Metal–Organic Framework-Based Nanostructures for Electrochemical Sensing of Sweat Biomarkers
Abstract
1. Introduction
2. Sweat Biomarkers
3. Electrochemical Detection of Sweat Biomarkers
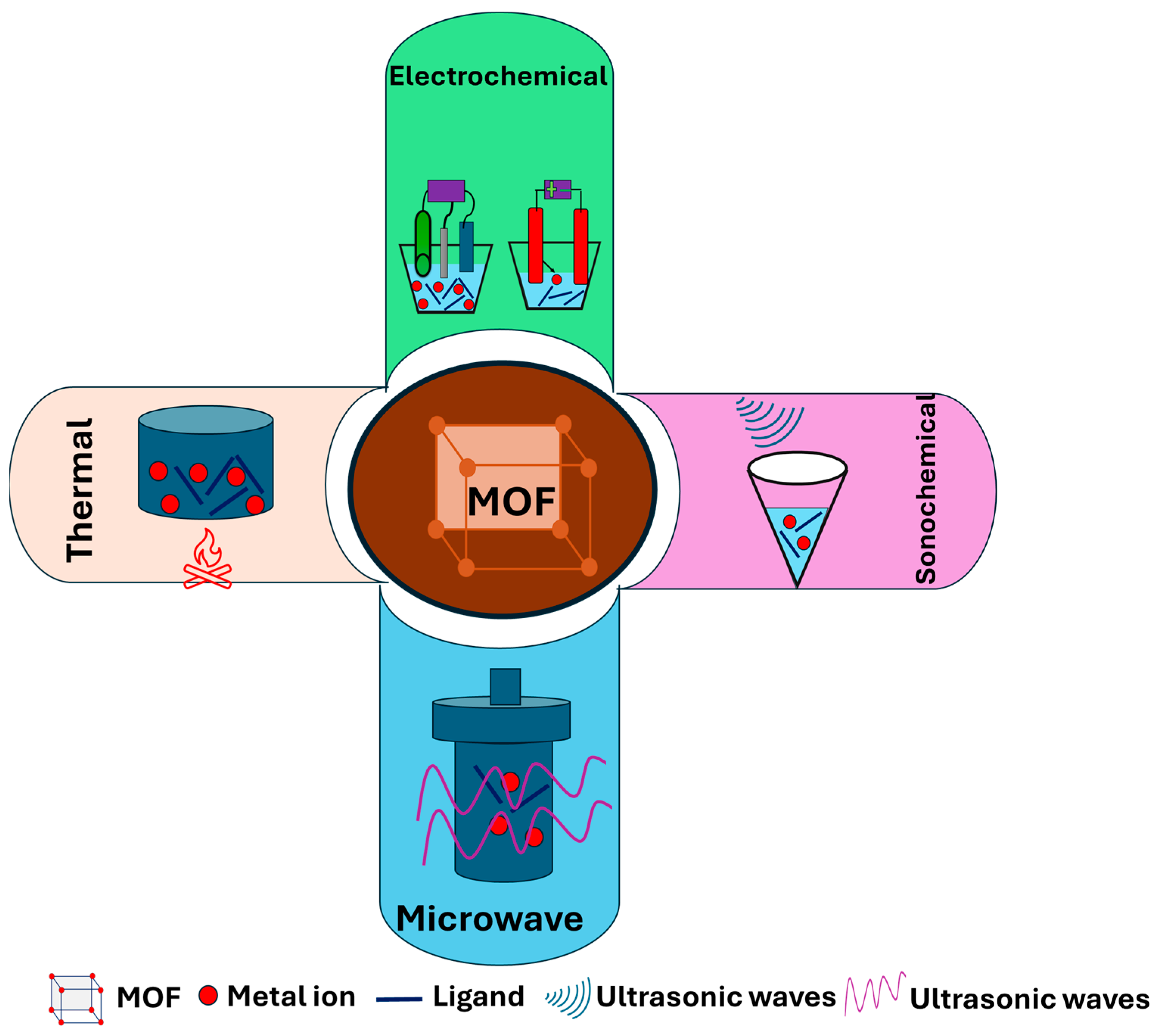
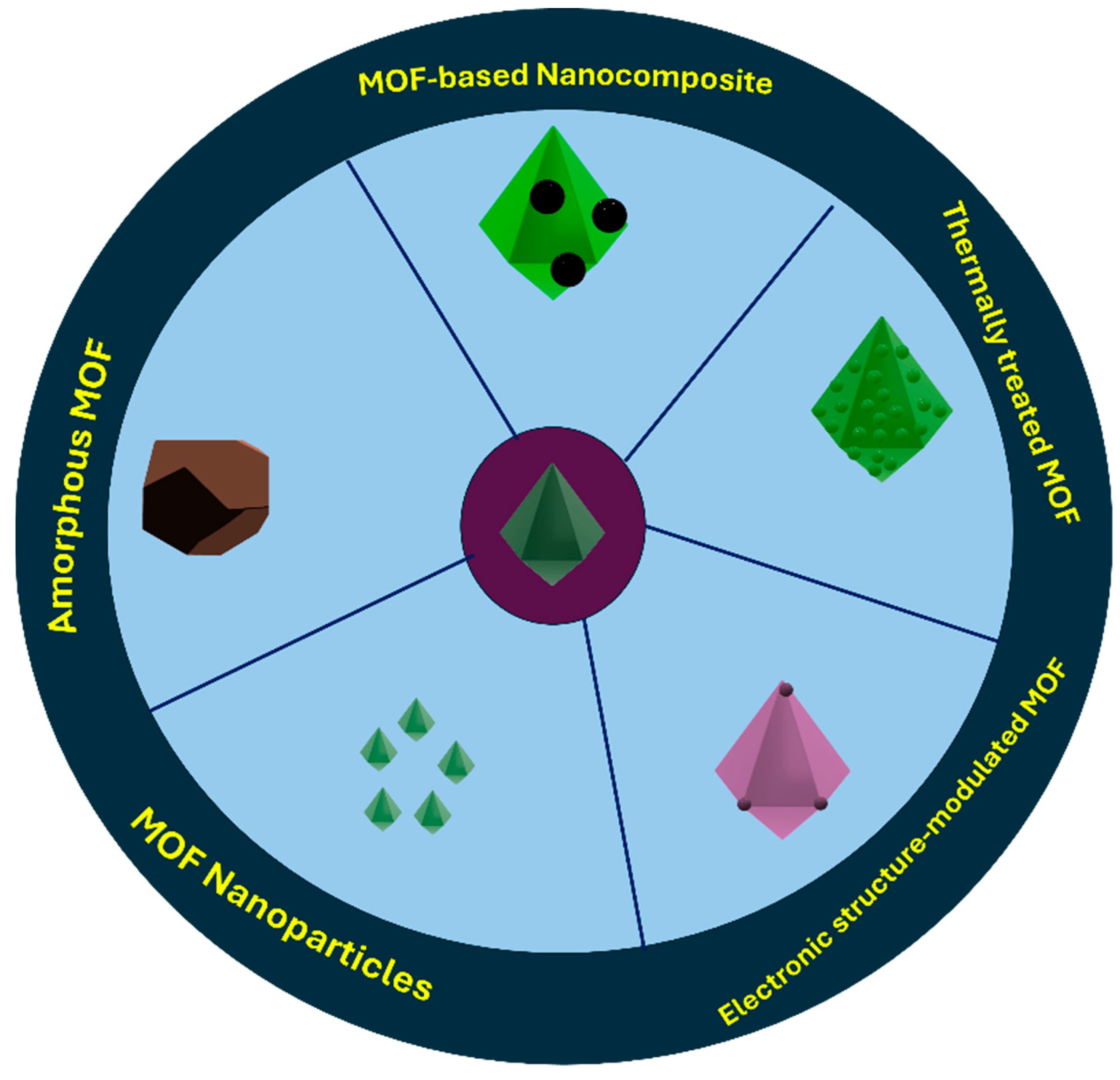
4. Fabrication and Characterization of MOFs and Their Nanostructures
5. MOFs for Electrochemical Detection of Sweat Biomarkers
6. MOF-Based NCs for Electrochemical Detection of Sweat Biomarkers
6.1. Cu-MOF
6.1.1. Cu-MOF Nanostructures
6.1.2. Cu-MOF/Pt NPs
6.1.3. Cu-CAT) Nanowires
6.1.4. Cu-MOF Nanosheets
6.2. Ni-MOF Nanorods
6.3. NiCo-MOF/CNTs
6.4. Zeolitic Imidazolate Framework (ZIF)
6.4.1. ZIF-8/Au NPs
6.4.2. ZIF-8/Ag NPs
6.4.3. ZIF-8 NPs/GO
6.4.4. ZIF-67-Derived NiCo
6.5. Zn-TCPP Nanosheets/MWCNTs
7. MOF NCs as Promising Electrode Modifiers for Detecting Sweat Biomarkers
7.1. Two-Dimensional Co-MOF Nanostructures
7.2. Cu-MOF
7.2.1. Cu-MOF Nanostructures
7.2.2. Cu-MOF/Ni NPs
7.2.3. Cu-MOF/Pt NPs
7.2.4. CuCo Nanostructures
7.2.5. CuCo-MOF/Cu NPs
7.2.6. CuCo-MOF/CuO NPs
7.3. MnCO-MOF Nanostructures
7.4. Nb-MOF Nanostructures
7.5. Ni-MOF
7.5.1. Ni-MOF Nanostructures
7.5.2. Ni-MOF NPs
7.5.3. Ni-MOF/Au NPs
7.5.4. Ni-MOF/CNTs
7.5.5. Ni2P/C NPs
7.5.6. Ni-P NPs
7.6. NiCo-MOF/Au NPs
7.7. NiMn-MOF Nanostructures
7.8. ZIF
7.8.1. ZIF Nanoporous Carbon
7.8.2. ZIF/Pt NPs
7.8.3. ZIF/ZnO NPs
7.8.4. ZIF-67 Derived NiCo LDH Nanosheets
7.9. Zn-MOF Nanoflower
7.10. Zr-MOF (Porous Carbon-ZrO2 NPs)
8. Perspective, Challenges, and Future Limitations
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bhardwaj, A.; Singh, A.K. Biogenic synthesis, characterization and photocatalytic activity of cerium nanoparticles for treatment of dyes contaminated water. Biocatal. Agric. Biotechnol. 2024, 57, 103075. [Google Scholar] [CrossRef]
- Özbek, O.; Altunoluk, O.C. Recent advances in nanoparticle–based potentiometric sensors. Adv. Sens. Energy Mater. 2023, 3, 100087. [Google Scholar] [CrossRef]
- Rao, G.N.M.; Vakkalagadda, M. A review on synthesis, characterization and applications of nanoparticles in polymer nanocomposites. Mater. Today Proc. 2023, 98, 68–80. [Google Scholar]
- Zahran, M.; Khalifa, Z.; Zahran, M.A.; Azzem, M.A. Abiotic Sensor for Electrochemical Determination of Chlorpyrifos in Natural Water Based on the Inhibition of Silver Nanoparticles Oxidation. Microchem. J. 2021, 165, 106173. [Google Scholar] [CrossRef]
- Zahran, M.; Khalifa, Z.; Zahran, M.A.; Azzem, M.A. Gum Arabic-capped silver nanoparticles for electrochemical amplification sensing of methylene blue in river water. Electrochim. Acta 2021, 394, 139152. [Google Scholar] [CrossRef]
- Zahran, M.; Khalifa, Z.; Zahran, M.A.-H.; Abdel Azzem, M. Dissolved Organic Matter-Capped Silver Nanoparticles for Electrochemical Aggregation Sensing of Atrazine in Aqueous Systems. ACS Appl. Nano Mater. 2020, 3, 3868–3875. [Google Scholar] [CrossRef]
- Zahran, M.; Khalifa, Z.; Zahran, M.A.-H.; Azzem, M.A. Natural latex-capped silver nanoparticles for two-way electrochemical displacement sensing of Eriochrome black T. Electrochim. Acta 2020, 356, 136825. [Google Scholar] [CrossRef]
- Murtala, N.; Rahman, R.; Said, K.A.M.B.; Mannan, M.A.; Patwary, A.M. A review of nanoparticle synthesis methods, classifications, applications, and characterization. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100900. [Google Scholar]
- Silverio, A.A.; Chung, W.-Y.; Tsai, V.F.; Cheng, C. Multi-parameter readout chip for interfacing with amperometric, potentiometric and impedometric sensors for wearable and point-of-care test applications. Microelectron. J. 2020, 100, 104769. [Google Scholar] [CrossRef]
- Han, E.; Pan, Y.; Li, L.; Cai, J. Bisphenol A detection based on nano gold-doped molecular imprinting electrochemical sensor with enhanced sensitivity. Food Chem. 2023, 426, 136608. [Google Scholar] [CrossRef]
- Zahran, M.; Azzem, M.A.; El-Attar, M. Red gum-capped gold nanoparticles for electrochemical sensing of bromocresol purple in water. Mater. Adv. 2024, 5, 1683–1690. [Google Scholar] [CrossRef]
- Zhou, C.; Feng, J.; Tian, Y.; Wu, Y.; He, Q.; Li, G.; Liu, J. Non-enzymatic electrochemical sensors based on nanomaterials for detection of organophosphorus pesticide residues. Environ. Sci. Adv. 2023, 2, 933–956. [Google Scholar] [CrossRef]
- Zahran, M. Carbohydrate polymer-supported metal and metal oxide nanoparticles for constructing electrochemical sensors. Mater. Adv. 2024, 5, 68–82. [Google Scholar] [CrossRef]
- Zahran, M.M.; Khalifa, Z.; Zahran, M.; Azzem, M.A. Recent advances in silver nanoparticle-based electrochemical sensors for determining organic pollutants in water: A review. Mater. Adv. 2021, 2, 7350–7365. [Google Scholar] [CrossRef]
- Shi, Y.; Zou, Y.; Khan, M.S.; Zhang, M.; Yan, J.; Zheng, X.; Wang, W.; Xie, Z. Metal–organic framework-derived photoelectrochemical sensors: Structural design and biosensing technology. J. Mater. Chem. C 2023, 11, 3692–3709. [Google Scholar] [CrossRef]
- Li, X.; Chi, F.; Huang, Y. A convenient, economical and excellent-yield method for the preparations of zeolite imidazolate frameworks (ZIFs) and the applications in environmental purification. J. Phys. Chem. Solids 2024, 188, 111895. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, W.; Wang, C.; Zhao, S.; Hao, Z.; Huang, X.; Miao, K.; Kang, X. MOF-Derived Fe2CoSe4@NC and Fe2NiSe4@NC Composite Anode Materials towards High-Performance Na-Ion Storage. Inorganics 2024, 12, 165. [Google Scholar] [CrossRef]
- El-Shamy, O.A.; Siddiqui, M.R.; Mele, G.; Mohsen, Q.; Alhajeri, M.M.; Deyab, M. Synthesis, Characterization and Application of CNTs@Co-MOF and FCNTs@Co-MOF as Superior Supercapacitor: Experimental and Theoretical studies. J. Mol. Struct. 2024, 1321, 140161. [Google Scholar] [CrossRef]
- Ye, R.-H.; Chen, J.-Y.; Huang, D.-H.; Wang, Y.-J.; Chen, S. Electrochemical sensor based on glassy-carbon electrode modified with dual-ligand EC-MOFs supported on rGO for BPA. Biosensors 2022, 12, 367. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, Y.; Ye, B. An efficient electrochemical glucose sensor based on porous nickel-based metal organic framework/carbon nanotubes composite (Ni-MOF/CNTs). J. Alloys Compd. 2018, 767, 651–656. [Google Scholar] [CrossRef]
- Ma, Y.-F.; Zhang, M.-L.; Lu, X.-Y.; Ren, Y.-X.; Yang, X.-G. Artificial light harvesting system of CM6@Zn-MOF nanosheets with highly enhanced photoelectric performance. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 325, 125152. [Google Scholar] [CrossRef] [PubMed]
- Cheraghi, H.; Jalali, F.; Sheikhi, S. Zirconium oxide-porous carbon derived from metal-organic frameworks as a new sensing platform: Application to simultaneous voltammetric determination of ascorbic acid, dopamine and uric acid. J. Iran. Chem. Soc. 2024, 21, 511–524. [Google Scholar] [CrossRef]
- Vikal, A.; Maurya, R.; Patel, P.; Paliwal, S.R.; Narang, R.K.; Gupta, G.D.; Kurmi, B.D. Exploring metal-organic frameworks (MOFs) in drug delivery: A concise overview of synthesis approaches, versatile applications, and current challenges. Appl. Mater. Today 2024, 41, 102443. [Google Scholar] [CrossRef]
- Xie, N.; Zhang, X.; Guo, Y.; Guo, R.; Wang, Y.; Sun, Z.; Li, H.; Jia, H.; Jiang, T.; Gao, J. Hollow Mn/Co-LDH produced by in-situ etching-growth of MOF: Nanoreactant for steady chemical immobilization of antimony. J. Taiwan Inst. Chem. Eng. 2021, 127, 197–207. [Google Scholar] [CrossRef]
