Aptamer-Based Gold Nanoparticle Lateral Flow Assay for Rapid Detection of Cardiac Troponin I
Abstract
1. Introduction
2. Materials and Methods
2.1. General Materials
2.2. Synthesis, Conjugation, and Characterization of AuNPs@Tro4
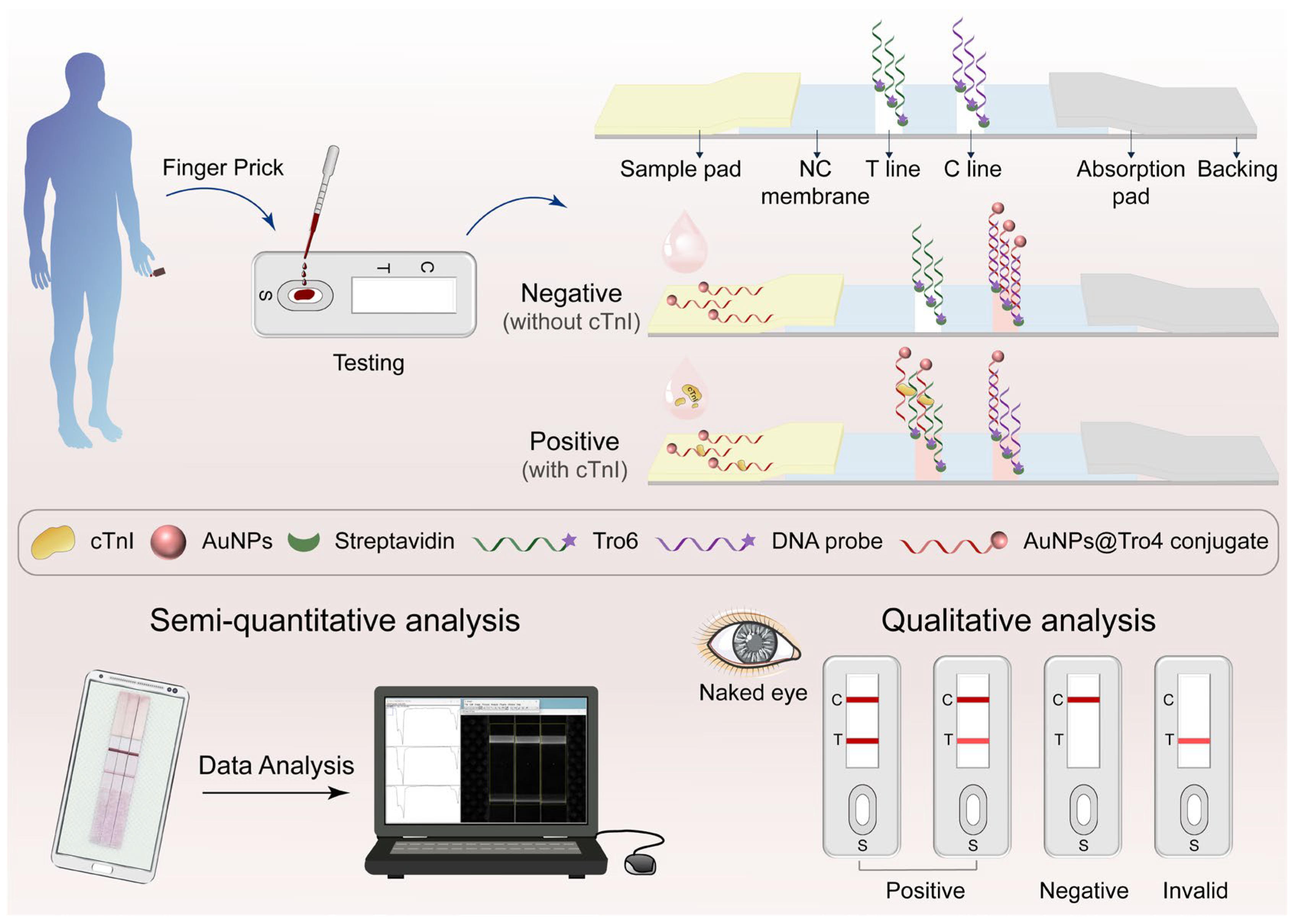
2.3. Preparation and Assembly of Aptamer-Based LFA
2.4. Aptamer-Based LFAs for cTnI Detection
3. Results and Discussion
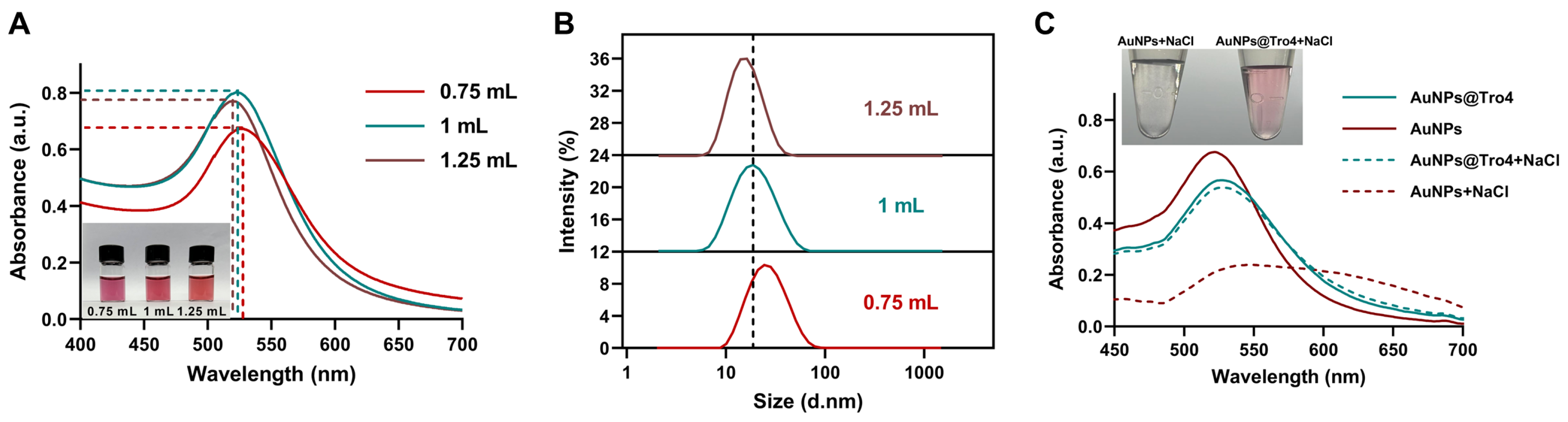
3.1. Characterization of AuNPs and AuNPs@Tro4
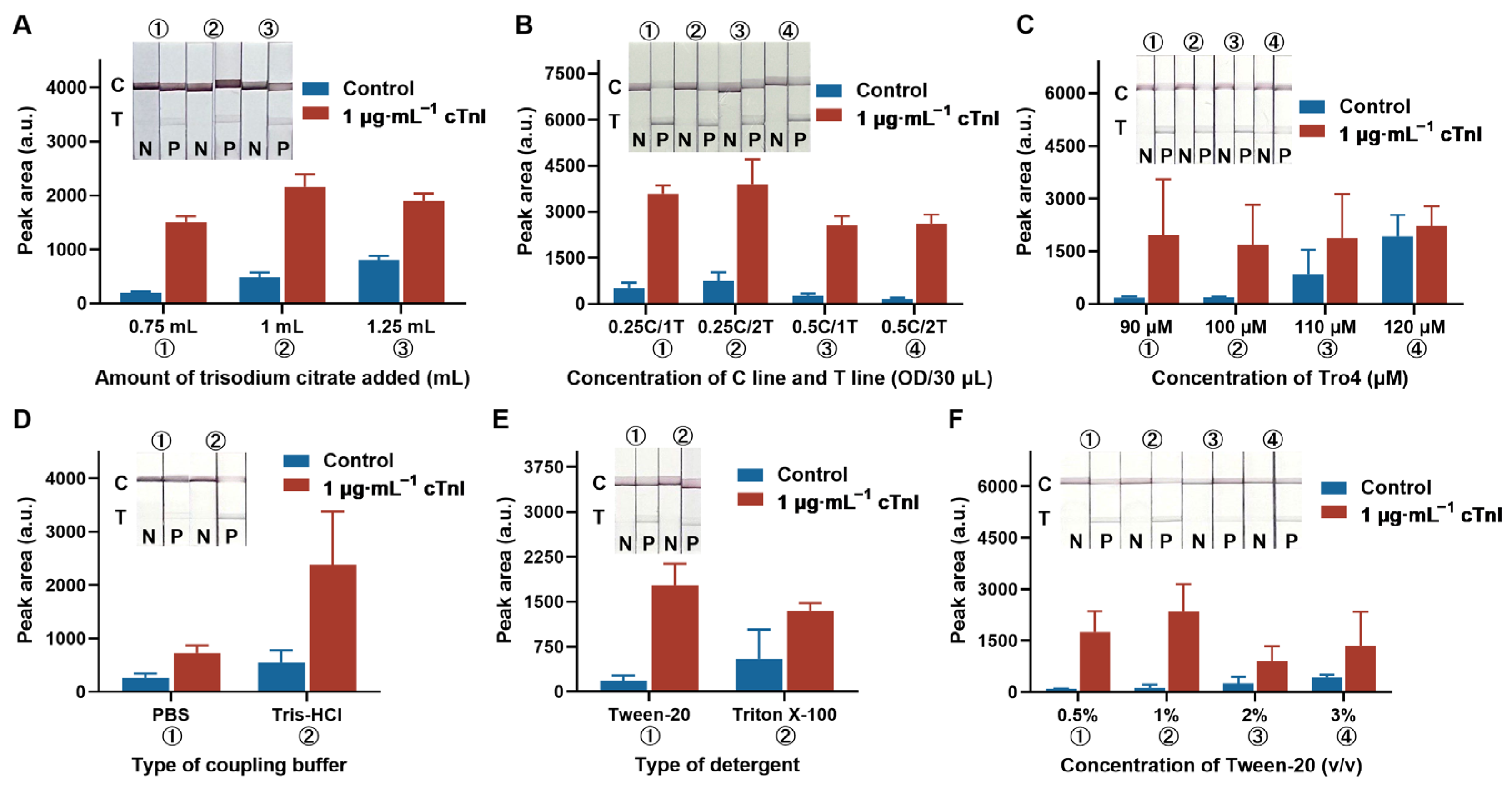
3.2. Optimization of Detection Parameters
3.3. Analytical Performance of the Proposed LFA
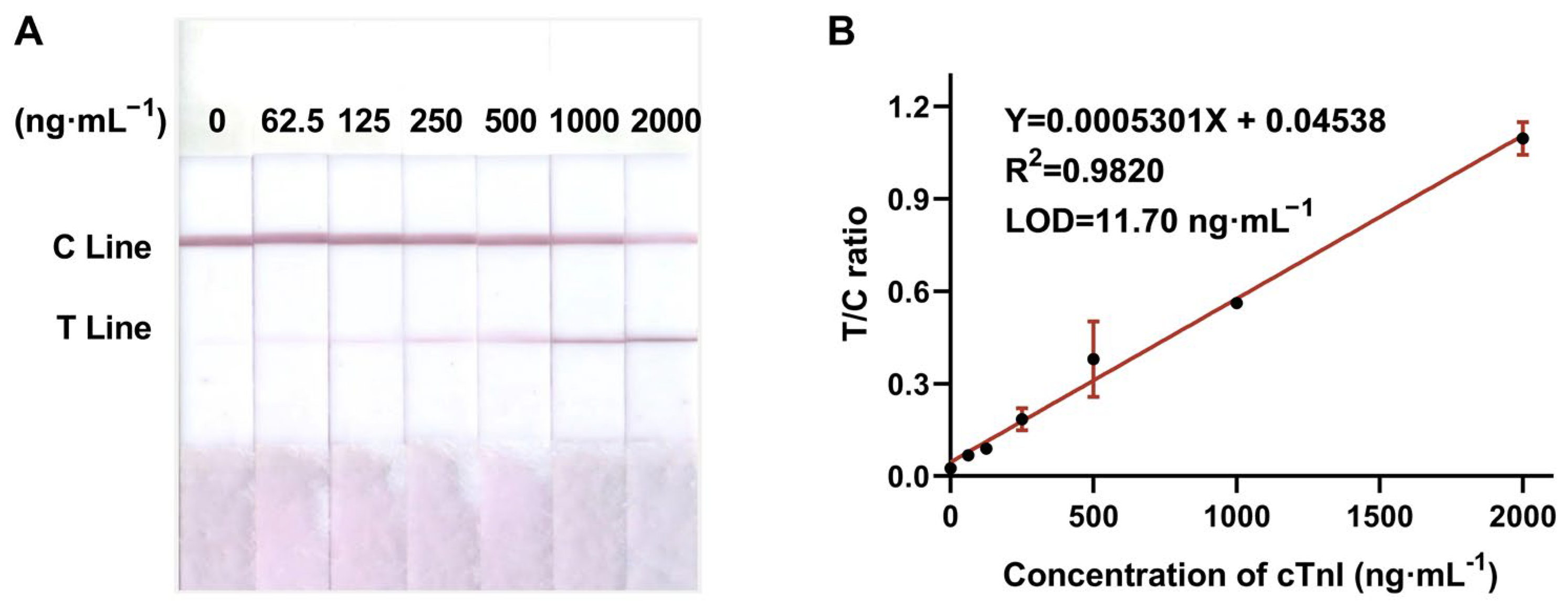
3.3.1. Sensitivity
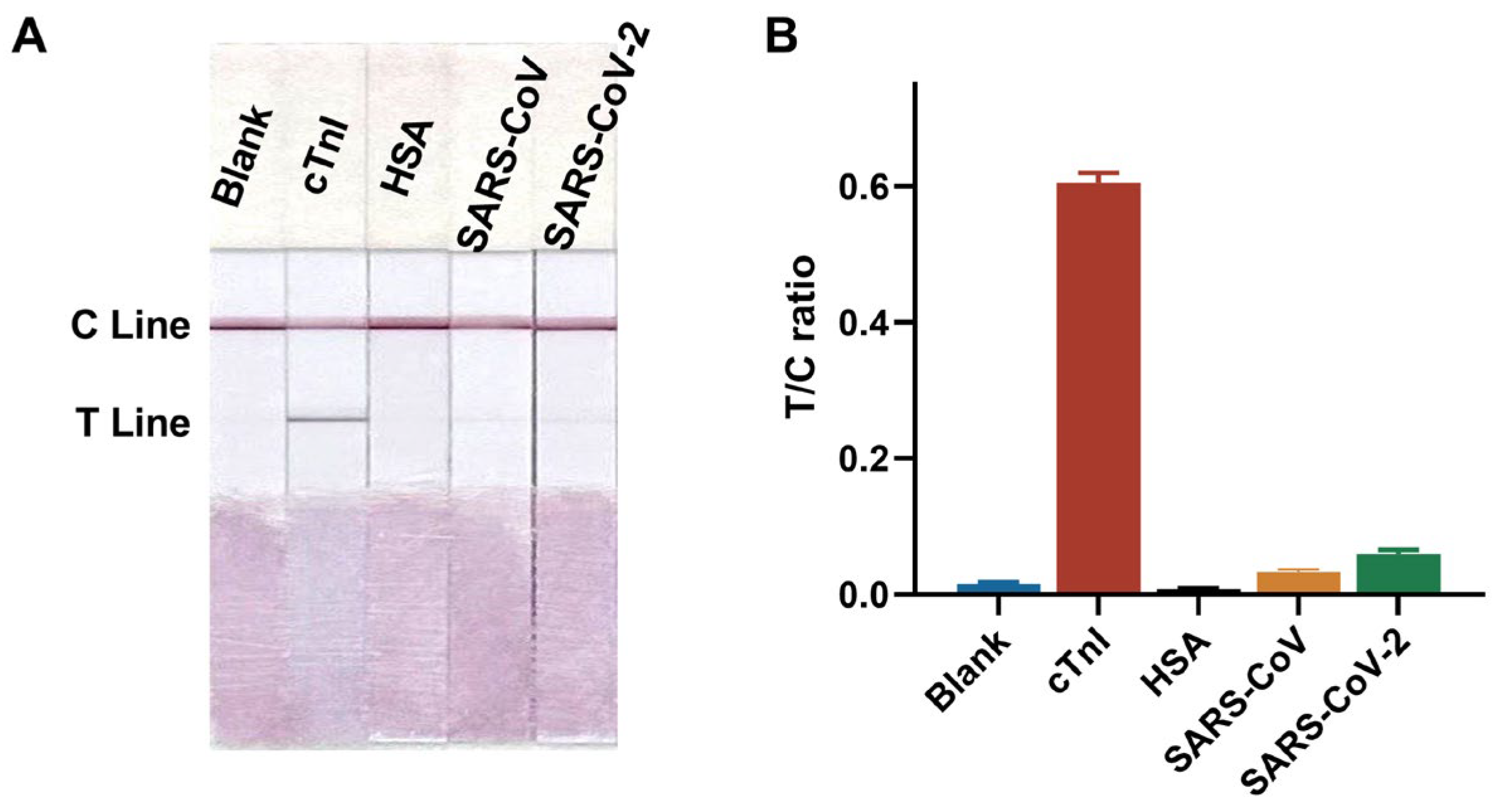
3.3.2. Specificity
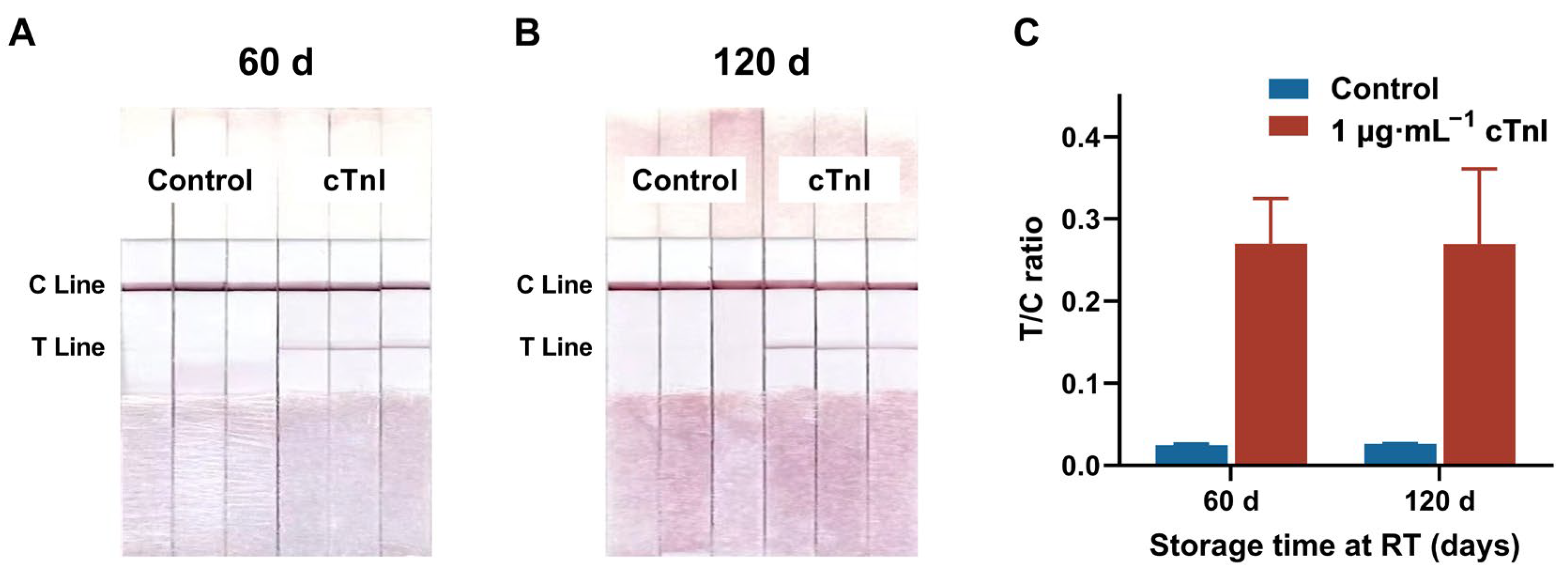
3.3.3. Repeatability and Stability
3.3.4. Accuracy and Precision
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.H.; Liu, J.J.; Qin, X.T.; Nie, X.; Dong, Y.F.; Liang, X.; Zhu, Z.W.; Yang, D.; Shao, Y.H. Electrochemiluminescence detection of cardiac troponin I based on Au-Ag alloy nanourchins. Analyst 2020, 145, 873–879. [Google Scholar] [CrossRef]
- Tuteja, S.K.; Chen, R.; Kukkar, M.; Song, C.K.; Mutreja, R.; Singh, S.; Paul, A.K.; Lee, H.; Kim, K.H.; Deep, A.; et al. A label-free electrochemical immunosensor for the detection of cardiac marker using graphene quantum dots (GQDs). Biosens. Bioelectron. 2016, 86, 548–556. [Google Scholar] [CrossRef]
