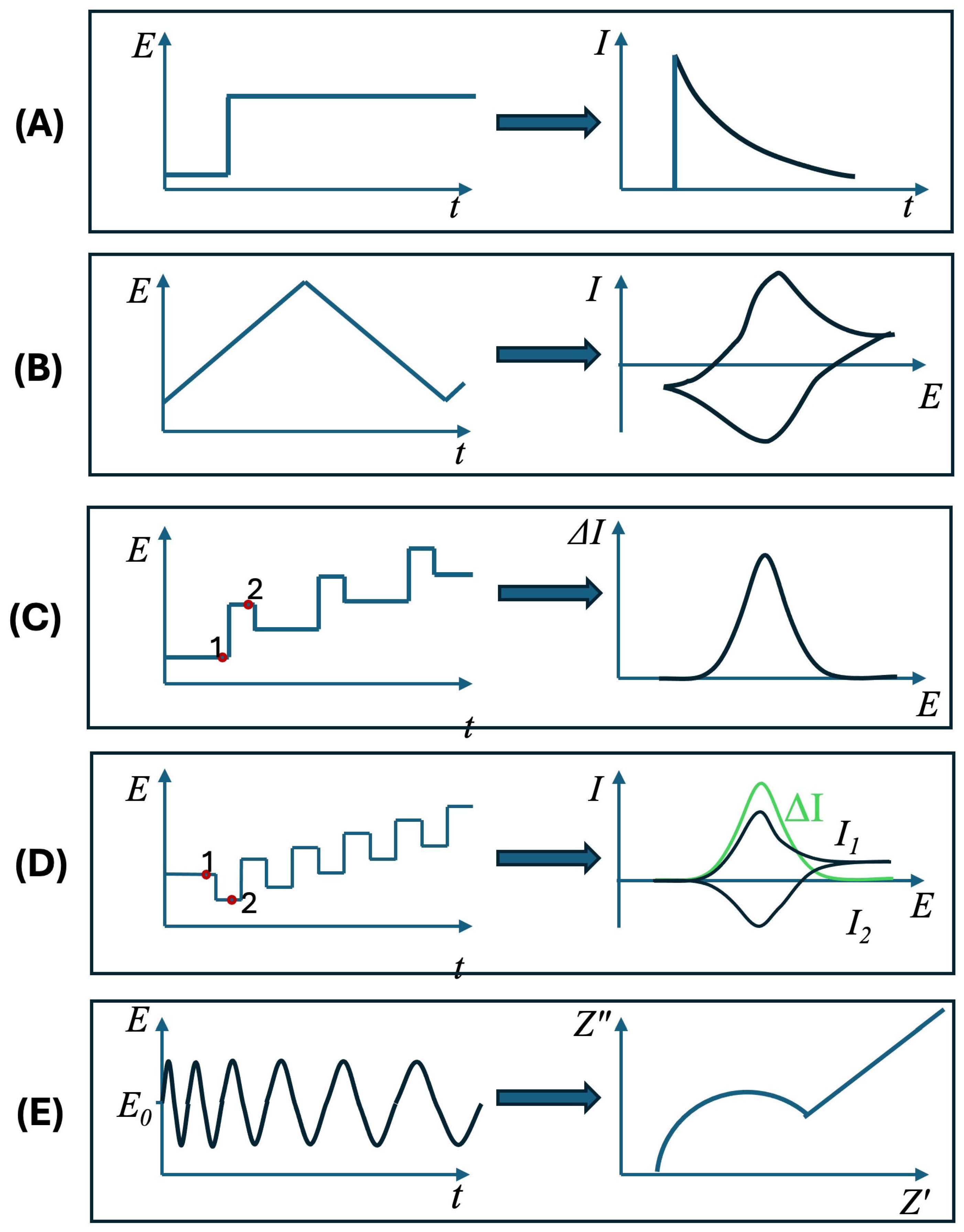
4. Electrochemical Techniques
5. Labels and Surface Enhancements
5.1. Metallic Nanoparticles
5.2. Carbon Materials
5.3. Enzymes
5.4. Redox Probes
6. Discussion/Perspective
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical Biosensors: Recommended Definitions and Classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C., Jr.; Lyons, C. Electrode Systems for Continuous Monitoring in Cardiovascular Surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Liu, H.; Chen, W.; Ma, B.; Ju, H. Device Integration of Electrochemical Biosensors. Nat. Rev. Bioeng. 2023, 1, 346–360. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef]
- Ricci, F.; Adornetto, G.; Palleschi, G. A Review of Experimental Aspects of Electrochemical Immunosensors. Electrochim. Acta 2012, 84, 74–83. [Google Scholar] [CrossRef]
- Liang, G.; He, Z.; Zhen, J.; Tian, H.; Ai, L.; Pan, L.; Gong, W. Development of the Screen-Printed Electrodes: A Mini Review on the Application for Pesticide Detection. Environ. Technol. Innov. 2022, 28, 102922. [Google Scholar] [CrossRef]
- Paimard, G.; Ghasali, E.; Baeza, M. Screen-Printed Electrodes: Fabrication, Modification, and Biosensing Applications. Chemosensors 2023, 11, 113. [Google Scholar] [CrossRef]
- Takaloo, S.; Moghimi Zand, M. Wearable Electrochemical Flexible Biosensors: With the Focus on Affinity Biosensors. Sens. Bio-Sens. Res. 2021, 32, 100403. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Garcia-Miranda Ferrari, A.; Dempsey, N.C.; Banks, C.E. Electroanalytical Overview: Screen-Printed Electrochemical Sensing Platforms for the Detection of Vital Cardiac, Cancer and Inflammatory Biomarkers. Sens. Diagn. 2022, 1, 405–428. [Google Scholar] [CrossRef]
- Metrohm Scientific Publications. Available online: https://metrohm-dropsens.com/publications/papers/ (accessed on 16 December 2024).
- PalmSens Scientific Publications. Available online: https://www.palmsens.com/publications/ (accessed on 16 December 2024).
- Micrux Scientific Publications. Available online: https://www.micruxfluidic.com/en/publications/ (accessed on 16 December 2024).
- iGii Scientific Publications. Available online: https://www.igii.uk/category/scientific-publications/ (accessed on 16 December 2024).
