Nanobiosensors for Single-Molecule Diagnostics: Toward Integration with Super-Resolution Imaging
Abstract
1. Introduction
2. Nanobiosensors: Overview
2.1. Biosensor
2.2. Nanobiosensors
2.2.1. Working Principle
2.2.2. Nanomaterials
2.3. Microfluidics and Nanobiosensors
2.4. Surface Functionalization
2.5. Nanobiosensor Components
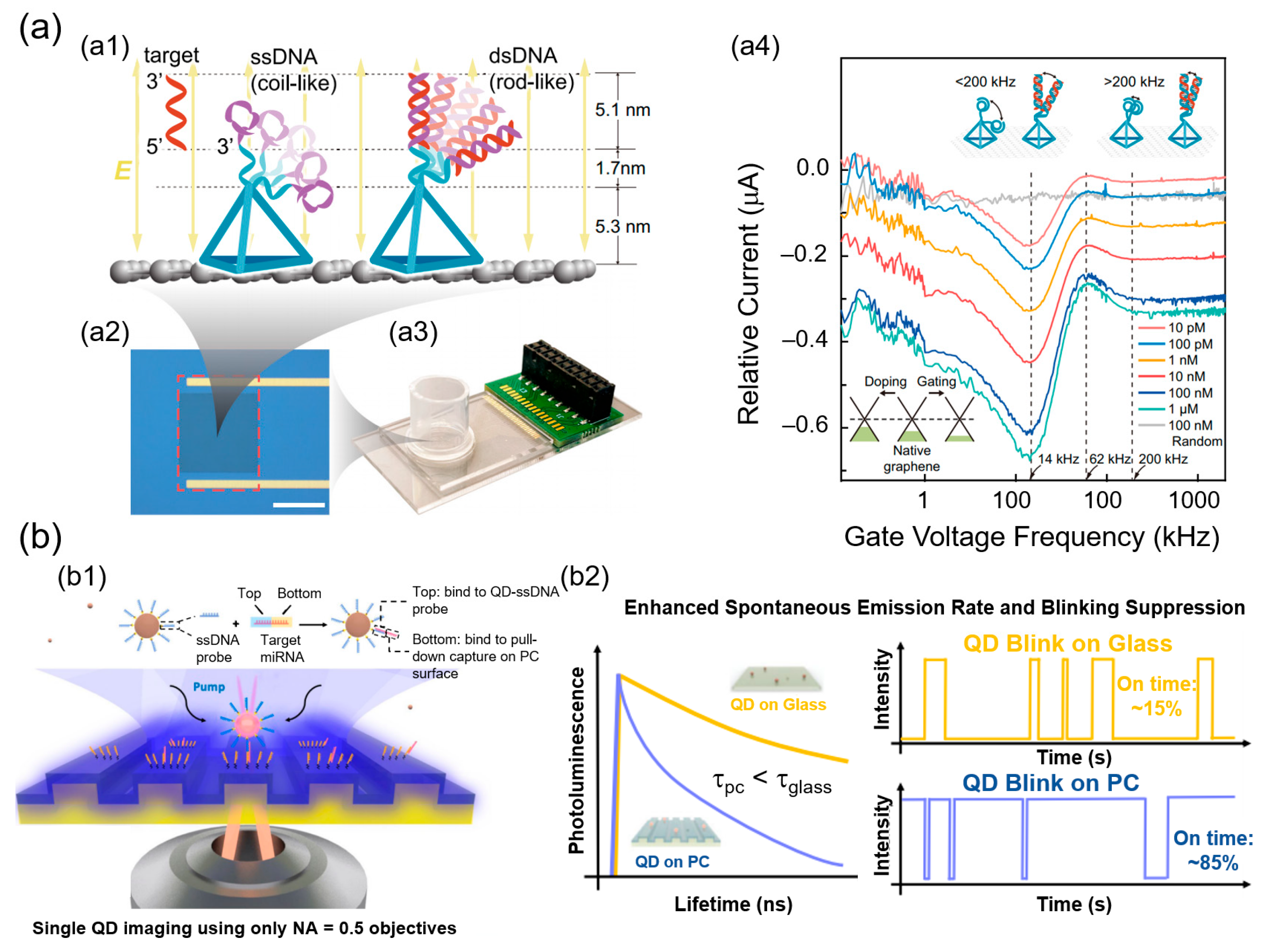
3. SMD Biosensors
3.1. Electrochemical Nanobiosensors
3.2. Optical Nanobiosensors
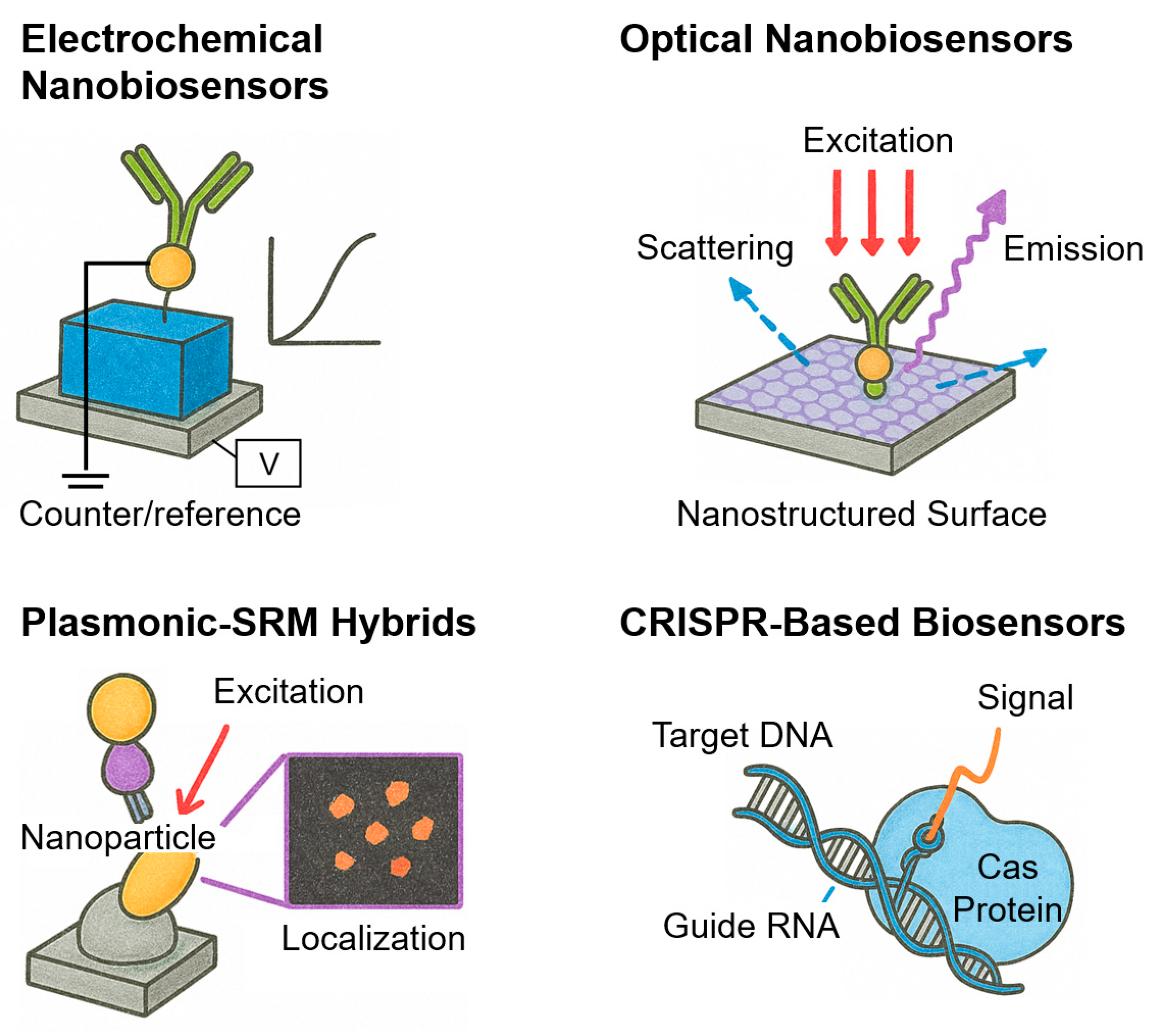
3.3. Plasmonic–SRM Hybrids
3.4. CRISPR-Based Biosensors
3.4.1. CRISPR/Cas-Based Fluorescent Biosensors
3.4.2. CRISPR/Cas-Based Nonfluorescent Biosensor
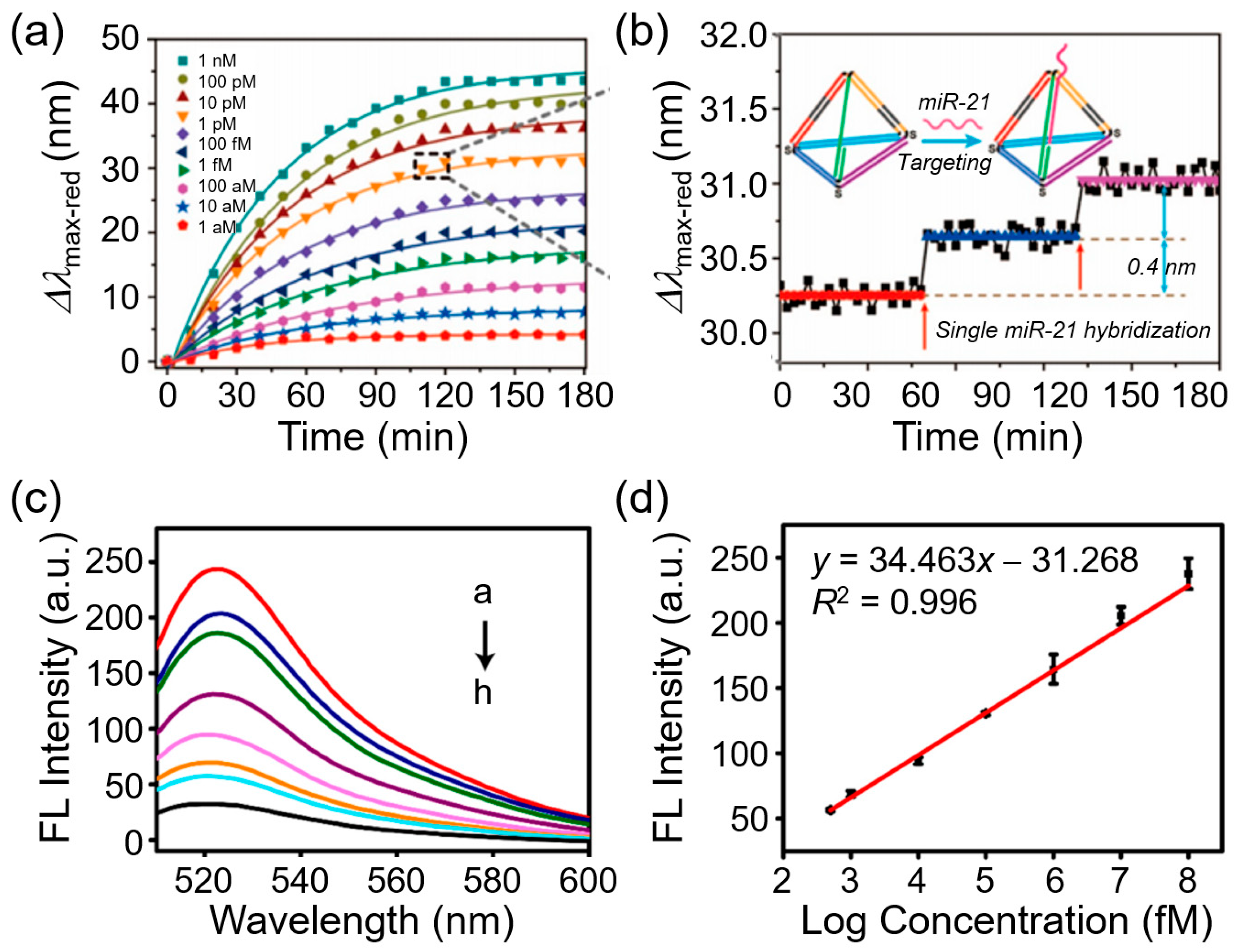
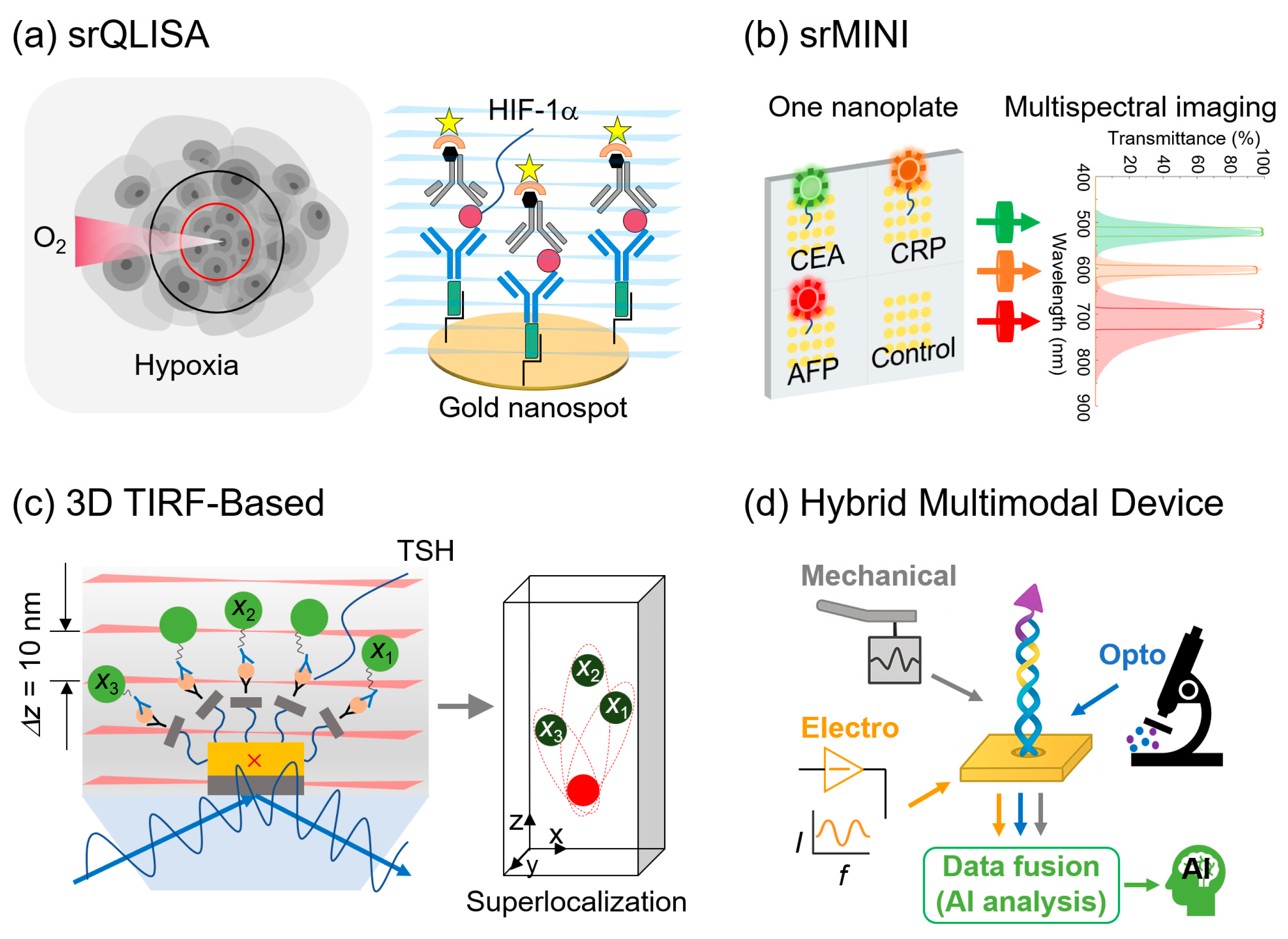
3.5. Fluorescent Nanoprobe–SRM Platforms
3.5.1. Conventional Single-Molecule Immunoassays with SRM
3.5.2. Advanced Nanoimmunosensors
3.6. Hybrid and Multimodal Devices
4. Integration of Nanobiosensors and SRM
4.1. Synergistic Signal Amplification and Spatial Resolution
4.2. Multiplexing and Spatiotemporal Profiling
4.3. Nanostructure-Assisted SRM
4.4. Technical Challenges and Opportunities in SRM Integration
4.5. Comparison with Previous Reviews (2018–2025)
5. Applications in Biomolecular Diagnostics
6. Clinical Translation Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AgNP | silver nanoparticle |
| AFP | alpha-fetoprotein |
| AI | artificial intelligence |
| AuNP | gold nanoparticle |
| BAs | biogenic amines |
| BSA | bovine serum albumin |
| CNTs | carbon nanotubes |
| CEA | carcinoembryonic antigen |
| CRP | C-reactive protein |
| DNA-PAINT | DNA points accumulation for imaging in nanoscale topography |
| dSTORM | direct stochastic optical reconstruction microscopy |
| ELISA | enzyme-linked immunosorbent assay |
| EV | extracellular vesicle |
| FET | field-effect transistor |
| GFET | graphene field-effect transistor |
| GO | graphene oxide |
| HBV | hepatitis B virus |
| HER2 | human epidermal growth factor receptor 2 |
| HIV | human immunodeficiency virus |
| HPV | human papillomavirus |
| IgG | Immunoglobulin G |
| iSCAT | interferometric scattering microscopy |
| ITO | indium tin oxide |
| LOD | limit of detection |
| LOC | lab-on-a-chip |
| LSPR | localized surface plasmon resonance |
| MEF | metal-enhanced fluorescence |
| MERS | Middle East respiratory syndrome |
| MIPs | molecularly imprinted polymers |
| miRNA | microRNA |
| MINFLUX | minimal photon fluxes |
| MXenes | transition-metal carbides/nitrides (2D nanomaterials) |
| NPs | nanoparticles |
| OECTs | organic electrochemical transistors |
| PALM | photoactivated localization microscopy |
| PC | photonic crystal |
| PCR | polymerase chain reaction |
| PEG | polyethylene glycol |
| POC | point-of-care |
| QDs | quantum dots |
| RT-PCR | reverse transcription polymerase chain reaction |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SERS | surface-enhanced Raman spectroscopy |
| SMD | single-molecule detection |
| smFRET | single-molecule fluorescence resonance energy transfer |
| SMLM | single-molecule localization microscopy |
| SPR | surface plasmon resonance |
| srQLISA | super-resolution quantum dot-linked immunosorbent assay |
| SRM | super-resolution microscopy |
| srMINI | super-resolution multispectral imaging nanoimmunosensor |
| STED | stimulated emission depletion microscopy |
| STORM | stochastic optical reconstruction microscopy |
| TIRF | total internal reflection fluorescence |
| TSH | thyroid-stimulating hormone |
| WGM | whispering-gallery mode |
References
- Herkert, E.K.; Bermeo Alvaro, D.R.; Recchia, M.; Langbein, W.; Borri, P.; Garcia-Parajo, M.F. Hybrid Plasmonic Nanostructures for Enhanced Single-Molecule Detection Sensitivity. ACS Nano 2023, 17, 8453–8464. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Kim, J.; Song, E.; Han, S.; Hohng, S. Analytical Techniques for Nucleic Acid and Protein Detection with Single-Molecule Sensitivity. Exp. Mol. Med. 2025, 57, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xu, Y.; Cao, M.; Chen, N.; Zeng, Q.; Lai, M.K.P.; Fan, D.; Sethi, G.; Cao, Y. Fluid-Based Biomarkers for Neurodegenerative Diseases. Ageing Res. Rev. 2025, 108, 102739. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; Cui, Y.; Yuan, J.; Fang, X. Deep Learning in Single-Molecule Imaging and Analysis: Recent Advances and Prospects. Chem. Sci. 2022, 13, 11964–11980. [Google Scholar] [CrossRef]
- Yan, J.; Cheng, L.; Li, Y.; Wang, R.; Wang, J. Advancements in Single-Molecule Fluorescence Detection Techniques and Their Expansive Applications in Drug Discovery and Neuroscience. Biosensors 2025, 15, 283. [Google Scholar] [CrossRef]
