Abstract
Cancer and aging are two distinct biological processes with shared cellular pathways, such as cellular senescence, DNA damage repair, and metabolic reprogramming. However, the outcomes of these processes differ in terms of proliferation. Understanding biomarkers related to aging and cancer opens a pathway for therapeutic interventions and more effective prevention, detection, and treatment strategies. Biomarkers, ranging from molecular to phenotypic indicators, play an important role in early detection, risk assessment, and prognosis in this endeavor. This review comprehensively examines key biomarkers associated with cancer and aging, highlighting their importance in early diagnostic strategies. The review discusses recent advances in biomarker-based diagnostic technologies, such as liquid biopsy, multi-omics integration, and artificial intelligence, and emphasizes their novel potential for early detection, accurate risk assessment, and personalized therapeutic interventions in cancer and aging science. We also explore the current state of biosensor development and clinical application cases. Finally, we discuss the limitations of current early diagnostic methods and propose future research directions to enhance biomarker-based diagnostic technologies.
1. Introduction
The global population is undergoing an unprecedented structural shift, with people aged 65 and over representing the fastest-growing segment [1,2,3]. This shift poses an urgent public health challenge, as aging is the most significant risk factor for chronic diseases, particularly cancer [1,4,5].
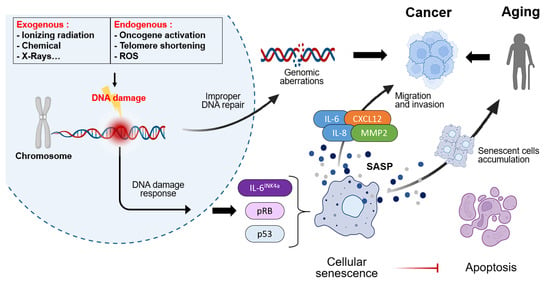
The cancer incidence in people over 65 is 11 times higher than in younger people. More than 60% of newly diagnosed cancer patients belong to this age group, highlining the inextricably linked between aging and cancer [6,7]. Both processes share common hallmarks, including genomic instability [8,9], cellular senescence [10,11], telomere dysfunction [12,13], chronic inflammation [14,15], autophagy [16,17], and dysregulated metabolic pathways [18,19]. Yet they diverge in their ultimate cellular fate and tissue-level outcomes, offering crucial insights into how aging and cancer overlap and differ [20].
Traditionally, cancer research has focused on chronological age. However, biological age is now recognized as a more meaningful measure of health and disease risk [21]. Biological age reflects the accumulation of molecular and physiological changes over time, providing a better indicator of an individual’s true vulnerability [22,23].
The heterogeneity of aging processes within age cohorts is a critical consideration. The concept of “ageotypes” highlights distinct aging patterns influenced by genetic predispositions and environmental factors [24]. This leads to varied health outcomes, even among people of similar ages. This inherent variability underscores the need for biomarkers that distinguish individuals of the same chronological age but with different biological aging profiles, enabling more precise and personalized interventions [24].
Biomarker discovery has become central to modern precision medicine [25]. Imaging technologies such as magnetic resonance imaging (MRI), positron emission tomography (PET), and computed tomography (CT) are increasingly being integrated with molecular biomarkers to refine diagnosis and treatment decisions [25]. Recent advances in high-throughput technologies, including genomics, proteomics, metabolomics, and multi-omics integration, as well as artificial intelligence (AI) have accelerated the discovery of novel biomarkers that can distinguish normal aging from disease-related changes [26].
Early cancer detection remains one of the most effective strategies for improved treatment outcomes, extended survival rates, and reduced patient suffering and economic burden [27]. Approximately 50% of cancers are still diagnosed at advanced stages, when treatment success is limited [27]. For example, when breast cancer (BC) is diagnosed at its earliest stage, the 5-year survival rate is approximately 100%. In contrast, late-stage diagnosis reduces survival to about 30% [28]. A similar trend is seen in colorectal cancer (CRC), where early detection ensures survival rates above 90%, but late detection drops survival to 10% [28]. These significant contrasts underscore the urgent global need for safe, cost-effective, accessible early diagnostic methods [29,30,31].
Throughout the entire cancer treatment process, biomarkers play indispensable roles. They support risk assessment, early detection, treatment selection, therapy monitoring, and recurrence prediction [25]. By aligning treatment with each patient’s molecular profile, biomarkers form the foundation of personalized medicine [32].
Importantly, cancer disproportionately affects older adults. The majority of cases occur in those over 50 [33]. However, this population often faces additional challenges, including multiple comorbidities, immune changes, and increased inflammation, all of which can obscure or complicate diagnosis [34]. Such factors can also affect the interpretation of biomarkers, making age-specific validation studies essential to maintain diagnostic sensitivity (SN) and specificity (SP).
This review provides a comprehensive overview of the shared and distinct biological mechanisms underlying aging and cancer, explores key biomarkers, details recent advancements in diagnostic technologies leveraging these biomarkers, and discusses current limitations and future research directions (Figure 1).
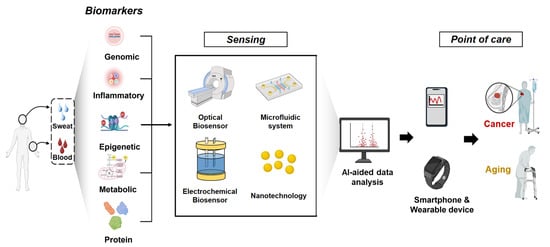
Figure 1.
Schematic illustration of the integrated non-invasive biomarker detection and AI-assisted biosensing workflow. The process begins with non-invasive sampling, such as liquid biopsy (blood, saliva, or urine collection), followed by biomarker sensing and signal transduction through advanced bio-sensing platforms. The detected signals are processed using AI-driven data analysis and transmitted to smartphones or wearable devices, enabling individuals to monitor disease biomarkers in real time and receive personalized medical guidance or early intervention.
3. Distinct Pathways: Where Aging and Cancer Diverge
Although aging and cancer share common mechanisms, such as genomic instability, chronic inflammation, and metabolic changes, they affect the fate of cells and tissues differently. Aging involves suppressing the proliferation of damaged cells and maintaining homeostasis. In contrast, cancer evades these inhibitory mechanisms, inducing unlimited proliferation and tissue destruction [205,206]. This section addresses the divergence points between aging and cancer, from the cellular to the tissue level (Figure 3).
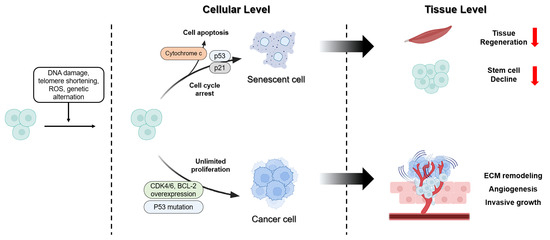
Figure 3.
Distinct pathway of cancer and aging in cellular level and tissue level. Cellular stress responses are induced by DNA damage, telomere shortening, ROS, and genetic alterations. In response to these stimuli, some cells undergo cell cycle arrest and apoptosis via the p53/p21 pathway, resulting in the formation of SnCs. Conversely, alterations such as p53 mutations and the overexpression of CDK4/6 and BCL-2 promote unlimited proliferation and drive the transition toward cancer cells. These dis-tinct cellular outcomes result in different tissue level. The accumulation of SnCs reduces tissue re-generation and stem cell decline. In contrast, the proliferation of cancer cells promotes ECM re-modeling, angiogenesis, and invasive growth, thereby enhancing cancer progression.
3.1. Cellular Fate Decisions
Cells do not divide indefinitely; they cease dividing once they reach a certain number of divisions. As they approach this limit, their division rate slows and they enter a quiescent state, halting division [207]. The process of aging is primarily induced by three distinct pathways. The initial damage to DNA and subsequent shortening of telomeres serves as crucial catalysts for the activation of the DNA damage response. This response prompts the relocation of the p53 transcription factor into the nucleus, where it facilitates the transcription of the cyclin-dependent kinase inhibitor 1A (CDKN1A) gene. This results in the production of p21 [206]. p21 binds to specific CDK proteins, preventing them from binding to cyclin proteins, and ultimately inducing cell cycle arrest. Thereafter, the cell cycle is arrested due to the presence of ROS. Increased internal ROS production activates the p38/MAPK signaling pathway, promoting TP53/p53 transcription. This upregulation of TP53/p53 transcription subsequently induces p21 and CDK activity, resulting in the arrest of the cell cycle [208]. Finally, the cell cycle is arrested through age-related CDKN2A depression. CDKN2A depression activates alternative splicing of CDKN2A mRNA, generating ARF tumor suppressor and p16 proteins. The resulting ARF activates the preceding p53 pathway, inducing p21 expression to arrest the cell cycle. Concurrently, p16 directly binds to specific CDK proteins, forming a complex that inhibits CDK-cyclin complex formation, thereby inducing cell cycle arrest [209].
Cancer cells possess the destiny of unlimited proliferation, unlike SnCs which undergo limited proliferation. Cancer forms through pathways involving the tumor suppressor protein p53, encoded by the p53 gene, and the tumor suppressor protein RB [210]. Both pathways induce arrest at checkpoints in the cell cycle [211,212]. However, when problems occur, such as p53 mutations or loss of the RB pathway, these checkpoints fail to function, allowing growth signals to be ignored. This can lead to cells becoming cancerous [213]. The disruption of cell arrest is a consequence of CDK4/6 expression. In cases of excessive CDK4/6 activation, the G1/S transition accelerates, causing cells entering the S phase prematurely. This occurs before the completion of DNA damage repair or replication stress checkpoint responses. This process can result in the accumulation of replication errors and DNA breaks. Furthermore, CDK4/6 has been shown to phosphorylate non-canonical substrates, thereby disrupting mitotic spindle assembly and increasing the risk of chromosome mis-separation [214,215].
When cells sustain damage from factors such as DNA damage, oxidative stress, and telomere shortening that is reparable, they induce cell cycle arrest. However, when the extent of cellular damage is irreparable, the cell undergoes cell death, such as apoptosis or necrosis [216,217]. Cell cycle arrest or apoptosis functions as a tumor suppression mechanism by halting the proliferation or eliminating damaged cells. The process of apoptosis can occur via two distinct pathways: the intrinsic and the extrinsic pathways [218]. The intrinsic pathway is induced by various stimuli such as oxidative stress, DNA damage, and kinase inhibition, and primarily involves mitochondria and the apoptosome. When apoptosis is induced, mitochondria release cytochrome c into the intermembrane space. Cytochrome c binds to the apoptotic peptidase activating factor 1 (Apaf-1) and procaspase-9 in the cytoplasm, forming a multiprotein complex called the apoptosome. This complex activates caspase-9 via a proximity-mediated mechanism in the presence of ATP or dATP. Activated caspase-9 then activates caspase-3, caspase-6, and caspase-7 [219]. The extrinsic pathway is initiated by the binding of Fas ligand to its receptor, thereby inducing apoptosis. Fas ligand binding to the Fas receptor (CD95) results in the trimerization of the Fas receptor. The cytoplasmic tail of the receptor has been observed to recruit FADD (Fas-associated protein with death domain) through interaction between the death domain of FADD and the Fas receptor [219]. Subsequently, FADD assembles the procaspase-8 molecule, and caspase-8 is activated via proximity-mediated activation. Activated caspase-8 can then activate caspase-3 and -7, which in turn initiate apoptosis [220].
However, in cancer, this process does not exert sufficient effects to kill cells through apoptosis. Cancer cells evade apoptosis through survival strategies, enabling continued proliferation [221]. The p53 mutation, a cause of cancer cell proliferation, is known to be a mechanism for evading apoptosis [222]. Additionally, in cancer, overexpression of B-cell lymphoma-2 (BCL-2) and BCL-XL inhibits apoptosis by blocking the mitochondrial regulator pathway, thereby increasing survival rates [223,224,225]. Furthermore, increased expression of inhibitors of apoptosis proteins suppresses caspase activity, thereby evading apoptosis.
The process of aging is characterized by a gradual decrease in the body’s ability to repair and replace itself. This decline is attributed to cell cycle arrest and a decrease in proliferative stem cells (SCs), which collectively result in a weakened capacity to maintain tissue integrity and function. However, it has been observed that tissue structure can maintain relative homeostasis until the terminal degenerative stage [226]. In contrast, cancer destroys normal tissue structure through invasive growth, angiogenesis, and ECM remodeling driven by unlimited proliferation, causing loss of normal function and replacement by cancerous tissue [221].
3.2. Tissue-Level Manifestations
The effects of aging manifest changes not only in cells but also in tissues. Aging is the most significant factor in age-related diseases such as neurodegenerative diseases, cardiovascular diseases (CVDs), and metabolic diseases [227]. These diseases can result in a loss of normal function, disability, or death in severe cases [228]. Aging is associated with a progressive decline in physiological functionality, which arises from diminished tissue regenerative capacity and SC depletion. This process has been shown to lead to a reduction in immune system function and an enhancement in inflammatory responses [229]. For instance, the process of aging is associated with a series of changes in the structure and function of skeletal muscle tissue. These changes are the result of a reduced energy metabolism rate and they lead to a loss of muscle function. In healthy, well-nourished individuals, this process typically progresses slowly. Among the numerous changes associated with aging, the reduction in muscle mass and function is a well-known and significant change, termed sarcopenia [230,231]. A notable finding is that heterochronic parabiosis can restore the proliferation and regenerative capacity of SCs in aged mice, suggesting that extrinsic factors in the blood, such as sex hormones, α-Klotho, and fibroblast growth factor (FGF), can reverse SC aging [232]. Kim et al. reported that SC depletion in induced paired box 7 (Pax7) knockout mice induces neuromuscular junction degeneration at a young age and exacerbates muscle fiber atrophy following neuromuscular deterioration [233]. The process of aging has been demonstrated to induce neurodegenerative diseases through the formation of misfolded proteins and reduced neuronal function. For instance, the process of aging is associated with the aberrant accumulation of amyloid β-proteins (Aβ) within neurons, resulting in the formation of senile plaques due to their progressive loss [234]. Aβ self-aggregates accumulate on neuronal membranes, generating ROS and causing membrane lipid peroxidation, which produces 4-hydroxy-2-nonenal. This results in impaired function of membrane ion pumps (ATPases), glucose and glutamate transporters, and disruption of neuronal Ca2+ homeostasis. Consequently, this induces neuronal hyperexcitability, excitotoxicity susceptibility, and metabolic depletion, ultimately leading to Aβ neurotoxicity [235,236]. Moreover, an increase in misfolded proteins and tau protein aggregation has been observed to induce Aβ oligomerization, which may contribute to the development of Alzheimer’s disease (AD) by impeding synaptic plasticity and signaling. The aggregation of tau protein can also result in the formation of neurofibrillary tangles. These phenomena progressively accumulate in the brain and can cause AD [237].
In cancer, cells evade cell cycle arrest by inhibiting the p53 and RB pathways, thereby repeating cell proliferation. This phenomenon can be considered a characteristic of cancer cells that survive beyond their normal lifespan [238]. The process by which normal cells transform into cancer cells occurs not only during early carcinogenesis but also during tumor metastasis. Cancer development manifests through multiple stages. Changes in signaling pathways due to epigenetic alterations—caused by mutations in tumor suppressor genes and oncogenes, DNA methylation (DNAm), or histone modifications—lead to cells becoming resistant to regulation by growth factors and hormones. This enables them to survive independently, resulting in malignant transformation [239,240,241]. Additionally, malignant transformation occurs due to decreased E-cadherin expression. This phenomenon is associated with changes in cellular phenotype, including EMT and cell migration [242]. Through metabolic reprogramming, cells shift their energy production and metabolic pathways, transitioning to a metabolic state prioritizing growth and proliferation [243]. Despite sufficient oxygen availability, cells maintain glycolysis-dependent pathways via the Warburg effect instead of mitochondrial oxidative phosphorylation [244]. This creates a hypoxic environment within the tumor, activating HIF-1α, promoting the survival and growth of tissue SCs, and even inducing angiogenesis [245].
4. Biomarkers in Aging and Cancer
Aging and cancer share common biological pathways as well as distinct ones. Along these pathways, various biomarkers are generated, which can serve as potential tools for early diagnosis (Figure 4).
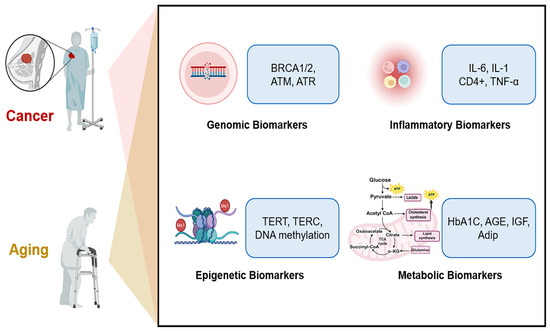
Figure 4.
Biomarkers in aging and cancer. Aging and cancer are interconnected through various mechanisms, including genomic instability, chronic inflammation, epigenetic alterations, and metabolic dysregulation. Along these pathways, specific biomarkers are generated—genomic biomarkers (e.g., BRCA 1/2, ATM, and ATR), inflammatory biomarkers (e.g., IL-6, IL-1, and TNF-α), epigenetic biomarkers (e.g., TERT, TERC, and DNAm), and metabolic biomarkers (e.g., HbA1C, AGE, and IGF).
4.1. Genomic Biomarkers
The genomes of organisms are continuously exposed to diverse external environments and endogenous stress, leading to molecular-level changes that induce aging and cancer [39,246]. Furthermore, many cancers exhibit genomic instability, which can lead to the loss or gain of large chromosomal regions. This instability can also result in base-level substitutions, insertions, and deletions, which can ultimately induce malignant transformation in cancer cells [247]. Ultimately, the continuous accumulation of genomic damage increases the incidence of age-related diseases and cancer [248].
Recent findings have identified mutations driven by genomic instability as a critical enabling characteristic that facilitates cancer development in most aging and cancers [39,246]. For example, mouse models with DNA repair defects exhibited an aged vascular phenotype. In smooth muscle cell-selective ERCC1 Knockout (SMC-KO) mice, the loss of the DNA repair protein ERCC1 in vascular smooth muscle cells (VSMCs) acts as a key aging factor. ERCC1 deficiency causes DNA damage accumulation, promoting increased p16 and p21 expression and inflammatory responses. These accelerated non-atherosclerotic vascular aging in SMC-KO mice [249]. Administration of the phosphodiesterase 1 (PDE1) inhibitor ITI-214 showed temporary improvement in vascular function [249,250]. In human, DNA repair defects are the underlying cause of progeria syndromes [251]. In contrast, breast epithelial tissue from women with germline mutations in the BRCA1 or BRCA2 genes exhibits DNA repair defects, leading to an elevated risk of breast and ovarian cancer, as well as premature aging [252]. Both ATM and ATR are indispensable kinases in the DNA damage response, each with specific roles in sensing and responding to different types of DNA lesions [253]. ATM primarily responds to DNA double-strand breaks, while ATR is crucial for replication stress and ssDNA. Their proper function is vital for genomic stability, development, and cancer prevention, and their inhibition or deletion can lead to severe developmental defects and genomic instability [253].
Beyond nuclear DNA, mitochondrial DNA (mtDNA) can also undergo mutations and deletions due to external or internal stressors, potentially contributing to aging and cancer [254]. Mouse models with mtDNA mutations exhibit premature aging phenomena such as osteoporosis, muscle atrophy, and respiratory disorders [255]. In mouse with mtDNA damage due to DNA polymerase γ deficiency, aging and shortened lifespan were observed [256]. Furthermore, human diseases associated with mtDNA damage also exhibit aging-like phenotypes, suggesting that mtDNA mutations could serve as biomarkers for aging [257].
A multitude of studies provide evidence that the process of aging and the development of cancer are associated with genomic instability. Additionally, interventions that target the reduction in DNA damage and the enhancement of repair processes may potentially postpone the onset of cancer, including age-related diseases.
A promising study has been reported that systematically investigated the genetic architecture of brain aging and identified potential drug targets and repurposed drugs capable of extending healthy lifespan, primarily utilizing brain aging gap (BAG) as a core biomarker. This study utilized MRI data from a large cohort of healthy individuals in UK Biobank and established a brain age model and systematically identified genes that could serve as therapeutic and prognostic markers for aging and related diseases such as AD, using the brain age gap (BAG) as an indicator [258]. The BAG is the difference between the predicted age from MRI data and chronological age and is considered a promising indicator of brain health [258]. A positive BAG value indicates accelerated brain aging, whereas a negative BAG value indicates slowed brain aging [258]. The subjects with brain disorders such as AD, dementia, and schizophrenia have higher BAG values than healthy subjects [258]. Identified genetic factors responsible for BAG offer insights into the genetic basis of brain aging and potential avenues for drug development to promote healthy aging [258]. Seven key genes and their relationships with BAP are summarized in Table 1 and genomic biomarkers are summarized in Table 2.

Table 1.
Seven Genetically Supported Biomarker Genes for Brain Aging [258].
4.2. Epigenetic Biomarkers
Epigenetic changes influence gene expression and key cellular functions, contributing to aging and cancer development. These changes include alterations in DNAm patterns, post-translational modifications of histones, and non-coding RNAs (ncRNAs), and are recognized as markers of aging and cancer [259].
DNAm in aging generally exhibits hypomethylation across genes. In contrast, many cancers show the opposite pattern of hypermethylation. Cancer has been observed to manifest epigenetic characteristics, such as the reprogramming of DNAm patterns, including the phenomenon of hypermethylation [260,261]. Thus, epimutations—epigenetic changes—primarily occur in intronic or intergenic regions, driving aging and cancer, but also arise through methylation and silencing of key tumor suppressor genes like p16 and p53 [262]. Notably, DNA (cytosine-5)-methyltransferase 3A (DNMT3A), which functions in de novo methylation, and TET methylcytosine dioxygenase 2 (TET2), which initiates demethylation, are frequently mutated in clonal hematopoiesis of indeterminate potential, known as an indicator for aging-related diseases like coronary heart disease and hematological cancers [263,264,265]. Recently, DNMT inhibitors are being studied for treating blood cancers and solid tumors. DNMT inhibitors feature a modified cytosine ring structurally similar to naturally occurring nucleosides. They are incorporated into DNA during replication in place of cytosine, thereby inactivating DNMTs [266,267]. For example, first-generation DNMT inhibitors include 5-azacytidine and 5-cytidine-2’-decitabine are first-generation DNMT inhibitors. Nucleoside analogs overcoming these limitations were subsequently developed [268]. Guadecitabine (SGI-110) is an improved second-generation form. Clinical studies for solid tumor treatment are underway using combination therapy with immunotherapy and anticancer agents [269,270,271]. Current studies are focused on the lysine (K)-specific demethylase (KDM) family, with a particular emphasis on exploring cancer treatments that involve the inhibition of KDM activity through the use of small molecules such as Iadademstat [272,273]. Promoter methylation is a significant regulatory element for TERT expression, correlating with both TERT mRNA levels and telomerase activity [274]. While the TERT promoter region around the transcription start site typically unmethylated in actively transcribed TERT, hypermethylation of the TERT gene has been shown to correlate with telomerase activity in various cancers [275]. The downregulation of TERT expression via epigenetic modifications such as TERT promoter methylation can significantly influence clinical study design [276].
In aged cells and cancerous tissues, a variety of post-translational modifications of histones, including acetylation, methylation, and phosphorylation, have been observed. These modifications arise in the N-terminal region of histones, leading to abnormalities in DNA binding and dynamically altering chromatin structure, which can disrupt gene transcription, metabolic regulation, and the maintenance of cellular homeostasis [277].
Histone methylation is the process of transferring a methyl group from S-adenosyl methionine to lysine or arginine residues. This process is catalyzed by lysine methyltransferases and arginine methyltransferases. Recent studies have indicated that histone methylation marks, including H3K4me3, H3K27me3, and H3K36me3, demonstrated significant alterations during the aging process [278]. These methylation abnormalities exhibit tissue-specific patterns in aging-related diseases. For example, Sun et al. has found that the level of H3K4me3 is increased in the HSCs of aging mice [279]. In neurodegenerative diseases such as AD, neurons in the prefrontal cortex show a significant increase in H3K9me2 and a decrease in H3K27me3, which leads to the silencing of genes associated with synaptic plasticity [280]. Similarly, in muscle atrophy, aging mouse muscles show elevated levels of H3K27me3, which inhibits the differentiation of muscle stem cells and muscle regeneration [281]. H3K36 methylation in the intestinal epithelium has several practical implications, particularly in understanding and potentially treating diseases related to cell plasticity, such as cancer, and in advancing regenerative medicine [282].
Histone acetylation and deacetylation are processes that add or remove acetyl groups, respectively, from lysine residues protruding from nucleosomes. These processes are closely associated with major cellular functions, including DNA replication, DNA damage repair, and RNA transcription. These reactions are primarily catalyzed by histone acetyltransferases (HATs) or histone deacetylases [283]. Qui et al. revealed that the expression and catalytic activity of major HATs, which is p300/CBP, decrease with aging and their nuclear localization also undergoes alterations [284]. In contrast, reducing p300/CBP, enzymes catalyzing H3K27ac, lowered homeostatic amyloid-reducing genes’ expression and increased secretion of toxic Aβ(1–42) in iPSC-derived neurons from familial AD patients carrying an amyloid precursor protein duplication. These results suggest that H3K27ac-driven transcriptional programs act as compensatory mechanisms to counteract APP-related pathology, indicating a protective role of H3K27ac in mitigating AD progression [285].
Histone phosphorylation is a process that adds negative charges to the side chains of serine, threonine, and tyrosine residues. This modification of the proteins affects the structure of the chromosomes, allowing them to interact with transcription factors. In turn, these factors regulate the expression of genes that are involved in the cell cycle and proliferation [286]. The dysregulation of histone phosphorylation, characterized by the abnormal accumulation of DNA damage-related phosphorylation markers and reduced efficiency of signal-responsive phosphorylation events, exhibits substantial reprogramming properties, emerging as a biomarker of aging. In aged cells, there is a marked increase in basal γ-H2AX levels, indicating heightened genomic instability [287]. Another phenomenon is a reduced ability to form new γ-H2AX foci upon damage, reflecting diminished repair efficiency [288]. The MAPK and Aurora kinase pathways have been identified as critical regulators of aging-associated phosphorylation processes. p38-MAPK has been observed to remain constitutively activated in aged cells, a process that has been shown to promote NF-κB influx and induce SASP production [289]. In contrast, Aurora B kinase activity exhibits a decline with aging, resulting in chromosome segregation errors and karyotype instability [290].
Mutations in chromatin remodeling complexes predict cancer aggressiveness. Various cancers frequently contain mutations in components of the ATP-dependent SWI/SNF chromatin remodeling complex (SWI/SNF complex) [291]. These mutations are found in approximately 20–25% of all human cancers and impact the complex’s ability to regulate gene expression by altering how DNA is packaged [292]. In PTEN-deficient cells, BRG1 (SMARCA4) regulates c-Myc and MAPK signaling, and stabilization of BRG1 maintains tumor cell growth [293]. Elevated BRG1 expression in PTEN-deficient prostate cancer (PCa) cells resulted in chromatin remodeling that facilitated a protumorigenic transcriptome, thereby increasing the cells’ dependency on BRG1 [293]. BRG1 inhibitors (e.g., PFI-3) suppress tumor progression in PTEN-deficient preclinical models, suggesting BRG1 is a promising target for these cancers [294].
Non-coding RNAs are characteristic features observed in aging and cancer. Examples include long ncRNAs (lncRNAs) (greater than 200 nt), microRNAs (miRNAs) (~22 nt), small nucleolar RNAs (sno RNAs), and small interfering RNAs (siRNAs) [295]. These elements do not function as templates for protein synthesis. Instead, they exert their influence on post-transcriptional pathways, with implications for both longevity and carcinogenesis [296]. Studies in cell and animal models demonstrating the acquisition of new functions or the loss of existing functions have proven that ncRNAs, including miRNAs, play a causally important role in aging and cancer. For example, miR-21 is found to be upregulated in T cells from older adults (65–85 years) compared to younger individuals, and in replicative senescent endothelial cells [295]. In age-related CVD, miR-21, miR-34a, miR-92a, and miR-146a are upregulated, whereas miR-125b, miR-126a, miR-142, and the miR-30 family are downregulated [295]. miR-455-3p deficiency causes cognitive decline and shortened lifespan in mice [297], while reduced miR-455-3p expression in human cancer cells increases cell proliferation and invasiveness [297]. Conversely, miR-455-3p overexpression protects neural function and extends lifespan, and this miRNA also inhibits tumor growth in a liver cancer transplant model [298]. In the molecular pathogenesis of PCa, miR-21, miR-221, and miR-1290 facilitate tumor proliferation, invasion, and therapeutic resistance, whereas miR-375 exerts tumor-suppressive effects by constraining EMT [296]. lncRNAs such as MALAT1, NEAT1, PCAT-1, and SCHLAP1 promote oncogenic signaling and metastasis, while PCAT-14 functions as a tumor suppressor [296]. The prostate-specific lncRNA PCA3 has been clinically implemented as a diagnostic biomarker [296]. Epigenetic biomarkers are summarized in Table 2.

Table 2.
Summary of genomic and epigenetic biomarkers in aging and cancer.
Table 2.
Summary of genomic and epigenetic biomarkers in aging and cancer.
| Biomarker Class | Representative Molecules | Sample Type | Detection Platform | Evidence Level |
|---|---|---|---|---|
| Genomic | ERCC1 defect | Mouse VSMC | SMC-KO | accelerated, nonatherosclerotic vascular aging in mouse model [249] |
| PDE1 | upregulated in aorta | qRT-PCR | Decreased vasodilation function in aging mice [249,250] | |
| BRCA1, BRCA2, | Normal breast tissue carrying germline mutations (BRCA1, BRCA2) | IF, Flow Cytometry, RNA-seq | Clinical, accelerated biological aging phenotypes [252], increase susceptibility to breast cancer [252], | |
| ATM, ATR | Mouse Models | knockout, knockin, transgenic mouse models | Preclinical Data [253] | |
| mtDNA | mtDNA deletions Human tissues (skeletal muscle, brain, colonic crypts); Mouse tissues | NGS, ddPCR | Fundamental Phenotype of aging in mouse, fly, and worm models [254] | |
| mtDNA | Mouse tissues, mouse cells, blood | Histochemical Analysis, RT PCR, Oxygen Electrode | Respiration defects, development of B-cell lymphoma, in vivo and in vitro [255] | |
| Primary Mitochondrial Diseases | Blood, Muscle DNA, Uroepithelial cells | NGS, WGS, and RFLP testing | age-related neurogenetic disorders [257] | |
| Brain Age Gap | MRI data in UK biobank Tissues, blood | MRI, DL | Genetically Supported Druggable Genes, a large cohort [258] | |
| Epigenetic | DNMT3A mutations, TET2 mutations | Blood | Targeted deep exome sequencing (custom panel), Illumina NovaSeq 6000 platform | Clinical, Associated with higher average age and increased risk of CVD [264] |
| DNMT1, DNMT3a, DNMT3b | Cancer tissues | DNA methylation assays | Aberrant expression associated with tumor development [266], in vitro, in vivo | |
| LINE-1, IL22RA1, PRAME, PAX8, GAGE2A, B2M | Blood, Tumor samples | Pyrosequencing, Illumina array | phase 1 dose-escalation study (NCT02998567) [269] | |
| KDM1A | HCC, Xenografts in Nude Mice | Western blot, flow cytometry qRT-PCR | role of KDM1A in sorafenib resistance of HCC, in vitro, in vivo [273] | |
| TERT | TERT Hypermethylated Oncological Region | DNA methylation assays, NGS | Human Tumors, cell lines, normal tissue and cells [275] | |
| H3K4me3; H3K27me3 | Aged hematopoietic stem cells (HSCs) | RNA-seq, ChIP-seq | H3K4me3 levels increase in aged HSCs; In vitro, in vivo [279] | |
| H3K9me2; H3K27me2 Catalyzed by KDM7A | Brain Regions; Cell Lines | Western blot, ChIP-qPCR qRT-PCR | in vitro, in vivo [280]. | |
| H3K27ac (Catalyzed by EP300/CBP) | AD patient brains; iPSC-derived neurons (AD model) | ChIP-seq; RNA-seq, ELISA | In vitro iPSC-neuron model; Comparison to Human Brain Data [285] | |
| H3K36me3 | Mouse small intestine, Intestinal organoids | RNA-seq | in vitro and in vivo models [282] | |
| SWI/SNF mutations | Tumor tissue samples | CRISPR screening | in vitro and in vivo models [291] | |
| BRG1 (component of SWI/SNF) | PTEN-deficient PCa cells (PCa model) | ChIP-Seq; RNA-Seq | In vitro, in vivo [293] | |
| miR-21 | Blood, tissue (breast, colorectal, leukemia, lung, prostate | qRT-PCR or sequencing | Diagnostic and prognostic biomarker for CRC; biomarker for CVD [295,296] | |
| miR-455-3p | Tumor tissues (Osteosarcoma, HCC, Esophageal Squamous Cell Carcinoma, BC) | qRT-PCR | Functions as a tumor suppressor (HCC); potential target for diagnosis and prognosis in Osteosarcoma (OS) [297,298] | |
| PCA3 (PCa Antigen 3) | urine | Molecular urine analysis | First FDA-approved ncRNA cancer biomarker test; used for diagnosis of PCa [296,299] |
Abbreviations: IF; Immunofluorescence, NGS; Next-generation sequencing, ddPCR; Droplet Digital PCR, RT-PCR; Reverse Transcription-PCR, WGS; whole genome sequencing, RFLP; Restriction Fragment Length Polymorphism, qRT-PCR; quantitative real time PCR, ELISA; Enzyme-Linked Immuno-sorbent Assay, ChIP-seq; Chromatin immunoprecipitation sequencing.
Epigenetic changes and aging share a complex relationship, and the epigenetic clock serves as a powerful tool for quantifying an individual’s biological age and rate of aging. The DNAm clock, often referred to as the epigenetic clock, is a powerful tool designed to estimate biological age by analyzing aging-related DNAm changes [300]. By examining genome-wide methylation profiles, these clocks offer predictive insights into mortality and age-related disease risks, effectively differentiating biological age from chronological age and addressing persistent inquiries in gerontology. Beyond blood, DNAm-based age prediction has been studied across diverse tissues and body fluids, with enhanced accuracy observed when integrating data from multiple sources [301]. The performance of DNAm clocks can vary depending on in vitro conditions, and challenges persist in their broad application and interpretation. Continued refinement is essential to establish these clocks as robust biomarkers of aging and functional decline [300]. DNAm clocks can be broadly categorized into first-generation clocks (primarily predicting chronological age) and second-generation clocks (predicting biological age and health-related factors) [302].
The first-generation clocks, including Hannum, Horvath, and Weidner, can predict chronological age more accurately [303]. Hannum et al. introduced apparent methylomic aging rates (AMARs), influenced by sex and genetic variants, highlighting DNAm as both a marker and potential regulator of human aging processes [304]. Horvath’s clock introduced the first multi-tissue DNAm age estimator applicable to all human tissue sources (except sperm) and the entire lifespan (from fetal samples to individuals over 100 years old) [305]. Weidner et al. showed that blood aging can be trackable using DNAm changes at as few as three specific CpG sites within or near the ITGA2B, ASPA, and PDE4C genes, highlighting the precision and efficiency of these markers [306].
While 1st-generation clocks accurately predict chronological age, they lacked insights into biological age measurement and disease prediction, leading to the development of 2nd -generation clocks such as GrimAge and PhenoAge [307,308]. They have proven valuable in predicting disease risk and mortality [309,310,311]. GrimAge, in particular, strongly predicts lifespan and healthspan [312]. PhenoAge is an algorithm that estimates biological age, utilizing nine blood biomarkers, including albumin, creatinine, glucose, C-reactive protein, Lymphocyte percent, mean cell volume, red cell distribution width, alkaline phosphatase, white blood cell count to measure an individual’s physiological health status, and degree of aging [308]. DunedinPACE was designed to quantify the pace of biological aging by analyzing 20 years of longitudinal data (19 indicators of multi-organ system integrity) [313].
While existing clocks simply select CpGs with the strongest correlation to age, CausAge, AdaptAge, and DamAge clocks represent an attempt to interpret the aging process more mechanistically by identifying CpG sites with causal relationships [314]. CausAge is a causality-enriched clock constructed using CpG sites causally related to aging. CausAge was developed using an ElasticNet regression model with 586 CpG sites. AdaptAge builds upon CausAge by being designed to capture CpG sites associated with adaptive responses to aging. DamAge is a clock constructed using only CpG sites associated with damage. The development of AdaptAge and DamAge focuses on enhancing predictive accuracy and interpretability for aging-related phenotypes by separating aging markers into ‘damage’ markers and ‘adaptation’ markers [314]. Meanwhile, traditional epigenetic clocks, trained on bulk tissues, are influenced by age-related changes in immune cell composition [315]. This makes it difficult to distinguish between true cellular aging and shifts in cell type proportions.
The IntrinClock developed by Tomusiak et al. addresses this by being designed to be unaffected by changes across 10 immune cell types, including CD8+ T-cell subsets [315]. This resistance to immune cell compositional changes makes it a more accurate measure of cell-intrinsic aging processes. A blood-based epigenetic clock for intrinsic capacity (IC) was developed [316]. This is a clock specifically trained to predict an individual’s Intrinsic Capacity (IC), a key indicator from the clinical perspective of aging [316]. The IC represents a composite score across five domains: cognition, mobility, psychological well-being, sensory function, and vitality. When analyzed using Framingham Heart Study data, the IC Clock was found to predict all-cause mortality risk more strongly than 1st- and 2nd-generation clocks (HR = 1.38). Furthermore, the CpG sites included in the IC Clock showed little overlap with existing clocks, indicating that it captures unique aspects of aging biology [316].
Jacques et al. has created an “Aging Atlas” mapping aging across 17 human tissues using DNAm analysis of 15,000 samples from adults aged 18–100 [317]. After mapping methylation changes across 900,000 potential locations within DNA, they created an open-access atlas. Most tissues exhibited age-associated hypermethylation, particularly in regions with low methylation in younger individuals, suggesting a coordinated increase in methylation at previously unmethylated sites. The exceptions are skeletal muscle and the lungs, where methylation loss is greater with aging. The overall global mean methylation across the analyzed tissues ranged from 38% to 63% [317]. The retina displayed the highest value, with a 63% average methylation rate, followed by the stomach (57%), heart (53%), muscles (51%), skin (48%), and cervix (35%), which suggests the retina ages fastest, in contrast to the cervix, which ages slowest [318]. The study identified PCDHGA1 as one of the key disruptors that exacerbates aging signals in various tissues, alongside MEST, HDAC4, and HOX genes, as well as a resilient NAD+ salvage pathway module [317]. The comparison of DNAm clocks is summarized in Table 3.

Table 3.
Comparison of DNAm clocks.
4.3. Inflammatory Biomarkers
An increase in inflammatory levels has been identified as a contributing factor in both the aging process and the development of cancer. In the process of aging, this phenomenon is known as inflammaging, which, when repeatedly accumulated, results in the development of inflammatory aging-related diseases, including osteoarthritis, sarcopenia, and neuroinflammation [319,320]. Similarly, inflammation establishes a tumor-promoting environment and is considered one of the enabling characteristics of cancer [321].
During aging, the sustained secretion of cytokines associated with SASP contributes to the transition toward inflammaging. Among these, IL-6 and IL-1β are the most representative cytokines [96]. IL-6 regulates immune cell differentiation and proliferation, and its elevated systemic levels contribute to chronic inflammatory states that underlie diseases such as sarcopenia, cardiovascular disorders, and insulin resistance. IL-1β, a major component of SASP, is strongly linked to systemic inflammatory responses [99]. In cancer, both IL-6 and IL-1β promote tumor growth and enhance invasiveness. Specifically, IL-6 activates the STAT3 signaling pathway, leading to anti-apoptotic effects and promoting cell proliferation, thereby enabling sustained tumor growth by evading cell death [102]. IL-1β increases invasiveness and fosters a pro-tumorigenic immune microenvironment, thus facilitating carcinogenesis. Another key cytokine involved in both aging and cancer is TNF-α. In aging, TNF-α promotes tissue damage and neuroinflammation, while in cancer, it activates NF-κB signaling, enhancing angiogenesis and metastasis, ultimately creating a microenvironment that supports tumor survival [168].
Tsukamoto et al. demonstrated that in aged environments, impaired Th1 differentiation of CD4+ T cells reduces antitumor immunity, which can be restored by IL-6 blockade or deficiency [322]. IL-6 inhibition further promotes CD8+ T cell-dependent tumor elimination through the restoration of CD4+ T cell function in an IFN-γ-dependent manner. Mechanistically, IL-6 has been shown to induce the transcription factor c-Maf expression, leading to increased production of IL-4 and IL-21, which have been observed to suppress Th1 differentiation. Additionally, IL-6 has been found to enhance IL-10 production by CD4+ T cells, thereby dampening CD8+ T cell responses [323]. Mei et al. demonstrated that pathological levels of TNFα, IL-6, and IL-10 suppress erythroid differentiation and impair hematopoiesis by inducing apoptosis through a ROS-mediated caspase-3 pathway [324]. The recent randomized phase II clinical trial, the NORDIC9 study, C-reactive protein (CRP) demonstrated the strongest prognostic value for overall survival of elderly metastatic CRC patients. CRP indicated it could guide decisions regarding palliative chemotherapy in vulnerable patient populations [325]. Chitinase-3-like protein-1 (CHI3L1) or YKL40 is a secreted glycoprotein involved in inflammation, macrophage polarization, apoptosis, and cancer [326]. Its expression increases in various inflammatory and immune diseases. Acting via cytokines like TNF-α and IL-6, CHI3L1 serves as a diagnostic and prognostic marker and a potential therapeutic target in inflammatory diseases. Inflammatory biomarkers are summarized in Table 4.
4.4. Metabolomic Biomarkers
In the body, glucose and its oxidative by-products permanently interact with the amino groups of both intra- and extracellular long-lived proteins, DNA, and lipids. This series of Maillard reactions is referred to as non-enzymatic glycosylation or glycation, resulting in the production of a diverse array of compounds termed advanced glycation end-products (AGEs) [327]. Glycation has emerged as a key metabolic hallmark of aging. It progressively alters the structure and function of long-lived macromolecules in skeletal muscle, skin, arteries, and nerves [328,329]. Structural proteins such as collagen, elastin, and myosin undergo glycation, leading to increased stiffness and loss of elasticity—a characteristic similar to age-related tissue degeneration [330]. Among measurable markers, glycated hemoglobin (HbA1c) is widely used as the diagnostic tool for diabetes and prediabetes [331]. Diagnostic thresholds for prediabetes are 5.7–6.4% (39–46 mmol/mol) and for diabetes are ≥6.5% (48 mmol/mol). To increase the diagnostic SN, 2 h post-load glucose measurement from an oral glucose tolerance test (OGTT) has been recommended for use alongside H1bA1c [331]. Several specific AGE molecules are commonly studied as biomarkers due to their prevalence and pathological significance [327]. Nε-Carboxymethyl-lysine (CML) is formed when methylglyoxal reacts with lysine. It is widely used as a general marker of glycation. Nε-(1-carboxyethyl)lysine (CEL) is found in human lens proteins and is a product of chemical modification by methylglyoxal. Glucosepane is identified as the most common AGE found in type I collagen. N2-Carboxyethyl-2’-deoxyguanosine is found in DNA and is considered a potential biomarker for chronic hyperglycemia [327]. AGEs interact with their receptor (RAGE) to induce oxidative stress and inflammation. The soluble RAGE (sRAGE) isoforms include endogenous secretory sRAGE (esRAGE) and cleaved RAGE (cRAGE) [332]. In healthy controls, cRAGE negatively correlated with age, while AGEs/sRAGE and AGEs/cRAGE ratios positively associated with age. An increase in the AGEs/cRAGE ratio was linked to a higher risk of all-cause mortality in T2D patients. sRAGE was associated with the development of Major Adverse Cardiovascular Events (MACE) in T2D patients [332].
In contrast to the metabolic slowing of aging, cancer cells exhibit metabolic reprogramming characterized by accelerated glucose uptake and glycolysis, even in the presence of oxygen—a phenomenon known as the Warburg effect. This metabolic shift fuels rapid proliferation by supplying ATP and biosynthetic intermediates [333]. These characteristics are particularly utilized as indicators for diagnosing and tracking solid tumors [334]. Key glycolytic enzymes such as hexokinase-2 (HK2) are overexpressed in many cancers. Genetic deletion of HK2 significantly reduced tumor burden and average tumor size in mouse models of LC and BC [335]. Zheng et al. demonstrated the efficacy of reducing glycolysis by selectively binding and inhibiting HK2 using the novel compound Benitrobenrazide (BNBZ) in tumor growth xenograft models [336]. The byproduct of enhanced glycolysis, lactate, accumulates in the TME, promoting cancer progression, angiogenesis, and metastasis, while suppressing antitumor immunity [337,338,339,340]. Lactic acid in the blood or within TME may serve as a predictive and prognostic biomarker for cancer [340]. Lactate dehydrogenase A (LDHA), the enzyme responsible for lactate production, is frequently upregulated in tumors [340]. Its genetic or pharmacologic inhibition suppresses KRAS- or epidermal growth factor receptor (EGFR)-mutant tumor growth [341]. Recent strategies using proteolysis-targeting chimeras (PROTACs) to degrade LDHA have shown promise in PC [342].
Aging and cancer are significantly influenced not only by carbohydrates but also by amino acid metabolism. Among various amino acids, changes in glutamine and tryptophan are particularly important indicators in aging and cancer [343,344]. In aging, glutamine levels decrease, inhibiting the synthesis of the antioxidant glutathione (GSH). This promotes oxidative stress and cellular aging within the body, potentially leading to age-related diseases [345]. Cancer exhibits an excessive dependence on glutamine, often termed glutamine addiction. Glutamine acts as a carbon and nitrogen donor, fueling the tricarboxylic acid (TCA) cycle, nucleotide biosynthesis, and amino acid production. Inhibition of glutaminase-1 (GLS1), the enzyme converting glutamine to glutamate, disrupts these anabolic pathways and attenuates tumor growth [346,347]. Accordingly, GLS1 inhibitors are being investigated as targeted anticancer therapeutics that exploit cancer’s metabolic vulnerabilities [348].
Both aging and cancer are characterized by upregulated tryptophan-degrading enzymes, including indoleamine-2,3-dioxygenase (IDO) and tryptophan-2,3-dioxygenase (TDO) [349]. Tryptophan depletion in the body suppresses T cell function or promotes the regulatory T (Treg) differentiation due to increased kynurenine, thereby inhibiting CD8+ T cells and impairing immune function [349]. In aging, chronic activation of this pathway contributes to immunosenescence, while in cancer it establishes an immunosuppressive microenvironment. For example, Du et al. demonstrated anti-glioma and anti-pancreatic efficacy by targeting IDO/TDO with a novel drug to inhibit the tryptophan/kynurenine pathway [350]. Kim et al. similarly demonstrated that targeting IDO and TDO can overcome checkpoint inhibitor resistance and activate CD8+ T cells [351].
The characteristics of aging and cancer also differ in growth hormone metabolism. GH/IGF-1 axis orchestrates cellular proliferation, metabolism, and lifespan regulation. [352]. Age-related decline in GH and IGF-1 signaling correlates with reduced anabolic activity but enhanced longevity in multiple models [353]. Individuals with growth hormone receptor mutations (Laron syndrome) exhibit markedly reduced risks of cancer, stroke, and T2D [354,355]. Preclinical evidence supports that targeted modulation of this pathway may extend healthspan. Administration of an anti-IGF-1R monoclonal antibody (L2-Cmu) in aged mice reduced tumor incidence, suppressed inflammation, and extended lifespan by approximately 9% [356]. Quipildor et al. reported that chronic IGF-1 overexpression improved motor function and mood in male mice but showed sex-specific metabolic responses, highlighting the complex, tissue-dependent regulation of IGF-1 in aging [357]. In cancer, the same GH/IGF-1 axis functions aberrantly, promoting mitogenesis and resistance to apoptosis. Overactivation of IGF-1R signaling contributes to tumor growth, metastasis, and therapeutic resistance. Thus, inhibitors targeting IGF-1R or downstream effectors are being explored as potential anticancer strategies [358,359].
Adiponectin, an adipocyte-derived hormone, enhances lipid oxidation and insulin SN. Its circulating levels decline under metabolically adverse conditions, correlating with increased oxidative stress [360]; conversely higher levels are associated with frailty in the elderly [361]. Choubey et al. demonstrated that declining adiponectin signaling contributes to age-related testicular dysfunction by impairing insulin SN, metabolism, steroidogenesis, and increasing oxidative stress. Furthermore, it showed that exogenous adiponectin treatment can significantly reverse these regressive changes, suggesting its potential as a therapeutic strategy for improving male reproductive health during aging [362].
Cancer cells reprogram lipid metabolism to sustain rapid proliferation [363]. Enhanced de novo fatty acid synthesis, mediated by fatty acid synthase (FASN), produces palmitate, a vital substrate for membrane synthesis and energy storage [364]. Overexpression of FASN suppresses TNF-α signaling and impairs immune surveillance, promoting tumor progression [365]. Elevated levels of phosphocholine and glycerophosphocholine, intermediates in phospholipid metabolism, have been detected by magnetic resonance spectroscopy (MRS) in BC and PCa serve as diagnostic metabolic signatures [366].
Dysregulated adipokine signaling also contributes to cancer risk. Hypoadiponectinemia is associated with insulin resistance, atherosclerosis, and colorectal tumor development [367]. Mutoh et al. demonstrated that hypoadiponectinemia promotes intestinal polyp formation and CRC, linking metabolic and inflammatory pathways in cancer development [368]. Metabolomic biomarkers are summarized in Table 4.

Table 4.
Summary of inflammatory and metabolomic biomarkers in aging and cancer.
Table 4.
Summary of inflammatory and metabolomic biomarkers in aging and cancer.
| Biomarker Class | Representative Molecules | Sample Type | Detection Platform | Evidence Level |
|---|---|---|---|---|
| Inflammatory | IL-6 | Blood/Serum/Plasma, Secreted media | Multiplex platforms, ELISA, Western Blot | Preclinical, clinical [96,102,319,322,324] |
| IL-1β | Blood/Serum/Plasma, Secreted media | Multiplex platforms, ELISA, Western Blot | Preclinical, clinical [96,99,319] | |
| TNF-α | Blood/Plasma, Serum | Multiplex platforms, ELISA | Preclinical, clinical [168,319,324] | |
| NF-κB | senescent cells | Western blot, Luciferase Assay | NF-κB activation has been observed in numerous age-related diseases [96,168] | |
| CD4+, CD8+ | T cell surface markers | RT-PCR, Flow Cytometry | in vivo mouse models, Elevated IL-6 in aged hosts impairs CD8+ T cell function, severely compromising CD4+ T cell-mediated antitumor responses [322] | |
| IL-10 | bone marrow cells, blood from mouse models | RT-PCR, ELISA, Microarray Analysis | age-related ineffective erythropoiesis animal models [324] | |
| CRP | Blood | ELISA | phase II clinical trial [325] | |
| CHI3L1(YKL-40) | Serum, CSF, tissues | ELISA | Preclinical, clinical [325,326] | |
| Metabolic | HbA1c | Blood | an immunoassay, HPLC | 6.5% for diabetes [331] |
| AGEs: CML, CEL, Glucosepane, N2-Carboxyethyl-2’-deoxyguanosine | Blood, tissue, urine, cell membranes | Electrophoresis, Spectroscopy, NMR, MS | In vitro, in vivo, clinical [327] | |
| sRAGE: esRAGE, cRAGE | plasma | ELISA | cohort study [332] | |
| HK2 | Lung and breast from Mouse models, human cancer cell lines | Immunoblot, IHC, PET, LC-MS/MS | In vitro, in vivo [335] | |
| Lactic acid, LDH | Blood, tumor tissues | Blood lactate test, MRS, MRI | Preclinical, Clinical [340] | |
| GSH | Blood, RBC, liver, muscle | HPLC, LC-MS | Rodent, human clinical trials [345] | |
| GLS1 | Tumor tissues, patients’ plasma, cell lines | The Cancer Genome Atlas (TCGA) database | Preclinical, bioinformatics analyses [348] | |
| IDO, TDO | Clinical samples, cell lines, animal models | IHC, HPLC, western blot, MRI | Preclinical, clinical [350] | |
| IGF-1, IGF-1R | Biopsy tissues, blood | IHC, genetic analysis | Preclinical, clinical [358,359] | |
| Adiponectin | Blood | ELISA | In vitro, in vivo [360] | |
| FASN | Tumor tissues, cell line | IHC, gene expression | Preclinical and clinical [364] |
Abbreviations: IHC; immunohistochemistry, CSF; Cerebrospinal Fluid, HPLC; High Performance Liquid Chromatography, MS; Mass spectrometry, LC-MS; Liquid Chromatography-Mass Spectrometry.
4.5. Protein Biomarkers
Protein biomarkers are fundamental to the diagnosis, prognosis, and treatment of cancer, as well as to the evaluation of biological aging. Multiple clinically validated and exploratory markers have been identified across tumor types and aging contexts, reflecting the complex molecular heterogeneity of disease [32].
Prostate-specific antigen (PSA) is the predominant biomarker for PCa, facilitating early detection and monitoring. Although highly SN, PSA lacks SP because benign prostatic hyperplasia, prostatitis, and malignancy can all elevate serum levels within the “gray zone” (2–10 ng/mL) [369]. To improve diagnostic accuracy, derivative indices such as the free-to-total PSA ratio, glycosylated PSA variants, and the Prostate Health Index (PHI) have been introduced [370]. The PHI, approved by the U.S. FDA in 2012, integrates total PSA, free PSA, and [−2]proPSA into a single composite score and outperforms individual measurements for clinically significant PCa [371].
In BC, testing for human epidermal growth factor receptor 2 (HER2), encoded by ERBB2, is essential for selecting patients eligible for HER2-targeted therapies such as trastuzumab [372]. IHC remains the principal diagnostic technique, with 3+ scores or 2+ scores accompanied by ERBB2-positive fluorescence in situ hybridization (FISH) results defining HER2-positive BC [372,373]. Recently, the classification of HER2-low tumors (IHC 1+ or 2+/FISH-negative) has gained clinical significance due to the responsiveness to antibody-drug conjugates (ADCs) (e.g., trastuzumab deruxtecan). Alongside HER2, estrogen receptor (ER) and progesterone receptor (PR) are utilized in diagnostic procedures. Triple-negative BC is characterized by the low levels of HER2 and the lack of ER and PR. These are negative for HER2, ER, and PR. Hormone therapy and HER2-targeted drug therapy are not effective in these tumors. Triple-positive BC is characterized by the presence of HER2, ER, and PR. This cancer type is managed through hormone therapy and HER2-targeted therapy. The HER2 status and diagnostic and prescribing flow for BC are summarized in Figure 5.
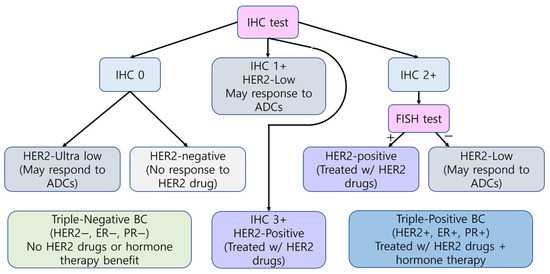
Figure 5.
Breast Cancer Diagnosis and Treatment Guidelines by HER2 status. The workflow was laid out by referring to “Breast Cancer HER2 status” in the American Cancer Society web site [373] and reference paper [372]. IHC results are graded from 0 to 3+, where 0 indicates an ultralow or negative expression (‘’) and higher scores represent increasing HER2 protein expression. FISH testing further classifies equivocal (2+) cases. HER2-positive (‘+’) tumors are eligible for HER2-targeted therapy (violet box), HER2-low cases may respond to ADCs (gray box), and HER2-negative tumors show no response (light gray box). Combined HER2, ER, and PR status defines triple-positive (blue box) or triple-negative (light green box) BC treatment strategies.
HER2 testing methods approved by the FDA, along with IHC methods, are summarized in Table 5. The HercepTest provide standardized HER2 assessment; however, their limited dynamic range and subjectivity highlight the need for improved quantitative methods, including FISH, chromogenic in situ hybridization (CISH), RT-PCR, quantitative immunofluorescence (QIF), and RNA-based assays [372]. However, resistance poses a significant hurdle [32].

Table 5.
FDA-approved HER2 assessment methods [372].
Carcinoma Antigen 15-3 (CA 15-3) serves as a tumor marker for various cancer types, particularly BC [374]. Elevated CA15-3, alongside alkaline phosphatase (ALP), was associated with a heightened likelihood of early recurrence in BC [375].
Cancer antigen 125 (CA-125) is an antigenic tumor marker of ovarian cancer (OC), used to track its development and recurrence using blood test [376]. However, its limited SN restricts its application in the early detection of OC [376]. To compensate for the low SN and SP of CA-125 alone, it is utilized alongside additional protein markers. The combinations of CA-125, human epididymis protein 4 (HE4), and soluble EGFR (SN 93.33% and SP 85.11% for alone vs. SN 83.3% and SP 100% for combination) [377], as well as CA-125, HE4, E-cadherin, and IL-6 (SN 90.4% and SP 87% for alone vs. SN 86.4% and SP 100% for combination) [378], demonstrated reduced SN and increased SP, meeting the ideal SN/SP criteria (SN 75% and SP 99.6%) [379]. Due to the low SN and SP of CA-125 for OC diagnosis, CA-125 alone is not used as a standalone test. Instead, some multivariate test methods including CA-125 have been cleared by the FDA for assessing the risk of malignant tumors in women with adnexal masses who are already scheduled for surgery (Table 6).
In the context of HCC, alpha-fetoprotein (AFP), des-gamma-carboxy prothrombin (DCP), and the glycosylated isoform AFP-L3 are clinically relevant [32]. ALP-L3 exhibits high SP for HCC but low SN when used alone. Of the three markers, AFP-L3 is the sole one cleared by the FDA for risk stratification [380]. Combining AFP, AFP-L3, and DCP significantly improves accuracy, especially in AFP-negative or small HCC lesion [380].
Carcinoembryonic antigen (CEA) and cancer antigen 19-9 (CA19-9) widely applied in the postoperative monitoring and prognosis of gastrointestinal (GI) malignancies [32]. In CRC patients, both elevated preoperative CEA and CA19-9 predicted shorter recurrence-free survival, while in PC both markers correlated with overall survival [381,382]. Combined CEA/CA19-9 measurement improved recurrence risk assessment following curative surgery.