- Smutok, O.; Katz, E. Biosensors: Electrochemical Devices—General Concepts and Performance. Biosensors 2022, 13, 44. [Google Scholar] [CrossRef]
- Mohan, J.M.; Amreen, K.; Javed, A.; Dubey, S.K.; Goel, S. Miniaturized PMMA Electrochemical Platform with Carbon Fiber for Multiplexed and Noninterfering Biosensing of Real Samples. IEEE Trans. Electron Devices 2021, 68, 769–774. [Google Scholar] [CrossRef]
- Nandhakumar, P.; Muñoz San Martín, C.; Arévalo, B.; Ding, S.; Lunker, M.; Vargas, E.; Djassemi, O.; Campuzano, S.; Wang, J. Redox Cycling Amplified Electrochemical Lateral-Flow Immunoassay: Toward Decentralized Sensitive Insulin Detection. ACS Sens. 2023, 8, 3892–3901. [Google Scholar] [CrossRef] [PubMed]
- Chittuam, K.; Jampasa, S.; Vilaivan, T.; Tangkijvanich, P.; Chuaypen, N.; Avihingsanon, A.; Sain, M.; Panraksa, Y.; Chailapakul, O. Electrochemical Capillary-Driven Microfluidic DNA Sensor for HIV-1 and HCV Coinfection Analysis. Anal. Chim. Acta 2023, 1265, 341257. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Wang, J.; Cinti, S. Review—An Overview on Recent Progress in Screen-Printed Electroanalytical (Bio)Sensors. ECS Sens. Plus 2022, 1, 023401. [Google Scholar] [CrossRef]
- Lakhera, P.; Chaudhary, V.; Jha, A.; Singh, R.; Kush, P.; Kumar, P. Recent Developments and Fabrication of the Different Electrochemical Biosensors Based on Modified Screen Printed and Glassy Carbon Electrodes for the Early Diagnosis of Diverse Breast Cancer Biomarkers. Mater. Today Chem. 2022, 26, 101129. [Google Scholar] [CrossRef]
- de Oliveira, R.A.G.; Materon, E.M.; Melendez, M.E.; Carvalho, A.L.; Faria, R.C. Disposable Microfluidic Immunoarray Device for Sensitive Breast Cancer Biomarker Detection. ACS Appl. Mater. Interfaces 2017, 9, 27433–27440. [Google Scholar] [CrossRef]
- Fletcher, S. Screen-Printed Carbon Electrodes. In Advances in Electrochemical Sciences and Engineering; Alkire, R.C., Bartlett, P.N., Lipkowski, J., Eds.; Wiley: Weinheim, Germany, 2015; pp. 425–444. ISBN 978-3-527-33732-3. [Google Scholar]
- Suresh, R.R.; Lakshmanakumar, M.; Arockia Jayalatha, J.B.B.; Rajan, K.S.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Fabrication of Screen-Printed Electrodes: Opportunities and Challenges. J. Mater. Sci. 2021, 56, 8951–9006. [Google Scholar] [CrossRef]
- Herrasti, Z.; Serna, E.D.L.; Ruiz-Vega, G.; Baldrich, E. Developing Enhanced Magnetoimmunosensors Based on Low-Cost Screen-Printed Electrode Devices. Rev. Anal. Chem. 2016, 35, 53–85. [Google Scholar] [CrossRef]
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Duato, P.; Castillo, J.R. An Electrochemical Immunosensor for Ochratoxin a Determination in Wines Based on a Monoclonal Antibody and Paramagnetic Microbeads. Anal. Bioanal. Chem. 2012, 403, 1585–1593. [Google Scholar] [CrossRef]
- Squissato, A.L.; Munoz, R.A.A.; Banks, C.E.; Richter, E.M. An Overview of Recent Electroanalytical Applications Utilizing Screen-Printed Electrodes Within Flow Systems. ChemElectroChem 2020, 7, 2211–2221. [Google Scholar] [CrossRef]
- García-González, R.; Fernández-Abedul, M.T.; Pernía, A.; Costa-García, A. Electrochemical Characterization of Different Screen-Printed Gold Electrodes. Electrochim. Acta 2008, 53, 3242–3249. [Google Scholar] [CrossRef]
- Norfun, P.; Suree, N.; Kungwan, N.; Punyodom, W.; Jakmunee, J.; Ounnunkad, K. Electrochemical Detection of Human Interleukin-15 Using a Graphene Oxide-Modified Screen-Printed Carbon Electrode. Anal. Lett. 2017, 50, 1112–1125. [Google Scholar] [CrossRef]
- Afonso, A.S.; Uliana, C.V.; Martucci, D.H.; Faria, R.C. Simple and Rapid Fabrication of Disposable Carbon-Based Electrochemical Cells Using an Electronic Craft Cutter for Sensor and Biosensor Applications. Talanta 2016, 146, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Maesa, J.-M.; Muñoz-Pascual, F.-X.; Baldrich, E. Voltammetric Discrimination of Skatole and Indole at Disposable Screen Printed Electrodes. Analyst 2013, 138, 1346–1352. [Google Scholar] [CrossRef] [PubMed]
- Taghdisi, S.M.; Danesh, N.M.; Emrani, A.S.; Ramezani, M.; Abnous, K. A Novel Electrochemical Aptasensor Based on Single-Walled Carbon Nanotubes, Gold Electrode and Complimentary Strand of Aptamer for Ultrasensitive Detection of Cocaine. Biosens. Bioelectron. 2015, 73, 245–250. [Google Scholar] [CrossRef]
- Ferreira, D.C.; Batistuti, M.R.; Bachour, B.; Mulato, M. Aptasensor Based on Screen-Printed Electrode for Breast Cancer Detection in Undiluted Human Serum. Bioelectrochemistry 2021, 137, 107586. [Google Scholar] [CrossRef]
- Singhal, C.; Khanuja, M.; Chaudhary, N.; Pundir, C.S.; Narang, J. Detection of Chikungunya Virus DNA Using Two-Dimensional MoS2 Nanosheets Based Disposable Biosensor. Sci. Rep. 2018, 8, 7734. [Google Scholar] [CrossRef]
- Tancharoen, C.; Sukjee, W.; Thepparit, C.; Jaimipuk, T.; Auewarakul, P.; Thitithanyanont, A.; Sangma, C. Electrochemical Biosensor Based on Surface Imprinting for Zika Virus Detection in Serum. ACS Sens. 2019, 4, 69–75. [Google Scholar] [CrossRef]
- Rafat, N.; Satoh, P.; Worden, R.M. Electrochemical Biosensor for Markers of Neurological Esterase Inhibition. Biosensors 2021, 11, 459. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Pereira, C.M.; Silva, A.F.; Sales, M.G.F. Electrochemical Detection of Cardiac Biomarker Myoglobin Using Polyphenol as Imprinted Polymer Receptor. Anal. Chim. Acta 2017, 981, 41–52. [Google Scholar] [CrossRef]
- Höfs, S.; Hülagü, D.; Bennet, F.; Carl, P.; Flemig, S.; Schmid, T.; Schenk, J.A.; Hodoroaba, V.; Schneider, R.J. Electrochemical Immunomagnetic Ochratoxin a Sensing: Steps Forward in the Application of 3,3′,5,5′-Tetramethylbenzidine in Amperometric Assays. ChemElectroChem 2021, 8, 2597–2606. [Google Scholar] [CrossRef]
- Doldán, X.; Fagúndez, P.; Cayota, A.; Laíz, J.; Tosar, J.P. Electrochemical Sandwich Immunosensor for Determination of Exosomes Based on Surface Marker-Mediated Signal Amplification. Anal. Chem. 2016, 88, 10466–10473. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.; Viezzi, S.; Mazerat, S.; Marks, R.S.; Vidic, J. Rapid and Label-Free Electrochemical DNA Biosensor for Detecting Hepatitis A Virus. Biosens. Bioelectron. 2018, 100, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, L.; Favero, G.; Tortolini, C.; Di Fusco, M.; Romagnoli, E.; Minisola, S.; Mazzei, F. Several Approaches for Vitamin D Determination by Surface Plasmon Resonance and Electrochemical Affinity Biosensors. Biosens. Bioelectron. 2013, 40, 350–355. [Google Scholar] [CrossRef]
- Zamani, M.; Yang, V.; Maziashvili, L.; Fan, G.; Klapperich, C.M.; Furst, A.L. Surface Requirements for Optimal Biosensing with Disposable Gold Electrodes. ACS Meas. Sci. Au 2022, 2, 91–95. [Google Scholar] [CrossRef]
