Application of Highly Sensitive Immunosensor Based on Optical Waveguide Light-Mode Spectroscopy (OWLS) Technique for the Detection of the Herbicide Active Ingredient Glyphosate
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. OWLS Instrument Set-Up
2.3. Functionalisation of the Sensor Surface
2.4. Derivatisation of the Glyphosate Standard
2.5. Fluorescent ELISA
3. Results and Discussion
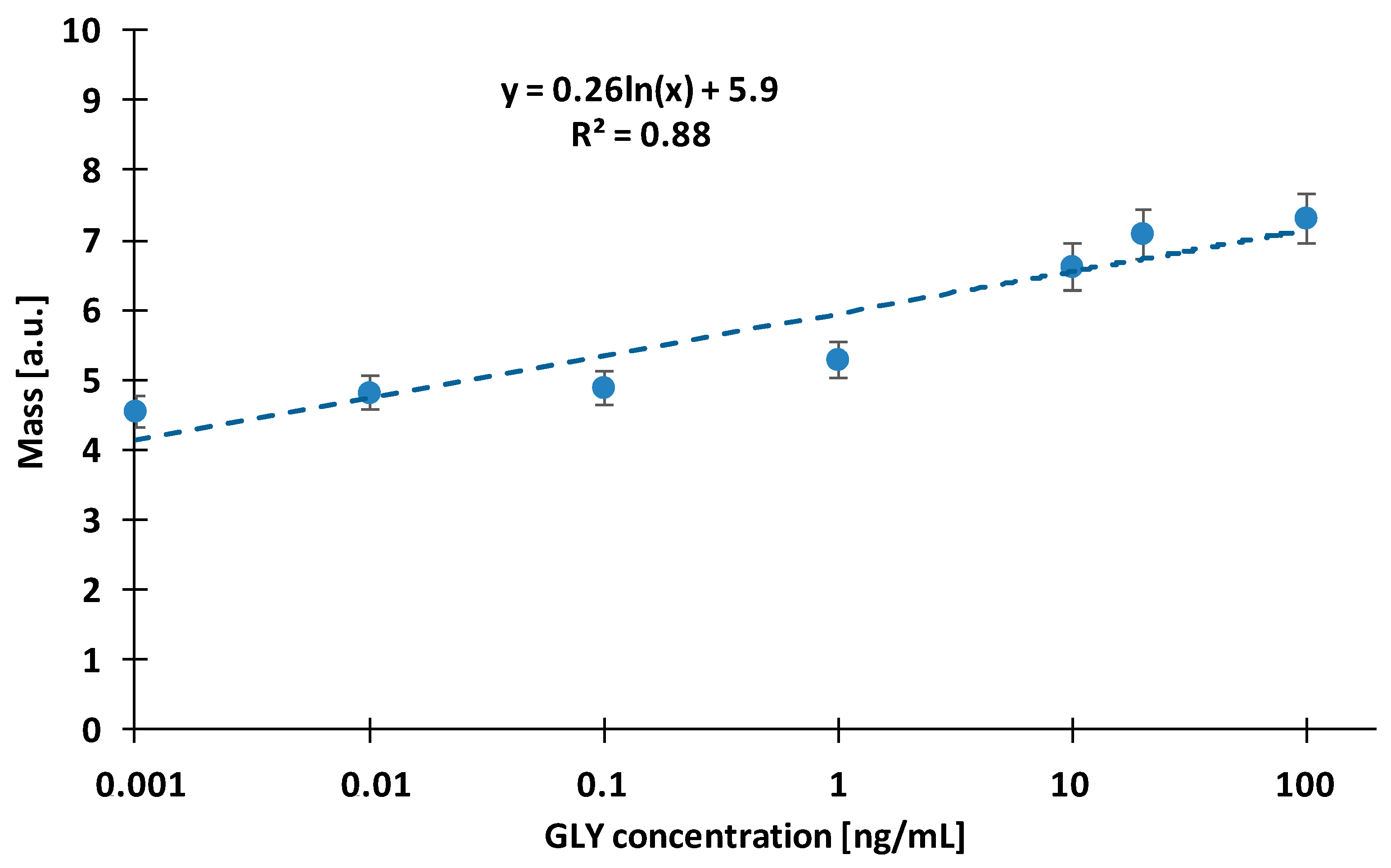
3.1. Direct Measurement of Glyphosate
3.2. Competitive Immunosensor
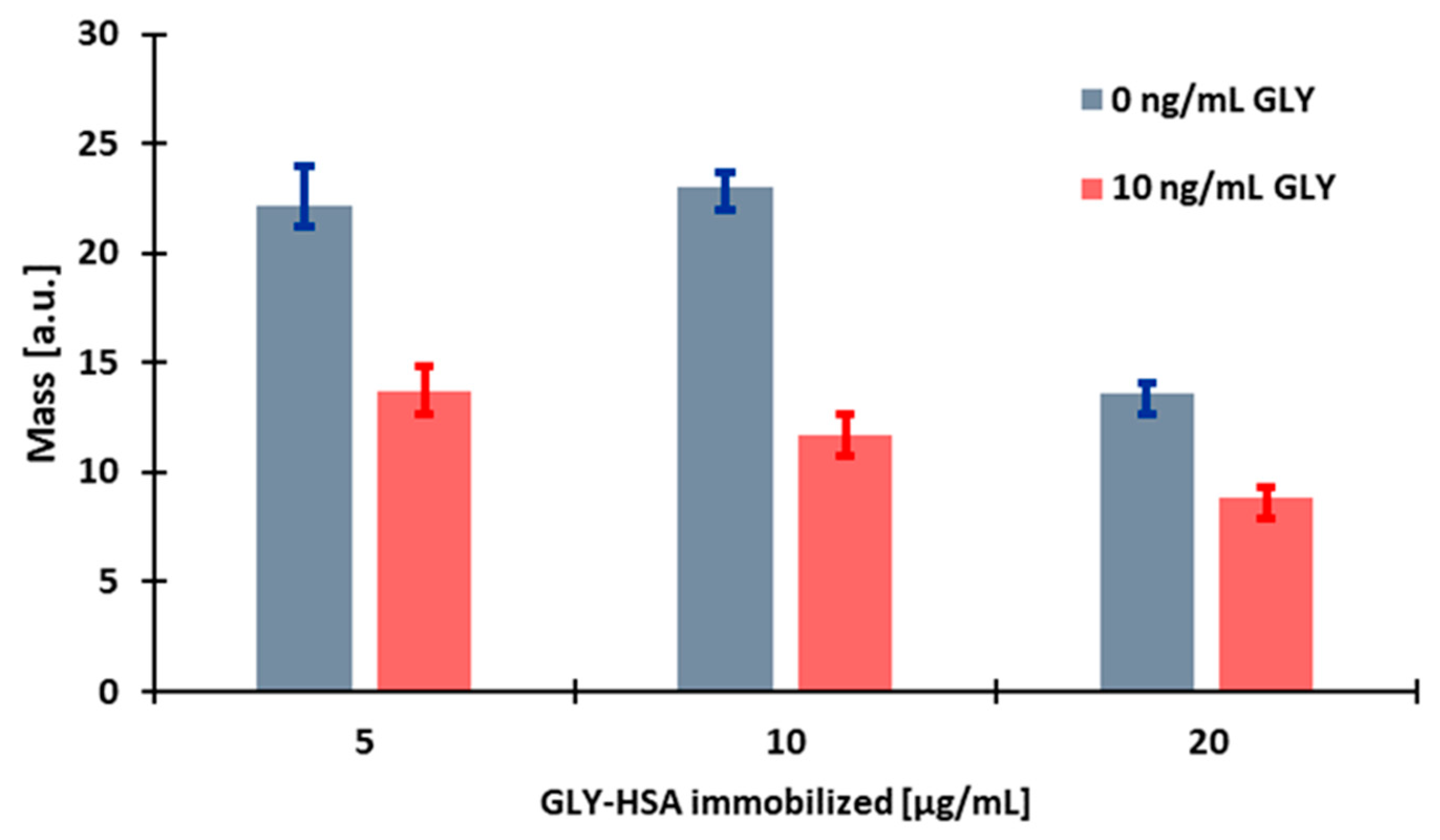
3.2.1. Determination of the Optimal Immobilised Antigen Conjugate Concentration
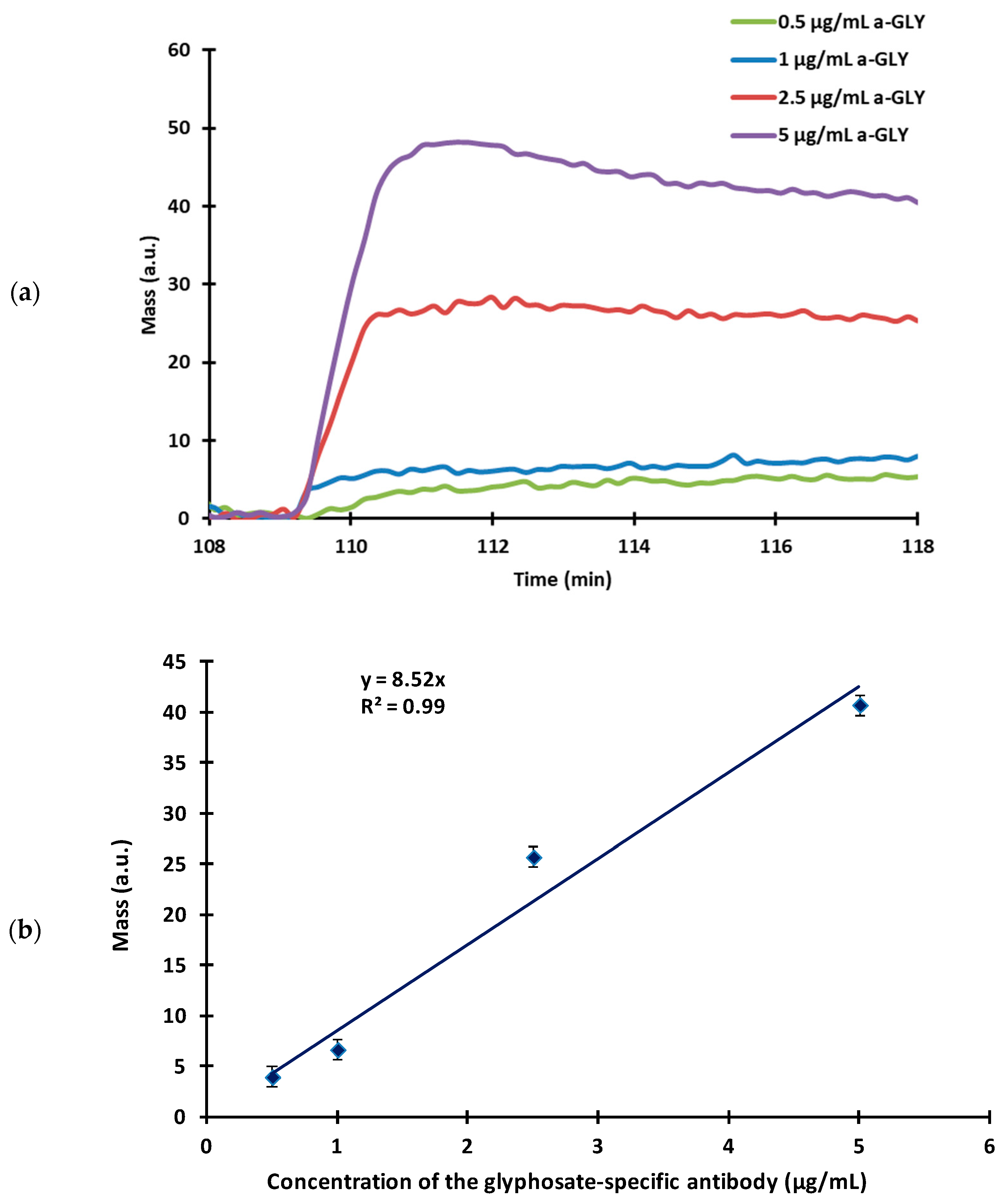
3.2.2. Determination of the Amount of the Applied Antibody
3.3. Cross-Reactivity of the Glyphosate-Specific Antibody
3.4. Determination of GLY Concentration in Surface Water Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duke, S.O.; Powles, S.B. Glyphosate: A Once-in-a-Century Herbicide. Pest Manag. Sci. 2008, 64, 319–325. [Google Scholar] [CrossRef]
- Antier, C.; Andersson, R.; Auskalnienė, O.; Barić, K.; Baret, P.; Besenhofer, G.; Calha, I.; Carrola Dos Santos, S.; De Cauwer, B.; Chachalis, D.; et al. A Survey on the Uses of Glyphosate in European Countries; INRAE: Paris, France, 2020. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Datta, S.; Wani, A.B.; Dhanjal, D.S.; Romero, R.; Singh, J. Glyphosate Uptake, Translocation, Resistance Emergence in Crops, Analytical Monitoring, Toxicity and Degradation: A Review. Environ. Chem. Lett. 2020, 18, 663–702. [Google Scholar] [CrossRef]
- Székács, I.; Fejes, Á.; Klátyik, S.; Takács, E.; Patkó, D.; Pomóthy, J.; Mörtl, M.; Horváth, R.; Madarász, E.; Darvas, B.; et al. Environmental and Toxicological Impacts of Glyphosate with Its Formulating Adjuvant. Int. J. Biol. Vet. Agric. Food Eng. 2014, 8, 212–218. [Google Scholar]
