The Quantitative Detection of Cystatin-C in Patient Samples Using a Colorimetric Lateral Flow Immunoassay
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
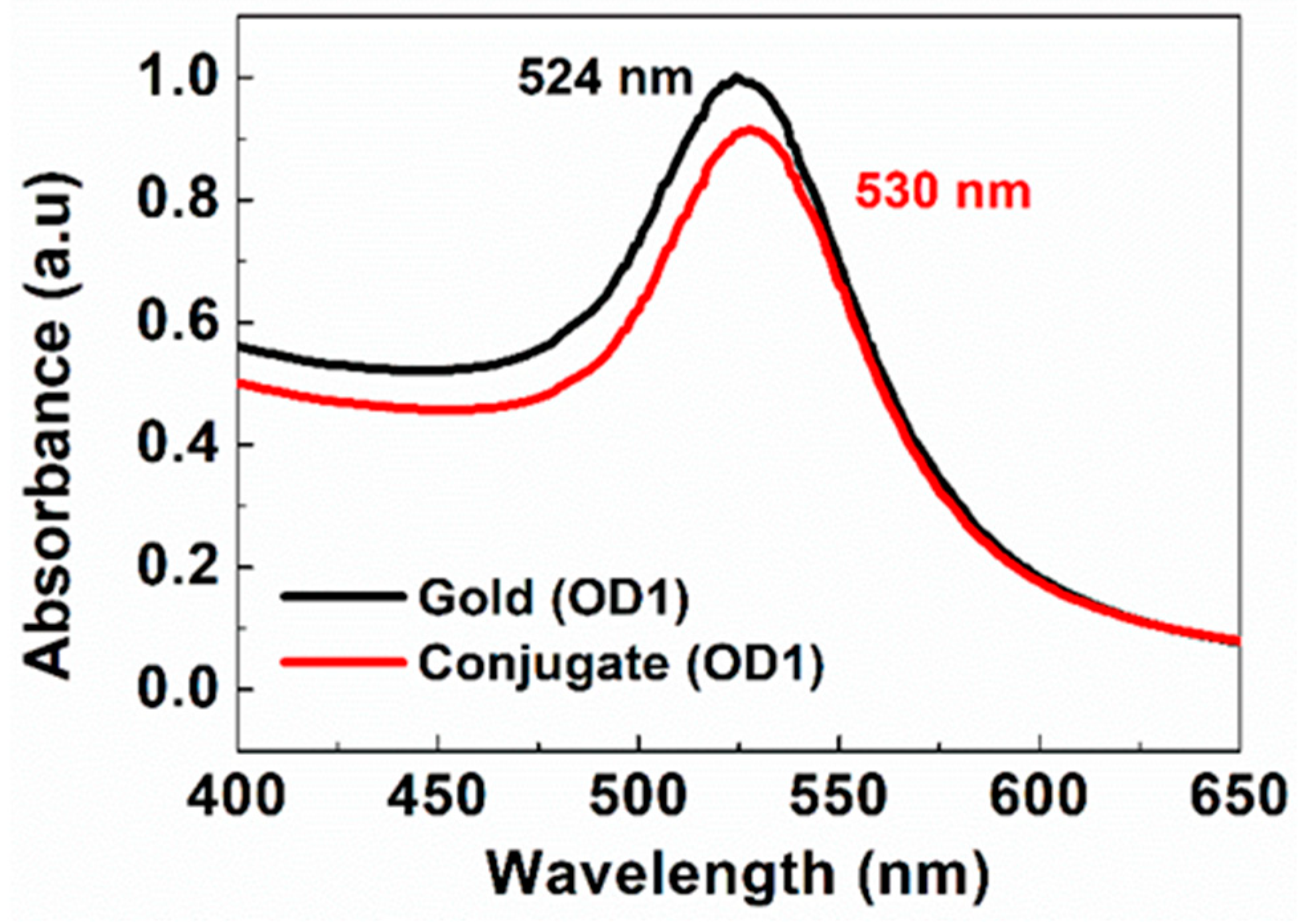
2.3. Antibody Conjugation onto Gold Nanoparticles
2.4. Preparation of Depleted Serum, Calibrators, Clinical Serum, and Blood Samples
2.5. Fabrication of LFAs
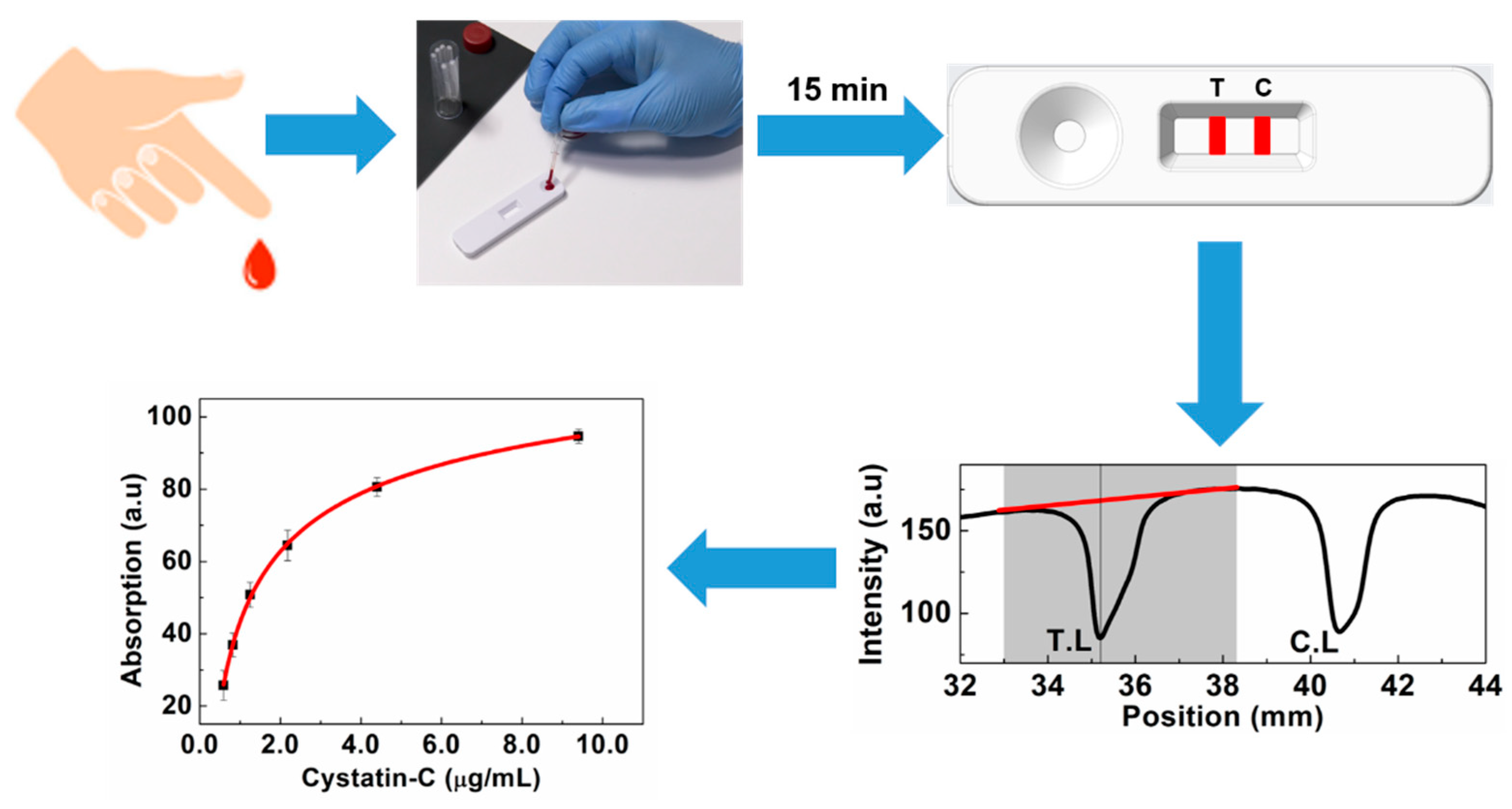
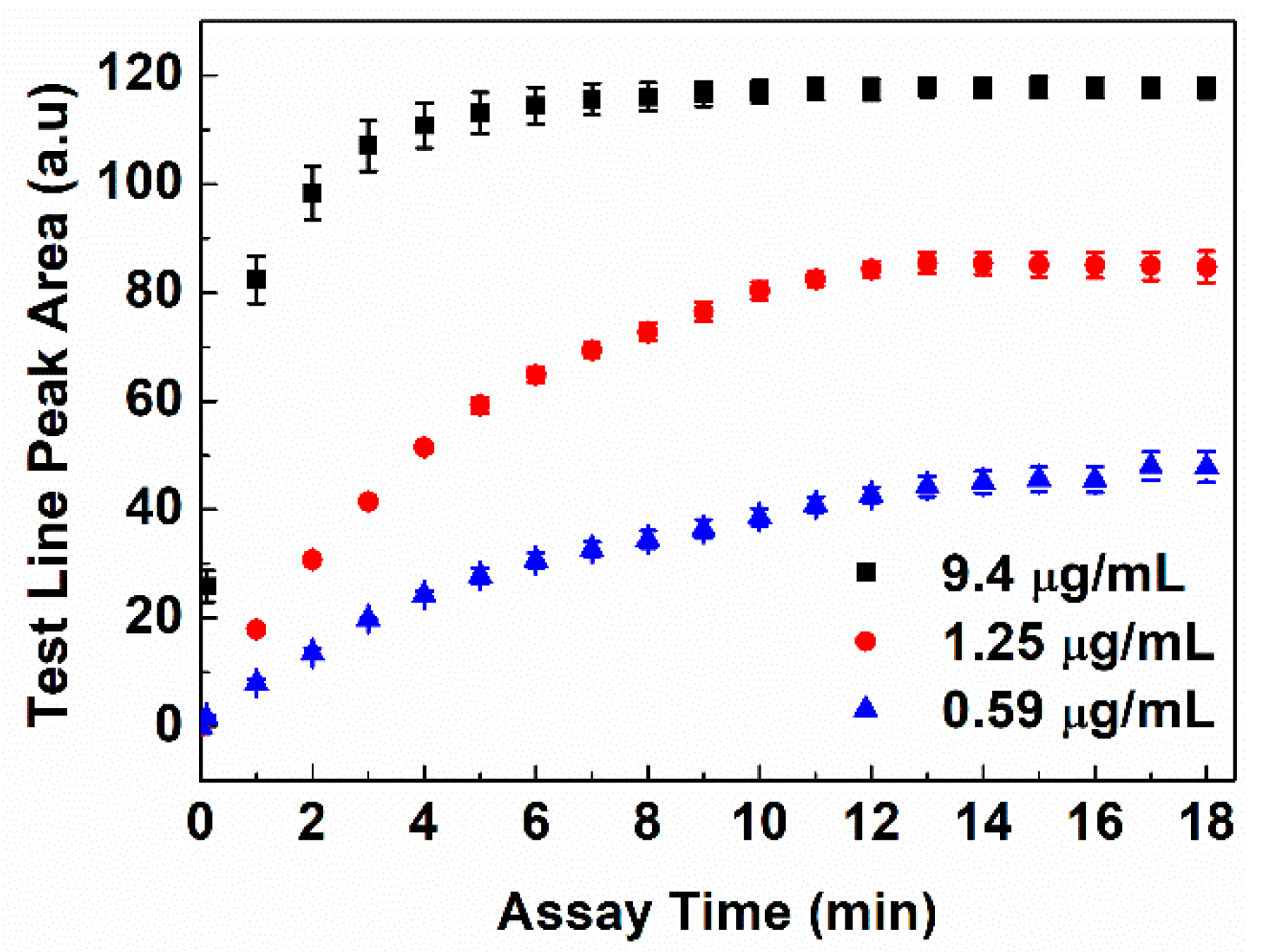
2.6. Colorimetric Lateral Flow Immunoassay Procedure
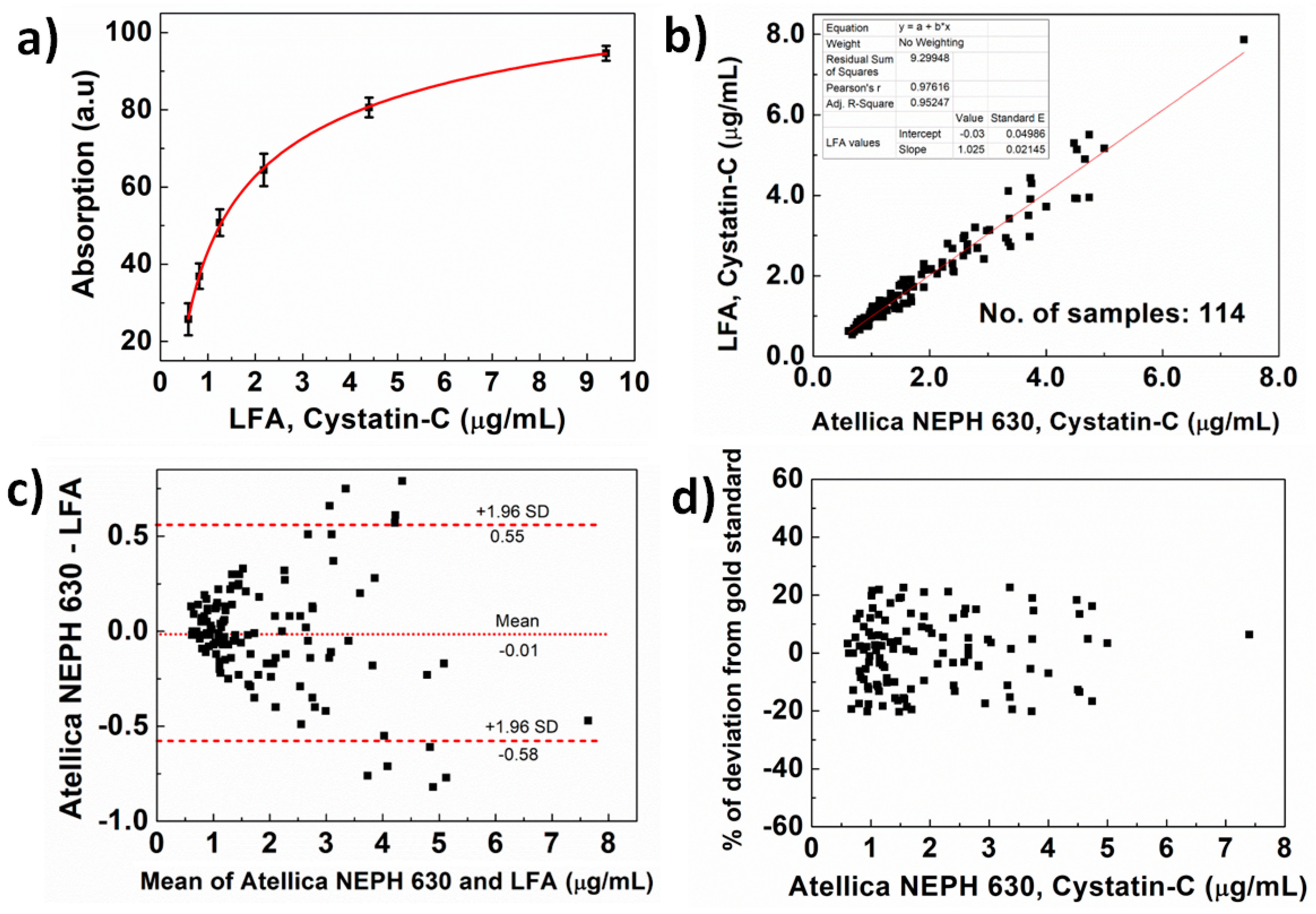
2.7. Validation of Developed LFA
2.8. Measurements with Atellica NEPH-630
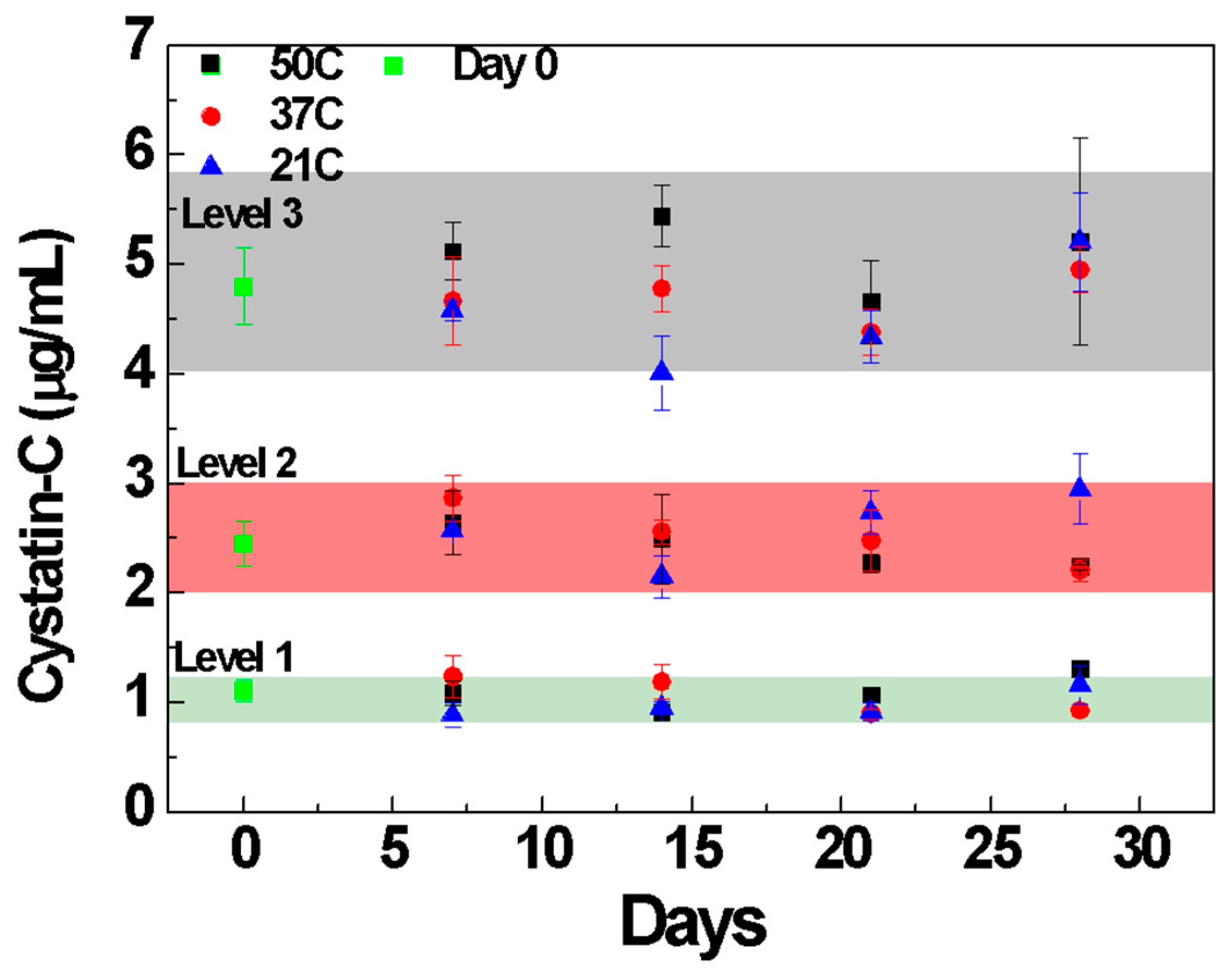
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Collard, D.; Brouwer, T.F.; Olde Engberink, R.H.G.; Zwinderman, A.H.; Vogt, L.; van den Born, B.-J.H. Initial Estimated Glomerular Filtration Rate Decline and Long-Term Renal Function During Intensive Antihypertensive Therapy. Hypertension 2020, 75, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Woodhouse, S.; Batten, W.; Hendrick, H.; Malek, P.A. The Glomerular Filtration Rate: An Important Test for Diagnosis, Staging, and Treatment of Chronic Kidney Disease. Lab. Med. 2006, 37, 244–246. [Google Scholar] [CrossRef]
