Biosensors with Boronic Acid-Based Materials as the Recognition Elements and Signal Labels
Abstract
1. Introduction
2. Boronate-Affinity-Based Electrochemical Biosensors
2.1. Boronic Acid-Based Electroactive Molecules for Electrochemical Biosensors
2.2. Boronic Acid-Based Nanomaterials for Electrochemical Biosensors

| Receptors | Target | Signal Label | Linear Range | LOD | Ref. |
|---|---|---|---|---|---|
| PNA | DNA | FcPBA | 1 × 10−5–10 nM | 2.9 fM | [104] |
| Aptamer | Mucin 1 | FcPBA | 5 × 10−2–50 U/mL | 0.021 U/mL | [105] |
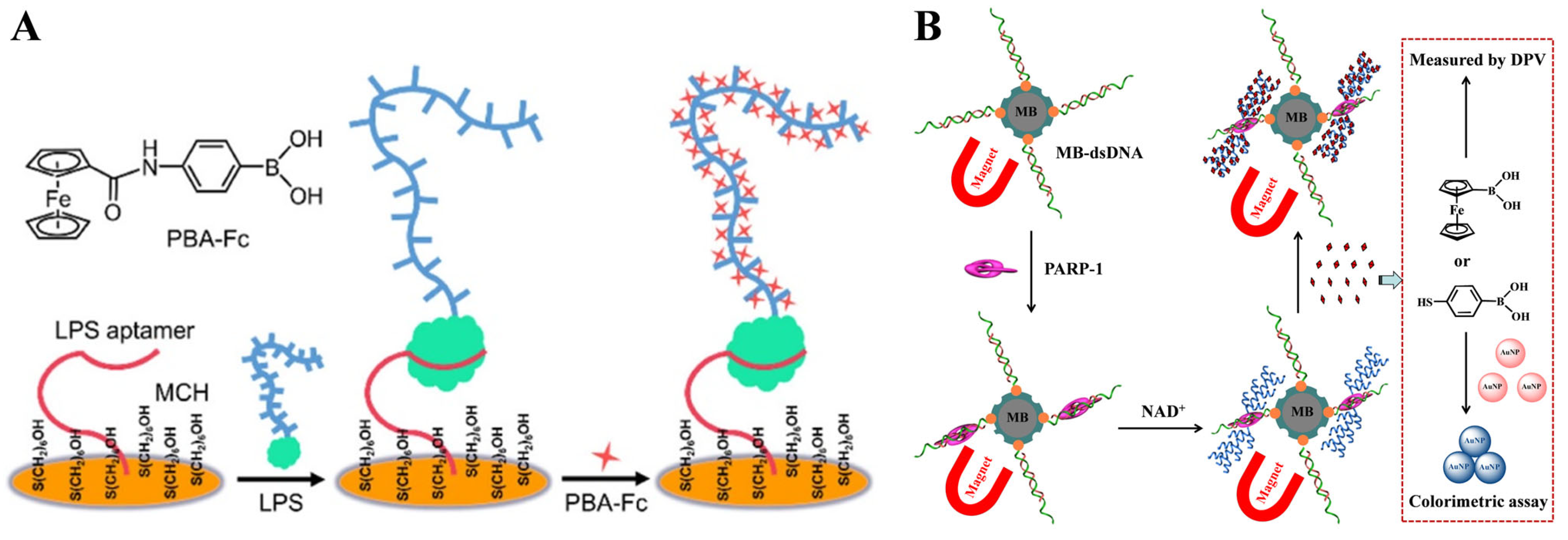
| Aptamer | LPS | FcPBA | 1 × 10−3–1 ng/mL | 0.34 pg/mL | [107] |
| dsDNA | PARP-1 | FcBA | 0.1–50 U | 0.1 U | [108] |
| Aptamer | CTCs | FcBA | 50–2 × 104 cells | 50 cells | [109] |
| IgY | E. coli | FcBA | 10–108 CFU/mL | 3 CFU/mL | [110] |
| Aptamer | Thrombin | Fc-MMA-based polymer | 5 × 10−2–100 pM | 35.3 fM | [106] |
| Aptamer | CEA | MPBA/Thi/SiO2 | 1 × 10−3–10 ng/mL | 0.49 pg/mL | [111] |
| Biotin | Avidin | Fc/MPBA-AuNPs | 0.75–19.6 pM | 0.2 pM | [112] |
| Aptamer | V.P | AuNPs/FcHT/MPBA | 10–109 CFU/mL | 3 CFU/mL | [114] |
| DNA | miRNA-21 | MPBA-AuNPs and DA-AuNPs | 0.1–10 pM | 45 fM | [115] |
| Aptamer | PSA | MPBA-AuNPs and DA-AuNPs | 0.125–3.65 pM | 50 fM | [116] |
| MIP | HRP | SiO2@Au/FcHT/MPBA | 1 × 10−3–100 ng/mL | 0.57 pg/mL | [117] |
| Aptamer | rHuEPO | MPBA-biotin-AuNPs and ALP | 0.02–2 pM | 8 fM | [118] |
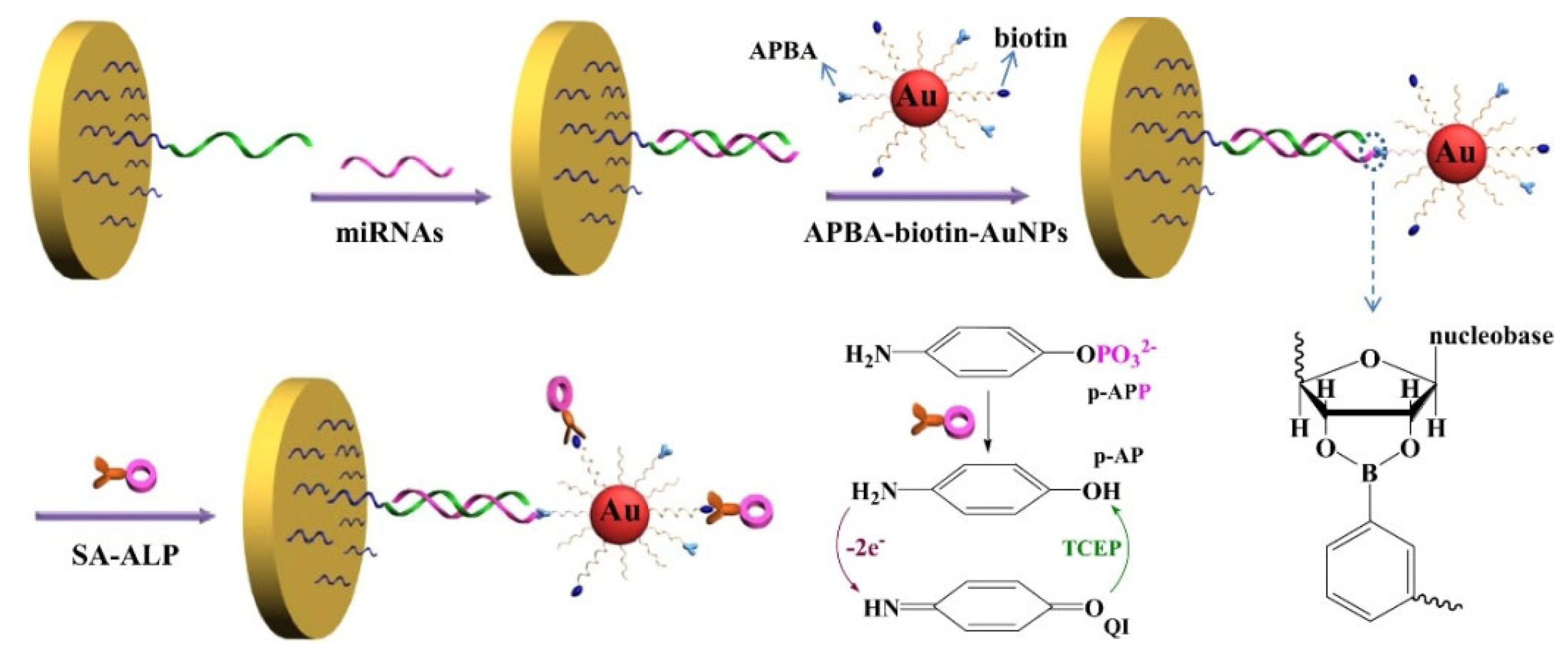
| DNA | miRNA-21 | APBA-biotin-AuNPs and ALP | 0.01–5 pM | 3 fM | [119] |
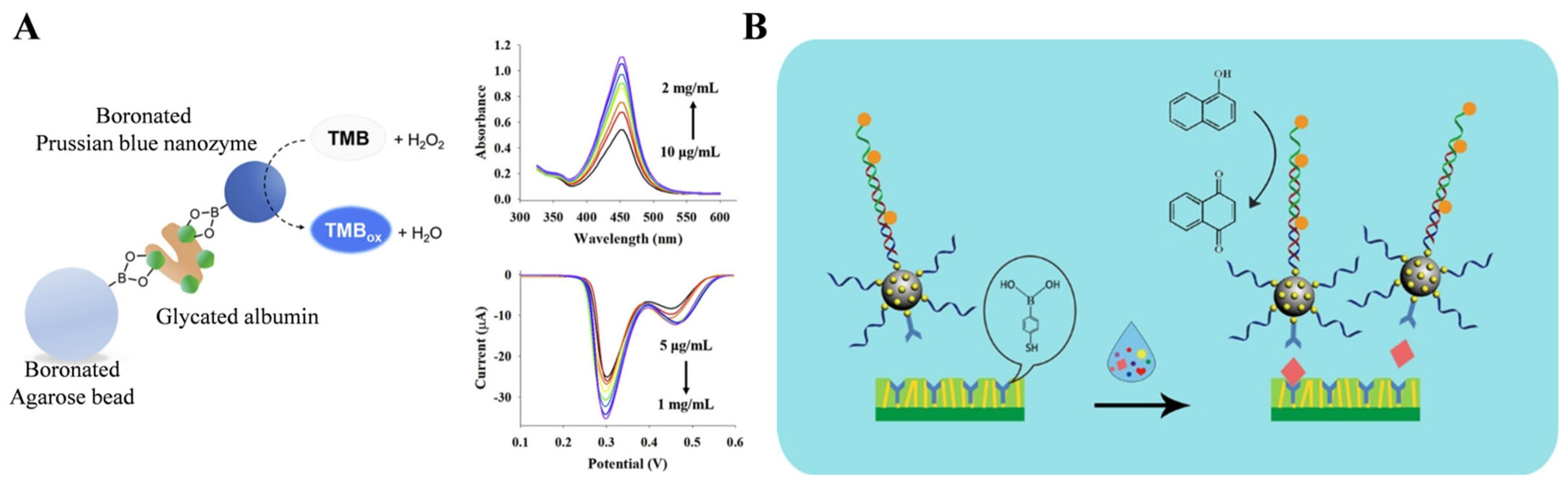
| APBA | glycated albumin | APBA-PBNPs | 5 × 10−3–1 mg/mL | 3.47μg/mL | [124] |
| MIP | OVA | MPBA-SiO2@Au/dsDNA/CeO2 | 1–1 × 106 pg/mL | 0.87 pg/mL | [125] |
| Antibody | CEA | BA-CPS@PANI@Au | 6 × 10−3–12 ng/mL | 1.56 pg/mL | [127] |
| Annexin-V | apoptotic Jurkat cells | ABA-AuNPs and silver | 1 × 102–3.5 × 103 cells | 38 cells | [129] |
| DNA | miRNA-21 | MPBA and citrate-AgNPs | 0.1–50 fM | 20 aM | [130] |
| Aptamer | PSA | MPBA and citrate-AgNPs | 0.5–200 pg/mL | 0.2 pg/mL | [131] |
| Peptide | Tyrosinase and thrombin | MPBA and citrate-AgNPs | 1 × 10−3–0.5 mU/mL and 0.025–5 ng/mL | 0.1 nU/mL and 0.02 ng/mL | [133] |
| Antibody | AFP-L3 and total AFP | MPBA-CuNPs and LCA-AgNPs | 50–1 × 105 pg/mL and 0.4–1 × 103ng/mL | 40 and 10 pg/mL | [134] |
| Aptamer | Exosomal glycoprotein | MPBA-SiO2@Ag | 4.2 × 102–4.2 × 109 particles/μL | 368 particles/μL | [135] |
| NTA-Ni2+ | rhuEPO | FPBA-Cu-MOFs | 0.01–50 ng/mL | 5.3 pg/mL | [138] |
3. Boronate-Affinity-Based Fluorescent Assays and Imaging
3.1. Binding-Induced Fluorescent Enhancement
3.2. Aggregation-Induced Quenching or Emission
3.3. Sandwich Fluorescence Assays
| Signal Probes | Target | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| APBA-CuNCs | Ovalbumin | 5–220 nM | 2.6 nM | [141] |
| UiO-66-NH2@B(OH)2 | SA | 0.05–2.5 mM | 0.025 mM | [142] |
| MPBA-QDs | TRF | 0.1–10 μM | 5.69 nM | [156] |
| BA-QDs | HRP and TRF | 0.3–0.7 μM and 0.4–0.9 μM | 0.144 nM and 0.336 nM | [157] |
| BA-g-C3N4 | IgG | 6.7–67 nM | 2.2 nM | [161] |
| BA-g-C3N4 | Exosome | 8 × 103–1 × 105 particles/mL | 2484 particles/mL | [162] |
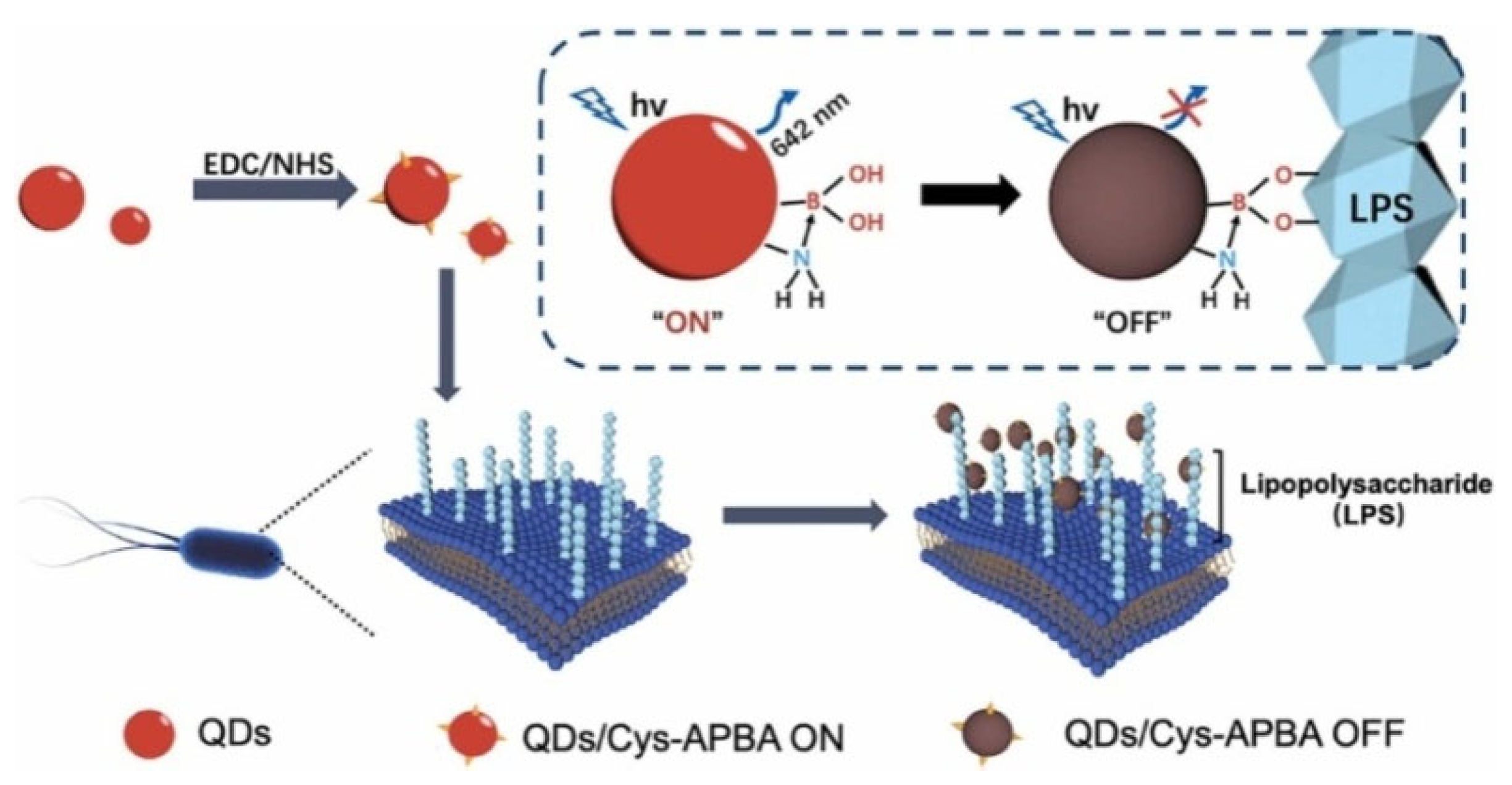
| APBA-QDs | Escherichia coli and P. aeruginosa | 1.12 × 103–1.12 × 108 CFU/mL | 58 and 97 CFU/mL | [167] |
| BA-CNDs | HRP | 3.3–333.3 μg/mL | 0.52 μg/mL | [168] |
| P-Glu/CuNCs | ALP | 0.56–30 U/L | 0.17 U/L | [173] |
| BA-QDs | HCG | 0.24–62.5 mIU/mL | 0.19 mIU/mL | [181] |
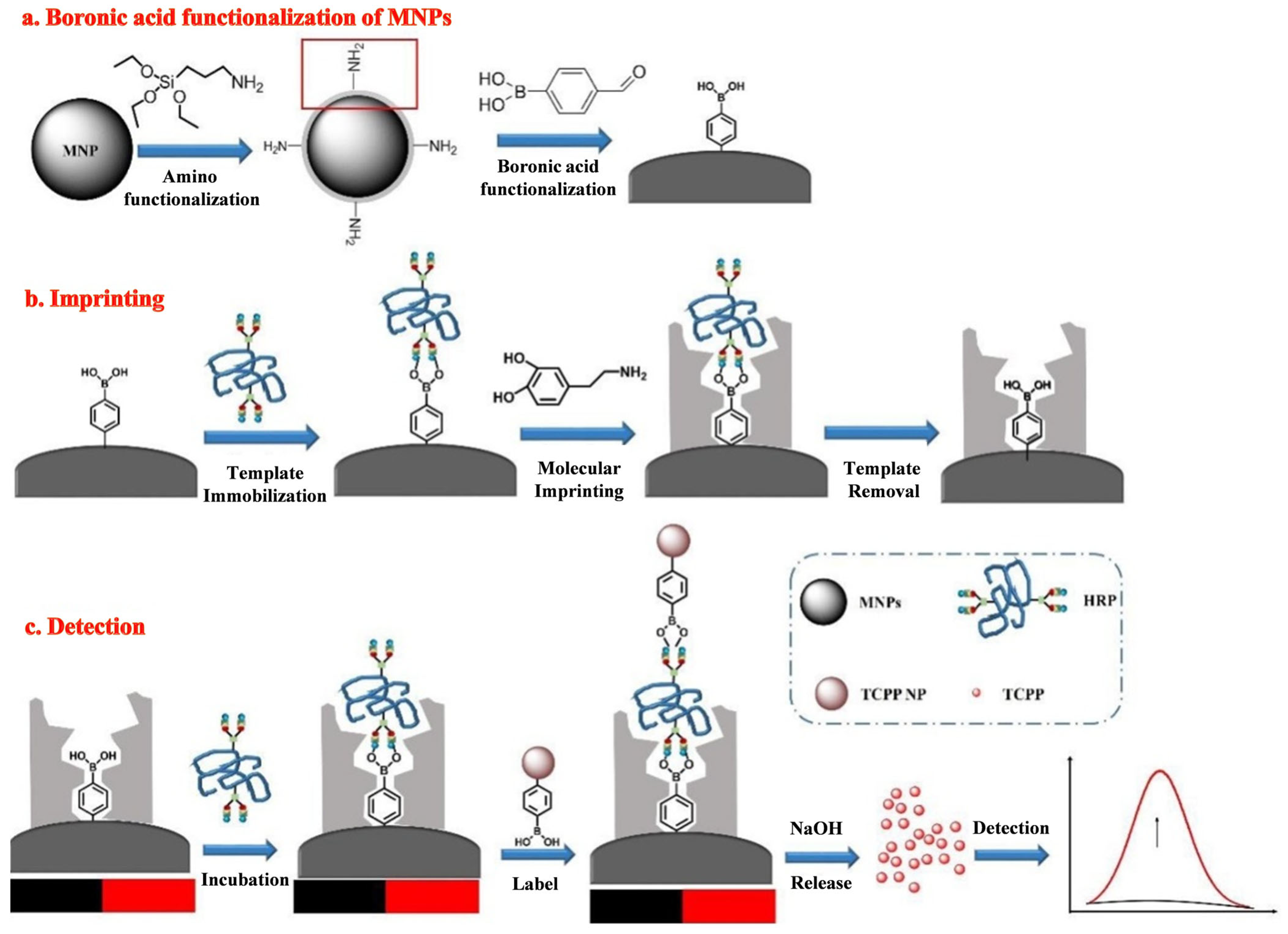
| BA-TCPPs | HRP | 0.1 μg/L–10 mg/L | 0.042 μg/L | [183] |
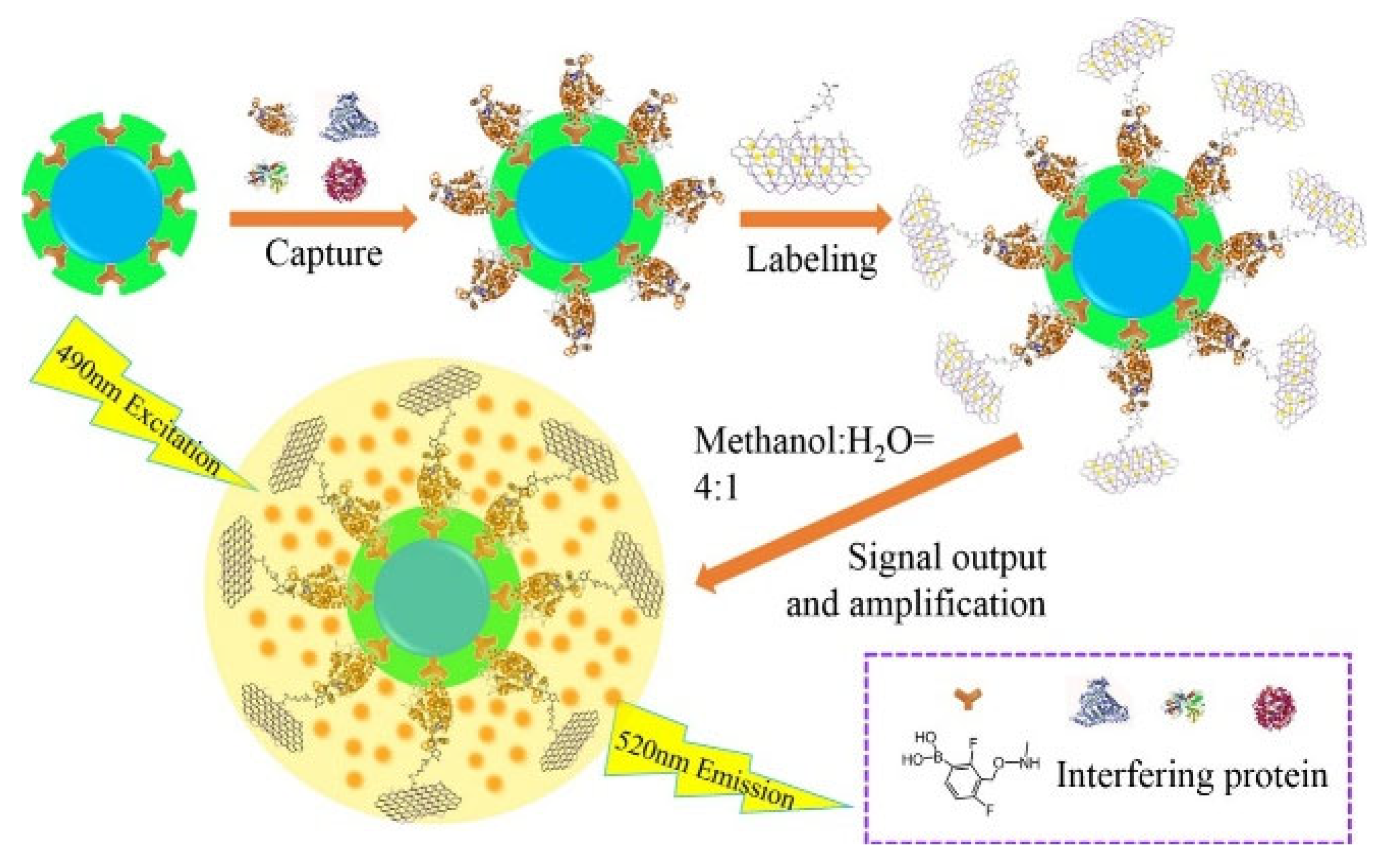
| BA-FITC-CNTs | HER2 | 31 fg/mL–5 μg/mL | 14 fg/mL | [184] |
| BA-FITC-GO | HRP and CEA | 1 × 10−7 pg/mL–5 μg/mL and 2.4 × 10−6–10 ng/mL | 23 and 1.2 fg/mL | [185] |
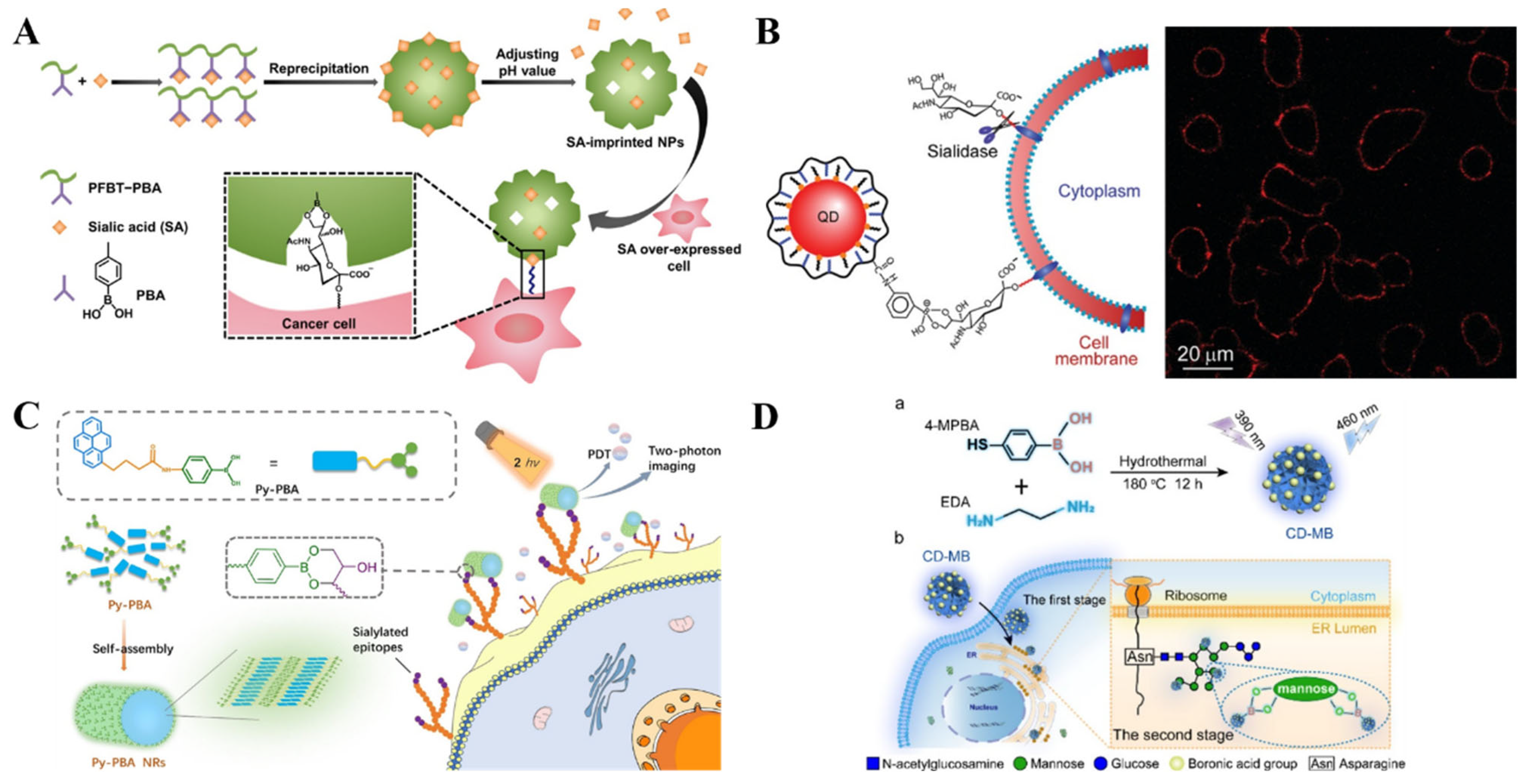
3.4. Fluorescent Imaging
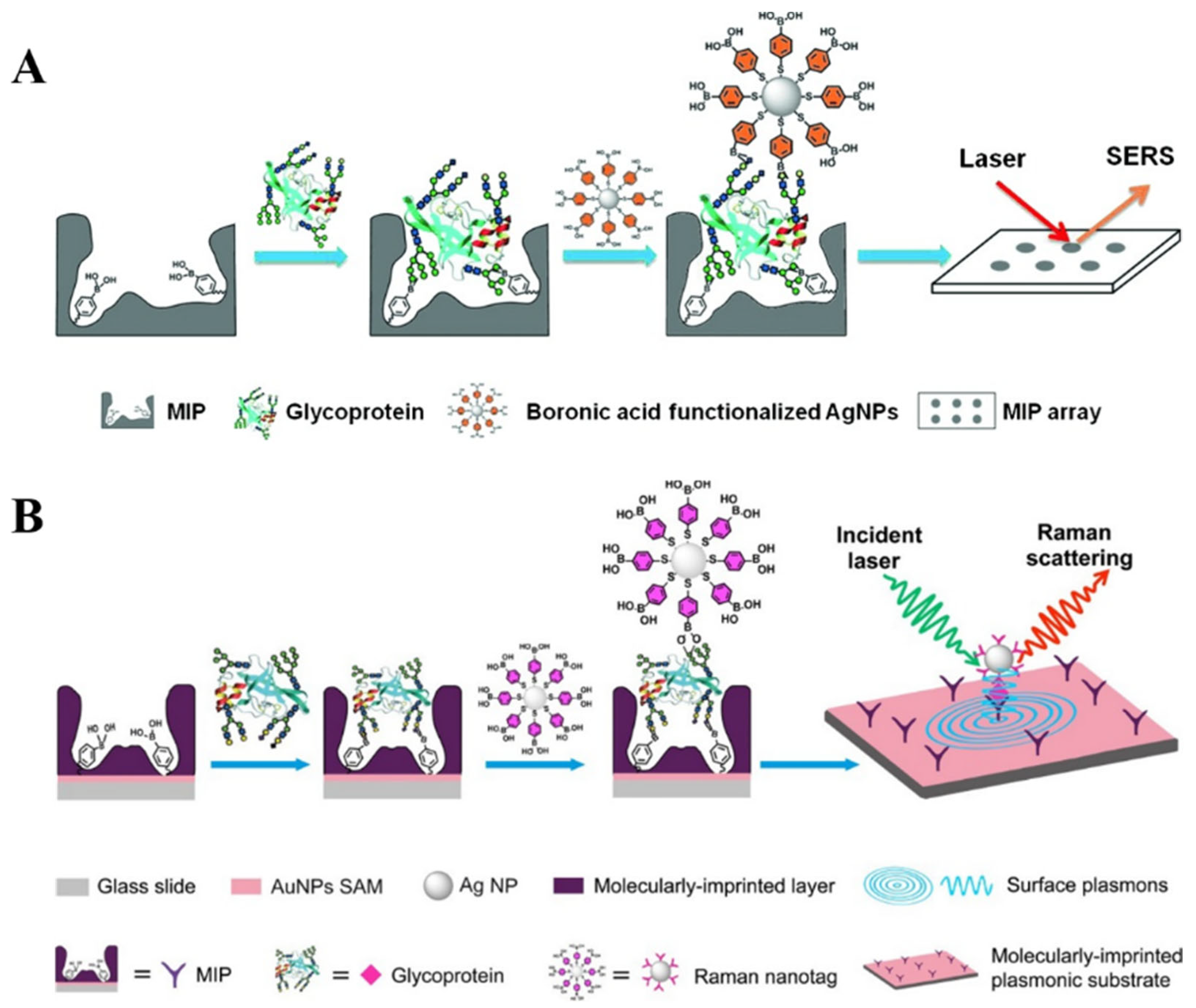
4. SERS Biosensors
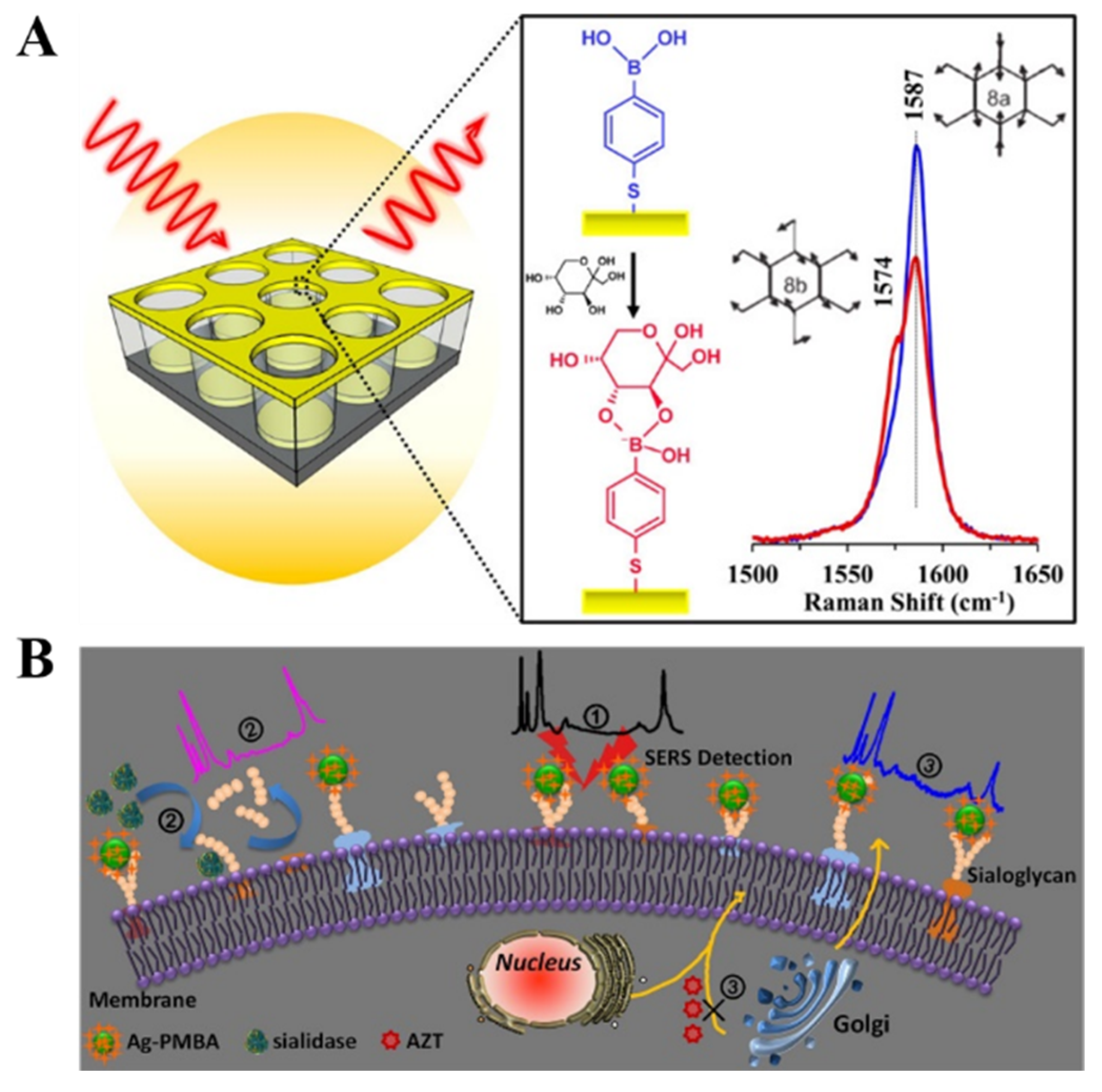
5. Boronate-Affinity-Based Colorimetric Assays
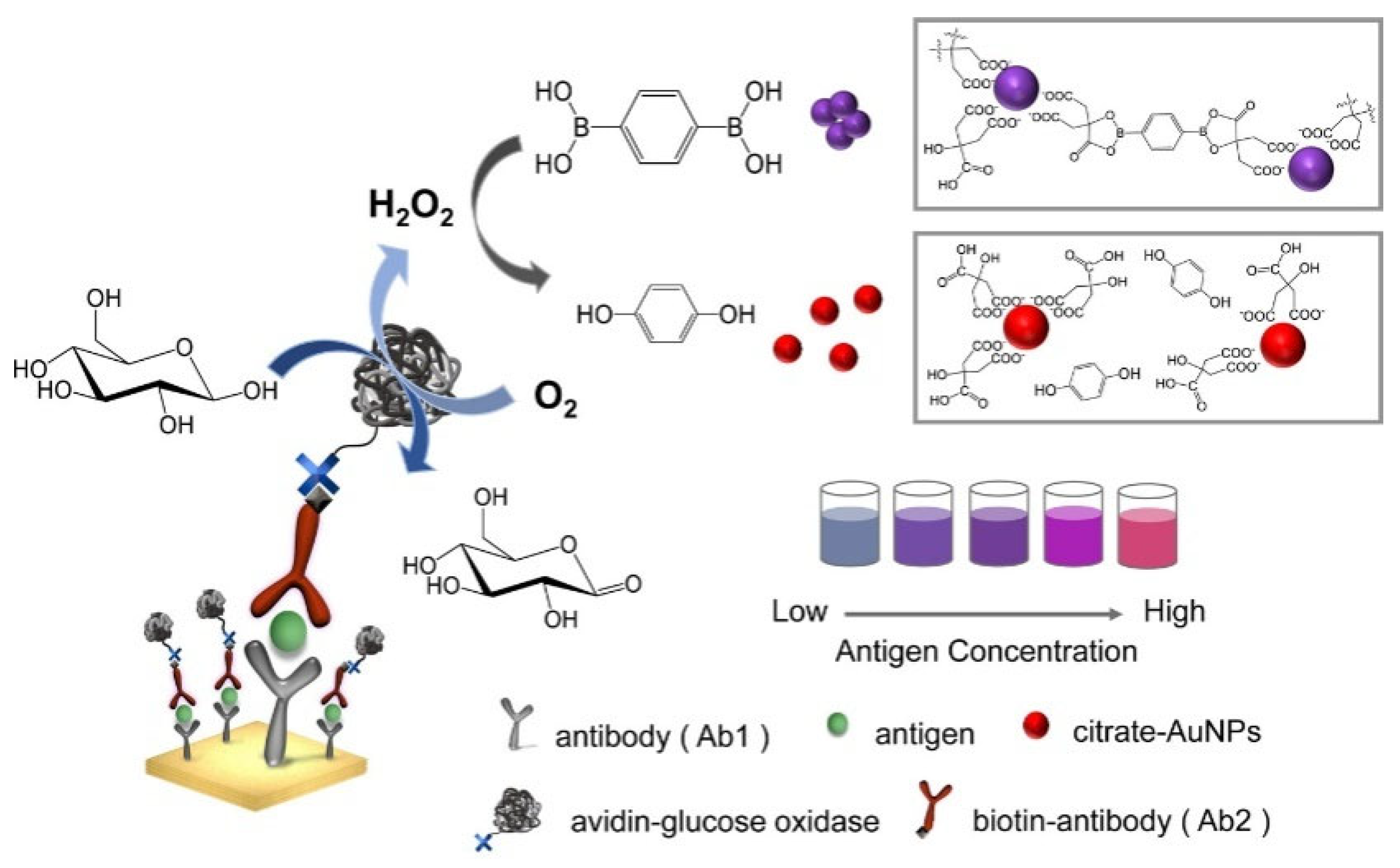
5.1. Boronic Acid-Based Plasmonic Colorimetric Biosensors
5.2. Boronic Acid-Based Lateral Flow Immunoassays
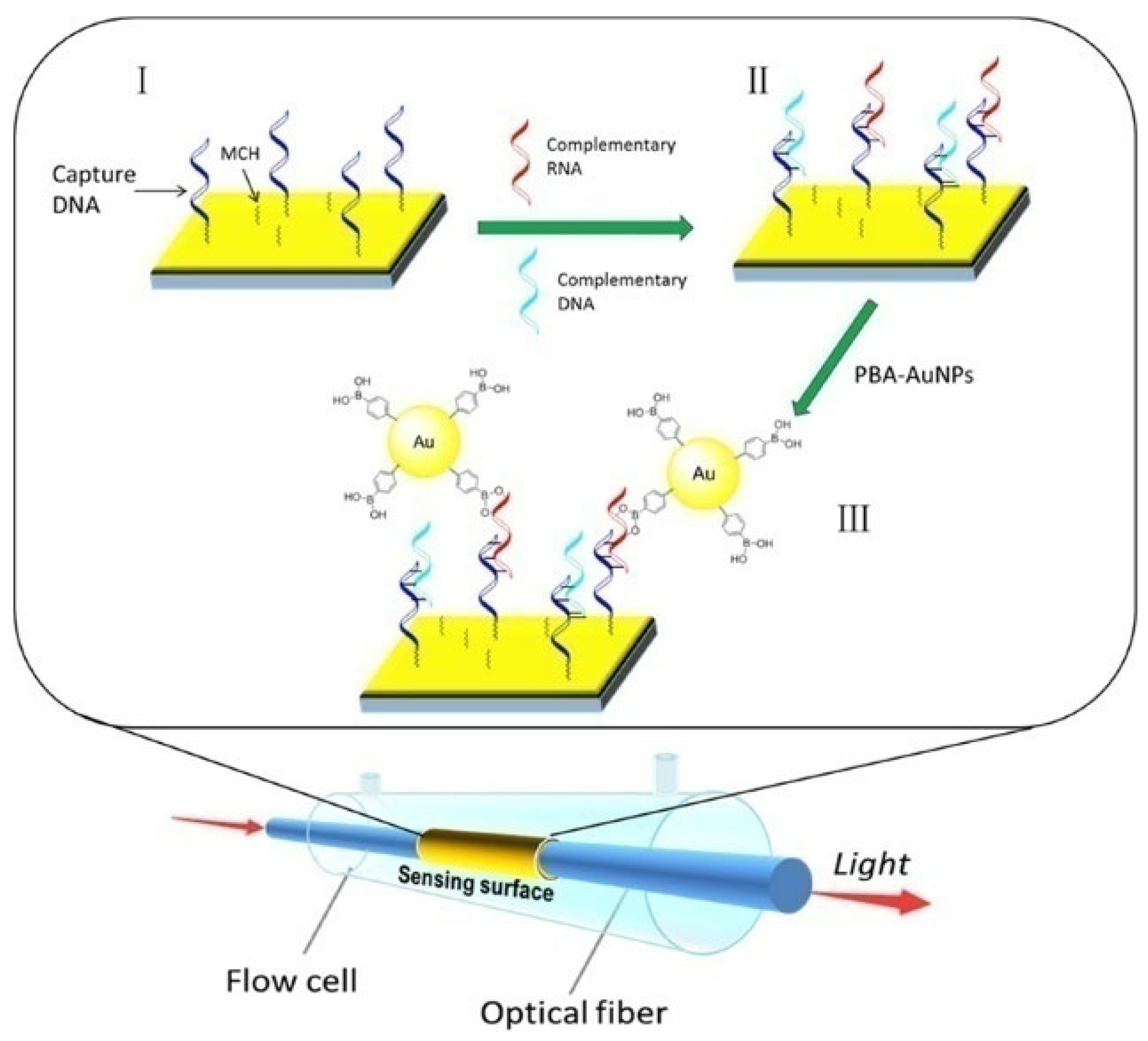
6. Others
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Turner, A.P. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [PubMed]
- Price, C.P. Application of chemistry to in vitro diagnostic tests. Chem. Soc. Rev. 2001, 30, 1–7. [Google Scholar] [CrossRef]
- Rusling, J.F.; Kumar, C.V.; Gutkind, J.S.; Patel, V. Measurement of biomarker proteins for point-of-care early detection and monitoring of cancer. Analyst 2010, 135, 2496–2511. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Freitas, A.C.; Pereira, R.; Duarte, A.C.; Rocha Santos, T.A.P. Recent developments in recognition elements for chemical sensors and biosensors. TrAC-Trends Anal. Chem. 2015, 68, 2–17. [Google Scholar] [CrossRef]
- Akiba, U.; Anzai, J.I. Recent progress in electrochemical biosensors for glycoproteins. Sensors 2016, 16, 2045. [Google Scholar] [CrossRef]
- Sugihara, J.M.; Bowman, C.M. Cyclic benzeneboronate esters. J. Am. Chem. Soc. 2002, 80, 2443–2446. [Google Scholar] [CrossRef]
- David, S. Applications of reversible covalent chemistry in analytical sample preparation. Analyst 2012, 137, 5457–5482. [Google Scholar]
- Zheng, H.; Lin, H.; Chen, X.; Tian, J.; Pavase, T.R.; Wang, R.; Sui, J.; Cao, L. Development of boronate affinity-based magnetic composites in biological analysis: Advances and future prospects. TrAC-Trends Anal. Chem. 2020, 129, 115952. [Google Scholar] [CrossRef]
- Wang, X.; Xia, N.; Liu, L. Boronic acid-based approach for separation and immobilization of glycoproteins and its application in sensing. Int. J. Mol. Sci. 2013, 14, 20890–20912. [Google Scholar] [CrossRef]
- Ding, Y.; Li, H.; Liu, T.; Liu, Y.; Yan, M.; Shan, L.; Liu, X.; Yan, T.; Wang, S. Boronate affinity-mediated magnetic solid phase extraction andbioactivities of polysaccharides from beverage plants. Beverage Plant Res. 2023, 3, 14. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, B.; Weng, Y.; Liu, J.; Li, S.; Hu, Y.; Yang, K.; Liang, Z.; Zhang, L.; Zhang, Y. 3-Carboxybenzoboroxole functionalized polyethylenimine modified magnetic graphene oxide nanocomposites for human plasma glycoproteins enrichment under physiological conditions. Anal. Chem. 2018, 90, 2671–2677. [Google Scholar] [CrossRef]
- Xiong, C.F.; Ding, J.; Zhu, Q.F.; Bai, Y.L.; Yin, X.M.; Ye, T.T.; Yu, Q.W.; Feng, Y.Q. Boron isotope tag-assisted ultrahigh-performance liquid chromatography coupled with high-resolution mass spectrometry for discovery and annotation of cis-diol-containing metabolites. Anal. Chem. 2021, 93, 3002–3009. [Google Scholar] [CrossRef]
- Zhu, M.; Xu, X.; Hou, Y.; Han, J.; Wang, J.; Zheng, Q.; Hao, H. Boronic derivatization of monoacylglycerol and monitoring in biofluids. Anal. Chem. 2019, 91, 6724–6729. [Google Scholar] [CrossRef]
- Chen, G.; Huang, S.; Kou, X.; Zhang, J.; Wang, F.; Zhu, F.; Ouyang, G. Novel magnetic microprobe with benzoboroxole-modified flexible multisite arm for high-efficiency cis-diol biomolecule detection. Anal. Chem. 2018, 90, 3387–3394. [Google Scholar] [CrossRef]
- Liu, L.; Xia, N.; Du, J.; He, Q.; Qin, Y.; Li, H.; Li, C. A simple and rapid method for probing of isomerization of glucose to fructose with ferroceneboronic acid. Int. J. Electrochem. Sci. 2013, 8, 9163–9170. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.X.; Song, B.N.; Huang, Y.J.; Ouyang, W.J.; Li, Z.; James, T.D.; Jiang, Y.B. Direct sensing of fluoride in aqueous solutions using a boronic acid based sensor. Chem. Commun. 2014, 50, 13987–13989. [Google Scholar] [CrossRef]
- Lee, S.A.; You, G.R.; Choi, Y.W.; Jo, H.Y.; Kim, A.R.; Noh, I.; Kim, S.J.; Kim, Y.; Kim, C. A new multifunctional Schiff base as a fluorescence sensor for Al3+ and a colorimetric sensor for CN− in aqueous media: An application to bioimaging. Dalton Trans. 2014, 43, 6650–6659. [Google Scholar] [CrossRef]
- Wang, S.-T.; Sie, Y.-W.; Wan, C.-F.; Wu, A.-T. A reaction-based fluorescent sensor for detection of cyanide in aqueous media. J. Lumin. 2016, 173, 25–29. [Google Scholar] [CrossRef]
- Hou, D.; You, Y.; Wu, X.; Li, C.; Wu, S.; Zhang, C.; Xian, Y. A nanosized metal–organic framework for visual detection of fluoride ions with smartphone via colorimetric test kit. Sens. Actuators B Chem. 2021, 332, 129508–129514. [Google Scholar] [CrossRef]
