Construction of Metal–Organic Framework as a Novel Platform for Ratiometric Determination of Cyanide
Abstract
1. Introduction
2. Materials and Methods
2.1. Main Reagents and Materials
2.2. Main Instruments and Equipment
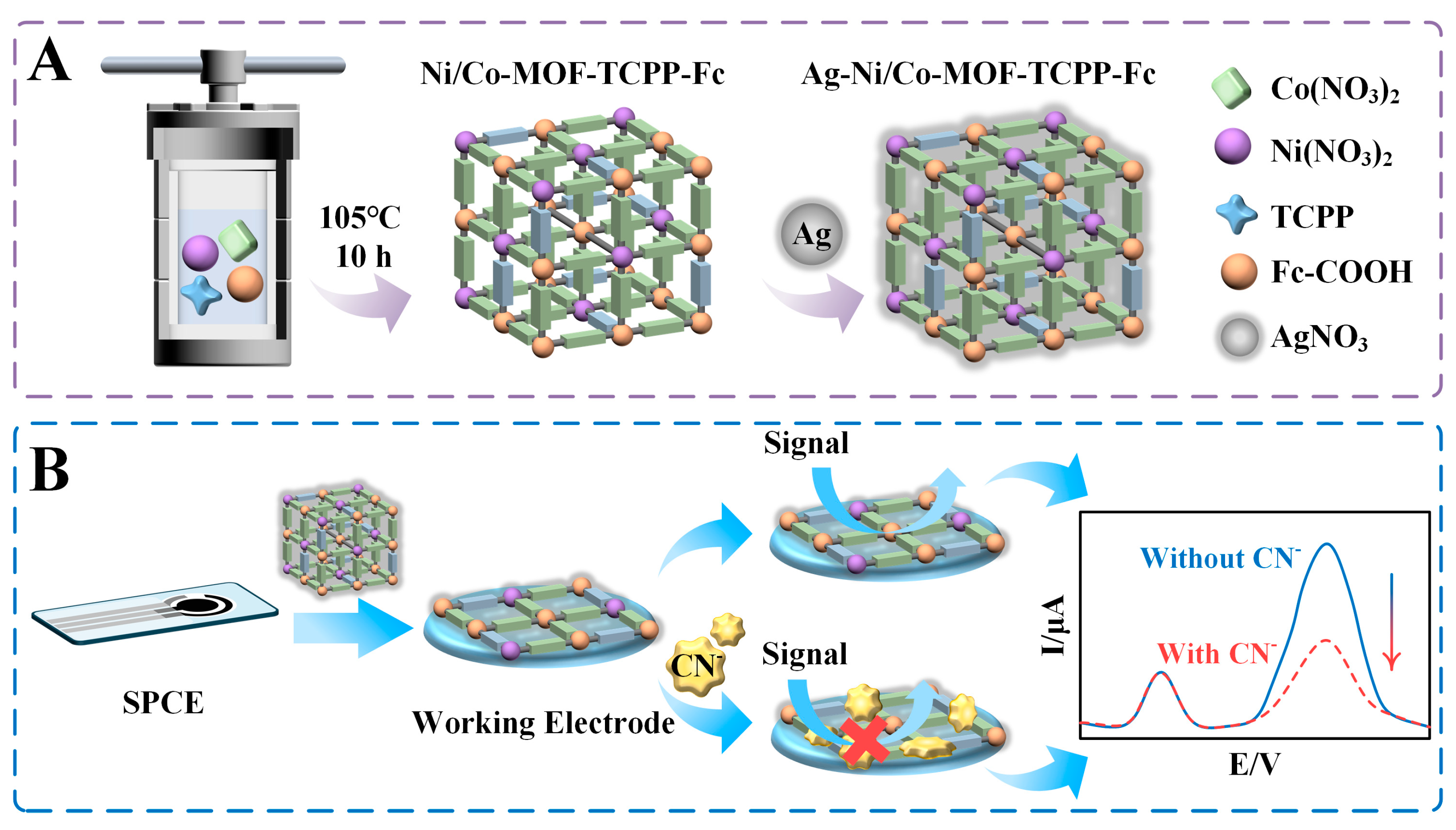
2.3. Preparation of Electrochemical Sensor
2.4. Electrochemical Experiment
2.5. Determination of Real Samples
3. Results and Discussion
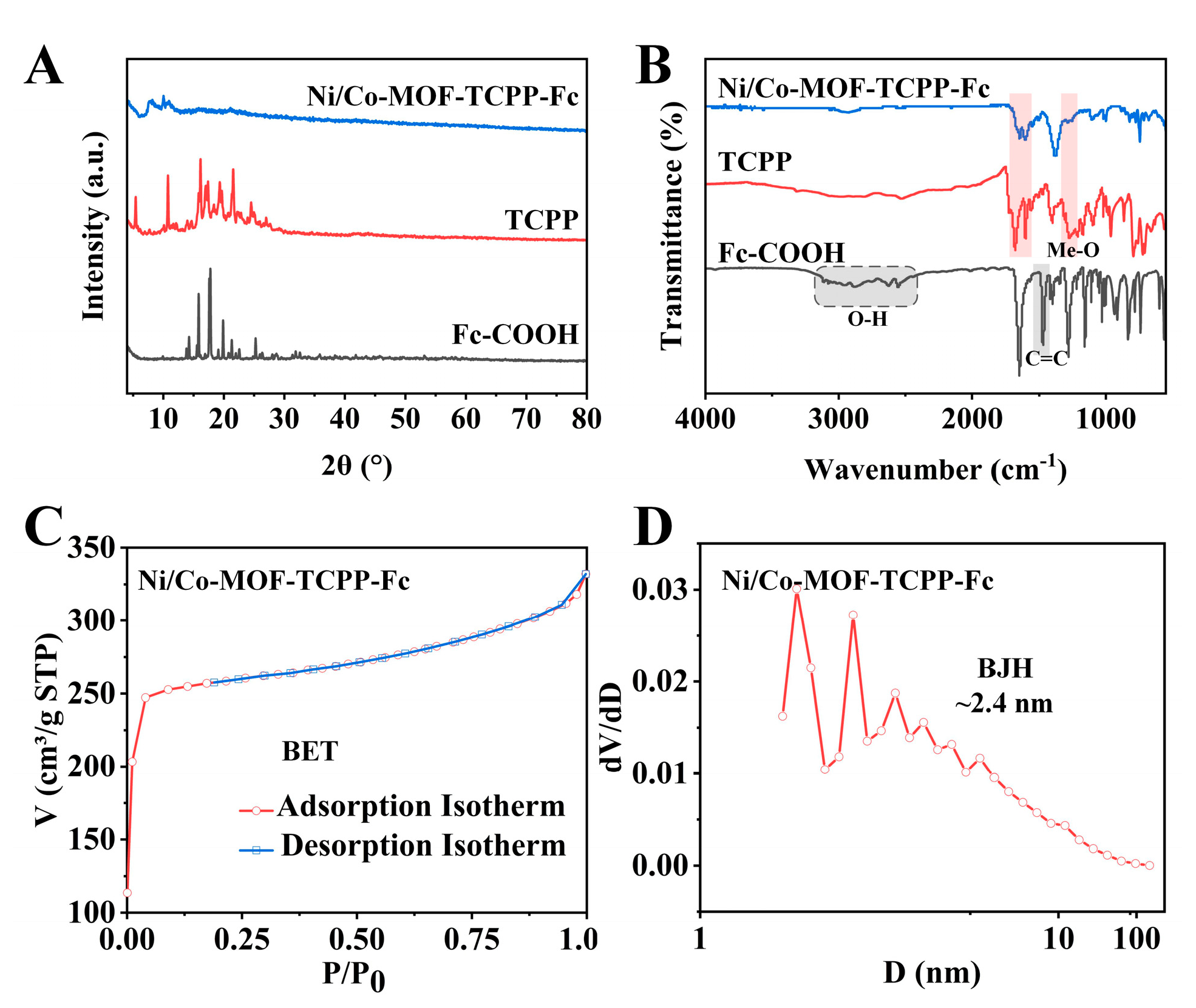
3.1. Morphological Study and Chemical Composition of Ni/Co−MOF−TCPP−Fc
3.2. Synthetic Properties of Ni/Co−MOF−TCPP−Fc
3.3. Electrochemical Characterization of Gradual Modification
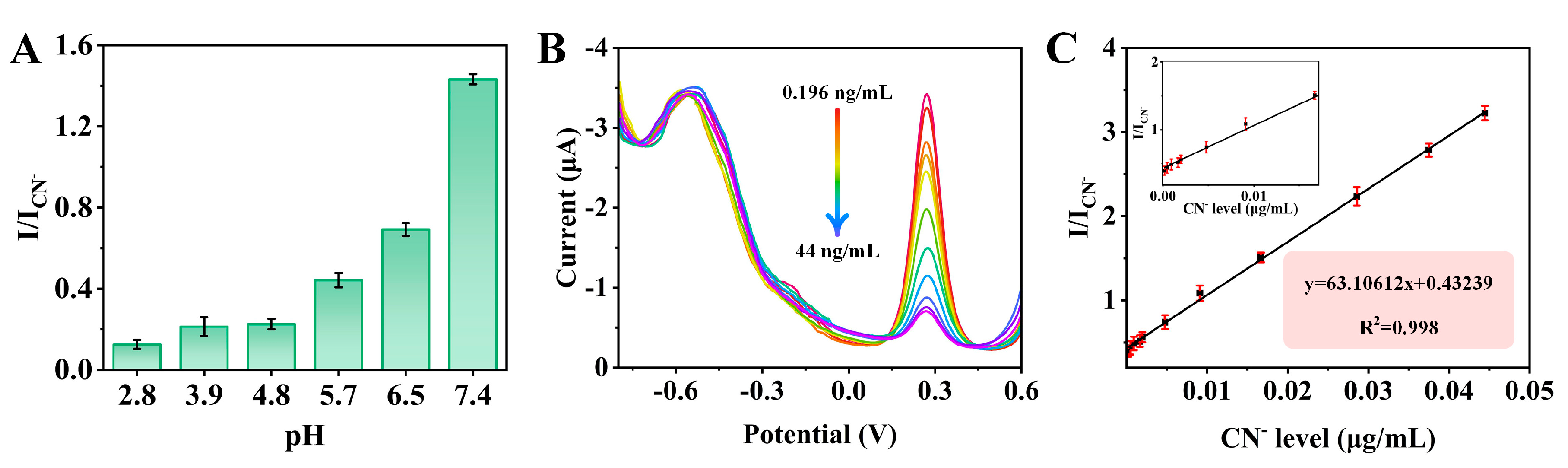
3.4. Electrochemical Determination of CN−
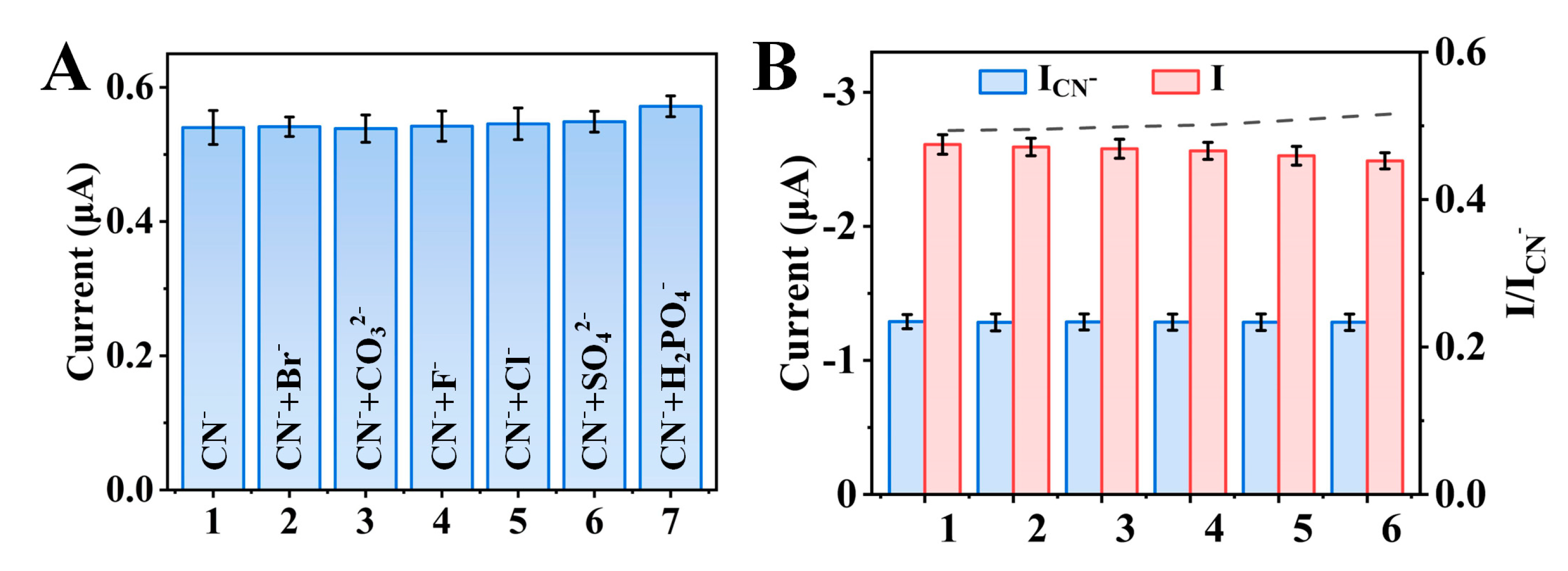
3.5. Specificity and Stability of the Sensor
3.6. Evaluation of CN− in Fermented Grains and Baijiu
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bai, C.B.; Zhang, J.; Qin, Y.X.; Huang, H.N.; Xia, Z.N.; Zheng, Q.J.; Dai, H.L.; Lu, P.K.; Miao, H.; Qu, C.Q.; et al. Precise probe design based ESIPT coupled AIE mechanism toward endogenous cyanide in food detection and bioimaging. Chem. Eng. J. 2022, 443, 136445. [Google Scholar] [CrossRef]
- Pacher, P. Cyanide emerges as an endogenous mammalian gasotransmitter. Proc. Natl. Acad. Sci. USA 2021, 118, e2108040118. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Wu, Q.; Xu, Y. Biodegradation of cyanide with Saccharomyces cerevisiae in Baijiu fermentation. Food Control 2021, 127, 108107. [Google Scholar] [CrossRef]
- Qin, Y.; Duan, B.; Shin, J.A.; So, H.J.; Hong, E.S.; Jeong, H.G.; Lee, J.H.; Lee, K.T. Effect of fermentation on cyanide and ethyl carbamate contents in cassava flour and evaluation of their mass balance during lab−scale continuous distillation. Foods 2021, 14, 1089. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Y.; Peng, Y.K.; Yan, J. Detection of cyanide in pollution−free livestock product breeding water by ion chromatography. Asian Agric. Res. 2018, 10, 34–36. [Google Scholar]
