Are Aptamer-Based Biosensors the Future of the Detection of the Human Gut Microbiome?—A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Links between the Gut Microbiome, Their Metabolites, and Diseases
3. Materials and Methods
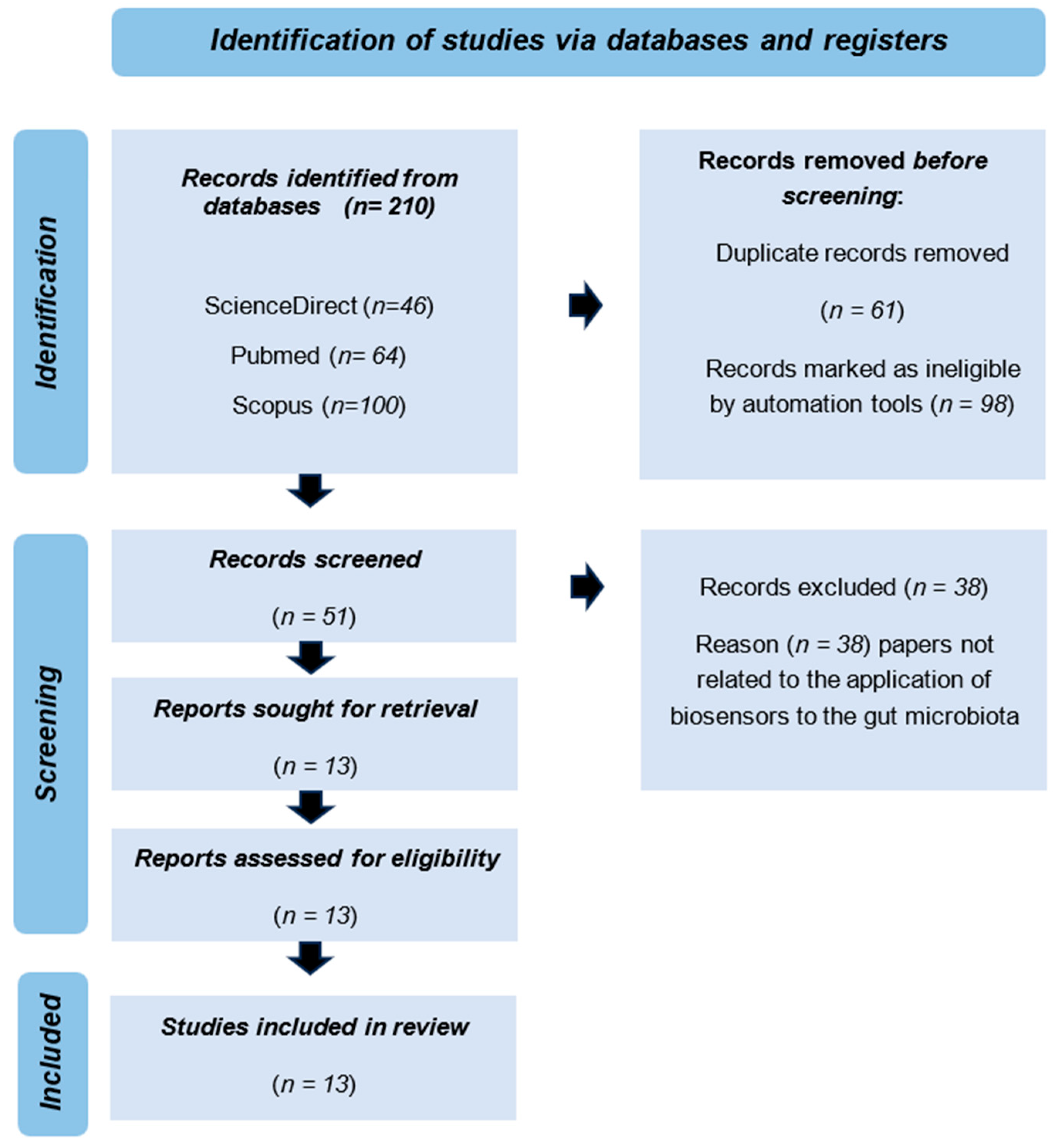
3.1. Selection of Eligibility and Exclusion Criteria
3.2. Results
4. From the Past to Present: Biosensors Applied to the Detection of Microorganisms and Metabolites
5. Biorecognition Elements Applied to Gut Microbiome
5.1. Microbial Sensor
5.2. Aptamers
6. Immobilization of Biorecognition Elements—An Important Technical Process
6.1. Covalent Binding
6.2. Adsorption
6.3. Self-Assembled Monolayer
6.4. Cross-Linking
6.5. Entrapment
7. Transducers
7.1. Electrochemical Transducing
7.1.1. Electrochemical Point-of-Care Devices Challenges
7.1.2. Amperometric Sensors
7.1.3. Conductometric Sensors
7.1.4. Potentiometric Sensors
7.1.5. Piezoelectric Sensors
7.2. Optical Transducing
7.2.1. Surface Plasmon Resonance
7.2.2. Surface-Enhanced Raman Spectroscopy
7.2.3. Fiber Optic Biosensors
8. Challenge and Future Perspectives: Why Is There a Continuous Need for Developing Biosensors for the Gut?
8.1. Developing Aptamer-Based Biosensors for the Detection of the Human Gut Microbiome
8.2. Developing Alternative Detection Platforms for the Complex Matrix—Gut Microbiome
9. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Madhogaria, B.; Bhowmik, P.; Kundu, A. Correlation between Human Gut Microbiome and Diseases. Infect. Med. 2022, 1, 180–191. [Google Scholar] [CrossRef]
- Panyod, S.; Wu, W.-K.; Chen, C.-C.; Wu, M.-S.; Ho, C.-T.; Sheen, L.-Y. Modulation of Gut Microbiota by Foods and Herbs to Prevent Cardiovascular Diseases. J. Tradit. Complement. Med. 2023, 13, 107–118. [Google Scholar] [CrossRef]
- Lebovich, M.; Andrews, L.B. Surveying the Genetic Design Space for Transcription Factor-Based Metabolite Biosensors: Synthetic Gamma-Aminobutyric Acid and Propionate Biosensors in E. coli Nissle 1917. Front. Bioeng. Biotechnol. 2022, 10, 938056. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Huang, W.; Yao, Y.-F. The Metabolites of Lactic Acid Bacteria: Classification, Biosynthesis and Modulation of Gut Microbiota. Microb. Cell 2023, 10, 49–62. [Google Scholar] [CrossRef]
- Colosimo, R.; Harris, H.C.; Ahn-Jarvis, J.; Troncoso-Rey, P.; Finnigan, T.J.A.; Wilde, P.J.; Warren, F.J. Colonic in Vitro Fermentation of Mycoprotein Promotes Shifts in Gut Microbiota, with Enrichment of Bacteroides Species. Commun. Biol. 2024, 7, 272. [Google Scholar] [CrossRef] [PubMed]
- Forner, E.; Ezenarro, J.J.; Pérez-Montero, M.; Vigués, N.; Asensio-Grau, A.; Andrés, A.; Mas, J.; Baeza, M.; Muñoz-Berbel, X.; Villa, R.; et al. Electrochemical Biosensor for Aerobic Acetate Detection. Talanta 2023, 265, 124882. [Google Scholar] [CrossRef]
- Serebrinsky-Duek, K.; Barra, M.; Danino, T.; Garrido, D. Engineered Bacteria for Short-Chain-Fatty-Acid-Repressed Expression of Biotherapeutic Molecules. Microbiol. Spectr. 2023, 11, e0004923. [Google Scholar] [CrossRef] [PubMed]
- Lippolis, T.; Cofano, M.; Caponio, G.R.; De Nunzio, V.; Notarnicola, M. Bioaccessibility and Bioavailability of Diet Polyphenols and Their Modulation of Gut Microbiota. Int. J. Mol. Sci. 2023, 24, 3813. [Google Scholar] [CrossRef]
- Song, Z.; Cheng, L.; Liu, Y.; Zhan, S.; Wu, Z.; Zhang, X. Plant-Derived Bioactive Components Regulate Gut Microbiota to Prevent Depression and Depressive-Related Neurodegenerative Diseases: Focus on Neurotransmitters. Trends Food Sci. Technol. 2022, 129, 581–590. [Google Scholar] [CrossRef]