- Yoon, J.; Czarnik, A.W. Fluorescent chemosensors of carbohydrates. A means of chemically communicating the binding of polyols in water based on chelation-enhanced quenching. J. Am. Chem. Soc. 2002, 114, 5874–5875. [Google Scholar] [CrossRef]
- Zhao, W.; Li, Y.; Yang, S.; Chen, Y.; Zheng, J.; Liu, C.; Qing, Z.; Li, J.; Yang, R. Target-activated modulation of dual-color and two-photon fluorescence of graphene quantum dots for in vivo imaging of hydrogen peroxide. Anal. Chem. 2016, 88, 4833–4840. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhu, M.S.; Luo, H.; Zhang, Q.; Guo, L.E.; Li, Z.; Jiang, Y.B. Aggregation-switching strategy for promoting fluorescent sensing of biologically relevant species: A simple near-infrared cyanine dye highly sensitive and selective for ATP. Anal. Chem. 2017, 89, 6210–6215. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Zhu, B.; Ji, D.K.; Chen, Q.; He, X.P.; Chen, G.R.; James, T.D. Selective fluorescence detection of monosaccharides using a material composite formed between graphene oxide and boronate-based receptors. ACS Appl. Mater. Interfaces 2014, 6, 10078–10082. [Google Scholar] [CrossRef]
- Kondo, N.; Aoki, E.; Takada, S.; Temma, T. A red-emitting fluorescence sensor for detecting boronic acid-containing agents in cells. Sensors 2022, 22, 7671. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Springsteen, G.; Deeter, S.; Wang, B. The relationship among pKa, pH, and binding constants in the interactions between boronic acids and diols—It is not as simple as it appears. Tetrahedron 2004, 60, 11205–11209. [Google Scholar] [CrossRef]
- Zhai, W.; Sun, X.; James, T.D.; Fossey, J.S. Boronic acid-based carbohydrate sensing. Chem. Asian J. 2015, 10, 1836–1848. [Google Scholar] [CrossRef]
- Morgenstern, D.; Wolf-Levy, H.; Tickotsky-Moskovitz, N.; Cooper, I.; Buchman, A.S.; Bennett, D.A.; Beeri, M.S.; Levin, Y. Optimized glycopeptide enrichment method–it is all about the sauce. Anal. Chem. 2022, 94, 10308–10313. [Google Scholar] [CrossRef]
- Li, Q.; Lu, C.; Liu, Z. Preparation and characterization of fluorophenylboronic acid-functionalized monolithic columns for high affinity capture of cis-diol containing compounds. J. Chromatogr. A 2013, 1305, 123–130. [Google Scholar] [CrossRef]
- Wulff, G.; Lauer, M.; Böhnke, H. Rapid proton transfer as cause of an unusually large neighboring group effect. Angew. Chem. Int. Ed. 1984, 23, 741–742. [Google Scholar] [CrossRef]
- Dowlut, M.; Hall, D.G. An improved class of sugar-binding boronic acids, soluble and capable of complexing glycosides in neutral water. J. Am. Chem. Soc. 2006, 128, 4226–4227. [Google Scholar] [CrossRef]
- Mohler, L.K.; Czarnik, A.W. Ribonucleoside membrane transport by a new class of synthetic carrier. J. Am. Chem. Soc. 2002, 115, 2998–2999. [Google Scholar] [CrossRef]
- Lu, C.; Li, H.; Wang, H.; Liu, Z. Probing the interactions between boronic acids and cis-diol-containing biomolecules by affinity capillary electrophoresis. Anal. Chem. 2013, 85, 2361–2369. [Google Scholar] [CrossRef]
- De Guzman, J.M.; Soper, S.A.; McCarley, R.L. Assessment of glycoprotein interactions with 4-[(2-aminoethyl)carbamoyl]phenylboronic acid surfaces using surface plasmon resonance spectroscopy. Anal. Chem. 2010, 82, 8970–8977. [Google Scholar] [CrossRef]
- Wang, Y.; Chalagalla, S.; Li, T.; Sun, X.L.; Zhao, W.; Wang, P.G.; Zeng, X. Multivalent interaction-based carbohydrate biosensors for signal amplification. Biosens. Bioelectron. 2010, 26, 996–1001. [Google Scholar] [CrossRef]
- Mujahid Ali, M.; Hussain, D.; Xu, B.; Sun, T.; Du, Z. Diethylenetriamine assisted functionalization of boronic acid on poly GMA-MAA-DVB for selective enrichment of glycoproteins and glycopeptides. Talanta 2020, 219, 121178–121186. [Google Scholar] [CrossRef]
- Nazemi, S.A.; Olesinska, M.; Pezzella, C.; Varriale, S.; Lin, C.W.; Corvini, P.F.; Shahgaldian, P. Immobilisation and stabilisation of glycosylated enzymes on boronic acid-functionalised silica nanoparticles. Chem. Commun. 2021, 57, 11960–11963. [Google Scholar] [CrossRef]
- Jiang, L.; Messing, M.E.; Ye, L. Temperature and pH dual-responsive core-brush nanocomposite for enrichment of glycoproteins. ACS Appl. Mater. Interfaces 2017, 9, 8985–8995. [Google Scholar] [CrossRef]
- Kong, S.; Zhang, Q.; Yang, L.; Huang, Y.; Liu, M.; Yan, G.; Zhao, H.; Wu, M.; Zhang, X.; Yang, P.; et al. Effective enrichment strategy using boronic acid-functionalized mesoporous graphene-silica composites for intact N- and O-linked glycopeptide analysis in human serum. Anal. Chem. 2021, 93, 6682–6691. [Google Scholar] [CrossRef]
- Weith, H.L.; Wiebers, J.L.; Gilham, P.T. Synthesis of cellulose derivatives containing the dihydroxyboryl group and a study of their capacity to form specific complexes with sugars and nucleic acid components. Biochemistry 1970, 9, 4396–4401. [Google Scholar] [CrossRef]
- Ali, M.M.; Hussain, D.; Tang, Y.; Sun, X.; Shen, Z.; Zhang, F.; Du, Z. Boronoisophthalic acid as a novel affinity ligand for the selective capture and release of glycoproteins near physiological pH. Talanta 2021, 225, 121896–121904. [Google Scholar] [CrossRef]
- Bai, M.; Tian, X.; Wang, Z.; Zhang, L.; Zhang, F.; Yang, Y.; Liu, L. Versatile dynamic bioactive lubricant-infused surface for effective isolation of circulating tumor cells. Anal. Chem. 2023, 95, 5307–5315. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Ma, Y.; Bie, Z.; Liu, B.; Liu, Z. Hybrid approach combining boronate affinity magnetic nanoparticles and capillary electrophoresis for efficient selection of glycoprotein-binding aptamers. Anal. Chem. 2016, 88, 9805–9812. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, M.; Xie, L.; Fang, C.; Xiong, H.; Lu, H. Highly efficient enrichment method for glycopeptide analyses: Using specific and nonspecific nanoparticles synergistically. Anal. Chem. 2014, 86, 2057–2064. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Wang, J.; Gao, M.; Zhang, X. Aminophenylboronic acid-functionalized thorny-trap-shaped monolayer microarray for efficient capture and release of circulating tumor cells. Anal. Chem. 2020, 92, 3403–3408. [Google Scholar] [CrossRef]
- Zhou, Y.; Huangfu, H.; Yang, J.; Dong, H.; Liu, L.; Xu, M. Potentiometric analysis of sialic acid with a flexible carbon cloth based on boronate affinity and molecularly imprinted polymers. Analyst 2019, 144, 6432–6437. [Google Scholar] [CrossRef] [PubMed]
- Bie, Z.; Xing, R.; He, X.; Ma, Y.; Chen, Y.; Liu, Z. Precision imprinting of glycopeptides for facile preparation of glycan-specific artificial antibodies. Anal. Chem. 2018, 90, 9845–9852. [Google Scholar] [CrossRef]
- Bi, X.; Liu, Z. Facile preparation of glycoprotein-imprinted 96-well microplates for enzyme-linked immunosorbent assay by boronate affinity-based oriented surface imprinting. Anal. Chem. 2014, 86, 959–966. [Google Scholar] [CrossRef]
- Bi, X.; Liu, Z. Enzyme activity assay of glycoprotein enzymes based on a boronate affinity molecularly imprinted 96-well microplate. Anal. Chem. 2014, 86, 12382–12389. [Google Scholar] [CrossRef]
- Xing, R.; Wang, S.; Bie, Z.; He, H.; Liu, Z. Preparation of molecularly imprinted polymers specific to glycoproteins, glycans and monosaccharides via boronate affinity controllable-oriented surface imprinting. Nat. Protoc. 2017, 12, 964–987. [Google Scholar] [CrossRef]
- Wang, S.; Ye, J.; Bie, Z.; Liu, Z. Affinity-tunable specific recognition of glycoproteins via boronate affinity-based controllable oriented surface imprinting. Chem. Sci. 2014, 5, 1135–1140. [Google Scholar] [CrossRef]
- Duan, R.; Peng, C.; Sun, L.; Zhang, L.-X.; Bai, C.-C.; Dong, L.-Y.; Wang, X.-H. Integrating boronate affinity controllable-oriented surface imprinting nylon wire and pH-triggered allochroic-graphene oxide for ultrasensitive detection of glycoprotein. Sens. Actuat. B Chem. 2021, 330, 129310–129320. [Google Scholar] [CrossRef]
- Ulrich, S. Growing prospects of dynamic covalent chemistry in delivery applications. Acc. Chem. Res. 2019, 52, 510–519. [Google Scholar] [CrossRef]
- Neubert, K.; Meister, S.; Moser, K.; Weisel, F.; Maseda, D.; Amann, K.; Wiethe, C.; Winkler, T.H.; Kalden, J.R.; Manz, R.A.; et al. The proteasome inhibitor bortezomib depletes plasma cells and protects mice with lupus-like disease from nephritis. Nat. Med. 2008, 14, 748–755. [Google Scholar] [CrossRef]
- Jay, J.I.; Lai, B.E.; Myszka, D.G.; Mahalingam, A.; Langheinrich, K.; Katz, D.F.; Kiser, P.F. Multivalent benzoboroxole functionalized polymers as gp120 glycan targeted microbicide entry inhibitors. Mol. Pharm. 2010, 7, 116–129. [Google Scholar] [CrossRef]
