Supramolecular Enzymatic Labeling for Aptamer Switch-Based Electrochemical Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Synthesis of Chemically Modified Biomolecules
2.3. Preparation of Aptamer-Functionalized Electrodes (Apt-ADA/AuNF/GO/SPE)
2.4. Electroanalytical Procedure
2.5. Real Sample Analysis
3. Results and Discussion
3.1. Characterization of the Electrode Surface Using Scanning Electronic Microscopy (SEM)
3.2. Selection of the Materials for the Sensor Construction
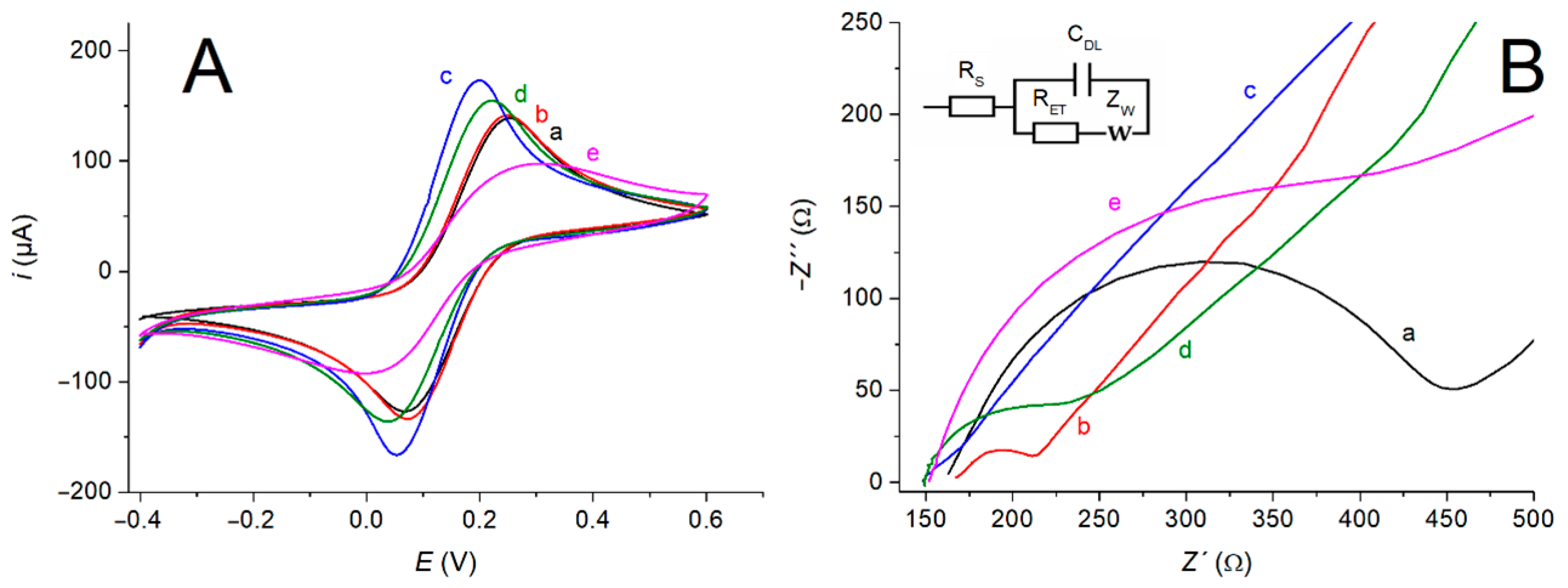
3.3. Electrochemical Characterization of the Assembling Steps
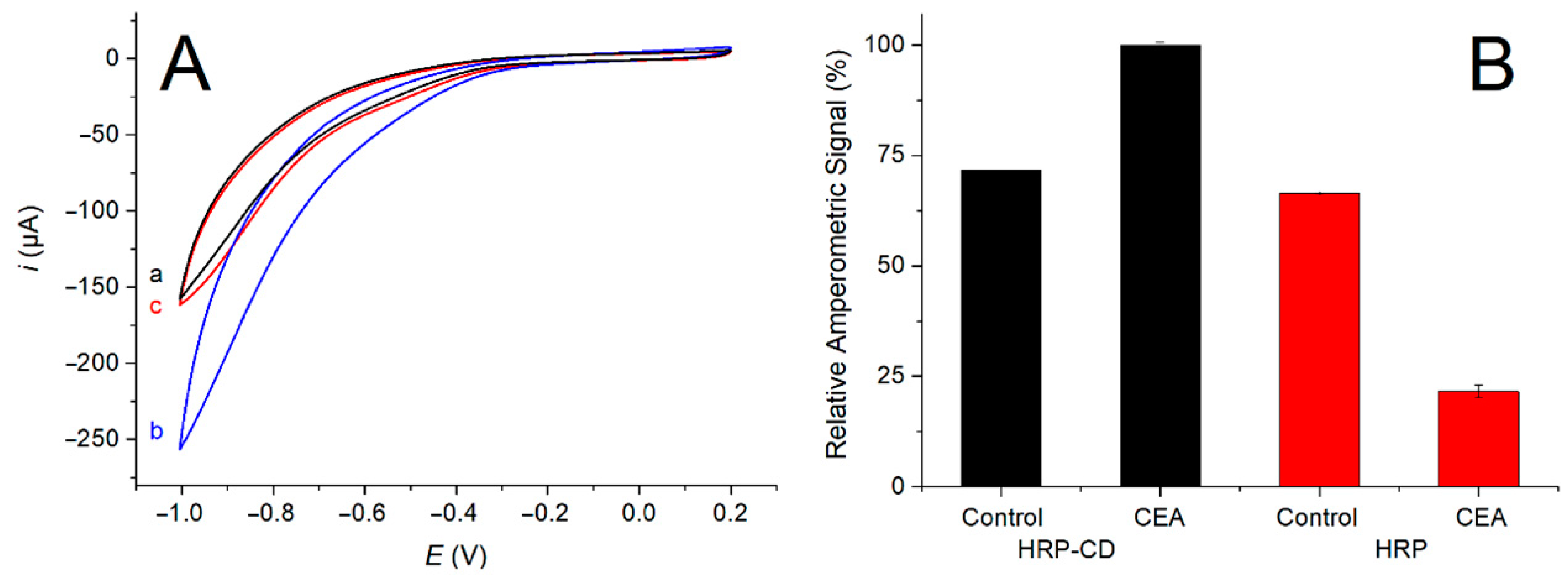
3.4. Demonstration of the Switching-Based Sensing Mechanism
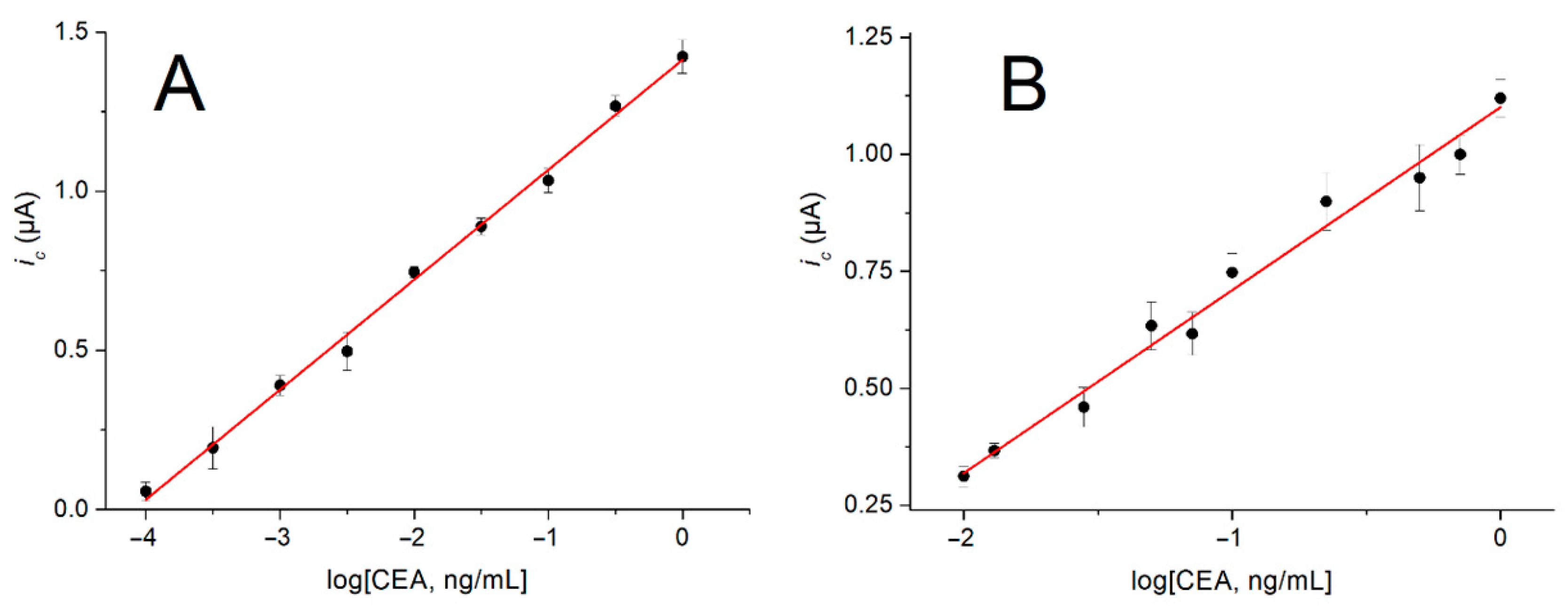
3.5. Analytical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, T.; Guo, Y.; Zhao, F. Electrochemical biosensors for the detection of cancer biomarkers with different signal amplification strategies. Int. J. Electrochem. Sci. 2021, 16, 210732. [Google Scholar] [CrossRef]
- Ding, L.; Bond, A.M.; Zhai, J.; Zhang, J. Utilization of nanoparticle labels for signal amplification in ultrasensitive electrochemical affinity biosensors: A review. Anal. Chim. Acta 2013, 797, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, M.; Shadjou, N.; de la Guardia, M. Iron and iron-oxide magnetic nanoparticles as signal-amplification elements in electrochemical biosensing. TrAC Trends Anal. Chem. 2015, 72, 1–9. [Google Scholar] [CrossRef]
- Jimenez-Falcao, S.; Parra-Nieto, J.; Perez-Cuadrado, H.; Martinez-Manez, R.; Martinez-Ruiz, P.; Villalonga, R. Avidin-gated mesoporous silica nanoparticles for signal amplification in electrochemical biosensor. Electrochem. Commun. 2019, 108, 106556. [Google Scholar] [CrossRef]