- Weng, C.-Y.C.; Suarez, C.; Cheang, S.E.; Couture, G.; Goodson, M.L.; Barboza, M.; Kalanetra, K.M.; Masarweh, C.F.; Mills, D.A.; Raybould, H.E.; et al. Quantifying Gut Microbial Short-Chain Fatty Acids and Their Isotopomers in Mechanistic Studies Using a Rapid, Readily Expandable LC–MS Platform. Anal. Chem. 2024, 96, 2415–2424. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Amaya, S.; Lin, L.-K.; Deering, A.J.; Stanciu, L.A. Aptamer-Based SERS Biosensor for Whole Cell Analytical Detection of E. coli O157:H7. Anal. Chim. Acta 2019, 1081, 146–156. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, M.; Zhao, F.; Han, S. Application of Nanomaterial Modified Aptamer-Based Electrochemical Sensor in Detection of Heavy Metal Ions. Foods 2022, 11, 1404. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Mittal, S.; Das, M.; Saharia, A.; Tiwari, M. Optical Biosensors: A Decade in Review. Alex. Eng. J. 2023, 67, 673–691. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Ayachit, N.H.; Aminabhavi, T.M. Biosensors and Microfluidic Biosensors: From Fabrication to Application. Biosensors 2022, 12, 543. [Google Scholar] [CrossRef]
- Mann, M.M.; Berger, B.W. A Genetically-Encoded Biosensor for Direct Detection of Perfluorooctanoic Acid. Sci. Rep. 2023, 13, 15186. [Google Scholar] [CrossRef] [PubMed]
- Papreja, P.; Thakur, A. A Review: Applications of Electrochemical Biosensors. In AIP Conference Proceedings; AIP Publishing: Long Island, NY, USA, 2023; p. 020008. [Google Scholar] [CrossRef]
- Lakshmanan, A.P.; Mingione, A.; Pivari, F.; Dogliotti, E.; Brasacchio, C.; Murugesan, S.; Cusi, D.; Lazzaroni, M.; Soldati, L.; Terranegra, A. Modulation of Gut Microbiota: The Effects of a Fruits and Vegetables Supplement. Front. Nutr. 2022, 9, 930883. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Watson, E.; Conlon, M.; Sanguansri, L.; Augustin, M.A. Impact of Co-Delivery of EGCG and Tuna Oil within a Broccoli Matrix on Human Gut Microbiota, Phenolic Metabolites and Short Chain Fatty Acids In Vitro. Molecules 2022, 27, 656. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Wu, J.; Jiang, X.; Tang, H.; Nielsen, O.H. Modulation of Gut Microbiota in Pathological States. Engineering 2017, 3, 83–89. [Google Scholar] [CrossRef]
- Zhao, J.; Zhao, F.; Yuan, J.; Liu, H.; Wang, Y. Gut Microbiota Metabolites, Redox Status, and the Related Regulatory Effects of Probiotics. Heliyon 2023, 9, e21431. [Google Scholar] [CrossRef]
- Fei, Y.; Fang, R.; Xiao, L.; Zhang, Y.; Fan, K.; Jiang, Y.; Lei, S.; Xu, R.; Yang, D.; Ye, Y.; et al. The Development of a Colorimetric Biosensing Assay for the Detection of Helicobacter pylori in Feces. Anal. Biochem. 2022, 651, 114737. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, H.; Cheng, X.; Geng, J.; Wang, L.; Dong, Q.; Liu, C.; Chi, Z.; Chi, Z. Aptamer-Superparamagnetic Nanoparticles Capture Coupling Siderophore-Fe3+ Scavenging Actuated with Carbon Dots to Confer an “off-on” Mechanism for the Ultrasensitive Detection of Helicobacter pylori. Biosens. Bioelectron. 2021, 193, 113551. [Google Scholar] [CrossRef]
- Khan, S.; Akrema; Qazi, S.; Ahmad, R.; Raza, K.; Rahisuddin. In Silico and Electrochemical Studies for a ZnO-CuO-Based Immunosensor for Sensitive and Selective Detection of E. coli. ACS Omega 2021, 6, 16076–16085. [Google Scholar] [CrossRef]
- Farsi, D.N.; Gallegos, J.L.; Finnigan, T.J.A.; Cheung, W.; Munoz, J.M.; Commane, D.M. The Effects of Substituting Red and Processed Meat for Mycoprotein on Biomarkers of Cardiovascular Risk in Healthy Volunteers: An Analysis of Secondary Endpoints from Mycomeat. Eur. J. Nutr. 2023, 62, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Zhang, Y.; Krämer, M.; Kissmann, A.-K.; Amann, V.; Raber, H.F.; Weil, T.; Stieger, K.R.; Knippschild, U.; Henkel, M.; et al. A Polyclonal Aptamer Library for the Specific Binding of the Gut Bacterium Roseburia Intestinalis in Mixtures with Other Gut Microbiome Bacteria and Human Stool Samples. Int. J. Mol. Sci. 2022, 23, 7744. [Google Scholar] [CrossRef] [PubMed]
- Raber, H.F.; Kubiczek, D.H.; Bodenberger, N.; Kissmann, A.-K.; D’souza, D.; Xing, H.; Mayer, D.; Xu, P.; Knippschild, U.; Spellerberg, B.; et al. FluCell-SELEX Aptamers as Specific Binding Molecules for Diagnostics of the Health Relevant Gut Bacterium Akkermansia Muciniphila. Int. J. Mol. Sci. 2021, 22, 425. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Chu, X.; Cheng, Y.; Tang, S.; Zogona, D.; Pan, S.; Xu, X. Modulation of Gut Microbiota by Lactobacillus Casei Fermented Raspberry Juice In Vitro and In Vivo. Foods 2021, 10, 3055. [Google Scholar] [CrossRef]
