Optical Interferometric Device for Rapid and Specific Detection of Biological Cells
Abstract
1. Introduction
2. Materials and Methods
3. Results
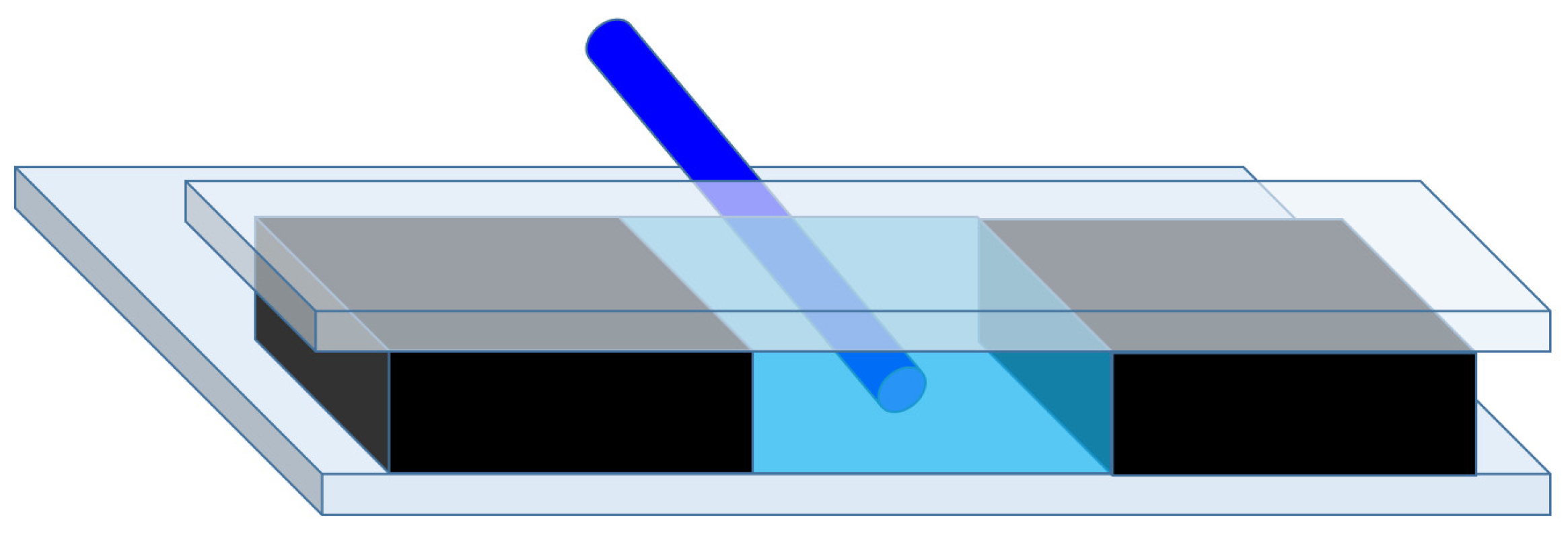
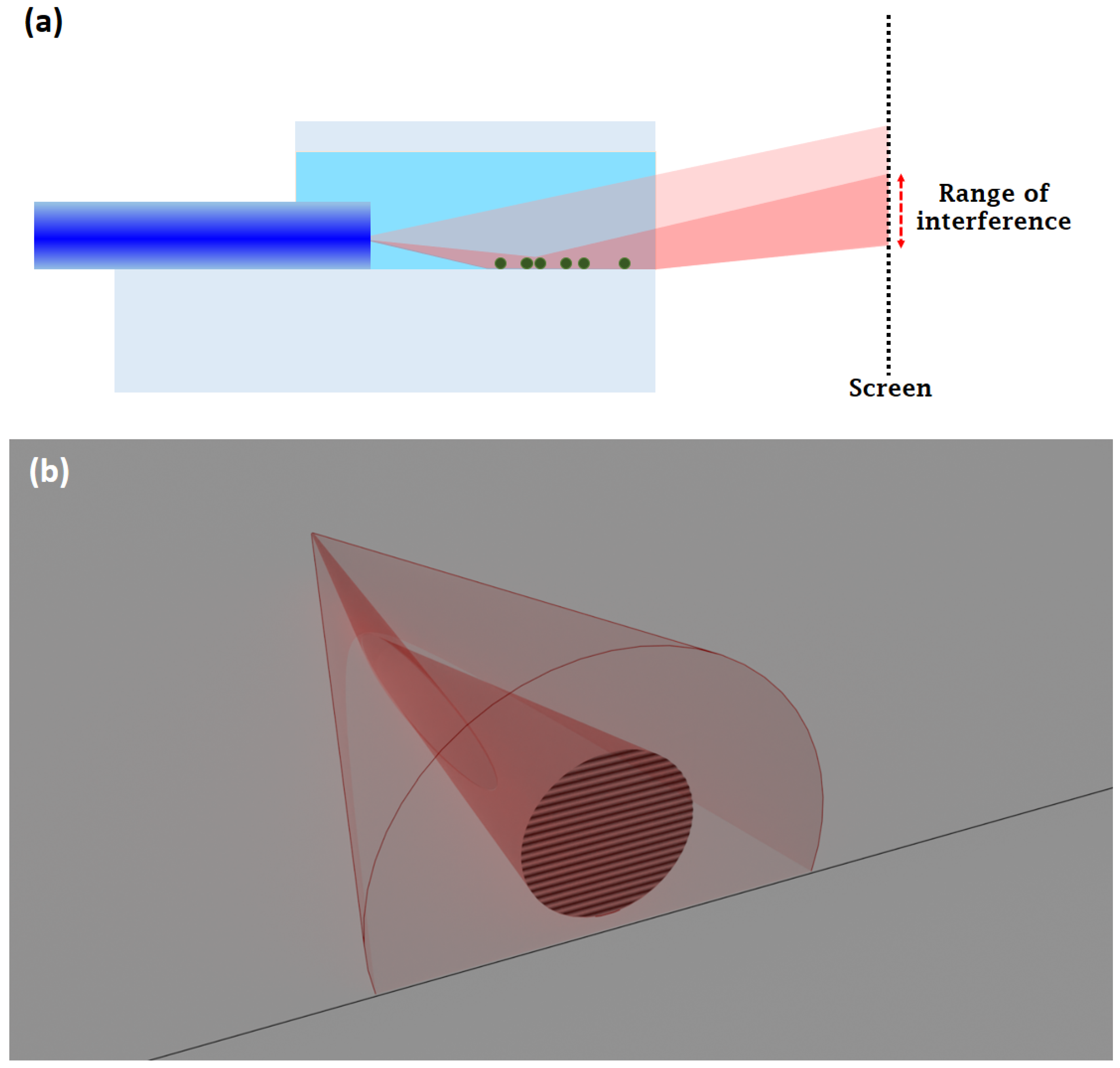
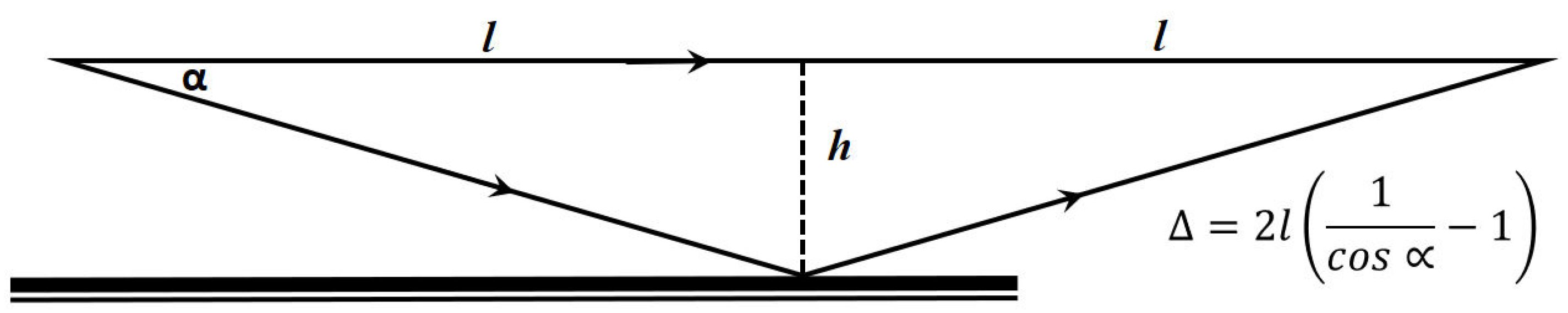
3.1. Working Principle
3.2. Evaluation Procedure
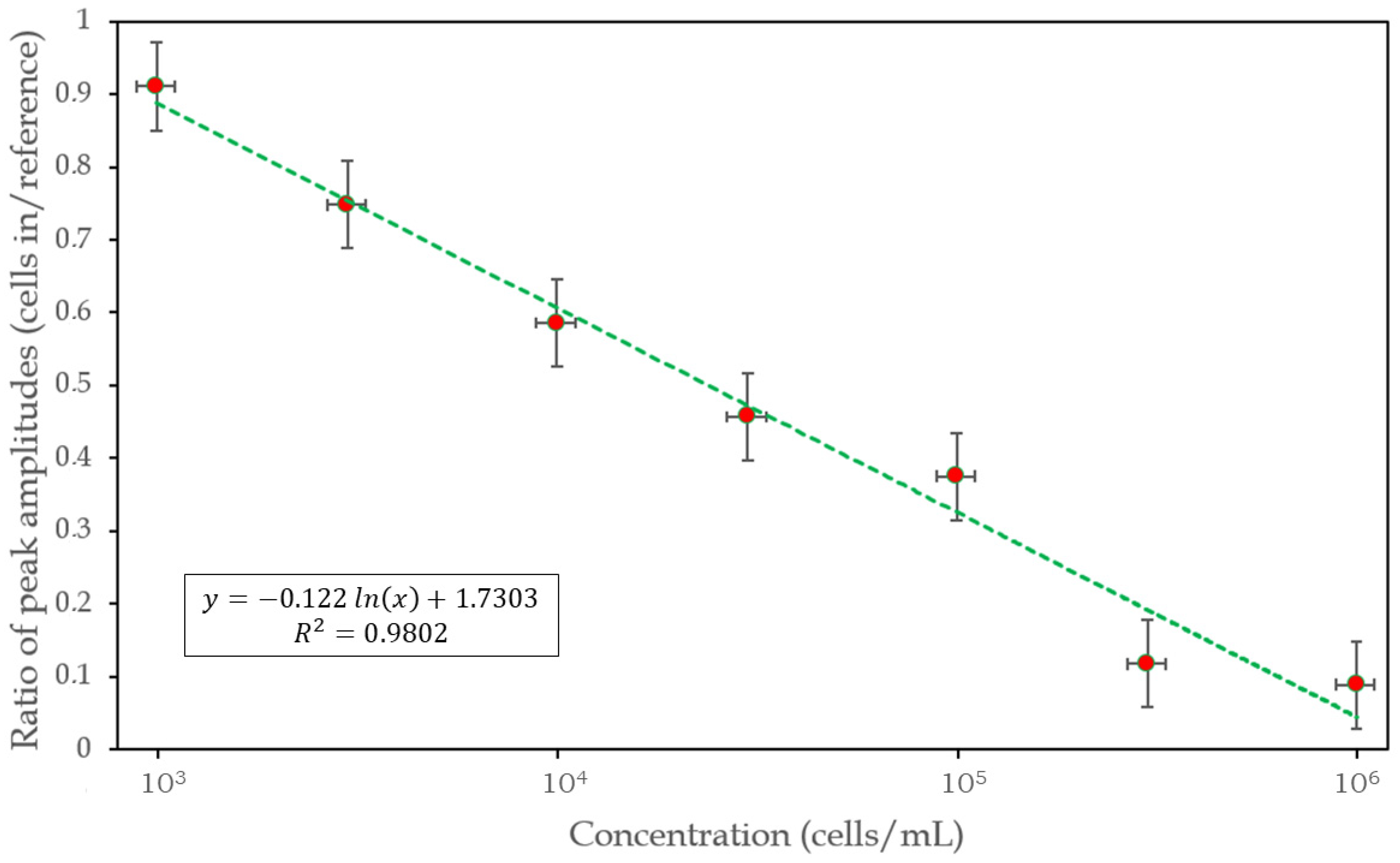
3.3. Calibration of the Device

4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engvall, E.; Perlmann, P. Enzyme-Linked Immunosorbent Assay, Elisa. J. Immunol. 1972, 109, 129–135. [Google Scholar] [CrossRef]
- Maldonado, J.; Estévez, M.-C.; Fernández-Gavela, A.; González-López, J.J.; González-Guerrero, A.B.; Lechuga, L.M. Label-Free Detection of Nosocomial Bacteria Using a Nanophotonic Interferometric Biosensor. Analyst 2020, 145, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Idil, N.; Aslıyüce, S.; Perçin, I.; Mattiasson, B. Recent Advances in Optical Sensing for the Detection of Microbial Contaminants. Micromachines 2023, 14, 1668. [Google Scholar] [CrossRef] [PubMed]
- Petrovszki, D.; Valkai, S.; Gora, E.; Tanner, M.; Bányai, A.; Fürjes, P.; Dér, A. An Integrated Electro-Optical Biosensor System for Rapid, Low-Cost Detection of Bacteria. Microelectron. Eng. 2021, 239–240, 111523. [Google Scholar] [CrossRef]
