2D Flower-like CdS@Co/Mo-MOF as Co-Reaction Accelerator of g-C3N4-Based Electrochemiluminescence Sensor for Chlorpromazine Hydrochloride
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Preparation of g-C3N4
2.3. Preparation of Co/Mo-MOF
2.4. Preparation of CdS@Co/Mo-MOF
2.5. Preparation of CdS NPs
2.6. Preparation of CdS@Co/Mo-MOF@g-C3N4
2.7. Preparation of M-ZIF-67, H-ZIF-67, and D-ZIF-67
2.8. Fabrication of the ECL Sensor
2.9. ECL Detection of CPH
3. Results and Discussion
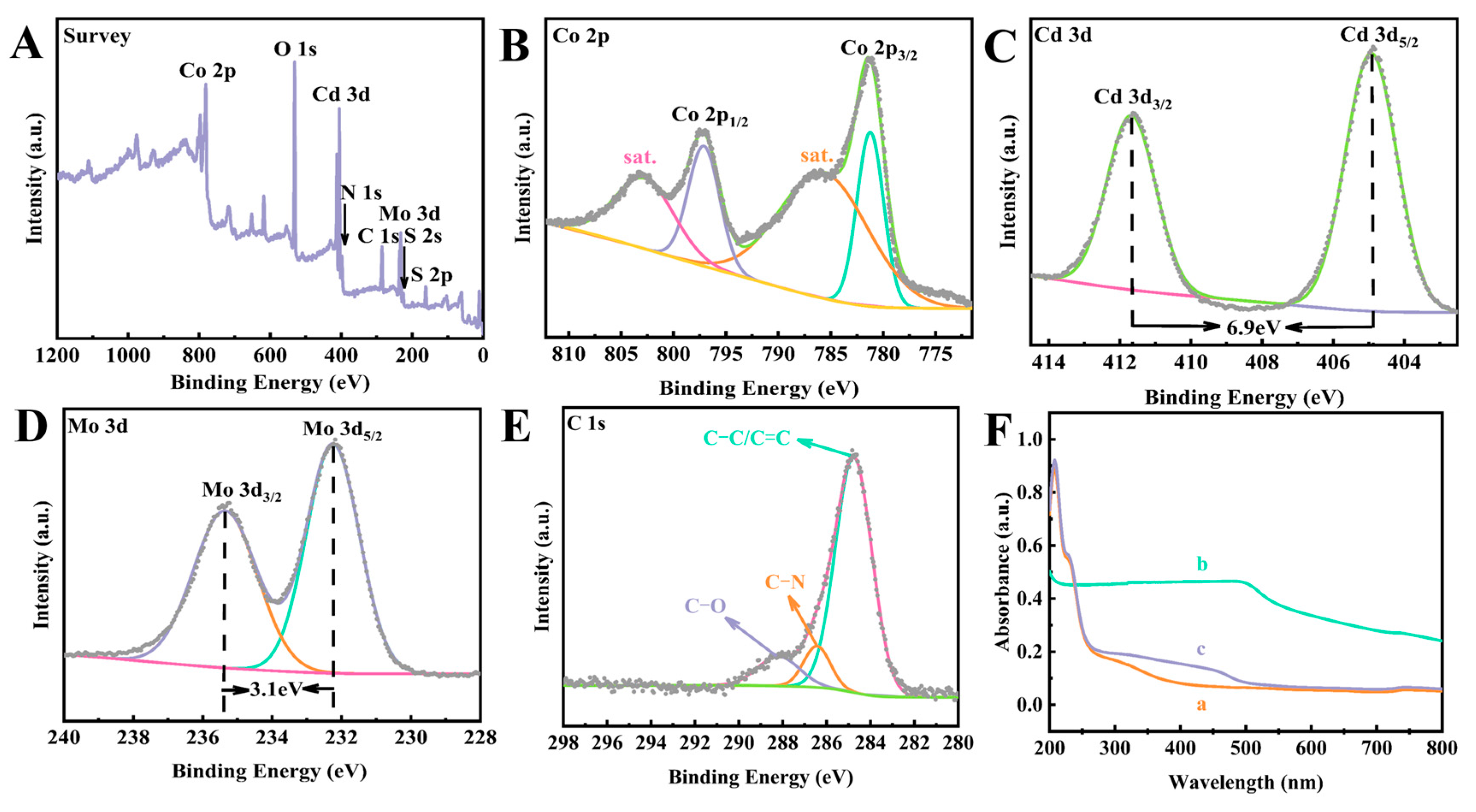
3.1. Characterization of the Different Nanomaterials
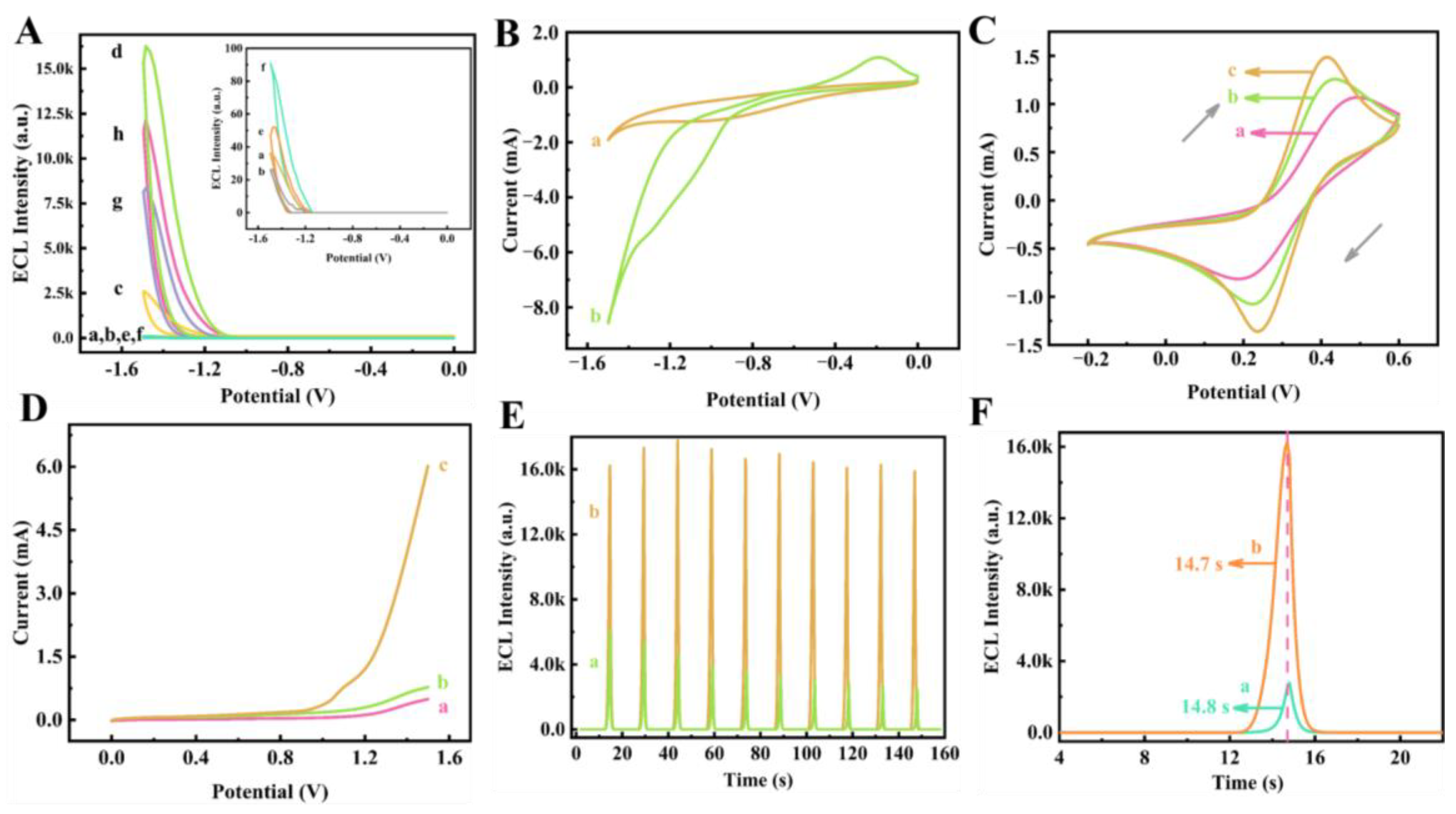
3.2. Possible ECL Mechanism of the CdS@Co/Mo-MOF/g-C3N4/S2O82− Ternary System
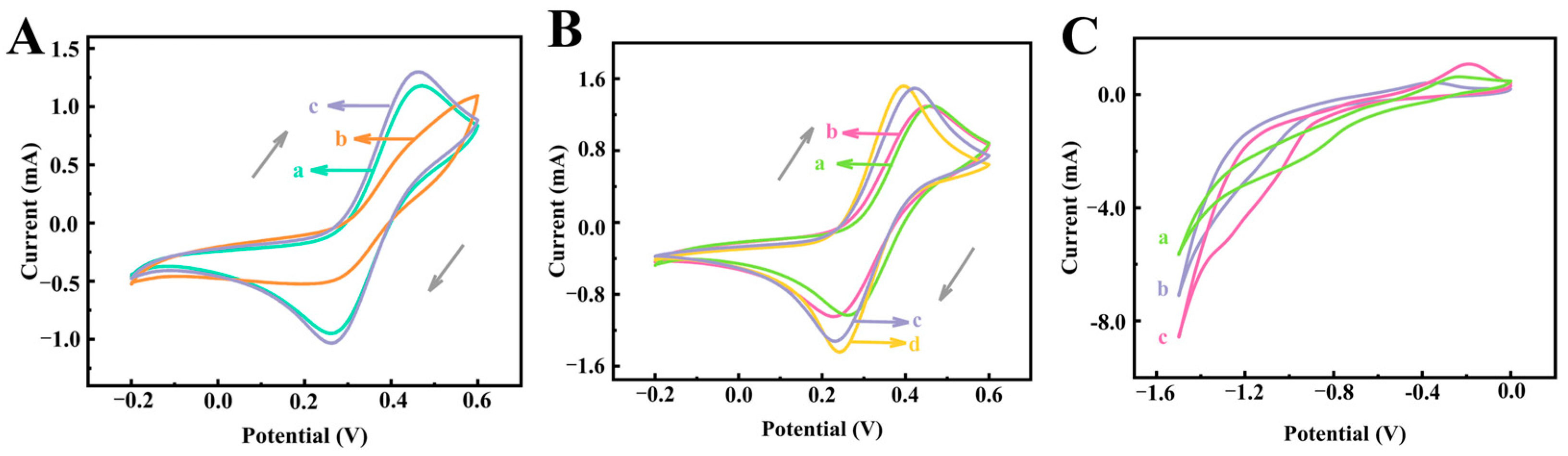
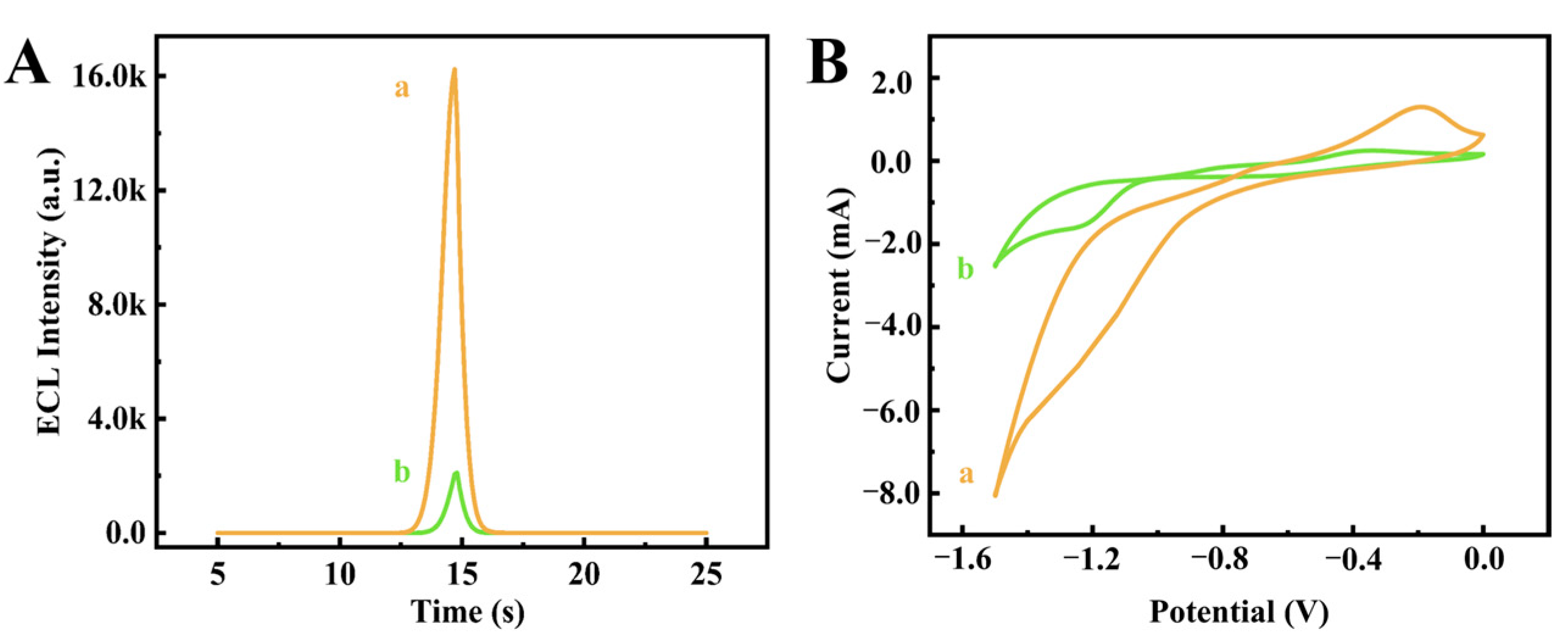
3.3. Investigation of the Electrochemical Differences
3.4. Quenching Effect of CPH Towards the ECL Sensor
3.5. Optimization of Experimental Conditions
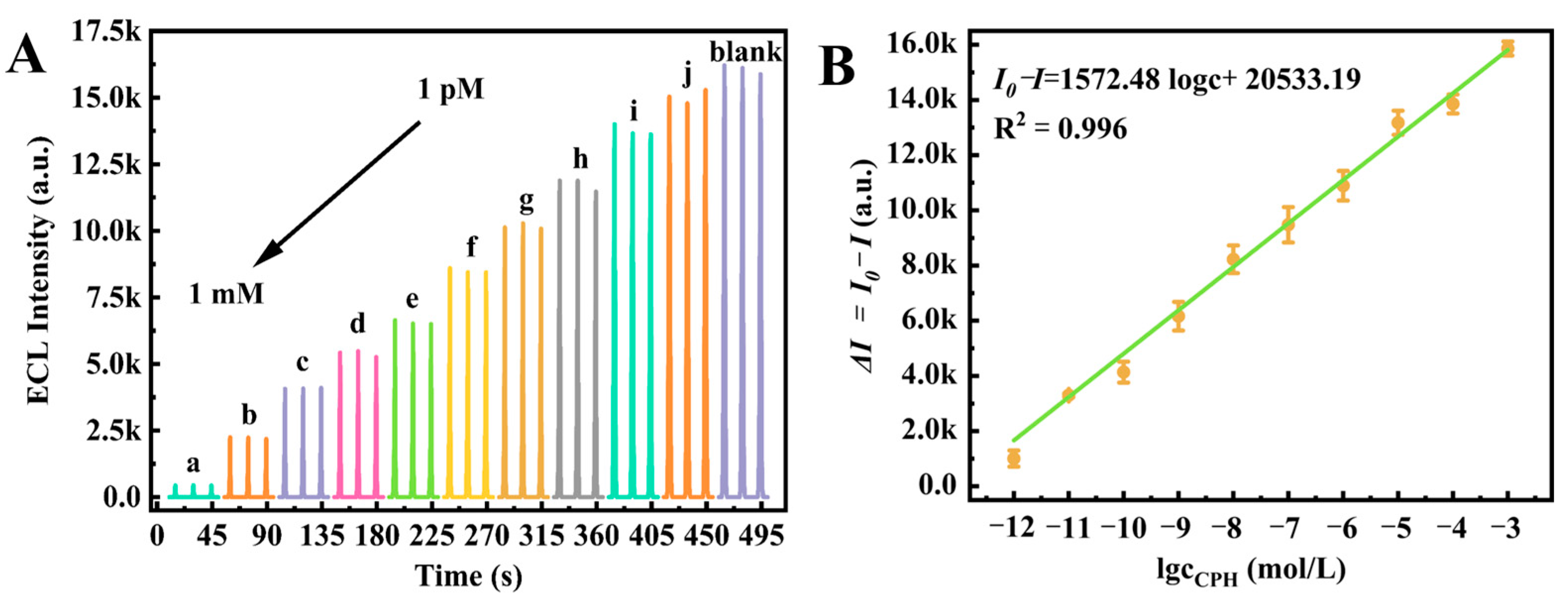
3.6. Analytical Performance of the Proposed ECL Biosensor
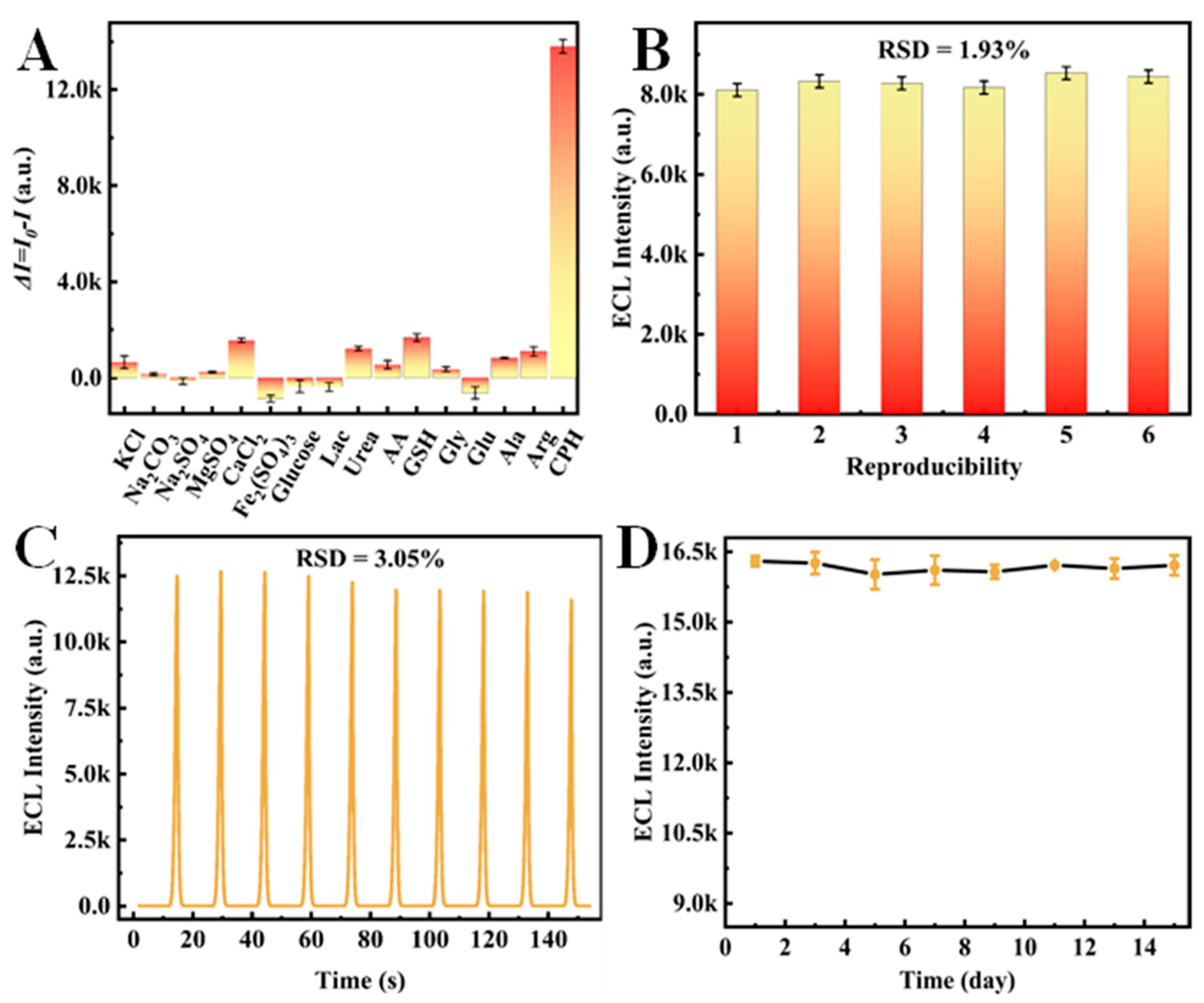
3.7. Performance of the Proposed Biosensor
3.8. Actual Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fiorani, A.; Merino, J.P.; Zanut, A.; Criado, A.; Valenti, G.; Prato, M.; Paolucci, F. Advanced carbon nanomaterials for electrochemiluminescent biosensor applications. Curr. Opin. Electrochem. 2019, 16, 66–74. [Google Scholar] [CrossRef]
- Ke, H.; Sha, H.; Wang, Y.; Guo, W.; Zhang, X.; Wang, Z.; Huang, C.; Jia, N. Electrochemiluminescence resonance energy transfer system between GNRs and Ru(bpy)32+: Application in magnetic aptasensor for β-amyloid. Biosens. Bioelectron. 2018, 100, 266–273. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.-Q.; Peng, L.-Z.; Lei, Y.-M.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y. Strong electrochemiluminescence from MOF accelerator enriched quantum dots for enhanced sensing of trace cTnI. Anal. Chem. 2018, 90, 3995–4002. [Google Scholar] [CrossRef]
- Alemu, Y.A.; Rampazzo, E.; Paolucci, F. Strategies of tailored nanomaterials for electrochemiluminescence signal enhancements. Curr. Opin. Colloid Interface Sci. 2022, 61, 101621. [Google Scholar] [CrossRef]
