Development and Evaluation of a Flexible PVDF-Based Balloon Sensor for Detecting Mechanical Forces at Key Esophageal Nodes in Esophageal Motility Disorders
Abstract
1. Introduction
2. Materials and Methods
2.1. Stress Detection System Design and Fabrication
2.2. Stress Reconstruction at Key Esophageal Nodes
2.2.1. Balloon Membrane Surface Stress Calculation
2.2.2. Catheter Bending Stress Calculation
2.2.3. Intra-Balloon Fluid Shear Stress
2.2.4. PVDF Force-Electric Conversion Calculation
2.2.5. Model of Balloon Input–Output Inverse Problem
2.3. Sensor Static Output Characteristic Test
2.4. Experimental Methods for Simulating Esophageal Peristalsis
2.4.1. Stress Reconstruction at Key Esophageal Nodes
2.4.2. Dynamic Output Test under Esophageal Peristalsis during Simulated Swallowing
3. Results and Discussion
3.1. Theoretical Value of Analytical Model of Joint Stress
3.2. Static Performance Curve and Analysis Results of PVDF Array
3.3. Esophageal Creep Simulation Test
3.3.1. Dynamic Performance Curve and Analysis
3.3.2. Dynamic Analysis under Simulated Esophageal Peristalsis
3.4. Robustness and Reproducibility
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vaezi, M.F.; Pandolfino, J.E.; Yadlapati, R.H.; Greer, K.B.; Kavitt, R.T. ACG clinical guidelines: Diagnosis and management of achalasia. Am. J. Gastroenterol. 2020, 115, 1393. [Google Scholar] [CrossRef]
- Yadlapati, R.; Kahrilas, P.J.; Fox, M.R.; Bredenoord, A.J.; Gyawaliet, C.P.; Roman, S.; Babaei, A.; Mittal, R.K.; Rommel, N.; Savarino, E.; et al. Esophageal motility disorders on high-resolution manometry: Chicago classification version 4.0. Neurogastroenterol. Motil. 2021, 33, e14058. [Google Scholar] [CrossRef]
- Savarino, E.; Marabotto, E.; Bodini, G.; Furnari, M.; Della Coletta, M.; Ghisa, M.; Barberio, B.; Frazzoni, M.; De Bortoli, N.; Zentilin, P.; et al. Advancements in the use of manometry and impedance testing for esophageal functional disorders. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 425–435. [Google Scholar] [CrossRef]
- Tutuian, R. Impedance technology for the management of esophageal disorders. Eur. Surg. 2008, 40, 50–57. [Google Scholar] [CrossRef]
