Recent Advances in DNA Nanotechnology-Enabled Biosensors for Virus Detection
Abstract
1. Introduction
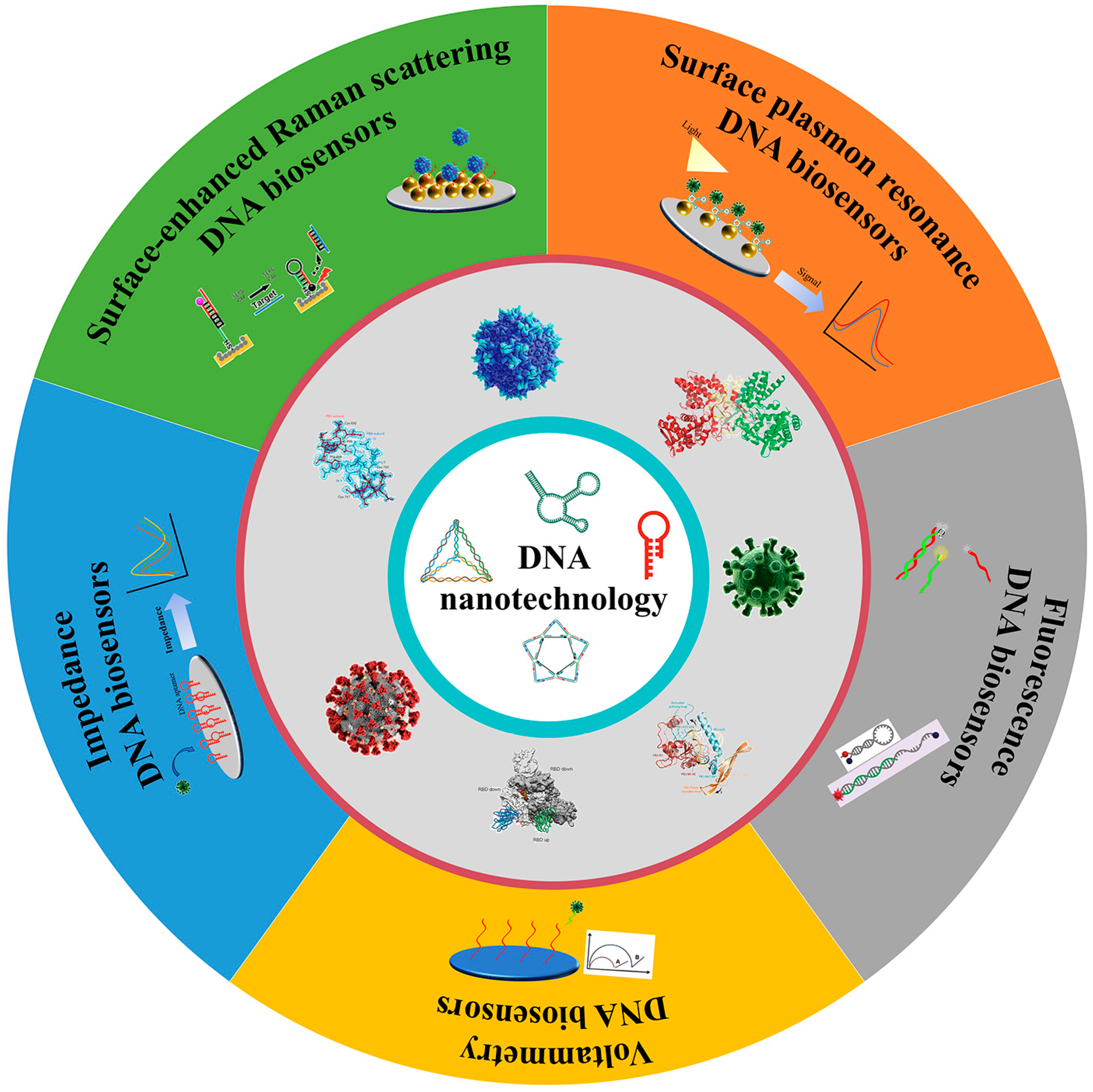
2. DNA Nanotechnology
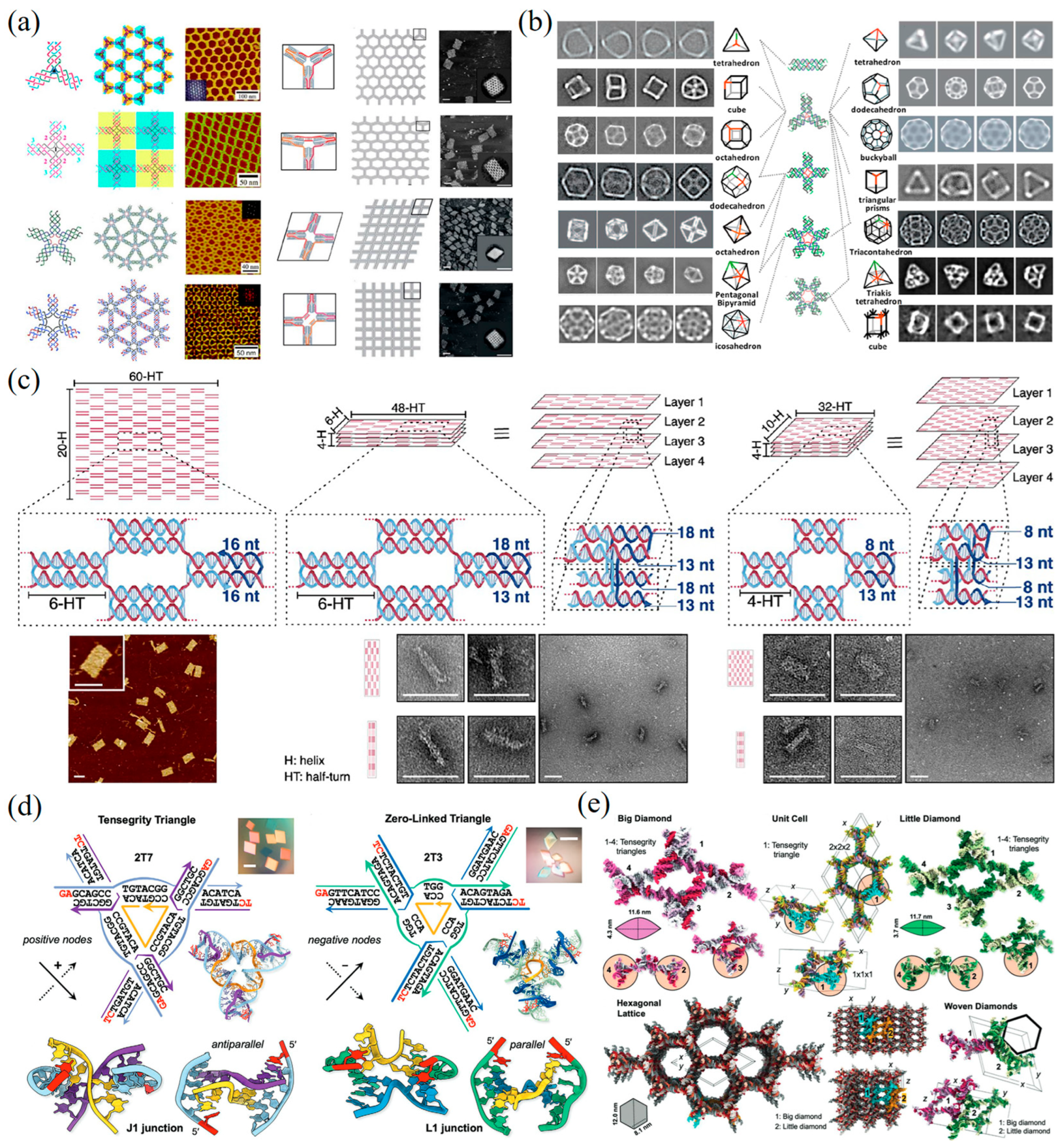
2.1. DNA Tile
2.2. DNA Aptamer
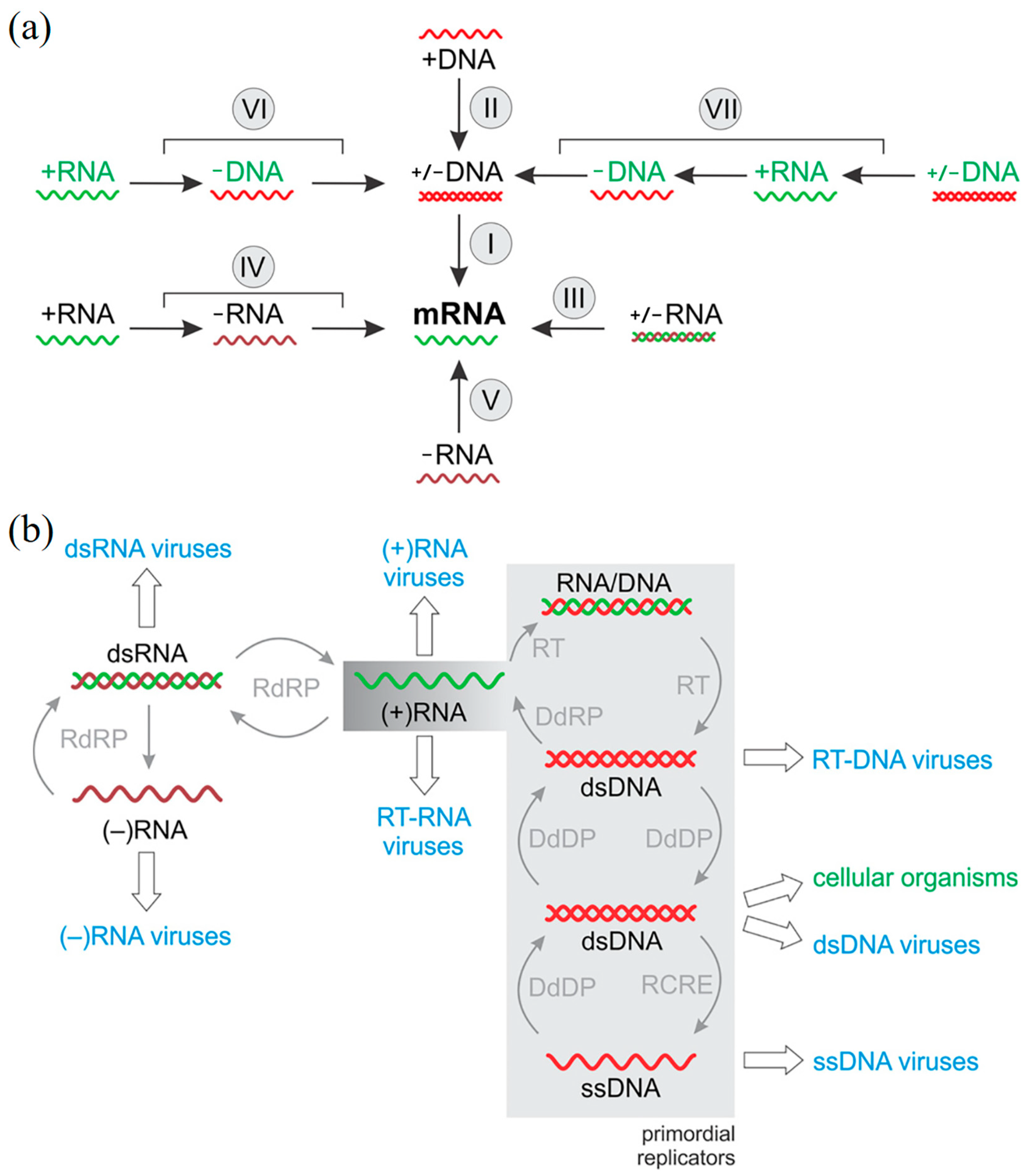
3. Virus Classification Based on Baltimore Classification System
3.1. Double-Stranded (ds) DNA Viruses
3.2. Single-Stranded (ss) DNA Viruses
3.3. Double-Stranded (ds) RNA Viruses
3.4. Positive-Sense (+) RNA Viruses
3.5. Negative-Sense (−) RNA Viruses
3.6. Reverse-Transcribing RNA Viruses
3.7. Double-Stranded DNA Retroviruses
4. Traditional Methods for the Detection of Viruses
4.1. Isolation and Culture Method
4.2. Immunological Detection Methods
4.3. Nucleic Acid Amplification-Based Assays
5. DNA Nanotechnology-Enabled Biosensor for Virus Detection
5.1. Optical DNA Nanobiosensors for Virus Detection
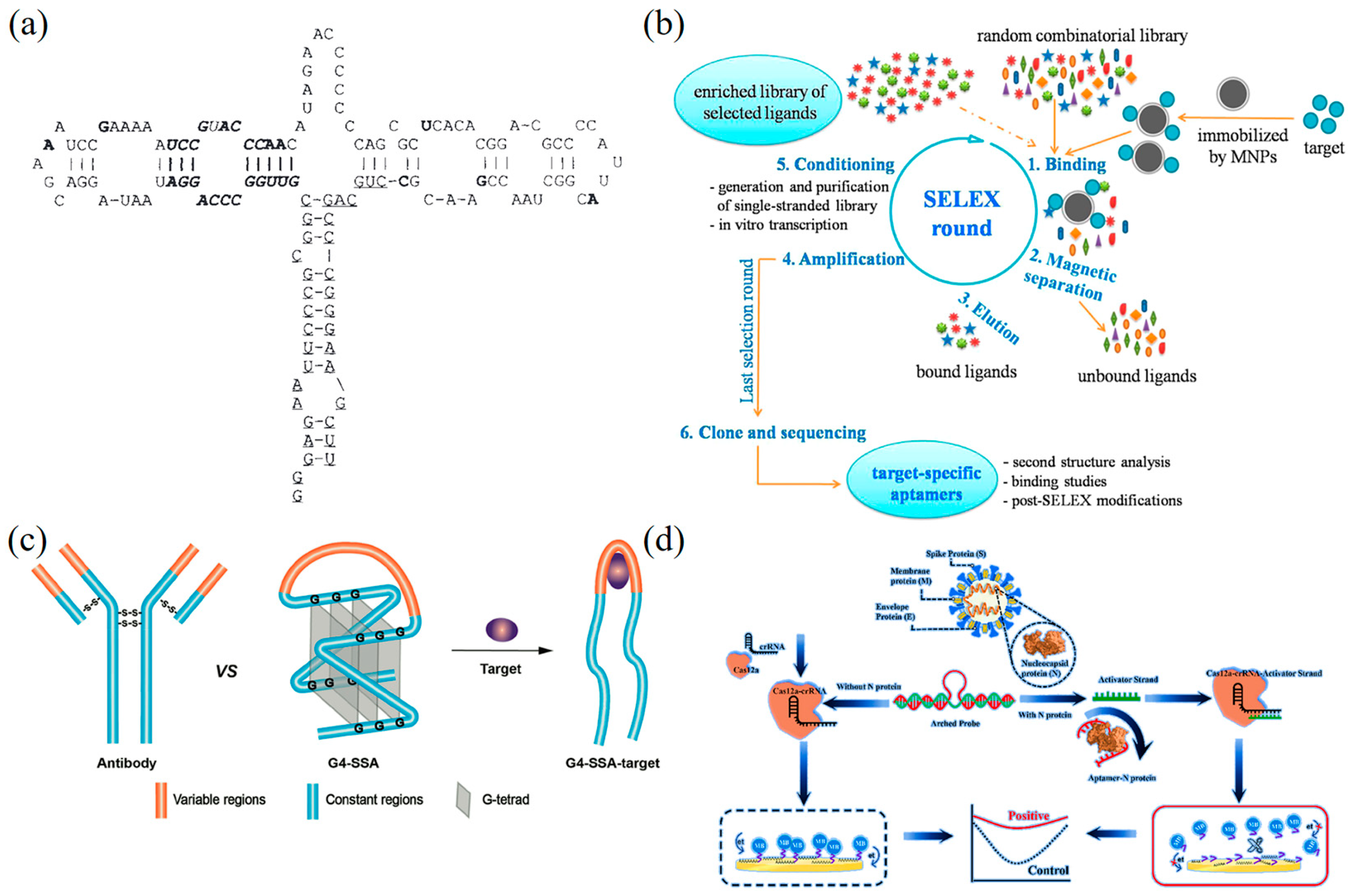
5.1.1. SERS DNA Nanobiosensors
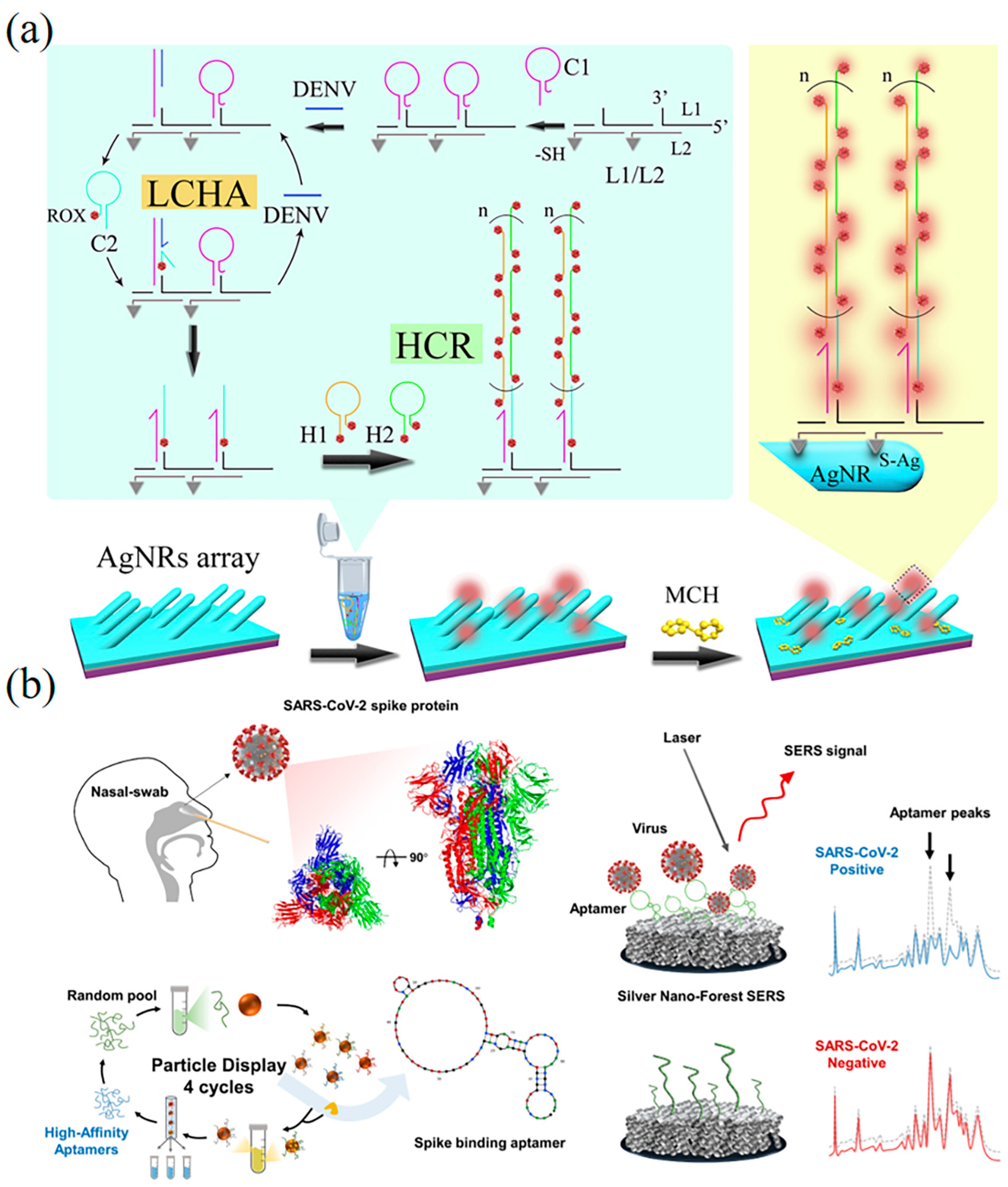
5.1.2. Surface Plasmon Resonance-Based DNA Nanobiosensors
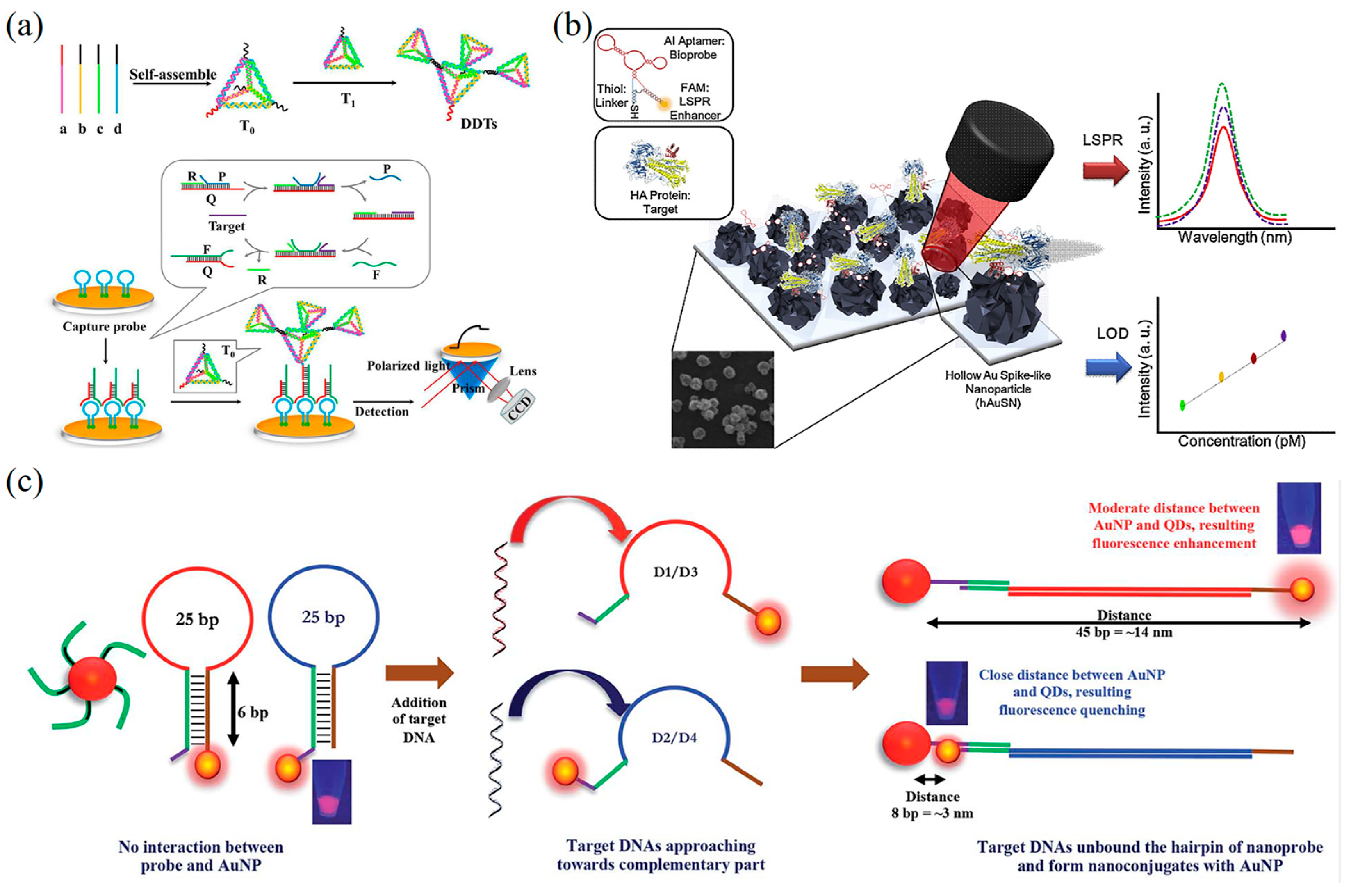
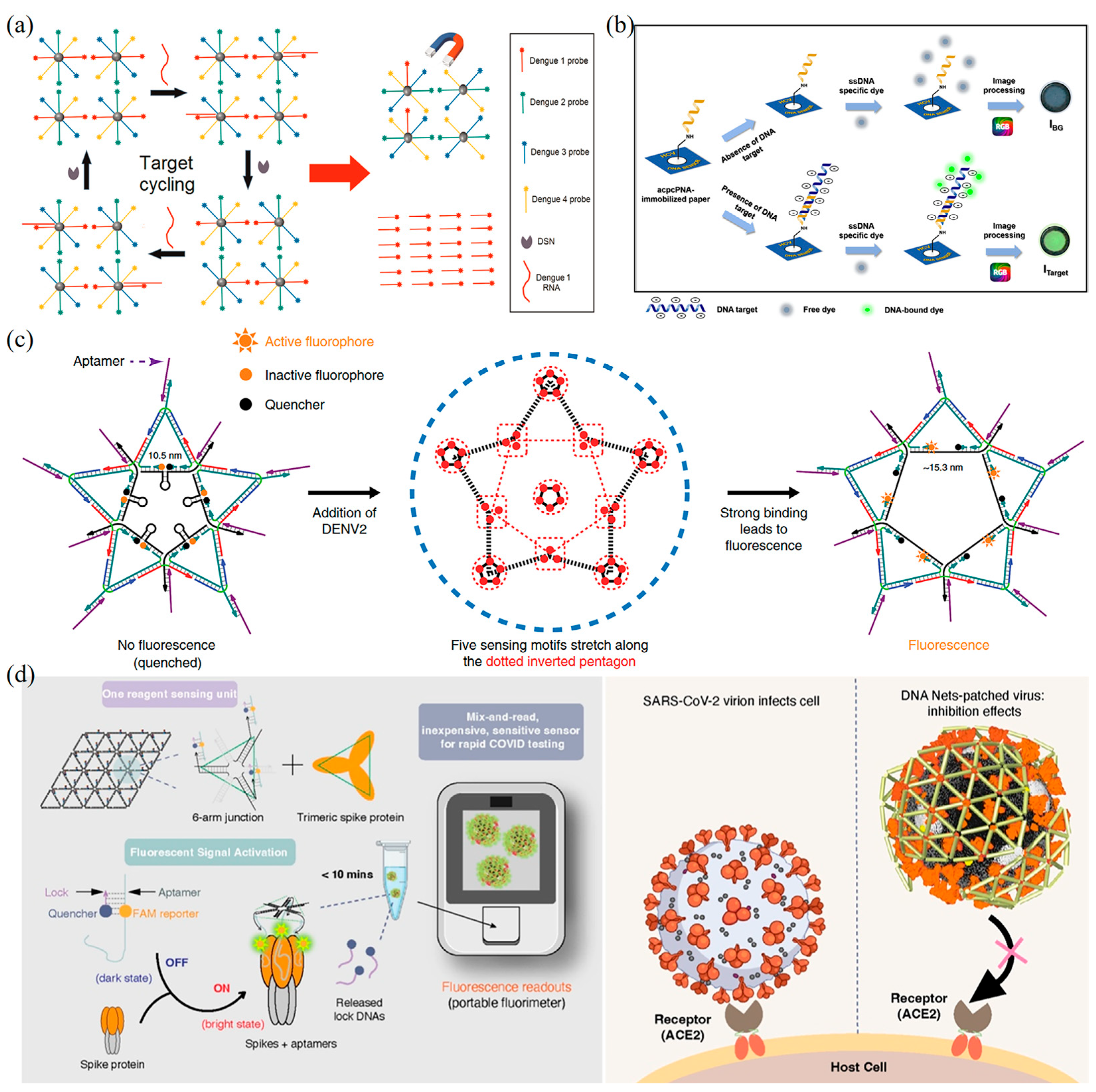
5.1.3. Fluorescence-Based DNA Nanobiosensors
| Detection Technique | Target Pathogen | DNA Nanoprobe | LOD | Reference |
|---|---|---|---|---|
| SERS nanobiosensors | DENV | DNA HCR probe | 0.49 fM | [148] |
| SARS-CoV-2 | DNA aptamer | 1 pM | [150] | |
| SPR nanobiosensors | HIV | Double-layer DNA tetrahedrons | 48 fM | [158] |
| AIV H5N1 | DNA 3 way-Junction | - | [159] | |
| DENV | DNA hairpin | 24.6 fM (DENV-1) 11.4 fM (DENV-2) 39.8 fM (DENV-3) 39.7 fM (DENV-4) | [160] | |
| Fluorescent nanobiosensors | SARS-CoV-2 | Net-Shaped DNA Nanostructures | 1 × 108 viral genome copies/mL | [31] |
| DENV | Quantum dot-capped DNA capture probes | 0.50 fM | [171] | |
| HVC | acpcPNA-DNA double helix | 5 pM | [173] | |
| DENV | DNA star | 1 × 102 p.f.u.mL−1 (serum) 1 × 103 p.f.u.mL−1 (plasma) | [175] | |
| SARS-CoV-2 | DNA nanoscaffold | 0.96 pM | [174] | |
| ZIKV | DNA nanoantenna | - | [176] | |
| DENV | DNA double helix | 9.4 fM | [177] | |
| HPV-16 | DNA-based microarray biochip | - | [178] |
5.2. Electrochemical DNA Nanobiosensors for Virus Detection
5.2.1. Voltammetry-Based DNA Nanobiosensors
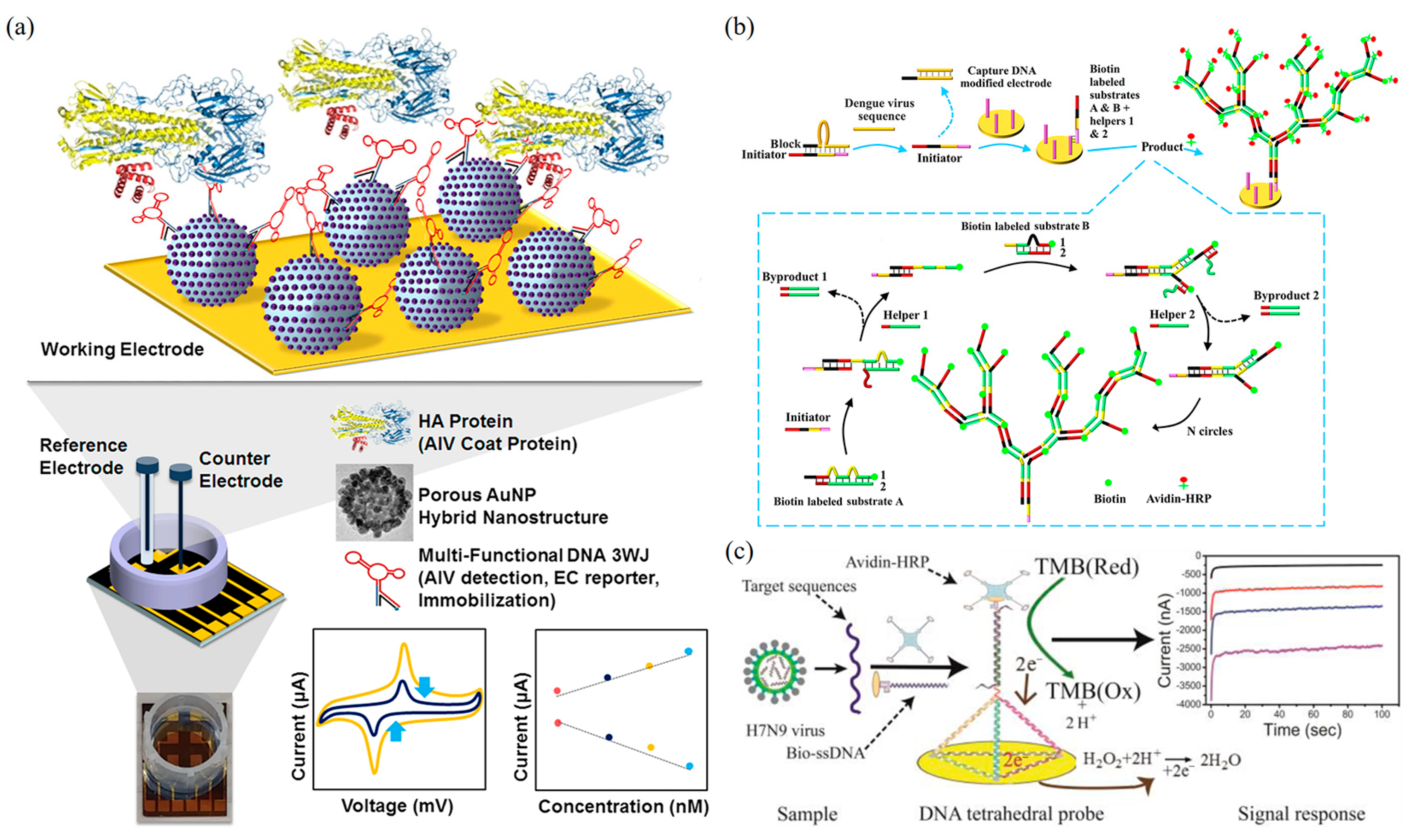
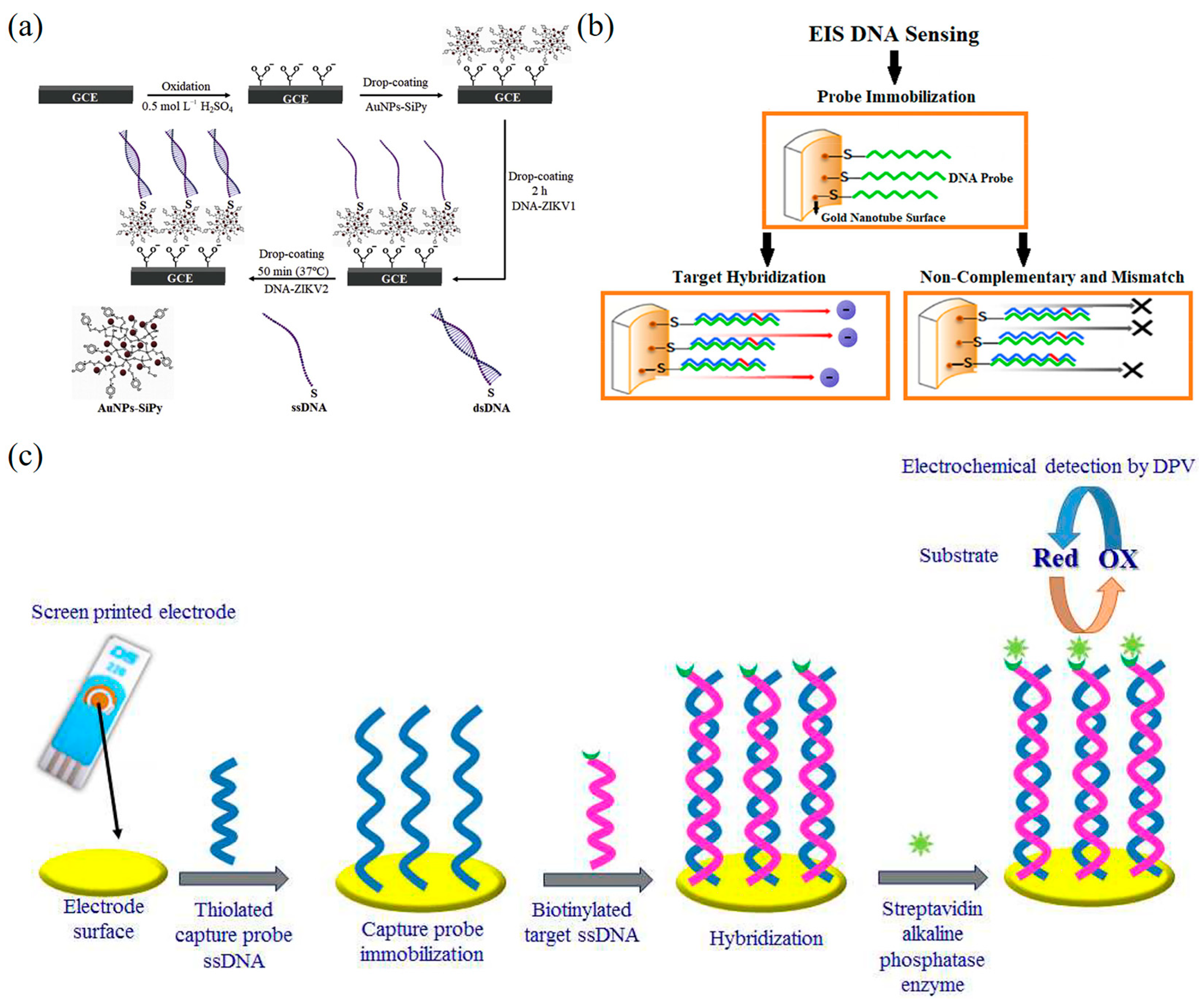
5.2.2. Impedance-Based DNA Nanobiosensors
| Detection Method | Target Pathogen | DNA Nanoprobe | LOD | Reference |
|---|---|---|---|---|
| Voltammetry biosensors | H5N1 | DNA 3 way-Junction | 1 pM | [186] |
| DENV | DNA double helix | 43 nM | [199] | |
| H1N1 | DNA aptamer | 3.7 PFU/mL | [200] | |
| DENV | DNA HCR | 188 fM | [187] | |
| H7N9 | DNA tetrahedral | 100 fM | [188] | |
| H5N1 | DNA double helix | 1.39 pM | [182] | |
| HPV-18 | Cys-AuNPs-DNA probe | 0.05 fM | [189] | |
| Impedance biosensors | HBV | ssDNA/AuNCs | 0.1 fM | [194] |
| HBV | SWCNTs/Au/ssDNA | 0.1 pM | [192] | |
| ZIKV | DNA double helix | 0.82 pM | [195] | |
| Ebola | DNA capture probe | 4.7 μM | [196] | |
| ZIKV | DNA HCR | 25 nM | [198] | |
| HPV | DNA aptamer | 1 fM | [197] |
6. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ke, Z.; Oton, J.; Qu, K.; Cortese, M.; Zila, V.; McKeane, L.; Nakane, T.; Zivanov, J.; Neufeldt, C.J.; Cerikan, B.; et al. Structures and Distributions of SARS-CoV-2 Spike Proteins on Intact Virions. Nature 2020, 588, 498–502. [Google Scholar] [CrossRef]
- Yao, H.; Song, Y.; Chen, Y.; Wu, N.; Xu, J.; Sun, C.; Zhang, J.; Weng, T.; Zhang, Z.; Wu, Z.; et al. Molecular Architecture of the SARS-CoV-2 Virus. Cell 2020, 183, 730–738.e13. [Google Scholar] [CrossRef]
- Notomi, T. Loop-Mediated Isothermal Amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Smyrlaki, I.; Ekman, M.; Lentini, A.; de Sousa, N.R.; Papanicolaou, N.; Vondracek, M.; Aarum, J.; Safari, H.; Muradrasoli, S.; Rothfuchs, A.G.; et al. Massive and Rapid COVID-19 Testing Is Feasible by Extraction-Free SARS-CoV-2 RT-PCR. Nat. Commun. 2020, 11, 4812. [Google Scholar] [CrossRef]
- Yelin, I.; Aharony, N.; Tamar, E.S.; Argoetti, A.; Messer, E.; Berenbaum, D.; Shafran, E.; Kuzli, A.; Gandali, N.; Shkedi, O.; et al. Evaluation of COVID-19 RT-QPCR Test in Multi Sample Pools. Clin. Infect. Dis. 2020, 71, 2073–2078. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, K.; Pt, U.; Kaduskar, O.; Vijay, N.; Rakhe, A.; Vidhate, S.; Khutwad, K.; Deshpande, G.R.; Tilekar, B.; Saka, S.; et al. Performance Assessment of Seven SARS-CoV-2 IgG Enzyme-linked Immunosorbent Assays. J. Med. Virol. 2021, 93, 6696–6702. [Google Scholar] [CrossRef] [PubMed]
- Dewald, F.; Suárez, I.; Johnen, R.; Grossbach, J.; Moran-Tovar, R.; Steger, G.; Joachim, A.; Rubio, G.H.; Fries, M.; Behr, F.; et al. Effective High-Throughput RT-QPCR Screening for SARS-CoV-2 Infections in Children. Nat. Commun. 2022, 13, 3640. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chan, K.-H.; Kang, Y.; Chen, H.; Luk, H.K.H.; Poon, R.W.S.; Chan, J.F.W.; Yuen, K.-Y.; Xia, N.; Lau, S.K.P.; et al. A Sensitive and Specific Antigen Detection Assay for Middle East Respiratory Syndrome Coronavirus. Emerg. Microbes Infect. 2015, 4, 1–5. [Google Scholar] [CrossRef]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A Serological Assay to Detect SARS-CoV-2 Seroconversion in Humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Shao, W.; Meng, H. Performance and Application Evaluation of SARS-CoV-2 Antigen Assay. J. Med. Virol. 2022, 94, 3548–3553. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, D.; Li, T.; Yan, J.; Zhu, J.; He, T.; Hu, R.; Li, Y.; Yang, Y.; Liu, M. Microfluidic Space Coding for Multiplexed Nucleic Acid Detection via CRISPR-Cas12a and Recombinase Polymerase Amplification. Nat. Commun. 2022, 13, 6480. [Google Scholar] [CrossRef] [PubMed]
- Jackson’, J.B.; Balfour, H.H. Practical Diagnostic Testing for Human Immunodeficiency Virus. Clin. Microbiol. Rev. 1988, 1, 124–138. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Smirnov, I.; Shafer, R.H. Effect of Loop Sequence and Size on DNA Aptamer Stability. Biochemistry 2000, 39, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Bishop, G.R.; Ren, J.; Polander, B.C.; Jeanfreau, B.D.; Trent, J.O.; Chaires, J.B. Energetic Basis of Molecular Recognition in a DNA Aptamer. Biophys. Chem. 2007, 126, 165–175. [Google Scholar] [CrossRef]