- Ding, Y.; Hu, X.; Piao, Y.; Huang, R.; Xie, L.; Yan, X.; Sun, H.; Li, Y.; Shi, L.; Liu, Y. Lipid prodrug nanoassemblies via dynamic covalent boronates. ACS Nano 2023, 17, 6601–6614. [Google Scholar] [CrossRef]
- Zegota, M.M.; Muller, M.A.; Lantzberg, B.; Kizilsavas, G.; Coelho, J.A.S.; Moscariello, P.; Martinez-Negro, M.; Morsbach, S.; Gois, P.M.P.; Wagner, M.; et al. Dual stimuli-responsive dynamic covalent peptide tags: Toward sequence-controlled release in tumor-like microenvironments. J. Am. Chem. Soc. 2021, 143, 17047–17058. [Google Scholar] [CrossRef]
- Estopina-Duran, S.; Donnelly, L.J.; McLean, E.B.; Hockin, B.M.; Slawin, A.M.Z.; Taylor, J.E. Aryl boronic acid catalysed dehydrative substitution of benzylic alcohols for C-O bond formation. Chem. Eur. J. 2019, 25, 3950–3956. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Wang, Y.; Liu, Y.; Liu, R.; Wang, X.; Tan, H.; Wang, T.; Kong, T. Oriented boronate affinity-imprinted inverse opal hydrogel for glycoprotein assay via colorimetry. Microchim. Acta 2020, 187, 348–356. [Google Scholar] [CrossRef]
- Gao, X.; Yin, Y.; Wu, H.; Hao, Z.; Li, J.; Wang, S.; Liu, Y. Integrated SERS platform for reliable detection and photothermal elimination of bacteria in whole blood samples. Anal. Chem. 2021, 93, 1569–1577. [Google Scholar] [CrossRef]
- Lin, P.C.; Chen, S.H.; Wang, K.Y.; Chen, M.L.; Adak, A.K.; Hwu, J.R.; Chen, Y.J.; Lin, C.C. Fabrication of oriented antibody-conjugated magnetic nanoprobes and their immunoaffinity application. Anal. Chem. 2009, 81, 8774–8782. [Google Scholar] [CrossRef]
- Hashemi, P.; Afkhami, A.; Baradaran, B.; Halabian, R.; Madrakian, T.; Arduini, F.; Nguyen, T.A.; Bagheri, H. Well-orientation strategy for direct immobilization of antibodies: Development of the immunosensor using the boronic acid-modified magnetic graphene nanoribbons for ultrasensitive detection of lymphoma cancer cells. Anal. Chem. 2020, 92, 11405–11412. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guisan, J.M.; Rocha-Martin, J. Oriented immobilization of antibodies onto sensing platforms—A critical review. Anal. Chim. Acta 2022, 1189, 338907. [Google Scholar] [CrossRef] [PubMed]
- Osawa, S.; Matsumoto, A.; Maejima, Y.; Suzuki, T.; Miyahara, Y.; Otsuka, H. Direct observation of cell surface sialylation by atomic force microscopy employing boronic acid-sialic acid reversible interaction. Anal. Chem. 2020, 92, 11714–11720. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Ye, Z.; Chen, L.; Xiao, L. Gold nanoparticles enumeration with dark-field optical microscope for the sensitive glycoprotein sandwich assay. Anal. Chim. Acta 2020, 1109, 53–60. [Google Scholar] [CrossRef]
- Hu, R.; Stevenson, A.C.; Lowe, C.R. An acoustic glucose sensor. Biosens. Bioelectron. 2012, 35, 425–428. [Google Scholar] [CrossRef]
- Ali, A.; Nouseen, S.; Saroj, S.; Shegane, M.; Majumder, P.; Puri, A.; Rakshit, T.; Mannac, D.; Pal, S. Repurposing pinacol esters of boronic acids for tuning viscoelastic properties of glucose-responsive polymer hydrogels: Effects on insulin release kinetics. J. Mater. Chem. B 2022, 10, 7591–7599. [Google Scholar] [CrossRef]
- Wang, H.-C.; Lee, A.-R. Recent developments in blood glucose sensors. J. Food Drug Anal. 2015, 23, 191–200. [Google Scholar] [CrossRef]
- Brooks, W.L.A.; Sumerlin, B.S. Synthesis and applications of boronic acid-containing polymers: From materials to medicine. Chem. Rev. 2016, 116, 1375–1397. [Google Scholar] [CrossRef]
- Guo, Z.; Shin, I.; Yoon, J. Recognition and sensing of various species using boronic acid derivatives. Chem. Commun. 2012, 48, 5956–5967. [Google Scholar] [CrossRef]
- Williams, G.T.; Kedge, J.L.; Fossey, J.S. Molecular boronic acid-based saccharide sensors. ACS Sens. 2021, 6, 1508–1528. [Google Scholar] [CrossRef]
- Qin, X.; Zhang, Z.; Shao, H.; Zhang, R.; Chen, L.; Yang, X. Boronate affinity material-based sensors for recognition and detection of glycoproteins. Analyst 2020, 145, 7511–7527. [Google Scholar] [CrossRef]
- Bian, Z.; Liu, A.; Li, Y.; Fang, G.; Yao, Q.; Zhang, G.; Wu, Z. Boronic acid sensors with double recognition sites: A review. Analyst 2020, 145, 719–744. [Google Scholar] [CrossRef]
- Wang, R.; Bian, Z.; Zhan, D.; Wu, Z.; Yao, Q.; Zhang, G. Boronic acid-based sensors for small-molecule reactive species: A review. Dyes Pigments 2021, 185, 108885. [Google Scholar] [CrossRef]
- Aung, Y.Y.; Kristanti, A.N.; Lee, H.V.; Fahmi, M.Z. Boronic-Acid-Modified Nanomaterials for Biomedical Applications. ACS Omega 2021, 6, 17750–17765. [Google Scholar] [CrossRef]
- Wu, X.; Chen, X.X.; Jiang, Y.B. Recent advances in boronic acid-based optical chemosensors. Analyst 2017, 142, 1403–1414. [Google Scholar] [CrossRef]
- Fang, G.; Wang, H.; Bian, Z.; Sun, J.; Liu, A.; Fang, H.; Liu, B.; Yao, Q.; Wu, Z. Recent development of boronic acid-based fluorescent sensors. RSC Adv. 2018, 8, 29400–29427. [Google Scholar] [CrossRef]
- Mader, H.S.; Wolfbeis, O.S. Boronic acid based probes for microdetermination of saccharides and glycosylated biomolecules. Microchim. Acta 2008, 162, 1–34. [Google Scholar] [CrossRef]
- Sun, X.; Zhai, W.; Fossey, J.S.; James, T.D. Boronic acids for fluorescence imaging of carbohydrates. Chem. Commun. 2016, 52, 3456–3469. [Google Scholar] [CrossRef]
- Anzai, J.I. Recent progress in electrochemical biosensors based on phenylboronic acid and derivatives. Mater. Sci. Eng. C 2016, 67, 737–746. [Google Scholar] [CrossRef]
- Li, M.; Zhu, W.; Marken, F.; James, T.D. Electrochemical sensing using boronic acids. Chem. Commun. 2015, 51, 14562–14573. [Google Scholar] [CrossRef]
- Li, D.; Chen, Y.; Liu, Z. Boronate affinity materials for separation and molecular recognition: Structure, properties and applications. Chem. Soc. Rev. 2015, 44, 8097–8123. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; He, H.; Liu, Z. Recent progress and application of boronate affinity materials in bioanalysis. TrAC-Trends Anal. Chem. 2021, 140, 116271–116295. [Google Scholar] [CrossRef]
- Xing, R.; Wen, Y.; He, H.; Guo, Z.; Liu, Z. Recent progress in the combination of molecularly imprinted polymer-based affinity extraction and mass spectrometry for targeted proteomic analysis. TrAC-Trends Anal. Chem. 2019, 110, 417–428. [Google Scholar] [CrossRef]
- Liu, Z.; He, H. Synthesis and applications of boronate affinity materials: From class selectivity to biomimetic specificity. Acc. Chem. Res. 2017, 50, 2185–2193. [Google Scholar] [CrossRef] [PubMed]
- Arimori, S.; Ushiroda, S.; Peter, L.M.; Jenkins, A.T.; James, T.D. A modular electrochemical sensor for saccharides. Chem. Commun. 2002, 20, 2368–2369. [Google Scholar] [CrossRef]
- Casulli, M.A.; Taurino, I.; Hashimoto, T.; Carrara, S.; Hayashita, T. Electrochemical assay for extremely selective recognition of fructose based on 4-ferrocene-phenylboronic acid probe and beta-cyclodextrins supramolecular complex. Small 2020, 16, e2003359–e2003368. [Google Scholar] [CrossRef]
- Wang, B.; Takahashi, S.; Du, X.; Anzai, J.-i. Electrochemical biosensors based on ferroceneboronic acid and its derivatives: A review. Biosensors 2014, 4, 243–256. [Google Scholar] [CrossRef]
- Lacina, K.; Skládal, P. Ferroceneboronic acid for the electrochemical probing of interactions involving sugars. Electrochim. Acta 2011, 56, 10246–10252. [Google Scholar] [CrossRef]
- Yang, M.; Ma, C.; Ding, S.; Zhu, Y.; Shi, G.; Zhu, A. Rational design of stimuli-responsive polymers modified nanopores for selective and sensitive determination of salivary glucose. Anal. Chem. 2019, 91, 14029–14035. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Bai, W.; Li, Y. Electrogenerated chemiluminescence biosensing method for DNA hydroxymethylation detection via glycosylation and a new multi-functional ECL signal compound. Sens. Actuators B Chem. 2020, 322, 12858–128587. [Google Scholar] [CrossRef]
- Su, L.; Chen, T.; Xue, T.; Sheng, A.; Cheng, L.; Zhang, J. Fabrication of pH-adjusted boronic acid-aptamer conjugate for electrochemical analysis of conjugated N-glycolylneuraminic acid. ACS Appl. Mater. Interfaces 2020, 12, 7650–7657. [Google Scholar] [CrossRef]
- Wang, H.C.; Zhou, H.; Chen, B.; Mendes, P.M.; Fossey, J.S.; James, T.D.; Long, Y.T. A bis-boronic acid modified electrode for the sensitive and selective determination of glucose concentrations. Analyst 2013, 138, 7146–7151. [Google Scholar] [CrossRef]