- Tran, H.V.; Piro, B.; Reisberg, S.; Tran, L.D.; Duc, H.T.; Pham, M.C. Label-free and reagentless electrochemical detection of microRNAs using a conducting polymer nanostructured by carbon nanotubes: Application to prostate cancer biomarker miR-141. Biosens. Bioelectron. 2013, 49, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Ravalli, A.; da Rocha, C.G.; Yamanaka, H.; Marrazza, G. A label-free electrochemical affisensor for cancer marker detection: The case of HER2. Bioelectrochemistry 2015, 106, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, B.; Angelomé, P.C.; Lechuga, L.M.; Liz-Marzán, L.M. LSPR-based nanobiosensors. Nano Today 2009, 4, 244–251. [Google Scholar] [CrossRef]
- Estevez, M.-C.; Alvarez, M.; Lechuga, L.M. Integrated optical devices for lab-on-a-chip biosensing applications. Laser Photonics Rev. 2012, 6, 463–487. [Google Scholar] [CrossRef]
- Tertis, M.; Leva, P.I.; Bogdan, D.; Suciu, M.; Graur, F.; Cristea, C. Impedimetric aptasensor for the label-free and selective detection of Interleukin-6 for colorectal cancer screening. Biosens. Bioelectron. 2019, 137, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Sándor, N.; Kristóf, K.; Paréj, K.; Pap, D.; Bajtay, Z. CR3 is the dominant phagocytotic complement receptor on human dendritic cells. Immunobiology 2013, 218, 652–663. [Google Scholar] [CrossRef] [PubMed]