- Xu, J.; Tong, H.W.; Yan, X.; Du, S.; Yao, Z.; Liu, S. Sensitive determination of cyanide in cigarette smoke by capillary GC with a microECD. Chromatographia 2006, 64, 609–612. [Google Scholar] [CrossRef]
- Kang, H.I.; Shin, H.S. Derivatization method of free cyanide including cyanogen chloride for the sensitive analysis of cyanide in chlorinated drinking water by liquid chromatography−tandem mass spectrometry. Anal. Chem. 2015, 87, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, X.A.; Tang, Q.Y.; Guo, Y.Q.; Zhang, Z.; Zhang, W.; Zou, X.B.; Sun, Z.B. Molecularly imprinted electrochemical sensor for ethyl carbamate detection in Baijiu based on “on−off” nanozyme−catalyzing process. Food Chem. 2024, 453, 139626. [Google Scholar] [CrossRef] [PubMed]
- Dowlatshahi, S.; Abdekhodaie, M.J. Electrochemical prostate−specific antigen biosensors based on electroconductive nanomaterials and polymers. Clin. Chim. Acta 2021, 516, 111–135. [Google Scholar] [CrossRef]
- Su, X.Y.; Wang, H.; Wang, C.Q.; Zhou, X.; Zou, X.B.; Zhang, W. Programmable dual−electric−field immunosensor using MXene−Au−based competitive signal probe for natural parathion−methyl detection. Biosens. Bioelectron. 2022, 214, 114546. [Google Scholar] [CrossRef]
- Monteiro, M.C.; Winiarski, J.P.; Santana, E.R.; Szpoganicz, B.; Vieira, I.C. Ratiometric electrochemical sensor for butralin determination using a quinazoline−engineered prussian blue analogue. Materials 2023, 16, 1024. [Google Scholar] [CrossRef] [PubMed]
- Lal, S.; Singh, P.; Singhal, A.; Kumar, S.; Gahlot, A.P.; Gandhi, N.; Kumari, P. Advances in metal–organic frameworks for water remediation applications. RSC Adv. 2024, 14, 3413–3446. [Google Scholar] [CrossRef] [PubMed]