- Fathil, M.F.M.; Arshad, M.K.M.; Gopinath, S.C.B.; Hashim, U.; Adzhri, R.; Ayub, R.M.; Ruslinda, A.R.; Nuzaihan, M.N.M.; Azman, A.H.; Zaki, M.; et al. Diagnostics on acute myocardial infarction: Cardiac troponin biomarkers. Biosens. Bioelectron. 2015, 70, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Park, K.C.; Gaze, D.C.; Collinson, P.O.; Marber, M.S. Cardiac troponins: From myocardial infarction to chronic disease. Cardiovasc. Res. 2017, 113, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Chekin, F.; Vasilescu, A.; Jijie, R.; Singh, S.K.; Kurungot, S.; Iancu, M.; Badea, G.; Boukherroub, R.; Szunerits, S. Sensitive electrochemical detection of cardiac troponin I in serum and saliva by nitrogen-doped porous reduced graphene oxide electrode. Sens. Actuators B Chem. 2018, 262, 180–187. [Google Scholar] [CrossRef]
- Szunerits, S.; Mishyn, V.; Grabowska, I.; Boukherroub, R. Electrochemical cardiovascular platforms: Current state of the art and beyond. Biosens. Bioelectron. 2019, 131, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Lucia, P.; Coppola, A.; Manetti, L.L.; Sebastiani, M.L.; Colliardo, A.; Cerroni, F.; Martinis, C.D.; Strappini, P.M. Cardiac troponin I in acute coronary ischemic syndromes. Epidemiological and clinical correlates. Int. J. Cardiol. 2001, 77, 215–222. [Google Scholar] [CrossRef]
- Char, D.M.; Israel, E.; Ladenson, J. Early laboratory indicators of acute myocardial infarction. Emerg. Med. Clin. N. Am. 1998, 16, 519–539. [Google Scholar] [CrossRef]
