Fluorescence Immunoassay of Prostate-Specific Antigen Using 3D Paddle Screw-Type Devices and Their Rotating System
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
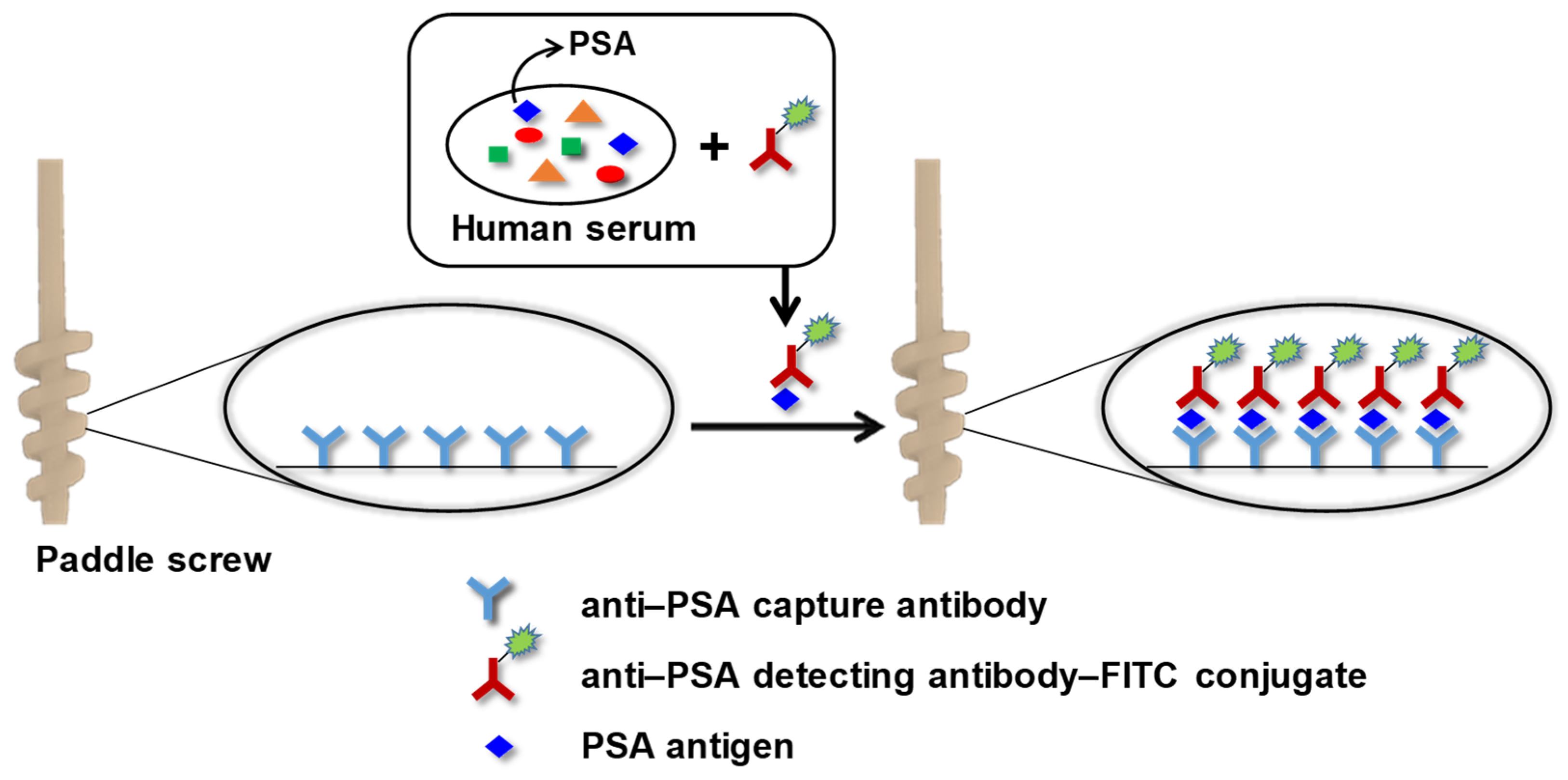
2.2. Preparation of Antibodies-Conjugated Paddle Screw
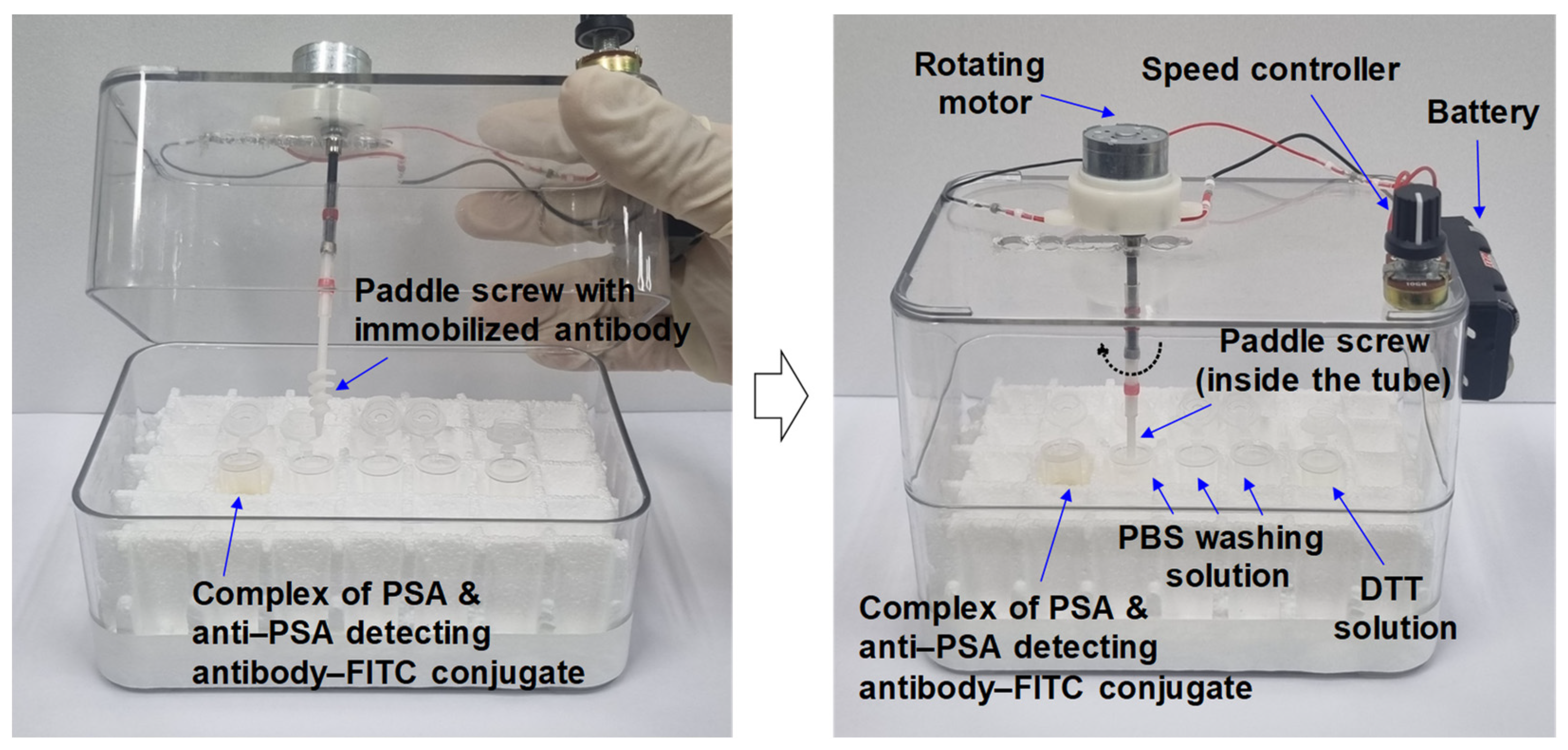
2.3. Fluorescence Measurement System
2.4. FITC Conjugation of Anti-PSA Detecting Antibody
2.5. Immunoassay for Detecting PSA Using a Paddle Screw Device and Its Rotating System
2.6. Fluorescence ELISAs for Detecting PSA Using Well Plates
3. Results and Discussion
3.1. PSA Immunoassay Using Paddle Crews and a Rotating System
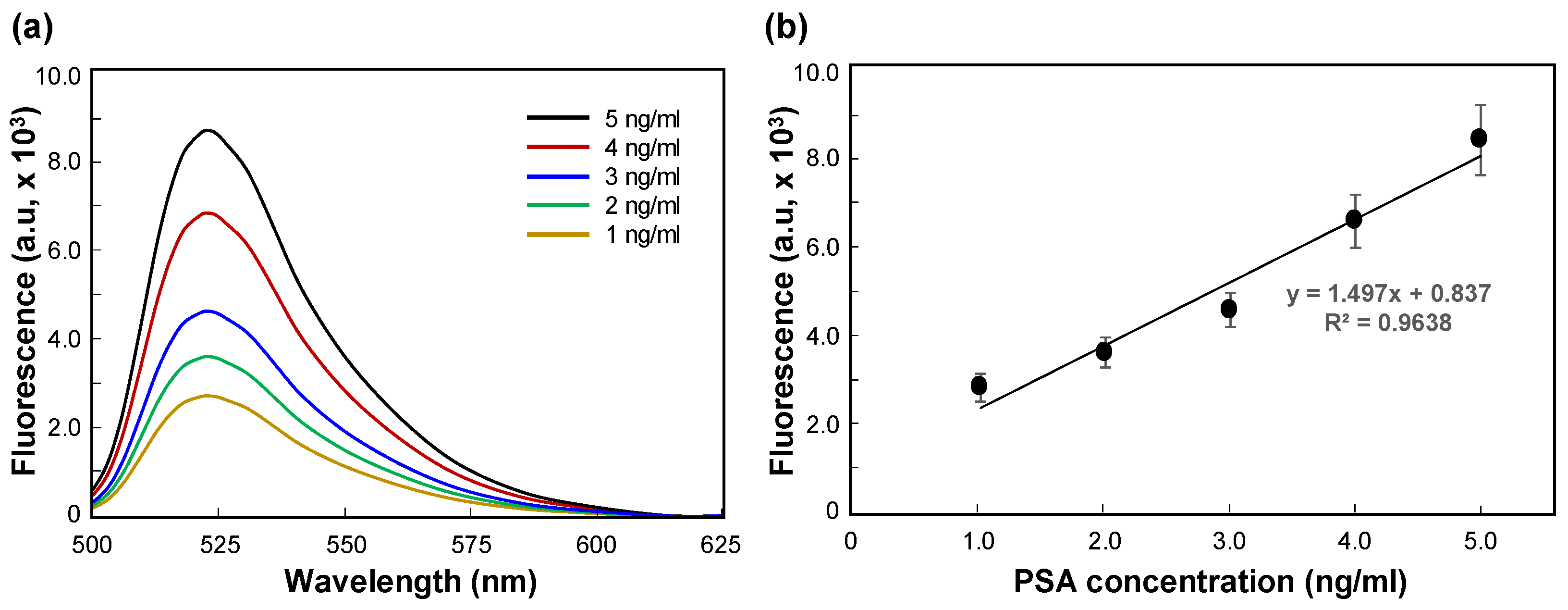
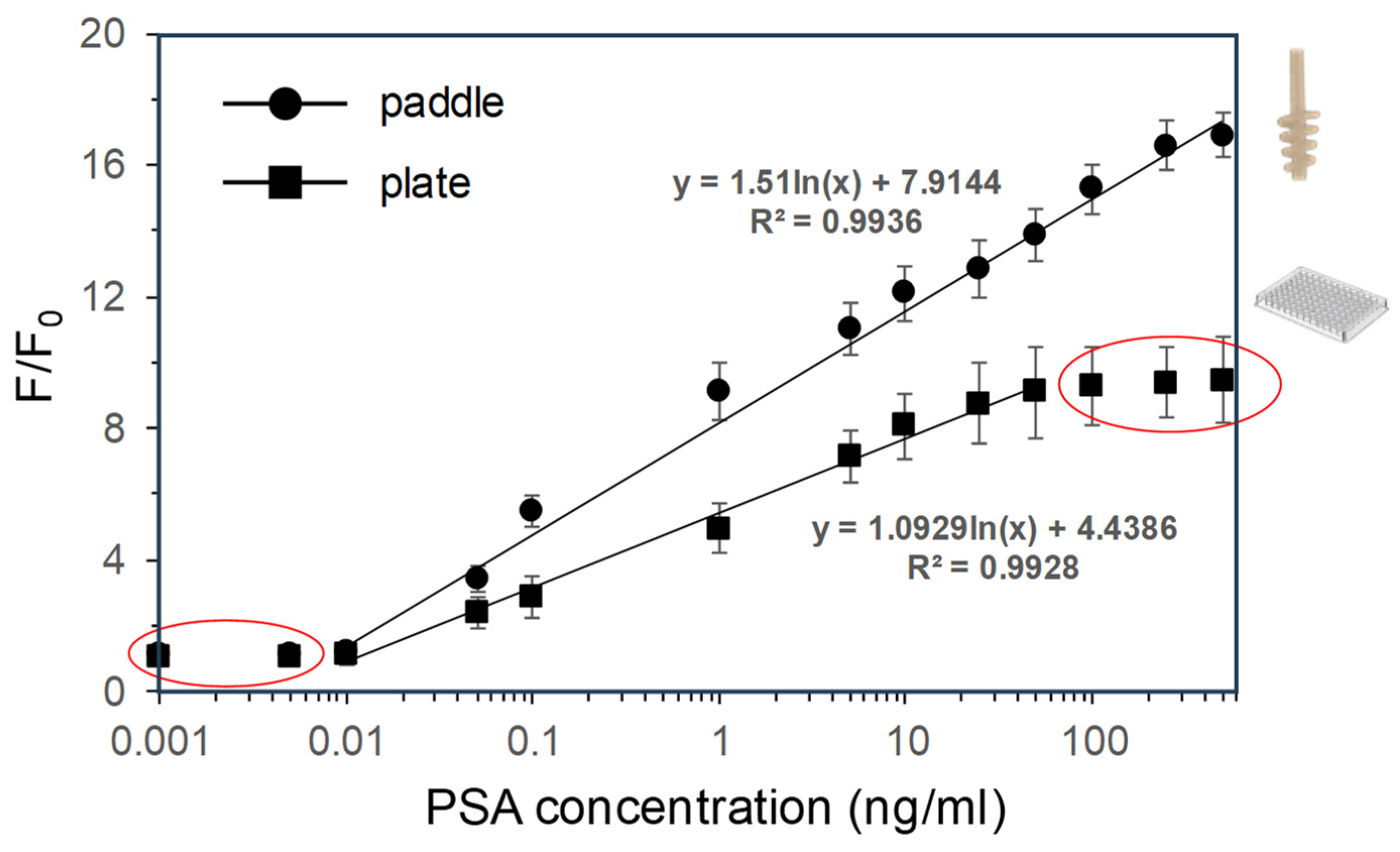
3.2. Dependence of Fluorescence Response on PSA Concentration
3.3. Blind Sample Analysis and Recovery Test for PSA Spiked in Human Serum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hage, D.S. Immunoassays. Anal. Chem. 1999, 71, 294–304. [Google Scholar] [CrossRef]
- Darwish, I.A. Immunoassay Methods and their applications in pharmaceutical analysis: Basic methodology and recent advances. Int. J. Biomed. Sci. 2006, 2, 217–235. [Google Scholar] [CrossRef]
- Koivunen, M.E.; Krogsrud, R.L. Principles of immunochemical techniques used in clinical laboratories. Lab. Med. 2006, 37, 490–497. [Google Scholar] [CrossRef]