- Leva-Bueno, J.; Meuskens, I.; Linke, D.; Millner, P.A.; Peyman, S.A. A Novel, Proof-of-Concept Electrochemical Impedimetric Biosensor Based on Extracellular Matrix Protein–Adhesin Interaction. Sens. Diagn. 2022, 1, 1003–1013. [Google Scholar] [CrossRef]
- Araujo, G.R.; Fujimura, P.T.; Vaz, E.R.; Silva, T.A.; Rodovalho, V.R.; Britto-Madurro, A.G.; Madurro, J.M.; Fonseca, J.E.; Silva, C.H.M.; Santos, P.S.; et al. A Novel Reactive Epitope-Based Antigen Targeted by Serum Autoantibodies in Oligoarticular and Polyarticular Juvenile Idiopathic Arthritis and Development of an Electrochemical Biosensor. Immunobiology 2016, 221, 634–640. [Google Scholar] [CrossRef]
- Zukauskas, S.; Rucinskiene, A.; Ratautaite, V.; Ramanaviciene, A.; Pilvenyte, G.; Bechelany, M.; Ramanavicius, A. Electrochemical Biosensor for the Determination of Specific Antibodies against SARS-CoV-2 Spike Protein. Int. J. Mol. Sci. 2022, 24, 718. [Google Scholar] [CrossRef]
- Damiati, S.; Küpcü, S.; Peacock, M.; Eilenberger, C.; Zamzami, M.; Qadri, I.; Choudhry, H.; Sleytr, U.B.; Schuster, B. Acoustic and Hybrid 3D-Printed Electrochemical Biosensors for the Real-Time Immunodetection of Liver Cancer Cells (HepG2). Biosens. Bioelectron. 2017, 94, 500–506. [Google Scholar] [CrossRef]
- Taufiq, S.; Waqar, M.; Sharif, M.N.; Abbas, S.R. Towards Portable Rapid TB Biosensor: Detecting Mycobacterium Tuberculosis in Raw Sputum Samples Using Functionalized Screen Printed Electrodes. Bioelectrochemistry 2023, 150, 108353. [Google Scholar] [CrossRef]
- Aydoğdu Tığ, G.; Pekyardımcı, Ş. An Electrochemical Sandwich-Type Aptasensor for Determination of Lipocalin-2 Based on Graphene Oxide/Polymer Composite and Gold Nanoparticles. Talanta 2020, 210, 120666. [Google Scholar] [CrossRef] [PubMed]
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Sun, J.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous Capillary-Flow Sensing of Glucose and Lactate in Sweat with an Electrochemical Sensor Based on Functionalized Graphene Oxide. Sens. Actuators B Chem. 2021, 344, 130253. [Google Scholar] [CrossRef]
- Guerrero, S.; Agüí, L.; Yáñez-Sedeño, P.; Pingarrón, J.M. Design of Electrochemical Immunosensors Using Electro-Click Chemistry. Application to the Detection of IL-1β Cytokine in Saliva. Bioelectrochemistry 2020, 133, 107484. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.Y.A.; Sultan, M.A.; El-Alamin, M.M.A.; Atia, M.A.; Aboul-Enein, H.Y. A Disposable Carbon Nanotubes-screen Printed Electrode (CNTs-SPE) for Determination of the Antifungal Agent Posaconazole in Biological Samples. Electroanalysis 2017, 29, 843–849. [Google Scholar] [CrossRef]
- Nassef, H.M.; Civit, L.; Fragoso, A.; O’Sullivan, C.K. Amperometric Sensing of Ascorbic Acid Using a Disposable Screen-Printed Electrode Modified with Electrografted o-Aminophenol Film. Analyst 2008, 133, 1736. [Google Scholar] [CrossRef]
- Hassan, M.H.; Omar, A.M.; Daskalakis, E.; Mohamed, A.A.; Boyd, L.A.; Blanford, C.; Grieve, B.; Bartolo, P.J. Multi-Layer Biosensor for Pre-Symptomatic Detection of Puccinia strifformis, the Causal Agent of Yellow Rust. Biosensors 2022, 12, 829. [Google Scholar] [CrossRef]
- Haghighian, N.; Kataky, R. Rapid Fingerprinting of Bacterial Species Using Nanocavities Created on Screen-Printed Electrodes Modified by β-Cyclodextrin. Sens. Diagn. 2023, 2, 1228–1235. [Google Scholar] [CrossRef]
- Rodríguez-Penedo, A.; Rioboó-Legaspi, P.; González-López, A.; Lores-Padín, A.; Pereiro, R.; García-Suárez, M.D.M.; Cima-Cabal, M.D.; Costa-Rama, E.; Fernández, B.; Fernández-Abedul, M.T. Electrocatalytic Palladium Nanoclusters as Versatile Indicators of Bioassays: Rapid Electroanalytical Detection of SARS-CoV-2 by Reverse Transcription Loop-Mediated Isothermal Amplification. Adv. Healthc. Mater. 2023, 12, 2202972. [Google Scholar] [CrossRef]
- Yaiwong, P.; Anuthum, S.; Sangthong, P.; Jakmunee, J.; Bamrungsap, S.; Ounnunkad, K. A New Portable Toluidine Blue/Aptamer Complex-on-Polyethyleneimine-Coated Gold Nanoparticles-Based Sensor for Label-Free Electrochemical Detection of Alpha-Fetoprotein. Front. Bioeng. Biotechnol. 2023, 11, 1182880. [Google Scholar] [CrossRef]
- Søpstad, S.; Imenes, K.; Johannessen, E.A. Hybrid Electrochemical Sensor Platform for Capsaicin Determination Using Coarsely Stepped Cyclic Squarewave Voltammetry. Biosens. Bioelectron. 2019, 130, 374–381. [Google Scholar] [CrossRef]
- Damiati, S.; Haslam, C.; Sopstad, S.; Peacock, M.; Whitley, T.; Davey, P.; Awan, S.A. Sensitivity Comparison of Macro- and Micro-Electrochemical Biosensors for Human Chorionic Gonadotropin (hCG) Biomarker Detection. IEEE Access 2019, 7, 94048–94058. [Google Scholar] [CrossRef]
- Malla, P.; Liao, H.-P.; Liu, C.-H.; Wu, W.-C.; Sreearunothai, P. Voltammetric Biosensor for Coronavirus Spike Protein Using Magnetic Bead and Screen-Printed Electrode for Point-of-Care Diagnostics. Microchim. Acta 2022, 189, 168. [Google Scholar] [CrossRef] [PubMed]
- Kadara, R.O.; Jenkinson, N.; Banks, C.E. Characterisation of Commercially Available Electrochemical Sensing Platforms. Sens. Actuators B Chem. 2009, 138, 556–562. [Google Scholar] [CrossRef]
- Reanpang, P.; Mool-am-kha, P.; Upan, J.; Jakmunee, J. A Novel Flow Injection Amperometric Sensor Based on Carbon Black and Graphene Oxide Modified Screen-Printed Carbon Electrode for Highly Sensitive Determination of Uric Acid. Talanta 2021, 232, 122493. [Google Scholar] [CrossRef]
- Kerr, E.; Alexander, R.; Francis, P.S.; Guijt, R.M.; Barbante, G.J.; Doeven, E.H. A Comparison of Commercially Available Screen-Printed Electrodes for Electrogenerated Chemiluminescence Applications. Front. Chem. 2021, 8, 628483. [Google Scholar] [CrossRef]
- Timilsina, S.S.; Ramasamy, M.; Durr, N.; Ahmad, R.; Jolly, P.; Ingber, D.E. Biofabrication of Multiplexed Electrochemical Immunosensors for Simultaneous Detection of Clinical Biomarkers in Complex Fluids. Adv. Healthc. Mater. 2022, 11, 2200589. [Google Scholar] [CrossRef]
- Li, M.; Abeyrathne, C.; Langley, D.P.; Cossins, L.R.; Samudra, A.N.; Green, G.W.; Moulton, S.E.; Silva, S.M. Highly Specific Lubricin-Lectin Electrochemical Sensor for Glycoprotein Cancer Biomarker Detection. Electrochim. Acta 2023, 457, 142508. [Google Scholar] [CrossRef]
- Zang, D.; Yan, M.; Ge, S.; Ge, L.; Yu, J. A Disposable Simultaneous Electrochemical Sensor Array Based on a Molecularly Imprinted Film at a NH2-Graphene Modified Screen-Printed Electrode for Determination of Psychotropic Drugs. Analyst 2013, 138, 2704. [Google Scholar] [CrossRef]