- Székács, A.; Darvas, B.; Székács, A.; Darvas, B. Forty Years with Glyphosate; IntechOpen: London, UK, 2012; Volume 14, ISBN 978-953-307-803-8. [Google Scholar]
- Vereecken, H. Mobility and Leaching of Glyphosate: A Review. Pest Manag. Sci. 2005, 61, 1139–1151. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Meyer, M.T.; Kuivila, K.M.; Dietze, J.E. Glyphosate and Its Degradation Product AMPA Occur Frequently and Widely in U.S. Soils, Surface Water, Groundwater, and Precipitation. JAWRA J. Am. Water Resour. Assoc. 2014, 50, 275–290. [Google Scholar] [CrossRef]
- Zhan, H.; Feng, Y.; Fan, X.; Chen, S. Recent Advances in Glyphosate Biodegradation. Appl. Microbiol. Biotechnol. 2018, 102, 5033–5043. [Google Scholar] [CrossRef]
- Mörtl, M.; Németh, G.; Juracsek, J.; Darvas, B.; Kamp, L.; Rubio, F.; Székács, A. Determination of Glyphosate Residues in Hungarian Water Samples by Immunoassay. Microchem. J. 2013, 107, 143–151. [Google Scholar] [CrossRef]
- Peruzzo, P.J.; Porta, A.A.; Ronco, A.E. Levels of Glyphosate in Surface Waters, Sediments and Soils Associated with Direct Sowing Soybean Cultivation in North Pampasic Region of Argentina. Environ. Pollut. 2008, 156, 61–66. [Google Scholar] [CrossRef]
- Rainio, M.J.; Ruuskanen, S.; Helander, M.; Saikkonen, K.; Saloniemi, I.; Puigbò, P. Adaptation of Bacteria to Glyphosate: A Microevolutionary Perspective of the Enzyme 5-Enolpyruvylshikimate-3-Phosphate Synthase. Environ. Microbiol. Rep. 2021, 13, 309–316. [Google Scholar] [CrossRef]
- Van Bruggen, A.H.C.; He, M.M.; Shin, K.; Mai, V.; Jeong, K.C.; Finckh, M.R.; Morris, J.G. Environmental and Health Effects of the Herbicide Glyphosate. Sci. Total Environ. 2018, 616–617, 255–268. [Google Scholar] [CrossRef]
- Kissane, Z.; Shephard, J.M. The Rise of Glyphosate and New Opportunities for Biosentinel Early-Warning Studies. Conserv. Biol. 2017, 31, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Tresnakova, N.; Stara, A.; Velisek, J. Effects of Glyphosate and Its Metabolite AMPA on Aquatic Organisms. Appl. Sci. 2021, 11, 9004. [Google Scholar] [CrossRef]