- Shu, H.; Lai, T.R.; Yang, Z.C.; Xiao, X.C.; Chen, X.M.; Wang, Y.D. High sensitivity electrochemical detection of ultra−trace imidacloprid in fruits and vegetables using a Fe−rich FeCoNi−MOF. Food Chem. 2023, 408, 135221. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Z.; Fu, Z.H.; Xu, G. Metal−organic framework nanosheets: Preparation and applications. Coord. Chem. Rev. 2019, 388, 79–106. [Google Scholar] [CrossRef]
- Ahmadi, M.; Ebrahimnia, M.; Shahbazi, M.A.; Keçili, R.; Ghorbani−Bidkorbeh, F. Microporous metal–organic frameworks: Synthesis and applications. J. Ind. Eng. Chem. 2022, 115, 1–11. [Google Scholar] [CrossRef]
- Yu, K.; Li, M.J.; Chai, H.N.; Liu, Q.; Hai, X.; Tian, M.W.; Qu, L.J.; Xu, T.L.; Zhang, G.Y.; Zhang, X.J. MOF−818 nanozyme−based colorimetric and electrochemical dual−mode smartphone sensing platform for in situ detection of H2O2 and H2S released from living cells. Chem. Eng. J. 2023, 451, 138321. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, J.H.; Wen, N.; Xiong, H.J.; Cai, S.D.; He, Q.Y.; Hu, Y.Q.; Peng, D.M.; Liu, Z.B.; Liu, Y.F. Metal−organic frameworks for stimuli−responsive drug delivery. Biomaterials 2020, 230, 119619. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C.Z.; Mei, C.T.; Sun, J.S.; Lee, J.; Wu, Q.L.; Hubbe, M.A.; Li, M.C. Recent advances in metal organic framework and cellulose nanomaterial composites. Coord. Chem. Rev. 2022, 461, 214496. [Google Scholar] [CrossRef]
- Shi, X.M.; Wang, Z.; Chen, M.H.; Wu, Q.Q.; Chen, F.Z.; Fan, G.C.; Zhao, W.W. Highly light−harvesting MOF−on−MOF heterostructure: Cascading functionality to flexible photogating of organic photoelectrochemical transistor and bienzyme cascade detection. Anal. Chem. 2024, 96, 3679–3685. [Google Scholar] [CrossRef]