- Clark, K.M.; Schenkel, M.S.; Pittman, T.W.; Samper, I.C.; Anderson, L.B.R.; Khamcharoen, W.; Elmegerhi, S.; Perera, R.; Siangproh, W.; Kennan, A.J.; et al. Electrochemical Capillary Driven Immunoassay for Detection of SARS-CoV-2. ACS Meas. Sci. Au 2022, 2, 584–594. [Google Scholar] [CrossRef]
- Araújo, D.A.G.; Camargo, J.R.; Pradela-Filho, L.A.; Lima, A.P.; Muñoz, R.A.A.; Takeuchi, R.M.; Janegitz, B.C.; Santos, A.L. A Lab-Made Screen-Printed Electrode as a Platform to Study the Effect of the Size and Functionalization of Carbon Nanotubes on the Voltammetric Determination of Caffeic Acid. Microchem. J. 2020, 158, 105297. [Google Scholar] [CrossRef]
- Wang, L.; Veselinovic, M.; Yang, L.; Geiss, B.J.; Dandy, D.S.; Chen, T. A Sensitive DNA Capacitive Biosensor Using Interdigitated Electrodes. Biosens. Bioelectron. 2017, 87, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Roy, S.; Nagabooshanam, S.; Wadhwa, S.; Dubey, S. Effect of Gap Size of Gold Interdigitated Electrodes on the Electrochemical Immunosensing of Cardiac Troponin-I for Point-of-Care Applications. Sens. Actuators Rep. 2022, 4, 100114. [Google Scholar] [CrossRef]
- Rodríguez, A.; Burgos-Flórez, F.; Posada, J.D.; Cervera, E.; Zucolotto, V.; Sanjuán, H.; Sanjuán, M.; Villalba, P.J. Electrochemical Immunosensor for the Quantification of S100B at Clinically Relevant Levels Using a Cysteamine Modified Surface. Sensors 2021, 21, 1929. [Google Scholar] [CrossRef] [PubMed]
- Lutsyk, P.M.; Shankar, P.; Rozhin, A.G.; Kulinich, S.A. Surface Sensitivity of Ultrasonically Treated Carbon Nanotube Network towards Ammonia. Surf. Interfaces 2019, 17, 100363. [Google Scholar] [CrossRef]
- Radwan, O.; Brothers, M.C.; Coyle, V.; Chapleau, M.E.; Chapleau, R.R.; Kim, S.S.; Ruiz, O.N. Electrochemical Biosensor for Rapid Detection of Fungal Contamination in Fuel Systems. Biosens. Bioelectron. 2022, 211, 114374. [Google Scholar] [CrossRef]
- Cui, F.; Jafarishad, H.; Zhou, Z.; Chen, J.; Shao, J.; Wen, Q.; Liu, Y.; Zhou, H.S. Batch Fabrication of Electrochemical Sensors on a Glycol-Modified Polyethylene Terephthalate-Based Microfluidic Device. Biosens. Bioelectron. 2020, 167, 112521. [Google Scholar] [CrossRef]
- Xie, Y.; Zhi, X.; Su, H.; Wang, K.; Yan, Z.; He, N.; Zhang, J.; Chen, D.; Cui, D. A Novel Electrochemical Microfluidic Chip Combined with Multiple Biomarkers for Early Diagnosis of Gastric Cancer. Nanoscale Res. Lett. 2015, 10, 477. [Google Scholar] [CrossRef]
- Guo, S.; Lakshmipriya, T.; Gopinath, S.C.B.; Anbu, P.; Feng, Y. Complementation of ELISA and an Interdigitated Electrode Surface in Gold Nanoparticle Functionalization for Effective Detection of Human Blood Clotting Defects. Nanoscale Res. Lett. 2019, 14, 222. [Google Scholar] [CrossRef]
- Pedersen, T.; Fojan, P.; Pedersen, A.K.N.; Magnusson, N.E.; Gurevich, L. Amperometric Biosensor for Quantitative Measurement Using Sandwich Immunoassays. Biosensors 2023, 13, 519. [Google Scholar] [CrossRef]
- Sondhi, P.; Neupane, D.; Bhattarai, J.K.; Demchenko, A.V.; Stine, K.J. Facile Fabrication of Hierarchically Nanostructured Gold Electrode for Bio-Electrochemical Applications. J. Electroanal. Chem. 2022, 924, 116865. [Google Scholar] [CrossRef]
- Triroj, N.; Saensak, R.; Porntheeraphat, S.; Paosawatyanyong, B.; Amornkitbamrung, V. Diamond-Like Carbon Thin Film Electrodes for Microfluidic Bioelectrochemical Sensing Platforms. Anal. Chem. 2020, 92, 3650–3657. [Google Scholar] [CrossRef] [PubMed]
- Hannah, S.; Al-Hatmi, M.; Gray, L.; Corrigan, D.K. Low-Cost, Thin-Film, Mass-Manufacturable Carbon Electrodes for Detection of the Neurotransmitter Dopamine. Bioelectrochemistry 2020, 133, 107480. [Google Scholar] [CrossRef] [PubMed]
- Whittingham, M.J.; Hurst, N.J.; Crapnell, R.D.; Garcia-Miranda Ferrari, A.; Blanco, E.; Davies, T.J.; Banks, C.E. Electrochemical Improvements Can Be Realized via Shortening the Length of Screen-Printed Electrochemical Platforms. Anal. Chem. 2021, 93, 16481–16488. [Google Scholar] [CrossRef] [PubMed]
- Søpstad, S.; Johannessen, E.A.; Seland, F.; Imenes, K. Long-Term Stability of Screen-Printed Pseudo-Reference Electrodes for Electrochemical Biosensors. Electrochim. Acta 2018, 287, 29–36. [Google Scholar] [CrossRef]
- Nazarpour, S. (Ed.) Thin Films and Coatings in Biology; Biological and Medical Physics, Biomedical Engineering; Springer: Dordrecht, The Netherlands, 2013; ISBN 978-94-007-2591-1. [Google Scholar]
- Yurttaş, B.; Maral, M.; Erdem, A.; Özyüzer, L. Development of Single-Use Thin Film Electrodes Based on Zn2SnO4 on In2O3:SnO2 Substrates with Their Biosensing Applications. Mater. Today Commun. 2022, 33, 104906. [Google Scholar] [CrossRef]
- Ebrahimi, G.; Samadi Pakchin, P.; Shamloo, A.; Mota, A.; De La Guardia, M.; Omidian, H.; Omidi, Y. Label-Free Electrochemical Microfluidic Biosensors: Futuristic Point-of-Care Analytical Devices for Monitoring Diseases. Microchim. Acta 2022, 189, 252. [Google Scholar] [CrossRef]
- Chakraborty, T.; Das, M.; Lin, C.Y.; Lei, K.F.; Kao, C.H. Highly Sensitive and Selective Electrochemical Detection of Lipocalin 2 by NiO Nanoparticles/Perovskite CeCuOx Based Immunosensor to Diagnose Renal Failure. Anal. Chim. Acta 2022, 1205, 339754. [Google Scholar] [CrossRef]
- Wang, Z.; Jinlong, L.; An, Z.; Kimura, M.; Ono, T. Enzyme Immobilization in Completely Packaged Freestanding SU-8 Microfluidic Channel by Electro Click Chemistry for Compact Thermal Biosensor. Process Biochem. 2019, 79, 57–64. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Foster, C.; Kelly, P.; Brownson, D.; Banks, C. Determination of the Electrochemical Area of Screen-Printed Electrochemical Sensing Platforms. Biosensors 2018, 8, 53. [Google Scholar] [CrossRef]
- Roslan, N.A.F.; Ab Rahim, R.; Md Ralib, A.A.; Za’bah, N.F.; Nordin, A.N.; Riza Bashri, M.S.; Suhaimi, M.I.; Samsudin, Z.; Ming, L.L.; Sugandi, G. Performance Analysis of Optimized Screen-Printed Electrodes for Electrochemical Sensing. Int. J. Integr. Eng. 2022, 14, 250–261. [Google Scholar] [CrossRef]
- Tangkuaram, T.; Ponchio, C.; Kangkasomboon, T.; Katikawong, P.; Veerasai, W. Design and Development of a Highly Stable Hydrogen Peroxide Biosensor on Screen Printed Carbon Electrode Based on Horseradish Peroxidase Bound with Gold Nanoparticles in the Matrix of Chitosan. Biosens. Bioelectron. 2007, 22, 2071–2078. [Google Scholar] [CrossRef] [PubMed]
- Zimmer and Peacock SPEs. Available online: https://shop.zimmerpeacock.com/collections/bare-electrodes (accessed on 20 December 2024).
- Hacking the Glucose Test Strip and Getting to Market. Available online: http://www.zimmerpeacocktech.com/2017/08/05/hacking-the-glucose-test-strip-and-getting-to-market/ (accessed on 20 December 2024).
