Recent Advances in the Detection of Aflatoxin M1 in Milk and Dairy Products
Abstract
1. Introduction
2. Antibody-Based Assays/Biosensors for AFM1 Determination
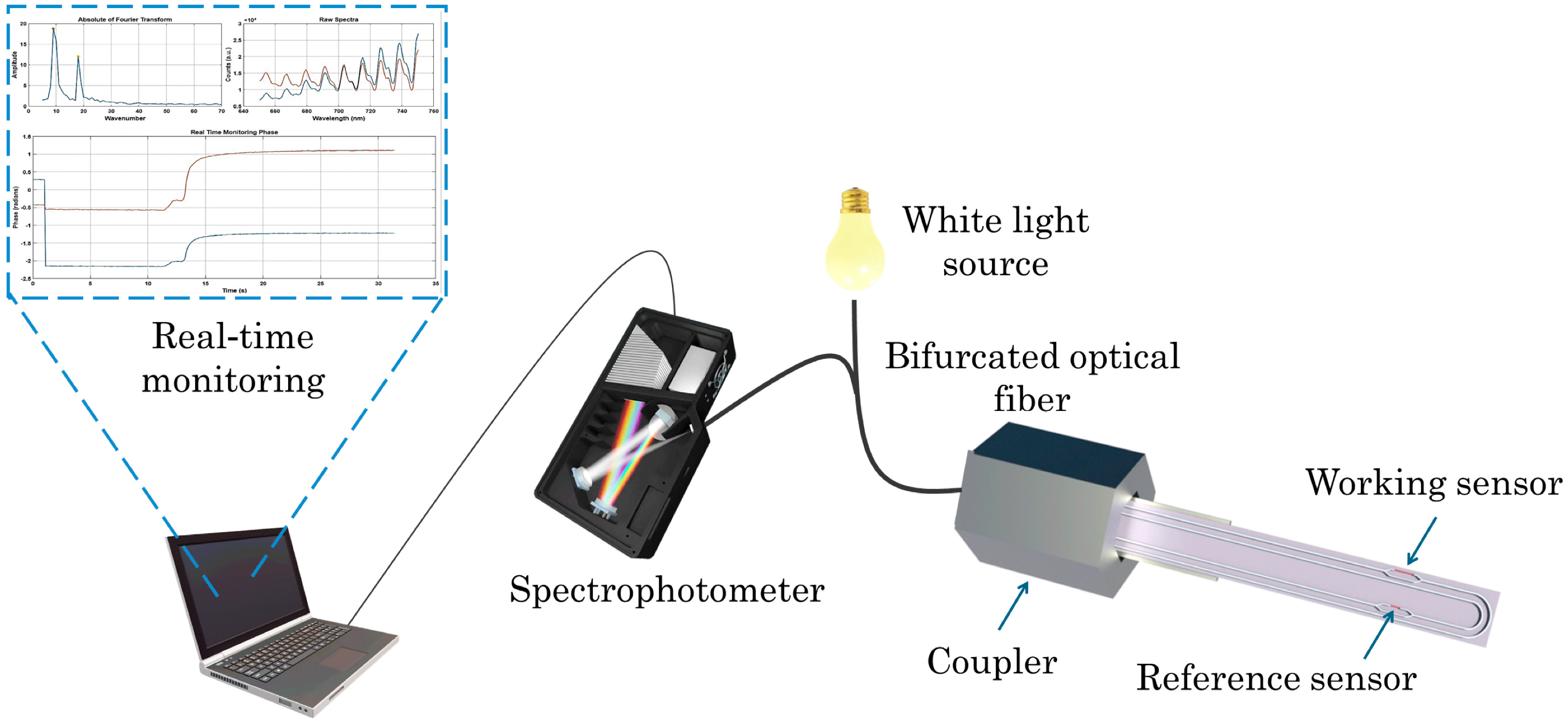
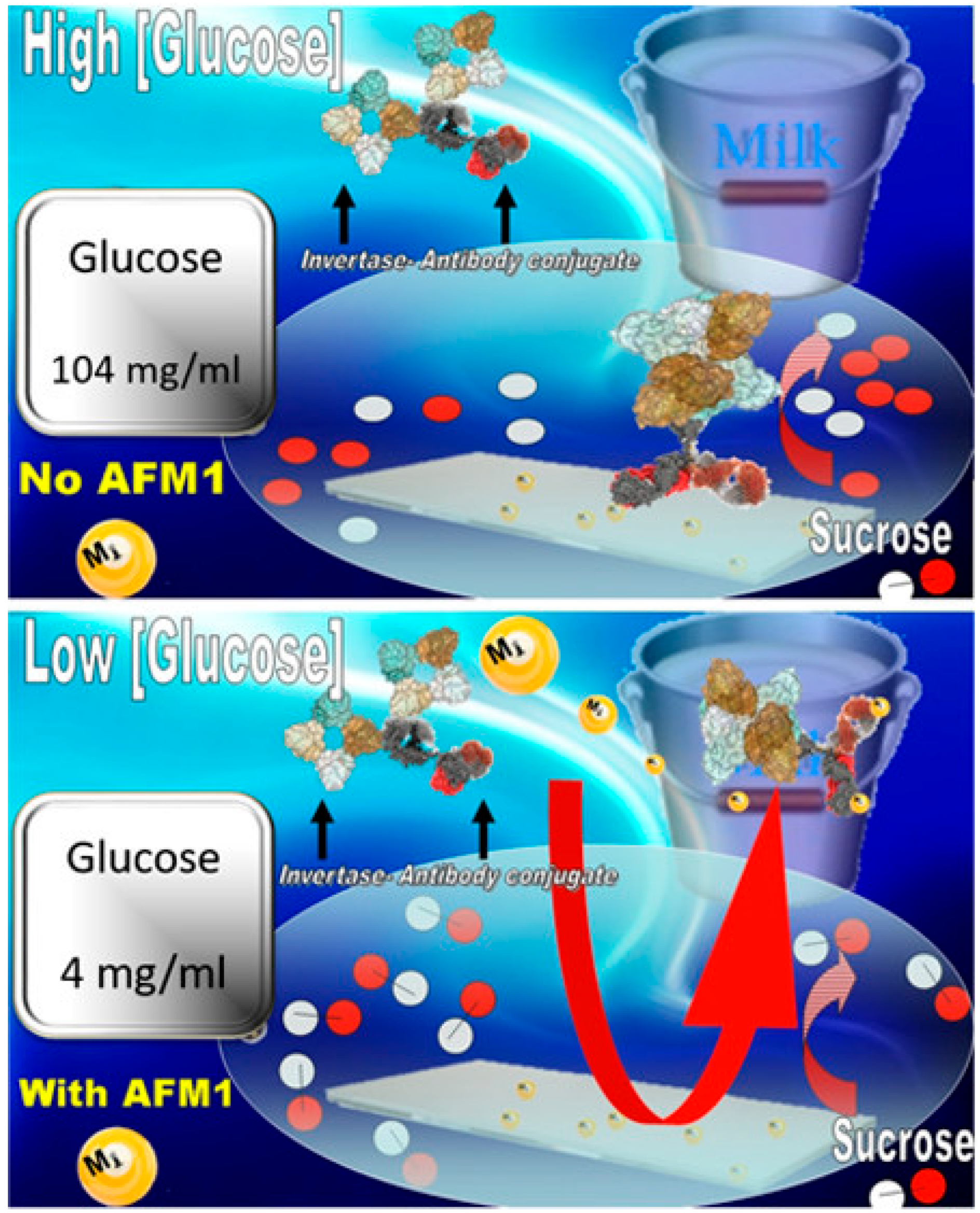
2.1. Optical Immunosensor for AFM1 Detection
2.2. Strip-Based Immunosensor for AFM1 Detection
2.3. Electrochemical-Based Immunosensor for AFM1 Detection
| Type | Sample Type | LOD (ng/mL) | Recovery (%) | Reference |
|---|---|---|---|---|
| Optical (Mach–Zehnder interferometer) | milk | 0.02 * | n.r. | [48] |
| cow milk | n.r. | 90.0–107.0 | ||
| sheep milk | n.r. | 93.3–107.0 | ||
| goat milk | n.r. | 86.7–112.0 | ||
| Optical (Mach–Zehnder interferometer) | full-fat milk | 0.005 | 90.0–110.0 | [49] |
| chocolate milk | 0.005 | 90.0–115.0 | ||
| yogurt | 0.01 | 86.7–106.0 | ||
| Immunostrip | optimized conditions | 0.1 ** | n.r. | [50] |
| milk | 0.25 ** | n.r. | ||
| Electrochemical | whole milk | 0.027 * | n.r. | [51] |
| Electrochemical | optimized conditions | 0.09 | n.r. | [53] |
| milk | n.r. | 82.0–108.0 |
3. Aptamer-Based Assays/Biosensors for AFM1 Determination
3.1. Colorimetric-Based Aptasensor for AFM1 Detection
3.2. Surface Plasmon Resonance-Based Aptasensor for AFM1 Detection
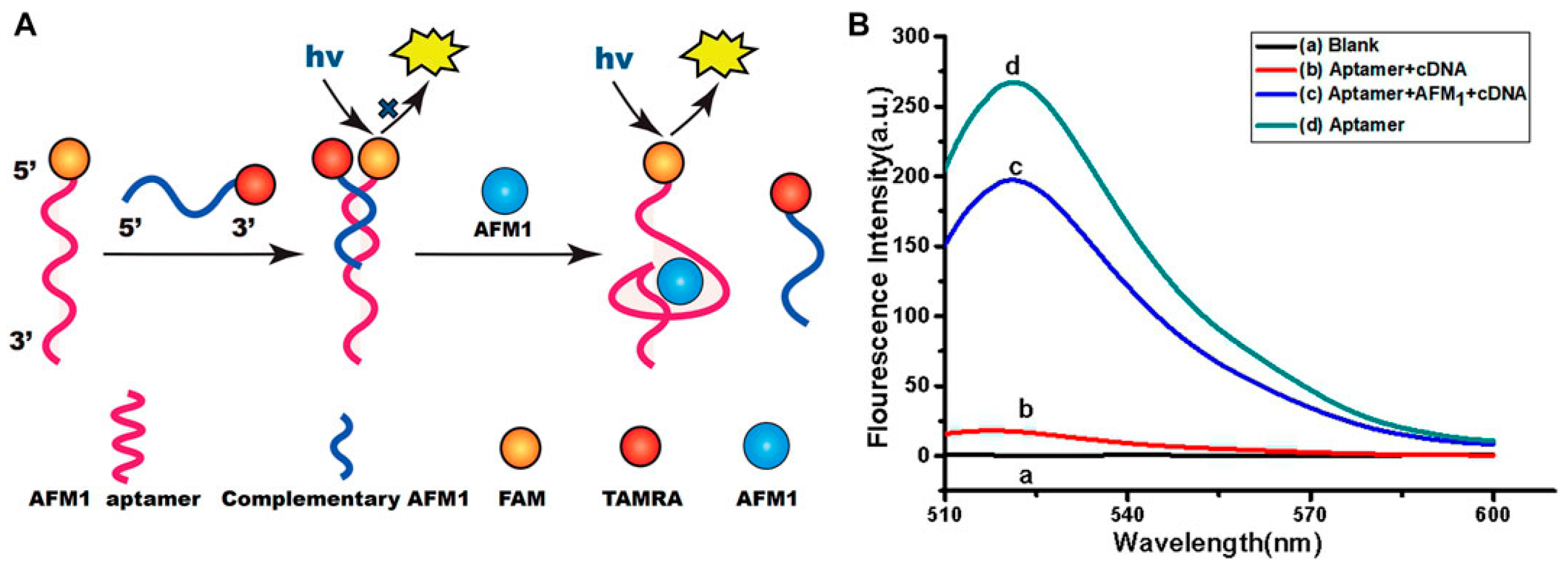
3.3. Fluorescence-Based Aptasensor for AFM1 Detection
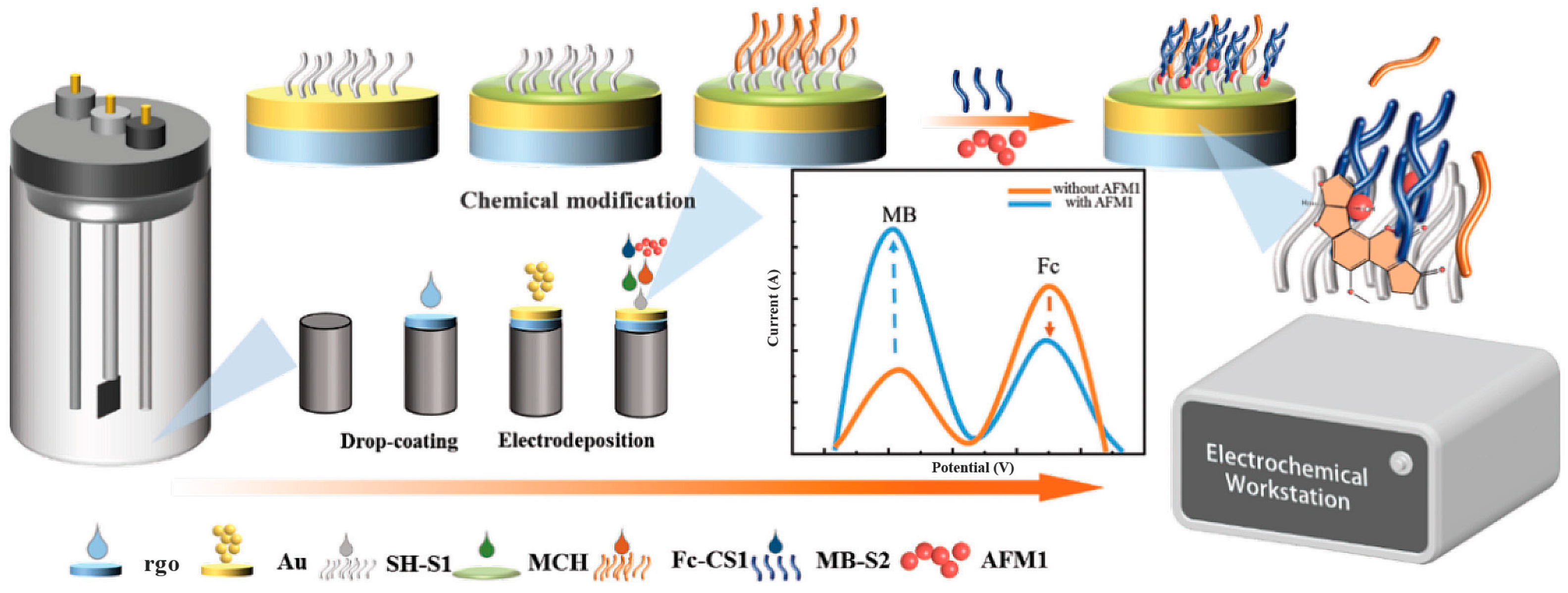
3.4. Electrochemical-Based Aptasensor for AFM1 Detection
| Type | Sample Type | LOD (ng/mL) | Recovery (%) | Reference |
|---|---|---|---|---|
| Colorimetric | water | 9.8 × 10−4 * | n.r. | [55] |
| milk | 3.28 * | n.r. | ||
| Colorimetric | optimized conditions | 0.03 * | n.r. | [54] |
