Research Progress of Biosensors in the Detection of Pesticide Residues and Heavy Metals in Tea Leaves
Abstract
1. Introduction
2. Types and Sources of Pesticide Residues and Heavy Metals in Tea
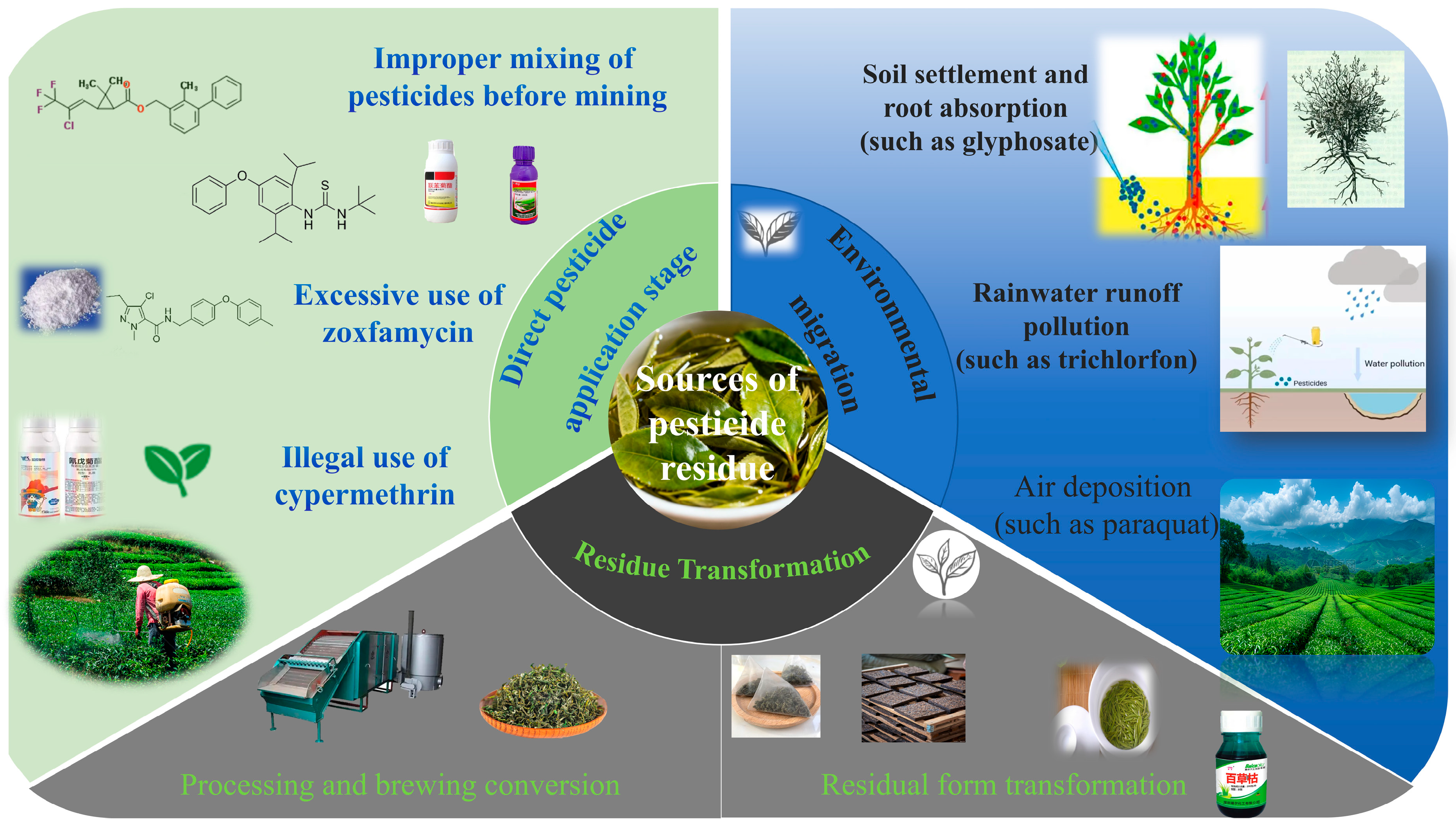
2.1. Classification and Properties of Tea Pesticide Residues
2.2. Primary Sources and Contamination Pathways
2.3. Heavy Metal Species in Tea
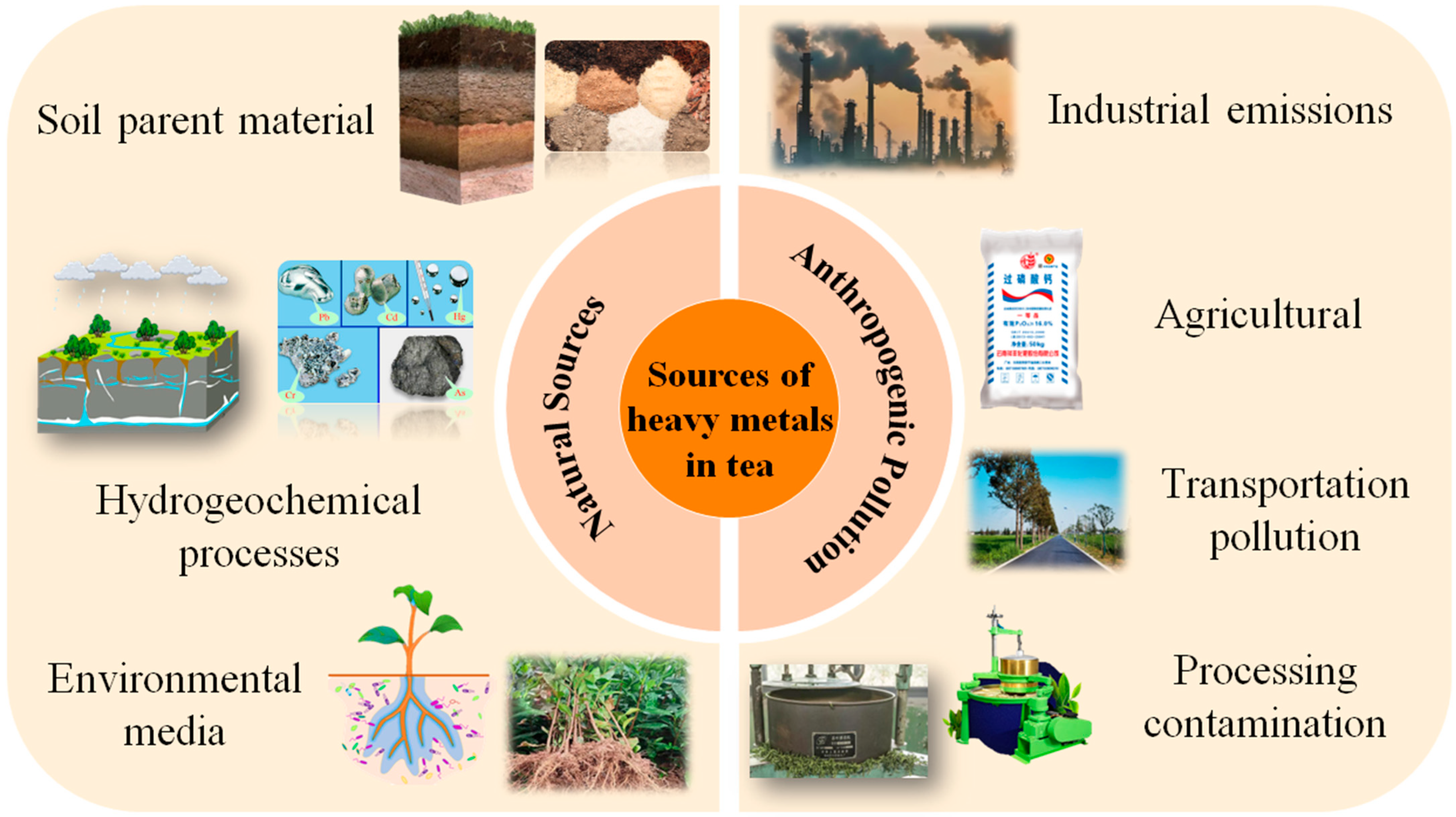
2.4. Source Analysis of Heavy Metals in Tea
2.4.1. Source of Natural Geological Background
2.4.2. Source of Pollutants
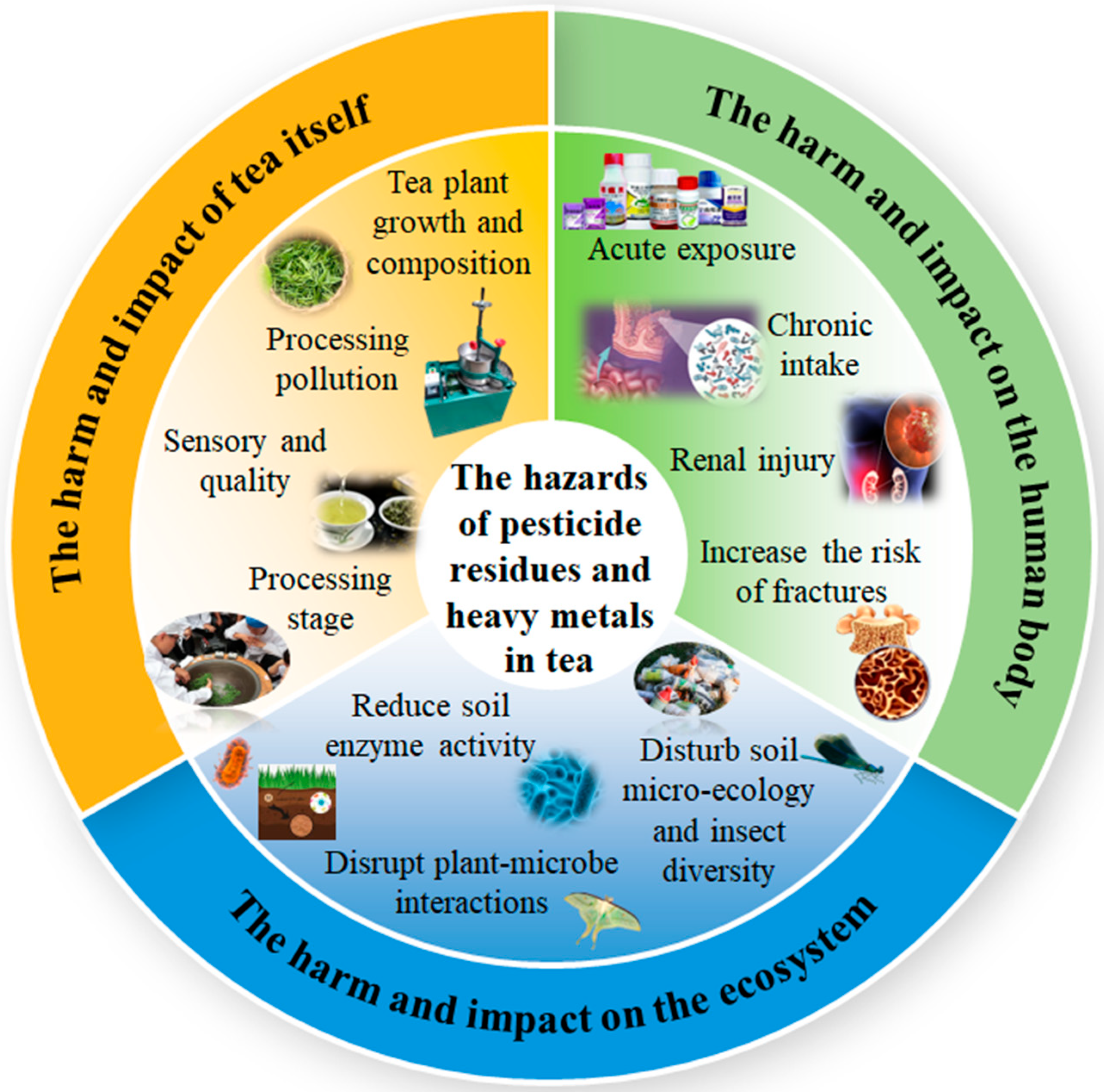
3. The Harm and Influence of Pesticide Residues and Heavy Metals in Tea Leaves
3.1. Hazards and Effects on Tea
3.2. Hazards and Effects on the Human Body
3.3. Hazards and Impacts on Ecosystems
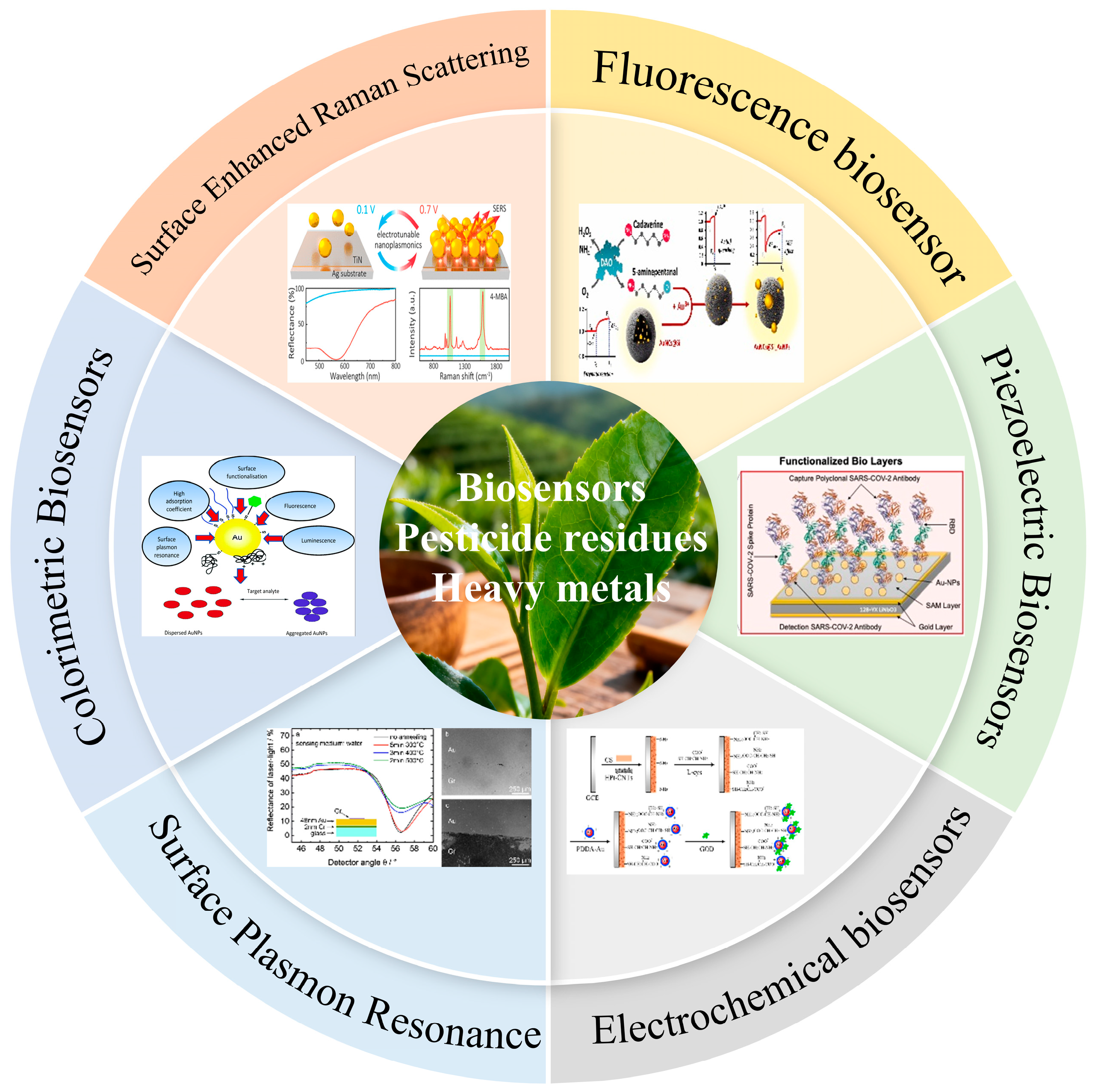
4. The Development and Application of Biosensors
4.1. Fluorescence Biosensor
4.1.1. Fluorescent Biosensor Based on the “Sign On” Strategy
4.1.2. Fluorescent Biosensor Utilizing the “Sign Off” Strategy
4.2. Surface Enhanced Raman Spectroscopy
4.3. Colorimetric Biosensors
4.4. Surface Plasmon Resonance Sensor
4.5. Electrochemical Biosensors
4.5.1. Label Free Electrochemical Biosensor
4.5.2. Label Type Electrochemical Biosensor
4.6. Piezoelectric Biosensors
4.7. Innovative Sensing Techniques and Microfluidic Integration
4.8. Technical Comparison, Challenges, and Translational Prospects
5. Summary and Prospect
5.1. Research Summary
5.2. Existing Challenges
5.3. Future Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, S.; Li, Z.; Ma, Y.; Liu, Y.; Lin, C.-C.; Li, S.; Zhan, J.; Ho, C.-T. Immunomodulatory Effects of Green Tea Polyphenols. Molecules 2021, 26, 3755. [Google Scholar] [CrossRef]
