A Novel Fluorescence-Based Microplate Assay for High-Throughput Screening of hSULT1As Inhibitors
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Expression and Purification of Recombinant Human SULT1As
2.3. hSULT1As Reaction Phenotyping Assay
2.4. Spectroscopic Changes in the Tested Fluorophores Catalyzed by hSULT1As
2.5. Fluorescence Properties of HN-241 and Its Sulfated Metabolite
2.6. Enzymatic Kinetics of hSULT1As-Catalyzed HN-241 4-O-Sulfation
2.7. Molecular Docking
2.8. Screening and Characterization of hSULT1As Inhibitors
2.9. Determination of Half-Maximal Inhibitory Concentration (IC50)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Seeking an Optimum Scaffold for Constructing hSULT1As Fluorogenic Substrates
3.2. Structural Optimizations of 4-HN to Improve Sulfation Rate
3.3. Fluorescence Properties of HN-241 and Its Sulfated Metabolite
3.4. Enzymatic Kinetics of hSULT1As-Catalyzed HN-241 4-O-Sulfation
3.5. Binding Modes of HN-241 in hSULT1As
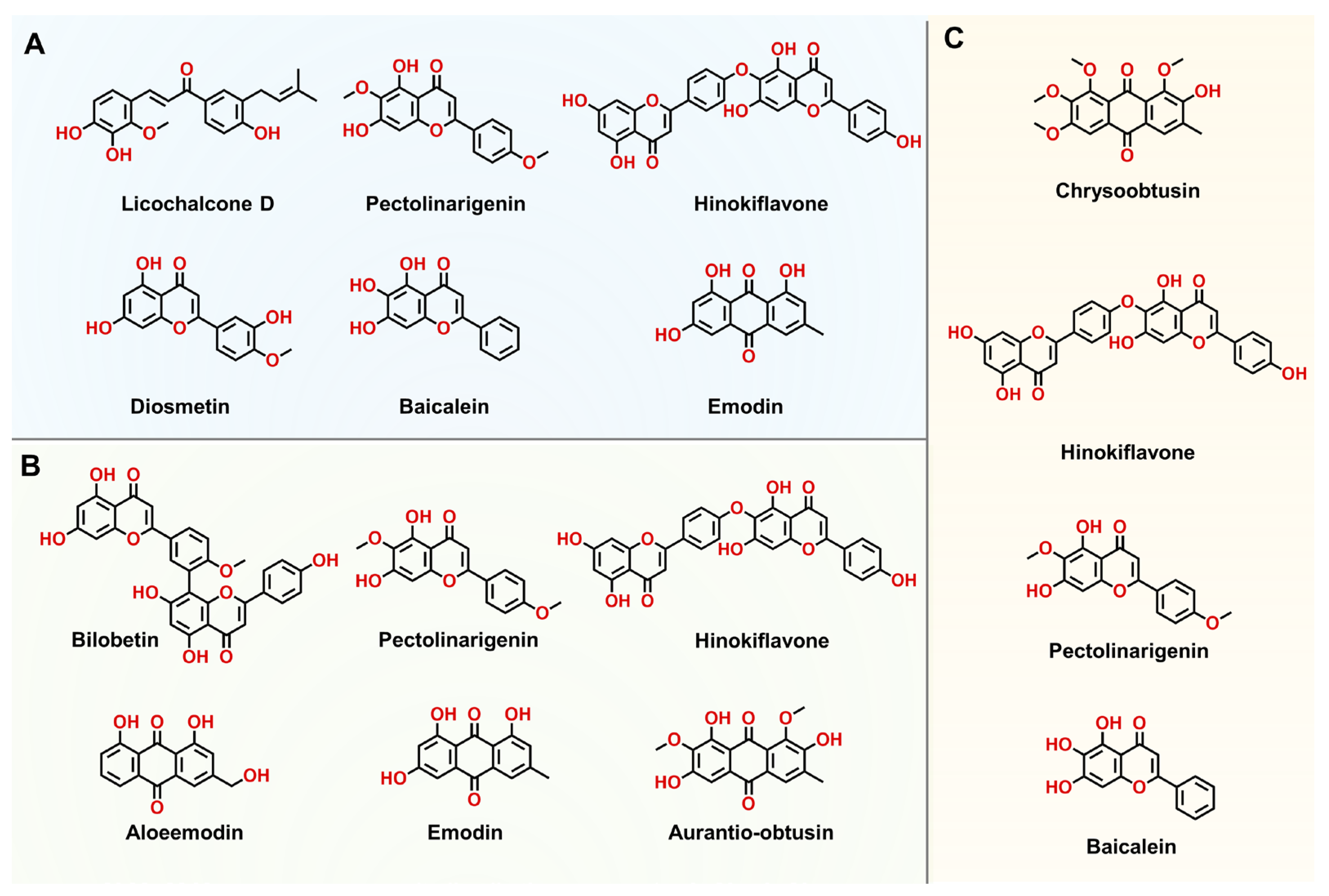
3.6. High-Throughput Screening and Characterization of hSULT1As Inhibitors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Glatt, H.; Meinl, W. Pharmacogenetics of soluble sulfotransferases (SULTs). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2004, 369, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Wang, L.L.; Li, Y.; Zhou, S.F. Structure, function and polymorphism of human cytosolic sulfotransferases. Curr. Drug Metab. 2008, 9, 99–105. [Google Scholar] [PubMed]
- Gamage, N.; Barnett, A.; Hempel, N.; Duggleby, R.G.; Windmill, K.F.; Martin, J.L.; McManus, M.E. Human sulfotransferases and their role in chemical metabolism. Toxicol. Sci. 2006, 90, 5–22. [Google Scholar] [CrossRef]