- Fdez-Sanromán, A.; Rosales, E.; Pazos, M.; Sanromán, A. One-pot synthesis of bimetallic Fe–Cu metal–organic frameworks composite for the elimination of organic pollutants via peroxymonosulphate activation. Environ. Sci. Pollut. Res. 2023, 1–16. [Google Scholar] [CrossRef]
- Gupta, M.; Tyagi, S.; Kumari, N. Electrochemical performance of hybrid spinel ferrite/carbon (NiFe2O4/C) nanocomposite derived from metal organic frameworks (MOF) as electrode material for supercapacitor application. J. Solid State Electrochem. 2024, 28, 169–180. [Google Scholar] [CrossRef]
- Ramu, S.; Kainthla, I.; Chandrappa, L.; Shivanna, J.M.; Kumaran, B.; Balakrishna, R.G. Recent advances in metal organic frameworks–based magnetic nanomaterials for waste water treatment. Environ. Sci. Pollut. Res. 2024, 31, 167–190. [Google Scholar] [CrossRef]
- Salunkhe, A.D.; Pawar, P.S.; Pagare, P.K.; Torane, A.P. Facile solvothermal synthesis of Ni-Co MOF/rGO nanoflakes for high-performance asymmetric supercapacitor. Electrochim. Acta 2024, 477, 143745. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Liang, Z.; Zhong, W.; Ma, Q. A novel Cu-MOFs nanosheet/BiVO4 nanorod-based ECL sensor for colorectal cancer diagnosis. Talanta 2024, 266, 124952. [Google Scholar] [CrossRef]
- Jia, Y.; Yan, B. Visual ratiometric fluorescence sensing of L-lactate in sweat by Eu-MOF and the design of logic devices. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 297, 122764. [Google Scholar] [CrossRef]
- Tang, M.-H.; Shi, Y.; Jiang, X.-L.; Xu, H.; Ma, Y.; Zhao, B. A high sensitivity luminescent sensor for the stress biomarker cortisol using four-fold interpenetrated europium–organic frameworks integrated with logic gates. J. Mater. Chem. C 2021, 9, 9643–9649. [Google Scholar] [CrossRef]
- Akmal, S.A.; Khalid, M.; Ahmad, M.S.; Shahid, M.; Ahmad, M. Interwoven Architectural Complexity in Ni (II) Ion-Based 3D MOF Using Bipyridine and Tetrabenzenecarboxylic Acid: Adsorption Insights in Highly Efficient Iodine and Cationic Dye Capture. Cryst. Growth Des. 2024, 24, 7173–7193. [Google Scholar] [CrossRef]
- Wang, X.; Ma, T.; Ma, J.-G.; Cheng, P. Integration of devices based on metal–organic frameworks: A promising platform for chemical sensing. Coord. Chem. Rev. 2024, 518, 216067. [Google Scholar] [CrossRef]
- Rodrigues, J.C.; Bezerra, C.S.; Lima, L.M.; Neves, D.D.; Paim, A.P.S.; Silva, W.E.; Lavorante, A.F. A multicommuted system using bacterial cellulose for urease immobilization and copper (II)-MOF colorimetric sensor for urea spectrophotometric determination in milk. Food Chem. 2024, 460, 140454. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Jiang, H.; Wang, W. Colorimetric sensor array for discriminating and determinating phenolic pollutants basing on different ratio of ligands in Cu/MOFs. J. Hazard. Mater. 2023, 460, 132418. [Google Scholar] [CrossRef]
- Ji, B.-T.; Liu, L.-P.; Chen, J.-H.; Gao, L.-L.; Sun, Y.; Wang, J.-J.; Deng, Z.-P.; Sun, Y.-X. Ratiometric fluorescence sensor based on dye-encapsulated Zn-MOF for highly sensitive detection of diquat in tap water and apple samples. Microchem. J. 2024, 207, 111663. [Google Scholar] [CrossRef]
- Chen, R.; Cheng, H.; Cao, X.; Huang, Z.; Zhan, Y.; Gao, S.; Huang, W.; Li, L.; Feng, J. Construction of selectively enhanced MOF-SERS sensor and highly sensitive detection of hCE1 in HepG2 cells. Microchem. J. 2024, 202, 110776. [Google Scholar] [CrossRef]
- Ahmad, M.M.; Roushani, M.; Farokhi, S. Ni-P nanosheets derived from a metal–organic framework containing triptycene ligand: A high-performance electrochemical sensor for glucose determination. Microchem. J. 2024, 197, 109737. [Google Scholar] [CrossRef]
- Wei, X.; Wang, Y.; Wang, Y.; Chai, Y.; Liu, N. Improved OER electrocatalytic performance of Co-MOF by forming heterojunction with lower-conductivityWO3 nanorods or Cr2O3 nanoparticles. Int. J. Hydrog. Energy 2024, 58, 1240–1248. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, M.; Wang, X.; Song, J.; Yang, X. Electrocatalysis in MOF Films for Flexible Electrochemical Sensing: A Comprehensive Review. Biosensors 2024, 14, 420. [Google Scholar] [CrossRef]
- Fan, J.; Wang, H.; Zeng, X.; Su, L.; Zhang, X. An electrochemical sensor based on ZIF-67/Ag nanoparticles (NPs)/polydopamine (PDA) nanocomposites for detecting chloride ion with good reproducibility. J. Electroanal. Chem. 2022, 914, 116323. [Google Scholar] [CrossRef]
- Kamal, S.; Khalid, M.; Khan, M.S.; Shahid, M. Metal organic frameworks and their composites as effective tools for sensing environmental hazards: An up to date tale of mechanism, current trends and future prospects. Coord. Chem. Rev. 2023, 474, 214859. [Google Scholar] [CrossRef]
- Liang, X.-H.; Yu, A.-X.; Bo, X.-J.; Du, D.-Y.; Su, Z.-M. Metal/covalent-organic frameworks-based electrochemical sensors for the detection of ascorbic acid, dopamine and uric acid. Coord. Chem. Rev. 2023, 497, 215427. [Google Scholar] [CrossRef]
- Zhu, R.; Liu, L.; Zhang, G.; Zhang, Y.; Jiang, Y.; Pang, H. Advances in Electrochemistry of Intrinsic Conductive Metal-Organic Frameworks and Their Composites: Mechanisms, Synthesis and Applications. Nano Energy 2024, 122, 109333. [Google Scholar] [CrossRef]
- Li, W.; Guo, X.; Shen, B.; Ge, H.; Zhang, J.; Liu, L.; Zheng, H.; Liu, W.; Wu, Z.; Yang, P. Facile synthesis of sub-10 nm Cu-MOF nanofibers as electrochemical sensor element for picomolar chloramphenicol detection. J. Electroanal. Chem. 2024, 958, 118160. [Google Scholar] [CrossRef]
- Pahade, P.; Bose, D.; Peris-Vicente, J.; Goberna-Bravo, M.Á.; Chiva, J.A.; Romero, J.E.; Carda-Broch, S.; Durgbanshi, A. Screening of some banned aromatic amines in textile products from Indian bandhani and gamthi fabric and in human sweat using micellar liquid chromatography. Microchem. J. 2021, 165, 106134. [Google Scholar] [CrossRef]
- Sakurada, K.; Akutsu, T.; Fukushima, H.; Watanabe, K.; Yoshino, M. Detection of dermcidin for sweat identification by real-time RT-PCR and ELISA. Forensic Sci. Int. 2010, 194, 80–84. [Google Scholar] [CrossRef]
- Nan, M.; Darmawan, B.A.; Go, G.; Zheng, S.; Lee, J.; Kim, S.; Lee, T.; Choi, E.; Park, J.-O.; Bang, D. Wearable localized surface plasmon resonance-based biosensor with highly sensitive and direct detection of cortisol in human sweat. Biosensors 2023, 13, 184. [Google Scholar] [CrossRef]
- Fiore, L.; Mazzaracchio, V.; Serani, A.; Fabiani, G.; Fabiani, L.; Volpe, G.; Moscone, D.; Bianco, G.M.; Occhiuzzi, C.; Marrocco, G. Microfluidic paper-based wearable electrochemical biosensor for reliable cortisol detection in sweat. Sens. Actuators B Chem. 2023, 379, 133258. [Google Scholar] [CrossRef]
- Yang, B.; Li, H.; Nong, C.; Li, X.; Feng, S. A novel electrochemical immunosensor based on SnS2/NiCo metal-organic frameworks loaded with gold nanoparticles for cortisol detection. Anal. Biochem. 2023, 669, 115117. [Google Scholar] [CrossRef]
- Yu, H.; Sun, J. Sweat detection theory and fluid driven methods: A review. Nanotechnol. Precis. Eng. 2020, 3, 126–140. [Google Scholar] [CrossRef]
- Mugo, S.M.; Robertson, S.V.; Lu, W. A molecularly imprinted electrochemical microneedle sensor for multiplexed metabolites detection in human sweat. Talanta 2023, 259, 124531. [Google Scholar] [CrossRef] [PubMed]