- Prakash, K.; Baddeley, D.; Eggeling, C.; Fiolka, R.; Heintzmann, R.; Manley, S.; Radenovic, A.; Shroff, H.; Smith, C.; Schermelleh, L. Resolution in Super-Resolution Microscopy—Facts, Artifacts, Technological Advancements and Biological Applications. J. Cell Sci. 2025, 138, jcs263567. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, Y.; Peng, Y.; Zhao, S.; Xu, M.; Zhang, L.; Huang, Z.; Shi, J.; Yang, Y. Recent Development of Surface-Enhanced Raman Scattering for Biosensing. J. Nanobiotechnol. 2023, 21, 149. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Cao, Y.; An, C.; Kang, S.H. Nanomaterial-Based Single-Molecule Optical Immunosensors for Supersensitive Detection. Biosens. Bioelectron. X 2022, 11, 100191. [Google Scholar] [CrossRef]
- Dey, S.; Dolci, M.; Zijlstra, P. Single-Molecule Optical Biosensing: Recent Advances and Future Challenges. ACS Phys. Chem. Au 2023, 3, 143–156. [Google Scholar] [CrossRef]
- Hemdan, M.; Abuelhaded, K.; Shaker, A.A.S.; Ashour, M.M.; Abdelaziz, M.M.; Dahab, M.I.; Nassar, Y.A.; Sarguos, A.M.M.; Zakaria, P.S.; Fahmy, H.A.; et al. Recent Advances in Nano-Enhanced Biosensors: Innovations in Design, Applications in Healthcare, Environmental Monitoring, and Food Safety, and Emerging Research Challenges. Sens. Bio-Sens. Res. 2025, 48, 100783. [Google Scholar] [CrossRef]
- Singh, N.; Dkhar, D.S.; Chandra, P.; Azad, U.P. Nanobiosensors Design Using 2D Materials: Implementation in Infectious and Fatal Disease Diagnosis. Biosensors 2023, 13, 166. [Google Scholar] [CrossRef]
- Singh, B.; Ma, S.; Hara, T.O.; Singh, S. Nanomaterials-Based Biosensors for the Detection of Prostate Cancer Biomarkers: Recent Trends and Future Perspective. Adv. Mater. Technol. 2023, 8, 2201860. [Google Scholar] [CrossRef]
- Darwish, M.A.; Abd-Elaziem, W.; Elsheikh, A.; Zayed, A.A. Advancements in Nanomaterials for Nanosensors: A Comprehensive Review. Nanoscale Adv. 2024, 6, 4015–4046. [Google Scholar] [CrossRef]
- Conte, R.; Foggia, R.; Valentino, A.; Salle, A.D.; Kandsi, F.; Calarco, A. Nanotechnology Advancements Transforming Molecular Diagnostics: Applications in Precision Healthcare. Int. J. Nano Dimens. 2024, 15. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Rebelo, R.; Reis, R.L.; Bhattacharya, M.; Correlo, V.M. Current Nanotechnology Advances in Diagnostic Biosensors. Med. Devices Sens. 2021, 4, e10156. [Google Scholar] [CrossRef]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef]
- Anker, J.N.; Hall, W.P.; Lyandres, O.; Shah, N.C.; Zhao, J.; Van Duyne, R.P. Biosensing with Plasmonic Nanosensors. Nat. Mater. 2008, 7, 442–453. [Google Scholar] [CrossRef]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors|Chemical Reviews. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef]
- Hell, S.W.; Wichmann, J. Breaking the Diffraction Resolution Limit by Stimulated Emission: Stimulated-Emission-Depletion Fluorescence Microscopy. Opt. Lett. 1994, 19, 780. [Google Scholar] [CrossRef] [PubMed]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging Intracellular Fluorescent Proteins at Nanometer Resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-Diffraction-Limit Imaging by Stochastic Optical Reconstruction Microscopy (STORM). Nat. Methods 2006, 3, 793–796. [Google Scholar] [CrossRef]
- Banerjee, A.; Maity, S.; Mastrangelo, C.H. Nanostructures for Biosensing, with a Brief Overview on Cancer Detection, IoT, and the Role of Machine Learning in Smart Biosensors. Sensors 2021, 21, 1253. [Google Scholar] [CrossRef]
- Gulati, S.; Yadav, R.; Kumari, V.; Nair, S.; Gupta, C.; Aishwari, M. Nanosensors in Healthcare: Transforming Real-Time Monitoring and Disease Management with Cutting-Edge Nanotechnology. RSC Pharm. 2025. [Google Scholar] [CrossRef]
- Ramesh, M.; Janani, R.; Deepa, C.; Rajeshkumar, L. Nanotechnology-Enabled Biosensors: A Review of Fundamentals, Design Principles, Materials, and Applications. Biosensors 2023, 13, 40. [Google Scholar] [CrossRef]
- Hassan, R.Y.A. Advances in Electrochemical Nano-Biosensors for Biomedical and Environmental Applications: From Current Work to Future Perspectives. Sensors 2022, 22, 7539. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, Y.; Kianfar, E. Nano Biosensors: Properties, Applications and Electrochemical Techniques. J. Mater. Res. Technol. 2021, 12, 1649–1672. [Google Scholar] [CrossRef]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Lutomia, D.; Poria, R.; Kala, D.; Kumar Singh, A.; K Gupta, M.; Kumar, D.; Kaushal, A.; Gupta, S. Unlocking the Potential of 2D Nanomaterial-Based Biosensors in Biomarker-Based Detection of Helicobacter Pylori. Mater. Adv. 2025, 6, 117–142. [Google Scholar] [CrossRef]
- Abid, N.; Khan, A.M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-down and Bottom-up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid Interface Sci. 2022, 300, 102597. [Google Scholar] [CrossRef]
- Al-Harbi, N.; Abd-Elrahman, N.K. Physical Methods for Preparation of Nanomaterials, Their Characterization and Applications: A Review. J. Umm Al-Qura Univ. Appl. Sci. 2025, 11, 356–377. [Google Scholar] [CrossRef]
- Dhaka, A.; Chand Mali, S.; Sharma, S.; Trivedi, R. A Review on Biological Synthesis of Silver Nanoparticles and Their Potential Applications. Results Chem. 2023, 6, 101108. [Google Scholar] [CrossRef]