- Gill, J.P.K.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate Toxicity for Animals. Environ. Chem. Lett. 2018, 16, 401–426. [Google Scholar] [CrossRef]
- Mesnage, R.; Defarge, N.; Spiroux de Vendômois, J.; Séralini, G.E. Potential Toxic Effects of Glyphosate and Its Commercial Formulations below Regulatory Limits. Food Chem. Toxicol. 2015, 84, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.P.; Antoniou, M.N.; Blumberg, B.; Carroll, L.; Colborn, T.; Everett, L.G.; Hansen, M.; Landrigan, P.J.; Lanphear, B.P.; Mesnage, R.; et al. Concerns Over Use of Glyphosate-Based Herbicides and Risks Associated with Exposures: A Consensus Statement. Environ. Health 2016, 15, 19. [Google Scholar] [CrossRef]
- Rivas-Garcia, T.; Espinosa-Calderón, A.; Hernández-Vázquez, B.; Schwentesius-Rindermann, R. Overview of Environmental and Health Effects Related to Glyphosate Usage. Sustainability 2022, 14, 6868. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Organophosphate Insecticides and Herbicides; IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2017; ISBN 978-92-832-0150-2. [Google Scholar]
- Commission Regulation (EU). No 441/2012 of 24 May 2012 Amending Annexes II and III to Regulation (EC) No 396/2005 of the European Parliament and of the Council as Regards Maximum Residue Levels for Bifenazate, Bifenthrin, Boscalid, Cadusafos, Chlorantraniliprole, Chlorothalonil, Clothianidin, Cyproconazole, Deltamethrin, Dicamba, Difenoconazole, Dinocap, Etoxazole, Fenpyroximate, Flubendiamide, Fludioxonil, Glyphosate, Metalaxyl-M, Meptyldinocap, Novaluron, Thiamethoxam, and Triazophos in or on Certain Products Text with EEA Relevance; Commission Regulation (EU): Brussels, Belgium, 2012; Volume 135. [Google Scholar]