- Li, Z.; Chen, G.-Y. Current conjugation methods for immunosensors. Nanomaterials 2018, 8, 278. [Google Scholar] [CrossRef] [PubMed]
- Farka, Z.; Jurik, T.; Kovar, D.; Trnkova, L.; Skladal, P. Nanoparticle-based immunochemical biosensors and assays: Recent advances and challenges. Chem. Rev. 2017, 117, 9973–10042. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef]
- Makhlynets, O.; Korendovych, I. Design of catalytically amplified sensors for small molecules. Biomolecules 2014, 4, 402–418. [Google Scholar] [CrossRef] [PubMed]
- Kabza, A.M.; Young, B.E.; Sczepanski, J.T. Heterochiral DNA strand-displacement circuits. J. Am. Chem. Soc. 2017, 139, 17715–17718. [Google Scholar] [CrossRef]
- Mize, P.D.; Hoke, R.A.; Lin, C.P.; Reardon, J.E.; Schulte, T.H. Dual-enzyme cascade—An amplified method for the detection of alkaline phosphatase. Anal. Biochem. 1989, 179, 229–235. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef]
- Chen, H.H.; Wu, C.H.; Tsai, M.L.; Huang, Y.J.; Chen, S.H. Detection of Total and A1c-Glycosylated Hemoglobin in Human Whole Blood Using Sandwich Immunoassays on Polydimethylsiloxane-Based Antibody Microarrays. Anal. Chem. 2012, 84, 8635–8641. [Google Scholar] [CrossRef] [PubMed]