- Petrovszki, D.; Walter, F.R.; Vigh, J.P.; Kocsis, A.; Valkai, S.; Deli, M.A.; Dér, A. Penetration of the SARS-CoV-2 Spike Protein across the Blood–Brain Barrier, as Revealed by a Combination of a Human Cell Culture Model System and Optical Biosensing. Biomedicines 2022, 10, 188. [Google Scholar] [CrossRef] [PubMed]
- Sundström, C.; Nilsson, K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int. J. Cancer 1976, 17, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Rustichelli, D.; Castiglia, S.; Gunetti, M.; Mareschi, K.; Signorino, E.; Muraro, M.; Castello, L.; Sanavio, F.; Leone, M.; Ferrero, I.; et al. Validation of analytical methods in compliance with good manufac-turing practice: A practical approach. J. Transl. Med. 2013, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zuckerman, S.T.; Kao, W.J. Intracellular protein phosphorylation in adherent U937 monocytes mediated by various culture conditions and fibronectin-derived surface ligands. Biomaterials 2005, 26, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Valkai, S.; Oroszi, L.; Ormos, P. Optical tweezers with tips grown at the end of fibers by photopolymerization. Appl. Opt. 2009, 48, 2880–2883. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S. The visual discrimination of intensity and the Weber-Fechner law. J. Gen. Physiol. 1924, 7, 235–267. [Google Scholar] [CrossRef] [PubMed]
- Prieto, F.; Sep Lveda, B.; Calle, A.; Llobera, A.; Dom Nguez, C.; Abad, A.; Montoya, A.; Lechuga, L.M. An Integrated Optical Interferometric Nanodevice Based on Silicon Technology for Biosensor Applications. Nanotechnology 2003, 14, 907–912. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Trends, Challenges, and Advances in Optical Sensing for Pathogenic Bacteria Detection (PathoBactD). Biosens. Bioelectron. X 2023, 14, 100352. [Google Scholar] [CrossRef]
- Nath, P.; Kabir, A.; Khoubafarin Doust, S.; Kreais, Z.J.; Ray, A. Detection of Bacterial and Viral Pathogens Using Photonic Point-of-Care Devices. Diagnostics 2020, 10, 841. [Google Scholar] [CrossRef] [PubMed]
- Dér, A.; Valkai, S.; Mathesz, A.; Andó, I.; Wolff, E.K.; Ormos, P. Protein-based all-optical sensor device. Sens. Actuators B 2010, 151, 26–29. [Google Scholar] [CrossRef]

| Concentration (Cells/mL) | Amplitude Ratio (Cells in/Reference) |
|---|---|
| 103 | 0.9105 |
| 3 × 103 | 0.7479 |
| 104 | 0.5857 |
| 3 × 104 | 0.4570 |
| 105 | 0.3749 |
| 3 × 106 | 0.1172 |
