Brillouin Biosensing of Viscoelasticity across Phase Transitions in Ovine Cornea
Abstract
1. Introduction
2. Materials and Methods
2.1. Cornea Samples Preparation
2.2. BLS Sensing
3. Results and Discussion
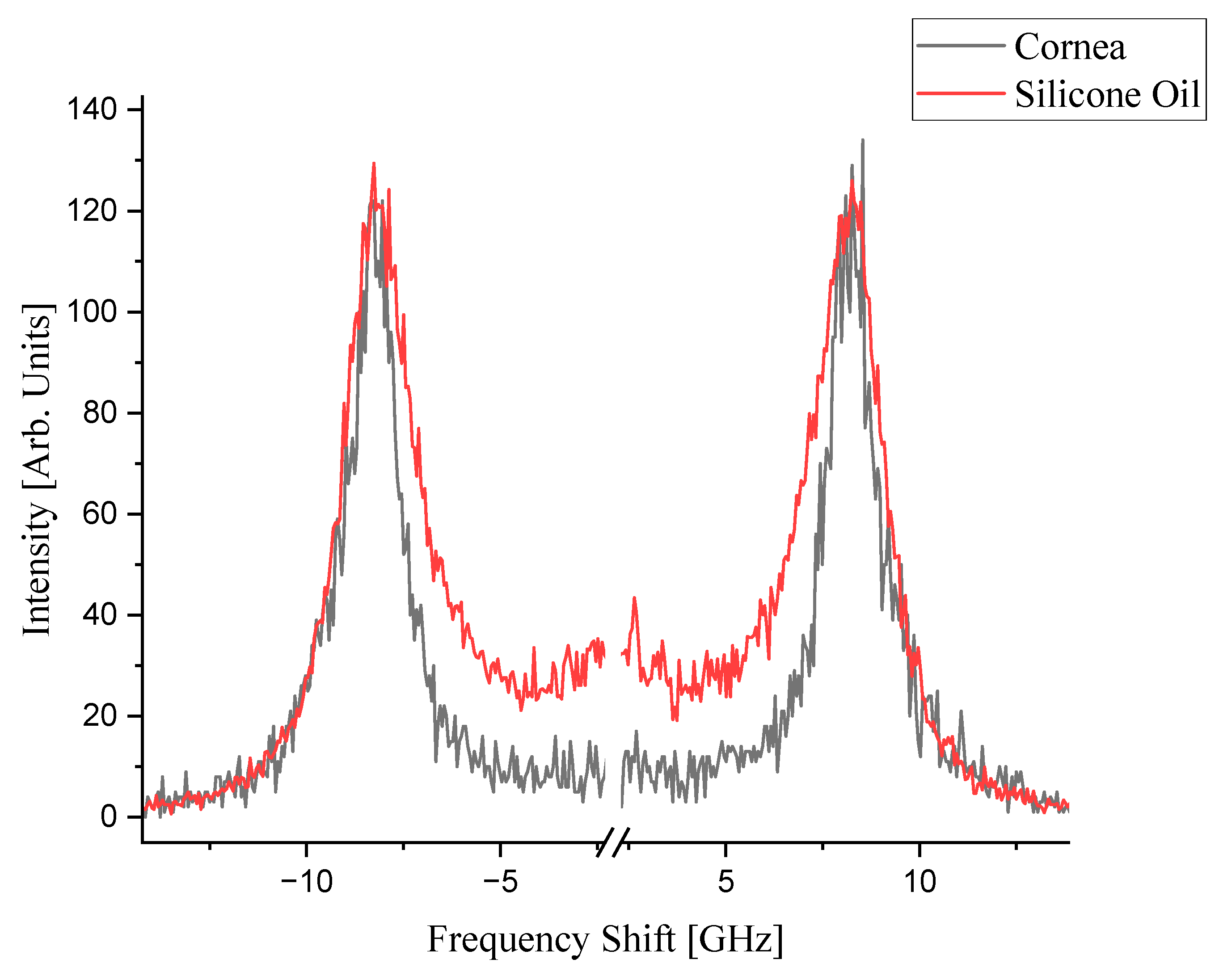
3.1. Brillouin Spectra of Cornea and Silicone Oil
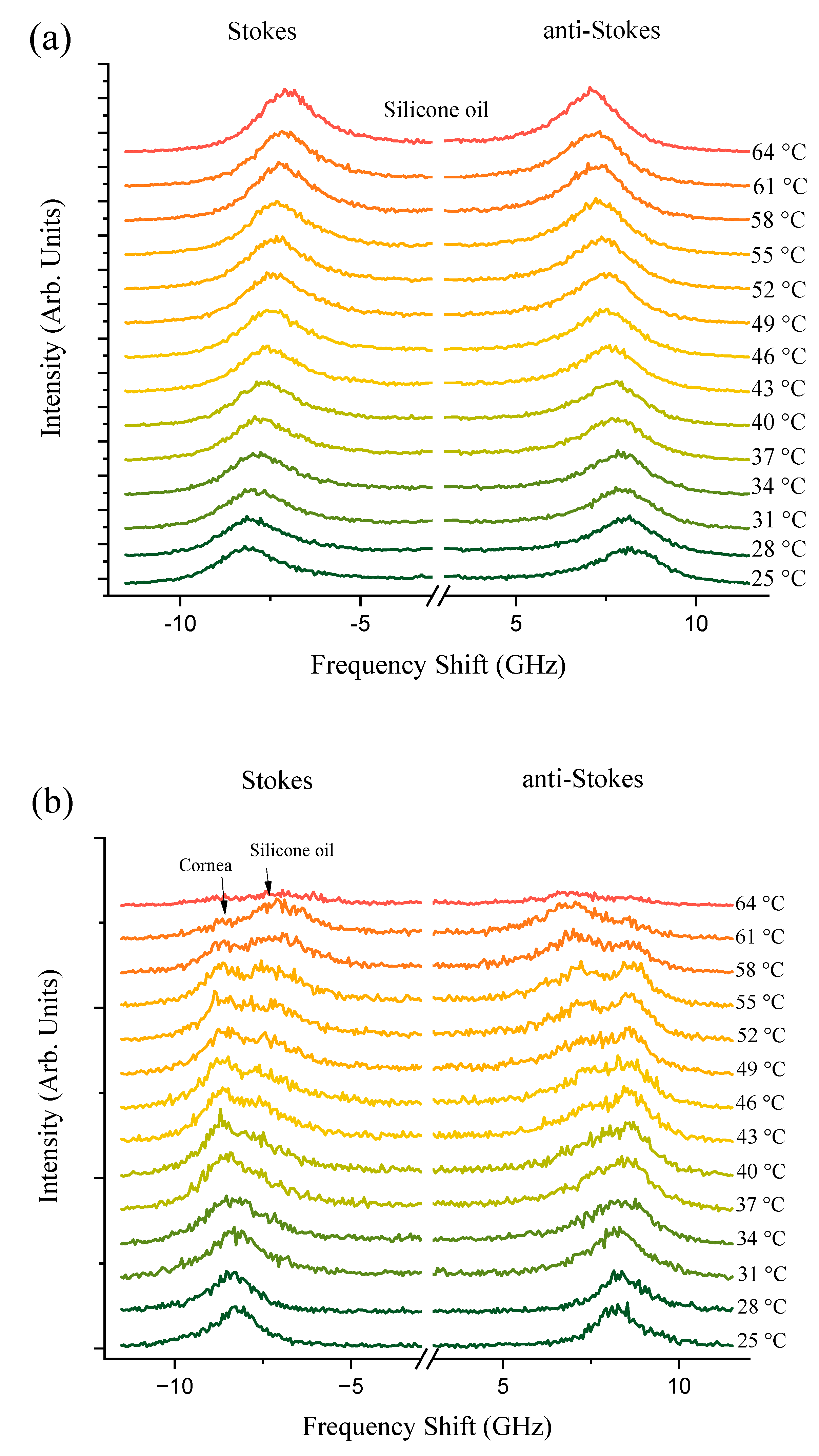
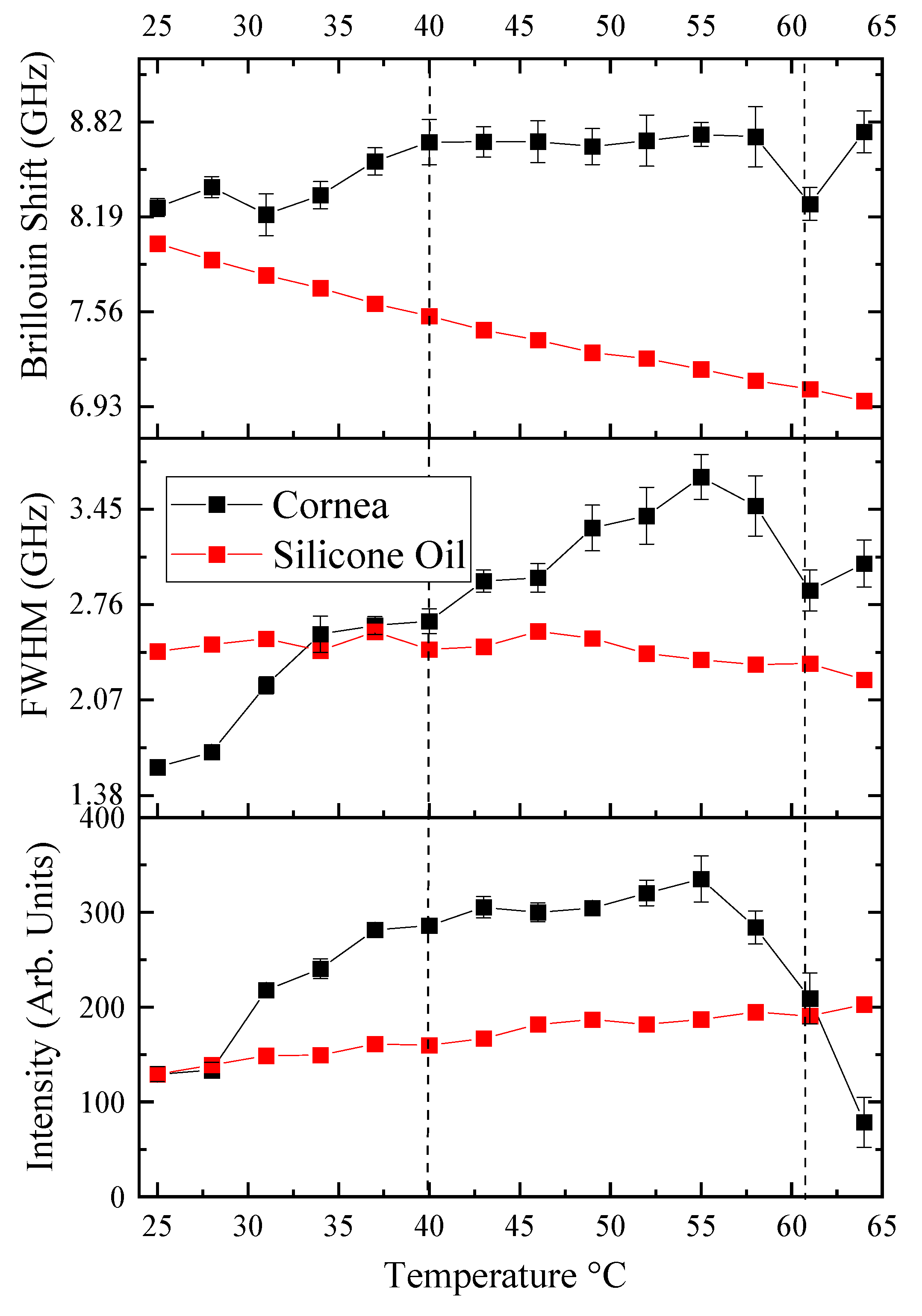
3.2. Phase Transitions: Characteristic Temperatures during Corneal Denaturation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
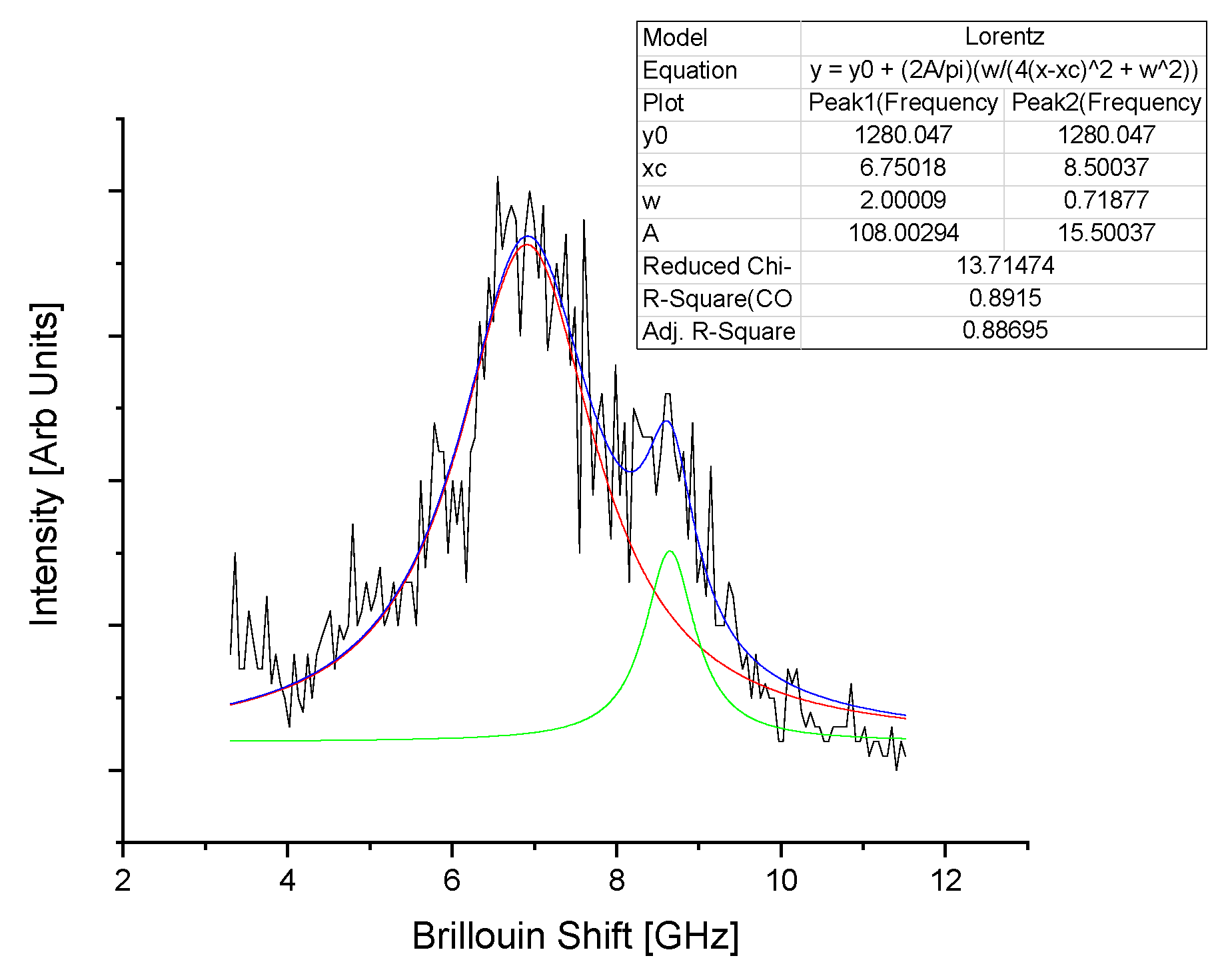
Appendix B
| Temperature (Celsius) | Standard Fitting Error | Standard Measurement Error |
|---|---|---|
| 25 | 0.06 | 0.59324 |
| 28 | 0.07 | 0.56231 |
| 31 | 0.14 | 0.38672 |
| 34 | 0.09 | 0.42943 |
| 37 | 0.09 | 0.34161 |
| 40 | 0.15 | 0.32695 |
| 43 | 0.1 | 0.41276 |
| 46 | 0.14 | 0.53032 |
| 49 | 0.12 | 0.4497 |
| 52 | 0.17 | 0.4606 |
| 55 | 0.08 | 0.64402 |
| 58 | 0.2 | 0.84993 |
| 61 | 0.11 | 0.71722 |
| 64 | 0.14 | 0.75961 |
References
- Munnerlyn, C.R.; Koons, S.J.; Marshall, J. Photorefractive Keratectomy: A Technique for Laser Refractive Surgery. J. Cataract. Refract. Surg. 1988, 14, 46–52. [Google Scholar] [CrossRef]
- Abdel-Radi, M.; Shehata, M.; Mostafa, M.M.; Aly, M.O.M. Transepithelial Photorefractive Keratectomy: A Prospective Randomized Comparative Study between the Two-Step and the Single-Step Techniques. Eye 2023, 37, 1545–1552. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, Y.; Zhang, X.; Shen, Y.; Zhou, X. Keratometry and Ultrastructural Changes after Microwave Thermokeratoplasty in Rabbit Eyes. Lasers Surg. Med. 2021, 54, 565–571. [Google Scholar] [CrossRef]