- Date, Y.; Aota, A.; Terakado, S.; Sasaki, K.; Matsumoto, N.; Watanabe, Y.; Matsue, T.; Ohmura, N. Trace-Level Mercury Ion (Hg2+) Analysis in Aqueous Sample Based on Solid-Phase Extraction Followed by Microfluidic Immunoassay. Anal. Chem. 2013, 85, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Dixit, C.K.; Vashist, S.K.; O’Neill, F.T.; O’Reilly, B.; MacCraith, B.D.; O’Kennedy, R. Development of a High Sensitivity Rapid Sandwich ELISA Procedure and Its Comparison with the Conventional Approach. Anal. Chem. 2010, 82, 7049–7052. [Google Scholar] [CrossRef] [PubMed]
- Terzapulo, X.; Kassenova, A.; Bulasov, R. Immunoassays: Analytical and Clinical Performance, Challenges, and Perspectives of SERS Detection in Comparison with Fluorescent Spectroscopic Detection. Int. J. Mol. Sci. 2024, 25, 2080. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, B.; García-Betancourt, M.-L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and nanostructure synthesis and controlled growth methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef]
- Haj-Ahmad, T.A.; Abdalla, M.A.K.; Haj-Ahmad, Y. Potential urinary protein biomarker candidates for the accurate detection of prostate cancer among benign prostatic hyperplasia patients. J. Cancer 2014, 5, 103–114. [Google Scholar] [CrossRef]