- Surya, G.; Anbarasan, P. Tea Tales: An Analytical Exploration of Branding Strategies and Consumer Trends in the Tea Industry. J. Agric. Food Res. 2025, 23, 102190. [Google Scholar] [CrossRef]
- Wan, D.; Jiao, C. Research on the Development of Tea Industry in Yibin City under Rural Revitalization. Front. Bus. Econ. Manag. 2023, 9, 20–23. [Google Scholar] [CrossRef]
- Li, H.; Fan, Y.; Zhou, H. During the “14th Five-Year Plan” Period, On Promoting the Construction of Ecological Hui Tea Under the Theory of Circular Economy and Helping Rural Revitalization: In-Depth Field Investigation and Analysis in Huoshan County. Front. Bus. Econ. Manag. 2023, 7, 289–294. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Barba, F.J.; Zhou, J.; Wang, M.; Altintas, Z. Electronic Sensor Technologies in Monitoring Quality of Tea: A Review. Biosensors 2022, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Zheng, K.; Lin, R.; Liu, X.; Wu, X.; Chen, R.; Yang, M. Multiresidue Pesticide Analysis in Tea Using GC–MS/MS to Determine 12 Pesticide Residues (GB 2763-2021). Molecules 2022, 27, 8419. [Google Scholar] [CrossRef] [PubMed]
- Xie, A.; Wu, W. Emerging Nanosensor Technologies for the Rapid Detection of Heavy Metal Contaminants in Agricultural Soils. Anal. Methods 2025, 17, 7846–7862. [Google Scholar] [CrossRef]
- GB 2763-2021; National Food Safety Standard-Maximum Residue Limits for Pesticides in Foods. National Health Commission of the People’s Republic of China, State Administration for Market Regulation: Beijing, China, 2021.