- Xia, T.; Yuan, J.; Fang, X. Conformational Dynamics of an ATP-Binding DNA Aptamer: A Single-Molecule Study. J. Phys. Chem. B 2013, 117, 14994–15003. [Google Scholar] [CrossRef]
- Tan, L.; Neoh, K.G.; Kang, E.-T.; Choe, W.-S.; Su, X. Affinity Analysis of DNA Aptamer–Peptide Interactions Using Gold Nanoparticles. Anal. Biochem. 2012, 421, 725–731. [Google Scholar] [CrossRef]
- Lai, J.-C.; Hong, C.-Y. Magnetic-Assisted Rapid Aptamer Selection (MARAS) for Generating High-Affinity DNA Aptamer Using Rotating Magnetic Fields. ACS Comb. Sci. 2014, 16, 321–327. [Google Scholar] [CrossRef]
- Minagawa, H.; Kataoka, Y.; Kuwahara, M.; Horii, K.; Shiratori, I.; Waga, I. A High Affinity Modified DNA Aptamer Containing Base-Appended Bases for Human β-Defensin. Anal. Biochem. 2020, 594, 113627. [Google Scholar] [CrossRef]
- Xue, C.; Zhang, S.; Yu, X.; Hu, S.; Lu, Y.; Wu, Z. Periodically Ordered, Nuclease-Resistant DNA Nanowires Decorated with Cell-Specific Aptamers as Selective Theranostic Agents. Angew. Chem. Int. Ed. 2020, 59, 17540–17547. [Google Scholar] [CrossRef]
- Xie, N.; Liu, S.; Yang, X.; He, X.; Huang, J.; Wang, K. DNA Tetrahedron Nanostructures for Biological Applications: Biosensors and Drug Delivery. Analyst 2017, 142, 3322–3332. [Google Scholar] [CrossRef]
- Sameiyan, E.; Bagheri, E.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. DNA Origami-Based Aptasensors. Biosens. Bioelectron. 2019, 143, 111662. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, J.; Sun, R.; Huang, Z.; Luo, Z.; Zhou, C.; Wu, M.; Duan, Y.; Li, Y. The Recent Development of Hybridization Chain Reaction Strategies in Biosensors. ACS Sens. 2020, 5, 2977–3000. [Google Scholar] [CrossRef] [PubMed]
- Loretan, M.; Domljanovic, I.; Lakatos, M.; Rüegg, C.; Acuna, G.P. DNA Origami as Emerging Technology for the Engineering of Fluorescent and Plasmonic-Based Biosensors. Materials 2020, 13, 2185. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, Y.; Liu, J. G-Quadruplex DNA for Construction of Biosensors. TrAC Trends Anal. Chem. 2020, 132, 116060. [Google Scholar] [CrossRef]
- Wang, S. Construction of DNA Biosensors for Mercury (II) Ion Detection Based on Enzyme-Driven Signal Amplification Strategy. Biomolecules 2021, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhang, Y.; Lu, M.; Du, Y.; Chen, M.; Meng, S.; Ji, W.; Sun, C. Near-Infrared Band Gold Nanoparticles-Au Film “Hot Spot” Model Based Label-Free Ultratrace Lead (II) Ions Detection via Fiber SPR DNAzyme Biosensor. Sens. Actuators B Chem. 2021, 337, 129816. [Google Scholar] [CrossRef]
- Zhai, J.; Cui, H.; Yang, R. DNA Based Biosensors. Biotechnol. Adv. 1997, 15, 43–58. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, G.; Pei, H.; Li, L.; Xu, Q.; Liang, W.; Li, Y.; Xu, L.; Ren, S. DNA Nanostructure-Based Ultrasensitive Electrochemical MicroRNA Biosensor. Methods 2013, 64, 276–282. [Google Scholar] [CrossRef]
- Li, Y.; Liu, S.; Deng, Q. A Sensitive Colorimetric DNA Biosensor for Specific Detection of the HBV Gene Based on Silver-Coated Glass Slide and G-Quadruplex-Hemin DNAzyme. J. Med. Virol. 2018, 90, 699–705. [Google Scholar] [CrossRef]
- Xie, B.; Qiu, G.; Hu, P.; Liang, Z.; Liang, Y.; Sun, B.; Bai, L.; Jiang, Z.; Chen, J. Simultaneous Detection of Dengue and Zika Virus RNA Sequences with a Three-Dimensional Cu-Based Zwitterionic Metal–Organic Framework, Comparison of Single and Synchronous Fluorescence Analysis. Sens. Actuators B Chem. 2018, 254, 1133–1140. [Google Scholar] [CrossRef]
- Chauhan, N.; Xiong, Y.; Ren, S.; Dwivedy, A.; Magazine, N.; Zhou, L.; Jin, X.; Zhang, T.; Cunningham, B.T.; Yao, S.; et al. Net-Shaped DNA Nanostructures Designed for Rapid/Sensitive Detection and Potential Inhibition of the SARS-CoV-2 Virus. J. Am. Chem. Soc. 2022. [Google Scholar] [CrossRef]
- Ito, K.; Hashimoto, K.; Ishimori, Y. Quantitative Analysis for Solid-Phase Hybridization Reaction and Binding Reaction of DNA Binder to Hybrids Using a Quartz Crystal Microbalance. Anal. Chim. Acta 1996, 327, 29–35. [Google Scholar] [CrossRef]
- Fawcett, N.C.; Evans, J.A.; Chien, L.-C.; Flowers, N. Nucleic Acid Hybridization Detected by Piezoelectric Resonance. Anal. Lett. 1988, 21, 1099–1114. [Google Scholar] [CrossRef]
- Ye, D.; Zuo, X.; Fan, C. DNA Nanotechnology-Enabled Interfacial Engineering for Biosensor Development. Annu. Rev. Anal. Chem. 2018, 11, 171–195. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wang, P.; Ke, Y. DNA Nanotechnology-Based Biosensors and Therapeutics. Adv. Healthc. Mater. 2021, 10, 2002205. [Google Scholar] [CrossRef]
- Hua, Y.; Ma, J.; Li, D.; Wang, R. DNA-Based Biosensors for the Biochemical Analysis: A Review. Biosensors 2022, 12, 183. [Google Scholar] [CrossRef]
- Seeman, N.C. Nucleic Acid Junctions and Lattices. J. Theor. Biol. 1982, 99, 237–247. [Google Scholar] [CrossRef]
- Ke, Y.; Ong, L.L.; Shih, W.M.; Yin, P. Three-Dimensional Structures Self-Assembled from DNA Bricks. Science 2012, 338, 1177–1183. [Google Scholar] [CrossRef]
- Gerling, T.; Wagenbauer, K.F.; Neuner, A.M.; Dietz, H. Dynamic DNA Devices and Assemblies Formed by Shape-Complementary, Non–Base Pairing 3D Components. Science 2015, 347, 1446–1452. [Google Scholar] [CrossRef]
- Rahbani, J.F.; Vengut-Climent, E.; Chidchob, P.; Gidi, Y.; Trinh, T.; Cosa, G.; Sleiman, H.F. DNA Nanotubes with Hydrophobic Environments: Toward New Platforms for Guest Encapsulation and Cellular Delivery. Adv. Healthc. Mater. 2018, 7, 1701049. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, J.; Zhou, C.; Wang, Q. Finite Assembly of Three-Dimensional DNA Hierarchical Nanoarchitectures through Orthogonal and Directional Bonding. Angew. Chem. Int. Ed. 2022, 61, e202116416. [Google Scholar] [CrossRef]
- Parsons, M.F.; Allan, M.F.; Li, S.; Shepherd, T.R.; Ratanalert, S.; Zhang, K.; Pullen, K.M.; Chiu, W.; Rouskin, S.; Bathe, M. 3D RNA-Scaffolded Wireframe Origami. Nat. Commun. 2023, 14, 382. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Yang, D.; Tan, Z.; Chen, S.; Xiang, Y.; Mi, Y.; Mao, C.; Wei, B. Self-Assembly of Wireframe DNA Nanostructures from Junction Motifs. Angew. Chem. Int. Ed. 2019, 58, 12123–12127. [Google Scholar] [CrossRef]
