Progress in Electrochemical Immunosensors with Alkaline Phosphatase as the Signal Label
Abstract
1. Introduction
2. Electrochemical Methods
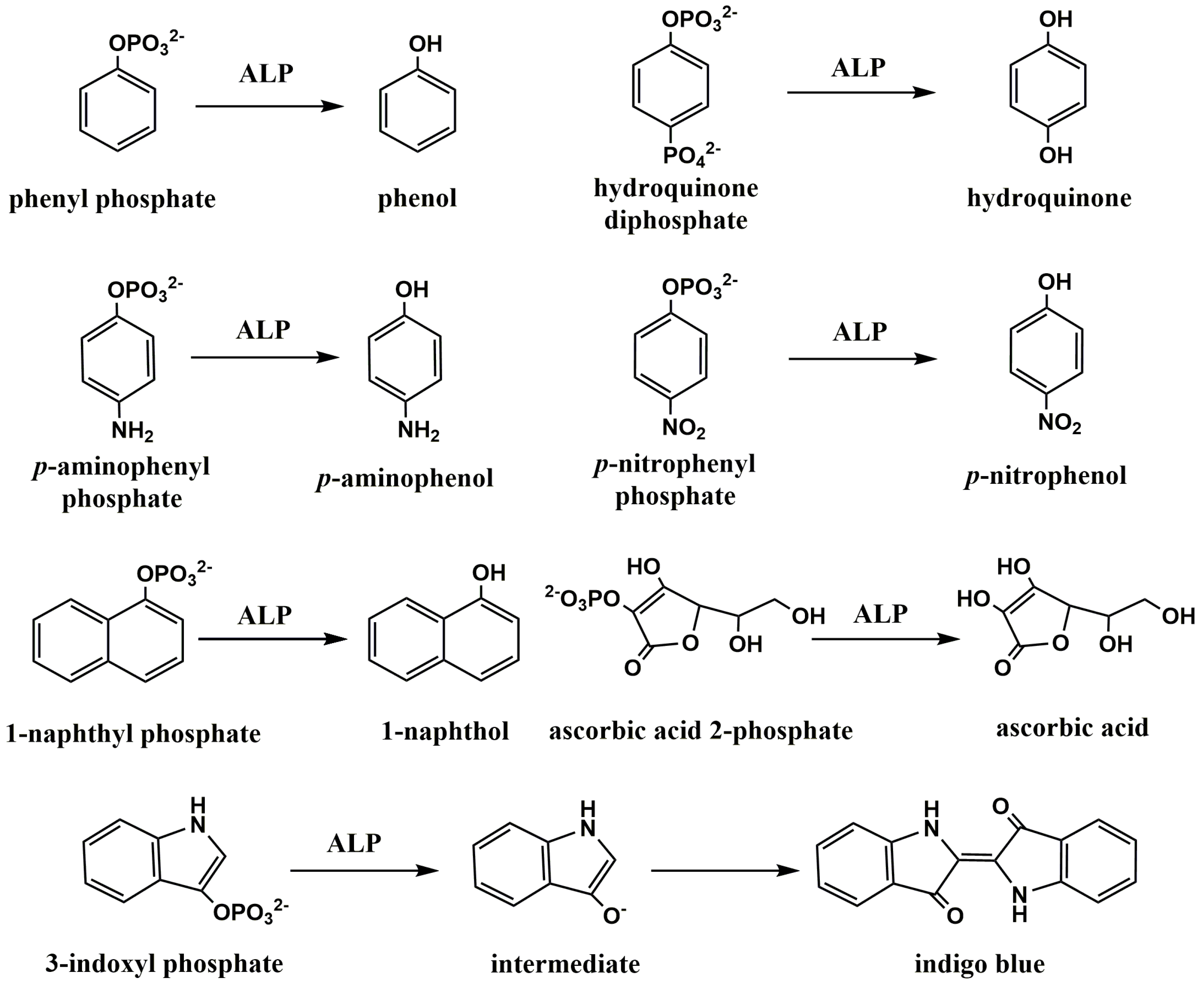
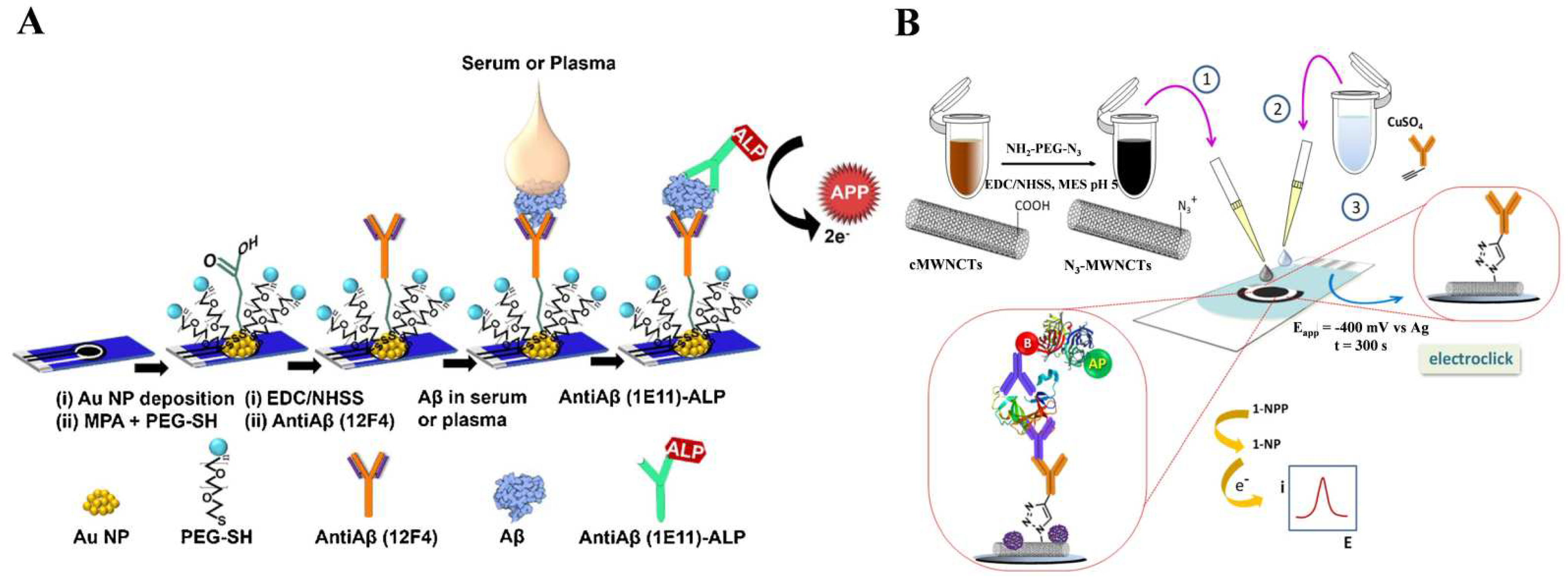
2.1. Direct Detection of ALP-Catalyzed Products
2.2. ALP Catalysis plus Redox Cycling
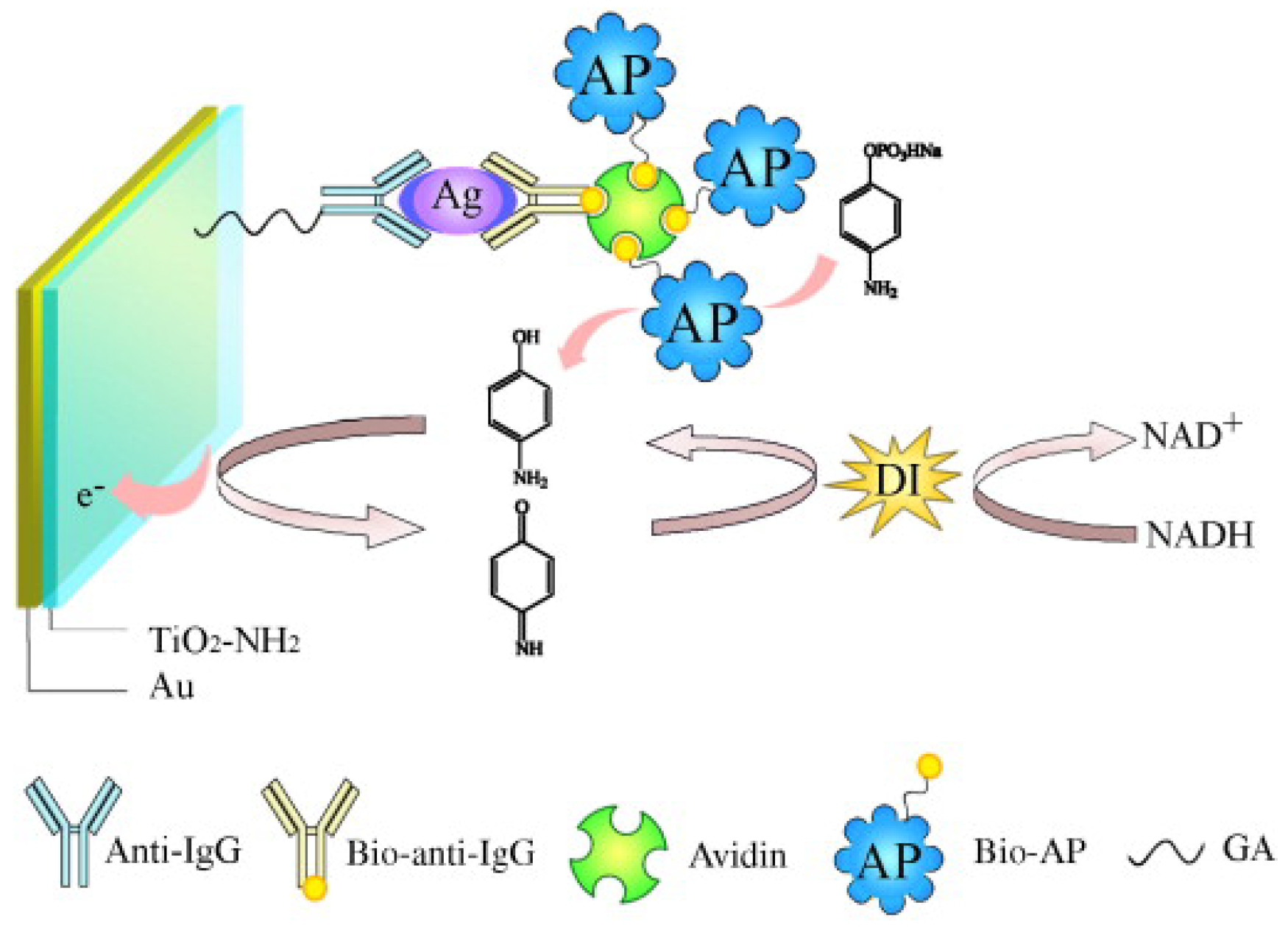
2.2.1. EC Redox Cycling
2.2.2. ECC Redox Cycling

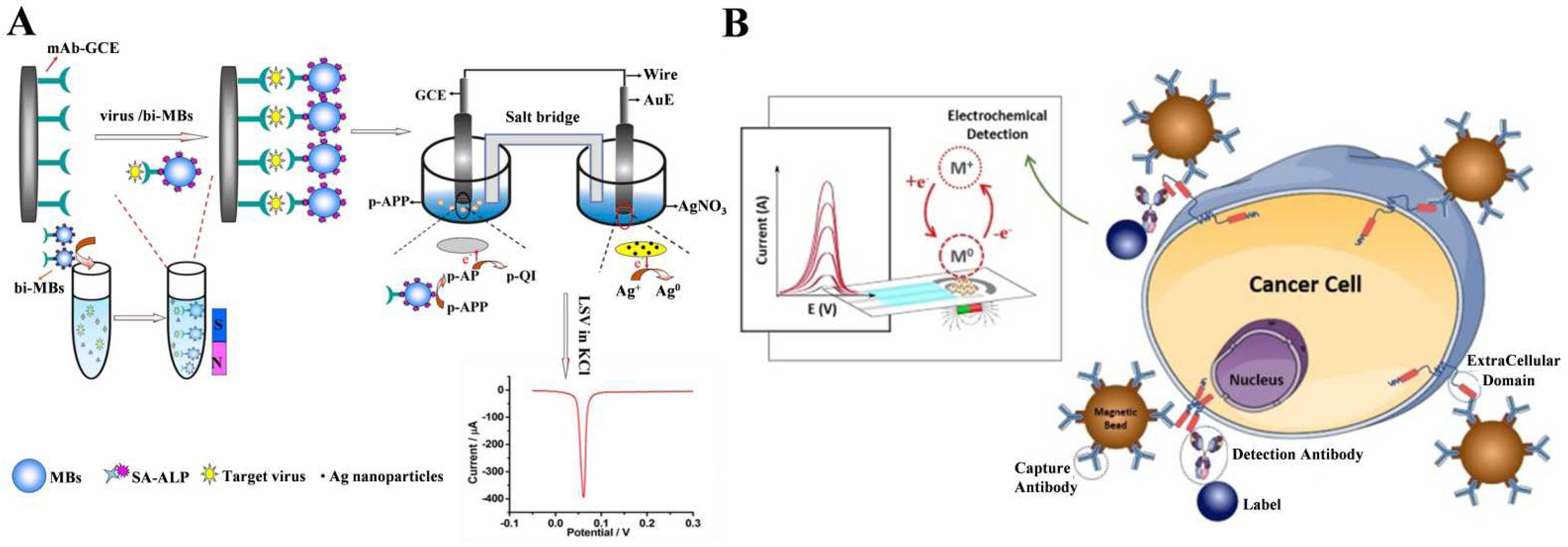
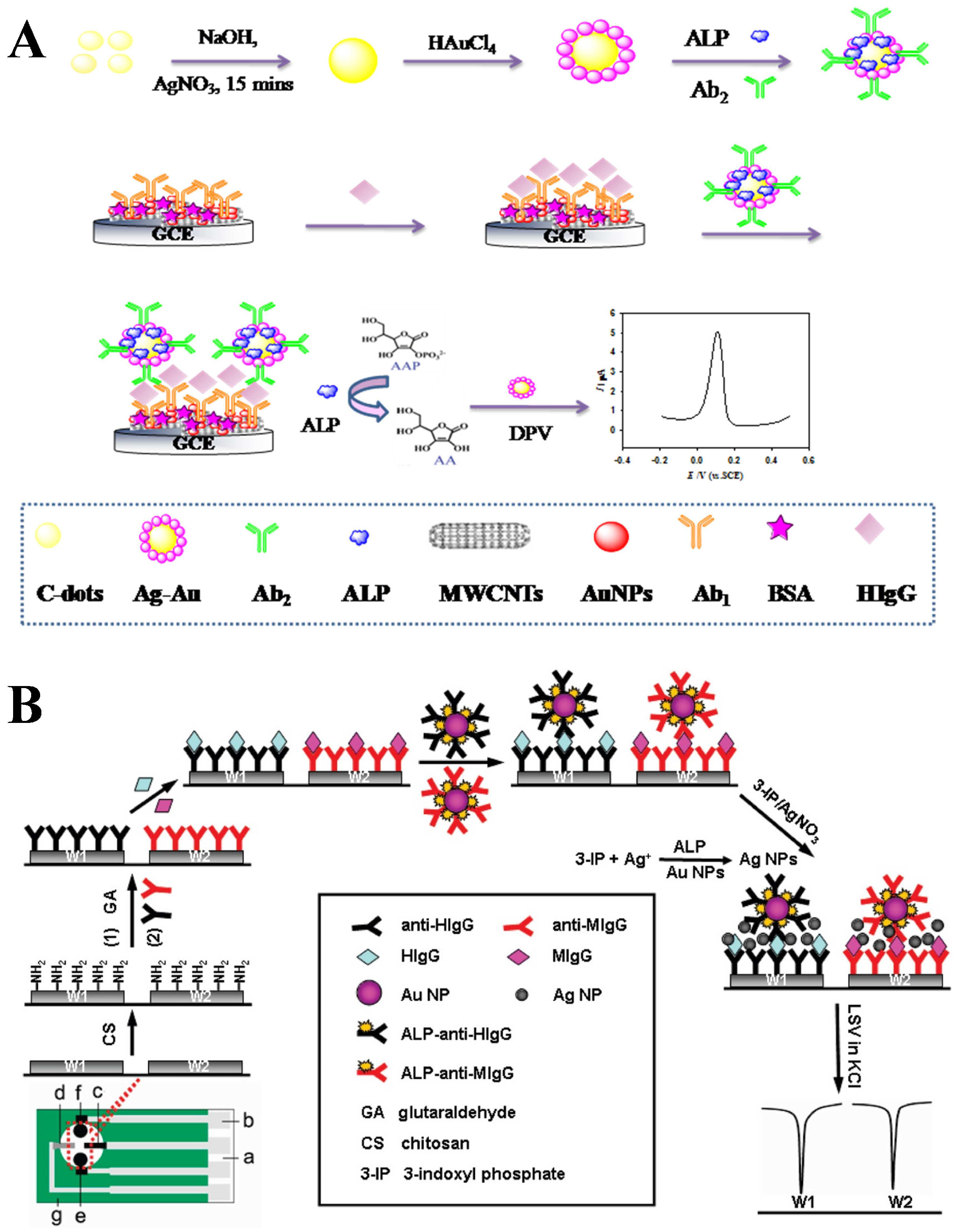
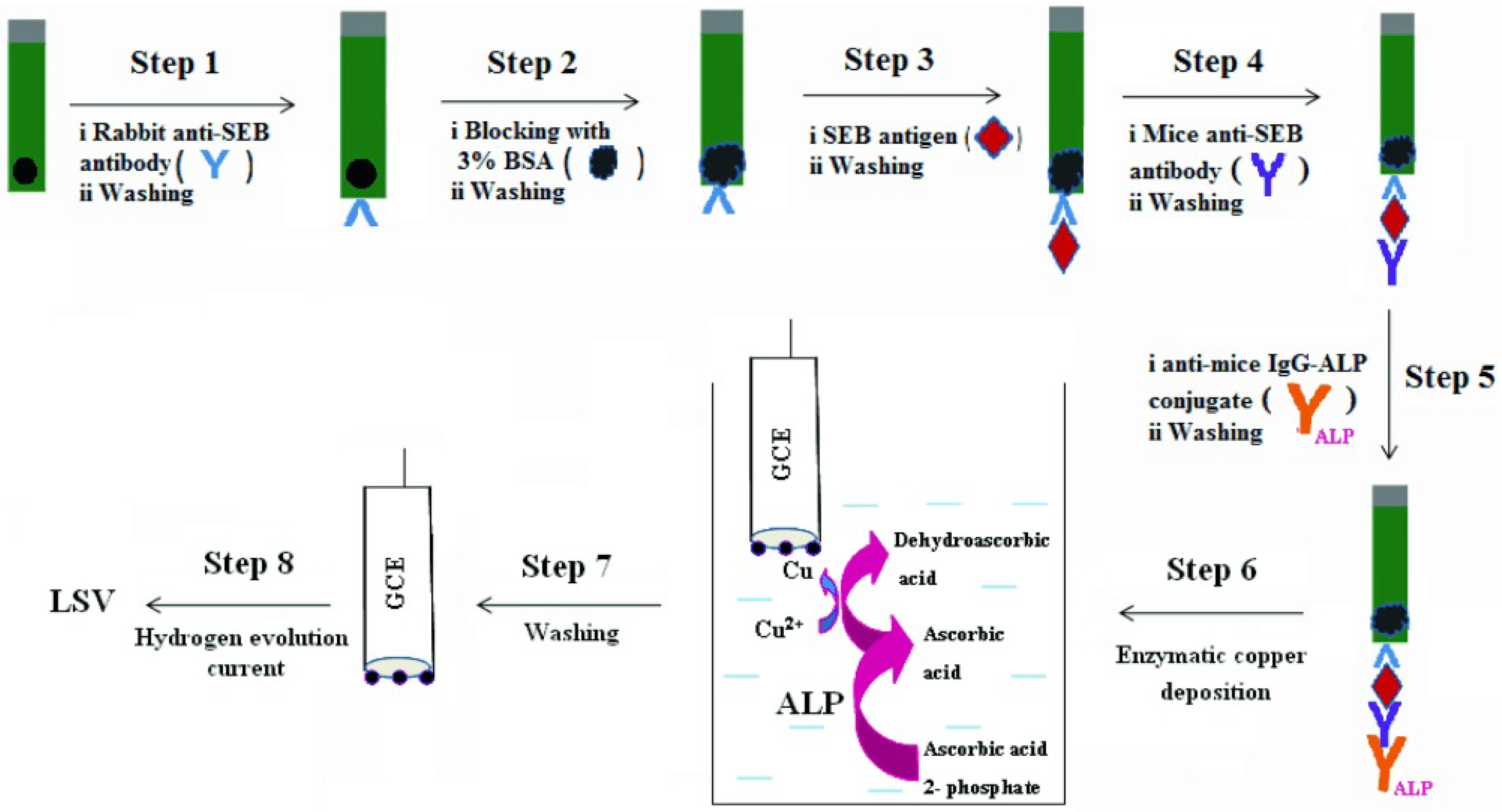
2.3. ALP-Catalyzed Metal Deposition
| Detection Principle | ALP Substrate | Target | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|
| Direct detection of enzymatic products | PAPP | Digoxin | 0.5–2.0 ng/mL | 30 pg/mL | [38] |
| PAPP | Mouse IgG | 10–1000 ng/mL | 10 ng/mL | [39] | |
| PAPP | hCG | 0.8–40 units/L | 0.8 units/L | [40] | |
| PAPP | TBG and cortisol | 31–1000 μg/L and 1 × 102–2000 nM | NA | [41] | |
| NPP | PCBs | 1 × 10−5–1 U/mL | 2.1 × 10−6 U/mL | [63] | |
| PNPP | 5-methylcytosine | 0.01–50 nM | 3.2 pM | [70] | |
| 3-IP | Escherichia coli O157:H7 | 6 × 104–6 × 107 cells/mL | 6 × 103 cells/mL | [89] | |
| AAP | Mouse IgG | 1–1000 ng/mL | 0.3 ng/mL | [99] | |
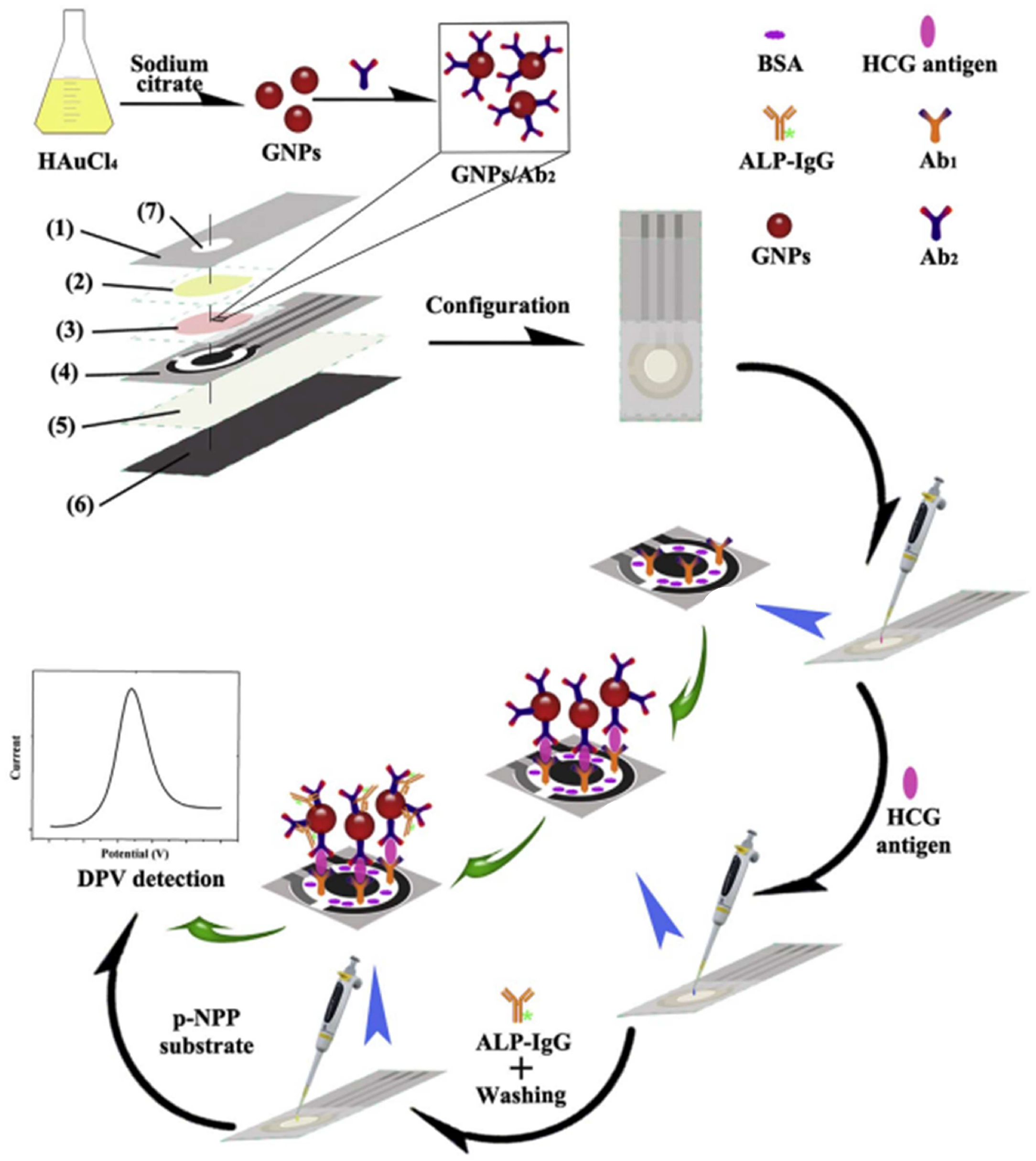
| PNPP | hCG | 1–100 mIU/mL | 0.36 mIU/mL | [109] | |
| ALP catalysis plus EC redox cycling | AAP | Troponin I | 100 fg/mL–1 μg/mL | 10 fg/mL | [118] |
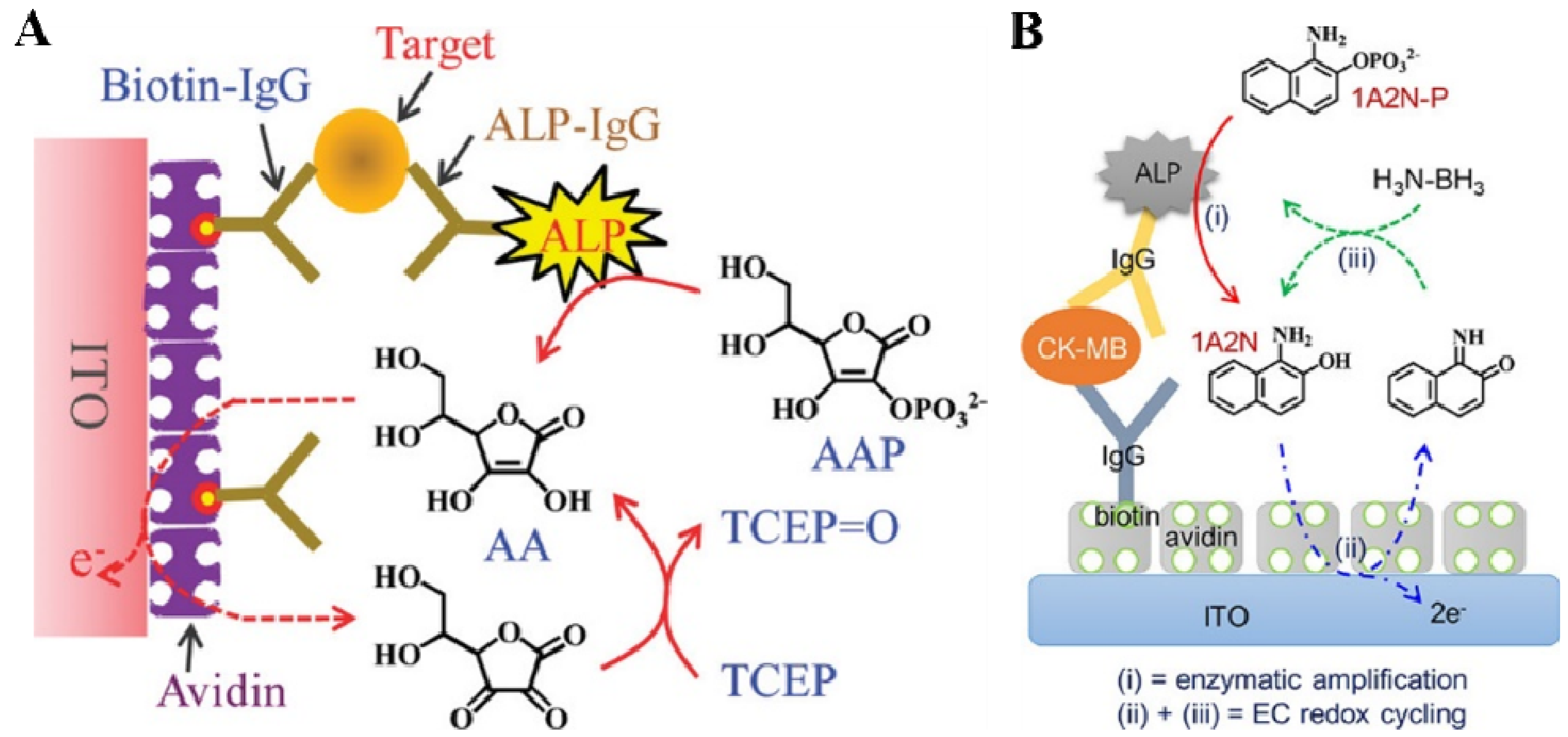
| 1A2N-P | CK-MB | 100 fg/mL–1 μg/mL | 80 fg/mL | [119] | |
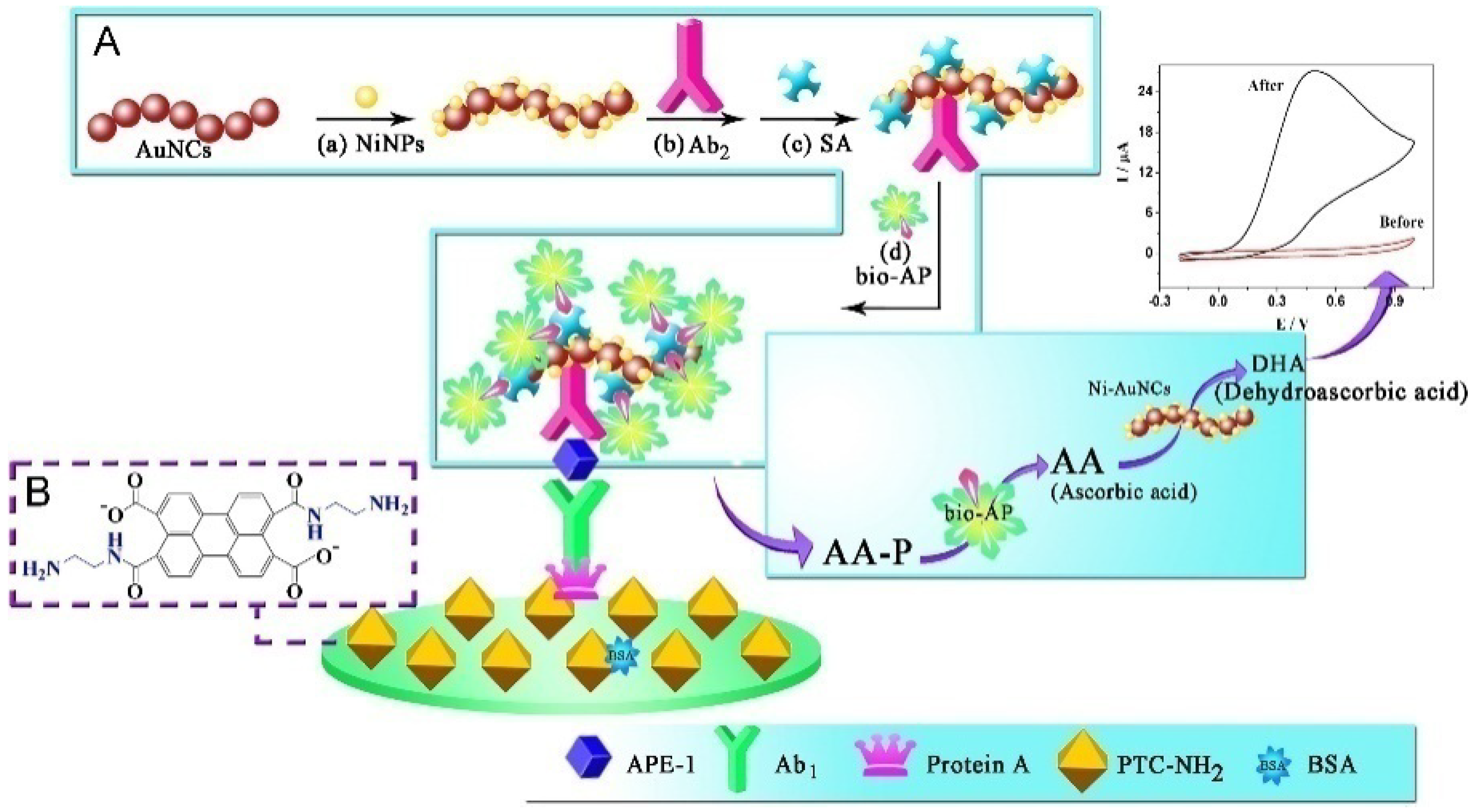
| AAP | APE-1 | 10 fg/mL–100 pg/mL | 3.9 fg/mL | [131] | |
| ALP catalysis plus ECC redox cycling | PAPP | Mouse IgG | 0.1–1 × 105 pg/mL | 100 fg/mL | [133] |
| PAPP | Mouse IgG | 1 pg/mL–1 μg/mL | 1 pg/mL | [134] | |
| PAPP | Troponin I | 10 fg/mL–1 μg/mL | 1 fg/mL | [135] | |
| ALP-based EE redox cycling | PAPP | CEA | 5 pg/mL–50 ng/mL | 2 pg/mL | [137] |
| PAPP | Human IgG | 10 fg/mL–1 μg/mL | 3.5 fg/mL | [138] | |
| ALP-catalyzed metal deposition | AAP | Human IgG | 0.1–50 ng/mL | 0.03 ng/mL | [142] |
| AAP | Human IgG | 5–1000 ng/mL | 2.2 ng/mL | [143] | |
| PAPP | H7N9 virus | 0.01–20 ng/mL | 6.8 pg/mL | [154] | |
| 3-IP | HER2 | 5–50 ng/mL | 2.8 ng/mL | [161] | |
| AAP | Human IgG | 0.005–100 ng/mL | 0.9 pg/mL | [170] | |
| 3-IP | Human and mouse IgG | 0.01–250 ng/mL | 4.8 pg/mL | [171] | |
| AAP | Human IgG | 10 pg/mL–1 μg/mL | 2 pg/mL | [173] | |
| AAP | SEB | 1 ng/mL–1 μg/mL | 1 ng/mL | [174] |
3. PEC Methods
3.1. ALP-Catalyzed Products as Electron Donors
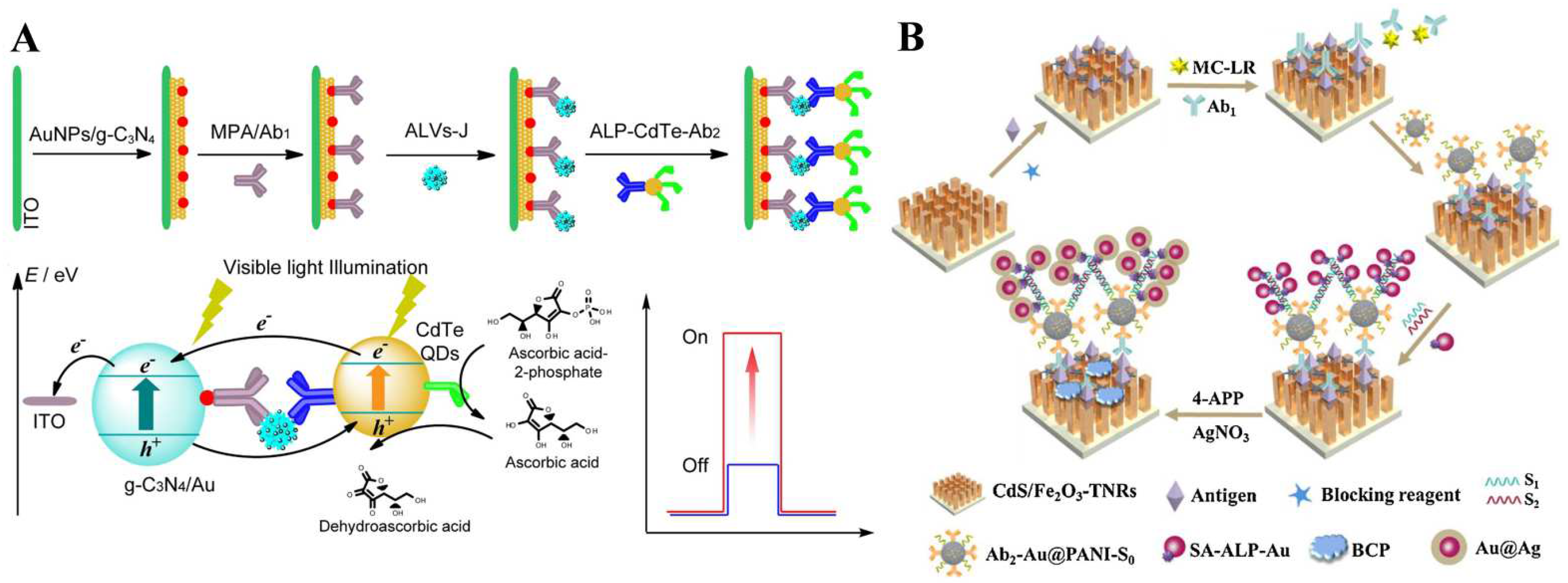
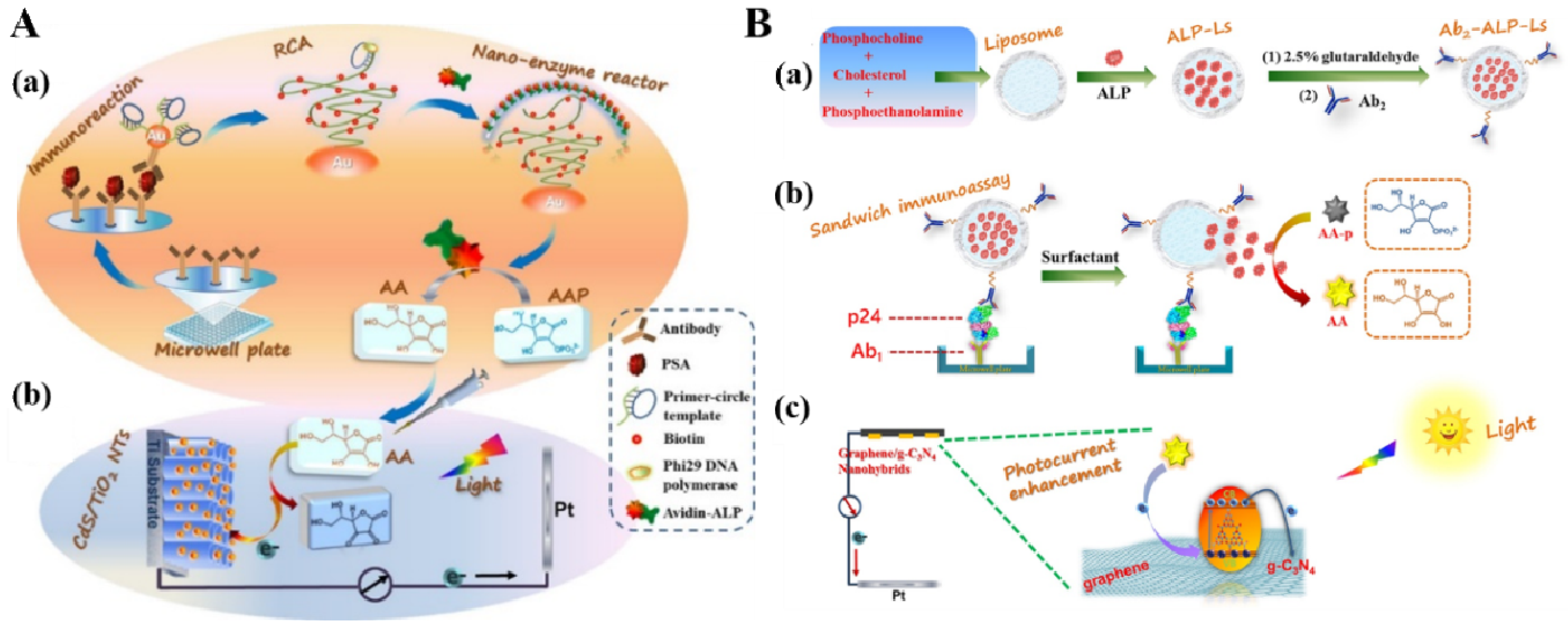
3.2. ALP-Mediated Redox Cycling
3.3. ALP-Mediated In Situ Growth or Bioetching of Photoelectrode
| Detection Principle | ALP Substrates | Target | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|
| ALP-catalyzed products as electron donors | AAP | M.SssIMTs | 1–50 unit/mL | 0.33 unit/mL | [186] |
| AAP | N6-methyladenosine | 0.005–35 nM | 2.57 pM | [188] | |
| AAP | MiRNA | 5–3000 fM | 2.26 fM | [192] | |
| AAP | AFP | 0.5–50 ng/mL | 37.9 pg/mL | [199] | |
| AAP | PSA | 0.001–3 ng/mL | 0.32 pg/mL | [201] | |
| AAP | HIV-p24 antigen | 1 pg/mL–50 ng/mL | 0.63 pg/mL | [207] | |
| AAP | Microcystin-LR | 0.05 ng/mL–5 μg/mL | 0.03 pg/mL | [212] | |
| ALP-mediated redox cycling | AAP | Myoglobin | 1 × 10−4–100 ng/mL | 0.1 pg/mL | [214] |
| AAP | Myoglobin | 1 × 10−4–100 ng/mL | 0.03 pg/mL | [215] | |
| AAP | Troponin I | 10 fg/mL–1 ng/mL | 3 fg/mL | [218] | |
| PAPP | Interleukin-6 | 50 fg/mL–10 ng/mL | 20 fg/mL | [219] | |
| ALP-mediated in situ growth or bioetching of photoelectrode | AAP | CRP | 1 pg/mL–200 ng/mL | 0.1 pg/mL | [221] |
| AAP | h-FABP | 0.5 pg/mL–50 ng/mL | 0.1 pg/mL | [222] | |
| thiophosphate | Troponin I | 0.01–10 ng/mL | 8.6 pg/mL | [226] | |
| thiophosphate | h-FABP | 0.1–1000 pg/mL | 55 fg/mL | [227] | |
| AAP | Aflatoxin B1 | 0.01–10 ng/mL | 2.6 pg/mL | [229] | |
| AAP | CEA | 0.01–100 ng/mL | 5.2 pg/mL | [230] | |
| AAP | Human IgG | 1 × 10−4–100 ng/mL | 25 fg/mL | [231] | |
| AAP | Alpha-fetoprotein | 0.01–100 ng/mL | 9.3 pg/mL | [234] |
4. ECL Methods
5. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Kokkinos, C.; Economou, A.; Prodromidis, M.I. Electrochemical immunosensors: Critical survey of different architectures and transduction strategies. TrAC-Trend. Anal. Chem. 2016, 79, 88–105. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Puiu, M.; Nativi, C.; Bala, C. Early detection of tumour-associated antigens: Assessment of point-of-care electrochemical immunoassays. TrAC-Trend. Anal. Chem. 2023, 160, 116981. [Google Scholar] [CrossRef]
- Kim, J.; Park, M. Recent progress in electrochemical immunosensors. Biosensors 2021, 11, 360. [Google Scholar] [CrossRef] [PubMed]
- Zherdev, A.V.; Dzantiev, B.B. Detection limits of immunoanalytical systems: Limiting factors and methods of reduction. J. Anal. Chem. 2022, 77, 391–401. [Google Scholar] [CrossRef]
- Campuzano, S.; Pedrero, M.; Yáñez-Sedeño, P.; Pingarrón, J.M. Nanozymes in electrochemical affinity biosensing. Microchim. Acta 2020, 187, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, J.; Zhou, B.; Ian, H.; Shao, H. Advanced sensitivity amplification strategies for voltammetric immunosensors of tumor marker: State of the art. Biosens. Bioelectron. 2021, 178, 113021–113042. [Google Scholar] [CrossRef]
- Police Patil, A.V.; Chuang, Y.S.; Li, C.; Wu, C.C. Recent advances in electrochemical immunosensors with nanomaterial assistance for signal amplification. Biosensors 2023, 13, 125. [Google Scholar] [CrossRef]
- Xia, N.; Huang, Y.; Zhao, Y.; Wang, F.; Liu, L.; Sun, Z. Electrochemical biosensors by in situ dissolution of self-assembled nanolabels into small monomers on electrode surface. Sens. Actuators B Chem. 2020, 325, 128777. [Google Scholar] [CrossRef]
- Jarczewska, M.; Malinowska, E. The application of antibody-aptamer hybrid biosensors in clinical diagnostics and environmental analysis. Anal. Methods 2020, 12, 3183–3199. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Deng, D.; Wu, D.; Hou, W.; Wang, L.; Li, N.; Sun, Z. Duplex-specific nuclease-based electrochemical biosensor for the detection of microRNAs by conversion of homogeneous assay into surface-tethered electrochemical analysis. Anal. Chim. Acta 2021, 1149, 338199. [Google Scholar] [CrossRef] [PubMed]
- Kuramitz, H. Magnetic microbead-based electrochemical immunoassays. Anal. Bioanal. Chem. 2009, 394, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Masud, M.K.; Na, J.; Younus, M.; Hossain, M.S.A.; Bando, Y.; Shiddiky, M.J.A.; Yamauchi, Y. Superparamagnetic nanoarchitectures for disease-specific biomarker detection. Chem. Soc. Rev. 2019, 48, 5717–5751. [Google Scholar] [CrossRef]
- Kavetskyy, T.; Alipour, M.; Smutok, O.; Mushynska, O.; Kiv, A.; Fink, D.; Farshchi, F.; Ahmadian, E.; Hasanzadeh, M. Magneto-immunoassay of cancer biomarkers: Recent progress and challenges in biomedical analysis. Microchem. J. 2021, 167, 106320–106332. [Google Scholar] [CrossRef]
- Li, X.-M.; Yang, X.-Y.; Zhang, S.-S. Electrochemical enzyme immunoassay using model labels. TrAC-Trend. Anal. Chem. 2008, 27, 543–553. [Google Scholar] [CrossRef]
- Yang, H. Enzyme-based ultrasensitive electrochemical biosensors. Curr. Opin. Chem. Biol. 2012, 16, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Pollap, A.; Kochana, J. Electrochemical immunosensors for antibiotic detection. Biosensors 2019, 9, 61. [Google Scholar] [CrossRef]
- Noji, H.; Minagawa, Y.; Ueno, H. Enzyme-based digital bioassay technology-key strategies and future perspectives. Lab. Chip 2022, 22, 3092–3109. [Google Scholar] [CrossRef]
- Xia, N.; Deng, D.; Yang, S.; Hao, Y.; Wang, L.; Liu, Y.; An, C.; Han, Q.; Liu, L. Electrochemical immunosensors with protease as the signal label for the generation of peptide-Cu(II) complexes as the electrocatalysts toward water oxidation. Sens. Actuators. B Chem. 2019, 291, 113–119. [Google Scholar] [CrossRef]
- Wignarajah, S.; Chianella, I.; Tothill, I.E. Development of electrochemical immunosensors for HER-1 and HER-2 analysis in serum for breast cancer patients. Biosensors 2023, 13, 355. [Google Scholar] [CrossRef] [PubMed]