- GB 31608-2023; National Food Safety Standard-Tea. National Health Commission of the People’s Republic of China, State Administration for Market Regulation: Beijing, China, 2023.
- European Food Safety Authority (EFSA); Anastassiadou, M.; Bernasconi, G.; Brancato, A.; Carrasco Cabrera, L.; Greco, L.; Jarrah, S.; Kazocina, A.; Leuschner, R.; Magrans, J.O.; et al. Review of the Existing Maximum Residue Levels for Ametoctradin According to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2020, 18, e05990. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Brancato, A.; Brocca, D.; De Lentdecker, C.; Erdos, Z.; Ferreira, L.; Greco, L.; Jarrah, S.; Kardassi, D.; Leuschner, R.; et al. Review of the Existing Maximum Residue Levels for Chlorpyrifos-Methyl According to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2017, 15, e04734. [Google Scholar] [CrossRef]
- Wang, C.; Du, X.; Nie, C.; Zhang, X.; Tan, X.; Li, Q. Evaluation of Sensory and Safety Quality Characteristics of “High Mountain Tea”. Food Sci. Nutr. 2022, 10, 3338–3354. [Google Scholar] [CrossRef]
- Ghorbani, F.; Abbaszadeh, H.; Mehdizadeh, A.; Ebrahimi-Warkiani, M.; Rashidi, M.-R.; Yousefi, M. Biosensors and Nanobiosensors for Rapid Detection of Autoimmune Diseases: A Review. Microchim. Acta 2019, 186, 838. [Google Scholar] [CrossRef] [PubMed]
- Rafique, B.; Iqbal, M.; Mehmood, T.; Shaheen, M.A. Electrochemical DNA Biosensors: A Review. Sens. Rev. 2019, 39, 34–50. [Google Scholar] [CrossRef]
- Du, X.; Niu, W.; Xin, Y.; Lou, T. Effect of Ultrasound Treatment on the Structure, Physicochemical Properties, and Functional Characteristics of Rice-Peanut Protein/EGCG Ternary Composite Nanoparticles. Int. J. Biol. Macromol. 2025, 312, 144128. [Google Scholar] [CrossRef]
- Fatunsin, O.T.; Oyeyiola, A.O.; Moshood, M.O.; Akanbi, L.M.; Fadahunsi, D.E. Dietary Risk Assessment of Organophosphate and Carbamate Pesticide Residues in Commonly Eaten Food Crops. Sci. Afr. 2020, 8, e00442. [Google Scholar] [CrossRef]
- Twagirayezu, G.; Cheng, H.; Wu, Y.; Lu, H.; Huang, S.; Fang, X.; Irumva, O. Insights into the Influences of Biochar on the Fate and Transport of Pesticides in the Soil Environment: A Critical Review. Biochar 2024, 6, 9. [Google Scholar] [CrossRef]
- Alnaimy, M.A.; Elrys, A.S.; Zelenakova, M.; Pietrucha-Urbanik, K.; Merwad, A.-R.M. The Vital Roles of Parent Material in Driving Soil Substrates and Heavy Metals Availability in Arid Alkaline Regions: A Case Study from Egypt. Water 2023, 15, 2481. [Google Scholar] [CrossRef]
- Leng, Y.; Zeng, Y.; Zhang, Y.; Zhang, J.; Xin, H.; Hou, M.; Ma, S.; Zhang, H. Neurotoxic Risks of Long-Term Environmental Exposure to Pesticides: A Review. Chem.-Biol. Interact. 2025, 418, 111626. [Google Scholar] [CrossRef]
- Wei, K.; Yang, Q.; Wei, Y.; Wang, Y.; Xu, N.; Wei, X. Preparation, Identification and Preliminary Application of the Fenvalerate Monoclonal Antibody in Six Kinds of Dark Tea. Foods 2023, 12, 1091. [Google Scholar] [CrossRef]
- Ding, Z.; Zhang, K.; Yang, W.; Fu, Q.; Jia, L. Magnetic Multi-Walled Carbon Nanotubes Assisted Solid Phase Extraction and Detection of Neonicotinoid Pesticides in Honey and Tea Samples. Microchem. J. 2023, 195, 109459. [Google Scholar] [CrossRef]
- Chang, T.-H.; Chen, Y.-C.; Lai, Y.-F.; Wu, T.-C.; Lai, C.-H.; Hsueh, H.-Y.; Chang, P.-F.L. Integrated Application of Grafted ZnO and Fungicide to Control the Fungicide-Resistant Colletotrichum spp. J. Taiwan Inst. Chem. Eng. 2024, 155, 105321. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, N.; Wang, C. Toxicity of the Pyrethroid Bifenthrin Insecticide. Env. Chem. Lett. 2018, 16, 1377–1391. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Bellisai, G.; Bernasconi, G.; Binaglia, M.; Brancato, A.; Carrasco Cabrera, L.; Castellan, I.; Castoldi, A.F.; Chiusolo, A.; Crivellente, F.; et al. Targeted Review of Maximum Residue Levels (MRLs) for Fenpropathrin. EFSA J. 2023, 21, e08057. [Google Scholar] [CrossRef]
- Yaseen, T.; Sun, D.-W.; Pu, H.; Pan, T.-T. Detection of Omethoate Residues in Peach with Surface-Enhanced Raman Spectroscopy. Food Anal. Methods 2018, 11, 2518–2527. [Google Scholar] [CrossRef]
- Hamill, A.S.; Penner, D. Interaction of Alachlor and Carbofuran. Weed Sci. 1973, 21, 330–335. [Google Scholar] [CrossRef]
- Yin, Q.; Fu, W.; Hu, X.; Xu, Z.; Li, Z.; Shao, X. Application of TNB in Dual Photo-Controlled Release of Phenamacril, Imidacloprid, and Imidacloprid Synergist. Photochem. Photobiol. 2024, 100, 1813–1826. [Google Scholar] [CrossRef]
- GB 23200.51-2016; National Food Safety Standard-Determination of Dinotefuran Residue in Foods Liquid Chromatography-Mass Spectrometry. National Health Commission of the People’s Republic of China, Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2016.
- Zhu, J.; Tao, Q.; Du, G.; Huang, L.; Li, M.; Wang, M.; Wang, Q. Mitochondrial Dynamics Disruption: Unraveling Dinotefuran’s Impact on Cardiotoxicity. Environ. Pollut. 2024, 343, 123238. [Google Scholar] [CrossRef]
- GB 23200.113-2021; National Food Safety Standard-Determination of 208 Pesticides and Metabolites Residues in Food of Plant Origin Gas Chromatography-Mass Spectrometry. National Health Commission of the People’s Republic of China, Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2021.