| milk | 0.045 ng/mL * | 92.0–109.5 | ||
| Surface plasmon resonance | MOPS buffer containing methanol (10%), pH 7.0 | 0.002 | n.r. | [58] |
| milk | n.r. | 80.5–89.7 | ||
| Colorimetric | binding buffer | 0.21 | n.r. | [56] |
| milk samples | n.r. | 92.34–104.35 | ||
| Colorimetric | optimized conditions | 0.50 | n.r. | [57] |
| milk powder | n.r. | 92.8–105.2 | ||
| Fluorescence | optimized conditions | 0.5 | n.r. | [60] |
| milk | n.r. | 93.4–101.3 | ||
| Fluorescence | optimized conditions | 0.56 * | n.r. | [61] |
| milk | n.r. | 91.3–110.2 | ||
| Fluorescence | pbs 1x | 3.28 × 10−4 * | n.r. | [62] |
| milk | 8.2 × 10−4 * | 96.67–101.65 | ||
| Fluorescence | optimized conditions | 0.010 | n.r. | [64] |
| raw milk | n.r. | 89.0–95.6 | ||
| raw goat milk | n.r. | 94.9–112.0 | ||
| pure milk | n.r. | 100.0–114.0 | ||
| Fluorescence | optimized conditions | 0.0201 * | n.r. | [63] |
| pure milk | n.r. | 97.1–101.0 | ||
| Colorimetric fluorescence | optimized conditions | 0.007 * | n.r. | [65] |
| milk | n.r. | 97.0–99.0 | ||
| optimized conditions | 0.005 * | n.r. | ||
| milk | n.r. | 96.0–101.0 | ||
| Capacitive signal | optimized conditions | 0.00714 * | n.r. | [67] |
| pasteurized cow milk | n.r. | 101.6–105.5 | ||
| Cyclic voltammetry | optimized conditions | 6 × 10−4 * | n.r. | [68] |
| milk | n.r. | 106–109 | ||
| Electrochemical aptasensor | optimized conditions | 0.02 | n.r. | [69] |
| cow, goat, and sheep milk | n.r. | 89.00–104.05 | ||
| Electrochemical aptasensor | optimized conditions | 3 × 10−4 * | n.r. | [70] |
| raw milk | n.r. | 92.0 | ||
| low-fat pasteurized milk | n.r. | 108.0 | ||
| full-fat pasteurized milk | n.r. | 90.0 | ||