- Chen, L.; Zeng, X.; Si, P.; Chen, Y.; Chi, Y.; Kim, D.-H.; Chen, G. Gold nanoparticle-graphite-like C3N4 nanosheet nanohybrids used for electrochemiluminescent immunosensor. Anal. Chem. 2014, 86, 4188–4195. [Google Scholar] [CrossRef]
- Feng, Y.; Shi, L.; Wu, H.; Chen, L.; Chi, Y. Detection of cyanide by etching-induced electrochemiluminescence recovery. Electrochim. Acta. 2018, 261, 29–34. [Google Scholar] [CrossRef]
- Cheng, C.; Huang, Y.; Tian, X.; Zheng, B.; Li, Y.; Yuan, H.; Xiao, D.; Xie, S.; Choi, M.M.F. Electrogenerated chemiluminescence behavior of graphite-like carbon nitride and its application in selective sensing Cu2+. Anal. Chem. 2012, 84, 4754–4759. [Google Scholar] [CrossRef]
- Irkham, T.; Watanabe, A.; Fiorani, G.; Valenti, F.; Paolucci, Y. Co-reactant-on-demand ECL: Electrogenerated chemiluminescence by the in situ production of S2O82– at boron-doped diamond electrodes. J. Am. Chem. Soc. 2016, 138, 15636–15641. [Google Scholar] [CrossRef]
- Wang, H. Advances in electrochemiluminescence co-reaction accelerator and its analytical applications. Anal. Bioanal. Chem. 2021, 413, 4119–4135. [Google Scholar] [CrossRef]
- Wu, Q.; Wang, P.; Yang, X.; Wei, M.; Zhou, M.; Pu, Y.; Zhang, M. Fe-Co-Co Prussian blue analogues as a novel co-reaction accelerator for ultrasensitive electrochemiluminescent biosensor construction. Sens. Actuat. B Chem. 2019, 297, 126767. [Google Scholar] [CrossRef]
- Yang, F.; Yang, F.; Tu, T.-T.; Liao, N.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y. A synergistic promotion strategy remarkably accelerated electrochemiluminescence of SnO2 QDs for microRNA detection using 3D DNA walker amplification. Biosens. Bioelectron. 2021, 173, 112820. [Google Scholar] [CrossRef]
- Lin, Z.; Li, P.; Zheng, D.; Huang, L.; Chen, Y.; Gao, W. Highly efficient synthesis of CeO2@g-C3N4 double-shelled hollow spheres for ultrasensitive self-enhanced electrochemiluminescence biosensors. Microchem. J. 2023, 190, 108588. [Google Scholar] [CrossRef]