- Pandolfino, J.E.; Bulsiewicz, W.J. Evaluation of esophageal motor disorders in the era of high-resolution manometry and intraluminal impedance. Curr. Gastroenterol. Rep. 2009, 11, 182–189. [Google Scholar] [CrossRef]
- Chen, C.-L. Esophageal Impedance: Role in the Evaluation of Esophageal Motility. Tzu Chi Med. J. 2009, 21, 110–117. [Google Scholar] [CrossRef]
- Carlson, D.A.; Omari, T.; Lin, Z.; Rommel, N.; Starkey, K.; Kahrilas, P.J.; Tack, J.; Pandolfino, J.E. High-resolution impedance manometry parameters enhance the esophageal motility evaluation in non-obstructive dysphagia patients without a major Chicago Classification motility disorder. Neurogastroenterol. Motil. 2016, 29, e12941. [Google Scholar] [CrossRef]
- Choksi, Y.; Lal, P.; Slaughter, J.C.; Sharda, R.; Parnell, J.; Higginbotham, T.; Vaezi, M.F. Esophageal Mucosal Impedance Patterns Discriminate Patients With Eosinophilic Esophagitis From Patients With GERD. Clin. Gastroenterol. Hepatol. 2018, 16, 664–671. [Google Scholar] [CrossRef]
- Chaudhry, N.A.; Zahid, K.; Keihanian, S.; Dai, Y.; Zhang, Q. Transmitted cardiovascular pulsations on high resolution esophageal impedance manometry, and their significance in dysphagia. World J. Gastroenterol. 2017, 23, 7840. [Google Scholar] [CrossRef]
- Tseng, P.H.; Rkm, W.; Wu, J.F.; Chen, C.C.; Tu, C.H.; Lee, Y.C.; Lee, H.C.; Wang, H.P.; Wu, M.S. Normative values and factors affecting water-perfused esophageal high-resolution impedance manometry for a Chinese population. Neurogastroenterol. Motil. 2017, 30, e13265. [Google Scholar] [CrossRef]
- Donnan, E.N.; Pandolfino, J.E. Applying the functional luminal imaging probe to esophageal disorders. Curr. Gastroenterol. Rep. 2020, 22, 1–5. [Google Scholar] [CrossRef]
- Clarke, J.O.; Ahuja, N.K.; Fernandez-Becker, N.Q.; Gregersen, H.; Kamal, A.N.; Khan, A.; Lynch, K.L.; Vela, M.F. The functional lumen imaging probe in gastrointestinal disorders: The past, present, and future. Ann. N. Y. Acad. Sci. 2020, 1482, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Desprez, C.; Roman, S.; Leroi, A.M.; Gourcerol, G. The use of impedance planimetry (Endoscopic Functional Lumen Imaging Probe, EndoFLIP®) in the gastrointestinal tract: A systematic review. Neurogastroenterol. Motil. 2020, 32, e13980. [Google Scholar] [CrossRef] [PubMed]
