Bisphenol A Imprinted Electrochemical Sensor Based on Graphene Quantum Dots with Boron Functionalized g-C3N4 in Food Samples
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Apparatus
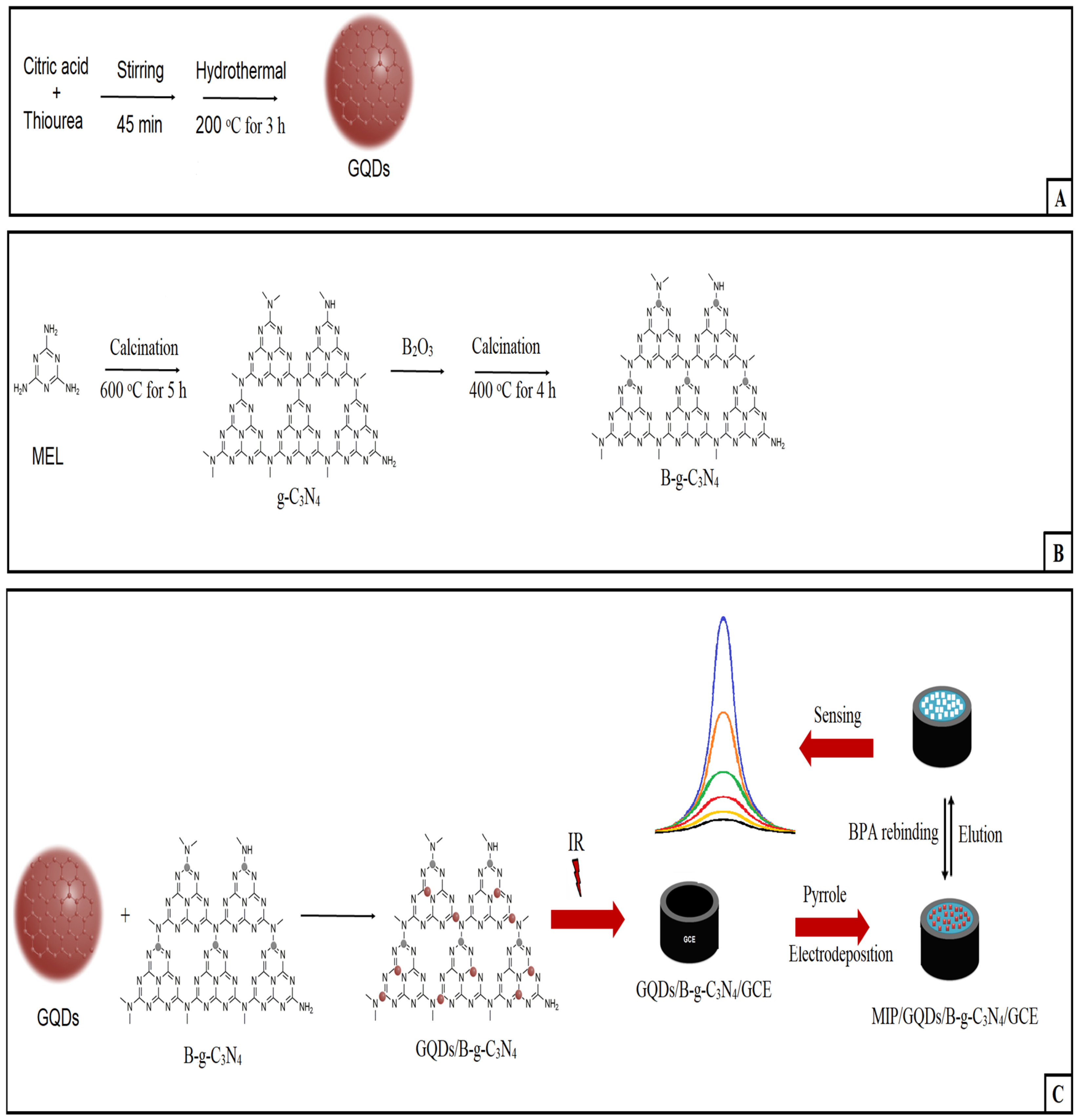
2.2. Production of g-C3N4, B-g-C3N4, and GQDs/B-g-C3N4 Nanocomposite
2.3. Production of GQDs/B-g-C3N4 Modified Glassy Carbon Electrode (GQDs/B-g-C3N4/GCE)
2.4. Development of BPA-Imprinted Sensor and BPA Removal
2.5. Sample Preparation
3. Results and Discussion
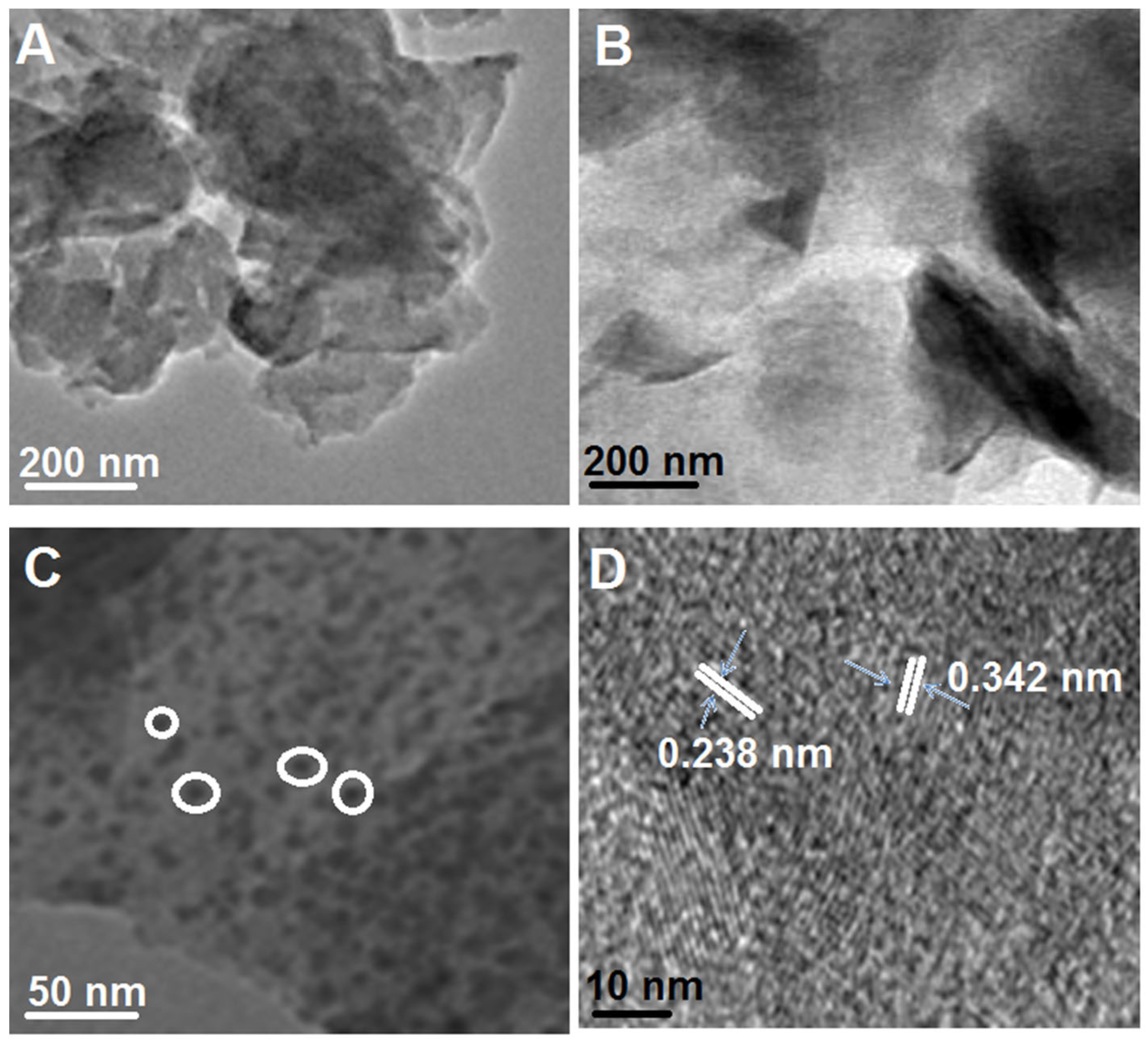
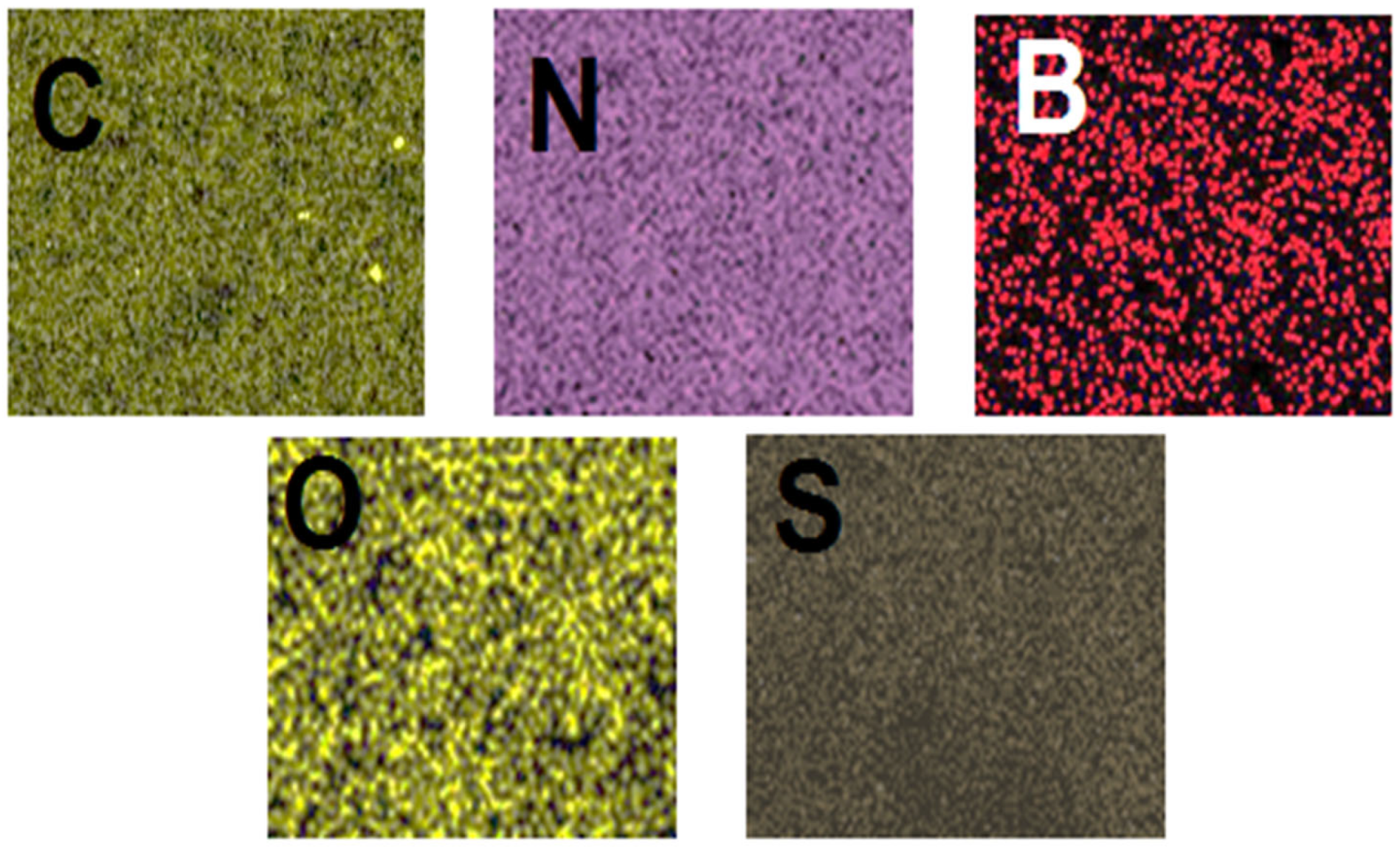
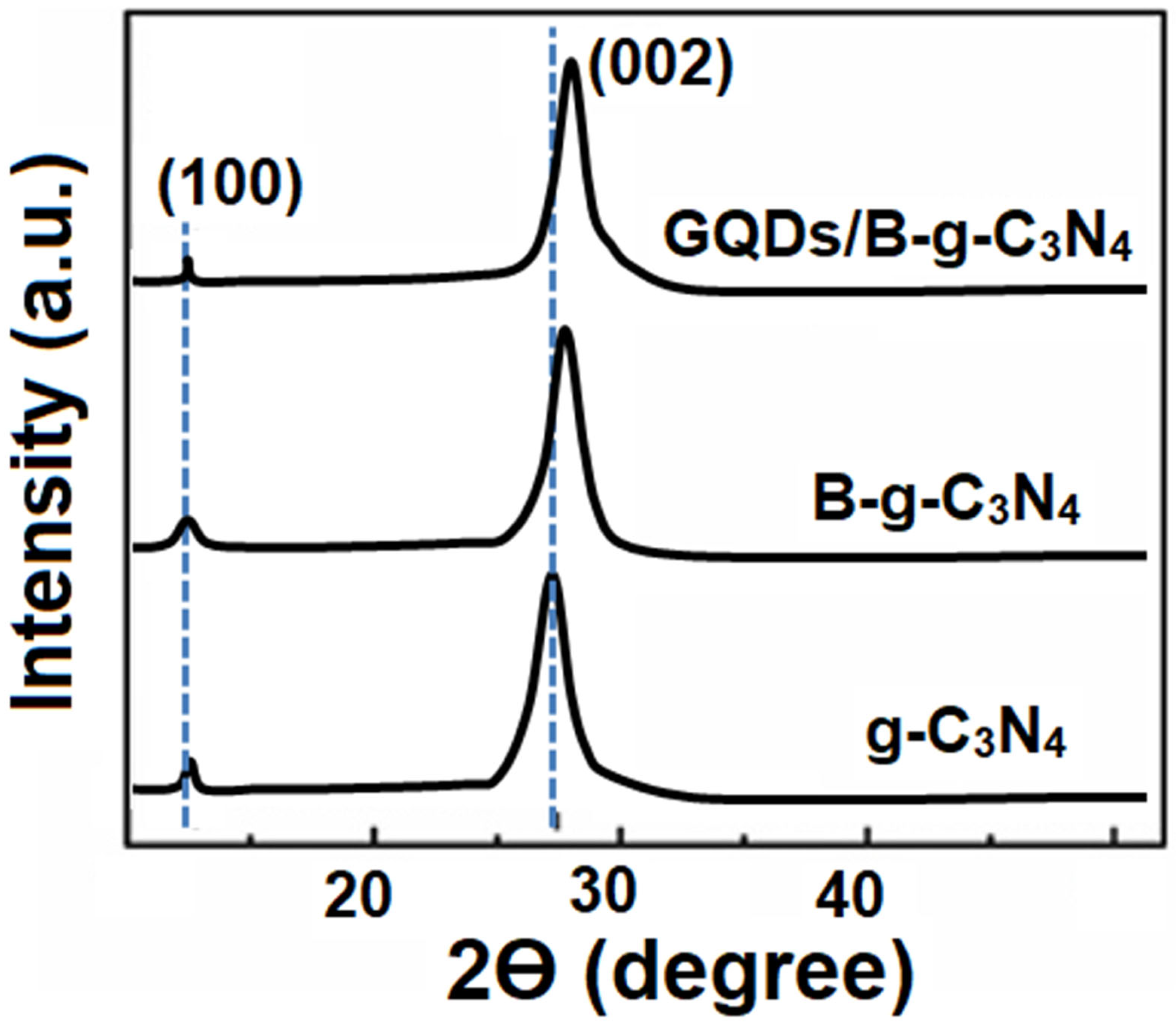
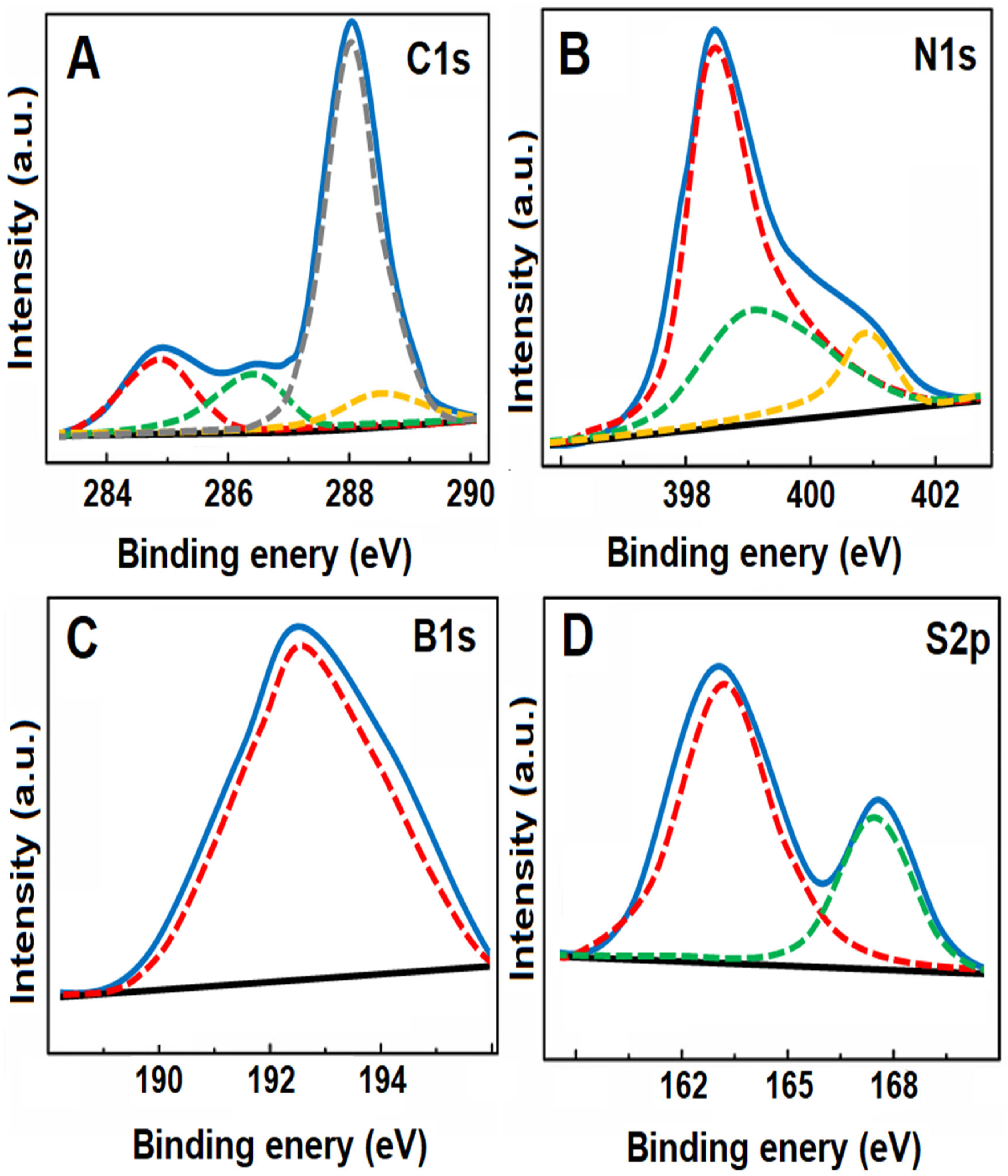
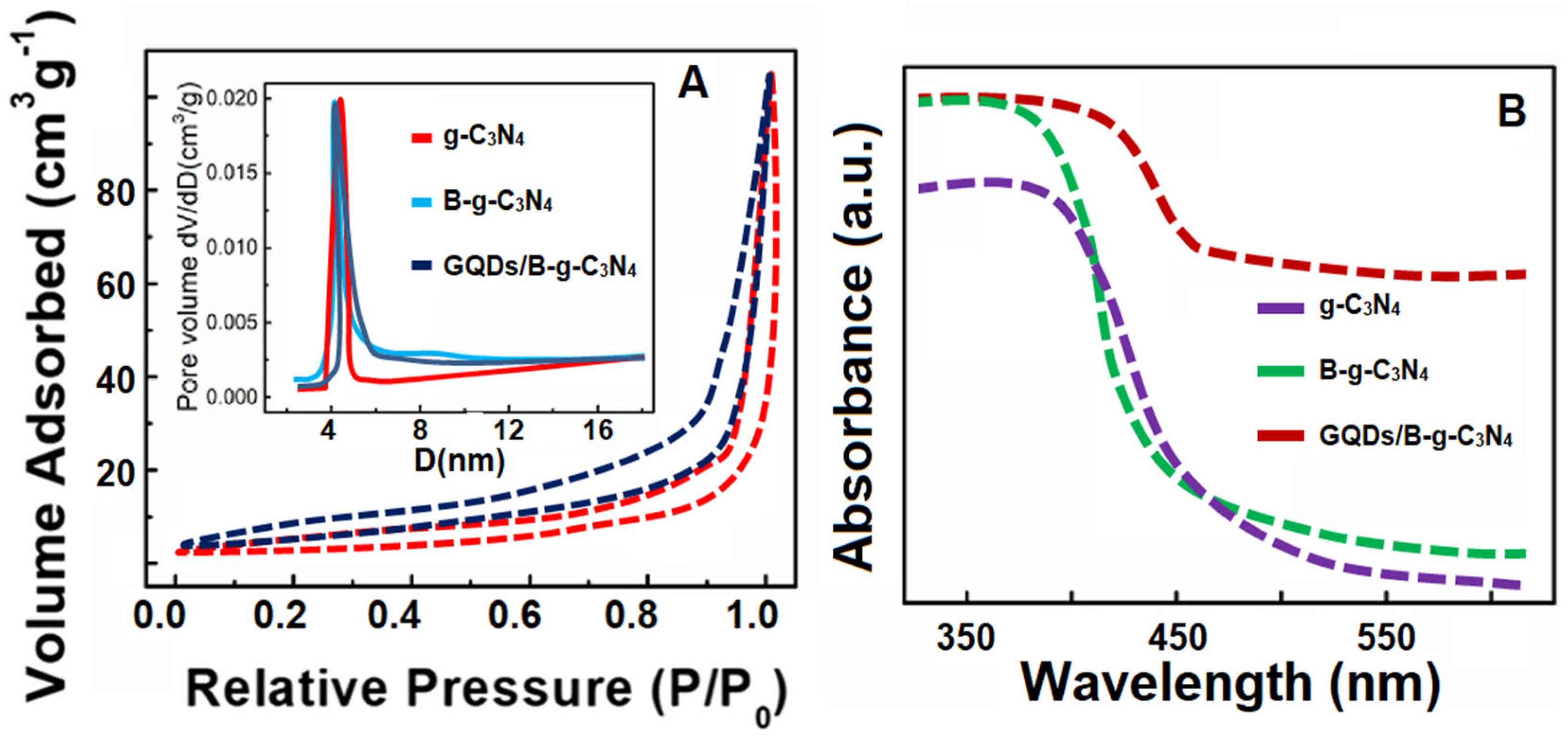
3.1. Characterizations of g-C3N4, B-g-C3N4, and GQDs/B-g-C3N4 Nanocomposite
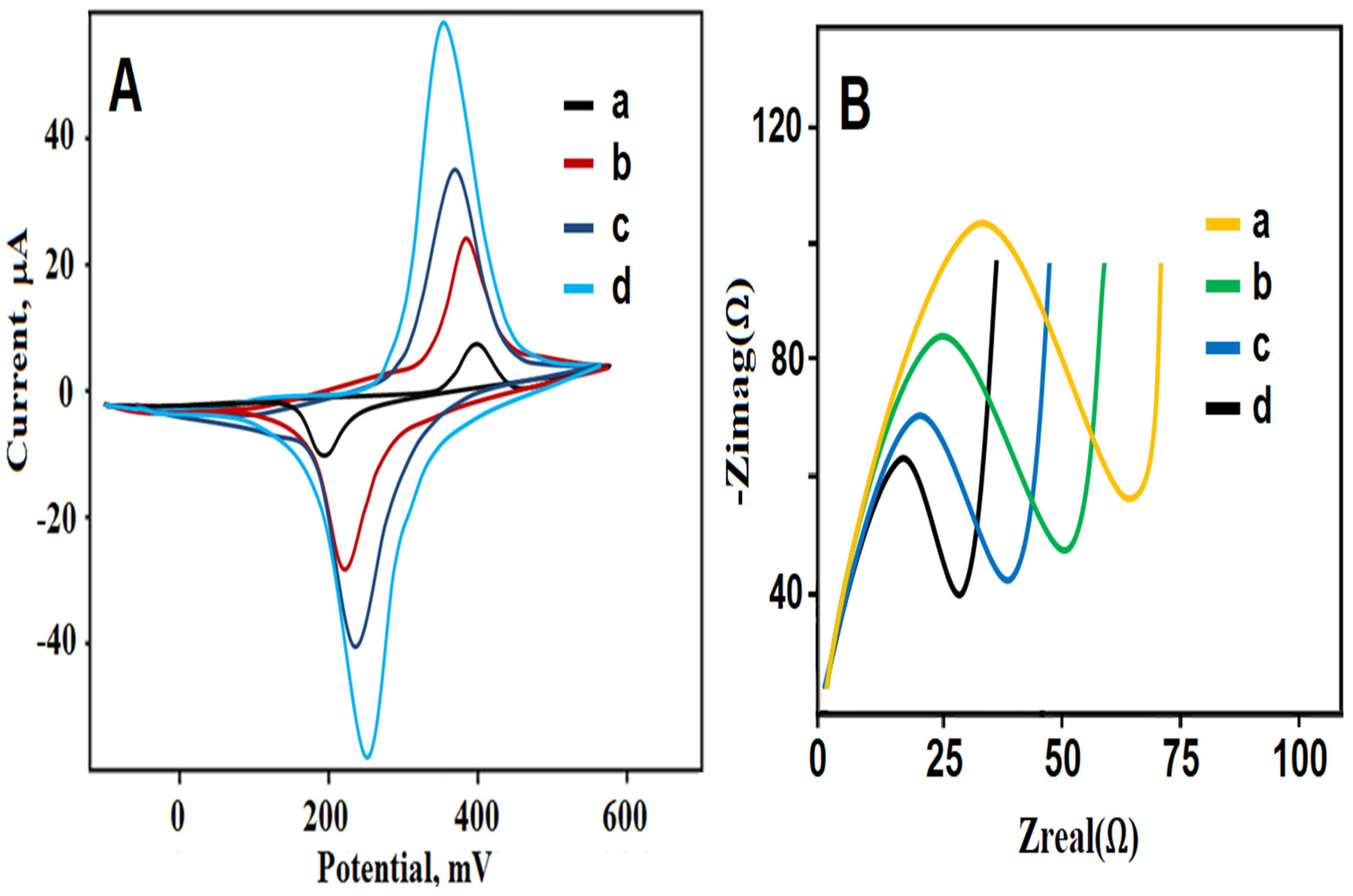
3.2. Electrochemical Characterizations of g-C3N4, B-g-C3N4, and GQDs/B-g-C3N4 Nanocomposite-Modified Electrodes
3.3. Fabrication of BPA-Imprinted Polymer on GQDs/B-g-C3N4/GCE
3.4. Optimization Studies
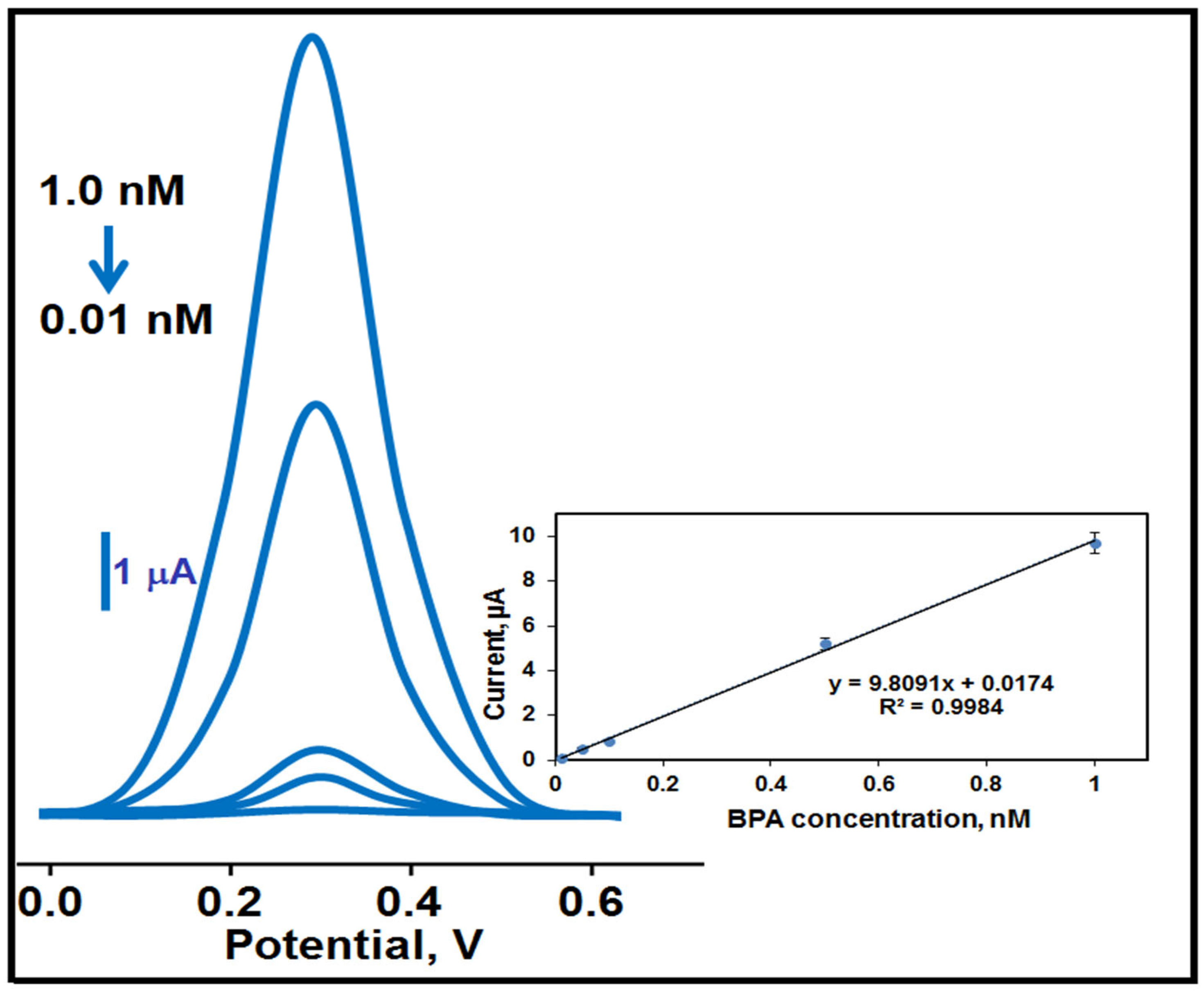
3.5. Quantification Limit (LOQ) and LOD Values
3.6. Recovery Assessment
3.7. Selectivity, Repeatability, and Stability Performances of MIP/GQDs/B-g-C3N4/GCE
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dekant, W.; Voelkel, W. Human exposure to bisphenol A by biomonitoring: Methods, results and assessment of environmental exposures. Toxicol. Appl. Pharmacol. 2008, 228, 114–134. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, J.; Wu, Y.; Zhao, Y.; Luo, F.; Li, S.; Yang, L.; Moez, E.K.; Dinu, I.; Martin, J.W. Bisphenol A Metabolites and Bisphenol S in Paired Maternal and Cord Serum. Environ. Sci. Technol. 2017, 51, 2456–2463. [Google Scholar] [CrossRef] [PubMed]