- Levey, A.S.; Perrone, R.D.; Madias, N.E. Serum Creatinine and Renal Function. Annu. Rev. Med. 1988, 39, 465–490. [Google Scholar] [CrossRef]
- Cimmino, C.J. Benefits of Direct Glomerular Filtration Rate (GFR) Determination versus Creatinine-Based Tests for Evaluating Renal Function. J. Am. Osteopath. Assoc. 1998, 98, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, O.; Golbetz, H.; Kriss, J.P.; Myers, B.D. Limitations of Creatinine as a Filtration Marker in Glomerulopathic Patients. Kidney Int. 1985, 28, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Stevens, L.A.; Schmid, C.H.; Greene, T.; Li, L.; Beck, G.J.; Joffe, M.M.; Froissart, M.; Kusek, J.W.; Zhang, Y.; Coresh, J.; et al. Factors Other than Glomerular Filtration Rate Affect Serum Cystatin C Levels. Kidney Int. 2009, 75, 652–660. [Google Scholar] [CrossRef]
- Kazama, J.J.; Kutsuwada, K.; Ataka, K.; Maruyama, H.; Gejyo, F. Serum Cystatin C Reliably Detects Renal Dysfunction in Patients with Various Renal Diseases. Nephron 2002, 91, 13–20. [Google Scholar] [CrossRef]
- Jung, K.; Jung, M. Cystatin C: A Promising Marker of Glomerular Filtration Rate to Replace Creatinine. Nephron 1995, 70, 370–371. [Google Scholar] [CrossRef]
