Highly Sensitive Qualitative and Quantitative Identification of Cashmere and Wool Based on Terahertz Electromagnetically Induced Transparent Metasurface Biosensor
Abstract
1. Introduction
2. Methods
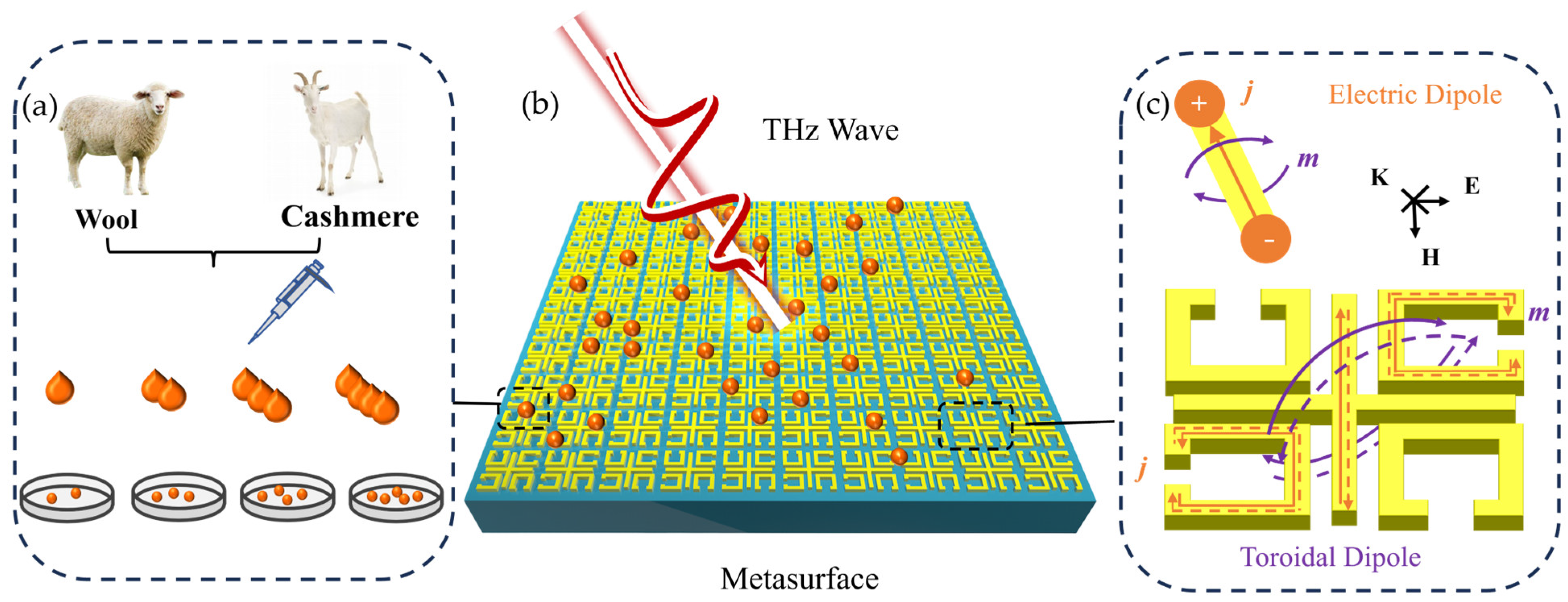
2.1. Terahertz Metasurface Sensing Scheme for the Detection of Cashmere and Wool