- Manes, T.; Hoylaerts, M.F.; Müller, R.; Lottspeich, F.; Holke, W.; Millán, J.L. Genetic complexity, structure, and characterization of highly active bovine intestinal alkaline phosphatases. J. Biol. Chem. 1998, 273, 23353–23360. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Gai, P.; Hou, T.; Li, H.; Xue, C.; Li, F. Enzymatic fuel cell-based self-powered homogeneous immunosensing platform via target-induced glucose release: An appealing alternative strategy for turn-on melamine assay. ACS Appl. Mater. Interfaces 2017, 9, 35721–35728. [Google Scholar] [CrossRef] [PubMed]
- Shaban, S.M.; Byeok Jo, S.; Hafez, E.; Ho Cho, J.; Kim, D.-H. A comprehensive overview on alkaline phosphatase targeting and reporting assays. Coordin. Chem. Rev. 2022, 465, 214567–214604. [Google Scholar] [CrossRef]
- Kanno, Y.; Zhou, Y.; Fukuma, T.; Takahashi, Y. Alkaline phosphatase-based electrochemical analysis for point-of-care testing. Electroanalysis 2021, 34, 161–167. [Google Scholar] [CrossRef]
- Nsabimana, A.; Lan, Y.; Du, F.; Wang, C.; Zhang, W.; Xu, G. Alkaline phosphatase-based electrochemical sensors for health applications. Anal. Methods 2019, 11, 1996–2006. [Google Scholar] [CrossRef]
- Xia, N.; Huang, Y.; Cui, Z.; Liu, S.; Deng, D.; Liu, L.; Wang, J. Impedimetric biosensor for assay of caspase-3 activity and evaluation of cell apoptosis using self-assembled biotin-phenylalanine network as signal enhancer. Sens. Actuators B Chem. 2020, 320, 128436. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The role of electrochemical immunosensors in clinical analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef]
- Plikusiene, I.; Ramanaviciene, A. Investigation of biomolecule interactions: Optical-, electrochemical-, and acoustic-based biosensors. Biosensors 2023, 13, 292. [Google Scholar] [CrossRef]
- Zhang, X.; Li, C.R.; Wang, W.C.; Xue, J.; Huang, Y.L.; Yang, X.X.; Tan, B.; Zhou, X.P.; Shao, C.; Ding, S.J.; et al. A novel electrochemical immunosensor for highly sensitive detection of aflatoxin B1 in corn using single-walled carbon nanotubes/chitosan. Food Chem. 2016, 192, 197–202. [Google Scholar] [CrossRef]
- Freitas, M.; Neves, M.; Nouws, H.P.A.; Delerue-Matos, C. Electrochemical immunosensor for the simultaneous determination of two main peanut allergenic proteins (Ara h 1 and Ara h 6) in food matrices. Foods 2021, 10, 1718. [Google Scholar] [CrossRef] [PubMed]
- El-Moghazy, A.Y.; Huo, J.; Amaly, N.; Vasylieva, N.; Hammock, B.D.; Sun, G. An innovative nanobody-based electrochemical immunosensor using decorated nylon nanofibers for point-of-care monitoring of human exposure to pyrethroid insecticides. ACS Appl. Mater. Interfaces 2020, 12, 6159–6168. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.G.; Eremenko, A.V.; Ehrentreich-Forster, E.; Bier, F.F.; Makower, A.; Halsall, H.B.; Heineman, W.R.; Scheller, F.W. Zeptomole-detecting biosensor for alkaline phosphatase in an electrochemical immunoassay for 2,4-dichlorophenoxyacetic acid. Anal. Chem. 1996, 68, 2453–2458. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bhalla, V.; Tuteja, S.; Kukkar, M.; Suri, C.R. Rapid extraction and quantitative detection of the herbicide diuron in surface water by a hapten-functionalized carbon nanotubes based electrochemical analyzer. Analyst 2012, 137, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Kanso, H.; Barthelmebs, L.; Inguimbert, N.; Noguer, T. Immunosensors for estradiol and ethinylestradiol based on new synthetic estrogen derivatives: Application to wastewater analysis. Anal. Chem. 2013, 85, 2397–2404. [Google Scholar] [CrossRef] [PubMed]
- Wehmeyer, K.R.; Halsall, H.B.; Heineman, W.R.; Volle, C.P.; Chen, I.W. Competitive heterogeneous enzyme immunoassay for digoxin with electrochemical detection. Anal. Chem. 1986, 58, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.P.; Pemberton, R.M.; Luxton, R.; Wedge, R. Studies towards a disposable screen-printed amperometric biosensor for progesterone. Biosens. Bioelectron. 1997, 12, 1113–1121. [Google Scholar] [CrossRef]
- Tang, H.T.; Lunte, C.E.; Halsall, H.B.; Heineman, W.R. p-Aminophenyl phosphate: An improved substrate for electrochemical enzyme immnoassay. Anal. Chim. Acta 1988, 214, 187–195. [Google Scholar] [CrossRef]
- Niwa, O.; Xu, Y.; Halsall, H.B.; Heineman, W.R. Small-volume voltammetric detection of 4-aminophenol with interdigitated array electrodes and its application to electrochemical enzyme immunoassay. Anal. Chem. 1993, 65, 1559–1563. [Google Scholar] [CrossRef]
- Duan, C.; Meyerhoff, M.E. Separation-free sandwich enzyme immunoassays using microporous gold electrodes and self-assembled monolayer/immobilized capture antibodies. Anal. Chem. 1994, 66, 1369–1377. [Google Scholar] [CrossRef]
- Treloar, P.H.; Nkohkwo, A.a.T.; Kane, J.W.; Barber, D.; Vadgama, P.M. Electrochemical immunoassay: Simple kinetic detection of alkaline phosphatase enzyme labels in limited and excess reagent systems. Electroanalysis 1994, 6, 561–566. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, Y.; Ip, M.P. An ultra-sensitive electrochemical enzyme immunoassay for thyroid stimulating hormone in human serum. J. Pharm. Biomed. Anal. 1994, 12, 787–793. [Google Scholar] [CrossRef][Green Version]
- Pemberton, R.M.; Hart, J.P.; Foulkes, J.A. Development of a sensitive, selective electrochemical immunoassay for progesterone in cow’s milk based on a disposable screen-printed amperometric biosensor. Electrochim. Acta 1998, 43, 3567–3574. [Google Scholar] [CrossRef]
- Wang, J.; Ibanez, A.; Chatrathi, M.P.; Escarpa, A. Electrochemical enzyme immunoassays on microchip platforms. Anal. Chem. 2001, 73, 5323–5327. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, T.M.; Pravda, M.; O’Sullivan, C.K.; Guilbault, G.G. Development of a disposable immunosensor for the detection of human heart fatty-acid binding protein in human whole blood using screen-printed carbon electrodes. Talanta 2002, 57, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.C.; Deng, A.; Huang, H.J. Immunoassay with a microtiter plate incorporated multichannel electrochemical detection system. Anal. Chem. 2002, 74, 2617–2621. [Google Scholar] [CrossRef] [PubMed]
- Rao, A.K.; Creager, S.E. Superporous agarose-reticulated vitreous carbon electrodes for electrochemical sandwich bioassays. Anal. Chim. Acta 2008, 628, 190–197. [Google Scholar] [CrossRef]
- Thompson, R.Q.; Porter, M.; Stuver, C.; Halsall, H.B.; Heineman, W.R.; Buckley, E.; Smyth, M.R. Zeptomole detection limit for alkaline phosphatase using 4-aminophenylphosphate, amperometric detection, and an optimal buffer system. Anal. Chim. Acta 1993, 271, 223–229. [Google Scholar] [CrossRef]
- Regiart, M.; Rinaldi-Tosi, M.; Aranda, P.R.; Bertolino, F.A.; Villarroel-Rocha, J.; Sapag, K.; Messina, G.A.; Raba, J.; Fernandez-Baldo, M.A. Development of a nanostructured immunosensor for early and in situ detection of Xanthomonas arboricola in agricultural food production. Talanta 2017, 175, 535–541. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Chen, J.; Liu, C.; Li, W.; Kong, J.; Yang, P.; Liu, B. A sensitive microchip-based immunosensor for electrochemical detection of low-level biomarker S100B. Electroanalysis 2013, 25, 1050–1055. [Google Scholar] [CrossRef]
- Serafin, V.; Ubeda, N.; Agui, L.; Yanez-Sedeno, P.; Pingarron, J.M. Ultrasensitive determination of human growth hormone (hGH) with a disposable electrochemical magneto-immunosensor. Anal. Bioanal. Chem. 2012, 403, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Pandey, B.; Demchenko, A.V.; Stine, K.J. Nanoporous gold as a solid support for protein immobilization and development of an electrochemical immunoassay for prostate specific antigen and carcinoembryonic antigen. Microchim. Acta 2012, 179, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Zhao, Z.; Wan, Q. Facile preparation of carbon nanotube-conducting polymer network for sensitive electrochemical immunoassay of Hepatitis B surface antigen in serum. Bioelectrochemistry 2011, 81, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S. Electrochemical immunosensors for the simultaneous detection of two tumor markers. Anal. Chem. 2005, 77, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Rauh, R.D. Hydroquinone diphosphate: An alkaline phosphatase substrate that does not produce electrode fouling in electrochemical immunoassays. Biosens. Bioelectron. 2004, 20, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Nie, W. Electrochemical multianalyte immunoassays using an array-based sensor. Anal. Chem. 2006, 78, 2507–2513. [Google Scholar] [CrossRef]