| Ratiometric electrochemical aptasensor | optimized conditions | 0.015 * | n.r. | [71] |
| milk | 0.05 * | n.r. | ||
| Electrochemical | optimized conditions | 0.023 * | n.r. | [73] |
| goat milk | n.r. | 97.9–105.0 | ||
| sheep milk | n.r. | 95.4–102.1 | ||
| cow milk | n.r. | 96.0–105.6 | ||
| Electrochemical | method I, in pbs | 0.0013 * | n.r. | [74] |
| method II, in acetate buffer | 0.00098 * | n.r. | ||
| milk | n.r. | 101.2–104.0 | ||
| Electrochemiluminescence | optimized conditions | 9 × 10−6 * | n.r. | [75] |
| defatted milk | n.r. | 93.3–104.0 |
4. Conclusions and Future Perspectives
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tola, M.; Kebede, B. Occurrence, importance and control of mycotoxins: A review. Cogent Food Agric. 2016, 2, 1191103. [Google Scholar] [CrossRef]
- Jahromi, A.S.; Jokar, M.; Abdous, A.; Rabiee, M.H.; Biglo, F.H.B.; Rahmanian, V. Prevalence and concentration of aflatoxin M1 in milk and dairy products: An umbrella review of meta-analyses. Int. Health 2025, 17, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Saha Turna, N.; Wu, F. Aflatoxin M1 in milk: A global occurrence, intake, & exposure assessment. Trends Food Sci. Technol. 2021, 110, 183–192. [Google Scholar] [CrossRef]
- Hsu, P.; Pokharel, A.; Scott, C.K.; Wu, F. Aflatoxin M1 in milk and dairy products: The state of the evidence for child growth impairment. Food Chem. Toxicol. 2024, 193, 115008. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Aflatoxins. In Chemical Agents and Related Occupations, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2012; Volume 100F, pp. 225–248. ISBN 978-92-832-1323-9. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Safety Evaluation of Certain Contaminants in Food; WHO Food Additives Series: 74, FAO JECFA Monographs 19bis; World Health Organization and Food and Agriculture Organization of United Nations: Geneva, Switzerland, 2018; pp. 3–280. ISBN 978-92-4-166074-7. [Google Scholar]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Risk assessment of aflatoxins in food. EFSA J. 2020, 18, 6040. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of United Nations (FAO). Worldwide regulations for mycotoxins in food and feed in 2003. In FAO Food and Nutrition Paper 81; FAO: Rome, Italy, 2004. [Google Scholar]
- European Commission. Commission Regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, L119, 103–157. [Google Scholar]
- Food and Drug Administration (FDA). 527.400 Whole Milk, L.f.m., Skim Milk–Aflatoxin M1 (CPG 7106.10). FDA/ORA Compliance Policy Guides. 2005. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-527400-whole-milk-lowfat-milk-skim-milk-aflatoxin-m1 (accessed on 13 November 2025).
- Codex Alimentarius, CXS 193-1995. General Standard for Contaminants and Toxins in Food and Feed. Adopted in 1995. Revised in 1997, 2006, 2008, 2009. Amended in 2010, 2012, 2013, 2014, 2015, 2016, 2017, 2018, 2019, 2021, 2022, 2023 and 2024. Available online: https://www.fao.org/fao-who-codexalimentarius (accessed on 13 November 2025).
- Italian Ministry of Health. DGISAN-MDS-P n.0070600. Conclusione dell’Attività del Gruppo di Lavoro per la Classificazione dei Formaggi e Definizione dei Fattori di Concentrazione (art. 2 del Regolamento CE 1881/2006 e s.m.i.) di Aflatossina M1. 23 December 2019. Available online: https://www.alimenti-salute.it/doc/11Nota_ministeriale.pdf (accessed on 13 November 2025).