- Liu, L.; Xia, N.; Xing, Y.; Deng, D. Boronic acid-based electrochemical sensors for detection of biomolecules. Int. J. Electrochem. Sci. 2013, 8, 11161–11174. [Google Scholar] [CrossRef]
- Tang, H.; Wang, H.; Zhao, D.; Cao, M.; Zhu, Y.; Li, Y. Nanopore-based single-entity electrochemistry for the label-free monitoring of single-molecule glycoprotein-boronate affinity interaction and its sensing application. Anal. Chem. 2022, 94, 5715–5722. [Google Scholar] [CrossRef]
- Liu, Z.; Yin, Z.Z.; Cai, W.; Wu, D.; Li, J.; Kong, Y. A surface protein-imprinted biosensor based on boronate affinity for the detection of anti-human immunoglobulin G. Microchim. Acta 2022, 189, 106–126. [Google Scholar] [CrossRef]
- Wang, Q.; Kaminska, I.; Niedziolka-Jonsson, J.; Opallo, M.; Li, M.; Boukherroub, R.; Szunerits, S. Sensitive sugar detection using 4-aminophenylboronic acid modified graphene. Biosens. Bioelectron. 2013, 50, 331–337. [Google Scholar] [CrossRef]
- Jiang, G.; Jiang, T.; Wang, Y.; Du, X.; Wei, Z.; Zhou, H. Facile preparation of novel Au–polydopamine nanoparticles modified by 4-mercaptophenylboronic acid for use in a glucose sensor. RSC Adv. 2014, 4, 33658–33661. [Google Scholar] [CrossRef]
- Thiruppathi, M.; Lee, J.-F.; Chen, C.C.; Ho, J.-A.A. A disposable electrochemical sensor designed to estimate glycated hemoglobin (HbA1c) level in whole blood. Sens. Actuators B Chem. 2021, 329, 129119–129127. [Google Scholar] [CrossRef]
- Hu, X.B.; Liu, Y.L.; Wang, W.J.; Zhang, H.W.; Qin, Y.; Guo, S.; Zhang, X.W.; Fu, L.; Huang, W.H. Biomimetic graphene-based 3D scaffold for long-term cell culture and real-time electrochemical monitoring. Anal. Chem. 2018, 90, 1136–1141. [Google Scholar] [CrossRef]
- Zhang, Y. Boronic acid-functionalized nanomaterials for the design of electrochemical biosensors. Int. J. Electrochem. Sci. 2022, 17, 220661. [Google Scholar] [CrossRef]
- Song, J.; He, K.; Xing, B.; Pei, Y.; Wang, D.; Wang, Y.; Li, S.; Li, J.; Huan, W.; Zhang, Y.; et al. Rapid measurement of residual kanamycin using highly specific biomimetic recognition paper-based chip. Anal. Chem. 2022, 94, 17567–17576. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wollenberger, U.; Katterle, M.; Scheller, F.W. Ferroceneboronic acid-based amperometric biosensor for glycated hemoglobin. Sens. Actuators B Chem. 2006, 113, 623–629. [Google Scholar] [CrossRef]
- Murakami, H.; Akiyoshi, H.; Wakamatsu, T.; Sagara, T.; Nakashima, N. Electrochemical saccharide recognition by a phenylboronic acid-terminated redox active self-assembled monolayer on a gold electrode. Chem. Lett. 2000, 29, 940–941. [Google Scholar] [CrossRef]
- Hu, Q.; Wan, J.; Luo, Y.; Li, S.; Cao, X.; Feng, W.; Liang, Y.; Wang, W.; Niu, L. Electrochemical detection of femtomolar DNA via boronate affinity-mediated decoration of polysaccharides with electroactive tags. Anal. Chem. 2022, 94, 12860–12865. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Wan, J.; Wang, H.; Cao, X.; Li, S.; Liang, Y.; Luo, Y.; Wang, W.; Niu, L. Boronate-affinity cross-linking-based ratiometric electrochemical detection of glycoconjugates. Anal. Chem. 2022, 94, 9481–9486. [Google Scholar] [CrossRef]
- Su, L.; Wan, J.; Hu, Q.; Qin, D.; Han, D.; Niu, L. Target-synergized biologically mediated RAFT polymerization for electrochemical aptasensing of femtomolar thrombin. Anal. Chem. 2023, 95, 4570–4575. [Google Scholar] [CrossRef]
- Hu, Q.; Feng, W.; Liang, Y.; Liang, Z.; Cao, X.; Li, S.; Luo, Y.; Wan, J.; Ma, Y.; Han, D.; et al. Boronate affinity-amplified electrochemical aptasensing of lipopolysaccharide. Anal. Chem. 2022, 94, 17733–17738. [Google Scholar] [CrossRef]
- Xia, N.; Wu, D.; Sun, T.; Wang, Y.; Ren, X.; Zhao, F.; Liu, L.; Yi, X. Magnetic bead-based electrochemical and colorimetric methods for the detection of poly(ADP-ribose) polymerase-1 with boronic acid derivatives as the signal probes. Sens. Actuators B Chem. 2021, 327, 128913–128920. [Google Scholar] [CrossRef]
- Xia, N.; Wu, D.; Yu, H.; Sun, W.; Yi, X.; Liu, L. Magnetic bead-based electrochemical and colorimetric assays of circulating tumor cells with boronic acid derivatives as the recognition elements and signal probes. Talanta 2021, 221, 121640–121647. [Google Scholar] [CrossRef]
- Xiao, S.; Yang, X.; Wu, J.; Liu, Q.; Li, D.; Huang, S.; Xie, H.; Yu, Z.; Gan, N. Reusable electrochemical biosensing platform based on egg yolk antibody-labeled magnetic covalent organic framework for on-site detection of Escherichia coli in foods. Sens. Actuators B Chem. 2022, 369, 132320–132329. [Google Scholar] [CrossRef]
- Chi, L.; Xu, C.; Li, S.; Wang, X.; Tang, D.; Xue, F. Thionine-doped nanometer-sized silica conjugated with phenylboronic acid: An innovative recognition/signal element for voltammetric aptasensing of colorectal cancer-related carcinoembryonic antigen. Anal. Chim. Acta 2020, 1136, 91–98. [Google Scholar] [CrossRef]
- Xing, Y.; Liu, L.; Zhao, D.; Yang, Y.; Chu, X. Synthesis of water-dispersed ferrecene/phenylboronic acid-modified bifunctional gold nanoparticles and the application in biosensing. Materials 2014, 7, 5554–5564. [Google Scholar] [CrossRef]
- Liu, L.; Du, J.; Li, S.; Yuan, B.; Han, H.; Jing, M.; Xia, N. Amplified voltammetric detection of dopamine using ferrocene-capped gold nanoparticle/streptavidin conjugates. Biosens. Bioelectron. 2013, 41, 730–735. [Google Scholar] [CrossRef]
- Wang, W.; Tan, L.; Wu, J.; Li, T.; Xie, H.; Wu, D.; Gan, N. A universal signal-on electrochemical assay for rapid on-site quantitation of vibrio parahaemolyticus using aptamer modified magnetic metal-organic framework and phenylboronic acid-ferrocene co-immobilized nanolabel. Anal. Chim. Acta 2020, 1133, 128–136. [Google Scholar] [CrossRef]
- Xia, N.; Zhang, L.; Wang, G.; Feng, Q.; Liu, L. Label-free and sensitive strategy for microRNAs detection based on the formation of boronate ester bonds and the dual-amplification of gold nanoparticles. Biosens. Bioelectron. 2013, 47, 461–466. [Google Scholar] [CrossRef]
- Xia, N.; Deng, D.; Zhang, L.; Yuan, B.; Jing, M.; Du, J.; Liu, L. Sandwich-type electrochemical biosensor for glycoproteins detection based on dual-amplification of boronic acid-gold nanoparticles and dopamine-gold nanoparticles. Biosens. Bioelectron. 2013, 43, 155–159. [Google Scholar] [CrossRef]
- You, M.; Yang, S.; Tang, W.; Zhang, F.; He, P.G. Ultrasensitive electrochemical detection of glycoprotein based on boronate affinity sandwich assay and signal amplification with functionalized SiO2@Au nanocomposites. ACS Appl. Mater. Interfaces 2017, 9, 13855–13864. [Google Scholar] [CrossRef]
- Liu, L.; Xing, Y.; Zhang, H.; Liu, R.; Liu, H.; Xia, N. Amplified voltammetric detection of glycoproteins using 4-mercaptophenylboronic acid/biotin-modified multifunctional gold nanoparticles as labels. Int. J. Nanomed. 2014, 9, 2619–2626. [Google Scholar]
- Liu, L.; Xia, N.; Liu, H.; Kang, X.; Liu, X.; Xue, C.; He, X. Highly sensitive and label-free electrochemical detection of microRNAs based on triple signal amplification of multifunctional gold nanoparticles, enzymes and redox-cycling reaction. Biosens. Bioelectron. 2014, 53, 399–405. [Google Scholar] [CrossRef]
- Wang, X.; Dong, S.; Wei, H. Recent advances on nanozyme-based electrochemical biosensors. Electroanalysis 2022, 35, 38–49. [Google Scholar] [CrossRef]
- Fu, C.; Sun, Y.; Huang, C.; Wang, F.; Li, N.; Zhang, L.; Ge, S.; Yu, J. Ultrasensitive sandwich-like electrochemical biosensor based on core-shell Pt@CeO2 as signal tags and double molecular recognition for cerebral dopamine detection. Talanta 2021, 223, 121719–121726. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Lv, W.X.; Yang, Q.T.; Li, H.Y.; Li, F. Label-free homogeneous electrochemical detection of MicroRNA based on target-induced anti-shielding against the catalytic activity of two-dimension nanozyme. Biosens. Bioelectron. 2021, 171, 112707. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.H.; Yang, Q.T.; Li, Q.; Li, H.Y.; Li, F. Two dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticide without interferences of H2O2 and color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef] [PubMed]
- Son, S.E.; Gupta, P.K.; Hur, W.; Choi, H.; Lee, H.B.; Park, Y.; Seong, G.H. Determination of glycated albumin using a Prussian blue nanozyme-based boronate affinity sandwich assay. Anal. Chim. Acta 2020, 1134, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jian, Y.; Wang, H.; Ge, S.; Yan, M.; Yu, J. Ultrasensitive microfluidic paper-based electrochemical biosensor based on molecularly imprinted film and boronate affinity sandwich assay for glycoprotein detection. ACS Appl. Mater. Interfaces 2019, 11, 16198–16206. [Google Scholar] [CrossRef]