- Gupta, D.; Boora, A.; Thakur, A.; Gupta, T.K. Green and Sustainable Synthesis of Nanomaterials: Recent Advancements and Limitations. Environ. Res. 2023, 231, 116316. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Kumar Sachan, R.S.; Devgon, I.; Devgon, J.; Pant, G.; Panchpuri, M.; Ahmad, A.; Alshammari, M.B.; Hossain, K.; Kumar, G. Gold Nanoparticles in Nanobiotechnology: From Synthesis to Biosensing Applications. ACS Omega 2024, 9, 29966–29982. [Google Scholar] [CrossRef]
- Hughes, K.J.; Iyer, K.A.; Bird, R.E.; Ivanov, J.; Banerjee, S.; Georges, G.; Zhou, Q.A. Review of Carbon Nanotube Research and Development: Materials and Emerging Applications. ACS Appl. Nano Mater. 2024, 7, 18695–18713. [Google Scholar] [CrossRef]
- Heydari-Bafrooei, E.; Ensafi, A.A. Nanomaterials-Based Biosensing Strategies for Biomarkers Diagnosis, a Review. Biosens. Bioelectron. X 2023, 13, 100245. [Google Scholar] [CrossRef]
- Banerjee, A.; Maity, S.; Mastrangelo, C.H. Nanotechnology for Biosensors: A Review. arXiv 2021, arXiv:2101.02430. [Google Scholar] [CrossRef]
- Huang, C.-C.; Kuo, Y.-H.; Chen, Y.-S.; Huang, P.-C.; Lee, G.-B. A Miniaturized, DNA-FET Biosensor-Based Microfluidic System for Quantification of Two Breast Cancer Biomarkers|Microfluidics and Nanofluidics. Microfluid. Nanofluid. 2021, 25, 33. [Google Scholar] [CrossRef]
- Jamiruddin, M.R.; Meghla, B.A.; Islam, D.Z.; Tisha, T.A.; Khandker, S.S.; Khondoker, M.U.; Haq, M.A.; Adnan, N.; Haque, M. Microfluidics Technology in SARS-CoV-2 Diagnosis and Beyond: A Systematic Review. Life 2022, 12, 649. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yu, N.; Zhang, J.; Yang, B. Advances in Microfluidic Single-Cell RNA Sequencing and Spatial Transcriptomics. Micromachines 2025, 16, 426. [Google Scholar] [CrossRef]
- Zou, S.; Peng, G.; Ma, Z. Surface-Functionalizing Strategies for Multiplexed Molecular Biosensing: Developments Powered by Advancements in Nanotechnologies. Nanomaterials 2024, 14, 2014. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Bonilla-Cruz, J. Review on Healthcare Biosensing Nanomaterials. ACS Appl. Nano Mater. 2023, 6, 5042–5074. [Google Scholar] [CrossRef]
- Ngernpimai, S.; Puangmali, T.; Kopwitthaya, A.; Tippayawat, P.; Chompoosor, A.; Teerasong, S. Enhanced Stability of Gold Nanoparticles with Thioalkylated Carboxyl-Terminated Ligands for Applications in Biosensing. ACS Appl. Nano Mater. 2024, 7, 13124–13133. [Google Scholar] [CrossRef]
- Wang, Z.; Dai, W.; Zhang, Z.; Wang, H. Aptamer-Based Graphene Field-Effect Transistor Biosensor for Cytokine Detection in Undiluted Physiological Media for Cervical Carcinoma Diagnosis. Biosensors 2025, 15, 138. [Google Scholar] [CrossRef]
- Zhang, T.; Liang, T.; Pan, Q.; Zhang, S.; Zhang, S.; Geng, Z.; Zhu, B. A Universal and Versatile Zwitterionic Coating for Blood-Contacting Catheters with Long Lengths and Complex Geometries. Adv. Sci. 2025, 12, 2502411. [Google Scholar] [CrossRef]
- Zhan, P.; Peil, A.; Jiang, Q.; Wang, D.; Mousavi, S.; Xiong, Q.; Shen, Q.; Shang, Y.; Ding, B.; Lin, C.; et al. Recent Advances in DNA Origami-Engineered Nanomaterials and Applications|Chemical Reviews. Chem. Rev. 2023, 123, 3976–4050. [Google Scholar] [CrossRef]
- Lee, J.-C.; Kim, S.Y.; Song, J.; Jang, H.; Kim, M.; Kim, H.; Choi, S.Q.; Kim, S.; Jolly, P.; Kang, T.; et al. Micrometer-Thick and Porous Nanocomposite Coating for Electrochemical Sensors with Exceptional Antifouling and Electroconducting Properties. Nat. Commun. 2024, 15, 711. [Google Scholar] [CrossRef]
- Manoharan Nair Sudha Kumari, S.; Thankappan Suryabai, X. Sensing the Future─Frontiers in Biosensors: Exploring Classifications, Principles, and Recent Advances. ACS Omega 2024, 9, 48918–48987. [Google Scholar] [CrossRef] [PubMed]
- Mpofu, K.; Chauke, S.; Thwala, L.; Mthunzi-Kufa, P. Aptamers and Antibodies in Optical Biosensing. Discov. Chem. 2025, 2, 23. [Google Scholar] [CrossRef]
- Roy, S.S.; Raj, D. Sensors and Modern Transducers. In Emerging Sensors for Environmental Monitoring; Elsevier: Amsterdam, The Netherlands, 2025; pp. 43–55. ISBN 978-0-443-13894-2. [Google Scholar]
- Polat, E.O.; Cetin, M.M.; Tabak, A.F.; Bilget Güven, E.; Uysal, B.Ö.; Arsan, T.; Kabbani, A.; Hamed, H.; Gül, S.B. Transducer Technologies for Biosensors and Their Wearable Applications. Biosensors 2022, 12, 385. [Google Scholar] [CrossRef]
- Hemdan, M.; Ali, M.A.; Doghish, A.S.; Mageed, S.S.A.; Elazab, I.M.; Khalil, M.M.; Mabrouk, M.; Das, D.B.; Amin, A.S. Innovations in Biosensor Technologies for Healthcare Diagnostics and Therapeutic Drug Monitoring: Applications, Recent Progress, and Future Research Challenges. Sensors 2024, 24, 5143. [Google Scholar] [CrossRef]
- Qazi, R.A.; Aman, N.; Ullah, N.; Jamila, N.; Bibi, N. Recent Advancement for Enhanced e. Coli Detection in Electrochemical Biosensors. Microchem. J. 2024, 196, 109673. [Google Scholar] [CrossRef]