- Wilson, M.S.; Nie, W. Multiplex measurement of seven tumor markers using an electrochemical protein chip. Anal. Chem. 2006, 78, 6476–6483. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.S.; Rauh, R.D. Novel amperometric immunosensors based on iridium oxide matrices. Biosens. Bioelectron. 2004, 19, 693–699. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Y.; Wu, J.; Wang, X.; Liu, Y. An amperometric immunosensor based on an ionic liquid and single-walled carbon nanotube composite electrode for detection of tetrodotoxin in pufferfish. J. Agric. Food Chem. 2016, 64, 6888–6894. [Google Scholar] [CrossRef]
- Yang, Z.; Jiang, W.; Liu, F.; Zhou, Y.; Yin, H.; Ai, S. A novel electrochemical immunosensor for the quantitative detection of 5-hydroxymethylcytosine in genomic DNA of breast cancer tissue. Chem. Commun. 2015, 51, 14671–14673. [Google Scholar] [CrossRef]
- Ding, J.; Wang, X.; Qin, W. Pulsed galvanostatic control of a polymeric membrane ion-selective electrode for potentiometric immunoassays. ACS Appl. Mater. Interfaces 2013, 5, 9488–9493. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Guzman, M.; Ojeda, I.; Villalonga, R.; Gonzalez-Cortes, A.; Yanez-Sedeno, P.; Pingarron, J.M. Ultrasensitive detection of adrenocorticotropin hormone (ACTH) using disposable phenylboronic-modified electrochemical immunosensors. Biosens. Bioelectron. 2012, 35, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Del Carlo, M.; Lionti, I.; Taccini, M.; Cagnini, A.; Mascini, M. Disposable screen-printed electrodes for the immunochemical detection of polychlorinated biphenyls. Anal. Chim. Acta 1997, 342, 189–197. [Google Scholar] [CrossRef]
- Masson, M.; Runarsson, O.V.; Johannson, F.; Aizawa, M. 4-Amino-1-naphthylphosphate as a substrate for the amperometric detection of alkaline phosphatase activity and its application for immunoassay. Talanta 2004, 64, 174–180. [Google Scholar] [CrossRef]
- Carralero, V.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Development of a progesterone immunosensor based on a colloidal gold-graphite-teflon composite electrode. Electroanalysis 2007, 19, 853–858. [Google Scholar] [CrossRef]
- Aziz, M.A.; Jo, K.; Qaium, M.A.; Huh, C.-H.; Hong, I.S.; Yang, H. Platform for highly sensitive alkaline phosphatase-based immunosensors using 1-naphthyl phosphate and an avidin-modified indium tin oxide electrode. Electroanalysis 2009, 21, 2160–2164. [Google Scholar] [CrossRef]
- Colozza, N.; Mazzaracchio, V.; Kehe, K.; Tsoutsoulopoulos, A.; Schioppa, S.; Fabiani, L.; Steinritz, D.; Moscone, D.; Arduini, F. Development of novel carbon black-based heterogeneous oligonucleotide-antibody assay for sulfur mustard detection. Sens. Actuators B Chem. 2021, 328, 129054–129064. [Google Scholar] [CrossRef]
- Yugender Goud, K.; Sunil Kumar, V.; Hayat, A.; Vengatajalabathy Gobi, K.; Song, H.; Kim, K.-H.; Marty, J.L. A highly sensitive electrochemical immunosensor for zearalenone using screen-printed disposable electrodes. J. Electroanal. Chem. 2019, 832, 336–342. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Migliorelli, D.; Muhlebach, L.; Generelli, S.; Stewart, L.; Elliott, C.T.; Campbell, K. Highly sensitive electrochemical detection of the marine toxins okadaic acid and domoic acid with carbon black modified screen printed electrodes. Talanta 2021, 228, 122215–122223. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, W.; Wu, H.; Liu, F.; Yin, H.; Lu, N.; Ai, S. Amplified electrochemical immunoassay for 5-methylcytosine using a nanocomposite prepared from graphene oxide, magnetite nanoparticles and β-cyclodextrin. Microchim. Acta 2019, 186, 488–497. [Google Scholar] [CrossRef]
- Lin, J.; He, C.; Zhang, S. Immunoassay channels for α-fetoprotein based on encapsulation of biorecognition molecules into SBA-15 mesopores. Anal. Chim. Acta 2009, 643, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.P.; Deng, Y.J.; Jin, X.Y.; Chen, L.G.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Ultrasensitive electrochemical immunosensor for ochratoxin A using gold colloid-mediated hapten immobilization. Anal. Biochem. 2009, 389, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Bonel, L.; Vidal, J.C.; Duato, P.; Castillo, J.R. Ochratoxin A nanostructured electrochemical immunosensors based on polyclonal antibodies and gold nanoparticles coupled to the antigen. Anal. Methods 2010, 2, 335–341. [Google Scholar] [CrossRef]
- Xu, W.; Qing, Y.; Chen, S.; Chen, J.; Qin, Z.; Qiu, J.; Li, C. Electrochemical indirect competitive immunoassay for ultrasensitive detection of zearalenone based on a glassy carbon electrode modified with carboxylated multi-walled carbon nanotubes and chitosan. Microchim. Acta 2017, 184, 3339–3347. [Google Scholar] [CrossRef]
- Karczmarczyk, A.; Baeumner, A.J.; Feller, K.-H. Rapid and sensitive inhibition-based assay for the electrochemical detection of ochratoxin A and aflatoxin M1 in red wine and milk. Electrochim. Acta 2017, 243, 82–89. [Google Scholar] [CrossRef]
- Yang, X.; Zhou, X.; Zhang, X.; Qing, Y.; Luo, M.; Liu, X.; Li, C.; Li, Y.; Xia, H.; Qiu, J. A highly sensitive electrochemical immunosensor for fumonisin B1 detection in corn using single-walled carbon nanotubes/chitosan. Electroanalysis 2015, 27, 2679–2687. [Google Scholar] [CrossRef]
- Ojeda, I.; Moreno-Guzmán, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. Electrochemical magnetic immunosensors for the determination of ceruloplasmin. Electroanalysis 2013, 25, 2166–2174. [Google Scholar] [CrossRef]
- Ojeda, I.; Moreno-Guzmán, M.; González-Cortés, A.; Yáñez-Sedeño, P.; Pingarrón, J.M. A disposable electrochemical immunosensor for the determination of leptin in serum and breast milk. Analyst 2013, 138, 4284–4291. [Google Scholar] [CrossRef]
- Al-Khafaji, Q.A.M.; Harris, M.; Tombelli, S.; Laschi, S.; Turner, A.P.F.; Mascini, M.; Marrazza, G. An electrochemical immunoassay for HER2 detection. Electroanalysis 2012, 24, 735–742. [Google Scholar] [CrossRef]
- Yin, Z.; Liu, Y.; Jiang, L.P.; Zhu, J.J. Electrochemical immunosensor of tumor necrosis factor α based on alkaline phosphatase functionalized nanospheres. Biosens. Bioelectron. 2011, 26, 1890–1894. [Google Scholar] [CrossRef]
- Chen, S.P.; Yu, X.D.; Xu, J.J.; Chen, H.Y. Gold nanoparticles-coated magnetic microspheres as affinity matrix for detection of hemoglobin A1c in blood by microfluidic immunoassay. Biosens. Bioelectron. 2011, 26, 4779–4784. [Google Scholar] [CrossRef]
- Liu, X.; Wu, H.; Zheng, Y.; Wu, Z.; Jiang, J.; Shen, G.; Yu, R. A sensitive electrochemical immunosensor for α-fetoprotein detection with colloidal gold-based dentritical enzyme complex amplification. Electroanalysis 2010, 22, 244–250. [Google Scholar] [CrossRef]
- Lin, J.; He, C.; Zhang, L.; Zhang, S. Sensitive amperometric immunosensor for α-fetoprotein based on carbon nanotube/gold nanoparticle doped chitosan film. Anal. Biochem. 2009, 384, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Li, C.; Wu, K.; Hu, C.; Yang, N. Detection of tumor marker using ZnO@reduced graphene oxide decorated with alkaline phosphatase-labeled magnetic beads. ACS Appl. Nano Mater. 2019, 2, 7747–7754. [Google Scholar] [CrossRef]
- Fernández-Sánchez, C.; Costa-García, A. 3-Indoxyl phosphate: An alkaline phosphatase substrate for enzyme immunoassays with voltammetric detection. Electroanalysis 1998, 10, 249–255. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Gonzalez-Garcia, M.B.; Costa-Garcia, A. Voltammetric determination of alkaline phosphatase and horseradish peroxidase activity using 3-indoxyl phosphate as substrate Application to enzyme immunoassay. Talanta 2004, 64, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gonzalez, M.; Hernandez-Santos, D.; Gonzalez-Garcia, M.B.; Costa-Garcia, A. Development of an immunosensor for the determination of rabbit IgG using streptavidin modified screen-printed carbon electrodes. Talanta 2005, 65, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Sanchez, C.; Gonzalez-Garcia, M.B.; Costa-Garcia, A. AC voltammetric carbon paste-based enzyme immunosensors. Biosens. Bioelectron. 2000, 14, 917–924. [Google Scholar] [CrossRef]
- Ruan, C.; Yang, L.; Li, Y. Immunobiosensor chips for detection of Escherichia coil O157:H7 using electrochemical impedance spectroscopy. Anal. Chem. 2002, 74, 4814–4820. [Google Scholar] [CrossRef]