- Hung, A.M.; Micheel, C.M.; Bozano, L.D.; Osterbur, L.W.; Wallraff, G.M.; Cha, J.N. Large-Area Spatially Ordered Arrays of Gold Nanoparticles Directed by Lithographically Confined DNA Origami. Nat. Nanotechnol. 2010, 5, 121–126. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Chen, Y.; Liu, H.; Ribbe, A.E.; Mao, C. Self-Assembly of Hexagonal DNA Two-Dimensional (2D) Arrays. J. Am. Chem. Soc. 2005, 127, 12202–12203. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Tian, Y.; Chen, Y.; Deng, Z.; Ribbe, A.E.; Mao, C. Sequence Symmetry as a Tool for Designing DNA Nanostructures. Angew. Chem. Int. Ed. 2005, 44, 6694–6696. [Google Scholar] [CrossRef]
- He, Y.; Tian, Y.; Ribbe, A.E.; Mao, C. Highly Connected Two-Dimensional Crystals of DNA Six-Point-Stars. J. Am. Chem. Soc. 2006, 128, 15978–15979. [Google Scholar] [CrossRef]
- Zhang, C.; Su, M.; He, Y.; Zhao, X.; Fang, P.-A.; Ribbe, A.E.; Jiang, W.; Mao, C. Conformational Flexibility Facilitates Self-Assembly of Complex DNA Nanostructures. Proc. Natl. Acad. Sci. USA 2008, 105, 10665–10669. [Google Scholar] [CrossRef]
- He, Y.; Ye, T.; Su, M.; Zhang, C.; Ribbe, A.E.; Jiang, W.; Mao, C. Hierarchical Self-Assembly of DNA into Symmetric Supramolecular Polyhedra. Nature 2008, 452, 198–201. [Google Scholar] [CrossRef]
- Zhang, C.; Ko, S.H.; Su, M.; Leng, Y.; Ribbe, A.E.; Jiang, W.; Mao, C. Symmetry Controls the Face Geometry of DNA Polyhedra. J. Am. Chem. Soc. 2009, 131, 1413–1415. [Google Scholar] [CrossRef]
- He, Y.; Su, M.; Fang, P.-A.; Zhang, C.; Ribbe, E.; Jiang, W.; Mao, C. On the Chirality of Self-Assembled DNA Octahedra. Angew. Chem. Int. Ed. 2010, 49, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, W.; Li, X.; Tian, C.; Qian, H.; Wang, G.; Jiang, W.; Mao, C. Controlling the Chirality of DNA Nanocages. Angew. Chem. Int. Ed. 2012, 51, 7999–8002. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Li, X.; Liu, Z.; Jiang, W.; Wang, G.; Mao, C. Directed Self-Assembly of DNA Tiles into Complex Nanocages. Angew. Chem. Int. Ed. 2014, 53, 8041–8044. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, C.; Liu, Z.; Jiang, W.; Structural, C.M. Transformation: Assembly of an Otherwise Inaccessible DNA Nanocage. Angew. Chem. Int. Ed. 2015, 54, 5990–5993. [Google Scholar] [CrossRef]
- Wang, P.; Wu, S.; Tian, C.; Yu, G.; Jiang, W.; Wang, G.; Mao, C. Retrosynthetic Analysis-Guided Breaking Tile Symmetry for the Assembly of Complex DNA Nanostructures. J. Am. Chem. Soc. 2016, 138, 13579–13585. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wu, C.; Ding, F.; Tian, C.; Jiang, W.; Mao, C.; Zhang, C. Binary Self-Assembly of Highly Symmetric DNA Nanocages via Sticky-End Engineering. Chin. Chem. Lett. 2017, 28, 851–856. [Google Scholar] [CrossRef]
- Wang, T.; Bai, T.; Tan, Z.; Ohayon, Y.P.; Sha, R.; Vecchioni, S.; Seeman, N.C.; Wei, B. Mesojunction-Based Design Paradigm of Structural DNA Nanotechnology. J. Am. Chem. Soc. 2023, 145, 2455–2460. [Google Scholar] [CrossRef]
- Vecchioni, S.; Lu, B.; Janowski, J.; Woloszyn, K.; Jonoska, N.; Seeman, N.C.; Mao, C.; Ohayon, Y.P.; Sha, R. The Rule of Thirds: Controlling Junction Chirality and Polarity in 3D DNA Tiles. Small 2023, 19, 2206511. [Google Scholar] [CrossRef]
- Lu, B.; Vecchioni, S.; Ohayon, Y.P.; Woloszyn, K.; Markus, T.; Mao, C.; Seeman, N.C.; Canary, J.W.; Sha, R. Highly Symmetric, Self-Assembling 3D DNA Crystals with Cubic and Trigonal Lattices. Small 2023, 19, 2205830. [Google Scholar] [CrossRef]
- Duckett, D.R.; Murchie, A.I.H.; Diekmann, S.; von Kitzing, E.; Kemper, B.; Lilley, D.M.J. The Structure of the Holliday Junction, and Its Resolution. Cell 1988, 55, 79–89. [Google Scholar] [CrossRef]
- Ma, R.-I.; Kallenbach, N.R.; Sheardy, R.D.; Petrillo, M.L.; Seeman, N.C. Three-Arm Nucleic Acid Junctions Are Flexible. Nucleic Acids Res. 1986, 14, 9745–9753. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mueller, J.E.; Kemper, B.; Seeman, N.C. Assembly and Characterization of Five-Arm and Six-Arm DNA Branched Junctions. Biochemistry 1991, 30, 5667–5674. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Seeman, N.C. Assembly and Characterization of 8-Arm and 12-Arm DNA Branched Junctions. J. Am. Chem. Soc. 2007, 129, 8169–8176. [Google Scholar] [CrossRef] [PubMed]
- Fu, T.J.; Seeman, N.C. DNA Double-Crossover Molecules. Biochemistry 1993, 32, 3211–3220. [Google Scholar] [CrossRef] [PubMed]
- LaBean, T.H.; Yan, H.; Kopatsch, J.; Liu, F.; Winfree, E.; Reif, J.H.; Seeman, N.C. Construction, Analysis, Ligation, and Self-Assembly of DNA Triple Crossover Complexes. J. Am. Chem. Soc. 2000, 122, 1848–1860. [Google Scholar] [CrossRef]
- Gu, H.; Chao, J.; Xiao, S.-J.; Seeman, N.C. A Proximity-Based Programmable DNA Nanoscale Assembly Line. Nature 2010, 465, 202–205. [Google Scholar] [CrossRef]
- Mathieu, F.; Liao, S.; Kopatsch, J.; Wang, T.; Mao, C.; Seeman, N.C. Six-Helix Bundles Designed from DNA. Nano Lett. 2005, 5, 661–665. [Google Scholar] [CrossRef]
- Dong, Y.; Yao, C.; Zhu, Y.; Yang, L.; Luo, D.; Yang, D. DNA Functional Materials Assembled from Branched DNA: Design, Synthesis, and Applications. Chem. Rev. 2020, 120, 9420–9481. [Google Scholar] [CrossRef]
- Goodman, R.P.; Schaap, I.A.T.; Tardin, C.F.; Erben, C.M.; Berry, R.M.; Schmidt, C.F.; Turberfield, A.J. Rapid Chiral Assembly of Rigid DNA Building Blocks for Molecular Nanofabrication. Science 2005, 310, 1661–1665. [Google Scholar] [CrossRef]
- Zhang, Y.; Seeman, N.C. Construction of a DNA-Truncated Octahedron. J. Am. Chem. Soc. 1994, 116, 1661–1669. [Google Scholar] [CrossRef]
- Hong, F.; Jiang, S.; Lan, X.; Narayanan, R.P.; Šulc, P.; Zhang, F.; Liu, Y.; Yan, H. Layered-Crossover Tiles with Precisely Tunable Angles for 2D and 3D DNA Crystal Engineering. J. Am. Chem. Soc. 2018, 140, 14670–14676. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, Q.; Liu, S.; Zhang, Y.; Tian, Y.; Song, C.; Wang, J.; Zou, Y.; Anderson, G.J.; Han, J.-Y.; et al. A DNA Nanorobot Functions as a Cancer Therapeutic in Response to a Molecular Trigger In Vivo. Nat. Biotechnol. 2018, 36, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic Evolution of Ligands by Exponential Enrichment: RNA Ligands to Bacteriophage T4 DNA Polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In Vitro Selection of RNA Molecules That Bind Specific Ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Jenison, R.D.; Gill, S.C.; Pardi, A.; Polisky, B. High-Resolution Molecular Discrimination by RNA. Science 1994, 263, 1425–1429. [Google Scholar] [CrossRef] [PubMed]