- Yun, J.; Keerthana, S.; Kwon, S.-R. Miniaturized Power-Integrated and Self-Powered Sensor Systems for Advanced Biomedical Applications. Sens. Actuators Rep. 2025, 9, 100260. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, S.; Li, H.; Yang, T.; Zheng, K.; Guo, Z.M.; Shi, J.; Huang, X.; Zou, X.; Picchetti, P.; et al. Design Principles of Nanosensors for Multiplex Detection of Contaminants in Food. Small 2025, 21, 2412271. [Google Scholar] [CrossRef]
- Varadharajan, S.; Gadre, M.; Mathur, V.; Vasanthan, K.S. Sustainable Integration of Nanobiosensors in Biomedical and Civil Engineering: A Comprehensive Review. ACS Omega 2025, 10, 25120–25157. [Google Scholar] [CrossRef] [PubMed]
- Sans, J.; Azevedo Gonçalves, I.; Quintana, R. Establishing Quartz Crystal Microbalance with Dissipation (QCM-D) Coupled with Spectroscopic Ellipsometry (SE) as an Advantageous Technique for the Characterization of Ultra-Thin Film Hydrogels. Small 2024, 20, 2312041. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Li, Z.; Gao, X.; Liu, W.; Zhao, M.; Ren, Y.; Ding, Q.; Li, B.; Song, Y.; Zheng, B.; et al. Enhancing Magnetic Bead Detection: Structural Innovations in GMR Sensors. IEEE Sens. J. 2025, 25, 13048–13054. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Surface-Enhanced Raman Scattering and Biophysics. J. Phys. Condens. Matter 2002, 14, R597–R624. [Google Scholar] [CrossRef]
- Camden, J.P.; Dieringer, J.A.; Wang, Y.; Masiello, D.J.; Marks, L.D.; Schatz, G.C.; Van Duyne, R.P. Probing the Structure of Single-Molecule Surface-Enhanced Raman Scattering Hot Spots. J. Am. Chem. Soc. 2008, 130, 12616–12617. [Google Scholar] [CrossRef]
- Akkilic, N.; Geschwindner, S.; Höök, F. Single-Molecule Biosensors: Recent Advances and Applications. Biosens. Bioelectron. 2020, 151, 111944. [Google Scholar] [CrossRef]
- Sumitha, M.S.; Xavier, T.S. Recent Advances in Electrochemical Biosensors—A Brief Review. Hybrid Adv. 2023, 2, 100023. [Google Scholar] [CrossRef]
- Zhu, W.; Dong, J.; Ruan, G.; Zhou, Y.; Feng, J. Quantitative Single-Molecule Electrochemiluminescence Bioassay. Angew. Chem. Int. Ed. Engl. 2023, 62, e202214419. [Google Scholar] [CrossRef]
- Iftikhar, F.J.; Shah, A.; Wali, Q.; Kokab, T. Advancements in Nanofiber-Based Electrochemical Biosensors for Diagnostic Applications—ProQuest. Biosensors 2023, 13, 416. [Google Scholar] [CrossRef]
- Atış, H.E.; Turan, K.; Aydoğdu Tığ, G. Development of a Cu@ERGO-p(L-Lys) Modified GCE Electrochemical Sensor for the Simultaneous Detection of Ascorbic Acid, Dopamine, and Uric Acid. J. Electrochem. Soc. 2024, 171, 077503. [Google Scholar] [CrossRef]
- Hwang, M.T.; Heiranian, M.; Kim, Y.; You, S.; Leem, J.; Taqieddin, A.; Faramarzi, V.; Jing, Y.; Park, I.; van der Zande, A.M.; et al. Ultrasensitive Detection of Nucleic Acids Using Deformed Graphene Channel Field Effect Biosensors. Nat. Commun. 2020, 11, 1543. [Google Scholar] [CrossRef]
- Zabitler, D.; Ülker, E.; Turan, K.; Erdoğan, N.Ö.; Aydoğdu Tığ, G. Electrochemical Sensor for Biological Samples Monitoring. Top. Catal. 2025. [Google Scholar] [CrossRef]
- Guo, K.; Wustoni, S.; Koklu, A.; Díaz-Galicia, E.; Moser, M.; Hama, A.; Alqahtani, A.A.; Ahmad, A.N.; Alhamlan, F.S.; Shuaib, M.; et al. Rapid Single-Molecule Detection of COVID-19 and MERS Antigens via Nanobody-Functionalized Organic Electrochemical Transistors. Nat. Biomed. Eng. 2021, 5, 666–677. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Bao, H.; Ping, J. Nanomechanoelectrical Approach to Highly Sensitive and Specific Label-Free DNA Detection. Proc. Natl. Acad. Sci. USA 2023, 120, e2306130120. [Google Scholar] [CrossRef] [PubMed]
- Mostufa, S.; Rezaei, B.; Ciannella, S.; Yari, P.; Gómez-Pastora, J.; He, R.; Wu, K. Advancements and Perspectives in Optical Biosensors. ACS Omega 2024, 9, 24181–24202. [Google Scholar] [CrossRef] [PubMed]
- Palounek, D.; Vala, M.; Bujak, Ł.; Kopal, I.; Jiříková, K.; Shaidiuk, Y.; Piliarik, M. Surpassing the Diffraction Limit in Label-Free Optical Microscopy. ACS Photonics 2024, 11, 3907–3921. [Google Scholar] [CrossRef] [PubMed]
- Houghton, M.C.; Kashanian, S.V.; Derrien, T.L.; Masuda, K.; Vollmer, F. Whispering-Gallery Mode Optoplasmonic Microcavities: From Advanced Single-Molecule Sensors and Microlasers to Applications in Synthetic Biology. ACS Photonics 2024, 11, 892–903. [Google Scholar] [CrossRef]
- Li, T.; Liu, G.; Kong, H.; Yang, G.; Wei, G.; Zhou, X. Recent Advances in Photonic Crystal-Based Sensors. Coord. Chem. Rev. 2023, 475, 214909. [Google Scholar] [CrossRef]
- Velasco, L.; Islam, A.N.; Kundu, K.; Oi, A.; Reinhard, B.M. Two-Color Interferometric Scattering (iSCAT) Microscopy Reveals Structural Dynamics in Discrete Plasmonic Molecules. Nanoscale 2024, 16, 11696–11704. [Google Scholar] [CrossRef]
- Yu, W.; Jiang, W.C.; Lin, Q.; Lu, T. Cavity Optomechanical Spring Sensing of Single Molecules. Nat. Commun. 2016, 7, 12311. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Huang, Q.; Canady, T.D.; Barya, P.; Liu, S.; Arogundade, O.H.; Race, C.M.; Che, C.; Wang, X.; Zhou, L.; et al. Photonic Crystal Enhanced Fluorescence Emission and Blinking Suppression for Single Quantum Dot Digital Resolution Biosensing. Nat. Commun. 2022, 13, 4647. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Kim, D.-H.; Zhou, K.; Jeong, M.G.; Park, S.; Kwon, Y.; Hong, T.M.; Noh, J.; Ryu, S.H. Improved Resolution in Single-Molecule Localization Microscopy Using QD-PAINT. Exp. Mol. Med. 2021, 53, 384–392. [Google Scholar] [CrossRef]