- Amor-Gutierrez, O.; Iglesias-Mayor, A.; Llano-Suarez, P.; Costa-Fernandez, J.M.; Soldado, A.; Podadera, A.; Parra, F.; Costa-Garcia, A.; de la Escosura-Muniz, A. Electrochemical quantification of Ag2S quantum dots: Evaluation of different surface coating ligands for bacteria determination. Microchim. Acta 2020, 187, 169–179. [Google Scholar] [CrossRef]
- Song, D.; Zheng, J.; Myung, N.V.; Xu, J.; Zhang, M. Sandwich-type electrochemical immunosensor for CEA detection using magnetic hollow Ni/C@SiO2 nanomatrix and boronic acid functionalized CPS@PANI@Au probe. Talanta 2021, 225, 122006–122016. [Google Scholar] [CrossRef]
- Hou, L.; Huang, Y.; Hou, W.; Yan, Y.; Liu, J.; Xia, N. Modification-free amperometric biosensor for the detection of wild-type p53 protein based on the in situ formation of silver nanoparticle networks for signal amplification. Int. J. Biol. Macromol. 2020, 158, 580–586. [Google Scholar] [CrossRef]
- Pan, Y.; Shan, W.; Fang, H.; Guo, M.; Nie, Z.; Huang, Y.; Yao, S. Sensitive and visible detection of apoptotic cells on Annexin-V modified substrate using aminophenylboronic acid modified gold nanoparticles (APBA-GNPs) labeling. Biosens. Bioelectron. 2014, 52, 62–68. [Google Scholar] [CrossRef]
- Liu, L.; Chang, Y.; Xia, N.; Peng, P.; Zhang, L.; Jiang, M.; Zhang, J.; Liu, L. Simple, sensitive and label-free electrochemical detection of microRNAs based on the in situ formation of silver nanoparticles aggregates for signal amplification. Biosens. Bioelectron. 2017, 94, 235–242. [Google Scholar] [CrossRef]
- Xia, N.; Cheng, C.; Liu, L.; Peng, P.; Liu, C.; Chen, J. Electrochemical glycoprotein aptasensors based on the in-situ aggregation of silver nanoparticles induced by 4-mercaptophenylboronic acid. Microchim. Acta 2017, 184, 4393–4400. [Google Scholar] [CrossRef]
- Liu, L.; Cheng, C.; Chang, Y.; Ma, H.; Hao, Y. Two sensitive electrochemical strategies for the detection of protein kinase activity based on the 4-mercaptophenylboronic acid-induced in situ assembly of silver nanoparticles. Sens. Actuators B Chem. 2017, 248, 178–186. [Google Scholar] [CrossRef]
- Xia, N.; Liu, L.; Chang, Y.; Hao, Y.; Wang, X. 4-Mercaptophenylboronic acid-induced in situ formation of silver nanoparticle aggregates as labels on an electrode surface. Electrochem. Commun. 2017, 74, 28–32. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, W.; Tan, Q.; Cui, X.; Dai, Z. Electrochemical assay of the alpha fetoprotein-L3 isoform ratio to improve the diagnostic accuracy of hepatocellular carcinoma. Anal. Chem. 2018, 90, 13051–13058. [Google Scholar] [CrossRef]
- An, Y.; Li, R.; Zhang, F.; He, P. A ratiometric electrochemical sensor for the determination of exosomal glycoproteins. Talanta 2021, 235, 122790–122797. [Google Scholar] [CrossRef]
- Chang, J.F.; Wang, X.; Wang, J.; Li, H.Y.; Li, F. Nucleic acid functionalized MOFs-based homogeneous electrochemical biosensor for simultaneous detection of multiple tumor biomarkers. Anal. Chem. 2019, 91, 3604–3610. [Google Scholar] [CrossRef]
- Chang, Y.; Lou, J.; Yang, L.; Liu, M.; Xia, N.; Liu, L. Design and application of electrochemical sensors with metal–organic frameworks as the electrode materials or signal tags. Nanomaterials 2022, 12, 3248. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, G.; Li, S.; Liu, L.; Song, Q. Biorecognition element-free electrochemical detection of recombinant glycoproteins using metal-organic frameworks as signal tags. Anal. Chim. Acta 2023, 1273, 341540. [Google Scholar] [CrossRef]
- Xie, H.; Dong, J.; Duan, J.; Waterhouse, G.I.N.; Hou, J.; Ai, S. Visual and ratiometric fluorescence detection of Hg2+ based on a dual-emission carbon dots-gold nanoclusters nanohybrid. Sens. Actuators B Chem. 2018, 259, 1082–1089. [Google Scholar] [CrossRef]
- Wang, L.L.; Qiao, J.; Liu, H.H.; Hao, J.; Qi, L.; Zhou, X.P.; Li, D.; Nie, Z.X.; Mao, L.Q. Ratiometric fluorescent probe based on gold nanoclusters and alizarin red-boronic acid for monitoring glucose in brain microdialysate. Anal. Chem. 2014, 86, 9758–9764. [Google Scholar] [CrossRef]
- Li, X.G.; Zhang, F.; Gao, Y.; Zhou, Q.M.; Zhao, Y.; Li, Y.; Huo, J.Z.; Zhao, X.J. Facile synthesis of red emitting 3-aminophenylboronic acid functionalized copper nanoclusters for rapid, selective and highly sensitive detection of glycoproteins. Biosens. Bioelectron. 2016, 86, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Li, Y.; Huang, A. Detection of sialic acid using boronic-acid-functionalized metal organic framework UiO-66-NH2@B(OH)2. Talanta 2021, 232, 122434. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Li, S.; Wang, H.; Meng, Q.; Fan, L.; Xie, H.; Cao, C.; Zhang, W. Simple boric acid-based fluorescent focusing for sensing of glucose and glycoprotein via multipath moving supramolecular boundary electrophoresis chip. Anal. Chem. 2013, 85, 5884–5891. [Google Scholar] [CrossRef] [PubMed]
- Han, E.; Ding, L.; Ju, H. Highly sensitive fluorescent analysis of dynamic glycan expression on living cells using glyconanoparticles and functionalized quantum dots. Anal. Chem. 2011, 83, 7006–7012. [Google Scholar] [CrossRef] [PubMed]
- Sang, L.J.; Wang, H.F. Aminophenylboronic-acid-conjugated polyacrylic acid-Mn-doped ZnS quantum dot for highly sensitive discrimination of glycoproteins. Anal. Chem. 2014, 86, 5706–5712. [Google Scholar] [CrossRef]
- Mandal, D.; Mandal, S.K.; Ghosh, M.; Das, P.K. Phenylboronic acid appended pyrene-based Low-molecular weight injectable hydrogel: Glucose-stimulated insulin release. Chem. Eur. J. 2015, 21, 12042–12052. [Google Scholar] [CrossRef]
- Mandal, D.; Das, S. Dissipation of pyrene-based phenylboronic acid-anchored vesicular self-assemblies: A motif for neurotransmitter recognition. ChemistrySelect 2019, 4, 1220–1226. [Google Scholar] [CrossRef]
- Deng, M.; Song, G.; Zhong, K.; Wang, Z.; Xia, X.; Tian, Y. Wearable fluorescent contact lenses for monitoring glucose via a smartphone. Sens. Actuators B Chem. 2022, 352, 131067. [Google Scholar] [CrossRef]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. A glucose sensing contact lens: A non-invasive technique for continuous physiological glucose monitoring. J. Fluoresc. 2003, 13, 371–374. [Google Scholar] [CrossRef]
- Badugu, R.; Lakowicza, J.R.; Geddes, C.D. Ophthalmic glucose sensing: A novel monosaccharide sensing disposable and colorless contact lens. Analyst 2004, 129, 516–521. [Google Scholar] [CrossRef]
- Badugu, R.; Lakowicz, J.R.; Geddes, C.D. Ophthalmic glucose monitoring using disposable contact lenses—A review. J. Fluoresc. 2004, 14, 617–633. [Google Scholar] [CrossRef]
- James, T.D.; Sandanayake, K.R.A.S.; Iguchi, R.; Shinkai, S. Novel saccharide-photoinduced electron transfer sensors based on the interaction of boronic acid and amine. J. Am. Chem. Soc. 1995, 117, 8982–8987. [Google Scholar] [CrossRef]
- James, T.D.; Sandanayake, K.R.A.S.; Shinkai, S. A glucose-selective molecular fluorescence sensor. Angew. Chem. Int. Ed. 1994, 33, 2207–2209. [Google Scholar] [CrossRef]
- Wu, X.; Meng, Q.; Zhang, Q.; Fan, L.; Xiao, H.; Cao, C. Glycoprotein fluorescent speed sensing by newly-synthesized boronic complex probe and chip supramolecular electrophoresis. Sens. Actuators B Chem. 2020, 309, 127773–127779. [Google Scholar] [CrossRef]
- Bi, X.; Li, D.; Liu, Z. Pattern recognition of monosaccharides via a virtual lectin array constructed by boronate affinity-based pH-featured encoding. Anal. Chem. 2015, 87, 4442–4447. [Google Scholar] [CrossRef]
- Chang, L.; He, X.; Chen, L.; Zhang, Y. Mercaptophenylboronic acid-capped Mn-doped ZnS quantum dots for highly selective and sensitive fluorescence detection of glycoproteins. Sens. Actuators B Chem. 2017, 243, 72–77. [Google Scholar] [CrossRef]
- Chang, L.; Wu, H.; He, X.; Chen, L.; Zhang, Y. A highly sensitive fluorescent turn-on biosensor for glycoproteins based on boronic acid functional polymer capped Mn-doped ZnS quantum dots. Anal. Chim. Acta 2017, 995, 91–98. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, X.; Li, P.; Zhang, W.; Wang, H.; Tang, B. In Situ fluorescence imaging of the levels of glycosylation and phosphorylation by a MOF-based nanoprobe in depressed mice. Anal. Chem. 2020, 92, 3716–3721. [Google Scholar] [CrossRef]