- Jurek, A.; Leitner, E. Analytical determination of bisphenol A (BPA) and bisphenol analogues in paper products by GC-MS/MS. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2017, 34, 1225–1238. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.H.; Wu, J.X.; Yuan, L.; Wang, Y.Q.; Du, X.D.; Wang, R.; Marwa, P.W.; Petlulu, P.; Chen, X.H.; et al. The adverse health effects of bisphenol A and related toxicity mechanisms. Environ. Res. 2019, 176, 108575. [Google Scholar] [CrossRef]
- Cerkvenik-Flajs, V.; Skibin, A.; Svara, T.; Gombac, M.; Pogacnik, M.; Sturm, S. Bisphenol A in edible tissues of rams exposed to repeated low-level dietary dose by high-performance liquid chromatography with fluorescence detection. Environ. Sci. Pollut. Res. 2022, 29, 76078–76090. [Google Scholar] [CrossRef]
- Ren, S.; Cho, S.; Lin, R.X.; Gedi, V.; Park, S.; Ahn, C.W.; Lee, D.K.; Lee, M.H.; Lee, S.; Kim, S. Nonbiodegradable Spiegelmer-Driven Colorimetric Biosensor for Bisphenol A Detection. Biosensors 2022, 12, 864. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.X.; Cao, Y.Q.; Han, J.J.; Xia, Y.T.; He, Z.X.; Sun, L.H.; Liang, J. A novel fluorescence sensor based on Zn porphyrin MOFs for the detection of bisphenol A with highly selectivity and sensitivity. Food Control 2022, 132, 108551. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W.; Zhang, L.; Feng, L.; Zhang, C.; Jiang, J.; Wang, H. Turning on the Photoelectrochemical Responses of Cd Probe-Deposited g-C3N4 Nanosheets by Nitrogen Plasma Treatment toward a Selective Sensor for H2S. ACS Appl. Mater. Interfaces 2021, 13, 2052–2061. [Google Scholar] [CrossRef]
- Yan, P.C.; Dong, J.T.; Mo, Z.; Xu, L.; Qian, J.C.; Xia, J.X.; Zhang, J.M.; Li, H.N. Enhanced photoelectrochemical sensing performance of graphitic carbon nitride by nitrogen vacancies engineering. Biosens. Bioelectron. 2020, 148, 111802. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.Q.; Xie, J.; Chen, X.B.