- Mair, J.; Lindahl, B.; Hammarsten, O.; Muller, C.; Giannitsis, E.; Huber, K.; Mockel, M.; Plebani, M.; Thygesen, K.; Jaffe, A.S. How is cardiac troponin released from injured myocardium? Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 553–560. [Google Scholar] [CrossRef]
- Willeit, P.; Welsh, P.; Evans, J.D.W.; Tschiderer, L.; Boachie, C.; Jukema, J.W.; Ford, I.; Trompet, S.; Stott, D.J.; Kearney, P.M.; et al. High-sensitivity cardiac troponin concentration and risk of first-ever cardiovascular outcomes in 154,052 participants. J. Am. Coll. Cardiol. 2017, 70, 558–568. [Google Scholar] [CrossRef]
- Zeng, L.Z.; Lin, C.J.; Liu, P.Q.; Sun, D.P.; Lu, J. Anisotropic aptamer-modified DNA tetrahedra/MOF nanoprobes for enhanced colorimetric aptasensing of cardiac troponin I. Chem. Eng. J. 2023, 474, 145525. [Google Scholar] [CrossRef]
- Zhong, L.L.; Gong, F.P.; Li, J.; Liu, G.Z.; Wen, X.; Yu, F.; Hou, C.J.; Yang, S.; Zhu, F.Y.; Wang, X.F. Synthesis of Cu(II)-loaded polydopamine to construct fluorescence sensor for detection of cTnI from myocardial infarction. Sens. Actuators B Chem. 2025, 435, 137649. [Google Scholar] [CrossRef]
- Pakkila, H.; Malmi, E.; Lahtinen, S.; Soukka, T. Rapid homogeneous immunoassay for cardiac troponin I using switchable lanthanide luminescence. Biosens. Bioelectron. 2014, 62, 201–207. [Google Scholar] [CrossRef]