- Wang, S.; Cui, J.; Fan, Q.; Gan, J.; Liu, C.; Wang, Y.; Yang, T.; Wang, J.; Yang, C. Reversible and highly ordered biointerfaces for efficient capture and nondestructive release of circulating tumor cells. Anal. Chem. 2022, 94, 9450–9458. [Google Scholar] [CrossRef]
- Wang, Y.T.; Wu, N.; Yang, T.; Wang, J.H. Unusual selective response to glycoprotein over sugar facilitates ultrafast universal fluorescent immunoassay of biomarkers. Anal. Chem. 2020, 92, 5540–5545. [Google Scholar] [CrossRef]
- Wang, Y.; Hai, X.; Shuang, E.; Chen, M.; Yang, T.; Wang, J. Boronic acid functionalized g-C3N4 nanosheets for ultrasensitive and selective sensing of glycoprotein in the physiological environment. Nanoscale 2018, 10, 4913–4920. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.T.; Cai, M.D.; Sun, L.L.; Hua, R.N. A rapid and facile separation-detection integrated strategy for exosome profiling based on boronic acid-directed coupling immunoaffinity. Anal. Chem. 2021, 93, 16059–16067. [Google Scholar] [CrossRef] [PubMed]
- Hao, T.; Wei, X.; Nie, Y.; Xu, Y.; Lu, K.; Yan, Y.; Zhou, Z. Surface modification and ratiometric fluorescence dual function enhancement for visual and fluorescent detection of glucose based on dual-emission quantum dots hybrid. Sens. Actuators B Chem. 2016, 230, 70–76. [Google Scholar] [CrossRef]
- Shen, P.; Xia, Y. Synthesis-modification integration: One-step fabrication of boronic acid functionalized carbon dots for fluorescent blood sugar sensing. Anal. Chem. 2014, 86, 5323–5329. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.B.; Zhou, X.; Gu, L.; Lan, R.; Sun, D.; Yu, D.; Shi, G. Boronic acid functionalized graphene quantum dots as a fluorescent probe for selective and sensitive glucose determination in microdialysate. Chem. Commun. 2013, 49, 9830–9832. [Google Scholar] [CrossRef]
- Zou, W.-S.; Ye, C.-H.; Wang, Y.-Q.; Li, W.-H.; Huang, X.-H. A hybrid ratiometric probe for glucose detection based on synchronous responses to fluorescence quenching and resonance light scattering enhancement of boronic acid functionalized carbon dots. Sens. Actuators B Chem. 2018, 271, 54–63. [Google Scholar] [CrossRef]
- Ye, S.; Han, T.; Cheng, M.; Dong, L. Wulff-type boronic acid-functionalized quantum dots for rapid and sensitive detection of Gram-negative bacteria. Sens. Actuators B Chem. 2022, 356, 131332–131339. [Google Scholar] [CrossRef]
- Zhang, X.; Chai, L.; Nie, S.; Lv, C.; Wang, Q.; Li, Z. Facile synthesis of boronic acid-decorated carbon nanodots as optical nanoprobes for glycoprotein sensing. Analyst 2019, 144, 1975–1981. [Google Scholar] [CrossRef]
- Peng, N.; Xu, R.; Si, M.; Victorious, A.; Ha, E.; Chang, C.-Y.; Xu, X.-D. Fluorescent probe with aggregation-induced emission characteristics for targeted labelling and imaging of cancer cells. RSC Adv. 2017, 7, 11282–11285. [Google Scholar] [CrossRef]
- Wang, D.; Tang, B.Z. Aggregation-induced emission luminogens for activity-based sensing. Acc. Chem. Res. 2019, 52, 2559–2570. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.Y.; Liang, R.P.; Li, Y.H.; Qiu, J.D. Boron-doped graphene quantum dots for selective glucose sensing based on the “abnormal” aggregation-induced photoluminescence enhancement. Anal. Chem. 2014, 86, 4423–4430. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, X.; Yu, Y.; Wang, J. Boronic acid-containing carbon dots array for sensitive identification of glycoproteins and cancer cells. Chin. Chem. Lett. 2021, 32, 3043–3047. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, L.; Ji, J.; Li, Y.; Liu, T.; Lei, J. Cleancap-regulated aggregation-induced emission strategy for highly specific analysis of enzyme. Anal. Chem. 2020, 92, 4726–4730. [Google Scholar] [CrossRef]
- Sheng, A.; Yang, J.; Cheng, L.; Zhang, J. Boronic ester-mediated dual recognition coupled with a CRISPR/Cas12a system for lipopolysaccharide analysis. Anal. Chem. 2022, 94, 12523–12530. [Google Scholar] [CrossRef]
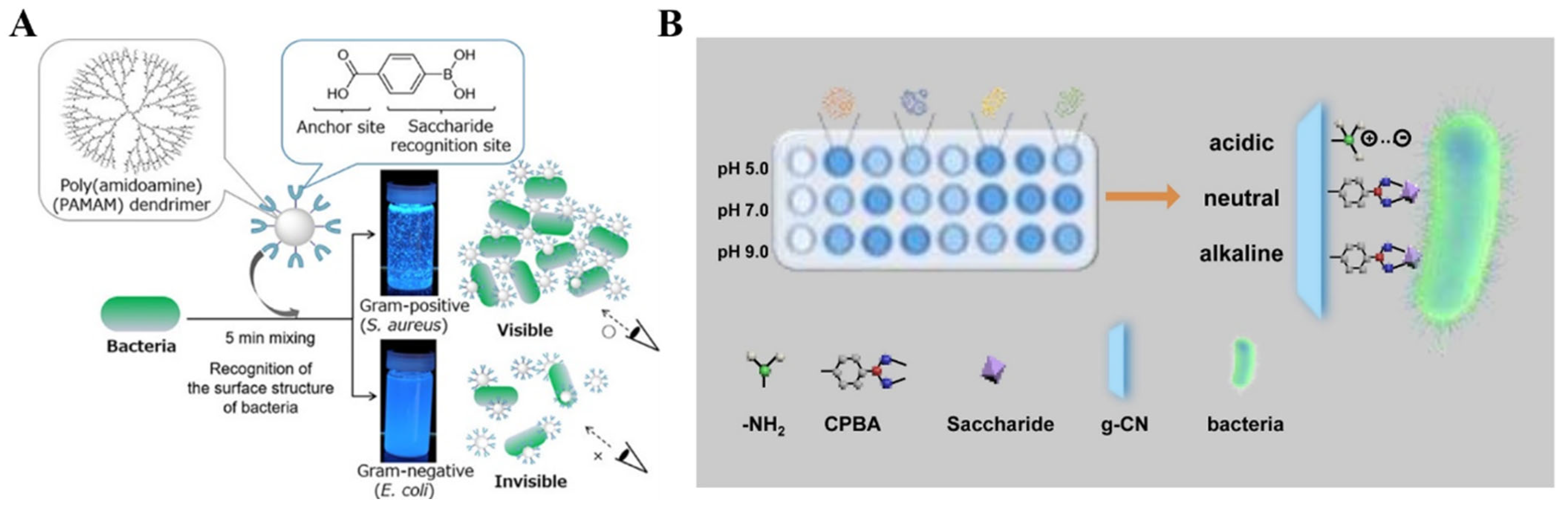
- Mikagi, A.; Manita, K.; Yoyasu, A.; Tsuchido, Y.; Kanzawa, N.; Hashimoto, T.; Hayashita, T. Rapid bacterial recognition over a wide pH range by boronic acid-based ditopic dendrimer probes for Gram-positive bacteria. Molecules 2021, 27, 256. [Google Scholar] [CrossRef]
- Tsuchido, Y.; Horiuchi, R.; Hashimoto, T.; Ishihara, K.; Kanzawa, N.; Hayashita, T. Rapid and selective discrimination of Gram-positive and Gram-negative bacteria by boronic acid-modified poly(amidoamine) dendrimer. Anal. Chem. 2019, 91, 3929–3935. [Google Scholar] [CrossRef]
- Mikagi, A.; Manita, K.; Tsuchido, Y.; Kanzawa, N.; Hashimoto, T.; Hayashita, T. Boronic acid-based dendrimers with various surface properties for bacterial recognition with adjustable selectivity. ACS Appl. Bio Mater. 2022, 5, 5255–5263. [Google Scholar] [CrossRef]
- Mikagi, A.; Tsurufusa, R.; Tsuchido, Y.; Hashimoto, T.; Hayashita, T. Fast and sensitive bacteria detection by boronic acid modified fluorescent dendrimer. Sensors 2021, 21, 3115. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Jia, X.-D.; Gao, R.-X.; Chen, M.-L.; Yang, T.; Wang, J.-H. Discrimination of pathogenic bacteria with boronic acid modified protonated g-C3N4 nanosheets at various pHs. Sens. Actuators B Chem. 2021, 340, 129951–129958. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Wei, Y. A sandwich boronate affinity sorbent assay for glucose detection facilitated by boronic acid-terminated fluorescent polymers. Sens. Actuators B Chem. 2017, 247, 595–601. [Google Scholar] [CrossRef]
- Chen, J.; Hao, L.; Wu, Y.; Lin, T.; Li, X.; Leng, Y.; Huang, X.; Xiong, Y. Integrated magneto-fluorescence nanobeads for ultrasensitive glycoprotein detection using antibody coupled boronate-affinity recognition. Chem. Commun. 2019, 55, 10312–10315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, W.; Li, P.; Xiao, H.; Wang, H.; Tang, B. A fluorescence nanosensor for glycoproteins with activity based on the molecularly imprinted spatial structure of the target and boronate affinity. Angew. Chem. Int. Ed. 2014, 53, 12489–12493. [Google Scholar]
- Lu, H.; Xu, S. Ultrasensitive turn on molecularly imprinted fluorescence sensor for glycoprotein detection based on nanoparticles signal amplification. Sens. Actuators B Chem. 2020, 306, 127566–127574. [Google Scholar] [CrossRef]
- Yu, S.S.; Shi, Y.J.; Wang, D.; Qiang, T.T.; Zhao, Y.Q.; Wang, X.Y.; Zhao, J.M.; Dong, L.Y.; Huang, Y.J.; Wang, X.H. Linking peptide-oriented surface imprinting magnetic nanoparticle with carbon nanotube-based fluorescence signal output device for ultrasensitive detection of glycoprotein. Anal. Chim. Acta 2023, 1259, 341202–341212. [Google Scholar] [CrossRef]
- Bai, C.-C.; Wang, D.; Liu, M.-X.; Ma, Y.-R.; Sun, Y.; Duan, R.; Dong, L.-Y.; Wang, X.-H. Ultrasensitive and specific detection of glycoprotein with boronic acid-modified/fluorescein isothiocyanate-loaded graphene oxide as signal amplification matrix. Sens. Actuators B Chem. 2021, 344, 130327–130337. [Google Scholar] [CrossRef]
- Chen, X.; Yu, S.; Yang, L.; Wang, J.; Jiang, C. Fluorescence and visual detection of fluoride ions using a photoluminescent graphene oxide paper sensor. Nanoscale 2016, 8, 13669–13677. [Google Scholar] [CrossRef]
- Xue, M.; Wang, X.; Duan, L.; Gao, W.; Ji, L.; Tang, B. A new nanoprobe based on FRET between functional quantum dots and gold nanoparticles for fluoride anion and its applications for biological imaging. Biosens. Bioelectron. 2012, 36, 168–173. [Google Scholar] [CrossRef]
- Yang, H.; Jie, X.; Wang, L.; Zhang, Y.; Wang, M.; Wei, W. An array consisting of glycosylated quantum dots conjugated to MoS2 nanosheets for fluorometric identification and quantitation of lectins and bacteria. Microchim. Acta 2018, 185, 512–521. [Google Scholar] [CrossRef]
- Wang, S.; Ye, J.; Li, X.; Liu, Z. Boronate affinity fluorescent nanoparticles for forster resonance energy transfer inhibition assay of cis-diol biomolecules. Anal. Chem. 2016, 88, 5088–5096. [Google Scholar] [CrossRef]
- Chang, L.; He, X.; Chen, L.; Zhang, Y. A fluorescent sensing for glycoproteins based on the FRET between quantum dots and Au nanoparticles. Sens. Actuators B Chem. 2017, 250, 17–23. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, X.; Zhang, Z.; Chen, H.; Zhang, S.; Kong, J. Multifunctional phenylboronic acid-tagged fluorescent silica nanoparticles via thiol-ene click reaction for imaging sialic acid expressed on living cells. Talanta 2013, 115, 823–829. [Google Scholar] [CrossRef]