- Savarino, E.; Di Pietro, M.; Bredenoord, A.J.; Carlson, D.A.; Clarke, J.A.; Khan, A.; Vela, M.F.; Yadlapati, R.; Pohl, D.; Pandolfino, J.E.; et al. Use of the functional lumen imaging probe in clinical esophagology. Am. J. Gastroenterol. 2020, 115, 1786. [Google Scholar] [CrossRef] [PubMed]
- Rohof, W.O.; Hirsch, D.P.; Kessing, B.F.; Boeckxstaens, G.E. Efficacy of treatment for patients with achalasia depends on the distensibility of the esophagogastric junction. Gastroenterology 2012, 143, 328–335. [Google Scholar] [CrossRef]
- Ponds, F.A.; Bredenoord, A.J.; Kessing, B.F.; Smout, A.J.P.M. Esophagogastric junction distensibility identifies achalasia subgroup with manometrically normal esophagogastric junction relaxation. Neurogastroenterol. Motil. 2017, 29, e12908. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.S.; Carlson, D.A.; Triggs, J.; Tye, M.; Kou, W.; Campagna, R.; Hungness, E.; Kim, D.; Kahrilas, P.J.; Pandolfino, J.E. Esophagogastric junction distensibility on functional lumen imaging probe topography predicts treatment response in achalasia–anatomy matters! Am. J. Gastroenterol. 2019, 114, 1455. [Google Scholar] [CrossRef]
- Carlson, D.A.; Kahrilas, P.J.; Lin, Z.; Hirano, I.; Gonsalves, N.; Listernick, Z.; Ritter, K.; Tye, M.; Ponds, F.A.; Wong, L.; et al. Evaluation of esophageal motility utilizing the functional lumen imaging probe (FLIP). Am. J. Gastroenterol. 2016, 111, 1726. [Google Scholar] [CrossRef]
- Okeke, F.C.; Raja, S.; Lynch, K.L.; Dhalla, S.; Nandwani, M.; Stein, E.M.; Roland, B.C.; Khashab, M.A.; Saxena, P.; Kumbhari, V.; et al. What is the clinical significance of esophagogastric junction outflow obstruction? evaluation of 60 patients at a tertiary referral center. Neurogastroenterol. Motil. 2017, 29, e13061. [Google Scholar] [CrossRef]
- Zikos, T.A.; Triadafilopoulos, G.; Clarke, J.O. Esophagogastric junction outflow obstruction: Current approach to diagnosis and management. Curr. Gastroenterol. Rep. 2020, 22, 1–9. [Google Scholar] [CrossRef]
- Nicodème, F.; Hirano, I.; Chen, J.; Robinson, K.; Lin, Z.; Xiao, Y.; Gonsalves, N.; Kwasny, M.J.; Kahrilas, P.J.; Pandolfino, J.E. Esophageal distensibility as a measure of disease severity in patients with eosinophilic esophagitis. Clin. Gastroenterol. Hepatol. 2013, 11, 1101–1107. [Google Scholar] [CrossRef]