- Song, X.; Li, D.; Wei, R.; Feng, T.; Yan, Y.; Wang, X.; Ren, B.; Du, H.; Ma, Q. CuS as co-reaction accelerator in PTCA-K2S2O8 system for enhancing electrochemiluminescence behavior of PTCA and its application in the detection of amyloid-β protein. Biosens. Bioelectron. 2019, 126, 222–229. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H.; Chai, Y.; Shi, W.; Yuan, R. High-efficiency CNNS@NH2-MIL(Fe) electrochemiluminescence emitters coupled with Ti3C2 nanosheets as a matrix for a highly sensitive cardiac troponin I assay. Anal. Chem. 2020, 92, 8992–9000. [Google Scholar] [CrossRef]
- Lee, S.; Kapustin, E.A.; Yaghi, O.M. Coordinative alignment of molecules in chiral metal-organic frameworks. Science 2016, 353, 808–811. [Google Scholar] [CrossRef]
- Liao, P.Q.; Shen, J.Q.; Zhang, J.P. Metal–organic frameworks for electrocatalysis. Coord. Chem. Rev. 2018, 373, 22–48. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-dimensional metal-organic framework nanosheets: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef]
- Zhong, H.; Ly, K.H.; Wang, M.; Krupskaya, Y.; Han, X.; Zhang, J.; Zhang, J.; Kataev, V.; Büchner, B.; Weidinger, I.M.; et al. A phthalocyanine-based layered two-dimensional conjugated metal-organic framework as a highly efficient electrocatalyst for the oxygen reduction reaction. Angew. Chem. 2019, 131, 10787–10792. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Y.; Teng, J.; Wong, W.-L.; Qiu, B. In situ deposition of MOF-74(Cu) nanosheet arrays onto carbon cloth to fabricate a sensitive and selective electrocatalytic biosensor and its application for the determination of glucose in human serum. Microchim. Acta. 2020, 187, 670. [Google Scholar] [CrossRef] [PubMed]
- Du, B.; Yan, F.; Lin, X.; Liang, C.; Guo, X.; Tan, Y.; Zhen, H.; Zhao, C.; Shi, Y.; Kibet, E.; et al. A bottom-up sonication-assisted synthesis of Zn-BTC MOF nanosheets and the ppb-level acetone detection of their derived ZnO nanosheets. Sensor. Actuat B Chem. 2023, 375, 132854. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Liu, X.; Chu, C.; Yao, D.; Mao, S. Modification strategies on 2D Ni-Fe MOF-based catalysts in peroxy-disulfate activation for efficient organic pollutant removal. Chin. Chem. Lett. 2023, 34, 107708. [Google Scholar] [CrossRef]