- Rovira, M.; Lafaye, C.; Wang, S.; Fernandez-Sanchez, C.; Saubade, M.; Liu, S.-C.; Jimenez-Jorquera, C. Analytical assessment of sodium ISFET based sensors for sweat analysis. Sens. Actuators B Chem. 2023, 393, 134135. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Z.; Wei, K.; Cao, Z.; Zhu, Z.; Chen, R. Sweat as a source of non-invasive biomarkers for clinical diagnosis: An overview. Talanta 2024, 273, 125865. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Lin, X.; Feng, J.; Min, X.; Ni, Y. NiNP/Cu-MOF-C/GCE for the the noninvasive detection of glucose in natural saliva samples. Microchem. J. 2023, 190, 108657. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, T.; Jiang, L.; Asif, M.; Qiu, X.; Yu, Y.; Xiao, F.; Liu, H. Assembling metal–organic frameworks into the fractal scale for sweat sensing. ACS Appl. Mater. Interfaces 2019, 11, 32310–32319. [Google Scholar] [CrossRef]
- Zhu, C.; Xu, Y.; Chen, Q.; Zhao, H.; Gao, B.; Zhang, T. A flexible electrochemical biosensor based on functionalized poly (3,4-ethylenedioxythiophene) film to detect lactate in sweat of the human body. J. Colloid Interface Sci. 2022, 617, 454–462. [Google Scholar] [CrossRef]
- Ma, G. Electrochemical sensing monitoring of blood lactic acid levels in sweat during exhaustive exercise. Int. J. Electrochem. Sci. 2023, 18, 100064. [Google Scholar] [CrossRef]
- Xu, Z.; Song, J.; Liu, B.; Lv, S.; Gao, F.; Luo, X.; Wang, P. A conducting polymer PEDOT: PSS hydrogel based wearable sensor for accurate uric acid detection in human sweat. Sens. Actuators B Chem. 2021, 348, 130674. [Google Scholar] [CrossRef]
- Xie, Q.; Li, R.; Li, Z. Designing of multifunctional graphene quantum dot-polyvinyl alcohol-polyglycerol luminescent film for fluorescence detection of pH in sweat. Anal. Chim. Acta 2024, 1292, 342224. [Google Scholar]
- Zhu, C.; Xue, H.; Zhao, H.; Fei, T.; Liu, S.; Chen, Q.; Gao, B.; Zhang, T. A dual-functional polyaniline film-based flexible electrochemical sensor for the detection of pH and lactate in sweat of the human body. Talanta 2022, 242, 123289. [Google Scholar] [CrossRef] [PubMed]
- Yan, T.; Zhang, G.; Yu, K.; Chai, H.; Tian, M.; Qu, L.; Dong, H.; Zhang, X. Smartphone light-driven zinc porphyrinic MOF nanosheets-based enzyme-free wearable photoelectrochemical sensor for continuous sweat vitamin C detection. Chem. Eng. J. 2023, 455, 140779. [Google Scholar] [CrossRef]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.; Oliveira, O.N., Jr. Wearable glove-embedded sensors for therapeutic drug monitoring in sweat for personalized medicine. Chem. Eng. J. 2022, 435, 135047. [Google Scholar] [CrossRef]
- Xiao, J.; Fan, C.; Xu, T.; Su, L.; Zhang, X. An electrochemical wearable sensor for levodopa quantification in sweat based on a metal–Organic framework/graphene oxide composite with integrated enzymes. Sens. Actuators B Chem. 2022, 359, 131586. [Google Scholar] [CrossRef]
- Mehmeti, E.; Kilic, T.; Laur, C.; Carrara, S. Electrochemical determination of nicotine in smokers’ sweat. Microchem. J. 2020, 158, 105155. [Google Scholar] [CrossRef]
- Silva, R.R.; Raymundo-Pereira, P.A.; Campos, A.M.; Wilson, D.; Otoni, C.G.; Barud, H.S.; Costa, C.A.; Domeneguetti, R.R.; Balogh, D.T.; Ribeiro, S.J. Microbial nanocellulose adherent to human skin used in electrochemical sensors to detect metal ions and biomarkers in sweat. Talanta 2020, 218, 121153. [Google Scholar] [CrossRef]
- Dalirirad, S.; Steckl, A.J. Aptamer-based lateral flow assay for point of care cortisol detection in sweat. Sens. Actuators B Chem. 2019, 283, 79–86. [Google Scholar] [CrossRef]
- Xi, P.; He, X.; Fan, C.; Zhu, Q.; Li, Z.; Yang, Y.; Du, X.; Xu, T. Smart Janus fabrics for one-way sweat sampling and skin-friendly colorimetric detection. Talanta 2023, 259, 124507. [Google Scholar] [CrossRef]
- Yang, M.; Sun, N.; Lai, X.; Zhao, X.; Zhou, W. Advances in Non-Electrochemical Sensing of Human Sweat Biomarkers: From Sweat Sampling to Signal Reading. Biosensors 2023, 14, 17. [Google Scholar] [CrossRef]
- Kim, J.; Lee, S.; Kim, S.; Jung, M.; Lee, H.; Han, M.S. Development of a fluorescent chemosensor for chloride ion detection in sweat using Ag+-benzimidazole complexes. Dye. Pigment. 2020, 177, 108291. [Google Scholar] [CrossRef]
- Wang, W.; Chen, Y.; Xiao, C.; Xiao, S.; Wang, C.; Nie, Q.; Xu, P.; Chen, J.; You, R.; Zhang, G. Flexible SERS wearable sensor based on nanocomposite hydrogel for detection of metabolites and pH in sweat. Chem. Eng. J. 2023, 474, 145953. [Google Scholar] [CrossRef]
- Zhang, Y.; Liao, J.; Li, Z.; Hu, M.; Bian, C.; Lin, S. All fabric and flexible wearable sensors for simultaneous sweat metabolite detection and high-efficiency collection. Talanta 2023, 260, 124610. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez-Redín, G.; Cagnani, G.R.; Gomes, N.O.; Raymundo-Pereira, P.A.; Machado, S.A.; Gutierrez, M.A.; Krieger, J.E.; Oliveira, O.N., Jr. Wearable potentiometric biosensor for analysis of urea in sweat. Biosens. Bioelectron. 2023, 223, 114994. [Google Scholar] [CrossRef] [PubMed]
- San Nah, J.; Barman, S.C.; Zahed, M.A.; Sharifuzzaman, M.; Yoon, H.; Park, C.; Yoon, S.; Zhang, S.; Park, J.Y. A wearable microfluidics-integrated impedimetric immunosensor based on Ti3C2Tx MXene incorporated laser-burned graphene for noninvasive sweat cortisol detection. Sens. Actuators B Chem. 2021, 329, 129206. [Google Scholar]
- Wei, C.; Wang, Z.; Li, S.; Li, T.; Du, X.; Wang, H.; Liu, Q.; Yu, Z. Hierarchical copper-based metal-organic frameworks nanosheet assemblies for electrochemical ascorbic acid sensing. Colloids Surf. B Biointerfaces 2023, 223, 113149. [Google Scholar] [CrossRef]
- Sadeghi, J. Amperometric biosensors. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 61–67. [Google Scholar]
- Vasiliou, F.; Plessas, A.K.; Economou, A.; Thomaidis, N.; Papaefstathiou, G.S.; Kokkinos, C. Graphite paste sensor modified with a Cu (II)-complex for the enzyme-free simultaneous voltammetric determination of glucose and uric acid in sweat. J. Electroanal. Chem. 2022, 917, 116393. [Google Scholar] [CrossRef]
- Shen, Y.; Chai, S.; Zhang, Q.; Zhang, M.; Mao, X.; Wei, L.; Zhou, F.; Sun, R.; Liu, C. PVF composite conductive nanofibers-based organic electrochemical transistors for lactate detection in human sweat. Chem. Eng. J. 2023, 475, 146008. [Google Scholar] [CrossRef]
- Huang, M.-R.; Li, X.-G. Highly sensing and transducing materials for potentiometric ion sensors with versatile applicability. Prog. Mater. Sci. 2022, 125, 100885. [Google Scholar] [CrossRef]
- Porfireva, A.; Goida, A.; Evtugyn, V.; Evtugyn, G. Impedimetric sensor based on molecularly imprinted polythionine from deep eutectic solvent for epinephrine determination. Green Anal. Chem. 2024, 9, 100113. [Google Scholar] [CrossRef]
- Şen, F.B.; Elmas, E.; Dilgin, Y.; Bener, M.; Apak, R. Amperometric sensor for total antioxidant capacity measurement using Cu (II)-neocuproine/carrageenan-MWCNT/GCE. Microchem. J. 2024, 199, 110081. [Google Scholar] [CrossRef]
- Luo, B.; Xi, X.; Wu, Y.; Wu, D.; Jiang, B.; Shen, C.; Wang, Z.; Zhang, F.; Su, Y.; Liu, R. Organic Electrochemical Transistors for Monitoring Dissolved Oxygen in Aqueous Electrolytes of Zinc Ion Batteries. Sens. Actuators B Chem. 2024, 409, 135601. [Google Scholar] [CrossRef]