- Tomás-Juan, J.; Murueta-Goyena Larrañaga, A.; Hanneken, L. Corneal Regeneration after Photorefractive Keratectomy: A Review. J. Optom. 2014, 8, 149–169. [Google Scholar] [CrossRef]
- Brinkmann, R.; Koop, N.; Geerling, G.; Kampmeier, J.; Borcherding, S.; Kamm, K.; Birngruber, R. Diode Laser Thermokeratoplasty: Application Strategy and Dosimetry. J. Cataract Refract. Surg. 1998, 24, 1195–1207. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Tang, M.; Shekhar, R. Mathematical Model of Corneal Surface Smoothing after Laser Refractive Surgery. Am. J. Ophthalmol. 2003, 135, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Karampatzakis, A.; Samaras, T. Numerical Model of Heat Transfer in the Human Eye with Consideration of Fluid Dynamics of the Aqueous Humour. Phys. Med. Biol. 2010, 55, 5653–5665. [Google Scholar] [CrossRef]
- Kharmyssov, C.; Abdildin, Y.G.; Kostas, K.V. Optic Nerve Head Damage Relation to Intracranial Pressure and Corneal Properties of Eye in Glaucoma Risk Assessment. Med Biol. Eng. Comput. 2019, 57, 1591–1603. [Google Scholar] [CrossRef]
- Nimni, M.E.; Harkness, R.D. Molecular Structure and Functions of Collagen. In Collagen: Volume I: Biochemistry; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Thomsen, S. Pathologic analysis of photothermal and photomechanical effects of laser–tissue interactions. Photochem. Photobiol. 1991, 53, 825–835. [Google Scholar] [CrossRef]