- Stojanovic, M.N.; De Prada, P.; Landry, D.W. Aptamer-Based Folding Fluorescent Sensor for Cocaine. J. Am. Chem. Soc. 2001, 123, 4928–4931. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Fast Colorimetric Sensing of Adenosine and Cocaine Based on a General Sensor Design Involving Aptamers and Nanoparticles. Angew. Chem. Int. Ed. 2006, 45, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Baker, B.R.; Lai, R.Y.; Wood, M.S.; Doctor, E.H.; Heeger, A.J.; Plaxco, K.W. An Electronic, Aptamer-Based Small-Molecule Sensor for the Rapid, Label-Free Detection of Cocaine in Adulterated Samples and Biological Fluids. J. Am. Chem. Soc. 2006, 128, 3138–3139. [Google Scholar] [CrossRef]
- Liu, J.; Cao, Z.; Lu, Y. Functional Nucleic Acid Sensors. Chem. Rev. 2009, 109, 1948–1998. [Google Scholar] [CrossRef]
- Santini, J.T., Jr.; Richards, A.C.; Scheidt, R.; Cima, M.J.; Langer, R. Microchips as Controlled Drug-Delivery Devices. Angew. Chem. Int. Ed. 2000, 39, 2396–2407. [Google Scholar] [CrossRef]
- Hamaguchi, N.; Ellington, A.; Stanton, M. Aptamer Beacons for the Direct Detection of Proteins. Anal. Biochem. 2001, 294, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Alkhamis, O.; Canoura, J.; Liu, Y.; Xiao, Y. Advances and Challenges in Small-Molecule DNA Aptamer Isolation, Characterization, and Sensor Development. Angew. Chem. Int. Ed. 2021, 60, 16800–16823. [Google Scholar] [CrossRef] [PubMed]
- Xi, Z.; Huang, R.; Li, Z.; He, N.; Wang, T.; Su, E.; Selection, Y.D. of HBsAg-Specific DNA Aptamers Based on Carboxylated Magnetic Nanoparticles and Their Application in the Rapid and Simple Detection of Hepatitis B Virus Infection. ACS Appl. Mater. Interfaces 2015, 7, 11215–11223. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zhao, J.; Huang, Y.; Cheng, X.; Wang, S.; Han, Y.; Xiao, Y.; Lou, X. Universal Design of Structure-Switching Aptamers with Signal Reporting Functionality. Anal. Chem. 2019, 91, 14514–14521. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Li, W.; Li, Q.; Xing, W.; Luo, H.; Ji, H.; Fang, X.; Luo, Z.; Zhang, L. CRISPR/Cas12a-Derived Electrochemical Aptasensor for Ultrasensitive Detection of COVID-19 Nucleocapsid Protein. Biosens. Bioelectron. 2022, 200, 113922. [Google Scholar] [CrossRef]
- Terracciano, M.; Rea, I.; Borbone, N.; Moretta, R.; Oliviero, G.; Piccialli, G.; De Stefano, L. Silicon-Based Aptasensors: The Next Generation of Label-Free Devices for Health Monitoring. Molecules 2019, 24, 2216. [Google Scholar] [CrossRef]
- Prante, M.; Segal, E.; Scheper, T.; Bahnemann, J.; Walter, J. Aptasensors for Point-of-Care Detection of Small Molecules. Biosensors 2020, 10, 108. [Google Scholar] [CrossRef]
- Yamamoto, R.; Kumar, P.K.R. Molecular Beacon Aptamer Fluoresces in the Presence of Tat Protein of HIV-1: Tat-Dependent Fluorescent Beacon Aptamer. Genes Cells 2000, 5, 389–396. [Google Scholar] [CrossRef]
- Tseng, Y.-T.; Wang, C.-H.; Chang, C.-P.; Lee, G.-B. Integrated Microfluidic System for Rapid Detection of Influenza H1N1 Virus Using a Sandwich-Based Aptamer Assay. Biosens. Bioelectron. 2016, 82, 105–111. [Google Scholar] [CrossRef]
- Wang, C.-H.; Chang, C.-P.; Lee, G.-B. Integrated Microfluidic Device Using a Single Universal Aptamer to Detect Multiple Types of Influenza Viruses. Biosens. Bioelectron. 2016, 86, 247–254. [Google Scholar] [CrossRef]
- Geng, Z.; Wang, L.; Liu, K.; Liu, J.; Tan, W. Enhancing Anti-PD-1 Immunotherapy by Nanomicelles Self-Assembled from Multivalent Aptamer Drug Conjugates. Angew. Chem. Int. Ed. 2021, 60, 15459–15465. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, A.; Weber, A.; Bayin, M.; Breuers, S.; Fieberg, V.; Famulok, M.; Mayer, G. A SARS-CoV-2 Spike Binding DNA Aptamer That Inhibits Pseudovirus Infection by an RBD-Independent Mechanism. Angew. Chem. Int. Ed. 2021, 60, 10279–10285. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, X.; Zhu, L.; Lin, S.; Li, C.; Li, X.; Huang, K.; Xu, W. Aptamer-Functionalized DNA–Silver Nanocluster Nanofilm for Visual Detection and Elimination of Bacteria. ACS Appl. Mater. Interfaces 2021, 13, 38647–38655. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Park, S.-K.; Joung, Y.; Kang, T.; Lee, M.-K.; Choo, J. SERS-Based Dual-Mode DNA Aptasensors for Rapid Classification of SARS-CoV-2 and Influenza A/H1N1 Infection. Sens. Actuators B Chem. 2022, 355, 131324. [Google Scholar] [CrossRef]
- Koonin, E.V.; Krupovic, M.; Agol, V.I. The Baltimore Classification of Viruses 50 Years Later: How Does It Stand in the Light of Virus Evolution? Microbiol. Mol. Biol. Rev. 2021, 85, e00053-21. [Google Scholar] [CrossRef] [PubMed]
- Rowe, W.P.; Huebner, R.J.; Gilmore, L.K.; Parrott, R.O.H.; Ward, T.G. Isolation of a Cytopathogenic Agent from Human Adenoids Undergoing Spontaneous Degeneration in Tissue Culture. Exp. Biol. Med. 1953, 84, 570–573. [Google Scholar] [CrossRef]
- Hllleman, M.R.; Werner, J.H. Recovery of New Agent from Patients with Acute Respiratory Illness. Exp. Biol. Med. 1954, 85, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Kulanayake, S.; Tikoo, S. Adenovirus Core Proteins: Structure and Function. Viruses 2021, 13, 388. [Google Scholar] [CrossRef]
- Gallardo, J.; Pérez-Illana, M.; Martín-González, N.; Martín, C.S. Adenovirus Structure: What Is New? Int. J. Mol. Sci. 2021, 22, 5240. [Google Scholar] [CrossRef]
- Lenman, A.; Liaci, A.M.; Liu, Y.; Frängsmyr, L.; Frank, M.; Blaum, B.S.; Chai, W.; Podgorski, I.I.; Harrach, B.; Benkő, M.; et al. Polysialic Acid Is a Cellular Receptor for Human Adenovirus 52. Proc. Natl. Acad. Sci. USA 2018, 115, E4264–E4273. [Google Scholar] [CrossRef]
- Badr, K.R.; Parente-Rocha, J.A.; Baeza, L.C.; Ficcadori, F.S.; Souza, M.; Soares, C.M.; Guissoni, A.C.P.; Almeida, T.N.; Cardoso, D.D. Quantitative Proteomic Analysis of A549 Cells Infected with Human Adenovirus Type 2. J. Med. Virol. 2019, 91, 1239–1249. [Google Scholar] [CrossRef] [PubMed]