Table 6.
FDA-Cleared CA-125-Based Tests [382].
Table 6.
FDA-Cleared CA-125-Based Tests [382].
| Test Name | Type | Components | FDA Clearance Year | Notes/Indications | SN | SP |
|---|---|---|---|---|---|---|
| ROMA (Risk of Ovarian Malignancy Algorithm) | Algorithm | CA125 + HE4 + menopausal status | 2011 | Preoperative risk stratification for epithelial ovarian cancer (EOC) in women with adnexal masses | ~94–95% | ~76–80% |
| OVA1 | Multivariate index assay | CA125-II + Transthyretin (TTR) + Apolipoprotein A-1 (ApoA-1) + Transferrin (TF) + β2-microglobulin | 2009 | Pre-surgical assessment of adnexal mass malignancy risk. | 96% (postmenopausal)/85% (premenopausal) | 28–40% |
| Overa (OVA2) | Second-generation OVA1 | CA125-II + HE4 + ApoA-1 + TF + Follicle-stimulating hormone (FSH) | 2016 | Improved version of OVA1 for adnexal mass risk assessment | 91% | 69% |
In small cell lung cancer (SCLC) patients, serum pro-gastrin-releasing peptide (ProGRP), neuron-specific enolase (NSE), and cytokeratin 19 fragment (CYFRA 21-1) levels closely correlated with treatment response and overall survival [383]. These markers effectively predicted prognosis, monitored disease progression, and often detected relapse months before radiological evidence, demonstrating strong value for continuous management of SCLC. Combined measurement of CEA, CA19-9, and CA72-4 improved the diagnosis and follow-up of advanced gastric cancer, particularly when CEA was undetectable [384]. While not effective for early screening, these markers served as prognostic markers, aiding in recurrence detection and treatment decisions.
In cervical cancer, serum proteins CEA, squamous cell carcinoma antigen (SCCA), high mobility group box chromosomal protein 1 (HMGB1), and CYFRA 21-1 were analyzed in 36 cervical cancer patients to identify reliable biomarkers associated with human papillomavirus (HPV) infection [385]. The results showed that CEA had the highest detection rate and exhibited the strongest association with HPV-16.
Immunotherapy-related markers such as programmed death-ligand 1 (PD-L1) have transformed treatment paradigms in lung, melanoma, and renal cancers by guiding checkpoint inhibitor therapy [386].
Beyond oncology, protein biomarkers of aging provide quantitative metrics for assessing biological age and predicting the onset of chronic and neurodegenerative diseases [387].
Blood-based proteins, including p-tau181, p-tau217, neurofilament light (NfL), and glial fibrillary acidic protein (GFAP), are strongly associated with AD and all-cause dementia, showing high predictive accuracy (AUC ≈ 0.71–0.83) and improved performance when combined, especially p-tau217 with NfL or GFAP [388]. Proteomic studies from the Framingham Heart Study identified circulating proteins such as GDF15, NT-proBNP, CRP, leptin, IGF-1, and sRAGE, which correlate with cardiovascular events, heart failure, and mortality, reflecting their involvement in metabolic and inflammatory pathways [389]. Representative protein biomarkers for cancer and aging diagnosis are summarized in Table 7.

Table 7.
Representative Protein Biomarkers for Cancer and Aging.
Comprehensive proteomic aging clocks, such as ProtAge, have extended this concept by integrating hundreds of proteins to predict biological age and multi-morbidity risk. The simplified ProtAge20 model maintains ~95% accuracy of the full model using only 20 proteins, representing pathways of extracellular-matrix (ELN), immune (GDF15, CXCL12), hormonal (FSHB, AGRP), and neural (GFAP, NEFL) function [387].
The ProtAgeGap, defined as the difference between predicted and chronological age, correlates with the risk of Alzheimer’s, chronic kidney disease (CKD), and all-cause mortality, demonstrating the clinical utility of proteomic age as a surrogate for biological health [387]. For each one-year increase in ProtAgeGap, the risk of developing AD rose by 1.16 times, all-cause dementia by 1.12 times, and CKD by 1.10 times. Individuals in the top 5% of ProtAgeGap had significantly greater odds of AD (2.6 times), CKD (1.8 times), and total mortality (1.9 times) [387].
Proteins such as ELN and GDF15 demonstrated the potential as therapeutic targets, providing the foundation for precision medicine to enable early intervention and promote healthy aging [387]. The selected 20 proteins are summarized in Table 8.

Table 8.
ProtAge20 Proteins by Tissue/Organ Source [387].
5. Early Detection Strategies for Cancer
Cancer ranks among the primary causes of mortality globally. Early detection of cancer in its initial stages or precancerous changes facilitates timely intervention, thereby slowing or preventing disease progression and mortality [27]. Late diagnosis significantly contributes to mortality rates in low- and middle-income countries. Equitable access to early diagnosis strategies is crucial to address this gap [27]. Recent literatures on the advancement of early cancer screening and diagnostic methods demonstrates significant progress through the integration of liquid biopsy technology, multi-omics biomarker analysis, and AI. These innovations have contributed to the development of multi-cancer early detection (MCED) tests, which exhibit high SN and SP in simultaneously identifying multiple cancer types. SN is defined as the proportion of correctly classified true positives among all samples assigned to the positive class, including true positive and false negative. In contrast, SP is defined as the proportion of correctly classified true negatives among all samples assigned to the negative class, including true negative and false positive [390]. Table 9 summarizes common evaluation metrics used in cancer diagnostics to aid understanding before discussing cancer diagnosis strategies.

Table 9.
Common evaluation metrics used in cancer diagnostics and biological aging prediction [391,392,393].
5.1. Liquid Biopsy Approaches
Liquid biopsies provide a novel and minimally invasive method for cancer diagnosis and monitoring. The analysis focuses on cancer-related markers present in various bodily fluids, including blood, urine, and cerebrospinal fluid [394]. This method presents a systematic and thorough alternative to conventional invasive tissue biopsies, offering an evolving perspective on disease progression over time [395]. Currently, liquid biopsies include various components, including circulating tumor cells (CTCs), circulating tumor DNA (ctDNA), EVs, miRNA, circulating RNA (cfRNA), tumor platelets, and tumor endothelial cells [394]. Despite promising developments, challenges remain in terms of SN, SP, and standardization of liquid biopsy technology, particularly in early-stage cancers where tumor-derived biomarkers are scarce [396,397,398]. Biomarkers found in liquid biopsies offer additional insights. ctDNA indicates genetic mutations present in tumors, CTCs signify viable tumor cells capable of metastasis, and exosomes transport molecular cargo that reflects tumor status [399,400]. Liquid biopsy detects various biomarkers associated with cancer in blood and other body fluids and, when combined with various advanced technologies, is expected to be a promising technology for early cancer diagnosis [401,402]. Liquid biopsy biomarkers are summarized in Table 10.

Table 10.
Summary of liquid biopsy biomarkers for cancer detection.
5.1.1. ctDNA
ctDNA is a piece of DNA released from tumors and present in the blood, which is used for detecting mutations, monitoring drug resistance, predicting recurrence, and other purposes [401]. Residual impurities can significantly affect DNA purity during ctDNA extraction. Cell-free DNA (cfDNA) is fragmented DNA found in biological fluids released from cells into the circulatory system [403]. It is present in both healthy individuals and patients with diseases, with ctDNA accounting for 1–2% of total cfDNA [404]. ctDNA can be differentiated from normal cfDNA fragments by the presence of epigenetic or genetic modifications, such as tumor-specific methylation markers and somatic point mutations [401,405]. Because the origin of ctDNA in the bloodstream is derived from CTC, exosomes secreted by tumor cells, apoptotic tumor cells and necrotic tumor cells, ctDNAm can aid in identifying clinical molecular subtypes of cancer and the tissue origin of the tumor [406].
The FDA has approved two blood-based tests (Shield and ColoHealth) for CRC screening in average-risk individuals. Shield test was performed to analyze cfDNA genomic variants, abnormal methylation status, and fragment patterns. This test demonstrated a SN of 83.1% for CRC and a SP of 89.6% for advanced neoplasia in an average-risk screening population [407]. These metrics met the prespecified acceptance criteria for FDA-approved screening tests for CRC. While its SN for advanced precancerous lesions was lower (13.2%), the test’s high SN for actual CRC, particularly for stage I, II, or III (87.5%), makes it a valuable tool for identifying existing cancers [407]. ColoHealth (Epi proColon) test detects methylated Septin 9 (mSEPT9) DNA as a screening tool for CRC [408]. mSEPT9 has shown higher SN in diagnosing CRC compared to conventional markers like CEA, CA19-9, or Fecal Occult Blood Test (FOBT) [409]. Combining mSEPT9 with other markers can further increase diagnostic SN, especially for early stages. mSEPT9, a representative marker for CRC diagnosis, is stable across disease stages, could potentially be repurposed for detecting other gastrointestinal adenocarcinomas [410].
PC is generally identified at a late stage, characterized by low survival rates and elevated recurrence rates. Therefore, early diagnosis and prognostic or predictive markers are essential for customized therapy [411]. Currently, CA19-9 is the sole FDA-approved biomarker for PC. However, its efficacy is constrained by low SN and SP [411]. Methylation of BNC1 and ADAMTS1 has been shown to detect PC at an earlier stage [412]. While generally less sensitive than CA19-9 for the early diagnosis of PC, the combination of ctDNA with mutant KRAS and CA 19-9 markedly increased the diagnostic SN to 91% [411]. The integration of ctDNA with mutant KRAS demonstrated an effective marker for assessing PC progression during or following chemoradiotherapy or surgical intervention [413].
Changes in ctDNAm patterns are very important for the diagnosis, staging, prognosis prediction, and recurrence detection of breast cancer (BC) and LC. Typical DNAm patterns found in early BC are hypermethylation of tumor suppressor genes such as BRCA1, ITIH5, and RASSF1A, which induce inactivation of these genes [414]. Other types reduce methylation, contributing to the development and progression of the disease. These specific methylation signatures may aid in distinguishing BC subtypes, which is important for patient classification and personalized treatment approaches. Methylation patterns are also associated with treatment response and play an important role in predicting the outcome of immunotherapy. In BC patients, immune cell-specific hypermethylation patterns have been identified, which may contribute to risk stratification and improved treatment management [415].
Nine hypermethylated genes were identified in early-stage lung tumors, and four of these genes (BCAT1, CDO1, TRIM58, and ZNF177) formed a diagnostic signature [416]. DNAm analysis of non-invasive samples from 83 LC patients revealed that BCAT1 is a candidate gene with high diagnostic efficacy in plasma-derived ctDNA [417]. A genome-wide DNAm profiling identified a panel of DNAm biomarkers (CLDN1, TP63, TBX5, TCF21, ADHFE1 and HNF1B) in squamous cell LC [418]. Next-Generation Sequencing (NGS) provided comprehensive genome-wide methylation profiling, allowing for the identification of seven differentially methylated regions corresponding to HOXB4, HOXA7, HOXD8, ITGA4, ZNF808, PTGER4, and B3GNTL1 genes associated with LC [419]. This panel achieved high diagnostic accuracy in distinguishing LC from benign diseases and healthy controls. SHOX2 and RASSF1A genes have demonstrated high SN and SP in clinical studies by showing distinct DNAm patterns in non-invasive samples like blood and sputum [420].
A new liquid biopsy strategy combining circulating cell-free mitochondrial DNA (mtDNA) and ctDNA has improved cancer detection [406,421]. mtDNA exhibits a significantly higher mutation rate compared to nuclear DNA, attributed to the lack of histone protection, inefficient DNA damage repair mechanisms, and the prevalence of highly reactive oxygen species in the surrounding environment. Somatic mutations in mtDNA have been reported across multiple cancer types [422]. Mitochondria contain hundreds to thousands of copies of mtDNA, with the quantity varying by cell type; it is specifically high in tissues with elevated metabolic activity, such as skeletal muscle and liver. These characteristics enable both qualitative and quantitative detection of cell-free mtDNA in patient blood samples [423]. mtDNA analysis can provide useful insights when cfDNA analysis is not feasible. This suggests that mtDNA analysis may reveal characteristics of cancers that are not detectable by ctDNA, such as the aggressiveness of cancer cells or metabolic changes [421].
5.1.2. CTCs
CTCs are cancer cells circulating in the bloodstream and are an important component of tumor spread and metastasis [401]. Evidence suggests that metastasis, the spread of cancer, is an early event in aggressive cancers but is usually detected late, often occurring even before primary tumors are clinically detectable [424]. The lack of CTCs in routine blood sample volumes is one of the main obstacles to using them for early cancer detection. CTC can be used not only for early detection of cancer but also for prognosis and treatment response monitoring [401]. It is challenging to accurately detect cancer in its early stages due to this low number, which restricts the SN of existing detection techniques [424]. In early-stage breast cancer (stage I-IIIA), the detection of more than one CTC is considered significant [425,426]. Although CTCs have been proven to be clinically useful biomarkers at metastatic BC [427], they are rarely detected in early BC, limiting their use as predictive or therapeutic diagnostic biomarkers or as indicators of minimal residual disease in this population [428]. However, utilizing nanostructured titanium oxide-coated slides, Krol et al. identified CTC clusters in a cohort of 28 BC patients, suggesting their role in early metastatic spread [425]. The presence of CTC clusters in early BC may serve as an additional, substantial risk factor for disease progression. Identifying patients with these clusters could help pinpoint high-risk individuals who might benefit from more aggressive or targeted early interventions.
Conventional methods for CRC diagnosis, such as colonoscopy and biopsy, are invasive and may miss asymptomatic patients. As a result, CTC analysis in CRC patients is gaining attention. Recent studies have detected CTCs using specific markers, including the adenomatous polyposis coli (APC) gene mutation, which is present in 60–70% of CRC patients [429]. Additionally, by utilizing established markers such as CK and vimentin in conjunction with the APC gene mutation, the origin of CTCs can be confirmed, thereby enhancing the accuracy, which indicates the proportion of correctly classified true positives and true negatives among all samples [390], and reliability of diagnosis [429]. The metabolic profiles of CTCs have shown potential for early and differential diagnosis of cancer. Yasmin et al. found that eicosanoids, acyl carnitine metabolites, and sterol lipids were specifically increased in CRC cells, suggesting their potential as diagnostic markers [430]. Quantifying mRNA levels of six CRC-related genes in the blood to detect CTCs has been demonstrated [431]. This study on 50 CRC patients using mRNA levels of six genes (CEA, mesenchymal–epithelial transition factor (c-Met), mucin 1(MUC1), CK19, EGFR, and Epithelial cell adhesion molecule (EpCAM)) showed a diagnostic SN of 87% and accuracy of 85%.
A study performed by Purcell et al. has shown that CTC load, PD-L1 expression, and gene expression profiles are valuable biomarkers to monitor and predict patient outcomes in stage III NSCLC [432]. A more substantial decrease in CTCs during chemoradiation therapy correlated with a significantly extended progression-free survival (PFS). Patients exhibiting a CTC reduction rate of less than 75% from baseline to 4 weeks post-treatment demonstrated a PFS of 7 m, markedly shorter than the 21 m average observed in the control group [432]. In patients receiving durvalumab, those who later experienced disease progression exhibited consistently elevated levels of PD-L1+ CTCs compared to patients with stable disease. Elevated PD-L1+ CTC ratios in patients receiving durvalumab were associated with reduced PFS [432]. CTCs isolated during chemoradiation therapy exhibited elevated gene expression linked to an aggressive and proliferative phenotype, indicating that surviving CTCs may possess enhanced metastatic and aggressive characteristics [432].
5.1.3. EVs and Plasma Proteomic Biomarkers
EVs are enclosed by a lipid bilayer membrane measuring 40–150 nm in size. They originate from the endosomal compartment within cells and are secreted by nearly all eukaryotic cells [433]. EVs are involved in cell-to-cell communication, and EVs derived from tumor cells carry important information related to tumor progression [434]. EVs are found in all body fluids, offering a non-invasive way to study originating cells, their oncogenic transformations, the TME, and immune system homeostasis [434]. EVs transport molecular components such as proteins, nucleic acids (RNA), and lipids from tumor-producing cells, carrying tumor-specific markers and providing tumor molecular information [435].
In PC, particularly in its early stages, a high prevalence of mutant KRAS in circulating exosomal DNA (exoDNA) has been observed [436]. This mutation serves as a pivotal signal for malignancy, given KRAS’s critical role in oncogenesis. The rate of detection of KRAS mutants in exosomes was found to be superior to cfDNA across all stages of PDAC studied [436]. This reflects that exoDNA originates from viable cancer cells rather than dying tissue (cfDNA), making it particularly useful in the early stages of cancer. Moreover, elevated levels of EVs containing a cell surface proteoglycan, glypican-1 (GPC1) have emerged as a potential biomarker, showing markedly higher concentrations in patients with PDAC [437] and CRC [438] than in healthy individuals. In CRC, miR-96-5p and miR-149 have been proposed as factors regulating GPC1 expression and exosome secretion [438]. The integration of EV-derived miRNA (miR-23a-3p, miR-92a-3p, and miR-150-5p with CEA has enabled high-precision early diagnosis of CRC [439].
Urine-derived exosome ncRNA demonstrated PCa classification with high precision [440]. Three platforms were used for analyzing ncRNA. The Sentinel™ PCa Test distinguishes PCa from controls, the Sentinel CS Test stratifies low-risk (Grade Group 1) and intermediate/high-risk (Grade Groups 2–5) patients, and the Sentinel HG Test stratifies low-risk/favorable intermediate-risk (Grade Groups 1–2) and high-risk (Grade Groups 3–5) patients. Validated in 1436 subjects, the PCa Test demonstrated 94% SN and 92% SP, the CS Test showed 93% and 90%, and the HG Test exhibited 94% and 96%, respectively.
Kim et al. identified SCLC-specific exosomal miRNAs through the analysis of serum samples from SCLC patients and healthy controls [441]. A trio of miRNAs (miR-200b-3p, miR-3124-5p, and miR-92b-5p) was identified as notably significant. The combination of these miRNAs enhances the SP of SCLC diagnosis relative to the utilization of individual miRNAs, evidenced by an AUC value of 0.93 for the combined miRNAs compared to 0.64–0.76 for single miRNAs. Furthermore, this study confirmed that a panel of three miRNA was significantly associated with poor prognosis in SCLC, suggesting that this panel may also act as prognostic biomarkers. miR-29a-3p demonstrated potential as a promising prognosis biomarker in early-stage NSCLC, which could play a key role in the treatment and management of patients at increased risk of metastasis [442]. While the paper primarily draws attention to the prognostic value of miR-29a-3p, the study found that the expression levels of miR-29a-3p in plasma EVs from NSCLC patients were significantly different compared to those in healthy donors (p = 0.004). This difference suggests that miR-29a-3p could potentially serve as a marker to differentiate between NSCLC patients and healthy individuals.
For NSCLC, detection of EVs-based EGFR T790M mutation resistant to EGFR inhibitors was carried out [443,444]. The exosomal nucleic acids (exoNA) from plasma enhanced detection, particularly for low T790M copy numbers, early-stage NSCLC and its early identification through EVs could inform personalized treatment strategies. In NSCLC, exosomal PD-L1 is known to promote tumor growth through immune evasion [445]. A substantial number of patients demonstrate inadequate responses to PD-1/PD-L1 inhibitors, underscoring a pivotal challenge in the treatment of NSCLC [446]. Shimada et al. demonstrated that serum exo PD-L1 levels could serve as a valuable quantitative factor to predict anti-PD-1 response in NSCLC patients [447]. Patients with serum exo PD-L1 levels ≥ 166 pg/mL demonstrated a 100% disease control rate when treated with anti-PD-1 inhibitors, indicating its potential as a strong predictor for favorable responses.
BC patients demonstrate significantly elevated phosphorylated protein levels of select EV proteins—including Ral GTPase-activating protein subunit alpha-2 (RALGAPA2), cGMP-dependent protein kinase1 (PKG1), tight junction protein 2 (TJP2), and nuclear transcription factor, X box-binding protein 1 (NFX1) [448]. OC diagnosis may benefit from a panel of seven EV protein markers: EGFR, HER2, CA125, folate receptor alpha (FRα), cluster of differentiation (CD)24, EpCAM, and the combination of CD9 and CD63 [449]. These markers have proven effective in differentiating early-stage OCs from healthy controls. Another independent study identified FGG, MUC16, and APOA4 as key EV proteins capable of distinguishing malignant ovarian tumors from benign cystadenomas or healthy tissue [450].
Circulating proteins have shown promising results in early-stage cancer detection for LC, CRC, BC, OC, and HCC. Gasparri et al. identified Raf Kinase Inhibitory Protein (RKIP) and its phosphorylated form (pRKIP) as potential biomarkers for early-stage LC. Serum levels of RKIP were found to be significantly higher in early-stage LC patients compared to healthy controls, allowing for discrimination with 93% accuracy (AUC 0.94) [451]. The ratio of RKIP to pRKIP further enhanced diagnostic accuracy, particularly in distinguishing early-stage LC patients from high-risk healthy subjects (AUC 0.79).
AFP and vitamin K absence or antagonist-II (PIVKA-II) in serum were analyzed for diagnosis of HBV-related HCC [452]. Diagnostic performance showed AUCs of 0.765 (AFP), 0.901 (PIVKA-II), and 0.917 (combined), with combined detection superior. Both markers correlated positively with tumor size, differentiation, and vascular invasion. PIVKA-II alone or combined with AFP improves HCC diagnosis. Another study demonstrated that the diagnostic accuracy for HBV-HCC is significantly improved when AFP, PIVKA-II, and golgi glycoprotein 73 (GP73) are used together, particularly in the chronic hepatitis B patient population [453].
While many circulating proteins serve as predictive and prognostic biomarkers in BC, fewer are specifically highlighted for early detection. Serum LRP6N, haptoglobin (Hp), and apolipoprotein C1 (APOC1) have been identified as potential biomarkers for the early detection of BC through blood proteomics [454]. In vivo, LRP6N inhibited SDF-1/CXCR4-driven lung metastasis and prolonged survival, whereas LRP6 knockdown enhanced metastasis [455]. A secreted serum form of LRP6N, reduced during metastasis, was identified in both mice and patients. Thus, LRP6N may serve as a diagnostic marker and therapeutic inhibitor of breast cancer metastasis. APOC1 was demonstrated as a potential diagnostic serum biomarker for breast cancer (BC) through a comprehensive proteomic analysis [456]. Validation confirmed progressively elevated APOC1 expression from healthy controls to fatty breast disease, mastopathy, and BC patients. Thus, APOC1 is a promising serum biomarker for BC diagnosis. Hp is a prevalent human plasma protein that effectively binds hemoglobin during hemolysis [457]. Hp expression was increased in breast cancer tissue and the circulatory system, and it was found to promote tumorigenesis by regulating the cell cycle and apoptosis [458]. The N-glycosylation of disease-specific Hp β (DSHp-β) was evaluated as a biomarker for distinguishing BC from benign breast lesions [459]. Analysis of serum samples from 497 patients demonstrated that the DSHp-β N-glycosylation signature can serve as an excellent serological biomarker for breast cancer diagnosis and may provide mechanistic insights into tumorigenesis.
CancerSEEK, an innovative multi-analyte blood test designed for the early detection and localization of cancers of the ovary, liver, stomach, pancreas, esophagus, colorectum, lung, or breast, has been developed. This test combines genetic and protein biomarkers to achieve high SP and SN, demonstrating particular effectiveness for cancers lacking existing screening tests [460]. Genetic markers (KRAS) and eight specific proteins, including CA-125, CEA, CA19-9, PRL, HGF, osteopontin (OPN), myeloperoxidase (MPO), and tissue metalloproteinase inhibitor 1 (TIMP-1), are used. The OncoSeek test uses a panel of seven protein tumor markers (PTMs), including AFP, CA125, CA15-3, CA19-9, CA72-4, CEA, and CYFRA 21-1 [461].
5.1.4. Non-Coding RNA
miRNAs in blood are increasingly recognized as non-invasive biomarkers for the diagnosis of various diseases, especially cancer, and for prognostic prediction [462]. miRNAs are rapidly released into bodily fluids from injured tissues, creating a profile that indicates particular disease conditions. This enables the early detection of diseases and the forecasting of recurrence risk.
miR-21 is a representative oncogenic microRNA that regulates post-transcriptional gene expression and is overexpressed in various cancers, including glioma, BC, and CRC [463]. Its major target genes include PTEN, proteolysis-targeting chimera, programmed cell death protein 4 (PDCD4), reversion-inducing cysteine-rich protein with Kazal motifs (RECK), and signal transducer activator of transcription 3 (STAT3), which are involved in apoptosis, invasion, metastasis, and proliferation. Consequently, miR-21 has been gaining attention as a diagnostic and prognostic biomarker for cancer, as well as a therapeutic target. Research and clinical trials are underway for anti-miR-21 oligonucleotides and a chemically modified miR-21 inhibitor (ADM-21)-based inhibition strategies. However, its potential utility as a tissue-specific biomarker requires careful consideration [463].
In OC, the combination of six miRNAs—miR-200a-3p, miR-766-3p, miR-26a-5p, miR-142-3p, let-7d-5p, and miR-328-3p—demonstrated the highest diagnostic performance, achieving an AUC of 0.994, SN of 98.4%, and SP of 95.6% [464]. This indicates that these miRNAs can distinguish malignant from benign OCs with very high accuracy, outperforming most single conventional markers. Furthermore, the combination of miRNAs with CA125 and HE4 yielded superior diagnostic results compared to using each marker alone. For instance, the combination of CA125, HE4, and exosomal miR-205 achieved 100% SN and 86.1% SP, which markedly exceeds the performance of CA125 or HE4 alone [465]. Similarly, the combination of miR-1307, CA125, and HE4 (AUC 0.970) and the combination of miR-375, CA125, and HE4 (AUC 0.945) demonstrated superior diagnostic accuracy compared to the individual markers: miR-1307 (AUC 0.671), miR-375 (AUC 0.788), CA125 (AUC 0.905), and HE4 (AUC 0.820) [466].
An integrated system combining an efficient EV separation platform and advanced AI analysis was used to analyze plasma samples collected from 80 CRC patients and 20 healthy individuals. The optimal combination of miR-23a-3p, miR-92a-3p, miR-150-5p, and the most commonly used CEA in relation to CRC, achieving an AUC of 0.9861 for overall CRC detection, surpassing individual markers and traditional CEA testing [440]. This biomarker combination demonstrated high SN (95.83%), SP (100%), and accuracy (96.67%) for overall CRC.
5.2. Advances in Diagnostic Technology
This session discusses DNAm detection methods in liquid biopsy and various advanced technologies recently developed to enhance the SN and SP of liquid biopsy.
5.2.1. DNAm/Tumor DNA Detection Methods
DNAm, a crucial epigenetic modification, can be detected using various techniques, primarily focusing on specific sites within the genome [412].
Methylation Specific PCR (MSP) technique is used to detect DNAm at specific sites. At first, DNA is treated with sodium bisulfite to convert unmethylated cytosines to uracil while methylated cytosines remain unchanged [467]. Subsequent PCR amplification with methylation-specific primers allows for the detection of methylated sequences [467]. This method enables early cancer detection with high SN, even at 0.1% methylation [468]. It is cost-effective, rapid, and compatible with liquid biopsy samples using minimal DNA, though it may yield false positives from incomplete bisulfite conversion [468].
Whole Genome Bisulfite Sequencing (WGBS) is a comprehensive technology that determines methylation status across all CpG sites in the entire genome. This approach allows for a thorough understanding of differential methylation patterns between cancer patients and healthy individuals [412]. WGBS offers extensive, single-base methylation analysis following bisulfite conversion and NGS, even with minimal DNA quantities [469]. It facilitated early BC detection with high precision (AUC ~ 0.97) [469], aided in the diagnosis of esophageal cancer through 5-hydroxymethylcytosine (5hmC) multimodal analysis [470,471], and provided methylation landscapes across various cancers. Despite its efficacy, the high cost frequently limits research to small cohorts, necessitating subsequent validation at specific loci within larger populations [412]. Specialized cfDNA library preparation is necessary; however, its accuracy renders WGBS an essential instrument in cancer epigenomics [472].
Digital PCR (dPCR) offers a highly sensitive and quantitative way to detect and measure DNAm, particularly useful for low-abundance DNA samples [412]. ddPCR is a reliable method for detecting nucleic acid markers from cf DNA/RNA, CTC, and exosomes in blood, plasma, or serum. It helps predict tumor relapse and reveals intratumor heterogeneity and clonal evolution, offering valuable insights into treatment response across various malignancies [473]. Several studies have demonstrated the utility of ddPCR for analyzing the genetic profile of LC. In sputum cytology-positive cases, ddPCR showed high SN for EGFR mutations in LC [474], and combining sputum and plasma improved the detection rate of EGFR T790M during disease progression in NSCLC [444,475]. Furthermore, ddPCR using bronchoalveolar lavage fluid (BWF) achieved higher SN and diagnostic accuracy than plasma, particularly in early-stage disease in LC, indicating that BWF is a superior alternative for detecting mEGFR mutations [476].
NGS rapidly, accurately, and cost-effectively analyzes large volumes of genetic information, aiding in identifying disease causes and developing personalized treatments [477]. In the first-generation sequencing methods, Sanger’s chain-termination method uses dideoxy nucleotides to generate fragments of varying lengths, while Maxam–Gilbert sequencing employs chemical cleavage of nucleotides and works best for short DNA fragments. These foundational methods enabled later technological advances [477,478]. To overcome their low throughput, the second-generation sequencing (or NGS) emerged around 2005. NGS involves DNA fragmentation, single-stranded library preparation, and clonal amplification on beads or slides, followed by massively parallel sequencing [477]. Major platforms include 454 Roche (Roche), which uses pyrosequencing and bioluminescent real-time detection, and Illumina Genome Analyzer from Illumina, which uses sequencing by synthesis and evolved into the HiSeq 2000 and MiSeq [479]. The ABI SOLiD system (Applied Biosystems) applies sequencing by ligation, offering high throughput but short reads, while the Ion Personal Genome Machine (PGM) from Ion Torrent detects pH changes for fast, cost-effective sequencing [479]. Later, the third-generation sequencing (TGS) addressed NGS limitations by producing long reads without PCR, enabling real-time single-molecule sequencing. Platforms like PacBio and MinION (Oxford Nanopore) generate kilobase-scale reads more quickly and cheaply, though with higher error rates that can be reduced by increasing coverage [477,480].
NGS is applied to WGS, targeted sequencing, ChIP-seq, and RNA-seq [477]. WGS provides complete genetic information for disease research and drug response prediction [480]. Targeted sequencing enables faster, more cost-effective analysis of specific genes with higher coverage. ChIP-seq maps DNA-protein interactions to elucidate gene regulation and epigenetic mechanisms, thereby advancing precision medicine and improving diagnostic accuracy [481]. RNA-seq provides information solely on transcribed sequences, including both coding and non-coding RNA, enabling precise identification of functionally relevant alterations, increasing diagnostic rate by up to 10–35% [482].
Both dPCR and NGS platform technologies are the two primary high-resolution nucleic acid quantification methodologies in biomarker-based diagnostics. However, owing to their foundational operational concepts, such as the partition-based quantification of digital PCR and the extensively parallel sequencing of next-generation sequencing, they exhibit unique analytical advantages and constraints. To quantitatively compare the two approaches, Table 11 summarizes representative performance parameters reported in the literature.

Table 11.
Comparative Analytical Performance of Digital PCR and NGS-Based Platforms for Circulating Biomarker Detection.
5.2.2. RNA-seq in Liquid Biopsy
RNA-seq provides deep analysis of the transcriptome, enabling the detection of novel RNA transcript variants, gene fusions, transcript isoforms, splice variants, and chimeric gene fusions involving previously unidentified genes or transcripts [498]. Specifically, RNA-seq can overcome technical limitations that DNA-based methods may have in detecting gene fusions (e.g., difficulty in detection due to long introns or repetitive sequences, lack of direct evidence for fusion expression at the mRNA level) [499]. RNA-seq can detect fusion events even in samples with low tumor cell content or unsuitable for DNA sequencing, thanks to the high expression of fusion mRNAs [499]. Furthermore, RNA-seq can determine whether mutations are expressed and their expression levels, filtering out cases where DNA mutations may be merely potential and clinically irrelevant, and directly assessing the functional impact of gene mutations [500].
RNA-seq is expanding its potential through the analysis of extracellular RNA (exRNA) in liquid biopsies [498]. ExRNA stably exists outside cells and is more readily accessible than tissue, enabling more frequent longitudinal sampling [498]. It offers significantly higher copy numbers than cell-free DNA, potentially providing greater SN, and differences in expression levels can serve as indicators of damage or disease states in specific organs or tissues [498]. The potential of exRNA has led companies like Exosome Diagnostics to develop ExoDx Lung (ALK), an exRNA-based clinical diagnostic test that measures the EML4-ALK transcript from exosomes in the plasma of NSCLC patients [498].
DNA sequencing and RNA-seq possess complementary characteristics, and when combined for analysis, they provide diagnostic information difficult to obtain from a single platform [499]. For example, in pediatric cancer patients, integrating WGS and RNA-seq identified one or more mutations in 93.7% of patients and therapeutic targets in 71.4%. This integrated approach also contributed to diagnostic changes [501].
Each of the RNA-seq pipeline’s components—sequence mapping algorithms, expression quantification algorithms, expression normalization methods, RNA extraction and quality control, data filtering and variant detection, splicing analysis algorithms, and bioinformatics pipeline integration—directly contributes to gene expression estimation quality (accuracy, precision, and reliability). To maximize the clinical utility of RNA-seq data, the pipeline must be carefully designed, optimized, and validated on a continuous basis [498,499,501,502,503].
5.2.3. Microfluidic Devices (MDs)
Microfluidics integrates mixers, actuators, reactors, separators, and sensors onto a chip to optimize detection processes [504,505]. Microfluidic devices’ sophisticated systems and miniaturization reduce sample consumption, reaction times, detection SN, and costs [506]. Microfluidic biosensors excel in the isolation, enrichment, and detection of rare cells within complex biological samples. The efficiency of CTC enrichment in liquid biopsies is significantly influenced by MD geometries and surface modifications. Various strategies have been developed to enhance CTC isolation, focusing on both physical properties and biological characteristics of the cells [507]. Combining physical and magnetophoretic separations has achieved high separation efficiencies, with reported rates of 100% for CTCs and 93.3% [508]. Using red blood cell membrane mimics for surface coating to prevent leukocyte contamination and achieve pure CTC separation, a surface was developed that excludes blood cells while capturing tumor cells, achieving a capture efficiency of 91% and a purity of 89% [509]. Furthermore, the incorporation of tumor-specific ligands, such as folate and RGD peptides, onto the surface improves CTC binding, markedly augmenting the enrichment factor [509].
Mishra et al. developed a novel high-throughput MD specifically designed for the enrichment of CTCs from large blood volumes, such as entire leukapheresis products [510]. Traditional liquid biopsies using standard blood draws (e.g., 10 mL) are limited by the extreme rarity of CTCs, which typically yield 0 to 10 CTCs per patient. This new technology can process complete leukapheresis products (averaging 5.83 L of blood equivalent), resulting in an average of 10,057 CTCs per patient. This significantly increased yield eliminates the statistical limitations of previous methods, allowing for more robust and reliable analyses [510].
The existing MD method frequently produces heterogeneous CTC clusters, necessitating further processing for thorough analysis and phenotype identification. Hyperuniform micropost MD has demonstrated potential efficacy in categorizing diverse CTC phenotypes via cell trajectory analysis and ML [511]. This device showed high accuracy in classifying two CTC phenotypes, including PC3 (PCa) and SKBR3 (BC) cells [511].
The μTASWako i30, a fully automated immunoanalyzer, was designed for in vitro diagnostics utilizing microfluidic technology [512]. Capillary electrophoresis on a microfluidic chip has facilitated the identification of various glycoform types of AFP, a serum biomarker for HCC. The glycoform AFP-L3 was allegedly more specific for HCC. This assay system offers high SN and delivers rapid results in 9 min.
5.2.4. Surface-Enhanced Raman Scattering (SERS) Biosensors
The integration of liquid biopsy with Surface-Enhanced Raman Scattering (SERS) biosensors significantly enhances the accuracy and SN of early cancer diagnosis [513]. SERS biosensors utilize the enhancement of Raman scattering signals by metallic nanostructures, typically gold or silver, to detect cancer biomarkers at very low concentrations [514,515,516,517]. This sensor, combined with nanotechnology, demonstrates improved detection capabilities. Gold nanorods and other nanoparticles (NPs) are employed to create hotspots that increase signal intensity, enabling the detection of low-abundance biomarker.
Circulating enzyme-mediated DNA amplification technology and gold nanoparticle@silicon-assisted SERS technology have enabled the reproducible identification of single-nucleotide variant ctDNA sequences in diffuse intrinsic pontine gliomas (DIPGs), with a low LoD as low as 9.1 fM in added blood samples [518]. Zhang et al. introduced an ‘immune-like sandwich multiple hotspots SERS biosensor’ designed for highly sensitive and stable CRC marker nucleoside diphosphate kinase A (NDKA) analysis in serum [519]. The practical utility of the biosensor was confirmed by detecting NDKA in serum, demonstrating a LoD of 0.25 pg/mL. Furthermore, it could detect NDKA at 10 ng/mL in mixed protein solutions, encompassing its high SN and SP.
Meanwhile, SERS biosensors face challenges in surface functionalization and signal fluctuation. To address these issues, Nguyen et al. used aluminum (Al) NPs to naturally immobilize DNA without surface functionalization and InGaN quantum wells to stabilize the SERS signals via abundant surface charges [520,521]. The combination of Al NPs for DNA immobilization and InGaN quantum wells for signal stabilization collectively enhances the SN of the biosensor to the single-molecule level.
A “sandwich” immunocomplex assembly strategy, entailing the pre-mixing of EpCAM-functionalized SERS nanotags with sEVs isolated from an OC cell line and the utilization of anti-CD9-conjugated magnetic particles as capture probes, exhibited optimal performance for sEV detection [522]. The enhanced SERS analytical approach was successfully implemented to profile surface protein biomarkers (EpCAM, CA125, and CD24) on the surface of sEVs derived from OC.
Hybrid SERS platforms have been developed to optimize the detection of cancer biomarkers in complex biological samples. Lu et al. developed a versatile microfluidic-SERS barcoding system by utilizing a single-layer, vertically oriented nanorod array, which generates a plasmonic coupling-based electromagnetic field [523]. The method is capable of achieving ideal sensitivities at subfemtomolar levels for four different miRNAs. The system also enables precise classification of tumor stages, metastatic conditions, and subtypes, achieving an overall accuracy of 94%.
Structurally unique molecules exhibit unique Raman scattering spectra. Label-free SERS analysis utilizes the unique Raman spectra of target substances to directly capture these unique spectral fingerprints from the sample itself, thereby identifying disease-related biomarkers [524]. The study performed by He et al. demonstrated a hybrid diagnostic approach that combines multiplexed Raman-tagged antibodies with label-free SERS and the Support Vector Machine (SVM) model, which significantly improved the accuracy, SN, and SP of OC biomarkers detection on EVs to over 95% [524].
5.2.5. Surface Plasmon Resonance (SPR) Biosensors
SPR biosensors have emerged as a powerful tool for cancer detection due to their ability to provide optical, label-free detection of cancer biomarkers with high SN and SP [525,526]. These biosensors leverage the interaction between light and metal surfaces to detect changes in the refractive index, which can indicate the presence of cancerous cells or biomarkers [527]. Recent advancements in SPR technology have focused on enhancing SN, selectivity, and the ability to detect a wide range of cancer types, making them a promising option for early cancer diagnosis and monitoring [526]. The incorporation of materials such as TiO2, graphene, and antimonene has significantly improved the performance of SPR biosensors. These materials enhance the SN and binding efficiency of the sensors, allowing for more accurate detection of cancerous cells [525,526,528]. Nanostructures, such as NPs, nanoholes, and nanopillars, facilitate SPR, resulting in localized electromagnetic “hot spots” due to the resonant vibration of free electrons in metallic nanostructures [529]. These hotspots enhance interactions with analytes, thereby improving SN and signal detection [530,531]. SPR biosensor is promising for detecting EV cancer biomarkers with low sample volume and high SN [532]. For instance, a fiber-optic SPR biosensor was developed to detect BC-specific EVs with an LoD of 2.1 × 107 particles/mL in buffer and 7 × 108 particles/mL in blood plasma, demonstrating high SN and SP [533].
5.2.6. Electrochemical Biosensors
Electrochemical biosensors for cancer marker detection are sophisticated devices that integrate various components to achieve high SN and SP. These biosensors primarily consist of a biorecognition element, a transducer, and signal amplification materials, which work together to detect tumor biomarkers effectively [534]. Common biorecognition molecules include antibodies, aptamers, and nucleic acids, which specifically bind to tumor biomarkers such as PSA, HER2, and CA19-9 [534,535]. These elements facilitate the selective recognition of cancer markers, crucial for accurate diagnosis. Electrochemical transducers convert the biological interaction into measurable electrochemical signals. Common types include glassy carbon electrodes modified with nanocomposites like reduced graphene oxide and metal oxides [536,537]. The transducer’s role is vital as it translates the binding event into an electrical signal, which can be quantified. Incorporation of nanomaterials such as gold NPs and metal oxides enhances the SN of the biosensor by increasing the surface area and improving electron transfer [537,538]. Signal amplification allows for the detection of low concentrations of biomarkers, with some biosensors achieving limits of detection as low as 1 cell/mL [539]. Electrochemical biosensors are capable of multiplexed detection, allowing simultaneous measurement of multiple cancer biomarkers. This capability is crucial for providing comprehensive diagnostic information and improving the accuracy of cancer diagnosis [540]. In addition, electrochemical biosensors can improve the vulnerability of false positive or false negative diagnoses caused by heterogeneity within and outside tumors (variations in genetic and protein expression profiles, epigenetic modifications, etc.) [540].
5.3. AI and Machine Learning
AI is the study of training machines to mimic human thinking, focusing on learning, reasoning, and self-correction [541]. Machine Learning (ML), a subset of AI, enables systems to automatically learn and improve from experience without explicit programming by analyzing data, recognizing patterns, and making better decisions [541]. Deep Learning (DL), a specialized branch of ML, uses neural networks inspired by the human brain to process information and classify patterns [541]. Unlike ML, DL handles larger datasets and performs self-directed predictions, making it highly effective in complex tasks such as image recognition, speech processing, and natural language understanding [541].
Mammography, CT, MRI, ultrasound, PET, SPECT, and X-ray are all essential imaging modalities for detecting cancer [542]. All are essential for identifying issues. Nonetheless, the interpretation of intricate images relies on the radiologist’s expertise and subjective judgment, potentially resulting in errors and discrepancies [542]. The risk of misdiagnosis and oversight of lesions is particularly elevated when lesions are subtle or when a radiologist is fatigued. Tissue-based biopsy remains the gold standard for definitive diagnosis but it presents certain challenges that render it invasive [394]. Consequently, AI is increasingly recognized as an essential complementary tool that enhances the precision and efficiency of diagnoses through objective and consistent analysis [543].
The integration of ML and DL in cancer early detection is revolutionizing the field of oncology by enhancing diagnostic accuracy, personalizing treatment strategies, and improving patient outcomes [29,544]. These technologies are particularly effective in analyzing complex datasets, such as medical images and genomic data, to identify early signs of cancer that might be missed by traditional methods [29,545,546].
5.3.1. Key Concepts of DL and Diagnostic Features
Unlike traditional ML methods that rely on manual feature engineering, DL excels at automatically learning complex patterns and extracting features from unstructured data such as medical images, text, and genomic data [547,548]. Traditional neural networks generally possess one or two hidden layers. Conversely, DL models utilize deep neural networks (DNNs) comprising multiple hidden layers to learn complex representations and hierarchical structures of data. This facilitates the automated extraction of features from raw data, including the identification of elementary features such as edges in images in the initial layers and the recognition of objects in the subsequent layers [549]. DL models can efficiently process vast amounts of high-dimensional data, which is particularly useful in cancer research that requires complex and heterogeneous biological data [548]. Various DL technologies are being used for early cancer diagnosis, each specializing in different data types and diagnostic objectives.
5.3.2. Convolutional Neural Networks (CNNs)
CNNs represent a category of DL models designed specifically for image data, such as CT, MRI, and histopathology, and are proficient in extracting spatial hierarchical structures of features. They achieve this by automatically learning spatial hierarchies and intricate patterns from raw image data, thereby bypassing the need for laborious manual feature engineering [550]. This indicates that they are capable of recognizing patterns irrespective of their precise location within an image [548]. Public datasets like the Curated Breast Imaging Subset of Digital Database for Screening Mammography (CBIS-DDSM) and The TCGA are commonly used for training CNN models, providing a diverse range of images for robust model development [551]. CNNs have revolutionized the detection of early-stage malignant tumors and tumor image classification [549].
CNN demonstrates elevated SN and SP in BC screening through the identification of microcalcifications and subtle architectural distortions. This significantly decreases false positive rates and increases the accuracy of cancer detection [552,553,554]. A prospective, multicenter cohort study evaluated the diagnostic accuracy of breast radiologists both with and without AI-based computer-aided detection (AI-CAD) for screening mammograms in a real-world, single-read setting [555]. The findings revealed a significant enhancement in the diagnostic accuracy of breast radiologists utilizing AI-CAD. While AI-based software such as the Lunit INSIGHT mammogram AI system + showed slightly lower SN, it outperformed radiologists in recall rate, SP, positive predictive value (PPV), and AUC [556]. In other comparative study, AI outperformed in terms of SP and PPV in low-density breast tissue [557]. However, radiologists showed greater SN than AI in diagnosing BC in dense breast tissue [557].
While CNNs are powerful for medical image analysis, several combinations and hybrid approaches have demonstrated superior performance for cancer detection, often by addressing the limitations of standalone CNNs or enhancing their capabilities. These superior combinations integrate CNNs with other DL architectures, ML techniques, or advanced preprocessing methods. CNN models have demonstrated high accuracy in LC detection, when combined with other neural network models [558,559,560]. A new combination model diversifies training data and improves model robustness by integrating differential augmentation (DA) into CNN and targeting specific elements like hue, brightness, saturation, and contrast [561]. The CNN+DA model outperformed DenseNet, ResNet, EfficientNetB0, and hybrid models like ensemble models.
The architectural variations in CNNs for PC detection exhibit distinct characteristics compared to those used for other cancers, primarily due to the unique challenges posed by pancreatic imaging. These variations are influenced by the pancreas’s small size, irregular shape, inaccessible location, and the necessity for precise segmentation in CT scans [562,563]. To tackle the complex challenges associated with pancreatic segmentation, including the pancreas’s varied morphology and the noise in medical CT images, a technique for automatic detection and segmentation classification of PC has been devised, employing the ASMO-ELC-Casnet-CNN model [564]. The developed model performs segmentation and classification by applying Spider Monkey Optimization through the fusion of UNET and CNN.
The combination of CNNs and Vision Transformers (ViTs) is a rapidly growing field in medical image analysis, as it effectively merges the strengths of both architectures to overcome their individual limitations [565]. CNNs excel at extracting local, fine-grained features due to their inherent inductive bias, while ViTs are adept at capturing global contextual relationships and long-range dependencies across the entire image. This hybrid approach has led to significant advancements in diagnostic accuracy. For instance, UnetTransCNN is a novel parallel architecture that integrates ViT encoder for global feature extraction with a CNN decoder for local detail restoration [566]. This design allows the model to simultaneously process both global and local information, which is crucial for complex anatomical structures. The model was specifically adapted for 3D medical image segmentation with volumetric convolutions and 3D positional encodings. The Dice score measures the extent of overlap between regions identified by the computer and those designated by experts; a higher value denotes a more precise correspondence, whereas a lower value reflects a discrepancy. The 95% Hausdorff distance (HD95) quantifies the spatial separation between boundaries; a diminished value signifies a strong alignment, whereas an elevated value denotes increased discrepancy. When tested on the Beyond the Cranial Vault (BTCV) data set for abdominal organ segmentation and brain tumor segmentation from MRI and CT data (MSD), along with the kidney tumors from CT scans (KiTS19) data set for generalization evaluation, UnetTransCNN outperformed existing models in abdominal organ segmentation on the BTCV dataset with an average Dice score of 85.3%, especially for difficult regions like the gallbladder and adrenal glands [566]. The model also demonstrated superior performance on the MSD (brain tumors) and KiTS19 (kidney tumors) datasets, achieving high Dice scores and low HD95, and notably faster inference times compared to other models [566].
5.3.3. Recurrent Neural Networks (RNNs) and Long Short-Term Memory (LSTM)
RNNs and their variants, including LSTM and Gated Recurrent Units (GRUs), are specifically designed for the analysis of sequential data, such as patient records, and are capable of modeling time-dependent health trajectories [548]. These methods have shown significant accuracy in gene expression analysis and the classification of cancer subtypes [567]. The integration of RNN and LSTM with CNN has shown promising results in improving the accuracy and efficiency of BC detection and classification. The hybrid CNN-LSTM model achieved high accuracy (99% for binary classification and 92.5% for multi-class classification) in identifying benign and malignant BC, as well as their subtypes [568]. Another hybrid CNN-LSTM model achieved further enhanced diagnostic accuracy and reliability for BC detection and classification [569].
The R-LSTM-CNN framework was proposed for detecting and classifying LC from CT images, outperforming traditional CNN and LSTM models by effectively segmenting and extracting features from lung images [570]. Bidirectional Long Short-Term Memory (Bi-LSTM) networks significantly improve LC detection by leveraging their ability to process sequential data and capture long-term dependencies, achieving very high accuracy in detecting LC [571]. This study showed they could identify cancer with nearly perfect accuracy (99.89%) and were excellent at correctly identifying both positive and negative cases.
5.3.4. Others
Rare cancers often lack sufficient annotated data due to their low prevalence and privacy constraints. To address this, researchers are increasingly turning to generative models such as Generative Adversarial Networks (GANs) and Variational Autoencoders (VAEs). These models can synthesize realistic medical data—ranging from histopathological images to gene expression profiles—thereby augmenting limited datasets and enhancing model generalization [572]. Sun et al. proposes Onto-CGAN, a novel generative framework that combines knowledge from disease ontologies with GANs to generate unseen diseases that are not present in the training data [573]. This study demonstrated that Onto-CGAN can generate new diseases with statistical characteristics similar to actual data and significantly improve ML model training. This novel approach addresses rare disease data shortages and has applications in data augmentation, hypothesis generation, and preclinical clinical model validation.
Transfer learning (TL) models are useful for accelerating model development and improving performance with a small amount of medical data by fine-tuning pre-trained models to medical data [574]. This model can also be particularly useful in cases where it is difficult to obtain large datasets, such as rare cancers. The RareNet framework, developed by Shao et al., demonstrated this by utilizing TL on DNAm data to classify rare cancers with high accuracy, outperforming traditional ML models [575]. TL utilizes insights acquired from training models on extensive datasets, like ImageNet, which comprises millions of images across various categories, and applies this knowledge to more specialized tasks with smaller datasets [576]. Consequently, TL offers a solid framework for enhancing the diagnosis of rare diseases in medical imaging.
Self-supervised learning (SSL) is a paradigm in ML that leverages unlabeled data to learn useful representations, which can be applied to various downstream tasks. This approach is particularly beneficial in scenarios where labeled data is scarce or expensive to obtain [577]. Pai et al. developed a foundation model for cancer imaging biomarker discovery by training a convolutional encoder with SSL using a dataset of 11,467 radiographic lesions [578]. A crucial finding is that the foundation model excels in reducing the demand for training samples in downstream applications, which is highly beneficial in medicine where large labeled datasets are often scarce. This allows for effective biomarker discovery even with limited data.
High-performing models alone are insufficient for clinical integration; interpretability is essential. The “black box” nature of DL models has spurred the development of Explainable AI (XAI) techniques, which aim to make model decisions transparent and trustworthy. This transparency is essential for fostering trust and understanding in AI applications across various domains, including healthcare [579]. Tools such as Gradient-weighted Class Activation Mapping (Grad-CAM), Shapley Additive Explanations (SHAP), and Logical Interpretable Model-agnostic Explanations (LIME) provide insights into feature importance and decision pathways, enabling clinicians and researchers to validate AI outputs [580,581]. Recent studies have applied XAI to cancer research with promising results. For example, Dalmolin et al. used SHAP to identify influential genes in cancer classification models, enhancing both interpretability and biological relevance [582]. In drug repurposing for rare diseases, Perdomo-Quinteiro et al. introduced a graph-based XAI framework that generates semantic explanations for predicted drug candidates, facilitating hypothesis generation and experimental validation [583].
5.3.5. AI-Based Early Cancer Diagnosis
AI technology is being used in modern healthcare systems to improve the effectiveness of cancer screening programs and methods. The use of AI in cancer screening can improve its accuracy, strengthen early detection through more sensitive and specific screening processes, optimize medical resource allocation through efficient targeting of high-risk groups, and enable personalized medical approaches tailored to the individual patient [584].
Image-Based Diagnosis
AI excel at detecting subtle patterns that may elude human observation, thereby assisting physicians in expediting and enhancing diagnostic accuracy [585]. CNNs have been developed to detect microcalcifications and architectural distortions in mammograms, which are critical indications of BC [551,552,553,554,556,569]. For example, in a cross-modality fusion technique that combined mammography and ultrasound, a specially created 17-layer CNN achieved an accuracy of 96.4% [554]. A newly developed CNN architecture using the Mammographic Image Analysis Society (MIAS) and Digital Database for Screening Mammography (DDSM) datasets for mammographic image analysis demonstrated accuracies of 99.175% and 98.44%, respectively, on mammographic images, clearly distinguishing the boundaries of cancerous regions in abnormal mammographic images [586]. A computational framework that classifies mammography images using ResNet-50 CNN to diagnose BC achieved an excellent classification accuracy of 93%, which is expected to facilitate the early diagnosis and classification of malignant and benign BC [587]. A hybrid CNN-LSTM model achieved high accuracy of 99.17% and 99.90% on two histological data set, demonstrating enhanced classification accuracy and resilience [569]. CNNs have been used to analyze infrared thermographic images for BC detection [588]. By integrating CNNs with Enhanced Particle Swarm Optimization (EPSO) and Generative Adversarial Networks (GANs) for data augmentation, achieving a recognition rate of 98.8%. and demonstrating the potential for early, reliable, and cost-effective BC screening in real-world clinical environments [588].
A novel Back Propagation Boosting Recurrent Wienmed model (BPBRW) with Hybrid Krill Herd African Buffalo Optimization (HKH-ABO) mechanism has been developed for detecting BC in an earlier stage using breast MRI images, achieving improved accuracy of 99.6% with a 0.12% lower error rate [589]. A new granular computing-based DL model was evaluated using ultrasound image datasets and breast tissue pathology image datasets [590]. The proposed model achieved accuracies of 93% and 95% on the two real-world datasets, respectively. A method that applies deep belief networks (DBN) to ROI images for BC diagnosis has been proposed [591]. The proposed DBN model achieved performance rates of 96.32%, 96.68%, 95.93%, and 96.40% in accuracy, SP, SN, and precision, respectively, suggesting DBN is an efficient and robust algorithm for classifying BC and normal tissue in mammograms [591].
The integration of low-dose CT scans with AI-driven analysis facilitates the detection and classification of early-stage LC lesions [558,559,560,561,592]. Google’s DL model looked at 3D CT scans and did better than expert radiologists in some cases, cutting down on false negatives by 5% and false alarms by more than 10%. These kinds of tools can pick up very small problems on scans that might be missed, which could help find LC at Stage I, when they are much easier to treat [592]. The combination of CNN for image analysis with XAI techniques, particularly Grad-CAM demonstrated a high accuracy up to 93.06% of LC detection [558]. The accuracy of cancer detection significantly increases by over 99% when CNNs are combined with a residual network architecture (ResNet), such as ResNet18 or SE-ResNeXt-50, primarily due to their advanced feature extraction capabilities, ability to handle complex patterns, and robust classification mechanisms [559,560]. CNNs applied to biparametric MRI have surpassed conventional techniques in the precise identification of significant PC lesions [593,594].
CAD–enhanced colonoscopies detected approximately 24% more adenomas than standard screening (44% vs. 36%), due to the capture of small polyps that are easily missed [595]. However, there was no significant improvement in the detection of advanced lesions, and the increase in SN was primarily due to the detection of very small polyps. The identification of small polyps necessitates meticulous evaluation of the potential for early adenoma diagnosis, which may progress to cancer, alongside the financial implications of the unwarranted excision of non-cancerous polyps [595].
Genomic and Molecular Diagnosis
AI can be used to make sense of the vast and complex data generated by genomic and molecular analyses, leading to the discovery of new biomarkers and more precise risk predictions [596,597]. The MCED testing is a technology that analyzes molecules such as DNA, RNA, and proteins derived from cancer cells in the blood to assess the possibility of cancer [598]. While some tests can estimate the origin of the cancer, additional tests such as imaging tests or tissue biopsies are required for a definitive diagnosis. MCED testing requires ongoing development to enable the early detection of various cancers before symptoms appear. Currently, these tests have not received FDA approval, and some are only available as laboratory-developed tests under the Clinical Laboratory Improvement Amendments (CLIA) regulations [598].
The integration of MCED with AI significantly enhances the accuracy of cancer early detection through various methodologies and technologies [599]. This multifaceted strategy improves predictive efficacy, enabling more reliable identification of tumors and facilitating clinical applications [600]. An AI-based MCED testing analyzes atypical methylation patterns using ctDNA and applies AI algorithms developed based on large datasets of cancer and non-cancer patients to detect more than 50 types of cancer at all stages with high SP and identify the primary tissue [597]. This test precisely detected two-thirds of the twelve lethal cancers lacking routine screening methods. Because advanced cancer has more tumor DNA in the blood, the detection rate increased with later stages of the disease [597]. The integration of large-scale WGS with real-world clinical data provides actionable insights for personalized cancer care, accelerates biomarker discovery, and demonstrates the potential to establish a robust framework for genomic medicine within national healthcare systems [601].
The SYMPLIFY study investigated the cancer detection performance of the GRAIL Galleri MCED blood test in patients referred for urgent testing for the possibility of female-specific conditions, LC, or lower or upper gastrointestinal cancer in the UK healthcare system, or in patients referred to rapid diagnostic centers with nonspecific symptoms [602]. At an advanced stage, it demonstrated effectiveness in detecting cancer signals with high SP (98.4%) and moderate SN (66.3%). The ML model analyzed cfDNAm patterns and showed stability across various patient subgroups. However, the moderate SN highlights the need to improve the test’s SN (negative predictive value) to enhance clinical utility [603].
The PATHFINDER Study performed in oncology and primary care outpatient clinics at seven US health networks, a convenience sample of adults aged 50 years or older without signs or symptoms of cancer consented to MCED testing [604]. This study utilized a ML model integrating cfDNAm patterns. It showed solid performance with a 99.1% SP and a 98.6% negative predictive value (NPV), effectively detecting multiple cancers, including those without routine screening, at early stages [604].
The SPOT-MAS test showed a non-invasive ctDNA-based test for the simultaneous early detection of multiple cancers in asymptomatic adults [605]. It identified the presence of cancer and its tissue of origin (TOO) through DNAm and fragment size analysis. This enables more precise support for subsequent diagnosis and treatment decisions, allowing healthcare professionals to efficiently determine the areas to focus on during testing. In the study, SPOT-MAS demonstrated NPV of 99.92%, indicating a very high likelihood that cancer will not be present for at least 12 months following a negative result [605]. This helps reduce patient anxiety and minimize unnecessary additional testing.
A recent study evaluated a new MCED test analyzing plasma cell-free DNA using genetic and fragmentomics features from whole-genome sequencing. This test was developed to identify cancer signals and tissue origins. [606]. The MCED test achieved an overall SN of 87.4% and SP of 97.8% in the independent validation cohort. Crucially, it maintained high SN for early-stage cancers (stages I and II), with 79.3% and 86.9% SN, respectively, indicating its potential to detect cancers when they are most treatable. The test demonstrated effectiveness in detecting cancers that currently lack established screening protocols, such as PC and OC. For instance, it achieved a SN of 76.9% for PC and 90.5% for OC, which are often diagnosed at advanced stages [606]. In addition, this study demonstrated an accurate prediction of TOO with 83.5% accuracy. The features help medical professionals to accurately identify the primary site of malignant tumors and take appropriate follow-up measures [606].
Homologous recombination deficiency (HRD) causes genomic instability in cancer and is associated with a higher risk of cancer in BRCA1/2 mutation carriers [607]. HRD reveals SN to Poly ADP-ribose polymerase (PARP) inhibitors. Therefore, accurate HRD detection improves mutational profiling, which aids in detecting genomic instability and guiding targeted therapies in cancer patients [608]. ML has been used to create algorithms such as ovaHRDscar and tnbcHRDscar, which enhance HRD detection in OC and BC, respectively [609]. These algorithms used a powerful combination of bioinformatics, ML, and statistical methods to analyze large genomic datasets and identify specific allelic imbalances, improving clinical outcome prediction and patient selection for HR-targeted therapies.
A DL framework, Genomic Status scan (GSscan), employing LSTM for the estimation of HRD status from low-pass whole genome sequencing data, has been developed [610]. The framework exhibited adaptability to low DNA input levels, suggesting potential applications in plasma cell-free DNA and minimal biopsy samples. Additionally, GSscan exhibited superior accuracy in predicting HRD status in breast and ovarian cancer samples, surpassing current methodologies, and HRD-positive patients identified by GSscan demonstrated enhanced clinical outcomes [610].
CancerSig, an evolutionary supervised learning method was proposed to identify cancer stage-specific miRNA signatures for early cancer predictions [611]. CancerSig demonstrated a mean performance across 15 different cancer types with a 10-fold cross-validation accuracy of 84.27% ± 6.31%, SN of 0.81 ± 0.12, SP of 0.80 ± 0.10 [611]. OncoSeek, a new protein analysis-based MCED approach that utilizes a panel of seven protein tumor markers, has been specifically designed to be cost-effective and practical for use in low- and middle-income countries, leveraging advanced AI models [461]. This method reduced false positive rates compared to traditional methods and improved SP to 92.9%.
A novel approach, the ZAHV-AI system, integrating EV isolation technology with AI-based analysis, has been introduced to enhance the early detection and monitoring of CRC [440]. By combining EV-derived miRNA with the conventional marker CEA through AI-based analysis, high-density early diagnosis was achieved across the entire CRC diagnostic spectrum (AUC 0.9861). Particularly, it demonstrated perfect performance with an AUC of 1.0 in detecting Stage 0–I CRC. This compensates for the low early-stage SN of conventional CEA testing, suggesting the potential for a practical clinical tool that significantly enhances the ability to diagnose and monitor CRC at an early stage.
RiboTIE is a state-of-the-art DL-based tool optimized for analyzing ribosome profiling (Ribo-seq) data to decipher RNA translation positions and identify translated open reading frames (ORFs) [503]. RiboTIE applies cutting-edge ML technologies such as transformer networks to process variable-length inputs (the number of reads mapped to the transcriptome) and perform automated feature extraction. It also utilizes pre-trained models optimized across numerous ribosome profiling samples to enhance overall performance [503]. RiboTIE outperformed other ORF detection tools in terms of SN and precision when identifying standard coding sequence (CDS). For example, in pancreatic progenitor cell samples, RiboTIE detected 64.9% more CDS than ORFquant, with 300% more small CDS under 300 bp in length [506]. It also detected the highest number of CDSs with non-canonical ORF (ncORF) (48, all CUG), outperforming other tools. These findings uncover the presence of previously unrecognized translated proteins or peptides. ncORFs may yield new insights into disease biology and facilitate the identification of novel biomarkers or therapeutic targets for cancer diagnosis and treatment [503].
The HCC Early detection Screening (HES) algorithm enhanced AFP surveillance for HCC by incorporating age, Alanine aminotransferase (ALT), platelet count, AFP rate of change, and cirrhosis etiology [612]. Validated in 7432 cirrhosis patients, it detected early-stage HCC within 6 months with 51.2% SN and 90% SP vs. 46.0% SN for AFP alone, identifying 136.46 vs. 118.01 early HCC cases per 1000 imaging analyses and detecting 56% vs. 50% of cases missed by ultrasound, offering modest but meaningful improvement at no added cost [612]. In the study, at a fixed 10% false-positive rate (FPR), HES V2.0 showed higher SN (~47% vs. 38% for AFP and 41% for GALAD), especially for early-stage HCC, which is the hardest to detect. Machine learning, as utilized in HES V2.0, can leverage dynamic trends, nonlinear effects, and multifactorial interactions, resulting in earlier and more precise detection of HCC in high-risk patients compared to logistic regression analyses such as GALAD and ASAP. These models are compared in Table 12.