- Song, S.; Kim, Y.J.; Shin, I.-S.; Kim, W.-H.; Lee, K.-N.; Seong, W.K. Electrochemical immunoassay based on indium tin oxide activity toward a alkaline phosphatase. BioChip J. 2019, 13, 387–393. [Google Scholar] [CrossRef]
- Kokado, A.; Arakawa, H.; Maeda, M. New electrochemical assay of alkaline phosphatase using ascorbic acid 2-phosphate and its application to enzyme immunoassay. Anal. Chim. Acta 2000, 407, 119–125. [Google Scholar] [CrossRef]
- Kim, S.-E.; Kim, Y.J.; Song, S.; Lee, K.-N.; Seong, W.K. A simple electrochemical immunosensor platform for detection of Apolipoprotein A1 (Apo-A1) as a bladder cancer biomarker in urine. Sens. Actuators B Chem. 2019, 278, 103–109. [Google Scholar] [CrossRef]
- Lee, S.E.; Jeong, S.E.; Hong, J.S.; Im, H.; Hwang, S.Y.; Oh, J.K.; Kim, S.E. Gold-nanoparticle-coated magnetic beads for ALP-enzyme-based electrochemical immunosensing in human plasma. Materials 2022, 15, 6875. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, J.; Xu, J. Ultrasensitive electrochemical immunoassay for screening the influenza A (H1N1) virus based on atomically Ru-dispersed nitrogen-doped carbon. New J. Chem. 2023, 47, 1685–1690. [Google Scholar] [CrossRef]
- Pemberton, R.M.; Hart, J.P.; Stoddard, P.; Foulkes, J.A. A comparison of 1-naphthyl phosphate and 4 aminophenyl phosphate as enzyme substrates for use with a screen-printed amperometric immunosensor for progesterone in cows’ milk. Biosens. Bioelectron. 1999, 14, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, M.P.; O’Sullivan, C.K.; Guilbault, G.G. Alkaline phosphatase as a label for immunoassay using amperometric detection with a variety of substrates and an optimal buffer system. Anal. Chim. Acta 1999, 393, 95–102. [Google Scholar] [CrossRef]
- Preechaworapun, A.; Dai, Z.; Xiang, Y.; Chailapakul, O.; Wang, J. Investigation of the enzyme hydrolysis products of the substrates of alkaline phosphatase in electrochemical immunosensing. Talanta 2008, 76, 424–431. [Google Scholar] [CrossRef]
- Chen, J.; Zou, G.; Zhang, X.; Jin, W. Ultrasensitive electrochemical immunoassay based on counting single magnetic nanobead by a combination of nanobead amplification and enzyme amplification. Electrochem. Commun. 2009, 11, 1457–1459. [Google Scholar] [CrossRef]
- Preechaworapun, A.; Ivandini, T.A.; Suzuki, A.; Fujishima, A.; Chailapakul, O.; Einaga, Y. Development of amperometric immunosensor using boron-doped diamond with poly(o-aminobenzoic acid). Anal. Chem. 2008, 80, 2077–2083. [Google Scholar] [CrossRef]
- Nam, E.J.; Kim, E.J.; Wark, A.W.; Rho, S.; Kim, H.; Lee, H.J. Highly sensitive electrochemical detection of proteins using aptamer-coated gold nanoparticles and surface enzyme reactions. Analyst 2012, 137, 2011–2016. [Google Scholar] [CrossRef]
- Safaei, T.S.; Mohamadi, R.M.; Sargent, E.H.; Kelley, S.O. In situ electrochemical ELISA for specific identification of captured cancer cells. ACS Appl. Mater. Interfaces 2015, 7, 14165–14169. [Google Scholar] [CrossRef]
- Diba, F.S.; Kim, S.; Lee, H.J. Electrochemical immunoassay for amyloid-beta 1–42 peptide in biological fluids interfacing with a gold nanoparticle modified carbon surface. Catal. Today 2017, 295, 41–47. [Google Scholar] [CrossRef]
- Guerrero, S.; Agui, L.; Yanez-Sedeno, P.; Pingarron, J.M. Design of electrochemical immunosensors using electro-click chemistry. Application to the detection of IL-1β cytokine in saliva. Bioelectrochemistry 2020, 133, 107484–107490. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yan, Y.; Zhong, L.; Liu, P.; Sang, Y.; Cheng, W.; Ding, S. Electrochemical sandwich immunoassay for the peptide hormone prolactin using an electrode modified with graphene, single walled carbon nanotubes and antibody-coated gold nanoparticles. Microchim. Acta 2015, 182, 1917–1924. [Google Scholar] [CrossRef]
- Thiruppathiraja, C.; Saroja, V.; Kamatchiammal, S.; Adaikkappan, P.; Alagar, M. Development of electrochemical based sandwich enzyme linked immunosensor for Cryptosporidium parvum detection in drinking water. J. Environ. Monit. 2011, 13, 2782–2787. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dzantiev, B.B. New and improved nanomaterials and approaches for optical bio- and immunosensors. Biosensors 2023, 13, 443. [Google Scholar] [CrossRef]
- Xia, N.; Wu, D.; Sun, T.; Wang, Y.; Ren, X.; Zhao, F.; Liu, L.; Yi, X. Magnetic bead-based electrochemical and colorimetric methods for the detection of poly(ADP-ribose) polymerase-1 with boronic acid derivatives as the signal probes. Sens. Actuators B. Chem. 2021, 327, 128913. [Google Scholar] [CrossRef]
- Xia, N.; Wu, D.; Yu, H.; Sun, W.; Yi, X.; Liu, L. Magnetic bead-based electrochemical and colorimetric assays of circulating tumor cells with boronic acid derivatives as the recognition elements and signal probes. Talanta 2021, 221, 121640. [Google Scholar] [CrossRef]
- Cao, L.; Fang, C.; Zeng, R.; Zhao, X.; Jiang, Y.; Chen, Z. Paper-based microfluidic devices for electrochemical immunofiltration analysis of human chorionic gonadotropin. Biosens. Bioelectron. 2017, 92, 87–94. [Google Scholar] [CrossRef]
- Hou, Y.; Li, T.; Huang, H.; Quan, H.; Miao, X.; Yang, M. Electrochemical immunosensor for the detection of tumor necrosis factor α based on hydrogel prepared from ferrocene modified amino acid. Sens. Actuators B Chem. 2013, 182, 605–609. [Google Scholar] [CrossRef]
- Xia, N.; Deng, D.; Mu, X.; Liu, A.; Xie, J.; Zhou, D.; Yang, P.; Xing, Y.; Liu, L. Colorimetric immunoassays based on pyrroloquinoline quinone-catalyzed generation of Fe(II)-ferrozine with tris(2-carboxyethyl)phosphine as the reducing reagent. Sens. Actuators B Chem. 2020, 306, 127571. [Google Scholar] [CrossRef]
- Kang, C.; Kang, J.; Lee, N.S.; Yoon, Y.H.; Yang, H. DT-diaphorase as a bifunctional enzyme label that allows rapid enzymatic amplification and electrochemical redox cycling. Anal. Chem. 2017, 89, 7974–7980. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.Y.; Inoue, K.; Ito-Sasaki, T.; Ino, K.; Shiku, H. Electrochemical immunoassay with dual-signal amplification for redox cycling within a nanoscale gap. ACS Appl. Nano Mater. 2021, 4, 12393–12400. [Google Scholar] [CrossRef]
- Ito, K.K.Y.I.; Ino, K.; Shiku, H. High-sensitivity amperometric dual immunoassay using two cascade reactions with signal amplification of redox cycling in nanoscale gap. Anal. Chem. 2022, 94, 16451–16460. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Singh, A.; Kim, S.; Yang, H. Electroreduction-based electrochemical-enzymatic redox cycling for the detection of cancer antigen 15-3 using graphene oxide-modified indium-tin oxide electrodes. Anal. Chem. 2014, 86, 1560–1566. [Google Scholar] [CrossRef]
- Kim, K.J.; Song, Y.; Park, S.; Oh, S.J.; Kwon, S.J. Immunosensor for human IgE detection using electrochemical redox cycling with ferrocene-mixed self-assembled monolayers modified Au electrode. Bull. Korean Chem. Soc. 2022, 44, 141–146. [Google Scholar] [CrossRef]
- Yan, K.; Liu, Y.; Guan, Y.; Bhokisham, N.; Tsao, C.Y.; Kim, E.; Shi, X.W.; Wang, Q.; Bentley, W.E.; Payne, G.F. Catechol-chitosan redox capacitor for added amplification in electrochemical immunoanalysis. Colloids Surf. B Biointerfaces 2018, 169, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.R.; Aziz, M.A.; Jo, K.; Tamilavan, V.; Hyun, M.H.; Kim, S.; Yang, H. Optimization of phosphatase- and redox cycling-based immunosensors and its application to ultrasensitive detection of troponin I. Anal. Chem. 2011, 83, 3926–3933. [Google Scholar] [CrossRef]
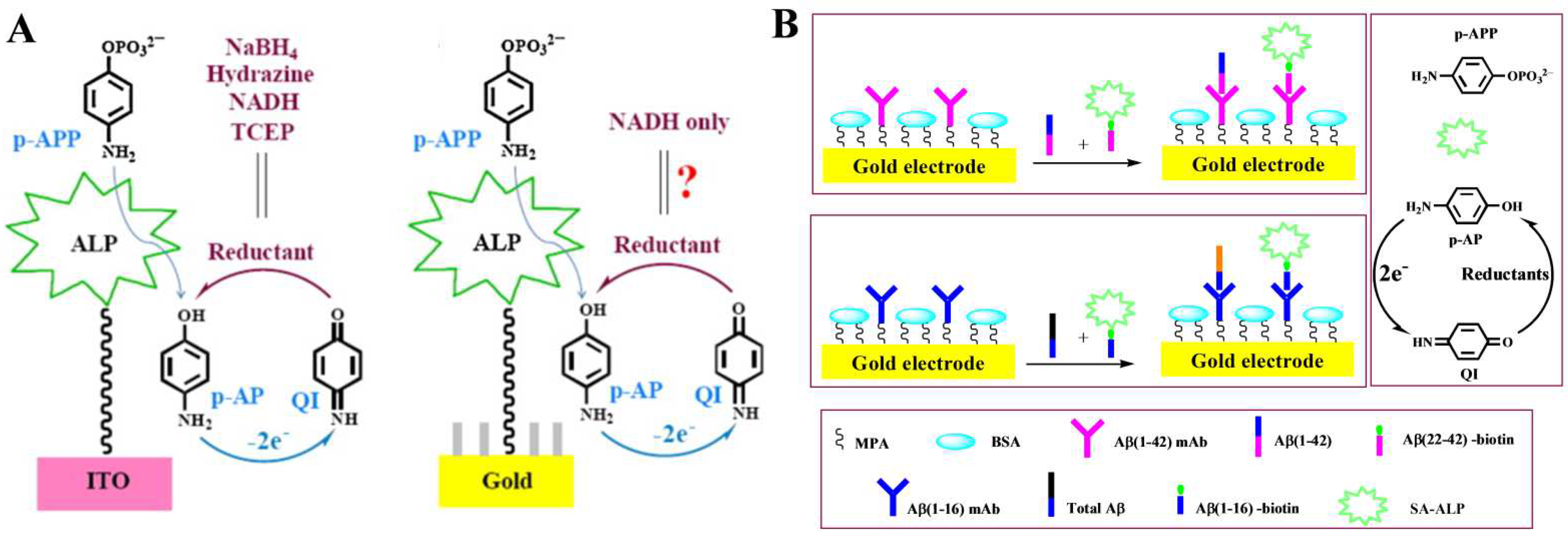
- Seo, J.; Ha, H.; Park, S.; Haque, A.J.; Kim, S.; Joo, J.M.; Yang, H. Immunosensor employing stable, solid 1-amino-2-naphthyl phosphate and ammonia-borane toward ultrasensitive and simple point-of-care testing. ACS Sens. 2017, 2, 1240–1246. [Google Scholar] [CrossRef]
- Xia, N.; Ma, F.; Zhao, F.; He, Q.; Du, J.; Li, S.; Chen, J.; Liu, L. Comparing the performances of electrochemical sensors using p-aminophenol redox cycling by different reductants on gold electrodes modified with self-assembled monolayers. Electrochim. Acta 2013, 109, 348–354. [Google Scholar] [CrossRef]