- Niu, S.; Li, M.; Jiang, Y.; Wang, Y.; Ren, Q. Removal Mechanism of Carbendazim in Water by Ozone and Remediation of Carbendazim Pollution in Soil. Land Degrad. Dev. 2025, 36, 4080–4092. [Google Scholar] [CrossRef]
- Liu, X.; Guo, S.; Xu, Y.; Zhang, Z.; Liu, Y.; Shao, Y.; Chen, K.; Ruan, L. Development of a Portable Biosensor for Glyphosate Detection Using Bacterial Surface-Displayed Glyphosate Oxidase. Biosens. Bioelectron. 2025, 292, 117974. [Google Scholar] [CrossRef]
- Liu, J.; Song, S.; Wu, A.; Kuang, H.; Liu, L.; Xiao, J.; Xu, C. Development of Immunochromatographic Strips for the Detection of Dicofol. Analyst 2021, 146, 2240–2247. [Google Scholar] [CrossRef]
- Giwa, A.S.; Waheed, M.; Khalid, H.V.; Shafique, E.; Rahman, S.U.; Ali, N. Mechanistic Insights into Fungal-Bacterial Synergy for DDT Biotransformation. Antonie Van Leeuwenhoek 2025, 118, 137. [Google Scholar] [CrossRef] [PubMed]
- Kyriakopoulos, G.L.; Sebos, I. Marketable and Banned Pesticides in Agriculture: Categorization, Simulation, and Crystallography Review. Int. J. Mol. Sci. 2024, 25, 11885. [Google Scholar] [CrossRef]
- Adams, R.E.; Brickel, J.A.; Bhat, V.S. Chemical-Specific Maximum Allowable Levels for Pesticide Residues in Dietary Supplements. Food Chem. Toxicol. 2019, 123, 511–519. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, L.; Wu, A.; Xu, X.; Liu, L.; Xu, C.; Kuang, H.; Xu, L. Immunochromatographic Test Strip for Quantitative and Rapid Detection of Tolfenpyrad in Food Samples. J. Chromatogr. B 2023, 1228, 123837. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, C.; Wang, S.; Zhang, T.; Tian, X. Temperature Influences Glyphosate Efficacy on Glyphosate-Resistant and -Susceptible Goosegrass (Eleusine Indica). Front. Plant Sci. 2023, 14, 1169726. [Google Scholar] [CrossRef]
- Décuq, C.; Bourdat-Deschamps, M.; Benoit, P.; Bertrand, C.; Benabdallah, R.; Esnault, B.; Durand, B.; Loubet, B.; Fritsch, C.; Pelosi, C.; et al. A Multiresidue Analytical Method on Air and Rainwater for Assessing Pesticide Atmospheric Contamination in Untreated Areas. Sci. Total Environ. 2022, 823, 153582. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Hao, Z.; Li, Y.; Zhou, Y.; Shao, R.; Chen, R.; Zheng, M.; Xu, Y.; Wang, H. Biochemical Toxicity and Transcriptome Aberration Induced by Dinotefuran in Bombyx mori. Environ. Pollut. 2022, 307, 119562. [Google Scholar] [CrossRef]
- Iqbal, N.; Agrawal, A.; Verma, A.; Kumar, J. Encapsulation of Water Soluble Pesticides for Extended Delivery of Pesticides without Contaminating Water Bodies. J. Environ. Sci. Health Part B 2021, 56, 458–466. [Google Scholar] [CrossRef]
- Deng, Q.; Liu, Y.; Liu, D.; Meng, Z.; Hao, X. Development of a Design of Experiments (DOE) Assistant Modified QuEChERS Method Coupled with HPLC-MS/MS Simultaneous Determination of Twelve Lipid-Soluble Pesticides and Four Metabolites in Chicken Liver and Pork. J. Food Compos. Anal. 2024, 133, 106379. [Google Scholar] [CrossRef]
- Carrasco Cabrera, L.; Di Piazza, G.; Dujardin, B.; Medina Pastor, P. The 2021 European Union Report on Pesticide Residues in Food. EFSA J. 2023, 21, e07939. [Google Scholar] [CrossRef] [PubMed]
- Kotnala, S.; Tiwari, S.; Nayak, A.; Bhushan, B.; Chandra, S.; Medeiros, C.R.; Coutinho, H.D.M. Impact of Heavy Metal Toxicity on the Human Health and Environment. Sci. Total Environ. 2025, 987, 179785. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef] [PubMed]
- Baruah, N.; Gogoi, N.; Roy, S.; Bora, P.; Chetia, J.; Zahra, N.; Ali, N.; Gogoi, P.; Farooq, M. Phytotoxic Responses and Plant Tolerance Mechanisms to Cadmium Toxicity. J. Soil Sci. Plant Nutr. 2023, 23, 4805–4826. [Google Scholar] [CrossRef]
- Nkansah, M.A.; Opoku, F.; Ackumey, A.A. Risk Assessment of Mineral and Heavy Metal Content of Selected Tea Products from the Ghanaian Market. Env. Monit. Assess. 2016, 188, 332. [Google Scholar] [CrossRef]
- Konate, A.; He, X.; Zhang, Z.; Ma, Y.; Zhang, P.; Alugongo, G.M.; Rui, Y. Magnetic (Fe3O4) Nanoparticles Reduce Heavy Metals Uptake and Mitigate Their Toxicity in Wheat Seedling. Sustainability 2017, 9, 790. [Google Scholar] [CrossRef]
- Zarshenas, N.; Tapsell, L.C.; Batterham, M.; Neale, E.P.; Talbot, M.L. Investigating the Prevalence of Copper and Zinc Abnormalities in Patients Pre and Post Bariatric Surgery—An Australian Experience. Obes. Surg. 2023, 33, 3437–3446. [Google Scholar] [CrossRef]
- Wang, Y.; Li, G.; Wang, Y.; Ding, Y.; Yan, G.; Han, D.; Wang, Z.; Zhao, X. The Evaluation of GI-Pill Gastrointestinal Electronic Capsule for Colonic Transit Test in Patients with Slow Transit Constipation. Int. J. Color. Dis. 2020, 35, 29–34. [Google Scholar] [CrossRef]
- Yang, H.; Kim, N.; Park, D. Ecotoxicity Study of Reduced-Cr(III) Generated by Cr(VI) Biosorption. Chemosphere 2023, 332, 138825. [Google Scholar] [CrossRef]
- Wu, X.; Xu, Z.; Tong, J.; Hu, B.X. Sensitivity Analysis for Modeling of Cr(VI) Transfer From Soil to Surface Runoff. Front. Environ. Sci. 2022, 10, 917103. [Google Scholar] [CrossRef]
- Dutta, S.; Jain, M.K.; Kumar, D. Evaluation of Soil Heavy Metals in Raniganj Open-Cast Coal Mines in India: Spatial Distribution, Positive Matrix Factorization and Monte Carlo Simulation. Process Saf. Environ. Prot. 2025, 194, 1038–1055. [Google Scholar] [CrossRef]
- Wen, J.; Wu, C.; Bi, X.; Zhang, S.; Ouyang, H.; Ye, J.; Ohnuki, T.; Yu, Q. Soil pH Change Induced by Smelting Activities Affects Secondary Carbonate Production and Long-Term Cd Activity in Subsoils. Appl. Geochem. 2023, 152, 105663. [Google Scholar] [CrossRef]
- Wang, J.; Dong, C.; Sun, S.; Peng, S.; Mu, L.; Zhang, N.; Bao, L. Characteristics and Risk Assessment of Heavy Metal Contamination in Arable Soils Developed from Different Parent Materials. Agriculture 2024, 14, 2010. [Google Scholar] [CrossRef]
- Pan, F.; Xiao, K.; Guo, Z.; Li, H. Effects of Fiddler Crab Bioturbation on the Geochemical Migration and Bioavailability of Heavy Metals in Coastal Wetlands. J. Hazard. Mater. 2022, 437, 129380. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.K.; Ramanathan, A.L. Geochemical Fractionation and Bioavailability of Heavy Metals in the Middle Gangetic Plain: Assessing Ecological Risks in Water and Sediment Systems. Sci. Total Environ. 2025, 964, 178564. [Google Scholar] [CrossRef]
- Zou, L.; Shang, Q.; Li, Z.; Xing, Z.; Chen, G.; Chen, Z.; Zhou, J.; Liu, X. Mediation of Crop Heavy Metal Uptake by Root Exudates in an Intercropping System of Heavy Metal Contaminated Soils. J. Sci. Food Agric. 2025, 105, 5780–5794. [Google Scholar] [CrossRef]