Table 12.
Comparison of HCC Detection Models [612].
Multi-Modal Data Integration
Electronic health record data are naturally rich in diverse features that span both time and space, including socioeconomic details, molecular data, and various modalities such as tabular data, images, time-series data, and free text. These data components can be structured or unstructured, providing a comprehensive view of patient information that aids in the development of effective AI-driven cancer detection and screening solutions [584].
A study published in Nature Medicine in 2023 utilized DL techniques on millions of patient records from Denmark and the United States to identify high-risk groups for PC, a challenging cancer to detect early, and successfully predicted its onset up to three years in advance [613]. The model, referred to as “CancerRiskNet,” employed electronic health data, including patients’ diagnostic records, test results, medication prescriptions, and family history. The system identified high-risk patients with notable accuracy by analyzing symptom and medical code combinations that mirrored the early progression pathways of PC. This method may facilitate the early identification of pancreatic tumors at a surgically operable stage by offering tailored screening for the designated high-risk patient population [613].
The Artificial intelligence-aided PCa (APCA) score, derived from routine health check-up data, demonstrated potential to significantly reduce the number of unnecessary prostate biopsies in Asian populations [614]. The APCA score predicts high grade PC (HGPC) using 18 features, including some previously unused markers, and achieved an AUC of 0.76 in the multicenter validation cohort. When applied, it could reduce unnecessary biopsies by 20–38%, but may miss 5–10% of HGPC cases [614].
Orpheus, a new multimodal DL tool that combines H&E whole-slide images and text-based pathology reports, accurately identified high-risk cases for BC (Recurrence Score (RS) > 25) in three separate test groups from different medical centers and countries [615]. With an AUC of 0.89, Orpheus found high-risk cases in TAILORx better than the current nomogram, which has an AUC of 0.73. It also accurately predicted the risk of metastasis recurrence in patients with RS ≤ 25, it achieved an AUC of 0.75 compared to 0.49 for the existing RS assay for hormone receptor-positive early BC, thus indicating potential for personalized treatment and monitoring [615].
Recent studies have employed multimodal data on BC to compare the clinical and molecular characteristics of the disease in African and South Asian women with those in European women, underscoring the necessity for customized diagnostic, therapeutic, and screening strategies that consider the distinct clinical and molecular traits linked to varying genetic backgrounds to guarantee equitable advantages for all communities [616].
Topological Data Analysis
In Topological Data Analysis (TDA), topological features (TFs) aim to quantify and describe information about the intrinsic “shape” and “connectivity” of data [617]. This focuses on identifying hidden patterns and structures within data that traditional statistical or ML methods may overlook [617]. Key TDA methods, Persistent Homology (PH) and Mapper, are used to extract and utilize TFs [617].
TFs, when combined with AI technologies, particularly ML and DL, have enabled significant advancements in cancer diagnostics. For example, when analyzing lung tumors in chest CT images, TFs can predict true histology more accurately than radiomic features [618]. This contributes to higher diagnostic accuracy by capturing complementary information in distinguishing benign from malignant tumors and adenocarcinoma from squamous cell carcinoma. It has also been used to classify patients with chronic obstructive pulmonary disease (COPD) [617,619] or detect COVID-19 infection [620] via CT images, demonstrating accuracy equal to or better than existing methods.
TDA is used in cancer research to capture important attributes such as a tumor’s shape, texture, and intensity variations [617]. In BC diagnosis, TFs enhanced DL model performance and help identify subgroups with cancer-related gene mutation profiles that were missed by existing models [621]. In OC and BC histopathology image analysis, the TopOC-1 and TopOC-CNN models improved tumor type discrimination accuracy by up to 8.35% (for TopOC-CNN) by integrating TFs into existing DL models [622]. Specifically, they extracted unique “topological fingerprints” by analyzing the evolution of topological patterns across each color channel [622]. For PC, TDA-based systems predicted tumor aggressiveness from histological images, replicated the Gleason grading system, and identified more refined disease subtypes [623].
DL models are often called ‘black box’ models, making their internal workings difficult to understand. TDA enhances model explainability by quantifying structural and geometric patterns in the data, providing insight into why the model makes specific decisions [624]. The Mapper uses the probability values from the ML classifier as a “lens function,” providing a visual representation of how the model classifies data, thereby offering insight into the model’s decision-making process [617]. For example, it visually shows whether certain data categories are easily identifiable or where the model experiences confusion [617].
Biomedical data is often difficult to process with traditional analytical tools due to its scale and complexity [625]. TDA effectively handles high dimensionality and is robust against noise and non-linearity, providing useful insights even in scenarios with data quality issues [626]. PH can compress large, high-dimensional data into compact, low-dimensional representations while preserving important topological information [617]. ML models require very large datasets for training, but in the biomedical field, it can be difficult to secure such large-scale labeled datasets. TDA can be used even with small datasets insufficient for ML model training, making it particularly important in the field of medical image diagnosis [617].
Beyond visual diagnostics, TDA can also be applied to gene regulatory network analysis. PH is utilized to understand the structural characteristics of cancer cell networks. By identifying significant deviations in topological features (e.g., loops, co-clusters) within gene interaction patterns between cancer and normal samples, it reveals higher-order connectivity relationships contributing to disease complexity [627,628].
6. Early Detection Strategies for Aging
Early diagnosis of aging and slowing its rate hold greater economic value than treating individual diseases. It has been reported that slowing aging to extend life expectancy by one year generates $38 trillion in value, while extending it by ten years generates $367 trillion in value [629]. In relation to early diagnosis of aging, this section discusses recently developed techniques for the early identification of neurodegenerative disorders such as AD and Parkinson’s disease (PD). AD is the most common cause of dementia and is a serious health problem that is expected to increase threefold by 2050 [630]. Early diagnosis of cognitive decline is crucial for implementing effective treatment strategies to mitigate disease progression. This can help alleviate symptoms and improve quality of life [630,631]. This section encompasses biomarkers, the significance of assessing cognitive function, MRI-based techniques, AI and ML models, and brain age prediction.
6.1. Key Biomarkers and Pathological Changes
6.1.1. Fluid-Based Biomarkers
When compared to CSF biomarkers and PET imaging, blood-based biomarkers offer advantages such as simple sampling, low invasiveness, and cost-effectiveness, making them the most promising early screening technology for AD [632]. Neurofibrillary Tangle (NfL) is a protein that is an important component of the cytoskeleton of neurons. It is released into CSF and plasma upon neural damage, leading to elevated levels in these bodily fluids. Plasma NfL levels were significantly higher in mild cognitive impairment (MCI) and AD groups compared to healthy controls, with the highest concentrations observed in the MCI group. This suggests that NfL levels begin to rise before the onset of significant cognitive decline [633,634]. In AD, abnormal aggregation of phosphorylated tau (p-tau) forms insoluble clumps, leading to synaptic dysfunction and brain cell death [635]. The subjective cognitive decline (SCD) group had higher plasma p-tau181 and plasma GFAP levels than the normal control (NC) group [632]. GFAP is an inflammatory molecule released by astrocytes and microglia, signaling neuronal damage due to reactive astrocytes [636]. A robust correlation between plasma p-tau217 levels and the severity of NfL in AD patients was demonstrated in a postmortem study [633]. miRNA-92a-3p, miRNA-486-5p, and miRNA-29a exhibited a significant correlation with the initial phases of AD. Also, levels of miRNA-483-5p were higher in people with MCI and AD than in NC [634]. Salivary levels of lactoferrin, and P. gingivalis IgG and specific urinary metabolites such as ApoC3 have been studied as non-invasive biomarkers for AD risk and disease diagnosis [634]. A study profiling human proteins throughout the entire 50-year lifespan identified 29 proteins that consistently increased in expression in six or more human tissues with age [637]. One of these, serum amyloid P component (SAP) is involved in amyloid formation, and its age-related increase may contribute to vascular aging, tissue damage, and inflammation. Amyloid protein aggregation has been identified as a common aging characteristic across human organs [637]. An intrinsic cellular aging clock (DNAm IC) derived from DNAm data based on the INSPIRE-T cohort has been constructed [316]. This clock has been validated as a more accurate predictor of mortality than existing epigenetic clocks and has been shown to be closely associated with aging indicators such as immune and inflammatory markers, functional and clinical outcomes, and lifestyle factors. Additionally, DNAm IC can be calculated using salivary samples, demonstrating its potential as a non-invasive alternative [316]. In contrast, a new proteomic aging clock measuring biological age using circulating plasma proteins has been developed [387]. Utilizing large-scale data from the UK Biobank (UKB), ProtAgeGap composed of 20 core protein models achieving approximately 95% of the age prediction performance of 204 protein models, demonstrated the ability to predict chronological age with high accuracy. This clock proved effective in predicting risks of age-related diseases, multiple morbidities, and mortality, showing comparable predictive power in other populations, including China (CKB) and Finland (FinnGen) [387].
6.1.2. Cell Aging and Neuroinflammation
There is growing evidence that terminally differentiated neurons in the brain can re-enter a cell cycle-like process during neuronal aging and disease states. Cell cycle-related events were predominantly observed in excitatory neurons, and it was demonstrated that their eventual fate is likely to be cellular senescence [638]. In normal brain aging, the number of neurons reentering the cell cycle and aging neurons diminished, whereas in late-onset AD, these cells accumulated [638]. Neuroinflammation, a persistent low-grade inflammation linked to aging, demonstrates an enhanced pro-inflammatory response. It originates from intricate interactions among immune cells in the aging brain, dystrophic glial cells, and the impairment of the blood–brain barrier [639]. When autophagy is impaired, damaged cellular components accumulate, particularly in neurons and microglia. The accumulation of these toxic proteins exacerbates inflammation and contributes to neurodegenerative diseases (NDDs) [639]. Chronic inflammation worsens neurodegenerative diseases (NDDs) and eventually causes cognitive decline [639]. NDD like AD and PD are strongly linked to impaired proteostasis, characterized by protein aggregates that signify a failure in the cellular mechanisms governing protein synthesis, folding, and degradation [640].
6.1.3. Cognitive Function Assessment
Dementia is an underdiagnosed syndrome, and there is a need to improve the early detection of cognitive decline. Early indicators of cognitive decline should be based on concerns from the individual or a reliable informant and can be objectified through standardized neuropsychological testing [641]. Repeatedly assessing learning using the multi-day learning curve (MDLC) can reveal disease-related memory decline in precliniciated with future cognitive decline relative to amyloid levels and standard measures [642]. In the preclinical AD stage, significant changes were found in tasks assessing various cognitive functions (e.g., episodic memory, semantic memory, language, and perception). Additionally, low cognitive performance in memory, attention, and executive function tasks predicts future cognitive decline even in global deterioration scale (GDS) stage 1 (i.e., when there is no subjective or objective cognitive decline) [641]. Gender-based research reveals significant gender-specific disparities in neurodegenerative biomarkers and their correlations with cognitive decline, especially concerning PD [643]. In both male and female PD patients, elevated CSF levels of IL-8 correlated with diminished Montreal Cognitive Assessment (MoCA) scores that identifies a sign of cognitive impairment, suggesting an association with cognitive decline. Elevated CSF levels of FABP in males and elevated MCP-1 and SCF levels in females were correlated with diminished MoCA scores, respectively [643]. Sensory deficits, including the deterioration of vision, hearing, taste, smell, and touch, may exacerbate the brain’s susceptibility to neuropathological alterations and increase the risk of AD onset [644]. Auditory processing deficits may worsen cognitive decline by elevating neural activity and resulting in tau accumulation [644]. Visual and tactile stimulation therapy has shown potential in alleviating cognitive decline in AD patients [644].
6.2. Neuroimaging Techniques
6.2.1. T1-Weighted MRI and Structural Changes
T1-weighted MRI scans, which enhance signals from fatty tissue and suppress signals from water, are particularly useful for identifying cognitive decline and early signs of AD, as well as monitoring cognitive decline over time [645]. Aging causes various changes in the brain that can be seen with an MRI including brain volume changes, fluid-attenuated inversion recovery (FLAIR) white matter hyperintensity lesions, and changes in tissue properties like relaxation rate, myelin, iron content, and neuronal density [646]. Changes in brain volume with age include a decrease in gray matter and an increase in ventricle volume, both of which are linked to a decline in cognitive function [646]. Structural brain changes such as hippocampal atrophy and cortical atrophy, can happen long before the onset of cognitive symptoms [645]. The left parahippocampal gyrus and the left inferior lateral ventricle are two important parts of the brain that are linked to early diagnosis of AD. The latter, which often reveals structural changes such as enlargement and shape distortion in AD patients, showed the highest accuracy for diagnosing AD, with a maximum accuracy of 0.86 [630].
6.2.2. White Matter Hyperintensity Lesions (WMH)
Vascular WMH are frequently observed in individuals aged 60 and above, with prevalence increasing with age. They are closely associated with cognitive decline, stroke risk, mental health, and brain structural deterioration [647]. WMH has been reported at high frequencies in young adults under 40 years of age. If these lesions represent an early form of subclinical cerebral small vessel disease (cSVD), it is important to investigate their emergence and progression to study pathophysiological correlates and associations with genetic, environmental, and behavioral risk factors [648]. Various tools exist for WMH segmentation, with DL-based methods increasing in number. However, there is a lack of validated automated methods in populations with low prevalence and low overall lesion burden [648]. The “SHIVA-WMH” detector, which was developed in the context of the SHIVA project (https://rhu-shiva.com/), is a 3D Unet-based tool optimized to detect the entire range of WMH severity, including very mild cases observable in young subjects. This tool demonstrated superior performance compared to existing methods in WMH segmentation [648].
6.2.3. Other MRI Techniques
Tissue relaxometry indicates changes in tissue relaxivity with age and provides reference values for distinguishing between normal aging and pathological conditions [649]. Myelin content quantified by MRI increases with age and later decreases, and is associated with cognitive decline and changes in walking speed in the elderly. In AD patients, age-related myelin destruction is more pronounced [646]. Brain iron accumulation detected using magnetization susceptibility MRI techniques increases with age in specific brain regions and may contribute to neurodegenerative processes [646]. Neurofluid imaging using MRI techniques such as gadolinium-based contrast agents (GBCA) and diffusion MRI demonstrates age-related changes in the dynamics of CSF and interstitial fluid (ISF), which are crucial for brain health and waste removal [646]. HIV infection accelerates brain aging and disrupts glymphatic clearance pathways in the aging brain, which may explain motor and executive dysfunction in HIV-infected individuals [650]. The assessment of glymphatic performance was conducted using the DTI-ALPS method, which is based on diffusion imaging of MRI data. Healthy individuals exhibit higher glymphatic function at younger ages, which significantly decreases with age. However, HIV-infected individuals exhibit impaired glymphatic clearance function regardless of age [650]. Functional MRI (fMRI) analyzes brain activity using BOLD contrast images, and image quality has been improved by introducing spin echo pulses and enhancing magnetic field strength. This allows comparison of differences between active and resting states, developmental and aging states, and normal and damaged brains. However, large sample sizes are required for interpretation in cognitive research [651].
6.2.4. Other Neuroimaging Data
Multimodal imaging is used for the diagnosis of AD, and T1-weighted MRI detects atrophy in the medial temporal lobe and other areas [652]. Position Emission Tomography (PET) provides information on functional changes in the brain, such as glucose metabolism (FDG-FET), amyloid plaque deposition, and tau pathology [652]. These various imaging techniques can contribute to the early diagnosis of AD by elucidating its characteristics and understanding the association between protein aggregates and pathophysiology [652]. Electroencephalography (EEG) is used to analyze brain activity patterns by recording synaptic activities and identifying changes associated with AD [653]. The diagnosis of AD is made on the basis of characteristic patterns such as a decline in oscillatory activity and a reduction in complexity levels in EEG signals [653]. Retinal imaging is being studied as a potential early diagnostic tool for AD by assessing retinal vascular thickness and characteristic changes noninvasively [654,655]. A study that employed mesoscale microscopy technology and in vivo imaging to elucidate the detailed changes occurring in the brain vascular network of aged mice revealed that vascular dysfunction, particularly impaired blood–brain barrier (BBB) function and reduced pericytes density, may precede neuronal damage associated with NDD and other forms of dementia [656]. This finding suggests that such alterations could function as early indicators or therapeutic targets.
6.2.5. Key Datasets
The Open Access Series of Imaging Studies (OASIS) is a project aimed at making neuroimaging data sets of the brain freely available to the scientific community [657]. OASIS-Cross-sectional and OASIS-Longitudinal have been used in neuroanatomical research and the development of segmentation algorithms. OASIS-3 includes long-term multimodal imaging, clinical, cognitive, and biomarker data from subjects with normal aging and AD, while OASIS-4 provides MR, clinical, cognitive, and biomarker data from patients with memory problems [657].
The AD Neuroimaging Initiative (ADNI) is a long-term, multi-center research dataset aimed at developing biomarkers for AD. It includes MRI, PET, and cognitive test data from patients with AD, CN (cognitively normal), and MCI [658]. The Internet Brain Segmentation Repository (IBSR) dataset has been developed for the purpose of evaluating brain image segmentation, and it comprises 20 real T1-weighted MRI scans as well as expert manual segmentation results [659]. The model consists of slices at various resolutions and volumes of 256 × 256 × 128, and also provides segmentation of 32 non-cortical structures [660].
The Human Connectome Project (HCP) is a large-scale international research project aimed at mapping the brain’s structural and functional connectivity in great detail [661,662]. It collected a wide range of brain imaging data, including structural MRI, diffusion MRI (dMRI), resting-state and task-based functional MRI (fMRI), as well as behavioral and cognitive data from approximately 1200 healthy adults [661,663,664]. The HCP provides standardized protocols and high-resolution images (e.g., dMRI 1.25 mm, fMRI 2 mm), as well as the ability to analyze genetic factors, including data from twins and families. This information is used in a variety of applications, including brain network analysis, cognitive function research, disease mechanism exploration, and predicting brain age [665,666,667]. An open platform makes data freely available, which is extremely useful in neuroscience and AI research [668].
The Cambridge Centre for Aging and Neuroscience (Cam-CAN) is a substantial public neuroscience dataset aimed to investigate the impact of aging on cognitive functions and the associated neural mechanisms [669,670]. It collected high-quality brain imaging data, including structural MRI, resting-state and task-based functional MRI, and MEG data, from 700 healthy adults aged 18 to 87 [669]. It also has information from a variety of cognitive and behavioral tests, including tests of memory, attention, and executive function [671]. This data, obtained through standardized protocols, is utilized to examine changes in brain structure and function, cognitive decline, and the mechanisms associated with age-related diseases [672,673]. Cam-CAN data supports the development of neuroimaging biomarkers for cognitive aging and early detection of decline [672]. Brain age prediction using multimodal neuroimaging data achieved high accuracy in distinguishing between chronological age and brain-predicted age [674]. Researchers can get free access by filling out an application.
6.3. Integration of AI
A range of data types have been leveraged for the early diagnosis and prediction of AD, with AI and ML/DL) models playing a pivotal role in the analysis of this data to discern subtle patterns indicative of the disease.
Various ML/DL Models and Performance
Traditional aging markers like SA-β-gal, p16, p21, HMGB1, and Lamin B1 for identifying SnCs have limitations in terms of variability and lack of consistency. However, the ML-based nuclear morphometric pipeline (NMP) demonstrated the quantification of nuclear features at single-cell resolution to generate an “aging gradient”, enabling objective and precise evaluation of SnCs [675]. NMP utilizes unsupervised learning, making it applicable to various aging-inducing factors, cell types, and tissue environments, and has demonstrated that major SnCs fractions change with age such as fibroadipogenic progenitors (FAPs) in young regenerating muscle versus satellite cells in aged muscle [675].
To facilitate early diagnosis of AD, 146 brain-based biomarkers (BBBMs) and 12 ML algorithms based on ADNI data were used [676]. The results indicated that Linear Discriminant analysis (LDA), Naive Bayes, and SVM showed promising performance, and an ensemble model integrating these algorithms was developed. Four BBBMs such as Immunoglobulin M (IGM), Placenta Growth Factor (PLGF), Serum Glutamic Oxaloacetic Transaminase (SGOT), and Alpha-1-Microglobulin (A1Micro) were found to be critical for early detection of AD, demonstrating a SN of 92.86% and SP of 82.35%, thereby validating that BBBMs and ML are effective diagnostic tools for clinical practice [676].
A study comparing traditional ML algorithms, including SVM, decision trees, and K nearest neighbor (K-NN), and DL techniques such as multilayer perceptron (MLP) and CNNs for early diagnosis of AD showed that ML is useful with limited data, while DL demonstrates superior performance with abundant data, suggesting the possibility of early and accurate diagnosis [677]. The primary strength of CNNs is their proficiency in automatic feature extraction from MRI images due to their ability to learn complex patterns in complex data [678]. CNNs have demonstrated excellence in MRI image processing, showing the highest accuracy in diagnosing AD [679]. However, their efficacy may be constrained by issues such as gradient vanishing or explosion, which can occur in deeper layers of the network [678,680]. Residual networks such as ResNet-50 demonstrated CNNs’ diagnostic accuracy of AD further by addressing vanishing gradient issues, allowing for deeper architectures and better feature extraction [681].
To overcome the limitations of existing large-capacity TL models, a lightweight CNN model integrating a squeeze and excitation (SE) block and an innovative Avg-TopK pooling layer has been reported [682]. Additionally, this model utilized SMOTE (Synthetic Minority Over-sampling Technique) to address data imbalance issues. This technique generates synthetic data for the minority class to balance the dataset and helps the model improve its prediction performance for the minority class. The CNNs model designed in this way achieved an improved accuracy of 99.84% in AD diagnosis [682].
A hybrid model combining features extracted from the ResNet-18 architecture and traditional image processing algorithms, including Local Binary Pattern (LBP), Discrete Wavelet Transform (DWT), Gray Level Co-occurrence Matrix (GLCM), demonstrated an accuracy of 99.8% and SP of 100% in the diagnosis of MRI images for early detection of AD [683]. Deep feature maps extracted by ResNet-18 were combined with traditional feature extraction algorithms such as LBP, DWT, and GLCM to generate rich feature vectors. These integrated features were classified using feedforward neural networks (FFNN) [683].
Through the utilization of previously trained models, TL provides the benefit of lowering the costs associated with training while simultaneously enhancing performance with a limited amount of training data [684]. The implementation of a fine-tuned CNN model was demonstrated by applying TL to CNN architectures (AlexNet, GoogleNet, and MobileNetV2) and combining these architectures with several solvers including Adam, Stochastic Gradient Descent (SGD), and Root Mean Square Propagation (RMSprop) [684]. This model achieved a high AD classification accuracy of 99.4% on the Kaggle MRI dataset and 98.2% on the OASIS dataset. These results demonstrate that the model has the potential for the early and accurate diagnosis of AD.
A new DL architecture combining a 3D convolutional block attention module (3D CBAM) with a video Swin Transformer in a 3D CNN, named 3D-CNN-VSwinFormer, demonstrated 92.92% accuracy and 0.9660 AUC in distinguishing AD patients from cognitively normal (CN) individuals by extracting local features from 3D MRI data and obtaining comprehensive information on brain structural changes through multi-scale features [685]. This model effectively avoided the data leakage issues that can occur in 2D MRI slice-based analyses while also capturing global spatial information about the brain [685]. Furthermore, it demonstrated superior computational efficiency.
DL can improve diagnostic accuracy by combining data from multiple sources, such as MRI, EEG signals, retinal images, cognitive test results, DNA analysis, blood-based biomarkers, and voice recordings [686]. For example, a multi-modal DL framework that utilizes the entire ADNI series, including T1-weighted MRI and 18F-FDG PET from the ADNI1 dataset, as well as Aβ PET and tau protein PET from ADNI2 and ADNI3 datasets, achieved a balanced accuracy of 1.00 in the AD versus CN task and 0.76 in the MCI versus CN task [687]. A multimodal classification model that combines clinical, cognitive, MRI, and EEG data has been proposed. This model uses the TimesBlock module to capture EEG time patterns and then fuses MRI spatial information and EEG time data via cross-modal attention to improve the distinction between AD, MCI, and CN groups [688]. Furthermore, the first AD classification dataset encompassing all three modalities has been constructed, increasing the potential for early detection and intervention [688]. A multimodal learning study incorporating MRI-derived brain atrophy, EEG irregular patterns, behavioral assessments, and amyloid beta (Aβ) markers demonstrated that a reduction in Aβ1-42/Aβ-40 correlates with frontal-temporal lobe atrophy and anomalies in EEG signal complexity and information transfer [689]. A random forest model that used structural, electrophysiological, and clinical data was able to accurately predict Aβ ratios 91.6% of the time. This suggests that it could be a useful and cheap way to diagnose problems early [689].
Given the importance of sleep disorders in AD, a multi-modal ML approach has been proposed to non-invasively predict core CSF biomarkers of AD using polysomnography (PSG) and clinical and blood data [690]. The study found significant correlations between sleep-related variables from PSG and the levels of AD CSF biomarkers. Poorer sleep quality and shorter sleep duration were associated with lower Aβ42 levels. The most effective predictors of CSF p-tau and t-tau levels were quantitative PSG features (PSGVAR), conventional PSG parameters, and clinical variables (ALL subset) [690]. These findings suggest that specific quantitative PSG features, such as sleep EEG, could serve as potential early biomarkers for AD. Furthermore, while CSF collection via lumbar puncture is invasive and expensive, making it unsuitable for widespread screening, PSG offers a less invasive alternative for detecting early AD markers [690].
To address the issue of the “black box” nature of DL models, which makes decision-making processes opaque and difficult to understand, explainable AI (XAI) frameworks such as Gradient-weighted Class Activation Mapping (Grad-CAM), Local Interpretable Model-Agnostic Explanations (LIME), SHapley Additive exPlanations (SHAP), Layer-wise Relevance Propagation (LRP), and Saliency Map have been developed [691]. For example, Grad-CAM improves model interpretability and clinician confidence by visually demonstrating that the model focuses on specific areas of the brain (e.g., parietal lobe, cerebral cortex, Broca’s area, medial temporal lobe) when making diagnostic decisions [691]. SHAP quantifies each variable’s contribution to the model’s prediction using numerical features such as brain volume and metabolic rate and points out the basis of predictions using numerical quantification or rule-based explanations, thereby increasing clinician confidence and encouraging clinical application of the model [691].
6.4. Brain Age Prediction and Brain Age Gap
The field of brain age prediction (BAP) is an emerging discipline, employs neuroimaging data and ML algorithms to estimate the biological age of an individual’s brain in relation to their chronological age [692]. This approach has attracted considerable attention as a potential biomarker for brain health, NDD, and cognitive aging [693]. The BAG is the difference between the predicted age and chronological age, reflects the rate at which the brain is aging relative to expectations [692]. The field encompasses a wide range of neuroimaging techniques, ML methodologies, and clinical applications.
6.4.1. Importance of BAP and BAG
Brain age refers to the biological age of the brain, estimated using MRI data [258]. MRI is important in the diagnosis of NDD and plays a key role in distinguishing normal aging from dementia [694]. Structural MRI is helpful in identifying early-stage AD by analyzing brain structural changes, such as hippocampal atrophy [695]. BAG is considered a promising indicator of brain health [258]. It provides indicators of age-related brain functions [258]. Identifying the genetic elements that contribute to BAG will enable the development of genetically validated targets and treatment strategies that may decelerate or potentially reverse brain aging, resulting in a prolonged lifetime [258].
6.4.2. Neuroimaging Techniques and Their Characteristics
Structural MRI, especially T1-weighted imaging, is the predominant method employed for predicting brain age [696]. Structural characteristics by T1 weighted MRI reflect age-related alterations in gray matter volume, cortical thickness, and brain morphology [697]. Regions exhibiting the most pronounced age-related alterations encompass the frontal and temporal cortices, with periventricular areas being especially important for age prediction [698]. The MAE tends to be lower in samples characterized by a narrower age range, as this reduces errors when the predicted brain age closely aligns with the mean chronological age of the population being studied [699]. The UK Biobank, ADNI, and Neurodegenerative Disease Cohort Study (NACC) datasets were employed to systematically assess the efficacy of these models as age prediction instruments for healthy individuals and their viability as biomarkers for diverse NDD [700]. The findings indicated that a penalized linear ML model utilizing Zhang’s BAP approach [701], which integrates age bias correction methods commonly applied in BAP models, attained highly precise BAP with MAE of under one year in external validation [700]. This model exhibited stable predictive performance across different age groups and subgroups, including race and MRI machine/manufacturer [700].
Recent studies demonstrate the enhanced efficacy of multimodal approaches compared to single-modality predictions [702]. Roibu et al. demonstrated that 57 distinct MRI contrasts, including structural, susceptibility-weighted, diffusion, and functional MRI, were capable of predicting brain age [702]. Ensemble methods that integrate multiple modalities offered greater accuracy compared to individual modalities and stronger correlations to non-imaging measurements [702].
Diffusion Tensor Imaging (DTI) offers distinct insights into alterations in white matter microstructure associated with aging [156,703]. Wang et al. proposed a 3D CNN model and trained it using fractional anisotropy (FA) data collected from six public datasets (n = 2406, age = 17–60) to estimate brain age, achieving robust BAP performance with a MAE of 2.785 years and a correlation coefficient of 0.932 [703].
PET imaging provides additional functional data for predicting brain age [704]. Dörfel et al. showed that 5-HT2AR binding assessed via PET exhibited performance similar to gray matter volume (MAE = 6.63 vs. 6.95 years), while the combining both measures enhanced prediction to MAE = 5.54 years [704]. This demonstrated the importance of integrating molecular imaging biomarkers with structural assessments.
6.4.3. ML/DL Performance of BAP
Traditional ML methods require manual feature extraction, which can be time-consuming and subjective [705]. However, it exhibits accelerated learning on small to medium-sized datasets and necessitates less computational power, making it appropriated for environments with constrained hardware resources [706]. ML models have shown promising results when combined with neuroimaging data in the early stages of diagnosing neurodegenerative diseases, particularly AD [707,708]. Xiong et al. evaluated the impact of ML algorithms and image modalities for predicting brain age in adults aged 44.6 to 82.3 years old [709]. Among six ML algorithms (Lasso, relevance vector regression (RVR), support vector regression (SVR), extreme gradient boosting (XgBoost), category boost (CatBoost), and multilayer perceptron (MLP)), Lasso achieved the lowest MAE in both uni- and multi-modality predictions, demonstrating it as the most accurate ML for BAP. Specifically, for multimodality data, Lasso recorded an MAE of 2.741 years [709]. Meanwhile, the ensemble model achieved an MAE of 2.338 years, outperforming Lasso, a single ML when computational efficiency was not a primary concern [709]. In addition, the study demonstrated that the choice of imaging modality, particularly T1-weighted MRI and DWI, plays a more significant role in prediction accuracy than the choice of ML algorithm [709]. While several ML algorithms can effectively predict brain age, the specific choice of algorithm significantly influences the observed associations between BAG and cognitive functions, particularly executive function and emotion processing [710]. SVR, RVR, and Gaussian Process Regression (GPR) demonstrated better performance in identifying BAG-Cognitive associations compared to other ML algorithms, emphasizing the critical role of algorithm selection in brain age research [710].
CNNs and other DL architectures have demonstrated exceptional efficacy in extracting intricate patterns and anomalies from imaging data for BAP. The Simple Fully Convolutional Network (SFCN) architecture has shown particular success, with studies reporting MAE values of 2.14 in UK Biobank data (n = 14,503) [711].
A number of DL models, including SFCN, ResNet, EfficientNet, VGGNet, DenseNet, GLT, and 3D-ViT, have been trained on T1-weighted MRI data from the UK Biobank [258]. Among them, the 3D-ViT (three-dimensional vision transformer) demonstrated the best performance, displaying an MAE of 2.64 and a Pearson correlation coefficient of 0.90 in 5-fold cross-validation [258].
The 3D DenseNet-169-based model was effectively trained using interpolated 2D axial T1-weighted MRI scans derived from research-grade 3D T1-weighted MRI scans [712]. The model achieved an MAE of 1.53 years with a high correlation to chronological age (Pearson’s r = 0.996). Furthermore, the model’s ability to achieve an MAE of 2.73 years (after age bias correction) on clinical grade 2D axial MRI scans, and even lower (2.23 years) with an ensemble approach, signified its high accuracy for practical application. For AD patients, the model predicted an MAE of 3.10, which was significantly higher than that of CU subjects (p < 0.001) [712]. This represents a significant improvement over existing models that could not accurately predict brain age using clinical grade 2D axial MRI scans because they have been trained using research-grade 3D T1-weighted MRI scans [713]. The study demonstrated that the 3D DenseNet-169-based model can accurately predict brain age from routine clinical 2D MRI scans and effectively identify increased brain age gaps associated with NDD such as AD, enhancing the technology’s broad clinical applicability [712].
Recent innovations include transformer-based approaches and hybrid CNN-transformer models [714]. The ds-FCRN (dual-stream fully convolutional residual networks) combined with transformer-based global–local feature learning achieved competitive performance while providing enhanced interpretability. In a test set comprising 3276 healthy subjects (mean age 64.15 ± 7.45 years, 1561 males), the 3D ds-FCRN model achieved an MAE of 2.2 years in BAP, outperforming existing models on the same dataset [714]. A Dense Transformer Foundation Model with Mixture of Experts (DenseFormer-MoE), which integrates dense convolutional network, a Vision Transformer, and MoE, was introduced, and improved BAP accuracy by effectively learning both local and global feature representations [715]. Pre-training the DenseFormer backbone using a masked autoencoder enabled the acquisition of robust and generalizable feature representations through the masking and reconstruction of input patch segments. This significantly enhanced performance, particularly in medical imaging, where labeled data is limited [715]. A global–local attention network model for BAP using multimodal neuroimaging data, including sMRI and dMRI, demonstrated an MAE of 2.44 years, showing a reduced MAE by 10–76% compared to uni-modality models [674]. In particular, this multimodal model revealed unique associations between predicted brain age and behavioral measures, such as walking endurance and loneliness, in the HCP dataset, which were not detected by chronological age alone. Specifically, higher predicted brain age was associated with lower walking endurance and loneliness scores, and higher odor identification ability and conscientiousness scores [674]. In the Cam-CAN dataset, brain age and chronological age exhibited a similar correlation with behavioral measures [674].
Quantum deep learning (QDL), an emerging DL sub-field, was assessed for brain data learning tasks [716]. A statistical analysis of brain tumor classification studies (n = 16) revealed that the QDL model achieved an average accuracy of 0.9701, slightly outperforming the classical model, which had an average accuracy of 0.9650. Similar trends were observed across multiple domains, including AD, stroke lesion detection, cognitive state monitoring, and BAP (MAE 3.302 vs. 3.31, RMSE 4.083 vs. 4.28) [716]. QDL models may offer advantages such as accelerated processing, enhanced performance, improved generalization, and more efficient parameter utilization. In addition, they could aid in the analysis of intricate neural data by using feature representations in high-dimensional quantum spaces. While QDL has great potential in neuroinformatics, it has fundamental limitations in data encoding, the need for robust and task-specific circuit design, current constraints in quantum hardware, and difficulties with model optimization and interpretability [716].
7. Point-of-Care Applications
Point-of-Care (POC) diagnostic technology plays a pivotal role in advancing healthcare services and precision medicine by enabling diagnosis and monitoring close to the patient [717]. POC devices, developed through advancements in nanotechnology, molecular diagnostics, AI, and smartphone integration, are revolutionizing healthcare by providing rapid, accurate, and highly accessible diagnostics [717,718,719]. This substantially influences the early detection of diseases like cancer and age-associated diseases, resulting in improved patient outcomes and increased efficiency within the healthcare system. In addition, it contributes to improving access to medical services, particularly in environments with scarce medical resources or in remote areas [717].
This review examines key technologies used in POC devices, clinically approved and developing point-of-care sensors for the diagnosis of cancer and geriatric diseases, emphasizing significant advancements in biosensor technology, and emerging innovations anticipated to transform early detection and monitoring.
7.1. Recent Developments for Enhancing Biosensor Performance
7.1.1. Nanotechnology (Nanomaterials and Nanostructures)
Nanomaterials play a crucial role in enhancing biosensor performance. Nano-structures contribute to lowering detection limits, reducing SN to contamination, and increasing mechanical strength [720,721]. Noble metal NPs (NM NPs) (e.g., gold, silver) and quantum dots (QDs) play a crucial role in signal amplification during POC biosensor development [722]. Gold NPs (AuNPs) generate colorimetric signals five orders of magnitude stronger than organic dyes [723,724]. QDs exhibit high resistance to photobleaching, making them advantageous for long-term and in vivo applications [725]. Carbon nanotubes (CNTs), and graphene are widely used. Combined with various sensing platforms, they enable rapid, cost-effective, and reliable POC diagnostics required for cancer diagnosis [720,726].
7.1.2. Wearable Sensors
Wearable biosensors are used to continuously and non-invasively monitor human physiological parameters through bodily fluids like sweat, tears, and saliva [727]. Designed to be flexible and integrated into multiple networks, enabling continuous biomarker monitoring. Integration with AI systems contributes to predictive model generation and remote clinical decision support [727]. Wearable platforms like smart contact lenses, sweat patches, and digital wristwatches provide valuable insights into an individual’s physiological state and health status [728]. Wireless smart contact lenses demonstrated potential for diabetes diagnosis and treatment [729,730,731], while wearable sweat-based blood glucose monitoring devices also measure blood sugar non-invasively [732,733].
7.1.3. Smartphone Integration
Smartphones have become a core component of POC diagnostic devices [734]. They leverage already widely available devices to reduce the cost of additional specialized equipment and provide functions such as data processing, image analysis, and result transmission [735]. Smartphones can be transformed into low-cost, high-precision colorimeters through image processing and data analysis capabilities, and are utilized for diagnosing various diseases [735]. They connect to POC devices via Bluetooth, Wi-Fi, or USB. Smartphones serve as readers for portable diagnostic devices, processing and quantifying images such as lateral flow immunoassay (LFIA) results [719], coffee ring biosensor patterns [726], and fluorescent signals [736].
7.1.4. Integration of AI in POC Testing (POCT)
AI and ML algorithms are integrated into POC devices to enhance diagnostic accuracy and efficiency [719]. They interpret complex multivariate patterns, generate predictive models, and automate image/signal analysis. AI can reduce the risk of misdiagnosis and lower dependence on skilled medical personnel, improving healthcare accessibility in resource-poor countries and remote areas [719]. AI models are used for image processing (e.g., cell type classification, anomaly detection), quantifying bioanalytical results, and predicting disease, achieving high accuracy and SN in diagnosing specific cancers (e.g., BC, LC) [717]. The convergence of these diverse technologies is expected to drive the evolution of POC devices into more accurate, affordable, and user-friendly forms, playing a crucial role in enhancing healthcare accessibility and delivering patient-centered medical services. Recently, a DL model combined multi-ultra-sensitive coffee ring biosensor has been proposed [726]. The system couples a generative neural network with a CNN to quantitatively analyze complex biomolecular patterns directly from smartphone-captured images. This AI-driven approach enabled highly precise protein detection down to 3 pg/mL within 12 min, eliminating the need for bulky analytical instruments [726]. By training the DL model on diverse optical interference and pattern datasets, the platform achieved a broad, multi-dimensional detection range for biomarkers related to sepsis (procalcitonin), COVID-19 (N-protein), and cancers (CEA, PSA). When validated using saliva samples, its signal-to-noise ratio exceeded that of conventional LFIA by more than twofold, demonstrating the transformative potential of AI-assisted biosensing for rapid, low-cost, and high-accuracy early disease diagnostics [726].
7.2. Microfluidics Systems and Chip-Based Devices
7.2.1. Concept and Features of Microfluidics Systems and Chip-Based Devices
Microfluidics, also known as lab-on-a-chip or micro total analytical systems manipulates minute fluids within microchannels [737]. MDs facilitate sample pretreatment, separation, dilution, mixing, chemical reactions, detection, and product extraction using minimal sample volumes within a singular, compact system. This enhances the speed and efficiency of analysis, reduces reagent consumption, and automates the entire process, thereby minimizing human intervention [504,506,738]. Due to their exceptional portability and user-friendly interfaces, MDs enable even inexperienced users to conduct analyses independently [738]. Furthermore, they provide multiplexing capabilities, which enable the simultaneous detection of multiple biomarkers in a single analysis, thus facilitating a more precise evaluation of the disease state [739].
7.2.2. Application of Microfluidic System for Cancer Diagnosis in POCT
An ISFET (Ion-Sensitive Field-Effect Transistor) sensor-based LoC (Lab-on-Chip) device was developed to detect cervical cancer by amplifying human papilloma virus (HPV) DNA and human TERT mRNA through the loop-mediated isothermal amplification (LAMP) assays [740]. This portable device effectively detected cervical cancer from biopsy samples, demonstrating the potential for large-scale cervical cancer screening in resource-limited settings. The HPV 16/18 E6 oncoprotein has been recognized as a potential biomarker for a more precise early diagnosis of cervical cancer [741]. The OncoE6™ Cervical Test (Arbor Vita) is a fast, simple lateral flow method that detects HPV16/18 E6 oncoproteins with high SP and detects high-grade cervical lesions. This technology could enable decentralized screening of hard-to-reach populations if it supports self-collection samples [742]. The diagnostic performance of the OncoE6™ cervical test kit for cervical precancer and cancer in the Amhara Region of northwestern Ethiopia was evaluated, revealing a SN of 57.14% and a SP of 98% in identifying cases of cervical precancer and cancer [743]. Its high SP renders it an appropriate screening choice in areas with high HPV prevalence and large high-risk populations.
He et al. developed a microfluidic chip that combines centrifugation pretreatment with a nano-sensing film to rapidly and accurately detect VEGF165, a crucial factor in angiogenesis and cancer progression, in clinical blood samples [744]. Plasma was separated and conveyed to the electrode within 5 min, where a dual-aptamer-based sandwich approach was employed. The Apt2 film on the Au electrode surface and the Apt1/Au NP nanoprobe selectively and sensitively bind to VEGF165 in plasma. This sensor exhibited a detection limit of 0.67 pg/mL and a broad linear range of 1 pg to 10 ng, suggesting its utility as a POC platform for the early clinical diagnosis and prognostic monitoring of VEGF165 [744].
Using nanoporous gold membranes and antibody-conjugated nanorods, a new MD demonstrated rapid, ultrasensitive isolation and in situ detection of LC-specific exosomes in urine [745]. Validated in patient samples, it distinguished early-stage cancer with high purity and low detection limits, indicating potential adaptability to other tumor types.
Inspired by the hunting efficiency of octopuses, a deterministic lateral displacement (DLD) microfluidic chip incorporating aptamer-functionalized nanospheres (AP-Octopus-Chip) has been developed [746]. The multivalent aptamer-antigen binding enhanced affinity by 100-fold and capture efficiency by over 300% compared with a monovalent aptamer-modified chip [746]. Captured cancer cells can be released with up to 80% efficiency and 96% survival rate, making it compatible with mutation detection and CTC culture. CTCs were successfully detected in all cancer samples [746]. In another similar study, aptamers were functionalized onto leukocyte membrane nanovesicles and attached to a microfluidic chip, implementing a fluidic multivalent nanointerface [747]. This biomimetic interface maximized ligand-receptor interactions, achieving a 7-fold higher capture efficiency and a 97.6% CTC survival rate compared to monovalent aptamer chips. Furthermore, it minimized background blood cell adsorption and successfully detected CTCs in 17/17 actual patient samples, demonstrating its clinical applicability [747]. Inspired by the predatory strategy of plate corals, a biomimetic CTC capture nanoprobe (MNPA-TCMMGO) has been developed by conjugating a multi-affinity aptamer to magnetic graphene oxide coated with tumor cell membranes [748]. The cell membrane provided homotypic tumor cell SP, while the multivalent aptamer significantly enhanced binding affinity and probability, enabling sensitive and specific capture of rare CTCs. Furthermore, it exhibited robustness and biocompatibility while maintaining captured cell viability, demonstrating promise for downstream analysis and clinical application [748].
Paper possesses unique properties such as capillary-based liquid transport, ease of design, and a high surface area-to-volume ratio, making it a widely used material in POC devices for its cost-effectiveness and ease of production and operation [749]. Paper-based MDs (µPADs) can be produced at low cost, providing accessibility to a wide range of patients. They can perform analysis on various bodily fluid samples (urine, saliva, blood, tears, etc.) [749]. Paper-based assays detecting HPV E7 tumor protein was developed, which assay operate without instruments through a simple 5-step process [750]. The HPV E7 paper-based assay demonstrated 95% accuracy in clinical performance when compared to histopathologic diagnosis of cervical intraepithelial neoplasia grade 2 or more severe (CIN2+) [750]. With additional clinical validation, they could enable highly specific POC testing in resource-limited settings [750]. A 3D platform has been developed to concurrently detect tumor markers such as CA125 and CEA in blood samples, as well as a wireless point-of-care system for detecting neuron-specific enolase (NSE) [749]. A label-free μPAD sensor for accurate electrochemical detection of PSA was developed using wax screen printing and gold NPs (AuNPs)/reduced graphene oxide (rGO)/thionine (THI) nano composites, demonstrating sensitive detection of PSA down to 10 pg/mL (linear range 0.05–200 ng/mL) [751]. This sensor showed the potential to provide a new platform for low-cost, sensitive POC diagnosis of PC [751].
7.2.3. Application of Microfluidic System for Age-Related/Chronic Diseases Diagnosis in POCT
A 3D μPAD capable of simultaneously measuring multiple cardiac biomarkers in blood, including heart-type fatty acid-binding protein (H-FABP), cardiac troponin I (cTnI), and copeptin, has been developed [752]. A high-performance optical sensing platform equipped with a passive blood filtration microfluidic cartridge for detecting CRP has also been developed, proving useful for monitoring patients with chronic diseases [753]. A microfluidic POC system integrated with a field-effect transistor sensor array capable of simultaneously analyzing multiple CVD biomarkers—including CRP, N-terminal pro-B-type natriuretic peptide (NT-proBNP), cTnI, and fibrinogen—has been developed, demonstrating promise for CVD monitoring [754]. The sensor assessed biomarkers over an extensive dynamic range: NT-proBNP (0.1–10,000 pg/mL), fibrinogen (50–1000 mg/dL), cTnI (0.1–10,000 pg/mL), and CRP (0.5–9 mg/L) [754].
A portable, low-cost sensor designed to measure serum phosphate in patients with advanced CKD and dialysis patients using a single drop of blood (less than 60 μL) was introduced [755]. This sensor combines a paper-based microfluidic platform with a smartphone reader and exhibited a strong correlation (r = 0.95) with laboratory tests in clinical trials [755]. This affordable and easy-to-use system can accurately measure serum phosphate levels in just 45 min in POC, allowing patients to monitor it more frequently at home [755].
Saliva is progressively utilized for diagnostic purposes. Saliva is an optimal non-invasive biological fluid for biomarker detection due to its capacity for large-volume collection and its precise reflection of blood analyte concentrations [756]. A cobalt-MOF (Co-MOF) modified carbon cloth/paper (CC/Paper) hybrid button-sensor was developed as a portable, robust, and user-friendly electrochemical analytical chip for the nonenzymatic quantitative detection of glucose, a classical biomarker of diabetes [757]. The flexible Co-MOF/CC interface, characterized by numerous catalytic sites and a large specific surface area, enabled the Co-MOF/CC/paper hybrid button sensor to accurately quantify glucose with high SN and selectivity in saliva as well as serum and urine, without the necessity of enzymes [757]. The paper-based chip demonstrated affordability, exceptional portability, and durability, rendering it appropriate for home healthcare and rapid POC diagnostics [757].
A POC diagnostic sensor utilizing paper-based fluorescent vertical flow analysis (fxVFA) successfully quantified three acute cardiac injury biomarkers (myoglobin, CK-MB, H-FABP) within 15 min using 50 µL of serum per patient [758]. Neural network-based analysis achieved a detection limit below 0.52 ng/mL, linearity above 0.9, and a coefficient of variation below 15%. Its low-cost, portable design demonstrated enhanced diagnostic accessibility even in resource-limited settings [758].
7.3. Electrochemical Biosensors in POCT
7.3.1. Concept and Features of Electrochemical Biosensors
Electrochemical sensors are gaining considerable attention in the POC field due to their high SN, miniaturization, and cost-effectiveness [759]. They are used in various applications ranging from disease diagnosis to health status monitoring. These sensors primarily detect biomarkers by immobilizing biomolecular recognition elements such as enzymes, antibodies, or aptamers onto electrode surfaces [759]. Electrochemical sensors provide rapid and quantitative results by directly reflecting material concentrations through various electrical signal changes [759]. Voltammetry is appropriate for trace analysis owing to its exceptional SN and precision, whereas amperometry facilitates rapid and accurate measurements by monitoring current responses to variations in analyte concentration [760]. Impedance measurement offers instantaneous assessment of electrode-analyte interactions, elucidating reaction rates and mechanisms [760]. Potentiometric titration provides a straightforward and expedient technique for determining concentration by measuring potential variations. These varied electrochemical methods concurrently fulfill high SN, cost-efficiency, and speed, rendering them ideal quantitative analytical instruments for biosensors [759].
Through microelectrode technology, they can maintain high SN even with very small sample volumes without significantly affecting measurement SN [761].
7.3.2. Application of Electrochemical Biosensors for Cancer Diagnosis in POCT
Exosome miRNA patterns reflect dysregulation in tumor cells, making them promising for cancer diagnosis. For examples, a photoelectrochemical (PEC) biosensor based on the hybrid nanomaterial MoS2@Ti3C2 was developed [762]. This biosensor exhibited high SN for miRNA detection, attributed to the superior electron transfer capability of Ti3C2. The detection limit for miR-92a-3p, an exosome miRNA linked to CRC, was 0.27 fM, with a linear detection range extending from 1 fM to 100 nM [762]. A significant characteristic was its selectivity, allowing for accurate differentiation between miR-92a-3p and other RNA molecules with erroneous sequences. In addition, by accurately detecting miR-92a-3p concentrations in exosomes from both patient groups and healthy controls, PEC biosensor demonstrated clinical applicability [762]. Another PEC immunosensor for detecting CEA utilized an organic-inorganic heterojunction photoactive material by combining the organic semiconductor Y6 with AuNPs [763]. ZnPO4 NPs with horseradish peroxidase (HRP) embedded was used as signal probe. The insulating film generated through HRP catalysis inhibited the photoelectrochemical current intensity, facilitating sensitive and selective detection of CEA within a linear range of 1 pg/mL to 1 ng/mL, with a detection limit of 0.5 pg/mL [763].
A screen-printed gold electrode-based electrochemical miRNA detection platform was developed [764]. Methylene blue-modified DNA sequences immobilized on the electrode enabled targeted detection of miRNA-21, achieving an LoD of 2 nM using square-wave voltammetry. After optimization for pH, interfering substances, and NaCl concentration, high selectivity, reproducibility, and nM-level performance were confirmed in urine samples [764].
A CRISPR-Cas12a-based electrochemical aptamer sensor was developed for ultra-high-SN detection of the cancer biomarker MUC1 [765]. The synergistic effects of aptamer dual recognition, Cas12a-mediated trans-cleavage, and glucose oxidase (GOD) enzyme-catalyzed signal amplification significantly enhanced selectivity and SN. The detection principle involves measuring the oxidation current change from H2O2 generated by the glucose oxidation reaction. This occurs when Cas12a activity cleaves probe single-stranded DNA (pDNA) within the GOD-pDNA/magnetic Fe3O4@Au (MGNP) complex, releasing GOD. The sensor exhibited excellent linearity in the range of 1.0 × 10−17–1.0 × 10−10 g/mL, with an extremely low LoD of 7.01 × 10−18 g/mL. Its applicability was also confirmed in real medical samples [765].
Nano-porous interdigital electrodes (NP-IDE) employing single-walled carbon nanotubes as transducers exhibited exceptional selectivity in detecting DNA (with fM SN) and BC biomarker proteins (with pg/L SN for p53) [766].
3D Microelectrode Arrays (3D MAs) provide superior detection characteristics (response time and detection levels) compared to conventional planar macroelectrodes [761]. 3D MAs demonstrated three-fold higher SN than ELISA (the optical-based gold standard) [767] and proved capable of detecting RNA down to picomolar concentrations [761]. Particularly in microfluidic environments, 3D MAs demonstrated high SN even with minimal samples of 0.3 µL, compared to ELISA’s 50 µL sample size [767]. The 3D morphology controls electrode tip discharge, enhances the diffusion profile of target species, and utilizes current concentration phenomena to reduce the active area of individual electrodes [761].
A portable disposable solid-contact ion-selective potentiometric sensor (SC-ISE) has been developed for rapid measurement of sarcosine, a PCa biomarker, with detection limits as low as 9.95 × 10−13 M [768]. Furthermore, it exhibited a Nernstian response in the 10−7–10−12 M range and successfully detected sarcosine in urine samples without pretreatment, demonstrating its clinical applicability as a point-of-care sensor meeting WHO ASSURED criteria [768].
A clinically applicable electrochemical micro-nano motor biosensor employing a compact MD for the multiplex detection and assessment of BC biomarkers has been developed [769]. The MOF-based micro-nano motor (MOFtor) biosensor simultaneously detected and quantified BC biomarkers, such as ER, PR, HER2, and Ki67 with elevated SN. MOFtor functionalized with aptamers and antibodies on SiO2@Co-Fe-MOF operated as a miniature swimmer under an electric field to capture targets, facilitating an automated sample-amplification-output system that enhances and transmits four electrochemical signals [769]. The biosensor achieved detection limits of HER2 0.01420 pg/mL, ER 0.03201 pg/mL, Ki67 0.01430 pg/mL, and PR 0.01229 pg/mL. Compared to conventional biopsy (approximately 1 week), this biosensor demonstrated a short detection time of 40 min, indicating significant potential as a rapid and precise auxiliary diagnostic tool in clinical settings [769].
7.3.3. Application of Electrochemical Biosensors for Age-Related/Chronic Diseases Diagnosis in POCT
An electrochemical platform integrating aptamers, magnetic NPs, and the CRISPR/Cas12a system has been developed for the rapid and precise detection of cTnI, a myocardial injury marker [770]. The principle involves complementary DNA (probe 2, P2) released upon aptamer–cTnI binding triggering Cas12a trans-cleavage, which reduces the electrode signal. This achieved an LoD of 10 pg/mL and a linear range of 100–50,000 pg/mL. This sensor demonstrated excellent selectivity and SN even in serum samples, suggesting its utility as a tool for CVD diagnosis, prognosis assessment, and treatment monitoring [770].
Detecting intracellular action potentials (Aps) is essential for a comprehensive understanding of cardiac pathogenesis and effective drug screening [771]. Microelectrode array (MEA) equipped with microelectrodes of various sizes, combined with microelectroporation technology, successfully recorded APs within cardiomyocytes [771]. Simulations and experiments demonstrated that large electrodes can also be utilized for intracellular AP acquisition. Large electrodes exhibited high amplitude and SNR, while small electrodes showed high perforation efficiency and single-cell signal yield [771]. Consequently, small electrodes were found suitable for densely packed cells, and large electrodes for dispersed cells. This confirms the suitability of each electrode type for different cell arrangements, presenting new possibilities for developing low-cost, high-quality intracellular AP recording electrodes [771].
The first glucose meters of the 1970s lacked precision. However, by 1980, the glucose meter enhanced functionality with a digital display. Inexpensive, low-blood-volume meters in the 1980s established self-monitoring of blood glucose as the standard [772]. The integration of A1C testing that measures the average amount of sugar in individual blood over the past three months and insulin pumps facilitated the Diabetes Control and Complications Trial, which validated the advantages of glucose regulation [773]. The proliferation of continuous glucose monitoring (CGM) devices has driven advancements in non-invasive and minimally invasive blood glucose measurement technologies. These offer the advantage of reducing pain, discomfort, and infection risks compared to fingerstick blood sampling [774]. Non-invasive methods measure glucose levels via skin-adhered sensors [775], while minimally invasive methods utilize bodily fluids like tears [776,777].
Remote-controlled smart contact lenses (SLCs) capable of simultaneously performing non-invasive glucose monitoring in tears and treating diabetes have been developed [729,730]. Keum et al. developed a wearable contact lens comprising a real-time electrochemical biosensor, an on-demand flexible drug delivery system (f-DDS), a resonant inductive wireless energy transfer system, a complementary integrated circuit (IC)-based microcontroller chip with a power management unit (PMU), and a remote radio frequency (RF) communication system [729]. It identifies glucose levels in tears instantaneously, eliminating the necessity for invasive blood tests. The GOD-based biosensor exhibited therapeutic effects comparable to Avastin injections by administering drugs on demand, demonstrating potential for advancement as a next-generation wearable therapeutic and diagnostic platform for the management of ophthalmic diseases and chronic conditions.
Tears show promise as an alternative means for diagnosing diabetes in blood, and SLCs are being utilized to measure glucose in tears. However, their clinical application remains limited due to controversy surrounding their correlation with blood glucose (BG) levels [730]. To resolve this controversy, a study was conducted utilizing wireless, flexible SCLs to examine the relationship between tear glucose (TG) and BG [730]. SCLs can continuously monitor basal TG at intervals shorter than one minute while eliminating the effects of reflex tears, thereby introducing the notion of “individual delay time,” which signifies variations in metabolic rates among individuals. Consequently, exact correlations between TG and BG were established among various subjects, including healthy individuals, diabetic patients, rabbits, and beagles. This study demonstrated an improved accuracy of TG-based BG prediction and the promise for the clinical utility of non-invasive diabetes monitoring [730].
Sweat, a biological fluid, is also emerging as a reliable surrogate indicator for blood analyte concentrations [733]. A wearable/disposable sweat-based glucose monitoring device integrated with a feedback-type transdermal drug delivery module has been developed [778]. The device integrates humidity, glucose, pH, and temperature sensors to detect changes in sweat composition in real time, while achieving high electrochemical SN and stability through a porous gold electrode and a fixed GOD-based structure. A combination of a stretchable heater and metformin-loaded phase-change NPs within hyaluronic acid hydrogel microneedles enabled stepwise and precise drug release according to temperature patterns. This multilayer patch system enhanced portability and accuracy through sensor calibration and a minimalist layout, representing a significant closed-loop therapeutic platform for non-invasive, personalized blood glucose management [778]. In a similar study, a bio-based cellulose paper glucose monitoring sensor utilizing methylated and phosphorylated microfibrillated cellulose (MFC) improved sweat capillary flow and enabled accurate glucose detection with just 1 µL of sweat [779]. Methylation provided chemical resistance, while the multilayer micro-patch design optimized sweat collection and detection efficiency. Integrated with an artificial transdermal drug delivery device (agarose gel), this system demonstrated stable performance under varying sweat volume, temperature, and pH conditions. Furthermore, temperature-dependent metformin release enabled a reliable closed-loop switch system for diabetes management [779].
7.4. Optical Biosensors
7.4.1. Concept and Features of Optical Biosensors
Optical biosensors are devices that generate and detect signals by utilizing the interaction between light and biomarkers. They include diverse technologies such as SERS, fluorescence, absorption/colorimetry, and SPR. Optical biosensors offer sensitive, economical, and broadly applicable diagnostic techniques, frequently integrated with smartphones for result delivery via picture analysis [780]. Nanoparticles are used for signal amplification in SERS, fluorescence, and colorimetric-based assays.
7.4.2. Application of Optical Biosensors for Cancer Diagnosis in POCT
The development of the DHOR portable fluorescence detection device demonstrated a significant advancement for rapid and cost-effective analysis of D-2-hydroxyglutaric acid (D-2-HG), a key biomarker for gliomas. acute myeloid leukemia, and chondrosarcoma [781]. Accumulation of D-2-HG due to mutant isocitrate dehydrogenase (IDH) activity competitively inhibits various α-ketoglutarate-dependent dioxygenase activities, thereby promoting tumorigenesis and carcinogenesis through epigenetic changes [782]. Unlike conventional methods such as liquid chromatography–tandem mass spectrometry (LC-MS/MS) and MRS, which are time-consuming and unsuitable for POC use, DHOR enables accurate D-2-HG detection from serum, urine, and brain tissues within approximately 5 min. Its low-cost design using off-the-shelf components allows rapid intraoperative assessment of IDH mutation status in tumor tissues, potentially improving real-time surgical decision-making and patient outcomes [781].
The smartphone-based optical microscope platform (EpiView-D4) directly quantified protein biomarkers in cell lysates using a polymer brush-based immunodiagnostic chip (D4) and simultaneously evaluated cell morphology and HER2 protein expression in fine needle aspiration (FNA) samples for BC diagnosis [736]. Pilot studies also accurately classified HER2-positive/negative tumors, demonstrating that this device can serve as a low-cost, rapid, and accurate alternative for BC diagnosis in settings with limited access to pathology services [736]. The buoyancy-lifted bio-interference-orthogonal organogel messenger (BLOOM) POC device achieved a SN of 88.8 percent and a SP of 88.9 percent for bladder cancer diagnosis, surpassing the SN of 20 percent of existing commercial kits [783]. It was also able to detect early-stage bladder cancer. The device measures fluorescence changes via smartphone without interference from patient hematuria [783]. An OLED-based active plasmon colorimeter biosensor detected LC biomarker neuron-specific enolase (NSE) in the 1–100 ng/mL range, demonstrating a low LoD of 200 pg/mL and high selectivity, showing potential for LC diagnosis [784].
SPR biosensor based on molecular aptamer beacon (MAB) conversion and tyramine signal amplification (TSA) was developed for the highly sensitive and specific detection of HER2-positive exosomes [785]. Following exosome capture, exposed G4-hemin induced tyramine-coated AuNPs deposition, amplifying the SPR signal. The use of non-enzymatic G4-hemin overcame enzyme limitations. Furthermore, high SP was achieved through dual recognition of protein and lipid membranes in exosomes. The sensor operated within the range of 1.0 × 104 to 1.0 × 107 particles/mL and accurately distinguishes patients from healthy individuals, demonstrating its clinical potential for HER2-positive BC diagnosis [785].
A SERS/electrochemical dual-mode biosensor was developed for the highly sensitive on-site detection of miR-106a, a marker associated with gastric cancer [786]. Based on a Ag nanorod array electrode coated with multi-functionalized MoS2 nanosheets, a sandwich-structured sensor was realized that simultaneously ensures stability and SN with a capability of distinguishing single-base mismatches, achieving low detection limits of 67.44 fM in SERS mode and 248.01 fM in electrochemical mode. Its excellent performance in human serum further enhances its applicability for early cancer diagnosis [786]. SERS has traditionally struggled to distinguish subtle spectral differences in complex matrices due to peak overlap, baseline noise, and intermolecular interactions. Integrating SERS with sophisticated AI data analysis effectively mitigates these challenges, enhancing analytical precision [787]. This method facilitates accurate differentiation of Raman spectra between healthy individuals and patients, significantly enhancing the potential of liquid biopsy in clinical applications [788,789].
The DNA-controlled protein fluorescence system represented a significant advance in biosensing technology due to its ability to accurately monitor intracellular ATP [790]. The system differentiated between cell types based on intracellular ATP levels, as seen in the significantly higher ATP levels in A549 LC cells compared to normal BEAS-2B cells, indicating its potential for developing cancer diagnostics [790].
A novel multi–ultra-sensitive coffee ring biosensor leveraging asymmetric nanoplasmonic structures has been recently developed [726]. Biomarkers were concentrated via droplet evaporation on a nanofiber membrane, and a second plasmonic droplet containing functionalized gold nanoshells was superimposed and dried to form an asymmetric nanoplasmonic pattern [726]. The device achieves protein detection down to 3 pg/mL within 12 min through a two-step droplet-based concentration mechanism: biomarker preconcentration via evaporation on a nanofiber membrane, followed by the deposition and drying of a secondary plasmonic droplet containing functionalized gold nanoshells. This process generates an asymmetric nanoplasmonic pattern that amplifies local electromagnetic fields, dramatically enhancing optical signal intensity [726].
7.4.3. Application of Optical Biosensors for Age-Related/Chronic Diseases Diagnosis in POCT
A smartphone-based POCT device utilizing fluorescence technology to detect cholesterol, glucose, lactate, and uric acid has been introduced for rapid monitoring of metabolic biomarkers [791]. Using an ANN-based smartphone app to analyze fluorescence RGB·HSV, it detected targets in serum within 50 min, demonstrating R-value of 95% or higher and performance comparable to standard methods. It also showed excellent normal/abnormal discrimination, indicating its potential as a portable, low-cost POCT device for rapid monitoring of metabolic biomarkers [791].
A sensor utilizing a plasmonic etalon nanostructure for TG detection in a SCL has been proposed. The proposed structure generates light interference and surface plasmon resonance between two metallic reflective surfaces, amplifying the electric field and consequently attaining heightened SN to variations in refractive index [792]. Constructive interference occurs when , linking light wavelength (λ), resonance order (m), layer distance (d), and refractive index (n). That is, if the interlayer distance or refractive index changes due to glucose binding to the etalon surface, the resonant wavelength (color) changes. The elevated quality factor (Q-factor) further amplifies detection efficacy. The engineered lens was capable of detecting glucose in PBS solution within the concentration range of 0.15–10 mM, exhibiting significant SN even at minimal levels [792].
Aptamer-based biosensors are emerging as promising tools for the early diagnosis of aging and age-related diseases due to their high SN and stability [793]. Optical aptasensors detect target molecules through changes in optical signals, including fluorescence, colorimetry, and biolayer interferometry (BLI) [793]. An aptasensor based on nitrogen-doped carbon dots (NCD) was developed for the detection of tau protein, a crucial biomarker for AD. The NCD fluorescence is suppressed by the aptamer; however, upon binding with tau protein, the aptamer is liberated, reinstating fluorescence and facilitating signal detection with an LoD of 3.64 ng/mL [794]. The aptasensor for detecting amyloid-β1-40 oligomer (Aβ40-O), a key biomarker for early diagnosis of AD, is a label-free colorimetric biosensor composed of an aptamer-AuNP conjugate. It relies on visible color changes arising from interactions among aptamers, the target molecules, and nanomaterials such as AuNPs [795]. This aptasensor demonstrated high SN with an LoD of 3.03 nM and a linear detection range from 10.00 nM to 100.0 nM [795]. Another dual-amplification colorimetric sensor for Aβo detection achieved an LoD of 0.23 pM [796]. A biosensing platform for GDF15 detection was developed, featuring automated, high-throughput, and real-time online monitoring capabilities using BLI- Enzyme-Linked Aptamer Sorbent Assay (ELASA) technology [797]. This platform detected growth differentiation factor-15 (GDF15), a biomarker for glaucoma and aging, with an exceptionally low LoD of 5–6 pg/mL [797].
7.5. Nucleic Acid Amplification Tests (NAATs)
7.5.1. Concept and Features of NAATs
NAATs are used to identify pathogens or disease biomarkers by extracting, amplifying, and detecting specific nucleic acid (DNA or RNA) sequences. PCR, LAMP and nanotechnology have been used.
7.5.2. Application of NAATs for Cancer Diagnosis in POCT
Loop-mediated isothermal amplification (LAMP) is a nucleic acid amplification technology that is faster than traditional PCR for nucleic acid amplification because it operates at a single, constant temperature, eliminating the need for a thermal cycler [798]. When combined with equipment-free sample preparation techniques such as ColdSHOT, LAMP facilitates highly sensitive and rapid on-site diagnostics in portable, POC settings [799]. An extraction-free HPV DNA test using LAMP-based detection of HPV DNA has been developed [800]. This test uses a low-cost benchtop heater/fluorescence reader to provide results within one hour, holding potential to expand cervical cancer screening in resource-limited settings [800].
ctDNA levels are present at extremely low concentrations in early stage of cancer. To address this issue, various amplification methods for detecting ctDNA have been developed. Surface-Limited Amplification (nSLAM) is a technology that directly amplifies and accumulates target ctDNA on the surfaces of Fe3O4-Au core–shell NPs (CSNPs) [801]. CSNPs exhibit dispersibility and superparamagnetism, acting as nanoelectrodes in PCR. Following magnetic collection, signals are reamplified via electrochemical measurement. This enabled ultra-high SN detection at approximately 3 aM levels and rapid detection within 7 min metastatic BC ctDNA detection in vitro [801].
The hybridization chain reaction (HCR) is an efficient, enzyme-free method for DNA amplification that runs at ambient temperature [802]. Two stable DNA hairpin species coexist in solution until the initiator strand triggers a series of hybridization events. This technology, combined with electrochemical DNA-based sensors, enables the measurement of nucleic acids in whole blood without calibration [802]. This approach enabled the quantitative detection of miR-21 with a SN of 1 pM and a dynamic range from 1 pM to 1 nM [803].
A dual-recognition-controlled electrochemical biosensor targeting CTCs has been developed [804]. In this sensor, two aptamer hairpin probes bind to adjacent proteins (EpCAM and MUC1) on the cell membrane, inducing a strand displacement reaction. This results in significant amplification of the electrochemical signal through dimer-like rolling circle amplification (RSA). Signal generation occurs only when the dual-expression protein is present, ensuring high analytical precision. When applying DNA nanostructure capture technology, the detection limit decreased to 3 cells/mL. Furthermore, stability and selectivity were demonstrated in whole blood matrices, indicating potential utility for cancer diagnosis and personalized treatment strategies [804].
DNA tetrahedron (DTN)-based logic signal amplification system has been developed [805]. This system utilized 3D DNA nanonetwork employing DTNs to perform catalytic hairpin assembly (CHA) reactions. It formed a hyperbranched 3D nanonetwork in the presence of target miRNAs (miR-21, miR-141), achieving a detection limit at the fM level, approximately 52 times lower than existing CHA systems [805]. This biosensor demonstrated the potential for distinguishing cancer cells from normal cells and identifying subtypes of BC cells based on modular logic gates [805].
7.5.3. Application of NAATs for Age-Related/Chronic Diseases Diagnosis in POCT
Musunuru et al. demonstrated that CRISPR base editors delivered to living cynomolgus monkeys (Macaca fascicularis) via lipid NPs can efficiently and precisely modify disease-related genes [806]. Specifically, using CRISPR technology and lipid NPs, nearly complete hepatic suppression of the proprotein convertase subtilisin/kexin type 9 (PCSK9) gene—a factor increasing cardiovascular disease risk—was observed. Concurrently, blood PCSK9 levels and PCSK9-mediated low-density lipoprotein cholesterol levels decreased by approximately 90% and 60%, respectively. This effect was maintained for 8 months after a single treatment [806].
A highly sensitive chemiluminescent immunoassay was developed, which employed a sandwich architecture comprising antibodies immobilized on magnetic beads and ssDNA detection antibodies to initiate RCA reactions and amplify signals [807]. Aβ42 and Aβ40 demonstrated minimal detection thresholds of 1.99 pg/mL and 3.14 pg/mL, respectively. The Aβ42/Aβ40 ratio distinguished AD patients from healthy controls with an SN of 90.48% and a SP of 63.64%, demonstrating significant potential for non-invasive diagnosis and assessment of AD [807].
7.6. FDA-Approved or Cleared POC Devices
In 2016, the cobas EGFR Mutation Test v2 became the first FDA-approved liquid biopsy test to detect specific EGFR gene mutations in cfDNA from plasma samples. This test is used as a companion diagnostic to determine which patients with metastatic NSCLC are eligible for first-line, maintenance, or second-line treatment with erlotinib, an EGFR-targeted medication [808].
Some MDs are used to isolate and analyze liquid biopsy markers such as CTCs and exosomes. The ClearCell® FX system (Biolidics, Singapore) is an automated, label-free device that enriches intact and viable CTCs from whole blood [809]. The system demonstrates over 80% SN and SP in detecting CTCs from clinical samples and has achieved multiple regulatory approvals, including CE-IVD, US FDA Class I, and CFDA Class I registrations [809].
In January 2019, the FDA approved the first POC total PSA test, the Silver Amplified NeoGold ImmunoAssay (Sangia), which gives results in under 15 min from a fingerstick blood sample [810]. With a digital rectal examination (DRE), it detects PC in men 50 and older, streamlining testing and patient care with rapid results outside of a lab [810].
7.7. Deployment Considerations of POC Versus Laboratory-Based Biosensing Platforms
The deployment of POC biosensing platforms presents a distinct paradigm from conventional laboratory-based systems in terms of analytical SN, robustness under ambient environments, and user interface design, particularly in the context of elderly populations requiring continuous or longitudinal health monitoring [811].
7.7.1. Analytical Sensitivity
From a SN standpoint, POC devices have made substantial advances through the integration of microfluidics, plasmonics, and nanomaterial-enhanced sensing layers, achieving detection limits that rival or surpass centralized assays such as ELISA or chemiluminescent immunoassays [504,726,811,812,813]. These innovations enable the quantification of ultra-low-abundance biomarkers, including circulating DNA, proteins or peptides at femtomolar to attomolar levels, critical for early disease detection in AD or cancer screening [801,814,815]. In contrast, laboratory-based platforms, though traditionally regarded as the gold standard for analytical precision, often rely on large-volume samples, batch processing, and controlled reaction conditions, which may limit their ability to capture transient or low-copy biological signals relevant in early pathophysiological changes [738,816].
7.7.2. Robustness
In terms of robustness, laboratory systems benefit from highly standardized protocols and controlled environmental conditions [717]. While highly reliable when conditions are met, the entire process is susceptible to delays from sample transport and errors associated with pre-analytical, analytical, and post-analytical phases [817]. POC devices, which must function reliably in diverse, non-clinical environments, prioritize robustness. To address this, modern POC biosensors emphasize mechanical and environmental stability as core design principles aligned with the WHO’s ASSURED (Affordable, Sensitive, Specific, User-friendly, Rapid, Equipment-free, and Deliverable) framework [818]. Challenges remain, however, as environmental fluctuations, sample matrix heterogeneity, and user-dependent variability can affect signal reproducibility [817]. POC systems incorporate solutions to mitigate these challenges. The integration of advanced sensors and microfluidic components helps in precise fluid control and reduces the system’s susceptibility to contamination [717]. Some optical sensing platforms are designed to be intrinsically stable, making them largely immune to positional changes and mechanical vibration, often allowing operation on a lab bench rather than a stabilized optical table [753]. Furthermore, the complexity and noisy nature of POC data can be effectively managed by integrating AI and ML algorithms, which are capable of learning complex patterns and improving diagnostic accuracy and reliability despite the imperfections of the sensors [717]. The BLOOM assay, for instance, enhances robustness against complex sample heterogeneity (like blood in urine) by using a biphasic system that spatially separates the biomarker recognition from the signal transduction [783].
7.7.3. Usability
The usability and interface design of POC platforms are central to their success, particularly for elderly patients managing chronic diseases. Conventional laboratory diagnostics require skilled personnel, complex instrumentation, and repeated patient visits, which can pose accessibility barriers for older adults with limited mobility or cognitive decline [813,819]. In contrast, POC platforms prioritize user-friendliness and accessibility, making them uniquely suitable for decentralized testing and self-monitoring, which is critical for the aging population [809]. POC solutions enable patients to potentially assess their health conditions without the need for regular physician visits [717]. They are designed to be operated feasibly by patients or in a “near-patient” format, generally requiring no specialized training [717]. Devices like the glucometer exemplify this success, dominating diabetes monitoring due to their affordability and ease of use for patients [820]. POC devices are portable and often leverage inexpensive and simple formats, such as paper-based analytical devices [813] or lateral flow assays [750]. Their simplicity often extends to sample preparation, employing non-invasive or minimally invasive sampling methods. Integration with ubiquitous smart technologies, like smartphones, facilitates reading and interpretation of results [755,777]. Digital tools offer cost savings and can shorten the administration time of certain tests, making the patient journey more patient-friendly. AI integration into POC automates complex result interpretation (e.g., classifying faint test lines) and data analysis, which reduces the dependency on untrained users and enhances diagnostic accuracy, empowering frontline health workers and potentially individual patients [719]. This is especially beneficial for ensuring equitable access and reliable delivery to diverse and low-literacy populations [821].
8. From Discovery to Clinical Application
From the discovery of a specific protein in the urine samples of patients with myeloma by Bence Jones in 1846 to today’s AI-enabled liquid biopsy systems, the trajectory of biomarker research reflects a shift from isolated biochemical observations to integrated, regulatory-validated clinical frameworks [822].
8.1. Historical Development of Biomarkers
From the 1960s to the 1980s, molecular identification transformed cancer diagnostics with biomarkers like AFP (HCC), CEA (CRC), and PSA (PCa) [823]. In the 1990s–2000s, genomic and proteomic tools advanced biomarker discovery. BRCA1/BRCA2 enabled hereditary cancer screening. CA 19-9 (PC) and microRNAs (CRC) revealed gene regulation and epigenetic mechanisms. Technologies like microarrays, MALDI-TOF, LC-MS/MS, and 2D PAGE supported high-throughput biomarker validation [822]. From the 2010s onward, clinical translation accelerated with FDA-approved markers: BTA and NMP22 (bladder cancer) [824], and CA-125/HE4 (ROMA test for ovarian cancer) [377]. Molecular diagnostics now routinely assess HER2, EGFR, KRAS, and ALK mutations to guide targeted therapies (e.g., trastuzumab, erlotinib, cetuximab) [372,825]. Liquid biopsy innovations have expanded their roles from early diagnosis to providing crucial information for patient prognosis and the real-time monitoring of treatment effectiveness and disease recurrence [395]. Integration of AI/ML-based multi-omics models began bridging laboratory discovery with real-time clinical decision support [826,827]. Proteomic clocks and AI-driven predictive models extend biomarker applications from cancer surveillance to aging-related disease prevention, representing a convergence of oncology, geroscience, and digital diagnostics [387].
8.2. Regulatory Frameworks
8.2.1. United States (FDA)
In vitro diagnostic (IVD) biomarker tests are regulated as medical devices via 510(k), De Novo, or Premarket Approval (PMA) pathways, depending on risk class and predicate availability. The following table summarizes their major distinctions and clinical implications for biomarker-based diagnostic devices [828] (Table 13).

