Electrochemical Analysis of Amyloid Plaques and ApoE4 with Chitosan-Coated Gold Nanostars for Alzheimer’s Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Materials, and Instrumentation
2.2. Synthesis of AuNS-CHI Nanostructures
2.3. Enhancement and Characterization of Working Electrodes
2.4. Experimental Protocol
3. Results and Discussion
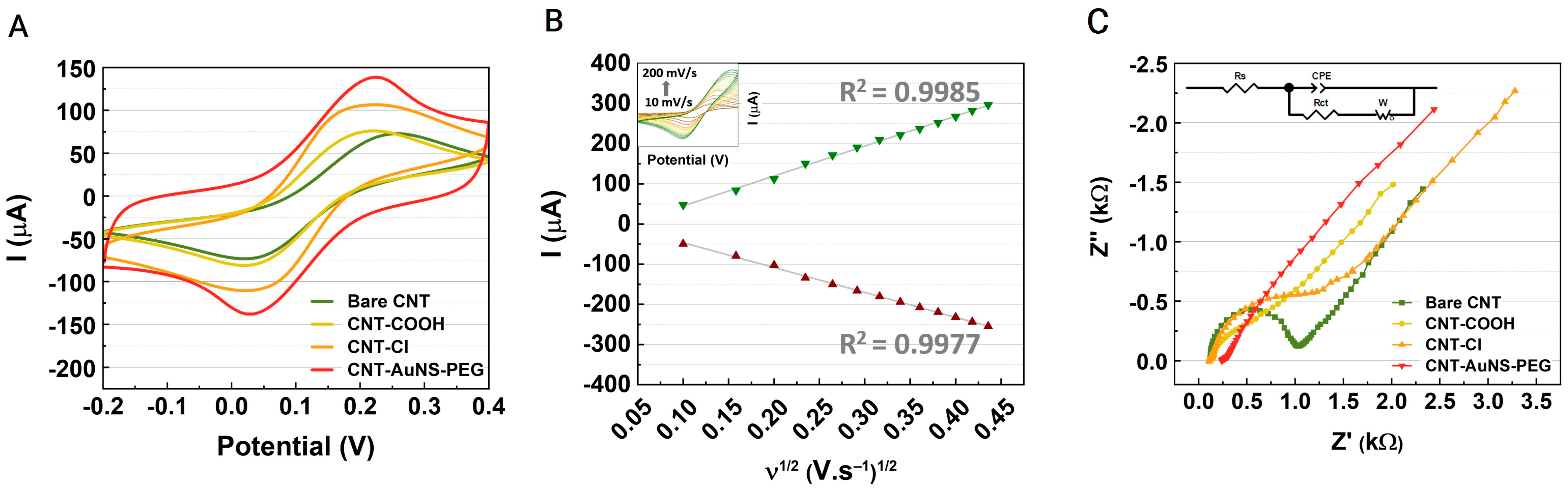
3.1. Characterization and Analysis of CNT-AuNS-PEG Devices
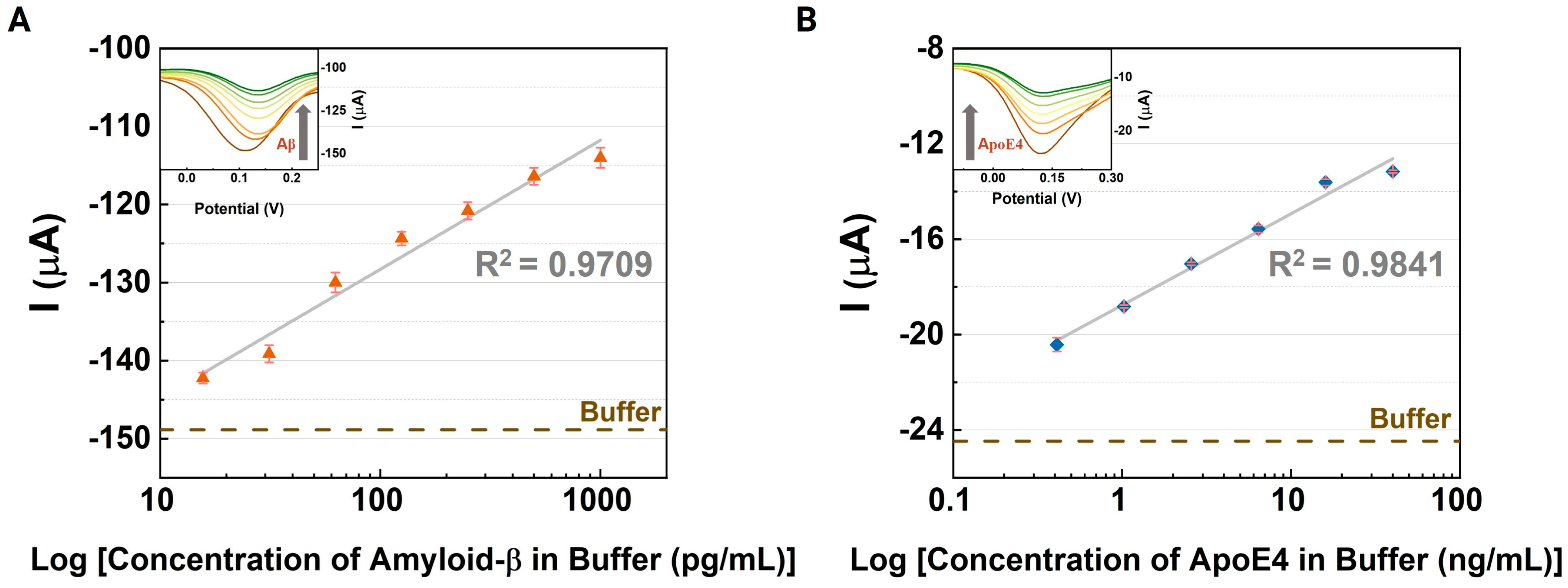
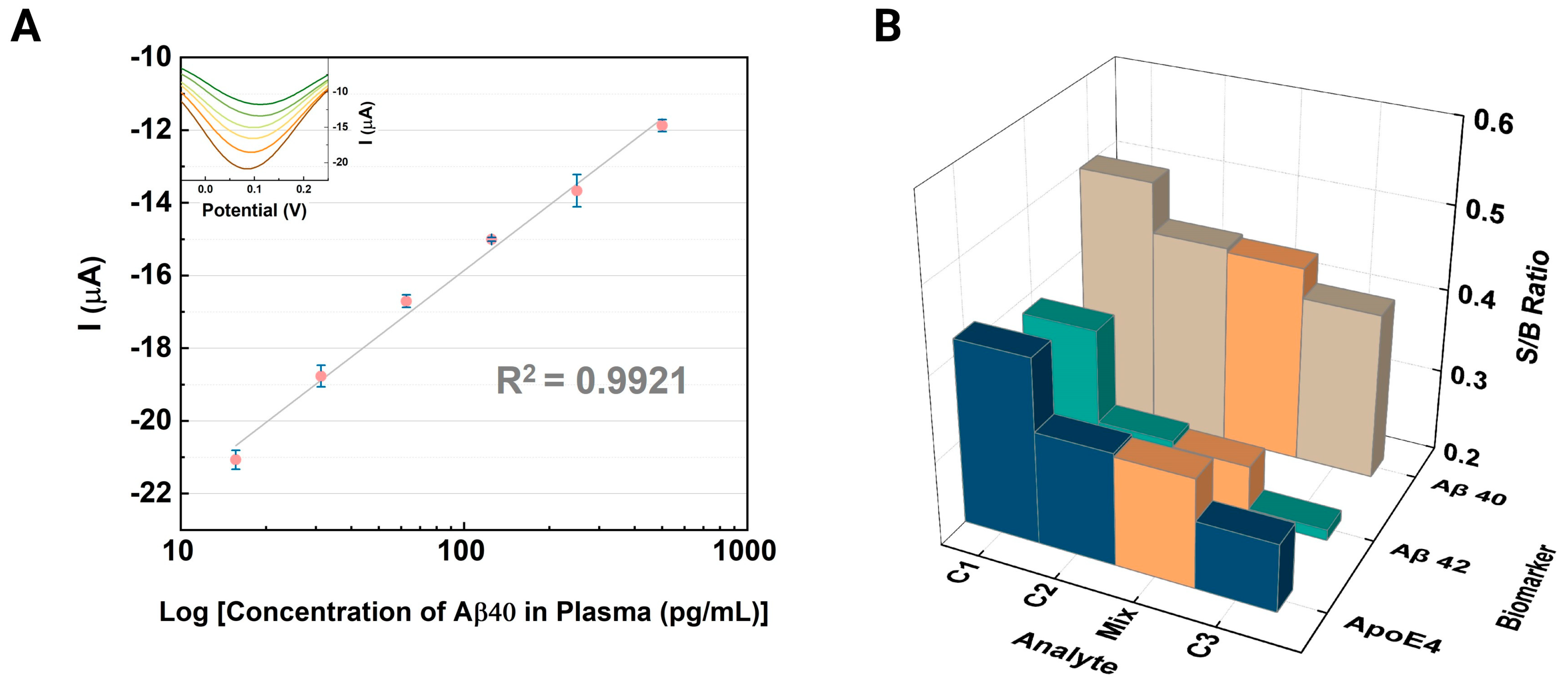
3.2. Electrochemical Immunoassays for Alzheimer’s Disease Biomarkers
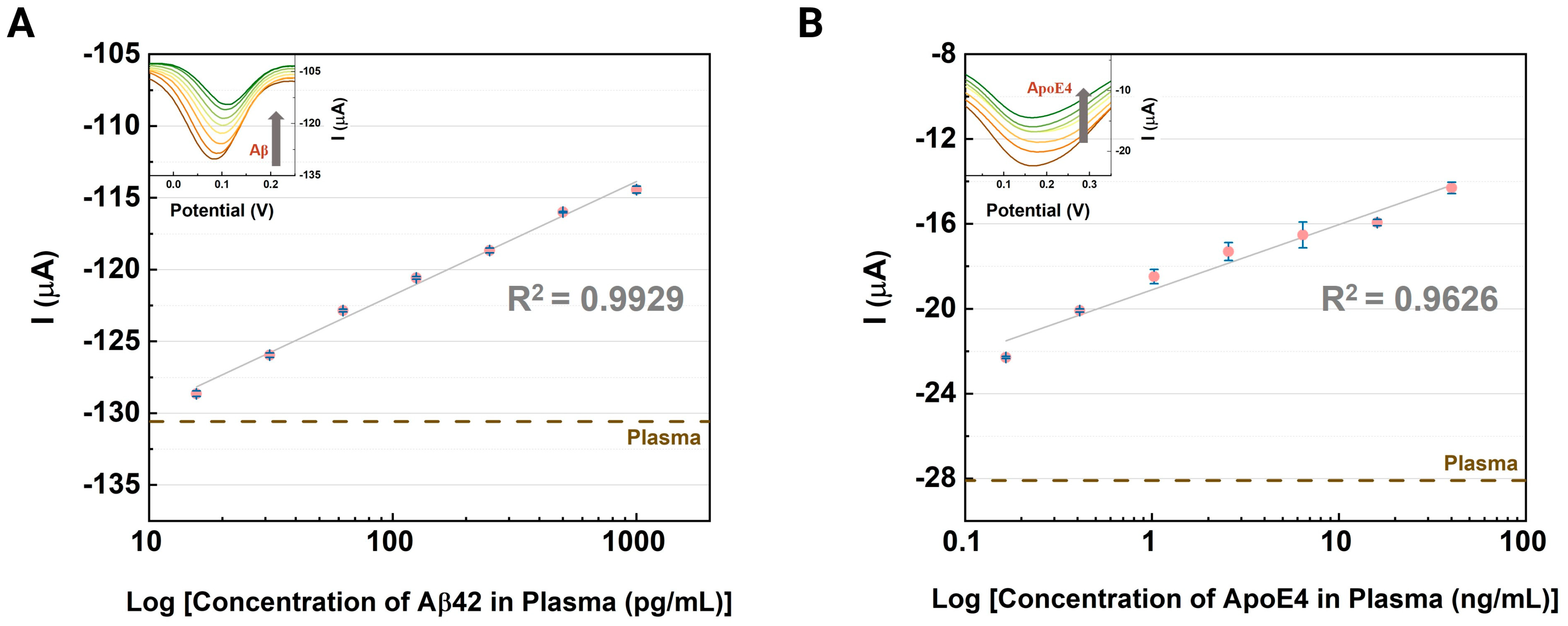
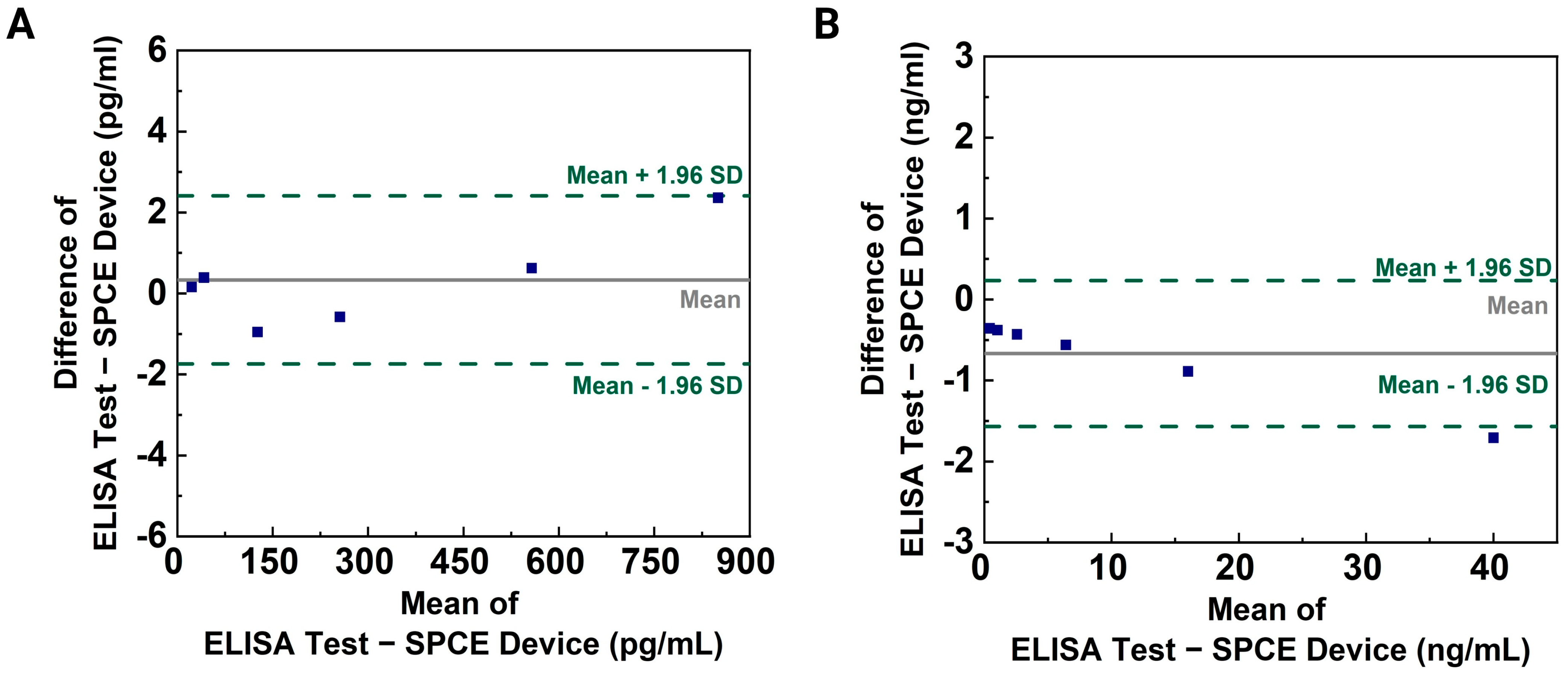
3.3. Evaluation of the CNT-AuNS-PEG Device Using Human Samples
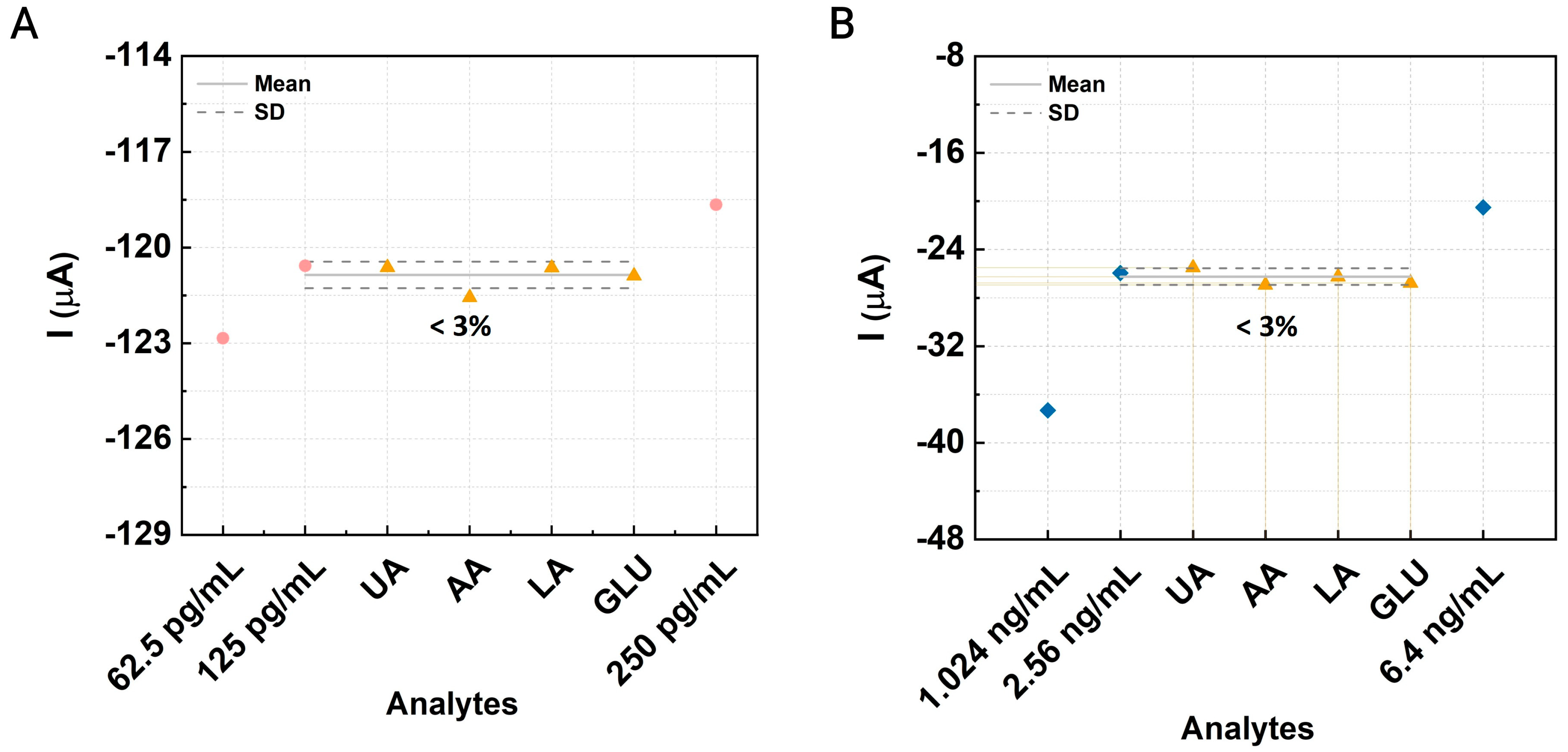
3.4. Interference and Cross-Reactivity Assessment
3.5. Validation and Recovery Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Schindler, S.E.; Bollinger, J.G.; Ovod, V.; Mawuenyega, K.G.; Weiner, M.W.; Shaw, L.M.; Masters, C.L.; Fowler, C.J.; Trojanowski, J.Q.; et al. Validation of Plasma Amyloid-β 42/40 for Detecting Alzheimer Disease Amyloid Plaques. Neurology 2022, 98, e688–e699. [Google Scholar] [CrossRef]
- Serra-Añó, P.; Pedrero-Sánchez, J.F.; Hurtado-Abellán, J.; Inglés, M.; Espí-López, G.V.; López-Pascual, J. Mobility assessment in people with Alzheimer disease using smartphone sensors. J. Neuroeng. Rehabil. 2019, 16, 103. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Song, X.; Wang, H.; Wang, J.; Wang, Y.; Huang, J.; Yu, J. Robust and Universal SERS Sensing Platform for Multiplexed Detection of Alzheimer’s Disease Core Biomarkers Using PAapt-AuNPs Conjugates. ACS Sens. 2019, 4, 2140–2149. [Google Scholar] [CrossRef]