- Poghossian, A.; Schöning, M.J. Capacitive Field-Effect EIS Chemical Sensors and Biosensors: A Status Report. Sensors 2020, 20, 5639. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Lee, G.-Y.; Song, Z.; Bong, J.-H.; Kim, H.-R.; Kang, M.-J.; Pyun, J.-C. A Vertically Paired Electrode for Redox Cycling and Its Application to Immunoassays. Analyst 2023, 148, 1349–1361. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.-Y.; Park, J.-H.; Chang, Y.W.; Cho, S.; Kang, M.-J.; Pyun, J.-C. Chronoamperometry-Based Redox Cycling for Application to Immunoassays. ACS Sens. 2018, 3, 106–112. [Google Scholar] [CrossRef]
- Jun, L.Q.; Bin Djaswadi, G.W.; Bin Hawari, H.F.; Bin Zakariya, M.A. Simulation of Interdigitated Electrodes (IDEs) Geometry Using COMSOL Multiphysics. In Proceedings of the 2018 International Conference on Intelligent and Advanced System (ICIAS), Kuala Lumpur, Malaysia, 13–14 August 2018; pp. 1–6. [Google Scholar]
- Min, J.; Baeumner, A.J. Characterization and Optimization of Interdigitated Ultramicroelectrode Arrays as Electrochemical Biosensor Transducers. Electroanalysis 2004, 16, 724–729. [Google Scholar] [CrossRef]
- Luppa, P.B.; Sokoll, L.J.; Chan, D.W. Immunosensors—Principles and Applications to Clinical Chemistry. Clin. Chim. Acta 2001, 314, 1–26. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef]
- Fischer, L.M.; Tenje, M.; Heiskanen, A.R.; Masuda, N.; Castillo, J.; Bentien, A.; Émneus, J.; Jakobsen, M.H.; Boisen, A. Gold Cleaning Methods for Electrochemical Detection Applications. Microelectron. Eng. 2009, 86, 1282–1285. [Google Scholar] [CrossRef]
- Lee, J.; Arrigan, D.W.M.; Silvester, D.S. Mechanical Polishing as an Improved Surface Treatment for Platinum Screen-Printed Electrodes. Sens. Bio-Sens. Res. 2016, 9, 38–44. [Google Scholar] [CrossRef]
- Stan, D.; Mirica, A.-C.; Iosub, R.; Stan, D.; Mincu, N.B.; Gheorghe, M.; Avram, M.; Adiaconita, B.; Craciun, G.; Bocancia Mateescu, A.L. What Is the Optimal Method for Cleaning Screen-Printed Electrodes? Processes 2022, 10, 723. [Google Scholar] [CrossRef]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, Techniques, and Applications of Biocatalyst Immobilization for Industrial Application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xie, Y.; Zhu, W.; Wei, T. Recent Advances in Functionalization Strategies for Biosensor Interfaces, Especially the Emerging Electro-Click: A Review. Chemosensors 2023, 11, 481. [Google Scholar] [CrossRef]
- Parkash, O.; Yean, C.; Shueb, R. Screen Printed Carbon Electrode Based Electrochemical Immunosensor for the Detection of Dengue NS1 Antigen. Diagnostics 2014, 4, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Parkash, O.; Abdullah, M.A.; Yean, C.Y.; Sekaran, S.D.; Shueb, R.H. Development and Evaluation of an Electrochemical Biosensor for Detection of Dengue-Specific IgM Antibody in Serum Samples. Diagnostics 2020, 11, 33. [Google Scholar] [CrossRef]
- Elshafey, R.; Tavares, A.C.; Siaj, M.; Zourob, M. Electrochemical Impedance Immunosensor Based on Gold Nanoparticles–Protein G for the Detection of Cancer Marker Epidermal Growth Factor Receptor in Human Plasma and Brain Tissue. Biosens. Bioelectron. 2013, 50, 143–149. [Google Scholar] [CrossRef]
- Kausaite-Minkstimiene, A.; Ramanaviciene, A.; Kirlyte, J.; Ramanavicius, A. Comparative Study of Random and Oriented Antibody Immobilization Techniques on the Binding Capacity of Immunosensor. Anal. Chem. 2010, 82, 6401–6408. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme Entrapment, Biocatalyst Immobilization without Covalent Attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Lipińska, W.; Siuzdak, K.; Karczewski, J.; Dołęga, A.; Grochowska, K. Electrochemical Glucose Sensor Based on the Glucose Oxidase Entrapped in Chitosan Immobilized onto Laser-Processed Au-Ti Electrode. Sens. Actuators B Chem. 2021, 330, 129409. [Google Scholar] [CrossRef]
- Moreira Gonçalves, L. Electropolymerized Molecularly Imprinted Polymers: Perceptions Based on Recent Literature for Soon-to-Be World-Class Scientists. Curr. Opin. Electrochem. 2021, 25, 100640. [Google Scholar] [CrossRef]
- Choi, E.J.; Drago, N.P.; Humphrey, N.J.; Van Houten, J.; Ahn, J.; Lee, J.; Kim, I.-D.; Ogata, A.F.; Penner, R.M. Electrodeposition-Enabled, Electrically-Transduced Sensors and Biosensors. Mater. Today 2023, 62, 129–150. [Google Scholar] [CrossRef]
- Frasco, M.F.; Truta, L.A.A.N.A.; Sales, M.G.F.; Moreira, F.T.C. Imprinting Technology in Electrochemical Biomimetic Sensors. Sensors 2017, 17, 523. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Liu, C.; Lin, J.; Zhu, Z.; Hu, B.; Wu, L. Towards Development of Molecularly Imprinted Electrochemical Sensors for Food and Drug Safety: Progress and Trends. Biosensors 2022, 12, 369. [Google Scholar] [CrossRef] [PubMed]
- Özcan, N.; Karaman, C.; Atar, N.; Karaman, O.; Yola, M.L. A Novel Molecularly Imprinting Biosensor Including Graphene Quantum Dots/Multi-Walled Carbon Nanotubes Composite for Interleukin-6 Detection and Electrochemical Biosensor Validation. ECS J. Solid State Sci. Technol. 2020, 9, 121010. [Google Scholar] [CrossRef]
- Cui, F.; Zhou, Z.; Zhou, H.S. Molecularly Imprinted Polymers and Surface Imprinted Polymers Based Electrochemical Biosensor for Infectious Diseases. Sensors 2020, 20, 996. [Google Scholar] [CrossRef]
- Sanati, A.; Jalali, M.; Raeissi, K.; Karimzadeh, F.; Kharaziha, M.; Mahshid, S.S.; Mahshid, S. A Review on Recent Advancements in Electrochemical Biosensing Using Carbonaceous Nanomaterials. Microchim. Acta 2019, 186, 773. [Google Scholar] [CrossRef]
- Danvirutai, P.; Ekpanyapong, M.; Tuantranont, A.; Bohez, E.; Anutrakulchai, S.; Wisitsoraat, A.; Srichan, C. Ultra-Sensitive and Label-Free Neutrophil Gelatinase-Associated Lipocalin Electrochemical Sensor Using Gold Nanoparticles Decorated 3D Graphene Foam towards Acute Kidney Injury Detection. Sens. Bio-Sens. Res. 2020, 30, 100380. [Google Scholar] [CrossRef]