- Blanchard, R.L.; Freimuth, R.R.; Buck, J.; Weinshilboum, R.M.; Coughtrie, M.W. A proposed nomenclature system for the cytosolic sulfotransferase (SULT) superfamily. Pharmacogenetics 2004, 14, 199–211. [Google Scholar] [CrossRef]
- Nagata, K.; Yamazoe, Y. Pharmacogenetics of sulfotransferase. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 159–176. [Google Scholar] [CrossRef] [PubMed]
- Gamage, N.U.; Tsvetanov, S.; Duggleby, R.G.; McManus, M.E.; Martin, J.L. The structure of human SULT1A1 crystallized with estradiol. An insight into active site plasticity and substrate inhibition with multi-ring substrates. Biol. Chem. 2005, 280, 41482–41486. [Google Scholar] [CrossRef] [PubMed]
- Gamage, N.U.; Duggleby, R.G.; Barnett, A.C.; Tresillian, M.; Latham, C.F.; Liyou, N.E.; McManus, M.E.; Martin, J.L. Structure of a human carcinogen-converting enzyme, SULT1A1. Structural and kinetic implications of substrate inhibition. Biol. Chem. 2003, 278, 7655–7662. [Google Scholar] [CrossRef]
- Chao, Y.; Ou, Q.; Shang, J. Expression and prognostic value of SULT1A2 in bladder cancer. Exp. Ther. Med. 2021, 22, 779. [Google Scholar] [CrossRef] [PubMed]
- Raftogianis, R.B.; Wood, T.C.; Weinshilboum, R.M. Human phenol sulfotransferases SULT1A2 and SULT1A1: Genetic polymorphisms, allozyme properties, and human liver genotype-phenotype correlations. Biochem. Pharmacol. 1999, 58, 605–616. [Google Scholar] [CrossRef]
- Negishi, M.; Pedersen, L.G.; Petrotchenko, E.; Shevtsov, S.; Gorokhov, A.; Kakuta, Y.; Pedersen, L.C. Structure and function of sulfotransferases. Arch. Biochem. Biophys. 2001, 390, 149–157. [Google Scholar] [CrossRef]