- Spoerl, E.; Wollensak, G.; Dittert, D.D.; Seiler, T. Thermomechanical Behavior of Collagen-Cross-Linked Porcine Cornea. Ophthalmologica 2004, 218, 136–140. [Google Scholar] [CrossRef]
- Knott, L.; Tarlton, J.F.; Bailey, A.J. Chemistry of Collagen Cross-Linking: Biochemical Changes in Collagen during the Partial Mineralization of Turkey Leg Tendon. Biochem. J. 1997, 322, 535–542. [Google Scholar] [CrossRef]
- Dai, C.A.; Chen, Y.F.; Liu, M.W. Thermal Properties Measurements of Renatured Gelatin Using Conventional and Temperature Modulated Differential Scanning Calorimetry. J. Appl. Polym. Sci. 2005, 99, 1795–1801. [Google Scholar] [CrossRef]
- Bozec, L.; Odlyha, M. Thermal Denaturation Studies of Collagen by Microthermal Analysis and Atomic Force Microscopy. Biophys. J. 2011, 101, 228–236. [Google Scholar] [CrossRef]
- Kurbanova, B.; Ashikbayeva, Z.; Amantayeva, A.; Sametova, A.; Blanc, W.; Gaipov, A.; Tosi, D.; Utegulov, Z. Thermo-Visco-Elastometry of RF-Wave-Heated and Ablated Flesh Tissues Containing Au Nanoparticles. Biosensors 2022, 13, 8. [Google Scholar] [CrossRef]
- Akilbekova, D.; Yakupov, T.; Ogay, V.; Umbayev, B.; Yakovlev, V.V.; Utegulov, Z.N. Brillouin Light Scattering Spectroscopy for Tissue Engineering Application. In Optical Elastography and Tissue Biomechanics V; SPIE: Bellingham, DC, USA, 2018. [Google Scholar]