- Tannenberg, R.; Paul, M.; Röder, B.; Gande, S.L.; Sreeramulu, S.; Saxena, K.; Richter, C.; Schwalbe, H.; Swart, C.; Weller, M.G. Chemiluminescence biosensor for the determination of cardiac troponin I (cTnI). Biosensors 2023, 13, 455. [Google Scholar] [CrossRef]
- Villalonga, A.; Estabiel, I.; Pérez-Calabuig, A.M.; Mayol, B.; Parrado, C.; Villalonga, R. Amperometric aptasensor with sandwich-type architecture for troponin I based on carboxyethylsilanetriol-modified graphene oxide coated electrodes. Biosens. Bioelectron. 2021, 183, 113203. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.F.; Wu, Q.; Song, D.Q.; Wang, X.H.; Ma, P.Y.; Sun, Y. Fe3O4@PDA immune probe-based signal amplification in surface plasmon resonance (SPR) biosensing of human cardiac troponin I. Colloids Surf. B Biointerfaces 2019, 177, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, W.B.; Yang, Q.H.; Gong, X.Q.; Guo, W.S.; Dong, C.H.; Liu, J.Q.; Xuan, L.X.; Chang, J. Rapid and quantitative detection of prostate specific antigen with a quantum dot nanobeads-based immunochromatography test strip. ACS Appl. Mater. Interfaces 2014, 6, 6406–6414. [Google Scholar] [CrossRef] [PubMed]
- Fenwick, C.; Croxatto, A.; Coste, A.T.; Pojer, F.; André, C.; Pellaton, C.; Farina, A.; Campos, J.; Hacker, D.; Lau, K.; et al. Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence studies. J. Virol. 2021, 95, e01828-20. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ren, X.L.; Chen, L.F.; Zou, J.; Li, T.; Tan, L.F.; Fu, C.H.; Wu, Q.; Li, C.H.; Wang, J.Y.; et al. Fluorescent hollow ZrO2@CdTe nanoparticles based lateral flow assay for simultaneous detection of C-reactive protein and troponin T. Microchim. Acta 2021, 188, 209. [Google Scholar] [CrossRef]
- Xin, Y.R.; Yang, R.L.; Qu, Y.; Liu, H.F.; Feng, Y.S.; Li, L.; Shi, W.J.; Liu, Q. Novel, highly sensitive, and specific assay to monitor acute myocardial infarction (AMI) by the determination of cardiac troponin I (cTnI) and heart-type fatty acid binding protein (H-FABP) by a colloidal gold-based immunochromatographic test strip. Anal. Lett. 2020, 54, 1329–1350. [Google Scholar] [CrossRef]
- Chen, A.L.; Yang, S.M. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens. Bioelectron. 2015, 71, 230–242. [Google Scholar] [CrossRef]
- Li, H.X.; Fu, X.Y.; You, Q.M.; Shi, D.W.; Su, L.X.; Song, M.H.; Peng, R.Z.; Fu, T.; Wang, P.; Tan, W.H. Multiple aptamer recognition-based quantum dot lateral flow platform: Ultrasensitive point-of-care testing of respiratory infectious diseases. J. Mater. Chem. B 2025, 13, 1681–1689. [Google Scholar] [CrossRef]
- Bachu, V.; Mili, M.; Dutta, A.; Thungon, P.D.; Goswami, P. Electrochemiluminescence-based lateral flow assay for detection of cardiac troponin T using aptamers developed through a modified SELEX technique. ACS Appl. Opt. Mater. 2024, 2, 1688–1698. [Google Scholar] [CrossRef]