- Menard-Katcher, C.; Benitez, A.J.; Pan, Z.; Ahmed, F.N.; Wilkins, B.J.; Capocelli, K.E.; Liacouras, C.A.; Verma, R.; Spergel, J.M.; Furuta, J.T.; et al. Influence of age and eosinophilic esophagitis on esophageal distensibility in a pediatric cohort. Am. J. Gastroenterol. 2017, 112, 1466. [Google Scholar] [CrossRef]
- Ilczyszyn, A.; Botha, A.J. Feasibility of esophagogastric junction distensibility measurement during Nissen fundoplication. Dis. Esophagus 2014, 27, 637–644. [Google Scholar] [CrossRef]
- Lottrup, C.; McMahon, B.P.; Ejstrud, P.; Ostapiuk, M.A.; Funch-Jensen, P.; Drewes, A.M. Esophagogastric junction distensibility in hiatus hernia. Dis. Esophagus 2016, 29, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Podboy, A.; Hwang, J.H.; Nguyen, L.A.; Garcia, P.; Zikos, T.A.; Kamal, A.; Triadafilopoulos, G.; Clarke, J.O. Gastric per-oral endoscopic myotomy: Current status and future directions. World J. Gastroenterol. 2019, 25, 2581. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Berean, K.J.; Ha, N.; Chrimes, A.F.; Xu, K.; Grando, D.; Ou, J.Z.; Pillai, N.; Campbell, J.L.; Brkljača, R.; et al. A Human Pilot Trial of Ingestible Electronic Capsules Capable of Sensing Different Gases in the Gut. Nat. Electron. 2018, 1, 79–87. [Google Scholar] [CrossRef]
- Thwaites, P.A.; Yao, C.K.; Maggo, J.; John, J.; Chrimes, A.F.; Burgell, R.E.; Muir, J.G.; Parke, F.C.; So, D.; Kalantar-Zadeh, K.; et al. Comparison of Gastrointestinal Landmarks Using the Gas-Sensing Capsule and Wireless Motility Capsule. Aliment. Pharmacol. Ther. 2022, 56, 1337. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Gao, X.; Zhao, S.; Liu, Y.-N.; Zhang, D.; Zhou, K.; Khanbareh, H.; Chen, W.; Zhang, Y.; Bowen, C. Construction of bio-piezoelectric platforms: From structures and synthesis to applications. Adv. Mater. 2021, 33, 2008452. [Google Scholar] [CrossRef]
- Safaei, M.; Sodano, H.A.; Anton, S.R. A review of energy harvesting using piezoelectric materials: State-of-the-art a decade later. Smart Mater. Struct. 2019, 28, 113001. [Google Scholar] [CrossRef]
- Ali, F.; Raza, W.; Li, X.; Gul, H.; Kim, K.-H. Piezoelectric energy harvesters for biomedical applications. Nano Energy 2019, 57, 879–902. [Google Scholar] [CrossRef]