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Shi, J.W.; Huang, Z.X.; Guan, X.J.; Zong, S.C.; Cheng, C.; Zheng, B.T.; Guo, L.J. Synchronous construction of CoS2 in-situ loading and S doping for g-C3N4: Enhanced photocatalytic H-2-evolution activity and mechanism insight. Chem. Eng. J. 2020, 401, 126135. [Google Scholar] [CrossRef]
- Li, Y.J.; Ding, L.; Guo, Y.C.; Liang, Z.Q.; Cui, H.Z.; Tian, J. Boosting the Photocatalytic Ability of g-C3N4 for Hydrogen Production by Ti3C2 MXene Quantum Dots. ACS Appl. Mater. Interfaces 2019, 11, 41440–41447. [Google Scholar] [CrossRef]
- Zhao, D.M.; Dong, C.L.; Bin, W.; Chen, C.; Huang, Y.C.; Diao, Z.D.; Li, S.Z.; Guo, L.J.; Shen, S.H. Synergy of Dopants and Defects in Graphitic Carbon Nitride with Exceptionally Modulated Band Structures for Efficient Photocatalytic Oxygen Evolution. Adv. Mater. 2019, 31, 1903545. [Google Scholar] [CrossRef]
- Kumar, P.S.; Prakash, P. Metal free nanocomposite of graphitic carbon nitride, boron nitride and chitosan for efficient evolution of hydrogen: A strategic approach to achieving sustainable and effective electrocatalysis. J. Environ. Chem. Eng. 2023, 11, 109045. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, R.; Wang, P.; Rao, L.; Wang, C. Developing a Novel Layered Boron Nitride–Carbon Nitride Composite with High Efficiency and Selectivity to Remove Protonated Dyes from Water. ACS Sustain. Chem. Eng. 2019, 7, 5727–5741. [Google Scholar] [CrossRef]
- Atar, N.; Yola, M.L. A novel QCM immunosensor development based on gold nanoparticles functionalized sulfur-doped graphene quantum dot and h-ZnS-CdS NC for Interleukin-6 detection. Anal. Chim. Acta 2021, 1148, 338202. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.C.; Yu, F.; Wang, L.C.; Xing, Q.J.; Liu, X.; Pei, Y.; Zou, J.P.; Dai, W.L.; Li, Y.; Suib, S.L. Photocatalytic degradation of organic pollutants coupled with simultaneous photocatalytic H-2 evolution over graphene quantum dots/Mn-N-TiO2/g-C3N4 composite catalysts: Performance and mechanism. Appl. Catal. B Environ. 2018, 227, 312–321. [Google Scholar] [CrossRef]