- Medina-Sánchez, M.; Miserere, S.; Morales-Narváez, E.; Merkoçi, A. On-chip magneto-immunoassay for Alzheimer’s biomarker electrochemical detection by using quantum dots as labels. Biosens. Bioelectron. 2014, 54, 279–284. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; Liu, L.; Li, C.; Chang, Z.; Zhu, X.; Ye, B.; Xu, M. Fabrication of an antibody-aptamer sandwich assay for electrochemical evaluation of levels of β-amyloid oligomers. Sci. Rep. 2016, 6, 35186. [Google Scholar] [CrossRef]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef]
- Kim, J.; Basak, J.M.; Holtzman, D.M. The Role of Apolipoprotein E in Alzheimer’s Disease. Neuron 2009, 63, 287–303. [Google Scholar] [CrossRef]
- Schmidt, M.; Sachse, C.; Richter, W.; Xu, C.; Fändrich, M.; Grigorieff, N. Comparison of Alzheimer Aβ(1–40) and Aβ(1–42) amyloid fibrils reveals similar protofilament structures. Proc. Natl. Acad. Sci. USA 2009, 106, 19813–19818. [Google Scholar] [CrossRef]
- Citron, M.; Diehl, T.S.; Gordon, G.; Biere, A.L.; Seubert, P.; Selkoe, D.J. Evidence that the 42- and 40-amino acid forms of amyloid β protein are generated from the β-amyloid precursor protein by different protease activities. Proc. Natl. Acad. Sci. USA 1996, 93, 13170–13175. [Google Scholar] [CrossRef]
- Cockerill, I.; Oliver, J.-A.; Xu, H.; Fu, B.M.; Zhu, D. Blood-Brain Barrier Integrity and Clearance of Amyloid-β from the BBB. Adv. Exp. Med. Biol. 2018, 1097, 261–278. [Google Scholar]
- Husain, M.A.; Laurent, B.; Plourde, M. APOE and Alzheimer’s Disease: From Lipid Transport to Physiopathology and Therapeutics. Front. Neurosci. 2021, 15, 630502. [Google Scholar] [CrossRef]