- Valle, A.L.; Mello, F.C.C.; Alves-Balvedi, R.P.; Rodrigues, L.P.; Goulart, L.R. Glyphosate Detection: Methods, Needs and Challenges. Environ. Chem. Lett. 2019, 17, 291–317. [Google Scholar] [CrossRef]
- Qian, K.; Tang, T.; Shi, T.; Wang, F.; Li, J.; Cao, Y. Residue Determination of Glyphosate in Environmental Water Samples with High-Performance Liquid Chromatography and UV Detection after Derivatization with 4-Chloro-3,5-Dinitrobenzotrifluoride. Anal. Chim. Acta 2009, 635, 222–226. [Google Scholar] [CrossRef]
- Steinborn, A.; Alder, L.; Michalski, B.; Zomer, P.; Bendig, P.; Martinez, S.A.; Mol, H.G.J.; Class, T.J.; Costa Pinheiro, N. Determination of Glyphosate Levels in Breast Milk Samples from Germany by LC-MS/MS and GC-MS/MS. J. Agric. Food Chem. 2016, 64, 1414–1421. [Google Scholar] [CrossRef]
- Ibáñez, M.; Pozo, Ó.J.; Sancho, J.V.; López, F.J.; Hernández, F. Residue Determination of Glyphosate, Glufosinate and Aminomethylphosphonic Acid in Water and Soil Samples by Liquid Chromatography Coupled to Electrospray Tandem Mass Spectrometry. J. Chromatogr. A 2005, 1081, 145–155. [Google Scholar] [CrossRef]
- Amberger, M.A.; Schröder, M.; Kuballa, J.; Jantzen, E. Direct Determination of Glyphosate, Aminomethylphosphonic Acid and Glufosinate in Food Samples with Ion Chromatography Coupled to Electrospray Ionization Tandem Mass Spectrometry. J. Chromatogr. A 2023, 1687, 463631. [Google Scholar] [CrossRef]
- Reynoso, E.C.; Torres, E.; Bettazzi, F.; Palchetti, I. Trends and Perspectives in Immunosensors for Determination of Currently-Used Pesticides: The Case of Glyphosate, Organophosphates, and Neonicotinoids. Biosensors 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Sequeira, R.; Starbird-Pérez, R.; Rojas-Carillo, O.; Vargas-Villalobos, S. What Are the Main Sensor Methods for Quantifying Pesticides in Agricultural Activities? A Review. Molecules 2019, 24, 2659. [Google Scholar] [CrossRef] [PubMed]