- Liu, J.Y.; Xu, H.; Xu, Y.G.; Song, Y.H.; Lian, J.B.; Zhao, Y.; Wang, L.; Huang, L.Y.; Ji, H.Y.; Li, H.M. Graphene quantum dots modified mesoporous graphite carbon nitride with significant enhancement of photocatalytic activity. Appl. Catal. B Environ. 2017, 207, 429–437. [Google Scholar] [CrossRef]
- Wang, L.; Pagett, M.; Zhang, W. Molecularly imprinted polymer (MIP) based electrochemical sensors and their recent advances in health applications. Sens. Actuators Rep. 2023, 5, 100153. [Google Scholar] [CrossRef]
- Rebelo, P.; Costa-Rama, E.; Seguro, I.; Pacheco, J.G.; Nouws, H.P.A.; Cordeiro, M.N.D.S.; Delerue-Matos, C. Molecularly imprinted polymer-based electrochemical sensors for environmental analysis. Biosens. Bioelectron. 2021, 172, 112719. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Samukaite-Bubniene, U.; Ratautaite, V.; Bechelany, M.; Ramanavicius, A. Electrochemical molecularly imprinted polymer based sensors for pharmaceutical and biomedical applications (review). J. Pharm. Biomed. Anal. 2022, 215, 114739. [Google Scholar] [CrossRef]
- Ayerdurai, V.; Cieplak, M.; Kutner, W. Molecularly imprinted polymer-based electrochemical sensors for food contaminants determination. TrAC Trends Anal. Chem. 2023, 158, 116830. [Google Scholar] [CrossRef]
- Rebocho, S.; Cordas, C.M.; Viveiros, R.; Casimiro, T. Development of a ferrocenyl-based MIP in supercritical carbon dioxide: Towards an electrochemical sensor for bisphenol A. J. Supercrit. Fluids 2018, 135, 98–104. [Google Scholar] [CrossRef]