- Mao, S. The Fitness Landscape of AAV. Science 2019, 366, 1090. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, F.; Su, R.; Li, X.; Chen, W.; Chen, Q.; Yang, T.; Wang, J.; Liu, H.; Fang, Q.; et al. Structure of RNA Polymerase Complex and Genome within a DsRNA Virus Provides Insights into the Mechanisms of Transcription and Assembly. Proc. Natl. Acad. Sci. USA 2018, 115, 7344–7349. [Google Scholar] [CrossRef]
- Inoue-Nagata, A.K.; Jordan, R.; Kreuze, J.; Li, F.; López-Moya, J.J.; Mäkinen, K.; Ohshima, K.; Wylie, S.J. ICTV Report Consortium. ICTV Virus Taxonomy Profile: Potyviridae 2022: This Article Is Part of the ICTV Virus Taxonomy Profiles Collection. J. Gen. Virol. 2022, 103, 1738. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 Entry into Cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-EM Structure of the 2019-NCoV Spike in the Prefusion Conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural Basis for the Recognition of SARS-CoV-2 by Full-Length Human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Xue, K.S.; Moncla, L.H.; Bedford, T.; Bloom, J.D. Within-Host Evolution of Human Influenza Virus. Trends Microbiol. 2018, 26, 781–793. [Google Scholar] [CrossRef]
- Ye, Q.; Krug, R.M.; Tao, Y.J. The Mechanism by Which Influenza A Virus Nucleoprotein Forms Oligomers and Binds RNA. Nature 2006, 444, 1078–1082. [Google Scholar] [CrossRef]
- Obayashi, E.; Yoshida, H.; Kawai, F.; Shibayama, N.; Kawaguchi, A.; Nagata, K.; Tame, J.R.H.; Park, S.-Y. The Structural Basis for an Essential Subunit Interaction in Influenza Virus RNA Polymerase. Nature 2008, 454, 1127–1131. [Google Scholar] [CrossRef]
- Sugiyama, K.; Obayashi, E.; Kawaguchi, A.; Suzuki, Y.; Tame, J.R.H.; Nagata, K.; Park, S.-Y. Structural Insight into the Essential PB1–PB2 Subunit Contact of the Influenza Virus RNA Polymerase. EMBO J. 2009, 28, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Wandzik, J.M.; Kouba, T.; Karuppasamy, M.; Pflug, A.; Drncova, P.; Provaznik, J.; Azevedo, N.; Cusack, S. A Structure-Based Model for the Complete Transcription Cycle of Influenza Polymerase. Cell 2020, 181, 877–893.e21. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, Z.; Tan, T.; Li, W.; Zhang, Z.; Song, S.; Zhang, X.; Hu, Q.; Zhou, P.; Wu, Y.; et al. Real-Time Imaging of Single HIV-1 Disassembly with Multicolor Viral Particles. ACS Nano 2016, 10, 6273–6282. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Dierich, M.P. Biological Function of H1V-1 Transmembrane Protein Gp41: A Study on a Putative Cellular Receptor of Gp41. Chin. Sci. Bull. 1998, 43, 1630–1635. [Google Scholar] [CrossRef]
- Chen, J.Y.; Feeney, E.R.; Chung, R.T. HCV and HIV Co-Infection: Mechanisms and Management. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Galibert, F.; Mandart, E.; Fitoussi, F.; Tiollais, P.; Charnay, P. Nucleotide Sequence of the Hepatitis B Virus Genome (Subtype Ayw) Cloned in E. Coli. Nature 1979, 281, 646–650. [Google Scholar] [CrossRef]
- Kay, A.; Zoulim, F. Hepatitis B Virus Genetic Variability and Evolution. Virus Res. 2007, 127, 164–176. [Google Scholar] [CrossRef]
- Kostyusheva, A.; Brezgin, S.; Glebe, D.; Kostyushev, D.; Chulanov, V. Host-Cell Interactions in HBV Infection and Pathogenesis: The Emerging Role of M6A Modification. Emerg. Microbes Infect. 2021, 10, 2264–2275. [Google Scholar] [CrossRef]
- Steinhardt, E.; Israeli, C.; Lambert, R.A. Studies on the Cultivation of the Virus of Vaccinia. J. Infect. Dis. 1913, 13, 294–300. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e19. [Google Scholar] [CrossRef]
- Feng, R.; Wan, K.; Sui, X.; Zhao, N.; Li, H.; Lei, W.; Yu, J.; Liu, X.; Shi, X.; Zhai, M.; et al. Anchoring Single Pt Atoms and Black Phosphorene Dual Co-Catalysts on CdS Nanospheres to Boost Visible-Light Photocatalytic H2 Evolution. Nano Today 2021, 37, 101080. [Google Scholar] [CrossRef]
- Bidwell, D.E.; Malaria, A.V. Diagnosis by Enzyme-Linked Immunosorbent Assays. BMJ 1981, 282, 1747–1748. [Google Scholar] [CrossRef]
- Lequin, R.M. Enzyme Immunoassay (EIA)/Enzyme-Linked Immunosorbent Assay (ELISA). Clin. Chem. 2005, 51, 2415–2418. [Google Scholar] [CrossRef]
- Faulk, W.P.; Taylor, G.M. Communication to the Editors. Immunochemistry 1971, 8, 1081–1083. [Google Scholar] [CrossRef]
- Spielberg, F.; Ryder, R.; Harris, J.; Heyward, W.; Kabeya, C.; Kifuani, N.K.; Bender, T.; Quinn, T. Filed Testing and Comparative Evaluation of Rapid, Visually Read Screening Assays for Antibody to Human Immunodeficiency Virus. Lancet 1989, 333, 580–584. [Google Scholar] [CrossRef]
- Xu, M.; Lu, F.; Lyu, C.; Wu, Q.; Zhang, J.; Tian, P.; Xue, L.; Xu, T.; Wang, D. Broad-Range and Effective Detection of Human Noroviruses by Colloidal Gold Immunochromatographic Assay Based on the Shell Domain of the Major Capsid Protein. BMC Microbiol. 2021, 21, 22. [Google Scholar] [CrossRef]
- Peng, P.; Liu, C.; Li, Z.; Xue, Z.; Mao, P.; Hu, J.; Xu, F.; Yao, C.; You, M. Emerging ELISA Derived Technologies for in Vitro Diagnostics. TrAC Trends Anal. Chem. 2022, 152, 116605. [Google Scholar] [CrossRef]
- Ho, S.N.; Hunt, H.D.; Horton, R.M.; Pullen, J.K.; Pease, L.R. Site-Directed Mutagenesis by Overlap Extension Using the Polymerase Chain Reaction. Gene 1989, 77, 51–59. [Google Scholar] [CrossRef]
- Tan, I.L.; Dimamay, M.P.S.; Buerano, C.C.; Alfon, J.A.R.; Tanig, C.Z.; Matias, R.R.; Natividad, F.F. Development and Evaluation of a Fluorogenic Real-Time RT-PCR for the Detection of Dengue 3 Virus. J. Med. Virol. 2010, 82, 2053–2063. [Google Scholar] [CrossRef]
- Pham, J.; Meyer, S.; Nguyen, C.; Williams, A.; Hunsicker, M.; McHardy, I.; Gendlina, I.; Goldstein, D.Y.; Fox, A.S.; Hudson, A.; et al. Performance Characteristics of a High-Throughput Automated Transcription-Mediated Amplification Test for SARS-CoV-2 Detection. J. Clin. Microbiol. 2020, 58, e01669-20. [Google Scholar] [CrossRef]