- Dubin, R.L.; Hall, C.M.; Pileri, C.L.; Kudlacek, P.E.; Li, X.Y.; Yee, J.A.; Johnson, M.L.; Anderson, R.J. Thermostable (SULT1A1) and thermolabile (SULT1A3) phenol sulfotransferases in human osteosarcoma and osteoblast cells. Bone 2001, 28, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Butcher, N.J.; Horne, M.K.; Mellick, G.D.; Fowler, C.J.; Masters, C.L.; Minchin, R.F. Sulfotransferase 1A3/4 copy number variation is associated with neurodegenerative disease. Pharmacogenomics J. 2018, 18, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Back, D.J.; Breckenridge, A.M.; Orme, M.L. Drug interactions with oral contraceptive steroids. IPPF Med. Bull. 1983, 17, 1–2. [Google Scholar] [PubMed]
- Helmer, E.; Karimian, N.; Van Assche, K.; Seghers, I.; Le Tallec, S.; Cherala, G.; Scott, G.; Namour, F.S. Ziritaxestat Drug-Drug Interaction with Oral Contraceptives: Role of SULT1E1 Inhibition. Clin. Pharmacol. Ther. 2022, 112, 901–908. [Google Scholar] [CrossRef]
- Back, D.J.; Breckenridge, A.M.; MacIver, M.; Orme, M.L.; Purba, H.; Rowe, P.H. Interaction of ethinyloestradiol with ascorbic acid in man. Br. Med. J. 1981, 282, 1516. [Google Scholar] [CrossRef] [PubMed]
- Glatt, H. Sulfotransferases in the bioactivation of xenobiotics. Chem.-Biol. Interact. 2000, 129, 141–170. [Google Scholar] [CrossRef] [PubMed]
- Waring, R.H.; Ayers, S.; Gescher, A.J.; Glatt, H.R.; Meinl, W.; Jarratt, P.; Kirk, C.J.; Pettitt, T.; Rea, D.; Harris, R.M. Phytoestrogens and xenoestrogens: The contribution of diet and environment to endocrine disruption. J. Steroid Biochem. Mol. Biol. 2008, 108, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Vietri, M.; De Santi, C.; Pietrabissa, A.; Mosca, F.; Pacifici, G.M. Inhibition of human liver phenol sulfotransferase by nonsteroidal anti-inflammatory drugs. Eur. J. Clin. Pharmacol. 2000, 56, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Kreis, P.; Brandner, S.; Coughtrie, M.W.; Pabel, U.; Meinl, W.; Glatt, H.; Andrae, U. Human phenol sulfotransferases hP-PST and hM-PST activate propane 2-nitronate to a genotoxicant. Carcinogenesis 2000, 21, 295–299. [Google Scholar] [CrossRef]