- O’Riordan, K.J.; Collins, M.K.; Moloney, G.M.; Knox, E.G.; Aburto, M.R.; Fülling, C.; Morley, S.J.; Clarke, G.; Schellekens, H.; Cryan, J.F. Short Chain Fatty Acids: Microbial Metabolites for Gut-Brain Axis Signalling. Mol. Cell. Endocrinol. 2022, 546, 111572. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.-Z. Gut Microbial Metabolite Butyrate and Its Therapeutic Role in Inflammatory Bowel Disease: A Literature Review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Kiela, P.R.; Salamon, A.; Barberán, A.; Chen, Y.; Yang, F.; Błaszczyk, M.K.; Sikora, A. Dynamics of Dark Fermentation Microbial Communities in the Light of Lactate and Butyrate Production. Microbiome 2021, 9, 158. [Google Scholar] [CrossRef]
- Gao, S.; Guo, Y.; Ma, C.; Ma, D.; Chen, K.; Ouyang, P.; Wang, X. Characterization and Application of a Recombinant Dopa Decarboxylase from Harmonia Axyridis for the Efficient Biosynthesis of Dopamine. Chin. J. Chem. Eng. 2022, 41, 449–456. [Google Scholar] [CrossRef]
- Braga, J.D.; Thongngam, M.; Kumrungsee, T. Gamma-Aminobutyric Acid as a Potential Postbiotic Mediator in the Gut–Brain Axis. NPJ Sci. Food 2024, 8, 16. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Liu, J.; Kong, J.; Deng, X.; Guo, X.; Shan, J.; Zhou, D.; Li, W.; Lin, Y.; et al. Gut Microbiota Dynamics and Fecal SCFAs after Colonoscopy: Accelerating Microbiome Stabilization by Clostridium butyricum. J. Transl. Med. 2024, 22, 222. [Google Scholar] [CrossRef]
- Barker-Tejeda, T.C.; Zubeldia-Varela, E.; Macías-Camero, A.; Alonso, L.; Martín-Antoniano, I.A.; Rey-Stolle, M.F.; Mera-Berriatua, L.; Bazire, R.; Cabrera-Freitag, P.; Shanmuganathan, M.; et al. Comparative Characterization of the Infant Gut Microbiome and Their Maternal Lineage by a Multi-Omics Approach. Nat. Commun. 2024, 15, 3004. [Google Scholar] [CrossRef] [PubMed]
- Ahallil, H.; Abdullah, A.; Maskat, M.Y.; Sarbini, S.R. Fermentation of Gum Arabic by Gut Microbiota Using in Vitro Colon Model. In AIP Conference Proceedings; AIP Publishing: Long Island, NY, USA, 2019; p. 050004. [Google Scholar] [CrossRef]
- Inriani, S.; Ansariadi; Abdullah, A.Z.; Birawida, A.B.; Saleh, L.M.; Bustan, N.; Wijaya, E. Fecal Myeloperoxidase Levels in Pregnant Women and Risk Factors to Low Birth Weight in A Makassar Slum Settlement: A Sub-Study of The Indonesian Birth Cohort Study. Natl. J. Community Med. 2023, 14, 549–555. [Google Scholar] [CrossRef]
- Yang, S.-M.; Kim, E.; Kim, D.; Baek, J.; Yoon, H.; Kim, H.-Y. Rapid Detection of Salmonella Enteritidis, Typhimurium, and Thompson by Specific Peak Analysis Using Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry. Foods 2021, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, K.J.; Simmons, L.A. Genome Editing Methods for Bacillus subtilis. In Recombineering: Methods and Protocols; Springer: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Yang, Q.; Jiang, X.; Li, Y.; Zhao, J.; Qu, K. Conductometric Sensor for Viable Escherichia coli and Staphylococcus aureus Based on Magnetic Analyte Separation via Aptamer. Microchim. Acta 2020, 187, 43. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, H.; Bolotnikov, G.; Krämer, M.; Gotzmann, N.; Knippschild, U.; Kissmann, A.-K.; Rosenau, F. Enriched Aptamer Libraries in Fluorescence-Based Assays for Rikenella Microfusus-Specific Gut Microbiome Analyses. Microorganisms 2023, 11, 2266. [Google Scholar] [CrossRef]
- Das, T.; Harshey, A.; Srivastava, A.; Nigam, K.; Yadav, V.K.; Sharma, K.; Sharma, A. Analysis of the Ex-Vivo Transformation of Semen, Saliva and Urine as They Dry out Using ATR-FTIR Spectroscopy and Chemometric Approach. Sci. Rep. 2021, 11, 11855. [Google Scholar] [CrossRef]
- Fadlelmoula, A.; Pinho, D.; Carvalho, V.H.; Catarino, S.O.; Minas, G. Fourier Transform Infrared (FTIR) Spectroscopy to Analyse Human Blood over the Last 20 Years: A Review towards Lab-on-a-Chip Devices. Micromachines 2022, 13, 187. [Google Scholar] [CrossRef]