- Park, D.Y.; Joe, D.J.; Kim, D.H.; Park, H.; Han, J.H.; Jeong, C.K.; Park, H.; Park, J.G.; Joung, B.; Lee, K.J. Self-powered real-time arterial pulse monitoring using ultrathin epidermal piezoelectric sensors. Adv. Mater. 2017, 29, 1702308. [Google Scholar] [CrossRef] [PubMed]
- Dagdeviren, C.; Javid, F.; Joe, P.; von Erlach, T.; Bensel, T.; Wei, Z.; Saxton, S.; Cleveland, C.; Booth, L.; McDonnell, S.; et al. Flexible piezoelectric devices for gastrointestinal motility sensing. Nat. Biomed. Eng. 2017, 1, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Guan, X.; Xu, B.; Gong, J. Hierarchically architected polydopamine modified BaTiO3@ P (VDF-TrFE) nanocomposite fiber mats for flexible piezoelectric nanogenerators and self-powered sensors. Nano Energy 2020, 70, 104516. [Google Scholar] [CrossRef]
- Jung, Y.H.; Hong, S.K.; Wang, H.S.; Han, J.H.; Pham, T.X.; Park, H.; Kim, J.; Kang, S.; Yoo, C.D.; Lee, K.J. Flexible piezoelectric acoustic sensors and machine learning for speech processing. Adv. Mater. 2020, 32, 1904020. [Google Scholar] [CrossRef] [PubMed]
- Sony, S.; Laventure, S.; Sadhu, A. A literature review of next-generation smart sensing technology in structural health monitoring. Struct. Control. Health Monit. 2019, 26, e2321. [Google Scholar] [CrossRef]
- Ventsel, E.; Krauthammer, T.; Carrera, E. Thin plates and shells: Theory, analysis, and applications. Appl. Mech. Rev. 2002, 55, B72–B73. [Google Scholar] [CrossRef]
- Calladine, C.R. The theory of thin shell structures 1888–1988. Proc. Inst. Mech. Eng. Part A Power Process Eng. 1988, 202, 141–149. [Google Scholar] [CrossRef]
- Sumelka, W.; Blaszczyk, T.; Liebold, C. Fractional Euler–Bernoulli beams: Theory, numerical study and experimental validation. Eur. J. Mech. -A/Solids 2015, 54, 243–251. [Google Scholar] [CrossRef]
- Masud, M.; Srivastava, A.K.L. Review on Euler-Bernoulli Beam subjected to Partially Distributed Load. J. Frankl. Inst. 2021, 257, 123. [Google Scholar]
- Effinger, H.; Grossmann, S. Static structure function of turbulent flow from the Navier-Stokes equations. Z. Für Phys. B Condens. Matter 1987, 66, 289–304. [Google Scholar] [CrossRef]