- Tan, A.Y.S.; Awan, H.T.A.; Cheng, F.; Zhang, M.; Tan, M.T.; Manickam, S.; Khalid, M.; Muthoosamy, K. Recent advances in the use of MXenes for photoelectrochemical sensors. Chem. Eng. J. 2024, 482, 148774. [Google Scholar] [CrossRef]
- Muratova, I.S.; Kartsova, L.A.; Mikhelson, K.N. Voltammetric vs. potentiometric sensing of dopamine: Advantages and disadvantages, novel cell designs, fundamental limitations and promising options. Sens. Actuators B Chem. 2015, 207, 900–906. [Google Scholar] [CrossRef]
- An, Q.; Gan, S.; Xu, J.; Bao, Y.; Wu, T.; Kong, H.; Zhong, L.; Ma, Y.; Song, Z.; Niu, L. A multichannel electrochemical all-solid-state wearable potentiometric sensor for real-time sweat ion monitoring. Electrochem. Commun. 2019, 107, 106553. [Google Scholar] [CrossRef]
- Cosio, M.; Scampicchio, M.; Benedetti, S. Electronic Noses and Tongues; Academic Press: Boston, MA, USA, 2012. [Google Scholar]
- Gajdosova, V.P.; Lorencova, L.; Blsakova, A.; Kasak, P.; Bertok, T.; Tkac, J. Challenges for impedimetric affinity sensors targeting protein detection. Curr. Opin. Electrochem. 2021, 28, 100717. [Google Scholar] [CrossRef]
- Devadoss, A.; Sudhagar, P.; Terashima, C.; Nakata, K.; Fujishima, A. Photoelectrochemical biosensors: New insights into promising photoelectrodes and signal amplification strategies. J. Photochem. Photobiol. C Photochem. Rev. 2015, 24, 43–63. [Google Scholar] [CrossRef]
- Liu, Y.; Zhong, L.; Zhang, S.; Wang, J.; Liu, Z. An ultrasensitive and wearable photoelectrochemical sensor for unbiased and accurate monitoring of sweat glucose. Sens. Actuators B Chem. 2022, 354, 131204. [Google Scholar] [CrossRef]
- Xu, A.; Xu, M.; Luo, F.; Lin, C.; Qiu, B.; Lin, Z.; Jiang, Z.; Wang, J. Realizing high-performance glucose sensing in sweat: Synergistic use of nickel oxide nanosheets as photoelectrodes and the masking effect of Mo-POM for photoelectrochemical detection. Sens. Actuators B Chem. 2024, 403, 135135. [Google Scholar] [CrossRef]
- Kanokpaka, P.; Chang, L.-Y.; Wang, B.-C.; Huang, T.-H.; Shih, M.-J.; Hung, W.-S.; Lai, J.-Y.; Ho, K.-C.; Yeh, M.-H. Self-powered molecular imprinted polymers-based triboelectric sensor for noninvasive monitoring lactate levels in human sweat. Nano Energy 2022, 100, 107464. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, W.; Liu, Y. A graphene-based electrochemical detection platform for monitoring the change of potassium content in sweat during exhaustive exercise. Int. J. Electrochem. Sci. 2023, 18, 100181. [Google Scholar] [CrossRef]
- Poletti, F.; Zanfrognini, B.; Favaretto, L.; Quintano, V.; Sun, J.; Treossi, E.; Melucci, M.; Palermo, V.; Zanardi, C. Continuous capillary-flow sensing of glucose and lactate in sweat with an electrochemical sensor based on functionalized graphene oxide. Sens. Actuators B Chem. 2021, 344, 130253. [Google Scholar] [CrossRef]
- Qing, X.; Wu, J.; Shu, Q.; Wang, D.; Li, M.; Liu, D.; Wang, X.; Lei, W. High gain fiber-shaped transistor based on rGO-mediated hierarchical polypyrrole for ultrasensitive sweat sensor. Sens. Actuators A Phys. 2023, 354, 114297. [Google Scholar] [CrossRef]
- Cui, X.; Bao, Y.; Han, T.; Liu, Z.; Ma, Y.; Sun, Z. A wearable electrochemical sensor based on β-CD functionalized graphene for pH and potassium ion analysis in sweat. Talanta 2022, 245, 123481. [Google Scholar] [CrossRef] [PubMed]
- Barber, R.; Davis, J.; Papakonstantinou, P. Stable chitosan and prussian blue-coated laser-induced graphene skin sensor for the electrochemical detection of hydrogen peroxide in sweat. ACS Appl. Nano Mater. 2023, 6, 10290–10302. [Google Scholar] [CrossRef]
- Li, P.; Ling, Z.; Liu, X.; Bai, L.; Wang, W.; Chen, H.; Yang, H.; Yang, L.; Wei, D. Nanocomposite hydrogels flexible sensors with functional cellulose nanocrystals for monitoring human motion and lactate in sweat. Chem. Eng. J. 2023, 466, 143306. [Google Scholar] [CrossRef]
- Liu, X.; Cai, Z.; Gao, N.; Ye, S.; Tao, T.; He, H.; Chang, G.; He, Y. Controllable preparation of (200) facets preferential oriented silver nanowires for non-invasive detection of glucose in human sweat. Smart Mater. Med. 2021, 2, 150–157. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, T.; Li, K.; Long, D.; Zhao, J.; Zhu, F.; Gong, W. A flexible nonenzymatic sweat glucose sensor based on Au nanoflowers coated carbon cloth. Sens. Actuators B Chem. 2023, 388, 133798. [Google Scholar] [CrossRef]
- Zhu, Y.; Qi, Y.; Xu, M.; Luo, J. Flexible biosensor based on signal amplification of gold nanoparticles-composite flower clusters for glucose detection in sweat. Colloids Surf. A Physicochem. Eng. Asp. 2023, 661, 130908. [Google Scholar] [CrossRef]
- Yamuna, A.; Chen, T.-W.; Akilarasan, M.; Chen, S.-M.; Lou, B.-S. Electrochemical determination of glucose in blood serum and sweat samples by the strontium doped Co3O4. J. Electroanal. Chem. 2022, 905, 115978. [Google Scholar] [CrossRef]
- Zhang, Q.; Ma, S.; Zhan, X.; Meng, W.; Wang, H.; Liu, C.; Zhang, T.; Zhang, K.; Su, S. Smartphone-based wearable microfluidic electrochemical sensor for on-site monitoring of copper ions in sweat without external driving. Talanta 2024, 266, 125015. [Google Scholar] [CrossRef]
- Laochai, T.; Yukird, J.; Promphet, N.; Qin, J.; Chailapakul, O.; Rodthongkum, N. Non-invasive electrochemical immunosensor for sweat cortisol based on L-cys/AuNPs/MXene modified thread electrode. Biosens. Bioelectron. 2022, 203, 114039. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Jiang, M.; Su, M.; Cao, X.; Jiang, Q.; Liu, Q.; Yu, C. Sweat cortisol determination utilizing MXene and multi-walled carbon nanotube nanocomposite functionalized immunosensor. Microchem. J. 2023, 185, 108172. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Liu, X.; Liu, Y.; Cheng, Y.; Cui, D.; Chen, F.; Cao, W. A MXene/MoS2 heterostructure based biosensor for accurate sweat ascorbic acid detection. FlatChem 2023, 39, 100503. [Google Scholar] [CrossRef]
- Mo, L.; Ma, X.; Fan, L.; Xin, J.H.; Yu, H. Weavable, large-scaled, rapid response, long-term stable electrochemical fabric sensor integrated into clothing for monitoring potassium ions in sweat. Chem. Eng. J. 2023, 454, 140473. [Google Scholar] [CrossRef]
- Tao, B.; Yang, W.; Miao, F.; Zang, Y.; Chu, P.K. A sensitive enzyme-free electrochemical sensor composed of Co3O4/CuO@MWCNTs nanocomposites for detection of L-lactic acid in sweat solutions. Mater. Sci. Eng. B 2023, 288, 116163. [Google Scholar] [CrossRef]
- Varsha, M.; Nageswaran, G. Direct electrochemical synthesis of metal organic frameworks. J. Electrochem. Soc. 2020, 167, 155527. [Google Scholar]
- Vaitsis, C.; Kanellou, E.; Pandis, P.K.; Papamichael, I.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Sonochemical synthesis of zinc adipate Metal-Organic Framework (MOF) for the electrochemical reduction of CO2: MOF and circular economy potential. Sustain. Chem. Pharm. 2022, 29, 100786. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, S.; Yan, X.; Lv, Y. Recent advances in metal-organic frameworks: Synthesis, application and toxicity. Sci. Total Environ. 2023, 902, 165944. [Google Scholar] [CrossRef]
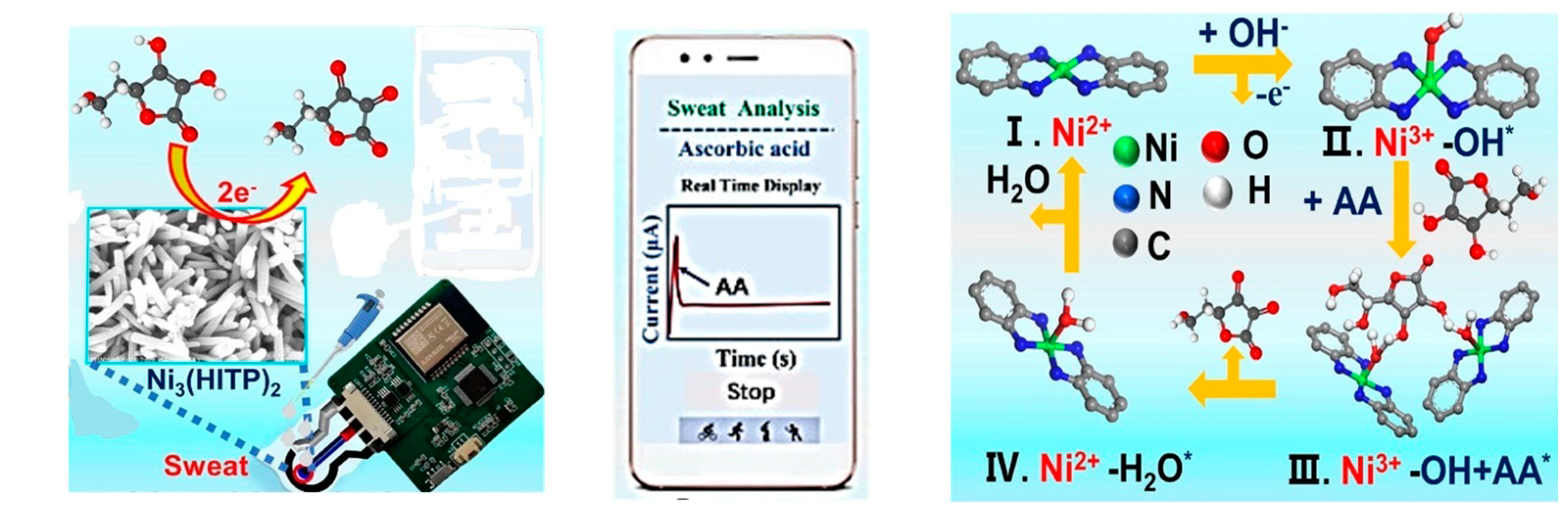
- Wang, L.; Pan, L.; Han, X.; Ha, M.N.; Li, K.; Yu, H.; Zhang, Q.; Li, Y.; Hou, C.; Wang, H. A portable ascorbic acid in sweat analysis system based on highly crystalline conductive nickel-based metal-organic framework (Ni-MOF). J. Colloid Interface Sci. 2022, 616, 326–337. [Google Scholar] [CrossRef]