- Silva, L.G.; Péres, A.F.S.; Freitas, D.L.D.; Morais, C.L.M.; Martin, F.L.; Crispim, J.C.O.; Lima, K.M.G. ATR-FTIR Spectroscopy in Blood Plasma Combined with Multivariate Analysis to Detect HIV Infection in Pregnant Women. Sci. Rep. 2020, 10, 20156. [Google Scholar] [CrossRef]
- Ghimire, H.; Garlapati, C.; Janssen, E.A.M.; Krishnamurti, U.; Qin, G.; Aneja, R.; Perera, A.G.U. Protein Conformational Changes in Breast Cancer Sera Using Infrared Spectroscopic Analysis. Cancers 2020, 12, 1708. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Kong, L.; Wang, Y.; Zhu, Y.; Zhang, C. In Situ Identification of Environmental Microorganisms with Raman Spectroscopy. Environ. Sci. Ecotechnol. 2022, 11, 100187. [Google Scholar] [CrossRef]
- Xu, J.-L.; Herrero-Langreo, A.; Lamba, S.; Ferone, M.; Scannell, A.G.M.; Caponigro, V.; Gowen, A.A. Characterisation and Classification of Foodborne Bacteria Using Reflectance FTIR Microscopic Imaging. Molecules 2021, 26, 6318. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Effah, C.Y.; He, S.; Drokow, E.K.; Agboyibor, C.; Sangmor, A.; Yuan, H.; Ding, L.; Li, X.; Sun, T.; et al. Surface-Enhanced Raman Spectroscopy: A Novel Diagnostic Method for Pathogenic Organisms. Vib. Spectrosc. 2023, 127, 103560. [Google Scholar] [CrossRef]
- Moreira, M.J.P.; Silva, A.; Saraiva, C.; Marques Martins de Almeida, J.M. Prediction of Adulteration of Game Meat Using FTIR and Chemometrics. Nutr. Food Sci. 2018, 48, 245–258. [Google Scholar] [CrossRef]
- Cao, X.; Chen, C.; Zhu, Q. Biosensors Based on Functional Nucleic Acids and Isothermal Amplification Techniques. Talanta 2023, 253, 123977. [Google Scholar] [CrossRef]
- Wesener, D.A.; Beller, Z.W.; Peters, S.L.; Rajabi, A.; Dimartino, G.; Giannone, R.J.; Hettich, R.L.; Gordon, J.I. Microbiota Functional Activity Biosensors for Characterizing Nutrient Metabolism in Vivo. ELife 2021, 10, e64478. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Trends, Challenges, and Advances in Optical Sensing for Pathogenic Bacteria Detection (PathoBactD). Biosens. Bioelectron. X 2023, 14, 100352. [Google Scholar] [CrossRef]
- Kaushik, S.; Pandey, A.; Tiwari, U.K.; Sinha, R.K. A Label-Free Fiber Optic Biosensor for Salmonella typhimurium Detection. Opt. Fiber Technol. 2018, 46, 95–103. [Google Scholar] [CrossRef]
- Kim, D.M.; Park, J.S.; Jung, S.-W.; Yeom, J.; Yoo, S.M. Biosensing Applications Using Nanostructure-Based Localized Surface Plasmon Resonance Sensors. Sensors 2021, 21, 3191. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, H.; Coelho, L.C.C.; Matias, A.; Saraiva, C.; Jorge, P.A.S.; de Almeida, J.M.M.M. Biosensors for Biogenic Amines: A Review. Biosensors 2021, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Sandhyarani, N. Surface Modification Methods for Electrochemical Biosensors. In Electrochemical Biosensors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–75. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, Z.; Ma, M.; Yang, J.; Liu, R.; Zhang, Y.; Ma, P.; Song, D. Electrochemiluminescence Biosensor for Specific Detection of Pancreatic Ductal Carcinoma through Dual Targeting of MUC1 and MiRNA-196a. Biosens. Bioelectron. 2024, 254, 116241. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Shen, Z.; Wang, G.; Gu, W.; Zhao, S.; Lin, Z.; Liu, W.; Cai, Y.; Mushtaq, G.; Jia, J.; et al. Research Progress of CRISPR-Based Biosensors and Bioassays for Molecular Diagnosis. Front. Bioeng. Biotechnol. 2022, 10, 986233. [Google Scholar] [CrossRef]
- Wang, M.; Yang, Y.; Min, J.; Song, Y.; Tu, J.; Mukasa, D.; Ye, C.; Xu, C.; Heflin, N.; McCune, J.S.; et al. A Wearable Electrochemical Biosensor for the Monitoring of Metabolites and Nutrients. Nat. Biomed. Eng. 2022, 6, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Özbek, O.; Berkel, C.; Isildak, Ö. Applications of Potentiometric Sensors for the Determination of Drug Molecules in Biological Samples. Crit. Rev. Anal. Chem. 2022, 52, 768–779. [Google Scholar] [CrossRef]