- Liu, L.; Xia, N.; Liu, H.; Kang, X.; Liu, X.; Xue, C.; He, X. Highly sensitive and label-free electrochemical detection of microRNAs based on triple signal amplification of multifunctional gold nanoparticles, enzymes and redox-cycling reaction. Biosens. Bioelectron. 2014, 53, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, Q.; Zhao, F.; Xia, N.; Liu, H.; Li, S.; Liu, R.; Zhang, H. Competitive electrochemical immunoassay for detection of beta-amyloid (1–42) and total beta-amyloid peptides using p-aminophenol redox cycling. Biosens. Bioelectron. 2014, 51, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, Z.; Chen, J.; Kinchla, A.J.; Nugen, S.R. Rapid detection of Salmonella using a redox cycling-based electrochemical method. Food Control 2016, 62, 81–88. [Google Scholar] [CrossRef]
- Kitani, A.; Miller, L.L. Fast oxidants for NADH and electrochemical discrimination between ascorbic acid and NADH. J. Am. Chem. Soc. 1981, 103, 3595–3597. [Google Scholar] [CrossRef]
- Lertanantawong, B.; O’Mullane, A.P.; Zhang, J.; Surareungchai, W.; Somasundrum, M.; Bond, A.M. Investigation of mediated oxidation of ascorbic acid by ferrocenemethanol using large-amplitude Fourier transformed ac voltammetry under quasi-reversible electron-transfer conditions at an indium tin oxide electrode. Anal. Chem. 2008, 80, 6515–6525. [Google Scholar] [CrossRef]
- Rahman, M.A.; Son, J.I.; Won, M.S.; Shim, Y.B. Gold nanoparticles doped conducting polymer nanorod electrodes: Ferrocene catalyzed aptamer-based thrombin immunosensor. Anal. Chem. 2009, 81, 6604–6611. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Li, M.; Qing, Y.; Dai, N.; Guan, W.; Liang, W.; Wang, D. Signal-on electrochemical immunoassay for APE1 using ionic liquid doped Au nanoparticle/graphene as a nanocarrier and alkaline phosphatase as enhancer. Analyst 2014, 139, 6563–6568. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhuo, Y.; Chai, Y.; Yuan, R.; Zhang, W.; Zhu, Q. Simultaneous electrochemical detection of multiple tumor markers based on dual catalysis amplification of multi-functionalized onion-like mesoporous graphene sheets. Anal. Chim. Acta 2012, 746, 70–76. [Google Scholar] [CrossRef]
- Yang, Z.H.; Zhuo, Y.; Yuan, R.; Chai, Y.Q. An amplified electrochemical immunosensor based on in situ-produced 1-naphthol as electroactive substance and graphene oxide and Pt nanoparticles functionalized CeO2 nanocomposites as signal enhancer. Biosens. Bioelectron. 2015, 69, 321–327. [Google Scholar] [CrossRef]
- Hayat, A.; Andreescu, S. Nanoceria particles as catalytic amplifiers for alkaline phosphatase assays. Anal. Chem. 2013, 85, 10028–10032. [Google Scholar] [CrossRef]
- Han, J.; Zhuo, Y.; Chai, Y.; Xiang, Y.; Yuan, R.; Yuan, Y.; Liao, N. Ultrasensitive electrochemical strategy for trace detection of APE-1 via triple signal amplification strategy. Biosens. Bioelectron. 2013, 41, 116–122. [Google Scholar] [CrossRef]
- Das, J.; Aziz, M.A.; Yang, H. A nanocatalyst-based assay for proteins: DNA-free ultrasensitive electrochemical detection using catalytic reduction of p-nitrophenol by gold-nanoparticle labels. J. Am. Chem. Soc. 2006, 128, 16022–16023. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Jo, K.; Lee, J.W.; Yang, H. Electrochemical immunosensor using p-aminophenol redox cycling by hydrazine combined with a low background current. Anal. Chem. 2007, 79, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.J.; Yang, H.; Jo, K.; Kwak, J. An electrochemical immunosensor using p-aminophenol redox cycling by NADH on a self-assembled monolayer and ferrocene-modified Au electrodes. Analyst 2008, 133, 1599–1604. [Google Scholar] [CrossRef] [PubMed]
- Akanda, M.R.; Choe, Y.L.; Yang, H. Outer-sphere to inner-sphere redox cycling for ultrasensitive immunosensors. Anal. Chem. 2012, 84, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Carralero, V.; Gonzalez-Cortes, A.; Yanez-Sedeno, P.; Pingarron, J.M. Nanostructured progesterone immunosensor using a tyrosinase-colloidal gold-graphite-Teflon biosensor as amperometric transducer. Anal. Chim. Acta 2007, 596, 86–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiang, Y.; Chai, Y.; Yuan, R.; Qian, X.; Zhang, H.; Chen, Y.; Su, J.; Xu, J. Gold nanolabels and enzymatic recycling dual amplification-based electrochemical immunosensor for the highly sensitive detection of carcinoembryonic antigen. Sci. China Chem. 2011, 54, 1770–1776. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, R.; Chai, Y.; Zhuo, Y.; Bai, L.; Liao, Y. An electrochemical enzyme bioaffinity electrode based on biotin-streptavidin conjunction and bienzyme substrate recycling for amplification. Anal. Biochem. 2010, 405, 121–126. [Google Scholar] [CrossRef]
- Fanjul-Bolado, P.; Hernandez-Santos, D.; Gonzalez-Garcia, M.B.; Costa-Garcia, A. Alkaline phosphatase-catalyzed silver deposition for electrochemical detection. Anal. Chem. 2007, 79, 5272–5277. [Google Scholar] [CrossRef]
- Hwang, S.; Kim, E.; Kwak, J. Electrochemical detection of DNA hybridization using biometallization. Anal. Chem. 2005, 77, 579–584. [Google Scholar] [CrossRef]
- Tan, Y.; Chu, X.; Shen, G.L.; Yu, R.Q. A signal-amplified electrochemical immunosensor for aflatoxin B1 determination in rice. Anal. Biochem. 2009, 387, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.P.; Peng, Z.F.; Luo, Y.; Qu, B.; Jiang, J.H.; Zhang, X.B.; Shen, G.L.; Yu, R.Q. Successively amplified electrochemical immunoassay based on biocatalytic deposition of silver nanoparticles and silver enhancement. Biosens. Bioelectron. 2007, 23, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-P.; Peng, Z.-F.; Jiang, J.-H.; Zhang, X.-B.; Shen, G.-L.; Yu, R.-Q. An electrochemical amplification immunoassay using biocatalytic metal deposition coupled with anodic stripping voltammetric detection. Sens. Actuators B Chem. 2008, 129, 146–151. [Google Scholar] [CrossRef]
- Ma, X.; Hao, Y.; Dong, X.; Xia, N. Biosensors with metal ion–phosphate chelation interaction for molecular recognition. Molecules 2023, 28, 4394. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chang, Y.; Li, Y.; Qiao, M.; Liu, L. Biosensors based on the binding events of nitrilotriacetic acid–metal complexes. Biosensors 2023, 13, 507. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Liu, G.; Li, S.; Liu, L.; Song, Q. Biorecognition element-free electrochemical detection of recombinant glycoproteins using metal-organic frameworks as signal tags. Anal. Chim. Acta 2023, 1273, 341540–341546. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Liu, M.; Wu, T.; Lin, R.; Liu, L.; Song, Q. Competitive electrochemical immunosensors by immobilization of hexahistidine-rich recombinant proteins on the signal labels. J. Electroanal. Chem. 2023, 944, 117662–117667. [Google Scholar] [CrossRef]
- Liu, L.; Ma, X.; Chang, Y.; Guo, H.; Wang, W. Biosensors with boronic acid-based materials as the recognition elements and signal labels. Biosensors 2023, 13, 785. [Google Scholar] [CrossRef]
- Qu, B.; Chu, X.; Shen, G.; Yu, R. A novel electrochemical immunosensor based on colabeled silica nanoparticles for determination of total prostate specific antigen in human serum. Talanta 2008, 76, 785–790. [Google Scholar] [CrossRef]
- Qu, B.; Guo, L.; Chu, X.; Wu, D.H.; Shen, G.L.; Yu, R.Q. An electrochemical immunosensor based on enzyme-encapsulated liposomes and biocatalytic metal deposition. Anal. Chim. Acta 2010, 663, 147–152. [Google Scholar] [CrossRef]
- Peng, X.; Luo, G.; Wu, Z.; Wen, W.; Zhang, X.; Wang, S. Fluorescent-magnetic-catalytic nanospheres for dual-modality detection of H9N2 avian influenza virus. ACS Appl. Mater. Interfaces 2019, 11, 41148–41156. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, X.; Yang, H.; Cao, L.; Deng, W.; Tan, Y.; Xie, Q. Three-dimensional macroporous gold electrodes superior to conventional gold disk electrodes in the construction of an electrochemical immunobiosensor for Staphylococcus aureus detection. Analyst 2020, 145, 2988–2994. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Wang, Y.; Zhang, J.; Xing, Y.; Li, G.; Deng, D.; Liu, L. Overview on the design of magnetically assisted electrochemical biosensors. Biosensors 2022, 12, 954. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Zhou, C.H.; Chen, J.J.; Xiong, C.; Chen, Z.; Pang, D.W.; Zhang, Z.L. Bifunctional magnetic nanobeads for sensitive detection of avian influenza A (H7N9) virus based on immunomagnetic separation and enzyme-induced metallization. Biosens. Bioelectron. 2015, 68, 586–592. [Google Scholar] [CrossRef] [PubMed]
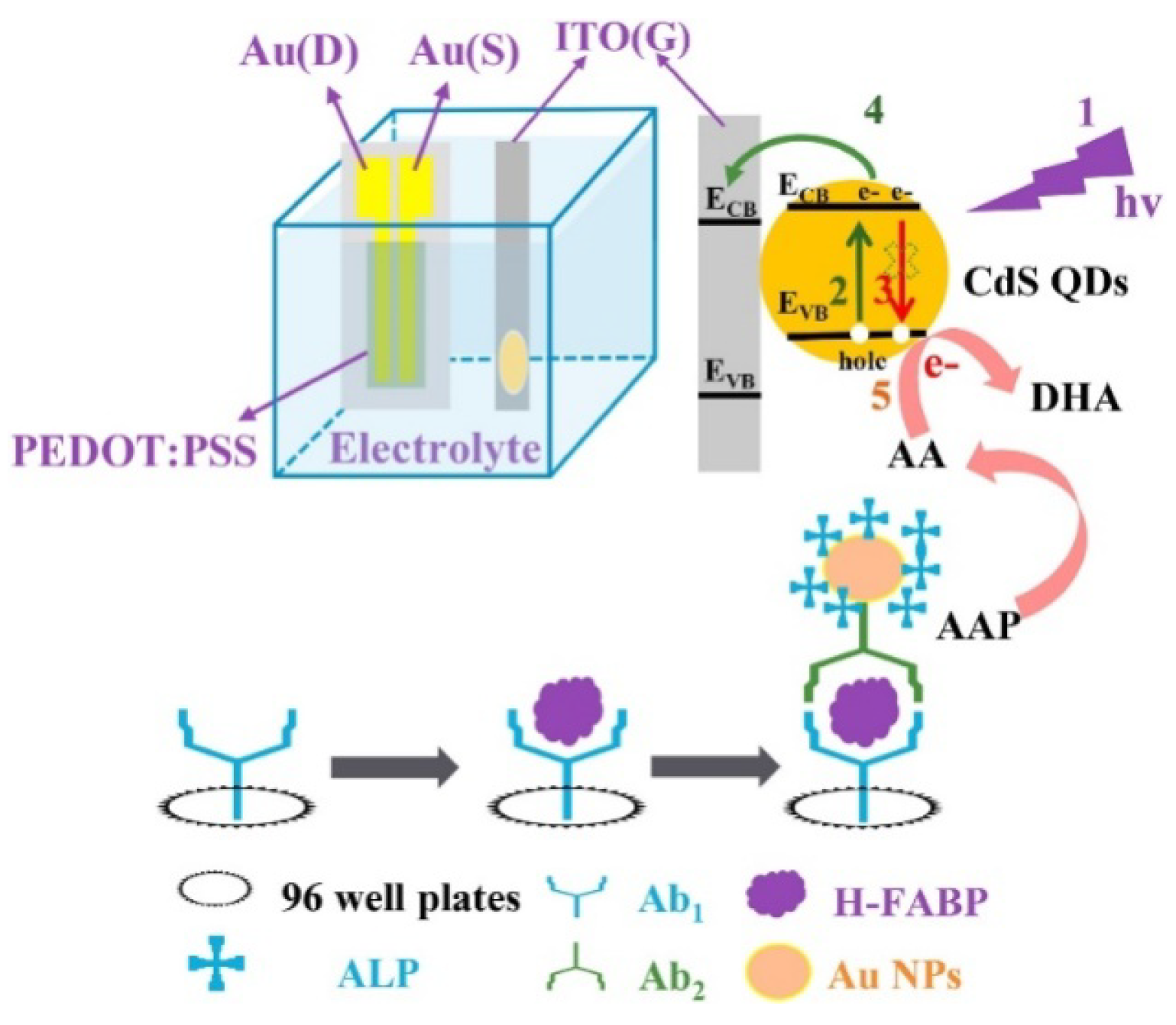