- Grubb, A. Cystatin C as a Biomarker in Kidney Disease. In Biomarkers of Kidney Disease; Elsevier: Amsterdam, The Netherlands, 2011; pp. 291–312. [Google Scholar]
- Onopiuk, A.; Tokarzewicz, A.; Gorodkiewicz, E. Cystatin C: A Kidney Function Biomarker. Adv. Clin. Chem. 2015, 68, 57–69. [Google Scholar] [CrossRef]
- Delanaye, P.; Rule, A.D. Assessing Kidney Function. In Chronic Renal Disease; Elsevier: Amsterdam, The Netherlands, 2015; pp. 31–42. [Google Scholar]
- Tenstad, O.; Roald, A.B.; Grubb, A.; Aukland, K. Renal Handling of Radiolabelled Human Cystatin C in the Rat. Scand. J. Clin. Lab. Investig. 1996, 56, 409–414. [Google Scholar] [CrossRef]
- Gurudas, S.; Frudd, K.; Maheshwari, J.J.; Revathy, Y.R.; Sivaprasad, S.; Ramanathan, S.M.; Pooleeswaran, V.; Prevost, A.T.; Karatsai, E.; Halim, S.; et al. Multicenter Evaluation of Diagnostic Circulating Biomarkers to Detect Sight-Threatening Diabetic Retinopathy. JAMA Ophthalmol. 2022, 140, 587–597. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.K.; Knicely, D.H.; Grams, M.E. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA 2019, 322, 1294–1304. [Google Scholar] [CrossRef] [PubMed]