2.2. Metasurface Design
2.3. Fabrication of Metasurface Sensor
2.4. Cashmere and Wool Sample Preparation and Terahertz Spectroscopic Measurement
3. Results and Discussion
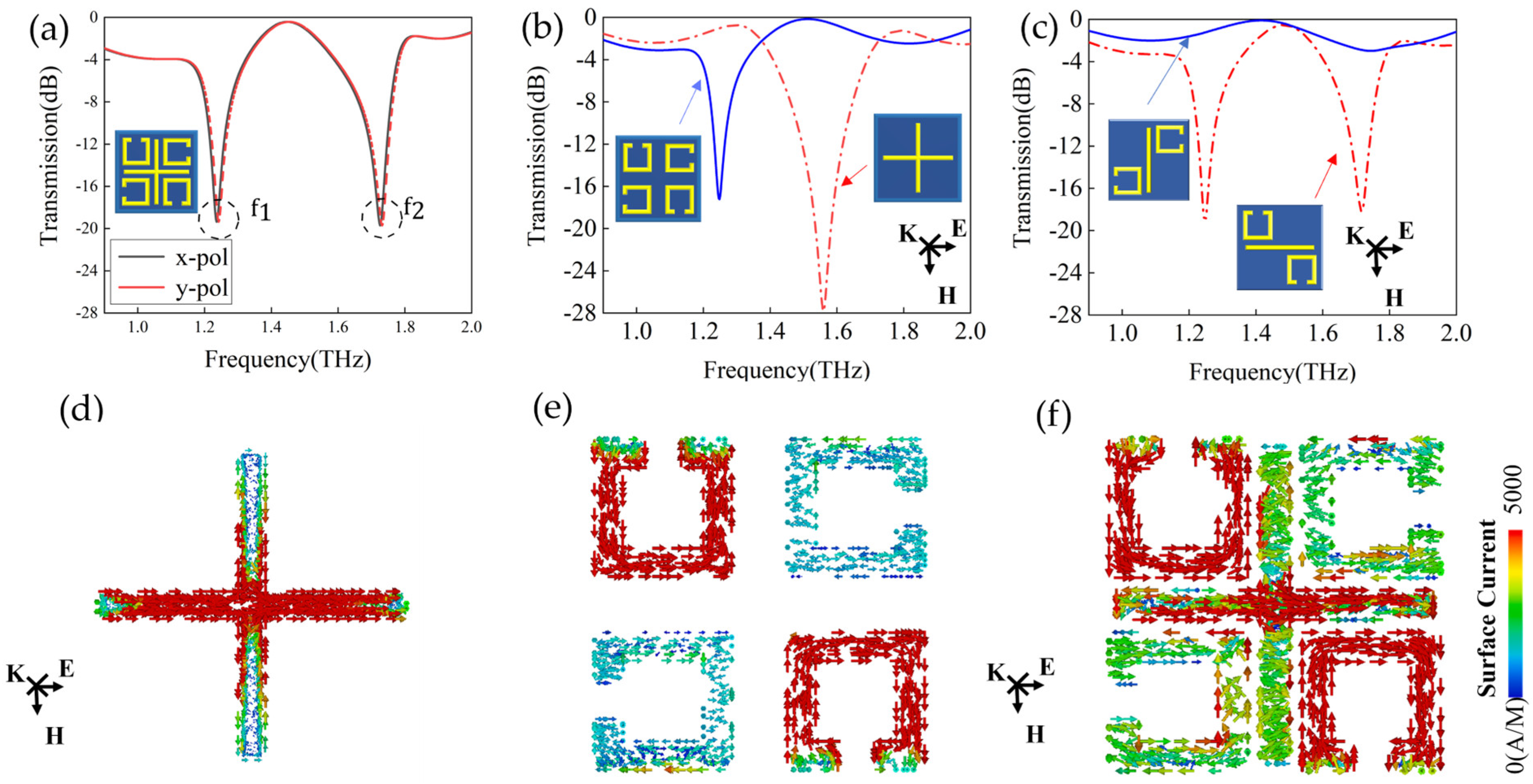
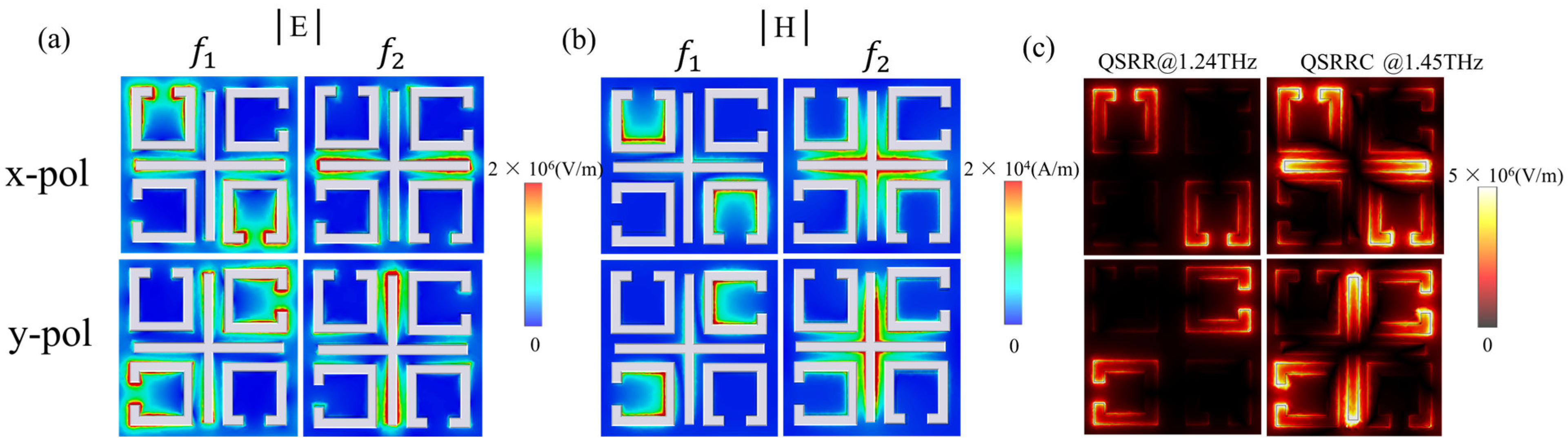