- Neves, M.M.P.S.; González-García, M.B.; Santos-Silva, A.; Costa-García, A. Voltammetric immunosensor for the diagnosis of celiac disease based on the quantification of anti-gliadin antibodies. Sens. Actuators B Chem. 2012, 163, 253–259. [Google Scholar] [CrossRef]
- Marques, R.C.B.; Costa-Rama, E.; Viswanathan, S.; Nouws, H.P.A.; Costa-García, A.; Delerue-Matos, C.; González-García, M.B. Voltammetric immunosensor for the simultaneous analysis of the breast cancer biomarkers CA 15-3 and HER2-ECD. Sens. Actuators B Chem. 2018, 255, 918–925. [Google Scholar] [CrossRef]
- Rama, E.C.; González-García, M.B.; Costa-García, A. Competitive electrochemical immunosensor for amyloid-beta 1–42 detection based on gold nanostructurated Screen-Printed Carbon Electrodes. Sens. Actuators B Chem. 2014, 201, 567–571. [Google Scholar] [CrossRef]
- Alves, R.C.; Pimentel, F.B.; Nouws, H.P.; Marques, R.C.; Gonzalez-Garcia, M.B.; Oliveira, M.B.; Delerue-Matos, C. Detection of Ara h 1 (a major peanut allergen) in food using an electrochemical gold nanoparticle-coated screen-printed immunosensor. Biosens. Bioelectron. 2015, 64, 19–24. [Google Scholar] [CrossRef]
- Neves, M.M.P.S.; Nouws, H.P.A.; Santos-Silva, A.; Delerue-Matos, C. Neutrophil gelatinase-associated lipocalin detection using a sensitive electrochemical immunosensing approach. Sens. Actuators B Chem. 2020, 304, 127285–127292. [Google Scholar] [CrossRef]
- Silva, N.F.D.; Neves, M.; Magalhaes, J.; Freire, C.; Delerue-Matos, C. Electrochemical immunosensor towards invasion-associated protein p60: An alternative strategy for Listeria monocytogenes screening in food. Talanta 2020, 216, 120976–120982. [Google Scholar] [CrossRef]
- Freitas, M.; Nouws, H.P.A.; Keating, E.; Delerue-Matos, C. High-performance electrochemical immunomagnetic assay for breast cancer analysis. Sens. Actuators B Chem. 2020, 308, 127667–127676. [Google Scholar] [CrossRef]
- Wang, J.; Polsky, R.; Xu, D. Silver-enhanced colloidal gold electrochemical stripping detection of DNA hybridization. Langmuir 2001, 17, 5739–5741. [Google Scholar] [CrossRef]
- Chu, X.; Fu, X.; Chen, K.; Shen, G.L.; Yu, R.Q. An electrochemical stripping metalloimmunoassay based on silver-enhanced gold nanoparticle label. Biosens. Bioelectron. 2005, 20, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Q.; Wang, K.; Tan, W.; Li, H. Enhanced surface plasmon resonance with the modified catalytic growth of Au nanoparticles. Biosens. Bioelectron. 2007, 22, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.-T.; Huang, H.-J. Femtomolar immunoassay based on coupling gold nanoparticle enlargement with square wave stripping voltammetry. Anal. Chim. Acta 2005, 538, 159–164. [Google Scholar] [CrossRef]
- Zhang, P.; Guo, X.; Wang, H.; Sun, Y.; Kang, Q.; Shen, D. An electrode-separated piezoelectric immunosensor array with signal enhancement based on enzyme catalytic deposition of palladium nanoparticles and electroless deposition nickel-phosphorus. Sens. Actuators B Chem. 2017, 248, 551–559. [Google Scholar] [CrossRef]
- Li, X.-Y.; Yi, Z.; Tang, H.; Chu, X.; Yu, R.-Q. A novel electrochemical immunosensor based on dual signal amplification of gold nanoparticles and telomerase extension reaction. Anal. Methods 2014, 6, 2221–2226. [Google Scholar] [CrossRef]
- Narayanan, J.; Sharma, M.K.; Ponmariappan, S.; Sarita; Shaik, M.; Upadhyay, S. Electrochemical immunosensor for botulinum neurotoxin type-E using covalently ordered graphene nanosheets modified electrodes and gold nanoparticles-enzyme conjugate. Biosens. Bioelectron. 2015, 69, 249–256. [Google Scholar] [CrossRef]
- Liu, C.; Dong, J.; Waterhouse, G.I.N.; Cheng, Z.; Ai, S. Electrochemical immunosensor with nanocellulose-Au composite assisted multiple signal amplification for detection of avian leukosis virus subgroup. J. Biosens. Bioelectron. 2018, 101, 110–115. [Google Scholar] [CrossRef]
- Zhang, S.; Li, R.; Liu, X.; Yang, L.; Lu, Q.; Liu, M.; Li, H.; Zhang, Y.; Yao, S. A novel multiple signal amplifying immunosensor based on the strategy of in situ-produced electroactive substance by ALP and carbon-based Ag-Au bimetallic as the catalyst and signal enhancer. Biosens. Bioelectron. 2017, 92, 457–464. [Google Scholar] [CrossRef]
- Lai, G.; Yan, F.; Wu, J.; Leng, C.; Ju, H. Ultrasensitive multiplexed immunoassay with electrochemical stripping analysis of silver nanoparticles catalytically deposited by gold nanoparticles and enzymatic reaction. Anal. Chem. 2011, 83, 2726–2732. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Tang, D.; Que, X.; Zhuang, J.; Fu, L.; Chen, G. Enzyme-catalyzed silver deposition on irregular-shaped gold nanoparticles for electrochemical immunoassay of alpha-fetoprotein. Anal. Chim. Acta 2012, 755, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Wen, Q.; Jiang, J.-H.; Shen, G.-L.; Yu, R.-Q. A novel electrochemical immunosensor based on hydrogen evolution inhibition by enzymatic copper deposition on platinum nanoparticle-modified electrode. Biosen. Bioelectron. 2008, 24, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kameswara Rao, V.; Vrat Kamboj, D.; Jain, R. Electrochemical immunosensor for Staphylococcal Enterotoxin B (SEB) based on platinum nanoparticles-modified electrode using hydrogen evolution inhibition approach. Electroanalysis 2014, 26, 2320–2327. [Google Scholar] [CrossRef]
- Lu, F.; Yang, L.; Hou, T.; Li, F. Label-free and “signal-on” homogeneous photoelectrochemical cytosensing strategy for ultrasensitive cancer cell detection. Chem. Commun. 2020, 56, 11126–11129. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Ma, H.; Zhang, Y.; Wang, Q.; Yang, Z.; Du, B.; Wu, D.; Wei, Q. Photoelectrochemical sensitive detection of insulin based on CdS/polydopamine co-sensitized WO3 nanorod and signal amplification of carbon nanotubes@polydopamine. Biosens. Bioelectron. 2017, 96, 345–350. [Google Scholar] [CrossRef]
- Gu, T.; Gu, M.; Liu, Y.L.; Dong, Y.; Zhu, L.B.; Li, Z.; Wang, G.L.; Zhao, W.W. In situ chemical redox and functionalization of graphene oxide: Toward new cathodic photoelectrochemical bioanalysis. Chem. Commun. 2019, 55, 10072–10075. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, F.; Wu, X.; Dong, Y.; Wang, G.L. Enzymatic in situ generation of covalently conjugated electron acceptor of PbSe quantum dots for high throughput and versatile photoelectrochemical bioanalysis. Anal. Chim. Acta 2019, 1058, 1–8. [Google Scholar] [CrossRef]
- Liu, K.; Deng, H.; Wang, Y.; Cheng, S.; Xiong, X.; Li, C. A sandwich-type photoelectrochemical immunosensor based on ReS2 nanosheets for high-performance determination of carcinoembryonic antigen. Sens. Actuators B Chem. 2020, 320, 128341–128348. [Google Scholar] [CrossRef]
- Su, L.; Tong, P.; Zhang, L.; Luo, Z.; Fu, C.; Tang, D.; Zhang, Y. Photoelectrochemical immunoassay of aflatoxin B1 in foodstuff based on amorphous TiO2 and CsPbBr3 perovskite nanocrystals. Analyst 2019, 144, 4880–4886. [Google Scholar] [CrossRef]
- Wang, F.X.; Ye, C.; Mo, S.; Liao, L.L.; Zhang, X.F.; Ling, Y.; Lu, L.; Luo, H.Q.; Li, N.B. A novel signal-on photoelectrochemical sensor for ultrasensitive detection of alkaline phosphatase activity based on a TiO2/g-C3N4 heterojunction. Analyst 2018, 143, 3399–3407. [Google Scholar] [CrossRef]
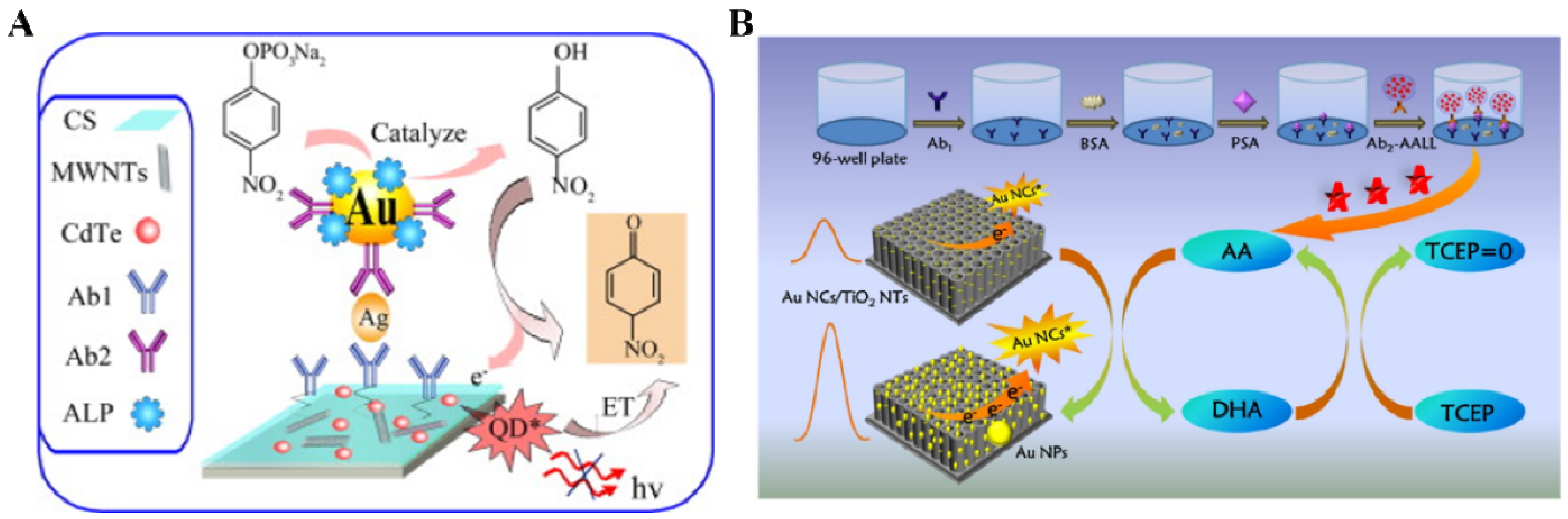
- Zhu, Y.C.; Zhang, N.; Ruan, Y.F.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Alkaline phosphatase tagged antibodies on gold nanoparticles/TiO2 nanotubes electrode: A plasmonic strategy for label-free and amplified photoelectrochemical immunoassay. Anal. Chem. 2016, 88, 5626–5630. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Yu, Z.; Lu, L.; Huang, X.; Wei, Q.; Tang, D. Smartphone-based photoelectrochemical immunoassay of prostate-specific antigen based on Co-doped Bi2O2S nanosheets. Biosens. Bioelectron. 2023, 230, 115260–115266. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; You, L.; He, Q.; Zhuang, W.; Huang, B.; Zheng, D. Surface plasmon resonance-enhanced photoelectrochemical immunoassay of prostate-specific antigen based on BiOCl-Au heterojunction. Electroanalysis 2023, 35, e202300001–e202300007. [Google Scholar] [CrossRef]