- Wang, S.; Wang, M.; Li, C.; Li, H.; Ge, C.; Zhang, X.; Jin, Y. A highly sensitive and stable electrochemiluminescence immunosensor for alpha-fetoprotein detection based on luminol-AgNPs@Co/Ni-MOF nanosheet microflowers. Sensor. Actuat B Chem. 2020, 311, 127919. [Google Scholar] [CrossRef]
- Duan, C.; Yu, Y.; Hu, H. Recent progress on the synthesis of ZIF-67-based materials and their application to heterogeneous catalysis. Green Energy Environ. 2022, 7, 3–15. [Google Scholar] [CrossRef]
- Zhong, G.; Liu, D.; Zhang, J. The application of ZIF-67 and its derivatives: Adsorption, separation, electrochemistry, and catalysts. J. Mater. 2018, 6, 1887–1899. [Google Scholar] [CrossRef]
- Sun, D.; Yang, D.; Wei, P.; Liu, B.; Chen, Z.; Zhang, L.; Lu, J. One-Step Electrodeposition of Silver Nanostructures on 2D/3D Metal-Organic Framework ZIF-67: Comparison and Application in Electrochemical Detection of Hydrogen Peroxide. ACS Appl. Mater. Interfaces 2020, 12, 41960–41968. [Google Scholar] [CrossRef]
- Butt, F.S.; Safdar, M.; Lewis, A.; Mazlan, N.A.; Radacsi, N.; Fan, X.; Arellano-García, H.; Huang, Y. Superhydrophobic ZIF-67 with exceptional hydrostability. Mater. Today Adv. 2023, 20, 100448. [Google Scholar] [CrossRef]
- Zhao, C.; Ma, C.; Zhang, F.; Li, W.; Hong, C.; Qi, Y. Two-dimensional metal-organic framework nanosheets: An efficient two-electron oxygen reduction reaction electrocatalyst for boosting cathodic luminol electrochemiluminescence. Chem. Eng. J. 2023, 466, 143156. [Google Scholar] [CrossRef]
- Li, G.; Zhang, X.; Zhang, H.; Liao, C.; Jiang, G. Bottom-up MOF-intermediated synthesis of 3D hierarchical flower-like cobalt-based homobimetallic phophide composed of ultrathin nanosheets for highly efficient oxygen evolution reaction. Appl. Catal. B Environ. 2019, 249, 147–154. [Google Scholar] [CrossRef]
- Ahn, C.H.; Yang, W.S.; Kim, J.J.; Priyanga, G.S.; Thomas, T.; Deshpande, N.G.; Lee, H.S.; Cho, H.K. Design of hydrangea-type Co/Mo bimetal MOFs and MOF-derived Co/Mo2C embedded carbon composites for highly efficient oxygen evolution reaction. Chem. Eng. J. 2022, 435, 134815. [Google Scholar] [CrossRef]
- Huo, X.-L.; Yang, H.; Li, M.-X.; Zhao, W.; Xu, J.-J.; Wang, Y.; Luo, X.-L.; Chen, H.-Y. Multi-segmented CdS-Au nanorods for electrochemiluminescence bioanalysis. Nanoscale 2018, 10, 19224–19230. [Google Scholar] [CrossRef]