- Kathiresan, V.; Thirumalai, D.; Rajarathinam, T.; Yeom, M.; Lee, J.; Kim, S.; Yoon, J.-H.; Chang, S.-C. A Simple One-Step Electrochemical Deposition of Bioinspired Nanocomposite for the Non-Enzymatic Detection of Dopamine. J. Anal. Sci. Technol. 2021, 12, 5. [Google Scholar] [CrossRef]
- Shu, H.; Cao, L.; Chang, G.; He, H.; Zhang, Y.; He, Y. Direct Electrodeposition of Gold Nanostructures onto Glassy Carbon Electrodes for Non-Enzymatic Detection of Glucose. Electrochim. Acta 2014, 132, 524–532. [Google Scholar] [CrossRef]
- Mistry, K.K.; Layek, K.; Mahapatra, A.; RoyChaudhuri, C.; Saha, H. A Review on Amperometric-Type Immunosensors Based on Screen-Printed Electrodes. Analyst 2014, 139, 2289. [Google Scholar] [CrossRef]
- Zaki, M.H.M.; Mohd, Y.; Chin, L.Y. Surface Properties of Nanostructured Gold Coatings Electrodeposited at Different Potentials. Int. J. Electrochem. Sci. 2020, 15, 11401–11415. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Shoaie, I.S.; Khalilzadeh, M.A.; Asl, M.S.; Le, Q.V.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent Developments in Conducting Polymers: Applications for Electrochemistry. RSC Adv. 2020, 10, 37834–37856. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Han, R.; Li, Q.; Han, Y.; Luo, X. Electrochemical Biosensors Capable of Detecting Biomarkers in Human Serum with Unique Long-Term Antifouling Abilities Based on Designed Multifunctional Peptides. Anal. Chem. 2020, 92, 7186–7193. [Google Scholar] [CrossRef]
- Stine, K.J. Biosensor Applications of Electrodeposited Nanostructures. Appl. Sci. 2019, 9, 797. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. A Comparative Study of Short Chain and Long Chain Mercapto Acids Used in Biosensor Fabrication: A VEGF-R1-Based Immunosensor as a Model System. Artif. Cells Nanomed. Biotechnol. 2016, 44, 462–470. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, N.; Comini, E. The Role of Self-Assembled Monolayers in Electronic Devices. J. Mater. Chem. C 2020, 8, 3938–3955. [Google Scholar] [CrossRef]
- Love, J.C.; Estroff, L.A.; Kriebel, J.K.; Nuzzo, R.G.; Whitesides, G.M. Self-Assembled Monolayers of Thiolates on Metals as a Form of Nanotechnology. Chem. Rev. 2005, 105, 1103–1170. [Google Scholar] [CrossRef]
- Choi, Y.; Tran, H.-V.; Lee, T.R. Self-Assembled Monolayer Coatings on Gold and Silica Surfaces for Antifouling Applications: A Review. Coatings 2022, 12, 1462. [Google Scholar] [CrossRef]
- Sheldon, R.A. Characteristic Features and Biotechnological Applications of Cross-Linked Enzyme Aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef]
- Šulek, F.; Fernández, D.P.; Knez, Ž.; Habulin, M.; Sheldon, R.A. Immobilization of Horseradish Peroxidase as Crosslinked Enzyme Aggregates (CLEAs). Process Biochem. 2011, 46, 765–769. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Li, J.-P.; Deng, H.; Pan, H.-C. Progress on Click Chemistry and Its Application in Chemical Sensors. Chin. J. Anal. Chem. 2015, 43, 609–617. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; González-Cortés, A.; Campuzano, S.; Pingarrón, J.M. Copper(I)-Catalyzed Click Chemistry as a Tool for the Functionalization of Nanomaterials and the Preparation of Electrochemical (Bio)Sensors. Sensors 2019, 19, 2379. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Finn, M.G. Introduction: Click Chemistry. Chem. Rev. 2021, 121, 6697–6698. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Dinolfo, P.H.; Chidsey, C.E.D.; Collman, J.P. Selective Functionalization of Independently Addressed Microelectrodes by Electrochemical Activation and Deactivation of a Coupling Catalyst. J. Am. Chem. Soc. 2006, 128, 1794–1795. [Google Scholar] [CrossRef]
- Krishnan, M.; Kathiresan, M.; Praveen, C. Electrochemically Generated Copper(I)-Catalyzed Click Chemistry: Triazole Synthesis and Insights into Their Photophysical Properties. Eur. J. Org. Chem. 2023, 26, e202201405. [Google Scholar] [CrossRef]
- Svalova, T.S.; Medvedeva, M.V.; Saigushkina, A.A.; Kozitsin, I.V.; Malysheva, N.N.; Zhdanovskikh, V.O.; Okhokhonin, A.V.; Kozitsina, A.N. A Label-Free Impedimetric Immunosensor Based on Covalent Immobilization of Anti-E. Coli Antibody via a Copper-Catalyzed Azide-Alkyne Cycloaddition Reaction. Anal. Bioanal. Chem. 2020, 412, 5077–5087. [Google Scholar] [CrossRef]
- Hein, C.D.; Liu, X.-M.; Wang, D. Click Chemistry, A Powerful Tool for Pharmaceutical Sciences. Pharm. Res. 2008, 25, 2216–2230. [Google Scholar] [CrossRef] [PubMed]
- Biesheuvel, P.M.; Porada, S.; Dykstra, J.E. The Difference between Faradaic and Non-Faradaic Electrode Processes. arXiv 2021, arXiv:1809.02930. [Google Scholar]
- Wang, J. Analytical Electrochemistry, 1st ed.; Wiley: New York, NY, USA, 2006; ISBN 978-0-471-67879-3. [Google Scholar]
- Vlamidis, Y.; Gualandi, I.; Tonelli, D. Amperometric Biosensors Based on Reduced GO and MWCNTs Composite for Polyphenols Detection in Fruit Juices. J. Electroanal. Chem. 2017, 799, 285–292. [Google Scholar] [CrossRef]
- Li, G.; Zeng, J.; Zhao, L.; Wang, Z.; Dong, C.; Liang, J.; Zhou, Z.; Huang, Y. Amperometric Cholesterol Biosensor Based on Reduction Graphene Oxide-Chitosan-Ferrocene/Platinum Nanoparticles Modified Screen-Printed Electrode. J. Nanopart. Res. 2019, 21, 162. [Google Scholar] [CrossRef]
- Villalonga, M.L.; Borisova, B.; Arenas, C.B.; Villalonga, A.; Arévalo-Villena, M.; Sánchez, A.; Pingarrón, J.M.; Briones-Pérez, A.; Villalonga, R. Disposable Electrochemical Biosensors for Brettanomyces Bruxellensis and Total Yeast Content in Wine Based on Core-Shell Magnetic Nanoparticles. Sens. Actuators B Chem. 2019, 279, 15–21. [Google Scholar] [CrossRef]