- Coker, Z.; Troyanova-Wood, M.; Traverso, A.J.; Yakupov, T.; Utegulov, Z.N.; Yakovlev, V.V. Assessing Performance of Modern Brillouin Spectrometers. Opt. Express 2018, 26, 2400–2409. [Google Scholar] [CrossRef]
- Kharmyssov, C.; Sekerbayev, K.; Nurekeyev, Z.; Gaipov, A.; Utegulov, Z.N. Mechano-Chemistry across Phase Transitions in Heated Albumin Protein Solutions. Polymers 2023, 15, 2039. [Google Scholar] [CrossRef]
- Scarcelli, G.; Pineda, R.; Yun, S.H. Brillouin Optical Microscopy for Corneal Biomechanics. Investig. Ophthalmol. Vis. Sci. 2012, 53, 185–190. [Google Scholar] [CrossRef]
- Scarcelli, G.; Kling, S.; Quijano, E.; Pineda, R.; Marcos, S.; Yun, S.H. Brillouin Microscopy of Collagen Crosslinking: Noncontact Depth-Dependent Analysis of Corneal Elastic Modulus. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1418–1425. [Google Scholar] [CrossRef]
- Scarcelli, G.; Besner, S.; Pineda, R.; Yun, S.H. Biomechanical Characterization of Keratoconus Corneas Ex Vivo with Brillouin Microscopy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4490–4495. [Google Scholar] [CrossRef]
- Zhang, H.; Roozbahani, M.; Piccinini, A.L.; Golan, O.; Hafezi, F.; Scarcelli, G.; Randleman, J.B. Depth-Dependent Reduction of Biomechanical Efficacy of Contact Lens–Assisted Corneal Cross-Linking Analyzed by Brillouin Microscopy. J. Refract. Surg. 2019, 35, 721–728. [Google Scholar] [CrossRef]