- Chen, D.D.; Sun, Q.H.; Han, C.; Guo, Y.Y.; Huang, Q.; Goddard, W.A.; Qian, J.J. Enhanced oxygen evolution catalyzed by in situ formed Fe−doped Ni oxyhydroxides in carbon nanotubes. J. Mater. Chem. A 2022, 10, 16007–16015. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Huang, Q.; Ding, J.Y.; Zhong, L.; Li, T.T.; Pan, J.Q.; Hu, Y.; Qian, J.J.; Huang, S.M. CoMo carbide/nitride from bimetallic MOF precursors for enhanced OER performance. Int. J. Hydrogen Energy 2021, 46, 22268–22276. [Google Scholar] [CrossRef]
- Zhou, N.; Su, F.F.; Guo, C.P.; He, L.H.; Jia, Z.K.; Wang, M.H.; Jia, Q.J.; Zhang, Z.H.; Lu, S.Y. Two−dimensional oriented growth of Zn−MOF−on−Zr−MOF architecture: A highly sensitive and selective platform for detecting cancer markers. Biosens. Bioelectron. 2019, 123, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.X.; Liu, J.H.; Liu, H.B.; Yi, J.L.; Xia, F.Q.; Tian, D.; Zhou, C.L. Multiplexed electrochemical aptasensor based on mixed valence Ce (III, IV)−MOF for simultaneous determination of malathion and chlorpyrifos. Anal. Chim. Acta 2022, 1230, 340364. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Abbasi-Azad, M.; Habibi, B.; Rouhani, F.; Moghanni-Bavil-Olyaei, H.; Liu, K.G.; Morsali, A. Electrochemical applications of ferrocene-based coordination polymers. ChemPlusChem 2020, 85, 2397–2418. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.K.; Yu, H.J.; Wang, L.; Liu, X.W.; Lin, T.F.; Haq, F.; Vatsadze, S.Z.; Lemenovskiy, D.A. Ferrocene−contained metal organic frameworks: From synthesis to applications. Coord. Chem. Rev. 2021, 430, 213737. [Google Scholar] [CrossRef]
- Zeng, Q.H.; Chen, P.P.; Li, Z.F.; Wen, X.; Wen, W.; Liu, Y.; Zhao, H.L.; Zhang, S.P.; Zhou, H.H.; Zhang, L.Y. Application of a modified porphyrin in a polymer electrolyte with superior properties for all−solid−state lithium batteries. ACS Appl. Mater. Interfaces 2021, 13, 48569–48581. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Wu, B.W.; Li, Z.Z.; Bai, Y.H.; Kan, L.; Wang, M.H.; He, L.H.; Du, M. Hierarchical CoCoPBA@ PCN−221 nanostructure for the highly sensitive detection of deoxynivalenol in foodstuffs. Food Chem. 2023, 403, 134370. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, J.X.; Huang, S.H.; Chen, Z.Y.; Lu, C.B.; Yang, C.Q.; Zhai, G.Q.; Zhu, J.H.; Zhuang, X.D. Porphyrinic conjugated microporous polymer anode for Li−ion batteries. J. Power Sources 2022, 531, 231340. [Google Scholar] [CrossRef]
- Yu, K.; Won, D.I.; Lee, W.I.; Ahn, W.S. Porphyrinic zirconium metal−organic frameworks: Synthesis and applications for adsorption/catalysis. Korean J. Chem. Eng. 2021, 38, 653–673. [Google Scholar] [CrossRef]
- Jiang, R.; Pang, Y.H.; Yang, Q.Y.; Wan, C.Q.; Shen, X.F. Copper porphyrin metal−organic framework modified carbon paper for electrochemical sensing of glyphosate. Sens. Actuators B 2022, 358, 131492. [Google Scholar] [CrossRef]
- Dong, J.B.; Zheng, J.L.; Hou, J.Z.; Zhao, P.; Liang, Y.; Lei, J.C.; Luo, X.G.; Hou, C.J.; Huo, D.Q. Au nanoparticle/CoFc/metal−organic framework as enzyme−free dual−signal ratiometric electrochemical sensor for in−situ determination of cell−secreted H2O2. ACS Appl. Nano Mater. 2023, 6, 11630–11639. [Google Scholar] [CrossRef]
- Ye, G.J.; Luo, P.; Zhao, Y.S.; Qiu, G.L.; Hu, Y.; Preis, S.; Wei, C.H. Three−dimensional Co/Ni bimetallic organic frameworks for high−efficient catalytic ozonation of atrazine: Mechanism, effect parameters, and degradation pathways analysis. Chemosphere 2020, 253, 126767. [Google Scholar] [CrossRef] [PubMed]
- Hang, X.X.; Zhao, J.W.; Xue, Y.D.; Yang, R.; Pang, H. Synergistic effect of Co/Ni bimetallic metal–organic nanostructures for enhanced electrochemical energy storage. J. Colloid Interface Sci. 2022, 628, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Tian, D.; Song, N.; Zhong, M.X.; Lu, X.F.; Wang, C. Bimetallic MOF nanosheets decorated on electrospun nanofibers for high−performance asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2019, 12, 1280–1291. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Q.; Wei, S.L.; Bai, P.Y.; Yang, C.C.; Xu, L. Robust coal matrix intensifies electron/substrate interaction of nickel−nitrogen (Ni−N) active sites for efficient CO2 electroreduction at industrial current density. Appl. Catal. B 2021, 299, 120661. [Google Scholar] [CrossRef]
- Liang, B.L.; Zhang, X.L.; Zhong, M.; Lv, C.C.; Li, K.X. Transition metal (Fe, Co, Ni) and sulfur codoped nitrogen−enriched hydrothermal carbon as high−performance cathode catalyst for microbial fuel cell. J. Power Sources 2021, 506, 230178. [Google Scholar] [CrossRef]
- Pang, L.Y.; Jia, X.; Wang, P.; Wang, Y.L.; Yang, Y.H.; Liu, H. Bimetallic synergy boost TCPP(Ni)−Co MOF as the high−performance electrochemical sensor for enhanced detection of trace theophylline. Microchem. J. 2022, 183, 107981. [Google Scholar] [CrossRef]
- Chen, G.X.; Dai, W.J.; Hu, C.Y.; Zang, H.; Sun, S.Y.; Zhen, S.J.; Zhan, L.; Huang, C.Z.; Li, Y.F. Ratiometric electrochemiluminescence of zirconium metal–organic framework as a single luminophore for sensitive detection of HPV−16 DNA. Anal. Chem. 2024, 96, 538–546. [Google Scholar] [CrossRef]
- Miao, J.C.; Du, K.; Li, X.; Xu, X.T.; Dong, X.; Fang, J.L.; Cao, W.; Wei, Q. Ratiometric electrochemical immunosensor for the detection of procalcitonin based on the ratios of SiO2−Fc–COOH–Au and UiO−66−TB complexes. Biosens. Bioelectron. 2021, 171, 112713. [Google Scholar] [CrossRef]
- Liu, D.Y.; Dai, Y.M.; Zou, J.Q.; Wang, S.Y.; Qi, L.; Ling, C. Sandwich−type ferrocene−functionalized magnetic nanoparticles: Synthesis, characterization, and the adsorption of Cr(VI). J. Mater. Sci. Mater. Electron. 2019, 30, 13924–13932. [Google Scholar]
- Gan, X.F.; Han, D.B.; Wang, J.M.; Liu, P.; Li, X.R.; Zheng, Q.Y.; Yan, Y.R. A highly sensitive electrochemiluminescence immunosensor for h−FABP determination based on self−enhanced luminophore coupled with ultrathin 2D nickel metal−organic framework nanosheets. Biosens. Bioelectron. 2021, 171, 112735. [Google Scholar] [CrossRef] [PubMed]