- Sahoo, S.L.; Liu, C.-H.; Kumari, M.; Wu, W.-C.; Wang, C.-C. Biocompatible quantum dot-antibody conjugate for cell imaging, targeting and fluorometric immunoassay: Crosslinking, characterization and applications. RSC Adv. 2019, 9, 32791–32803. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the Determination of Limit of Detection and Limit of Quantitation of the Analytical Methods. Chron. Young Sci. 2011, 2, 15–21. [Google Scholar] [CrossRef]
- Gong, K.; Gai, S.; Tao, Y.; Du, Y.; Wang, Q.; Ansari, A.A.; Ding, K.; Wang, Q.; Yang, P. Colorimetric and photothermal dual-modal switching lateral flow immunoassay based on a forced dispersion prussian blue nanocomposite for the sensitive detection of Prostate-Specific Antigen. Anal. Chem. 2024, 96, 8665–8673. [Google Scholar] [CrossRef]
- Korram, J.; Anbalagan, C.; Banerjee, A.; Sawant, S.N. Bio-conjugated carbon dots for the bimodal detection of prostate cancer biomarkers via sandwich fluorescence and electrochemical immunoassays. J. Mater. Chem. B 2024, 12, 742–751. [Google Scholar] [CrossRef]
- Miglione, A.; Nardo, F.D.; Cavalera, S.; Serra, T.; Baggiani, C.; Cinti, S.; Anfossi, L. Merging lateral flow immunoassay with electroanalysis as a novel sensing platform: Prostate Specific Antigen detection as case of study. Anal. Chem. 2024, 96, 2297–2302. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Sangachin, E.; Mohammadnejad, J.; Hosseini, M. Fluorescence self-assembled DNA hydrogel for the determination of prostate specific antigen by aggregation induced emission. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2023, 303, 123234. [Google Scholar] [CrossRef] [PubMed]
- Flora, F.C.; Relvas, S.B.; e Silva, F.A.; Freire, M.G.; Chu, V.; Conde, J.P. Combined use of ionic liquid-based aqueous biphasic systems and microfluidic devices for the detection of Prostate-Specific Antigen. Biosensors 2023, 13, 334. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.; Lee, S.S. Highly sensitive piezoelectric immunosensors employing signal amplification with gold nanoparticles. Nanotechnology 2019, 30, 445502. [Google Scholar] [CrossRef]
- Karami, P.; Khoshsafar, H.; Ahar, M.J.; Arduini, F.; Afkhami, A.; Bagheri, H. Colorimetric immunosensor for determination of prostate specific antigen using surface plasmon resonance band of colloidal triangular shape gold nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117218. [Google Scholar] [CrossRef]
- Ma, K.; Zheng, Y.; An, L.; Liu, J. Ultrasensitive Immunosensor for Prostate-Specific Antigen based on enhanced electrochemiluminescence by vertically ordered mesoporous silica-nanochannel film. Front. Chem. 2022, 10, 851178. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, E.; Addona, T.; Keshishian, H.; Burgess, M.; Mani, D.R.; Lee, R.T.; Sabatine, M.S.; Gerszten, R.E.; Carr, S.A. Developing multiplexed assays for troponin I and interleukin-33 in plasma by peptide immunoaffinity enrichment and targeted mass spectrometry. Clin. Chem. 2009, 55, 1108–1117. [Google Scholar] [CrossRef]
- Liu, J.; Li, G. Application of biosensors for diagnostic analysis and bioprocess monitoring. Sens. Actuators B Chem. 2000, 65, 26–31. [Google Scholar] [CrossRef]



| Detection Technique | LOD (pg/mL) | Dynamic Range (ng/mL) | Reference |
|---|---|---|---|
| Absorbance | 202 | 1–40 | [19] |
| Electrochemistry | 38 | 0.1–100 | [20] |
| Electrochemistry | 150 | 0.01–20 | [21] |
| Fluorescence | 4.4 | 0.05–8.0 | [22] |
| Fluorescence | 5.6 | 0–25 | [23] |
| QCM 1 | 48 | 0.1–25 | [24] |
| SPR 2 | 9 | 0.01–20 | [25] |
| ECL 3 | 0.1 | 0.001–100 | [26] |
| Fluorescence | 13.7 | 0.01–500 | This work |
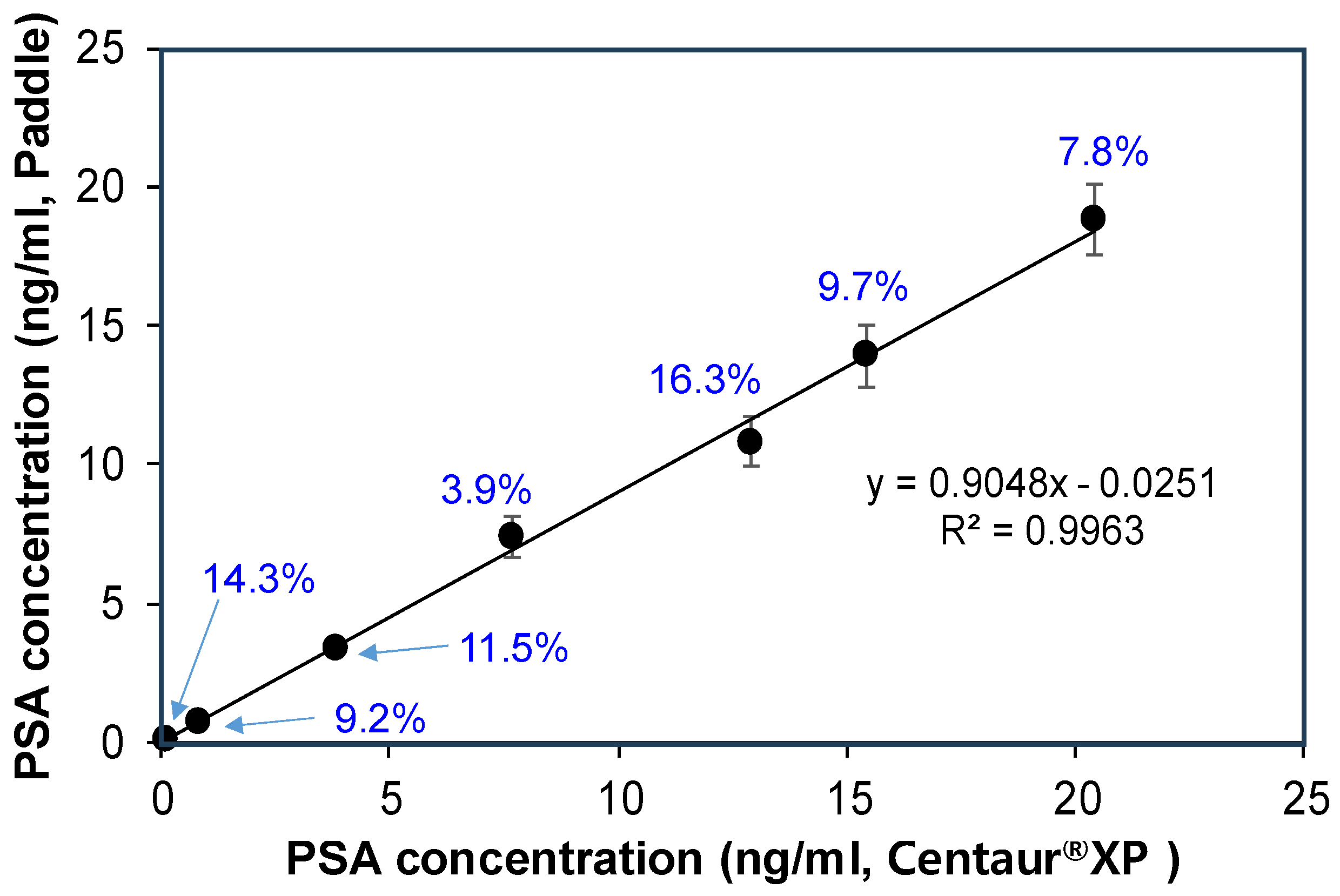
| Sample No. | Centaur®XP (ng/mL) | Paddle Screw (ng/mL) | Relative Error (%) 1 |
|---|---|---|---|
| 1 | 0.0914 | 0.0783 ± 0.00487 | 14.3 |
| 2 | 0.841 | 0.764 ± 0.0594 | 9.2 |
| 3 | 3.85 | 3.41 ± 0.298 | 11.5 |
| 4 | 7.69 | 7.39 ± 0.715 | 3.9 |
| 5 | 12.9 | 10.8 ± 0.894 | 16.3 |