- Fowler, V.L.; Armson, B.; Gonzales, J.L.; Wise, E.L.; Howson, E.L.A.; Vincent-Mistiaen, Z.; Fouch, S.; Maltby, C.J.; Grippon, S.; Munro, S.; et al. A Highly Effective Reverse-Transcription Loop-Mediated Isothermal Amplification (RT-LAMP) Assay for the Rapid Detection of SARS-CoV-2 Infection. J. Infect. 2021, 82, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, J.; Xia, Y.; Rui, Y.; Yang, X.; Xie, G.; Jiang, G.; Wang, H. Rolling Circle Extension-Assisted Loop-Mediated Isothermal Amplification (Rol-LAMP) Method for Locus-Specific and Visible Detection of RNA N6-Methyladenosine. Nucleic Acids Res. 2023, 51, e51. [Google Scholar] [CrossRef]
- Glökler, J.; Lim, T.S.; Ida, J.; Frohme, M. Isothermal Amplifications—A Comprehensive Review on Current Methods. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 543–586. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, J.M.; Barnewall, R.J.; Marsh, I.B.; Szentirmay, A.N.; Quinn, J.C.; Van Houdt, R.; Gunst, Q.D.; Van Den Hoff, M.J.B. Efficiency Correction Is Required for Accurate Quantitative PCR Analysis and Reporting. Clin. Chem. 2021, 67, 829–842. [Google Scholar] [CrossRef]
- Liu, Y.; Kumar, S.; Taylor, R.E. Mix-and-match Nanobiosensor Design: Logical and Spatial Programming of Biosensors Using Self-assembled DNA Nanostructures. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2018, 10, e1518. [Google Scholar] [CrossRef]
- Fan, S.; Cheng, J.; Liu, Y.; Wang, D.; Luo, T.; Dai, B.; Zhang, C.; Cui, D.; Ke, Y.; Song, J. Proximity-Induced Pattern Operations in Reconfigurable DNA Origami Domino Array. J. Am. Chem. Soc. 2020, 142, 14566–14573. [Google Scholar] [CrossRef]
- Terracciano, M.; De Stefano, L.; Borbone, N.; Politi, J.; Oliviero, G.; Nici, F.; Casalino, M.; Piccialli, G.; Dardano, P.; Varra, M.; et al. Solid Phase Synthesis of a Thrombin Binding Aptamer on Macroporous Silica for Label Free Optical Quantification of Thrombin. RSC Adv. 2016, 6, 86762–86769. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Pashazadeh-Panahi, P.; Baradaran, B.; de la Guardia, M.; Hejazi, M.; Sohrabi, H.; Mokhtarzadeh, A.; Maleki, A. Recent Progress in Optical and Electrochemical Biosensors for Sensing of Clostridium Botulinum Neurotoxin. TrAC Trends Anal. Chem. 2018, 103, 184–197. [Google Scholar] [CrossRef]
- Tong, L.; Xu, H.; Käll, M. Nanogaps for SERS Applications. MRS Bull. 2014, 39, 163–168. [Google Scholar] [CrossRef]
- Song, C.; Jiang, X.; Yang, Y.; Zhang, J.; Larson, S.; Zhao, Y.; Wang, L. High-Sensitive Assay of Nucleic Acid Using Tetrahedral DNA Probes and DNA Concatamers with a Surface-Enhanced Raman Scattering/Surface Plasmon Resonance Dual-Mode Biosensor Based on a Silver Nanorod-Covered Silver Nanohole Array. ACS Appl. Mater. Interfaces 2020, 12, 31242–31254. [Google Scholar] [CrossRef]
- Khalil, I.; Yehye, W.A.; Julkapli, N.M.; Rahmati, S.; Sina, A.A.I.; Basirun, W.J.; Johan, M.R. Graphene Oxide and Gold Nanoparticle Based Dual Platform with Short DNA Probe for the PCR Free DNA Biosensing Using Surface-Enhanced Raman Scattering. Biosens. Bioelectron. 2019, 131, 214–223. [Google Scholar] [CrossRef]
- Das, G.M.; Managò, S.; Mangini, M.; De Luca, A.C. Biosensing Using SERS Active Gold Nanostructures. Nanomaterials 2021, 11, 2679. [Google Scholar] [CrossRef]
- Li, Q.; Huo, H.; Wu, Y.; Chen, L.; Su, L.; Zhang, X.; Song, J.; Yang, H. Design and Synthesis of SERS Materials for In Vivo Molecular Imaging and Biosensing. Adv. Sci. 2023, 10, 2202051. [Google Scholar] [CrossRef]
- Seymour, E.; Kanik, F.E.; Gür, S.D.; Bakhshpour-Yucel, M.; Araz, A.; Ünlü, N.L.; Ünlü, M.S. Solid-Phase Optical Sensing Techniques for Sensitive Virus Detection. Sensors 2023, 23, 5018. [Google Scholar] [CrossRef]
- Xia, J.; Li, W.; Sun, M.; Wang, H. Application of SERS in the Detection of Fungi, Bacteria and Viruses. Nanomaterials 2022, 12, 3572. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Rahmanian, V.; Kalashgrani, M.Y.; Gholami, A.; Omidifar, N.; Chiang, W.-H. Highly Sensitive Flexible SERS-Based Sensing Platform for Detection of COVID-19. Biosensors 2022, 12, 466. [Google Scholar] [CrossRef] [PubMed]
- Saviñon-Flores, F.; Méndez, E.; López-Castaños, M.; Carabarin-Lima, A.; López-Castaños, K.A.; González-Fuentes, M.A.; Méndez-Albores, A. A Review on SERS-Based Detection of Human Virus Infections: Influenza and Coronavirus. Biosensors 2021, 11, 66. [Google Scholar] [CrossRef]
- Song, C.; Zhang, J.; Liu, Y.; Guo, X.; Guo, Y.; Jiang, X.; Wang, L. Highly Sensitive SERS Assay of DENV Gene via a Cascade Signal Amplification Strategy of Localized Catalytic Hairpin Assembly and Hybridization Chain Reaction. Sens. Actuators B Chem. 2020, 325, 128970. [Google Scholar] [CrossRef]
- Huh, Y.S.; Chung, A.J.; Cordovez, B.; Erickson, D. Enhanced On-Chip SERS Based Biomolecular Detection Using Electrokinetically Active Microwells. Lab Chip 2009, 9, 433–439. [Google Scholar] [CrossRef]
- Park, K.S.; Choi, A.; Kim, H.J.; Park, I.; Eom, M.-S.; Yeo, S.-G.; Son, R.G.; Park, T.-I.; Lee, G.; Soh, H.T.; et al. Ultra-Sensitive Label-Free SERS Biosensor with High-Throughput Screened DNA Aptamer for Universal Detection of SARS-CoV-2 Variants from Clinical Samples. Biosens. Bioelectron. 2023, 228, 115202. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A.; Ishak, N.S.; Omar, N.A.S.; Zainudin, A.A. Preparation, Characterization and Optical Properties of Ionophore Doped Chitosan Biopolymer Thin Film and Its Potential Application for Sensing Metal Ion. Optik 2015, 126, 4688–4692. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S. Structural, Optical and Sensing Properties of Ionophore Doped Graphene Based Bionanocomposite Thin Film. Optik 2017, 144, 308–315. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W. Recent Development of SPR Spectroscopy as Potential Method for Diagnosis of Dengue Virus E-Protein. Sens. Rev. 2018, 38, 106–116. [Google Scholar] [CrossRef]
- Murali, S.; Rustandi, R.; Zheng, X.; Payne, A.; Shang, L. Applications of Surface Plasmon Resonance and Biolayer Interferometry for Virus–Ligand Binding. Viruses 2022, 14, 717. [Google Scholar] [CrossRef]
- Takemura, K. Surface Plasmon Resonance (SPR)- and Localized SPR (LSPR)-Based Virus Sensing Systems: Optical Vibration of Nano- and Micro-Metallic Materials for the Development of Next-Generation Virus Detection Technology. Biosensors 2021, 11, 250. [Google Scholar] [CrossRef]
