Ultra-Sensitive Detection of Mercury by Using Field-Effect Transistor Biosensors Based on Single-Walled Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents and Experimental Materials
2.2. Instruments
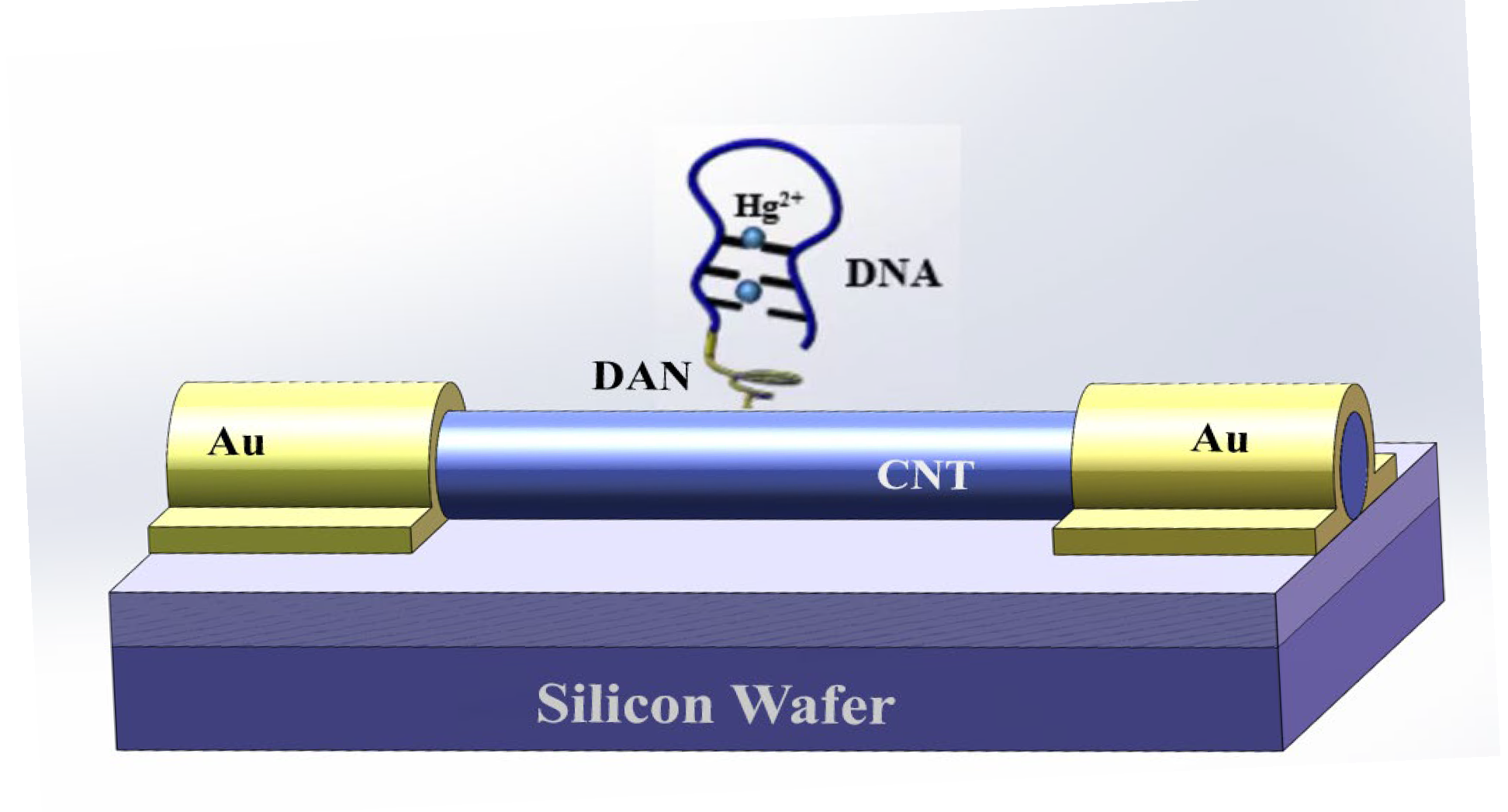
2.3. Principle of the Biosensor
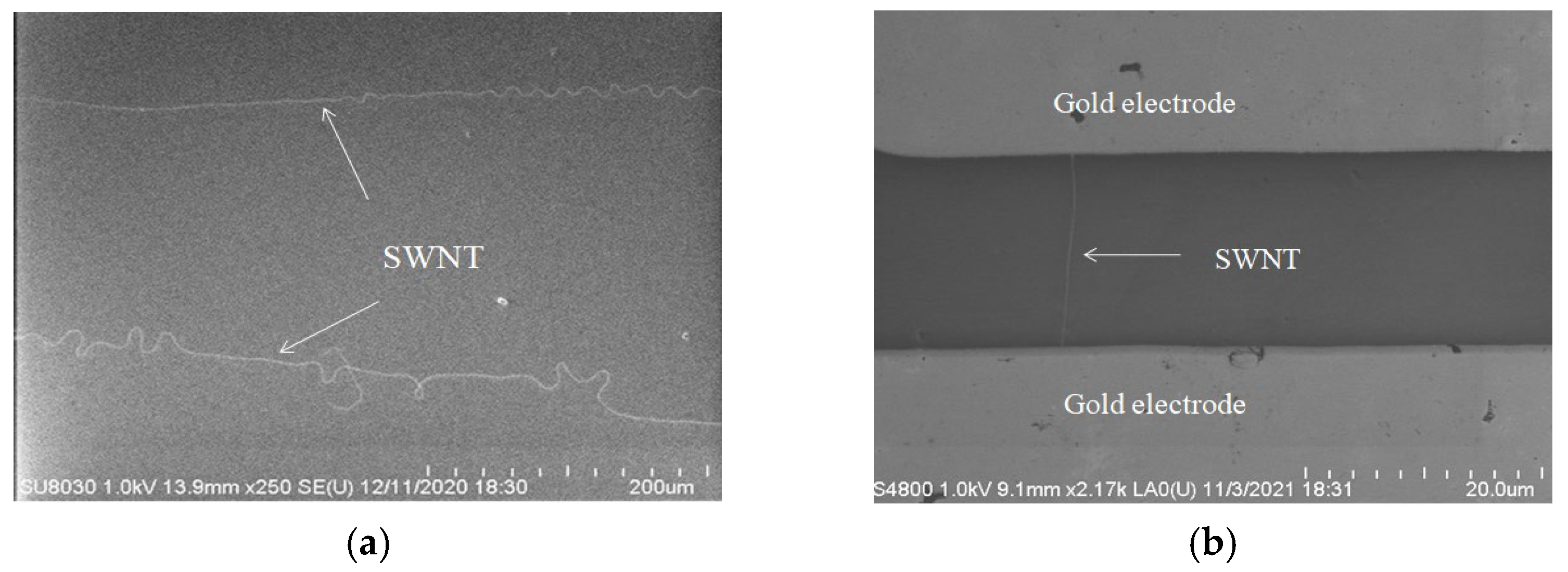
2.4. Single-Walled Carbon Nanotube Preparation