- Elashery, S.E.; Attia, N.F.; Oh, H. Design and fabrication of novel flexible sensor based on 2D Ni-MOF nanosheets as a preliminary step toward wearable sensor for onsite Ni (II) ions detection in biological and environmental samples. Anal. Chim. Acta 2022, 1197, 339518. [Google Scholar] [CrossRef]
- Srinivasan, P.; Sethuraman, M.G.; Govindasamy, M.; Gokulkumar, K.; Alothman, A.A.; Alanazi, H.A.; Huang, C.-H. Nanomolar Detection of Organic Pollutant-Tartrazine in Food Samples Using Zinc-Metal Organic Framework/Carbon Nano Fiber-Composite Modified Screen-Printed Electrode. J. Food Compos. Anal. 2024, 133, 106462. [Google Scholar] [CrossRef]
- Manquian, C.; Navarrete, A.; Vivas, L.; Troncoso, L.; Singh, D.P. Synthesis and Optimization of Ni-Based Nano Metal–Organic Frameworks as a Superior Electrode Material for Supercapacitor. Nanomaterials 2024, 14, 353. [Google Scholar] [CrossRef] [PubMed]
- Poudel, M.B.; Awasthi, G.P.; Kim, H.J. Novel insight into the adsorption of Cr (VI) and Pb (II) ions by MOF derived Co-Al layered double hydroxide@hematite nanorods on 3D porous carbon nanofiber network. Chem. Eng. J. 2021, 417, 129312. [Google Scholar] [CrossRef]
- Guo, X.; Feng, S.; Peng, Y.; Li, B.; Zhao, J.; Xu, H.; Meng, X.; Zhai, W.; Pang, H. Emerging insights into the application of metal-organic framework (MOF)-based materials for electrochemical heavy metal ion detection. Food Chem. 2024, 463, 141387. [Google Scholar] [CrossRef]
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Metal organic frameworks for electrochemical sensor applications: A review. Environ. Res. 2022, 204, 112320. [Google Scholar] [CrossRef]
- Li, W.-H.; Deng, W.-H.; Wang, G.-E.; Xu, G. Conductive MOFs. EnergyChem 2020, 2, 100029. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Y.; Xu, M.; Ly, A.; Gili, A.; Murphy, E.; Asset, T.; Liu, Y.; De Andrade, V.; Segre, C.U. Catalysts by pyrolysis: Transforming metal-organic frameworks (MOFs) precursors into metal-nitrogen-carbon (MNC) materials. Mater. Today 2023, 69, 66–78. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Zhang, Y.; Luo, D.; Yu, A.; Wang, X.; Chen, Z. Amorphizing metal-organic framework towards multifunctional polysulfide barrier for high-performance lithium-sulfur batteries. Nano Energy 2021, 86, 106094. [Google Scholar] [CrossRef]
- Subramaniyam, V.; Thangadurai, D.T.; Ravi, P.V.; Pichumani, M. Do the acid/base modifiers in solvothermal synthetic conditions influence the formation of Zr-Tyr MOFs to be amorphous? J. Mol. Struct. 2022, 1267, 133611. [Google Scholar] [CrossRef]
- Wang, W.; Chai, M.; Lin, R.; Yuan, F.; Wang, L.; Chen, V.; Hou, J. Amorphous MOFs for next generation supercapacitors and batteries. Energy Adv. 2023, 2, 1591–1603. [Google Scholar] [CrossRef]
- Liu, K.-K.; Meng, Z.; Fang, Y.; Jiang, H.-L. Conductive MOFs for electrocatalysis and electrochemical sensor. eScience 2023, 3, 100133. [Google Scholar] [CrossRef]
- Xia, Y.; Su, T.; Mi, Z.; Feng, Z.; Hong, Y.; Hu, X.; Shu, Y. Wearable electrochemical sensor based on bimetallic MOF coated CNT/PDMS film electrode via a dual-stamping method for real-time sweat glucose analysis. Anal. Chim. Acta 2023, 1278, 341754. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Mi, Z.; Xia, Y.; Jin, D.; Xu, Q.; Hu, X.; Shu, Y. A wearable sweat electrochemical aptasensor based on the Ni–Co MOF nanosheet-decorated CNTs/PU film for monitoring of stress biomarker. Talanta 2023, 260, 124620. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Chen, T.; Hu, Y.; Son, K. Ultra-thin 2D bimetallic MOF nanosheets for highly sensitive and stable detection of glucose in sweat for dancer. Discov. Nano 2023, 18, 62. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Liu, Y.; Zeng, X.; Jin, C.; Huo, D.; Hou, J.; Hou, C. A wearable flexible electrochemical biosensor with CuNi-MOF@rGO modification for simultaneous detection of uric acid and dopamine in sweat. Anal. Chim. Acta 2024, 1299, 342441. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Tsao, P.-K.; Rinawati, M.; Chen, K.-J.; Chen, K.-Y.; Chang, C.Y.; Yeh, M.-H. Designing ZIF-67 derived NiCo layered double hydroxides with 3D hierarchical structure for Enzyme-free electrochemical lactate monitoring in human sweat. Chem. Eng. J. 2022, 427, 131687. [Google Scholar] [CrossRef]
- Cunha-Silva, H.; Arcos-Martinez, M.J. Dual range lactate oxidase-based screen printed amperometric biosensor for analysis of lactate in diversified samples. Talanta 2018, 188, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, T.; Asif, M.; Yu, Y.; Wang, W.; Wang, H.; Xiao, F.; Liu, H. Rimelike structure-inspired approach toward in situ-oriented self-assembly of hierarchical porous MOF films as a sweat biosensor. ACS Appl. Mater. Interfaces 2018, 10, 27936–27946. [Google Scholar] [CrossRef]
- Paul, A.; Vyas, G.; Paul, P.; Srivastava, D.N. Gold-nanoparticle-encapsulated ZIF-8 for a mediator-free enzymatic glucose sensor by amperometry. ACS Appl. Nano Mater. 2018, 1, 3600–3607. [Google Scholar] [CrossRef]
- Ganash, A.; Othman, S.; Al-Moubaraki, A.; Ganash, E. An electrodeposition of Cu-MOF on platinum electrode for efficient electrochemical degradation of tartrazine dye with parameter control and degradation mechanisms: Experimental and theoretical findings. Appl. Surf. Sci. Adv. 2024, 19, 100577. [Google Scholar] [CrossRef]
- Wang, H.; Dai, Y.; Wang, Y.; Yin, L. One-pot solvothermal synthesis of Cu–Fe-MOF for efficiently activating peroxymonosulfate to degrade organic pollutants in water: Effect of electron shuttle. Chemosphere 2024, 352, 141333. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, K.; Fan, L.; Song, Z.; Han, H.; Wang, Q.; Song, S.; Liu, D.; Feng, S. Modification of carnation-like CuInS2 with Cu-MOF nanoparticles for efficient photocatalytic hydrogen production. Int. J. Hydrog. Energy 2024, 59, 551–560. [Google Scholar] [CrossRef]
- Gao, Z.; Guan, J.; Wang, M.; Liu, S.; Chen, K.; Liu, Q.; Chen, X. A novel laccase-like Cu-MOF for colorimetric differentiation and detection of phenolic compounds. Talanta 2024, 272, 125840. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wu, Y.; Xu, Z.; Wang, Y. In situ anchoring Cu nanoclusters on Cu-MOF: A new strategy for a combination of catalysis and fluorescence toward the detection of H2O2 and 2, 4-DNP. Chem. Eng. J. 2024, 479, 147508. [Google Scholar] [CrossRef]
- Liu, L.; Ma, T.; Xu, L.; Wang, B.; Zhang, L.; Fu, Y.; Yang, H.; Ji, W. Dense pyridine nitrogen as surface decoration of interwoven Cu-MOFs for chloramphenicol-specific electrochemical sensor. Microchem. J. 2024, 200, 110318. [Google Scholar] [CrossRef]
- Kiran, S.; Yasmeen, G.; Shafiq, Z.; Abbas, A.; Manzoor, S.; Hussain, D.; Pashameah, R.A.; Alzahrani, E.; Alanazi, A.K.; Ashiq, M.N. Nickel-based nitrodopamine MOF and its derived composites functionalized with multi-walled carbon nanotubes for efficient OER applications. Fuel 2023, 331, 125881. [Google Scholar] [CrossRef]
- Shin, S.; Shin, M.W. Nickel metal–organic framework (Ni-MOF) derived NiO/C@CNF composite for the application of high performance self-standing supercapacitor electrode. Appl. Surf. Sci. 2021, 540, 148295. [Google Scholar] [CrossRef]