- Diao, X.; Pang, X.; Xie, C.; Guo, Z.; Zhong, D.; Chen, X. Bioactivation of 3-n-butylphthalide via sulfation of its major metabolite 3-hydroxy-NBP: Mediated mainly by sulfotransferase 1A1. Drug Metab. Dispos. 2014, 42, 774–781. [Google Scholar] [CrossRef]
- He, L.; Chen, C.; Duan, S.; Li, Y.; Li, C.; Yao, X.; Gonzalez, F.J.; Qin, Z.; Yao, Z. Inhibition of estrogen sulfation by Xian-Ling-Gu-Bao capsule. J. Steroid Biochem. Mol. Biol. 2023, 225, 106182. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Yan, T.M.; Chen, J.M.; Li, X.Y.; Zou, J.; Zhu, L.J.; Lu, L.L.; Wang, Y.; Zhou, F.Y.; Liu, Z.Q.; et al. LC-MS/MS quantification of sulfotransferases is better than conventional immunogenic methods in determining human liver SULT activities: Implication in precision medicine. Sci. Rep. 2017, 7, 3858. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, E.B.; More, V.; Neira, K.L.; Lu, Z.J.; Cherrington, N.J.; Slitt, A.L.; King, R.S. Downregulation of sulfotransferase expression and activity in diseased human livers. Drug Metab. Dispos. 2013, 41, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- Falany, J.L.; Pilloff, D.E.; Leyh, T.S.; Falany, C.N. Sulfation of raloxifene and 4-hydroxytamoxifen by human cytosolic sulfotransferases. Drug Metab. Dispos. 2006, 34, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Riches, Z.; Stanley, E.L.; Bloomer, J.C.; Coughtrie, M.W. Quantitative evaluation of the expression and activity of five major sulfotransferases (SULTs) in human tissues: The SULT “pie”. Drug Metab. Dispos. 2009, 37, 2255–2261. [Google Scholar] [CrossRef]
- Wysocki, L.M.; Lavis, L.D. Advances in the chemistry of small molecule fluorescent probes. Curr. Opin. Chem. Biol. 2011, 15, 752–759. [Google Scholar] [CrossRef]