- Wang, F.X.; Ye, C.; Mo, S.; Liao, L.L.; Luo, H.Q.; Li, N.B. A novel photoelectrochemical sensing platform based on Fe2O3@Bi2S3 heterojunction for an enzymatic process and enzyme activity inhibition reaction. Sens. Actuators B Chem. 2019, 288, 202–209. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Wang, M.; Yin, H.; Ai, S. A novel signal-on strategy for M.SssI methyltransfease activity analysis and inhibitor screening based on photoelectrochemical immunosensor. Biosens. Bioelectron. 2015, 66, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ma, Z.Y.; Ruan, Y.F.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Simultaneous photoelectrochemical immunoassay of dual cardiac markers using specific enzyme tags: A proof of principle for multiplexed bioanalysis. Anal. Chem. 2016, 88, 1990–1994. [Google Scholar] [CrossRef]
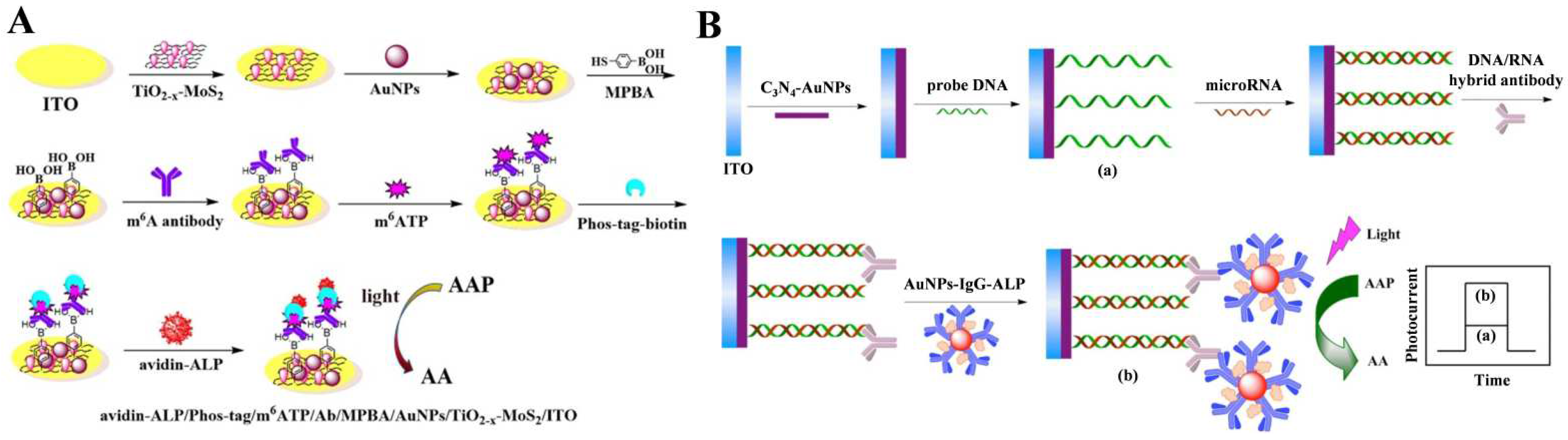
- Ai, L.; Wang, Y.; Zhou, Y.; Yin, H. Photoelectrochemical biosensor for N6-methyladenosine detection based on enhanced photoactivity of TiO2−X and MoS2 nanocomposite. J. Electroanal. Chem. 2021, 895, 115444–115452. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Zhang, Y.; Du, B.; Wei, Q. Ultrasensitive photoelectrochemical immunosensor based on Cu-doped TiO2 and carbon nitride for detection of carcinoembryonic antigen. Carbon 2019, 146, 276–283. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, C.; Li, P.; Wu, T.; Yang, N.; Wang, X.; Wang, Y.; Li, C. ZnS/C/MoS2 nanocomposite derived from metal-organic framework for high-performance photo-electrochemical immunosensing of carcinoembryonic antigen. Small 2019, 15, e1902086–e1902095. [Google Scholar] [CrossRef]
- Sun, B.; Qiao, F.; Chen, L.; Zhao, Z.; Yin, H.; Ai, S. Effective signal-on photoelectrochemical immunoassay of subgroup J avian leukosis virus based on Bi2S3 nanorods as photosensitizer and in situ generated ascorbic acid for electron donating. Biosens. Bioelectron. 2014, 54, 237–243. [Google Scholar] [CrossRef]
- Yin, H.; Zhou, Y.; Li, B.; Li, X.; Yang, Z.; Ai, S.; Zhang, X. Photoelectrochemical immunosensor for microRNA detection based on gold nanoparticles-functionalized g-C3N4 and anti-DNA:RNA antibody. Sens. Actuators B Chem. 2016, 222, 1119–1126. [Google Scholar] [CrossRef]
- Zeng, X.; Bao, J.; Han, M.; Tu, W.; Dai, Z. Quantum dots sensitized titanium dioxide decorated reduced graphene oxide for visible light excited photoelectrochemical biosensing at a low potential. Biosens. Bioelectron. 2014, 54, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Dong, J.; Cui, L.; Feng, T.; Zhu, J.; Liu, X.; Ai, S. A dual signal-on photoelectrochemical immunosensor for sensitively detecting target avian viruses based on AuNPs/g-C3N4 coupling with CdTe quantum dots and in situ enzymatic generation of electron donor. Biosens. Bioelectron. 2019, 124–125, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Ruan, Y.F.; Ma, Z.Y.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Simultaneous photoelectrochemical and visualized immunoassay of β-human chorionic gonadotrophin. Biosens. Bioelectron. 2016, 85, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Y.; Xu, F.; Qin, Y.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Invoking direct exciton-plasmon interactions by catalytic Ag deposition on Au nanoparticles: Photoelectrochemical bioanalysis with high efficiency. Anal. Chem. 2016, 88, 4183–4187. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Xie, X.; Chang, W.; Yang, Z.; Liu, Y. Ultrasensitive photoelectrochemical detection of microcystin-LR based on hybridization chain reaction assisted exciton-plasmon interaction and enzymatic biocatalytic precipitation. Sens. Actuators B Chem. 2018, 276, 180–188. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, Y.; Liao, W.; Yin, H.; Ai, S. A novel signal-on photoelectrochemical biosensor for detection of 5-hydroxymethylcytosine based on in situ electron donor producing strategy and all wavelengths of light irradiation. Sens. Actuators B Chem. 2016, 223, 621–625. [Google Scholar] [CrossRef]
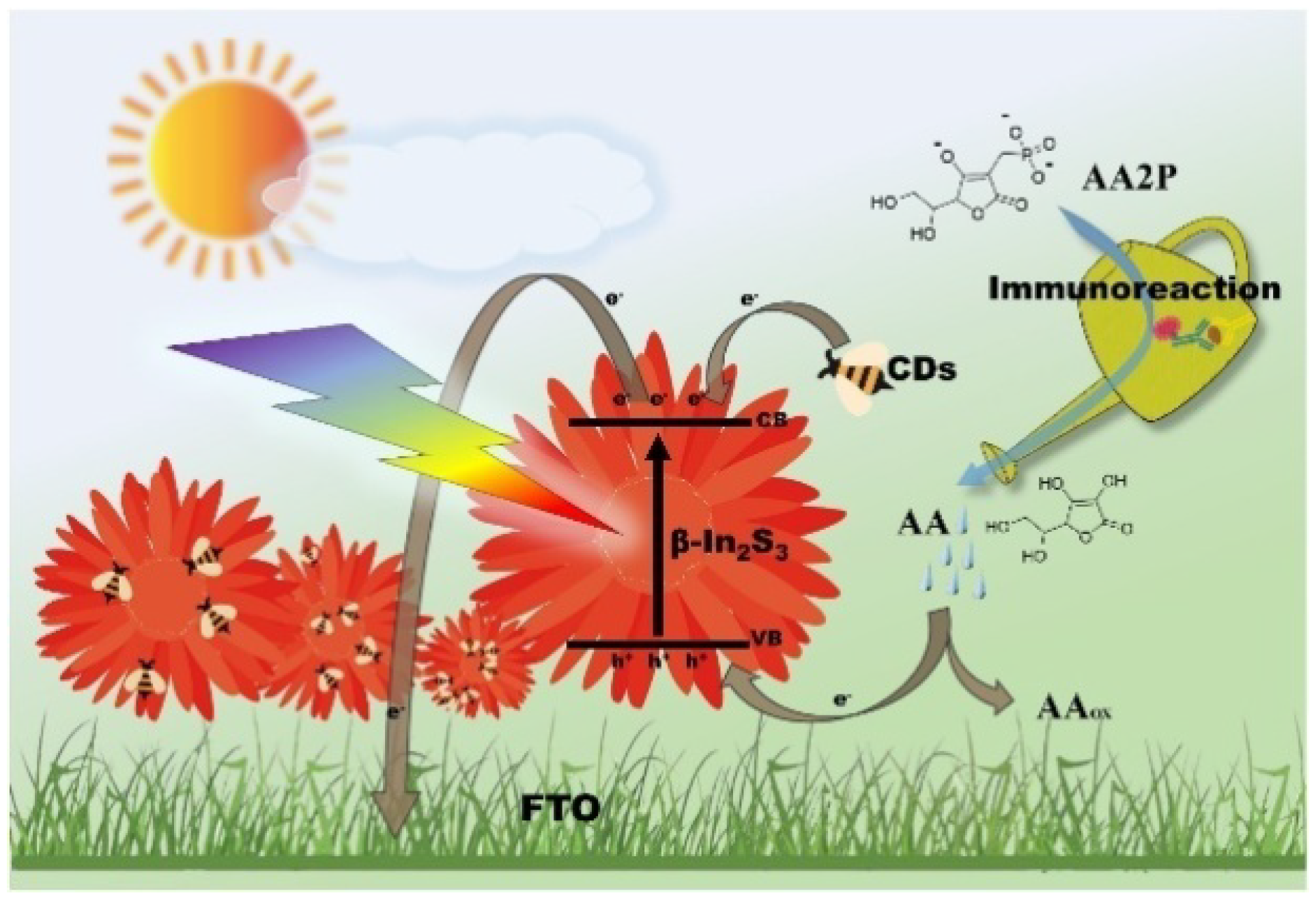
- Yu, Z.; Huang, L.; Chen, J.; Tang, Y.; Xia, B.; Tang, D. Full-spectrum responsive photoelectrochemical immunoassay based on β-In2S3@carbon dot nanoflowers. Electrochim. Acta 2020, 332, 135473–135480. [Google Scholar] [CrossRef]
- Li, N.; Fu, C.; Wang, F.; Sun, Y.; Zhang, L.; Ge, S.; Zhu, P.; Yu, J. Photoelectrochemical detection of let-7a based on toehold-mediated strand displacement reaction and Bi2S3 nanoflower for signal amplification. Sens. Actuators B Chem. 2020, 323, 128655–128661. [Google Scholar] [CrossRef]
- Zhuang, J.; Tang, D.; Lai, W.; Xu, M.; Tang, D. Target-induced nano-enzyme reactor mediated hole-trapping for high-throughput immunoassay based on a split-type photoelectrochemical detection strategy. Anal. Chem. 2015, 87, 9473–9480. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.P.; Liu, F.; Pan, J.B.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Enediol-ligands-encapsulated liposomes enables sensitive immunoassay: A proof-of-concept for general liposomes-based photoelectrochemical bioanalysis. Anal. Chem. 2017, 89, 6300–6304. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhou, Q.; Tang, D. Dopamine-loaded liposomes for in-situ amplified photoelectrochemical immunoassay of AFB1 to enhance photocurrent of Mn2+-doped Zn3OH2V2O7 nanobelts. Anal. Chem. 2017, 89, 11803–11810. [Google Scholar] [CrossRef]
- Wei, J.; Chen, H.; Chen, H.; Cui, Y.; Qileng, A.; Qin, W.; Liu, W.; Liu, Y. Multifunctional peroxidase-encapsulated nanoliposomes: Bioetching-induced photoelectrometric and colorimetric immunoassay for broad-spectrum detection of ochratoxins. ACS Appl. Mater. Interfaces 2019, 11, 23832–23839. [Google Scholar] [CrossRef]
- Yu, Z.; Gong, H.; Li, M.; Tang, D. Hollow prussian blue nanozyme-richened liposome for artificial neural network-assisted multimodal colorimetric-photothermal immunoassay on smartphone. Biosens. Bioelectron. 2022, 218, 114751–114759. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gong, H.; Xu, J.; Li, Y.; Xue, F.; Zeng, Y.; Liu, X.; Tang, D. Liposome-embedded Cu2−xAgxS nanoparticle-mediated photothermal immunoassay for daily monitoring of cTnI protein using a portable thermal imager. Anal. Chem. 2022, 94, 7408–7416. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Han, B.; Liu, W.; Zhou, J.; Liu, K.; Yang, D.; Tang, D. Liposome-amplified photoelectrochemical immunoassay for highly sensitive monitoring of disease biomarkers based on a split-type strategy. Biosens. Bioelectron. 2018, 99, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, J.; Gao, G.; Liu, X.-N.; Wu, J.-Q.; Xu, Y.-T.; Zhou, H.; Zhao, W.-W.; Xu, J.-J.; Chen, H.-Y. Organic photoelectrochemical transistor detection of tear lysozyme. Sens. Diagn. 2022, 1, 294–300. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, Z.; Hu, J.; Wei, W.; Zeng, X.; Zhao, W.W.; Lin, P. Ascorbic acid-mediated organic photoelectrochemical transistor sensing strategy for highly sensitive detection of heart-type fatty acid binding protein. Biosens. Bioelectron. 2022, 201, 113958–113964. [Google Scholar] [CrossRef]
- Mei, L.P.; Jiang, X.Y.; Yu, X.D.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Cu nanoclusters-encapsulated liposomes: Toward sensitive liposomal photoelectrochemical immunoassay. Anal. Chem. 2018, 90, 2749–2755. [Google Scholar] [CrossRef]
- Wei, J.; Liu, S.; Qileng, A.; Qin, W.; Liu, W.; Wang, K.; Liu, Y. A photoelectrochemical/colorimetric immunosensor for broad-spectrum detection of ochratoxins using bifunctional copper oxide nanoflowers. Sens. Actuators B Chem. 2021, 330, 129380–129389. [Google Scholar] [CrossRef]
- Wei, J.; Chang, W.; Qileng, A.; Liu, W.; Zhang, Y.; Rong, S.; Lei, H.; Liu, Y. Dual-modal split-type immunosensor for sensitive detection of microcystin-LR: Enzyme-induced photoelectrochemistry and colorimetry. Anal. Chem. 2018, 90, 9606–9613. [Google Scholar] [CrossRef] [PubMed]
- Qileng, A.; Zhu, H.; Liu, S.; He, L.; Qin, W.; Liu, W.; Xu, Z.; Liu, Y. Machine learning: Assisted multivariate detection and visual image matching to build broad-specificity immunosensor. Sens. Actuators B Chem. 2021, 339, 129872–129881. [Google Scholar] [CrossRef]