- Verheggen, I.C.M.; Van Boxtel, M.P.J.; Verhey, F.R.J.; Jansen, J.F.A.; Backes, W.H. Interaction between blood-brain barrier and glymphatic system in solute clearance. Neurosci. Biobehav. Rev. 2018, 90, 26–33. [Google Scholar] [CrossRef]
- Blennow, K.; Zetterberg, H. Biomarkers for Alzheimer’s disease: Current status and prospects for the future. J. Intern. Med. 2018, 284, 643–663. [Google Scholar] [CrossRef]
- Mahley, R.W.; Weisgraber, K.H.; Huang, Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5644–5651. [Google Scholar] [CrossRef]
- Raulin, A.-C.; Doss, S.V.; Trottier, Z.A.; Ikezu, T.C.; Bu, G.; Liu, C.-C. ApoE in Alzheimer’s disease: Pathophysiology and therapeutic strategies. Mol. Neurodegener. 2022, 17, 72. [Google Scholar] [CrossRef]
- Taddei, K.; Clarnette, R.; Gandy, S.E.; Martins, R.N. Increased plasma apolipoprotein E (apoE) levels in Alzheimer’s disease. Neurosci. Lett. 1997, 223, 29–32. [Google Scholar] [CrossRef]
- Toyos-Rodríguez, C.; García-Alonso, F.J.; de la Escosura-Muñiz, A. Electrochemical biosensors based on nanomaterials for early detection of alzheimer’s disease. Sensors 2020, 20, 4748. [Google Scholar] [CrossRef]
- Wang, C.; Yu, J.T.; Wang, H.F.; Jiang, T.; Tan, C.C.; Meng, X.F.; Soares, H.D.; Tan, L. Meta-analysis of peripheral blood apolipoprotein E levels in alzheimer’s disease. PLoS ONE 2014, 9, e89041. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, L.-P.; Wang, S.; Yang, W.; Wen, Y.; Zhang, X. An ultrasensitive electrochemical immunosensor for apolipoprotein E4 based on fractal nanostructures and enzyme amplification. Biosens. Bioelectron. 2015, 71, 396–400. [Google Scholar] [CrossRef]
- Budde, B.; Schartner, J.; Tönges, L.; Kötting, C.; Nabers, A.; Gerwert, K. Reversible immuno-infrared sensor for the detection of Alzheimer’s disease related biomarkers. ACS Sens. 2019, 4, 1851–1856. [Google Scholar] [CrossRef]