- Chai, R.; Kan, X. Au-polythionine nanocomposites: A novel mediator for bisphenol A dual-signal assay based on imprinted electrochemical sensor. Anal. Bioanal. Chem. 2019, 411, 3839–3847. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Ma, Y.; Zeng, Q.; Wang, M.; Wang, L.S. An Electropolymerized Molecularly Imprinted Electrochemical Sensor for the Selective Determination of Bisphenol A Diglycidyl Ether. Chemistryselect 2020, 5, 3574–3580. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Development of molecular imprinted sensor including graphitic carbon nitride/N-doped carbon dots composite for novel recognition of epinephrine. Compos. Part B Eng. 2019, 175, 107113. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, H.; Won, M.; Kim, E.; Li, M.; Kim, J.S. Codoping g-C3N4 with boron and graphene quantum dots: Enhancement of charge transfer for ultrasensitive and selective photoelectrochemical detection of dopamine. Biosens. Bioelectron. 2023, 224, 115050. [Google Scholar] [CrossRef] [PubMed]
- Yola, M.L.; Atar, N. A novel detection approach for serotonin by graphene quantum dots/two-dimensional (2D) hexagonal boron nitride nanosheets with molecularly imprinted polymer. Appl. Surf. Sci. 2018, 458, 648–655. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z.C.; Zheng, M.; Li, J.; Zhang, Y.Q.; Zhang, G.Q.; Zhao, H.F.; Liu, X.Y.; Xie, Z.G. Three Colors Emission from S,N Co-doped Graphene Quantum Dots for Visible Light H-2 Production and Bioimaging. Adv. Opt. Mater. 2015, 3, 360–367. [Google Scholar] [CrossRef]
- Huang, C.F.; Wen, Y.P.; Ma, J.; Dong, D.D.; Shen, Y.F.; Liu, S.Q.; Ma, H.B.; Zhang, Y.J. Unraveling fundamental active units in carbon nitride for photocatalytic oxidation reactions. Nat. Commun. 2021, 12, 320. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Li, L.L.; Shi, D.; Jiang, H.H.; Ai, Z.Z.; Wang, S.Z.; Shao, Y.L.; Shen, J.X.; Wu, Y.Z.; Li, Y.L.; et al. Metal-free boron carbonitride with tunable boron Lewis acid sites for enhanced nitrogen electroreduction to ammonia. Appl. Catal. B Environ. 2021, 283, 119622. [Google Scholar] [CrossRef]