- de Frutos, J.; García-Archilla, B.; Novo, J. Static two-grid mixed finite-element approximations to the Navier-Stokes equations. J. Sci. Comput. 2012, 52, 619–637. [Google Scholar] [CrossRef][Green Version]
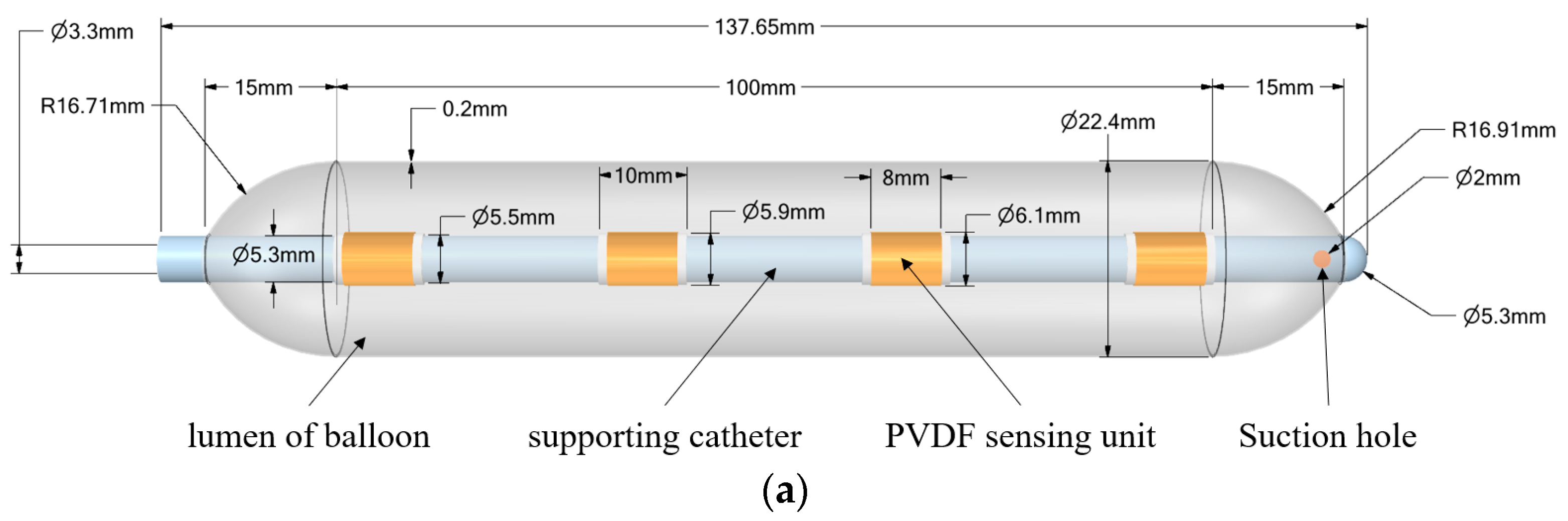
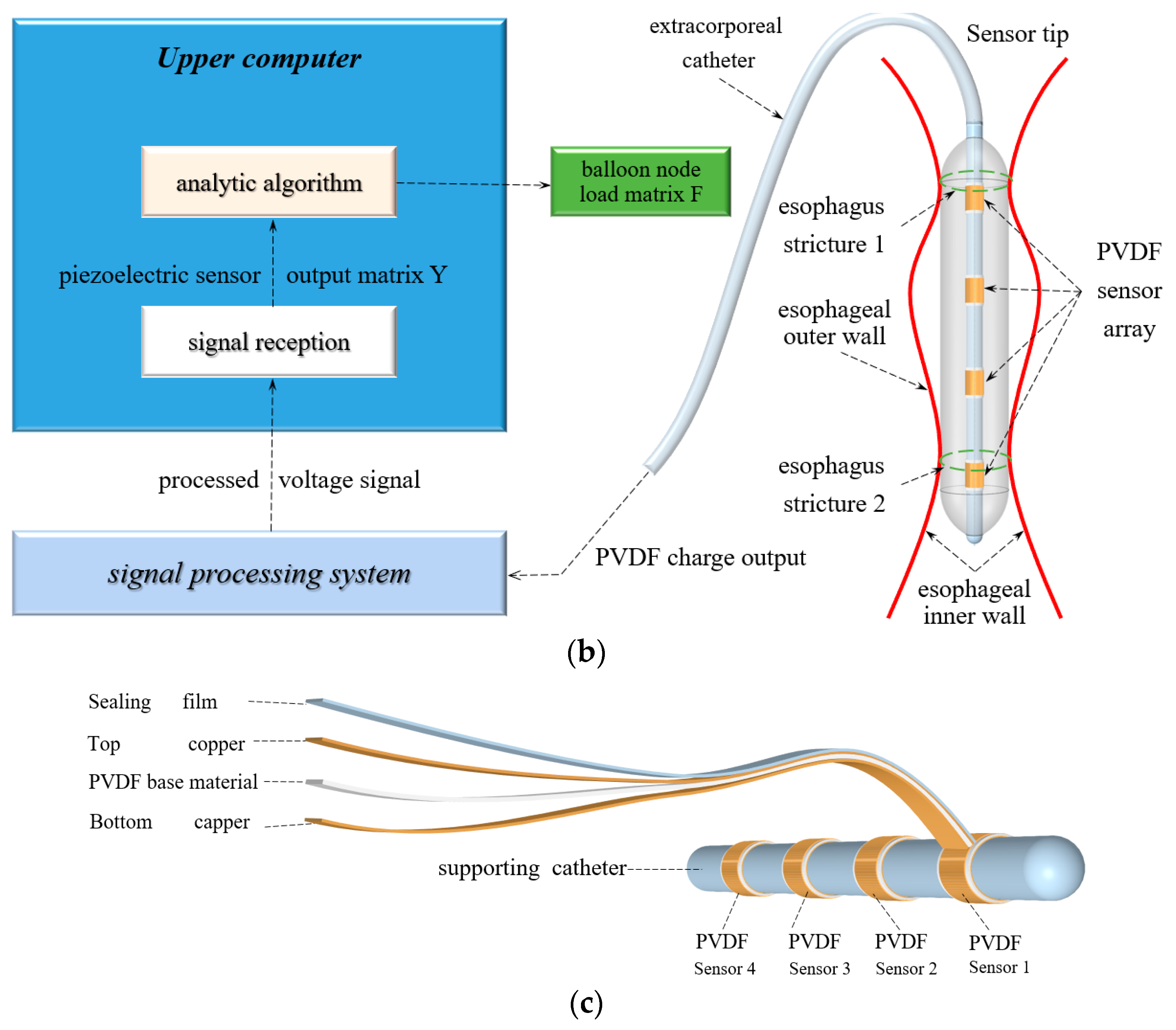


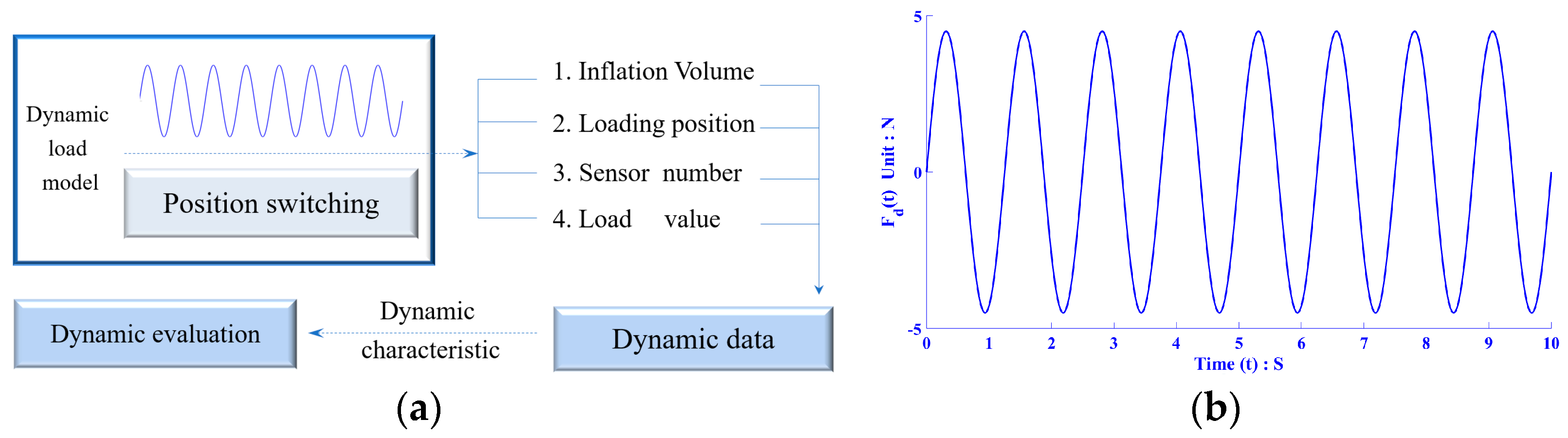
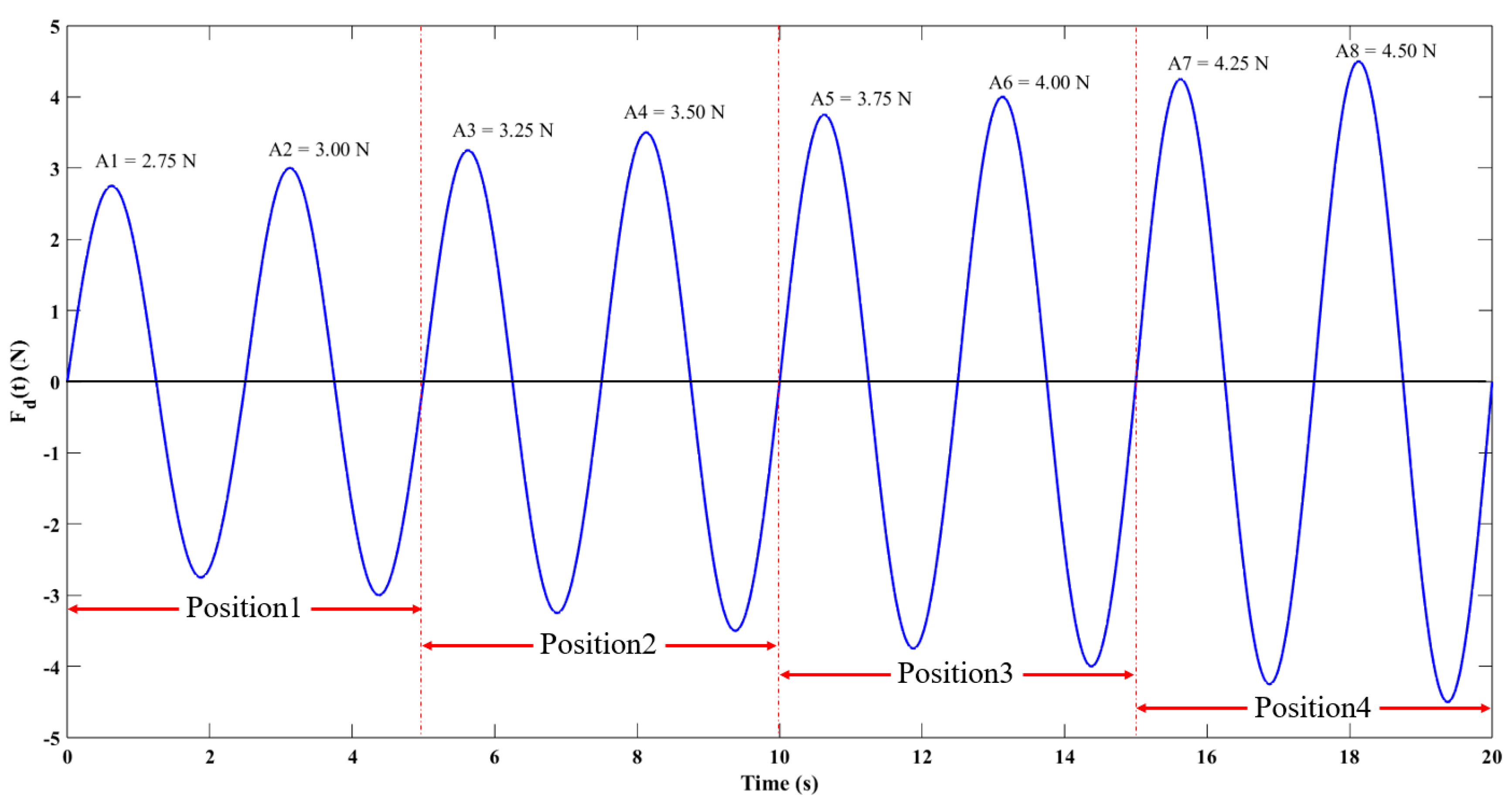
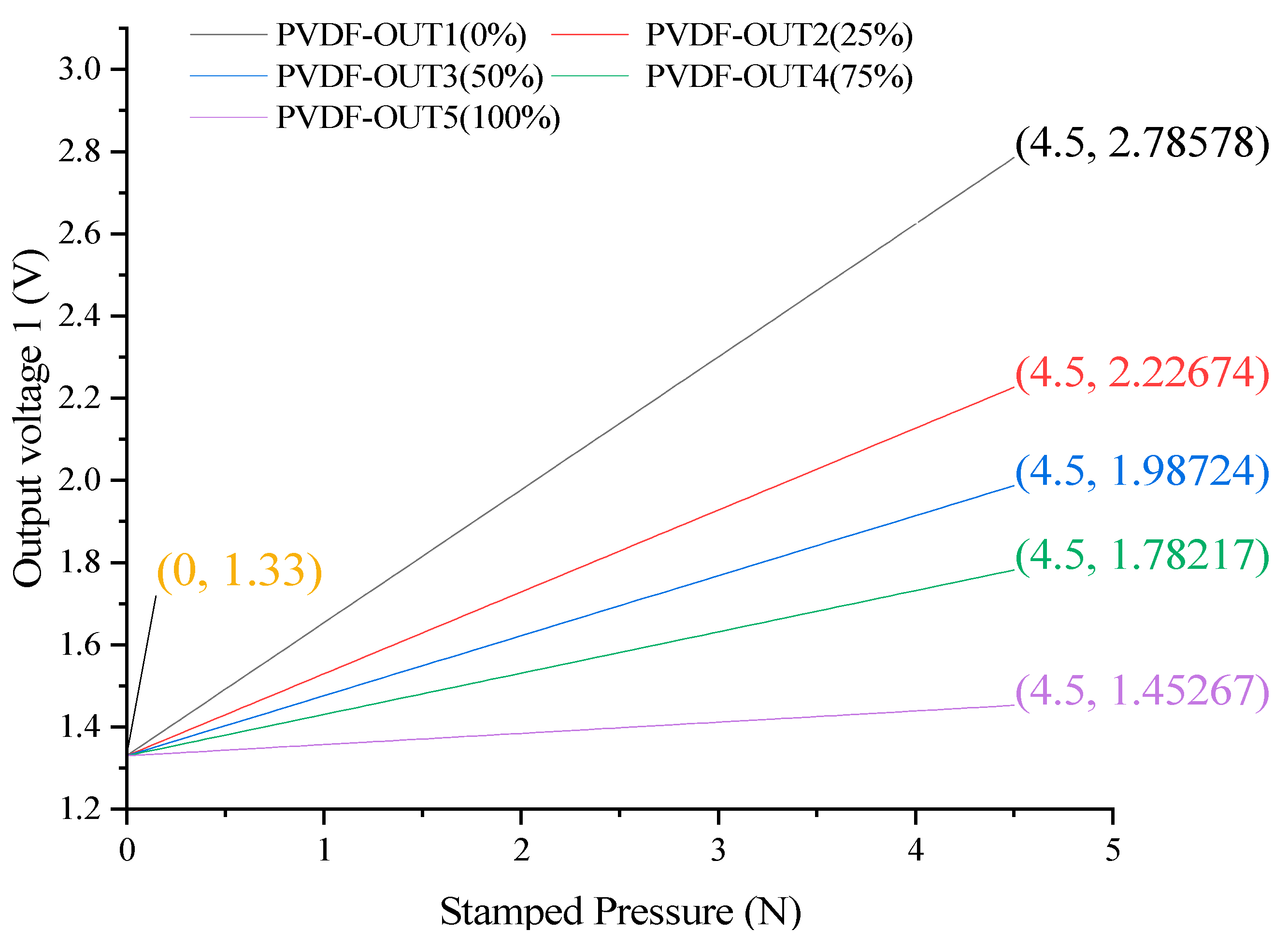
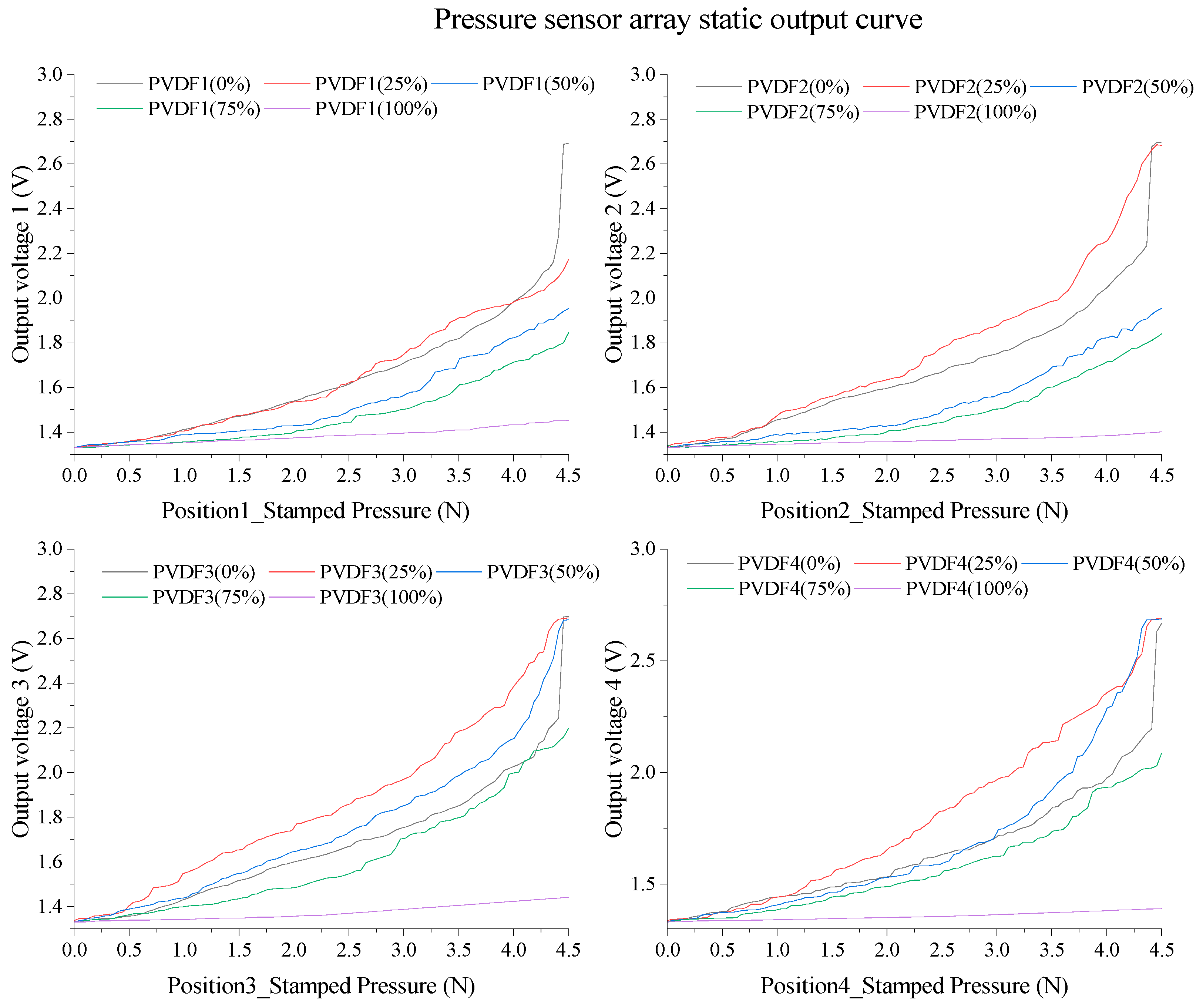
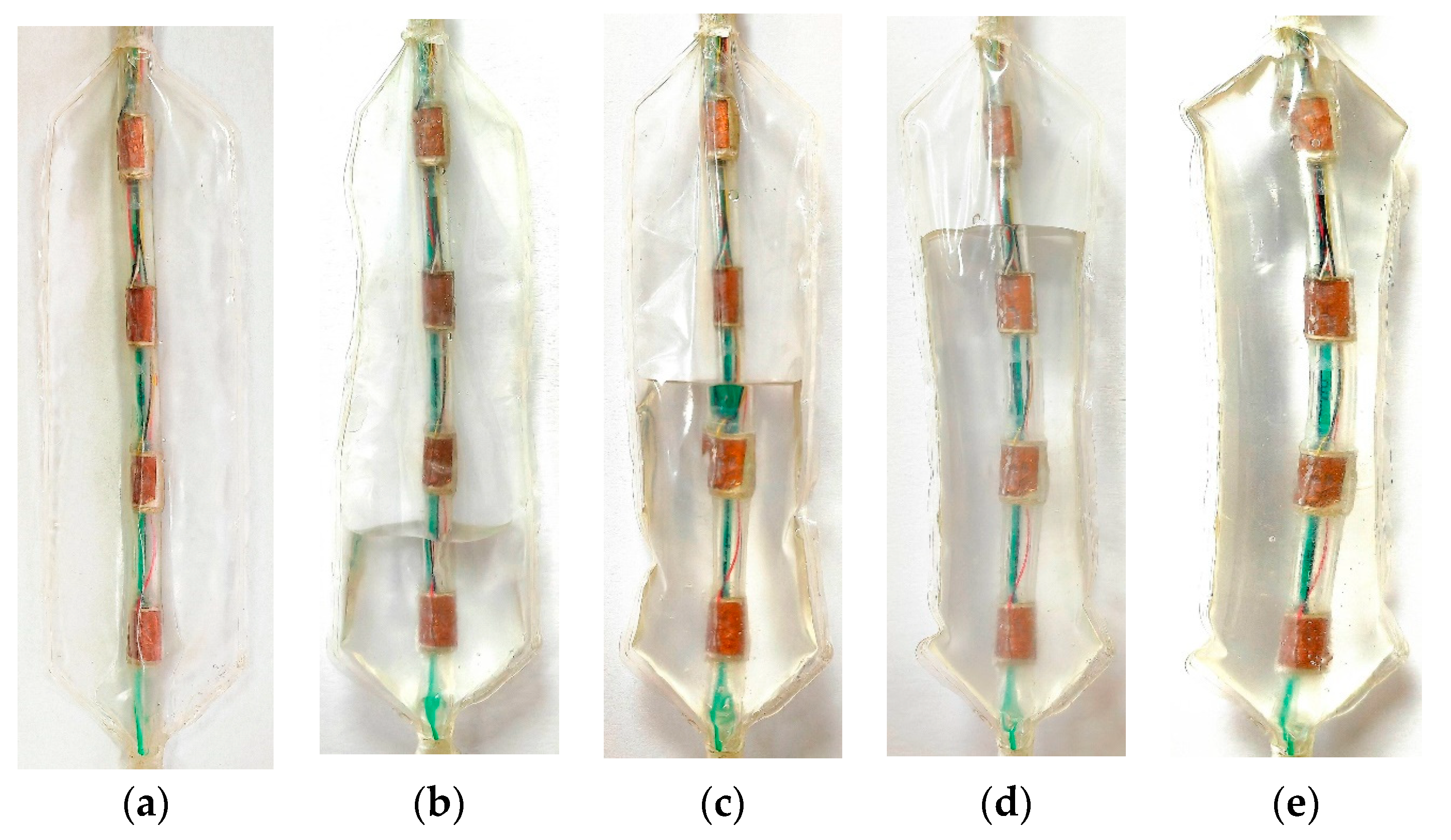

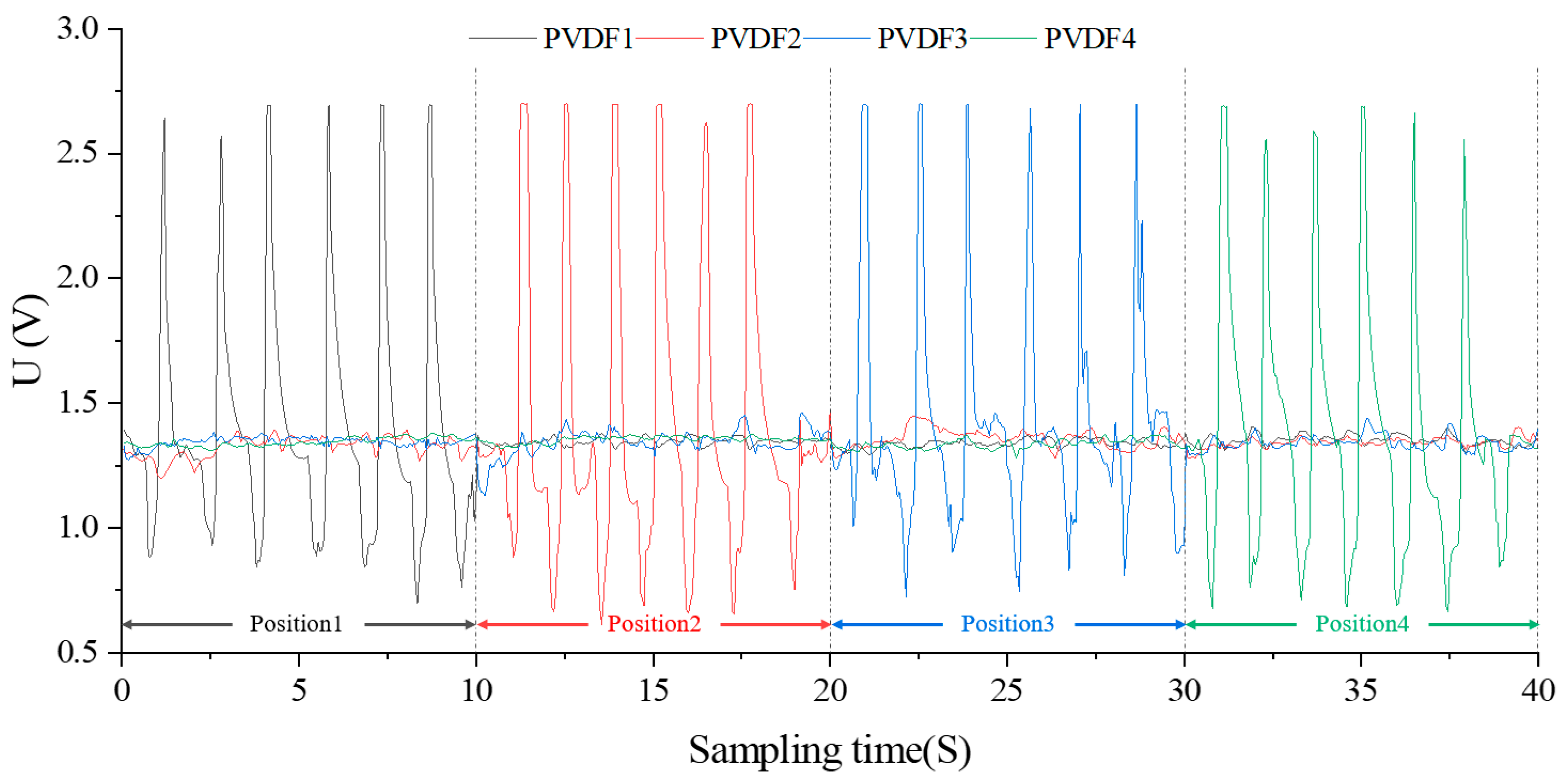




| Parameter\Filling Degree | 0% | 25% | 50% | 75% | 100% |
|---|---|---|---|---|---|
| 1. Elasticity modulus (E, MPa) | 20 | 20 | 20 | 20 | 20 |
| 2. Calculated diameter of balloon (R, mm) | 0.5 | 5.6 | 11.2 | 16.8 | 22.2 |
| 3. Balloon thickness (h, mm) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| 4. Poisson’s Ratio (v, \) | 0.35 | 0.35 | 0.35 | 0.35 | 0.35 |
| 5. Diameter of load surface (dn, mm) | 15 | 15 | 15 | 15 | 15 |
| 6. Inner tube radius (Ri, mm) | 3.3 | 3.3 | 3.3 | 3.3 | 3.3 |
| 7. Outer tube radius(Ro, mm) | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| 8. Distance to the central axis of the balloon membrane in the filled state (d, mm) | 45 | 45 | 45 | 45 | 45 |
| 9. Tube cross-sectional area (A, mm2) | 11.07 | 11.07 | 11.07 | 11.07 | 11.07 |
| 10. Volume modulus (K, MPa) | 2200 | 2200 | 2200 | 2200 | 2200 |
| 11. Fluid dynamic viscosity (μ, cP) | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 |
| 12. Piezoelectric coefficient (g31, mV/N) | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| 13. PVDF surface area(s, cm2) | 1 | 1 | 1 | 1 | 1 |
| 14. PVDF Zero Input Response (PVDF0, V) | 1.33 | 1.33 | 1.33 | 1.33 | 1.33 |
| Steady-State Performance\Filling Degree | 0% | 25% | 50% | 75% | 100% |
|---|---|---|---|---|---|
| 1. Linearity_PVDF1(LD_P1, %) | 29.59382 | 36.4666 | 46.11891 | 44.44342 | 1.38517 |
| Sensitivity_PVDF1(SC_P1, V/N) | 0.18967 | 0.18496 | 0.13494 | 0.10848 | 0.02607 |
| Zero-input_PVDF1(PVDF0_P1, V) | 1.33055 | 1.33015 | 1.33096 | 1.33015 | 1.33015 |
| 2. Linearity_PVDF2(LD_P2, %) | 27.63658 | 21.69366 | 46.32882 | 43.31854 | 4.02154 |
| Sensitivity_PVDF2(SC_P2, V/N)) | 0.19472 | 0.27715 | 0.13105 | 0.10608 | 0.01425 |
| Zero-input_PVDF2(PVDF0_P2, V) | 1.33203 | 1.33337 | 1.33305 | 1.34124 | 1.33015 |