- Arghavan, B.; Kordkatuli, K.; Mardani, H.; Jafari, A. A Comprehensive Systematic Review and Meta-Analysis on the Prevalence of Aflatoxin M1 in Dairy Products in Selected Middle East Countries. Vet. Med. Sci. 2025, 11, e70204. [Google Scholar] [CrossRef]
- Fakhri, Y.; Ranaei, V.; Pilevar, Z.; Sarkhosh, M.; Sarafraz, M.; Abdi-Moghadam, Z.; Javid, R. Prevalence and Concentration of Aflatoxin M1 in Mother Milk: A Meta-analysis, Meta-regression, and Infants’ Health Risk Assessment. J. Food Prot. 2025, 88, 100462. [Google Scholar] [CrossRef]
- Hassen, J.Y.; Debella, A.; Eyeberu, A.; Mussa, I. Prevalence and concentration of aflatoxin M1 in breast milk in Africa: A meta-analysis and implication for the interface of agriculture and health. Sci. Rep. 2024, 14, 16611. [Google Scholar] [CrossRef]
- Malissiova, E.; Tsinopoulou, G.; Gerovasileiou, E.S.; Meleti, E.; Soultani, G.; Koureas, M.; Maisoglou, I.; Manouras, A. A 20-Year Data Review on the Occurrence of Aflatoxin M1 in Milk and Dairy Products in Mediterranean Countries—Current Situation and Exposure Risks. Dairy 2024, 5, 491–514. [Google Scholar] [CrossRef]
- Summa, S.; Lo Magro, S.; Vita, V.; Franchino, C.; Scopece, V.; D’Antini, P.; Iammarino, M.; De Pace, R.; Muscarella, M. Occurrence of Aflatoxin M1 in Raw and Processed Milk: A Contribution to Human Exposure Assessment After 12 Years of Investigation. Appl. Sci. 2025, 15, 853. [Google Scholar] [CrossRef]
- Aranda, C.; Rodriguez, R.; Fernández-Baldo, M.A.; Durán, P. Mycotoxins in Cheese: Assessing Risks, Fungal Contaminants, and Control Strategies for Food Safety. Foods 2025, 14, 351. [Google Scholar] [CrossRef] [PubMed]
- Vaz, A.; Cabral Silva, A.C.; Rodrigues, P.; Venâncio, A. Detection Methods for Aflatoxin M1 in Dairy Products. Microorganisms 2020, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, D.; Morsi, R.; Usman, M.; Meetani, M.A. Recent Advances in the Chromatographic Analysis of Emerging Pollutants in Dairy Milk: A Review (2018–2023). Molecules 2024, 29, 1296. [Google Scholar] [CrossRef]
- Kolarič, L.; Šimko, P. Development and validation of HPLC-FLD method for aflatoxin M1 determination in milk and dairy products. Acta Chim. Slovaca 2023, 16, 99–108. [Google Scholar] [CrossRef]
- Stella, R.; Bovo, D.; Noviello, S.; Contiero, L.; Barberio, A.; Angeletti, R.; Biancotto, G. Fate of aflatoxin M1 from milk to typical Italian cheeses: Validation of an HPLC method based on aqueous buffer extraction and immune-affinity clean up with limited use of organic solvents. Food Control 2024, 157, 110149. [Google Scholar] [CrossRef]
- Pecorelli, I.; Guarducci, N.; von Holst, C.; Bibi, R.; Pascale, M.; Ciasca, B.; Logrieco, A.F.; Lattanzio, V.M.T. Critical Comparison of Analytical Performances of Two Immunoassay Methods for Rapid Detection of Aflatoxin M1 in Milk. Toxins 2020, 12, 270. [Google Scholar] [CrossRef]
- Maggira, M.; Ioannidou, M.; Sakaridis, I.; Samouris, G. Determination of Aflatoxin M1 in Raw Milk Using an HPLC-FL Method in Comparison with Commercial ELISA Kits—Application in Raw Milk Samples from Various Regions of Greece. Vet. Sci. 2021, 8, 46. [Google Scholar] [CrossRef]
- Kourti, D.; Angelopoulou, M.; Petrou, P.; Kakabakos, S. Sensitive Aflatoxin M1 Detection in Milk by ELISA: Investigation of Different Assay Configurations. Toxins 2024, 16, 515. [Google Scholar] [CrossRef]
- Morais, D.N.; Massarolo, K.C.; Ardohain, E.N.G.; Lima, J.F.; Ferreira, F.D.; Drunkler, D.A. Method for Determination of Multi-mycotoxins in Milk: QuEChERS Extraction Modified Followed by HPLC-FL Analysis. Food Anal. Methods 2024, 17, 47–60. [Google Scholar] [CrossRef]
- Pavicich, M.A.; Compagnoni, S.; Meerpoel, C.; Raes, K.; De Saeger, S. Ochratoxin A and AFM1 in Cheese and Cheese Substitutes: LC-MS/MS Method Validation, Natural Occurrence, and Risk Assessment. Toxins 2024, 16, 547. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Sahu, P.P. Biosensors in Food Safety and Quality: Fundamentals and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2022; Taylor & Francis Group, LLC: Oxfordshire, UK, 2022. [Google Scholar]
- Inês, A.; Cosme, F. Biosensors for Detecting Food Contaminants—An Overview. Processes 2025, 13, 380. [Google Scholar] [CrossRef]
- Xiao, X.; Hu, S.; Lai, X.; Peng, J.; Lai, W. Developmental trend of immunoassays for monitoring hazards in food samples: A review. Trends Food Sci. Technol. 2021, 111, 68–88. [Google Scholar] [CrossRef]