- Yola, M.L. Sensitive sandwich-type voltammetric immunosensor for breast cancer biomarker HER2 detection based on gold nanoparticles decorated Cu-MOF and Cu2ZnSnS4 NPs/Pt/g-C3N4 composite. Microchim. Acta 2021, 188, 78. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Zheng, M.; Du, P.; Zhou, Y.; Zhang, L.G.; Li, D.; Tan, H.Q.; Zhao, Z.; Xie, Z.G.; Sun, Z.C. Highly luminescent S, N co-doped graphene quantum dots with broad visible absorption bands for visible light photocatalysts. Nanoscale 2013, 5, 12272–12277. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Chen, F.; Wang, Y.G.; Tian, N.; Ma, T.Y.; Zhang, Y.H.; Huang, H.W. Exceptional Cocatalyst-Free Photo-Enhanced Piezocatalytic Hydrogen Evolution of Carbon Nitride Nanosheets from Strong In-Plane Polarization. Adv. Mater. 2021, 33, 2101751. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Yong, K. Boron doping induced charge transfer switching of a C3N4/ZnO photocatalyst from Z-scheme to type II to enhance photocatalytic hydrogen production. Appl. Catal. B Environ. 2021, 282, 119538. [Google Scholar] [CrossRef]
- Zhu, J.H.; Gou, H.W.; Zhao, T.J.; Mei, L.P.; Wang, A.J.; Feng, J.J. Ultrasensitive photoelectrochemical aptasensor for detecting telomerase activity based on Ag2S/Ag decorated ZnIn2S4/C3N4 3D/2D Z-scheme heterostructures and amplified by Au/Cu2+-boron-nitride nanozyme. Biosens. Bioelectron. 2022, 203, 114048. [Google Scholar] [CrossRef]
- Eftekhari, A.; Dalili, M.; Karimi, Z.; Rouhani, S.; Hasanzadeh, A.; Rostamnia, S.; Khaksar, S.; Idris, A.O.; Karimi-Maleh, H.; Yola, M.L.; et al. Sensitive and selective electrochemical detection of bisphenol A based on SBA-15 like Cu-PMO modified glassy carbon electrode. Food Chem. 2021, 358, 129763. [Google Scholar] [CrossRef]