- Kyhse-Andersen, J.; Schmidt, C.; Nordin, G.; Andersson, B.; Nilsson-Ehle, P.; Lindström, V.; Grubb, A. Serum Cystatin C, Determined by a Rapid, Automated Particle-Enhanced Turbidimetric Method, Is a Better Marker than Serum Creatinine for Glomerular Filtration Rate. Clin. Chem. 1994, 40, 1921–1926. [Google Scholar] [CrossRef] [PubMed]
- Makris, K.; Nikolaki, E.; Nanopoulos, K.; Pirgakis, K.M.; Maltezos, C.K. Measurement of Cystatin C in Human Urine by Particle-Enhanced Turbidimetric Immunoassay on an Automated Biochemistry Analyzer. Clin. Biochem. 2013, 46, 1128–1130. [Google Scholar] [CrossRef] [PubMed]
- Mussap, M.; Ruzzante, N.; Varagnolo, M.; Plebani, M. Quantitative Automated Particle-Enhanced Immunonephelometric Assay for the Routinary Measurement of Human Cystatin C. CCLM 1998, 36, 859–865. [Google Scholar] [CrossRef]
- Tomaschitz, A.; Pilz, S.; Ritz, E.; Grammer, T.; Drechsler, C.; Boehm, B.O.; März, W. Association of Plasma Aldosterone With Cardiovascular Mortality in Patients With Low Estimated GFR: The Ludwigshafen Risk and Cardiovascular Health (LURIC) Study. Am. J. Kidney Dis. 2011, 57, 403–414. [Google Scholar] [CrossRef]
- Benoit, S.W.; Ciccia, E.A.; Devarajan, P. Cystatin C as a Biomarker of Chronic Kidney Disease: Latest Developments. Expert. Rev. Mol. Diagn. 2020, 20, 1019–1026. [Google Scholar] [CrossRef]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and Highly Specific Lateral Flow Assays for Point-of-Care Diagnosis. ACS Nano 2021, 15, 3593–3611. [Google Scholar] [CrossRef]