- Khan, J.; Shakeel, N.; Alam, S.; Iqbal, M.Z.; Ahmad, Z.; Yusuf, K. Exploring the potency of EDTA-based Ni-Co-MOF nanospheres for highly durable battery-supercapacitor hybrids. Electrochim. Acta 2024, 486, 143970. [Google Scholar] [CrossRef]
- Dong, P.; Pan, J.; Zhang, L.; Yang, X.-L.; Xie, M.-H.; Zhang, J. Regulation of electron delocalization between flower-like (Co, Ni)-MOF array and WO3/W photoanode for effective photoelectrochemical water splitting. Appl. Catal. B Environ. Energy 2024, 350, 123925. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Geng, Q.; Gao, X. Co/Ni-MOF as a bifunctional electrode material for electrochemical sensor and oxygen evolution reaction. Mater. Lett. 2024, 355, 135538. [Google Scholar] [CrossRef]
- Iravani, S.; Zare, E.N.; Zarrabi, A.; Khosravi, A.; Makvandi, P. MXene/zeolitic imidazolate framework (ZIF) composites: A perspective on their emerging applications. FlatChem 2024, 44, 100631. [Google Scholar] [CrossRef]
- Li, Y.-R.; Dong, X.; Pan, S.-Y.; Luo, L.; Lei, H.-T.; Xu, Z.-L. Design to enhance sensing performance of ZIF-8 crystals. Prog. Nat. Sci. Mater. Int. 2024, 34, 240–250. [Google Scholar] [CrossRef]
- Nazir, M.A.; Shaik, M.R.; Ullah, S.; Hossain, I.; Najam, T.; Ullah, S.; Muhammad, N.; Shah, S.S.A.; ur Rehman, A. Synthesis of bimetallic Mn@ZIF–8 nanostructure for the adsorption removal of methyl orange dye from water. Inorg. Chem. Commun. 2024, 165, 112294. [Google Scholar] [CrossRef]
- Hu, L.; Xu, W.; Jiang, Q.; Ji, R.; Yan, Z.; Wu, G. Recent progress on CO2 cycloaddition with epoxide catalyzed by ZIFs and ZIFs-based materials. J. CO2 Util. 2024, 81, 102726. [Google Scholar] [CrossRef]
- Kumar, A.; Arya, K.; Mehra, S.; Kumar, A.; Mehta, S.K.; Kataria, R. Luminescent Zn-MOF@COF hybrid for selective decontamination of Cu (II) ions and methylene blue dye in aqueous media. Sep. Purif. Technol. 2024, 340, 126756. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, M.; Shu, Y.; Han, Q.; Chen, B.; Liu, B.; Wang, Z.; Tang, C.Y. Precisely regulated in-plane pore sizes of Co-MOF nanosheet membranes for efficient dye recovery. Desalination 2023, 567, 116979. [Google Scholar] [CrossRef]
- Gurav, S.R.; Chodankar, G.R.; Sawant, S.A.; Shembade, U.V.; Moholkar, A.V.; Sonkawade, R.G. Exploring the potential of simultaneous nanoarchitectonics and utilization of Co-MOFs electrode as well as powder for aqueous supercapacitors. J. Energy Storage 2023, 73, 109254. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, L.; Xu, W. Biologically inspired Co-MOF as catalase mimics with an unexpected ROS production ability for the efficient removal of organic dyes. J. Environ. Chem. Eng. 2024, 12, 112358. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, L.; Hong, P.; He, X.; Gao, S.; Yu, Y.; Liao, B.; Pang, H. Densely stacked lamellate Co-MOF for boosting the recycling performance in ofloxacin degradation. J. Environ. Chem. Eng. 2023, 11, 111480. [Google Scholar] [CrossRef]
- Xu, F.; Wang, L.; Wu, M.; Ma, G. Vertical growth of leaf-like Co-metal organic framework on carbon fiber cloth as integrated electrode for sensitive detection of dopamine and uric acid. Sens. Actuators B Chem. 2023, 386, 133734. [Google Scholar] [CrossRef]
- Moallem, Q.A.; Beitollahi, H. Electrochemical sensor for simultaneous detection of dopamine and uric acid based on a carbon paste electrode modified with nanostructured Cu-based metal-organic frameworks. Microchem. J. 2022, 177, 107261. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Li, C.-H.; Guo, X.-M. Multimode determination of uric acid based on porphyrinic MOFs thin films by electrochemical and photoelectrochemical methods. Microchem. J. 2022, 175, 107198. [Google Scholar] [CrossRef]
- Chen, X.; Feng, X.-Z.; Zhan, T.; Xue, Y.-T.; Li, H.-X.; Han, G.-C.; Chen, Z.; Kraatz, H.-B. Construction of a portable enzyme-free electrochemical glucose detection system based on the synergistic interaction of Cu-MOF and PtNPs. Sens. Actuators B Chem. 2023, 395, 134498. [Google Scholar] [CrossRef]
- Tian, Y.; Xie, L.; Liu, X.; Geng, Y.; Wang, J.; Ma, M. In situ synthesis of self-supporting conductive CuCo-based bimetal organic framework for sensitive nonenzymatic glucose sensing in serum and beverage. Food Chem. 2024, 437, 137875. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Jin, S.; Wang, H.; Pang, J.; Peng, W.; Chen, L. Copper-based bimetallic metal–organic framework in-situ embedded of heterogeneous Cu nanoparticles for enhanced nonenzymatic glucose sensing. Mater. Today Chem. 2024, 35, 101884. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Tang, Z.; Liao, G.; Nie, L. In-situ co-precipitation of Bi-MOF derivatives for highly sensitive electrochemical glucose sensing. Microchem. J. 2024, 199, 109897. [Google Scholar] [CrossRef]
- Moradi, M.; Afkhami, A.; Madrakian, T.; Moazami, H.R. Electrosynthesis of CoMn layered-double-hydroxide as a precursor for Co-Mn-MOFs and subsequent electrochemical sulfurization for supercapacitor application. J. Energy Storage 2023, 71, 108177. [Google Scholar] [CrossRef]
- Feng, X.; Chen, C.; He, C.; Chai, S.; Yu, Y.; Cheng, J. Non-thermal plasma coupled with MOF-74 derived Mn-Co-Ni-O porous composite oxide for toluene efficient degradation. J. Hazard. Mater. 2020, 383, 121143. [Google Scholar] [CrossRef]
- Li, C.; Chai, Q.; Liu, X.; Song, L.; Peng, T.; Lin, C.; Zhang, Y.; Qiu, W.; Sun, S.; Zheng, X. Mn/Co bimetallic MOF-derived hexagonal multilayered oxide catalysts with rich interface defects for propane total oxidation: Characterization, catalytic performance and DFT calculation. Appl. Catal. A Gen. 2023, 668, 119449. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Gao, P.; Yin, W.; Yin, M.; Pu, H.; Sun, Q.; Liang, X.; Fa, H.-b. Bimetal-organic frameworks MnCo-MOF-74 derived Co/MnO@HC for the construction of a novel enzyme-free glucose sensor. Microchem. J. 2022, 175, 107097. [Google Scholar] [CrossRef]
- Ahmad, M.W.; Choudhury, A.; Dey, B.; Anand, S.; Al Saidi, A.K.A.; Lee, G.H.; Yang, D.-J. Three-dimensional core-shell niobium-metal organic framework@carbon nanofiber mat as a binder-free positive electrode for asymmetric supercapacitor. J. Energy Storage 2022, 55, 105484. [Google Scholar] [CrossRef]
- Nizamidin, P.; Yang, Q.; Du, X.; Guo, C. Optical gas sensing and kinetics of azobenzene modified niobium metal-organic framework film composite optical waveguides. Opt. Laser Technol. 2023, 167, 109708. [Google Scholar] [CrossRef]
- Dey, B.; Ahmad, M.W.; Sarkhel, G.; Lee, G.H.; Choudhury, A. Fabrication of niobium metal organic frameworks anchored carbon nanofiber hybrid film for simultaneous detection of xanthine, hypoxanthine and uric acid. Microchem. J. 2023, 186, 108295. [Google Scholar] [CrossRef]
- Manivel, P.; Suryanarayanan, V.; Nesakumar, N.; Velayutham, D.; Madasamy, K.; Kathiresan, M.; Kulandaisamy, A.J.; Rayappan, J.B.B. A novel electrochemical sensor based on a nickel-metal organic framework for efficient electrocatalytic oxidation and rapid detection of lactate. New J. Chem. 2018, 42, 11839–11846. [Google Scholar] [CrossRef]
- Koyappayil, A.; Yeon, S.-h.; Chavan, S.G.; Jin, L.; Go, A.; Lee, M.-H. Efficient and rapid synthesis of ultrathin nickel-metal organic framework nanosheets for the sensitive determination of glucose. Microchem. J. 2022, 179, 107462. [Google Scholar] [CrossRef]