- Scarcelli, G.; Besner, S.; Pineda, R.; Kalout, P.; Yun, S.H. In Vivo Biomechanical Mapping of Normal and Keratoconus CorneasIn Vivo Biomechanical Mapping of CorneasLetters. JAMA Ophthalmol. 2015, 133, 480–482. [Google Scholar] [CrossRef]
- Akilbekova, D.; Ogay, V.; Yakupov, T.; Sarsenova, M.; Umbayev, B.; Nurakhmetov, A.; Tazhin, K.; Yakovlev, V.V.; Utegulov, Z.N. Brillouin Spectroscopy and Radiography for Assessment of Viscoelastic and Regenerative Properties of Mammalian Bones. J. Biomed. Opt. 2018, 23, 097004. [Google Scholar] [CrossRef]
- Seiler, T.G.; Shao, P.; Frueh, B.E.; Yun, S.H.; Seiler, T. The Influence of Hydration on Different Mechanical Moduli of the Cornea. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 1653–1660. [Google Scholar] [CrossRef]
- Shao, P.; Seiler, T.G.; Eltony, A.M.; Ramier, A.; Kwok, S.J.J.; Scarcelli, G.; Pineda, R.; Yun, S.H.A. Effects of Corneal Hydration on Brillouin Microscopy in Vivo. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3020–3027. [Google Scholar] [CrossRef]
- Darracq, G.; Couvert, A.; Couriol, C.; Amrane, A.; Thomas, D.; Dumont, E.; Andres, Y.; Le Cloirec, P. Silicone Oil: An Effective Absorbent for the Removal of Hydrophobic Volatile Organic Compounds. J. Chem. Technol. Biotechnol. 2010, 85, 309–313. [Google Scholar] [CrossRef]
- Hatami-Marbini, H.; Rahimi, A. Effects of Bathing Solution on Tensile Properties of the Cornea. Exp. Eye Res. 2014, 120, 103–108. [Google Scholar] [CrossRef]
- Wang, X.; Chen, C.; Huang, X.; Wang, J.; Yao, M.; Wang, K.; Huang, F.; Han, B.; Zhou, Q.; Li, F. Acoustic and Elastic Properties of Silicone Oil under High Pressure. RSC Adv. 2015, 5, 38056–38060. [Google Scholar] [CrossRef]