- Wang, D.E.; Yan, J.; Jiang, J.; Liu, X.; Tian, C.; Xu, J.; Yuan, M.S.; Han, X.; Wang, J. Polydiacetylene liposomes with phenylboronic acid tags: A fluorescence turn-on sensor for sialic acid detection and cell-surface glycan imaging. Nanoscale 2018, 10, 4570–4578. [Google Scholar] [CrossRef]
- Liu, R.; Cui, Q.; Wang, C.; Wang, X.; Yang, Y.; Li, L. Preparation of sialic acid-imprinted fluorescent conjugated nanoparticles and their application for targeted cancer cell imaging. ACS Appl. Mater. Interfaces 2017, 9, 3006–3015. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y. Self-assembled nanorods of phenylboronic acid functionalized pyrene for in situ two-photon imaging of cell surface sialic acids and photodynamic therapy. Anal. Chem. 2021, 93, 7029–7036. [Google Scholar] [CrossRef]
- Liu, A.; Peng, S.; Soo, J.C.; Kuang, M.; Chen, P.; Duan, H. Quantum dots with phenylboronic acid tags for specific labeling of sialic acids on living cells. Anal. Chem. 2011, 83, 1124–1130. [Google Scholar] [CrossRef]
- Wu, M.S.; Zhou, Z.R.; Wang, X.Y.; Chen, B.B.; Hafez, M.E.; Shi, J.F.; Li, D.W.; Qian, R.C. Dynamic visualization of endoplasmic reticulum stress in living cells via a two-stage cascade recognition process. Anal. Chem. 2022, 94, 2882–2890. [Google Scholar] [CrossRef]
- Todaro, B.; Begarani, F.; Sartori, F.; Luin, S. Is Raman the best strategy towards the development of non-invasive continuous glucose monitoring devices for diabetes management? Front. Chem. 2022, 10, 994272. [Google Scholar] [CrossRef]
- Ahmadianyazdi, A.; Nguyen, N.H.L.; Xu, J.; Berry, V. Glucose measurement via Raman spectroscopy of graphene: Principles and operation. Nano Res. 2022, 15, 8697–8704. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Q.; Chu, W.; Zhao, W.; Zheng, J. Surface-enhanced Raman scattering behaviour of 4-mercaptophenyl boronic acid on assembled silver nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 17638–17645. [Google Scholar] [CrossRef]
- Sun, F.; Bai, T.; Zhang, L.; Ella-Menye, J.R.; Liu, S.; Nowinski, A.K.; Jiang, S.; Yu, Q. Sensitive and fast detection of fructose in complex media via symmetry breaking and signal amplification using surface-enhanced Raman spectroscopy. Anal. Chem. 2014, 86, 2387–2394. [Google Scholar] [CrossRef]
- Liang, L.; Qu, H.; Zhang, B.; Zhang, J.; Deng, R.; Shen, Y.; Xu, S.; Liang, C.; Xu, W. Tracing sialoglycans on cell membrane via surface-enhanced Raman scattering spectroscopy with a phenylboronic acid-based nanosensor in molecular recognition. Biosens. Bioelectron. 2017, 94, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hassan, M.M.; Zhu, A.; Li, H.; Chen, Q. Dual-mode of magnetic assisted Au@Ag SERS tags and cationic conjugated UCNPs for qualitative and quantitative analysis of multiple foodborne pathogens. Sens. Actuators B Chem. 2021, 344, 130305–130313. [Google Scholar] [CrossRef]
- He, X.N.; Wang, Y.N.; Wang, Y.; Xu, Z.R. Accurate quantitative detection of cell surface sialic acids with a background-free SERS probe. Talanta 2020, 209, 120579–120585. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.V.; Ho, C.J.; Gong, T.; Lau, W.K.; Olivo, M. Sensitive SERS glucose sensing in biological media using alkyne functionalized boronic acid on planar substrates. Biosens. Bioelectron. 2014, 56, 186–191. [Google Scholar] [CrossRef]
- Bi, X.; Du, X.; Jiang, J.; Huang, X. Facile and sensitive glucose sandwich assay using in situ-generated Raman reporters. Anal. Chem. 2015, 87, 2016–2021. [Google Scholar] [CrossRef]
- Feng, J.; Lu, H.; Yang, Y.; Huang, W.; Cheng, H.; Kong, H.; Li, L. SERS-ELISA determination of human carboxylesterase 1 using metal-organic framework doped with gold nanoparticles as SERS substrate. Microchim. Acta 2021, 188, 280–290. [Google Scholar] [CrossRef]
- Ma, Y.; Promthaveepong, K.; Li, N. Gold superparticles functionalized with azobenzene derivatives: SERS nanotags with strong signals. ACS Appl. Mater. Interfaces 2017, 9, 10530–10536. [Google Scholar] [CrossRef]
- Muhammad, P.; Liu, J.; Xing, R.; Wen, Y.; Wang, Y.; Liu, Z. Fast probing of glucose and fructose in plant tissues via plasmonic affinity sandwich assay with molecularly-imprinted extraction microprobes. Anal. Chim. Acta 2017, 995, 34–42. [Google Scholar] [CrossRef]
- Li, X.; Li, B.; Hong, J.; Zhou, X. Highly selective determination of acid phosphatase in biological samples using a biomimetic recognition-based SERS sensor. Sens. Actuators B Chem. 2018, 276, 421–428. [Google Scholar] [CrossRef]
- Ye, J.; Chen, Y.; Liu, Z. A boronate affinity sandwich assay: An appealing alternative to immunoassays for the determination of glycoproteins. Angew. Chem. Int. Ed. 2014, 53, 10386–10389. [Google Scholar] [CrossRef]
- Muhammad, P.; Tu, X.; Liu, J.; Wang, Y.; Liu, Z. Molecularly imprinted plasmonic substrates for specific and ultrasensitive immunoassay of trace glycoproteins in biological samples. ACS Appl. Mater. Interfaces 2017, 9, 12082–12091. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Zhang, R.; Tang, K.; Ding, C.; Yu, S. SERS-based immunocapture and detection of pathogenic bacteria using a boronic acid-functionalized polydopamine-coated Au@Ag nanoprobe. Microchim. Acta 2020, 187, 290–298. [Google Scholar] [CrossRef]
- Zhuang, X.; Hu, Y.; Wang, J.; Hu, J.; Wang, Q.; Yu, X. A colorimetric and SERS dual-readout sensor for sensitive detection of tyrosinase activity based on 4-mercaptophenyl boronic acid modified AuNPs. Anal. Chim. Acta 2021, 1188, 339172–339181. [Google Scholar] [CrossRef]
- Usta, D.D.; Salimi, K.; Pinar, A.; Coban, I.; Tekinay, T.; Tuncel, A. A boronate affinity-assisted SERS tag equipped with a sandwich system for detection of glycated hemoglobin in the hemolysate of human erythrocytes. ACS Appl. Mater. Interfaces 2016, 8, 11934–11944. [Google Scholar] [CrossRef]
- Hu, C.; Peng, F.; Mi, F.; Wang, Y.; Geng, P.; Pang, L.; Ma, Y.; Li, G.; Li, Y.; Guan, M. SERS-based boronate affinity biosensor with biomimetic specificity and versatility: Surface-imprinted magnetic polymers as recognition elements to detect glycoproteins. Anal. Chim. Acta 2022, 1191, 339289–339298. [Google Scholar] [CrossRef]
- Solís-Delgado, L.E.; Ochoa-Terán, A.; Yatsimirsky, A.K.; Pina-Luis, G. Colorimetric and fluorescent determination of fluoride using a novel naphthalene diimide boronic acid derivative. Anal. Lett. 2016, 49, 2301–2311. [Google Scholar] [CrossRef]
- Ghosh, K.K.; Yap, E.; Kim, H.; Lee, J.-S.; Chang, Y.-T. A colorimetric pH indicators and boronic acids ensemble array for quantitative sugar analysisw. Chem. Commun. 2011, 47, 4001–4003. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, J.-S.; Chang, Y.-T. Colorimetric identification of carbohydrates by a pH indicator/pH change inducer ensemble. Angew. Chem. Int. Ed. 2006, 45, 6485–6487. [Google Scholar] [CrossRef]
- Hu, Y.; Shao, L.; Fan, Y.; Lu, L.; Zhou, C.; Fu, H.; She, Y. Colorimetric discrimination of tea polyphenols based on boronic acid sensor assembled with pH indicator. Dyes Pigments 2022, 203, 110326. [Google Scholar] [CrossRef]
- Egawa, Y.; Miki, R.; Seki, T. Colorimetric sugar sensing using boronic acid-substituted azobenzenes. Materials 2014, 7, 1201–1220. [Google Scholar] [CrossRef]
- Sikora, A.; Zielonka, J.; Debowska, K.; Michalski, R.; Smulik-Izydorczyk, R.; Pieta, J.; Podsiadły, R.; Artelska, A.; Pierzchała, K.; Kalyanaraman, B. Boronate-based probes for biological oxidants: A novel class of molecular tools for redox biology. Front. Chem. 2020, 8, 580899. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Y.; He, X.; Chen, L.; Zhang, Y. Colorimetric sensor based on 4-mercaptophenylboronic modified gold nanoparticles for rapid and selective detection of fluoride anion. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 214, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Jayeoye, T.J.; Rujiralai, T. Sensitive and selective colorimetric probe for fluoride detection based on the interaction between 3-aminophenylboronic acid and dithiobis(succinimidylpropionate) modified gold nanoparticles. New J. Chem. 2020, 44, 5711–5719. [Google Scholar] [CrossRef]
- Borsley, S.; Kay, E.R. Dynamic covalent assembly and disassembly of nanoparticle aggregates. Chem. Commun. 2016, 52, 9117–9120. [Google Scholar] [CrossRef]
- Nair, P.A.; Sreenivasan, K. Non enzymatic colorimetric detection of glucose using cyanophenyl boronic acid included β-cyclodextrin stabilized gold nanoparticles. Anal. Methods 2016, 8, 2082–2087. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Q.; Huang, C.; Qin, S.; Han, S.; Huo, Z.; Li, Y.; Sun, X.; Chen, J. 3-Aminophenylboronic acid-mediated aggregation of gold nanoparticles for colorimetric sensing of iohexol in environmental and biological samples. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 261, 120004–120011. [Google Scholar] [CrossRef]