- Cao, J.T.; Wang, B.; Dong, Y.X.; Wang, Q.; Ren, S.W.; Liu, Y.M.; Zhao, W.W. Photogenerated hole-induced chemical redox cycling on Bi2S3/Bi2Sn2O7 heterojunction: Toward general amplified split-type photoelectrochemical immunoassay. ACS Sens. 2018, 3, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Mei, L.P.; Ma, Y.; Xu, Y.T.; Ren, S.W.; Cao, J.T.; Liu, Y.M.; Zhao, W.W. Photoelectrochemical-chemical-chemical redox cycling for advanced signal amplification: Proof-of-concept toward ultrasensitive photoelectrochemical bioanalysis. Anal. Chem. 2018, 90, 12347–12351. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.T.; Lv, J.L.; Liao, X.J.; Ma, S.H.; Liu, Y.M. A membraneless self-powered photoelectrochemical biosensor based on Bi2S3/BiPO4 heterojunction photoanode coupling with redox cycling signal amplification strategy. Biosens. Bioelectron. 2022, 195, 113651–113656. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, Y.T.; Lv, J.L.; Xue, T.Y.; Ren, S.W.; Cao, J.T.; Liu, Y.M.; Zhao, W.W. Ru(NH3)63+/Ru(NH3)62+-mediated redox cycling: Toward enhanced triple signal amplification for photoelectrochemical immunoassay. Anal. Chem. 2019, 91, 3768–3772. [Google Scholar] [CrossRef]
- Liao, X.J.; Xiao, H.J.; Cao, J.T.; Ren, S.W.; Liu, Y.M. A novel split-type photoelectrochemical immunosensor based on chemical redox cycling amplification for sensitive detection of cardiac troponin I. Talanta 2021, 233, 122564–122570. [Google Scholar] [CrossRef]
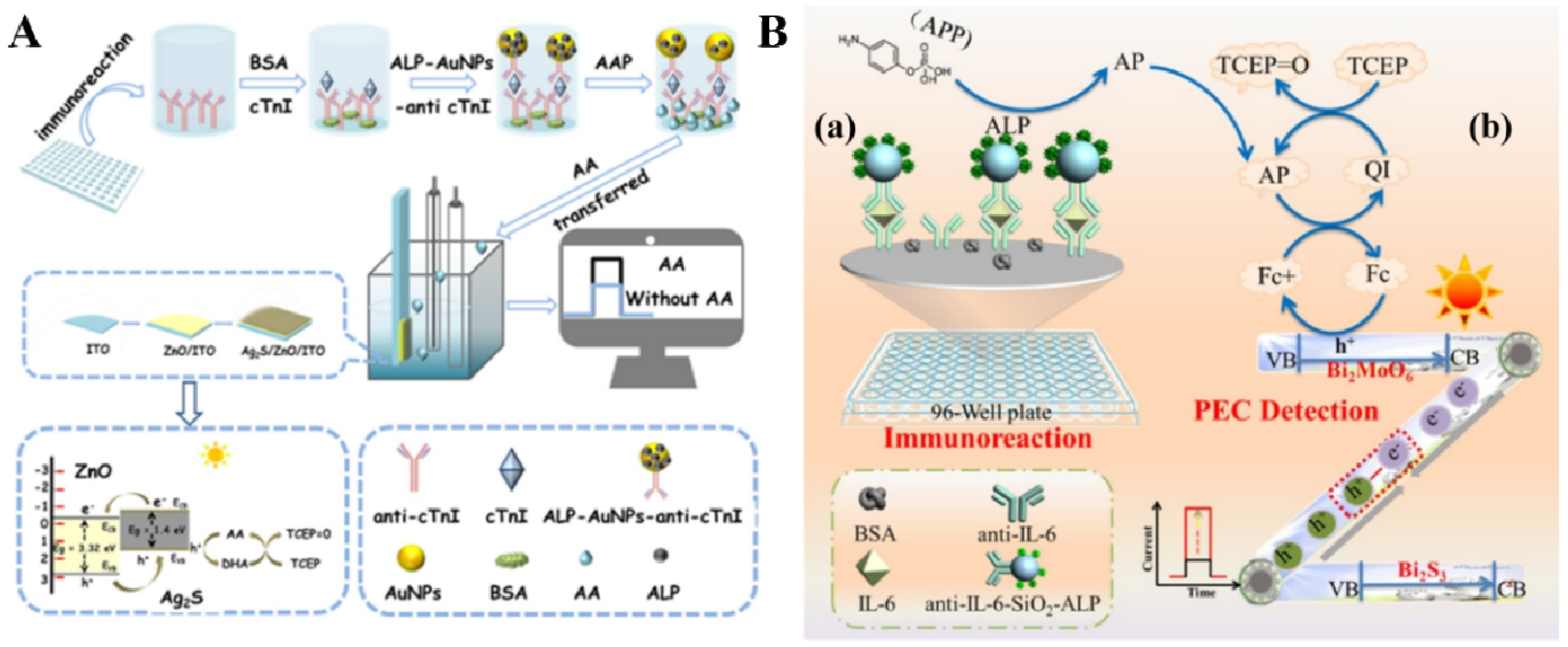
- Cao, J.T.; Lv, J.L.; Liao, X.J.; Ma, S.H.; Liu, Y.M. Photogenerated hole-induced chemical-chemical redox cycling strategy on a direct Z-scheme Bi2S3/Bi2MoO6 heterostructure photoelectrode: Toward an ultrasensitive photoelectrochemical immunoassay. Anal. Chem. 2021, 93, 9920–9926. [Google Scholar] [CrossRef]
- Lv, J.L.; Wang, B.; Liao, X.J.; Ren, S.W.; Cao, J.T.; Liu, Y.M. Chemical-chemical redox cycling amplification strategy in a self-powered photoelectrochemical system: A proof of concept for signal amplified photocathodic immunoassay. Chem. Commun. 2021, 57, 1883–1886. [Google Scholar] [CrossRef]
- Lu, M.-J.; Chen, F.-Z.; Hu, J.; Zhou, H.; Chen, G.; Yu, X.-D.; Ban, R.; Lin, P.; Zhao, W.-W. Regulating light-sensitive gate of organic photoelectrochemical transistor toward sensitive biodetection at Zero gate bias. Small Struct. 2021, 2, 2100087–2100093. [Google Scholar] [CrossRef]
- Chen, F.Z.; Han, D.M.; Chen, H.Y. Liposome-assisted enzymatic modulation of plasmonic photoelectrochemistry for immunoassay. Anal. Chem. 2020, 92, 8450–8458. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Wang, C.S.; Li, Z.; Hu, J.; Yu, S.Y.; Xu, Y.T.; Lin, P.; Zhao, W.W. Dual functional conjugated acetylenic polymers: High-efficacy modulation for organic photoelectrochemical transistors and structural evolution for bioelectronic detection. Anal. Chem. 2023, 95, 4243–4250. [Google Scholar] [CrossRef] [PubMed]
- Barroso, J.; Saa, L.; Grinyte, R.; Pavlov, V. Photoelectrochemical detection of enzymatically generated CdS nanoparticles: Application to development of immunoassay. Biosens. Bioelectron. 2016, 77, 323–329. [Google Scholar] [CrossRef]
- Diez-Buitrago, B.; Fernandez-San Argimiro, F.J.; Lorenzo, J.; Bijelic, G.; Briz, N.; Pavlov, V. Design of a photoelectrochemical lab-on-a-chip immunosensor based on enzymatic production of quantum dots in situ. Analyst 2022, 147, 3470–3477. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, M.; Zeng, Y.; Liu, X.; Tang, D. Tunable competitive absorption-induced signal-on photoelectrochemical immunoassay for cardiac troponin I based on Z-scheme metal-organic framework heterojunctions. Anal. Chem. 2022, 94, 13582–13589. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zeng, Y.; Liu, X.; Tang, D. Liposome-mediated in situ formation of type-I heterojunction for amplified photoelectrochemical immunoassay. Anal. Chem. 2022, 94, 4859–4865. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, C.; Ge, S.; Yu, J.; Yan, M. Platelike WO3 sensitized with CdS quantum dots heterostructures for photoelectrochemical dynamic sensing of H2O2 based on enzymatic etching. Biosens. Bioelectron. 2016, 85, 205–211. [Google Scholar] [CrossRef]
- Su, L.; Song, Y.; Fu, C.; Tang, D. Etching reaction-based photoelectrochemical immunoassay of aflatoxin B1 in foodstuff using cobalt oxyhydroxide nanosheets-coating cadmium sulfide nanoparticles as the signal tags. Anal. Chim. Acta 2019, 1052, 49–56. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, S.; Zhou, Q.; Tang, D. CoOOH nanosheets-coated g-C3N4/CuInS2 nanohybrids for photoelectrochemical biosensor of carcinoembryonic antigen coupling hybridization chain reaction with etching reaction. Sens. Actuators B Chem. 2020, 307, 127631–127638. [Google Scholar] [CrossRef]
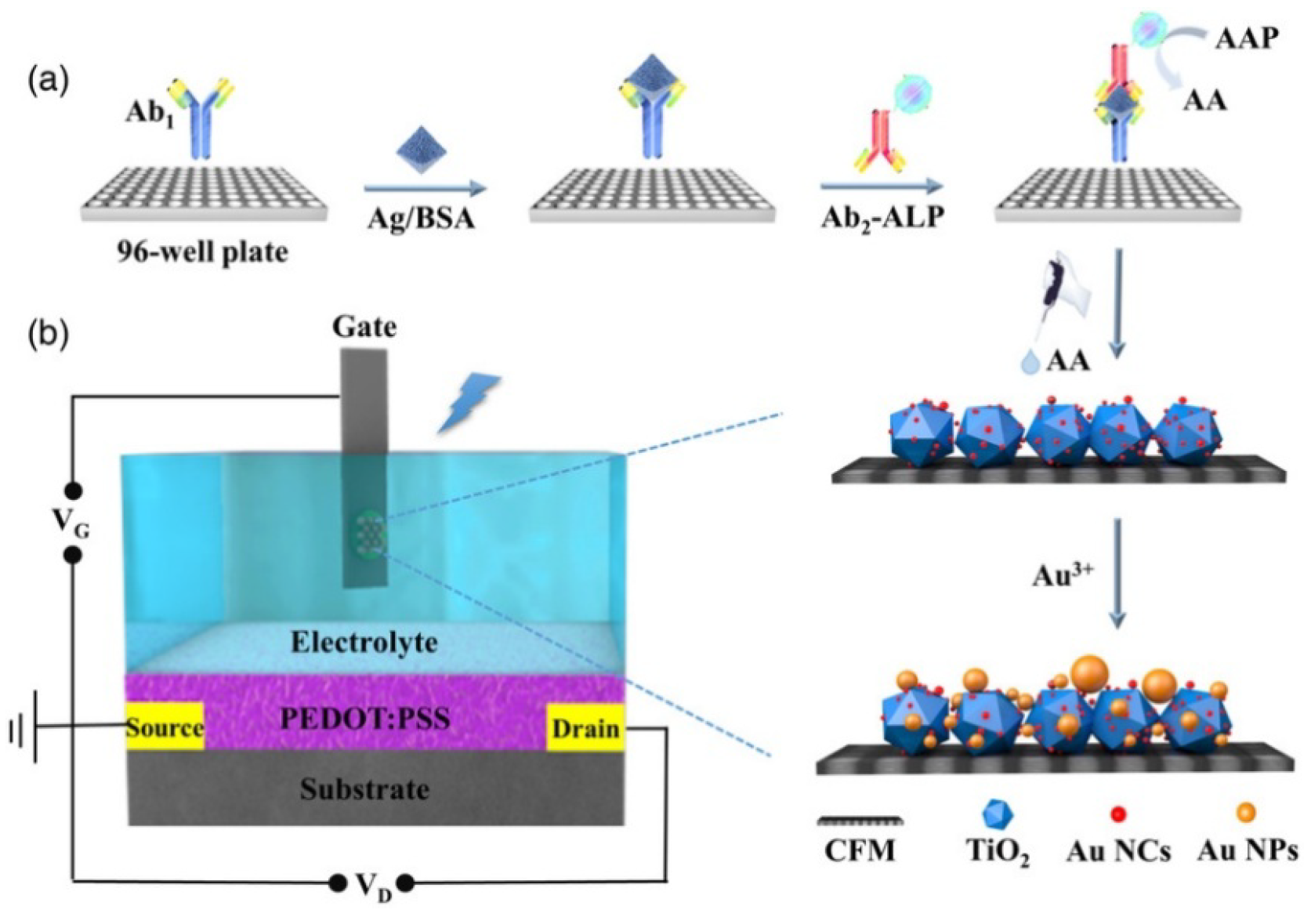
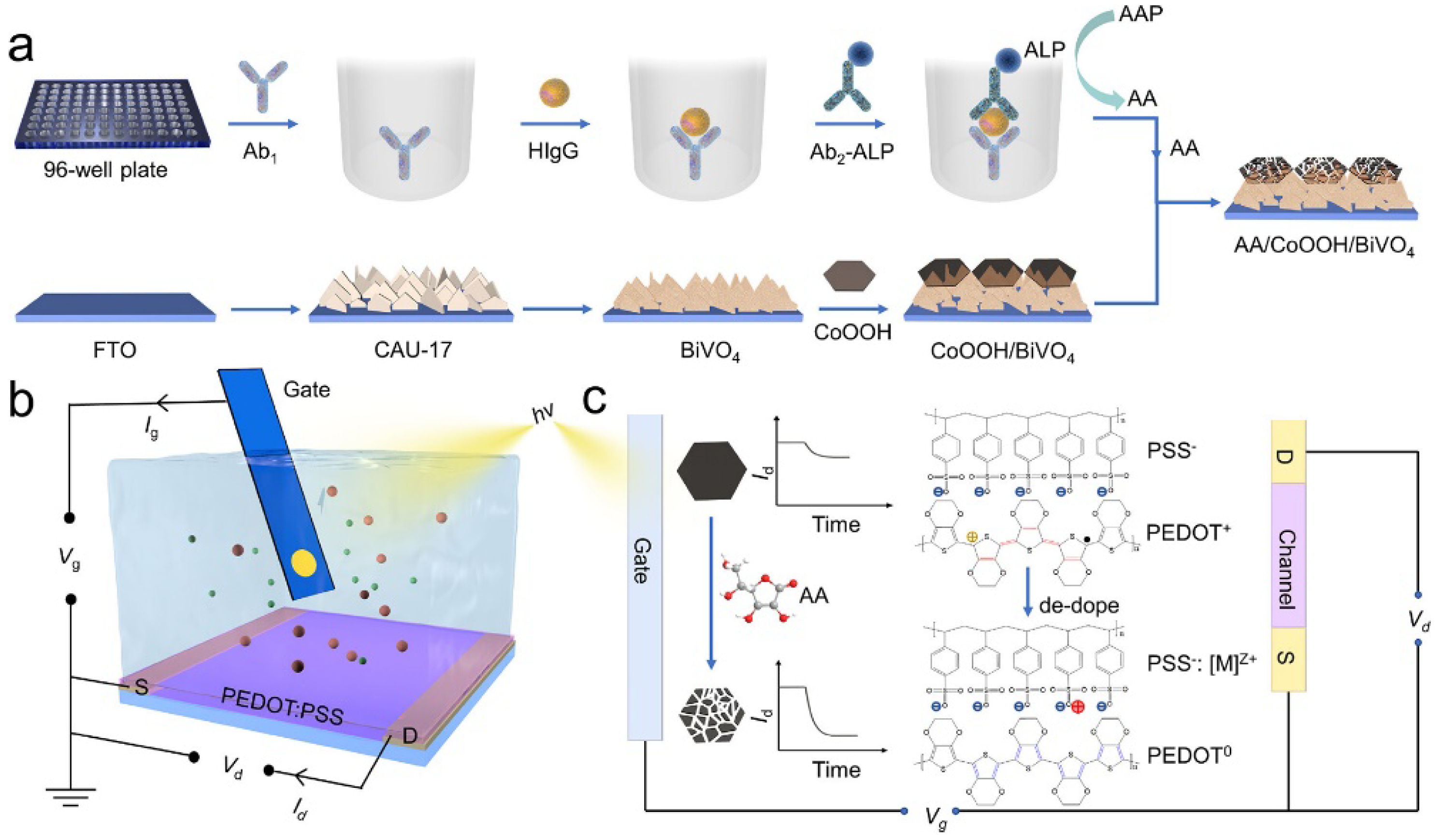
- Ban, R.; Li, C.J.; Xu, Y.T.; Zhu, Y.Y.; Ju, P.; Li, Y.M.; Du, H.J.; Hu, J.; Chen, G.; Lin, P.; et al. Alkaline phosphatase-nediated bioetching of CoOOH/BiVO4 for signal-on organic photoelectrochemical transistor bioanalysis. Anal. Chem. 2023, 95, 1454–1460. [Google Scholar] [PubMed]
- Lin, Y.; Zhou, Q.; Tang, D.; Niessner, R.; Knopp, D. Signal-on photoelectrochemical immunoassay for aflatoxin B1 based on enzymatic product-etching MnO2 nanosheets for dissociation of carbon dots. Anal. Chem. 2017, 89, 5637–5645. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Zhu, L.; Yin, Z.; Li, M.; Tang, D. Signal-on photoelectrochemical immunoassay mediated by the etching reaction of oxygen/phosphorus co-doped g-C3N4/AgBr/MnO2 nanohybrids. Anal. Chim. Acta 2021, 1171, 338680–338687. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Liu, G.; Qiu, Z.; Huang, L.; Weng, S. Etching reaction of carbon quantum dot-functionalized MnO2 nanosheets with an enzymatic product for photoelectrochemical immunoassay of alpha-fetoprotein. New J. Chem. 2022, 46, 12836–12843. [Google Scholar] [CrossRef]
- Fu, Y.Z.; Liu, X.M.; Ma, S.H.; Cao, J.T.; Liu, Y.M. Liposome-assisted enzyme catalysis: Toward signal amplification for sensitive split-type electrochemiluminescence immunoassay. Analyst 2021, 146, 3918–3923. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, R.; Xue, Y.; Jie, G. Versatile Au nanoclusters/Au-MnO2 nanoflowers electrochemiluminescence energy transfer platform coupled with rolling circle amplification for dual-targets biosensing of PSA and Let-7a. Sens. Actuators B Chem. 2022, 369, 132397–132404. [Google Scholar] [CrossRef]
- Lv, W.; Yang, Q.; Li, Q.; Li, H.; Li, F. Quaternary ammonium salt-functionalized tetraphenylethene derivative boosts electrochemiluminescence for highly sensitive aqueous-phase biosensing. Anal. Chem. 2020, 92, 11747–11754. [Google Scholar] [CrossRef]
- Lin, Z.; Cheng, W.; Liu, C.; Zhao, M.; Ding, S.; Deng, Z. Coordination-induced self-assembly based carbon dot dendrimers as efficient signal labels for electrochemiluminescent immunosensor construction. Talanta 2023, 254, 124101–124110. [Google Scholar] [CrossRef]
- Qi, W.; Fu, Y.; Zhao, M.; He, H.; Tian, X.; Hu, L.; Zhang, Y. Electrochemiluminescence resonance energy transfer immunoassay for alkaline phosphatase using p-nitrophenyl phosphate as substrate. Anal. Chim. Acta 2020, 1097, 71–77. [Google Scholar] [CrossRef]
- Yang, M.; Chen, Y.; Xiang, Y.; Yuan, R.; Chai, Y. In situ energy transfer quenching of quantum dot electrochemiluminescence for sensitive detection of cancer biomarkers. Biosens. Bioelectron. 2013, 50, 393–398. [Google Scholar] [CrossRef]
- Cao, J.T.; Fu, Y.Z.; Wang, Y.L.; Zhang, H.D.; Liu, X.M.; Ren, S.W.; Liu, Y.M. Liposome-assisted chemical redox cycling strategy for advanced signal amplification: A proof-of-concept toward sensitive electrochemiluminescence immunoassay. Biosens. Bioelectron. 2022, 214, 114514–114520. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; La, M.; Yi, X.; Huang, M.; Xia, N.; Zhou, Y. Progress in Electrochemical Immunosensors with Alkaline Phosphatase as the Signal Label. Biosensors 2023, 13, 855. https://doi.org/10.3390/bios13090855
Chen C, La M, Yi X, Huang M, Xia N, Zhou Y. Progress in Electrochemical Immunosensors with Alkaline Phosphatase as the Signal Label. Biosensors. 2023; 13(9):855. https://doi.org/10.3390/bios13090855
Chicago/Turabian StyleChen, Changdong, Ming La, Xinyao Yi, Mengjie Huang, Ning Xia, and Yanbiao Zhou. 2023. "Progress in Electrochemical Immunosensors with Alkaline Phosphatase as the Signal Label" Biosensors 13, no. 9: 855. https://doi.org/10.3390/bios13090855
APA StyleChen, C., La, M., Yi, X., Huang, M., Xia, N., & Zhou, Y. (2023). Progress in Electrochemical Immunosensors with Alkaline Phosphatase as the Signal Label. Biosensors, 13(9), 855. https://doi.org/10.3390/bios13090855