Table 13.
Comparative Summary of 510(k), De Novo, and PMA Regulatory Pathways [828].
8.2.2. Europe
The European Medicines Agency (EMA) operates a formal Biomarker Qualification process through Committee for Medicinal Products for Human Use (CHMP), providing qualification advice, letters of support, or a qualification opinion for specific contexts of use, facilitating consistent uptake across trials and submissions [831].
8.3. Regulatory, Validation, and Cost-Effectiveness for New Biomarkers
Introducing a new biomarker into routine clinical practice involves navigating complex regulatory requirements, ensuring robust multi-center validation, and addressing critical cost-effectiveness issues. These factors are crucial for successful adoption and widespread use [832].
8.3.1. CLIA/LDT Considerations in the U.S.
In the United States, many early-stage biomarker tests are initially introduced as laboratory-developed tests (LDTs) under the CLIA, which are regulated by the Centers for Medicare and Medicaid Services to ensure analytical quality [833]. Although the FDA has intermittently sought to expand its oversight of LDTs as medical devices, a 2024 final rule was vacated by a federal court in March 2025 [834]. Subsequently, in September 2025, the FDA reverted to the pre-rule regulatory framework, while broader policy discussions continue [835]. As a result, developers are advised to pursue CLIA validation in the early stages and prepare for potential FDA submissions such as 510(k), De Novo, or PMA, when aiming to scale beyond a single laboratory or to secure national coverage and standardization.
8.3.2. Multi-Center Validation Pathways and Evidentiary Standards
Translational readiness of biomarker-based diagnostics is significantly enhanced through prospective, multi-center studies that rigorously establish three key pillars: analytical validity across different sites and instruments, clinical validity against appropriate comparators, and clinical utility in terms of impact on clinical decision-making and patient outcomes [836]. To ensure transparency and reproducibility, study designs should adhere to established consensus standards for diagnostic accuracy, such as the Standards for Reporting of Diagnostic Accuracy Studies (STARD) guidelines [837]. These standards support the generation of robust estimates for SN, SP, AUC, LoD, and overall reproducibility, all within the framework of pre-specified analysis plans and external validation cohorts.
8.3.3. Cost-Effectiveness and Implementation
From an implementation perspective, cost-effectiveness is a critical consideration for payers and health technology assessment (HTA) bodies. Evaluations typically incorporate quality-adjusted life years (QALYs), budget impact, downstream resource utilization (e.g., confirmatory imaging or biopsy), and diagnostic turnaround time [838]. Strategies to enhance value include enrichment approaches (such as applying age or risk thresholds and multi-analyte triage), reflex testing algorithms (initial screening followed by confirmatory diagnostics), optimizing site-of-care delivery (point-of-care versus centralized laboratories), and leveraging longitudinal monitoring to enable earlier detection and reduce overtreatment [839,840]. Ultimately, biomarker tests that demonstrate clear clinical actionability and a favorable economic profile in real-world, multi-center settings are more likely to gain payer coverage and widespread clinical adoption.
8.3.4. FDA-Recognized Examples
Examples directly relevant to early detection include: (i) Epi proColon® (methylated SEPT9)—the first FDA-approved blood-based CRC screening assay (PMA, 2016) [841]; (ii) Cologuard® (multi-target stool DNA, mt-sDNA) for CRC screening (PMA, 2014) [842]; (iii) comprehensive genomic CDx panels such as FoundationOne CDx and Guardant360 CDx (PMA) for tumor profiling to guide therapy (not screening) [843]; and (iv) CellSearch® for CTCs detection (FDA-cleared) for monitoring [428]. For aging-related conditions, the FDA has cleared the first blood test to aid in diagnosing AD—the Lumipulse G p-tau217/β-amyloid 1-42 plasma ratio (May 2025) and previously cleared a CSF β-amyloid 1-42/1-40 ratio test via De Novo (2022) [844]. At present, there is no FDA-approved MCED screening test. Several are under investigation, and many assays are available only as CLIA LDTs. Table 14 summarizes representative cancer and aging biomarkers that have obtained FDA or EMA approval, or are under regulatory review, illustrating the translation pipeline from discovery to clinical application.

Table 14.
Regulatory Status of Representative Cancer and Aging Biomarkers and Diagnostic Platforms.
9. Current Limitations and Future Directions for Early Diagnosis of Cancer and Aging
9.1. Current Limitations in Early Diagnosis of Cancer and Aging
9.1.1. Biomarker-Related Limitations
The early detection of cancer and age-associated diseases can markedly enhance patient outcomes, decrease mortality, and provide prompt therapies. However, despite significant technological progress, several critical limitations currently hinder their clinical translation. One major challenge is the low abundance and biological heterogeneity of biomarkers. Early disease signals, such as ctDNA, CTCs, and EVs detected through liquid biopsy, are present at extremely low concentrations and exhibit high intra- and inter-tumor variability. The presence of tumor heterogeneity significantly increases the probability of false negatives and false positives. Similarly, aging-related markers such as p16, p21, and Lamin B1 show substantial inter-individual variation, and there is no clear distinction between normal and pathological aging states. This variability reduces SN and contributes to inconsistent results.
Another major barrier is the lack of established pre-analytical and analytical techniques impedes cross-study comparisons and clinical application. No commonly agreed cut-offs or reference values exist for aging-related biomarkers, specifically BAG indices obtained from neuroimaging, which complicates clinical interpretation. Moreover, confounding by age-related comorbidities such as chronic inflammation, immune dysfunction, and metabolic disorders can obscure true biomarker signals and increase the risk of false positives, especially in older populations.
9.1.2. Technological and Analytical Limitations
Current techniques are constrained by technological and analytical restrictions. Many platforms confront an inherent trade-off between SN and SP, with ultra-sensitive detection increasing the possibility of false positives, which is especially challenging in population-wide screening.
AI and ML models have demonstrated transformative potential in cancer diagnosis, prognosis prediction, and biomarker discovery. However, despite their impressive analytical capabilities, several critical challenges must be addressed before these technologies can be fully integrated into clinical practice. The main barriers include data bias and representativeness, lack of external validation and generalizability, and limited interpretability of DL models, all of which undermine clinical reliability, regulatory acceptance, and physician trust. Table 15 summarizes the key technical and ethical challenges limiting the clinical adoption of AI/ML models and presents potential strategies to enhance reliability, transparency, and regulatory compliance.
In addition, existing early diagnostic tools, such as NGS and high-resolution imaging, require significant infrastructure, cost, and skill, limiting their use at POC settings. Crucially, most systems are still in the proof-of-concept or pilot-study stages, with no large-scale, multicenter prospective clinical validation.
Regarding POCT, despite these advantages, miniaturization that enables portability and low power consumption introduces inherent trade-offs. Reduced optical path lengths, limited reagent volumes, and microfabrication tolerances can amplify noise and inter-device variability [845]. Furthermore, miniaturized components are more sensitive to environmental perturbations such as temperature, humidity, or vibration [846]. Therefore, precise instrument calibration remains a critical challenge in POC deployment. Unlike laboratory analyzers that undergo regular professional calibration, POC systems must maintain long-term stability through self-calibration algorithms, embedded reference standards, or sensor redundancy [847]. Some platforms employ built-in calibration cartridges or cloud-linked quality control feedback loops to ensure consistent analytical performance across distributed devices [848].

Table 15.
Limitations and Improvement Strategies of AI/ML Models for Clinical Translation.
Table 15.
Limitations and Improvement Strategies of AI/ML Models for Clinical Translation.
| Category | Key Limitations | Improved Strategy/Future Directions | Ref. |
|---|---|---|---|
| Dataset Bias and Representativeness | Many AI/ML models are trained on single-center or demographically narrow datasets, resulting in poor generalizability across populations. Imbalanced datasets (e.g., underrepresentation of minority groups, rare cancer subtypes) cause biased model predictions and reduced diagnostic fairness. | Implement fairness-aware training algorithms to ensure demographic balance. Employ FL to enable data diversity without centralizing sensitive data. Adopt model cards to disclose dataset composition, bias sources, and subgroup performance. | [849,850] |
| Lack of Cross-Cohort Validation and Overfitting | Many models perform well in internal validation but fail in external datasets due to overfitting. Lack of multi-institutional testing limits generalization. Medical data drift over time reduces algorithmic robustness. | Conduct multi-site, prospective validation and external benchmarking. Apply TL and domain adaptation to enhance robustness across cohorts. Establish continuous model monitoring and recalibration pipelines in clinical use. | [851,852] |
| Interpretability and Black-Box Barriers | DL models often act as “black boxes,” providing limited insight into decision-making. Lack of interpretability decreases clinician trust and impedes regulatory approval. | Develop XAI frameworks (e.g., SHAP, LIME, integrated gradients) to visualize key features influencing model outputs. Combine interpretable hybrid architectures (e.g., attention-based + explainability layers). Include clinician-in-the-loop validation for model reasoning. | [853] |
| FL and Distributed Model Deployment | Despite privacy advantages, FL faces challenges with data heterogeneity, communication overhead, and explanation consistency across institutions. Lack of harmonization hinders reproducibility. | Use FED-XAI frameworks integrating explainability with FL. Standardize communication protocols and ontology alignment among institutions. Implement federated evaluation benchmarks to harmonize model performance reporting. | [854] |
| Clinical Integration and Regulatory Adoption | Limited clinical trial evidence for efficacy and safety. Absence of standardized regulatory pathways and real-time post-market surveillance for AI tools. | Conduct prospective clinical trials aligned with FDA/EMA digital health guidelines. Establish AI-QMS for traceability and auditability. Encourage regulator–developer collaboration for explainability and patient safety. | [855] |
Abbreviation: AI; artificial intelligence; ML; machine learning, DL; deep learning, FL; federated learning, TL; transfer learning, XAI; Explainable AI, SHAP; SHapley Additive exPlanations, LIME; Local Interpretable Model-Agnostic Explanations, FED-XAI; Federated Explainable Artificial Intelligence, QMS/AI-QMS; Quality Management System/Artificial Intelligence Quality Management System, FDA; U.S. Food and Drug Administration, EMA; European Medicines Agency.
9.2. Future Directions for Early Diagnosis of Cancer and Aging
We discuss prospective development directions based on significant instances of technology that showed notable improvements in early cancer and aging diagnosis compared to existing techniques (Table 16).