- Song, K.; Saleh, R.O.; Kadhum, W.R.; Saleh, E.A.M.; Kassem, A.F.; Noori, S.D.; Alawady, A.h.; Kumar, A.; Ghildiyal, P.; Kadhim, A.J. Research progress on aptamer-based electrochemiluminescence sensors for detection of mycotoxins in food and environmental samples. J. Environ. Chem. Eng. 2024, 12, 113313. [Google Scholar] [CrossRef]
- Booth, M.A.; Karaosmanoglu, H.; Wu, Y.; Partridge, A. Biosensor Platforms for Detecting Target Species in Milk. In Food Biosensors; Ahmed, M.U., Zourob, M., Tamiya, E., Eds.; Special Collection: 2016 Ebook Collection, Series: Food Chemistry, Function and Analysis; The Royal Society of Chemistry: London, UK, 2016; pp. 71–103. [Google Scholar]
- Matabaro, E.; Ishimwe, N.; Uwimbabazi, E.; Lee, B.H. Current Immunoassay Methods for the Rapid Detection of Aflatoxin in Milk and Dairy Products. Compr. Rev. Food Sci. Food Saf. 2017, 16, 808–820. [Google Scholar] [CrossRef]
- Chen, Q.; Meng, M.; Li, W.; Xiong, Y.; Fang, Y.; Lin, Q. Emerging biosensors to detect aflatoxin M1 in milk and dairy products. Food Chem. 2023, 398, 133848. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, M.; Wen, X.; Zhang, G.; Zhang, T.; Lou, X.; Wang, M.; Fauconnier, M.-L.; Xie, K. Aptamers for aflatoxin M1: From aptasensing technology to commercialization. Crit. Rev. Food Sci. Nutr. 2025, 65, 8823–8841. [Google Scholar] [CrossRef]
- Malhotra, S.; Pandey, A.K.; Rajput, Y.S.; Sharma, R. Selection of aptamers for aflatoxin M1 and their characterization. J. Mol. Recognit. 2014, 27, 493–500. [Google Scholar] [CrossRef]
- Nguyen, B.H.; Tran, L.D.; Do, Q.P.; Nguyen, H.L.; Tran, N.H.; Nguyen, P.X. Label-free detection of aflatoxin M1 with electrochemical Fe3O4/polyaniline-based aptasensor. Mater. Sci. Eng. C 2013, 33, 2229–2234. [Google Scholar] [CrossRef]
- Gurban, A.-M.; Epure, P.; Oancea, F.; Doni, M. Achievements and Prospects in Electrochemical-Based Biosensing Platforms for Aflatoxin M1 Detection in Milk and Dairy Products. Sensors 2017, 17, 2951. [Google Scholar] [CrossRef]
- Beitollahi, H.; Tajik, S.; Dourandish, Z.; Zhang, K.; Le, Q.V.; Jang, H.W.; Kim, S.Y.; Shokouhimehr, M. Recent Advances in the Aptamer-Based Electrochemical Biosensors for Detecting Aflatoxin B1 and Its Pertinent Metabolite Aflatoxin M1. Sensors 2020, 20, 3256. [Google Scholar] [CrossRef] [PubMed]
- Thurner, F.; Alatraktchi, F.A.a. Recent advances in electrochemical biosensing of aflatoxin M1 in milk—A mini review. Microchem. J. 2023, 190, 108594. [Google Scholar] [CrossRef]
- Danesh, N.M.; Bostan, H.B.; Abnous, K.; Ramezani, M.; Youssefi, K.; Taghdisi, S.M.; Karimi, G. Ultrasensitive detection of aflatoxin B1 and its major metabolite aflatoxin M1 using aptasensors: A review. TrAC Trends Anal. Chem. 2018, 99, 117–128. [Google Scholar] [CrossRef]
- Janeway, C.A.J.; Travers, P.; Walport, M. Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science, N.Y., Ed.; Garland Publishing: New York, NY, USA, 2001. [Google Scholar]
- Litman, G.W.; Rast, J.P.; Shamblott, M.J.; Haire, R.N.; Hulst, M.; Roess, W.; Litman, R.T.; Hinds-Frey, K.R.; Zilch, A.; Amemiya, C.T. Phylogenetic diversification of immunoglobulin genes and the antibody repertoire. Mol. Biol. Evol. 1993, 10, 60–72. [Google Scholar] [CrossRef]
- Wilson, I.A.; Stanfield, R.L. 50 Years of structural immunology. J. Biol. Chem. 2021, 296, 100745. [Google Scholar] [CrossRef]
- Staiano, M.; Bazzicalupo, P.; Rossi, M.; D’Auria, S. Glucose biosensors as models for the development of advanced protein-based biosensors. Mol. Biosyst. 2005, 1, 354–362. [Google Scholar] [CrossRef]
- Edite Bezerra da Rocha, M.; Freire, F.d.C.O.; Erlan Feitosa Maia, F.; Izabel Florindo Guedes, M.; Rondina, D. Mycotoxins and their effects on human and animal health. Food Control 2014, 36, 159–165. [Google Scholar] [CrossRef]
- Forcada, S.; Sánchez-Visedo, A.; Melendreras, C.; Menéndez-Miranda, M.; Costa-Fernández, J.M.; Royo, L.J.; Soldado, A. Design and Evaluation of a Competitive Phosphorescent Immunosensor for Aflatoxin M1 Quantification in Milk Samples Using Mn:ZnS Quantum Dots as Antibody Tags. Chemosensors 2022, 10, 41. [Google Scholar] [CrossRef]
- Kourti, D.; Angelopoulou, M.; Makarona, E.; Economou, A.; Petrou, P.; Misiakos, K.; Kakabakos, S. Aflatoxin M1 Determination in Whole Milk with Immersible Silicon Photonic Immunosensor. Toxins 2025, 17, 165. [Google Scholar] [CrossRef]