- Xiang, M.; Wu, J.; Lu, T.; Lin, W.; Quan, M.; Ye, H.; Dong, S.; Yang, Z. Ni-MOFs grown on carbonized loofah sponge for electrochemical glucose detection: Effects of different carboxylic acid ligands and reaction temperatures on electrochemical performance. J. Taiwan Inst. Chem. Eng. 2024, 155, 105269. [Google Scholar] [CrossRef]
- Li, G.; Chen, D.; Chen, Y.; Dong, L. MOF Ni-BTC derived Ni/C/graphene composite for highly sensitive non-enzymatic electrochemical glucose detection. ECS J. Solid State Sci. Technol. 2021, 9, 121014. [Google Scholar] [CrossRef]
- Li, F.; Liu, L.; Liu, T.; Zhang, M. Ni-MOF nanocomposites decorated by au nanoparticles: An electrochemical sensor for detection of uric acid. Ionics 2022, 28, 4843–4851. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Liu, D.; Zhang, N. MOF-derived porous nanostructured Ni2P/C material with highly sensitive electrochemical sensor for uric acid. Inorg. Chem. Commun. 2021, 130, 108713. [Google Scholar] [CrossRef]
- Hao, Y.; Guo, H.; Yang, F.; Zhang, J.; Wu, N.; Wang, M.; Li, C.; Yang, W. Hydrothermal synthesis of MWCNT/Ni-Mn-S composite derived from bimetallic MOF for high-performance electrochemical energy storage. J. Alloys Compd. 2022, 911, 164726. [Google Scholar] [CrossRef]
- Meng, T.-L.; Wang, J.-W.; Ma, Y.-X.; Chen, X.-Q.; Lei, L.; Li, J.; Ran, F. Fabrication of a novel nanohybrid via the introduction of Ni/Mn-LDH into Ni-MOFs/MWCNTs for high-performance electrochemical supercapacitor. Diam. Relat. Mater. 2024, 143, 110901. [Google Scholar] [CrossRef]
- Lin, S.; Zhang, T. Electrochemical in-situ generation of Ni-Mn MOF nanomaterials as anode materials for lithium-ion batteries. J. Alloys Compd. 2023, 942, 168926. [Google Scholar] [CrossRef]
- Wei, Y.; Hui, Y.; Lu, X.; Liu, C.; Zhang, Y.; Fan, Y.; Chen, W. One-pot preparation of NiMn layered double hydroxide-MOF material for highly sensitive electrochemical sensing of glucose. J. Electroanal. Chem. 2023, 933, 117276. [Google Scholar] [CrossRef]
- Liu, L.; Liu, L.; Wang, Y.; Ye, B.-C. A novel electrochemical sensor based on bimetallic metal–organic framework-derived porous carbon for detection of uric acid. Talanta 2019, 199, 478–484. [Google Scholar] [CrossRef]
- Shanmugam, R.; Aniruthan, S.; Yamunadevi, V.; Nellaiappan, S.; Amali, A.J.; Suresh, D. Co-N/Zn@NPC derived from bimetallic zeolitic imidazolate frameworks: A dual mode simultaneous electrochemical sensor for uric acid and ascorbic acid. Surf. Interfaces 2023, 40, 103103. [Google Scholar] [CrossRef]
- Chen, W.; Wang, S.; Yang, M. Encapsulation of Pt nanoparticles in ZIF-8 derived porous carbon for uric acid sensing. Mater. Lett. 2023, 331, 133408. [Google Scholar] [CrossRef]
- Zhao, Z.; Kong, Y.; Liu, C.; Huang, G.; Xiao, Z.; Zhu, H.; Bao, Z.; Mei, Y. Atomic layer deposition-assisted fabrication of 3D Co-doped carbon framework for sensitive enzyme-free lactic acid sensor. Chem. Eng. J. 2021, 417, 129285. [Google Scholar] [CrossRef]
- Song, D.; Wang, L.; Qu, Y.; Wang, B.; Li, Y.; Miao, X.; Yang, Y.; Duan, C. A high-performance three-dimensional hierarchical structure MOF-derived NiCo LDH nanosheets for non-enzymatic glucose detection. J. Electrochem. Soc. 2019, 166, B1681. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, L.; Zhang, Y.; Guo, R.; Hu, T. A heterometallic sensor based on Ce@Zn-MOF for electrochemical recognition of uric acid. Microporous Mesoporous Mater. 2021, 322, 111126. [Google Scholar] [CrossRef]
- Liu, T.-H.; Li, Q.; Yin, H.-Y.; Cai, X.-B.; Wang, Z.-G.; Wu, Z.-Q.; Li, D.; Fan, Z.-L.; Zhu, W. Tuning band structures at metal sulfides/Zr-MOF heterojunctions: Modulate optical-electronic properties to boost photocatalytic detoxification of Cr (VI) and degradation of reactive dyes. J. Mol. Struct. 2024, 1298, 137081. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, Z.; Deng, Y.; Deng, W.; Xiao, H.; Wu, W. Fluorescent bacterial cellulose@Zr-MOF via in-situ synthesis for efficient enrichment and sensitive detection of Cr (VI). Int. J. Biol. Macromol. 2024, 262, 129854. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Qian, X.; Jin, Y.; Miao, X.; Deng, A.; Li, J. Ultrasensitive detection of zearalenone based on electrochemiluminescent immunoassay with Zr-MOF nanoplates and Au@MoS2 nanoflowers. Anal. Chim. Acta 2024, 1299, 342451. [Google Scholar] [CrossRef] [PubMed]
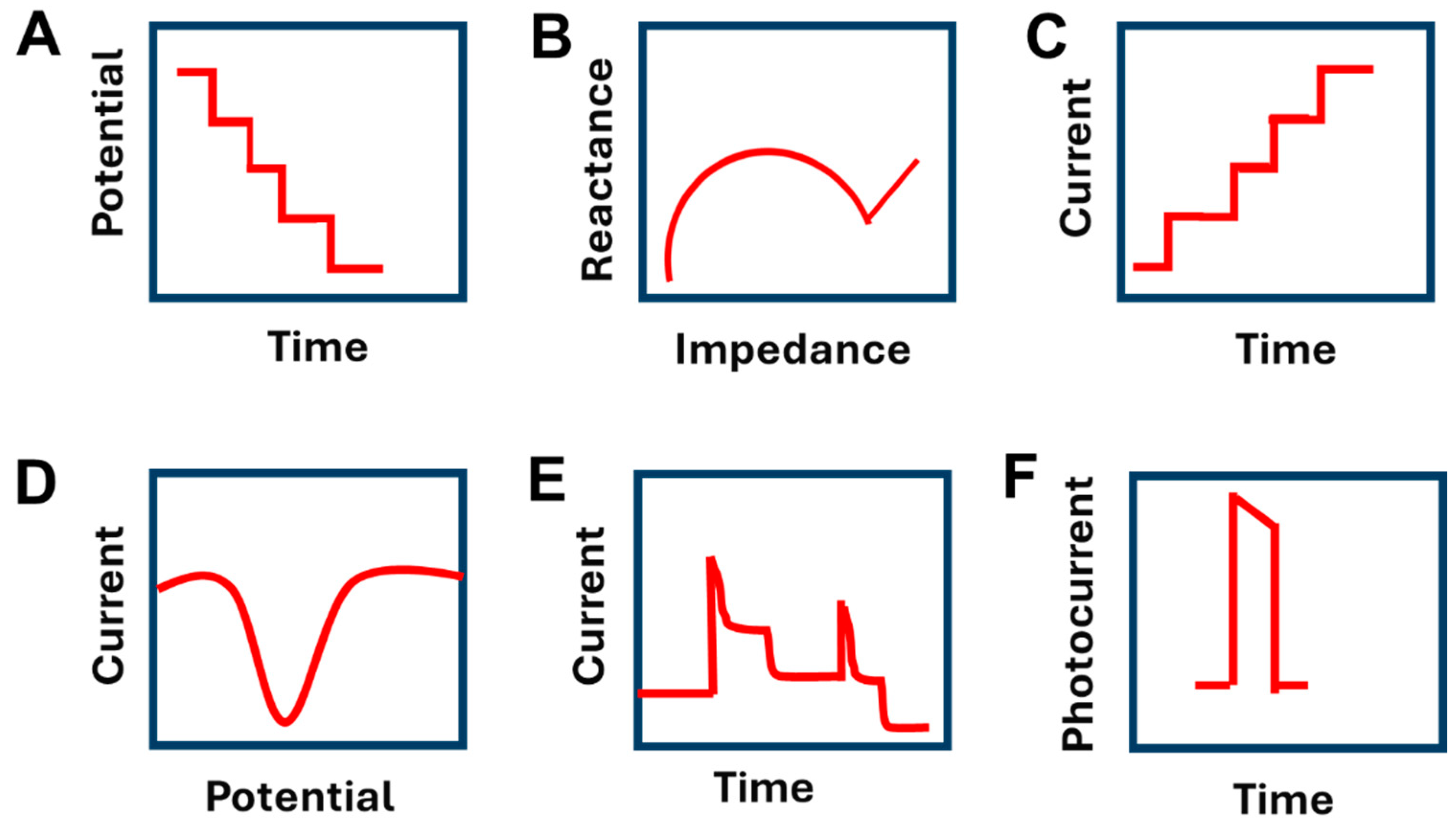


| Type of Electrochemical Sensor | Advantages | Disadvantages | Comments | Ref |
|---|---|---|---|---|
| Amperometric | The fixed potential during amperometric detection results in a negligible charging current. | It leads to the consumption of the analyte that may result in hindering the measurements at low concentrations. | [76,84] | |
| Impedimetric | It is a simple, sensitive, and nondestructive method. The sensor can be miniaturized into devices. | The nonspecific interaction of biomolecules affects the sensor response. | [87] | |
| Organic electrochemical transistor | It is easy to be designed and does not need a high voltage for operation. | It has high hardness and a large mass. | The fiber-based organic electrochemical transistors have been used to overcome the limitation of large mass. | [78] |
| Potentiometric | It has a fast response and an ability for miniaturization and integration. There is no consumption of the target analyte during the electroanalysis. | The signal of the potentiometric sensor depends on temperature and determines the free ions only. | [73,84,85,86] | |
| Photoelectrochemical | It is known for its simple instrumentation, low cost, and miniaturization, low background, and high sensitivity. | It has poor stability for bioanalysis. | This stability limitation can be overcome by using an enzyme-free system | [88,89,90] |
| Voltametric | It shows superior sensitivity, robustness, and selectivity | It leads to the consumption of the analyte that may result in hindering the measurements at low concentrations | [5,84] |
| Electrocatalyst | Example | Sweat Biomarker | Catalytic Mechanism | Comments | Ref |
|---|---|---|---|---|---|
| Conductive polymers | PEDOT | Lactate | Lactate was oxidized by lactate oxidase to form pyruvate and hydrogen peroxide. Then, hydrogen peroxide was decomposed into hydrogen ions, which were oxidized at the PEDOT interface producing a current response. | Conductive polymers are flexible and conductive with acceptable mechanical properties. | [57] |
| MIPs | Lactate | They are specific and inexpensive with high reproducibility. | [92] | ||
| Graphene-based materials | Graphene | K+ | The electrocatalytic activity of graphene is attributed to its superior conductivity and large surface area. | [93] | |
| Graphene oxide | Glucose -Lactate | The high number of oxidized moieties are considered active sites for the interaction with the target analytes. | [94] | ||
| Reduced -graphene oxide | pH—K+ | [95] | |||
| Dyes | Prussian Blue | H2O2 | The reduced form of Prussian blue, Prussian white, catalyzed the reduction of hydrogen peroxide at low potential. | [96] | |
| Metal complexes | Cu(II)-complex | Glucose | 1-Reduction of Cu(II) in the complex to metallic Cu by applying a negative potential 2-Oxidation of glucose by the electrogenerated Cu(II) during DPV scan | [77] |