- Kong, B.; Zhu, A.; Luo, Y.; Tian, Y.; Yu, Y.; Shi, G. Sensitive and selective colorimetric visualization of cerebral dopamine based on double molecular recognition. Angew. Chem. Int. Ed. 2011, 50, 1837–1840. [Google Scholar] [CrossRef]
- Godoy-Reyes, T.M.; Costero, A.M.; Gaviña, P.; Martínez-Máñez, R.; Sancenón, F. A colorimetric probe for the selective detection of norepinephrine based on a double molecular recognition with functionalized gold nanoparticles. ACS Appl. Nano Mater. 2019, 2, 1367–1373. [Google Scholar] [CrossRef]
- Jayeoye, T.J.; Cheewasedtham, W.; Putson, C.; Rujiralai, T. A selective probe based on 3-aminophenyl boronic acid assembly on dithiobis(succinimidylpropionate) functionalized gold nanoparticles for sialic acid detection in human serum. J. Mol. Liq. 2019, 281, 407–414. [Google Scholar] [CrossRef]
- Na, W.; Liu, H.; Wang, M.; Su, X. A boronic acid based glucose assay based on the suppression of the inner filter effect of gold nanoparticles on the orange fluorescence of graphene oxide quantum dots. Microchim. Acta 2017, 184, 1463–1470. [Google Scholar] [CrossRef]
- Zhang, J.; He, L.; Zhang, X.; Wang, J.; Yang, L.; Liu, B.; Jiang, C.; Zhang, Z. Colorimetric and SERS dual-readout for assaying alkaline phosphatase activity by ascorbic acid induced aggregation of Ag coated Au nanoparticles. Sens. Actuators B Chem. 2017, 253, 839–845. [Google Scholar] [CrossRef]
- Korich, A.L.; Iovine, P.M. Boroxine chemistry and applications: A perspective. Dalton Trans. 2010, 39, 1423–1431. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, H.; Liu, L.; Li, M.; Xiao, K.; Xu, M. Selective and sensitive colorimetric sensor of mercury (II) based on gold nanoparticles and 4-mercaptophenylboronic acid. Sens. Actuators B Chem. 2014, 196, 106–111. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, J.; Li, X. Light-switchable self-assembly of non-photoresponsive gold nanoparticles. Langmuir 2018, 34, 6117–6124. [Google Scholar] [CrossRef]
- Jiang, G.; Zhu, W.; Shen, X.; Xu, L.; Li, X.; Wang, R.; Liu, C.; Zhou, X. Colorimetric and visual determination of adenosine triphosphate using a boronic acid as the recognition element, and based on the deaggregation of gold nanoparticles. Microchim. Acta 2017, 184, 4305–4312. [Google Scholar] [CrossRef]
- Liu, S.; Du, Z.; Li, P.; Li, F. Sensitive colorimetric visualization of dihydronicotinamide adenine dinucleotide based on anti-aggregation of gold nanoparticles via boronic acid-diol binding. Biosens. Bioelectron. 2012, 35, 443–446. [Google Scholar] [CrossRef]
- Yan, P.; Ding, Z.; Li, X.; Dong, Y.; Fu, T.; Wu, Y. Colorimetric sensor array based on Wulff-type boronate functionalized AgNPs at various pH for bacteria identification. Anal. Chem. 2019, 91, 12134–12137. [Google Scholar] [CrossRef]
- Zheng, L.; Qi, P.; Zhang, D. A simple, rapid and cost-effective colorimetric assay based on the 4-mercaptophenylboronic acid functionalized silver nanoparticles for bacteria monitoring. Sens. Actuators B Chem. 2018, 260, 983–989. [Google Scholar] [CrossRef]
- Yang, Y.C.; Tseng, W.L. 1,4-Benzenediboronic-acid-induced aggregation of gold nanoparticles: Application to hydrogen peroxide detection and biotin-avidin-mediated immunoassay with naked-eye detection. Anal. Chem. 2016, 88, 5355–5362. [Google Scholar] [CrossRef]
- Li, R.; Gu, X.; Liang, X.; Hou, S.; Hu, D. Aggregation of gold nanoparticles caused in two different ways involved in 4-mercaptophenylboronic acid and hydrogen peroxide. Materials 2019, 12, 1802. [Google Scholar] [CrossRef]
- Quesada-Gonzalez, D.; Merkoci, A. Nanoparticle-based lateral flow biosensors. Biosens. Bioelectron. 2015, 73, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chung, D.R.; Kang, M. A new point-of-care test for the diagnosis of infectious diseases based on multiplex lateral flow immunoassays. Analyst 2019, 144, 2460–2466. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, Z.; Zhang, L.; Wang, C.; Deng, S.; Huo, X.; Tian, X.; Zhang, B.; Ma, X. Highly selective and sensitive visualization and identification of glycoproteins using multi-functionalized soluble dendrimer. Anal. Chim. Acta. 2017, 988, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Xue, F.; Zuo, W.; Yang, J.; Liu, X.; Jiang, H.; Dai, J.; Ju, Y. A universal bacterial catcher Au-PMBA-nanocrab-based lateral flow immunoassay for rapid pathogens detection. Anal. Chem. 2022, 94, 4277–4285. [Google Scholar] [CrossRef]
- Liu, X.; Dai, Q.; Austin, L.; Coutts, J.; Knowles, G.; Zou, J.; Chen, H.; Huo, Q. A one-step homogeneous immunoassay for cancer biomarker detection using gold nanoparticle probes coupled with dynamic light scattering. J. Am. Chem. Soc. 2008, 130, 2780–2782. [Google Scholar] [CrossRef]
- Kalluri, J.R.; Arbneshi, T.; AfrinKhan, S.; Neely, A.; Candice, P.; Varisli, B.; Washington, M.; McAfee, S.; Robinson, B.; Banerjee, S.; et al. Use of gold nanoparticles in a simple colorimetric and ultrasensitive dynamic light scattering assay: Selective detection of arsenic in groundwater. Angew. Chem. 2009, 121, 9848–9851. [Google Scholar] [CrossRef]
- Zheng, T.; Pierre-Pierre, N.; Yan, X.; Huo, Q.; Almodovar, A.J.; Valerio, F.; Rivera-Ramirez, I.; Griffith, E.; Decker, D.D.; Chen, S.; et al. Gold nanoparticle-enabled blood test for early stage cancer detection and risk assessment. ACS Appl. Mater. Interfaces 2015, 7, 6819–6827. [Google Scholar] [CrossRef]
- Huang, X.; Xu, Z.; Mao, Y.; Ji, Y.; Xu, H.; Xiong, Y.; Li, Y. Gold nanoparticle-based dynamic light scattering immunoassay for ultrasensitive detection of Listeria monocytogenes in lettuces. Biosens. Bioelectron. 2015, 66, 184–190. [Google Scholar] [CrossRef]
- Hu, J.Q.; Ding, L.; Chen, J.; Fu, J.H.; Zhu, K.; Guo, Q.; Huang, X.L.; Xiong, Y.H. Ultrasensitive dynamic light scattering immunosensing platform for NT-proBNP detection using boronate affinity amplification. J. Nanobiotechnol. 2022, 20, 21–30. [Google Scholar] [CrossRef]
- Zhu, K.; Zou, H.; Chen, J.; Hu, J.; Xiong, S.; Fu, J.; Xiong, Y.; Huang, X. Rapid and sensitive determination of lactoferrin in milk powder by boronate affinity amplified dynamic light scattering immunosensor. Food Chem. 2023, 405, 134983–134989. [Google Scholar] [CrossRef]
- Chen, J.; Hao, L.; Hu, J.; Zhu, K.; Li, Y.; Xiong, S.; Huang, X.; Xiong, Y.; Tang, B.Z. A universal boronate-affinity crosslinking-amplified dynamic light scattering immunoassay for point-of-care glycoprotein detection. Angew. Chem. Int. Ed. 2022, 61, e202112031–e202112039. [Google Scholar]
- Zou, F.; Wu, B.; Wang, X.; Chen, Y.; Koh, K.; Wang, K.; Chen, H. Signal amplification and dual recognition strategy for small-molecule detection by surface plasmon resonance based on calix[4]arene crown ether-modified gold nanoparticles. Sens. Actuators B Chem. 2017, 241, 160–167. [Google Scholar] [CrossRef]
- Qian, S.; Lin, M.; Ji, W.; Yuan, H.; Zhang, Y.; Jing, Z.; Zhao, J.; Masson, J.F.; Peng, W. Boronic acid functionalized Au nanoparticles for selective microRNA signal amplification in fiber-optic surface plasmon resonance sensing system. ACS Sens. 2018, 3, 929–935. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, Q.; Meng, X.; Zhao, L.; Liu, Z.; Huang, Y. Boronate avidity assisted by dendrimer-like polyhedral oligomeric silsesquioxanes for a microfluidic platform for selective enrichment of ubiquitination and glycosylation. Anal. Chem. 2023, 95, 1241–1250. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Ma, X.; Chang, Y.; Guo, H.; Wang, W. Biosensors with Boronic Acid-Based Materials as the Recognition Elements and Signal Labels. Biosensors 2023, 13, 785. https://doi.org/10.3390/bios13080785
Liu L, Ma X, Chang Y, Guo H, Wang W. Biosensors with Boronic Acid-Based Materials as the Recognition Elements and Signal Labels. Biosensors. 2023; 13(8):785. https://doi.org/10.3390/bios13080785
Chicago/Turabian StyleLiu, Lin, Xiaohua Ma, Yong Chang, Hang Guo, and Wenqing Wang. 2023. "Biosensors with Boronic Acid-Based Materials as the Recognition Elements and Signal Labels" Biosensors 13, no. 8: 785. https://doi.org/10.3390/bios13080785
APA StyleLiu, L., Ma, X., Chang, Y., Guo, H., & Wang, W. (2023). Biosensors with Boronic Acid-Based Materials as the Recognition Elements and Signal Labels. Biosensors, 13(8), 785. https://doi.org/10.3390/bios13080785