- Eerqing, N.; Subramanian, S.; Rubio, J.; Lutz, T.; Wu, H.-Y.; Anders, J.; Soeller, C.; Vollmer, F. Comparing Transient Oligonucleotide Hybridization Kinetics Using DNA-PAINT and Optoplasmonic Single-Molecule Sensing on Gold Nanorods. ACS Photonics 2021, 8, 2882–2888. [Google Scholar] [CrossRef]
- Lee, S.; Moussa, N.A.M.; Kang, S.H. Plasmonic Nanostructures for Exosome Biosensing: Enabling High-Sensitivity Diagnostics. Nanomaterials 2025, 15, 1153. [Google Scholar] [CrossRef]
- Liu, J.; Jalali, M.; Mahshid, S.; Wachsmann-Hogiu, S. Are Plasmonic Optical Biosensors Ready for Use in Point-of-Need Applications? Analyst 2020, 145, 364–384. [Google Scholar] [CrossRef]
- Mcoyi, M.P.; Mpofu, K.T.; Sekhwama, M.; Mthunzi-Kufa, P. Developments in Localized Surface Plasmon Resonance. Plasmonics 2025, 20, 5481–5520. [Google Scholar] [CrossRef]
- Kim, D.M.; Park, J.S.; Jung, S.-W.; Yeom, J.; Yoo, S.M. Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors. Sensors 2021, 21, 3191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shuai, Z.; Zhou, H.; Luo, Z.; Liu, B.; Zhang, Y.; Zhang, L.; Chen, S.; Chao, J.; Weng, L.; et al. Single-Molecule Analysis of MicroRNA and Logic Operations Using a Smart Plasmonic Nanobiosensor. J. Am. Chem. Soc. 2018, 140, 3988–3993. [Google Scholar] [CrossRef]
- Jeong, Y.; Kook, Y.-M.; Lee, K.; Koh, W.-G. Metal Enhanced Fluorescence (MEF) for Biosensors: General Approaches and a Review of Recent Developments. Biosens. Bioelectron. 2018, 111, 102–116. [Google Scholar] [CrossRef]
- Fu, W.; Chi, H.; Dai, X.; Zhu, H.; Mesias, V.S.D.; Liu, W.; Huang, J. Efficient Optical Plasmonic Tweezer-Controlled Single-Molecule SERS Characterization of pH-Dependent Amylin Species in Aqueous Milieus. Nat. Commun. 2023, 14, 6996. [Google Scholar] [CrossRef]
- Zhao, Y.; Hubarevich, A.; De Fazio, A.F.; Iarossi, M.; Huang, J.-A.; De Angelis, F. Plasmonic Bowl-Shaped Nanopore for Raman Detection of Single DNA Molecules in Flow-Through. Nano Lett. 2023, 23, 4830–4836. [Google Scholar] [CrossRef]
- Macchia, E.; Franco, C.D.; Scandurra, C.; Sarcina, L.; Piscitelli, M.; Catacchio, M.; Caputo, M.; Bollella, P.; Scamarcio, G.; Torsi, L. Plasmonic Single-Molecule Affinity Detection at 10−20 Molar. Adv. Mater. 2025, 37, 2418610. [Google Scholar] [CrossRef]
- Cortés, E.; Huidobro, P.A.; Sinclair, H.G.; Guldbrand, S.; Peveler, W.J.; Davies, T.; Parrinello, S.; Görlitz, F.; Dunsby, C.; Neil, M.A.A.; et al. Plasmonic Nanoprobes for Stimulated Emission Depletion Nanoscopy. ACS Nano 2016, 10, 10454–10461. [Google Scholar] [CrossRef]
- Balzarotti, F.; Eilers, Y.; Gwosch, K.C.; Gynnå, A.H.; Westphal, V.; Stefani, F.D.; Elf, J.; Hell, S.W. Nanometer Resolution Imaging and Tracking of Fluorescent Molecules with Minimal Photon Fluxes. Science 2017, 355, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Wani, A.K.; Akhtar, N.; ul Gani Mir, T.; Chopra, C.; Singh, R.; Hong, J.C.; Kadam, U.S. CRISPR/Cas12a-Based Biosensors for Environmental Monitoring and Diagnostics. Environ. Technol. Innov. 2024, 34, 103625. [Google Scholar] [CrossRef]
- Liu, X.; Hussain, M.; Dai, J.; Li, Y.; Zhang, L.; Yang, J.; Ali, Z.; He, N.; Tang, Y. Programmable Biosensors Based on RNA-Guided CRISPR/Cas Endonuclease. Biol. Proced. Online 2022, 24, 2. [Google Scholar] [CrossRef]
- He, Y.; Hu, Q.; San, S.; Kasputis, T.; Splinter, M.G.D.; Yin, K.; Chen, J. CRISPR-Based Biosensors for Human Health: A Novel Strategy to Detect Emerging Infectious Diseases. Trends Anal. Chem. TRAC 2023, 168, 117342. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.-H.; Yoon, J.; Chen, M.; Shin, M.; Goldston, L.L.; Lee, K.-B.; Choi, J.-W. CRISPR/Cas-Based Nanobiosensor Using Plasmonic Nanomaterials to Detect Disease Biomarkers. BioChip J. 2025, 19, 167–181. [Google Scholar] [CrossRef]
- Sun, X.-M.; Kang, Y.-F.; He, J.-W.; Tang, H.-W.; Liu, D.; Li, C.-Y. Near-Infrared Light Activated and Hybridization Chain Reaction Cascaded CRISPR/Cas12a System under the Enhancement of Mn2+ for Intracellular Biosensing. Sens. Actuators B Chem. 2024, 398, 134777. [Google Scholar] [CrossRef]
- Zhu, D.; Su, T.; Sun, T.; Qin, X.; Su, S.; Bai, Y.; Li, F.; Zhao, D.; Shao, G.; Chao, J.; et al. Enhancing Point-of-Care Diagnosis of African Swine Fever Virus (ASFV) DNA with the CRISPR-Cas12a-Assisted Triplex Amplified Assay. Anal. Chem. 2024, 96, 5178–5187. [Google Scholar] [CrossRef]
- Tian, F.; Jiang, L.; Wang, Z.; Peng, L.; Zhang, Z.; Huang, Y. Mn2+-Activated CRISPR-Cas12a Strategy for Fluorescence Detection of the Insecticide Carbaryl. Sens. Actuators B Chem. 2024, 398, 134695. [Google Scholar] [CrossRef]