- Villalonga, A.; Pérez-Calabuig, A.M.; Villalonga, R. Electrochemical biosensors based on nucleic acid aptamers. Anal. Bioanal. Chem. 2020, 412, 55–72. [Google Scholar] [CrossRef]
- Zhu, Q.; Chai, Y.; Zhuo, Y.; Yuan, R. Ultrasensitive simultaneous detection of four biomarkers based on hybridization chain reaction and biotin–streptavidin signal amplification strategy. Biosens. Bioelectron. 2015, 68, 42–48. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Jiang, L.; Chu, P.K.; Dong, Y.; Wei, Q. A sandwich-type electrochemical immunosensor based on the biotin- streptavidin-biotin structure for detection of human immunoglobulin G. Sci. Rep. 2016, 6, 22694. [Google Scholar] [CrossRef]
- Hyre, D.E.; Le Trong, I.; Merritt, E.A.; Eccleston, J.F.; Green, N.M.; Stenkamp, R.E.; Stayton, P.S. Cooperative hydrogen bond interactions in the streptavidin–biotin system. Protein Sci. 2006, 15, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Dundas, C.M.; Demonte, D.; Park, S. Streptavidin–biotin technology: Improvements and innovations in chemical and biological applications. Appl. Microbiol. Biotechnol. 2013, 97, 9343–9353. [Google Scholar] [CrossRef]
- Luong, J.H.; Male, K.B.; Glennon, J.D. Biotin interference in immunoassays based on biotin-strept(avidin) chemistry: An emerging threat. Biotechnol. Adv. 2019, 37, 634–641. [Google Scholar] [CrossRef]
- Gifford, J.L.; de Koning, L.; Sadrzadeh, S.H. Strategies for mitigating risk posed by biotin interference on clinical immunoassays. Clin. Biochem. 2019, 65, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Yi, Y.; Chen, J. Recent advances for cyclodextrin-based materials in electrochemical sensing. TrAC Trends Anal. Chem. 2016, 80, 232–241. [Google Scholar] [CrossRef]