Table 16.
Improved Technologies for Early Diagnosis.
9.2.1. Multi-Omics Integration and Personalized Biomarker Panels
The next generation of diagnostic systems will integrate genomics, epigenomics (including DNA methylation), transcriptomics, proteomics, metabolomics, and immune markers into comprehensive biomarker panels. This multi-omics approach will elucidate complex biological networks underlying cancer and aging, overcoming the sensitivity and specificity limitations of single-marker approaches. These integrated analyses will enable accurate early diagnosis and distinguish diverse aging pathways (“ageotypes”) within same-age cohorts, facilitating personalized risk prediction and intervention strategies.
9.2.2. AI/ML Advancement
AI and ML algorithms will enable pattern recognition from large-scale, high-dimensional datasets, detecting early disease signals imperceptible to human analysis. Next-generation explainable AI (XAI), self-supervised learning (SSL), and foundation model methodologies will enhance diagnostic accuracy, reduce algorithmic bias, and increase clinician trust and adoption. When integrated into POCT platforms, these algorithms will enhance biomarker detection precision and reliability, particularly through advanced colorimetric and electrochemical sensing methods [719]. This is described in greater detail in Table 14.
9.2.3. Ultra-Sensitive Detection Technologies
Next-generation high-sensitivity detection platforms are being developed to achieve picogram-level and single-cell resolution while maintaining minimal invasiveness. These systems integrate multiple complementary technologies to enhance diagnostic accuracy and responsiveness. Rapid screening can be accomplished through lateral flow assays and LAMP, while SERS enables molecular fingerprinting with exceptional specificity [719]. Electrochemical and plasmonics-based sensors provide real-time quantitative monitoring of dynamic biological processes, and FET sensors allow highly sensitive, label-free detection of biomolecular interactions [856]. Furthermore, nanomaterial-based sensors enable multiplexed, high-sensitivity analyses within miniaturized systems. The convergence of electrochemical, immunological, plasmonic, and aptamer-based sensing modalities represents a transformative step toward faster, more precise, and integrated diagnostic platforms.
9.2.4. Miniaturized and Portable Diagnostic Platforms
Microfluidic lab-on-a-chip (LoC) systems are poised to revolutionize diagnostic testing by miniaturizing complex immunoassay workflows into highly integrated single-chip platforms. By combining microfluidic control, surface-enhanced nanostructures, and advanced photonic sensing modules, these systems enable the simultaneous detection of multiple biomarkers using only minimal sample volumes [857,858]. Such integration significantly improves assay speed, portability, and real-time quantification while reducing sample handling and reagent consumption. Moreover, their compact and automated design enhances accessibility in resource-limited settings. Emerging sensor architectures, including dual-core D-shaped photonic crystal fiber-based SPR sensors [859] and dielectric-grating near-field sensors [860], offer exceptional chemical stability, high sensitivity, and robust multiplexed performance, making them ideally suited for portable POC applications.
9.2.5. Organ-on-Chip Systems for Dynamic Disease Modeling
Organ-on-a chip (OoC) platforms will enable tissue-specific modeling of disease microenvironments for longitudinal protein monitoring [861,862]. These microphysiological systems utilize living human cells within microfluidic environments to replicate organ-level physiology and pathology, offering ethical and predictive alternatives to animal models [863]. Cancer-on-a-chip models incorporating patient-derived cancer cells and stromal components will track secretion patterns of VEGF and cytokines throughout drug response cycles, bridging in vitro discovery with in vivo validation [864,865,866]. These platforms will recreate TMEs and organ interactions, facilitating personalized drug testing and metastasis modeling. Supported by regulatory frameworks such as the 2022 FDA Modernization Act 2.0, this “clinical-trial-on-a-chip” approach will advance precision oncology, drug safety evaluation, and translational cancer research [867].
9.2.6. Longitudinal Monitoring and Digital Integration
Future biomarker research will prioritize longitudinal tracking [868,869] and multiplexed biosensing [870] for dynamic monitoring of biomarker fluctuations, enabling early relapse detection and therapy response assessment. These systems will permit continuous surveillance of aging-related proteins under controlled flow and stress conditions, providing mechanistic insights into proteomic aging clocks and therapy-response trajectories. Integration of AI-driven analytics with cloud-linked longitudinal databases will enable trend analysis, anomaly detection, and predictive modeling of biomarker evolution across time and organ systems [871]. This digital-biosensing synergy will transform episodic diagnostics into continuous, personalized disease monitoring, particularly for cancer and age-associated chronic diseases.
9.2.7. Manufacturing and Quality Assurance
Nanotechnology will redefine POCT device fabrication through nanometrology techniques such as atomic force microscopy (AFM) and scanning tunneling microscopy (STM), ensuring device integrity and performance at the nanoscale. These quality assurance approaches will be essential for ultra-miniature devices, including those deployed in Internet of Things (IoT) healthcare applications. Regulatory harmonization will ensure consistent standards and safe deployment across healthcare settings [872].
9.2.8. Paradigm Shift Toward Preventive Medicine
A fundamental transition from single-point testing to longitudinal and preventive screening will identify pre-disease states and enable early interventions. This includes tracking early deviations in brain aging trajectories BAG measures and monitoring circulating biomarkers for cancer recurrence risk.
9.2.9. Clinical Translation Framework
Achieving clinical translation will require robust regulatory and standardization frameworks, including clearly defined performance criteria, quality control systems, and privacy safeguards. Successful implementation will depend on collaborative efforts across industry, healthcare institutions, and academic research centers to establish unified standards for these emerging technologies [317].
10. Conclusions
Cancer and aging are distinct but interconnected biological processes that share common mechanisms such as genomic instability, telomere dysfunction, cellular senescence, chronic inflammation, and metabolic abnormalities. This overlap offers opportunities for biomarker discovery enabling early detection and targeted interventions. Early diagnosis is critical for improving treatment outcomes, reducing mortality, and enabling timely therapeutic interventions. However, conventional diagnostic methods often rely on invasive biopsies, imaging tests with low SN, and single-protein analyses, potentially missing early signals and delaying intervention.
Emerging biomarker-based approaches are shifting this paradigm. Liquid biopsy enables non-invasive detection of ctDNA, cfDNA, CTCs, EVs, and miRNA in blood or other biofluids. However, its clinical performance is limited by low analyte concentrations and high biological variability. Advanced analytical and detection platforms, including NGS, ddPCR, LAMP, MDs, SERS sensors, FET biosensors, and electrochemical sensors, along with nanotechnology further enhance SN with minimal samples. Concurrently, AI and ML/DL enable the identification of subtle, high-dimensional patterns, improving diagnostic accuracy and risk evaluation.
Furthermore, wearable biosensors and miniature lab-on-a-chip systems provide real-time POCT. Emerging biomarkers, such as biological age and BAG, provide new means to identify at-risk populations before symptom onset. The convergence of liquid biopsy, multi-omics, AI, and smart biosensors promises a shift from single-point diagnostics to continuous personalized monitoring.
To apply these advances to routine clinical practice, additional efforts are required to establish standardized protocols, conduct large-scale clinical validation, and develop accessible POC diagnostic platforms. These innovations hold the potential to revolutionize the early detection and personalized management of cancer and age-related diseases.
Author Contributions
Conceptualization, S.P.P. and M.-R.K.; methodology, M.-R.K., D.H.K. and M.A.A.A.; investigation, M.-R.K., D.H.K. and M.A.A.A.; writing—original draft preparation, M.-R.K. and D.H.K.; writing—review and editing, M.-R.K., D.H.K., M.A.A.A. and S.P.P.; visualization, M.-R.K. and D.H.K.; supervision, S.P.P. and M.-R.K.; project administration, S.P.P.; funding acquisition, S.P.P. and M.-R.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea funded by the Korean Ministry of Science and ICT (RS-2021-NR060107). This work was also supported by the National Research Foundation of Korea funded by the Korean Ministry of Education (RS-2021-NR065961).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Abbreviation | Full Term | Category/Context |
| AD | Alzheimer’s Disease | Neurodegenerative disorder/aging research |
| AFP | Alpha-Fetoprotein | Clinical protein biomarker (HCC, germ cell tumors) |
| AGEs | Advanced Glycation End Products | Metabolic/oxidative stress biomarkers |
| AI | Artificial Intelligence | Computational analysis/biosensing |
| ATM | Ataxia Telangiectasia Mutated | DNA damage response/telomere dysfunction |
| ATR | ATM and Rad3-Related | DNA damage response/telomere dysfunction |
| AUC | Area Under the Curve | Diagnostic performance metric |
| AuNPs | Gold Nanoparticles | Probe for biosensing |
| Aβ | Amyloid Beta Protein | Neurodegeneration/Alzheimer’s biomarker |
| BAG | Brain Age Gap | Neuroimaging biomarker for biological aging |
| BAP | Brain Age Prediction | AI-based neuroaging estimation |
| BC | Breast Cancer | Solid tumor type |
| BRCA | Breast Cancer Gene (BRCA1/2) | Tumor suppressor genes |
| CA125 | Cancer Antigen 125 | Clinical protein biomarker (Ovarian cancer) |
| CA19-9 | Carbohydrate Antigen 19-9 | Clinical protein biomarker (Pancreatic cancer) |
| CAD | Computer-Aided Detection | AI-assisted image analysis |
| CD4 | Cluster of Differentiation 4 | Immune cell surface marker (T-helper cells) |
| CD8 | Cluster of Differentiation 8 | Cytotoxic T-cell surface glycoprotein |
| CDK | Cyclin-Dependent Kinase | Cell cycle regulation |
| CCL | Chemokine (C–C Motif) Ligand | Inflammatory/immune signaling |
| CEA | Carcinoembryonic Antigen | Clinical tumor biomarker (CRC, BC) |
| cfDNA | Cell-Free DNA | Liquid biopsy/genetic biomarker |
| cfRNA | Cell-Free RNA | Liquid biopsy/transcriptional biomarker |
| CLIA | Clinical Laboratory Improvement Amendments | a U.S. law that sets quality standards |
| ChIP-seq | Chromatin immunoprecipitation sequencing | Analysis of Protein-DNA interactions |
| CK | Cytokeratin | Structural epithelial protein |
| CKD | Chronic Kidney Disease | Metabolic/renal disorder |
| CNN | Convolutional Neural Network | Deep learning model architecture |
| CRC | Colorectal Cancer | Solid tumor type |
| CRP | C-Reactive Protein | Inflammatory/cardiovascular biomarker |
| CT | Computed Tomography | Diagnostic imaging modality |
| CTCs | Circulating Tumor Cells | Liquid biopsy/cancer detection |
| ctDNA | Circulating Tumor DNA | Liquid biopsy/genetic biomarker |
| cTnI | Cardiac Troponin I | Cardiac injury biomarker |
| CVD | Cardiovascular Disease | Chronic disease/heart pathology |
| CXCL | Chemokine (C–X–C Motif) Ligand | Immune/inflammatory signaling |
| CXCR | Chemokine (C–X–C Motif) Receptor | Immune/inflammatory signaling |
| ddPCR | Droplet Digital Polymerase Chain Reaction | Genomic detection platform |
| DL | Deep Learning | Subfield of AI for data modeling |
| DNAm | DNA Methylation | Epigenetic biomarker |
| DNMT3A | DNA (Cytosine-5)-Methyltransferase 3A | Epigenetic modification enzyme |
| ECM | Extracellular Matrix | Tumor microenvironment structure |
| EGFR | Epidermal Growth Factor Receptor | Oncogenic driver/therapeutic target |
| ELISA | Enzyme-Linked Immunosorbent Assay | Protein quantification assay |
| EMT | Epithelial-to-Mesenchymal Transition | Cancer invasion/metastasis mechanism |
| EpCAM | Epithelial Cell Adhesion Molecule | Diagnostic and prognostic marker |
| ER | Estrogen Receptor | Hormone signaling/BC subtype |
| ERCC1 | Excision Repair Cross-Complementation Group 1 | DNA repair enzyme |
| EVs | Extracellular Vesicles | Intercellular communication/biomarker source |
| FDA | U.S. Food and Drug Administration | Regulatory agency |
| FET | Field-Effect Transistor | Electronic biosensing platform |
| GFAP | Glial Fibrillary Acidic Protein | Neurodegeneration biomarker |
| GDF15 | Growth Differentiation Factor 15 | Aging/metabolic biomarker |
| HbA1c | Hemoglobin A1c | Glycemic control/diabetes biomarker |
| HCC | Hepatocellular Carcinoma | Solid tumor type |
| HER2 | Human Epidermal Growth Factor Receptor 2 | Breast/gastric cancer biomarker |
| HK2 | Hexokinase 2 | Glycolytic enzyme/metabolic marker |
| HPV | Human Papillomavirus | Viral oncogenic infection |
| HSCs | Hematopoietic Stem Cells | Aging and regeneration biology |
| IDO | Indoleamine 2,3-Dioxygenase | Immune-metabolic enzyme |
| IFN | Interferon | Immune signaling cytokine |
| IGF | Insulin-Like Growth Factor | Growth/metabolic regulation |
| IL | Interleukin | Inflammatory cytokine family |
| IVD | In Vitro Diagnostic | Regulatory classification |
| KRAS | Kirsten Rat Sarcoma Viral Oncogene Homolog | Oncogenic mutation marker |
| LAMP | Loop-Mediated Isothermal Amplification | Rapid nucleic acid detection |
| LC | Lung Cancer | Solid tumor type |
| LC–MS/MS | Liquid Chromatography–Tandem Mass Spectrometry | Proteomic/metabolomic detection |
| LDHA | Lactate Dehydrogenase A | Metabolic enzyme/hypoxia biomarker |
| LDTs | Laboratory-developed tests | Diagnostic tests |
| lncRNAs | long non-coding RNA | Non coding RNA |
| LoD | Limit of Detection | Analytical sensitivity parameter |
| MAE | Mean Absolute Error | Statistical model evaluation metric |
| MAPK | Mitogen-Activated Protein Kinase | Signal transduction pathway |
| MCED | Multi-Cancer Early Detection | Blood-based screening assay |
| MD | Microfluidics Device | Technology used in POCT |
| miRNA | MicroRNA | Post-transcriptional regulatory molecule |
| ML | Machine Learning | AI method for pattern recognition |
| MMP | Matrix Metalloproteinase | Proteolytic/tumor invasion enzyme |
| MRI | Magnetic Resonance Imaging | Diagnostic imaging technique |
| MRS | Magnetic Resonance Spectroscopy | Detecting tool |
| mTOR | Mechanistic Target of Rapamycin | Nutrient-sensing/autophagy regulation |
| mtDNA | Mitochondrial DNA | Oxidative stress/aging biomarker |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of Activated B Cells | Inflammatory transcription factor |
| NGS | Next-Generation Sequencing | Genomic profiling platform |
| NSCLC | Non-Small Cell Lung Cancer | Solid tumor type |
| NT-proBNP | N-terminal Pro-B-type Natriuretic Peptide | Heart failure biomarker |
| OC | Ovarian Cancer | Gynecologic malignancy |
| PC | Pancreatic Cancer | Solid tumor type |
| PCa | Prostate Cancer | Solid tumor type/biomarker target |
| PDAC | Pancreatic Ductal Adenocarcinoma | Pancreatic cancer subtype |
| PD-L1 | Programmed Death Ligand-1 | Immune checkpoint biomarker |
| PET | Positron Emission Tomography | Functional imaging technique |
| PI3K | Phosphoinositide 3-Kinase | Cell survival/growth signaling |
| PMA | Premarket Approval | FDA regulatory pathway |
| POC | Point-of-Care | Decentralized diagnostic setting |
| POCT | Point-of-Care Testing | Decentralized diagnostic setting |
| PSA | Prostate-Specific Antigen | FDA-approved diagnostic biomarker |
| PTEN | Phosphatase and Tensin Homolog | Tumor suppressor gene |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction | Gene expression quantification |
| RAGE | Receptor for Advanced Glycation End Products | Oxidative stress/aging biomarker |
| RB | Retinoblastoma Protein | Tumor suppressor/cell cycle control |
| SASP | Senescence-Associated Secretory Phenotype | Aging/inflammatory response |
| SCL | Smart Contact Lens | Glucose sensing |
| SCLC | Small Cell Lung Cancer | Pulmonary malignancy subtype |
| SCs | Stem Cells | Regenerative medicine context |
| SERS | Surface-Enhanced Raman Spectroscopy | Plasmonic biosensing method |
| SN | Sensitivity | Diagnostic performance metric |
| SP | Specificity | Diagnostic performance metric |
| SPR | Surface Plasmon Resonance | Optical biosensing platform |
| STAT | Signal Transducer and Activator of Transcription | Cytokine/growth factor signaling |
| T2D | Type 2 Diabetes | Metabolic disease |
| TCGA | The Cancer Genome Atlas | Genomic data resource |
| TERT | Telomerase Reverse Transcriptase | Telomere maintenance enzyme |
| TF | Transcription Factor | Gene expression regulator |
| TG | Tear Glucose | Glucose sensing |
| TME | Tumor Microenvironment | Cancer biology/pathology |
| TNF-α | Tumor Necrosis Factor Alpha | Inflammatory cytokine |
| VEGF | Vascular Endothelial Growth Factor | Angiogenesis/vascular signaling |
| WGS | Whole Genome Sequencing | Genomic profiling platform |
References
- Jones, C.H.; Dolsten, M. Healthcare on the brink: Navigating the challenges of an aging society in the United States. NPJ Aging 2024, 10, 22. [Google Scholar] [CrossRef]
- Pais-Magalhães, V.; Moutinho, V.; Robaina, M. Is an ageing population impacting energy use in the European Union? Drivers, lifestyles, and consumption patterns of elderly households. Energy Res. Soc. Sci. 2022, 85, 102443. [Google Scholar] [CrossRef]
- Jarzebski, M.P.; Elmqvist, T.; Gasparatos, A.; Fukushi, K.; Eckersten, S.; Haase, D.; Goodness, J.; Khoshkar, S.; Saito, O.; Takeuchi, K.; et al. Ageing and population shrinking: Implications for sustainability in the urban century. NPJ Urban Sustain. 2021, 1, 17. [Google Scholar] [CrossRef]
- Furrer, R.; Handschin, C. Biomarkers of aging: From molecules and surrogates to physiology and function. Physiol. Rev. 2025, 105, 1609–1694. [Google Scholar] [CrossRef]
- Chatsirisupachai, K.; Lesluyes, T.; Paraoan, L.; Van Loo, P.; de Magalhaes, J.P. An integrative analysis of the age-associated multi-omic landscape across cancers. Nat. Commun. 2021, 12, 2345. [Google Scholar] [CrossRef]
- Ki, M.R.; Youn, S.; Kim, D.H.; Pack, S.P. Natural Compounds for Preventing Age-Related Diseases and Cancers. Int. J. Mol. Sci. 2024, 25, 7530. [Google Scholar] [CrossRef]
- Marosi, C.; Köller, M. Challenge of cancer in the elderly. ESMO Open 2016, 1, e000020. [Google Scholar] [CrossRef]
- Guo, S.; Zhu, X.; Huang, Z.; Wei, C.; Yu, J.; Zhang, L.; Feng, J.; Li, M.; Li, Z. Genomic instability drives tumorigenesis and metastasis and its implications for cancer therapy. Biomed. Pharmacother. 2023, 157, 114036. [Google Scholar] [CrossRef]
- Lopez-Gil, L.; Pascual-Ahuir, A.; Proft, M. Genomic Instability and Epigenetic Changes during Aging. Int. J. Mol. Sci. 2023, 24, 14279. [Google Scholar] [CrossRef]
- Schmitt, C.A.; Wang, B.; Demaria, M. Senescence and cancer—Role and therapeutic opportunities. Nat. Rev. Clin. Oncol. 2022, 19, 619–636. [Google Scholar] [CrossRef]
- Zhang, L.; Pitcher, L.E.; Yousefzadeh, M.J.; Niedernhofer, L.J.; Robbins, P.D.; Zhu, Y. Cellular senescence: A key therapeutic target in aging and diseases. J. Clin. Investig. 2022, 132, e158450. [Google Scholar] [CrossRef]
- Gao, J.; Pickett, H.A. Targeting telomeres: Advances in telomere maintenance mechanism-specific cancer therapies. Nat. Rev. Cancer 2022, 22, 515–532. [Google Scholar] [CrossRef]
- Rossiello, F.; Jurk, D.; Passos, J.F.; d’Adda di Fagagna, F. Telomere dysfunction in ageing and age-related diseases. Nat. Cell Biol. 2022, 24, 135–147. [Google Scholar] [CrossRef]
- Baechle, J.J.; Chen, N.; Makhijani, P.; Winer, S.; Furman, D.; Winer, D.A. Chronic inflammation and the hallmarks of aging. Mol. Metab. 2023, 74, 101755. [Google Scholar] [CrossRef]
- Fernandes, Q.; Inchakalody, V.P.; Bedhiafi, T.; Mestiri, S.; Taib, N.; Uddin, S.; Merhi, M.; Dermime, S. Chronic inflammation and cancer; the two sides of a coin. Life Sci. 2024, 338, 122390. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, L.D.; Narita, M. Autophagy at the intersection of aging, senescence, and cancer. Mol. Oncol. 2022, 16, 3259–3275. [Google Scholar] [CrossRef] [PubMed]
- Zapateria, B.; Arias, E. Aging, cancer, and autophagy: Connections and therapeutic perspectives. Front. Mol. Biosci. 2024, 11, 1516789. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sun, C.; Qin, Z. Metabolic reprogramming of cancer-associated fibroblasts and its effect on cancer cell reprogramming. Theranostics 2021, 11, 8322–8336. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target. Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Lopez-Otin, C.; Pietrocola, F.; Roiz-Valle, D.; Galluzzi, L.; Kroemer, G. Meta-hallmarks of aging and cancer. Cell Metab. 2023, 35, 12–35. [Google Scholar] [CrossRef]
- DeGregori, J.; Seidl, K.J.; Montano, M. Aging and Cancer-Inextricably Linked Across the Lifespan. Aging Cell 2025, 24, e14483. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H. Epigenetic Clocks and EpiScore for Preventive Medicine: Risk Stratification and Intervention Models for Age-Related Diseases. J. Clin. Med. 2025, 14, 3604. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Garrett, J.W.; Liu, D.; Pickhardt, P.J. CT Biomarkers for Phenotypic Biological Aging: Emerging Concepts and Advantages. RadioGraphics 2025, 45, e250007. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, S.; Zhou, W.; Schüssler-Fiorenza Rose, S.M.; Sailani, M.R.; Contrepois, K.; Avina, M.; Ashland, M.; Brunet, A.; Snyder, M. Personal aging markers and ageotypes revealed by deep longitudinal profiling. Nat. Med. 2020, 26, 83–90. [Google Scholar] [CrossRef]
- Tenchov, R.; Sapra, A.K.; Sasso, J.; Ralhan, K.; Tummala, A.; Azoulay, N.; Zhou, Q.A. Biomarkers for Early Cancer Detection: A Landscape View of Recent Advancements, Spotlighting Pancreatic and Liver Cancers. ACS Pharmacol. Transl. Sci. 2024, 7, 586–613. [Google Scholar] [CrossRef]
- Ren, F.; Wei, J.; Chen, Q.; Hu, M.; Yu, L.; Mi, J.; Zhou, X.; Qin, D.; Wu, J.; Wu, A. Artificial intelligence-driven multi-omics approaches in Alzheimer’s disease: Progress, challenges, and future directions. Acta Pharm. Sin. B 2025, 15, 4327–4385. [Google Scholar] [CrossRef]
- Crosby, D.; Bhatia, S.; Brindle, K.M.; Coussens, L.M.; Dive, C.; Emberton, M.; Esener, S.; Fitzgerald, R.C.; Gambhir, S.S.; Kuhn, P.; et al. Early detection of cancer. Science 2022, 375, eaay9040. [Google Scholar] [CrossRef]
- Cancer Research UK. Why is Early Cancer Diagnosis Important? Available online: https://www.cancerresearchuk.org/about-cancer/spot-cancer-early/why-is-early-diagnosis-important (accessed on 23 July 2025).
- Alum, E.U. AI-driven biomarker discovery: Enhancing precision in cancer diagnosis and prognosis. Discov. Oncol. 2025, 16, 313. [Google Scholar] [CrossRef]
- Imai, M.; Nakamura, Y.; Yoshino, T. Transforming cancer screening: The potential of multi-cancer early detection (MCED) technologies. Int. J. Clin. Oncol. 2025, 30, 180–193. [Google Scholar] [CrossRef]
- Eledkawy, A.; Hamza, T.; El-Metwally, S. Precision cancer classification using liquid biopsy and advanced machine learning techniques. Sci. Rep. 2024, 14, 5841. [Google Scholar] [CrossRef]
- Kedzierska, M.; Bankosz, M. Role of Proteins in Oncology: Advances in Cancer Diagnosis, Prognosis, and Targeted Therapy—A Narrative Review. J. Clin. Med. 2024, 13, 7131. [Google Scholar] [CrossRef]
- National Cancer Institute. Age and Cancer Risk. National Cancer Institute. Updated: 2 May 2025. Available online: https://www.cancer.gov/about-cancer/causes-prevention/risk/age (accessed on 23 July 2025).
- Montegut, L.; Lopez-Otin, C.; Kroemer, G. Aging and cancer. Mol. Cancer 2024, 23, 106. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Wang, J.; Shao, F.; Yu, Q.X.; Ye, L.; Wusiman, D.; Wu, R.; Tuo, Z.; Wang, Z.; Li, D.; Cho, W.C.; et al. The Common Hallmarks and Interconnected Pathways of Aging, Circadian Rhythms, and Cancer: Implications for Therapeutic Strategies. Research 2025, 8, 612. [Google Scholar] [CrossRef]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef] [PubMed]
- Vijg, J.; Montagna, C. Genome instability and aging: Cause or effect? Transl. Med. Aging 2017, 1, 5–11. [Google Scholar] [CrossRef]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Yousefzadeh, M.; Henpita, C.; Vyas, R.; Soto-Palma, C.; Robbins, P.; Niedernhofer, L. DNA damage-how and why we age? Elife 2021, 10, e62852. [Google Scholar] [CrossRef]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Sherr, C.J.; McCormick, F. The RB and p53 pathways in cancer. Cancer Cell 2002, 2, 103–112. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, X.; Yi, Y.; Wang, X.; Zhu, L.; Shen, Y.; Lin, D.; Wu, C. Tumor initiation and early tumorigenesis: Molecular mechanisms and interventional targets. Signal Transduct. Target. Ther. 2024, 9, 149. [Google Scholar] [CrossRef] [PubMed]
- Salavoura, A. Mechanisms of Aging and Cancer. In Chemical Environmental Pollutants and Their Effect on Health; Springer: Berlin/Heidelberg, Germany, 2025; pp. 311–328. [Google Scholar]
- Abubakar, M.; Hameed, Y.; Kiani, M.N.; Aftab, A. Common features between aging and cancer: A narrative review. Aging Adv. 2024, 1, 118–134. [Google Scholar] [CrossRef]
- Trastus, L.A.; d’Adda di Fagagna, F. The complex interplay between aging and cancer. Nat. Aging 2025, 5, 350–365. [Google Scholar] [CrossRef] [PubMed]
- d’Adda di Fagagna, F. Living on a break: Cellular senescence as a DNA-damage response. Nat. Rev. Cancer 2008, 8, 512–522. [Google Scholar] [CrossRef]
- Roos, W.P.; Kaina, B. DNA damage-induced cell death by apoptosis. Trends Mol. Med. 2006, 12, 440–450. [Google Scholar] [CrossRef]
- O’Hagan, H.M.; Wang, W.; Sen, S.; Shields, C.D.; Lee, S.S.; Zhang, Y.W.; Clements, E.G.; Cai, Y.; Van Neste, L.; Easwaran, H.; et al. Oxidative damage targets complexes containing DNA methyltransferases, SIRT1, and polycomb members to promoter CpG Islands. Cancer Cell 2011, 20, 606–619. [Google Scholar] [CrossRef]
- Goriuc, A.; Cojocaru, K.-A.; Luchian, I.; Ursu, R.-G.; Butnaru, O.; Foia, L. Using 8-Hydroxy-2′-Deoxiguanosine (8-OHdG) as a Reliable Biomarker for Assessing Periodontal Disease Associated with Diabetes. Int. J. Mol. Sci. 2024, 25, 1425. [Google Scholar] [CrossRef]
- Lee, J.W.; Ong, E.B.B. Genomic Instability and Cellular Senescence: Lessons From the Budding Yeast. Front. Cell Dev. Biol. 2020, 8, 619126. [Google Scholar] [CrossRef]
- Sasaki, M.; Kobayashi, T. Transcription near arrested DNA replication forks triggers ribosomal DNA copy number changes. Nucleic Acids Res. 2025, 53, gkaf014. [Google Scholar] [CrossRef]
- Kasselimi, E.; Pefani, D.-E.; Taraviras, S.; Lygerou, Z. Ribosomal DNA and the nucleolus at the heart of aging. Trends Biochem. Sci. 2022, 47, 328–341. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Rysz-Górzyńska, M.; Gluba-Brzózka, A. Ageing, Age-Related Cardiovascular Risk and the Beneficial Role of Natural Components Intake. Int. J. Mol. Sci. 2021, 23, 183. [Google Scholar] [CrossRef]
- Miklikova, S.; Trnkova, L.; Plava, J.; Bohac, M.; Kuniakova, M.; Cihova, M. The Role of BRCA1/2-Mutated Tumor Microenvironment in Breast Cancer. Cancers 2021, 13, 575. [Google Scholar] [CrossRef]
- Pietragalla, A.; Arcieri, M.; Marchetti, C.; Scambia, G.; Fagotti, A. Ovarian cancer predisposition beyond BRCA1 and BRCA2 genes. Int. J. Gynecol. Cancer 2020, 30, 1803–1810. [Google Scholar] [CrossRef]
- Wang, S.; Min, Z.; Ji, Q.; Geng, L.; Su, Y.; Liu, Z.; Hu, H.; Wang, L.; Zhang, W.; Suzuiki, K.; et al. Rescue of premature aging defects in Cockayne syndrome stem cells by CRISPR/Cas9-mediated gene correction. Protein Cell 2020, 11, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Pekhale, K.; Tiwari, V.; Hussain, M.; Bridges, C.C.; Croteau, D.L.; Levi, M.; Rosenberg, A.Z.; Santo, B.; Yang, X.; Kulikowicz, T.; et al. Cockayne syndrome mice reflect human kidney disease and are defective in de novo NAD biosynthesis. Cell Death Differ. 2025. [Google Scholar] [CrossRef] [PubMed]
- Rizza, E.R.H.; DiGiovanna, J.J.; Khan, S.G.; Tamura, D.; Jeskey, J.D.; Kraemer, K.H. Xeroderma Pigmentosum: A Model for Human Premature Aging. J. Investig. Dermatol. 2021, 141, 976–984. [Google Scholar] [CrossRef] [PubMed]
- Danter, W.R. Age-Dependent Effects of UV Exposure and Xeroderma Pigmentosum Group A on DNA Damage, Repair Mechanisms, Genomic Instability, Cancer Risk, and Neurological Disorders. medRxiv 2024. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, J.; Flanagan, M.; Martinez, J.A.; Cunniff, C.; Kucine, N.; Lu, A.T.; Haghani, A.; Gordevičius, J.; Horvath, S.; et al. Bloom syndrome patients and mice display accelerated epigenetic aging. Aging Cell 2023, 22, e13964. [Google Scholar] [CrossRef]
- Aguado, J.; Gómez-Inclán, C.; Leeson, H.C.; Lavin, M.F.; Shiloh, Y.; Wolvetang, E.J. The hallmarks of aging in Ataxia-Telangiectasia. Ageing Res. Rev. 2022, 79, 101653. [Google Scholar] [CrossRef]
- Agrelo, R.; Cheng, W.-H.; Setien, F.; Ropero, S.; Espada, J.; Fraga, M.F.; Herranz, M.; Paz, M.F.; Sanchez-Cespedes, M.; Artiga, M.J.; et al. Epigenetic inactivation of the premature aging Werner syndrome gene in human cancer. Proc. Natl. Acad. Sci. USA 2006, 103, 8822–8827. [Google Scholar] [CrossRef]
- Velleuer, E.; Carlberg, C. A Nutrigenomic View on the Premature-Aging Disease Fanconi Anemia. Nutrients 2024, 16, 2271. [Google Scholar] [CrossRef]
- Zhang, C.; Gao, X.; Li, M.; Yu, X.; Huang, F.; Wang, Y.; Yan, Y.; Zhang, H.; Shi, Y.; He, X. The role of mitochondrial quality surveillance in skin aging: Focus on mitochondrial dynamics, biogenesis and mitophagy. Ageing Res. Rev. 2023, 87, 101917. [Google Scholar] [CrossRef] [PubMed]
- Tamashiro, H.; Ishikawa, K.; Sadotomo, K.; Ogasawara, E.; Nakada, K. Mitochondrial Respiratory Dysfunction Is Not Correlated With Mitochondrial Genotype in Premature Aging Mice. Aging Cell 2025, 24, e70085. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, H.; Liang, R.; Huang, Y.; Tang, Q. Aging through the lens of mitochondrial DNA mutations and inheritance paradoxes. Biogerontology 2025, 26, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Vodicka, P.; Vodenkova, S.; Danesova, N.; Vodickova, L.; Zobalova, R.; Tomasova, K.; Boukalova, S.; Berridge, M.V.; Neuzil, J. Mitochondrial DNA damage, repair, and replacement in cancer. Trends Cancer 2025, 11, 62–73. [Google Scholar] [CrossRef]
- Nguyen, B.; Fong, C.; Luthra, A.; Smith, S.A.; DiNatale, R.G.; Nandakumar, S.; Walch, H.; Chatila, W.K.; Madupuri, R.; Kundra, R. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell 2022, 185, 563–575.e11. [Google Scholar] [CrossRef]
- van Dijk, E.; van den Bosch, T.; Lenos, K.J.; El Makrini, K.; Nijman, L.E.; van Essen, H.F.B.; Lansu, N.; Boekhout, M.; Hageman, J.H.; Fitzgerald, R.C.; et al. Chromosomal copy number heterogeneity predicts survival rates across cancers. Nat. Commun. 2021, 12, 3188. [Google Scholar] [CrossRef]
- Ippolito, M.R.; Martis, V.; Martin, S.; Tijhuis, A.E.; Hong, C.; Wardenaar, R.; Dumont, M.; Zerbib, J.; Spierings, D.C.; Fachinetti, D. Gene copy-number changes and chromosomal instability induced by aneuploidy confer resistance to chemotherapy. Dev. Cell 2021, 56, 2440–2454.e6. [Google Scholar] [CrossRef]
- Lukow, D.A.; Sausville, E.L.; Suri, P.; Chunduri, N.K.; Wieland, A.; Leu, J.; Smith, J.C.; Girish, V.; Kumar, A.A.; Kendall, J. Chromosomal instability accelerates the evolution of resistance to anti-cancer therapies. Dev. Cell 2021, 56, 2427–2439.e4. [Google Scholar] [CrossRef]
- Boccardi, V.; Marano, L. The telomere connection between aging and cancer: The burden of replication stress and dysfunction. Mech. Ageing Dev. 2025, 223, 112026. [Google Scholar] [CrossRef]
- Milán, M. Chromosomal instability in development and disease: Beyond cancer evolution. Curr. Opin. Cell Biol. 2025, 95, 102537. [Google Scholar] [CrossRef]
- Santaguida, S.; Richardson, A.; Iyer, D.R.; M’Saad, O.; Zasadil, L.; Knouse, K.A.; Wong, Y.L.; Rhind, N.; Desai, A.; Amon, A. Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the immune system. Dev. Cell 2017, 41, 638–651.e5. [Google Scholar] [CrossRef]
- Macedo, J.C.; Vaz, S.; Bakker, B.; Ribeiro, R.; Bakker, P.L.; Escandell, J.M.; Ferreira, M.G.; Medema, R.; Foijer, F.; Logarinho, E. FoxM1 repression during human aging leads to mitotic decline and aneuploidy-driven full senescence. Nat. Commun. 2018, 9, 2834. [Google Scholar] [CrossRef]
- Barroso-Vilares, M.; Macedo, J.C.; Reis, M.; Warren, J.D.; Compton, D.; Logarinho, E. Small-molecule inhibition of aging-associated chromosomal instability delays cellular senescence. EMBO Rep. 2020, 21, e49248. [Google Scholar] [CrossRef]
- Clemente-Ruiz, M.; Murillo-Maldonado, J.M.; Benhra, N.; Barrio, L.; Perez, L.; Quiroga, G.; Nebreda, A.R.; Milán, M. Gene dosage imbalance contributes to chromosomal instability-induced tumorigenesis. Dev. Cell 2016, 36, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Dekanty, A.; Barrio, L.; Muzzopappa, M.; Auer, H.; Milán, M. Aneuploidy-induced delaminating cells drive tumorigenesis in Drosophila epithelia. Proc. Natl. Acad. Sci. USA 2012, 109, 20549–20554. [Google Scholar] [CrossRef] [PubMed]
- Benhra, N.; Barrio, L.; Muzzopappa, M.; Milán, M. Chromosomal instability induces cellular invasion in epithelial tissues. Dev. Cell 2018, 47, 161–174.e4. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Linstra, R.; van Vugt, M.A. Genomic instability, inflammatory signaling and response to cancer immunotherapy. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2022, 1877, 188661. [Google Scholar] [CrossRef]
- Tsantoulis, P.; Kotsinas, A.; Sfikakis, P.; Evangelou, K.; Sideridou, M.; Levy, B.; Mo, L.; Kittas, C.; Wu, X.; Papavassiliou, A. Oncogene-induced replication stress preferentially targets common fragile sites in preneoplastic lesions. A genome-wide study. Oncogene 2008, 27, 3256–3264. [Google Scholar] [CrossRef]
- Liao, P.; Yan, B.; Wang, C.; Lei, P. Telomeres: Dysfunction, maintenance, aging and cancer. Aging Dis. 2023, 15, 2595. [Google Scholar] [CrossRef]
- Yang, L.; Huang, M.; Chen, Y.; Wu, Y. Philadelphia chromosome-positive mixed-phenotype acute leukemia: A case report and literature review. Front. Oncol. 2025, 15, 1623528. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, D.; Eladl, E.; Capo-Chichi, J.-M.; Kim, D.D.H.; Chang, H. Molecular genetic characterization of Philadelphia chromosome-positive acute myeloid leukemia. Leuk. Res. 2023, 124, 107002. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.H.; Stiller, C.A.; Walker, L.; Rahman, N. Syndromes and constitutional chromosomal abnormalities associated with Wilms tumour. J. Med. Genet. 2006, 43, 705–715. [Google Scholar] [CrossRef] [PubMed]
- Aschero, R.; Francis, J.H.; Ganiewich, D.; Gomez-Gonzalez, S.; Sampor, C.; Zugbi, S.; Ottaviani, D.; Lemelle, L.; Mena, M.; Winter, U.; et al. Recurrent Somatic Chromosomal Abnormalities in Relapsed Extraocular Retinoblastoma. Cancers 2021, 13, 673. [Google Scholar] [CrossRef] [PubMed]
- Alimbetov, D.; Davis, T.; Brook, A.J.C.; Cox, L.S.; Faragher, R.G.A.; Nurgozhin, T.; Zhumadilov, Z.; Kipling, D. Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2. Biogerontology 2016, 17, 305–315. [Google Scholar] [CrossRef]
- Dierick, J.-F.; Eliaers, F.; Remacle, J.; Raes, M.; Fey, S.J.; Larsen, P.M.; Toussaint, O. Stress-induced premature senescence and replicative senescence are different phenotypes, proteomic evidence. Biochem. Pharmacol. 2002, 64, 1011–1017. [Google Scholar] [CrossRef]
- Fang, Y.; Gong, A.Y.; Haller, S.T.; Dworkin, L.D.; Liu, Z.; Gong, R. The ageing kidney: Molecular mechanisms and clinical implications. Ageing Res. Rev. 2020, 63, 101151. [Google Scholar] [CrossRef]
- Kim, K.W.; Ha, K.Y.; Lee, J.S.; Na, K.H.; Kim, Y.Y.; Woo, Y.K. Senescence of nucleus pulposus chondrocytes in human intervertebral discs. Asian Spine J. 2008, 2, 1–8. [Google Scholar] [CrossRef]
- Pizzul, P.; Rinaldi, C.; Bonetti, D. The multistep path to replicative senescence onset: Zooming on triggering and inhibitory events at telomeric DNA. Front. Cell Dev. Biol. 2023, 11, 1250264. [Google Scholar] [CrossRef]
- Muthamil, S.; Kim, H.-Y.; Jang, H.-J.; Lyu, J.-H.; Shin, U.C.; Go, Y.; Park, S.-H.; Lee, H.G.; Park, J.H. Biomarkers of Cellular Senescence and Aging: Current State-of-the-Art, Challenges and Future Perspectives. Adv. Biol. 2024, 8, 2400079. [Google Scholar] [CrossRef]
- Campisi, J. Aging, cellular senescence, and cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef]
- Ohtani, N. The roles and mechanisms of senescence-associated secretory phenotype (SASP): Can it be controlled by senolysis? Inflamm. Regen. 2022, 42, 11. [Google Scholar] [CrossRef] [PubMed]
- Herbstein, F.; Sapochnik, M.; Attorresi, A.; Pollak, C.; Senin, S.; Gonilski-Pacin, D.; Giudice, N.C.D.; Fiz, M.; Elguero, B.; Fuertes, M.; et al. The SASP factor IL-6 sustains cell-autonomous senescent cells via a cGAS-STING-NFκB intracrine senescent noncanonical pathway. Aging Cell 2024, 23, e14258. [Google Scholar] [CrossRef] [PubMed]
- Özcan, S.; Alessio, N.; Acar, M.B.; Mert, E.; Omerli, F.; Peluso, G.; Galderisi, U. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging 2016, 8, 1316–1329. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Luo, Y.; Yuan, Z.; Tian, Y.; Jin, T.; Xu, F. Cellular senescence and SASP in tumor progression and therapeutic opportunities. Mol. Cancer 2024, 23, 181. [Google Scholar] [CrossRef]
- Lau, L.; Porciuncula, A.; Yu, A.; Iwakura, Y.; David, G. Uncoupling the Senescence-Associated Secretory Phenotype from Cell Cycle Exit via Interleukin-1 Inactivation Unveils Its Protumorigenic Role. Mol. Cell. Biol. 2019, 39, e00586-18. [Google Scholar] [CrossRef]
- Leon, K.E.; Buj, R.; Lesko, E.; Dahl, E.S.; Chen, C.W.; Tangudu, N.K.; Imamura-Kawasawa, Y.; Kossenkov, A.V.; Hobbs, R.P.; Aird, K.M. DOT1L modulates the senescence-associated secretory phenotype through epigenetic regulation of IL1A. J. Cell Biol. 2021, 220, 8101. [Google Scholar] [CrossRef]
- Giuliani, K.T.K.; Nag, P.; Adams, B.C.; Wang, X.; Hong, S.; Grivei, A.; Johnston, R.L.; Waddell, N.; Ho, K.K.C.; Tian, Y.; et al. Human proximal tubular epithelial cell interleukin-1 receptor signalling triggers G2/M arrest and cellular senescence during hypoxic kidney injury. Cell Death Dis. 2025, 16, 61. [Google Scholar] [CrossRef]
- Wang, S.-T.; Huang, S.-W.; Liu, K.-T.; Lee, T.-Y.; Shieh, J.-J.; Wu, C.-Y. Atorvastatin-induced senescence of hepatocellular carcinoma is mediated by downregulation of hTERT through the suppression of the IL-6/STAT3 pathway. Cell Death Discov. 2020, 6, 17. [Google Scholar] [CrossRef]
- Shriki, A.; Lanton, T.; Sonnenblick, A.; Levkovitch-Siany, O.; Eidelshtein, D.; Abramovitch, R.; Rosenberg, N.; Pappo, O.; Elgavish, S.; Nevo, Y.; et al. Multiple Roles of IL6 in Hepatic Injury, Steatosis, and Senescence Aggregate to Suppress Tumorigenesis. Cancer Res. 2021, 81, 4766–4777. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, X.; Ma, Y.; Du, Y.; Feng, J. A new emerging target in cancer immunotherapy: Galectin-9 (LGALS9). Genes. Dis. 2023, 10, 2366–2382. [Google Scholar] [CrossRef]
- Tarallo, D.; Martínez, J.; Leyva, A.; Mónaco, A.; Perroni, C.; Tassano, M.; Gambini, J.P.; Cappetta, M.; Durán, R.; Moreno, M.; et al. Mitofusin 1 silencing decreases the senescent associated secretory phenotype, promotes immune cell recruitment and delays melanoma tumor growth after chemotherapy. Sci. Rep. 2024, 14, 909. [Google Scholar] [CrossRef]
- Daley, D.; Mani, V.R.; Mohan, N.; Akkad, N.; Ochi, A.; Heindel, D.W.; Lee, K.B.; Zambirinis, C.P.; Pandian, G.S.B.; Savadkar, S.; et al. Dectin 1 activation on macrophages by galectin 9 promotes pancreatic carcinoma and peritumoral immune tolerance. Nat. Med. 2017, 23, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, C.; Li, Y.; Li, H.; Zhang, W.; Liu, J.; Wang, L.; Sun, C. Galectin-9 in cancer therapy: From immune checkpoint ligand to promising therapeutic target. Front. Cell Dev. Biol. 2024, 11, 1332205. [Google Scholar] [CrossRef] [PubMed]
- Van Niel, G.; d’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Hanley, S.; Chen, Y.-Y.; Hazeldine, J.; Lord, J.M. Senescent cell-derived extracellular vesicles as potential mediators of innate immunosenescence and inflammaging. Exp. Gerontol. 2024, 187, 112365. [Google Scholar] [CrossRef]
- Misawa, T.; Tanaka, Y.; Okada, R.; Takahashi, A. Biology of extracellular vesicles secreted from senescent cells as senescence-associated secretory phenotype factors. Geriatr. Gerontol. Int. 2020, 20, 539–546. [Google Scholar] [CrossRef]
- Wallis, R.; Mizen, H.; Bishop, C.L. The bright and dark side of extracellular vesicles in the senescence-associated secretory phenotype. Mech. Ageing Dev. 2020, 189, 111263. [Google Scholar] [CrossRef]
- Rao, S.G.; Jackson, J.G. SASP: Tumor Suppressor or Promoter? Yes! Trends Cancer 2016, 2, 676–687. [Google Scholar] [CrossRef]
- Krtolica, A.; Parrinello, S.; Lockett, S.; Desprez, P.-Y.; Campisi, J. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Proc. Natl. Acad. Sci. USA 2001, 98, 12072–12077. [Google Scholar] [CrossRef]
- Di, G.H.; Liu, Y.; Lu, Y.; Liu, J.; Wu, C.; Duan, H.F. IL-6 secreted from senescent mesenchymal stem cells promotes proliferation and migration of breast cancer cells. PLoS ONE 2014, 9, e113572. [Google Scholar] [CrossRef]
- Al-Khalaf, H.H.; Ghebeh, H.; Inass, R.; Aboussekhra, A. Senescent Breast Luminal Cells Promote Carcinogenesis through Interleukin-8-Dependent Activation of Stromal Fibroblasts. Mol. Cell Biol. 2019, 39, e00359-18. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, M.; Okada, R.; Takahashi, A.; Chen, D.V.; Watanabe, S.; Hara, E. Small extracellular vesicles secreted from senescent cells promote cancer cell proliferation through EphA2. Nat. Commun. 2017, 8, 15729. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6, 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hornsby, P.J. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007, 67, 3117–3126. [Google Scholar] [CrossRef]
- Ohuchida, K.; Mizumoto, K.; Murakami, M.; Qian, L.W.; Sato, N.; Nagai, E.; Matsumoto, K.; Nakamura, T.; Tanaka, M. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004, 64, 3215–3222. [Google Scholar] [CrossRef]
- Parrinello, S.; Coppe, J.P.; Krtolica, A.; Campisi, J. Stromal-epithelial interactions in aging and cancer: Senescent fibroblasts alter epithelial cell differentiation. J. Cell Sci. 2005, 118, 485–496. [Google Scholar] [CrossRef]
- Eggert, T.; Wolter, K.; Ji, J.; Ma, C.; Yevsa, T.; Klotz, S.; Medina-Echeverz, J.; Longerich, T.; Forgues, M.; Reisinger, F.; et al. Distinct Functions of Senescence-Associated Immune Responses in Liver Tumor Surveillance and Tumor Progression. Cancer Cell 2016, 30, 533–547. [Google Scholar] [CrossRef]
- Noh, J.Y.; Lee, I.P.; Han, N.R.; Kim, M.; Min, Y.K.; Lee, S.Y.; Yun, S.H.; Kim, S.I.; Park, T.; Chung, H.; et al. Additive Effect of CD73 Inhibitor in Colorectal Cancer Treatment With CDK4/6 Inhibitor Through Regulation of PD-L1. Cell Mol. Gastroenterol. Hepatol. 2022, 14, 769–788. [Google Scholar] [CrossRef]
- Liu, B.; Peng, Z.; Zhang, H.; Zhang, N.; Liu, Z.; Xia, Z.; Huang, S.; Luo, P.; Cheng, Q. Regulation of cellular senescence in tumor progression and therapeutic targeting: Mechanisms and pathways. Mol. Cancer 2025, 24, 106. [Google Scholar] [CrossRef]
- Milanovic, M.; Fan, D.N.Y.; Belenki, D.; Däbritz, J.H.M.; Zhao, Z.; Yu, Y.; Dörr, J.R.; Dimitrova, L.; Lenze, D.; Monteiro Barbosa, I.A.; et al. Senescence-associated reprogramming promotes cancer stemness. Nature 2018, 553, 96–100. [Google Scholar] [CrossRef]
- Saleh, T.; Tyutyunyk-Massey, L.; Gewirtz, D.A. Tumor Cell Escape from Therapy-Induced Senescence as a Model of Disease Recurrence after Dormancy. Cancer Res. 2019, 79, 1044–1046. [Google Scholar] [CrossRef] [PubMed]
- Kobashigawa, S.; Sakaguchi, Y.M.; Masunaga, S.; Mori, E. Stress-induced cellular senescence contributes to chronic inflammation and cancer progression. Therm. Med. 2019, 35, 41–58. [Google Scholar] [CrossRef]
- Kortlever, R.M.; Higgins, P.J.; Bernards, R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat. Cell Biol. 2006, 8, 877–884. [Google Scholar] [CrossRef] [PubMed]
- Undas, A.; Ariëns, R.A. Fibrin clot structure and function: A role in the pathophysiology of arterial and venous thromboembolic diseases. Arterioscler. Thromb. Vasc. Biol. 2011, 31, e88–e99. [Google Scholar] [CrossRef]
- Orjalo, A.V.; Bhaumik, D.; Gengler, B.K.; Scott, G.K.; Campisi, J. Cell surface-bound IL-1alpha is an upstream regulator of the senescence-associated IL-6/IL-8 cytokine network. Proc. Natl. Acad. Sci. USA 2009, 106, 17031–17036. [Google Scholar] [CrossRef]
- Guo, H.; Liu, Z.; Xu, B.; Hu, H.; Wei, Z.; Liu, Q.; Zhang, X.; Ding, X.; Wang, Y.; Zhao, M.; et al. Chemokine receptor CXCR2 is transactivated by p53 and induces p38-mediated cellular senescence in response to DNA damage. Aging Cell 2013, 12, 1110–1121. [Google Scholar] [CrossRef]
- Miller, K.N.; Li, B.; Pierce-Hoffman, H.R.; Patel, S.; Lei, X.; Rajesh, A.; Teneche, M.G.; Havas, A.P.; Gandhi, A.; Macip, C.C.; et al. p53 enhances DNA repair and suppresses cytoplasmic chromatin fragments and inflammation in senescent cells. Nat. Commun. 2025, 16, 2229. [Google Scholar] [CrossRef]
- Klepacki, H.; Kowalczuk, K.; Łepkowska, N.; Hermanowicz, J.M. Molecular Regulation of SASP in Cellular Senescence: Therapeutic Implications and Translational Challenges. Cells 2025, 14, 942. [Google Scholar] [CrossRef]
- Abbas, T.; Dutta, A. p21 in cancer: Intricate networks and multiple activities. Nat. Rev. Cancer 2009, 9, 400–414. [Google Scholar] [CrossRef]
- de Renty, C.; DePamphilis, M.L.; Ullah, Z. Cytoplasmic localization of p21 protects trophoblast giant cells from DNA damage induced apoptosis. PLoS ONE 2014, 9, e97434. [Google Scholar] [CrossRef]
- Chambers, C.R.; Ritchie, S.; Pereira, B.A.; Timpson, P. Overcoming the senescence-associated secretory phenotype (SASP): A complex mechanism of resistance in the treatment of cancer. Mol. Oncol. 2021, 15, 3242–3255. [Google Scholar] [CrossRef]
- Sharpless, N.E.; Sherr, C.J. Forging a signature of in vivo senescence. Nat. Rev. Cancer 2015, 15, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Rodier, F.; Patil, C.K.; Freund, A.; Desprez, P.-Y.; Campisi, J. Tumor suppressor and aging biomarker p16INK4a induces cellular senescence without the associated inflammatory secretory phenotype. J. Biol. Chem. 2011, 286, 36396–36403. [Google Scholar] [CrossRef] [PubMed]
- Buj, R.; Chen, C.-W.; Dahl, E.S.; Leon, K.E.; Kuskovsky, R.; Maglakelidze, N.; Navaratnarajah, M.; Zhang, G.; Doan, M.T.; Jiang, H. Suppression of p16 induces mTORC1-mediated nucleotide metabolic reprogramming. Cell Rep. 2019, 28, 1971–1980.e8. [Google Scholar] [CrossRef] [PubMed]
- Yin, K.; Patten, D.; Gough, S.; Gonçalves, S.d.B.; Chan, A.; Olan, I.; Cassidy, L.; Poblocka, M.; Zhu, H.; Lun, A.; et al. Senescence-induced endothelial phenotypes underpin immune-mediated senescence surveillance. Genes Dev. 2022, 36, 533–549. [Google Scholar] [CrossRef]
- Ruscetti, M.; Morris, J.P.t.; Mezzadra, R.; Russell, J.; Leibold, J.; Romesser, P.B.; Simon, J.; Kulick, A.; Ho, Y.J.; Fennell, M.; et al. Senescence-Induced Vascular Remodeling Creates Therapeutic Vulnerabilities in Pancreas Cancer. Cell 2020, 181, 424–441.e21. [Google Scholar] [CrossRef]
- Krizhanovsky, V.; Yon, M.; Dickins, R.A.; Hearn, S.; Simon, J.; Miething, C.; Yee, H.; Zender, L.; Lowe, S.W. Senescence of activated stellate cells limits liver fibrosis. Cell 2008, 134, 657–667. [Google Scholar] [CrossRef]
- Chen, H.A.; Ho, Y.J.; Mezzadra, R.; Adrover, J.M.; Smolkin, R.; Zhu, C.; Woess, K.; Bernstein, N.; Schmitt, G.; Fong, L.; et al. Senescence Rewires Microenvironment Sensing to Facilitate Antitumor Immunity. Cancer Discov. 2023, 13, 432–453. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef]
- Kang, T.W.; Yevsa, T.; Woller, N.; Hoenicke, L.; Wuestefeld, T.; Dauch, D.; Hohmeyer, A.; Gereke, M.; Rudalska, R.; Potapova, A.; et al. Senescence surveillance of pre-malignant hepatocytes limits liver cancer development. Nature 2011, 479, 547–551. [Google Scholar] [CrossRef]
- Hasegawa, T.; Oka, T.; Son, H.G.; Oliver-García, V.S.; Azin, M.; Eisenhaure, T.M.; Lieb, D.J.; Hacohen, N.; Demehri, S. Cytotoxic CD4+ T cells eliminate senescent cells by targeting cytomegalovirus antigen. Cell 2023, 186, 1417–1431.e20. [Google Scholar] [CrossRef] [PubMed]
- Majewska, J.; Krizhanovsky, V. Immune surveillance of senescent cells in aging and disease. Nat. Aging 2025, 5, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.W.; Johmura, Y.; Suzuki, N.; Omori, S.; Migita, T.; Yamaguchi, K.; Hatakeyama, S.; Yamazaki, S.; Shimizu, E.; Imoto, S.; et al. Blocking PD-L1-PD-1 improves senescence surveillance and ageing phenotypes. Nature 2022, 611, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, V.; Paldino, G.; Kunkl, M.; Paroli, M.; Sorrentino, R.; Tuosto, L.; Fiorillo, M.T. CD8(+) T Cell Senescence: Lights and Shadows in Viral Infections, Autoimmune Disorders and Cancer. Int. J. Mol. Sci. 2022, 23, 3374. [Google Scholar] [CrossRef]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef]
- Wu, L.; Fidan, K.; Um, J.Y.; Ahn, K.S. Telomerase: Key regulator of inflammation and cancer. Pharmacol. Res. 2020, 155, 104726. [Google Scholar] [CrossRef]
- Demanelis, K.; Jasmine, F.; Chen, L.S.; Chernoff, M.; Tong, L.; Delgado, D.; Zhang, C.; Shinkle, J.; Sabarinathan, M.; Lin, H.; et al. Determinants of telomere length across human tissues. Science 2020, 369, eaaz6876. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Kuk, M.U.; Kim, J.W.; Lee, Y.H.; Lee, Y.-S.; Choy, H.E.; Park, S.C.; Park, J.T. ATM mediated-p53 signaling pathway forms a novel axis for senescence control. Mitochondrion 2020, 55, 54–63. [Google Scholar] [CrossRef]
- Xiong, H.; Hua, F.; Dong, Y.; Lin, Y.; Ying, J.; Liu, J.; Wang, X.; Zhang, L.; Zhang, J. DNA damage response and GATA4 signaling in cellular senescence and aging-related pathology. Front. Aging Neurosci. 2022, 14, 933015. [Google Scholar] [CrossRef]
- Herbig, U.; Jobling, W.A.; Chen, B.P.; Chen, D.J.; Sedivy, J.M. Telomere shortening triggers senescence of human cells through a pathway involving ATM, p53, and p21(CIP1), but not p16(INK4a). Mol. Cell 2004, 14, 501–513. [Google Scholar] [CrossRef]
- Shay, J.W. Role of telomeres and telomerase in aging and cancer. Cancer Discov. 2016, 6, 584–593. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, X.; Yuan, Z.; Qiu, J.; Lu, W. A comparison between diffusion tensor imaging and generalized q-sampling imaging in the age prediction of healthy adults via machine learning approaches. J. Neural Eng. 2022, 19, 016013. [Google Scholar] [CrossRef] [PubMed]
- Campa, D.; Mergarten, B.; De Vivo, I.; Boutron-Ruault, M.-C.; Racine, A.; Severi, G.; Nieters, A.; Katzke, V.A.; Trichopoulou, A.; Yiannakouris, N. Leukocyte telomere length in relation to pancreatic cancer risk: A prospective study. Cancer Epidemiol. Biomark. Prev. 2014, 23, 2447–2454. [Google Scholar] [CrossRef] [PubMed]
- Duell, E.J. Telomere length and pancreatic cancer risk: Breaking down the evidence. Gut 2017, 66, 1. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Shi, L.; Lei, J.; Li, B.; Jin, Y. Advances in optical assays for detecting telomerase activity. Luminescence 2019, 34, 136–152. [Google Scholar] [CrossRef]
- Hata, T.; Ishida, M.; Motoi, F.; Yamaguchi, T.; Naitoh, T.; Katayose, Y.; Egawa, S.; Unno, M. Telomerase activity in pancreatic juice differentiates pancreatic cancer from chronic pancreatitis: A meta-analysis. Pancreatology 2016, 16, 372–381. [Google Scholar] [CrossRef]
- Dong, H.; Qian, D.; Wang, Y.; Meng, L.; Chen, D.; Ji, X.; Feng, W. Survivin expression and serum levels in pancreatic cancer. World J. Surg. Oncol. 2015, 13, 189. [Google Scholar] [CrossRef]
- Mormile, R. Telomere Length and Pancreatic Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2017, 26, 1157. [Google Scholar] [CrossRef]
- Sharma, V.; Mehdi, M.M. Oxidative stress, inflammation and hormesis: The role of dietary and lifestyle modifications on aging. Neurochem. Int. 2023, 164, 105490. [Google Scholar] [CrossRef]
- Bogeska, R.; Mikecin, A.M.; Kaschutnig, P.; Fawaz, M.; Büchler-Schäff, M.; Le, D.; Ganuza, M.; Vollmer, A.; Paffenholz, S.V.; Asada, N.; et al. Inflammatory exposure drives long-lived impairment of hematopoietic stem cell self-renewal activity and accelerated aging. Cell Stem Cell 2022, 29, 1273–1284.e8. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.R.; Soneji, S.; Adolfsson, S.; Bryder, D.; Pronk, C.J. Human and Murine Hematopoietic Stem Cell Aging Is Associated with Functional Impairments and Intrinsic Megakaryocytic/Erythroid Bias. PLoS ONE 2016, 11, e0158369. [Google Scholar] [CrossRef][Green Version]
- Jahandideh, B.; Derakhshani, M.; Abbaszadeh, H.; Movassaghpour, A.A.; Mehdizadeh, A.; Talebi, M.; Yousefi, M. The pro-Inflammatory cytokines effects on mobilization, self-renewal and differentiation of hematopoietic stem cells. Hum. Immunol. 2020, 81, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, M.; Passegué, E. TNF-α Coordinates Hematopoietic Stem Cell Survival and Myeloid Regeneration. Cell Stem Cell 2019, 25, 357–372.e7. [Google Scholar] [CrossRef]
- Ho, N.P.; Takizawa, H. Inflammation Regulates Haematopoietic Stem Cells and Their Niche. Int. J. Mol. Sci. 2022, 23, 1125. [Google Scholar] [CrossRef]
- Shi, Y.; Riese, D.J., 2nd; Shen, J. The Role of the CXCL12/CXCR4/CXCR7 Chemokine Axis in Cancer. Front. Pharmacol. 2020, 11, 574667. [Google Scholar] [CrossRef]
- Mistry, J.J.; Hellmich, C.; Moore, J.A.; Jibril, A.; Macaulay, I.; Moreno-Gonzalez, M.; Di Palma, F.; Beraza, N.; Bowles, K.M.; Rushworth, S.A. Free fatty-acid transport via CD36 drives β-oxidation-mediated hematopoietic stem cell response to infection. Nat. Commun. 2021, 12, 7130. [Google Scholar] [CrossRef]
- Ribeiro, C.; Ferreirinha, P.; Landry, J.J.M.; Macedo, F.; Sousa, L.G.; Pinto, R.; Benes, V.; Alves, N.L. Foxo3 regulates cortical and medullary thymic epithelial cell homeostasis with implications in T cell development. Cell Death Dis. 2024, 15, 352. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, L.; Nie, L.; Lin, H. Unraveling the molecular mechanisms between inflammation and tumor angiogenesis. Am. J. Cancer Res. 2021, 11, 301–317. [Google Scholar]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Marroquin-Muciño, M.; Perez-Medina, M.; Benito-Lopez, J.J.; Camarena, A.; Rumbo-Nava, U.; Lopez-Gonzalez, J.S. The systemic-level repercussions of cancer-associated inflammation mediators produced in the tumor microenvironment. Front. Endocrinol. 2022, 13, 929572. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, X.; Zhang, Y.; Zhu, M.; Wang, F.X.C.; Mu, J.; Li, J.; Yao, H.; Chen, K. Role of tumor microenvironment in cancer progression and therapeutic strategy. Cancer Med. 2023, 12, 11149–11165. [Google Scholar] [CrossRef]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in healthy aging and disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Lim, S.H.Y.; Hansen, M.; Kumsta, C. Molecular Mechanisms of Autophagy Decline during Aging. Cells 2024, 13, 1364. [Google Scholar] [CrossRef]