- Dong, Y.-X.; Cao, J.-T.; Wang, B.; Ma, S.-H.; Liu, Y.-M. Spatial-resolved photoelectrochemical biosensing array based on CdS@g-C3N4 heterojunction: A universal immunosensing platform for accurate detection. ACS Appl. Mater. 2018, 10, 3723–3731. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Liu, X.-P.; Chen, J.-S.; Mao, C.-J.; Niu, H.-L.; Jin, B.-K. Electrochemiluminescence immunoassay for the prostate-specific antigen by using a CdS/chitosan/g-C3N4 nanocomposite. Microchim. Acta. 2020, 187, 155. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Liu, D.; Wang, C.; Li, J.; Yu, R.; Wang, Y.; Yin, J.; Wang, X.; Du, Y. Nanosheet-assembled transition metal sulfides nanoflowers derived from CoMo-MOF for efficient oxygen evolution reaction. J. Colloid Interface Sci. 2024, 653, 1464–1477. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, L.; Wang, Z.; Gao, P.; Li, G.K. Synthesis and application of mesoporous materials: Process status, technical problems, and development prospects: A mini-review. Energy Fuel. 2023, 37, 3413–3427. [Google Scholar] [CrossRef]
- Kaleeswarran, P.; Sriram, B.; Wang, S.-F.; Baby, J.N.; Arumugam, A.; Bilgrami, A.L.; Hashsham, S.A.; Sayegh, F.A.; Liu, C.-J. Electrochemical detection of antipsychotic drug in water samples based on nano/sub-microrod-like CuBi2-xInxO4 electrocatalysts. Microchem. J. 2021, 163, 105886. [Google Scholar] [CrossRef]
- Woods, S.W. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J. Clin. Psychiatry 2003, 64, 663–667. [Google Scholar] [CrossRef]
- Dudley, K.; Liu, X.; Haan, S.D. Chlorpromazine dose for people with schizophrenia. Cochrane Db. Syst. Rev. 2017, 4, CD007778. [Google Scholar] [CrossRef]
- Liu, F.; Shao, J.; Zhao, Y. Electrochemiluminescence detection of chlorpromazine hydrochloride at bare and graphene oxide modified glassy carbon electrodes. Anal. Methods 2014, 6, 6483–6487. [Google Scholar] [CrossRef]
- Li, M.; Wang, C.; Liu, D. A novel “off-on” electrochemiluminescence sensor based on highly efficient resonance energy transfer in C-g-C3N4/CuO nanocomposite. Anal. Chim. Acta. 2020, 1138, 30–37. [Google Scholar] [CrossRef]
