Revolutionizing Precision Medicine: Exploring Wearable Sensors for Therapeutic Drug Monitoring and Personalized Therapy
Abstract
1. Introduction
2. Wearable Monitoring of Drugs
2.1. Anti-Parkinson’s Drugs

2.2. Antimicrobial Drugs

2.3. Analgesic Drugs
2.4. Psychoactive Drugs
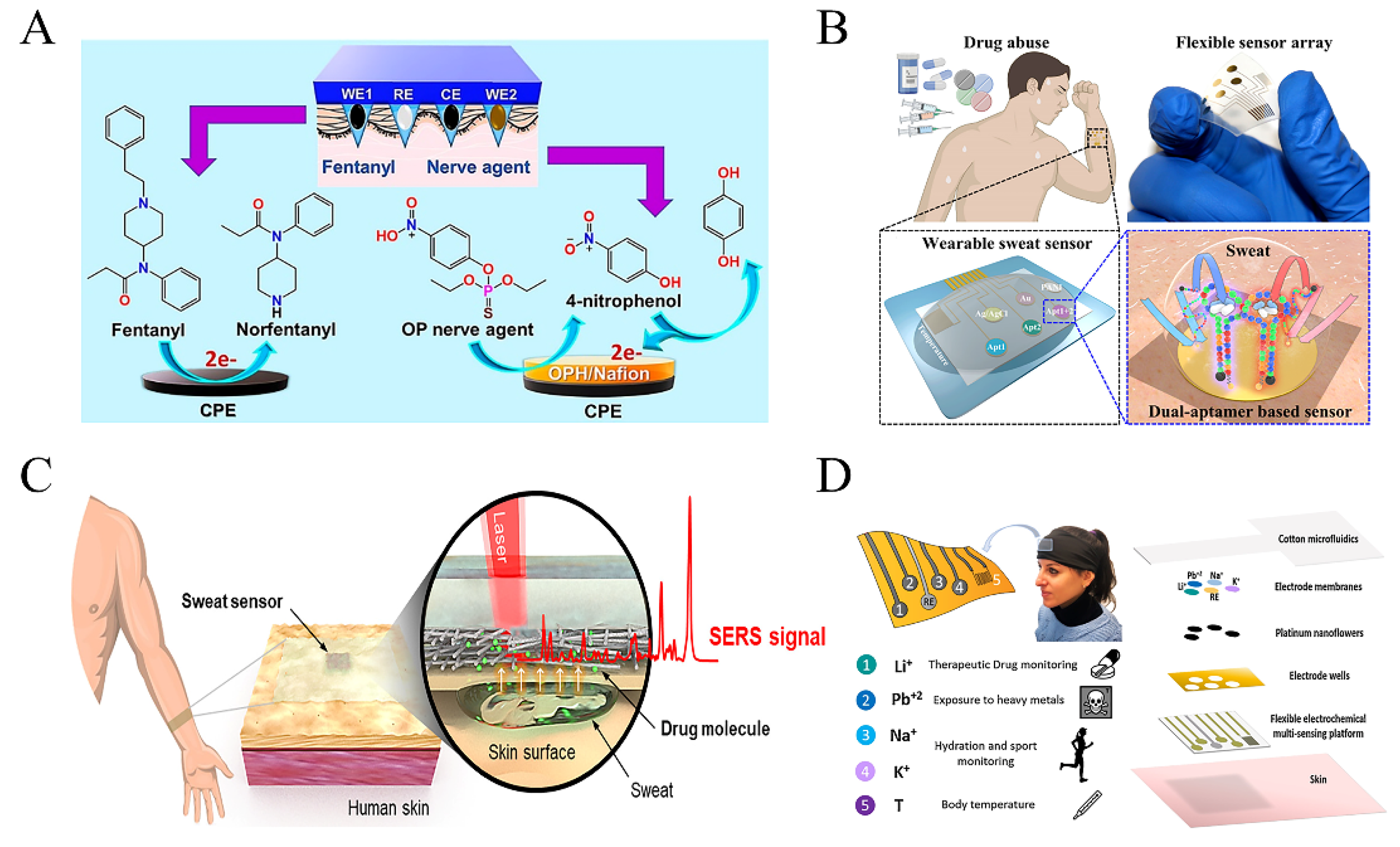
3. The Source of Biofluids and Continuous Sensing Technologies
3.1. Non-Contact Sensing Technology
3.1.1. Optical Sensing Technology
3.1.2. Electromagnetic Sensing Technology
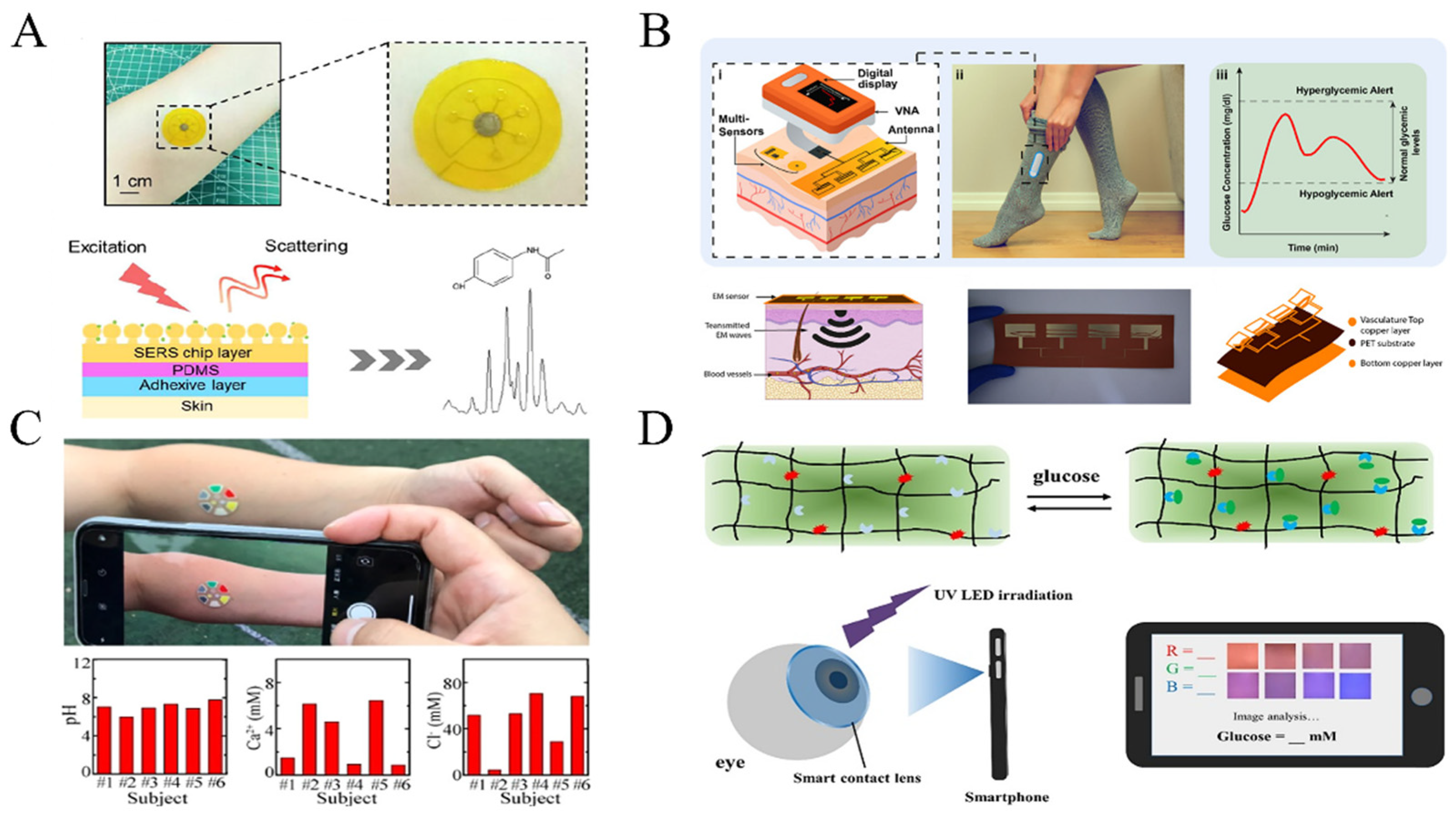
3.2. Epidermal Sensing Technology
3.2.1. The Source of Biofluids
Correlation of Compound Concentration between Biofluids and Blood
3.2.2. Optical Sensing Technology
3.2.3. Electrochemical Sensing Technology
Conventional Electrodes without Recognition Element Sensing Technology
Ion-Selective Electrodes Sensing Technology
Enzyme-Based Electrochemical Sensing Technology
Electrochemical Immunosensing Technology

Electrochemical Aptamer-Based (E-AB) Sensing Technology
3.3. Invasive Sensing Technology
3.3.1. The Source of Biofluids
3.3.2. Optical and Electrochemical Sensing Technology
3.4. Emerging Sensing Technologies and Application Barriers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Böhm, R.; Cascorbi, I. Pharmacogenetics and Predictive Testing of Drug Hypersensitivity Reactions. Front. Pharmacol. 2016, 7, 396. [Google Scholar] [CrossRef]
- Krähenbühl, S. Adverse drug reaction—Definitions, risk factors and pharmacovigilance. Ther. Umsch. 2015, 72, 669–671. [Google Scholar] [CrossRef]
- Cacabelos, R.; Naidoo, V.; Corzo, L.; Cacabelos, N.; Carril, J.C. Genophenotypic Factors and Pharmacogenomics in Adverse Drug Reactions. Int. J. Mol. Sci. 2021, 22, 13302. [Google Scholar] [CrossRef]