- Leikina, E.; Mertts, M.V.; Kuznetsova, N.; Leikin, S. Type I Collagen Is Thermally Unstable at Body Temperature. Proc. Natl. Acad. Sci. USA 2002, 99, 1314–1318. [Google Scholar] [CrossRef]
- Kampmeier, J.; Radt, B.; Birngruber, R.; Brinkmann, R. Thermal and Biomechanical Parameters of Porcine Cornea. Cornea 2000, 19, 355–363. [Google Scholar] [CrossRef]
- Iannucci, L.E.; Riak, M.B.; Meitz, E.; Bersi, M.R.; Gruev, V.; Lake, S.P. Effect of Matrix Properties on Transmission and Reflectance Mode Division-of-Focal-Plane Stokes Polarimetry. J. Biomed. Opt. 2023, 28, 102902. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Yu, S.M.; Li, Y. The Chemistry and Biology of Collagen Hybridization. J. Am. Chem. Soc. 2023, 145, 10901–10916. [Google Scholar] [CrossRef]
- Li, Y.; Qiao, C.; Shi, L.; Jiang, Q.; Li, T. Viscosity of Collagen Solutions: Influence of Concentration, Temperature, Adsorption, and Role of Intermolecular Interactions. J. Macromol. Sci. Part B Phys. 2014, 53, 893–901. [Google Scholar] [CrossRef]
- Brinkmann, R.; Koop, N.; Ksmpmeier, J.; Bruhns, A.; Asiyo-Vogel, M.; Engelhardt, R.; Birngruber, R. Corneal Collagen Denaturation in Laser Thermokeratoplasty (LTK). Investig. Ophthalmol. Vis. Sci. 1997, 38. [Google Scholar] [CrossRef]
- Na, G.C. Monomer and Oligomer of Type I Collagen: Molecular Properties and Fibril Assembly. Biochemistry 1989, 28, 7161–7167. [Google Scholar] [CrossRef]