- Miao, X.; Lei, H.; Dong, S. Facile fabrication of highly efficient g-C3N4/Ag2O heterostructured photocatalysts with enhanced visible-light photocatalytic activity. ACS Appl. Mater. 2013, 5, 12533–12540. [Google Scholar] [CrossRef]
- Xu, H.-Q.; Yang, S.; Ma, X.; Huang, J.; Jiang, H.-L. Unveiling charge-separation dynamics in CdS/metal-organic framework composites for enhanced photocatalysis. ACS Catal. 2018, 8, 11615–11621. [Google Scholar] [CrossRef]
- Xu, M.; Sun, M.; Zhao, X.; Jiang, H.; Wang, H.; Huo, P. Fabricated hierarchical CdS/Ni-MOF heterostructure for promoting the photocatalytic reduction of CO2. Appl. Surf. Sci. 2022, 576, 151792. [Google Scholar] [CrossRef]
- Chu, X.; Meng, F.; Deng, T.; Lu, Y.; Bondarchuk, O.; Sui, M.; Feng, M.; Li, H.; Zhang, W. Mechanistic insight into bimetallic CoNi-MOF arrays with enhanced performance for supercapacitors. Nanoscale 2020, 12, 5669–5677. [Google Scholar] [CrossRef]
- Ge, L.; Zuo, F.; Liu, J.; Ma, Q.; Wang, C.S.; Sun, D.; Bartels, L.; Feng, P. Synthesis and efficient visible light photocatalytic hydrogen evolution of polymeric g-C3N4 coupled with CdS quantum dots. J. Phys. Chem. C 2012, 116, 13708–13714. [Google Scholar] [CrossRef]
- Xu, J.; Yan, X.; Qi, Y.; Fu, Y.; Wang, C.; Wang, L. Novel phosphidated MoS2 nanosheets modified CdS semiconductor for an efficient photocatalytic H2 evolution. Chem. Eng. J. 2019, 375, 122053. [Google Scholar] [CrossRef]
- Du, J.; Chai, J.; Li, Q.; Zhang, W.; Tang, B. Application of two-dimensional layered Mo-MOF@ppy with high valency molybdenum in lithium-ion batteries. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127810. [Google Scholar] [CrossRef]
- Lei, L.; Huang, D.; Lai, C.; Zhang, C.; Deng, R.; Chen, Y.; Chen, S.; Wang, W. Interface modulation of Mo2C@foam Nickel via MoS2 quantum dots for the electrochemical oxygen evolution reaction. J. Mater. 2020, 8, 15074–15085. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Gao, P.; Yin, W.; Yin, M.; Pu, H.; Sun, Q.; Liang, X.; Fa, H. Bimetal-organic frameworks MnCo-MOF-74 derived Co/MnO@HC for the construction of a novel enzyme-free glucose sensor. Microchem. J. 2022, 175, 107097. [Google Scholar] [CrossRef]