- Villalonga, R.; Cao, R.; Fragoso, A. Supramolecular chemistry of cyclodextrins in enzyme technology. Chem. Rev. 2007, 107, 3088–3116. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, H.; Zhang, L.; Li, H.; Yan, M.; Song, X.; Yu, J. Multiplexed aptasensor for simultaneous detection of carcinoembryonic antigen and mucin-1 based on metal ion electrochemical labels and Ru(NH3)63+ electronic wires. Biosens. Bioelectron. 2018, 99, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.; Fragoso, A.; Cao, R.; Villalonga, R. Improved functional properties of trypsin modified by monosubstituted amino-β-cyclodextrins. J. Mol. Catal. B Enzym. 2003, 21, 133–141. [Google Scholar] [CrossRef]
- Childs, R.E.; Bardsley, W.G. The steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethyl-benzthiazoline-6-sulphonic acid) as chromogen. Biochem. J. 1975, 145, 93–103. [Google Scholar] [CrossRef]
- Villalonga, A.; Vegas, B.; Paniagua, G.; Eguílaz, M.; Mayol, B.; Parrado, C.; Rivas, G.; Díez, P.; Villalonga, R. Amperometric aptasensor for carcinoembryonic antigen based on a reduced graphene oxide/gold nanoparticles modified electrode. J. Electroanal. Chem. 2020, 877, 114511. [Google Scholar] [CrossRef]
- Cherevko, S.; Chung, C.H. Direct electrodeposition of nanoporous gold with controlled multimodal pore size distribution. Electrochem. Commun. 2011, 13, 16–19. [Google Scholar] [CrossRef]
- Chen, X.; Tian, X.; Su, B.; Huang, Z.; Chen, X.; Oyama, M. Au nanoparticles on citrate-functionalized graphene nanosheets with a high peroxidase-like performance. Dalton Trans. 2014, 43, 7449–7454. [Google Scholar] [CrossRef]
- Zhaoyang, W.; Liguo, C.; Guoli, S.; Ruqin, Y. Platinum nanoparticle-modified carbon fiber ultramicroelectrodes for mediator-free biosensing. Sens. Actuators B Chem. 2006, 119, 295–301. [Google Scholar] [CrossRef]