- Kim, K.; Kim, M.J.; Kim, D.W.; Kim, S.Y.; Park, S.; Park, C.B. Clinically accurate diagnosis of Alzheimer’s disease via multiplexed sensing of core biomarkers in human plasma. Nat. Commun. 2020, 11, 119. [Google Scholar] [CrossRef]
- Park, Y.M.; Ahn, J.; Choi, Y.S.; Jeong, J.-M.; Lee, S.J.; Lee, J.J.; Choi, B.G.; Lee, K.G. Flexible nanopillar-based immunoelectrochemical biosensor for noninvasive detection of Amyloid beta. Nano Converg. 2020, 7, 29. [Google Scholar] [CrossRef]
- Park, D.; Kim, J.H.; Kim, H.J.; Lee, D.; Lee, D.S.; Yoon, D.S.; Hwang, K.S. Multiplexed femtomolar detection of Alzheimer’s disease biomarkers in biofluids using a reduced graphene oxide field-effect transistor. Biosens. Bioelectron. 2020, 167, 112505. [Google Scholar] [CrossRef]
- Liu, Y.; He, G.; Liu, H.; Yin, H.; Gao, F.; Chen, J.; Zhang, S.; Yang, B. Electrochemical immunosensor based on AuBP@Pt nanostructure and AuPd-PDA nanozyme for ultrasensitive detection of APOE4. RSC Adv. 2020, 10, 7912–7917. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef]
- Yoo, Y.K.; Kim, J.; Kim, G.; Kim, Y.S.; Kim, H.Y.; Lee, S.; Cho, W.W.; Kim, S.; Lee, S.-M.; Lee, B.C.; et al. A highly sensitive plasma-based amyloid-β detection system through medium-changing and noise cancellation system for early diagnosis of the Alzheimer’s disease. Sci. Rep. 2017, 7, 8882. [Google Scholar] [CrossRef]
- Razzino, C.A.; Serafín, V.; Gamella, M.; Pedrero, M.; Montero-Calle, A.; Barderas, R.; Calero, M.; Lobo, A.O.; Yáñez-Sedeño, P.; Campuzano, S.; et al. An electrochemical immunosensor using gold nanoparticles-PAMAM-nanostructured screen-printed carbon electrodes for tau protein determination in plasma and brain tissues from Alzheimer patients. Biosens. Bioelectron. 2020, 163, 112238. [Google Scholar] [CrossRef]