- Shi, D.L.; Liu, W.; Wang, G.W.; Guo, Y.; Li, J. Small-molecule fluorescence-based probes for aging diagnosis. Acta Mater. Med. 2022, 1, 4–23. [Google Scholar] [CrossRef]
- Sakakibara, Y.; Takami, Y.; Nakayama, T.; Suiko, M.; Liu, M.C. Localization and functional analysis of the substrate specificity/catalytic domains of human M-form and P-form phenol sulfotransferases. J. Biol. Chem. 1998, 273, 6242–6247. [Google Scholar] [CrossRef] [PubMed]
- Suiko, M.; Sakakibara, Y.; Liu, M.C. Sulfation of environmental estrogen-like chemicals by human cytosolic sulfotransferases. Biochem. Biophys. Res. Commun. 2000, 267, 80–84. [Google Scholar] [CrossRef]
- Liang, S.C.; Xia, Y.L.; Hou, J.; Ge, G.B.; Zhang, J.W.; He, Y.Q.; Wang, J.Y.; Qi, X.Y.; Yang, L. Methylation, Glucuronidation, and Sulfonation of Daphnetin in Human Hepatic Preparations In Vitro: Metabolic Profiling, Pathway Comparison, and Bioactivity Analysis. J. Pharm. Sci. 2016, 105, 808–816. [Google Scholar] [CrossRef]
- Gardner, S.H.; Reinhardt, C.J.; Chan, J. Advances in Activity-Based Sensing Probes for Isoform-Selective Imaging of Enzymatic Activity. Angew. Chem. Int. Ed. Engl. 2021, 60, 5000–5009. [Google Scholar] [CrossRef] [PubMed]
- Sedgwick, A.C.; Wu, L.; Han, H.H.; Bull, S.D.; He, X.P.; James, T.D.; Sessler, J.L.; Tang, B.Z.; Tian, H.; Yoon, J. Excited-state intramolecular proton-transfer (ESIPT) based fluorescence sensors and imaging agents. Chem. Soc. Rev. 2018, 47, 8842–8880. [Google Scholar] [CrossRef]
- Cao, D.; Liu, Z.; Verwilst, P.; Koo, S.; Jangjili, P.; Kim, J.S.; Lin, W. Coumarin-Based Small-Molecule Fluorescent Chemosensors. Chem. Rev. 2019, 119, 10403–10519. [Google Scholar] [CrossRef]