- Cheng, X.; Yan, Y.; Chen, X.; Duan, J.; Zhang, D.; Yang, T.; Gou, X.; Zhao, M.; Ding, S.; Cheng, W. CRISPR/Cas12a-Modulated Fluorescence Resonance Energy Transfer with Nanomaterials for Nucleic Acid Sensing. Sens. Actuators B Chem. 2021, 331, 129458. [Google Scholar] [CrossRef]
- Choi, J.-H.; Shin, M.; Yang, L.; Conley, B.; Yoon, J.; Lee, S.-N.; Lee, K.-B.; Choi, J.-W. Clustered Regularly Interspaced Short Palindromic Repeats-Mediated Amplification-Free Detection of Viral DNAs Using Surface-Enhanced Raman Spectroscopy-Active Nanoarray. ACS Nano. ACS Nano 2021, 15, 13475–13485. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Gockel, N.; Arizono, M.; Dembitskaya, Y.; Nägerl, U.V.; Pennacchietti, F.; Damenti, M.; Testa, I.; Willig, K.I. Super-Resolution Microscopy Opens New Doors to Life at the Nanoscale. J. Neurosci. 2022, 42, 8488–8497. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Kaminski Schierle, G.S.; Lei, B.; Liu, Y.; Kaminski, C.F. Fluorescent Nanoparticles for Super-Resolution Imaging|Chemical Reviews. Chem. Rev. 2022, 122, 12495–12543. [Google Scholar] [CrossRef]
- Valli, J.; Garcia-Burgos, A.; Rooney, L.M.; Oliveira, B.V. de M. e; Duncan, R.R.; Rickman, C. Seeing beyond the Limit: A Guide to Choosing the Right Super-Resolution Microscopy Technique. J. Biol. Chem. 2021, 297, 100791. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Sauer, M.; Geis, C. Super-Resolving Microscopy in Neuroscience. Chem. Rev. 2021, 121, 11971–12015. [Google Scholar] [CrossRef]
- Akbari-Alavijeh, S.; Shaddel, R.; Lee, C.-C.; Pourjafar, H.; Ansari, F.; Alizadeh Sani, M.; Ajili, N.; Assadpour, E.; Zhang, F.; Jafari, S.M. Nano-Immunosensors for the Rapid and Sensitive Detection of Foodborne Toxins; Recent Advances. Ind. Crops Prod. 2025, 228, 120879. [Google Scholar] [CrossRef]
- Lee, S.; Lee, J.; Batjikh, I.; Yu, H.; Kang, S.H. Ultrasensitive Hypoxia Sensing at the Single-Molecule Level via Super-Resolution Quantum Dot-Linked Immunosandwich Assay. ACS Sens. 2022, 7, 1372–1380. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.; Kang, S.H. Super-Resolution Multispectral Imaging Nanoimmunosensor for Simultaneous Detection of Diverse Early Cancer Biomarkers. ACS Sens. 2024, 9, 3652–3659. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Kang, S.H. Wavelength-Dependent Three-Dimensional Single-Molecule Superlocalization Imaging for Yoctomole Detection of Thyroid-Stimulating Hormone on a Quantum Dot Nanobiosensor. Chin. Chem. Lett. 2023, 34, 108383. [Google Scholar] [CrossRef]
- Mauriz, E.; Lechuga, L.M. Plasmonic Biosensors for Single-Molecule Biomedical Analysis. Biosensors 2021, 11, 123. [Google Scholar] [CrossRef]
- Bahlol, H.S.; Li, J.; Deng, J.; Foda, M.F.; Han, H. Recent Progress in Nanomaterial-Based Surface-Enhanced Raman Spectroscopy for Food Safety Detection. Nanomaterials 2024, 14, 1750. [Google Scholar] [CrossRef]
- Liu, H.-L.; Zhan, K.; Wang, K.; Xia, X.-H. Recent Advances in Nanotechnologies Combining Surface-Enhanced Raman Scattering and Nanopore. TrAC Trends Anal. Chem. 2023, 159, 116939. [Google Scholar] [CrossRef]
- Cao, Y.; Lee, D.; Lee, S.; Lin, J.-M.; Kang, S.H. One-Shot Dual-Detection-Based Single-Molecule Super-Resolution Imaging Method for Real-Time Observation of Spatiotemporal Catalytic Activity Variations on the Plasmonic Gold Nanoparticle Surface. Anal. Chem. 2024, 96, 1957–1964. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, X.; Zhang, Y.; Yang, X.; Guo, L.; Man, C.; Jiang, Y.; Zhang, W.; Zhang, X. Emerging Biosensors Integrated with Microfluidic Devices: A Promising Analytical Tool for On-Site Detection of Mycotoxins. npj Sci. Food 2025, 9, 84. [Google Scholar] [CrossRef]
- Verschueren, D.V.; Pud, S.; Shi, X.; De Angelis, L.; Kuipers, L.; Dekker, C. Label-Free Optical Detection of DNA Translocations through Plasmonic Nanopores. ACS Nano 2019, 13, 61–70. [Google Scholar] [CrossRef]
- Spitzberg, J.D.; Zrehen, A.; van Kooten, X.F.; Meller, A. Plasmonic-Nanopore Biosensors for Superior Single-Molecule Detection. Adv. Mater. 2019, 31, 1900422. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, S.; Lee, G.; Kang, S.H. Simultaneous Quantification of Thyroid Hormones Using an Ultrasensitive Single-Molecule Fourplex Nanoimmunosensor in an Evanescent Field. Biosens. Bioelectron. 2023, 220, 114894. [Google Scholar] [CrossRef]
- Cao, Y.; Lee, S.; Kim, K.; Kang, S.H. Minimizing the Optical Illusion of Nanoparticles in Single Cells Using Four-Dimensional Cuboid Multiangle Illumination-Based Light-Sheet Super-Resolution Imaging. Anal. Chem. 2022, 94, 17877–17884. [Google Scholar] [CrossRef]
- Cao, Y.; Lee, S.; Kim, K.; Kwak, J.-Y.; Kang, S.H. Real-Time Six-Dimensional Spatiotemporal Tracking of Single Anisotropic Nanoparticles in Live Cells by Integrated Multifunctional Light-Sheet Nanoscopy. Microchim. Acta 2023, 190, 54. [Google Scholar] [CrossRef]