| MOF | NP/NC | Electrochemical Behavior of MOF NC | Comments | Ref |
|---|---|---|---|---|
| Cu-MOF | Pt | Cu-MOF enhanced the signal’s sensitivity and stability, while Pt allowed higher sensitivity for detecting sweat biomarker due to its high electrocatalytic activity. | The chitosan layer was added as a biocompatible cationic polymer for enzyme immobilization. | [129,130] |
| Cu-MOF | Cu-MOF acted as an electrocatalyst by increasing the electrochemically active surface area and enhancing the electron transfer rate. | [56,75,130] | ||
| Ni-MOF | Ni3(HITP)2 | Ni-MOF NPs showed high electrocatalytic activity resulting from the effects of highly active Ni-N4 catalytic sites, the two-dimensional superimposed honeycomb lattice of Ni-MOF, and the increased surface area. | [111] | |
| Porous carbon, polypyrrole, Ni-MOF | Ni-MOF NC acted as an electroactive material and adsorbent for the sweat biomarker. | Ni-MOF was incorporated with porous carbon cloth decorated with nitrogen. | [112] | |
| NiCO-MOF | NiCo-MOF, CNTs | NiCo-MOF efficiently captured the aptamer of sweat cortisol owing to its high specific surface area. | [125] | |
| CNTs, MWCNT | NiCo-MOF exhibited high electrocatalytic activity and optimized sensitivity for sweat biomarkers. | NiCo-MOF NC showed high stability under stretching and bending conditions. | [124] | |
| ZIF-8 | Au NPs | The thermally and chemically stable ZIF-8 was used to encapsulate the enzyme and NPs. The presence of conductive Au NPs boosted the activity of the enzyme and improved electron transfer. | [131] | |
| ZIF-67 | ZIF-67 derived NiCo LDH | ZIF-67 acted as an electrocatalyst for enhancing the oxidation of sweat biomarker. | [128] | |
| ZIF-67, Ag NPs | ZIF-67 provided good dispersity as well as a protective effect for Ag NPs. | [41] | ||
| Zn-MOF | Zn-TCPP- MOF, MWCNT | MOF NC generated electron-hole pairs under the stimulation of a light source. | [62] |
| MOF | NPs | Electrode Material | Sweat Biomarker | Electrochemical Technique | LOD | Ref |
|---|---|---|---|---|---|---|
| Cu-MOF | Pt | Lactate oxidase/Cu-MOF/chitosan/Pt NPs/SPE | Lactate | Amperometry | 0.75 μM | [129] |
| Cu-MOF | ACF-rGO/Cu(INA)2 | Lactate | Amperometry | 500 nM | [130] | |
| Glucose | 50 nM | |||||
| Cu-MOF | Cu-CAT nanowires/CP | Lactate | Amperometry | 10 μM | [56] | |
| Glucose | 2 μM | |||||
| Cu-MOF | Cu-BDC/GCE | Ascorbic acid | Amperometry | 0.1 μM | [75] | |
| Ni-MOF (Ni3(HITP)2) | Ni3(HITP)2 | Ni3(HITP)2 nanorods/SPCE | Ascorbic acid | Amperometry | 1 μM | [111] |
| Ni-MOF | Porous carbon, polypyrrole, Ni-MOF | AFCC-polypyrrole NPs/2D Ni-MOF | Ni (II) ions | Potentiometry | 2.7 × 10−6 M | [112] |
| NiCO-MOF | NiCo-MOF, CNTs | Cortisol/apt/NiCo-MOF/CNTs/PVA/CP | Cortisol | DPV | 0.032 ng/mL | [125] |
| CNTs, MWCNT | NiCo-MOF/CNTs/MWCNT/PDMS | Glucose | Amperometry | 6.78 μM | [124] | |
| ZIF-8 | Au NPs | Glucose oxidase/Au NPs/ZIF-8/GCE | Glucose | Amperometry | 50 nM | [131] |
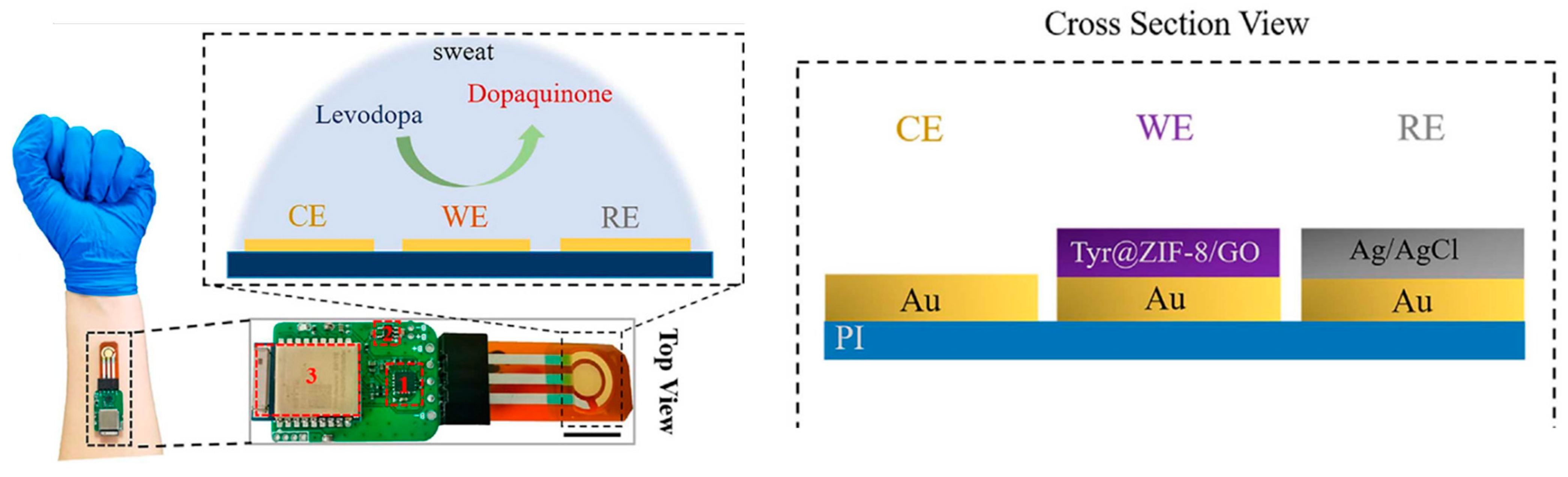
| ZIF-8 | Tyrosinase/ZIF-8 NPs/GO/Au SPE | Levodopa drug | Amperometry | 0.45 μM | [64] | |
| ZIF-67 | ZIF-67 derived NiCo LDH | ZIF-67 derived NiCo LDH nanocage/SPCE | Lactate | Amperometry | 0.399 mM | [128] |
| ZIF-67, Ag NPs | ZIF-67/Ag NPs/PDA/GCE | Cl- | DPV | 1 mM | [41] | |
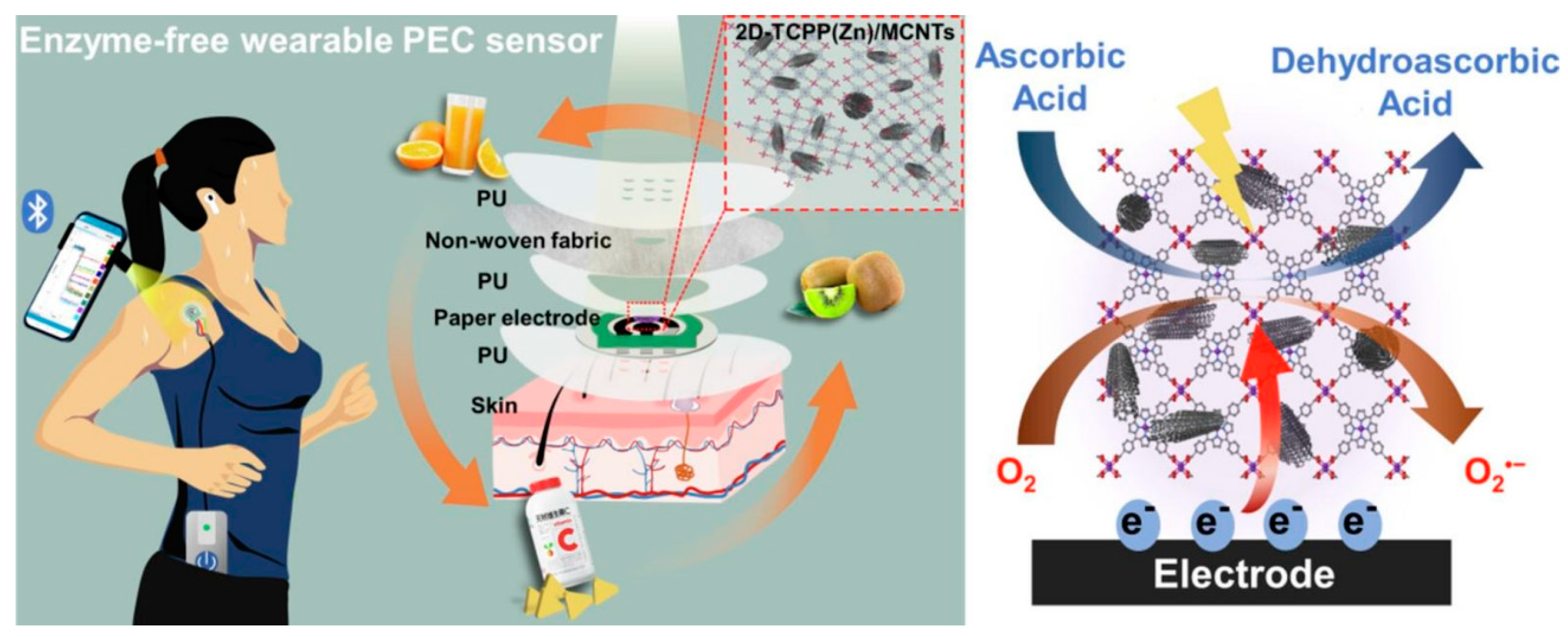
| Zn-TCPP-MOF | Zn-TCPP- MOF, MWCNT | Zn-TCPP- MOF nanosheets/MWCNT/SPPE | Ascorbic acid | PEC | 3.61 μM | [62] |
| MOF | NPs | Electrode Material | Analyte | Role of MOF | Electrochemical Technique | LOD | Ref |
|---|---|---|---|---|---|---|---|
| Co-MOF | Co-MOF | Co-MOF/CC | Uric acid | Electrocatalyst | DPV | 7 nM | [152] |
| Cu-MOF | Cu-BTC | Cu-BTC MOF/CPE | Uric acid | Electrocatalyst | DPV | 0.2 μM | [153] |
| Cu-MOF | Cu-TCPP | Cu-TCPP MOF/GCE | Uric acid | Electrocatalyst | CV | 0.03 μM | [154] |
| DPV | 1.37 μM | ||||||
| Amperometry | 0.3 μM | ||||||
| Photoelectrochemical | 0.01 μM | ||||||
| Cu-MOF | Ni | Ni NPs/Cu-MOF-C/GCE | Glucose | Electrocatalyst | Amperometry | 0.090 μM | [55] |
| Cu-MOF | Pt | Pt NPs/Cu-MOF/Au electrode | Glucose | Electrocatalyst | DPV | 0.06 mM | [155] |
| CuCO-MOF | CuCO-MOF | CuCO MOF/CC | Glucose | Electrocatalyst | Amperometry | 0.27 μM | [156] |
| CuCo-MOF | CuO | CuCo-BTC derivative/CC | Glucose | Electrocatalyst | Amperometry | 0.09 μM | [158] |
| CuCo-MOF | Cu | Cu/CuCo MOF | Glucose | Electrocatalyst | Amperometry | 0.27 μM | [157] |
| MnCo-MOF-74 | MnCo | Co/MnO/hierarchical carbon/GCE | Glucose | Electrocatalyst | Amperometry | 1.31 μM | [162] |
| Nb-MOF | Nb(BTC) MOF, CNF | Nb(BTC) MOF/CNF/GCE | Uric acid | Electrocatalyst | DPV | 70 nM | [165] |
| Ni-MOF | Ni-MOF | Ni-MOF/Pt electrode | Lactate | Electrocatalyst | Amperometry | 5 μM | [166] |
| Ni-MOF | Ni-MOF | Ni-MOF nanosheet/GCE | Glucose | Electrocatalyst | Amperometry | 0.6 μM | [167] |
| Ni-MOF | Ni-MOF | CLS/Ni-MOF/GCE | Glucose | Electrocatalyst | Amperometry | 0.4 μM | [168] |
| Ni-MOF | Ni | Ni/C/graphene | Glucose | Electrocatalyst | Amperometry | 0.6 μM | [169] |
| Ni-MOF | Au | Au/Ni-MOF/GCE | Uric acid | Electrocatalyst | DPV | 5.6 μM | [170] |
| Ni-MOF | CNTs | CNTs/Ni-MOF/GCE | Glucose | Electrocatalyst | Amperometry | 0.82 μM | [20] |
| Ni-MOF-74 | Ni2P/C | Ni2P/C/GCE | Uric acid | Electrocatalyst | DPV | 70 nM | [171] |
| Ni-MOF | Ni-P | Ni-P/Ni foam | Glucose | Electrocatalyst | Amperometry | 0.15 μM | [38] |
| Ni-CO MOF | SnS2, Ni-CO MOF, Au | BSA/anti-cortisol (C-Mab)/Au NPs/SnS2/Ni-CO MOF | Cortisol | Providing active sites for the deposition of Au NPs | SWV | 29 fg/mL | [50] |
| Ni-Mn MOF | Ni-Mn MOF | Ni-Mn LDH MOF/GCE | Glucose | Electrocatalyst | Amperometry | 0.87 μM | [175] |
| ZIF | Zn, Co | Co-N/Zn nanoporous carbon/GCE | Ascorbic acid | Electrocatalyst | DPV | 7.65 nM | [177] |
| Uric acid | 0.21 nM | ||||||
| ZIF-8 | Pt | ZIF-8/Pt NPs/GCE | Uric acid | Distribution of Pt NPs in the porous carbon | DPV | 5 μM | [178] |
| ZIF-67 | Co, C, ZnO | Co-NCF/ZnO/GCE | Lactic acid | Electrocatalyst | Amperometry | 13.7 μM | [179] |
| ZIF-67 | ZIF-67 derived NiCo LDH, cobalt carbonate | ZIF-67 derived NiCo LDH/cobalt carbonate/CC | Glucose | Electrocatalyst | Amperometry | 110 nM | [180] |
| ZIF-8/ZIF-67 | ZIF-8/ZIF-67, Co | ZIF-8/ZIF-67/GCE | Uric acid | Electrocatalyst | DPV | 0.83 μM | [176] |
| Zn-MOF | Zn-MOF | Ce/Zn-MOF/GCE | Uric acid | Electrocatalyst | DPV | 0.51 ng/mL | [181] |
| Zr-MOF | ZrO2 | ZrO2 porous carbon/GCE | Uric acid | Electrocatalyst | DPV | 0.1 μM | [22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, J.; Zahran, M.; Li, X. Metal–Organic Framework-Based Nanostructures for Electrochemical Sensing of Sweat Biomarkers. Biosensors 2024, 14, 495. https://doi.org/10.3390/bios14100495
Meng J, Zahran M, Li X. Metal–Organic Framework-Based Nanostructures for Electrochemical Sensing of Sweat Biomarkers. Biosensors. 2024; 14(10):495. https://doi.org/10.3390/bios14100495
Chicago/Turabian StyleMeng, Jing, Moustafa Zahran, and Xiaolin Li. 2024. "Metal–Organic Framework-Based Nanostructures for Electrochemical Sensing of Sweat Biomarkers" Biosensors 14, no. 10: 495. https://doi.org/10.3390/bios14100495
APA StyleMeng, J., Zahran, M., & Li, X. (2024). Metal–Organic Framework-Based Nanostructures for Electrochemical Sensing of Sweat Biomarkers. Biosensors, 14(10), 495. https://doi.org/10.3390/bios14100495