- Masteller, A.; Sankar, S.; Kim, H.B.; Ding, K.; Liu, X.; All, A.H. Recent Developments in Prosthesis Sensors, Texture Recognition, and Sensory Stimulation for Upper Limb Prostheses. Ann. Biomed. Eng. 2021, 49, 57–74. [Google Scholar] [CrossRef]
- Herrera-Domínguez, M.; Morales-Luna, G.; Mahlknecht, J.; Cheng, Q.; Aguilar-Hernández, I.; Ornelas-Soto, N. Optical Biosensors and Their Applications for the Detection of Water Pollutants. Biosensors 2023, 13, 370. [Google Scholar] [CrossRef]
- Justino, C.; Duarte, A.; Rocha-Santos, T. Recent Progress in Biosensors for Environmental Monitoring: A Review. Sensors 2017, 17, 2918. [Google Scholar] [CrossRef]
- Ye, W.; Liu, T.; Zhang, W.; Zhu, M.; Liu, Z.; Kong, Y.; Liu, S. Marine Toxins Detection by Biosensors Based on Aptamers. Toxins 2019, 12, 1. [Google Scholar] [CrossRef]
- Adane, W.D.; Chandravanshi, B.S.; Tessema, M. A Novel Electrochemical Sensor for the Detection of Metronidazole Residues in Food Samples. Chemosphere 2024, 359, 142279. [Google Scholar] [CrossRef] [PubMed]
- Maas, M.B.; Maybery, G.H.C.; Perold, W.J.; Neveling, D.P.; Dicks, L.M.T. Borosilicate Glass Fiber-Optic Biosensor for the Detection of Escherichia coli. Curr. Microbiol. 2018, 75, 150–155. [Google Scholar] [CrossRef]
- Jin, L.; Cai, X.; Ren, F.; Yang, J. An Aptamer-Based SERS Method for Rapid Screening and Identification of Pathogens Assisted by Machine Learning Technique with Robustness Evaluation. Sens. Actuators B Chem. 2024, 405, 135356. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Xiao, R.; Tang, L.; Huang, J.; Wu, D.; Liu, S.; Wang, Y.; Zhang, D.; Wang, S.; et al. Sensitive and Specific Detection of Clinical Bacteria via Vancomycin-Modified Fe3O4 @Au Nanoparticles and Aptamer-Functionalized SERS Tags. J. Mater. Chem. B 2018, 6, 3751–3761. [Google Scholar] [CrossRef]
- Das, R.; Chaterjee, B.; Kapil, A.; Sharma, T.K. Aptamer-NanoZyme Mediated Sensing Platform for the Rapid Detection of Escherichia coli in Fruit Juice. Sens. Biosensing Res. 2020, 27, 100313. [Google Scholar] [CrossRef]
- Wang, X.; Li, W.; Dai, S.; Dou, M.; Jiao, S.; Yang, J.; Li, W.; Su, Y.; Li, Q.; Li, J. High-Throughput, Highly Sensitive and Rapid SERS Detection of Escherichia coli O157:H7 Using Aptamer-Modified Au@macroporous Silica Magnetic Photonic Microsphere Array. Food Chem. 2023, 424, 136433. [Google Scholar] [CrossRef] [PubMed]
- Zúñiga, A.; Muñoz-Guamuro, G.; Boivineau, L.; Mayonove, P.; Conejero, I.; Pageaux, G.-P.; Altwegg, R.; Bonnet, J. A Rapid and Standardized Workflow for Functional Assessment of Bacterial Biosensors in Fecal Samples. Front. Bioeng. Biotechnol. 2022, 10, 859600. [Google Scholar] [CrossRef]
- Rehman, A.; Di Benedetto, G.; Bird, J.K.; Dabene, V.; Vadakumchery, L.; May, A.; Schyns, G.; Sybesma, W.; Mak, T.N. Development of a Workflow for the Selection, Identification and Optimization of Lactic Acid Bacteria with High γ-Aminobutyric Acid Production. Sci. Rep. 2023, 13, 13663. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Xu, J.; Ye, C. Development of Multiple Cross Displacement Amplification Label-Based Gold Nanoparticles Lateral Flow Biosensor for Detection of Shigella spp. Front. Microbiol. 2016, 7, 1834. [Google Scholar] [CrossRef]
- Nuñez, S.; Barra, M.; Garrido, D. Developing a Fluorescent Inducible System for Free Fucose Quantification in Escherichia coli. Biosensors 2023, 13, 388. [Google Scholar] [CrossRef]
- Vaaben, T.H.; Vazquez-Uribe, R.; Sommer, M.O.A. Characterization of Eight Bacterial Biosensors for Microbial Diagnostic and Therapeutic Applications. ACS Synth. Biol. 2022, 11, 4184–4192. [Google Scholar] [CrossRef]
- Matulis, P.; Kutraite, I.; Augustiniene, E.; Valanciene, E.; Jonuskiene, I.; Malys, N. Development and Characterization of Indole-Responsive Whole-Cell Biosensor Based on the Inducible Gene Expression System from Pseudomonas Putida KT2440. Int. J. Mol. Sci. 2022, 23, 4649. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.-G.; Moon, S.-J.; Kim, S.K.; Kim, T.H.; Lim, H.S.; Yeon, G.-H.; Sung, B.H.; Lee, C.-H.; Lee, S.-G.; Hwang, J.H.; et al. A Designed Whole-Cell Biosensor for Live Diagnosis of Gut Inflammation through Nitrate Sensing. Biosens. Bioelectron. 2020, 168, 112523. [Google Scholar] [CrossRef]