- Shao, H.; Chung, J.; Balaj, L.; Charest, A.; Bigner, D.D.; Carter, B.S.; Hochberg, F.H.; Breakefield, X.O.; Weissleder, R.; Lee, H. Protein Typing of Circulating Microvesicles Allows Real-Time Monitoring of Glioblastoma Therapy. Nat. Med. 2012, 18, 1835–1840. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, J.E.; Stratton, C.W.; Persing, D.H.; Tang, Y.-W. Forty Years of Molecular Diagnostics for Infectious Diseases. J. Clin. Microbiol. 2022, 60, e02446-21. [Google Scholar] [CrossRef]
- Bruijns, B.; Folkertsma, L.; Tiggelaar, R. FDA Authorized Molecular Point-of-Care SARS-CoV-2 Tests: A Critical Review on Principles, Systems and Clinical Performances. Biosens. Bioelectron. X 2022, 11, 100158. [Google Scholar] [CrossRef]
- Sadr, S.; Hajjafari, A.; Sazmand, A.; Santucciu, C.; Masala, G.; Soroushianfar, M.; Nazemian, S.; Rahdar, A.; Pandey, S.; Guettari, M.; et al. Nanobiosensors for Revolutionizing Parasitic Infections Diagnosis: A Critical Review to Improve Global Health with an Update on Future Challenges Prospect. Eur. J. Med. Res. 2025, 30, 484. [Google Scholar] [CrossRef]
- Lee, S.; Ahn, S.; Chakkarapani, S.K.; Kang, S.H. Supersensitive Detection of the Norovirus Immunoplasmon by 3D Total Internal Reflection Scattering Defocus Microscopy with Wavelength-Dependent Transmission Grating. ACS Sens. 2019, 4, 2515–2523. [Google Scholar] [CrossRef]
- Dhahi, T.S.; Yousif Dafhalla, A.K.; Al-Mufti, A.W.; Elobaid, M.E.; Adam, T.; Gopinath, S.C.B. Application of Nanobiosensor Engineering in the Diagnosis of Neurodegenerative Disorders. Results Eng. 2024, 24, 102790. [Google Scholar] [CrossRef]
- Yadav, S.; Bukke, S.P.N.; Prajapati, S.; Singh, A.P.; Chettupalli, A.K.; Nicholas, B. Nanobiosensors in Neurodegenerative Disease Diagnosis: A Promising Pathway for Early Detection. Digit. Health 2025, 11, 20552076251342457. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, G.; Yang, X. Electrochemical Immunosensor Based on Fe3O4/MWCNTs-COOH/AuNPs Nanocomposites for Trace Liver Cancer Marker Alpha-Fetoprotein Detection. Talanta 2023, 259, 124492. [Google Scholar] [CrossRef]
- Makableh, Y.; Athamneh, T.; Ajlouni, M.; Hijazi, S.; Alnaimi, A. Enhanced Response and Selective Gold Nanoparticles/Carbon Nanotubes Biosensor for the Early Detection of HER2 Biomarker. Sens. Actuators Rep. 2023, 5, 100158. [Google Scholar] [CrossRef]
- Ahn, S.; Yu, H.; Kang, S.H. Enhanced Detection Sensitivity of Carcinoembryonic Antigen on a Plasmonic Nanoimmunosensor by Transmission Grating-Based Total Internal Reflection Scattering Microscopy. Biosens. Bioelectron. 2017, 96, 159–166. [Google Scholar] [CrossRef]
- Meskher, H.; Mustansar, H.C.; Thakur, A.K.; Sathyamurthy, R.; Lynch, I.; Singh, P.; Han, T.K.; Saidur, R. Recent Trends in Carbon Nanotube (CNT)-Based Biosensors for the Fast and Sensitive Detection of Human Viruses: A Critical Review. Nanoscale Adv. 2023, 5, 992–1010. [Google Scholar] [CrossRef]
- Curulli, A. Functional Nanomaterials Enhancing Electrochemical Biosensors as Smart Tools for Detecting Infectious Viral Diseases. Molecules 2023, 28, 3777. [Google Scholar] [CrossRef]
- Liang, Y.; Xiao, M.; Xie, J.; Li, J.; Zhang, Y.; Liu, H.; Zhang, Y.; He, J.; Zhang, G.; Wei, N.; et al. Amplification-Free Detection of SARS-CoV-2 Down to Single Virus Level by Portable Carbon Nanotube Biosensors. Small 2023, 19, 2208198. [Google Scholar] [CrossRef]
- Shoaib, A.; Darraj, A.; Khan, M.E.; Azmi, L.; Alalwan, A.; Alamri, O.; Tabish, M.; Khan, A.U. A Nanotechnology-Based Approach to Biosensor Application in Current Diabetes Management Practices. Nanomaterials 2023, 13, 867. [Google Scholar] [CrossRef] [PubMed]
- Saddique, Z.; Faheem, M.; Habib, A.; UlHasan, I.; Mujahid, A.; Afzal, A. Electrochemical Creatinine (Bio)Sensors for Point-of-Care Diagnosis of Renal Malfunction and Chronic Kidney Disorders. Diagnostics 2023, 13, 1737. [Google Scholar] [CrossRef]
- Lee, S.; Cao, Y.; Kwak, J.-Y.; Kang, S.H. Multidimensional Spatiotemporal Tracking of Intracellular Fucoidan via Plasmon-Enhanced Dark-Field Superresolution Imaging. Sens. Actuators B Chem. 2026, 446, 138647. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of Circulating Tumor DNA in Early- and Late-Stage Human Malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and Future Perspectives of Liquid Biopsies in Genomics-Driven Oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Vaidyanathan, R.; Naghibosadat, M.; Rauf, S.; Korbie, D.; Carrascosa, L.G.; Shiddiky, M.J.A.; Trau, M. Detecting Exosomes Specifically: A Multiplexed Device Based on Alternating Current Electrohydrodynamic Induced Nanoshearing. Anal. Chem. 2014, 86, 11125–11132. [Google Scholar] [CrossRef] [PubMed]
- Niemz, A.; Ferguson, T.M.; Boyle, D.S. Point-of-Care Nucleic Acid Testing for Infectious Diseases. Trends Biotechnol. 2011, 29, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Regulation (EU) 2017/746 of the European Parliament and of the Council of 5 April 2017 on In Vitro Diagnostic Medical Devices and Repealing Directive 98/79/EC and Commission Decision 2010/227/EU. Off. J. Eur. Union L 117, 5 May 2017, 176–332. Available online: https://eur-lex.europa.eu/eli/reg/2017/746/oj/eng (accessed on 5 October 2025).