- Takács, E.; Gémes, B.; Szendrei, F.; Keszei, C.; Barócsi, A.; Lenk, S.; Domján, L.; Mörtl, M.; Székács, A. Utilization of a Novel Immunofluorescence Instrument Prototype for the Determination of the Herbicide Glyphosate. Molecules 2022, 27, 6514. [Google Scholar] [CrossRef]
- Adányi, N.; Majer-Baranyi, K.; Székács, A. 11—Evanescent Field Effect–Based Nanobiosensors for Agro-Environmental and Food Safety. In Nanobiosensors; Grumezescu, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 429–474. ISBN 978-0-12-804301-1. [Google Scholar]
- Erdélyi, K.; Frutos, A.G.; Ramsden, J.J.; Szendrő, I.; Voirin, G. Grating-Based Optical Biosensors. In Handbook of Biosensors and Biochips; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2008; ISBN 978-0-470-06156-5. [Google Scholar]
- Székács, A.; Trummer, N.; Adányi, N.; Váradi, M.; Szendrő, I. Development of a Non-Labeled Immunosensor for the Herbicide Trifluralin via Optical Waveguide Lightmode Spectroscopic Detection. Anal. Chim. Acta 2003, 487, 31–42. [Google Scholar] [CrossRef]
- Székács, I.; Adányi, N.; Szendrő, I.; Székács, A. Direct and Competitive Optical Grating Immunosensors for Determination of Fusarium Mycotoxin Zearalenone. Toxins 2021, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Majer-Baranyi, K.; Adányi, N.; Nagy, A.; Bukovskaya, O.; Szendrő, I.; Székács, A. Label-Free Immunosensor for Monitoring Vitellogenin as a Biomarker for Exogenous Oestrogen Compounds in Amphibian Species. Int. J. Environ. Anal. Chem. 2015, 95, 481–493. [Google Scholar] [CrossRef]
- Adányi, N.; Váradi, M.; Kim, N.; Szendrö, I. Development of New Immunosensors for Determination of Contaminants in Food. Curr. Appl. Phys. 2006, 6, 279–286. [Google Scholar] [CrossRef]