- Parolo, C.; Sena-Torralba, A.; Bergua, J.F.; Calucho, E.; Fuentes-Chust, C.; Hu, L.; Rivas, L.; Álvarez-Diduk, R.; Nguyen, E.P.; Cinti, S.; et al. Tutorial: Design and Fabrication of Nanoparticle-Based Lateral-Flow Immunoassays. Nat. Protoc. 2020, 15, 3788–3816. [Google Scholar] [CrossRef]
- Xiao, W.; Huang, C.; Xu, F.; Yan, J.; Bian, H.; Fu, Q.; Xie, K.; Wang, L.; Tang, Y. A Simple and Compact Smartphone-Based Device for the Quantitative Readout of Colloidal Gold Lateral Flow Immunoassay Strips. Sens. Actuators B Chem. 2018, 266, 63–70. [Google Scholar] [CrossRef]
- Raj, S.; McCafferty, D.; Lubrasky, G.; Johnston, S.; Skillen, K.-L.; McLaughlin, J. Point-of-Care Monitoring of Respiratory Diseases Using Lateral Flow Assay and CMOS Camera Reader. IEEE J. Transl. Eng. Health Med. 2022, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.G.; Nordman, E.S.; Johnson, M.D.; Oldham, M.F. A Low-Cost, High-Performance System for Fluorescence Lateral Flow Assays. Biosensors 2013, 3, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Xiong, Y.; Zhao, H.; Guo, H.; Li, Z.; Ye, K.; Zhang, Z.; Liu, T.; Wu, Y.; Lin, G. Europium (III) Chelate Nanoparticle-Based Lateral Flow Immunoassay Strips for Rapid and Quantitative Detection of Cystatin C in Serum. J. Chromatogr. B 2022, 1194, 123133. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.; DeRosa, M.C.; Shah, M.I.; Jayaraj, J. Development and Evaluation of a Quantitative Fluorescent Lateral Flow Immunoassay for Cystatin-C, a Renal Dysfunction Biomarker. Sensors 2021, 21, 3178. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fishlock, S.; Sharpe, P.; McLaughlin, J. Development of Colorimetric Lateral Flow Assays with Gold Nanostructures for Cystatin C Detection. Sens. Actuators Rep. 2022, 4, 100121. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; McNamee, S.E.; McGregor, S.; Vance, P.; McGhee, H.; Marlow, E.L.; McLaughlin, J. A Lateral Flow Immunoassay with Self-Sufficient Microfluidic System for Enhanced Detection of Thyroid-Stimulating Hormone. AIP Adv. 2020, 10, 125316. [Google Scholar] [CrossRef]
- Carter, P. Preparation of Ligand-Free Human Serum for Radioimmunoassay by Adsorption on Activated Charcoal. Clin. Chem. 1978, 24, 362–364. [Google Scholar] [CrossRef]
- Choi, S.; Hwang, J.; Lee, S.; Lim, D.W.; Joo, H.; Choo, J. Quantitative Analysis of Thyroid-Stimulating Hormone (TSH) Using SERS-Based Lateral Flow Immunoassay. Sens. Actuators B Chem. 2017, 240, 358–364. [Google Scholar] [CrossRef]
- Bikkarolla, S.K.; McNamee, S.E.; Vance, P.; McLaughlin, J. High-Sensitive Detection and Quantitative Analysis of Thyroid-Stimulating Hormone Using Gold-Nanoshell-Based Lateral Flow Immunoassay Device. Biosensors 2022, 12, 182. [Google Scholar] [CrossRef]
- Fagúndez, P.; Botasini, S.; Tosar, J.P.; Méndez, E. Systematic process evaluation of the conjugation of proteins to gold nanoparticles. Heliyon 2021, 7, 7392–7402. [Google Scholar] [CrossRef]
| Cystatin-C Levels (µg/mL) | Average (µg/mL) | Within Run CV (%) | Between Run CV (%) | Total CV (%) |
|---|---|---|---|---|
| Level 1 (1.0) | 0.97 | 6.78 | 9.28 | 10.3 |
| Level 2 (2.43) | 2.39 | 7.43 | 10.87 | 11.64 |
| Level 3 (5.0) | 5.17 | 7.55 | 9.76 | 10.58 |
| Interferent | Concentration of Interferent | Cystatin-C (µg/mL) | Lateral Flow Result (µg/mL) | Recovery (%) |
|---|---|---|---|---|
| Control | - | 2.0 | 2.2 ± 0.19 | 110 |
| Hemoglobin | 5 mg/mL | 2.0 | 2.4 ± 0.089 | 120 |
| Bilirubin | 0.2 mg/mL | 2.0 | 2.3 ± 0.18 | 115 |
| Triglycerides | 10 mg/mL | 2.0 | 2.3 ± 0.15 | 115 |