- He, R.J.; Tian, Z.H.; Huang, J.; Sun, M.R.; Wei, F.; Li, C.Y.; Zeng, H.R.; Zhang, F.; Guan, X.Q.; Feng, Y.; et al. Rationally Engineered CYP3A4 Fluorogenic Substrates for Functional Imaging Analysis and Drug-Drug Interaction Studies. J. Med. Chem. 2023, 66, 6743–6755. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.F.; Zhang, T.T.; Song, Y.Q.; Sang, Z.P.; Zeng, H.R.; Liu, P.Q.; Wang, P.; Ge, G.B. Rationally Engineered hCES2A Near-Infrared Fluorogenic Substrate for Functional Imaging and High-Throughput Inhibitor Screening. Anal. Chem. 2023, 95, 15665–15672. [Google Scholar] [CrossRef]
- Fan, Y.F.; Wu, Y.; Hou, J.; Wang, P.; Peng, X.J.; Ge, G.B. Coumarin-based near-infrared fluorogenic probes: Recent advances, challenges and future perspectives. Coord. Chem. Rev. 2023, 480, 215020. [Google Scholar] [CrossRef]
- Jin, Q.; Ma, H.Y.; Feng, L.; Wang, P.; He, R.J.; Ning, J.; Yang, L.; Ge, G.B. Sensing cytochrome P450 1A1 activity by a resorufin-based isoform-specific fluorescent probe. Chin. Chem. Lett. 2020, 31, 2945–2949. [Google Scholar] [CrossRef]
- Ding, L.L.; Tian, Z.H.; Hou, J.; Dou, T.Y.; Jin, Q.; Wang, D.D.; Zou, L.W.; Zhu, Y.; Song, Y.; Cui, J.; et al. Sensing carboxylesterase 1 in living systems by a practical and isoform specific fluorescent probe. Chin. Chem. Lett. 2019, 30, 558–562. [Google Scholar] [CrossRef]
- Sun, M.R.; Song, L.L.; Wei, H.Z.; Shi, J.H.; Zhao, B.; Tian, T.; Song, Y.Q.; Tian, Z.H.; Zhu, G.H.; Ge, G.B. Rational construction of a practical enzyme-activatable fluorogenic substrate for hNotum and its applications in functional imaging and inhibitor screening. Sens. Actuators B Chem. 2023, 393, 134145. [Google Scholar] [CrossRef]
- Wu, M.Y.; Li, K.; Li, C.Y.; Hou, J.T.; Yu, X.Q. A water-soluble near-infrared probe for colorimetric and ratiometric sensing of SO2 derivatives in living cells. Chem. Commun. 2014, 50, 183–185. [Google Scholar] [CrossRef]