- Talap, J.; Zhao, J.; Shen, M.; Song, Z.; Zhou, H.; Kang, Y.; Sun, L.; Yu, L.; Zeng, S.; Cai, S. Recent Advances in Therapeutic Nucleic Acids and Their Analytical Methods. J. Pharm. Biomed. Anal. 2021, 206, 114368. [Google Scholar] [CrossRef] [PubMed]
- Sinitsyna, V.V.; Vetcher, A.A. Nucleic Acid Aptamers in Nanotechnology. Biomedicines 2022, 10, 1079. [Google Scholar] [CrossRef]
- Xing, H.; Zhang, Y.; Krämer, M.; Kissmann, A.-K.; Henkel, M.; Weil, T.; Knippschild, U.; Rosenau, F. A Polyclonal Selex Aptamer Library Directly Allows Specific Labelling of the Human Gut Bacterium Blautia Producta without Isolating Individual Aptamers. Molecules 2022, 27, 5693. [Google Scholar] [CrossRef]
- Xing, H.; Kissmann, A.-K.; Raber, H.F.; Krämer, M.; Amann, V.; Kohn, K.; Weil, T.; Rosenau, F. Polyclonal Aptamers for Specific Fluorescence Labeling and Quantification of the Health Relevant Human Gut Bacterium Parabacteroides Distasonis. Microorganisms 2021, 9, 2284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xing, H.; Bolotnikov, G.; Krämer, M.; Bozdogan, A.; Kissmann, A.-K.; Weil, T.; Spellerberg, B.; Stenger, S.; Rosenau, F. Robust Fluorometric Aptamer Assay for Direct and Rapid Detection of Clinical Isolates of Candida Spec. Int. J. Mol. Sci. 2024, 25, 3444. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of Bio-Electrochemical Sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- Liébana, S.; Drago, G.A. Bioconjugation and Stabilisation of Biomolecules in Biosensors. Essays Biochem. 2016, 60, 59–68. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.; Goodge, K.; Delaney, M.; Struzyk, A.; Tansey, N.; Frey, M. A Comprehensive Review of the Covalent Immobilization of Biomolecules onto Electrospun Nanofibers. Nanomaterials 2020, 10, 2142. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Wang, H. Bioreceptors as the Key Components for Electrochemical Biosensing in Medicine. Cell Rep. Phys. Sci. 2024, 5, 101801. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Lee, S.H.; Lee, U.J.; Fermin, C.D.; Kim, M. Immobilized Enzymes in Biosensor Applications. Materials 2019, 12, 121. [Google Scholar] [CrossRef]
- YazdanYar, A.; Liu, S.S.Y.; Fyta, M. Evidence of Molecular Clicking on Self-Assembled Monolayers on Au (111) and Their Properties. Comput. Mater. Sci. 2023, 216, 111809. [Google Scholar] [CrossRef]
- Kim, S.; Yoo, H. Self-Assembled Monolayers: Versatile Uses in Electronic Devices from Gate Dielectrics, Dopants, and Biosensing Linkers. Micromachines 2021, 12, 565. [Google Scholar] [CrossRef]
- Wu, H.; Fujii, Y.; Nakano, T.; Arimoto, T.; Murata, M.; Matsumoto, H.; Yoshiura, Y.; Ohnuki, H.; Endo, H. Development of a Novel Enhanced Biosensor System for Real-Time Monitoring of Fish Stress Using a Self-Assembled Monolayer. Sensors 2019, 19, 1518. [Google Scholar] [CrossRef] [PubMed]
- Niknejad, H.; Mahmoudzadeh, R. Comparison of Different Crosslinking Methods for Preparation of Docetaxel-Loaded Albumin Nanoparticles. Iran. J. Pharm. Res. 2015, 14, 385–394. [Google Scholar]
- Ionescu, R.E. Use of Cysteamine and Glutaraldehyde Chemicals for Robust Functionalization of Substrates with Protein Biomarkers—An Overview on the Construction of Biosensors with Different Transductions. Biosensors 2022, 12, 581. [Google Scholar] [CrossRef]
- Imam, H.T.; Marr, P.C.; Marr, A.C. Enzyme Entrapment, Biocatalyst Immobilization without Covalent Attachment. Green Chem. 2021, 23, 4980–5005. [Google Scholar] [CrossRef]
- Lim, R.R.X.; Sofer, Z.; Bonanni, A. Functionalized 2D Germanene and Its Derivatives for Electrochemical Detection of Gut-Derived Metabolites in Human Serum. Eng. Proc. 2023, 48, 45. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Gatto, G.; Broncova, G.; Kumar, A. Electrochemical Sensors and Their Applications: A Review. Chemosensors 2022, 10, 363. [Google Scholar] [CrossRef]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable Electrochemical Sensing Methodologies for On-Site Detection of Pesticide Residues in Fruits and Vegetables. Coord. Chem. Rev. 2022, 453, 214305. [Google Scholar] [CrossRef]