- Xie, X.; Zhang, Y.; Zhang, L.; Zheng, J.; Huang, Y.; Fa, H. Plasmon-driven interfacial catalytic reactions in plasmonic MOF nanoparticles. Anal. Chem. 2021, 93, 13219–13225. [Google Scholar] [CrossRef]
- Lei, Y.-M.; Wen, R.-X.; Zhou, J.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y. Silver ions as novel co-reaction accelerator for remarkably enhanced electrochemiluminescence in a PTCA-S2O82− system and its application in an ultrasensitive assay for mercury ions. Anal. Chem. 2018, 90, 6851–6858. [Google Scholar] [CrossRef]
- Mohammad, A.; Khan, M.E.; Alarifi, I.M.; Cho, M.H.; Yoon, T. A sensitive electrochemical detection of hydrazine based on SnO2/CeO2 nanostructured oxide. Microchem. J. 2021, 171, 106784. [Google Scholar] [CrossRef]
- Khana, F.; Misra, R. Recent advances in the development of phenothiazine and its fluorescent derivatives for optoelectronic applications. J. Mater. 2023, 11, 2786–2825. [Google Scholar] [CrossRef]
- Jaberi, S.Y.S.; Ghaffarinejad, A.; Kamalifar, M.; Heidari, M. Determination of chlorpromazine hydrochloride with a layered double hydroxide modified glassy carbon electrode as a nanocatalyst. Electroanalysis 2020, 32, 2065–2071. [Google Scholar] [CrossRef]
- Bozokalfa, G.; Akbulut, H.; Demir, B.; Guler, E.; Gumus, Z.P.; Demirkol, D.O.; Aldemir, E.; Yamada, S.; Endo, T.; Coskunol, H.; et al. Polypeptide functional surface for the aptamer immobilization: Electrochemical cocaine biosensing. Anal. Chem. 2016, 88, 4161–4167. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qin, J.; Yang, Q.; Wei, S.; Yang, R. Cost-effective and facile fluorescent probes for label-free recognition of chlorpromazine hydrochloride and logic gate operation. J. Photochem. Photobiol. A Chem. 2019, 382, 111918. [Google Scholar] [CrossRef]
- Shi, W.; Yang, J.; Huang, Y. Ion-pair complex-based solvent extraction combined with chemiluminescence determination of chlorpromazine hydrochloride with luminol in reverse micelles. J. Pharm. Biomed. Anal. 2004, 36, 197–203. [Google Scholar] [CrossRef]