- Hassan, M.M.; Sium, F.S.; Islam, F.; Choudhury, S.M. A Review on Plasmonic and Metamaterial Based Biosensing Platforms for Virus Detection. Sens. Bio-Sens. Res. 2021, 33, 100429. [Google Scholar] [CrossRef]
- Pandey, P.S.; Raghuwanshi, S.K.; Shadab, A.; Ansari, M.T.I.; Tiwari, U.K.; Kumar, S. SPR Based Biosensing Chip for COVID-19 Diagnosis—A Review. IEEE Sens. J. 2022, 22, 13800–13810. [Google Scholar] [CrossRef]
- Diao, W.; Tang, M.; Ding, S.; Li, X.; Cheng, W.; Mo, F.; Yan, X.; Ma, H.; Yan, Y. Highly Sensitive Surface Plasmon Resonance Biosensor for the Detection of HIV-Related DNA Based on Dynamic and Structural DNA Nanodevices. Biosens. Bioelectron. 2018, 100, 228–234. [Google Scholar] [CrossRef]
- Lee, T.; Kim, G.H.; Kim, S.M.; Hong, K.; Kim, Y.; Park, C.; Sohn, H.; Min, J. Label-Free Localized Surface Plasmon Resonance Biosensor Composed of Multi-Functional DNA 3 Way Junction on Hollow Au Spike-like Nanoparticles (HAuSN) for Avian Influenza Virus Detection. Colloids Surf. B Biointerfaces 2019, 182, 110341. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Takemura, K.; Khorish, I.M.; Nasrin, F.; Tun, M.M.N.; Morita, K.; Park, E.Y. The Detection and Identification of Dengue Virus Serotypes with Quantum Dot and AuNP Regulated Localized Surface Plasmon Resonance. Nanoscale Adv. 2020, 2, 699–709. [Google Scholar] [CrossRef]
- Oliveira, N.; Souza, E.; Ferreira, D.; Zanforlin, D.; Bezerra, W.; Borba, M.; Arruda, M.; Lopes, K.; Nascimento, G.; Martins, D.; et al. A Sensitive and Selective Label-Free Electrochemical DNA Biosensor for the Detection of Specific Dengue Virus Serotype 3 Sequences. Sensors 2015, 15, 15562–15577. [Google Scholar] [CrossRef] [PubMed]
- Damborský, P.; Švitel, J.; Katrlík, J. Optical Biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Celiker, T.; Ghorbanizamani, F.; Moulahoum, H.; Celik, E.G.; Tok, K.; Zihnioglu, F.; Cicek, C.; Sertoz, R.; Arda, B.; Goksel, T.; et al. Fluorescent Bioassay for SARS-CoV-2 Detection Using Polypyrene-g-Poly(ε-Caprolactone) Prepared by Simultaneous Photoinduced Step-Growth and Ring-Opening Polymerizations. Microchim. Acta 2022, 189, 202. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Mishra, R.K.; Goud, K.Y.; Mohamed, M.A.; Kummari, S.; Tiwari, S.; Li, Z.; Narayan, R.; Stanciu, L.A.; Marty, J.L. Optical Biosensors for Diagnostics of Infectious Viral Disease: A Recent Update. Diagnostics 2021, 11, 2083. [Google Scholar] [CrossRef] [PubMed]
- Kulzer, F.; Orrit, M. Single-Molecule Optics. Annu. Rev. Phys. Chem. 2004, 55, 585–611. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.; Kalantari, H.; Khodayar, M.J.; Kouchak, M.; Rahbar, N. A Novel Strategy for Detection of Small Molecules Based on Aptamer/Gold Nanoparticles/Graphitic Carbon Nitride Nanosheets as Fluorescent Biosensor. Talanta 2020, 219, 121235. [Google Scholar] [CrossRef]
- Salama, A.M.; Yasin, G.; Zourob, M.; Lu, J. Fluorescent Biosensors for the Detection of Viruses Using Graphene and Two-Dimensional Carbon Nanomaterials. Biosensors 2022, 12, 460. [Google Scholar] [CrossRef] [PubMed]
- Ekiz-Kanik, F.; Sevenler, D.D.; Ünlü, N.L.; Chiari, M.; Ünlü, M.S. Surface Chemistry and Morphology in Single Particle Optical Imaging. Nanophotonics 2017, 6, 713–730. [Google Scholar] [CrossRef]
- Sharma, A.; Khan, R.; Catanante, G.; Sherazi, T.; Bhand, S.; Hayat, A.; Marty, J. Designed Strategies for Fluorescence-Based Biosensors for the Detection of Mycotoxins. Toxins 2018, 10, 197. [Google Scholar] [CrossRef]
- Maddali, H.; Miles, C.E.; Kohn, J.; O’Carroll, D.M. Optical Biosensors for Virus Detection: Prospects for SARS-CoV-2/COVID-19. ChemBioChem 2021, 22, 1176–1189. [Google Scholar] [CrossRef]
- Shen, W.; Gao, Z. Quantum Dots and Duplex-Specific Nuclease Enabled Ultrasensitive Detection and Serotyping of Dengue Viruses in One Step in a Single Tube. Biosens. Bioelectron. 2015, 65, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Yan, X.-P. Doped Quantum Dots for Chemo/Biosensing and Bioimaging. Chem. Soc. Rev. 2013, 42, 5489. [Google Scholar] [CrossRef] [PubMed]
- Teengam, P.; Nisab, N.; Chuaypen, N.; Tangkijvanich, P.; Vilaivan, T.; Chailapakul, O. Fluorescent Paper-Based DNA Sensor Using Pyrrolidinyl Peptide Nucleic Acids for Hepatitis C Virus Detection. Biosens. Bioelectron. 2021, 189, 113381. [Google Scholar] [CrossRef]
- Jiao, J.; Duan, C.; Xue, L.; Liu, Y.; Sun, W.; Xiang, Y. DNA Nanoscaffold-Based SARS-CoV-2 Detection for COVID-19 Diagnosis. Biosens. Bioelectron. 2020, 167, 112479. [Google Scholar] [CrossRef] [PubMed]
- Kwon, P.S.; Ren, S.; Kwon, S.-J.; Kizer, M.E.; Kuo, L.; Xie, M.; Zhu, D.; Zhou, F.; Zhang, F.; Kim, D.; et al. Designer DNA Architecture Offers Precise and Multivalent Spatial Pattern-Recognition for Viral Sensing and Inhibition. Nat. Chem. 2020, 12, 26–35. [Google Scholar] [CrossRef]
- Ochmann, S.E.; Vietz, C.; Trofymchuk, K.; Acuna, G.P.; Lalkens, B.; Tinnefeld, P. Optical Nanoantenna for Single Molecule-Based Detection of Zika Virus Nucleic Acids without Molecular Multiplication. Anal. Chem. 2017, 89, 13000–13007. [Google Scholar] [CrossRef]
- Chowdhury, A.D.; Ganganboina, A.B.; Nasrin, F.; Takemura, K.; Doong, R.-A.; Utomo, D.I.S.; Lee, J.; Khoris, I.M.; Park, E.Y. Femtomolar Detection of Dengue Virus DNA with Serotype Identification Ability. Anal. Chem. 2018, 90, 12464–12474. [Google Scholar] [CrossRef]
- Gogianu, L.; Popescu, M.C.; Vasile, B.S.; Mihalache, I.; Anghel, E.M.; Damian, C.M.; Salceanu, A.; Boldeiu, A.; Constantin, E.; Radoi, A.; et al. Microarray Biochip Fabricated on Silicon Nanowires/Carbon Dots Heterostructures for Enhanced Viral DNA Detection. Appl. Surf. Sci. 2023, 636, 157878. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Wang, Y.; Li, H.; Xia, F. Re-Engineering Electrochemical Aptamer-Based Biosensors to Tune Their Useful Dynamic Range via Distal-Site Mutation and Allosteric Inhibition. Anal. Chem. 2020, 92, 13427–13433. [Google Scholar] [CrossRef]
- Huang, H.; Bai, W.; Dong, C.; Guo, R.; Liu, Z. An Ultrasensitive Electrochemical DNA Biosensor Based on Graphene/Au Nanorod/Polythionine for Human Papillomavirus DNA Detection. Biosens. Bioelectron. 2015, 68, 442–446. [Google Scholar] [CrossRef] [PubMed]