- Loock, H.P.; Wentzell, P.D. Detection limits of chemical sensors: Applications and misapplications. Sens. Actuators B Chem. 2012, 173, 157–163. [Google Scholar] [CrossRef] [Green Version]
- Rivas, G.A.; Rodríguez, M.C.; Rubianes, M.D.; Gutierrez, F.A.; Eguílaz, M.; Dalmasso, P.R.; Primo, E.N.; Tettamanti, C.; Ramírez, M.L.; Montemerlo, A.; et al. Carbon nanotubes-based electrochemical (bio)sensors for biomarkers. Appl. Mater. Today 2017, 9, 566–588. [Google Scholar] [CrossRef]
- Yen, Y.-K.; Chao, C.-H.; Yeh, Y.-S. A Graphene-PEDOT:PSS Modified Paper-Based Aptasensor for Electrochemical Impedance Spectroscopy Detection of Tumor Marker. Sensors 2020, 20, 1372. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, L.; Ai, Y.; Liu, Y.; Sun, H.; Liang, Q. Self-Polymerized Dopamine-Decorated Au NPs and Coordinated with Fe-MOF as a Dual Binding Sites and Dual Signal-Amplifying Electrochemical Aptasensor for the Detection of CEA. ACS Appl. Mater. Interfaces 2020, 12, 5500–5510. [Google Scholar] [CrossRef]
- Song, J.; Teng, H.; Xu, Z.; Liu, N.; Xu, L.; Liu, L.; Gao, F.; Luo, X. Free-standing electrochemical biosensor for carcinoembryonic antigen detection based on highly stable and flexible conducting polypyrrole nanocomposite. Microchim. Acta 2021, 188, 217. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, J.; Song, D.; Xu, J.; Zhang, M. Electrochemical Aptasensor of Carcinoembryonic Antigen Based on Concanavalin A-Functionalized Magnetic Copper Silicate Carbon Microtubes and Gold-Nanocluster-Assisted Signal Amplification. ACS Appl. Nano Mater. 2020, 3, 3449–3458. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Y.; Hou, Q.; Xu, Q.; Ding, C. Magnetic antifouling material based ratiometric electrochemical biosensor for the accurate detection of CEA in clinical serum. Biosens. Bioelectron. 