- Di Malta, C.; Cinque, L.; Settembre, C. Transcriptional Regulation of Autophagy: Mechanisms and Diseases. Front. Cell Dev. Biol. 2019, 7, 114. [Google Scholar] [CrossRef]
- Anderson, C.M.; Macleod, K.F. Autophagy and cancer cell metabolism. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 347, pp. 145–190. [Google Scholar]
- Pandey, A.; Goswami, A.; Jithin, B.; Shukla, S. Autophagy: The convergence point of aging and cancer. Biochem. Biophys. Rep. 2025, 42, 101986. [Google Scholar] [CrossRef]
- Park, S.J.; Frake, R.A.; Karabiyik, C.; Son, S.M.; Siddiqi, F.H.; Bento, C.F.; Sterk, P.; Vicinanza, M.; Pavel, M.; Rubinsztein, D.C. Vinexin contributes to autophagic decline in brain ageing across species. Cell Death Differ. 2022, 29, 1055–1070. [Google Scholar] [CrossRef] [PubMed]
- Stavoe, A.K.; Gopal, P.P.; Gubas, A.; Tooze, S.A.; Holzbaur, E.L. Expression of WIPI2B counteracts age-related decline in autophagosome biogenesis in neurons. Elife 2019, 8, e44219. [Google Scholar] [CrossRef] [PubMed]
- Nixon, R.A. The aging lysosome: An essential catalyst for late-onset neurodegenerative diseases. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2020, 1868, 140443. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xu, L.; Li, Y.; Jia, S.; Wang, G.; Cen, X.; Xu, Y.; Cao, Z.; Wang, J.; Shen, N. Mitophagy defect mediates the aging-associated hallmarks in Hutchinson–Gilford progeria syndrome. Aging Cell 2024, 23, e14143. [Google Scholar] [CrossRef]
- Kim, Y.-M.; Park, J.-M.; Grunwald, D.; Kim, D.-H. An expanded role for mTORC1 in autophagy. Mol. Cell. Oncol. 2016, 3, e1010958. [Google Scholar] [CrossRef]
- Burrinha, T.; Cunha, C.; Hall, M.J.; Lopes-da-Silva, M.; Seabra, M.C.; Guimas Almeida, C. Deacidification of endolysosomes by neuronal aging drives synapse loss. Traffic 2023, 24, 334–354. [Google Scholar] [CrossRef] [PubMed]
- Babbar, M.; Basu, S.; Yang, B.; Croteau, D.L.; Bohr, V.A. Mitophagy and DNA damage signaling in human aging. Mech. Ageing Dev. 2020, 186, 111207. [Google Scholar] [CrossRef] [PubMed]
- Palikaras, K.; Tavernarakis, N. Mitochondrial homeostasis: The interplay between mitophagy and mitochondrial biogenesis. Exp. Gerontol. 2014, 56, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Kubli, D.A.; Gustafsson, Å.B. Mitochondria and mitophagy: The yin and yang of cell death control. Circ. Res. 2012, 111, 1208–1221. [Google Scholar] [CrossRef]
- Palmer, J.E.; Wilson, N.; Son, S.M.; Obrocki, P.; Wrobel, L.; Rob, M.; Takla, M.; Korolchuk, V.I.; Rubinsztein, D.C. Autophagy, aging, and age-related neurodegeneration. Neuron 2025, 113, 29–48. [Google Scholar] [CrossRef]
- Kelly, G.; Kataura, T.; Panek, J.; Ma, G.; Salmonowicz, H.; Davis, A.; Kendall, H.; Brookes, C.; Ayine-Tora, D.M.; Banks, P. Suppressed basal mitophagy drives cellular aging phenotypes that can be reversed by a p62-targeting small molecule. Dev. Cell 2024, 59, 1924–1939.e7. [Google Scholar] [CrossRef]
- Eisenberg-Lerner, A.; Kimchi, A. The paradox of autophagy and its implication in cancer etiology and therapy. Apoptosis 2009, 14, 376–391. [Google Scholar] [CrossRef]
- White, E. Deconvoluting the context-dependent role for autophagy in cancer. Nat. Rev. Cancer 2012, 12, 401–410. [Google Scholar] [CrossRef]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef]
- Shen, Y.; Li, D.-D.; Wang, L.-L.; Deng, R.; Zhu, X.-F. Decreased expression of autophagy-related proteins in malignant epithelial ovarian cancer. Autophagy 2008, 4, 1067–1068. [Google Scholar] [CrossRef]
- Liang, X.H.; Jackson, S.; Seaman, M.; Brown, K.; Kempkes, B.; Hibshoosh, H.; Levine, B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature 1999, 402, 672–676. [Google Scholar] [CrossRef]
- Takahashi, Y.; Coppola, D.; Matsushita, N.; Cualing, H.D.; Sun, M.; Sato, Y.; Liang, C.; Jung, J.U.; Cheng, J.Q.; Mulé, J.J.; et al. Bif-1 interacts with Beclin 1 through UVRAG and regulates autophagy and tumorigenesis. Nat. Cell Biol. 2007, 9, 1142–1151. [Google Scholar] [CrossRef]
- Furuya, N.; Yu, J.; Byfield, M.; Pattingre, S.; Levine, B. The evolutionarily conserved domain of Beclin 1 is required for Vps34 binding, autophagy and tumor suppressor function. Autophagy 2005, 1, 46–52. [Google Scholar] [CrossRef]
- Noguchi, M.; Hirata, N.; Suizu, F. The links between AKT and two intracellular proteolytic cascades: Ubiquitination and autophagy. Biochim. Biophys. Acta 2014, 1846, 342–352. [Google Scholar] [CrossRef]
- Roy, S.; Debnath, J. Autophagy and tumorigenesis. Semin. Immunopathol. 2010, 32, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Kausar, M.A.; Anwar, S.; Khan, Y.S.; Saleh, A.A.; Ahmed, M.A.A.; Kaur, S.; Iqbal, N.; Siddiqui, W.A.; Najm, M.Z. Autophagy and Cancer: Insights into Molecular Mechanisms and Therapeutic Approaches for Chronic Myeloid Leukemia. Biomolecules 2025, 15, 215. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.Y.; Ryan, K.M. Autophagy and cancer—Issues we need to digest. J. Cell Sci. 2012, 125, 2349–2358. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhang, Z.; Xu, R.; Zhang, Y.; Wang, Z. Contribution of HIF-1α/BNIP3-mediated autophagy to lipid accumulation during irinotecan-induced liver injury. Sci. Rep. 2023, 13, 6528. [Google Scholar] [CrossRef]
- Kim, M.J.; Woo, S.J.; Yoon, C.H.; Lee, J.S.; An, S.; Choi, Y.H.; Hwang, S.G.; Yoon, G.; Lee, S.J. Involvement of autophagy in oncogenic K-Ras-induced malignant cell transformation. J. Biol. Chem. 2011, 286, 12924–12932. [Google Scholar] [CrossRef]
- Tabibzadeh, S. Signaling pathways and effectors of aging. Front. Biosci. 2021, 26, 50–96. [Google Scholar] [CrossRef]
- Ajoolabady, A.; Pratico, D.; Bahijri, S.; Tuomilehto, J.; Uversky, V.N.; Ren, J. Hallmarks of cellular senescence: Biology, mechanisms, regulations. Exp. Mol. Med. 2025, 57, 1482–1491. [Google Scholar] [CrossRef]
- Simon, M.; Konrath, F.; Wolf, J. From regulation of cell fate decisions towards patient-specific treatments, insights from mechanistic models of signalling pathways. Curr. Opin. Syst. Biol. 2024, 39, 100533. [Google Scholar] [CrossRef]
- Chang, W.-L.; Peng, J.-Y.; Hong, C.-L.; Li, P.-C.; Chye, S.M.; Lu, F.-J.; Lin, H.-Y.; Chen, C.-H. Piperine Induces Apoptosis and Cell Cycle Arrest via Multiple Oxidative Stress Mechanisms and Regulation of PI3K/Akt and MAPK Signaling in Colorectal Cancer Cells. Antioxidants 2025, 14, 892. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Han, W.; Zhang, J.; Liu, G.; Li, H.; Xu, Y.; Liu, F.; Sun, S. Repurposing HIV protease inhibitors as senotherapeutic agents in cervical cancer: Dual targeting of CDK1/6-cell cycle arrest and p53/p21/p16 signaling axis. Biochem. Biophys. Res. Commun. 2025, 771, 152040. [Google Scholar] [CrossRef] [PubMed]
- Sedrak, M.S.; Cohen, H.J. The Aging-Cancer Cycle: Mechanisms and Opportunities for Intervention. J. Gerontol. A Biol. Sci. Med. Sci. 2023, 78, 1234–1238. [Google Scholar] [CrossRef]
- Engeland, K. Cell cycle regulation: p53-p21-RB signaling. Cell Death Differ. 2022, 29, 946–960. [Google Scholar] [CrossRef]
- Indovina, P.; Pentimalli, F.; Casini, N.; Vocca, I.; Giordano, A. RB1 dual role in proliferation and apoptosis: Cell fate control and implications for cancer therapy. Oncotarget 2015, 6, 17873–17890. [Google Scholar] [CrossRef]
- Sasaki, K.; Takahashi, S.; Ouchi, K.; Otsuki, Y.; Wakayama, S.; Ishioka, C. Different impacts of TP53 mutations on cell cycle-related gene expression among cancer types. Sci. Rep. 2023, 13, 4868. [Google Scholar] [CrossRef]
- Jirawatnotai, S.; Dalton, S.; Wattanapanitch, M. Role of cyclins and cyclin-dependent kinases in pluripotent stem cells and their potential as a therapeutic target. Semin. Cell Dev. Biol. 2020, 107, 63–71. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, Y.; Pi, H.; Sheng, Y. Current therapeutic progress of CDK4/6 inhibitors in breast cancer. Cancer Manag. Res. 2020, 12, 3477–3487. [Google Scholar] [CrossRef] [PubMed]
- Terracina, S.; Ferraguti, G.; Petrella, C.; Bruno, S.M.; Blaconà, G.; Di Certo, M.G.; Minni, A.; Greco, A.; Musacchio, A.; Ralli, M. Characteristic hallmarks of aging and the impact on carcinogenesis. Curr. Cancer Drug Targets 2023, 23, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Jan, N.; Sofi, S.; Mir, A.A.; Masoodi, G.; Mir, M.A. Balancing senescence and apoptosis: Therapeutic insights into aging and cancer. Mol. Cell. Biochem. 2025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Herman, B. Ageing and apoptosis. Mech. Ageing Dev. 2002, 123, 245–260. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Xu, C.-J.; Yuan, J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 1998, 94, 491–501. [Google Scholar] [CrossRef]
- Muzio, M.; Stockwell, B.R.; Stennicke, H.R.; Salvesen, G.S.; Dixit, V.M. An induced proximity model for caspase-8 activation. J. Biol. Chem. 1998, 273, 2926–2930. [Google Scholar] [CrossRef]
- Lowe, S.W.; Lin, A.W. Apoptosis in cancer. Carcinogenesis 2000, 21, 485–495. [Google Scholar] [CrossRef]
- Vazquez, A.; Bond, E.E.; Levine, A.J.; Bond, G.L. The genetics of the p53 pathway, apoptosis and cancer therapy. Nat. Rev. Drug Discov. 2008, 7, 979–987. [Google Scholar] [CrossRef]
- Fulda, S.; Meyer, E.; Debatin, K.-M. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene 2002, 21, 2283–2294. [Google Scholar] [CrossRef]
- Soni, S.; Anang, V.; Zhao, Y.; Horowitz, J.C.; Nho, R.S.; Mebratu, Y.A. A new era in cancer therapy: Targeting the Proteasome-Bcl-2 axis. J. Exp. Clin. Cancer Res. 2025, 44, 246. [Google Scholar] [CrossRef]
- Lopez, A.; Reyna, D.E.; Gitego, N.; Kopp, F.; Zhou, H.; Miranda-Roman, M.A.; Nordstrøm, L.U.; Narayanagari, S.-R.; Chi, P.; Vilar, E. Co-targeting of BAX and BCL-XL proteins broadly overcomes resistance to apoptosis in cancer. Nat. Commun. 2022, 13, 1199. [Google Scholar] [CrossRef]
- Higami, Y.; Shimokawa, I. Apoptosis in the aging process. Cell Tissue Res. 2000, 301, 125–132. [Google Scholar] [CrossRef]
- Joaquin, A.M.; Gollapudi, S. Functional decline in aging and disease: A role for apoptosis. J. Am. Geriatr. Soc. 2001, 49, 1234–1240. [Google Scholar] [CrossRef]
- Cai, Y.; Xiong, M.; Xin, Z.; Liu, C.; Ren, J.; Yang, X.; Lei, J.; Li, W.; Liu, F.; Chu, Q.; et al. Decoding aging-dependent regenerative decline across tissues at single-cell resolution. Cell Stem Cell 2023, 30, 1674–1691.e1678. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, X.; Dou, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Signal Transduct. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.i.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, T.; Ulfhake, B. Sarcopenia: What Is the Origin of This Aging-Induced Disorder? Front. Genet. 2021, 12, 688526. [Google Scholar] [CrossRef]
- Wang, N.; Zheng, A.; Yan, Y.; He, T.; Wu, X.; Xian, M.; Luo, J.; Li, C.; Wei, J.; Wang, Y.; et al. Aging induces sarcopenia by disrupting the crosstalk between the skeletal muscle microenvironment and myofibers. J. Adv. Res. 2025. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, I.; Shin, H.R.; Rhee, J.; Seo, J.Y.; Jo, Y.W.; Yoo, K.; Hann, S.H.; Kang, J.S.; Park, J. The hypothalamic–pituitary–gonadal axis controls muscle stem cell senescence through autophagosome clearance. J. Cachexia Sarcopenia Muscle 2021, 12, 177–191. [Google Scholar] [CrossRef]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Nixon, R.A.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Primers 2021, 7, 33. [Google Scholar] [CrossRef]
- Nisbet, R.M.; Götz, J. Amyloid-β and Tau in Alzheimer’s disease: Novel pathomechanisms and non-pharmacological treatment strategies. J. Alzheimer’s Dis. 2018, 64, S517–S527. [Google Scholar] [CrossRef]
- Tong, B.C.-K.; Wu, A.J.; Li, M.; Cheung, K.-H. Calcium signaling in Alzheimer’s disease & therapies. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2018, 1865, 1745–1760. [Google Scholar]
- Ashrafian, H.; Zadeh, E.H.; Khan, R.H. Review on Alzheimer’s disease: Inhibition of amyloid beta and tau tangle formation. Int. J. Biol. Macromol. 2021, 167, 382–394. [Google Scholar] [CrossRef]
- Pucci, B.; Kasten, M.; Giordano, A. Cell cycle and apoptosis. Neoplasia 2000, 2, 291–299. [Google Scholar] [CrossRef]
- Gao, X.; Lin, S.H.; Ren, F.; Li, J.T.; Chen, J.J.; Yao, C.B.; Yang, H.B.; Jiang, S.X.; Yan, G.Q.; Wang, D.; et al. Acetate functions as an epigenetic metabolite to promote lipid synthesis under hypoxia. Nat. Commun. 2016, 7, 11960. [Google Scholar] [CrossRef]
- Lakshminarasimhan, R.; Liang, G. The Role of DNA Methylation in Cancer. Adv. Exp. Med. Biol. 2016, 945, 151–172. [Google Scholar] [CrossRef]
- Espinoza Pereira, K.N.; Shan, J.; Licht, J.D.; Bennett, R.L. Histone mutations in cancer. Biochem. Soc. Trans. 2023, 51, 1749–1763. [Google Scholar] [CrossRef]
- Lau, M.T.; Klausen, C.; Leung, P.C. E-cadherin inhibits tumor cell growth by suppressing PI3K/Akt signaling via β-catenin-Egr1-mediated PTEN expression. Oncogene 2011, 30, 2753–2766. [Google Scholar] [CrossRef]
- Chawla, S.P.; Cranmer, L.D.; Van Tine, B.A.; Reed, D.R.; Okuno, S.H.; Butrynski, J.E.; Adkins, D.R.; Hendifar, A.E.; Kroll, S.; Ganjoo, K.N. Phase II study of the safety and antitumor activity of the hypoxia-activated prodrug TH-302 in combination with doxorubicin in patients with advanced soft tissue sarcoma. J. Clin. Oncol. 2014, 32, 3299–3306. [Google Scholar] [CrossRef]
- Hoefflin, R.; Harlander, S.; Schäfer, S.; Metzger, P.; Kuo, F.; Schönenberger, D.; Adlesic, M.; Peighambari, A.; Seidel, P.; Chen, C.Y.; et al. HIF-1α and HIF-2α differently regulate tumour development and inflammation of clear cell renal cell carcinoma in mice. Nat. Commun. 2020, 11, 4111. [Google Scholar] [CrossRef]
- Chen, Z.; Han, F.; Du, Y.; Shi, H.; Zhou, W. Hypoxic microenvironment in cancer: Molecular mechanisms and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 70. [Google Scholar] [CrossRef]
- Vijg, J.; Dong, X. Pathogenic mechanisms of somatic mutation and genome mosaicism in aging. Cell 2020, 182, 12–23. [Google Scholar] [CrossRef]
- Yoshioka, K.-I.; Kusumoto-Matsuo, R.; Matsuno, Y.; Ishiai, M. Genomic instability and cancer risk associated with erroneous DNA repair. Int. J. Mol. Sci. 2021, 22, 12254. [Google Scholar] [CrossRef]
- Stead, E.R.; Bjedov, I. Balancing DNA repair to prevent ageing and cancer. Exp. Cell Res. 2021, 405, 112679. [Google Scholar] [CrossRef]
- Ataabadi, E.A.; Golshiri, K.; van der Linden, J.; de Boer, M.; Duncker, D.J.; Jüttner, A.; de Vries, R.; Van Veghel, R.; van der Pluijm, I.; Dutheil, S.; et al. Vascular Ageing Features Caused by Selective DNA Damage in Smooth Muscle Cell. Oxid. Med. Cell Longev. 2021, 2021, 2308317. [Google Scholar] [CrossRef]
- Golshiri, K.; Ataabadi, E.A.; Rubio-Beltran, E.; Dutheil, S.; Yao, W.; Snyder, G.L.; Davis, R.E.; van der Pluijm, I.; Brandt, R.; Van den Berg-Garrelds, I.M.; et al. Selective Phosphodiesterase 1 Inhibition Ameliorates Vascular Function, Reduces Inflammatory Response, and Lowers Blood Pressure in Aging Animals. J. Pharmacol. Exp. Ther. 2021, 378, 173–183. [Google Scholar] [CrossRef]
- Meng, F.; Liu, B.; Zhou, Z. Progeria and Genome Instability. In Aging Mechanisms: Longevity, Metabolism, and Brain Aging; Mori, N., Mook-Jung, I., Eds.; Springer: Tokyo, Japan, 2015; pp. 51–63. [Google Scholar]
- Shalabi, S.F.; Miyano, M.; Sayaman, R.W.; Lopez, J.C.; Jokela, T.A.; Todhunter, M.E.; Hinz, S.; Garbe, J.C.; Stampfer, M.R.; Kessenbrock, K. Evidence for accelerated aging in mammary epithelia of women carrying germline BRCA1 or BRCA2 mutations. Nat. Aging 2021, 1, 838–849. [Google Scholar] [CrossRef]
- Menolfi, D.; Zha, S. ATM, ATR and DNA-PKcs kinases—The lessons from the mouse models: Inhibition ≠ deletion. Cell Biosci. 2020, 10, 8. [Google Scholar] [CrossRef]
- Sanchez-Contreras, M.; Kennedy, S.R. The Complicated Nature of Somatic mtDNA Mutations in Aging. Front. Aging 2022, 2, 805126. [Google Scholar] [CrossRef]
- Mito, T.; Kikkawa, Y.; Shimizu, A.; Hashizume, O.; Katada, S.; Imanishi, H.; Ota, A.; Kato, Y.; Nakada, K.; Hayashi, J.-I. Mitochondrial DNA mutations in mutator mice confer respiration defects and B-cell lymphoma development. PLoS ONE 2013, 8, e55789. [Google Scholar] [CrossRef]
- Lujan, S.A.; Longley, M.J.; Humble, M.H.; Lavender, C.A.; Burkholder, A.; Blakely, E.L.; Alston, C.L.; Gorman, G.S.; Turnbull, D.M.; McFarland, R. Ultrasensitive deletion detection links mitochondrial DNA replication, disease, and aging. Genome Biol. 2020, 21, 248. [Google Scholar] [CrossRef]
- Macken, W.L.; Vandrovcova, J.; Hanna, M.G.; Pitceathly, R.D. Applying genomic and transcriptomic advances to mitochondrial medicine. Nat. Rev. Neurol. 2021, 17, 215–230. [Google Scholar] [CrossRef]
- Yi, F.; Yuan, J.; Somekh, J.; Peleg, M.; Zhu, Y.C.; Jia, Z.; Wu, F.; Huang, Z. Genetically supported targets and drug repurposing for brain aging: A systematic study in the UK Biobank. Sci. Adv. 2025, 11, eadr3757. [Google Scholar] [CrossRef]
- Fraga, M.F.; Agrelo, R.; Esteller, M. Cross-talk between aging and cancer: The epigenetic language. Ann. N. Y. Acad. Sci. 2007, 1100, 60–74. [Google Scholar] [CrossRef]
- Seale, K.; Horvath, S.; Teschendorff, A.; Eynon, N.; Voisin, S. Making sense of the ageing methylome. Nat. Rev. Genet. 2022, 23, 585–605. [Google Scholar] [CrossRef]
- Agarwal, N.; Jha, A.K. DNA hypermethylation of tumor suppressor genes among oral squamous cell carcinoma patients: A prominent diagnostic biomarker. Mol. Biol. Rep. 2025, 52, 44. [Google Scholar] [CrossRef]
- Zabransky, D.J.; Jaffee, E.M.; Weeraratna, A.T. Shared genetic and epigenetic changes link aging and cancer. Trends Cell Biol. 2022, 32, 338–350. [Google Scholar] [CrossRef]
- Chen, B.-F.; Chan, W.-Y. The de novo DNA methyltransferase DNMT3A in development and cancer. Epigenetics 2014, 9, 669–677. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, S.; Zeng, M.; Liu, M.; Zhao, C.; Yi, B.; Hu, S.; Yu, B.; Jia, H. Impact of Carrying DNMT3A or TET2 Mutations on Plaque Characteristics and Prognosis in Patients With STEMI Based on OCT. Circ. Cardiovasc. Imaging 2025, 18, e017915. [Google Scholar] [CrossRef]
- Vargas-Alarcón, G.; Avilés-Jiménez, F.; Mejía-Sánchez, F.; Pérez-Hernández, N.; Cardoso-Saldaña, G.; Posadas-Sánchez, R. Helicobacter pylori infection and DNMT3a polymorphism are associated with the presence of premature coronary artery disease and subclinical atherosclerosis. Data from the GEA Mexican Study. Microb. Pathog. 2022, 170, 105719. [Google Scholar] [CrossRef]
- Uddin, M.G.; Fandy, T.E. DNA methylation inhibitors: Retrospective and perspective view. Adv. Cancer Res. 2021, 152, 205–223. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Li, Y.; Lei, D.; Xiang, J.; Ouyang, L.; Wang, Y.; Yang, J. Recent progress in DNA methyltransferase inhibitors as anticancer agents. Front. Pharmacol. 2022, 13, 1072651. [Google Scholar] [CrossRef]
- Laranjeira, A.B.A.; Hollingshead, M.G.; Nguyen, D.; Kinders, R.J.; Doroshow, J.H.; Yang, S.X. DNA damage, demethylation and anticancer activity of DNA methyltransferase (DNMT) inhibitors. Sci. Rep. 2023, 13, 5964. [Google Scholar] [CrossRef]
- Papadatos-Pastos, D.; Yuan, W.; Pal, A.; Crespo, M.; Ferreira, A.; Gurel, B.; Prout, T.; Ameratunga, M.; Chénard-Poirier, M.; Curcean, A.; et al. Phase 1, dose-escalation study of guadecitabine (SGI-110) in combination with pembrolizumab in patients with solid tumors. J. Immunother. Cancer 2022, 10, e004495. [Google Scholar] [CrossRef]
- Amaro, A.; Reggiani, F.; Fenoglio, D.; Gangemi, R.; Tosi, A.; Parodi, A.; Banelli, B.; Rigo, V.; Mastracci, L.; Grillo, F.; et al. Guadecitabine increases response to combined anti-CTLA-4 and anti-PD-1 treatment in mouse melanoma in vivo by controlling T-cells, myeloid derived suppressor and NK cells. J. Exp. Clin. Cancer Res. 2023, 42, 67. [Google Scholar] [CrossRef]
- Jang, H.J.; Hostetter, G.; Macfarlane, A.W.; Madaj, Z.; Ross, E.A.; Hinoue, T.; Kulchycki, J.R.; Burgos, R.S.; Tafseer, M.; Alpaugh, R.K.; et al. A Phase II Trial of Guadecitabine plus Atezolizumab in Metastatic Urothelial Carcinoma Progressing after Initial Immune Checkpoint Inhibitor Therapy. Clin. Cancer Res. 2023, 29, 2052–2065. [Google Scholar] [CrossRef]
- Tayari, M.M.; Santos, H.G.D.; Kwon, D.; Bradley, T.J.; Thomassen, A.; Chen, C.; Dinh, Y.; Perez, A.; Zelent, A.; Morey, L. Clinical responsiveness to all-trans retinoic acid is potentiated by LSD1 inhibition and associated with a quiescent transcriptome in myeloid malignancies. Clin. Cancer Res. 2021, 27, 1893–1903. [Google Scholar] [CrossRef]
- Huang, M.; Chen, C.; Geng, J.; Han, D.; Wang, T.; Xie, T.; Wang, L.; Wang, Y.; Wang, C.; Lei, Z. Targeting KDM1A attenuates Wnt/β-catenin signaling pathway to eliminate sorafenib-resistant stem-like cells in hepatocellular carcinoma. Cancer Lett. 2017, 398, 12–21. [Google Scholar] [CrossRef]
- Dratwa, M.; Wysoczańska, B.; Łacina, P.; Kubik, T.; Bogunia-Kubik, K. TERT—Regulation and Roles in Cancer Formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef]
- Lee, D.D.; Leão, R.; Komosa, M.; Gallo, M.; Zhang, C.H.; Lipman, T.; Remke, M.; Heidari, A.; Nunes, N.M.; Apolónio, J.D.; et al. DNA hypermethylation within TERT promoter upregulates TERT expression in cancer. J. Clin. Investig. 2018, 129, 223–229. [Google Scholar] [CrossRef]
- Dogan, F.; Forsyth, N.R. Telomerase Regulation: A Role for Epigenetics. Cancers 2021, 13, 1213. [Google Scholar] [CrossRef]
- Oh, E.S.; Petronis, A. Origins of human disease: The chrono-epigenetic perspective. Nat. Rev. Genet. 2021, 22, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Al Ojaimi, M.; Banimortada, B.J.; Othman, A.; Riedhammer, K.M.; Almannai, M.; El-Hattab, A.W. Disorders of histone methylation: Molecular basis and clinical syndromes. Clin. Genet. 2022, 102, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Luo, M.; Jeong, M.; Rodriguez, B.; Xia, Z.; Hannah, R.; Wang, H.; Le, T.; Faull, K.F.; Chen, R.; et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell 2014, 14, 673–688. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.J.; Yu, D.Y.; Gao, F.F.; Zhou, D.Y.; Wu, Y.N.; Yang, X.X.; Chen, J.; Yang, J.S.; Shen, M.Q.; Zhang, Y.X. The Histone Lysine Demethylase KDM7A Contributes to Reward Memory via Fscn1-Induced Synaptic Plasticity in the Medial Prefrontal Cortex. Adv. Sci. 2025, 12, 2405352. [Google Scholar] [CrossRef]
- Xiao, L.; Qiao, J.; Huang, Y.; Tan, B.; Hong, L.; Li, Z.; Cai, G.; Wu, Z.; Zheng, E.; Wang, S. RASGRP1 targeted by H3K27me3 regulates myoblast proliferation and differentiation in mice and pigs: RASGRP1 regulates myoblast proliferation and differentiation. Acta Biochim. Biophys. Sin. 2024, 56, 452. [Google Scholar]
- Pashos, A.R.S.; Meyer, A.R.; Bussey-Sutton, C.; O’Connor, E.S.; Coradin, M.; Coulombe, M.; Riemondy, K.A.; Potlapelly, S.; Strahl, B.D.; Hansson, G.C.; et al. H3K36 methylation regulates cell plasticity and regeneration in the intestinal epithelium. Nat. Cell Biol. 2025, 27, 202–217. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, W.; Qu, J.; Liu, G.-H. Emerging epigenetic insights into aging mechanisms and interventions. Trends Pharmacol. Sci. 2024, 45, 157–172. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, X.; Xia, H.; Xu, C. Downregulation of P300/CBP-associated factor protects from vascular aging via Nrf2 signal pathway activation. Int. J. Mol. Sci. 2022, 23, 12574. [Google Scholar] [CrossRef]
- Xu, D.C.; Sas-Nowosielska, H.; Donahue, G.; Huang, H.; Pourshafie, N.; Good, C.R.; Berger, S.L. Histone acetylation in an Alzheimer’s disease cell model promotes homeostatic amyloid-reducing pathways. Acta Neuropathol. Commun. 2024, 12, 3. [Google Scholar] [CrossRef]
- Maity, S.; Farrell, K.; Navabpour, S.; Narayanan, S.N.; Jarome, T.J. Epigenetic mechanisms in memory and cognitive decline associated with aging and Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 12280. [Google Scholar] [CrossRef] [PubMed]
- Georgoulis, A.; Vorgias, C.E.; Chrousos, G.P.; Rogakou, E.P. Genome instability and γH2AX. Int. J. Mol. Sci. 2017, 18, 1979. [Google Scholar] [CrossRef] [PubMed]
- Oizumi, T.; Suzuki, T.; Kobayashi, J.; Nakamura, A.J. Senescence-associated heterochromatin foci suppress γ-H2AX focus formation induced by radiation exposure. Int. J. Mol. Sci. 2024, 25, 3355. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, A.; Innes, A.J.; Roux, P.-F.; Buisman, S.C.; Jung, J.; Ortet, L.; Moiseeva, V.; Wagner, V.; Robinson, L.; Ausema, A. 3-Deazaadenosine alleviates senescence to promote cellular fitness and cell therapy efficiency in mice. Nat. Aging 2022, 2, 851–866. [Google Scholar] [CrossRef]
- Kim, H.-J.; Cho, J.H.; Quan, H.; Kim, J.-R. Down-regulation of Aurora B kinase induces cellular senescence in human fibroblasts and endothelial cells through a p53-dependent pathway. FEBS Lett. 2011, 585, 3569–3576. [Google Scholar] [CrossRef][Green Version]
- Hao, F.; Zhang, Y.; Hou, J.; Zhao, B. Chromatin remodeling and cancer: The critical influence of the SWI/SNF complex. Epigenetics Chromatin 2025, 18, 22. [Google Scholar] [CrossRef]
- Wanior, M.; Kramer, A.; Knapp, S.; Joerger, A.C. Exploiting vulnerabilities of SWI/SNF chromatin remodelling complexes for cancer therapy. Oncogene 2021, 40, 3637–3654. [Google Scholar] [CrossRef]
- Ding, Y.; Li, N.; Dong, B.; Guo, W.; Wei, H.; Chen, Q.; Yuan, H.; Han, Y.; Chang, H.; Kan, S.; et al. Chromatin remodeling ATPase BRG1 and PTEN are synthetic lethal in prostate cancer. J. Clin. Investig. 2019, 129, 759–773. [Google Scholar] [CrossRef]
- Muthuswami, R.; Bailey, L.; Rakesh, R.; Imbalzano, A.N.; Nickerson, J.A.; Hockensmith, J.W. BRG1 is a prognostic indicator and a potential therapeutic target for prostate cancer. J. Cell Physiol. 2019, 234, 15194–15205. [Google Scholar] [CrossRef]
- Jusic, A.; Thomas, P.B.; Wettinger, S.B.; Dogan, S.; Farrugia, R.; Gaetano, C.; Tuna, B.G.; Pinet, F.; Robinson, E.L.; Tual-Chalot, S. Noncoding RNAs in age-related cardiovascular diseases. Ageing Res. Rev. 2022, 77, 101610. [Google Scholar] [CrossRef]
- Slack, F.J.; Chinnaiyan, A.M. The role of non-coding RNAs in oncology. Cell 2019, 179, 1033–1055. [Google Scholar] [CrossRef]
- Yi, X.; Wang, Y.; Xu, S. MiR-455-3p downregulation facilitates cell proliferation and invasion and predicts poor prognosis of osteosarcoma. J. Orthop. Surg. Res. 2020, 15, 454. [Google Scholar] [CrossRef]
- Ma, F.; Huang, J.; Li, W.; Li, P.; Liu, M.; Xue, H. MicroRNA-455-3p functions as a tumor suppressor by targeting HDAC2 to regulate cell cycle in hepatocellular carcinoma. Environ. Toxicol. 2022, 37, 1675–1685. [Google Scholar] [CrossRef] [PubMed]
- Hessels, D.; Gunnewiek, J.M.T.K.; van Oort, I.; Karthaus, H.F.M.; van Leenders, G.J.L.; van Balken, B.; Kiemeney, L.A.; Witjes, J.A.; Schalken, J.A. DD3PCA3-based Molecular Urine Analysis for the Diagnosis of Prostate Cancer. Eur. Urol. 2003, 44, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Tang, Q.; Chen, J.; Zhu, L. Epigenetic Clocks: Beyond Biological Age, Using the Past to Predict the Present and Future. Aging Dis. 2024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Vallerga, C.L.; Walker, R.M.; Lin, T.; Henders, A.K.; Montgomery, G.W.; He, J.; Fan, D.; Fowdar, J.; Kennedy, M.; et al. Improved precision of epigenetic clock estimates across tissues and its implication for biological ageing. Genome Med. 2019, 11, 54. [Google Scholar] [CrossRef]
- You, Y.; Chen, Y.; Ding, H.; Liu, Q.; Wang, R.; Xu, K.; Wang, Q.; Gasevic, D.; Ma, X. Relationship between physical activity and DNA methylation-predicted epigenetic clocks. NPJ Aging 2025, 11, 27. [Google Scholar] [CrossRef]
- Izadi, M.; Sadri, N.; Abdi, A.; Serajian, S.; Jalalei, D.; Tahmasebi, S. Epigenetic biomarkers in aging and longevity: Current and future application. Life Sci. 2024, 351, 122842. [Google Scholar] [CrossRef]
- Hannum, G.; Guinney, J.; Zhao, L.; Zhang, L.; Hughes, G.; Sadda, S.; Klotzle, B.; Bibikova, M.; Fan, J.B.; Gao, Y.; et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol. Cell 2013, 49, 359–367. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef]
- Weidner, C.I.; Lin, Q.; Koch, C.M.; Eisele, L.; Beier, F.; Ziegler, P.; Bauerschlag, D.O.; Jockel, K.H.; Erbel, R.; Muhleisen, T.W.; et al. Aging of blood can be tracked by DNA methylation changes at just three CpG sites. Genome Biol. 2014, 15, R24. [Google Scholar] [CrossRef]
- Bao, C.; Zhu, W.; Bao, T.; Hou, Y.; Wu, T.; Huang, J.; He, C. The application of epigenetic clocks in degenerative musculoskeletal diseases: A systematic review. Osteoarthr. Cartil. 2025, 33, 1052–1065. [Google Scholar] [CrossRef]
- Levine, M.E.; Lu, A.T.; Quach, A.; Chen, B.H.; Assimes, T.L.; Bandinelli, S.; Hou, L.; Baccarelli, A.A.; Stewart, J.D.; Li, Y.; et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging 2018, 10, 573–591. [Google Scholar] [CrossRef]
- McCrory, C.; Fiorito, G.; Hernandez, B.; Polidoro, S.; O’Halloran, A.M.; Hever, A.; Ni Cheallaigh, C.; Lu, A.T.; Horvath, S.; Vineis, P.; et al. GrimAge Outperforms Other Epigenetic Clocks in the Prediction of Age-Related Clinical Phenotypes and All-Cause Mortality. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Protsenko, E.; Yang, R.; Nier, B.; Reus, V.; Hammamieh, R.; Rampersaud, R.; Wu, G.W.Y.; Hough, C.M.; Epel, E.; Prather, A.A.; et al. “GrimAge,” an epigenetic predictor of mortality, is accelerated in major depressive disorder. Transl. Psychiatry 2021, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Horvath, S.; Raj, K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet. 2018, 19, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.T.; Quach, A.; Wilson, J.G.; Reiner, A.P.; Aviv, A.; Raj, K.; Hou, L.; Baccarelli, A.A.; Li, Y.; Stewart, J.D.; et al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging 2019, 11, 303–327. [Google Scholar] [CrossRef]
- Belsky, D.W.; Caspi, A.; Corcoran, D.L.; Sugden, K.; Poulton, R.; Arseneault, L.; Baccarelli, A.; Chamarti, K.; Gao, X.; Hannon, E.; et al. DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife 2022, 11, e73420. [Google Scholar] [CrossRef]
- Ying, K.; Liu, H.; Tarkhov, A.E.; Sadler, M.C.; Lu, A.T.; Moqri, M.; Horvath, S.; Kutalik, Z.; Shen, X.; Gladyshev, V.N. Causality-enriched epigenetic age uncouples damage and adaptation. Nat. Aging 2024, 4, 231–246. [Google Scholar] [CrossRef]
- Tomusiak, A.; Floro, A.; Tiwari, R.; Riley, R.; Matsui, H.; Andrews, N.; Kasler, H.G.; Verdin, E. Development of an epigenetic clock resistant to changes in immune cell composition. Commun. Biol. 2024, 7, 934. [Google Scholar] [CrossRef]
- Fuentealba, M.; Rouch, L.; Guyonnet, S.; Lemaitre, J.-M.; de Souto Barreto, P.; Vellas, B.; Andrieu, S.; Furman, D. A blood-based epigenetic clock for intrinsic capacity predicts mortality and is associated with clinical, immunological and lifestyle factors. Nat. Aging 2025, 5, 1207–1216. [Google Scholar] [CrossRef] [PubMed]
- Jacques, M.; Seale, K.; Voisin, S.; Lysenko, A.; Grolaux, R.; Jones-Freeman, B.; Lamon, S.; Levinger, I.; Bauer, C.; Sharples, A.P.; et al. DNA Methylation Ageing Atlas Across 17 Human Tissues. bioRxiv 2025. [Google Scholar] [CrossRef]
- Simms, C. How ageing changes our genes—Huge epigenetic atlas gives clearest picture yet. Nature 2025, 645, 292–293. [Google Scholar] [CrossRef] [PubMed]
- Walker, K.A.; Basisty, N.; Wilson, D.M., 3rd; Ferrucci, L. Connecting aging biology and inflammation in the omics era. J. Clin. Investig. 2022, 132, e158448. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Tsukamoto, H.; Senju, S.; Matsumura, K.; Swain, S.L.; Nishimura, Y. IL-6-mediated environmental conditioning of defective Th1 differentiation dampens antitumour immune responses in old age. Nat. Commun. 2015, 6, 6702. [Google Scholar] [CrossRef]
- Haura, E.B.; Turkson, J.; Jove, R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat. Clin. Pract. Oncol. 2005, 2, 315–324. [Google Scholar] [CrossRef]
- Mei, Y.; Zhao, B.; Basiorka, A.; Yang, J.; Cao, L.; Zhang, J.; List, A.; Ji, P. Age-related inflammatory bone marrow microenvironment induces ineffective erythropoiesis mimicking del (5q) MDS. Leukemia 2018, 32, 1023–1033. [Google Scholar] [CrossRef]
- Liposits, G.; Skuladottir, H.; Ryg, J.; Winther, S.B.; Möller, S.; Hofsli, E.; Shah, C.-H.; Poulsen, L.Ø.; Berglund, Å.; Qvortrup, C. The prognostic value of pre-treatment circulating biomarkers of systemic inflammation (CRP, dNLR, YKL-40, and IL-6) in vulnerable older patients with metastatic colorectal cancer receiving palliative chemotherapy—The Randomized NORDIC9-Study. J. Clin. Med. 2022, 11, 5603. [Google Scholar] [CrossRef]
- Yu, J.E.; Yeo, I.J.; Han, S.-B.; Yun, J.; Kim, B.; Yong, Y.J.; Lim, Y.-S.; Kim, T.H.; Son, D.J.; Hong, J.T. Significance of chitinase-3-like protein 1 in the pathogenesis of inflammatory diseases and cancer. Exp. Mol. Med. 2024, 56, 1–18. [Google Scholar] [CrossRef]
- Uceda, A.B.; Mariño, L.; Casasnovas, R.; Adrover, M. An overview on glycation: Molecular mechanisms, impact on proteins, pathogenesis, and inhibition. Biophys. Rev. 2024, 16, 189–218. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthy, B.; Höök, P.; Jones, A.D.; Larsson, L. Changes in myosin structure and function in response to glycation. FASEB J. 2001, 15, 2415–2422. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Lin, B.; Ortwerth, B.J. Rate of formation of AGEs during ascorbate glycation and during aging in human lens tissue. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2002, 1587, 65–74. [Google Scholar] [CrossRef][Green Version]
- Brownlee, M. Advanced protein glycosylation in diabetes and aging. Annu. Rev. Med. 1994, 154, 2473–2479. [Google Scholar] [CrossRef]
- Bergman, M.; Abdul-Ghani, M.; Neves, J.S.; Monteiro, M.P.; Medina, J.L.; Dorcely, B.; Buysschaert, M. Pitfalls of HbA1c in the Diagnosis of Diabetes. J. Clin. Endocrinol. Metab. 2020, 105, 2803–2811. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Castiglione, S.; Macrì, F.; Giuliani, A.; Ramini, D.; Vinci, M.C.; Tortato, E.; Bonfigli, A.R.; Olivieri, F.; Raucci, A. Circulating levels of AGEs and soluble RAGE isoforms are associated with all-cause mortality and development of cardiovascular complications in type 2 diabetes: A retrospective cohort study. Cardiovasc. Diabetol. 2022, 21, 95. [Google Scholar] [CrossRef]
- De Leon-Oliva, D.; González-Prieto, P.; De Castro-Martinez, P.; Boaru, D.L.; Laguna-Hernández, P.; Fraile-Martinez, O.; García-Montero, C.; Guijarro, L.G.; López-González, L.; Díaz-Pedrero, R.; et al. Revisiting the biological role of the Warburg effect: Evolving perspectives on cancer metabolism. Pathol. Res. Pract. 2025, 273, 156151. [Google Scholar] [CrossRef]
- Walker-Samuel, S.; Ramasawmy, R.; Torrealdea, F.; Rega, M.; Rajkumar, V.; Johnson, S.P.; Richardson, S.; Gonçalves, M.; Parkes, H.G.; Arstad, E.; et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat. Med. 2013, 19, 1067–1072. [Google Scholar] [CrossRef]
- Patra, K.C.; Wang, Q.; Bhaskar, P.T.; Miller, L.; Wang, Z.; Wheaton, W.; Chandel, N.; Laakso, M.; Muller, W.J.; Allen, E.L.; et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 2013, 24, 213–228. [Google Scholar] [CrossRef]
- Zheng, M.; Wu, C.; Yang, K.; Yang, Y.; Liu, Y.; Gao, S.; Wang, Q.; Li, C.; Chen, L.; Li, H. Novel selective hexokinase 2 inhibitor Benitrobenrazide blocks cancer cells growth by targeting glycolysis. Pharmacol. Res. 2021, 164, 105367. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, Z.; Chen, Y.; Tian, H.; Chai, P.; Shen, Y.; Yao, Y.; Xu, S.; Ge, S.; Jia, R. Lactate and lactylation in cancer. Signal Transduct. Target. Ther. 2025, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Baltazar, F.; Afonso, J.; Costa, M.; Granja, S. Lactate Beyond a Waste Metabolite: Metabolic Affairs and Signaling in Malignancy. Front. Oncol. 2020, 10, 231. [Google Scholar] [CrossRef] [PubMed]
- de la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [CrossRef]
- Hayes, C.; Donohoe, C.L.; Davern, M.; Donlon, N.E. The oncogenic and clinical implications of lactate induced immunosuppression in the tumour microenvironment. Cancer Lett. 2021, 500, 75–86. [Google Scholar] [CrossRef]
- Xie, H.; Hanai, J.; Ren, J.G.; Kats, L.; Burgess, K.; Bhargava, P.; Signoretti, S.; Billiard, J.; Duffy, K.J.; Grant, A.; et al. Targeting lactate dehydrogenase—A inhibits tumorigenesis and tumor progression in mouse models of lung cancer and impacts tumor-initiating cells. Cell Metab. 2014, 19, 795–809. [Google Scholar] [CrossRef]
- Sun, N.; Kabir, M.; Lee, Y.; Xie, L.; Hu, X.; Velez, J.; Chen, X.; Kaniskan, H.Ü.; Jin, J. Discovery of the First Lactate Dehydrogenase Proteolysis Targeting Chimera Degrader for the Treatment of Pancreatic Cancer. J. Med. Chem. 2023, 66, 596–610. [Google Scholar] [CrossRef]
- Timmerman, K.L.; Volpi, E. Amino acid metabolism and regulatory effects in aging. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 45–49. [Google Scholar] [CrossRef]
- Chen, J.; Cui, L.; Lu, S.; Xu, S. Amino acid metabolism in tumor biology and therapy. Cell Death Dis. 2024, 15, 42. [Google Scholar] [CrossRef]
- Sekhar, R.V. GlyNAC Supplementation Improves Glutathione Deficiency, Oxidative Stress, Mitochondrial Dysfunction, Inflammation, Aging Hallmarks, Metabolic Defects, Muscle Strength, Cognitive Decline, and Body Composition: Implications for Healthy Aging. J. Nutr. 2021, 151, 3606–3616. [Google Scholar] [CrossRef]
- Yang, L.; Chu, Z.; Liu, M.; Zou, Q.; Li, J.; Liu, Q.; Wang, Y.; Wang, T.; Xiang, J.; Wang, B. Amino acid metabolism in immune cells: Essential regulators of the effector functions, and promising opportunities to enhance cancer immunotherapy. J. Hematol. Oncol. 2023, 16, 59. [Google Scholar] [CrossRef]
- Song, M.; Sandoval, T.A.; Chae, C.S.; Chopra, S.; Tan, C.; Rutkowski, M.R.; Raundhal, M.; Chaurio, R.A.; Payne, K.K.; Konrad, C.; et al. IRE1α-XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature 2018, 562, 423–428. [Google Scholar] [CrossRef]
- Yu, W.; Yang, X.; Zhang, Q.; Sun, L.; Yuan, S.; Xin, Y. Targeting GLS1 to cancer therapy through glutamine metabolism. Clin. Transl. Oncol. 2021, 23, 2253–2268. [Google Scholar] [CrossRef]
- Seo, S.-K.; Kwon, B. Immune regulation through tryptophan metabolism. Exp. Mol. Med. 2023, 55, 1371–1379. [Google Scholar] [CrossRef]
- Du, L.; Xing, Z.; Tao, B.; Li, T.; Yang, D.; Li, W.; Zheng, Y.; Kuang, C.; Yang, Q. Both IDO1 and TDO contribute to the malignancy of gliomas via the Kyn–AhR–AQP4 signaling pathway. Signal Transduct. Target. Ther. 2020, 5, 10. [Google Scholar] [CrossRef]
- Kim, C.; Kim, J.H.; Kim, J.S.; Chon, H.J.; Kim, J.-H. A novel dual inhibitor of IDO and TDO, CMG017, potently suppresses the kynurenine pathway and overcomes resistance to immune checkpoint inhibitors. J. Clin. Oncol. 2019, 37, e14228. [Google Scholar] [CrossRef]
- Baserga, R.; Peruzzi, F.; Reiss, K. The IGF-1 receptor in cancer biology. Int. J. Cancer 2003, 107, 873–877. [Google Scholar] [CrossRef] [PubMed]
- Thissen, J.-P.; Ketelslegers, J.-M.; Underwood, L.E. Nutritional regulation of the insulin-like growth factors. Endocr. Rev. 1994, 15, 80–101. [Google Scholar] [PubMed]
- Gardner, E.J.; Kentistou, K.A.; Stankovic, S.; Lockhart, S.; Wheeler, E.; Day, F.R.; Kerrison, N.D.; Wareham, N.J.; Langenberg, C.; O’Rahilly, S. Damaging missense variants in IGF1R implicate a role for IGF-1 resistance in the etiology of type 2 diabetes. Cell Genom. 2022, 2, 100208. [Google Scholar] [CrossRef]
- Kasprzak, A. Insulin-like growth factor 1 (IGF-1) signaling in glucose metabolism in colorectal cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef]
- Mao, K.; Quipildor, G.F.; Tabrizian, T.; Novaj, A.; Guan, F.; Walters, R.O.; Delahaye, F.; Hubbard, G.B.; Ikeno, Y.; Ejima, K. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nat. Commun. 2018, 9, 2394. [Google Scholar] [CrossRef]
- Quipildor, G.E.F.; Mao, K.; Hu, Z.; Novaj, A.; Cui, M.-H.; Gulinello, M.; Branch, C.A.; Gubbi, S.; Patel, K.; Moellering, D.R. Central IGF-1 protects against features of cognitive and sensorimotor decline with aging in male mice. Geroscience 2019, 41, 185–208. [Google Scholar] [CrossRef]
- Christopoulos, P.F.; Msaouel, P.; Koutsilieris, M. The role of the insulin-like growth factor-1 system in breast cancer. Mol. Cancer 2015, 14, 43. [Google Scholar] [CrossRef]
- Soni, U.K.; Jenny, L.; Hegde, R.S. IGF-1R targeting in cancer—Does sub-cellular localization matter? J. Exp. Clin. Cancer Res. 2023, 42, 273. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.N.; Burhans, M.S.; Clark, J.P.; Howell, P.R.; Polewski, M.A.; DeMuth, T.M.; Eliceiri, K.W.; Lindstrom, M.J.; Ntambi, J.M.; Anderson, R.M. Aging and caloric restriction impact adipose tissue, adiponectin, and circulating lipids. Aging Cell 2017, 16, 497–507. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, M.; Takami, Y.; Akasaka, H.; Kabayama, M.; Maeda, S.; Yokoyama, S.; Fujimoto, T.; Nozato, Y.; Imaizumi, Y.; Takeda, M. High plasma adiponectin levels are associated with frailty in a general old-old population: The septuagenarians, octogenarians, nonagenarians investigation with centenarians study. Geriatr. Gerontol. Int. 2018, 18, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Choubey, M.; Ranjan, A.; Bora, P.S.; Baltazar, F.; Martin, L.J.; Krishna, A. Role of adiponectin as a modulator of testicular function during aging in mice. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2019, 1865, 413–427. [Google Scholar] [CrossRef]
- Jin, H.-R.; Wang, J.; Wang, Z.-J.; Xi, M.-J.; Xia, B.-H.; Deng, K.; Yang, J.-L. Lipid metabolic reprogramming in tumor microenvironment: From mechanisms to therapeutics. J. Hematol. Oncol. 2023, 16, 103. [Google Scholar] [CrossRef]
- Broadfield, L.A.; Pane, A.A.; Talebi, A.; Swinnen, J.V.; Fendt, S.-M. Lipid metabolism in cancer: New perspectives and emerging mechanisms. Dev. Cell 2021, 56, 1363–1393. [Google Scholar] [CrossRef]
- Liu, H.; Wu, X.; Dong, Z.; Luo, Z.; Zhao, Z.; Xu, Y.; Zhang, J.-T. Fatty acid synthase causes drug resistance by inhibiting TNF-α and ceramide production[S]. J. Lipid Res. 2013, 54, 776–785. [Google Scholar] [CrossRef]
- Sharma, U.; Jagannathan, N.R. In vivo MR spectroscopy for breast cancer diagnosis. BJR Open 2019, 1, 20180040. [Google Scholar] [CrossRef]
- Dalamaga, M.; Diakopoulos, K.N.; Mantzoros, C.S. The role of adiponectin in cancer: A review of current evidence. Endocr. Rev. 2012, 33, 547–594. [Google Scholar] [CrossRef]
- Mutoh, M.; Teraoka, N.; Takasu, S.; Takahashi, M.; Onuma, K.; Yamamoto, M.; Kubota, N.; Iseki, T.; Kadowaki, T.; Sugimura, T. Loss of adiponectin promotes intestinal carcinogenesis in Min and wild-type mice. Gastroenterology 2011, 140, 2000–2008.e2002. [Google Scholar] [CrossRef]
- David, M.K.; Leslie, S.W. Prostate-Specific Antigen. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557495/ (accessed on 14 September 2025).
- Ahamed, Y.; Hossain, M.; Baral, S.; Al-Raiyan, A.U.; Ashraf, S.B.; Sun, W. The research progress on diagnostic indicators related to prostate-specific antigen gray-zone prostate cancer. BMC Cancer 2025, 25, 1264. [Google Scholar] [CrossRef]
- Lepor, A.; Catalona, W.J.; Loeb, S. The Prostate Health Index: Its Utility in Prostate Cancer Detection. Urol. Clin. N. Am. 2016, 43, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.J.; Bates, K.M.; Rimm, D.L. HER2 testing: Evolution and update for a companion diagnostic assay. Nat. Rev. Clin. Oncol. 2025, 22, 408–423. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Breast Cancer HER2 Status. Available online: https://www.cancer.org/cancer/types/breast-cancer/understanding-a-breast-cancer-diagnosis/breast-cancer-her2-status.html#:~:text=If%20the%20IHC%20is%201,the%20cancer%20is%20HER2%2Dpositive (accessed on 12 September 2025).
- Fejzic, H.; Mujagic, S.; Azabagic, S.; Burina, M. Tumor marker CA 15-3 in breast cancer patients. Acta Med. Acad. 2015, 44, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Keshaviah, A.; Dellapasqua, S.; Rotmensz, N.; Lindtner, J.; Crivellari, D.; Collins, J.; Colleoni, M.; Thürlimann, B.; Mendiola, C.; Aebi, S.; et al. CA15-3 and alkaline phosphatase as predictors for breast cancer recurrence: A combined analysis of seven International Breast Cancer Study Group trials. Ann. Oncol. 2007, 18, 701–708. [Google Scholar] [CrossRef]
- Gandhi, T.; Zubair, M.; Bhatt, H. Cancer Antigen 125. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/nBK562245/ (accessed on 14 September 2025).
- Li, Y.; Wang, Z.C.; Luo, L.; Mu, C.Y.; Xu, J.; Feng, Q.; Li, S.B.; Gu, B.; Ma, P.; Lan, T. The clinical value of the combined detection of sEGFR, CA125 and HE4 for epithelial ovarian cancer diagnosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 604–610. [Google Scholar] [CrossRef]
- Han, C.; Bellone, S.; Siegel, E.R.; Altwerger, G.; Menderes, G.; Bonazzoli, E.; Egawa-Takata, T.; Pettinella, F.; Bianchi, A.; Riccio, F.; et al. A novel multiple biomarker panel for the early detection of high-grade serous ovarian carcinoma. Gynecol. Oncol. 2018, 149, 585–591. [Google Scholar] [CrossRef]
- Englisz, A.; Smycz-Kubanska, M.; Mielczarek-Palacz, A. Sensitivity and Specificity of Selected Biomarkers and Their Combinations in the Diagnosis of Ovarian Cancer. Diagnostics 2024, 14, 949. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-T.; Zhang, C.; Wu, J.; Lu, P.; Xu, L.; Yuan, H.; Feng, Y.; Chen, Z.-S.; Wang, N. Biomarkers for diagnosis and therapeutic options in hepatocellular carcinoma. Mol. Cancer 2024, 23, 189. [Google Scholar] [CrossRef] [PubMed]
- Gramkow, M.H.; Mosgaard, C.S.; Schou, J.V.; Nordvig, E.H.; Dolin, T.G.; Lykke, J.; Nielsen, D.L.; Pfeiffer, P.; Qvortrup, C.; Yilmaz, M.K.; et al. The prognostic role of circulating CA19-9 and CEA in patients with colorectal cancer. Cancer Treat. Res. Commun. 2025, 43, 100907. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, P.; Wang, F.; Li, D.; Xie, E.; Zhang, Y.; Pan, S. Relationship between serum CA19-9 and CEA levels and prognosis of pancreatic cancer. Ann. Transl. Med. 2015, 3, 328. [Google Scholar] [CrossRef]
- Muley, T.; Herth, F.J.; Heussel, C.P.; Kriegsmann, M.; Thomas, M.; Meister, M.; Schneider, M.A.; Wehnl, B.; Mang, A.; Holdenrieder, S. Prognostic value of tumor markers ProGRP, NSE and CYFRA 21-1 in patients with small cell lung cancer and chemotherapy-induced remission. Tumour Biol. 2024, 46, S219–S232. [Google Scholar] [CrossRef]
- Rosu, M.C.; Ardelean, A.; Moldovan, S.D.; Faur, F.I.; Nesiu, A.; Totoloci, B.D. The importance of CA 72-4 and CA 19-9 dosing in gastric cancer. J. Med. Life 2023, 16, 186–188. [Google Scholar] [CrossRef]
- Sharmin, S.; Jamiruddin, M.; Jamiruddin, M.R.; Islam, A.; Ahsan, C.R.; Yasmin, M. Detection of Protein Markers From Blood Samples of Cervical Cancer Patients. Cureus 2024, 16, e72365. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hsu, J.-M.; Sun, L.; Wang, S.-C.; Hung, M.-C. Advances and prospects of biomarkers for immune checkpoint inhibitors. Cell Rep. Med. 2024, 5, 101621. [Google Scholar] [CrossRef]
- Argentieri, M.A.; Xiao, S.; Bennett, D.; Winchester, L.; Nevado-Holgado, A.J.; Ghose, U.; Albukhari, A.; Yao, P.; Mazidi, M.; Lv, J.; et al. Proteomic aging clock predicts mortality and risk of common age-related diseases in diverse populations. Nat. Med. 2024, 30, 2450–2460. [Google Scholar] [CrossRef]
- Grande, G.; Valletta, M.; Rizzuto, D.; Xia, X.; Qiu, C.; Orsini, N.; Dale, M.; Andersson, S.; Fredolini, C.; Winblad, B.; et al. Blood-based biomarkers of Alzheimer’s disease and incident dementia in the community. Nat. Med. 2025, 31, 2027–2035. [Google Scholar] [CrossRef]
- Ho, J.E.; Lyass, A.; Courchesne, P.; Chen, G.; Liu, C.; Yin, X.; Hwang, S.J.; Massaro, J.M.; Larson, M.G.; Levy, D. Protein Biomarkers of Cardiovascular Disease and Mortality in the Community. J. Am. Heart Assoc. 2018, 7, e008108. [Google Scholar] [CrossRef]
- Hicks, S.A.; Strumke, I.; Thambawita, V.; Hammou, M.; Riegler, M.A.; Halvorsen, P.; Parasa, S. On evaluation metrics for medical applications of artificial intelligence. Sci. Rep. 2022, 12, 5979. [Google Scholar] [CrossRef]
- Shreffler, J.; Huecker, M.R. Diagnostic Testing Accuracy: Sensitivity, Specificity, Predictive Values and Likelihood Ratios. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557491/ (accessed on 14 September 2025).
- Ahmad, I.; Alqurashi, F. Early cancer detection using deep learning and medical imaging: A survey. Crit. Rev. Oncol. Hematol. 2024, 204, 104528. [Google Scholar] [CrossRef]
- Nahm, F.S. Receiver operating characteristic curve: Overview and practical use for clinicians. Korean J. Anesth. Anesthesiol. 2022, 75, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Guo, H.; Zhao, Y.; Liu, Z.; Wang, C.; Bu, J.; Sun, T.; Wei, J. Liquid biopsy in cancer current: Status, challenges and future prospects. Signal Transduct. Target. Ther. 2024, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.N.; Nisar, S.; Masoodi, T.; Singh, M.; Rizwan, A.; Hashem, S.; El-Rifai, W.; Bedognetti, D.; Batra, S.K.; Haris, M.; et al. Liquid biopsy: A step closer to transform diagnosis, prognosis and future of cancer treatments. Mol. Cancer 2022, 21, 79. [Google Scholar] [CrossRef]
- Heidrich, I.; Ackar, L.; Mohammadi, P.M.; Pantel, K. Liquid biopsies: Potential and challenges. Int. J. Cancer 2021, 148, 528–545. [Google Scholar] [CrossRef]
- Vandekerckhove, O.; Cuppens, K.; Pat, K.; Du Pont, B.; Froyen, G.; Maes, B. Liquid Biopsy in Early-Stage Lung Cancer: Current and Future Clinical Applications. Cancers 2023, 15, 2702. [Google Scholar] [CrossRef]
- Shbeer, A.M.; Robadi, I.A. liquid biopsy holds a promising approach for the early detection of cancer: Current information and future perspectives. Pathol. Res. Pract. 2024, 254, 155082. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Lin, H.; Zhu, Y.; Huang, D.; Lai, M.; Xi, X.; Huang, J.; Zhang, W.; Zhong, T. Research progress of CTC, ctDNA, and EVs in cancer liquid biopsy. Front. Oncol. 2024, 14, 1303335. [Google Scholar] [CrossRef]
- Bonanno, L.; Dal Maso, A.; Pavan, A.; Zulato, E.; Calvetti, L.; Pasello, G.; Guarneri, V.; Conte, P.; Indraccolo, S. Liquid biopsy and non-small cell lung cancer: Are we looking at the tip of the iceberg? Br. J. Cancer 2022, 127, 383–393. [Google Scholar] [CrossRef]
- Connal, S.; Cameron, J.M.; Sala, A.; Brennan, P.M.; Palmer, D.S.; Palmer, J.D.; Perlow, H.; Baker, M.J. Liquid biopsies: The future of cancer early detection. J. Transl. Med. 2023, 21, 118. [Google Scholar] [CrossRef]
- Hegade, K.P.N.; Bhat, R.B.; Packirisamy, M. Microfluidic Liquid Biopsy Minimally Invasive Cancer Diagnosis by Nano-Plasmonic Label-Free Detection of Extracellular Vesicles: Review. Int. J. Mol. Sci. 2025, 26, 6352. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Guo, Q.R.; Wang, F.H.; Adhikari, R.; Zhu, Z.Y.; Zhang, H.Y.; Zhou, W.M.; Yu, H.; Li, J.Q.; Zhang, J.Y. Cell-Free DNA: Hope and Potential Application in Cancer. Front. Cell Dev. Biol. 2021, 9, 639233. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Dai, F.; Mei, L.; Huang, D.; Shen, X.; Zhang, H.; She, X.; Ma, Z. The Potential Use of Dynamics Changes of ctDNA and cfDNA in the Perioperative Period to Predict the Recurrence Risk in Early NSCLC. Front. Oncol. 2021, 11, 671963. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Garrastacho, M.; Bajo-Santos, C.; Line, A.; Martens-Uzunova, E.S.; de la Fuente, J.M.; Moros, M.; Soekmadji, C.; Tasken, K.A.; Llorente, A. Extracellular vesicles as a source of prostate cancer biomarkers in liquid biopsies: A decade of research. Br. J. Cancer 2022, 126, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, Y. Circulating tumor DNA methylation detection as biomarker and its application in tumor liquid biopsy: Advances and challenges. Medcomm (2020) 2024, 5, e766. [Google Scholar] [CrossRef]
- Chung, D.C.; Gray, D.M.; Singh, H.; Issaka, R.B.; Raymond, V.M.; Eagle, C.; Hu, S.; Chudova, D.I.; Talasaz, A.; Greenson, J.K.; et al. A Cell-free DNA Blood-Based Test for Colorectal Cancer Screening. N. Engl. J. Med. 2024, 390, 973–983. [Google Scholar] [CrossRef]
- Rasmussen, S.L.; Krarup, H.B.; Sunesen, K.G.; Johansen, M.B.; Stender, M.T.; Pedersen, I.S.; Madsen, P.H.; Thorlacius-Ussing, O. Hypermethylated DNA, a circulating biomarker for colorectal cancer detection. PLoS ONE 2017, 12, e0180809. [Google Scholar] [CrossRef]
- Nassar, F.J.; Msheik, Z.S.; Nasr, R.R.; Temraz, S.N. Methylated circulating tumor DNA as a biomarker for colorectal cancer diagnosis, prognosis, and prediction. Clin. Epigenet 2021, 13, 111. [Google Scholar] [CrossRef]
- Laven-Law, G.; Kichenadasse, G.; Young, G.P.; Symonds, E.L.; Winter, J.M. BCAT1, IKZF1 and SEPT9: Methylated DNA biomarkers for detection of pan-gastrointestinal adenocarcinomas. Biomarkers 2024, 29, 194–204. [Google Scholar] [CrossRef]
- Yang, H.; Li, W.; Ren, L.; Yang, Y.; Zhang, Y.; Ge, B.; Li, S.; Zheng, X.; Liu, J.; Zhang, S.; et al. Progress on diagnostic and prognostic markers of pancreatic cancer. Oncol. Res. 2023, 31, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Fan, Z.; Meng, Y.; Liu, S.; Zhan, H. Blood-based DNA methylation signatures in cancer: A systematic review. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166583. [Google Scholar] [CrossRef] [PubMed]
- Del Re, M.; Vivaldi, C.; Rofi, E.; Vasile, E.; Miccoli, M.; Caparello, C.; d’Arienzo, P.D.; Fornaro, L.; Falcone, A.; Danesi, R. Early changes in plasma DNA levels of mutant KRAS as a sensitive marker of response to chemotherapy in pancreatic cancer. Sci. Rep. 2017, 7, 7931. [Google Scholar] [CrossRef] [PubMed]
- Hum, M.; Lee, A.S.G. DNA methylation in breast cancer: Early detection and biomarker discovery through current and emerging approaches. J. Transl. Med. 2025, 23, 465. [Google Scholar] [CrossRef]
- Chen, Z.; Jiang, H.; Qin, Q.; Li, Q.; Hong, L. DNA methylation signatures provide novel diagnostic biomarkers and predict responses of immune therapy for breast cancer. Front. Genet. 2024, 15, 1403907. [Google Scholar] [CrossRef]
- Diaz-Lagares, A.; Mendez-Gonzalez, J.; Hervas, D.; Saigi, M.; Pajares, M.J.; Garcia, D.; Crujerias, A.B.; Pio, R.; Montuenga, L.M.; Zulueta, J.; et al. A Novel Epigenetic Signature for Early Diagnosis in Lung Cancer. Clin. Cancer Res. 2016, 22, 3361–3371. [Google Scholar] [CrossRef]
- Palanca-Ballester, C.; Hervas, D.; Villalba, M.; Valdes-Sanchez, T.; Garcia, D.; Alcoriza-Balaguer, M.I.; Benet, M.; Martinez-Tomas, R.; Briones-Gomez, A.; Galbis-Caravajal, J.; et al. Translation of a tissue epigenetic signature to circulating free DNA suggests BCAT1 as a potential noninvasive diagnostic biomarker for lung cancer. Clin. Epigenet 2022, 14, 116. [Google Scholar] [CrossRef]
- Shi, Y.X.; Wang, Y.; Li, X.; Zhang, W.; Zhou, H.H.; Yin, J.Y.; Liu, Z.Q. Genome-wide DNA methylation profiling reveals novel epigenetic signatures in squamous cell lung cancer. BMC Genom. 2017, 18, 901. [Google Scholar] [CrossRef]
- Hu, S.; Tao, J.; Peng, M.; Ye, Z.; Chen, Z.; Chen, H.; Yu, H.; Wang, B.; Fan, J.B.; Ni, B. Accurate detection of early-stage lung cancer using a panel of circulating cell-free DNA methylation biomarkers. Biomark. Res. 2023, 11, 45. [Google Scholar] [CrossRef]
- Kontic, M.; Markovic, F. Use of DNA methylation patterns for early detection and management of lung cancer: Are we there yet? Oncol. Res. 2025, 33, 781–793. [Google Scholar] [CrossRef] [PubMed]
- van der Pol, Y.; Moldovan, N.; Ramaker, J.; Bootsma, S.; Lenos, K.J.; Vermeulen, L.; Sandhu, S.; Bahce, I.; Pegtel, D.M.; Wong, S.Q.; et al. The landscape of cell-free mitochondrial DNA in liquid biopsy for cancer detection. Genome Biol. 2023, 24, 229. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Ju, Y.S.; Kim, Y.; Li, J.; Wang, Y.; Yoon, C.J.; Yang, Y.; Martincorena, I.; Creighton, C.J.; Weinstein, J.N.; et al. Comprehensive molecular characterization of mitochondrial genomes in human cancers. Nat. Genet. 2020, 52, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Wang, S.; Feng, Z.; Zhou, K.; Zhang, H.; Guo, X.; Xing, J.; Liu, Y. Circulating cell-free mtDNA as a new biomarker for cancer detection and management. Cancer Biol. Med. 2023, 21, 105–110. [Google Scholar] [CrossRef]