3.1. Modes Analysis
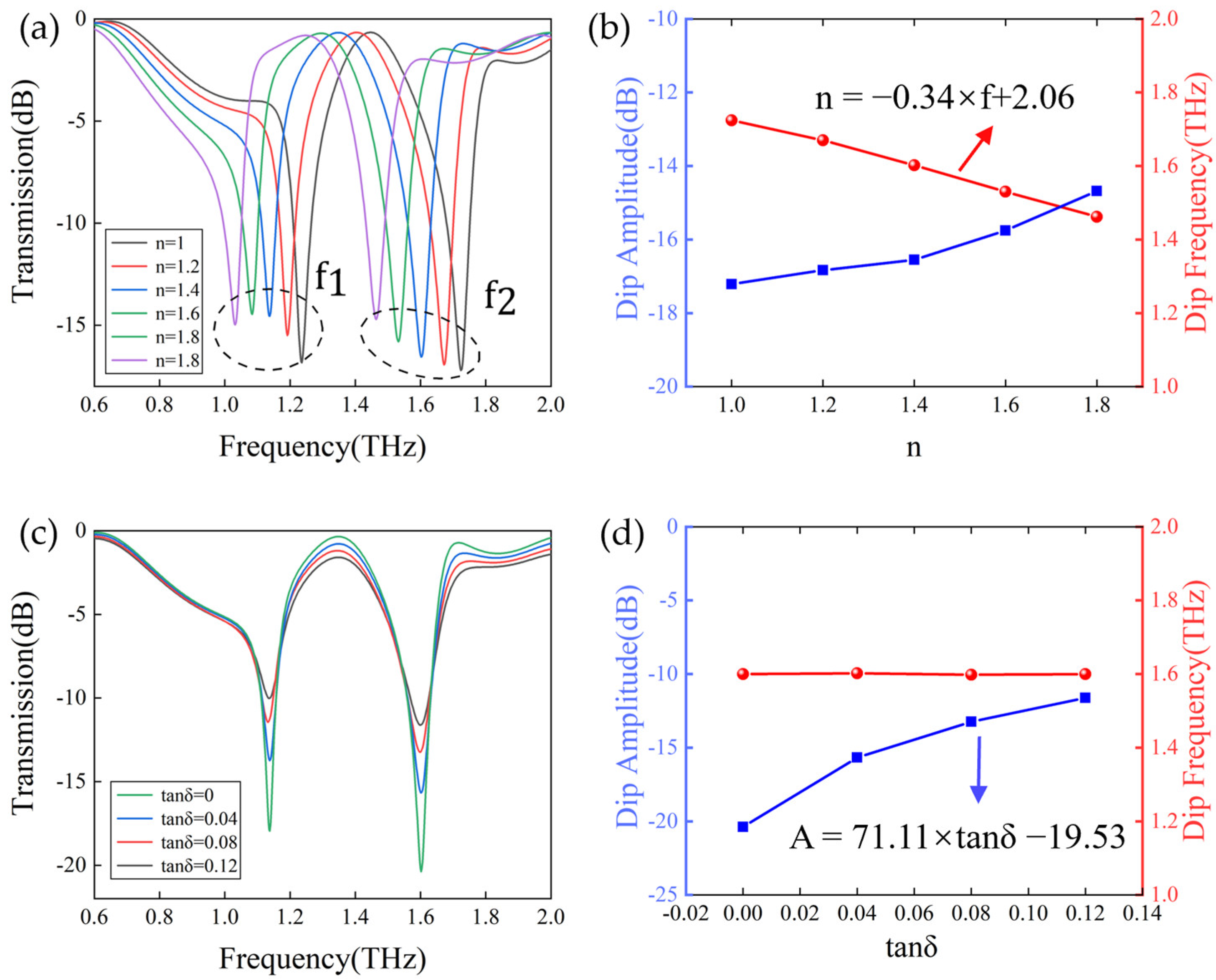
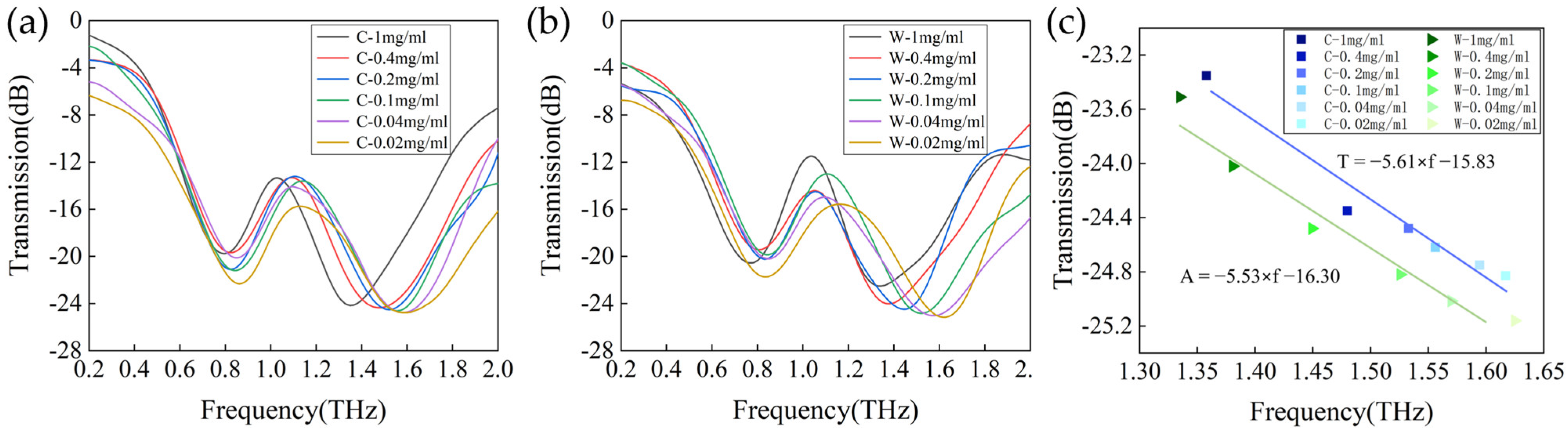
3.2. Factors That Influence Sensitivity of the Metasurface Sensor Covered with Analytes
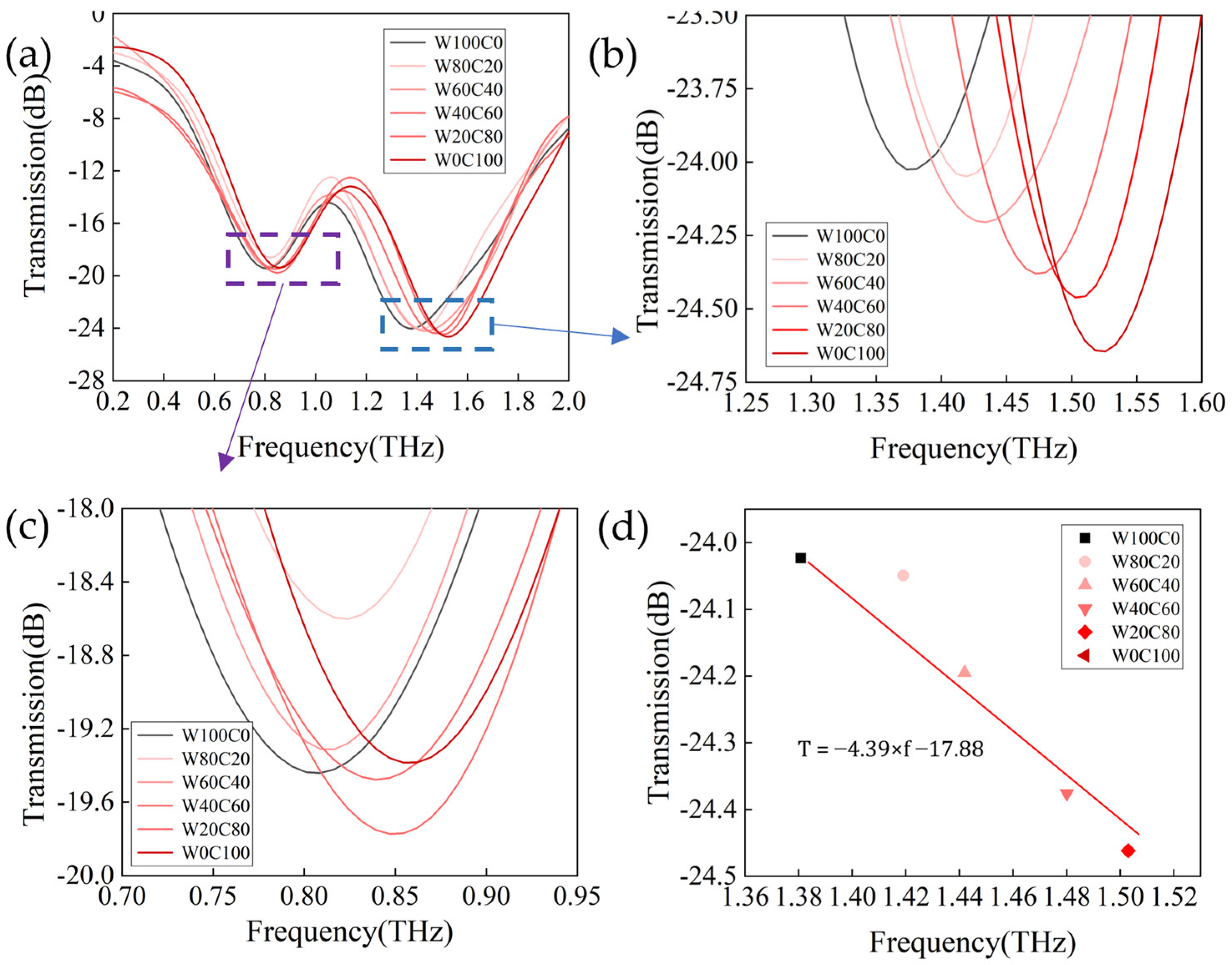
3.3. Application of Metasurface Sensors for Terahertz Spectroscopic Detection of Cashmere and Wool
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hunter, L. 10—Mohair, Cashmere and Other Animal Hair Fibres. In Handbook of Natural Fibres, 2nd ed.; Kozłowski, R.M., Mackiewicz-Talarczyk, M., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2020; pp. 279–383. ISBN 978-0-12-818398-4. [Google Scholar]
- Cashmere Clothing Market Overview, Trends, Segmentation, Scenario, and Forecast Analysis to 2029. Available online: https://www.databridgemarketresearch.com/reports/global-cashmere-clothing-market (accessed on 18 March 2024).