- Trummer, N.; Adányi, N.; Váradi, M.; Szendrö, I. Modification of the Surface of Integrated Optical Wave-Guide Sensors for Immunosensor Applications. Fresenius J. Anal. Chem. 2001, 371, 21–24. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Lu, M.; Zhang, M.; Yang, C.; Ma, R.; Memon, A.G.; Shi, H.; Qian, Y. An Array Fluorescent Biosensor Based on Planar Waveguide for Multi-Analyte Determination in Water Samples. Sens. Actuators B Chem. 2017, 240, 107–113. [Google Scholar] [CrossRef]
- Tan, J.; Liu, L.; Li, F.; Chen, Z.; Chen, G.Y.; Fang, F.; Guo, J.; He, M.; Zhou, X. Screening of Endocrine Disrupting Potential of Surface Waters via an Affinity-Based Biosensor in a Rural Community in the Yellow River Basin, China. Environ. Sci. Technol. 2022, 56, 14350–14360. [Google Scholar] [CrossRef] [PubMed]
- González-Martínez, M.Á.; Brun, E.M.; Puchades, R.; Maquieira, Á.; Ramsey, K.; Rubio, F. Glyphosate Immunosensor. Application for Water and Soil Analysis. Anal. Chem. 2005, 77, 4219–4227. [Google Scholar] [CrossRef] [PubMed]

| Compound | Nominal Conc. (ng/mL) | Detected Conc. OWLS (ng/mL) | Detected Conc. OWLS (%) | Detected Conc. Fluorescent ELISA (ng/mL) [28] | Detected Conc. Fluorescent ELISA (%) |
|---|---|---|---|---|---|
| Glyphosate * | 100 | 97.6 ± 1.3 | 100 | 99.3 ± 0.8 | 100 |
| 50 | 51.7 ± 2.1 | 100 | 50.4 ± 1.1 | 100 | |
| AMPA * | 6700 | <0.1 | <0.1 | <0.01 | <0.0015 |
| 100 | <0.1 | <0.1 | <0.01 | <0.01 | |
| PMIDA * | 1650 | 0.22 | 0.2 | 0.31 | 0.018 |
| Glycine | 100 | <0.1 | 0.15 | <0.1 | <0.01 |
| Acetylglycine | 100 | <0.1 | 0.15 | <0.1 | <0.01 |
| Sample ID | Spiked Conc. (ng/mL) | Conc. OWLS (ng/mL) | Recovery OWLS (%) | Concentration Fluorescent ELISA (ng/mL) [28] | Recovery Fluorescent ELISA (%) |
|---|---|---|---|---|---|
| 1 | 0 | <0.01 | <0.09 | ||
| 2 | 0 | <0.01 | <0.09 | ||
| 3 | 0 | <0.01 | <0.09 | ||
| 4 | 0.1 | 0.080 ± 0.009 | 80.0 ± 9.0 | 0.112 ± 0.012 | 112.0 ± 12.0 |
| 5 | 1.56 | 1.67 ± 0.24 | 107.1 ± 15.4 | 1.46 ± 0.22 | 93.6 ± 14.1 |
| 6 | 12.5 | 11.8 ± 0.9 | 94.4 ± 7.2 | 14.1 ± 1.04 | 112.8 ± 8.3 |
| 7 | 100 | 106.3 ± 7.6 | 106,3 ± 7.6 | 108.1 ± 6.3 | 108.1 ± 6.3 |