2.5. Thermal Evaporation of Metal Electrode
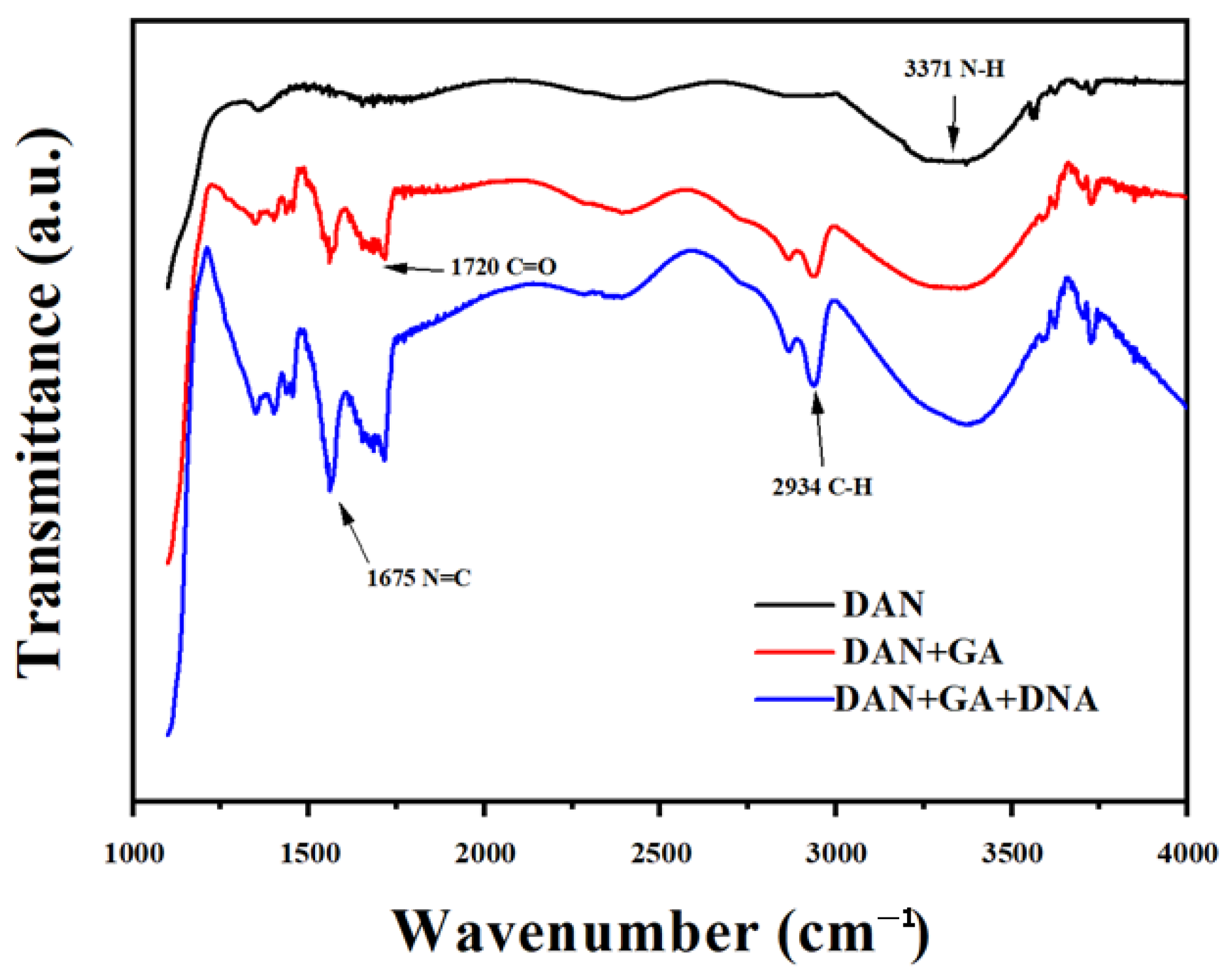
2.6. 1,5-Diaminonaphthalene Connection
2.7. DTT Reaction with Hg2+
3. Results and Discussion
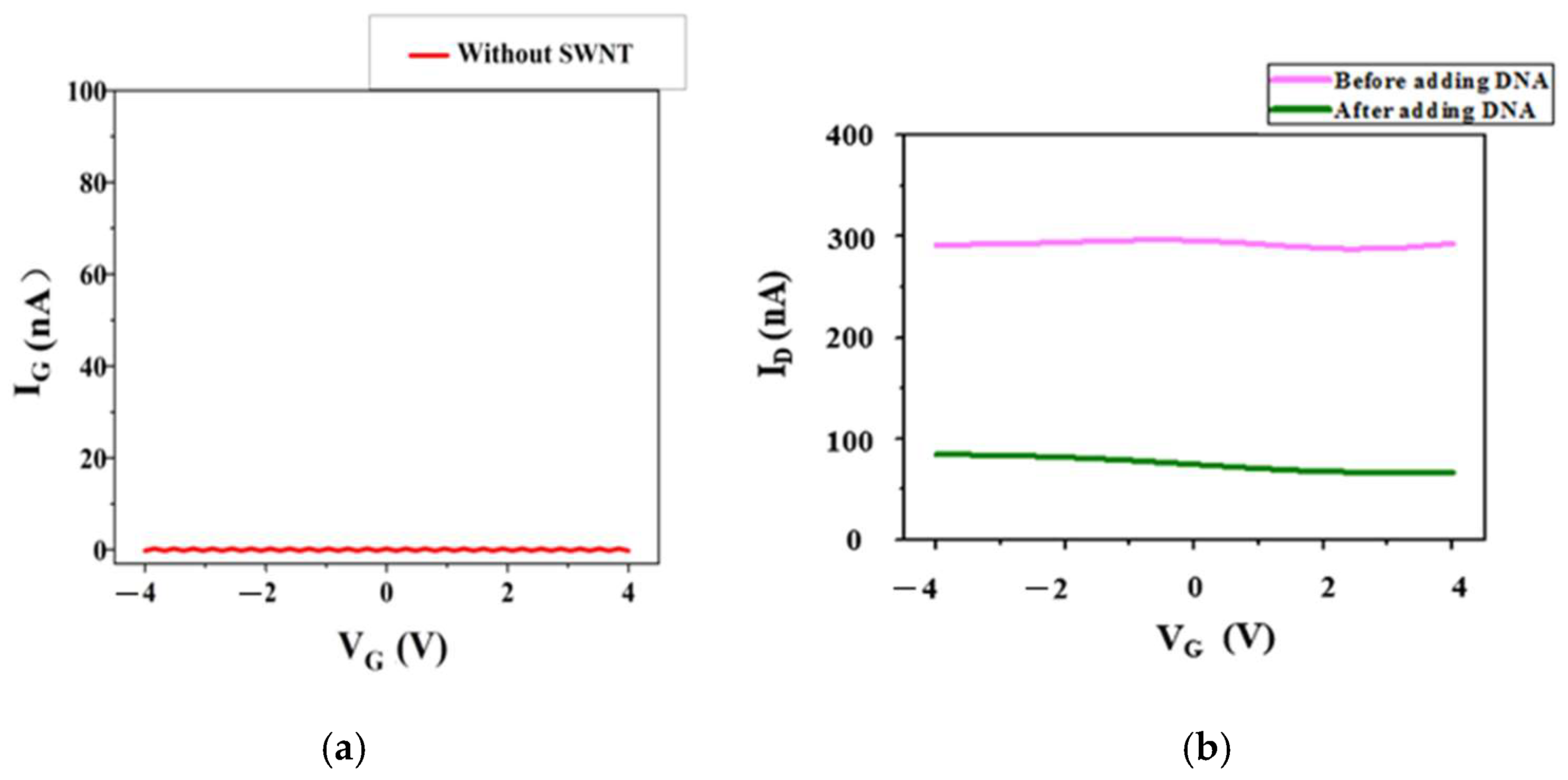
3.1. Current Changes Before and After Connection of DNA
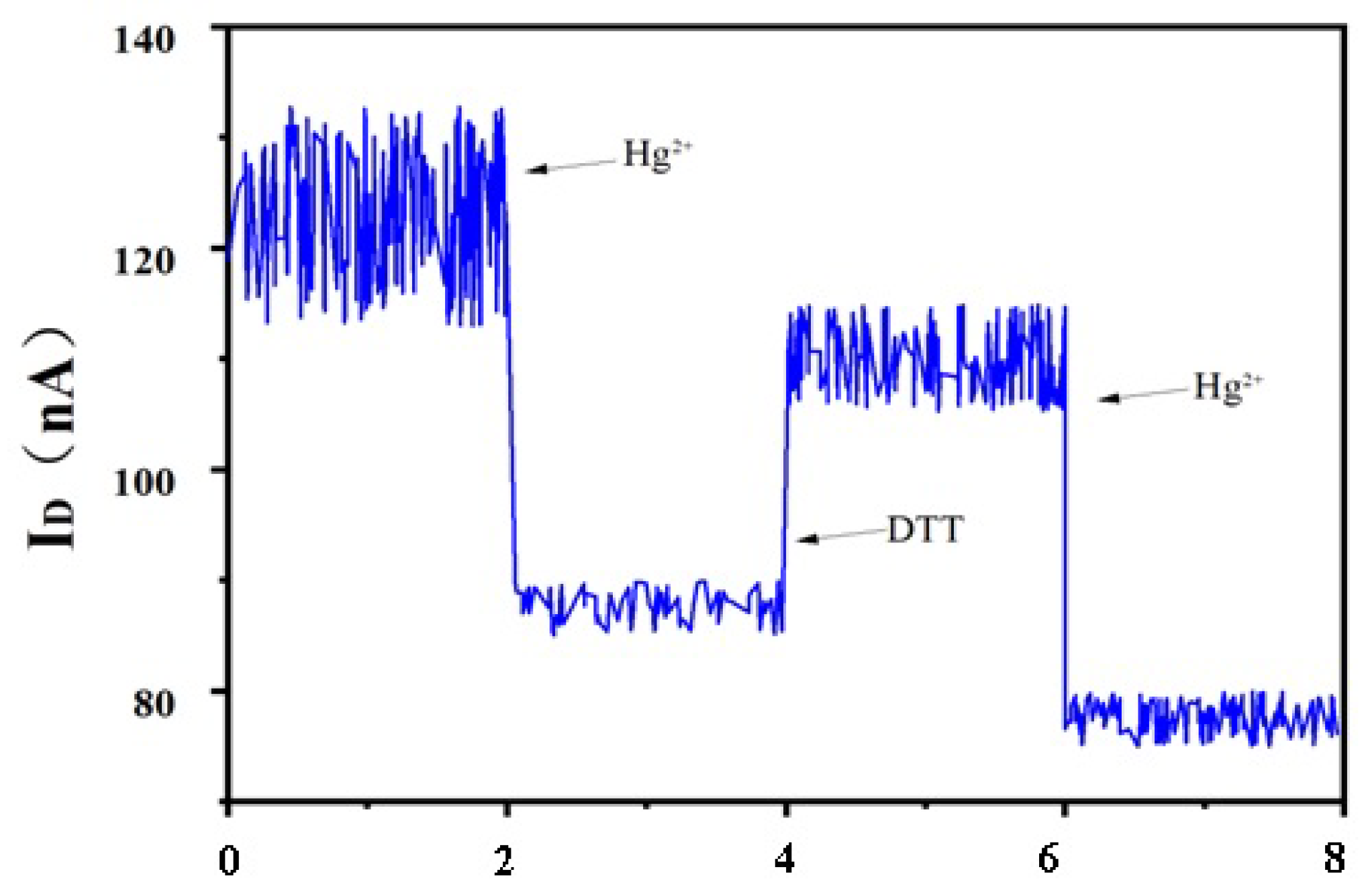
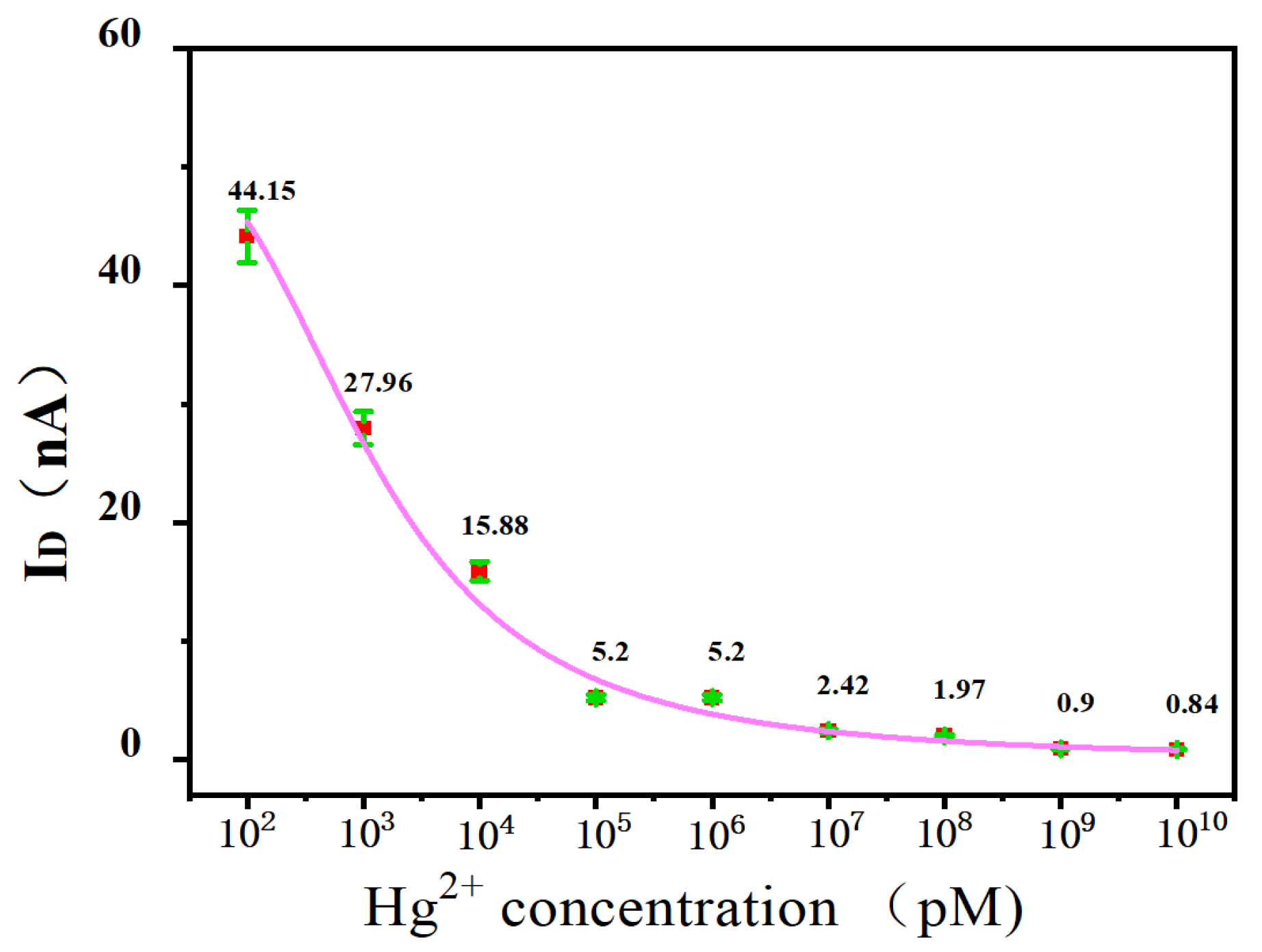
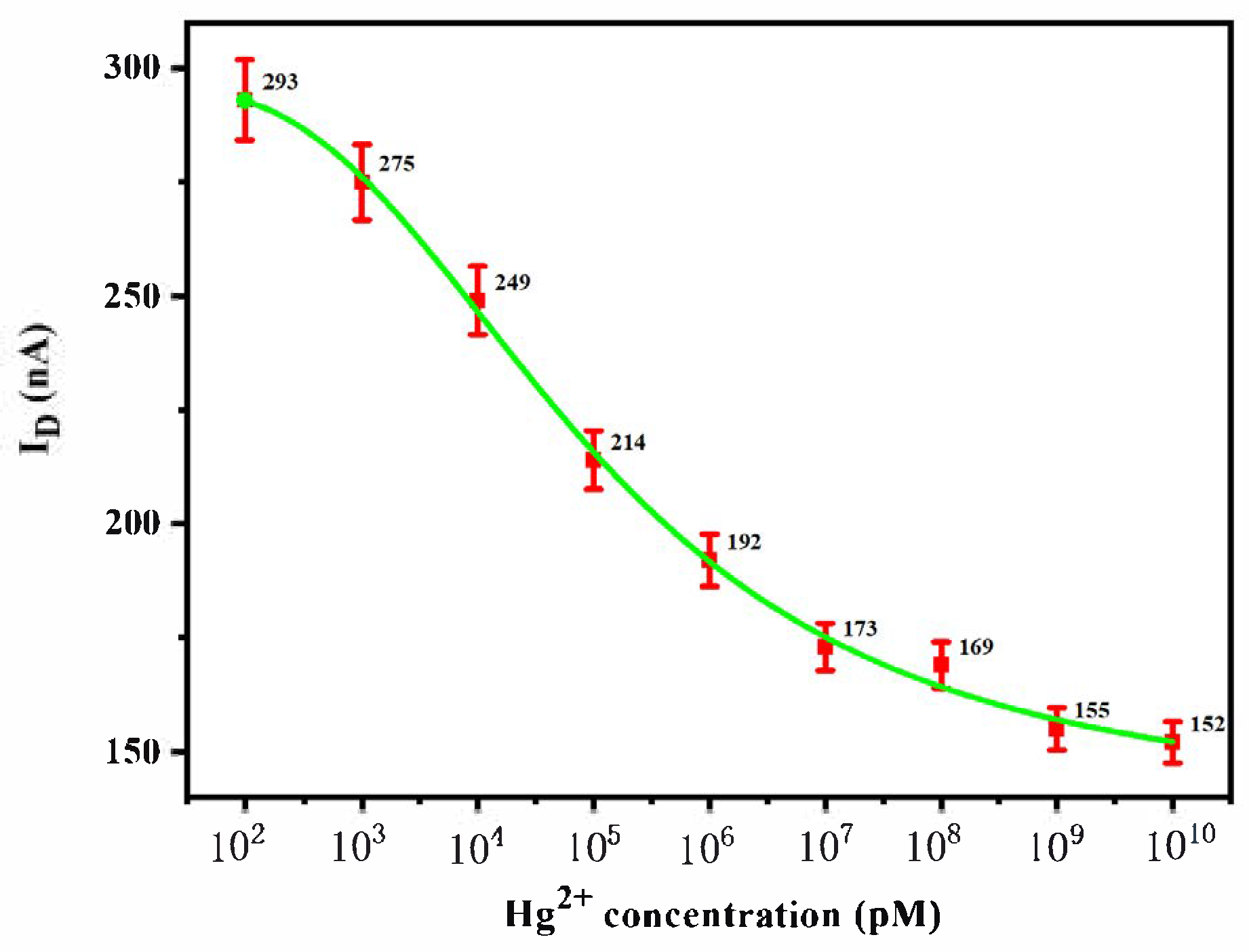