- Li, X.x.; Yin, J.; Tang, J.; Li, Y.; Yang, Q.; Xiao, Z.; Zhang, R.; Wang, Y.; Hong, J.; Tao, L.; et al. Determining the Balance Between Drug Efficacy and Safety by the Network and Biological System Profile of Its Therapeutic Target. Front. Pharmacol. 2018, 9, 1245. [Google Scholar] [CrossRef]
- Holford, N.H.G.; Sheiner, L.B. Understanding the Dose-Effect Relationship. Clin. Pharmacokinet. 1981, 6, 429–453. [Google Scholar] [CrossRef]
- Zeng, L.; Yi, Q.; Huang, L.; Chen, W.; Men, P.; Zhang, J.; Jiang, Z.; Miao, L.; Zhao, R.; Zhang, X.; et al. The guideline for therapeutic drug monitoring guidelines development. J. Evid.-Based Med. 2022, 15, 272–283. [Google Scholar] [CrossRef]
- Irving, P.M.; Gecse, K.B. Optimizing Therapies Using Therapeutic Drug Monitoring: Current Strategies and Future Perspectives. Gastroenterology 2022, 162, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Tuzimski, T.; Petruczynik, A. Review of chromatographic methods coupled with modern detection techniques applied in the therapeutic drugs monitoring (TDM). Molecules 2020, 25, 4026. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Jiang, X.; Wang, X.; Bao, X. Pharmacokinetic Study of Zhebeirine in Mouse Blood by Ultra- Performance Liquid Chromatography/tandem Mass Spectrometry. Curr. Pharm. Anal. 2021, 17, 547–553. [Google Scholar] [CrossRef]
- Losoya-Leal, A.; Estevez, M.-C.; Martínez-Chapa, S.O.; Lechuga, L.M. Design of a surface plasmon resonance immunoassay for therapeutic drug monitoring of amikacin. Talanta 2015, 141, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Rafalskiy, V.V.; Zyubin, A.Y.; Moiseeva, E.M.; Kupriyanova, G.S.; Mershiev, I.G.; Kryukova, N.O.; Kon, I.I.; Samusev, I.G.; Belousova, Y.D.; Doktorova, S.A. Application of vibrational spectroscopy and nuclear magnetic resonance methods for drugs pharmacokinetics research. Drug Metab. Pers. Ther. 2022, 38, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-Y.; Wang, J.