| Method | Linear Measuring Range | LOD | Matrix | Reference |
|---|---|---|---|---|
| HPLC-UV | 0.3–48.5 µg/mL | 0.009 µg/mL | Water | [22] |
| LC-MS/MS | 0–50 ng/mL | 0.5 ng/mL | Breast milk | [23] |
| LC-ESI-MS/MS | 50–500 pg/mL 0.05–0.5 mg/kg | 5 pg/mL 5 µg/kg | Water Soil | [24] |
| LC-ESI-MS/MS | 10–400 µg/kg or 10–800 µg/kg | 0.09–0.8 µg/kg | Apple Mushrooms Grapefruit Linseed Red lentils Wheat | [25] |
| OWLS | 0.01–100 ng/mL | 0.0001 ng/mL | Water | Present study |
| Fluorescent ELISA | 0–100 ng/mL | 0.09 ng/mL | Water | [28] |
| FLIS * | 0–1 ng/mL | 0.021 ng/mL | Water | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majer-Baranyi, K.; Szendrei, F.; Adányi, N.; Székács, A. Application of Highly Sensitive Immunosensor Based on Optical Waveguide Light-Mode Spectroscopy (OWLS) Technique for the Detection of the Herbicide Active Ingredient Glyphosate. Biosensors 2023, 13, 771. https://doi.org/10.3390/bios13080771
Majer-Baranyi K, Szendrei F, Adányi N, Székács A. Application of Highly Sensitive Immunosensor Based on Optical Waveguide Light-Mode Spectroscopy (OWLS) Technique for the Detection of the Herbicide Active Ingredient Glyphosate. Biosensors. 2023; 13(8):771. https://doi.org/10.3390/bios13080771
Chicago/Turabian StyleMajer-Baranyi, Krisztina, Fanni Szendrei, Nóra Adányi, and András Székács. 2023. "Application of Highly Sensitive Immunosensor Based on Optical Waveguide Light-Mode Spectroscopy (OWLS) Technique for the Detection of the Herbicide Active Ingredient Glyphosate" Biosensors 13, no. 8: 771. https://doi.org/10.3390/bios13080771
APA StyleMajer-Baranyi, K., Szendrei, F., Adányi, N., & Székács, A. (2023). Application of Highly Sensitive Immunosensor Based on Optical Waveguide Light-Mode Spectroscopy (OWLS) Technique for the Detection of the Herbicide Active Ingredient Glyphosate. Biosensors, 13(8), 771. https://doi.org/10.3390/bios13080771