- Jo, H.; Gu, H.; Jeon, W.J.; Youn, H.J.; Her, J.; Kim, S.-K.; Lee, J.B.; Shin, J.H.; Ban, C. Electrochemical aptasensor of cardiac troponin I for the early diagnosis of acute myocardial infarction. Anal. Chem. 2015, 87, 9869–9875. [Google Scholar] [CrossRef]
- Jo, H.; Her, J.; Lee, H.H.; Shim, Y.-B.; Ban, C. Highly sensitive amperometric detection of cardiac troponin I using sandwich aptamers and screen-printed carbon electrodes. Talanta 2017, 165, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lv, X.F.; Feng, W.; Li, X.Q.; Li, K.J.; Deng, Y.L. Aptamer-based fluorometric lateral flow assay for creatine kinase MB. Microchim. Acta 2018, 185, 364. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yao, M.D.; Feng, W.; Lv, X.F.; Deng, Y.L. Aptamer-based colloidal gold chromatography strips for rapid detection of creatine kinase-MB. Trans. Beijing Inst. Technol. 2019, 39, 108–115. [Google Scholar] [CrossRef]
- Phung, N.L.; Walter, J.G.; Jonczyk, R.; Seiler, L.K.; Scheper, T.; Blume, C. Development of an aptamer-based lateral flow assay for the detection of C-reactive protein using microarray technology as a prescreening platform. ACS Comb. Sci. 2020, 22, 617–629. [Google Scholar] [CrossRef]
- Jiang, X.X.; Wang, Y.F.; Zhao, L.H.; Li, X.T.; Dong, Y.Y. Development of Aptamer-based Au nanoparticle lateral flow test strips for the detection of deoxynivalenol in corn. ACS Food Sci. Technol. 2023, 3, 182–190. [Google Scholar] [CrossRef]