- Yoo, Y.K.; Kim, G.; Park, D.; Kim, J.; Kim, Y.; Yun Kim, H.; Yang, S.H.; Lee, J.H.; Hwang, K.S. Gold nanoparticles assisted sensitivity improvement of interdigitated microelectrodes biosensor for amyloid-β detection in plasma sample. Sens. Actuators B Chem. 2020, 308, 127710. [Google Scholar] [CrossRef]
- Risacher, S.L.; Fandos, N.; Romero, J.; Sherriff, I.; Pesini, P.; Saykin, A.J.; Apostolova, L.G. Plasma amyloid beta levels are associated with cerebral amyloid and tau deposition. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2019, 11, 510–519. [Google Scholar] [CrossRef]
- Kang, M.K.; Lee, J.; Nguyen, A.H.; Sim, S.J. Label-free detection of ApoE4-mediated β-amyloid aggregation on single nanoparticle uncovering Alzheimer’s disease. Biosens. Bioelectron. 2015, 72, 197–204. [Google Scholar] [CrossRef]
- Mars, A.; Hamami, M.; Bechnak, L.; Patra, D.; Raouafi, N. Curcumin-graphene quantum dots for dual mode sensing platform: Electrochemical and fluorescence detection of APOe4, responsible of Alzheimer’s disease. Anal. Chim. Acta 2018, 1036, 141–146. [Google Scholar] [CrossRef]
- Simon, R.; Girod, M.; Fonbonne, C.; Salvador, A.; Clément, Y.; Lantéri, P.; Amouyel, P.; Lambert, J.C.; Lemoine, J. Total ApoE and ApoE4 isoform assays in an Alzheimer’s disease case-control study by targeted mass spectrometry (n = 669): A pilot assay for methionine-containing proteotypic peptides. Mol. Cell. Proteom. 2012, 11, 1389–1403. [Google Scholar] [CrossRef]
- Phiri, M.M.; Mulder, D.W.; Vorster, B.C. Seedless gold nanostars with seed-like advantages for biosensing applications. R. Soc. Open Sci. 2019, 6, 181971. [Google Scholar] [CrossRef]