- Shanmugam, R.; Ganesamurthi, J.; Chen, T.W.; Chen, S.M.; Balamurugan, M.; Ali, M.A.; Al-Mohaimeed, A.M.; Al-onazi, W.A.; Alagumalai, K. Preparation and fabrication of porous-Fe2O3/carbon black nanocomposite: A portable electrochemical sensor for psychotropic drug detection in environmental samples. Mater. Today Chem. 2022, 25, 100982. [Google Scholar] [CrossRef]
- Priscillal, I.J.D.; Wang, S.F. Highly sensitive amperometric determination of chlorpromazine hydrochloride in blood serum sample employing antimony vanadate nanospheres as electrode modifier. Microchem. J. 2023, 187, 108396. [Google Scholar] [CrossRef]
- Purushothama, H.T.; Nayaka, Y.A.; Manjunatha, P.; Yathisha, R.O.; Vinay, M.M.; Basavarajappa, K.V. Electrochemical determination of Chlorpromazine using l-Cysteine modified carbon paste electrode. Chem. Data Collect. 2019, 23, 100268. [Google Scholar] [CrossRef]
- Chen, R.; Chen, Q.; Wang, Y.; Feng, Z.; Xu, Z.; Zhou, P.; Huang, W.; Cheng, H.; Li, L.; Feng, J. Ultrasensitive SERS substrate for label-free therapeutic drug monitoring of chlorpromazine hydrochloride and aminophylline in human serum. Anal. Bioanal. Chem. 2023, 415, 1803–1815. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, X.; Zhang, G.; Li, X.; Wang, Y.; Wang, Y.; Hao, S.; Liu, D. 2D Flower-like CdS@Co/Mo-MOF as Co-Reaction Accelerator of g-C3N4-Based Electrochemiluminescence Sensor for Chlorpromazine Hydrochloride. Biosensors 2024, 14, 586. https://doi.org/10.3390/bios14120586
Fan X, Zhang G, Li X, Wang Y, Wang Y, Hao S, Liu D. 2D Flower-like CdS@Co/Mo-MOF as Co-Reaction Accelerator of g-C3N4-Based Electrochemiluminescence Sensor for Chlorpromazine Hydrochloride. Biosensors. 2024; 14(12):586. https://doi.org/10.3390/bios14120586
Chicago/Turabian StyleFan, Xiaowei, Guping Zhang, Xiaodi Li, Yao Wang, Yi Wang, Shilei Hao, and Defang Liu. 2024. "2D Flower-like CdS@Co/Mo-MOF as Co-Reaction Accelerator of g-C3N4-Based Electrochemiluminescence Sensor for Chlorpromazine Hydrochloride" Biosensors 14, no. 12: 586. https://doi.org/10.3390/bios14120586
APA StyleFan, X., Zhang, G., Li, X., Wang, Y., Wang, Y., Hao, S., & Liu, D. (2024). 2D Flower-like CdS@Co/Mo-MOF as Co-Reaction Accelerator of g-C3N4-Based Electrochemiluminescence Sensor for Chlorpromazine Hydrochloride. Biosensors, 14(12), 586. https://doi.org/10.3390/bios14120586