- Kim, H.; Lee, M.; Lee, J.-H.; Kim, K.-H.; Owens, G.; Kim, K.-R. Distribution and Extent of Heavy Metal(Loid) Contamination in Agricultural Soils as Affected by Industrial Activity. Appl. Biol. Chem. 2020, 63, 31. [Google Scholar] [CrossRef]
- Chi, G.; Qin, F.; Zhu, B.; Chen, X. Long-Term Wetland Reclamation Affects the Accumulation and Profile Distribution of Heavy Metals in Soils. J. Soils Sediments 2023, 23, 1706–1717. [Google Scholar] [CrossRef]
- Stojić, N.; Štrbac, S.; Ćurčić, L.; Pucarević, M.; Prokić, D.; Stepanov, J.; Stojić, G. Exploring the Impact of Transportation on Heavy Metal Pollution: A Comparative Study of Trains and Cars. Transp. Res. Part D Transp. Environ. 2023, 125, 103966. [Google Scholar] [CrossRef]
- Altun, O.; Darılmaz, Ö.; Karahan, E.; Sert, T.; Altun, D.; Toprak, A.; Hür, A. Understanding HIGMill Operation at Copper Regrind Application; Operating Parameters, Wear and Mineral Liberation. Miner. Eng. 2023, 191, 107964. [Google Scholar] [CrossRef]
- Shinde, A.; Guduru, N.S.S.C.S.; Das, A.; Bitra, K.S.; Bobbili, H.B.; Mukkollu, K.S.; Allu, C.S.; Vanga, S.; Shyam, R.; Kallepalli, R.; et al. SUVN-L3307032, A Positive Allosteric Modulator (PAM) at Muscarinic M4 Receptor for the Treatment of Neuropsychiatric Symptoms. Alzheimer’s Dement. 2024, 20, e087845. [Google Scholar] [CrossRef]
- Sheng, M.; Hamel, C.; Fernandez, M.R. Cropping Practices Modulate the Impact of Glyphosate on Arbuscular Mycorrhizal Fungi and Rhizosphere Bacteria in Agroecosystems of the Semiarid Prairie. Can. J. Microbiol. 2012, 58, 990–1001. [Google Scholar] [CrossRef]
- Moldovan, R.; Iacob, B.-C.; Farcău, C.; Bodoki, E.; Oprean, R. Strategies for SERS Detection of Organochlorine Pesticides. Nanomaterials 2021, 11, 304. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.; Luo, M.; Hu, J.; Wang, H.; He, Y.; Li, D.; Yuan, Y.; Hou, J.; Song, Y.; et al. Tea Polyphenols Attenuate Glufosinate-Induced Breast Injury by Reducing Endoplasmic Reticulum Stress and Autophagy. J. Hazard. Mater. 2025, 495, 138823. [Google Scholar] [CrossRef] [PubMed]
- Muth, F.; Francis, J.S.; Leonard, A.S. Modality-Specific Impairment of Learning by a Neonicotinoid Pesticide. Biol. Lett. 2019, 15, 20190359. [Google Scholar] [CrossRef]
- Lei, M.; Ding, X.; Liu, J.; Tang, Y.; Chen, H.; Zhou, Y.; Zhu, C.; Yan, H. Trace Amount of Bi-Doped Core–Shell Pd@Pt Mesoporous Nanospheres with Specifically Enhanced Peroxidase-Like Activity Enable Sensitive and Accurate Detection of Acetylcholinesterase and Organophosphorus Nerve Agents. Anal. Chem. 2024, 96, 6072–6078. [Google Scholar] [CrossRef] [PubMed]
- Oyovwi, M.O.; Atere, A.D.; Chimwuba, P.; Joseph, U.G. Implication of Pyrethroid Neurotoxicity for Human Health: A Lesson from Animal Models. Neurotox. Res. 2024, 43, 1. [Google Scholar] [CrossRef]
- Takahashi, T.; Shimohata, T. Vascular Dysfunction Induced by Mercury Exposure. Int. J. Mol. Sci. 2019, 20, 2435. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, B.; Qian, X.; Xu, G.; Jin, X.; Chen, D.; Tang, J.; Xu, L. Transcriptomics-Based Analysis of Co-Exposure of Cadmium (Cd) and 2,2’,4,4’-Tetrabromodiphenyl Ether (BDE-47) Indicates Mitochondrial Dysfunction Induces NLRP3 Inflammasome and Inflammatory Cell Death in Renal Tubular Epithelial Cells. Ecotoxicol. Environ. Saf. 2022, 241, 113790. [Google Scholar] [CrossRef]
- Moreira, R.A.; dos Santos Silva, E.; Sanches, A.L.M.; Freitas, E.C.; Vieira, B.H.; Reghini, M.V.; de Mello Batista, H.; da Silva Pinto, T.J.; dos Santos Wisniewski, M.J.; Espindola, E.L.G.; et al. Impact of Simulated Pesticide Spray Drift and Runoff Events on the Structural and Functional Zooplankton Diversity in Tropical Freshwater Microcosms. Water Air Soil Pollut. 2021, 232, 315. [Google Scholar] [CrossRef]
- Haider, B.; Imran, M.; Ashraf, M.; Mahmood, S. Mechanistic Insights into Pressmud-Mediated Improvement in Wheat Growth, Quality and Productivity under Lead Contaminated Salt-Affected Soil. J. Soil Sci. Plant Nutr. 2025, 25, 3018–3028. [Google Scholar] [CrossRef]
- Javed, M.T.; Akram, M.S.; Tanwir, K.; Javed Chaudhary, H.; Ali, Q.; Stoltz, E.; Lindberg, S. Cadmium Spiked Soil Modulates Root Organic Acids Exudation and Ionic Contents of Two Differentially Cd Tolerant Maize (Zea mays L.) Cultivars. Ecotoxicol. Environ. Saf. 2017, 141, 216–225. [Google Scholar] [CrossRef]
- Dwivedi, A.; Srivastava, M.; Srivastava, A.; Kumar, A.; Chaurasia, R.N.; Srivastava, S.K. A Eu3+doped Functional Core-Shell Nanophosphor as Fluorescent Biosensor for Highly Selective and Sensitive Detection of dsDNA. J. Photochem. Photobiol. B Biol. 2023, 249, 112802. [Google Scholar] [CrossRef] [PubMed]
- Hybrid Sol-Gel Surface-Enhanced Raman Sensor for Xylene Detection in Solution. Available online: https://www.mdpi.com/1424-8220/21/23/7912 (accessed on 14 February 2025).
- Li, X.; Li, S.; Lv, Q.; Wang, C.; Liang, J.; Zhou, Z.; Li, G. Colorimetric Biosensor for Visual Determination of Golgi Protein 73 Based on Reduced Graphene Oxide-Carboxymethyl Chitosan-Hemin/Platinum@palladium Nanozyme with Peroxidase-like Activity. Microchim. Acta 2022, 189, 392. [Google Scholar] [CrossRef]
- Li, Z.; Lu, S.; Jin, J.; Wang, T. Preparation of a New Cellulose Magnetic Molecularly Imprinted Polymer Micro-Spheres to Extract and Analyze the Indole-3-Acetic Acid in Plant Tissues. J. Chromatogr. B 2018, 1092, 343–349. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Xie, H.; Sun, R.; Cao, T.; Paudyal, N.; Fang, W.; Song, H. Development of a Magnetic Nanoparticles-Based Screen-Printed Electrodes (MNPs-SPEs) Biosensor for the Quantification of Ochratoxin A in Cereal and Feed Samples. Toxins 2018, 10, 317. [Google Scholar] [CrossRef]
- Skládal, P. Piezoelectric Biosensors: Shedding Light on Principles and Applications. Microchim. Acta 2024, 191, 184. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, C.; Kang, X.; Zhang, X.; Xue, B.; Li, C.; Wang, S.; Yang, X.; Li, C.; Qiu, Z.; et al. A Cell-Free Fluorescence Biosensor Based on Allosteric Transcription Factor NalC for Detection of Pentachlorophenol. Biotechnol. Lett. 2024, 46, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yu, H.; Li, Q.; Tian, Y.; Gao, X.; Zhang, W.; Sun, Z.; Mou, Y.; Sun, X.; Guo, Y.; et al. Development of a Fluorescent Sensor Based on TPE-Fc and GSH-AuNCs for the Detection of Organophosphorus Pesticide Residues in Vegetables. Food Chem. 2024, 431, 137067. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, J.; Wang, X.; Jiang, B.; Niu, Q. A New Oligothiophene-Derivatized Fluorescent Sensor for Detecting and Imaging of Hg2+ in Water/Soil/Urine/Tea/Seafood Samples and Living Plants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2025, 329, 125585. [Google Scholar] [CrossRef]