- Hallaj, R.; Haghighi, N. Photoelectrochemical amperometric sensing of cyanide using a glassy carbon electrode modified with graphene oxide and titanium dioxide nanoparticles. Microchim. Acta 2017, 184, 3581–3590. [Google Scholar] [CrossRef]
- Kang, J.; Huo, F.J.; Zhang, Y.B.; Chao, J.B.; Glass, T.E.; Yin, C.X. A novel near−infrared ratiometric fluorescent probe for cyanide and its bioimaging applications. Spectrochim. Acta Part A 2019, 209, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Achadu, O.J.; Nyokong, T. Fluorescence “turn−ON” nanosensor for cyanide ion using supramolecular hybrid of graphene quantum dots and cobalt pyrene−derivatized phthalocyanine. Dyes Pigm. 2019, 160, 328–335. [Google Scholar] [CrossRef]
- Feng, Y.; Deng, D.Y.; Zhang, L.C.; Liu, R.; Lv, Y. LRET−based functional persistent luminescence nanoprobe for imaging and detection of cyanide ion. Sens. Actuators B 2019, 279, 189–196. [Google Scholar] [CrossRef]


| Method | Response Time | Sensitivity | Determination Limit | Linear Range | Reference |
|---|---|---|---|---|---|
| Ion chromatography | − | 0.0907 | 1 μg/L | 10~160 μg/L | [5] |
| HPLC−MS | − | 0.0053 | 0.07 μg/L | 0.2~8.42 μg/L | [7] |
| Photoelectrochemical amperometry | − | 0.0518 | 0.1 μM | 0.1~60 μM | [42] |
| Fluorescence | 4.8 min | 2.45 | 2.41 μM | 0~30 μM | [43] |
| Fluorescence | − | − | 0.5 nM | 1~50 nM | [44] |
| Fluorescence | − | − | 0.11 μM | 0.3~40 μM | [45] |
| Electrochemical | 4 min | 63.10612 | 0.052 ng/mL | 0.196~44 ng/mL | This method |
| Species | Method Comparison (µg/mL) | |||
|---|---|---|---|---|
| This Method | GC−MS | RSD | ||
| Fermented grains | Sample 1 | 0.021 ± 0.005 a | 0.020 ± 0.007 a | 5.6% |
| Sample 2 | 0.020 ± 0.003 a | 0.021 ± 0.005 a | 5.9% | |