- Saha, T.; Del Caño, R.; Mahato, K.; De La Paz, E.; Chen, C.; Ding, S.; Yin, L.; Wang, J. Wearable Electrochemical Glucose Sensors in Diabetes Management: A Comprehensive Review. Chem. Rev. 2023, 123, 7854–7889. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Liu, N.; Liang, Y.; Bin, J.; Zhang, Z.; Huang, J.; Shu, R.; Yang, K. Classification of Green and Black Teas by PCA and SVM Analysis of Cyclic Voltammetric Signals from Metallic Oxide-Modified Electrode. Food Anal. Methods 2014, 7, 472–480. [Google Scholar] [CrossRef]
- Faura, G.; González-Calabuig, A.; del Valle, M. Analysis of Amino Acid Mixtures by Voltammetric Electronic Tongues and Artificial Neural Networks. Electroanalysis 2016, 28, 1894–1900. [Google Scholar] [CrossRef]
- Roselló, A.; Serrano, N.; Díaz-Cruz, J.M.; Ariño, C. Discrimination of Beers by Cyclic Voltammetry Using a Single Carbon Screen-printed Electrode. Electroanalysis 2021, 33, 864–872. [Google Scholar] [CrossRef]
- Liu, J.; Xu, N.; Men, H.; Li, S.; Lu, Y.; Low, S.S.; Li, X.; Zhu, L.; Cheng, C.; Xu, G.; et al. Salivary Cortisol Determination on Smartphone-Based Differential Pulse Voltammetry System. Sensors 2020, 20, 1422. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, P.; Ramachandran, K.; Maheshvaran, K.; Senthil, T.S.; Manivel, P. Simultaneous Electrochemical Detection of Ascorbic Acid, Dopamine and Uric Acid Using Au Decorated Carbon Nanofibers Modified Screen Printed Electrode. Carbon Lett. 2024, 34, 2325–2341. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical Impedance Spectroscopy. Nat. Rev. Methods Primers 2021, 1, 41. [Google Scholar] [CrossRef]
- Bahner, N.; Reich, P.; Frense, D.; Menger, M.; Schieke, K.; Beckmann, D. An Aptamer-Based Biosensor for Detection of Doxorubicin by Electrochemical Impedance Spectroscopy. Anal. Bioanal. Chem. 2018, 410, 1453–1462. [Google Scholar] [CrossRef]
- Panagopoulou, C.; Skotadis, E.; Aslanidis, E.; Tzourmana, G.; Rapesi, A.; Tsioustas, C.; Kainourgiaki, M.; Kleitsiotis, G.; Tsekenis, G.; Tsoukalas, D. Non-Faradaic Impedimetric Detection of Heavy Metal Ions via a Hybrid Nanoparticle-DNAzyme Biosensor. Biosensors 2024, 14, 321. [Google Scholar] [CrossRef]
- Cho, I.-H.; Kim, D.H.; Park, S. Electrochemical Biosensors: Perspective on Functional Nanomaterials for on-Site Analysis. Biomater. Res. 2020, 24, 6. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. A Paper-Based Electrochemical Immunosensor with Reduced Graphene Oxide/Thionine/Gold Nanoparticles Nanocomposites Modification for the Detection of Cancer Antigen 125. Biosens. Bioelectron. 2019, 135, 1–7. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. Smartphone-Based Electrochemical System with Multi-Walled Carbon Nanotubes/Thionine/Gold Nanoparticles Modified Screen-Printed Immunosensor for Cancer Antigen 125 Detection. Microchem. J. 2022, 174, 107044. [Google Scholar] [CrossRef]
- Liu, P.; Li, C.; Zhang, R.; Tang, Q.; Wei, J.; Lu, Y.; Shen, P. An Ultrasensitive Electrochemical Immunosensor for Procalcitonin Detection Based on the Gold Nanoparticles-Enhanced Tyramide Signal Amplification Strategy. Biosens. Bioelectron. 2019, 126, 543–550. [Google Scholar] [CrossRef]
- Tang, J.; Su, B.; Tang, D.; Chen, G. Conductive Carbon Nanoparticles-Based Electrochemical Immunosensor with Enhanced Sensitivity for α-Fetoprotein Using Irregular-Shaped Gold Nanoparticles-Labeled Enzyme-Linked Antibodies as Signal Improvement. Biosens. Bioelectron. 2010, 25, 2657–2662. [Google Scholar] [CrossRef]
- Awan, M.; Rauf, S.; Abbas, A.; Nawaz, M.H.; Yang, C.; Shahid, S.A.; Amin, N.; Hayat, A. A Sandwich Electrochemical Immunosensor Based on Antibody Functionalized-Silver Nanoparticles (Ab-Ag NPs) for the Detection of Dengue Biomarker Protein NS1. J. Mol. Liq. 2020, 317, 114014. [Google Scholar] [CrossRef]
- Khristunova, E.; Barek, J.; Kratochvil, B.; Korotkova, E.; Dorozhko, E.; Vyskocil, V. Electrochemical Immunoassay for the Detection of Antibodies to Tick-Borne Encephalitis Virus by Using Various Types of Bioconjugates Based on Silver Nanoparticles. Bioelectrochemistry 2020, 135, 107576. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yu, J.; Li, L.; Zhang, Y.; Xin, S.; Ni, X.; Sun, Y.; Song, K. Recent Progress of the Practical Applications of the Platinum Nanoparticle-Based Electrochemistry Biosensors. Front. Chem. 2021, 9, 677876. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Li, Y.; Wang, Y.; Luo, X.; Wei, D.; Feng, R.; Yan, T.; Ren, X.; Du, B.; Wei, Q. A Prostate-Specific Antigen Electrochemical Immunosensor Based on Pd NPs Functionalized Electroactive Co-MOF Signal Amplification Strategy. Biosens. Bioelectron. 2019, 132, 97–104. [Google Scholar] [CrossRef]
- Dorozhko, E.V.; Gashevskay, A.S.; Korotkova, E.I.; Barek, J.; Vyskocil, V.; Eremin, S.A.; Galunin, E.V.; Saqib, M. A Copper Nanoparticle-Based Electrochemical Immunosensor for Carbaryl Detection. Talanta 2021, 228, 122174. [Google Scholar] [CrossRef]
- Singh, P.; Katkar, P.K.; Patil, U.M.; Bohara, R.A. A Robust Electrochemical Immunosensor Based on Core–Shell Nanostructured Silica-Coated Silver for Cancer (Carcinoembryonic-Antigen-CEA) Diagnosis. RSC Adv. 2021, 11, 10130–10143. [Google Scholar] [CrossRef]
- Akbari Nakhjavani, S.; Afsharan, H.; Khalilzadeh, B.; Ghahremani, M.H.; Carrara, S.; Omidi, Y. Gold and Silver Bio/Nano-Hybrids-Based Electrochemical Immunosensor for Ultrasensitive Detection of Carcinoembryonic Antigen. Biosens. Bioelectron. 2019, 141, 111439. [Google Scholar] [CrossRef]
- Białas, K.; Moschou, D.; Marken, F.; Estrela, P. Electrochemical Sensors Based on Metal Nanoparticles with Biocatalytic Activity. Mikrochim. Acta 2022, 189, 172. [Google Scholar] [CrossRef]
- Shi, X.; Gu, W.; Li, B.; Chen, N.; Zhao, K.; Xian, Y. Enzymatic Biosensors Based on the Use of Metal Oxide Nanoparticles. Microchim. Acta 2014, 181, 1–22. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Bytesnikova, Z.; Barek, J.; Richtera, L.; Adam, V. A Critical Comparison of Natural Enzymes and Nanozymes in Biosensing and Bioassays. Biosens. Bioelectron. 2021, 192, 113494. [Google Scholar] [CrossRef]