- Wang, Y.; He, L.; Wei, W.; Zhang, X.; Zeng, L.; Jiang, T.; Li, J.; Chen, S.; Gao, Z. A Review of Fluorescent Covalent Organic Frameworks for Heavy Metal Ion Sensors. Chem. Eng. J. 2025, 505, 159249. [Google Scholar] [CrossRef]
- Feng, H.; Wang, P.; Bai, H.; Shao, Y. Ratiometric Fluorescence Sensor Prepared by Silica-Based Fluorescent Surface Molecularly Imprinted Polymer for Efficient Detection of Bisphenol A. Mater. Lett. 2024, 365, 136490. [Google Scholar] [CrossRef]
- Guo, X.; Xiao, J.; Zhang, Y.; Zhang, Q.; Yang, J.; Wei, Y.; Wang, L.; Yao, W. Portable Analysis of Tract Mercury Ions by a Hydrogel-Based Ratiometric Fluorescence Sensor Using C3N4-CdTe0.16S0.84 QDs Nanocomposites. Sens. Actuators B Chem. 2024, 413, 135846. [Google Scholar] [CrossRef]
- Huang, Q.; Han, L.; Ma, H.; Lan, W.; Tu, K.; Peng, J.; Su, J.; Pan, L. An Aptamer Sensor Based on Alendronic Acid-Modified Upconversion Nanoparticles Combined with Magnetic Separation for Rapid and Sensitive Detection of Thiamethoxam. Foods 2025, 14, 182. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Cheng, J.; Zhou, N.; Zhang, L.; Mao, H.; Huang, C. SERS Devices with Hedgehog-like Nanosphere Arrays for Detection of Trace Pesticides. J. Innov. Opt. Health Sci. 2021, 14, 2141005. [Google Scholar] [CrossRef]
- Liu, J.; Hong, Z.; Yang, W.; Liu, C.; Lu, Z.; Wu, L.; Foda, M.F.; Yang, Z.; Han, H.; Zhao, Y. Bacteria Inspired Internal Standard SERS Substrate for Quantitative Detection. ACS Appl. Bio Mater. 2021, 4, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Wang, H.; Shao, X.; Yu, X.; Xu, X. Au@Ag Core-Shell Nanorods Enhance Surface-Enhanced Raman Scattering Aptasensor for Ultrasensitive Detection of Salmonella Typhimurium. Food Control 2024, 161, 110379. [Google Scholar] [CrossRef]
- Terry, L.R.; Kruel, J.W.; Jain, M.; Lara, A.; Sharma, P.; Hsiao, B.S.; Guo, H. Detection of Pesticides in Sprayed Droplets by Using Biowaste-Derived Nanocellulose-Based SERS Nanosubstrate. Cellulose 2024, 31, 10915–10929. [Google Scholar] [CrossRef]
- He, Q.; Han, Y.; Huang, Y.; Gao, J.; Gao, Y.; Han, L.; Zhang, Y. Reusable Dual-Enhancement SERS Sensor Based on Graphene and Hybrid Nanostructures for Ultrasensitive Lead (II) Detection. Sens. Actuators B Chem. 2021, 341, 130031. [Google Scholar] [CrossRef]
- Dikmen, G. Surface Enhanced Raman Spectroscopy Sensor Based on Silver Nanoparticles/Multi Wall Carbon Nanotubes for Ultrasensitive Detection of Cholesterol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 303, 123235. [Google Scholar] [CrossRef]
- Zhou, J.; Qi, X.; Zhang, J.X.J. Controlled Synthesis of Metal–Insulator–Metal Nanoparticles for Enhanced Raman Spectroscopy. Nanoscale 2025, 17, 23654–23666. [Google Scholar] [CrossRef]
- Cucuiet, V.; Maniu, D.; Astilean, S.; Lamy de la Chapelle, M.; Focsan, M. Graphene-Mediated Surface Enhanced Raman Spectroscopy for DNA Detection&hybridization: Breakthroughs and Challenges. Biosens. Bioelectron. 2025, 286, 117610. [Google Scholar] [CrossRef]
- He, Q.; Koster, H.J.; O’Sullivan, J.; Ono, S.G.; O’Toole, H.J.; Leiserowitz, G.S.; Heffern, M.C.; Carney, R.P. Integration of Label-Free Surface Enhanced Raman Spectroscopy (SERS) of Extracellular Vesicles (EVs) with Raman Tagged Labels to Enhance Ovarian Cancer Diagnostics. Biosens. Bioelectron. 2025, 288, 117800. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Chen, C.; Wang, R.; Qiao, X.; Waterhouse, G.I.N.; Xu, Z. A Surface-Enhanced Raman Scattering Sensor for the Detection of Benzo[a]Pyrene in Foods Based on a Gold Nanostars@reduced Graphene Oxide Substrate. Food Chem. 2023, 421, 136171. [Google Scholar] [CrossRef]
- Qi, L.; Xiao, M.; Wang, F.; Wang, L.; Ji, W.; Man, T.; Aldalbahi, A.; Khan, M.N.; Periyasami, G.; Rahaman, M.; et al. Poly-Cytosine-Mediated Nanotags for SERS Detection of Hg2+. Nanoscale 2017, 9, 14184–14191. [Google Scholar] [CrossRef]
- Chen, Q.; Yao, L.; Yao, B.; Meng, X.; Wu, Q.; Chen, Z.; Chen, W. Low-Cost Signal Enhanced Colorimetric and SERS Dual-Mode Paper Sensor for Rapid and Ultrasensitive Screening of Mercury Ions in Tea. Food Chem. 2025, 463, 141375. [Google Scholar] [CrossRef]
- Kaur, J.; Bandyopadhyay, D.; Singh, P.K. A Simple and Convenient Choline Oxidase Inhibition Based Colorimetric Biosensor for Detection of Organophosphorus Class of Pesticides. J. Mol. Liq. 2022, 347, 118258. [Google Scholar] [CrossRef]
- Shayesteh, O.H.; Derakhshandeh, K.; Ranjbar, A.; Mahjub, R.; Farmany, A. Development of a Label-Free, Sensitive Gold Nanoparticles–Poly(Adenine) Aptasensing Platform for Colorimetric Determination of Aflatoxin B1 in Corn. Anal. Methods 2024, 16, 3030–3038. [Google Scholar] [CrossRef]
- Xie, L.; Guo, C.; Yang, L.; He, Y. Harnessing Gold Nanomaterials for Advanced Multicolor Colorimetric Biosensors in Food Hazards Detection. J. Food Drug Anal. 2024, 32, 274–295. [Google Scholar] [CrossRef]
- Hu, S.; Hui, C.; Wu, C.; Gao, C.; Huang, Z.; Guo, Y. Dual-Colored Bacterial Biosensor Responsive to Cadmium, Mercury, and Lead for Detecting Heavy Metal Pollution in Seawater. Ecol. Indic. 2024, 166, 112244. [Google Scholar] [CrossRef]
- El barghouti, M.; Houari, F.; Talbi, A.; Mir, A.; Akjouj, A. Surface Plasmon Resonance Sensors: Temperature Effects. Opt. Mater. 2024, 155, 115865. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, G.; Bi, J.; Bao, K.; Wang, P. In-Situ and Ultrasensitive Detection of Mercury (II) Ions (Hg2+) Using the Localized Surface Plasmon Resonance (LSPR) Nanosensor and the Microfluidic Chip. Sens. Actuators A Phys. 2023, 349, 114074. [Google Scholar] [CrossRef]
- Zhong, X.; Ma, L.; Yin, G. Ion-Imprinted Chitosan-Based Localized Surface Plasmon Resonance Sensor for Ni2+ Detection. Sensors 2022, 22, 9005. [Google Scholar] [CrossRef]
- Bakhshpour, M.; Denizli, A. Highly Sensitive Detection of Cd(II) Ions Using Ion-Imprinted Surface Plasmon Resonance Sensors. Microchem. J. 2020, 159, 105572. [Google Scholar] [CrossRef]
- Çakır, O.; Baysal, Z. Pesticide Analysis with Molecularly Imprinted Nanofilms Using Surface Plasmon Resonance Sensor and LC-MS/MS: Comparative Study for Environmental Water Samples. Sens. Actuators B Chem. 2019, 297, 126764. [Google Scholar] [CrossRef]
- Li, D.; Cai, K.; Wu, L.; Zuo, Y.; Yin, W.; Zhang, H.; Lu, Z.; Zhu, G.; Han, H. Ammonia Mediated One-Step Synthesis of Three-Dimensional Porous PtxCu100–x Nanochain Networks with Enhanced Electrocatalytic Activity toward Polyhydric Alcohol Oxidation. ACS Sustain. Chem. Eng. 2017, 5, 11086–11095. [Google Scholar] [CrossRef]