| 3. Linearity_PVDF3(LD_P3, %) | 28.31165 | 15.49573 | 20.0427 | 25.09424 | 2.12105 |
| Sensitivity_PVDF3(SC_P3, V/N)) | 0.19786 | 0.29016 | 0.26229 | 0.18705 | 0.02494 |
| Zero-input_PVDF3(PVDF0_P3, V) | 1.33337 | 1.3374 | 1.33096 | 1.33096 | 1.33015 |
| 4. Linearity_PVDF4(LD_P4, %) | 30.09618 | 16.30085 | 23.40208 | 30.11328 | 4.45227 |
| Sensitivity_PVDF4(SC_P4, V/N)) | 0.19206 | 0.29136 | 0.28059 | 0.16042 | 0.01287 |
| Zero-input_PVDF4(PVDF0_P4, V) | 1.33176 | 1.3374 | 1.33176 | 1.33257 | 1.33096 |
| Loading Condition\Filling Degree | 0% | 25% | 50% | 75% | 100% |
|---|---|---|---|---|---|
| 1. Pressure_2 times (%) | 0.81545 | 0.94521 | 1.10525 | 1.21594 | \ |
| Pressure_3 times (%) | 0.99452 | 1.08452 | 1.18752 | 1.28751 | \ |
| Pressure_4 times (%) | 1.23015 | 1.43541 | 1.59627 | 1.65873 | \ |
| Pressure_5 times (%) | 1.69854 | 1.85463 | 1.93309 | 2.10548 | \ |
| 2. Tensile_2 times (%) | 1.56248 | 1.86326 | 1.96247 | 2.05478 | \ |
| Tensile_3 times (%) | 1.76236 | 1.89631 | 1.99632 | 2.18236 | \ |
| Tensile_4 times (%) | 1.89544 | 1.93325 | 2.16548 | 2.36514 | \ |
| Tensile_5 times (%) | 1.95421 | 2.15659 | 2.35623 | 2.59874 | \ |
| 3. Torsion_2 times (%) | 1.71219 | 1.89654 | 1.96587 | 2.16594 | \ |
| Torsion_3 times (%) | 1.90548 | 1.95631 | 2.14856 | 2.23658 | \ |
| Torsion_4 times (%) | 2.10585 | 2.15698 | 2.19658 | 2.37892 | \ |
| Torsion_5 times (%) | 2.43289 | 2.56612 | 2.69878 | 2.72364 | \ |
| Temperature\Filling Degree | 0% | 25% | 50% | 75% | 100% |
|---|---|---|---|---|---|
| 1. Temperature _10 °C (%) | 2.41202 | 1.93325 | 2.16548 | 2.36514 | \ |
| 2. Temperature _12 °C (%) | 2.25456 | 1.89631 | 1.99632 | 2.18236 | \ |
| 3. Temperature _14 °C (%) | 2.19455 | 1.86326 | 1.96247 | 2.05478 | \ |
| 4. Temperature _16 °C (%) | 2.11483 | 2.19878 | 2.30154 | 2.41254 | \ |
| 5. Temperature _18 °C (%) | 1.98457 | 2.15664 | 2.18965 | 2.32549 | \ |
| 6. Temperature _20 °C (%) | 1.82365 | 1.92345 | 1.98544 | 2.08934 | \ |
| 7. Temperature _22 °C (%) | 1.65452 | 1.78953 | 1.85412 | 1.92302 | \ |
| 8. Temperature _24 °C (%) | 0.659514 | 0.79862 | 0.84512 | 0.98754 | \ |
| 9. Temperature _26 °C (%) | 1.14854 | 1.23144 | 1.25478 | 1.28656 | \ |
| 10. Temperature _28 °C (%) | 1.28563 | 1.37894 | 1.48951 | 1.56334 | \ |