- Zoccola, M.; Lu, N.; Mossotti, R.; Innocenti, R.; Montarsolo, A. Identification of Wool, Cashmere, Yak, and Angora Rabbit Fibers and Quantitative Determination of Wool and Cashmere in Blend: A near Infrared Spectroscopy Study. Fibers Polym. 2013, 14, 1283–1289. [Google Scholar] [CrossRef]
- Zoccola, M.; Bhavsar, P.; Anceschi, A.; Patrucco, A. Analytical Methods for the Identification and Quantitative Determination of Wool and Fine Animal Fibers: A Review. Fibers 2023, 11, 67. [Google Scholar] [CrossRef]
- Wortmann, F.; Wortmann, F.J.; Wortmann, G. Scanning Elecron Microscopy as a Tool for the Analysis of Wool/Speciality Fiber Blends; EEC Comett: Guimarães, Portugal, 1991. [Google Scholar]
- Tang, M.; Zhang, W.; Zhou, H.; Fei, J.; Yang, J.; Lu, W.; Zhang, S.; Ye, S.; Wang, X. A Real-Time PCR Method for Quantifying Mixed Cashmere and Wool Based on Hair Mitochondrial DNA. Text. Res. J. 2014, 84, 1612–1621. [Google Scholar] [CrossRef]
- Sun, M.R.; Fei, J.; Cai, J.S.; Zhao, J. Application of DNA Analysis in Quantifying Cashmere and Wool Binary Blend. Key Eng. Mater. 2016, 671, 378–384. [Google Scholar] [CrossRef]
- Liu, H.; Liu, C.; Yang, G.; Li, H.; Dai, J.; Cong, Y.; Li, X. DNA Polymorphism of Insulin-like Growth Factor-Binding Protein-3 Gene and Its Association with Cashmere Traits in Cashmere Goats. Asian-Australas. J. Anim. Sci. 2012, 25, 1515–1520. [Google Scholar] [CrossRef]
- Enzyme-Aided Wool Dyeing: Influence of Internal Lipids|Fibers and Polymers. Available online: https://link.springer.com/article/10.1007/s12221-015-0363-8 (accessed on 18 March 2024).
- Zhu, Y.; JiaYI, H.; Li, Y.; Li, W. Image Identification of Cashmere and Wool Fibers Based on the Improved Xception Network. J. King Saud Univ.—Comput. Inf. Sci. 2022, 34, 9301–9310. [Google Scholar] [CrossRef]
- Luo, J.; Lu, K.; Chen, Y.; Zhang, B. Automatic Identification of Cashmere and Wool Fibers Based on Microscopic Visual Features and Residual Network Model. Micron 2021, 143, 103023. [Google Scholar] [CrossRef]
- Xing, W.; Liu, Y.; Deng, N.; Xin, B.; Wang, W.; Chen, Y. Automatic Identification of Cashmere and Wool Fibers Based on the Morphological Features Analysis. Micron 2020, 128, 102768. [Google Scholar] [CrossRef]
- He, J.; He, X.; Dong, T.; Wang, S.; Fu, M.; Zhang, Y. Recent Progress and Applications of Terahertz Metamaterials. J. Phys. D Appl. Phys. 2021, 55, 123002. [Google Scholar] [CrossRef]
- Foteinopoulou, S.; Economou, E.N.; Soukoulis, C.M. Refraction in Media with a Negative Refractive Index. Phys. Rev. Lett. 2003, 90, 107402. [Google Scholar] [CrossRef]
- Korolkov, A.I.; Nazarov, S.A.; Shanin, A.V. Stabilizing Solutions at Thresholds of the Continuous Spectrum and Anomalous Transmission of Waves. ZAMM—J. Appl. Math. Mech. Z. Angew. Math. Mech. 2016, 96, 1245–1260. [Google Scholar] [CrossRef]
- Treacy, M.M.J. Dynamical Diffraction Explanation of the Anomalous Transmission of Light through Metallic Gratings. Phys. Rev. B 2002, 66, 195105. [Google Scholar] [CrossRef]
- Paul, T.; Rockstuhl, C.; Menzel, C.; Lederer, F. Anomalous Refraction, Diffraction, and Imaging in Metamaterials. Phys. Rev. B 2009, 79, 115430. [Google Scholar] [CrossRef]
- Chen, H.; Wu, B.-I.; Zhang, B.; Kong, J.A. Electromagnetic Wave Interactions with a Metamaterial Cloak. Phys. Rev. Lett. 2007, 99, 063903. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Yang, Z.; Pan, H.; Wu, R. Electromagnetic Absorption Materials: Current Progress and New Frontiers. Prog. Mater. Sci. 2022, 127, 100946. [Google Scholar] [CrossRef]