- Stiufiuc, R.; Iacovita, C.; Nicoara, R.; Stiufiuc, G.; Florea, A.; Achim, M.; Lucaciu, C.M. One-step synthesis of PEGylated gold nanoparticles with tunable surface charge. J. Nanomater. 2013, 2013, 146031. [Google Scholar] [CrossRef]
- Yu, S.; Xu, X.; Feng, J.; Liu, M.; Hu, K. Chitosan and chitosan coating nanoparticles for the treatment of brain disease. Int. J. Pharm. 2019, 560, 282–293. [Google Scholar] [CrossRef]
- Mohan, C.O.; Gunasekaran, S.; Ravishankar, C.N. Chitosan-capped gold nanoparticles for indicating temperature abuse in frozen stored products. NPJ Sci. Food 2019, 3, 2. [Google Scholar] [CrossRef]
- Dong, X.-X.; Yang, J.-Y.; Luo, L.; Zhang, Y.-F.; Mao, C.; Sun, Y.-M.; Lei, H.-T.; Shen, Y.-D.; Beier, R.C.; Xu, Z.-L. Portable amperometric immunosensor for histamine detection using Prussian blue-chitosan-gold nanoparticle nanocomposite films. Biosens. Bioelectron. 2017, 98, 305–309. [Google Scholar] [CrossRef]
- Fan, G.-C.; Ren, X.-L.; Zhu, C.; Zhang, J.-R.; Zhu, J.-J. A new signal amplification strategy of photoelectrochemical immunoassay for highly sensitive interleukin-6 detection based on TiO2/CdS/CdSe dual co-sensitized structure. Biosens. Bioelectron. 2014, 59, 45–53. [Google Scholar] [CrossRef]
- Schuck, A.; Kim, H.E.; Kang, M.; Kim, Y.-S. Gold nanostar-modified electrochemical sensor for highly sensitive renin quantification as a marker of tissue-perfusion. MRS Commun. 2023, 13, 1150–1155. [Google Scholar] [CrossRef]