3.2. Hg2+ Sensitivity Detection
3.3. Hg2+ Sensitivity Detection with Phase-Locked Amplifier
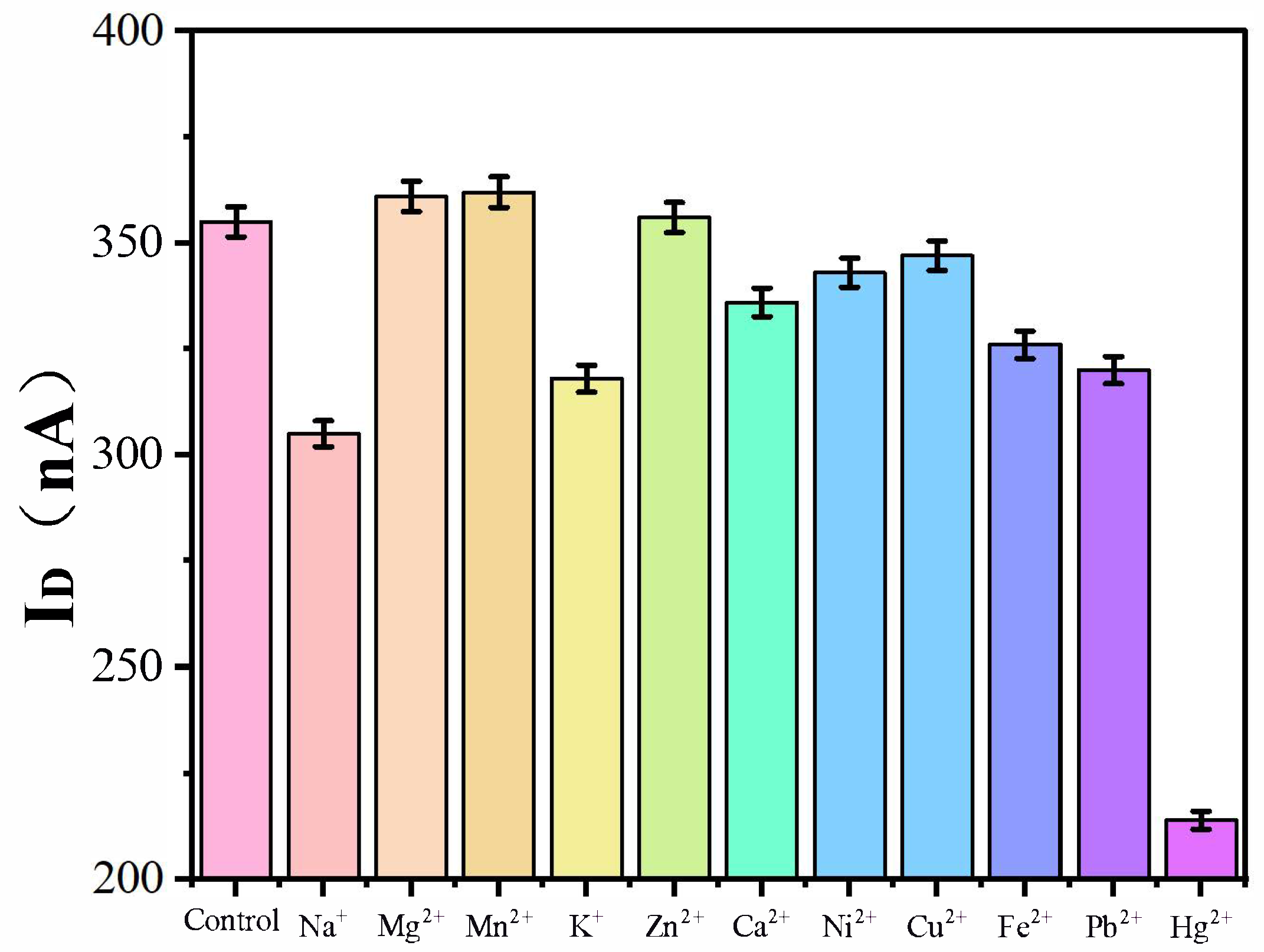
3.4. Selectivity Analysis
3.5. Detection in Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xing, X.; Zhang, Y.; Tan, Y.; Hu, T.; Chen, Z.; Liu, B. A multi-Well SERS chip for the detection of Hg2+. ACS Appl. Appl. Appl. Nano