- Lawrence, R.; Watters, M.; Davies, C.R.; Pantel, K.; Lu, Y.-J. Circulating tumour cells for early detection of clinically relevant cancer. Nat. Rev. Clin. Oncol. 2023, 20, 487–500. [Google Scholar] [CrossRef]
- Krol, I.; Schwab, F.D.; Carbone, R.; Ritter, M.; Picocci, S.; De Marni, M.L.; Stepien, G.; Franchi, G.M.; Zanardi, A.; Rissoglio, M.D.; et al. Detection of clustered circulating tumour cells in early breast cancer. Br. J. Cancer 2021, 125, 23–27. [Google Scholar] [CrossRef]
- Trapp, E.; Janni, W.; Schindlbeck, C.; Juckstock, J.; Andergassen, U.; de Gregorio, A.; Alunni-Fabbroni, M.; Tzschaschel, M.; Polasik, A.; Koch, J.G.; et al. Presence of Circulating Tumor Cells in High-Risk Early Breast Cancer During Follow-Up and Prognosis. J. Natl. Cancer Inst. 2019, 111, 380–387. [Google Scholar] [CrossRef]
- Hayes, D.F. Defining Clinical Utility of Tumor Biomarker Tests: A Clinician’s Viewpoint. J. Clin. Oncol. 2021, 39, 238–248. [Google Scholar] [CrossRef]
- Thomas-Bonafos, T.; Pierga, J.Y.; Bidard, F.-C.; Cabel, L.; Kiavue, N. Circulating tumor cells in breast cancer: Clinical validity and utility. NPJ Breast Cancer 2024, 10, 103. [Google Scholar] [CrossRef]
- Takahashi, Y.; Ijiri, Y.; Fujino, S.; Elnaz, N.; Kishimoto, A.; Shirai, K.; Iwanaga, S.; Yanagida, M.; Bhagat, A.A.S.; Miyoshi, N. Detection and Characterization of Circulating Tumor Cells in Colorectal Cancer Patients via Epithelial-Mesenchymal Transition Markers. Cancers 2025, 17, 303. [Google Scholar] [CrossRef]
- Abouleila, Y.; Onidani, K.; Ali, A.; Shoji, H.; Kawai, T.; Lim, C.T.; Kumar, V.; Okaya, S.; Kato, K.; Hiyama, E.; et al. Live single cell mass spectrometry reveals cancer-specific metabolic profiles of circulating tumor cells. Cancer Sci. 2019, 110, 697–706. [Google Scholar] [CrossRef]
- Shou, X.; Li, Y.; Hu, W.; Ye, T.; Wang, G.; Xu, F.; Sui, M.; Xu, Y. Six-gene Assay as a new biomarker in the blood of patients with colorectal cancer: Establishment and clinical validation. Mol. Oncol. 2019, 13, 781–791. [Google Scholar] [CrossRef]
- Purcell, E.; Niu, Z.; Owen, S.; Grzesik, M.; Radomski, A.; Kaehr, A.; Onukwugha, N.-E.; Winkler, H.F.; Ramnath, N.; Lawrence, T.; et al. Circulating tumor cells reveal early predictors of disease progression in patients with stage III NSCLC undergoing chemoradiation and immunotherapy. Cell Rep. 2024, 43, 113687. [Google Scholar] [CrossRef]
- Murakami, M. Extracellular vesicles as a hydrolytic platform of secreted phospholipase A(2). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2024, 1869, 159536. [Google Scholar] [CrossRef] [PubMed]
- Semeradtova, A.; Liegertova, M.; Herma, R.; Capkova, M.; Brignole, C.; Del Zotto, G. Extracellular vesicles in cancer’s communication: Messages we can read and how to answer. Mol. Cancer 2025, 24, 86. [Google Scholar] [CrossRef] [PubMed]
- Jia, E.; Ren, N.; Shi, X.; Zhang, R.; Yu, H.; Yu, F.; Qin, S.; Xue, J. Extracellular vesicle biomarkers for pancreatic cancer diagnosis: A systematic review and meta-analysis. BMC Cancer 2022, 22, 573. [Google Scholar] [CrossRef] [PubMed]
- Allenson, K.; Castillo, J.; San Lucas, F.A.; Scelo, G.; Kim, D.U.; Bernard, V.; Davis, G.; Kumar, T.; Katz, M.; Overman, M.J.; et al. High prevalence of mutant KRAS in circulating exosome-derived DNA from early-stage pancreatic cancer patients. Ann. Oncol. 2017, 28, 741–747. [Google Scholar] [CrossRef]
- Melo, S.A.; Luecke, L.B.; Kahlert, C.; Fernandez, A.F.; Gammon, S.T.; Kaye, J.; LeBleu, V.S.; Mittendorf, E.A.; Weitz, J.; Rahbari, N.; et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 2015, 523, 177–182. [Google Scholar] [CrossRef]
- Li, J.; Chen, Y.; Guo, X.; Zhou, L.; Jia, Z.; Peng, Z.; Tang, Y.; Liu, W.; Zhu, B.; Wang, L.; et al. GPC1 exosome and its regulatory miRNAs are specific markers for the detection and target therapy of colorectal cancer. J. Cell Mol. Med. 2017, 21, 838–847. [Google Scholar] [CrossRef]
- Koo, B.; Kim, Y.I.; Lee, M.; Lim, S.B.; Shin, Y. Enhanced Early Detection of Colorectal Cancer via Blood Biomarker Combinations Identified Through Extracellular Vesicle Isolation and Artificial Intelligence Analysis. J. Extracell. Vesicles 2025, 14, e70088. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.W.; Sorokin, I.; Aleksic, I.; Fisher, H.; Kaufman, R.P.; Winer, A.; McNeill, B.; Gupta, R.; Tilki, D.; Fleshner, N.; et al. Expression of Small Noncoding RNAs in Urinary Exosomes Classifies Prostate Cancer into Indolent and Aggressive Disease. J. Urol. 2020, 204, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, H.; Choi, Y.J.; Im, K.; Lee, C.W.; Kim, D.-S.; Pack, C.-G.; Kim, H.-Y.; Choi, C.-M.; Lee, J.C.; et al. Identification of exosomal microRNA panel as diagnostic and prognostic biomarker for small cell lung cancer. Biomark. Res. 2023, 11, 80. [Google Scholar] [CrossRef]
- Bafiti, V.; Thanou, E.; Ouzounis, S.; Kotsakis, A.; Georgoulias, V.; Lianidou, E.; Katsila, T.; Markou, A. Profiling Plasma Extracellular Vesicle Metabotypes and miRNAs: An Unobserved Clue for Predicting Relapse in Patients with Early-Stage NSCLC. Cancers 2024, 16, 3729. [Google Scholar] [CrossRef] [PubMed]
- Castellanos-Rizaldos, E.; Grimm, D.G.; Tadigotla, V.; Hurley, J.; Healy, J.; Neal, P.L.; Sher, M.; Venkatesan, R.; Karlovich, C.; Raponi, M.; et al. Exosome-Based Detection of EGFR T790M in Plasma from Non-Small Cell Lung Cancer Patients. Clin. Cancer Res. 2018, 24, 2944–2950. [Google Scholar] [CrossRef]
- Kim, Y.; Shin, S.; Lee, K.A. Exosome-based detection of EGFR T790M in plasma and pleural fluid of prospectively enrolled non-small cell lung cancer patients after first-line tyrosine kinase inhibitor therapy. Cancer Cell Int. 2021, 21, 50. [Google Scholar] [CrossRef]
- Kim, D.H.; Kim, H.; Choi, Y.J.; Kim, S.Y.; Lee, J.-E.; Sung, K.J.; Sung, Y.H.; Pack, C.-G.; Jung, M.-K.; Han, B.; et al. Exosomal PD-L1 promotes tumor growth through immune escape in non-small cell lung cancer. Exp. Mol. Med. 2019, 51, 1–13. [Google Scholar] [CrossRef]
- Tarin, M.; Oryani, M.A.; Javid, H.; Karimi-Shahri, M. Exosomal PD-L1 in non-small cell lung Cancer: Implications for immune evasion and resistance to immunotherapy. Int. Immunopharmacol. 2025, 155, 114519. [Google Scholar] [CrossRef]
- Shimada, Y.; Matsubayashi, J.; Kudo, Y.; Maehara, S.; Takeuchi, S.; Hagiwara, M.; Kakihana, M.; Ohira, T.; Nagao, T.; Ikeda, N. Serum-derived exosomal PD-L1 expression to predict anti-PD-1 response and in patients with non-small cell lung cancer. Sci. Rep. 2021, 11, 7830. [Google Scholar] [CrossRef]
- Chen, I.H.; Xue, L.; Hsu, C.C.; Paez, J.S.; Pan, L.; Andaluz, H.; Wendt, M.K.; Iliuk, A.B.; Zhu, J.K.; Tao, W.A. Phosphoproteins in extracellular vesicles as candidate markers for breast cancer. Proc. Natl. Acad. Sci. USA 2017, 114, 3175–3180. [Google Scholar] [CrossRef]
- Zhang, P.; Zhou, X.; Zeng, Y. Multiplexed immunophenotyping of circulating exosomes on nano-engineered ExoProfile chip towards early diagnosis of cancer. Chem. Sci. 2019, 10, 5495–5504. [Google Scholar] [CrossRef]
- Lai, H.; Guo, Y.; Tian, L.; Wu, L.; Li, X.; Yang, Z.; Chen, S.; Ren, Y.; He, S.; He, W.; et al. Protein Panel of Serum-Derived Small Extracellular Vesicles for the Screening and Diagnosis of Epithelial Ovarian Cancer. Cancers 2022, 14, 3719. [Google Scholar] [CrossRef]
- Gasparri, R.; Papale, M.; Sabalic, A.; Catalano, V.; Deleonardis, A.; De Luca, F.; Ranieri, E.; Spaggiari, L. Circulating RKIP and pRKIP in Early-Stage Lung Cancer: Results from a Pilot Study. J. Clin. Med. 2024, 13, 5830. [Google Scholar] [CrossRef]
- Si, Y.Q.; Wang, X.Q.; Fan, G.; Wang, C.Y.; Zheng, Y.W.; Song, X.; Pan, C.C.; Chu, F.L.; Liu, Z.F.; Lu, B.R.; et al. Value of AFP and PIVKA-II in diagnosis of HBV-related hepatocellular carcinoma and prediction of vascular invasion and tumor differentiation. Infect. Agent. Cancer 2020, 15, 70. [Google Scholar] [CrossRef]
- Rui, H.; Yueqin, N.; Wei, W.; Bangtao, L.; Li, X. Combining AFP, PIVKA-II, and GP73 has diagnostic utility for hepatitis B-associated hepatocellular carcinoma and is consistent with liver pathology results. Sci. Rep. 2025, 15, 14869. [Google Scholar] [CrossRef]
- Veyssiere, H.; Bidet, Y.; Penault-Llorca, F.; Radosevic-Robin, N.; Durando, X. Circulating proteins as predictive and prognostic biomarkers in breast cancer. Clin. Proteom. 2022, 19, 25. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J.; Wo, D.; Yan, H.; Liu, P.; Ma, E.; Li, L.; Zheng, L.; Chen, D.; Yu, Z.; et al. LRP6 Ectodomain Prevents SDF-1/CXCR4-Induced Breast Cancer Metastasis to Lung. Clin. Cancer Res. 2019, 25, 4832–4845. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, Y.; Wang, K.; Qiao, Y.; Zhao, L.; Chen, M. Apolipoprotein C1 (APOC1), A Candidate Diagnostic Serum Biomarker for Breast Cancer Identified by Serum Proteomics Study. Crit. Rev. Eukaryot. Gene Expr. 2022, 32, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.B.F.; Stodkilde, K.; Saederup, K.L.; Kuhlee, A.; Raunser, S.; Graversen, J.H.; Moestrup, S.K. Haptoglobin. Antioxid. Redox Signal 2017, 26, 814–831. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cheuk, I.W.; Siu, M.T.; Yang, W.; Cheng, A.S.; Shin, V.Y.; Kwong, A. Human haptoglobin contributes to breast cancer oncogenesis through glycolytic activity modulation. Am. J. Cancer Res. 2020, 10, 2865–2877. [Google Scholar]
- Li, L.; Xu, Y.; Lai, Z.; Li, D.; Sun, Q.; Li, Z.; Zhou, Y. Development and validation of a model and nomogram for breast cancer diagnosis based on quantitative analysis of serum disease-specific haptoglobin N-glycosylation. J. Transl. Med. 2024, 22, 331. [Google Scholar] [CrossRef]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef] [PubMed]
- Luan, Y.; Zhong, G.; Li, S.; Wu, W.; Liu, X.; Zhu, D.; Feng, Y.; Zhang, Y.; Duan, C.; Mao, M. A panel of seven protein tumour markers for effective and affordable multi-cancer early detection by artificial intelligence: A large-scale and multicentre case-control study. EClinicalMedicine 2023, 61, 102041. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ba, Y.; Ma, L.; Cai, X.; Yin, Y.; Wang, K.; Guo, J.; Zhang, Y.; Chen, J.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Bautista-Sánchez, D.; Arriaga-Canon, C.; Pedroza-Torres, A.; De La Rosa-Velázquez, I.A.; González-Barrios, R.; Contreras-Espinosa, L.; Montiel-Manríquez, R.; Castro-Hernández, C.; Fragoso-Ontiveros, V.; Álvarez-Gómez, R.M.; et al. The Promising Role of miR-21 as a Cancer Biomarker and Its Importance in RNA-Based Therapeutics. Mol. Ther. Nucleic Acids 2020, 20, 409–420. [Google Scholar] [CrossRef]
- Yokoi, A.; Yoshioka, Y.; Hirakawa, A.; Yamamoto, Y.; Ishikawa, M.; Ikeda, S.I.; Kato, T.; Niimi, K.; Kajiyama, H.; Kikkawa, F.; et al. A combination of circulating miRNAs for the early detection of ovarian cancer. Oncotarget 2017, 8, 89811–89823. [Google Scholar] [CrossRef]
- Zhu, Z.; Chen, Z.; Wang, M.; Zhang, M.; Chen, Y.; Yang, X.; Zhou, C.; Liu, Y.; Hong, L.; Zhang, L. Detection of plasma exosomal miRNA-205 as a biomarker for early diagnosis and an adjuvant indicator of ovarian cancer staging. J. Ovarian Res. 2022, 15, 27. [Google Scholar] [CrossRef]
- Su, Y.Y.; Sun, L.; Guo, Z.R.; Li, J.C.; Bai, T.T.; Cai, X.X.; Li, W.H.; Zhu, Y.F. Upregulated expression of serum exosomal miR-375 and miR-1307 enhance the diagnostic power of CA125 for ovarian cancer. J. Ovarian Res. 2019, 12, 6. [Google Scholar] [CrossRef]
- Ku, J.L.; Jeon, Y.K.; Park, J.G. Methylation-specific PCR. Methods Mol. Biol. 2011, 791, 23–32. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Mikeska, T.; Krypuy, M.; Dobrovic, A. Sensitive Melting Analysis after Real Time- Methylation Specific PCR (SMART-MSP): High-throughput and probe-free quantitative DNA methylation detection. Nucleic Acids Res. 2008, 36, e42. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, H.; An, K.; Liu, Z.; Hai, L.; Li, R.; Zhou, Y.; Zhao, W.; Jia, Y.; Wu, N.; et al. Whole-genome bisulfite sequencing analysis of circulating tumour DNA for the detection and molecular classification of cancer. Clin. Transl. Med. 2022, 12, e1014. [Google Scholar] [CrossRef]
- Liu, J.; Dai, L.; Wang, Q.; Li, C.; Liu, Z.; Gong, T.; Xu, H.; Jia, Z.; Sun, W.; Wang, X.; et al. Multimodal analysis of cfDNA methylomes for early detecting esophageal squamous cell carcinoma and precancerous lesions. Nat. Commun. 2024, 15, 3700. [Google Scholar] [CrossRef]
- Lu, D.; Wu, X.; Wu, W.; Wu, S.; Li, H.; Zhang, Y.; Yan, X.; Zhai, J.; Dong, X.; Feng, S.; et al. Plasma cell-free DNA 5-hydroxymethylcytosine and whole-genome sequencing signatures for early detection of esophageal cancer. Cell Death Dis. 2023, 14, 843. [Google Scholar] [CrossRef] [PubMed]
- Maggi, E.C.; Gravina, S.; Cheng, H.; Piperdi, B.; Yuan, Z.; Dong, X.; Libutti, S.K.; Vijg, J.; Montagna, C. Development of a Method to Implement Whole-Genome Bisulfite Sequencing of cfDNA from Cancer Patients and a Mouse Tumor Model. Front. Genet. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Olmedillas-López, S.; Olivera-Salazar, R.; García-Arranz, M.; García-Olmo, D. Current and Emerging Applications of Droplet Digital PCR in Oncology: An Updated Review. Mol. Diagn. Ther. 2021, 26, 61–87. [Google Scholar] [CrossRef] [PubMed]
- Isaka, T.; Yokose, T.; Ito, H.; Nakayama, H.; Miyagi, Y.; Saito, H.; Masuda, M. Detection of EGFR mutation of pulmonary adenocarcinoma in sputum using droplet digital PCR. BMC Pulm. Med. 2021, 21, 100. [Google Scholar] [CrossRef]
- Hackner, K.; Buder, A.; Hochmair, M.J.; Strieder, M.; Grech, C.; Fabikan, H.; Burghuber, O.C.; Errhalt, P.; Filipits, M. Detection of EGFR Activating and Resistance Mutations by Droplet Digital PCR in Sputum of EGFR-Mutated NSCLC Patients. Clin. Med. Insights Oncol. 2021, 15, 1179554921993072. [Google Scholar] [CrossRef]
- Lee, S.H.; Kim, E.Y.; Kim, T.; Chang, Y.S. Compared to plasma, bronchial washing fluid shows higher diagnostic yields for detecting EGFR-TKI sensitizing mutations by ddPCR in lung cancer. Respir. Res. 2020, 21, 142. [Google Scholar] [CrossRef]
- Mandlik, J.S.; Patil, A.S.; Singh, S. Next-Generation Sequencing (NGS): Platforms and Applications. J. Pharm. Bioallied Sci. 2024, 16, S41–S45. [Google Scholar] [CrossRef]
- Cheng, C.; Fei, Z.; Xiao, P. Methods to improve the accuracy of next-generation sequencing. Front. Bioeng. Biotechnol. 2023, 11, 982111. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Li, S.; Hu, N.; He, Y.; Pong, R.; Lin, D.; Lu, L.; Law, M. Comparison of next-generation sequencing systems. J. Biomed. Biotechnol. 2012, 2012, 251364. [Google Scholar] [CrossRef]
- Goodwin, S.; McPherson, J.D.; McCombie, W.R. Coming of age: Ten years of next-generation sequencing technologies. Nat. Rev. Genet. 2016, 17, 333–351. [Google Scholar] [CrossRef] [PubMed]
- Shah, A. Chromatin immunoprecipitation sequencing (ChIP-Seq) on the SOLiD™ system. Nat. Methods 2009, 6, ii–iii. [Google Scholar] [CrossRef]
- Marco-Puche, G.; Lois, S.; Benitez, J.; Trivino, J.C. RNA-Seq Perspectives to Improve Clinical Diagnosis. Front. Genet. 2019, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.R. Next-Generation Sequencing in High-Sensitive Detection of Mutations in Tumors: Challenges, Advances, and Applications. J. Mol. Diagn. 2020, 22, 994–1007. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, K.; Jing, C.; Cao, H.; Ma, R.; Wu, J. Comparison of droplet digital PCR and direct Sanger sequencing for the detection of the BRAF(V600E) mutation in papillary thyroid carcinoma. J. Clin. Lab. Anal. 2019, 33, e22902. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.; Fu, B.; Wang, J. Evaluation of droplet digital PCR and next generation sequencing for characterizing DNA reference material for KRAS mutation detection. Sci. Rep. 2018, 8, 9650. [Google Scholar] [CrossRef]
- Qiagen. dPCR and NGS: Better Together? Available online: https://www.qiagen.com/us/knowledge-and-support/knowledge-hub/science-matters/pcr-solutions/dpcr-and-ngs (accessed on 9 October 2025).
- Crucitta, S.; Ruglioni, M.; Novi, C.; Manganiello, M.; Arici, R.; Petrini, I.; Pardini, E.; Cucchiara, F.; Marmorino, F.; Cremolini, C.; et al. Comparison of digital PCR systems for the analysis of liquid biopsy samples of patients affected by lung and colorectal cancer. Clin. Chim. Acta 2023, 541, 117239. [Google Scholar] [CrossRef]
- Mattox, A.K.; D’Souza, G.; Khan, Z.; Allen, H.; Henson, S.; Seiwert, T.Y.; Koch, W.; Pardoll, D.M.; Fakhry, C. Comparison of next generation sequencing, droplet digital PCR, and quantitative real-time PCR for the earlier detection and quantification of HPV in HPV-positive oropharyngeal cancer. Oral. Oncol. 2022, 128, 105805. [Google Scholar] [CrossRef]
- Trouchet, A.; Gines, G.; Benhaim, L.; Taly, V. Digital PCR: From early developments to its future application in clinics. Lab. Chip 2025, 25, 3921–3961. [Google Scholar] [CrossRef]
- Liao, P.; Jiang, M.; Chen, Z.; Zhang, F.; Sun, Y.; Nie, J.; Du, M.; Wang, J.; Fei, P.; Huang, Y. Three-dimensional digital PCR through light-sheet imaging of optically cleared emulsion. Proc. Natl. Acad. Sci. USA 2020, 117, 25628–25633. [Google Scholar] [CrossRef]
- Katara, A.; Chand, S.; Chaudhary, H.; Chaudhry, V.; Chandra, H.; Dubey, R.C. Evolution and applications of Next Generation Sequencing and its intricate relations with chromatographic and spectrometric techniques in modern day sciences. J. Chromatogr. Open 2024, 5, 100121. [Google Scholar] [CrossRef]
- Dobbs, F.M.; van Eijk, P.; Fellows, M.D.; Loiacono, L.; Nitsch, R.; Reed, S.H. Precision digital mapping of endogenous and induced genomic DNA breaks by INDUCE-seq. Nat. Commun. 2022, 13, 3989. [Google Scholar] [CrossRef]
- Barbany, G.; Arthur, C.; Lieden, A.; Nordenskjold, M.; Rosenquist, R.; Tesi, B.; Wallander, K.; Tham, E. Cell-free tumour DNA testing for early detection of cancer—A potential future tool. J. Intern. Med. 2019, 286, 118–136. [Google Scholar] [CrossRef]
- Szeto, S.; Kytola, S.; Erkan, E.P.; Ahtiainen, M.; Mecklin, J.P.; Kuopio, T.; Sallinen, V.; Lepisto, A.; Koskenvuo, L.; Renkonen-Sinisalo, L.; et al. Performance Comparison of Droplet Digital PCR and Next-Generation Sequencing for Circulating Tumor DNA Detection in Non-Metastatic Rectal Cancer. Cancer Med. 2025, 14, e70943. [Google Scholar] [CrossRef] [PubMed]
- Excedr. How Much Does a Next-Generation Sequencer Cost? Available online: https://www.excedr.com/blog/how-much-does-a-next-generation-sequencer-cost (accessed on 9 October 2025).
- Ip, B.B.K.; Wong, A.T.C.; Law, J.H.Y.; Au, C.H.; Ma, S.Y.; Chim, J.C.S.; Liang, R.H.S.; Leung, A.Y.H.; Wan, T.S.K.; Ma, E.S.K. Application of droplet digital PCR in minimal residual disease monitoring of rare fusion transcripts and mutations in haematological malignancies. Sci. Rep. 2024, 14, 6400. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, H. Next-generation sequencing in liquid biopsy: Cancer screening and early detection. Hum. Genom. 2019, 13, 34. [Google Scholar] [CrossRef] [PubMed]
- Byron, S.A.; Van Keuren-Jensen, K.R.; Engelthaler, D.M.; Carpten, J.D.; Craig, D.W. Translating RNA sequencing into clinical diagnostics: Opportunities and challenges. Nat. Rev. Genet. 2016, 17, 257–271. [Google Scholar] [CrossRef]
- Benayed, R.; Offin, M.; Mullaney, K.; Sukhadia, P.; Rios, K.; Desmeules, P.; Ptashkin, R.; Won, H.; Chang, J.; Halpenny, D.; et al. High Yield of RNA Sequencing for Targetable Kinase Fusions in Lung Adenocarcinomas with No Mitogenic Driver Alteration Detected by DNA Sequencing and Low Tumor Mutation Burden. Clin. Cancer Res. 2019, 25, 4712–4722. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Johann, D.J., Jr.; Butler, D.; Chen, G.; Foox, J.; Gong, B.; Jones, W.; Kreil, D.P.; Kusko, R.; et al. Augmenting precision medicine via targeted RNA-Seq detection of expressed mutations. NPJ Precis. Oncol. 2025, 9, 182. [Google Scholar] [CrossRef]
- Wong, M.; Mayoh, C.; Lau, L.M.S.; Khuong-Quang, D.A.; Pinese, M.; Kumar, A.; Barahona, P.; Wilkie, E.E.; Sullivan, P.; Bowen-James, R.; et al. Whole genome, transcriptome and methylome profiling enhances actionable target discovery in high-risk pediatric cancer. Nat. Med. 2020, 26, 1742–1753. [Google Scholar] [CrossRef]
- Tong, L.; Wu, P.Y.; Phan, J.H.; Hassazadeh, H.R.; Consortium, S.; Tong, W.; Wang, M.D. Impact of RNA-seq data analysis algorithms on gene expression estimation and downstream prediction. Sci. Rep. 2020, 10, 17925. [Google Scholar] [CrossRef]
- Clauwaert, J.; McVey, Z.; Gupta, R.; Yannuzzi, I.; Basrur, V.; Nesvizhskii, A.I.; Menschaert, G.; Prensner, J.R. Deep learning to decode sites of RNA translation in normal and cancerous tissues. Nat. Commun. 2025, 16, 1275. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhang, Y.; Fan, Y.; Liu, Y.; Yang, M. Recent advances in droplet-based microfluidics in liquid biopsy for cancer diagnosis. Droplet 2024, 3, e92. [Google Scholar] [CrossRef]
- Culbertson, C.T.; Mickleburgh, T.G.; Stewart-James, S.A.; Sellens, K.A.; Pressnall, M. Micro total analysis systems: Fundamental advances and biological applications. Anal. Chem. 2014, 86, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Prajapati, P. Navigating pharmaceuticals: Microfluidic devices in analytical and formulation sciences. Discov. Chem. 2025, 2, 49. [Google Scholar] [CrossRef]
- Qiao, Z.; Teng, X.; Liu, A.; Yang, W. Novel Isolating Approaches to Circulating Tumor Cell Enrichment Based on Microfluidics: A Review. Micromachines 2024, 15, 706. [Google Scholar] [CrossRef]
- Lee, J.; Sul, O.; Lee, S.B. Enrichment of Circulating Tumor Cells from Whole Blood Using a Microfluidic Device for Sequential Physical and Magnetophoretic Separations. Micromachines 2020, 11, 481. [Google Scholar] [CrossRef]
- Li, T.; Li, N.; Ma, Y.; Bai, Y.-J.; Xing, C.-M.; Gong, Y.-K. A blood cell repelling and tumor cell capturing surface for high-purity enrichment of circulating tumor cells. J. Mater. Chem. B 2019, 7, 6087–6098. [Google Scholar] [CrossRef]
- Mishra, A.; Huang, S.-B.; Dubash, T.; Burr, R.; Edd, J.F.; Wittner, B.S.; Cunneely, Q.E.; Putaturo, V.R.; Deshpande, A.; Antmen, E.; et al. Tumor cell-based liquid biopsy using high-throughput microfluidic enrichment of entire leukapheresis product. Nat. Commun. 2025, 16, 32. [Google Scholar] [CrossRef]
- Rejuan, R.; Aulisa, E.; Li, W.; Thompson, T.; Kumar, S.; Canic, S.; Wang, Y. Validation of a Microfluidic Device Prototype for Cancer Detection and Identification: Circulating Tumor Cells Classification Based on Cell Trajectory Analysis Leveraging Cell-Based Modeling and Machine Learning. Int. J. Numer. Method. Biomed. Eng. 2025, 41, e70037. [Google Scholar] [CrossRef]
- Kurosawa, T.; Watanabe, M. Development of on-chip fully automated immunoassay system “muTASWako i30” to measure the changes in glycosylation profiles of alpha-fetoprotein in patients with hepatocellular carcinoma. Proteomics 2016, 16, 3056–3061. [Google Scholar] [CrossRef] [PubMed]
- Lyu, N.; Hassanzadeh-Barforoushi, A.; Rey Gomez, L.M.; Zhang, W.; Wang, Y. SERS biosensors for liquid biopsy towards cancer diagnosis by detection of various circulating biomarkers: Current progress and perspectives. Nano Converg. 2024, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Xiang, Y.; Mao, L.; Yang, Y.; Wei, B.; Lu, H.; Li, X.; Zhang, Y.; Yang, F. Buoyant Metal–Organic Framework Corona-Driven Fast Isolation and Ultrasensitive Profiling of Circulating Extracellular Vesicles. ACS Nano 2024, 18, 14569–14582. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Kwon, S.; Lee, J.-J. Highly Dense and Accessible Nanogaps in Au–Ag Alloy Patterned Nanostructures for Surface-Enhanced Raman Spectroscopy Analysis. ACS Appl. Nano Mater. 2020, 3, 5920–5927. [Google Scholar] [CrossRef]
- Cheng, H.; Chen, R.; Zhan, Y.; Dong, W.; Chen, Q.; Wang, Y.; Zhou, P.; Gao, S.; Huang, W.; Li, L.; et al. Novel Ratiometric Surface-Enhanced Raman Scattering (SERS) Biosensor for Ultrasensitive Quantitative Monitoring of Human Carboxylesterase-1 in Hepatocellular Carcinoma Cells Using Ag–Au Nanoflowers as SERS Substrate. Anal. Chem. 2024, 96, 18555–18563. [Google Scholar] [CrossRef]
- Park, K.S.; Choi, A.; Kim, H.J.; Park, I.; Eom, M.-S.; Yeo, S.-G.; Son, R.G.; Park, T.-I.; Lee, G.; Soh, H.T.; et al. Ultra-sensitive label-free SERS biosensor with high-throughput screened DNA aptamer for universal detection of SARS-CoV-2 variants from clinical samples. Biosens. Bioelectron. 2023, 228, 115202. [Google Scholar] [CrossRef]
- Miao, X.; Fang, Q.; Xiao, X.; Liu, S.; Wu, R.; Yan, J.; Nie, B.; Liu, J. Integrating Cycled Enzymatic DNA Amplification and Surface-Enhanced Raman Scattering for Sensitive Detection of Circulating Tumor DNA. Front. Mol. Biosci. 2021, 8, 676065. [Google Scholar] [CrossRef]
- Zhang, X.; Gan, T.; Xu, Z.; Zhang, H.; Wang, D.; Zhao, X.; Huang, Y.; Liu, Q.; Fu, B.; Dai, Z.; et al. Immune-like sandwich multiple hotspots SERS biosensor for ultrasensitive detection of NDKA biomarker in serum. Talanta 2024, 271, 125630. [Google Scholar] [CrossRef]
- Nguyen, T.A.N.; Yu, Y.-L.; Chang, Y.C.; Wang, Y.-H.; Woon, W.-Y.; Wu, C.-T.; Lin, K.-L.; Liu, C.-Y.; Chien, F.-C.; Lai, K.-Y. Controlling the Electron Concentration for Surface-Enhanced Raman Spectroscopy. ACS Photonics 2021, 8, 2410–2416. [Google Scholar] [CrossRef]
- Nguyen, T.A.N.; Luo, C.-L.; Chien, F.-C.; Lai, K.-Y. Single-Base Detection of DNA with Simplified Steps on InGaN Quantum Wells. J. Phys. Chem. B 2025, 129, 4366–4372. [Google Scholar] [CrossRef]
- Ngo, L.; Zhang, W.; Hnit, S.S.T.; Wang, Y. Improving SERS biosensors for the analysis of ovarian cancer-derived small extracellular vesicles. Analyst 2023, 148, 3074–3086. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhang, D.; Chen, X.; Yao, C.; Li, Z. Interfacial Profiling of MicroRNAs at Patterned Nanogaps for an Integrated Microfluidic-SERS Liquid Biopsy. Anal. Chem. 2023, 95, 16049–16053. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Koster, H.J.; O’Sullivan, J.; Ono, S.G.; O’Toole, H.J.; Leiserowitz, G.S.; Heffern, M.C.; Carney, R.P. Integration of label-free surface enhanced Raman spectroscopy (SERS) of extracellular vesicles (EVs) with Raman tagged labels to enhance ovarian cancer diagnostics. Biosens. Bioelectron. 2025, 288, 117800. [Google Scholar] [CrossRef] [PubMed]
- Mostufa, S.; Akib, T.B.A.; Rana, M.M.; Islam, M.R. Highly Sensitive TiO(2)/Au/Graphene Layer-Based Surface Plasmon Resonance Biosensor for Cancer Detection. Biosensors 2022, 12, 603. [Google Scholar] [CrossRef]
- Azzouz, A.; Hejji, L.; Kim, K.H.; Kukkar, D.; Souhail, B.; Bhardwaj, N.; Brown, R.J.C.; Zhang, W. Advances in surface plasmon resonance-based biosensor technologies for cancer biomarker detection. Biosens. Bioelectron. 2022, 197, 113767. [Google Scholar] [CrossRef]
- Topor, C.V.; Puiu, M.; Bala, C. Strategies for Surface Design in Surface Plasmon Resonance (SPR) Sensing. Biosens 2023, 13, 465. [Google Scholar] [CrossRef]
- Vikas Saccomandi, P. Antimonene-Coated Uniform-Waist Tapered Fiber Optic Surface Plasmon Resonance Biosensor for the Detection of Cancerous Cells: Design and Optimization. ACS Omega 2023, 8, 4627–4638. [Google Scholar] [CrossRef]
- Zhou, Z.; Tang, Q.; Yao, B.; Ma, G. Advancing cancer diagnosis through surface plasmon resonance detection of extracellular vesicles. TrAC Trends Anal. Chem. 2025, 191, 118298. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, H.; Xia, J.; Zhu, Z.; Koh, K.; Chen, H. Controllable synthesis of multi-tip spatial gold nanostructures to facilitate SPR enhancement for exosomal PD-L1 assay. Chem. Eng. J. 2024, 481, 148137. [Google Scholar] [CrossRef]
- Takaloo, S.; Xu, A.H.; Zaidan, L.; Irannejad, M.; Yavuz, M. Towards Point-of-Care Single Biomolecule Detection Using Next Generation Portable Nanoplasmonic Biosensors: A Review. Biosensors 2024, 14, 593. [Google Scholar] [CrossRef]
- Lee, S.; Moussa, N.A.M.; Kang, S.H. Plasmonic Nanostructures for Exosome Biosensing: Enabling High-Sensitivity Diagnostics. Nanomaterials 2025, 15, 1153. [Google Scholar] [CrossRef]
- Yildizhan, Y.; Driessens, K.; Tsao, H.S.K.; Boiy, R.; Thomas, D.; Geukens, N.; Hendrix, A.; Lammertyn, J.; Spasic, D. Detection of Breast Cancer-Specific Extracellular Vesicles with Fiber-Optic SPR Biosensor. Int. J. Mol. Sci. 2023, 24, 3764. [Google Scholar] [CrossRef]
- Ghahramani, Y.; Mokhberi, M.; Mousavi, S.M.; Hashemi, S.A.; Lai, C.W. Electrochemical biosensors for determination of tumor biomarkers. In Semiconducting Polymer Materials for Biosensing Applications; Woodhead Publishing: Cambridge, UK, 2024; pp. 351–377. [Google Scholar]
- Li, M.; Jiang, F.; Xue, L.; Peng, C.; Shi, Z.; Zhang, Z.; Li, J.; Pan, Y.; Wang, X.; Feng, C.; et al. Recent Progress in Biosensors for Detection of Tumor Biomarkers. Molecules 2022, 27, 7327. [Google Scholar] [CrossRef] [PubMed]
- Hosseine, M.; Naghib, S.M.; Khodadadi, A. Label-free electrochemical biosensor based on green-synthesized reduced graphene oxide/Fe(3)O(4)/nafion/polyaniline for ultrasensitive detection of SKBR3 cell line of HER2 breast cancer biomarker. Sci. Rep. 2024, 14, 11928. [Google Scholar] [CrossRef] [PubMed]
- Eskandarinezhad, S.; Wani, I.A.; Nourollahileilan, M.; Khosla, A.; Ahmad, T. Review—Metal and Metal Oxide Nanoparticles/Nanocomposites as Electrochemical Biosensors for Cancer Detection. J. Electrochem. Soc. 2022, 169, 047504. [Google Scholar] [CrossRef]
- Kangarshahi, B.M.; Naghib, S.M.; Rabiee, N. DNA/RNA-based electrochemical nanobiosensors for early detection of cancers. Crit. Rev. Clin. Lab. Sci. 2024, 61, 473–495. [Google Scholar] [CrossRef]
- Sridharan, G.; Atchudan, R.; Magesh, V.; Arya, S.; Ganapathy, D.; Nallaswamy, D.; Sundramoorthy, A.K. Advanced electrocatalytic materials based biosensors for cancer cell detection—A review. Electroanalysis 2023, 35, e202300093. [Google Scholar] [CrossRef]
- Cao, Y.; Xia, J.; Li, L.; Zeng, Y.; Zhao, J.; Li, G. Electrochemical Biosensors for Cancer Diagnosis: Multitarget Analysis to Present Molecular Characteristics of Tumor Heterogeneity. JACS Au 2024, 4, 4655–4672. [Google Scholar] [CrossRef]
- Geeksforgeeks. Difference Between Artificial Intelligence vs Machine Learning vs Deep Learning. Available online: https://www.geeksforgeeks.org/artificial-intelligence/difference-between-artificial-intelligence-vs-machine-learning-vs-deep-learning/ (accessed on 4 September 2025).
- Malik, M.; Alqahtani, M.M.; Hadadi, I.; Kanbayti, I.; Alawaji, Z.; Aloufi, B.A. Molecular Imaging Biomarkers for Early Cancer Detection: A Systematic Review of Emerging Technologies and Clinical Applications. Diagnostics 2024, 14, 2459. [Google Scholar] [CrossRef]
- Shobayo, O.; Saatchi, R. Developments in Deep Learning Artificial Neural Network Techniques for Medical Image Analysis and Interpretation. Diagnostics 2025, 15, 1072. [Google Scholar] [CrossRef]
- AnandKumar, C. Artificial intelligence and machine learning for early cancer prediction and response. World J. Adv. Eng. Technol. Sci. 2024, 12, 35–40. [Google Scholar] [CrossRef]
- Bi, W.L.; Hosny, A.; Schabath, M.B.; Giger, M.L.; Birkbak, N.J.; Mehrtash, A.; Allison, T.; Arnaout, O.; Abbosh, C.; Dunn, I.F.; et al. Artificial intelligence in cancer imaging: Clinical challenges and applications. CA Cancer J. Clin. 2019, 69, 127–157. [Google Scholar] [CrossRef]
- Frasca, M.; La Torre, D.; Repetto, M.; De Nicolò, V.; Pravettoni, G.; Cutica, I. Artificial intelligence applications to genomic data in cancer research: A review of recent trends and emerging areas. Discov. Anal. 2024, 2, 10. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Tiwari, A.; Mishra, S.; Kuo, T.R. Current AI technologies in cancer diagnostics and treatment. Mol. Cancer 2025, 24, 159. [Google Scholar] [CrossRef]
- Yao, I.Z.; Dong, M.; Hwang, W.Y.K. Deep Learning Applications in Clinical Cancer Detection: A Review of Implementation Challenges and Solutions. Mayo Clin. Proc. Digit. Health 2025, 3, 100253. [Google Scholar] [CrossRef]
- Mienye, I.D.; Swart, T.G.; Obaido, G.; Jordan, M.; Ilono, P. Deep Convolutional Neural Networks in Medical Image Analysis: A Review. Information 2025, 16, 195. [Google Scholar] [CrossRef]
- Nayak, N.; Kumar, D.; Malhotra, A. A CNN-Based Approach for Early Detection of Breast Cancer Using Infrared Imaging. In Proceedings of the 2024 International Conference on Intelligent Systems and Advanced Applications (ICISAA), Pune, India, 25–26 October 2024; pp. 1–4. [Google Scholar]
- Ahn, J.S.; Shin, S.; Yang, S.A.; Park, E.K.; Kim, K.H.; Cho, S.I.; Ock, C.Y.; Kim, S. Artificial Intelligence in Breast Cancer Diagnosis and Personalized Medicine. J. Breast Cancer 2023, 26, 405–435. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Das, P.K.; Meher, S. High accuracy hybrid CNN classifiers for breast cancer detection using mammogram and ultrasound datasets. Biomed. Signal Process Control 2023, 80, 104292. [Google Scholar] [CrossRef]
- Wang, Y.M.; Wang, C.Y.; Liu, K.Y.; Huang, Y.H.; Chen, T.B.; Chiu, K.N.; Liang, C.Y.; Lu, N.H. CNN-Based Cross-Modality Fusion for Enhanced Breast Cancer Detection Using Mammography and Ultrasound. Tomography 2024, 10, 2038–2057. [Google Scholar] [CrossRef]
- Chang, Y.W.; Ryu, J.K.; An, J.K.; Choi, N.; Park, Y.M.; Ko, K.H.; Han, K. Artificial intelligence for breast cancer screening in mammography (AI-STREAM): Preliminary analysis of a prospective multicenter cohort study. Nat. Commun. 2025, 16, 2248. [Google Scholar] [CrossRef]
- Kwon, M.-R.; Chang, Y.; Ham, S.-Y.; Cho, Y.; Kim, E.Y.; Kang, J.; Park, E.K.; Kim, K.H.; Kim, M.; Kim, T.S.; et al. Screening mammography performance according to breast density: A comparison between radiologists versus standalone intelligence detection. Breast Cancer Res. 2024, 26, 68. [Google Scholar] [CrossRef] [PubMed]
- Caldas, F.A.A.; Caldas, H.C.; Henrique, T.; Jordão, P.H.F.; Fernandes-Ferreira, R.; Souza, D.R.S.; Bauab, S.d.P. Evaluating the performance of artificial intelligence and radiologists accuracy in breast cancer detection in screening mammography across breast densities. Eur. J. Radiol. Artif. Intell. 2025, 2, 100013. [Google Scholar] [CrossRef]
- Hammad, M.; ElAffendi, M.; El-Latif, A.A.A.; Ateya, A.A.; Ali, G.; Plawiak, P. Explainable AI for lung cancer detection via a custom CNN on CT images. Sci. Rep. 2025, 15, 12707. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, S.; Ali, S.; Alahmadi, T.J.; Assam, M.; Ansari, G.J.; Bhatti, U.A. Pretrained Model with CNN for Lung Cancer Identification and Classification. In Proceedings of the 2024 7th International Conference on Pattern Recognition and Artificial Intelligence (PRAI), Hangzhou, China, 15–17 August 2024; pp. 811–816. [Google Scholar]
- Priya, A.; Bharathi, P.S. SE-ResNeXt-50-CNN: A deep learning model for lung cancer classification. Appl. Soft Comput. 2025, 171, 112696. [Google Scholar] [CrossRef]
- Shariff, V.; Paritala, C.; Ankala, K.M. Optimizing non small cell lung cancer detection with convolutional neural networks and differential augmentation. Sci. Rep. 2025, 15, 15640. [Google Scholar] [CrossRef]
- Mekala, S.; Kumar, S.P. Enhancing pancreatic cancer detection in CT images through secretary wolf bird optimization and deep learning. Sci. Rep. 2025, 15, 19787. [Google Scholar] [CrossRef]
- Podina, N.; Gheorghe, E.C.; Constantin, A.; Cazacu, I.; Croitoru, V.; Gheorghe, C.; Balaban, D.V.; Jinga, M.; Tieranu, C.G.; Saftoiu, A. Artificial Intelligence in Pancreatic Imaging: A Systematic Review. United Eur. Gastroenterol. J. 2025, 13, 55–77. [Google Scholar] [CrossRef]
- Chaithanyadas, K.V.; King, D.R.G.R.G. Automated detection of pancreatic cancer with segmentation and classification using fusion of UNET and CNN through spider monkey optimization. Biomed. Signal Process Control 2025, 102, 107413. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Tang, Y.; Wang, C.; Landman, B.A.; Zhou, S.K. Transforming medical imaging with Transformers? A comparative review of key properties, current progresses, and future perspectives. Med. Image Anal. 2023, 85, 102762. [Google Scholar] [CrossRef]
- Xie, Y.-H.; Huang, B.-S.; Li, F. UnetTransCNN: Integrating transformers with convolutional neural networks for enhanced medical image segmentation. Front. Oncol. 2025, 15, 1467672. [Google Scholar] [CrossRef] [PubMed]
- Sokouti, M.; Sokouti, B. Cancer genetics and deep learning applications for diagnosis, prognosis, and categorization. J. Biol. Methods 2024, 11, e99010017. [Google Scholar] [CrossRef] [PubMed]
- Srikantamurthy, M.M.; Rallabandi, V.P.S.; Dudekula, D.B.; Natarajan, S.; Park, J. Classification of benign and malignant subtypes of breast cancer histopathology imaging using hybrid CNN-LSTM based transfer learning. BMC Med. Imaging 2023, 23, 19. [Google Scholar] [CrossRef] [PubMed]
- Kaddes, M.; Ayid, Y.M.; Elshewey, A.M.; Fouad, Y. Breast cancer classification based on hybrid CNN with LSTM model. Sci. Rep. 2025, 15, 4409. [Google Scholar] [CrossRef]
- Pohl, M.; Uesaka, M.; Takahashi, H.; Demachi, K.; Bhusal Chhatkuli, R. Prediction of the position of external markers using a recurrent neural network trained with unbiased online recurrent optimization for safe lung cancer radiotherapy. Comput. Methods Programs Biomed. 2022, 222, 106908. [Google Scholar] [CrossRef]
- Rasheed, J.; Diao, S.; Wan, Y.; Huang, D.; Huang, S.; Sadiq, T.; Khan, M.S.; Hussain, L.; Alkahtani, B.S.; Mazhar, T. Optimizing Bi-LSTM networks for improved lung cancer detection accuracy. PLoS ONE 2025, 20, e0316136. [Google Scholar] [CrossRef]
- Mendes, J.M.; Barbar, A.; Refaie, M. Synthetic data generation: A privacy-preserving approach to accelerate rare disease research. Front. Digit. Health 2025, 7, 1563991. [Google Scholar] [CrossRef]
- Sun, C.; Dumontier, M. Generating unseen diseases patient data using ontology enhanced generative adversarial networks. NPJ Digit. Med. 2025, 8, 4. [Google Scholar] [CrossRef]
- Zhao, Z.; Alzubaidi, L.; Zhang, J.; Duan, Y.; Gu, Y. A comparison review of transfer learning and self-supervised learning: Definitions, applications, advantages and limitations. Expert. Syst. Appl. 2024, 242, 122807. [Google Scholar] [CrossRef]
- Shao, D.; Addagudi, S.; Cowles, J.; Jain, A.; D’Souza, L.; Gore, S.; Azad, R.K. RareNet: A deep learning model for rare cancer diagnosis. Sci. Rep. 2025, 15, 22732. [Google Scholar] [CrossRef]
- Kaur, N.; Hans, R. Transfer Learning for Cancer Diagnosis in Medical Images: A Compendious Study. Int. J. Comput. Intell. Syst. 2025, 18, 62. [Google Scholar] [CrossRef]
- Gui, J.; Chen, T.; Zhang, J.; Cao, Q.; Sun, Z.; Luo, H.; Tao, D. A Survey on Self-Supervised Learning: Algorithms, Applications, and Future Trends. IEEE Trans. Pattern Anal. Mach. Intell. 2024, 46, 9052–9071. [Google Scholar] [CrossRef]
- Pai, S.; Bontempi, D.; Hadzic, I.; Prudente, V.; Sokač, M.; Chaunzwa, T.L.; Bernatz, S.; Hosny, A.; Mak, R.H.; Birkbak, N.J.; et al. Foundation model for cancer imaging biomarkers. Nat. Mach. Intell. 2024, 6, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, Z.; Alizadehsani, R.; Cifci, M.A.; Kausar, S.; Rehman, R.; Mahanta, P.; Bora, P.K.; Almasri, A.; Alkhawaldeh, R.S.; Hussain, S.; et al. A review of Explainable Artificial Intelligence in healthcare. Comput. Electr. Eng. 2024, 118, 109370. [Google Scholar] [CrossRef]
- Agrawal, R.; Agrawal, R. Explainable AI in early autism detection: A literature review of interpretable machine learning approaches. Discov. Ment. Health 2025, 5, 98. [Google Scholar] [CrossRef] [PubMed]
- Houssein, E.H.; Gamal, A.M.; Younis, E.M.G.; Mohamed, E. Explainable artificial intelligence for medical imaging systems using deep learning: A comprehensive review. Clust. Comput. 2025, 28, 469. [Google Scholar] [CrossRef]
- Dalmolin, M.; Azevedo, K.S.; Souza, L.C.d.; de Farias, C.B.; Lichtenfels, M.; Fernandes, M.A.C. Feature Selection in Cancer Classification: Utilizing Explainable Artificial Intelligence to Uncover Influential Genes in Machine Learning Models. AI 2024, 6, 2. [Google Scholar] [CrossRef]
- Perdomo-Quinteiro, P.; Belmonte-Hernández, A. Knowledge Graphs and Explainable AI for Drug Repurposing: A review of databases and methods. Brief. Bioinform. 25. [CrossRef]
- Haue, A.D.; Hjaltelin, J.X.; Holm, P.C.; Placido, D.; Brunak, S.R. Artificial intelligence-aided data mining of medical records for cancer detection and screening. Lancet Oncol. 2024, 25, e694–e703. [Google Scholar] [CrossRef]
- Lang, K.; Josefsson, V.; Larsson, A.M.; Larsson, S.; Hogberg, C.; Sartor, H.; Hofvind, S.; Andersson, I.; Rosso, A. Artificial intelligence-supported screen reading versus standard double reading in the Mammography Screening with Artificial Intelligence trial (MASAI): A clinical safety analysis of a randomised, controlled, non-inferiority, single-blinded, screening accuracy study. Lancet Oncol. 2023, 24, 936–944. [Google Scholar] [CrossRef]
- Raaj, R.S. Breast cancer detection and diagnosis using hybrid deep learning architecture. Biomed. Signal Process Control 2023, 82, 104558. [Google Scholar] [CrossRef]
- Rahman, H.; Bukht, T.F.N.; Ahmad, R.; Almadhor, A.; Javed, A.R.; Aoun, N.B. Efficient Breast Cancer Diagnosis from Complex Mammographic Images Using Deep Convolutional Neural Network. Comput. Intell. Neurosci. 2023, 2023, 7717712. [Google Scholar] [CrossRef]
- Alzahrani, R.M.; Sikkandar, M.Y.; Begum, S.S.; Babetat, A.F.S.; Alhashim, M.; Alduraywish, A.; Prakash, N.B.; Ng, E.Y.K. Early breast cancer detection via infrared thermography using a CNN enhanced with particle swarm optimization. Sci. Rep. 2025, 15, 25290. [Google Scholar] [CrossRef]
- Dewangan, K.K.; Dewangan, D.K.; Sahu, S.P.; Janghel, R. Breast cancer diagnosis in an early stage using novel deep learning with hybrid optimization technique. Multimed. Tools Appl. 2022, 81, 13935–13960. [Google Scholar] [CrossRef] [PubMed]
- Zakareya, S.; Izadkhah, H.; Karimpour, J. A New Deep-Learning-Based Model for Breast Cancer Diagnosis from Medical Images. Diagnostics 2023, 13, 1944. [Google Scholar] [CrossRef] [PubMed]
- Altan, G. Breast cancer diagnosis using deep belief networks on ROI images. Pamukkale Univ. J. Eng. Sci. 2022, 28, 286–291. [Google Scholar] [CrossRef]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019, 25, 954–961. [Google Scholar] [CrossRef]
- Lu, F.; Zhao, Y.; Wang, Z.; Feng, N. Biparametric MRI-based radiomics for prediction of clinically significant prostate cancer of PI-RADS category 3 lesions. BMC Cancer 2025, 25, 615. [Google Scholar] [CrossRef]
- Hiremath, A.; Shiradkar, R.; Fu, P.; Mahran, A.; Rastinehad, A.R.; Tewari, A.; Tirumani, S.H.; Purysko, A.; Ponsky, L.; Madabhushi, A. An integrated nomogram combining deep learning, Prostate Imaging–Reporting and Data System (PI-RADS) scoring, and clinical variables for identification of clinically significant prostate cancer on biparametric MRI: A retrospective multicentre study. Lancet Digit. Health 2021, 3, e445–e454. [Google Scholar] [CrossRef]
- Soleymanjahi, S.; Huebner, J.; Elmansy, L.; Rajashekar, N.; Ludtke, N.; Paracha, R.; Thompson, R.; Grimshaw, A.A.; Foroutan, F.; Sultan, S.; et al. Artificial Intelligence-Assisted Colonoscopy for Polyp Detection: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2024, 177, 1652–1663. [Google Scholar] [CrossRef]
- Unger, M.; Loeffler, C.M.L.; Zigutyte, L.; Sainath, S.; Lenz, T.; Vibert, J.; Mock, A.; Frohling, S.; Graham, T.A.; Carrero, Z.I.; et al. Deep Learning for Biomarker Discovery in Cancer Genomes. bioRxiv 2025. [Google Scholar] [CrossRef]
- Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Swanton, C.; Seiden, M.V.; Liu, M.C.; Oxnard, G.R.; Klein, E.A.; Smith, D.; Richards, D.; et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann. Oncol. 2020, 31, 745–759. [Google Scholar] [CrossRef]
- The American Cancer Society Medical and Editorial Content Team, Multi-Cancer Early Detection (MCED) Tests. Available online: https://www.cancer.org/cancer/screening/multi-cancer-early-detection-tests.html (accessed on 12 August 2025).
- Hajjar, M.; Albaradei, S.; Aldabbagh, G. Machine Learning Approaches in Multi-Cancer Early Detection. Information 2024, 15, 627. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lin, W.Y.; Zhou, C.; Yang, Z.A.; Kalpana, S.; Lebowitz, M.S. Integrating Artificial Intelligence for Advancing Multiple-Cancer Early Detection via Serum Biomarkers: A Narrative Review. Cancers 2024, 16, 862. [Google Scholar] [CrossRef] [PubMed]
- Sosinsky, A.; Ambrose, J.; Cross, W.; Turnbull, C.; Henderson, S.; Jones, L.; Hamblin, A.; Arumugam, P.; Chan, G.; Chubb, D.; et al. Insights for precision oncology from the integration of genomic and clinical data of 13,880 tumors from the 100,000 Genomes Cancer Programme. Nat. Med. 2024, 30, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, B.D.; Oke, J.; Virdee, P.S.; Harris, D.A.; O’Doherty, C.; Park, J.E.; Hamady, Z.; Sehgal, V.; Millar, A.; Medley, L.; et al. Multi-cancer early detection test in symptomatic patients referred for cancer investigation in England and Wales (SYMPLIFY): A large-scale, observational cohort study. Lancet Oncol. 2023, 24, 733–743. [Google Scholar] [CrossRef]
- Tie, J. Triaging suspected cancer with a multi-cancer early detection blood test. Lancet Oncol. 2023, 24, 710–711. [Google Scholar] [CrossRef]
- Schrag, D.; Beer, T.M.; McDonnell, C.H., 3rd; Nadauld, L.; Dilaveri, C.A.; Reid, R.; Marinac, C.R.; Chung, K.C.; Lopatin, M.; Fung, E.T.; et al. Blood-based tests for multicancer early detection (PATHFINDER): A prospective cohort study. Lancet 2023, 402, 1251–1260. [Google Scholar] [CrossRef]
- Nguyen, L.H.D.; Nguyen, T.H.H.; Le, V.H.; Bui, V.Q.; Nguyen, L.H.; Pham, N.H.; Phan, T.H.; Nguyen, H.T.; Tran, V.S.; Bui, C.V.; et al. Prospective validation study: A non-invasive circulating tumor DNA-based assay for simultaneous early detection of multiple cancers in asymptomatic adults. BMC Med. 2025, 23, 90. [Google Scholar] [CrossRef]
- Bao, H.; Yang, S.; Chen, X.; Dong, G.; Mao, Y.; Wu, S.; Cheng, X.; Wu, X.; Tang, W.; Wu, M.; et al. Early detection of multiple cancer types using multidimensional cell-free DNA fragmentomics. Nat. Med. 2025, 31, 2737–2745. [Google Scholar] [CrossRef]
- Batalini, F.; Madison, R.W.; Sokol, E.S.; Jin, D.X.; Chen, K.T.; Decker, B.; Pavlick, D.C.; Frampton, G.M.; Wulf, G.M.; Garber, J.E.; et al. Homologous Recombination Deficiency Landscape of Breast Cancers and Real-World Effectiveness of Poly ADP-Ribose Polymerase Inhibitors in Patients With Somatic BRCA1/2, Germline PALB2, or Homologous Recombination Deficiency Signature. JCO Precis. Oncol. 2023, 7, e2300091. [Google Scholar] [CrossRef]
- Kim, Y.N.; Gulhan, D.C.; Jin, H.; Glodzik, D.; Park, P.J. Recent Advances in Genomic Approaches for the Detection of Homologous Recombination Deficiency. Cancer Res. Treat. 2024, 56, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Perez-Villatoro, F.; Oikkonen, J.; Casado, J.; Chernenko, A.; Gulhan, D.C.; Tumiati, M.; Li, Y.; Lavikka, K.; Hietanen, S.; Hynninen, J.; et al. Optimized detection of homologous recombination deficiency improves the prediction of clinical outcomes in cancer. NPJ Precis. Oncol. 2022, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Bi, X.; Leng, Y.; Chen, D.; Wang, J.; Ma, Y.; Zhang, M.Z.; Han, B.W.; Li, Y. A deep-learning-based genomic status estimating framework for homologous recombination deficiency detection from low-pass whole genome sequencing. Heliyon 2024, 10, e26121. [Google Scholar] [CrossRef] [PubMed]
- Sathipati, S.Y.; Tsai, M.J.; Shukla, S.K.; Ho, S.Y. Artificial intelligence-driven pan-cancer analysis reveals miRNA signatures for cancer stage prediction. HGG Adv. 2023, 4, 100190. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Jin, Q.; Tayob, N.; Salem, E.; Luster, M.; Alsarraj, A.; Khaderi, S.; Singal, A.G.; Marrero, J.A.; Asrani, S.K.; et al. HES V2.0 outperforms GALAD for detection of HCC: A phase 3 biomarker study in the United States. Hepatology 2025, 81, 465–475. [Google Scholar] [CrossRef]
- Placido, D.; Yuan, B.; Hjaltelin, J.X.; Zheng, C.; Haue, A.D.; Chmura, P.J.; Yuan, C.; Kim, J.; Umeton, R.; Antell, G.; et al. A deep learning algorithm to predict risk of pancreatic cancer from disease trajectories. Nat. Med. 2023, 29, 1113–1122. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, W.; Jiang, Q.; Deng, L.; Du, L.; Mou, W.; Lai, Y.; Zhang, W.; Yang, Y.; Lim, J.; et al. Artificial intelligence-aided detection for prostate cancer with multimodal routine health check-up data: An Asian multi-center study. Int. J. Surg. 2023, 109, 3848–3860. [Google Scholar] [CrossRef]
- Boehm, K.M.; El Nahhas, O.S.M.; Marra, A.; Waters, M.; Jee, J.; Braunstein, L.; Schultz, N.; Selenica, P.; Wen, H.Y.; Weigelt, B.; et al. Multimodal histopathologic models stratify hormone receptor-positive early breast cancer. Nat. Commun. 2025, 16, 2106. [Google Scholar] [CrossRef]
- Thorn, G.J.; Gadaleta, E.; Dayem Ullah, A.Z.M.; James, L.G.E.; Abdollahyan, M.; Barrow-McGee, R.; Jones, L.J.; Chelala, C. The clinical and molecular landscape of breast cancer in women of African and South Asian ancestry. Nat. Commun. 2025, 16, 4237. [Google Scholar] [CrossRef]
- Skaf, Y.; Laubenbacher, R. Topological data analysis in biomedicine: A review. J. Biomed. Inf. Inform. 2022, 130, 104082. [Google Scholar] [CrossRef]
- Vandaele, R.; Mukherjee, P.; Selby, H.M.; Shah, R.P.; Gevaert, O. Topological data analysis of thoracic radiographic images shows improved radiomics-based lung tumor histology prediction. Patterns 2023, 4, 100657. [Google Scholar] [CrossRef]
- Belchi, F.; Pirashvili, M.; Conway, J.; Bennett, M.; Djukanovic, R.; Brodzki, J. Lung Topology Characteristics in patients with Chronic Obstructive Pulmonary Disease. Sci. Rep. 2018, 8, 5341. [Google Scholar] [CrossRef]
- Iqbal, S.; Ahmed, H.F.; Qaiser, T.; Qureshi, M.I.; Rajpoot, N. Classification of COVID-19 via Homology of CT-SCAN. Comput. Biol. Med. 2025, 193, 110226. [Google Scholar] [CrossRef]
- Yadav, A.; Ahmed, F.; Daescu, O.; Gedik, R.; Coskunuzer, B. Histopathological Cancer Detection with Topological Signatures. In Proceedings of the 2023 IEEE International Conference on Bioinformatics and Biomedicine (BIBM), Istanbul, Turkey, 5–8 December 2023; pp. 1610–1619. [Google Scholar]
- Fatema, S.; Nuwagira, B.; Chakraborty, S.; Gedik, R.; Coskunuzer, B. Diagnosis. In Topology- and Graph-Informed Imaging Informatics. Lecture Notes in Computer Science; Springer: Berlin/Heidelberg, Germany, 2025; Volume 15239, pp. 22–32. [Google Scholar]
- Ward, A.D.; Tomaszewski, J.E.; Lawson, P.J.; Sheppard, J.W.; Fasy, B.T.; Schupbach, J. Persistent homology for the automatic classification of prostate cancer aggressiveness in histopathology images. In Proceedings of the Medical Imaging 2019: Digital Pathology, San Diego, CA, USA, 9–21 February 2019; p. 109560. [Google Scholar]
- Singh, Y.; Farrelly, C.M.; Hathaway, Q.A.; Leiner, T.; Jagtap, J.; Carlsson, G.E.; Erickson, B.J. Topological data analysis in medical imaging: Current state of the art. Insights Imaging 2023, 14, 58. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, C.F.; Fitzpatrick, P.; Orr, N.; Jurek-Loughrey, A.; Wren, J. The topology of data: Opportunities for cancer research. Bioinform 2021, 37, 3091–3098. [Google Scholar] [CrossRef] [PubMed]
- Bukkuri, A.; Andor, N.; Darcy, I.K. Applications of Topological Data Analysis in Oncology. Front. Artif. Intell. 2021, 4, 659037. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.H.; Bardelotte, Y.A.; Ferreira, C.d.O.L.; Simao, A. Identifying key genes in cancer networks using persistent homology. Sci. Rep. 2025, 15, 2751. [Google Scholar] [CrossRef]
- Masoomy, H.; Askari, B.; Tajik, S.; Rizi, A.K.; Jafari, G.R. Topological analysis of interaction patterns in cancer-specific gene regulatory network: Persistent homology approach. Sci. Rep. 2021, 11, 16414. [Google Scholar] [CrossRef]
- Scott, A.J.; Ellison, M.; Sinclair, D.A. The economic value of targeting aging. Nat. Aging 2021, 1, 616–623. [Google Scholar] [CrossRef]
- Jytzler, J.A.; Lysdahlgaard, S. Radiomics evaluation for the early detection of Alzheimer’s dementia using T1-weighted MRI. Radiography 2024, 30, 1427–1433. [Google Scholar] [CrossRef]
- Mahavar, A.; Patel, A.; Patel, A. A Comprehensive Review on Deep Learning Techniques in Alzheimer’s Disease Diagnosis. Curr. Top. Med. Chem. 2025, 25, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, D.; Cai, Y.; Li, A.; Lan, G.; Sun, P.; Liu, L.; Zhu, Y.; Yang, J.; Zhou, Y.; et al. Pathophysiology characterization of Alzheimer’s disease in South China’s aging population: For the Greater-Bay-Area Healthy Aging Brain Study (GHABS). Alzheimer’s Res. Ther. 2024, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Wang, L.; Song, M.; Geng, H.; Li, W.; Huo, Y.; Huang, A.; Wang, X.; An, C. Serum neurofilament light chain and inflammatory cytokines as biomarkers for early detection of mild cognitive impairment. Sci. Rep. 2024, 14, 9072. [Google Scholar] [CrossRef] [PubMed]
- Chimthanawala, N.M.A.; Haria, A.; Sathaye, S. Non-invasive Biomarkers for Early Detection of Alzheimer’s Disease: A New-Age Perspective. Mol. Neurobiol. 2023, 61, 212–223. [Google Scholar] [CrossRef]
- Guo, T.; Noble, W.; Hanger, D.P. Roles of tau protein in health and disease. Acta Neuropathol. 2017, 133, 665–704. [Google Scholar] [CrossRef]
- d’Abramo, C.; D’Adamio, L.; Giliberto, L. Significance of Blood and Cerebrospinal Fluid Biomarkers for Alzheimer’s Disease: Sensitivity, Specificity and Potential for Clinical Use. J. Pers. Med. 2020, 10, 116. [Google Scholar] [CrossRef]
- Ding, Y.; Zuo, Y.; Zhang, B.; Fan, Y.; Xu, G.; Cheng, Z.; Ma, S.; Fang, S.; Tian, A.; Gao, D.; et al. Comprehensive human proteome profiles across a 50-year lifespan reveal aging trajectories and signatures. Cell 2025, 188, 5763–5784.e26. [Google Scholar] [CrossRef]
- Wu, D.; Sun, J.K.; Chow, K.H. Neuronal cell cycle reentry events in the aging brain are more prevalent in neurodegeneration and lead to cellular senescence. PLoS Biol. 2024, 22, e3002559. [Google Scholar] [CrossRef]
- Tamatta, R.; Pai, V.; Jaiswal, C.; Singh, I.; Singh, A.K. Neuroinflammaging and the Immune Landscape: The Role of Autophagy and Senescence in Aging Brain. Biogerontology 2025, 26, 52. [Google Scholar] [CrossRef]
- Zhong, Y.; Jin, C.; Luo, X.; Huang, J.; Wu, F.; Chen, H.; Wang, J.; Tian, M.; Zhang, H. PET molecular imaging-based prevention for brain aging. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 1611–1613. [Google Scholar] [CrossRef]
- Alzola, P.; Carnero, C.; Bermejo-Pareja, F.; Sánchez-Benavides, G.; Peña-Casanova, J.; Puertas-Martín, V.; Fernández-Calvo, B.; Contador, I. Neuropsychological Assessment for Early Detection and Diagnosis of Dementia: Current Knowledge and New Insights. J. Clin. Med. 2024, 13, 3442. [Google Scholar] [CrossRef]
- Papp, K.V.; Jutten, R.J.; Soberanes, D.; Weizenbaum, E.; Hsieh, S.; Molinare, C.; Buckley, R.; Betensky, R.A.; Marshall, G.A.; Johnson, K.A.; et al. Early Detection of Amyloid-Related Changes in Memory among Cognitively Unimpaired Older Adults with Daily Digital Testing. Ann. Neurol. 2024, 95, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Lerche, S.; Zimmermann, M.; Wurster, I.; Roeben, B.; Fries, F.L.; Deuschle, C.; Waniek, K.; Lachmann, I.; Gasser, T.; Jakobi, M.; et al. CSF and Serum Levels of Inflammatory Markers in PD: Sparse Correlation, Sex Differences and Association With Neurodegenerative Biomarkers. Front. Neurol. 2022, 13, 834580. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Baek, S.-H.; Lai, M.K.P.; Arumugam, T.V.; Jo, D.-G. Aging-associated sensory decline and Alzheimer’s disease. Mol. Neurodegener. 2024, 19, 93. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, I. Brain Age: A Promising Biomarker for Understanding Aging in the Context of Cognitive Reserve. medRxiv 2025. [Google Scholar] [CrossRef]