- U.S. Food and Drug Administration (FDA). Center for Devices and Radiological Health (CDRH). In Vitro Diagnostics EUAs—Molecular Diagnostic Tests for SARS-CoV-2. FDA. 2025. Available online: https://www.fda.gov/medical-devices/covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-molecular-diagnostic-tests-sars-cov-2 (accessed on 5 October 2025).
- Lee, S.; Moussa, N.A.M.; Kang, S.H. Deep Learning-Enhanced Nanozyme-Based Biosensors for Next-Generation Medical Diagnostics. Biosensors 2025, 15, 571. [Google Scholar] [CrossRef]
| Detection Method | Principle/Advantages | Limitations | SRM Integration | Applications |
|---|---|---|---|---|
| Electrochemical | · Electron transfer at electrode · Miniaturization, low cost | · Electrode fouling · Limited reproducibility | Correlative STORM–electrochemical imaging | DNA hybridization, viral antigen detection |
| Optical | · Light–matter interactions (fluorescence, scattering, interferometry) · High sensitivity, multiplexing | · Heterogeneous signal stability · Photobleaching · Labeling required | Protein–DNA mapping (STORM/PALM), nucleic acid multiplexing (DNA-PAINT) | miRNA assays, protein–DNA interactions |
| Plasmonic (SPR, LSPR, SERS, MEF) | · SPR and field enhancement · Real-time, label-free, single-molecule sensitivity | · Fabrication variability · High cost | STED (improved photostability), MINFLUX (<5 nm localization) | Protein–ligand binding, biomarker detection |
| Hybrid/ Multimodal | · Combines optical + electrochemical or plasmonic modalities · Reduced false positives, robust sensing | · Device complexity · Limited scalability | Nanopore–SRM dual readout | Rare analyte detection, EV analysis |
| Fluorescent Immunoassay | · Antigen–antibody binding with nanoprobes · High specificity, multiplex detection | · Photobleaching · Probe cost · Limited photostability for long-term tracking | srQLISA (HIF-1α), srMINI (tumor markers) | Ultrasensitive immunodiagnostics |
| Characteristic | Nanobiosensor-Only Devices | Biosensors Integrated with SRM Techniques |
|---|---|---|
| Sensitivity/LOD | Femtomolar to picomolar; limited by background noise | Attomolar to zeptomolar detection; single-molecule precision [97,98] |
| Spatial resolution | Diffraction-limited (~200 nm); ensemble measurements | Super-resolution (20–50 nm lateral; <10 nm in advanced SRM); nanoscale localization [82,83,109] |
| Multiplexing capability | Limited by spectral overlap and signal crosstalk | Expanded via spatial/temporal discrimination (e.g., srMINI multiplexed assays) [98] |
| Information output | Bulk signal intensity; limited structural information | Quantitative, spatial mapping, and dynamic tracking of biomolecular events |
| Reproducibility/ Scalability | Prone to variability in nanofabrication and assay conditions | Improved validation by correlating nanosensor signals with localization accuracy; however, complexity and cost are high |
| Translational Potential | Early diagnostic potential, limited clinical validation | Enhanced diagnostic confidence; case studies in ctDNA, exosomes, and FDA-authorized (EUA) POC references indicate a pathway to clinical translation [118] |
| Disease Area | Biomarkers | Sensor Type | Key Advantages |
|---|---|---|---|
| Infectious Diseases | Malaria antigens, Leishmania DNA, SARS-CoV-2 RNA | AuNPs/CNTs/QDs/GO-based LOC | Attomolar sensitivity, multiplex POC screening |
| Neurodegenerative | Amyloid-β, Tau | Molecular beacon-functionalized nanomaterial sensors | Early, noninvasive, personalized monitoring |
| Oncology | HER2, PSA, AFP | AuNP-based nanoimmunosensors | Sub-ng of LOD, early screening |
| Viral Infections | HIV RNA, HBV antigens, SARS-CoV-2 | QD/CNT-based portable sensors | High-sensitivity, rapid on-site testing |
| Metabolic and Renal | HbA1c, Insulin, Creatinine, Cystatin C | Electrochemical and enzyme-linked nanoplatforms | Accurate glycemic/renal monitoring |
| Challenges | Future Directions | Examples/Clinical Progress |
|---|---|---|
| Complex sample prep and calibration | · Standardized protocols · Automated calibration | POC systems validated for SARS-CoV-2 detection (Abbott ID NOW and Cepheid GeneXpert) |
| Multiplexing limitations | · Spatial/spectral separation · Computational unmixing | srMINI multiplexed biomarker detection in serum (proof-of-concept) |
| Photobleaching and phototoxicity | · Photostable QDs · Hybrid plasmonic–fluorophore probes · Low-light SRM | Validated use of long-lifetime QDs in live-cell tracking studies |
| Reproducibility and standardization | · Scalable · Reproducible nanomaterial synthesis · Robust sensor fabrication | Exosome nanoplasmonic chips tested with patients’ plasma (preclinical) |
| High cost and clinical translation gap | · Cost-effective fabrication · Industrial–academic partnerships · Integration into regulatory science frameworks | ctDNA plasmonic sensors undergoing preclinical validation |
| Regulatory hurdles | · Early engagement with agencies · Large-scale clinical trials | FDA emergency-use authorization for molecular diagnostics |
| Data complexity | · AI-driven image reconstruction · Multimodal data fusion · Cloud-based workflows and automated pipelines | Deep learning methods applied to nanozyme-based biosensors |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Rafiq, S.; Kang, S.H. Nanobiosensors for Single-Molecule Diagnostics: Toward Integration with Super-Resolution Imaging. Biosensors 2025, 15, 705. https://doi.org/10.3390/bios15100705
Lee S, Rafiq S, Kang SH. Nanobiosensors for Single-Molecule Diagnostics: Toward Integration with Super-Resolution Imaging. Biosensors. 2025; 15(10):705. https://doi.org/10.3390/bios15100705
Chicago/Turabian StyleLee, Seungah, Sobia Rafiq, and Seong Ho Kang. 2025. "Nanobiosensors for Single-Molecule Diagnostics: Toward Integration with Super-Resolution Imaging" Biosensors 15, no. 10: 705. https://doi.org/10.3390/bios15100705
APA StyleLee, S., Rafiq, S., & Kang, S. H. (2025). Nanobiosensors for Single-Molecule Diagnostics: Toward Integration with Super-Resolution Imaging. Biosensors, 15(10), 705. https://doi.org/10.3390/bios15100705