Material 2024, 7, 17287–17294. [Google Scholar] [CrossRef]
- Yu, X.; Chang, W.; Cai, Z.; Yu, C.; Lai, L.; Zhou, Z.; Li, P.; Yang, Y.; Zeng, C. Hg2+detection and information encryption of new [1+1] lanthanide cluster. Talanta 2024, 26, 125105. [Google Scholar] [CrossRef]
- Hu, P.; Liu, J.; Xia, C.; Liu, B.; Zhu, H.; Niu, X. Matrix redox interference-free nanozyme-amplified detection of Hg2+ using thiol-modified phosphatase-mimetic nanoceria. Sens. Actuators B-Chem. 2024, 401, 135030. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Hu, C.; Li, S.; Liu, Y.; Chen, Z.; Li, S.; Chen, H.; Sami, R.; Deng, Y. Electrochemical aptasensor based on black phosphorus-porous graphene nanocomposites for high-performance detection of Hg2+. Chin. Chem. Lett. 2024, 35, 109561. [Google Scholar] [CrossRef]
- European Parliament and Council of the European Union, Directive 2010/75/EU of the European Parliament and Council of 24 November 2010 on Industrial Emissions (Integrated Pollution Prevention and Control) (Recast). Available online: https://www.legislation.gov.uk/eudr/2010/75 (accessed on 10 October 2025).