| 6 | 15.4 | 13.9 ± 1.095 | 9.7 |
| 7 | 20.4 | 18.8 ± 1.248 | 7.8 |
| Sample No. | Added (pg/mL) | Found (pg/mL) | Recovery Rate (%) 1 |
|---|---|---|---|
| 1 | 25 | 22.7 ± 0.33 | 90.8 |
| 2 | 50 | 45.9 ± 0.62 | 91.8 |
| 3 | 100 | 93.1 ± 0.96 | 93.1 |
| 4 | 250 | 230.6 ± 3.28 | 92.2 |
| 5 | 500 | 466.9 ± 5.42 | 93.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, S.B.; Kim, H.S.; Jo, Y.J.; Lee, S.S. Fluorescence Immunoassay of Prostate-Specific Antigen Using 3D Paddle Screw-Type Devices and Their Rotating System. Biosensors 2024, 14, 494. https://doi.org/10.3390/bios14100494
Han SB, Kim HS, Jo YJ, Lee SS. Fluorescence Immunoassay of Prostate-Specific Antigen Using 3D Paddle Screw-Type Devices and Their Rotating System. Biosensors. 2024; 14(10):494. https://doi.org/10.3390/bios14100494
Chicago/Turabian StyleHan, Su Bin, Han Sol Kim, Young Ju Jo, and Soo Suk Lee. 2024. "Fluorescence Immunoassay of Prostate-Specific Antigen Using 3D Paddle Screw-Type Devices and Their Rotating System" Biosensors 14, no. 10: 494. https://doi.org/10.3390/bios14100494
APA StyleHan, S. B., Kim, H. S., Jo, Y. J., & Lee, S. S. (2024). Fluorescence Immunoassay of Prostate-Specific Antigen Using 3D Paddle Screw-Type Devices and Their Rotating System. Biosensors, 14(10), 494. https://doi.org/10.3390/bios14100494