- Umapathi, R.; Ghoreishian, S.M.; Rani, G.M.; Cho, Y.; Huh, Y.S. Review—Emerging Trends in the Development of Electrochemical Devices for the On-Site Detection of Food Contaminants. ECS Sens. Plus 2022, 1, 044601. [Google Scholar] [CrossRef]
- Wang, X.; Shi, S.; Zhang, F.; Li, S.; Tan, J.; Su, B.; Cheng, Q.; Gou, Y.; Zhang, Y. Application of a Nanotip Array-Based Electrochemical Sensing Platform for Detection of Indole Derivatives as Key Indicators of Gut Microbiota Health. Alex. Eng. J. 2023, 85, 294–299. [Google Scholar] [CrossRef]
- Ngashangva, L.; Chattopadhyay, S. Biosensors for Point-of-Care Testing and Personalized Monitoring of Gastrointestinal Microbiota. Front. Microbiol. 2023, 14, 1114707. [Google Scholar] [CrossRef]
- Ibadullaeva, S.Z.; Appazov, N.O.; Tarahovsky, Y.S.; Zamyatina, E.A.; Fomkina, M.G.; Kim, Y.A. Amperometric Multi-Enzyme Biosensors: Development and Application, a Short Review. Biophysics 2019, 64, 696–707. [Google Scholar] [CrossRef]
- Zouaoui, F.; Zine, N.; Errachid, A.; Jaffrezic-Renault, N. Mathematical Model and Numerical Simulation of Conductometric Biosensor of Urea. Electroanalysis 2022, 34, 1131–1140. [Google Scholar] [CrossRef]
- Hou, K.; Zhao, P.; Chen, Y.; Li, G.; Lin, Y.; Chen, D.; Zhu, D.; Wu, Z.; Lian, D.; Huang, X.; et al. Rapid Detection of Bifidobacterium Bifidum in Feces Sample by Highly Sensitive Quartz Crystal Microbalance Immunosensor. Front. Chem. 2020, 8, 548. [Google Scholar] [CrossRef]
- Zhou, S.; Lu, C.; Li, Y.; Xue, L.; Zhao, C.; Tian, G.; Bao, Y.; Tang, L.; Lin, J.; Zheng, J. Gold Nanobones Enhanced Ultrasensitive Surface-Enhanced Raman Scattering Aptasensor for Detecting Escherichia coli O157:H7. ACS Sens. 2020, 5, 588–596. [Google Scholar] [CrossRef]
- Song, X.; Wang, H.; Shao, X.; Yu, X.; Xu, X. Au@Ag Core-Shell Nanorods Enhance Surface-Enhanced Raman Scattering Aptasensor for Ultrasensitive Detection of Salmonella typhimurium. Food Control 2024, 161, 110379. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Ning, W.; Yang, E.; Li, Y.; Luo, Z.; Duan, Y. Sandwich Method-Based Sensitivity Enhancement of Ω-Shaped Fiber Optic LSPR for Time-Flexible Bacterial Detection. Biosens. Bioelectron. 2022, 201, 113911. [Google Scholar] [CrossRef] [PubMed]
- Basaran, S.; Dey, S.; Bhusari, S.; Sankaran, S.; Kraus, T. Plasmonic Stimulation of Gold Nanorods for the Photothermal Control of Engineered Living Materials. Biomater. Adv. 2023, 147, 213332. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Wang, Q.; Zhao, W.-M. A Novel SPR Sensor Sensitivity-Enhancing Method for Immunoassay by Inserting MoS2 Nanosheets between Metal Film and Fiber. Opt. Lasers Eng. 2020, 132, 106135. [Google Scholar] [CrossRef]
- Akanny, E.; Bonhommé, A.; Bois, L.; Minot, S.; Bourgeois, S.; Bordes, C.; Bessueille, F. Development and Comparison of Surface-Enhanced Raman Scattering Gold Substrates for In Situ Characterization of ‘Model’ Analytes in Organic and Aqueous Media. Chem. Afr. 2019, 2, 309–320. [Google Scholar] [CrossRef]
- Akanny, E.; Bonhommé, A.; Commun, C.; Doleans-Jordheim, A.; Farre, C.; Bessueille, F.; Bourgeois, S.; Bordes, C. Surface-enhanced Raman Spectroscopy Using Uncoated Gold Nanoparticles for Bacteria Discrimination. J. Raman Spectrosc. 2020, 51, 619–629. [Google Scholar] [CrossRef]
- Tavakkoli Yaraki, M.; Tukova, A.; Wang, Y. Emerging SERS Biosensors for the Analysis of Cells and Extracellular Vesicles. Nanoscale 2022, 14, 15242–15268. [Google Scholar] [CrossRef]
- Fang, S.; Wu, S.; Chen, Z.; He, C.; Lin, L.L.; Ye, J. Recent Progress and Applications of Raman Spectrum Denoising Algorithms in Chemical and Biological Analyses: A Review. TrAC Trends Anal. Chem. 2024, 172, 117578. [Google Scholar] [CrossRef]