- Abjameh, R.; Moradi, O.; Amani, J. The study of synthesis and functionalized single-walled carbon nanotubes with amide group. Int. Nano Lett. 2014, 4, 97. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Jha, A.; Chattopadhyay, K.K. Thionyl chloride assisted functionalization of amorphous carbon nanotubes: A better field emitter and stable nanofluid with better thermal conductivity. Mater. Res. Bull. 2015, 66, 1–8. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, J.K.; Huh, J.H.; Kim, Y.H.; Kim, G.T.; Roth, S.; Dettlaff-Weglikowska, U. Effect of SOCl2 doping on electronic properties of single-walled carbon nanotube thin film transistors. Phys. Status Solidi Basic Res. 2011, 248, 2668–2671. [Google Scholar] [CrossRef]
- Zarrabi, H.; Yekavalangi, M.E.; Vatanpour, V.; Shockravi, A.; Safarpour, M. Improvement in desalination performance of thin film nanocomposite nanofiltration membrane using amine-functionalized multiwalled carbon nanotube. Desalination 2016, 394, 83–90. [Google Scholar] [CrossRef]
- Aydoğdu Tığ, G. Gold nanoparticle and poly(arginine) modified GCE for simultaneous determination of hydroquinone and catechol. Hacet. J. Biol. Chem. 2017, 3, 443–451. [Google Scholar] [CrossRef]
- Schuck, A.; Kim, H.E.; Kang, M.; Kim, Y.-S. Comparison and Analysis of Polymer-Functionalized Carbon Nanotubes for Enhancement of the Quantitative Detection of Procalcitonin Levels in Human Plasma. BioChip J. 2023, 17, 274–283. [Google Scholar] [CrossRef]


| Sensing Material a | Target | Method b | Sample c | Concentration | LOD | Reference |
|---|---|---|---|---|---|---|
| Au Nanopillars | Aβ42 | SWV | Artificial Tear | 0.1 to 1 ng/mL | 0.14 ng/mL | [23] |
| CdSe@ZnS QDs | ApoE4 | SWV | Diluted plasma | 10 to 200 ng/mL | 12.5 ng/mL | [4] |
| GO/Au | Aβ42 | Transfer Curves | CSF/Plasma | 10−1 to 105 pg/mL | 9990 pg/mL (Plasma) | [24] |
| CNT | Aβ42 | Transfer Curves | Diluted plasma | 6.75~4500 pg/mL | 9.585 pg/mL | [22] |