- Xue, L.; Jin, N.; Guo, R.; Wang, S.; Qi, W.; Liu, Y.; Li, Y.; Lin, J. Microfluidic Colorimetric Biosensors Based on MnO 2 Nanozymes and Convergence–Divergence Spiral Micromixers for Rapid and Sensitive Detection of Salmonella. ACS Sens. 2021, 6, 2883–2892. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Son, S.E.; Hur, W.; Tran, V.-K.; Lee, H.B.; Park, Y.; Han, D.K.; Seong, G.H. Electrochemical Immunoassay for Determination of Glycated Albumin Using Nanozymes. Sci. Rep. 2020, 10, 9513. [Google Scholar] [CrossRef] [PubMed]
- López-Domene, R.; Kumar, K.; Barcelon, J.E.; Guedes, G.; Beloqui, A.; Cortajarena, A.L. Nanozymes with Versatile Redox Capabilities Inspired in Metalloenzymes. Nanoscale 2023, 15, 16959–16966. [Google Scholar] [CrossRef] [PubMed]
- Kudr, J.; Haddad, Y.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Lee, C.-Y.; Wu, L.-P.; Chou, T.-T.; Hsieh, Y.-Z. Functional Magnetic Nanoparticles–Assisted Electrochemical Biosensor for Eosinophil Cationic Protein in Cell Culture. Sens. Actuators B Chem. 2018, 257, 672–677. [Google Scholar] [CrossRef]
- Iglesias-Mayor, A.; Amor-Gutiérrez, O.; Costa-García, A.; de la Escosura-Muñiz, A. Nanoparticles as Emerging Labels in Electrochemical Immunosensors. Sensors 2019, 19, 5137. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic Nanoparticles: Preparation, Physical Properties, and Applications in Biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Meskher, H.; Mustansar, H.C.; Thakur, A.K.; Sathyamurthy, R.; Lynch, I.; Singh, P.; Han, T.K.; Saidur, R. Recent Trends in Carbon Nanotube (CNT)-Based Biosensors for the Fast and Sensitive Detection of Human Viruses: A Critical Review. Nanoscale Adv. 2023, 5, 992–1010. [Google Scholar] [CrossRef]
- Gergeroglu, H.; Yildirim, S.; Ebeoglugil, M.F. Nano-Carbons in Biosensor Applications: An Overview of Carbon Nanotubes (CNTs) and Fullerenes (C60). SN Appl. Sci. 2020, 2, 603. [Google Scholar] [CrossRef]
- Krishnan, S.K.; Singh, E.; Singh, P.; Meyyappan, M.; Nalwa, H.S. A Review on Graphene-Based Nanocomposites for Electrochemical and Fluorescent Biosensors. RSC Adv. 2019, 9, 8778–8881. [Google Scholar] [CrossRef] [PubMed]
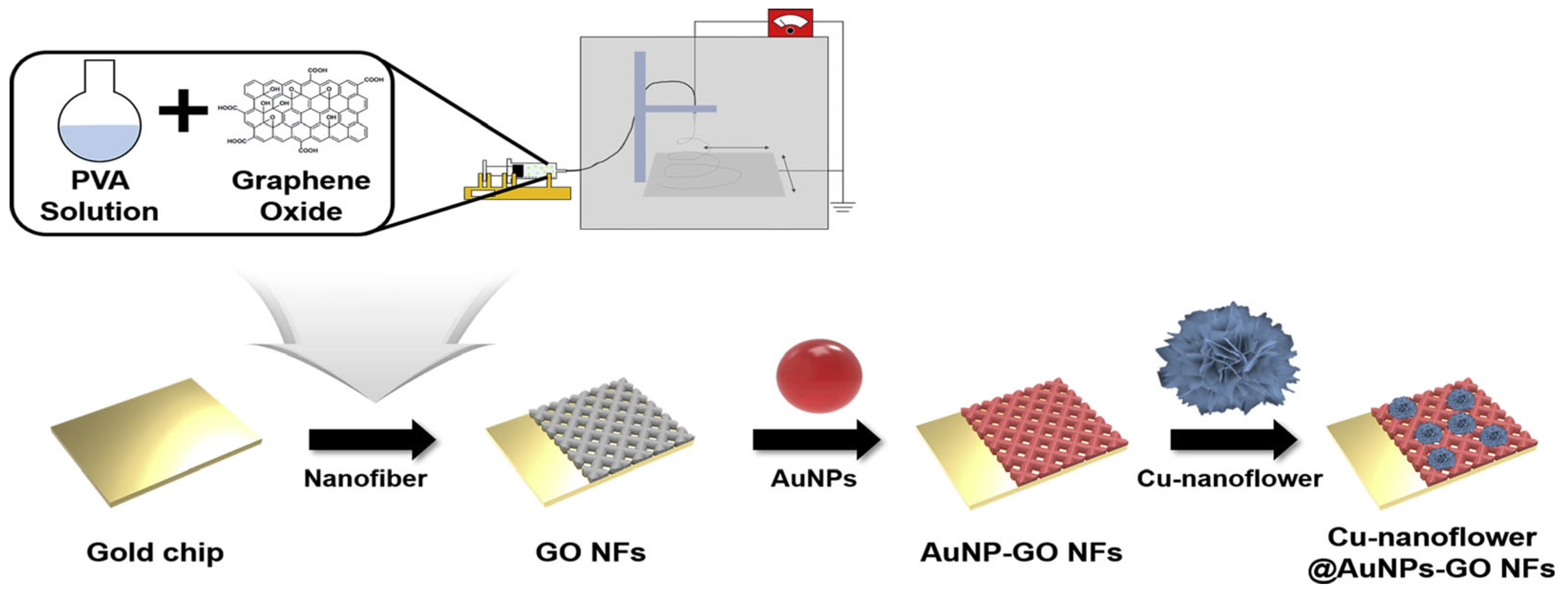
- Baek, S.H.; Roh, J.; Park, C.Y.; Kim, M.W.; Shi, R.; Kailasa, S.K.; Park, T.J. Cu-Nanoflower Decorated Gold Nanoparticles-Graphene Oxide Nanofiber as Electrochemical Biosensor for Glucose Detection. Mater. Sci. Eng. C 2020, 107, 110273. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Murthy, C.N.; Prabha, C.R. Recent Advances in Carbon Nanotube Based Electrochemical Biosensors. Int. J. Biol. Macromol. 2018, 108, 687–703. [Google Scholar] [CrossRef] [PubMed]
- Nsabimana, A.; Lan, Y.; Du, F.; Wang, C.; Zhang, W.; Xu, G. Alkaline Phosphatase-Based Electrochemical Sensors for Health Applications. Anal. Methods 2019, 11, 1996–2006. [Google Scholar] [CrossRef]
- Xia, N.; Deng, D.; Yang, S.; Hao, Y.; Wang, L.; Liu, Y.; An, C.; Han, Q.; Liu, L. Electrochemical Immunosensors with Protease as the Signal Label for the Generation of Peptide-Cu(II) Complexes as the Electrocatalysts toward Water Oxidation. Sens. Actuators B Chem. 2019, 291, 113–119. [Google Scholar] [CrossRef]
- Mahmudunnabi, R.G.; Farhana, F.Z.; Kashaninejad, N.; Firoz, S.H.; Shim, Y.-B.; Shiddiky, M.J.A. Nanozyme-Based Electrochemical Biosensors for Disease Biomarker Detection. Analyst 2020, 145, 4398–4420. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Kilic, N.M.; Singh, S.; Keles, G.; Cinti, S.; Kurbanoglu, S.; Odaci, D. Novel Approaches to Enzyme-Based Electrochemical Nanobiosensors. Biosensors 2023, 13, 622. [Google Scholar] [CrossRef]
- Kucherenko, I.S.; Soldatkin, O.O.; Dzyadevych, S.V.; Soldatkin, A.P. Electrochemical Biosensors Based on Multienzyme Systems: Main Groups, Advantages and Limitations—A Review. Anal. Chim. Acta 2020, 1111, 114–131. [Google Scholar] [CrossRef]
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme Immobilized Nanomaterials as Electrochemical Biosensors for Detection of Biomolecules. Enzym. Microb. Technol. 2022, 156, 110006. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Jampasa, S.; Siangproh, W.; Laocharoensuk, R.; Vilaivan, T.; Chailapakul, O. Electrochemical Detection of C-Reactive Protein Based on Anthraquinone-Labeled Antibody Using a Screen-Printed Graphene Electrode. Talanta 2018, 183, 311–319. [Google Scholar] [CrossRef] [PubMed]
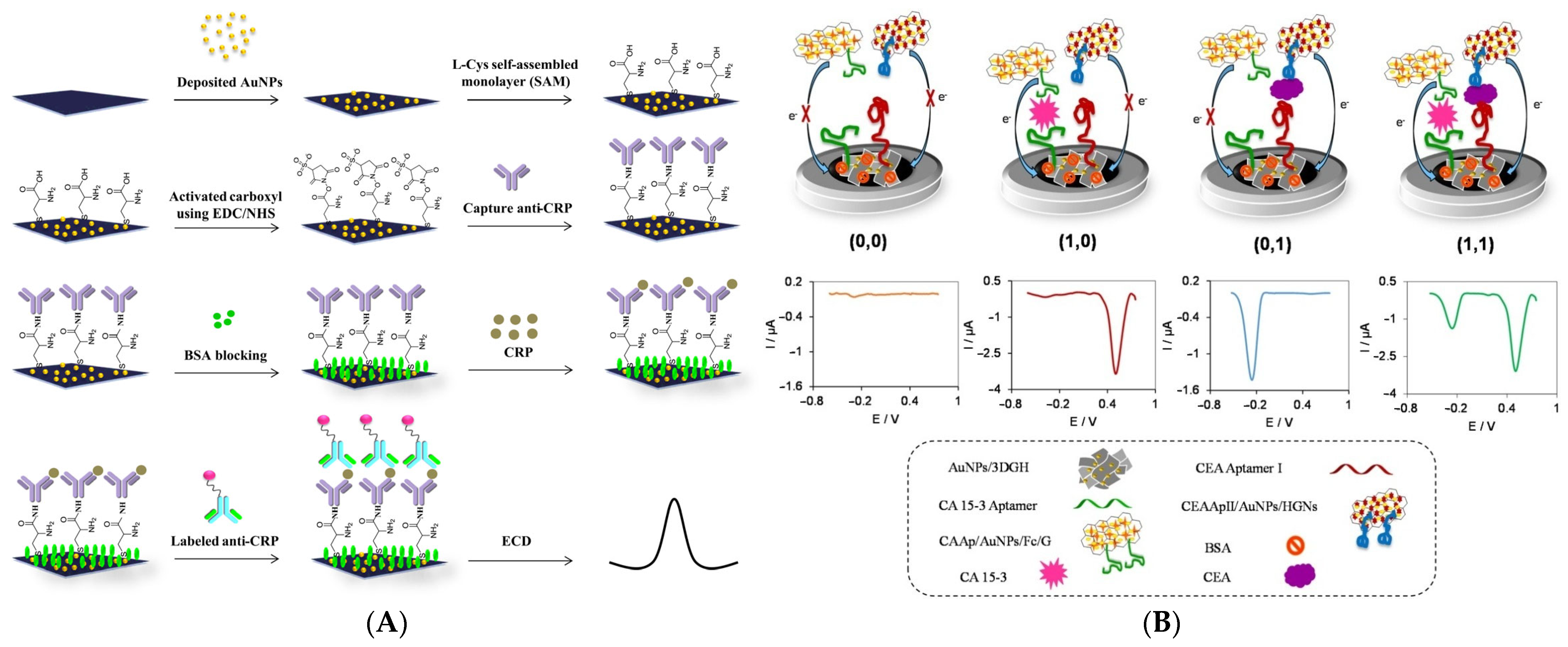
- Shekari, Z.; Zare, H.R.; Falahati, A. Dual Assaying of Breast Cancer Biomarkers by Using a Sandwich–Type Electrochemical Aptasensor Based on a Gold Nanoparticles–3D Graphene Hydrogel Nanocomposite and Redox Probes Labeled Aptamers. Sens. Actuators B Chem. 2021, 332, 129515. [Google Scholar] [CrossRef]
- McCreery, R.L. Advanced Carbon Electrode Materials for Molecular Electrochemistry. Chem. Rev. 2008, 108, 2646–2687. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedersen, T.; Gurevich, L.; Magnusson, N.E. Aspects of Electrochemical Biosensors Using Affinity Assays. Biosensors 2025, 15, 166. https://doi.org/10.3390/bios15030166
Pedersen T, Gurevich L, Magnusson NE. Aspects of Electrochemical Biosensors Using Affinity Assays. Biosensors. 2025; 15(3):166. https://doi.org/10.3390/bios15030166
Chicago/Turabian StylePedersen, Thor, Leonid Gurevich, and Nils E. Magnusson. 2025. "Aspects of Electrochemical Biosensors Using Affinity Assays" Biosensors 15, no. 3: 166. https://doi.org/10.3390/bios15030166
APA StylePedersen, T., Gurevich, L., & Magnusson, N. E. (2025). Aspects of Electrochemical Biosensors Using Affinity Assays. Biosensors, 15(3), 166. https://doi.org/10.3390/bios15030166