| 106 | 0.0888 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valkai, S.; Petrovszki, D.; Fáskerti, Z.; Baumgärtner, M.; Biczók, B.; Dakos, K.; Dósa, K.; Kirner, B.B.; Kocsis, A.E.; Nagy, K.; et al. Optical Interferometric Device for Rapid and Specific Detection of Biological Cells. Biosensors 2024, 14, 421. https://doi.org/10.3390/bios14090421
Valkai S, Petrovszki D, Fáskerti Z, Baumgärtner M, Biczók B, Dakos K, Dósa K, Kirner BB, Kocsis AE, Nagy K, et al. Optical Interferometric Device for Rapid and Specific Detection of Biological Cells. Biosensors. 2024; 14(9):421. https://doi.org/10.3390/bios14090421
Chicago/Turabian StyleValkai, Sándor, Dániel Petrovszki, Zsombor Fáskerti, Margaréta Baumgärtner, Brigitta Biczók, Kira Dakos, Kevin Dósa, Berill B. Kirner, Anna E. Kocsis, Krisztina Nagy, and et al. 2024. "Optical Interferometric Device for Rapid and Specific Detection of Biological Cells" Biosensors 14, no. 9: 421. https://doi.org/10.3390/bios14090421
APA StyleValkai, S., Petrovszki, D., Fáskerti, Z., Baumgärtner, M., Biczók, B., Dakos, K., Dósa, K., Kirner, B. B., Kocsis, A. E., Nagy, K., Andó, I., & Dér, A. (2024). Optical Interferometric Device for Rapid and Specific Detection of Biological Cells. Biosensors, 14(9), 421. https://doi.org/10.3390/bios14090421