- Zhou, Q.H.; Lv, X.; Tian, Z.H.; Finel, M.; Feng, L.; Huo, P.C.; Zhu, Y.D.; Lu, Y.; Hou, J.; Ge, G.B. A fluorescence-based microplate assay for high-throughput screening and evaluation of human UGT inhibitors. Anal. Chim. Acta 2021, 1153, 338305. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Ge, G.B.; Feng, L.; Troberg, J.; Hu, L.H.; Hou, J.; Cheng, H.L.; Wang, P.; Liu, Z.M.; Finel, M.; et al. An optimized ratiometric fluorescent probe for sensing human UDP-glucuronosyltransferase 1A1 and its biological applications. Biosens. Bioelectron. 2015, 72, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Shibata, H.; Ebine, M.; Tsuchikawa, H.; Matsumori, N.; Murata, M.; Yoshida, M.; Morisawa, M.; Lin, S.; Yamauchi, K.; et al. Synthesis and complete structure determination of a sperm-activating and -attracting factor isolated from the ascidian ascidia sydneiensis. J. Nat. Prod. 2018, 81, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, F.; De Santi, C.; Vietri, M.; Pietrabissa, A.; Spisni, R.; Mosca, F.; Pacifici, G.M. Differential inhibition of human liver and duodenum sulphotransferase activities by quercetin, a flavonoid present in vegetables, fruit and wine. Xenobiotica 2001, 31, 841–847. [Google Scholar] [CrossRef]
- Wang, L.Q.; James, M.O. Inhibition of sulfotransferases by xenobiotics. Curr. Drug Metab. 2006, 7, 83–104. [Google Scholar] [CrossRef]
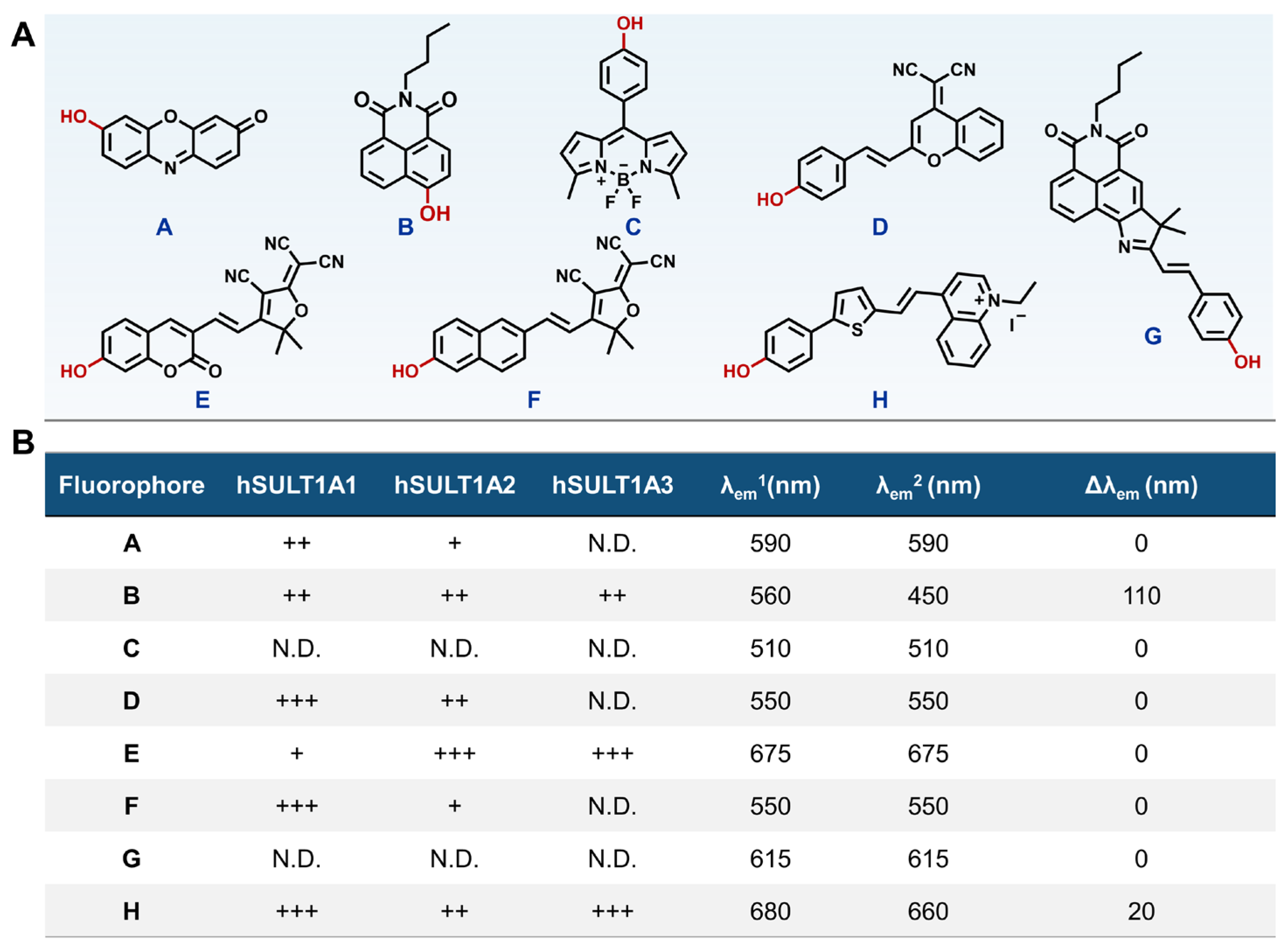
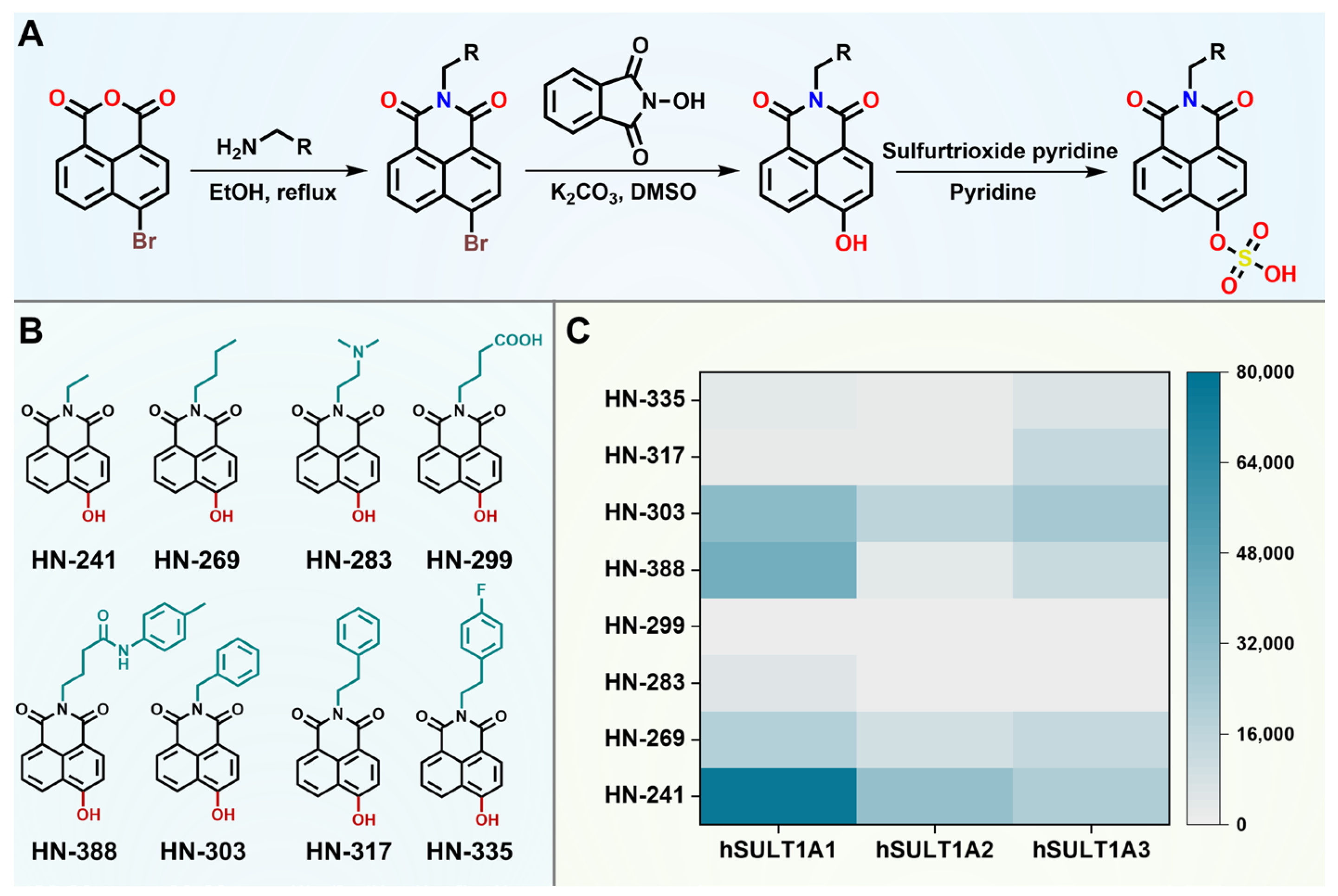
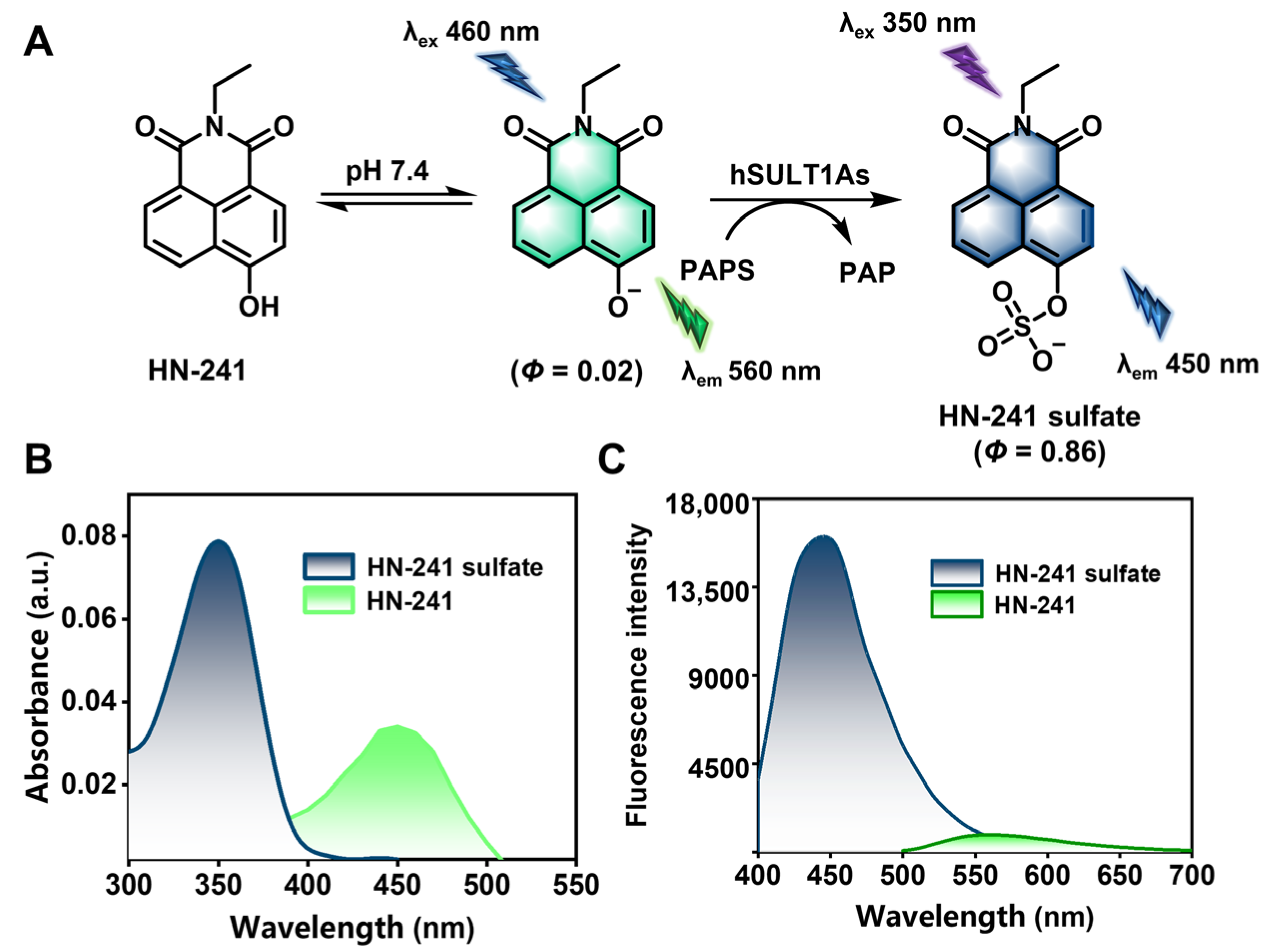
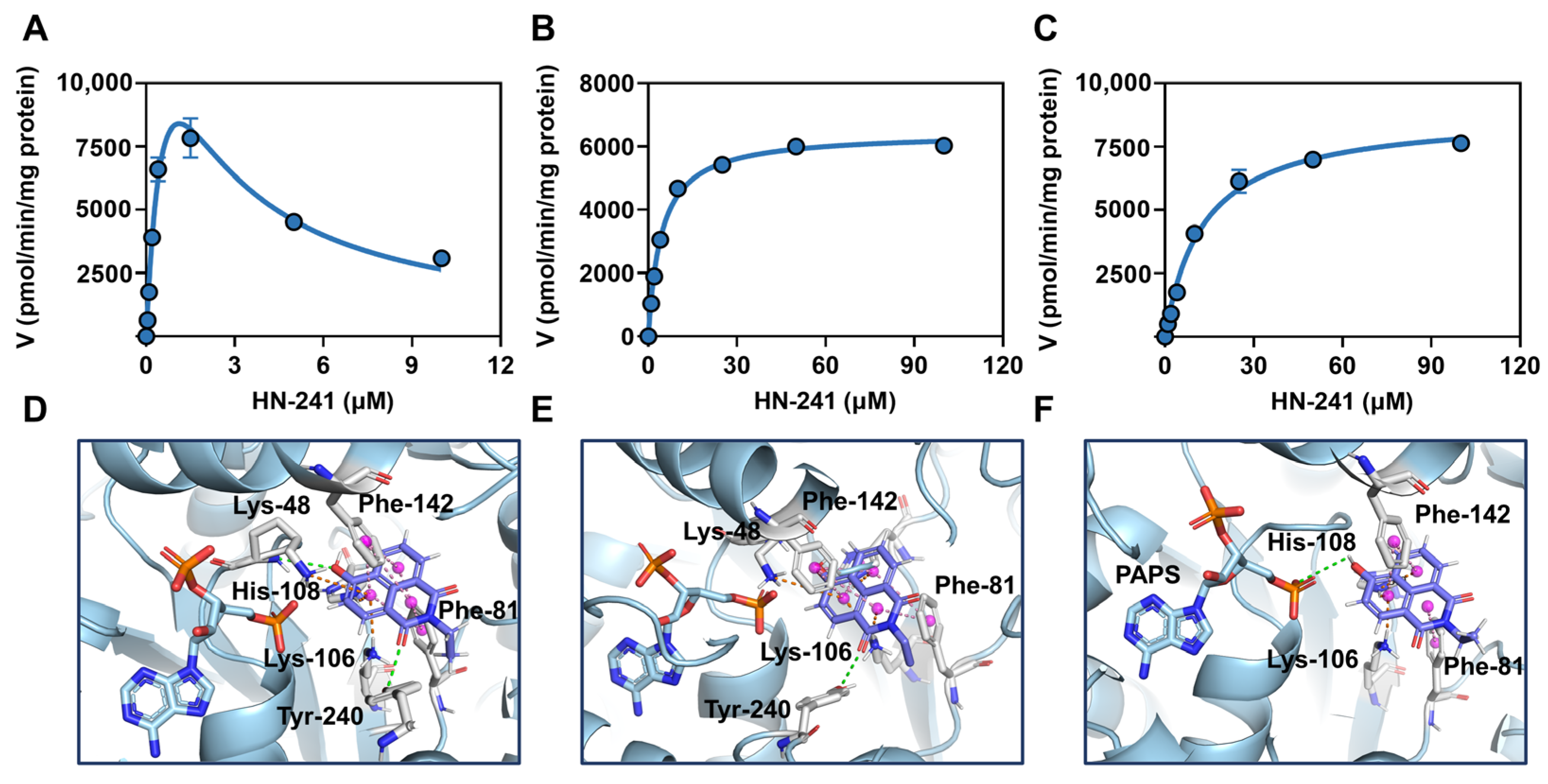
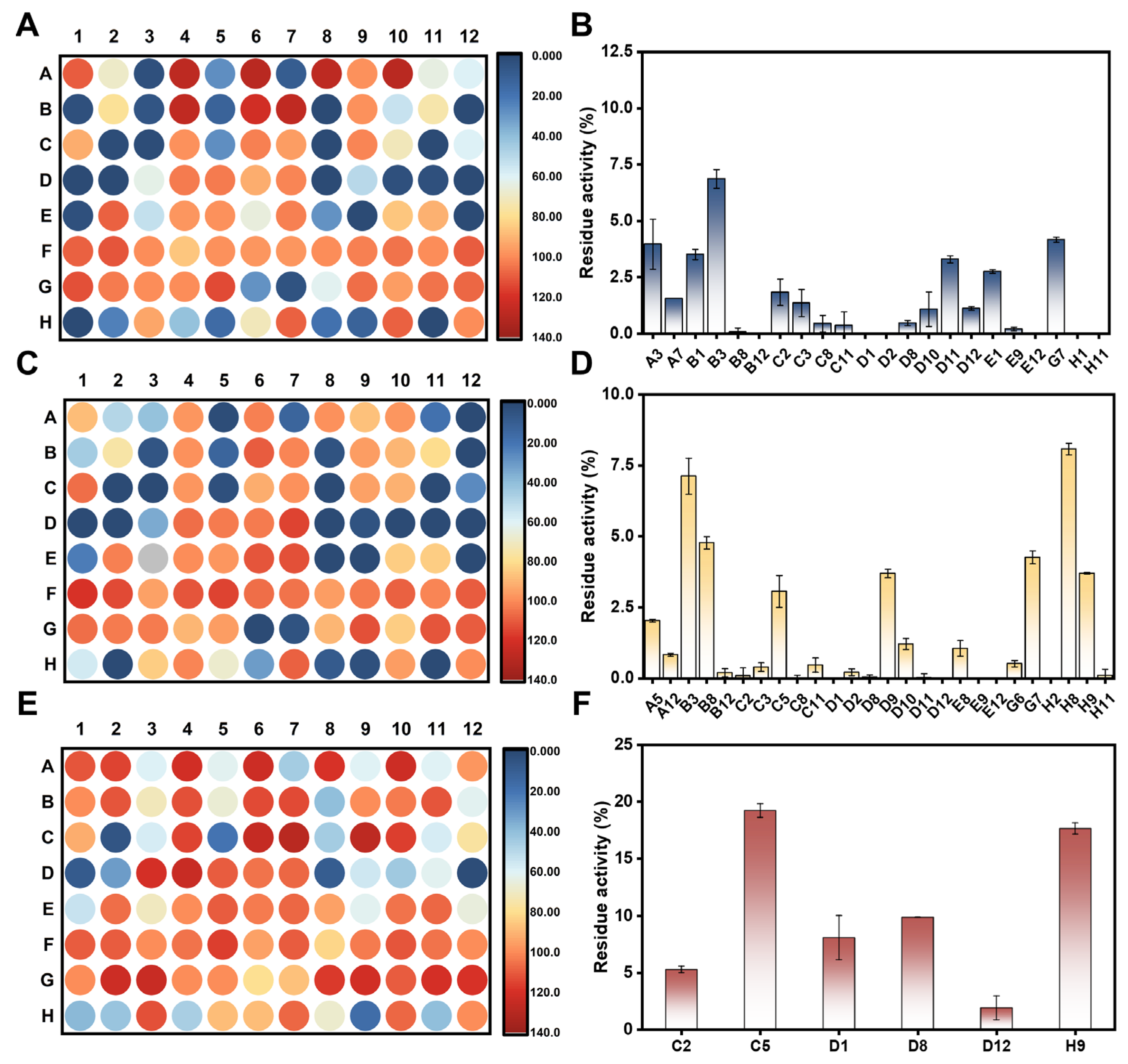

| Enzyme | Vmax (pmol/min/mg Protein) | Km (μM) | Clint (μL/min/mg Protein) | Ki (μM) |
| hSULT1A1 | 22,082.00 ± 4426.00 | 0.91 ± 0.24 | 24,265.93 | 1.37 ± 0.40 |