| Rheumatoid Factor | 600 IU/mL | 2.0 | 1.8 ± 0.25 | 80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bikkarolla, S.K.; Venkatesan, K.; Revathy, Y.R.; Parameswaran, S.; Krishnakumar, S.; Dendukuri, D. The Quantitative Detection of Cystatin-C in Patient Samples Using a Colorimetric Lateral Flow Immunoassay. Biosensors 2024, 14, 30. https://doi.org/10.3390/bios14010030
Bikkarolla SK, Venkatesan K, Revathy YR, Parameswaran S, Krishnakumar S, Dendukuri D. The Quantitative Detection of Cystatin-C in Patient Samples Using a Colorimetric Lateral Flow Immunoassay. Biosensors. 2024; 14(1):30. https://doi.org/10.3390/bios14010030
Chicago/Turabian StyleBikkarolla, Santosh Kumar, Kavipriya Venkatesan, Yeddula Rebecca Revathy, Sowmya Parameswaran, Subramanian Krishnakumar, and Dhananjaya Dendukuri. 2024. "The Quantitative Detection of Cystatin-C in Patient Samples Using a Colorimetric Lateral Flow Immunoassay" Biosensors 14, no. 1: 30. https://doi.org/10.3390/bios14010030
APA StyleBikkarolla, S. K., Venkatesan, K., Revathy, Y. R., Parameswaran, S., Krishnakumar, S., & Dendukuri, D. (2024). The Quantitative Detection of Cystatin-C in Patient Samples Using a Colorimetric Lateral Flow Immunoassay. Biosensors, 14(1), 30. https://doi.org/10.3390/bios14010030