- Hagiwara, A.; Kamio, S.; Kikuta, J.; Nakaya, M.; Uchida, W.; Fujita, S.; Nikola, S.; Akasahi, T.; Wada, A.; Kamagata, K.; et al. Decoding Brain Development and Aging: Pioneering Insights From MRI Techniques. Investig. Radiol. 2025, 60, 162–174. [Google Scholar] [CrossRef]
- Torres-Simon, L.; del Cerro-León, A.; Yus, M.; Bruña, R.; Gil-Martinez, L.; Dolado, A.M.; Maestú, F.; Arrazola-Garcia, J.; Cuesta, P. Decoding the best automated segmentation tools for vascular white matter hyperintensities in the aging brain: A clinician’s guide to precision and purpose. GeroScience 2024, 46, 5485–5504. [Google Scholar] [CrossRef]
- Tsuchida, A.; Boutinaud, P.; Verrecchia, V.; Tzourio, C.; Debette, S.; Joliot, M. Early detection of white matter hyperintensities using SHIVA-WMH detector. Hum. Brain Mapp. 2024, 45, e26548. [Google Scholar] [CrossRef]
- Hagiwara, A.; Fujimoto, K.; Kamagata, K.; Murata, S.; Irie, R.; Kaga, H.; Someya, Y.; Andica, C.; Fujita, S.; Kato, S.; et al. Age-Related Changes in Relaxation Times, Proton Density, Myelin, and Tissue Volumes in Adult Brain Analyzed by 2-Dimensional Quantitative Synthetic Magnetic Resonance Imaging. Investig. Radiol. 2021, 56, 163–172. [Google Scholar] [CrossRef]
- Nguchu, B.A.; Zhao, J.; Lu, Y.; Han, Y.; Jin, H.; Wang, X.; Li, H.; Shaw, P. Human immunodeficiency virus accelerates brain aging and disrupts the trajectory of glymphatic clearance in aging brain. Front. Psychiatry 2025, 16, 1509093. [Google Scholar] [CrossRef]
- Chow, M.S.; Wu, S.L.; Webb, S.E.; Gluskin, K.; Yew, D.T. Functional magnetic resonance imaging and the brain: A brief review. World J. Radiol. 2017, 9, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Maschio, C.; Ni, R. Amyloid and Tau Positron Emission Tomography Imaging in Alzheimer’s Disease and Other Tauopathies. Front. Aging Neurosci. 2022, 14, 838034. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Srivastava, R. Enhanced EEG-based Alzheimer’s disease detection using synchrosqueezing transform and deep transfer learning. Neuroscience 2025, 576, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, G.; McGuinness, M.; Khan, M.A.; Obtinalla, C.; Hadoux, X.; van Wijngaarden, P. Retinal imaging biomarkers of Alzheimer’s disease: A systematic review and meta-analysis of studies using brain amyloid beta status for case definition. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2023, 15, e12421. [Google Scholar] [CrossRef]
- Zabel, P.; Przekoracka-Krawczyk, A.; Jaworski, D.; Zabel, K.; Suwala, K.; Gebska-Toloczko, M.; Kucharski, R.; Kaluzny, J.J. Assessment of individual retinal layer thickness and vascular changes in Alzheimer’s disease. Sci. Rep. 2025, 15, 17287. [Google Scholar] [CrossRef]
- Bennett, H.C.; Zhang, Q.; Wu, Y.-T.; Manjila, S.B.; Chon, U.; Shin, D.; Vanselow, D.J.; Pi, H.-J.; Drew, P.J.; Kim, Y. Aging drives cerebrovascular network remodeling and functional changes in the mouse brain. Nat. Commun. 2024, 15, 6398. [Google Scholar] [CrossRef]
- Open Access Series of Imaging Studies (OASIS). Available online: https://sites.wustl.edu/oasisbrains/ (accessed on 19 August 2025).
- Jack, C.R., Jr.; Bernstein, M.A.; Fox, N.C.; Thompson, P.; Alexander, G.; Harvey, D.; Borowski, B.; Britson, P.J.; Whitwell, J.L.; Ward, C.; et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J. Magn. Reson. Imaging 2008, 27, 685–691. [Google Scholar] [CrossRef]
- NeuroImaging Tool. Resources Collaboratory. The Internet Brain Segmentation Repository (IBSR). Available online: https://www.nitrc.org/plugins/mwiki/index.php/ibsr:MainPage (accessed on 19 August 2025).
- Yamanakkanavar, N.; Choi, J.Y.; Lee, B. MRI Segmentation and Classification of Human Brain Using Deep Learning for Diagnosis of Alzheimer’s Disease: A Survey. Sensors 2020, 20, 3243. [Google Scholar] [CrossRef]
- Elam, J.S.; Glasser, M.F.; Harms, M.P.; Sotiropoulos, S.N.; Andersson, J.L.R.; Burgess, G.C.; Curtiss, S.W.; Oostenveld, R.; Larson-Prior, L.J.; Schoffelen, J.M.; et al. The Human Connectome Project: A retrospective. Neuroimage 2021, 244, 118543. [Google Scholar] [CrossRef]
- Glasser, M.F.; Smith, S.M.; Marcus, D.S.; Andersson, J.L.; Auerbach, E.J.; Behrens, T.E.; Coalson, T.S.; Harms, M.P.; Jenkinson, M.; Moeller, S.; et al. The Human Connectome Project’s neuroimaging approach. Nat. Neurosci. 2016, 19, 1175–1187. [Google Scholar] [CrossRef]
- NIH Blueprint for Neuroscience Research. 1200 Subjects Data Release. Available online: https://www.humanconnectome.org/study/hcp-young-adult/document/1200-subjects-data-release (accessed on 28 August 2025).
- NIH Blueprint for Neuroscience Research. Components of the Human Connectome Project—Task fMRI. Available online: https://www.humanconnectome.org/study/hcp-young-adult/project-protocol/task-fmri (accessed on 28 August 2025).
- Van Essen, D.C.; Smith, S.M.; Barch, D.M.; Behrens, T.E.; Yacoub, E.; Ugurbil, K.; Consortium, W.U.-M.H. The WU-Minn Human Connectome Project: An overview. Neuroimage 2013, 80, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Battiston, F.; Lucas, M.; Petri, G.; Amico, E. Higher-order connectomics of human brain function reveals local topological signatures of task decoding, individual identification, and behavior. Nat. Commun. 2024, 15, 10244. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, N.A.; Bauer, C.C.C.; Siless, V.; Auerbach, R.P.; Elam, J.S.; Frosch, I.R.; Henin, A.; Hofmann, S.G.; Hodge, M.R.; Jones, R.; et al. The Human Connectome Project of adolescent anxiety and depression dataset. Sci. Data 2024, 11, 837. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Cocuzza, C.V.; Lawhead, C.; Ricard, J.A.; Labache, L.; Patrick, L.M.; Kumar, P.; Rubenstein, A.; Moses, J.; Chen, L.; et al. The Transdiagnostic Connectome Project: An open dataset for studying brain-behavior relationships in psychiatry. Sci. Data 2025, 12, 923. [Google Scholar] [CrossRef]
- Shafto, M.A.; Tyler, L.K.; Dixon, M.; Taylor, J.R.; Rowe, J.B.; Cusack, R.; Calder, A.J.; Marslen-Wilson, W.D.; Duncan, J.; Dalgleish, T.; et al. The Cambridge Centre for Ageing and Neuroscience (Cam-CAN) study protocol: A cross-sectional, lifespan, multidisciplinary examination of healthy cognitive ageing. BMC Neurol. 2014, 14, 204. [Google Scholar] [CrossRef]
- University of Cambridge. Cambridge Centre for Ageing and Neuroscience. Available online: https://cam-can.mrc-cbu.cam.ac.uk/ (accessed on 28 August 2025).
- Shafto, M.A.; Henson, R.N.; Matthews, F.E.; Taylor, J.R.; Emery, T.; Erzinclioglu, S.; Hanley, C.; Rowe, J.B.; Cusack, R.; Calder, A.J.; et al. Cognitive Diversity in a Healthy Aging Cohort: Cross-Domain Cognition in the Cam-CAN Project. J. Aging Health 2020, 32, 1029–1041. [Google Scholar] [CrossRef]
- Jauny, G.; Mijalkov, M.; Canal-Garcia, A.; Volpe, G.; Pereira, J.; Eustache, F.; Hinault, T. Linking structural and functional changes during aging using multilayer brain network analysis. Commun. Biol. 2024, 7, 239. [Google Scholar] [CrossRef]
- Mijalkov, M.; Storm, L.; Zufiria-Gerbolés, B.; Veréb, D.; Xu, Z.; Canal-Garcia, A.; Sun, J.; Chang, Y.-W.; Zhao, H.; Gómez-Ruiz, E.; et al. Computational memory capacity predicts aging and cognitive decline. Nat. Commun. 2025, 16, 2748. [Google Scholar] [CrossRef]
- Moon, S.; Lee, J.; Lee, W.H. Predicting brain age with global-local attention network from multimodal neuroimaging data: Accuracy, generalizability, and behavioral associations. Comput. Biol. Med. 2025, 184, 109411. [Google Scholar] [CrossRef]
- Mapkar, S.A.; Bliss, S.A.; Carbajal, E.E.P.; Murray, S.H.; Li, Z.; Wilson, A.K.; Piprode, V.; Lee, Y.J.; Kirsch, T.; Petroff, K.S.; et al. Nuclear morphometrics coupled with machine learning identifies dynamic states of senescence across age. Nat. Commun. 2025, 16, 6231. [Google Scholar] [CrossRef]
- Ali, A.-N. Developing an Artificial Intelligence Framework for Identifying Fusion Blood-Based Biomarkers in Alzheimer’s Disease. J. Inf. Syst. Eng. Manag. 2025, 10, 443–458. [Google Scholar] [CrossRef]
- Elgandelwar, S.M.; Bairagi, V.; Vasekar, S.; Nanthaamornphong, A.; Tupe-Waghmare, P. Analyzing electroencephalograph signals for early Alzheimer’s disease detection: Deep learning vs. traditional machine learning approaches. Int. J. Elec Comp. Eng. 2024, 14, 2602–2615. [Google Scholar] [CrossRef]
- Li, Z.; Li, J.; Li, J.; Wang, M.; Xu, A.; Huang, Y.; Yu, Q.; Zhang, L.; Li, Y.; Li, Z.; et al. Development and Validation of a Brain Aging Biomarker in Middle-Aged and Older Adults: Deep Learning Approach. JMIR Aging 2025, 8, e73004. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, I.U.; Hossain, A.-A.; Imam, T.; Islam, J. A Multimodal Analytical Approach to Alzheimer’s Disease Diagnosis Using Machine Learning and Convolutional Neural Networks on MRI Datasets. In Proceedings of the 2024 IEEE Asia Pacific Conference on Wireless and Mobile (APWiMob), Bandung, Indonesia, 28–30 November 2024; pp. 32–37. [Google Scholar]
- Ahamed, I.U.; Hossain, A.-A.; Gupta, U.D.; Ahamed, I.U.; Saha, U. Confronting the Challenges of Alzheimer’s Diagnosis: A Deep Dive into MRI-Based Early Detection Methods. In Proceedings of the 2024 4th International Conference of Science and Information Technology in Smart Administration (ICSINTESA), Balikpapan, Indonesia, 12–13 July 2024; pp. 696–701. [Google Scholar]
- Awotunde, J.B.; Sur, N.S.; Imoize, A.L.; Misra, S.; Gaber, T. An Enhanced Residual Networks Based Framework for Early Alzheimer’s Disease Classification and Diagnosis. In Advances in Communication, Devices and Networking. ICCDN 2022; Dhar, S., Do, D.T., Sur, S.N., Liu, C.M., Eds.; Lecture Notes in Electrical Engineering; Springer: Singapore, 2023; Volume 1037, pp. 335–348. [Google Scholar] [CrossRef]
- Ozdemir, C.; Dogan, Y. Advancing early diagnosis of Alzheimer’s disease with next-generation deep learning methods. Biomed. Signal Process. Control 2024, 96, 106614. [Google Scholar] [CrossRef]
- Abunadi, I. Deep and hybrid learning of MRI diagnosis for early detection of the progression stages in Alzheimer’s disease. Conn. Sci. 2022, 34, 2395–2430. [Google Scholar] [CrossRef]
- Hussain, M.Z.; Shahzad, T.; Mehmood, S.; Akram, K.; Khan, M.A.; Tariq, M.U.; Ahmed, A. A fine-tuned convolutional neural network model for accurate Alzheimer’s disease classification. Sci. Rep. 2025, 15, 11616. [Google Scholar] [CrossRef]
- Zhou, J.; Wei, Y.; Li, X.; Zhou, W.; Tao, R.; Hua, Y.; Liu, H. A deep learning model for early diagnosis of alzheimer’s disease combined with 3D CNN and video Swin transformer. Sci. Rep. 2025, 15, 23311. [Google Scholar] [CrossRef]
- Babu, B.; Parvathy, G.; Bawa, F.S.M.; Gill, G.S.; Patel, J.; Sibia, D.S.; Sureddi, J.; Patel, V. Comparing the Artificial Intelligence Detection Models to Standard Diagnostic Methods and Alternative Models in Identifying Alzheimer’s Disease in At-Risk or Early Symptomatic Individuals: A Scoping Review. Cureus 2024, 16, e75389. [Google Scholar] [CrossRef]
- Kim, S.K.; Duong, Q.A.; Gahm, J.K. Multimodal 3D Deep Learning for Early Diagnosis of Alzheimer’s Disease. IEEE Access 2024, 12, 46278–46289. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, S.; Fang, Z.; Liu, C.; Zou, B.; Qiu, L.; Wang, Y.; Chang, S.; Jia, F.; Qin, F.; et al. Toward Robust Early Detection of Alzheimer’s Disease via an Integrated Multimodal Learning Approach. In Proceedings of the ICASSP 2025 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Hyderabad, India, 6–11 April 2025; pp. 1–5. [Google Scholar]
- Sun, J.; Xie, Z.; Sun, Y.; Shen, A.; Li, R.; Yuan, X.; Lu, B.; Li, Y. Precise prediction of cerebrospinal fluid amyloid beta protein for early Alzheimer’s disease detection using multimodal data. MedComm (2020) 2024, 5, e532. [Google Scholar] [CrossRef]
- Gaeta, A.M.; Quijada-Lopez, M.; Barbe, F.; Vaca, R.; Pujol, M.; Minguez, O.; Sanchez-de-la-Torre, M.; Munoz-Barrutia, A.; Pinol-Ripoll, G. Predicting Alzheimer’s disease CSF core biomarkers: A multimodal Machine Learning approach. Front. Aging Neurosci. 2024, 16, 1369545. [Google Scholar] [CrossRef]
- Viswan, V.; Shaffi, N.; Mahmud, M.; Subramanian, K.; Hajamohideen, F. Explainable Artificial Intelligence in Alzheimer’s Disease Classification: A Systematic Review. Cogn. Comput. 2023, 16, 1–44. [Google Scholar] [CrossRef]
- Kumari, L.K.S.; Sundarrajan, R. A review on brain age prediction models. Brain Res. 2024, 1823, 148668. [Google Scholar] [CrossRef] [PubMed]
- Baecker, L.; Garcia-Dias, R.; Vieira, S.; Scarpazza, C.; Mechelli, A. Machine learning for brain age prediction: Introduction to methods and clinical applications. EBioMedicine 2021, 72, 103600. [Google Scholar] [CrossRef] [PubMed]
- Zivanovic, M.; Trenkic, A.A.; Milosevic, V.; Stojanov, D.; Misic, M.; Radovanovic, M.; Radovanovic, V. The role of magnetic resonance imaging in the diagnosis and prognosis of dementia. Biomol. Biomed. 2023, 23, 209–224. [Google Scholar] [CrossRef]
- Srividhya, L.; Sowmya, V.; Ravi, V.; Gopalakrishnan, E.A.; Soman, K.P. Deep learning-based approach for multi-stage diagnosis of Alzheimer’s disease. Multimed. Tools Appl. 2023, 83, 16799–16822. [Google Scholar] [CrossRef]
- Han, J.; Kim, S.Y.; Lee, J.; Lee, W.H. Brain Age Prediction: A Comparison between Machine Learning Models Using Brain Morphometric Data. Sensors 2022, 22, 8077. [Google Scholar] [CrossRef]
- Lockhart, S.N.; DeCarli, C. Structural imaging measures of brain aging. Neuropsychol. Rev. 2014, 24, 271–289. [Google Scholar] [CrossRef]
- Dartora, C.; Marseglia, A.; Martensson, G.; Rukh, G.; Dang, J.; Muehlboeck, J.S.; Wahlund, L.O.; Moreno, R.; Barroso, J.; Ferreira, D.; et al. A deep learning model for brain age prediction using minimally preprocessed T1w images as input. Front. Aging Neurosci. 2023, 15, 1303036. [Google Scholar] [CrossRef]
- Yu, Y.; Cui, H.Q.; Haas, S.S.; New, F.; Sanford, N.; Yu, K.; Zhan, D.; Yang, G.; Gao, J.H.; Wei, D.; et al. Brain-age prediction: Systematic evaluation of site effects, and sample age range and size. Hum. Brain Mapp. 2024, 45, e26768. [Google Scholar] [CrossRef]
- Capó, M.; Vitali, S.; Athanasiou, G.; Cusimano, N.; García, D.; Cruickshank, G.; Patel, B. UK Biobank MRI data can power the development of generalizable brain clocks: A study of standard ML/DL methodologies and performance analysis on external databases. NeuroImage 2025, 308, 121064. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, S.; Feng, J.; Zhang, S. Age-level bias correction in brain age prediction. Neuroimage Clin. 2023, 37, 103319. [Google Scholar] [CrossRef] [PubMed]
- Roibu, A.-C.; Adaszewski, S.; Schindler, T.; Smith, S.M.; Namburete, A.I.L.; Lange, F.J. Brain Ages Derived from Different MRI Modalities are Associated with Distinct Biological Phenotypes. In Proceedings of the 2023 10th IEEE Swiss Conference on Data Science (SDS), Zurich, Switzerland, 22–23 June 2023; pp. 17–25. [Google Scholar]
- Wang, Y.; Wen, J.; Xin, J.; Zhang, Y.; Xie, H.; Tang, Y. 3DCNN predicting brain age using diffusion tensor imaging. Med. Biol. Eng. Comput. 2023, 61, 3335–3344. [Google Scholar] [CrossRef] [PubMed]
- Dorfel, R.P.; Arenas-Gomez, J.M.; Svarer, C.; Ganz, M.; Knudsen, G.M.; Svensson, J.E.; Plaven-Sigray, P. Multimodal brain age prediction using machine learning: Combining structural MRI and 5-HT2AR PET-derived features. Geroscience 2024, 46, 4123–4133. [Google Scholar] [CrossRef]
- Dardouri, S. An efficient method for early Alzheimer’s disease detection based on MRI images using deep convolutional neural networks. Front. Artif. Intell. 2025, 8, 1563016. [Google Scholar] [CrossRef]
- Umirzakova, S.; Muksimova, S.; Iskhakova, N.; Anorova, S.; Cho, Y.I. Enhancing Early Alzheimer′s Disease Detection: Integrative Approaches Using Machine Learning and Deep Learning in Neuroimaging. J. Artif. Intell. Res. Appl. 2024, 1, 113–126. [Google Scholar]
- Li, Y.; Chang, X.; Wu, J.; Liu, Y.; Wang, H.; Zhang, Y. Machine learning in early diagnosis of neurological diseases: Advancing accuracy and overcoming challenges. Brain Netw. Disord. 2025, 1, 132–139. [Google Scholar] [CrossRef]
- Ahsan, M.M.; Luna, S.A.; Siddique, Z. Machine-Learning-Based Disease Diagnosis: A Comprehensive Review. Healthcare 2022, 10, 541. [Google Scholar] [CrossRef]
- Xiong, M.; Lin, L.; Jin, Y.; Kang, W.; Wu, S.; Sun, S. Comparison of Machine Learning Models for Brain Age Prediction Using Six Imaging Modalities on Middle-Aged and Older Adults. Sensors 2023, 23, 3622. [Google Scholar] [CrossRef]
- Lee, W.H. The Choice of Machine Learning Algorithms Impacts the Association between Brain-Predicted Age Difference and Cognitive Function. Mathematics 2023, 11, 1229. [Google Scholar] [CrossRef]
- Peng, H.; Gong, W.; Beckmann, C.F.; Vedaldi, A.; Smith, S.M. Accurate brain age prediction with lightweight deep neural networks. Med. Image Anal. 2021, 68, 101871. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, S.; Seo, S.W.; Na, D.L.; Jang, H.; Kim, J.P.; Kim, H.J.; Kang, S.H.; Kwak, K. A novel deep learning-based brain age prediction framework for routine clinical MRI scans. NPJ Aging 2025, 11, 70. [Google Scholar] [CrossRef] [PubMed]
- Valdes-Hernandez, P.A.; Nodarse, C.L.; Cole, J.H.; Cruz-Almeida, Y. Feasibility of brain age predictions from clinical T1-weighted MRIs. Brain Res. Bull. 2023, 205, 110811. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, C.; Ma, X.; Zhu, X.; Lin, L.; Tian, M. ds-FCRN: Three-dimensional dual-stream fully convolutional residual networks and transformer-based global-local feature learning for brain age prediction. Brain Struct. Funct. 2025, 230, 32. [Google Scholar] [CrossRef]
- Ding, R.; Lu, H.; Liu, M. DenseFormer-MoE: A Dense Transformer Foundation Model with Mixture of Experts for Multi-Task Brain Image Analysis. IEEE Trans. Med. Imaging 2025, Online ahead of print. [Google Scholar] [CrossRef]
- Orka, N.A.; Awal, M.A.; Liò, P.; Pogrebna, G.; Ross, A.G.; Moni, M.A. Quantum deep learning in neuroinformatics: A systematic review. Artif. Intell. Rev. 2025, 58, 134. [Google Scholar] [CrossRef]
- Haghayegh, F.; Norouziazad, A.; Haghani, E.; Feygin, A.A.; Rahimi, R.H.; Ghavamabadi, H.A.; Sadighbayan, D.; Madhoun, F.; Papagelis, M.; Felfeli, T.; et al. Revolutionary Point-of-Care Wearable Diagnostics for Early Disease Detection and Biomarker Discovery through Intelligent Technologies. Adv. Sci. 2024, 11, e2400595. [Google Scholar] [CrossRef]
- Syedmoradi, L.; Daneshpour, M.; Alvandipour, M.; Gomez, F.A.; Hajghassem, H.; Omidfar, K. Point of care testing: The impact of nanotechnology. Biosens. Bioelectron. 2017, 87, 373–387. [Google Scholar] [CrossRef]
- Han, G.-R.; Goncharov, A.; Eryilmaz, M.; Ye, S.; Palanisamy, B.; Ghosh, R.; Lisi, F.; Rogers, E.; Guzman, D.; Yigci, D.; et al. Machine learning in point-of-care testing: Innovations, challenges, and opportunities. Nat. Commun. 2025, 16, 3165. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Goyat, R.; Saharan, Y.; Chaudhry, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Ameen, S.; Baskoutas, S. Nanomaterials-based biosensor and their applications: A review. Heliyon 2023, 9, e19929. [Google Scholar] [CrossRef]
- Tripathi, A.; Bonilla-Cruz, J. Review on Healthcare Biosensing Nanomaterials. ACS Appl. Nano Mater. 2023, 6, 5042–5074. [Google Scholar] [CrossRef]
- Burlec, A.F.; Corciova, A.; Boev, M.; Batir-Marin, D.; Mircea, C.; Cioanca, O.; Danila, G.; Danila, M.; Bucur, A.F.; Hancianu, M. Current Overview of Metal Nanoparticles’ Synthesis, Characterization, and Biomedical Applications, with a Focus on Silver and Gold Nanoparticles. Pharmaceuticals 2023, 16, 1410. [Google Scholar] [CrossRef]
- Sadiq, Z.; Tali, S.H.S.; Hajimiri, H.; Al-Kassawneh, M.; Jahanshahi-Anbuhi, S. Gold Nanoparticles-Based Colorimetric Assays for Environmental Monitoring and Food Safety Evaluation. Crit. Rev. Anal. Chem. 2023, 54, 2209–2244. [Google Scholar] [CrossRef]
- Wang, J.; Giordani, S.; Marassi, V.; Placci, A.; Roda, B.; Reschiglian, P.; Zattoni, A. Multi-environment and multi-parameter screening of stability and coating efficiency of gold nanoparticle bioconjugates in application media. Sci. Rep. 2024, 14, 31568. [Google Scholar] [CrossRef] [PubMed]
- Hanmante, P.S.; Lohiya, R.T.; Wankhede, A.G.; Undirwade, D.S.; Lade, S.N.; Burle, S.S.; Umekar, M.J. Quantum dot nanotechnology: Advancing target drug delivery in Oncology. Next Nanotechnol. 2025, 7, 100172. [Google Scholar] [CrossRef]
- Behrouzi, K.; Fard, Z.K.; Chen, C.-M.; He, P.; Teng, M.; Lin, L. Plasmonic coffee-ring biosensing for AI-assisted point-of-care diagnostics. Nat. Commun. 2025, 16, 4597. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, Y.; Jiang, N.; Yetisen, A.K. Wearable artificial intelligence biosensor networks. Biosens. Bioelectron. 2023, 219, 114825. [Google Scholar] [CrossRef]
- Smith, A.A.; Li, R.; Tse, Z.T.H. Reshaping healthcare with wearable biosensors. Sci. Rep. 2023, 13, 4998. [Google Scholar] [CrossRef]
- Keum, D.H.; Kim, S.K.; Koo, J.; Lee, G.H.; Jeon, C.; Mok, J.W.; Mun, B.H.; Lee, K.J.; Kamrani, E.; Joo, C.K.; et al. Wireless smart contact lens for diabetic diagnosis and therapy. Sci. Adv. 2020, 6, eaba3252. [Google Scholar] [CrossRef]
- Park, W.; Seo, H.; Kim, J.; Hong, Y.-M.; Song, H.; Joo, B.J.; Kim, S.; Kim, E.; Yae, C.-G.; Kim, J.; et al. In-depth correlation analysis between tear glucose and blood glucose using a wireless smart contact lens. Nat. Commun. 2024, 15, 2828. [Google Scholar] [CrossRef]
- Youn, S.; Ki, M.R.; Abdelhamid, M.A.A.; Pack, S.P. Biomimetic Materials for Skin Tissue Regeneration and Electronic Skin. Biomimetics 2024, 9, 278. [Google Scholar] [CrossRef]
- Promphet, N.; Thanawattano, C.; Buekban, C.; Laochai, T.; Lormaneenopparat, P.; Sukmas, W.; Rattanawaleedirojn, P.; Puthongkham, P.; Potiyaraj, P.; Leewattanakit, W.; et al. Smartphone based wearable sweat glucose sensing device correlated with machine learning for real-time diabetes screening. Anal. Chim. Acta 2024, 1312, 342761. [Google Scholar] [CrossRef]
- Luo, R.; Yang, Y.; Huang, S.; Huang, Z. Wearable devices for monitoring sweat glucose: An integrated strategy for efficient electrochemical sensors. Sens. Actuators Rep. 2025, 9, 100339. [Google Scholar] [CrossRef]
- Xing, E.; Chen, H.; Xin, X.; Cui, H.; Dou, Y.; Song, S. Recent Advances in Smart Phone-Based Biosensors for Various Applications. Chemosensors 2025, 13, 221. [Google Scholar] [CrossRef]
- Zarei, M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. TrAC Trends Anal. Chem. 2017, 91, 26–41. [Google Scholar] [CrossRef]
- Joh, D.Y.; Heggestad, J.T.; Zhang, S.; Anderson, G.R.; Bhattacharyya, J.; Wardell, S.E.; Wall, S.A.; Cheng, A.B.; Albarghouthi, F.; Liu, J.; et al. Cellphone enabled point-of-care assessment of breast tumor cytology and molecular HER2 expression from fine-needle aspirates. NPJ Breast Cancer 2021, 7, 85. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, Y.; Lu, J.; Gong, T.; Ibáñez, E.; Cifuentes, A.; Lu, W. Microfluidic biosensors for biomarker detection in body fluids: A key approach for early cancer diagnosis. Biomark. Res. 2024, 12, 153. [Google Scholar] [CrossRef]
- Yang, S.M.; Lv, S.; Zhang, W.; Cui, Y. Microfluidic Point-of-Care (POC) Devices in Early Diagnosis: A Review of Opportunities and Challenges. Sensors 2022, 22, 1620. [Google Scholar] [CrossRef]
- Chen, C.; Ran, B.; Liu, B.; Liu, X.; Zhang, Z.; Li, Y.; Li, H.; Lan, M.; Zhu, Y. Multiplexed detection of biomarkers using a microfluidic chip integrated with mass-producible micropillar array electrodes. Anal. Chim. Acta 2023, 1272, 341450. [Google Scholar] [CrossRef]
- Wormald, B.W.; Moser, N.; deSouza, N.M.; Mantikas, K.-T.; Malpartida-Cardenas, K.; Pennisi, I.; Ind, T.E.J.; Vroobel, K.; Kalofonou, M.; Rodriguez-Manzano, J.; et al. Lab-on-chip assay of tumour markers and human papilloma virus for cervical cancer detection at the point-of-care. Sci. Rep. 2022, 12, 8750. [Google Scholar] [CrossRef]
- Mwai, L.M.; Kyama, M.C.; Ngugi, C.W.; Walong, E. Development of HPV 16/18 E6 Oncoprotein Paperbased Nanokit for Enhanced Detection of HPV 16/18 E6 Oncoprotein in Cervical Cancer Screening. J. Nanotechnol. Nanomater. 2020, 1, 31–44. [Google Scholar] [CrossRef]
- Krings, A.; Dückelmann, A.M.; Moser, L.; Gollrad, J.; Wiegerinck, M.; Schweizer, J.; Kaufmann, A.M. Performance of OncoE6 cervical test with collection methods enabling self-sampling. BMC Women’s Health 2018, 18, 68. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Munshea, A.; Nibret, E.; Bezabih, B.; Amare, B.; Yismaw, G. Evaluation of Onco E6 point of care rapid diagnostic test for human papilloma virus in Bahir Dar, Amhara Regional State, Ethiopia. PLoS ONE 2025, 20, e0321076. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, X.; Ge, C.; Li, S.; Wang, L.; Xu, Y. Detection of VEGF165 in Whole Blood by Differential Pulse Voltammetry Based on a Centrifugal Microfluidic Chip. ACS Sens. 2022, 7, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Cheng, L.; Hu, L.; Lou, D.; Zhang, T.; Li, J.; Zhu, Q.; Liu, F. An integrative microfluidic device for isolation and ultrasensitive detection of lung cancer-specific exosomes from patient urine. Biosens. Bioelectron. 2020, 163, 112290. [Google Scholar] [CrossRef]
- Song, Y.; Shi, Y.; Huang, M.; Wang, W.; Wang, Y.; Cheng, J.; Lei, Z.; Zhu, Z.; Yang, C. Bioinspired Engineering of a Multivalent Aptamer-Functionalized Nanointerface to Enhance the Capture and Release of Circulating Tumor Cells. Angew. Chem. Int. Ed. Engl. 2019, 58, 2236–2240. [Google Scholar] [CrossRef]
- Wu, L.; Ding, H.; Qu, X.; Shi, X.; Yang, J.; Huang, M.; Zhang, J.; Zhang, H.; Song, J.; Zhu, L.; et al. Fluidic Multivalent Membrane Nanointerface Enables Synergetic Enrichment of Circulating Tumor Cells with High Efficiency and Viability. J. Am. Chem. Soc. 2020, 142, 4800–4806. [Google Scholar] [CrossRef]
- Jia, L.; Zhen, X.; Chen, L.; Feng, Q.; Yuan, W.; Bu, Y.; Wang, S.; Xie, X. Bioinspired nano-plate-coral platform enabled efficient detection of circulating tumor cells via the synergistic capture of multivalent aptamer and tumor cell membrane. J. Colloid. Interface Sci. 2023, 631, 55–65. [Google Scholar] [CrossRef]
- Erkocyigit, B.A.; Ozufuklar, O.; Yardim, A.; Celik, E.G.; Timur, S. Biomarker Detection in Early Diagnosis of Cancer: Recent Achievements in Point-of-Care Devices Based on Paper Microfluidics. Biosensors 2023, 13, 387. [Google Scholar] [CrossRef]
- Smith, C.A.; Paul, S.; Haney, K.E.; Parra, S.G.; Bond, M.; Lopez, L.; Maza, M.; Felix, J.; Ramalingam, P.; Escobar, P.; et al. A paper-based HPV E7 oncoprotein assay for cervical precancer detection at the point of care. Sci. Rep. 2025, 15, 3041. [Google Scholar] [CrossRef]
- Wei, B.; Mao, K.; Liu, N.; Zhang, M.; Yang, Z. Graphene nanocomposites modified electrochemical aptamer sensor for rapid and highly sensitive detection of prostate specific antigen. Biosens. Bioelectron. 2018, 121, 41–46. [Google Scholar] [CrossRef]
- Li, F.; Guo, L.; Hu, Y.; Li, Z.; Liu, J.; He, J.; Cui, H. Multiplexed chemiluminescence determination of three acute myocardial infarction biomarkers based on microfluidic paper-based immunodevice dual amplified by multifunctionalized gold nanoparticles. Talanta 2020, 207, 120346. [Google Scholar] [CrossRef]
- Gilroy, C.; Silver, C.D.; Kunstmann-Olsen, C.; Miller, L.M.; Johnson, S.D.; Krauss, T.F. A passive blood separation sensing platform for point-of-care devices. NPJ Biosens. 2025, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Li, P.R.; Boilla, S.K.; Wang, C.H.; Lin, P.C.; Kuo, C.N.; Tsai, T.H.; Lee, G.B. A self-driven, microfluidic, integrated-circuit biosensing chip for detecting four cardiovascular disease biomarkers. Biosens. Bioelectron. 2024, 249, 115931. [Google Scholar] [CrossRef] [PubMed]
- Ray, A.; Esparza, S.; Wu, D.; Hanudel, M.R.; Joung, H.A.; Gales, B.; Tseng, D.; Salusky, I.B.; Ozcan, A. Measurement of serum phosphate levels using a mobile sensor. Analyst 2020, 145, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Pittman, T.W.; Decsi, D.B.; Punyadeera, C.; Henry, C.S. Saliva-based microfluidic point-of-care diagnostic. Theranostics 2023, 13, 1091–1108. [Google Scholar] [CrossRef]
- Wei, X.; Guo, J.; Lian, H.; Sun, X.; Liu, B. Cobalt metal-organic framework modified carbon cloth/paper hybrid electrochemical button-sensor for nonenzymatic glucose diagnostics. Sens. Actuators B Chem. 2021, 329, 129205. [Google Scholar] [CrossRef]
- Goncharov, A.; Joung, H.A.; Ghosh, R.; Han, G.R.; Ballard, Z.S.; Maloney, Q.; Bell, A.; Aung, C.T.Z.; Garner, O.B.; Carlo, D.D.; et al. Deep Learning-Enabled Multiplexed Point-of-Care Sensor using a Paper-Based Fluorescence Vertical Flow Assay. Small 2023, 19, 2300617. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, J.; Ko, S.H. Electrochemical biosensors for point-of-care testing. Bio-Des. Manuf. 2024, 7, 548–565. [Google Scholar] [CrossRef]
- Madadelahi, M.; Romero-Soto, F.O.; Kumar, R.; Tlaxcala, U.B.; Madou, M.J. Electrochemical sensors: Types, applications, and the novel impacts of vibration and fluid flow for microfluidic integration. Biosens. Bioelectron. 2025, 272, 117099. [Google Scholar] [CrossRef]
- Ko, D.H.; Bates, D.; Karaosmanoglu, H.; Taredun, K.; Elton, C.; Jones, L.; Hosseini, A.; Partridge, A. 3D microelectrode arrays, pushing the bounds of sensitivity toward a generic platform for point-of-care diagnostics. Biosens. Bioelectron. 2023, 227, 115154. [Google Scholar] [CrossRef]
- Sun, Z.; Tong, Y.; Zhao, L.; Li, J.; Gao, F.; Wang, C.; Li, H.; Du, L.; Jiang, Y. MoS2@Ti3C2 nanohybrid-based photoelectrochemical biosensor: A platform for ultrasensitive detection of cancer biomarker exosomal miRNA. Talanta 2022, 238, 123077. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ye, Q.; Yang, M. Sensitive photoelectrochemical sensor for detection of cancer biomarker based on enzyme embedded nanoparticles as signal probe. Mater. Lett. 2023, 331, 133479. [Google Scholar] [CrossRef]
- Cimmino, W.; Migliorelli, D.; Singh, S.; Miglione, A.; Generelli, S.; Cinti, S. Design of a printed electrochemical strip towards miRNA-21 detection in urine samples: Optimization of the experimental procedures for real sample application. Anal. Bioanal. Chem. 2023, 415, 4511–4520. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Xiao, W.; Shen, L.; Shi, X.; Li, J. An electrochemical method based on CRISPR-Cas12a and enzymatic reaction for the highly sensitive detection of tumor marker MUC1 mucin. Analyst 2024, 149, 3920–3927. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Kargupta, R.; Ghoshal, D.; Li, Z.; Chande, C.; Feng, L.; Chatterjee, S.; Koratkar, N.; Motkuri, R.K.; Basuray, S. ESSENCE—A rapid, shear-enhanced, flow-through, capacitive electrochemical platform for rapid detection of biomolecules. Biosens. Bioelectron. 2021, 182, 113163. [Google Scholar] [CrossRef]
- Ko, D.H.; Hosseini, A.; Karaosmanoglu, H.; Taredun, K.; Jones, L.; Partridge, A. Microfluidic separation of capture from detection and its application for determination of COVID-19 antibodies. Sens. Actuators B Chem. 2022, 351, 130918. [Google Scholar] [CrossRef]
- Yamani, H.Z.; Safwat, N.; Mahmoud, A.M.; Ayad, M.F.; Abdel-Ghany, M.F.; Gomaa, M.M. Point-of-care diagnostics for rapid determination of prostate cancer biomarker sarcosine: Application of disposable potentiometric sensor based on oxide-conductive polymer nanocomposite. Anal. Bioanal. Chem. 2023, 415, 5451–5462. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, W.; Li, N.; Yang, M.; Hou, C.; Huo, D. A Clinically Feasible Diagnostic Typing of Breast Cancer Built on a Homogeneous Electrochemical Biosensor for Simultaneous Multiplex Detection. Anal. Chem. 2024, 96, 12316–12322. [Google Scholar] [CrossRef]
- Chen, H.; Li, Z.Y.; Chen, J.; Yu, H.; Zhou, W.; Shen, F.; Chen, Q.; Wu, L. CRISPR/Cas12a-based electrochemical biosensor for highly sensitive detection of cTnI. Bioelectrochemistry 2022, 146, 108167. [Google Scholar] [CrossRef]
- Xu, X.; Liu, Z.; Liu, J.; Yao, C.; Chen, X.; Huang, X.; Huang, S.; Shi, P.; Li, M.; Wang, L.; et al. Multi-sized microelectrode array coupled with micro-electroporation for effective recording of intracellular action potential. Microsyst. Nanoeng. 2025, 11, 85. [Google Scholar] [CrossRef]
- Hirsch, I.B. Introduction: History of Glucose Monitoring. In Role of Continuous Glucose Monitoring in Diabetes Treatment; ADA Clinical Compendia Series; American Diabetes Association: Arlington, VA, USA, 2018; p. 1. [Google Scholar] [CrossRef]
- Diabetes, C.; Research, G.C.T.; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef]
- Mittal, R.; Koutras, N.; Maya, J.; Lemos, J.R.N.; Hirani, K. Blood glucose monitoring devices for type 1 diabetes: A journey from the food and drug administration approval to market availability. Front. Endocrinol. 2024, 15, 1352302. [Google Scholar] [CrossRef]
- Lin, P.H.; Sheu, S.C.; Chen, C.W.; Huang, S.C.; Li, B.R. Wearable hydrogel patch with noninvasive, electrochemical glucose sensor for natural sweat detection. Talanta 2022, 241, 123187. [Google Scholar] [CrossRef]
- Xu, J.; Tao, X.; Liu, X.; Yang, L. Wearable Eye Patch Biosensor for Noninvasive and Simultaneous Detection of Multiple Biomarkers in Human Tears. Anal. Chem. 2022, 94, 8659–8667. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Song, G.; Zhong, K.; Wang, Z.; Xia, X.; Tian, Y. Wearable fluorescent contact lenses for monitoring glucose via a smartphone. Sens. Actuators B Chem. 2022, 352, 131067. [Google Scholar] [CrossRef]
- Lee, H.; Song, C.; Hong, Y.S.; Kim, M.; Cho, H.R.; Kang, T.; Shin, K.; Choi, S.H.; Hyeon, T.; Kim, D.-H. Wearable/disposable sweat-based glucose monitoring device with multistage transdermal drug delivery module. Sci. Adv. 2017, 3, e1601314. [Google Scholar] [CrossRef] [PubMed]
- Karim, Z.; Khan, M.J.; Hussain, A.; Ahmed, F.; Khan, Z.H. Multilayer patch functionalized microfibrillated cellulosic paper sensor for sweat glucose monitoring. Sci. Rep. 2024, 14, 23434. [Google Scholar] [CrossRef]
- Duy Mac, K.; Su, J. Optical biosensors for diagnosing neurodegenerative diseases. NPJ Biosens. 2025, 2, 20. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, Z.; Xu, R.; Hou, S.; Gong, L.; Guo, L.; Gao, K.; Xu, X.; Tan, X.; Xiao, D.; et al. A genetically encoded biosensor for point-of-care and live-cell detection of D-2-hydroxyglutarate. Nat. Commun. 2025, 16, 6913. [Google Scholar] [CrossRef]
- Chou, F.J.; Liu, Y.; Lang, F.; Yang, C. D-2-Hydroxyglutarate in Glioma Biology. Cells 2021, 10, 2345. [Google Scholar] [CrossRef] [PubMed]
- Keum, C.; Yeom, H.; Noh, T.I.; Yi, S.Y.; Jin, S.; Kim, C.; Shim, J.S.; Yoon, S.G.; Kim, H.; Lee, K.H.; et al. Diagnosis of early-stage bladder cancer via unprocessed urine samples at the point of care. Na Biomed. Eng. 2024, 9, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Ming-Jung, T.; Nan-Fu, C. Active plasmonic colorimetric biosensor for detecting lung cancer proteins. In Proceedings of the SPIE Optics + Optoelectronics Exhibition 2025, Prague, Czech Republic, 7–10 April 2025; p. 135270Q. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Cheng, W.; Wu, T.; Li, J.; Li, X.; Liu, L.; Bai, H.; Ding, S.; Li, X.; et al. Surface plasmon resonance biosensor for exosome detection based on reformative tyramine signal amplification activated by molecular aptamer beacon. J. Nanobiotechnol. 2021, 19, 450. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Li, X.; Zhang, J.; Pan, H.; Peng, Q.; Gan, H.; Su, S.; Yuwen, L.; Song, C. SERS/electrochemical dual-mode biosensor based on multi-functionalized molybdenum disulfide nanosheet probes and SERS-active Ag nanorods array electrodes for reliable detection of cancer-related miRNA. Sens. Actuators B Chem. 2022, 368, 132245. [Google Scholar] [CrossRef]
- Ding, Y.; Sun, Y.; Liu, C.; Jiang, Q.Y.; Chen, F.; Cao, Y. SERS-Based Biosensors Combined with Machine Learning for Medical Application. ChemistryOpen 2023, 12, e202200192. [Google Scholar] [CrossRef]
- Lin, D.; Wu, Q.; Qiu, S.; Chen, G.; Feng, S.; Chen, R.; Zeng, H. Label-free liquid biopsy based on blood circulating DNA detection using SERS-based nanotechnology for nasopharyngeal cancer screening. Nanomedicine 2019, 22, 102100. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zeng, Q.Y.; Li, L.F.; Qi, M.N.; Qi, Q.C.; Li, S.X.; Xu, J.F. Label-free rapid identification of tumor cells and blood cells with silver film SERS substrate. Opt. Express 2018, 26, 33044–33056. [Google Scholar] [CrossRef]
- Park, K.S.; Cha, H.; Niu, J.; Soh, H.T.; Lee, J.H.; Pack, S.P. DNA-controlled protein fluorescence: Design of aptamer-split peptide hetero-modulator for GFP to respond to intracellular ATP levels. Nucleic Acids Res. 2024, 52, 8063–8071. [Google Scholar] [CrossRef]
- Chen, H.; Zhuang, Z.; Guo, S.; Xie, S.; Xin, Y.; Chen, Y.; Ouyang, S.; Zhao, W.; Shen, K.; Tao, J.; et al. Artificial neural network processed linear-light tristimulus and hue parameters of fluorescence for smartphone assisted point-of-care testing device. Sens. Actuators B Chem. 2023, 384, 133659. [Google Scholar] [CrossRef]
- Roostaei, N.; Hamidi, S.M. Plasmonic smart contact lens based on etalon nanostructure for tear glucose sensing. Sci. Rep. 2025, 15, 14948. [Google Scholar] [CrossRef]
- Park, T.I.; Yang, A.H.; Kanth, B.K.; Pack, S.P. Aptamers as Diagnostic and Therapeutic Agents for Aging and Age-Related Diseases. Biosensors 2025, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Phan, L.M.T.; Cho, S. Fluorescent Aptasensor and Colorimetric Aptablot for p-tau231 Detection: Toward Early Diagnosis of Alzheimer’s Disease. Biomedicines 2022, 10, 93. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wu, J.; Chai, K.; Hu, X.; Hu, Y.; Shi, S.; Yao, T. A turn-on unlabeled colorimetric biosensor based on aptamer-AuNPs conjugates for amyloid-beta oligomer detection. Talanta 2023, 260, 124649. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Man, Y.; Xu, S.; Wu, H.; Ling, P.; Gao, F. A label-free dually-amplified aptamer sensor for the specific detection of amyloid-beta peptide oligomers in cerebrospinal fluids. Anal. Chim. Acta 2023, 1266, 341298. [Google Scholar] [CrossRef]
- Gao, S.; Li, Q.; Zhang, S.; Sun, X.; Zhou, H.; Wang, Z.; Wu, J. A novel biosensing platform for detection of glaucoma biomarker GDF15 via an integrated BLI-ELASA strategy. Biomaterials 2023, 294, 121997. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, H.; Han, X.; Liu, Z.; Lu, Y. Advancements and applications of loop-mediated isothermal amplification technology: A comprehensive overview. Front. Microbiol. 2024, 15, 1406632. [Google Scholar] [CrossRef]
- Manning, J.C.; Boza, J.M.; Cesarman, E.; Erickson, D. Rapid, equipment-free extraction of DNA from skin biopsies for point-of-care diagnostics. Sci. Rep. 2024, 14, 13782. [Google Scholar] [CrossRef]
- Barra, M.J.; Wilkinson, A.F.; Ma, A.E.; Goli, K.; Atif, H.; Osman, N.; Lorenzoni, C.; Tivir, G.; Lathrop, E.H.; Castle, P.E.; et al. One-hour extraction-free loop-mediated isothermal amplification HPV DNA assay for point-of-care testing in Maputo, Mozambique. Nat. Commun. 2025, 16, 7295. [Google Scholar] [CrossRef]
- Park, B.C.; Soh, J.O.; Choi, H.-J.; Park, H.S.; Lee, S.M.; Fu, H.E.; Kim, M.S.; Ko, M.J.; Koo, T.M.; Lee, J.-Y.; et al. Ultrasensitive and Rapid Circulating Tumor DNA Liquid Biopsy Using Surface-Confined Gene Amplification on Dispersible Magnetic Nano-Electrodes. ACS Nano 2024, 18, 12781–12794. [Google Scholar] [CrossRef]
- Yang, C.H.; Wu, T.H.; Chang, C.C.; Lo, H.Y.; Liu, H.W.; Huang, N.T.; Lin, C.W. Biosensing Amplification by Hybridization Chain Reaction on Phase-Sensitive Surface Plasmon Resonance. Biosensors 2021, 11, 75. [Google Scholar] [CrossRef]
- Hosseinzadeh, E.; Ravan, H.; Mohammadi, A.; Pourghadamyari, H. Colorimetric detection of miRNA-21 by DNAzyme-coupled branched DNA constructs. Talanta 2020, 216, 12913. [Google Scholar] [CrossRef]
- Peng, Y.; Lu, B.; Deng, Y.; Yang, N.; Li, G. A dual-recognition-controlled electrochemical biosensor for accurate and sensitive detection of specific circulating tumor cells. Biosens. Bioelectron. 2022, 201, 113973. [Google Scholar] [CrossRef]
- Jia, Y.-L.; Li, X.-Q.; Wang, Z.-X.; Gao, H.; Chen, H.-Y.; Xu, J.-J. Logic Signal Amplification System for Sensitive Electrochemiluminescence Detection and Subtype Identification of Cancer Cells. Anal. Chem. 2024, 96, 7172–7178. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Chadwick, A.C.; Mizoguchi, T.; Garcia, S.P.; DeNizio, J.E.; Reiss, C.W.; Wang, K.; Iyer, S.; Dutta, C.; Clendaniel, V.; et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 2021, 593, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Dai, Y.; Wang, X.; Yu, P.; Qu, S.; Liu, Z.; Cao, Y.; Zhang, L.; Ping, Y.; Liu, W.; et al. Determination of plasma beta-amyloids by rolling circle amplification chemiluminescent immunoassay for noninvasive diagnosis of Alzheimer’s disease. Mikrochim. Acta 2021, 188, 24. [Google Scholar] [CrossRef]
- Kwapisz, D. The first liquid biopsy test approved. Is it a new era of mutation testing for non-small cell lung cancer? Ann. Transl. Med. 2017, 5, 46. [Google Scholar] [CrossRef]
- Lim, S.B.; Menon, N.V.; Lim, C.T. Microfluidic tools for probing micro-culprits: Opportunities and challenges in microfluidic diagnostics. EMBO Rep. 2020, 21, e49749. [Google Scholar] [CrossRef]
- Meyer, A.R.; Gorin, M.A. First point-of-care PSA test for prostate cancer detection. Nat. Rev. Urol. 2019, 16, 331–332. [Google Scholar] [CrossRef]
- Xu, H.; Li, S.; Liu, Y.S. Nanoparticles in the diagnosis and treatment of vascular aging and related diseases. Signal Transduct. Target. Ther. 2022, 7, 231. [Google Scholar] [CrossRef]
- Ratinho, L.; Meyer, N.; Greive, S.; Cressiot, B.; Pelta, J. Nanopore sensing of protein and peptide conformation for point-of-care applications. Nat. Commun. 2025, 16, 3211. [Google Scholar] [CrossRef]
- Prasad, K.S.; Cao, X.; Gao, N.; Jin, Q.; Sanjay, S.T.; Henao-Pabon, G.; Li, X. A Low-Cost Nanomaterial-based Electrochemical Immunosensor on Paper for High-Sensitivity Early Detection of Pancreatic Cancer. Sens. Actuators B Chem. 2020, 305, 127516. [Google Scholar] [CrossRef]
- Resmi, A.N.; Nazeer, S.S.; Dhushyandhun, M.E.; Paul, W.; Chacko, B.P.; Menon, R.N.; Jayasree, R.S. Ultrasensitive Detection of Blood-Based Alzheimer’s Disease Biomarkers: A Comprehensive SERS-Immunoassay Platform Enhanced by Machine Learning. ACS Chem. Neurosci. 2024, 15, 4390–4401. [Google Scholar] [CrossRef]
- Palacio, I.; Moreno, M.; Nanez, A.; Purwidyantri, A.; Domingues, T.; Cabral, P.D.; Borme, J.; Marciello, M.; Mendieta-Moreno, J.I.; Torres-Vazquez, B.; et al. Attomolar detection of hepatitis C virus core protein powered by molecular antenna-like effect in a graphene field-effect aptasensor. Biosens. Bioelectron. 2023, 222, 115006. [Google Scholar] [CrossRef] [PubMed]
- Demeritte, T.; Nellore, B.P.V.; Kanchanapally, R.; Sinha, S.S.; Pramanik, A.; Chavva, S.R.; Ray, P.C. Hybrid Graphene Oxide Based Plasmonic-Magnetic Multifunctional Nanoplatform for Selective Separation and Label-Free Identification of Alzheimer’s Disease Biomarkers. ACS Appl. Mater. Interfaces 2015, 7, 13693–13700. [Google Scholar] [CrossRef] [PubMed]
- Daoutakou, M.; Kintzios, S. Point-of-Care Testing (POCT) for Cancer and Chronic Disease Management in the Workplace: Opportunities and Challenges in the Era of Digital Health Passports. Appl. Sci. 2025, 15, 6906. [Google Scholar] [CrossRef]
- Behera, P.P.; Kumar, N.; Kumari, M.; Kumar, S.; Mondal, P.K.; Arun, R.K. Integrated microfluidic devices for point-of-care detection of bio-analytes and disease. Sens. Diagn. 2023, 2, 1437–1459. [Google Scholar] [CrossRef]
- Heydari, A.; Sharifi, M.; Moghaddam, A.B. Challenges and Barriers to Providing Care to Older Adult Patients in the Intensive Care Unit: A Qualitative Research. Open Access Maced. J. Med. Sci. 2019, 7, 3682–3690. [Google Scholar] [CrossRef]
- Pullano, S.A.; Greco, M.; Bianco, M.G.; Foti, D.; Brunetti, A.; Fiorillo, A.S. Glucose biosensors in clinical practice: Principles, limits and perspectives of currently used devices. Theranostics 2022, 12, 493–511. [Google Scholar] [CrossRef]
- van der Flier, W.M.; de Vugt, M.E.; Smets, E.M.A.; Blom, M.; Teunissen, C.E. Towards a future where Alzheimer’s disease pathology is stopped before the onset of dementia. Nat. Aging 2023, 3, 494–505. [Google Scholar] [CrossRef]
- Naseem, M.S. Tumor biomarkers from discovery to clinical practice. J. Cancer Biol. 2022, 3, 19–32. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Qiu, J.; Xu, J.; Yang, X.; Zhang, Y.; Tian, X.; Guan, X.; Cen, X.; Zhao, Y. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Sig Transduct. Target. Ther. 2024, 9, 132. [Google Scholar] [CrossRef] [PubMed]
- Sugeeta, S.S.; Sharma, A.; Ng, K.; Nayak, A.; Vasdev, N. Biomarkers in Bladder Cancer Surveillance. Front. Surg. 2021, 8, 735868. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Li, Y.; Huang, Y.; Wu, J.; Bao, W.; Xue, C.; Li, X.; Dong, S.; Dong, Z.; Hu, S. Advances in molecular pathology and therapy of non-small cell lung cancer. Sig Transduct. Target. Ther. 2025, 10, 186. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.J.; Zhong, X.H.; Duan, L.X. Integration of artificial intelligence and multi-omics in kidney diseases. Fundam. Res. 2023, 3, 126–148. [Google Scholar] [CrossRef]
- Zhang, J.; Che, Y.; Liu, R.; Wang, Z.; Liu, W. Deep learning–driven multi-omics analysis: Enhancing cancer diagnostics and therapeutics. Brief. Bioinform. 2025, 26, bbaf440. [Google Scholar] [CrossRef]
- Purnama, A.; Drago, D. MEDICAL DEVICES: FDA Regulatory Pathways for Medical Devices. Available online: https://www.topra.org/topra/topra_member/pdfs/CPD-May-2019-Medical-Devices-and-FDA.pdf (accessed on 9 October 2025).
- FDA. 510(k) Clearances. Available online: https://www.fda.gov/medical-devices/device-approvals-and-clearances/510k-clearances?utm_source=chatgpt.com (accessed on 15 October 2025).
- FDA. Premarket Approval (PMA). Available online: https://www.fda.gov/medical-devices/premarket-submissions-selecting-and-preparing-correct-submission/premarket-approval-pma?utm_source=chatgpt.com (accessed on 15 October 2025).
- EMA’s Committee. Qualification of Novel Methodologies for Medicine Development. Available online: https://www.ema.europa.eu/en/qualification-novel-methodologies-medicine-development?utm_source=chatgpt.com (accessed on 9 October 2025).
- Sturgeon, C.; Hill, R.; Hortin, G.L.; Thompson, D. Taking a new biomarker into routine use--a perspective from the routine clinical biochemistry laboratory. Proteom. Clin. Appl. 2010, 4, 892–903. [Google Scholar] [CrossRef]
- Budelier, M.M.; Hubbard, J.A. The regulatory landscape of laboratory developed tests: Past, present, and a perspective on the future. J. Mass. Spectrom. Adv. Clin. Lab. 2023, 28, 67–69. [Google Scholar] [CrossRef]
- Gonzalez, S.J. Federal District Court Vacates FDA’s Laboratory Developed Tests Final Rule. Available online: https://www.thefdalawblog.com/2025/04/federal-district-court-vacates-fdas-laboratory-developed-tests-final-rule/ (accessed on 9 October 2025).
- FDA. Regulation Identification Number 0910-AJ05 Medical Devices; Laboratory Developed Tests; Implementation of Vacatur. Available online: https://www.federalregister.gov/documents/2024/05/06/2024-08935/medical-devices-laboratory-developed-tests (accessed on 9 October 2025).
- McShane, L.M.; Lively, T.G.; Makhlouf, H.R. Translation of Biomarkers into Clinical Practice. In Molecular Pathology of Breast Cancer; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–18. [Google Scholar]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 guidelines for reporting diagnostic accuracy studies: Explanation and elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef]
- Sittimart, M.; Rattanavipapong, W.; Mirelman, A.J.; Hung, T.M.; Dabak, S.; Downey, L.E.; Jit, M.; Teerawattananon, Y.; Turner, H.C. An overview of the perspectives used in health economic evaluations. Cost. Eff. Resour. Alloc. 2024, 22, 41. [Google Scholar] [CrossRef]
- Schunemann, H.J.; Reinap, M.; Piggott, T.; Laidmae, E.; Kohler, K.; Pold, M.; Ens, B.; Irs, A.; Akl, E.A.; Cuello, C.A.; et al. The ecosystem of health decision making: From fragmentation to synergy. Lancet Public Health 2022, 7, e378–e390. [Google Scholar] [CrossRef]
- Schroll, M.M.; Quinn, E.; Pritchard, D.; Chang, A.; Amanti, K.G.; Perez, O.; Agarwal, A.; Gustavsen, G. Perspectives on Clinical Adoption Barriers to Blood-Based Multi-Cancer Early Detection Tests across Stakeholders. J. Pers. Med. 2024, 14, 593. [Google Scholar] [CrossRef]
- Anghel, S.A.; Ionita-Mindrican, C.B.; Luca, I.; Pop, A.L. Promising Epigenetic Biomarkers for the Early Detection of Colorectal Cancer: A Systematic Review. Cancers 2021, 13, 4965. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. FDA Approves First Non-Invasive DNA Screening Test for Colorectal cancer. Available online: https://wayback.archive-it.org/7993/20170112222835/http:/www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm409021.htm (accessed on 9 October 2025).
- Milbury, C.A.; Creeden, J.; Yip, W.K.; Smith, D.L.; Pattani, V.; Maxwell, K.; Sawchyn, B.; Gjoerup, O.; Meng, W.; Skoletsky, J.; et al. Clinical and analytical validation of FoundationOne(R)CDx, a comprehensive genomic profiling assay for solid tumors. PLoS ONE 2022, 17, e0264138. [Google Scholar] [CrossRef]
- Wisch, J.K.; Ances, B.M. Ruling in, ruling out: The clinical utility of plasma biomarkers in diagnosis of Alzheimer’s disease. J. Clin. Investig. 2025, 135, e198725. [Google Scholar] [CrossRef] [PubMed]
- Argueta-Diaz, V.; Owens, M.; Al Ramadan, A. Increasing Optical Path Lengths in Micro-Fluidic Devices Using a Multi-Pass Cell. Micromachines 2024, 15, 820. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dou, W.; Zhou, C.; Wang, X.; Yang, A.; Zhang, Y.; Qiao, D. A Microtester for Measuring the Reliability of Microdevices in Controlled Environmental Conditions. Micromachines 2021, 12, 585. [Google Scholar] [CrossRef]
- Gerken, E.; Konig, A. Enhancing Reliability in Redundant Homogeneous Sensor Arrays with Self-X and Multidimensional Mapping. Sensors 2025, 25, 3841. [Google Scholar] [CrossRef]
- Gunasegaram, D.R.; Barnard, A.S.; Matthews, M.J.; Jared, B.H.; Andreaco, A.M.; Bartsch, K.; Murphy, A.B. Machine learning-assisted in-situ adaptive strategies for the control of defects and anomalies in metal additive manufacturing. Addit. Manuf. 2024, 81, 104013. [Google Scholar] [CrossRef]
- Bernhardt, M.; Jones, C.; Glocker, B. Potential sources of dataset bias complicate investigation of underdiagnosis by machine learning algorithms. Nat. Med. 2022, 28, 1157–1158. [Google Scholar] [CrossRef]
- Poulain, R.; Tarek, M.F.B.; Beheshti, R. Improving Fairness in AI Models on Electronic Health Records: The Case for Federated Learning Methods. FAccT 23 (2023) 2023, 2023, 1599–1608. [Google Scholar] [CrossRef]
- Chen, R.J.; Wang, J.J.; Williamson, D.F.K.; Chen, T.Y.; Lipkova, J.; Lu, M.Y.; Sahai, S.; Mahmood, F. Algorithmic fairness in artificial intelligence for medicine and healthcare. Nat. Biomed. Eng. 2023, 7, 719–742. [Google Scholar] [CrossRef]
- Guo, L.L.; Pfohl, S.R.; Fries, J.; Johnson, A.E.W.; Posada, J.; Aftandilian, C.; Shah, N.; Sung, L. Evaluation of domain generalization and adaptation on improving model robustness to temporal dataset shift in clinical medicine. Sci. Rep. 2022, 12, 2726. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, D.; Bendechache, M. Unveiling the black box: A systematic review of Explainable Artificial Intelligence in medical image analysis. Comput. Struct. Biotechnol. J. 2024, 24, 542–560. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Verma, C.; Illés, Z. Federated learning with explainable AI for liver disease prediction: A privacy-preserving approach. Intell. Based Med. 2025, 12, 100285. [Google Scholar] [CrossRef]
- Erdemli, G.; Murphy, T.; Walinsky, S. Regulatory considerations for successful implementation of digital endpoints in clinical trials for drug development. NPJ Digit. Med. 2025, 8, 142. [Google Scholar] [CrossRef]
- Zu, Y.; Chang, H.; Cui, Z. Molecular point-of-care testing technologies: Current status and challenges. Nexus 2025, 2, 100059. [Google Scholar] [CrossRef]
- Chong, S.W.; Shen, Y.; Palomba, S.; Vigolo, D. Nanofluidic Lab-On-A-Chip Systems for Biosensing in Healthcare. Small 2024, 21, 2407478. [Google Scholar] [CrossRef]
- Siavashy, S.; Soltani, M.; Rahimi, S.; Hosseinali, M.; Guilandokht, Z.; Raahemifar, K. Recent advancements in microfluidic-based biosensors for detection of genes and proteins: Applications and techniques. Biosens. Bioelectron. X 2024, 19, 100489. [Google Scholar] [CrossRef]
- Monfared, Y.E. Refractive Index Sensor Based on Surface Plasmon Resonance Excitation in a D-Shaped Photonic Crystal Fiber Coated by Titanium Nitride. Plasmonics 2019, 15, 535–542. [Google Scholar] [CrossRef]
- Toma, A.d.; Brunetti, G.; Colapietro, P.; Ciminelli, C. High-Resolved Near-Field Sensing by Means of Dielectric Grating With a Box-Like Resonance Shape. IEEE Sens. J. 2024, 24, 6045–6053. [Google Scholar] [CrossRef]
- Ramadan, Q.; Zourob, M. Organ-on-a-chip engineering: Toward bridging the gap between lab and industry. Biomicrofluidics 2020, 14, 041501. [Google Scholar] [CrossRef]
- Shinde, A.; Illath, K.; Kasiviswanathan, U.; Nagabooshanam, S.; Gupta, P.; Dey, K.; Chakrabarty, P.; Nagai, M.; Rao, S.; Kar, S.; et al. Recent Advances of Biosensor-Integrated Organ-on-a-Chip Technologies for Diagnostics and Therapeutics. Anal. Chem. 2023, 95, 3121–3146. [Google Scholar] [CrossRef] [PubMed]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Cauli, E.; Polidoro, M.A.; Marzorati, S.; Bernardi, C.; Rasponi, M.; Lleo, A. Cancer-on-chip: A 3D model for the study of the tumor microenvironment. J. Biol. Eng. 2023, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Holman, J.B.; Shi, Z.; Qiu, B.; Ding, W. On-chip modeling of tumor evolution: Advances, challenges and opportunities. Mater. Today Bio 2023, 21, 100724. [Google Scholar] [CrossRef]
- Yang, H.; Li, J.; Wang, Z.; Khutsishvili, D.; Tang, J.; Zhu, Y.; Cai, Y.; Dai, X.; Ma, S. Bridging the organoid translational gap: Integrating standardization and micropatterning for drug screening in clinical and pharmaceutical medicine. Life Med. 2024, 3, lnae016. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, R.; Song, Y.; Wei, W.; Baek, D.; Gillin, M.; Kurabayashi, K.; Chen, W. Cancer-on-a-chip for precision cancer medicine. Lab. Chip 2025, 25, 3314–3347. [Google Scholar] [CrossRef]
- Han, Y.; Albert, P.S.; Berg, C.D.; Wentzensen, N.; Katki, H.A.; Liu, D. Statistical approaches using longitudinal biomarkers for disease early detection: A comparison of methodologies. Stat. Med. 2020, 39, 4405–4420. [Google Scholar] [CrossRef]
- He, S.; Zhu, C.; Liu, Y.; Xu, Z.; Sun, R.; Yang, B.; Guo, X.; Herrmann, I.M.; Muñoz, L.E.; Gjertsson, I.; et al. A longitudinal cohort study uncovers plasma protein biomarkers predating clinical onset and treatment response of rheumatoid arthritis. Nat. Commun. 2025, 16, 6692. [Google Scholar] [CrossRef]
- Chu, S.S.; Nguyen, H.A.; Zhang, J.; Tabassum, S.; Cao, H. Towards Multiplexed and Multimodal Biosensor Platforms in Real-Time Monitoring of Metabolic Disorders. Sensors 2022, 22, 5200. [Google Scholar] [CrossRef]
- Huang, G.; Chen, X.; Liao, C. AI-Driven Wearable Bioelectronics in Digital Healthcare. Biosensors 2025, 15, 410. [Google Scholar] [CrossRef]
- Chandel, M.; Rosenkranz, A.; Moraru, D.; Woźniak, A.; Jastrzębska, A.M. Measuring the Future—Nanometrology for Advanced Manufacturing of Miniaturized Devices. Adv. Funct. Mater. 2025, 2501663. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).