- Angelopoulou, M.; Kourti, D.; Misiakos, K.; Economou, A.; Petrou, P.; Kakabakos, S. Mach-Zehnder Interferometric Immunosensor for Detection of Aflatoxin M1 in Milk, Chocolate Milk, and Yogurt. Biosensors 2023, 13, 592. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-W.; Ko, J.-L.; Liu, B.-H.; Yu, F.-Y. A Sensitive Two-Analyte Immunochromatographic Strip for Simultaneously Detecting Aflatoxin M1 and Chloramphenicol in Milk. Toxins 2020, 12, 637. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, S.; Zambrini, V.; Varriale, A.; D’Auria, S. Sweet Sensor for the Detection of Aflatoxin M1 in Whole Milk. ACS Omega 2019, 4, 12803–12807. [Google Scholar] [CrossRef] [PubMed]
- Erdil, K.; Akcan, Ö.G.; Gül, Ö.; Gökdel, Y.D. A disposable MEMS biosensor for aflatoxin M1 molecule detection. Sens. Actuators A Phys. 2022, 338, 113438. [Google Scholar] [CrossRef]
- Tang, X.; Catanante, G.; Huang, X.; Marty, J.-L.; Wang, H.; Zhang, Q.; Li, P. Screen-printed electrochemical immunosensor based on a novel nanobody for analyzing aflatoxin M1 in milk. Food Chem. 2022, 383, 132598. [Google Scholar] [CrossRef] [PubMed]
- Jalalian, S.H.; Lavaee, P.; Ramezani, M.; Danesh, N.M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. An optical aptasensor for aflatoxin M1 detection based on target-induced protection of gold nanoparticles against salt-induced aggregation and silica nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 246, 119062. [Google Scholar] [CrossRef]
- Kasoju, A.; Shahdeo, D.; Khan, A.A.; Shrikrishna, N.S.; Mahari, S.; Alanazi, A.M.; Bhat, M.A.; Giri, J.; Gandhi, S. Author Correction: Fabrication of microfluidic device for Aflatoxin M1 detection in milk samples with specific aptamers. Sci. Rep. 2020, 10, 9222. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, M.; Chen, P.; Li, Y.; Lu, X.; Sun, A.; Wang, Z.; Yang, J. A lateral flow assay based on aptamer for the detection of AFM1 in milk samples. Food Biosci. 2025, 66, 106175. [Google Scholar] [CrossRef]
- Wei, X.; Ma, P.; Imran Mahmood, K.; Zhang, Y.; Wang, Z. Screening of a High-Affinity Aptamer for Aflatoxin M1 and Development of Its Colorimetric Aptasensor. J. Agric. Food Chem. 2023, 71, 7546–7556. [Google Scholar] [CrossRef]
- Lerdsri, J.; Soongsong, J.; Laolue, P.; Jakmunee, J. Reliable colorimetric aptasensor exploiting 72-Mers ssDNA and gold nanoprobes for highly sensitive detection of aflatoxin M1 in milk. J. Food Compos. Anal. 2021, 102, 103992. [Google Scholar] [CrossRef]
- Chen, L.; Wen, F.; Li, M.; Guo, X.; Li, S.; Zheng, N.; Wang, J. A simple aptamer-based fluorescent assay for the detection of Aflatoxin B1 in infant rice cereal. Food Chem. 2017, 215, 377–382. [Google Scholar] [CrossRef]
- Qiao, Q.; Guo, X.; Wen, F.; Chen, L.; Xu, Q.; Zheng, N.; Cheng, J.; Xue, X.; Wang, J. Aptamer-Based Fluorescence Quenching Approach for Detection of Aflatoxin M1 in Milk. Front. Chem. 2021, 9, 653869. [Google Scholar] [CrossRef]
- Aran, G.C.; Bayraç, C. Simultaneous Dual-Sensing Platform Based on Aptamer-Functionalized DNA Hydrogels for Visual and Fluorescence Detection of Chloramphenicol and Aflatoxin M1. Bioconjug. Chem. 2023, 34, 922–933. [Google Scholar] [CrossRef]
- Yadav, K.; Moovendaran, K.; Dhenadhayalan, N.; Lee, S.-F.; Leung, M.-K.; Sankar, R. From food toxins to biomarkers: Multiplexed detection of aflatoxin B1 and aflatoxin M1 in milk and human serum using PEGylated ternary transition metal sulfides. Sens. Actuators Rep. 2023, 5, 100156. [Google Scholar] [CrossRef]
- Cai, Y.; Guo, G.; Fu, Y.; Huang, X.; Wang, T.; Li, T. A fluorescent aptasensor based on functional graphene oxide and FRET strategy simultaneously detects aflatoxins B1 and aflatoxins M1. Chin. J. Anal. Chem. 2024, 52, 100408. [Google Scholar] [CrossRef]
- Lan, Y.; Ma, Y.; Xu, Y.; Zhang, Y.; Deng, R.; Liu, L.; Hou, B.; Cui, H.; Yun, K.; Wei, Z.; et al. A label-free fluorescent aptasensor for AFB1 and AFM1 based on the aptamer tailoring strategy and synergistic signal amplification of HCR and MoS2 nanosheets. Sens. Actuators B Chem. 2025, 434, 137591. [Google Scholar] [CrossRef]
- Naz, I.; Alanazi, S.J.F.; Hayat, A.; Jubeen, F. Covalent organic framework-based aptananozyme (COF@NH2 apt-AFM1): A novel platform for colorimetric and fluorescent aptasensing of AFM1 in milk. Food Chem. 2025, 484, 144478. [Google Scholar] [CrossRef]
- Kordasht, H.K.; Hasanzadeh, M. Specific monitoring of aflatoxin M1 in real samples using aptamer binding to DNFS based on turn-on method: A novel biosensor. J. Mol. Recognit. 2020, 33, e2832. [Google Scholar] [CrossRef] [PubMed]