- Vermisoglou, E.; Panáček, D.; Jayaramulu, K.; Pykal, M.; Frébort, I.; Kolář, M.; Hajdúch, M.; Zbořil, R.; Otyepka, M. Human Virus Detection with Graphene-Based Materials. Biosens. Bioelectron. 2020, 166, 112436. [Google Scholar] [CrossRef]
- Kurzątkowska, K.; Sirko, A.; Zagórski-Ostoja, W.; Dehaen, W.; Radecka, H.; Radecki, J. Electrochemical Label-Free and Reagentless Genosensor Based on an Ion Barrier Switch-off System for DNA Sequence-Specific Detection of the Avian Influenza Virus. Anal. Chem. 2015, 87, 9702–9709. [Google Scholar] [CrossRef]
- Li, C.; Sun, L.; Xu, Z.; Wu, X.; Liang, T.; Shi, W. Experimental Investigation and Error Analysis of High Precision FBG Displacement Sensor for Structural Health Monitoring. Int. J. Struct. Stab. Dyn. 2020, 20, 2040011. [Google Scholar] [CrossRef]
- Kianfar, E.; Salimi, M.; Koohestani, B. Methanol to Gasoline Conversion over CuO/ZSM-5 Catalyst Synthesized and Influence of Water on Conversion. Fine Chem. Eng. 2020, 75–82. [Google Scholar] [CrossRef]
- Kianfar, E. An Experimental Study PVDF and PSF Hollow Fiber Membranes for Chemical Absorption Carbon Dioxide. Fine Chem. Eng. 2020, 92–103. [Google Scholar] [CrossRef]
- Lee, T.; Park, S.Y.; Jang, H.; Kim, G.-H.; Lee, Y.; Park, C.; Mohammadniaei, M.; Lee, M.-H.; Min, J. Fabrication of Electrochemical Biosensor Consisted of Multi-Functional DNA Structure/Porous Au Nanoparticle for Avian Influenza Virus (H5N1) in Chicken Serum. Mater. Sci. Eng. C 2019, 99, 511–519. [Google Scholar] [CrossRef]
- Fu, J.; Wu, J.; Zhang, R.; Wu, Q.; Ju, H. Electrochemical Biosensing of DENV Nucleic Acid Amplified with Triplet Nanostructure-Mediated Dendritic Hybridization Chain Reaction. Sens. Actuators B Chem. 2021, 345, 130436. [Google Scholar] [CrossRef]
- Dong, S.; Zhao, R.; Zhu, J.; Lu, X.; Li, Y.; Qiu, S.; Jia, L.; Jiao, X.; Song, S.; Fan, C.; et al. Electrochemical DNA Biosensor Based on a Tetrahedral Nanostructure Probe for the Detection of Avian Influenza A (H7N9) Virus. ACS Appl. Mater. Interfaces 2015, 7, 8834–8842. [Google Scholar] [CrossRef]
- Mahmoodi, P.; Rezayi, M.; Rasouli, E.; Avan, A.; Gholami, M.; Mobarhan, M.G.; Karimi, E.; Alias, Y. Early-Stage Cervical Cancer Diagnosis Based on an Ultra-Sensitive Electrochemical DNA Nanobiosensor for HPV-18 Detection in Real Samples. J. Nanobiotechnol. 2020, 18, 11. [Google Scholar] [CrossRef]
- Weng, X.; Li, C.; Chen, C.; Wang, G.; Xia, C.; Zheng, L. A Microfluidic Device for Tobacco Ringspot Virus Detection by Electrochemical Impedance Spectroscopy. Micromachines 2023, 14, 1118. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Huang, C.-H.; Pai, P.-C.; Seo, J.; Lei, K.F. A Review on Microfluidics-Based Impedance Biosensors. Biosensors 2023, 13, 83. [Google Scholar] [CrossRef]
- Wang, S.; Li, L.; Jin, H.; Yang, T.; Bao, W.; Huang, S.; Wang, J. Electrochemical Detection of Hepatitis B and Papilloma Virus DNAs Using SWCNT Array Coated with Gold Nanoparticles. Biosens. Bioelectron. 2013, 41, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Štukovnik, Z.; Bren, U. Recent Developments in Electrochemical-Impedimetric Biosensors for Virus Detection. Int. J. Mol. Sci. 2022, 23, 15922. [Google Scholar] [CrossRef] [PubMed]
- Shariati, M. Impedimetric Biosensor for Monitoring Complementary DNA from Hepatitis B Virus Based on Gold Nanocrystals. J. Electrochem. Soc. 2021, 168, 016512. [Google Scholar] [CrossRef]
- Steinmetz, M.; Lima, D.; Viana, A.G.; Fujiwara, S.T.; Pessôa, C.A.; Etto, R.M.; Wohnrath, K. A Sensitive Label-Free Impedimetric DNA Biosensor Based on Silsesquioxane-Functionalized Gold Nanoparticles for Zika Virus Detection. Biosens. Bioelectron. 2019, 141, 111351. [Google Scholar] [CrossRef]
- Ilkhani, H.; Farhad, S. A Novel Electrochemical DNA Biosensor for Ebola Virus Detection. Anal. Biochem. 2018, 557, 151–155. [Google Scholar] [CrossRef]
- Shariati, M.; Ghorbani, M.; Sasanpour, P.; Karimizefreh, A. An Ultrasensitive Label Free Human Papilloma Virus DNA Biosensor Using Gold Nanotubes Based on Nanoporous Polycarbonate in Electrical Alignment. Anal. Chim. Acta 2019, 1048, 31–41. [Google Scholar] [CrossRef]
- Faria, H.A.M.; Zucolotto, V. Label-Free Electrochemical DNA Biosensor for Zika Virus Identification. Biosens. Bioelectron. 2019, 131, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Singhal, C.; Pundir, C.S.; Narang, J. A Genosensor for Detection of Consensus DNA Sequence of Dengue Virus Using ZnO/Pt-Pd Nanocomposites. Biosens. Bioelectron. 2017, 97, 75–82. [Google Scholar] [CrossRef]
- Bhardwaj, J.; Chaudhary, N.; Kim, H.; Jang, J. Subtyping of Influenza A H1N1 Virus Using a Label-Free Electrochemical Biosensor Based on the DNA Aptamer Targeting the Stem Region of HA Protein. Anal. Chim. Acta 2019, 1064, 94–103. [Google Scholar] [CrossRef]
- Ding, H.; Li, J.; Chen, N.; Hu, X.; Yang, X.; Guo, L.; Li, Q.; Zuo, X.; Wang, L.; Ma, Y.; et al. DNA Nanostructure-Programmed Like-Charge Attraction at the Cell-Membrane Interface. ACS Cent. Sci. 2018, 4, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, F.; Kick, B.; Behler, K.L.; Honemann, M.N.; Weuster-Botz, D.; Dietz, H. Biotechnological Mass Production of DNA Origami. Nature 2017, 552, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Huo, S.; Li, H.; Boersma, A.J.; Herrmann, A. DNA Nanotechnology Enters Cell Membranes. Adv. Sci. 2019, 6, 1900043. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuwen, L.; Zhang, S.; Chao, J. Recent Advances in DNA Nanotechnology-Enabled Biosensors for Virus Detection. Biosensors 2023, 13, 822. https://doi.org/10.3390/bios13080822
Yuwen L, Zhang S, Chao J. Recent Advances in DNA Nanotechnology-Enabled Biosensors for Virus Detection. Biosensors. 2023; 13(8):822. https://doi.org/10.3390/bios13080822
Chicago/Turabian StyleYuwen, Lihui, Shifeng Zhang, and Jie Chao. 2023. "Recent Advances in DNA Nanotechnology-Enabled Biosensors for Virus Detection" Biosensors 13, no. 8: 822. https://doi.org/10.3390/bios13080822
APA StyleYuwen, L., Zhang, S., & Chao, J. (2023). Recent Advances in DNA Nanotechnology-Enabled Biosensors for Virus Detection. Biosensors, 13(8), 822. https://doi.org/10.3390/bios13080822