- Pederson, A.W.; Ruberti, J.W.; Messersmith, P.B. Thermal Assembly of a Biomimetic Mineral/Collagen Composite. Biomaterials 2003, 24, 4881–4890. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Qiao, C.; Li, J.; Zhang, H.; Li, T. Viscometric Study of the Gelatin Solutions Ranging from Dilute to Extremely Dilute Concentrations. J. Macromol. Sci. Part B Phys. 2011, 50, 1481–1490. [Google Scholar] [CrossRef]
- Abrusci, C.; Martín-González, A.; Del Amo, A.; Corrales, T.; Catalina, F. Biodegradation of Type-B Gelatine by Bacteria Isolated from Cinematographic Films. A Viscometric Study. Polym. Degrad. Stab. 2004, 86, 283–291. [Google Scholar] [CrossRef]
- Raub, C.B.; Suresh, V.; Krasieva, T.; Lyubovitsky, J.; Mih, J.D.; Putnam, A.J.; Tromberg, B.J.; George, S.C. Noninvasive Assessment of Collagen Gel Microstructure and Mechanics Using Multiphoton Microscopy. Biophys. J. 2007, 92, 2212–2222. [Google Scholar] [CrossRef]
- Jansen, K.A.; Licup, A.J.; Sharma, A.; Rens, R.; MacKintosh, F.C.; Koenderink, G.H. The Role of Network Architecture in Collagen Mechanics. Biophys. J. 2018, 114, 2665–2678. [Google Scholar] [CrossRef]
- Taufalele, P.V.; VanderBurgh, J.A.; Muñoz, A.; Zanotelli, M.R.; Reinhart-King, C.A. Fiber Alignment Drives Changes in Architectural and Mechanical Features in Collagen Matrices. PLoS ONE 2019, 14, e0216537. [Google Scholar] [CrossRef]