- Çam Derin, D.; Gültekin, E.; Gündüz, E.; Otlu, B. Comparison of six aptamer-aptamer pairs on rapid detection of SARS-CoV-2 by lateral flow assay. J. AOAC Int. 2024, 107, 464–470. [Google Scholar] [CrossRef]
- Du, F.X.; Dong, Z.Y.; Guan, Y.R.; Zeid, A.M.; Ma, D.; Feng, J.C.; Yang, D.; Xu, G.B. Single-electrode electrochemical system for the visual and high-throughput electrochemiluminescence immunoassay. Anal. Chem. 2022, 94, 2189–2194. [Google Scholar] [CrossRef] [PubMed]
- Bayoumy, S.; Martiskainen, I.; Heikkilä, T.; Rautanen, C.; Hedberg, P.; Hyytiä, H.; Wittfooth, S.; Pettersson, K. Sensitive and quantitative detection of cardiac troponin I with upconverting nanoparticle lateral flow test with minimized interference. Sci. Rep. 2021, 11, 18698. [Google Scholar] [CrossRef] [PubMed]
| Name | DNA Sequence (5′ to 3′) | Modification |
|---|---|---|
| C line | AAAAAAAAAAAAAAAAAAAA | 5′Biotin |
| T line | CGCATGCCAAACGTTGCCTCATAGTTCCCTCCCCGTGTCC | 5′Biotin |
| Tro4 | CGTGCAGTACGCCAACCTTTCTCATGCGCTGCCCCTCTTATTTTTTTTTTTTTTTTTTTT | 3′SH C6 |
| Targets | Methods | LOD | Time | Reporter | Reference |
|---|---|---|---|---|---|
| Creatine kinase-MB | aptamer-based LFA | 3.6 μg·mL−1 | 15 min | AuNPs | [27] |
| C-reactive protein | aptamer-based LFA | 10 μg·mL−1 | 30 min | AuNPs | [28] |
| Deoxynivalenol | aptamer-based LFA | 24.11 ng·mL−1 | 10 min | AuNPs | [29] |
| SARS-CoV-2 | aptamer-based LFA | – | 5–6 min | AuNPs | [30] |
| cTnI | aptamer-based LFA | 11.70 ng·mL−1 | 10 min | AuNPs | This study |