- Naik, T.; Singh, S.; Pavithra, N.; Varshney, R.; Uppara, B.; Singh, J.; Khan, N.A.; Singh, L.; Arshad, M.Z.; Ramamurthy, P.C. Advanced experimental techniques for the sensitive detection of a toxic bisphenol A using UiO-66-NDC/GO-based electrochemical sensor. Chemosphere 2023, 311, 137104. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, H.; Zha, Q.; Li, J.; Zhu, M. In-Situ Construction Molecular Imprinting Electrocatalyst of Au-MoO3/Graphene for Bisphenol A Determination with Long-Term Stability. Catalysts 2023, 13, 91. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, T.Y.; Khan, A.; Chen, Z.J.; Liu, P.; Li, X.K. A novel electrochemical biosensor for bisphenol A detection based on engineered Escherichia coli cells with a surface-display of tyrosinase. Sens. Actuators B Chem. 2022, 353, 131063. [Google Scholar] [CrossRef]
- Sanko, V.; Senocak, A.; Tumay, S.O.; Orooji, Y.; Demirbas, E.; Khataee, A. An electrochemical sensor for detection of trace-level endocrine disruptor bisphenol A using Mo2Ti2AlC3 MAX phase/MWCNT composite modified electrode. Environ. Res. 2022, 212, 113071. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, K.; Kumar, D.R.; Bhargav, P.B.; Manigandan, R.; Ahmed, N.; Balaji, C.; Shim, J.J. Carbon dot-V2O5 layered nanoporous architectures for electrochemical detection of Bisphenol A: An analytical approach. J. Environ. Chem. Eng. 2022, 10, 108206. [Google Scholar] [CrossRef]
- Wang, K.P.; Hu, J.M.; Zhang, X. Sensitive electrochemical detection of endocrine disruptor bisphenol A (BPA) in milk based on iodine-doped graphene. Microchem. J. 2022, 173, 107047. [Google Scholar] [CrossRef]
| Material | Linear Range (M) | LOD (M) | Ref. |
|---|---|---|---|
| UiO-66-NDC/GO | 1.0 × 10−5–7.0 × 10−5 | 2.5 × 10−8 | [38] |