| AuBP@Pt | ApoE4 | Amp | Goat Serum | 0.05 to 2000 ng/ml | 0.015 ng/mL | [25] |
| AuNPs | Aβ42 | LSPR | CSF Buffer | 4500~450,000 pg/mL | 6750 pg/mL | [31] |
| ITO/FracAu | ApoE4 | Amp | PBS | 1.0 to 10,000 ng/mL | 0.30 ng/mL | [20] |
| CNT-AuNS-PEG | Aβ42 | DPV | Plasma | 15.63 to 500 pg/mL | 0.2042 pg/mL | This work. |
| ApoE4 | 0.41 to 40 ng/mL | 0.6366 ng/mL |
| Biomarker | Added | Detected | Recovery |
|---|---|---|---|
| Amyloid-β 42 | 31.25 pg/mL | 33.74 pg/mL | 108% |
| 125 pg/mL | 131.37 pg/mL | 105% | |
| 250 pg/mL | 236.35 pg/mL | 95% | |
| ApoE4 | 1.024 ng/mL | 0.966 ng/mL | 94% |
| 2.56 ng/mL | 2.832 ng/mL | 111% | |
| 6.40 ng/mL | 6.81 ng/mL | 106% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, M.-K.; Schuck, A.; Kang, M.; Kim, Y.-S. Electrochemical Analysis of Amyloid Plaques and ApoE4 with Chitosan-Coated Gold Nanostars for Alzheimer’s Detection. Biosensors 2024, 14, 510. https://doi.org/10.3390/bios14100510
Shin M-K, Schuck A, Kang M, Kim Y-S. Electrochemical Analysis of Amyloid Plaques and ApoE4 with Chitosan-Coated Gold Nanostars for Alzheimer’s Detection. Biosensors. 2024; 14(10):510. https://doi.org/10.3390/bios14100510
Chicago/Turabian StyleShin, Min-Kyung, Ariadna Schuck, Minhee Kang, and Yong-Sang Kim. 2024. "Electrochemical Analysis of Amyloid Plaques and ApoE4 with Chitosan-Coated Gold Nanostars for Alzheimer’s Detection" Biosensors 14, no. 10: 510. https://doi.org/10.3390/bios14100510
APA StyleShin, M.-K., Schuck, A., Kang, M., & Kim, Y.-S. (2024). Electrochemical Analysis of Amyloid Plaques and ApoE4 with Chitosan-Coated Gold Nanostars for Alzheimer’s Detection. Biosensors, 14(10), 510. https://doi.org/10.3390/bios14100510