- You, S.-M.; Luo, K.; Jung, J.-Y.; Jeong, K.-B.; Lee, E.-S.; Oh, M.-H.; Kim, Y.-R. Gold Nanoparticle-Coated Starch Magnetic Beads for the Separation, Concentration, and SERS-Based Detection of E. coli O157:H7. ACS Appl. Mater. Interfaces 2020, 12, 18292–18300. [Google Scholar] [CrossRef]
- Duan, N.; Chang, B.; Zhang, H.; Wang, Z.; Wu, S. Salmonella Typhimurium Detection Using a Surface-Enhanced Raman Scattering-Based Aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhao, Z.; Lian, K.; Yin, L.; Wang, J.; Man, S.; Liu, G.; Ma, L. SERS-Based CRISPR/Cas Assay on Microfluidic Paper Analytical Devices for Supersensitive Detection of Pathogenic Bacteria in Foods. Biosens. Bioelectron. 2022, 207, 114167. [Google Scholar] [CrossRef]
- Duan, N.; Shen, M.; Qi, S.; Wang, W.; Wu, S.; Wang, Z. A SERS Aptasensor for Simultaneous Multiple Pathogens Detection Using Gold Decorated PDMS Substrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 230, 118103. [Google Scholar] [CrossRef]
- Jin, L.; Wang, S.; Shao, Q.; Cheng, Y. A Rapid and Facile Analytical Approach to Detecting Salmonella Enteritidis with Aptamer-Based Surface-Enhanced Raman Spectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120625. [Google Scholar] [CrossRef] [PubMed]
- Cano Perez, J.L.; Gutiérrez-Gutiérrez, J.; Perezcampos Mayoral, C.; Pérez-Campos, E.L.; Pina Canseco, M.d.S.; Tepech Carrillo, L.; Mayoral, L.P.-C.; Vargas Treviño, M.; Apreza, E.L.; Rojas Laguna, R. Fiber Optic Sensors: A Review for Glucose Measurement. Biosensors 2021, 11, 61. [Google Scholar] [CrossRef]
- Song, D.; Han, X.; Xu, W.; Liu, J.; Zhuo, Y.; Zhu, A.; Long, F. Target Nucleic Acid Amplification-Free Detection of Escherichia coli O157:H7 by CRISPR/Cas12a and Hybridization Chain Reaction Based on an Evanescent Wave Fluorescence Biosensor. Sens. Actuators B Chem. 2023, 376, 133005. [Google Scholar] [CrossRef]
- Uniyal, A.; Srivastava, G.; Pal, A.; Taya, S.; Muduli, A. Recent Advances in Optical Biosensors for Sensing Applications: A Review. Plasmonics 2023, 18, 735–750. [Google Scholar] [CrossRef]
- Zhu, Q.; Liu, L.; Wang, R.; Zhou, X. A Split Aptamer (SPA)-Based Sandwich-Type Biosensor for Facile and Rapid Detection of Streptomycin. J. Hazard. Mater. 2021, 403, 123941. [Google Scholar] [CrossRef]
- Ma, H.; Pan, S.-Q.; Wang, W.-L.; Yue, X.; Xi, X.-H.; Yan, S.; Wu, D.-Y.; Wang, X.; Liu, G.; Ren, B. Surface-Enhanced Raman Spectroscopy: Current Understanding, Challenges, and Opportunities. ACS Nano 2024, 18, 14000–14019. [Google Scholar] [CrossRef]
- Barhoum, A.; Sadak, O.; Ramirez, I.A.; Iverson, N. Stimuli-Bioresponsive Hydrogels as New Generation Materials for Implantable, Wearable, and Disposable Biosensors for Medical Diagnostics: Principles, Opportunities, and Challenges. Adv. Colloid Interface Sci. 2023, 317, 102920. [Google Scholar] [CrossRef]
- Ayati, M.H.; Araj-Khodaei, M.; Haghgouei, T.; Ahmadalipour, A.; Mobed, A.; Sanaie, S. Biosensors: The Nanomaterial-Based Method in Detection of Human Gut Microbiota. Mater. Chem. Phys. 2023, 307, 127854. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, M.J.; Pintado, M.; Almeida, J.M.M.M.D. Are Aptamer-Based Biosensors the Future of the Detection of the Human Gut Microbiome?—A Systematic Review and Meta-Analysis. Biosensors 2024, 14, 423. https://doi.org/10.3390/bios14090423
Moreira MJ, Pintado M, Almeida JMMMD. Are Aptamer-Based Biosensors the Future of the Detection of the Human Gut Microbiome?—A Systematic Review and Meta-Analysis. Biosensors. 2024; 14(9):423. https://doi.org/10.3390/bios14090423
Chicago/Turabian StyleMoreira, Maria João, Manuela Pintado, and José M. M. M. De Almeida. 2024. "Are Aptamer-Based Biosensors the Future of the Detection of the Human Gut Microbiome?—A Systematic Review and Meta-Analysis" Biosensors 14, no. 9: 423. https://doi.org/10.3390/bios14090423
APA StyleMoreira, M. J., Pintado, M., & Almeida, J. M. M. M. D. (2024). Are Aptamer-Based Biosensors the Future of the Detection of the Human Gut Microbiome?—A Systematic Review and Meta-Analysis. Biosensors, 14(9), 423. https://doi.org/10.3390/bios14090423