- Xia, N.; Feng, F.; Liu, C.; Li, R.; Xiang, W.; Shi, H.; Gao, L. The detection of mercury ion using DNA as sensors based on fluorescence resonance energy transfer. Talanta 2019, 192, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; He, M.; Chen, B.; Hu, B. Facile green synthesis of magnetic porous organic polymers for fast preconcentration of trace lead andmercuryfrom environmental water followed by graphite urnace atomic absorption spectrometry detection. Spectrochim. Acta Part B-At. Spectrosc. 2022, 196, 106524. [Google Scholar] [CrossRef]
- Yang, H.; Qi, L.; Zhou, J.; Li, Q.; Yuan, X.; Zhang, M.; He, Y.; Huang, K.; Chen, P. Metal ions-regulated chemical vapor generation of Hg2+: Mechanism and application in miniaturized point discharge atomic emission spectrometry assay of oxalate in clinical urolithiasis samples. Anal. Chim. Acta 2023, 1262, 341223. [Google Scholar] [CrossRef] [PubMed]
- Ilmiah, K.; Sumranjit, J.; Wutikhun, T.; Siripinyanond, A. Tracking silver nanoparticles during their synthesis by inductively coupled plasmamassspectrometry: Implications for colorimetric sensing of mercury ions. ACS Appl. Nano Mater. 2023, 6, 1250–1260. [Google Scholar] [CrossRef]
- Gao, L.; Lv, Q.; Xia, N.; Lin, Y.; Lin, F.; Han, B. Detection of mercury ion with high sensitivity and selectivity using a DNA/graphene oxide hybrid immobilized on glass slides. Biosensors 2021, 11, 300. [Google Scholar] [CrossRef]
- Ouyang, J.; Li, Y.; Yang, F.; Wu, X.; Qiu, Z.; Shu, J. Electrochemical and field effect transistor dual-mode biosensor chip for label-free detection of cytokine storm biomarker with high sensitivity within a wide range. Adv. Funct. Mater. 2024, 34, 2405212. [Google Scholar] [CrossRef]
- Howe, L.; Wang, Y.; Ellepola, K.; Ho, V.; Dohmen, R.; Pinto, M.; Hoff, W.; Cooney, M.; Vinh, N. Interfacial photogating of graphene field-effect transistor for photosensorybiomolecular detection. Adv. Electron. Mater. 2025, 11, 2400716. [Google Scholar] [CrossRef]
- Li, D.; Ren, Y.; Chen, R.; Wu, H.; Zhuang, S.; Zhang, M. Label-free MXene-assisted field effect transistor for the determination of IL-6 in patients with kidney transplantation infection. Microchim. Acta 2023, 190, 284. [Google Scholar] [CrossRef]
- Guo, L.; Zhang, Q.; Chan, K. A dual-gate field-effect transistor in graphene heterojunctions. Superlattices Microstruct. 2021, 150, 106778. [Google Scholar] [CrossRef]
- Hu, J.; Liu, X.; Li, F.; Qiu, Y.; Hu, Y.; Zhou, Y.; Wang, P.; Wan, H. Ultrasensitive graphene field-effect transistor biosensor for rapidly detecting miRNA-208a. Sens. Actuators B-Chem. 2024, 418, 136262. [Google Scholar] [CrossRef]
- Gwyther, R.; Côté, S.; Lee, C.; Miao, H.; Ramakrishnan, K.; Palma, M.; Jones, D. Optimising CNT-FET biosensor design through modelling of biomolecular electrostatic gating and its application to β-lactamase detection. Nat. Commun. 2024, 15, 7482. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Wei, C.; Li, Y.; Peng, Z.; Chuang, H.; Pearce, L.; Boon, A.; Huang, Y.; Kim, D.; et al. Advanced detection of SARS-CoV-2 and omicron variants via MXene-graphene hybrid biosensors utilizing nucleic acid probes. ACS Appl. Nano Mater. 2024, 24, 28255–28272. [Google Scholar] [CrossRef]
- Huang, K.; Geng, Y.; Zhang, X.; Chen, D.; Cai, Z.; Wang, M.; Zhu, Z.; Wang, Z. A wide-band digital lock-in amplifier and its application in microfluidic impedance measurement. Sensors 2019, 19, 3519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, Z.; Li, Y.; Li, S.; Lin, T. A lock-in amplifier modeling recovery method to extract the surface nuclear magnetic resonance signal from residual noise. Rev. Sci. Instrum. 2019, 90, 114710. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhu, R.; Zhang, X.; Du, C.; Chen, J. Ultrasensitive detection and efficient removal of mercury ions based on covalent organic framework spheres with double active sites. Anal. Chim. Acta 2023, 1278, 341751. [Google Scholar] [CrossRef]