- Hamami, M.; Mars, A.; Raouafi, N. Biosensor based on antifouling PEG/Gold nanoparticles composite for sensitive detection of aflatoxin M1 in milk. Microchem. J. 2021, 165, 106102. [Google Scholar] [CrossRef]
- Rahmani, H.R.; Adabi, M.; Bagheri, K.P.; Karim, G. Development of electrochemical aptasensor based on gold nanoparticles and electrospun carbon nanofibers for the detection of aflatoxin M1 in milk. J. Food Meas. Charact. 2021, 15, 1826–1833. [Google Scholar] [CrossRef]
- Hui, Y.; Peng, H.; Zhang, F.; Zhang, L.; Yufang, L.; Zhao, A.; Jia, R.; Wang, B.; Song, Y. A novel electrochemical aptasensor based on layer-by-layer assembly of DNA-Au@Ag conjugates for rapid detection of aflatoxin M1 in milk samples. J. Dairy Sci. 2022, 105, 1966–1977. [Google Scholar] [CrossRef]
- Ahmadi, S.F.; Hojjatoleslamy, M.; Kiani, H.; Molavi, H. Monitoring of Aflatoxin M1 in milk using a novel electrochemicalaptasensorbased on reduced graphene oxide and gold nanoparticles. Food Chem. 2022, 373, 131321. [Google Scholar] [CrossRef]
- Li, H.; Du, C.; Guo, T.; Zhou, H.; Zhou, Y.; Huang, X.; Zhang, Y.H.; Wang, S.; Liu, X.; Ma, L. Ratiometric electrochemical aptasensor based on split aptamer and Au-rGO for detection of aflatoxin M1. J. Dairy Sci. 2024, 107, 2748–2759. [Google Scholar] [CrossRef]
- Chen, L.; Wang, X.; Lu, W.; Wu, X.; Li, J. Molecular imprinting: Perspectives and applications. Chem. Soc. Rev. 2016, 45, 2137–2211. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Hui, Y.; Liu, Y.; Wang, W.; He, C.; Zhao, A.; Wei, L.; Wang, B. Novel dual-recognition electrochemical biosensor for the sensitive detection of AFM1 in milk. Food Chem. 2024, 433, 137362. [Google Scholar] [CrossRef]
- Huma, Z.-E.; Nazli, Z.-I.H.; Gokce, G.; Ali, M.; Jubeen, F.; Hayat, A. A novel and universal dual-functionalized Hazo-POPs@COOH-apt/PGE-based electrochemical biosensor for detection of aflatoxin M1 (AFM1) in raw milk sample: A versatile peroxidase-mimicking aptananozyme approach. Mater. Chem. Phys. 2025, 341, 130887. [Google Scholar] [CrossRef]
- Zeng, W.-J.; Wang, K.; Liang, W.-B.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y. Covalent organic frameworks as micro-reactors: Confinement-enhanced electrochemiluminescence. Chem. Sci. 2020, 11, 5410–5414. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Asumadu, P.; Zhou, S.; Wang, M.; Liu, C.; Zhang, Q.; Zhong, J.; Guan, H.; Ye, H. Recognition mechanism of split T-2 toxin aptamer coupled with reliable dual-mode detection in peanut and beer. Food Biosci. 2024, 60, 104268. [Google Scholar] [CrossRef]
- Shoaib, M.; Li, H.; Zareef, M.; Khan, I.M.; Iqbal, M.W.; Niazi, S.; Raza, H.; Yan, Y.; Chen, Q. Recent Advances in Food Safety Detection: Split Aptamer-Based Biosensors Development and Potential Applications. J. Agric. Food Chem. 2025, 73, 4397–4424. [Google Scholar] [CrossRef]
| Antibody-Based Biosensors/Assays | Aptamer-Based Biosensors/Assays | |
|---|---|---|
| Advantages | Quick and Continuous Measurements High Specificity Rapid Response High/Good Sensitivity Minimal Reagent Usage Cost-Effectiveness Portability and Ease of Use | Low Immunogenicity Low Toxicity Low Production Cost Ease of Production Ease of Modification High Affinity Low/Good Sensitivity * High Chemical and Thermal Stability Good Specificity |
| Disadvantages | Sensitive to Organic Solvent Sensor Regeneration Problems | Low/Good Sensitivity * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maurelli, A.M.; Catucci, L.; Pascale, M.; D’Auria, S.; Staiano, M. Recent Advances in the Detection of Aflatoxin M1 in Milk and Dairy Products. Biosensors 2025, 15, 775. https://doi.org/10.3390/bios15120775
Maurelli AM, Catucci L, Pascale M, D’Auria S, Staiano M. Recent Advances in the Detection of Aflatoxin M1 in Milk and Dairy Products. Biosensors. 2025; 15(12):775. https://doi.org/10.3390/bios15120775
Chicago/Turabian StyleMaurelli, Anna Maria, Lucia Catucci, Michelangelo Pascale, Sabato D’Auria, and Maria Staiano. 2025. "Recent Advances in the Detection of Aflatoxin M1 in Milk and Dairy Products" Biosensors 15, no. 12: 775. https://doi.org/10.3390/bios15120775
APA StyleMaurelli, A. M., Catucci, L., Pascale, M., D’Auria, S., & Staiano, M. (2025). Recent Advances in the Detection of Aflatoxin M1 in Milk and Dairy Products. Biosensors, 15(12), 775. https://doi.org/10.3390/bios15120775