-H.; Chang, T.-Y.; Shih, C.-L. Mass defect filter technique combined with stable isotope tracing for drug metabolite identification using high-resolution mass spectrometry. Anal. Chim. Acta 2022, 1208, 339814. [Google Scholar] [CrossRef]
- Nishi, H.; Terabe, S. Optical resolution of drugs by capillary electrophoretic techniques. J. Chromatogr. A 1995, 694, 245–276. [Google Scholar] [CrossRef]
- Eliasson, E.; Lindh, J.D.; Malmström, R.E.; Beck, O.; Dahl, M.-L. Therapeutic drug monitoring for tomorrow. Eur. J. Clin. Pharmacol. 2013, 69, 25–32. [Google Scholar] [CrossRef] [PubMed]
- González-Gross, M.; Breidenassel, C.; Gómez-Martínez, S.; Ferrari, M.; Beghin, L.; Spinneker, A.; Diaz, L.; Maiani, G.; Demailly, A.; Al-Tahan, J. Sampling and processing of fresh blood samples within a European multicenter nutritional study: Evaluation of biomarker stability during transport and storage. Int. J. Obes. 2008, 32, S66–S75. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Zhu, B.; Rong, G.; Sawan, M. Towards wearable and implantable continuous drug monitoring: A review. J. Pharm. Anal. 2021, 11, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Heikenfeld, J.; Jajack, A.; Rogers, J.; Gutruf, P.; Tian, L.; Pan, T.; Li, R.; Khine, M.; Kim, J.; Wang, J. Wearable sensors: Modalities, challenges, and prospects. Lab Chip 2018, 18, 217–248. [Google Scholar] [CrossRef]
- Zhu, B.Y.; Li, X.R.; Zhou, L.; Su, B. An Overview of Wearable and Implantable Electrochemical Glucose Sensors. Electroanalysis 2022, 34, 237–245. [Google Scholar] [CrossRef]
- Kwon, S.; Kim, H.; Yeo, W.H. Recent advances in wearable sensors and portable electronics for sleep monitoring. iScience 2021, 24, 102461. [Google Scholar] [CrossRef]