2022, 208, 114216. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Luo, J.; Liu, J.; Wang, L.; Fan, Y.; Yan, S.; Yang, Y.; Cai, X. A novel label-free microfluidic paper-based immunosensor for highly sensitive electrochemical detection of carcinoembryonic antigen. Biosens. Bioelectron. 2016, 83, 319–326. [Google Scholar] [CrossRef]
- Lee, S.X.; Lim, H.N.; Ibrahim, I.; Jamil, A.; Pandikumar, A.; Huang, N.M. Horseradish peroxidase-labeled silver/reduced graphene oxide thin film-modified screen-printed electrode for detection of carcinoembryonic antigen. Biosens. Bioelectron. 2017, 89, 673–680. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalonga, A.; Parrado, C.; Díaz, R.; Sánchez, A.; Mayol, B.; Martínez-Ruíz, P.; Vilela, D.; Villalonga, R. Supramolecular Enzymatic Labeling for Aptamer Switch-Based Electrochemical Biosensor. Biosensors 2022, 12, 514. https://doi.org/10.3390/bios12070514
Villalonga A, Parrado C, Díaz R, Sánchez A, Mayol B, Martínez-Ruíz P, Vilela D, Villalonga R. Supramolecular Enzymatic Labeling for Aptamer Switch-Based Electrochemical Biosensor. Biosensors. 2022; 12(7):514. https://doi.org/10.3390/bios12070514
Chicago/Turabian StyleVillalonga, Anabel, Concepción Parrado, Raúl Díaz, Alfredo Sánchez, Beatriz Mayol, Paloma Martínez-Ruíz, Diana Vilela, and Reynaldo Villalonga. 2022. "Supramolecular Enzymatic Labeling for Aptamer Switch-Based Electrochemical Biosensor" Biosensors 12, no. 7: 514. https://doi.org/10.3390/bios12070514
APA StyleVillalonga, A., Parrado, C., Díaz, R., Sánchez, A., Mayol, B., Martínez-Ruíz, P., Vilela, D., & Villalonga, R. (2022). Supramolecular Enzymatic Labeling for Aptamer Switch-Based Electrochemical Biosensor. Biosensors, 12(7), 514. https://doi.org/10.3390/bios12070514