| Au-MoO3/graphene | 1.0 × 10−8–1.0 × 10−4 | 3.0 × 10−9 | [39] |
| Escherichia coli/tyrosinase | 1.0 × 10−11–1.0 × 10−7 | 1.0 × 10−11 | [40] |
| Mo2Ti2AlC3 MAX phase/MWCNT | 1.0 × 10−8–8.5 × 10−6 | 2.7 × 10−9 | [41] |
| Carbon dot-V2O5 | 5.0 × 10−9–9.2 × 10−3 | 8.0 × 10−10 | [42] |
| Iodine-doped graphene | 4.0 × 10−8–4.5 × 10−6 | 2.0 × 10−8 | [43] |
| MIP/GQDs/B-g-C3N4/GCE | 1.0 × 10−11–1.0 × 10−9 | 3.0 × 10−12 | This study |
| Sample | Added BPA (nM) | Found BPA (nM) | * Recovery (%) |
|---|---|---|---|
| Orange Juice | - | 0.203 ± 0.003 | - |
| 0.100 | 0.304 ± 0.002 | 100.33 ± 0.02 | |
| 0.300 | 0.502 ± 0.001 | 99.80 ± 0.05 | |
| 0.500 | 0.701 ± 0.004 | 99.72 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deveci, H.A.; Mavioğlu Kaya, M.; Kaya, İ.; Bankoğlu Yola, B.; Atar, N.; Yola, M.L. Bisphenol A Imprinted Electrochemical Sensor Based on Graphene Quantum Dots with Boron Functionalized g-C3N4 in Food Samples. Biosensors 2023, 13, 725. https://doi.org/10.3390/bios13070725
Deveci HA, Mavioğlu Kaya M, Kaya İ, Bankoğlu Yola B, Atar N, Yola ML. Bisphenol A Imprinted Electrochemical Sensor Based on Graphene Quantum Dots with Boron Functionalized g-C3N4 in Food Samples. Biosensors. 2023; 13(7):725. https://doi.org/10.3390/bios13070725
Chicago/Turabian StyleDeveci, Haci Ahmet, Müge Mavioğlu Kaya, İnan Kaya, Bahar Bankoğlu Yola, Necip Atar, and Mehmet Lütfi Yola. 2023. "Bisphenol A Imprinted Electrochemical Sensor Based on Graphene Quantum Dots with Boron Functionalized g-C3N4 in Food Samples" Biosensors 13, no. 7: 725. https://doi.org/10.3390/bios13070725
APA StyleDeveci, H. A., Mavioğlu Kaya, M., Kaya, İ., Bankoğlu Yola, B., Atar, N., & Yola, M. L. (2023). Bisphenol A Imprinted Electrochemical Sensor Based on Graphene Quantum Dots with Boron Functionalized g-C3N4 in Food Samples. Biosensors, 13(7), 725. https://doi.org/10.3390/bios13070725