| hSULT1A2 | 6441.00 ± 72.43 | 4.47 ± 0.20 | 1440.94 | -- |
| hSULT1A3 | 8814.00 ± 242.80 | 12.90 ± 1.16 | 683.26 | -- |
| Enzyme | Compound | MW | IC50 (nM) |
| hSULT1A1 | licochalcone D | 354.40 | 5.37 ± 0.18 |
| pectolinarigenin | 314.29 | 9.52 ± 0.39 | |
| hinokiflavone | 538.46 | 18.88 ± 1.17 | |
| diosmetin | 300.27 | 34.73 ± 1.34 | |
| baicalein | 270.24 | 45.19 ± 3.91 | |
| emodin | 270.24 | 76.53 ± 5.28 | |
| hSULT1A2 | bilobetin | 552.49 | 55.47 ± 1.30 |
| pectolinarigenin | 314.29 | 87.24 ± 3.16 | |
| hinokiflavone | 538.46 | 110.50 ± 14.70 | |
| aloeemodin | 270.24 | 117.70 ± 7.20 | |
| emodin | 270.24 | 359.80 ± 22.57 | |
| aurantio-obtusin | 330.29 | 491.90 ± 15.18 | |
| hSULT1A3 | chrysoobtusin | 358.35 | 1515.00 ± 79.14 |
| hinokiflavone | 538.46 | 2127.00 ± 182.10 | |
| pectolinarigenin | 314.29 | 4364.00 ±252.20 | |
| baicalein | 270.24 | 6319.00 ± 194.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, X.; Fan, Y.; Zou, L.; Ge, G. A Novel Fluorescence-Based Microplate Assay for High-Throughput Screening of hSULT1As Inhibitors. Biosensors 2024, 14, 275. https://doi.org/10.3390/bios14060275
Niu X, Fan Y, Zou L, Ge G. A Novel Fluorescence-Based Microplate Assay for High-Throughput Screening of hSULT1As Inhibitors. Biosensors. 2024; 14(6):275. https://doi.org/10.3390/bios14060275
Chicago/Turabian StyleNiu, Xiaoting, Yufan Fan, Liwei Zou, and Guangbo Ge. 2024. "A Novel Fluorescence-Based Microplate Assay for High-Throughput Screening of hSULT1As Inhibitors" Biosensors 14, no. 6: 275. https://doi.org/10.3390/bios14060275
APA StyleNiu, X., Fan, Y., Zou, L., & Ge, G. (2024). A Novel Fluorescence-Based Microplate Assay for High-Throughput Screening of hSULT1As Inhibitors. Biosensors, 14(6), 275. https://doi.org/10.3390/bios14060275