- Xiao, S.; Wang, T.; Liu, T.; Zhou, C.; Jiang, X.; Zhang, J. Active Metamaterials and Metadevices: A Review. J. Phys. D Appl. Phys. 2020, 53, 503002. [Google Scholar] [CrossRef]
- Ra’di, Y.; Simovski, C.R.; Tretyakov, S.A. Thin Perfect Absorbers for Electromagnetic Waves: Theory, Design, and Realizations. Phys. Rev. Appl. 2015, 3, 037001. [Google Scholar] [CrossRef]
- Li, M.; Yang, H.-L.; Hou, X.-W.; Tian, Y.; Hou, D.-Y. Perfect metamaterial absorber with dual bands. Prog. Electromagn. Res. 2010, 108, 37–49. [Google Scholar] [CrossRef]
- Li, A.; Luo, Z.; Wakatsuchi, H.; Kim, S.; Sievenpiper, D.F. Nonlinear, Active, and Tunable Metasurfaces for Advanced Electromagnetics Applications. IEEE Access 2017, 5, 27439–27452. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, L. Electromagnetically Induced Transparency Metamaterials: Theories, Designs and Applications. J. Phys. D Appl. Phys. 2022, 55, 263003. [Google Scholar] [CrossRef]
- Yang, Y.; Kravchenko, I.I.; Briggs, D.P.; Valentine, J. All-Dielectric Metasurface Analogue of Electromagnetically Induced Transparency. Nat. Commun. 2014, 5, 5753. [Google Scholar] [CrossRef]
- Wang, B.-X.; Duan, G.; Lv, W.; Tao, Y.; Xiong, H.; Zhang, D.-Q.; Yang, G.; Shu, F.-Z. Design and Experimental Realization of Triple-Band Electromagnetically Induced Transparency Terahertz Metamaterials Employing Two Big-Bright Modes for Sensing Applications. Nanoscale 2023, 15, 18435–18446. [Google Scholar] [CrossRef]
- Ziemkiewicz, D.; Słowik, K.; Zielińska-Raczyńska, S. Ultraslow Long-Living Plasmons with Electromagnetically Induced Transparency. Opt. Lett. 2018, 43, 490–493. [Google Scholar] [CrossRef]
- Yang, M.; Liang, L.; Zhang, Z.; Xin, Y.; Wei, D.; Song, X.; Zhang, H.; Lu, Y.; Wang, M.; Zhang, M.; et al. Electromagnetically Induced Transparency-like Metamaterials for Detection of Lung Cancer Cells. Opt. Express 2019, 27, 19520–19529. [Google Scholar] [CrossRef]
- Yan, X.; Yang, M.; Zhang, Z.; Liang, L.; Wei, D.; Wang, M.; Zhang, M.; Wang, T.; Liu, L.; Xie, J.; et al. The Terahertz Electromagnetically Induced Transparency-like Metamaterials for Sensitive Biosensors in the Detection of Cancer Cells. Biosens. Bioelectron. 2019, 126, 485–492. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, X.Q.; Yan, Y.H.; Xiao, F.; Khan, M.T.; Zhang, S. Noncontact Group-Delay-Based Sensor for Metal Deformation and Crack Detection. IEEE Trans. Ind. Electron. 2021, 68, 7613–7619. [Google Scholar] [CrossRef]
- Zhu, L.; Li, H.; Dong, L.; Zhou, W.; Rong, M.; Zhang, X.; Guo, J. Dual-Band Electromagnetically Induced Transparency (EIT) Terahertz Metamaterial Sensor. Opt. Mater. Express 2021, 11, 2109–2121. [Google Scholar] [CrossRef]
- Zhu, L.; Guo, J.; Dong, L.; Meng, F.-Y.; Wu, Q. Coherent Perfect Absorption in an Electromagnetically Induced Transparency-like (EIT-like) System. J. Opt. 2016, 18, 095104. [Google Scholar] [CrossRef]
- Zhu, L.; Dong, L.; Guo, J.; Meng, F.Y.; He, X.J.; Wu, T.H. Polarization-Independent Transparent Effect in Windmill-like Metasurface. J. Phys. D Appl. Phys. 2018, 51, 265101. [Google Scholar] [CrossRef]
- Guo, Z.; Jiang, H.; Li, Y.; Chen, H.; Agarwal, G.S. Enhancement of Electromagnetically Induced Transparency in Metamaterials Using Long Range Coupling Mediated by a Hyperbolic Material. Opt. Express 2018, 26, 627–641. [Google Scholar] [CrossRef]