- Yao, J.; Ma, J.; Zhao, J.; Qi, P.; Li, M.; Lin, L.; Sun, L.; Wang, X.; Liu, W.; Wang, Y. Corneal Hydration Assessment Indicator Based on Terahertz Time Domain Spectroscopy. Biomed. Opt. Express 2020, 11, 2073–2084. [Google Scholar] [CrossRef] [PubMed]
- Kurpakus-Wheater, M.; Kernacki, K.A.; Hazlett, L.D. Maintaining Corneal Integrity How the “Window” Stays Clear. Prog. Histochem. Cytochem. 2001, 36, 179–259. [Google Scholar] [CrossRef]
- Miles, C.A.; Bailey, A.J. Thermal Denaturation of Collagen Revisited. Proc. Indian Acad. Sci. Chem. Sci. 1999, 111, 71–80. [Google Scholar] [CrossRef]
- Elsheikh, A.; Alhasso, D. Mechanical anisotropy of porcine cornea and correlation with stromal microstructure. Exp. Eye Res. 2009, 88, 1084–1091. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kharmyssov, C.; Utegulov, Z. Brillouin Biosensing of Viscoelasticity across Phase Transitions in Ovine Cornea. Biosensors 2024, 14, 371. https://doi.org/10.3390/bios14080371
Kharmyssov C, Utegulov Z. Brillouin Biosensing of Viscoelasticity across Phase Transitions in Ovine Cornea. Biosensors. 2024; 14(8):371. https://doi.org/10.3390/bios14080371
Chicago/Turabian StyleKharmyssov, Chingis, and Zhandos Utegulov. 2024. "Brillouin Biosensing of Viscoelasticity across Phase Transitions in Ovine Cornea" Biosensors 14, no. 8: 371. https://doi.org/10.3390/bios14080371
APA StyleKharmyssov, C., & Utegulov, Z. (2024). Brillouin Biosensing of Viscoelasticity across Phase Transitions in Ovine Cornea. Biosensors, 14(8), 371. https://doi.org/10.3390/bios14080371