- Liu, C.; Wu, T.; Zeng, W.; Liu, J.; Hu, B.; Wu, L. Dual-Signal Electrochemical Aptasensor Involving Hybridization Chain Reaction Amplification for Aflatoxin B1 Detection. Sens. Actuators B Chem. 2022, 371, 132494. [Google Scholar] [CrossRef]
- Madianos, L.; Tsekenis, G.; Skotadis, E.; Patsiouras, L.; Tsoukalas, D. A Highly Sensitive Impedimetric Aptasensor for the Selective Detection of Acetamiprid and Atrazine Based on Microwires Formed by Platinum Nanoparticles. Biosens. Bioelectron. 2018, 101, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, W.; Shi, J.; Li, Z.; Huang, X.; Zou, X.; Tan, W.; Zhang, X.; Hu, X.; Wang, X.; et al. Impedimetric Aptasensor Based on Highly Porous Gold for Sensitive Detection of Acetamiprid in Fruits and Vegetables. Food Chem. 2020, 322, 126762. [Google Scholar] [CrossRef]
- Ali, M.R.; Bacchu, M.S.; Das, S.; Akter, S.; Rahman, M.M.; Saad Aly, M.A.; Khan, M.Z.H. Label Free Flexible Electrochemical DNA Biosensor for Selective Detection of Shigella flexneri in Real Food Samples. Talanta 2023, 253, 123909. [Google Scholar] [CrossRef]
- Pérez-Fernández, B.; Mercader, J.V.; Checa-Orrego, B.I.; de la Escosura-Muñiz, A.; Costa-García, A. A Monoclonal Antibody-Based Immunosensor for the Electrochemical Detection of Imidacloprid Pesticide. Analyst 2019, 144, 2936–2941. [Google Scholar] [CrossRef]
- Yang, Y.; Cheng, J.; Wang, B.; Guo, Y.; Dong, X.; Zhao, J. An Amino-Modified Metal-Organic Framework (Type UiO-66-NH2) Loaded with Cadmium(II) and Lead(II) Ions for Simultaneous Electrochemical Immunosensing of Triazophos and Thiacloprid. Microchim. Acta 2019, 186, 101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, H.; Chen, M.; Fang, X.; Pang, P.; Wang, H.; Wu, Z.; Yang, W. Ultrasensitive Electrochemical Biosensor for Silver Ion Based on Magnetic Nanoparticles Labeling with Hybridization Chain Reaction Amplification Strategy. Sens. Actuators B Chem. 2017, 249, 431–438. [Google Scholar] [CrossRef]
- Wei, J.; Liu, C.; Wu, T.; Zeng, W.; Hu, B.; Zhou, S.; Wu, L. A Review of Current Status of Ratiometric Molecularly Imprinted Electrochemical Sensors: From Design to Applications. Anal. Chim. Acta 2022, 1230, 340273. [Google Scholar] [CrossRef]
- Fathizadeh, S.; Behnia, S. Control of a DNA Based Piezoelectric Biosensor. J. Phys. Soc. Jpn. 2020, 89, 024004. [Google Scholar] [CrossRef]
- Mészáros, G.; Akbarzadeh, S.; De La Franier, B.; Keresztes, Z.; Thompson, M. Advances in Electromagnetic Piezoelectric Acoustic Sensor Technology for Biosensor-Based Detection. Chemosensors 2021, 9, 58. [Google Scholar] [CrossRef]
- Obořilová, R.; Kučerová, E.; Botka, T.; Vaisocherová-Lísalová, H.; Skládal, P.; Farka, Z. Piezoelectric Biosensor with Dissipation Monitoring Enables the Analysis of Bacterial Lytic Agent Activity. Sci. Rep. 2025, 15, 3419. [Google Scholar] [CrossRef]
- D’Ambrogio, G.; Zahhaf, O.; Le, M.-Q.; Bordet, M.; Lermusiaux, P.; Della Schiava, N.; Liang, R.; Cottinet, P.-J.; Capsal, J.-F. Piezoelectric Biosensor for Smart Cardiovascular Grafts Based on NaNbO3 Fibers/PDMS Structured Composite. Mater. Des. 2022, 223, 111195. [Google Scholar] [CrossRef]
- Morrison, K.; Tincher, M.; Rothchild, A.; Yehl, K. Fingerprinting DNAzyme Cross-Reactivity for Pattern-Based Detection of Heavy Metals. Anal. Chem. 2024, 96, 11780–11789. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Wang, Z.; Fu, Y.; Jiang, J.; Chen, Y.-C.; Liu, T. High-Sensitive Hydrogel Optofluidic Microcavities for Heavy Metal Ion Detection. ACS Sens. 2025, 10, 2330–2338. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhuang, Y.; Guo, S.; Sohan, A.S.M.M.F.; Yin, B. Advances in Microfluidics Techniques for Rapid Detection of Pesticide Residues in Food. Foods 2023, 12, 2868. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Z.; Gao, F.; Song, D.; Lu, X. Selection of Representative Matrices for the Multiresidue Analysis of Pesticides in Tea by GC-MS/MS. Anal. Methods 2018, 10, 855–866. [Google Scholar] [CrossRef]
- Feng, J.; Fan, J.; Zhang, Y.; Ma, H. Preparation and Characterization of In-House Reference Material for Four Organochlorine Pesticides in Tea Matrix. Accredit. Qual. Assur. 2018, 23, 269–275. [Google Scholar] [CrossRef]
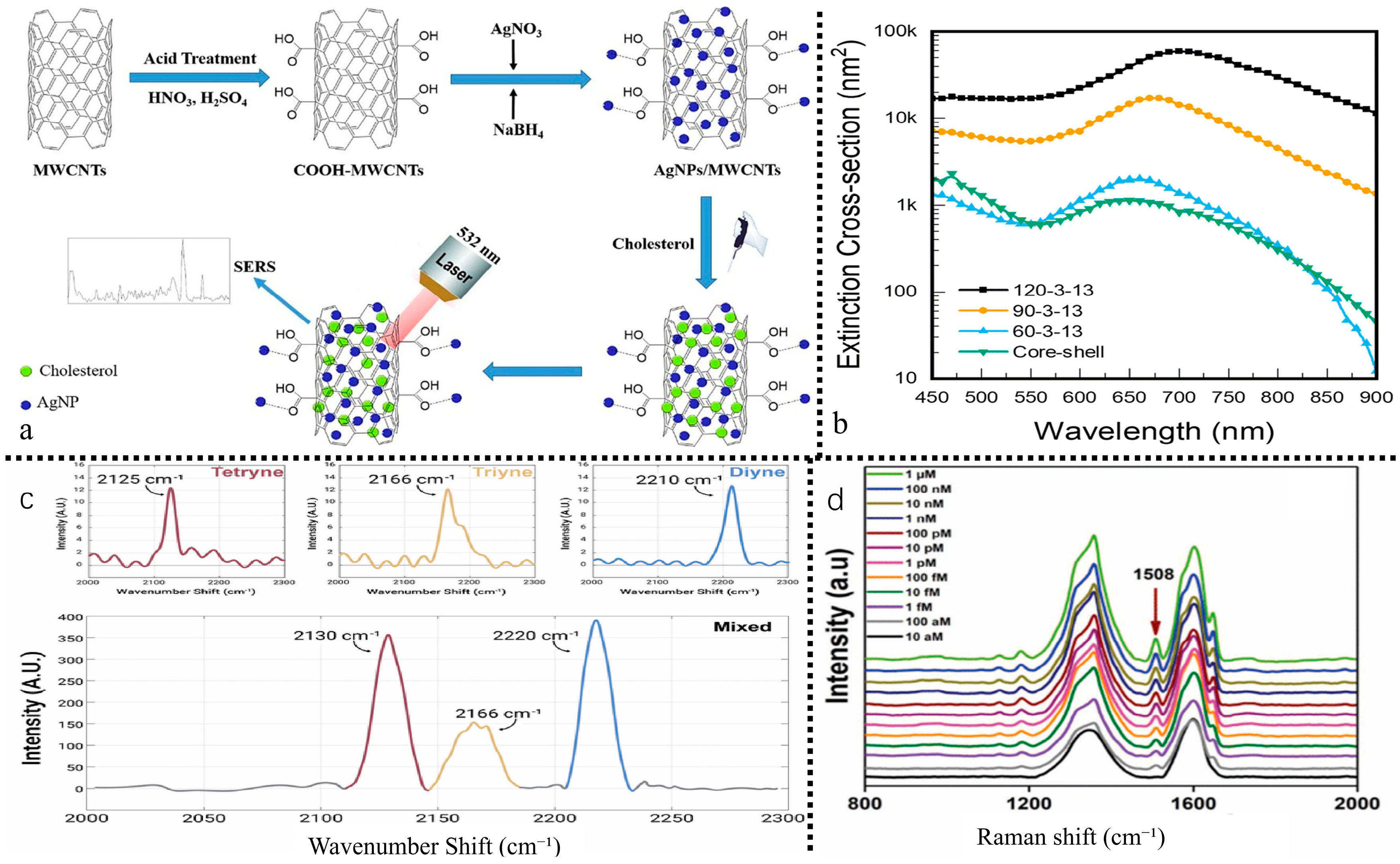
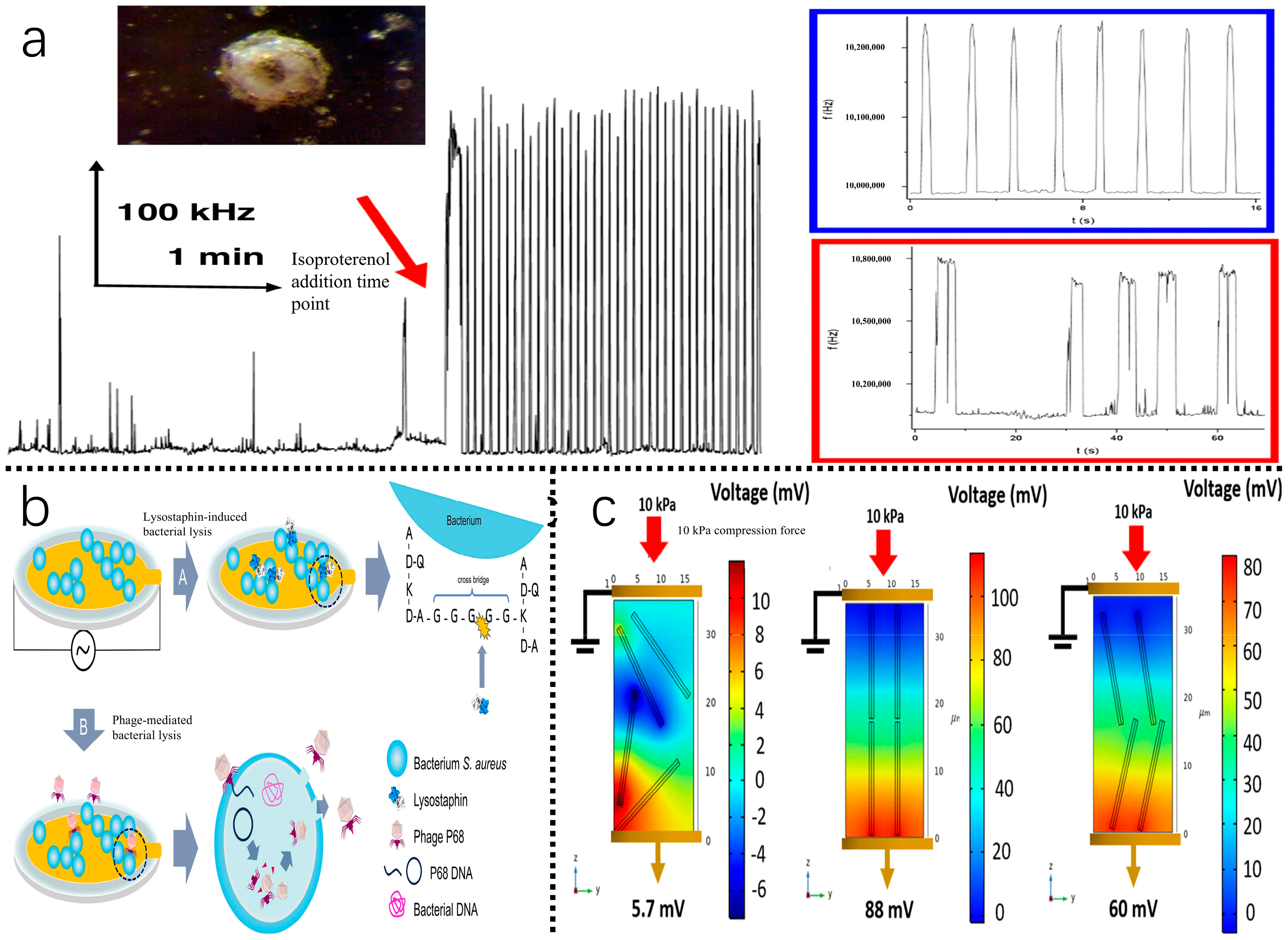
| Category | Specific Name | Positive Rate | Mechanism of Action/Characteristics | Usage Regulation | Pollution Pathway | Reference |
|---|---|---|---|---|---|---|
| Pyrethroids | Bifenthrin | 26.4% | Interferes with insect nerve conduction | Permitted, must comply with GB 2763-2021 standard | Direct pollution (foliar spray) | [23] |
| Fenpropathrin | 21.7% | Interferes with insect nerve conduction | Permitted, must comply with GB 2763-2021 standard | Direct pollution (foliar spray) | [24] | |
| Fenvalerate | - | Interferes with insect nerve conduction | Banned pesticide | Direct pollution (foliar spray) | [20] | |
| Organophosphorus and Carbamates | Omethoate | - | Inhibits acetylcholinesterase activity in the insect nervous system | Restricted use | Exhibits both direct and indirect pollution characteristics | [25] |
| Carbofuran | - | Inhibits acetylcholinesterase activity and interferes with nerve signal transmission in the insect nervous system | Restricted use | Exhibits both direct and indirect pollution characteristics | [26] | |
| Neonicotinoids and Heterocyclics | Imidacloprid | 15.1% | Interferes with the function of nicotinic acetylcholine receptors in the insect nervous system | Permitted, must comply with GB 2763-2021 standard | Exhibits both direct and indirect pollution characteristics | [27] |
| Dinotefuran | 6.67% | Interferes with insect nervous system function | Permitted, must comply with GB 23200.51-2016 standard | Exhibits both direct and indirect pollution characteristics | [28,29] | |
| Fungicides and Herbicides | Carbendazim | 15.1% | Interferes with fungal cell division and metabolic processes | Permitted, must comply with GB 23200.113-2021 standard | Exhibits both direct and indirect pollution characteristics | [30,31] |
| Glyphosate | 9.4% | Inhibits specific enzyme activity in plants, blocking the biosynthesis of aromatic amino acids | Restricted pesticide | Indirect pollution (migration via soil, water) | [32] | |
| Organochlorines | Dicofol | - | Interferes with nervous system function of mites, ultimately leading to death | Banned pesticide | Indirect pollution (migration via soil, water) | [33] |
| DDT | - | Interferes with insect nervous system function | Banned pesticide | Indirect pollution (migration via soil, water) | [34] |