- Xing, Y.; Han, J.; Wu, X.; Pierce, D.; Zhao, J. Aggregation-based determination of mercury (II) using DNA-modified single gold nanoparticle, T-Hg (II)-T interaction, and single-particle ICP-MS. Microchim. Acta 2019, 187, 56. [Google Scholar] [CrossRef]
- Tu, J.; Gan, Y.; Liang, T.; Hu, Q.; Wang, Q.; Ren, T.; Sun, Q.; Wan, H.; Wang, P. Graphene FET array biosensor based on ssDNA aptamer for ultrasensitive Hg2+ detection in environmental pollutants. Front. Chem. 2018, 6, 333. [Google Scholar] [CrossRef] [PubMed]
- Dang, W.; Li, Y.; Zhang, J. Highly sensitive detection of Hg2+ based on imprinting sensor modified DNA. IEEE Sens. J. 2024, 24, 23369–23375. [Google Scholar] [CrossRef]
- Wang, M.; Guan, J.; Liu, S.; Chen, K.; Gao, Z.; Liu, Q.; Chen, X. Dual-ligand lanthanide metal-organic framework probe for ratiometric fluorescence detection of mercury ions in wastewater. Microchim. Acta 2023, 190, 359. [Google Scholar] [CrossRef]
- Gao, L.; Liu, C.; Li, R.; Xia, N.; Xiong, Y. Highly sensitive detection of Hg2+ using covalent linking single-strand DNA to the surface of graphene oxide with co-anchor strand. Anal. Methods 2019, 11, 4416–4420. [Google Scholar] [CrossRef]
- Hu, H.; Yin, Z.; Cui, H.; Xiong, W.; Yu, F.; Zhang, J.; Liao, F.; Wei, G.; Yang, L.; Zhang, J.; et al. A novel dual-detection electrochemiluminescence sensor for the selective detection of Hg2+ and Zn2+: Signal suppression and activation mechanisms. Anal. Chim. Acta 2024, 1330, 343283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deng, Z.; Feng, H.; Shao, B.; Liu, D. A multifunctional fluorescent sensor for Ag+ and Hg2+ detection in seawater. Environ. Monit. Assess. 2024, 196, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Feng, J.; Tan, Z.; Wang, H. Electrochemical impedance spectroscopy aptasensor for ultrasensitive detection of adenosine with dual backfillers. Biosens. Bioelectron. 2014, 60, 218–223. [Google Scholar] [CrossRef]
| Method | Linear Range | DD OD | Reference |
|---|---|---|---|
| Imprinting sensor | 0.01~100,000 nM | 0.006 nM | [23] |
| Fluorescence sensor using metal–organic framework probe | 10–60 nM | 1.62 nM | [24] |
| Fluorescence sensor based on graphene oxide sensor | 2~ 20 μM | 40 nM | [25] |
| Electrochemiluminescence sensor | 0.02 μM~0.1μM | 2.52 nM | [26] |
| Fluorescent sensor using gold nanoparticles | 1 uM~1nM | 4.71 nM | [27] |
| Electrochemical impedance spectroscopy aptasensor | 100–900 nM | 5.59 nM | [28] |
| SWNT FET sensor | 0.1~100 nM | 5.14pM | This study |
| Samples | Added (nM) | Obtained (nM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| 1 | 0.1 | 0.102 | 102.05 | 4.22 |
| 2 | 1 | 0.98 | 97.81 | 3.08 |
| 3 | 10 | 9.60 | 95.98 | 5.06 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, C.; Lv, Q.; Lin, Y.; Gao, L. Ultra-Sensitive Detection of Mercury by Using Field-Effect Transistor Biosensors Based on Single-Walled Carbon Nanotubes. Biosensors 2025, 15, 779. https://doi.org/10.3390/bios15120779
Lu C, Lv Q, Lin Y, Gao L. Ultra-Sensitive Detection of Mercury by Using Field-Effect Transistor Biosensors Based on Single-Walled Carbon Nanotubes. Biosensors. 2025; 15(12):779. https://doi.org/10.3390/bios15120779
Chicago/Turabian StyleLu, Chao, Qiuxiang Lv, Yuanwei Lin, and Li Gao. 2025. "Ultra-Sensitive Detection of Mercury by Using Field-Effect Transistor Biosensors Based on Single-Walled Carbon Nanotubes" Biosensors 15, no. 12: 779. https://doi.org/10.3390/bios15120779
APA StyleLu, C., Lv, Q., Lin, Y., & Gao, L. (2025). Ultra-Sensitive Detection of Mercury by Using Field-Effect Transistor Biosensors Based on Single-Walled Carbon Nanotubes. Biosensors, 15(12), 779. https://doi.org/10.3390/bios15120779