| Sample 3 | 0.018 ± 0.005 a | 0.017 ± 0.006 a | 4.7% | |
| Baijiu | Sample 1 | 0.024 ± 0.007 a | 0.025 ± 0.006 a | 3.4% |
| Sample 2 | 0.029 ± 0.003 a | 0.030 ± 0.004 a | 3.8% | |
| Sample 3 | 0.021 ± 0.004 a | 0.020 ± 0.002 a | 4.2% | |
| River water | Sample 1 | 0.019 ± 0.003 a | 0.020 ± 0.002 a | 5.3% |
| Sample 2 | 0.020 ± 0.002 a | 0.021 ± 0.002 a | 3.3% | |
| Sample 3 | 0.021 ± 0.003 a | 0.020 ± 0.004 a | 2.5% | |
| Mineral water | Sample 1 | Not detected | Not detected | − |
| Sample 2 | Not detected | Not detected | − | |
| Sample 3 | Not detected | Not detected | − | |
| Samples | Detected (µg/mL) | Added (µg/mL) | Found | Recovery |
|---|---|---|---|---|
| Fermented grains | 0.021 ± 0.005 | 0.1 | 0.119 ± 0.005 | 98.9% |
| Baijiu | 0.024 ± 0.007 | 0.128 ± 0.009 | 103.3% | |
| River water | 0.019 ± 0.006 | 0.121 ± 0.004 | 101.1% | |
| Mineral water | Not detected | 0.098 ± 0.003 | 98.5% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Z.; Wu, Z.; Zong, Y.; Li, C.; Guo, W.; Guo, Y.; Zou, X. Construction of Metal–Organic Framework as a Novel Platform for Ratiometric Determination of Cyanide. Biosensors 2024, 14, 276. https://doi.org/10.3390/bios14060276
Sun Z, Wu Z, Zong Y, Li C, Guo W, Guo Y, Zou X. Construction of Metal–Organic Framework as a Novel Platform for Ratiometric Determination of Cyanide. Biosensors. 2024; 14(6):276. https://doi.org/10.3390/bios14060276
Chicago/Turabian StyleSun, Zongbao, Zhiwei Wu, Yiran Zong, Chen Li, Wang Guo, Yiqing Guo, and Xiaobo Zou. 2024. "Construction of Metal–Organic Framework as a Novel Platform for Ratiometric Determination of Cyanide" Biosensors 14, no. 6: 276. https://doi.org/10.3390/bios14060276
APA StyleSun, Z., Wu, Z., Zong, Y., Li, C., Guo, W., Guo, Y., & Zou, X. (2024). Construction of Metal–Organic Framework as a Novel Platform for Ratiometric Determination of Cyanide. Biosensors, 14(6), 276. https://doi.org/10.3390/bios14060276