| Classification Dimension | Specific Type | Metal Name | Key Characteristics |
|---|---|---|---|
| Chemical Property and Toxicity Grade | Highly toxic heavy metals | Lead (Pb) | Exposure results in neurotoxicity, anemia, nephrotoxicity, hypertension, and developmental toxicity |
| Cadmium (Cd) | Renal accumulation include irreversible renal damage and bone disease, notably osteoporosis accompanied by severe pain | ||
| Mercury (Hg) | Tremors and cognitive decline, and is a developmental toxicant that causes severe defects upon fetal exposure | ||
| Chromium (Cr6+) | Exposure via inhalation is carcinogenic, oral exposure leads to systemic organ damage, and dermal contact causes skin ulcers | ||
| Metalloid Arsenic (As) | Human carcinogen and a chronic health hazard, associated with skin lesions, neuropathy, and multi-organ toxicity | ||
| Moderately toxic heavy metals | Copper (Cu) | A toxicant capable of inducing gastrointestinal distress, abdominal pain, and, at high doses, hemolytic anemia and organ toxicity | |
| Zinc (Zn) | A disruptor of essential metal metabolism, leading to anemia and impaired immune function | ||
| Nickel (Ni) | Contact dermatitis and an elevated cancer risk from chronic inhalation | ||
| Environmental Migration Characteristics | High mobility heavy metals | Cadmium (Cd) | High mobility and ion exchange enable its efficient uptake by tea roots from soil |
| Zinc (Zn) | Ahigh soil-to-tea plant transfer factor due to efficient root uptake | ||
| Low mobility heavy metals | Lead (Pb) | Immobile in soil, contamination of tea via adhesion and surface deposition | |
| Chromium (Cr) | Low solubility and mobility in soil limit root uptake, posing potential risks through alternative pathways | ||
| Pollution Source Pathway | Primary pollution source | Lead (Pb), etc. | Originating from geological weathering and soil parent materials |
| Secondary pollution source | Cadmium (Cd), Mercury (Hg), Chromium (Cr6+), etc. | An anthropogenic pollutant primarily derived from industrial and agricultural activities |
| Pollutant Name | Tea Production Area Soil Environmental Limit | Screening Value for Soil Risk in Agricultural Land |
|---|---|---|
| Cd | pH ≤ 6.5:0.30; pH > 6.5:0.40 | pH ≤ 5.5:0.3; 5.5 < pH ≤ 6.5:0.3 |
| Pb | pH ≤ 6.5:250; pH > 6.5:300 | pH ≤ 5.5:70; 5.5 < pH ≤ 6.5:90 |
| Hg | pH ≤ 6.5:0.30; pH > 6.5:0.50 | pH ≤ 5.5:1.3; 5.5 < pH ≤ 6.5:1.8 |
| As | pH ≤ 6.5:40; pH > 6.5:30 | pH ≤ 5.5:40; 5.5 < pH ≤ 6.5:49 |
| Cr | pH ≤ 6.5:150; pH > 6.5:200 | pH ≤ 5.5:150; 5.5 < pH ≤ 6.5:150 |
| Detection Method | Linear Range | LOD (μg/L) | Recognition Material | Detection Time (T)/Reproducibility (RSD or CV)/Stability (S) | Real Samples | Equipment Cost | Material Cost | Reference |
|---|---|---|---|---|---|---|---|---|
| Fluorescence | 0.01–10 μg/L (Cd2+) | 0.001 | Cd2+ aptamer | T = 25 min; RSD ≤ 5.0%; tea spiked recovery 88.2–102.5% | Tea, rice | $8000–15,000 | $50–100 | [75] |
| SERS | 0.1–100 μg/L (imidacloprid) | 0.05 | Imidacloprid antibody | T = 5 min; RSD = 6.2%; tea spiked recovery 94.3–101.5% | Tea, fruits/vegetables | $150,000–180,000 | $50–200 | [76] |
| Colorimetric assay | 0.01–50 μg/L (Hg2+) | 0.005 | Horseradish peroxidase | T = 20 min; RSD = 3.8%; tea spiked recovery 95.2–103.7% | Tea, environmental water | $5000–10,000 | $30–80 | [77] |
| SPR | 0.01–1000 μg/L (Cd2+) | 1.25 | Ion-imprinted polymer | T = 15 min; RSD = 5.3%; tea/soil spiked recovery 92.6–100.8% | Tea, soil | $200,000–250,000 | $500–800 | [78] |
| Electrochemical Biosensors | 0.01–100 μg/L (acetamiprid) | 0.005 | Acetamiprid aptamer | T = 20 min; RSD = 4.5%; tea/fruits spiked recovery 89.4–102.1% | Tea, fruits/vegetables | $6000–10,000 | $30–80 | [79] |
| Piezoelectric Biosensors | 0.1–1000 μg/L (carbaryl) | 0.1 | Carbaryl antibody | T = 30 min; RSD = 7.5%; tea/pesticide samples recovery 91.3–101.2% | Tea, pesticide samples | $100,000–150,000 | $150–300 | [80] |
| Sensing Technology | Key Advantages | Key Limitations/Challenges | Typical Targets | Suitable Scenarios |
|---|---|---|---|---|
| Fluorescence | High sensitivity (nM-pM), rapid response, multiplexing potential | Photobleaching, matrix background interference, biotoxicity of some nanomaterials | Cd2+, Hg2+, Organophosphates | Laboratory precision analysis, High-throughput screening |
| SERS | “Fingerprint” specificity, single-molecule level sensitivity | Substrate reproducibility and cost, difficulty in signal quantification | Imidacloprid, other pesticides | Laboratory trace identification and quantification |
| Colorimetric | Visual readout, simple operation, very low cost | Limited sensitivity and accuracy, prone to sample color interference | Hg2+, Cu2+, Organophosphates | On-site rapid preliminary screening, Grassroots testing |
| SPR | Label-free, real-time kinetic monitoring, high precision | Expensive equipment, sensitive to nonspecific adsorption, poor portability | Cd2+, Ni2+, small molecule pesticides | Laboratory molecular interaction studies, High-precision detection |
| Electrochemical | High portability, excellent sensitivity, low cost, easy miniaturization | Electrode modification stability, interference from complex matrices (e.g., tea polyphenols) | Acetamiprid, Neonicotinoids | On-site rapid, portable quantitative detection |
| Piezoelectric | Label-free, real-time, highly sensitive to mass change | Long baseline stabilization time, high instrument cost, vulnerable to environmental vibration | Carbaryl, other pesticides | Laboratory real-time mass change monitoring |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, P.; Chen, M.; Yao, T.; Wu, L.; Wang, S.; Han, Y.; Song, Y.; Yin, J. Research Progress of Biosensors in the Detection of Pesticide Residues and Heavy Metals in Tea Leaves. Biosensors 2025, 15, 778. https://doi.org/10.3390/bios15120778
Li P, Chen M, Yao T, Wu L, Wang S, Han Y, Song Y, Yin J. Research Progress of Biosensors in the Detection of Pesticide Residues and Heavy Metals in Tea Leaves. Biosensors. 2025; 15(12):778. https://doi.org/10.3390/bios15120778
Chicago/Turabian StyleLi, Pin, Miaopeng Chen, Tianle Yao, Long Wu, Shanran Wang, Yu Han, Ying Song, and Jia Yin. 2025. "Research Progress of Biosensors in the Detection of Pesticide Residues and Heavy Metals in Tea Leaves" Biosensors 15, no. 12: 778. https://doi.org/10.3390/bios15120778
APA StyleLi, P., Chen, M., Yao, T., Wu, L., Wang, S., Han, Y., Song, Y., & Yin, J. (2025). Research Progress of Biosensors in the Detection of Pesticide Residues and Heavy Metals in Tea Leaves. Biosensors, 15(12), 778. https://doi.org/10.3390/bios15120778