| cTnI | ECL aptasensor | 24 pg·mL−1 | 10 min | hydrazine | [25] |
| cTnI | colorimetric aptasensor | 27 pg·mL−1 | 65 min | horseradish peroxidase | [11] |
| cTnI | ECL immunosensor | 0.94 ng·mL−1 | 65 min | luminol | [31] |
| cTnI | antibody-based LFA | 30 pg·mL−1 | 30 min | upconverting nanoparticle | [32] |
| cTnI (ng·mL−1) | Intra-Assay | Inter-Assay | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Recovery | CV (%) | Mean | SD | Recovery | CV (%) | |
| 200 | 190 | 7.49 | 95.11% | 3.94 | 199 | 3.85 | 99.48% | 1.93 |
| 600 | 619 | 8.17 | 103.17% | 1.32 | 607 | 9.73 | 101.21% | 1.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Pang, J.; Cui, C. Aptamer-Based Gold Nanoparticle Lateral Flow Assay for Rapid Detection of Cardiac Troponin I. Biosensors 2025, 15, 776. https://doi.org/10.3390/bios15120776
Zhang J, Pang J, Cui C. Aptamer-Based Gold Nanoparticle Lateral Flow Assay for Rapid Detection of Cardiac Troponin I. Biosensors. 2025; 15(12):776. https://doi.org/10.3390/bios15120776
Chicago/Turabian StyleZhang, Jing, Jiayi Pang, and Cheng Cui. 2025. "Aptamer-Based Gold Nanoparticle Lateral Flow Assay for Rapid Detection of Cardiac Troponin I" Biosensors 15, no. 12: 776. https://doi.org/10.3390/bios15120776
APA StyleZhang, J., Pang, J., & Cui, C. (2025). Aptamer-Based Gold Nanoparticle Lateral Flow Assay for Rapid Detection of Cardiac Troponin I. Biosensors, 15(12), 776. https://doi.org/10.3390/bios15120776