- Chen, K.; Ruan, C.; Zhan, F.; Song, X.; Fahad, A.K.; Zhang, T.; Shi, W. Ultra-Sensitive Terahertz Metamaterials Biosensor Based on Luxuriant Gaps Structure. iScience 2023, 26, 105781. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, M.; Yan, X.; Guo, X.; Li, J.; Yang, Y.; Wei, D.; Liu, L.; Xie, J.; Liu, Y.; et al. The Antibody-Free Recognition of Cancer Cells Using Plasmonic Biosensor Platforms with the Anisotropic Resonant Metasurfaces. ACS Appl. Mater. Interfaces 2020, 12, 11388–11396. [Google Scholar] [CrossRef]
- Lin, S.; Xu, X.; Hu, F.; Chen, Z.; Wang, Y.; Zhang, L.; Peng, Z.; Li, D.; Zeng, L.; Chen, Y.; et al. Using Antibody Modified Terahertz Metamaterial Biosensor to Detect Concentration of Carcinoembryonic Antigen. IEEE J. Sel. Top. Quantum Electron. 2021, 27, 6900207. [Google Scholar] [CrossRef]
- Tang, C.; Yang, J.; Wang, Y.; Cheng, J.; Li, X.; Chang, C.; Hu, J.; Lü, J. Integrating Terahertz Metamaterial and Water Nanodroplets for Ultrasensitive Detection of Amyloid β Aggregates in Liquids. Sens. Actuators B Chem. 2021, 329, 129113. [Google Scholar] [CrossRef]
- Cheng, R.; Xu, L.; Yu, X.; Zou, L.; Shen, Y.; Deng, X. High-Sensitivity Biosensor for Identification of Protein Based on Terahertz Fano Resonance Metasurfaces. Opt. Commun. 2020, 473, 125850. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Hu, F.; Li, D.; Teng, H.; Rong, Q.; Zhang, W.; Han, J.; Liang, H. Four Resonators Based High Sensitive Terahertz Metamaterial Biosensor Used for Measuring Concentration of Protein. J. Phys. D Appl. Phys. 2019, 52, 095105. [Google Scholar] [CrossRef]

| Resonance Type | Sensitivity (GHz/RIU) | Detection Target |
Metal and Reference |
|---|---|---|---|
| EIT−like resonance | 131.05 | Cancer Cells | Gold [36] |
| Electric dipole Resonance | 76.5 | carcinoembryonic antigen | Al [37] |
| Dipole resonance | 200 | amyloid β aggregates | Gold [38] |
| Fano resonance | 240 | Protein | Cu [39] |
| Electric dipole Resonance | 85 | Protein | Al [40] |
| EIT−like resonance | 342 | cashmere/wool | Gold This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, D.; Xu, L.; Jia, L.; Cheng, L.; Tang, P.; Zhou, J. Highly Sensitive Qualitative and Quantitative Identification of Cashmere and Wool Based on Terahertz Electromagnetically Induced Transparent Metasurface Biosensor. Biosensors 2024, 14, 240. https://doi.org/10.3390/bios14050240
Luo D, Xu L, Jia L, Cheng L, Tang P, Zhou J. Highly Sensitive Qualitative and Quantitative Identification of Cashmere and Wool Based on Terahertz Electromagnetically Induced Transparent Metasurface Biosensor. Biosensors. 2024; 14(5):240. https://doi.org/10.3390/bios14050240
Chicago/Turabian StyleLuo, Dongpeng, Limin Xu, Lifeng Jia, Lianglun Cheng, Ping Tang, and Jinyun Zhou. 2024. "Highly Sensitive Qualitative and Quantitative Identification of Cashmere and Wool Based on Terahertz Electromagnetically Induced Transparent Metasurface Biosensor" Biosensors 14, no. 5: 240. https://doi.org/10.3390/bios14050240
APA StyleLuo, D., Xu, L., Jia, L., Cheng, L., Tang, P., & Zhou, J. (2024). Highly Sensitive Qualitative and Quantitative Identification of Cashmere and Wool Based on Terahertz Electromagnetically Induced Transparent Metasurface Biosensor. Biosensors, 14(5), 240. https://doi.org/10.3390/bios14050240