| 11. Temperature _30 °C (%) | 1.36578 | 1.54872 | 1.66891 | 1.89541 | \ |
| 12. Temperature _32 °C (%) | 1.64214 | 1.89651 | 1.99842 | 2.14523 | \ |
| 13. Temperature _34 °C (%) | 1.85415 | 2.05998 | 2.25486 | 2.32786 | \ |
| 14. Temperature _36 °C (%) | 2.08654 | 2.30154 | 2.48965 | 2.55483 | \ |
| 15. Temperature _38 °C (%) | 2.28124 | 2.51243 | 2.64877 | 2.89654 | \ |
| 16. Temperature _40 °C (%) | 2.68872 | 2.87541 | 2.98545 | 3.15568 | \ |
| Times of Repetition\Filling Degree | 0% | 25% | 50% | 75% | 100% |
|---|---|---|---|---|---|
| 1. 1 time (%) | 4.23124 | 4.92346 | 5.25442 | 5.85621 | \ |
| 2. 2 times (%) | 3.86042 | 4.56211 | 4.98847 | 5.13234 | \ |
| 3. 4 times (%) | 3.24427 | 3.98551 | 4.26591 | 4.86214 | \ |
| 4. 8 times (%) | 2.87563 | 3.15478 | 3.45337 | 4.23668 | \ |
| 5. 16 times (%) | 2.12567 | 2.56448 | 2.89314 | 3.54120 | \ |
| 6. 32 times (%) | 1.42588 | 1.59846 | 2.04518 | 2.13354 | \ |
| 7. 64 times (%) | \ | \ | \ | \ | \ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ran, P.; Li, M.; Zhang, K.; Sun, D.; Lai, Y.; Liu, W.; Zhong, Y.; Li, Z. Development and Evaluation of a Flexible PVDF-Based Balloon Sensor for Detecting Mechanical Forces at Key Esophageal Nodes in Esophageal Motility Disorders. Biosensors 2023, 13, 791. https://doi.org/10.3390/bios13080791
Ran P, Li M, Zhang K, Sun D, Lai Y, Liu W, Zhong Y, Li Z. Development and Evaluation of a Flexible PVDF-Based Balloon Sensor for Detecting Mechanical Forces at Key Esophageal Nodes in Esophageal Motility Disorders. Biosensors. 2023; 13(8):791. https://doi.org/10.3390/bios13080791
Chicago/Turabian StyleRan, Peng, Minchuan Li, Kunlin Zhang, Daming Sun, Yingbing Lai, Wei Liu, Ying Zhong, and Zhangyong Li. 2023. "Development and Evaluation of a Flexible PVDF-Based Balloon Sensor for Detecting Mechanical Forces at Key Esophageal Nodes in Esophageal Motility Disorders" Biosensors 13, no. 8: 791. https://doi.org/10.3390/bios13080791
APA StyleRan, P., Li, M., Zhang, K., Sun, D., Lai, Y., Liu, W., Zhong, Y., & Li, Z. (2023). Development and Evaluation of a Flexible PVDF-Based Balloon Sensor for Detecting Mechanical Forces at Key Esophageal Nodes in Esophageal Motility Disorders. Biosensors, 13(8), 791. https://doi.org/10.3390/bios13080791