- Lee, S.J.; Bae, J.; Kwon, J.E.; Lee, Y.J.; Yoon, J.Y.; Kang, J.S. Comparison of the Initial Concentration of Tacrolimus according to Weight Application Criteria after Hematopoietic Stem Cell Transplantation in Obese Patients. J. Korean Soc. Health-Syst. Pharm. 2021, 38, 195–205. [Google Scholar]
- Brunet, M.; van Gelder, T.; Åsberg, A.; Haufroid, V.; Hesselink, D.A.; Langman, L.; Lemaitre, F.; Marquet, P.; Seger, C.; Shipkova, M.; et al. Therapeutic Drug Monitoring of Tacrolimus-Personalized Therapy: Second Consensus Report. Ther. Drug Monit. 2019, 41, 261–307. [Google Scholar] [CrossRef]
- Ogawa, H.; Kameda, H.; Amano, K.; Takeuchi, T. Efficacy and safety of cyclosporine A in patients with refractory systemic lupus erythematosus in a daily clinical practice. Lupus 2010, 19, 162–169. [Google Scholar] [CrossRef]
- Kholodov, L.E.; Prityko, A.G.; Ivanova, E.S.; Sokolov, A.V.; Tishchenkova, I.F.; Dubrovina, L.; Postnikov, S.S.; Tatarinov, P.A.; Kopanev Iu, A.; Veselov, N.K.; et al. Therapeutic drug monitoring and the pharmacokinetics of carbamazepine in children with a convulsive syndrome. Zhurnal Nevrol. Psikhiatrii Im. SS Korsakova 1995, 95, 35–40. [Google Scholar]
- Schmidt, D.; Einicke, I.; Haenel, F. The influence of seizure type on the efficacy of plasma concentrations of phenytoin, phenobarbital, and carbamazepine. Arch. Neurol. 1986, 43, 263–265. [Google Scholar] [CrossRef] [PubMed]
- Buchthal, F.; Svensmark, O.; Schiller, P.J. Clinical and electroencephalographic correlations with serum levels of diphenylhydanotin. Arch. Neurol. 1960, 2, 624–630. [Google Scholar] [CrossRef]
- Karaźniewicz-Łada, M.; Główka, A.K.; Mikulska, A.A.; Główka, F.K. Pharmacokinetic Drug-Drug Interactions among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients. Int. J. Mol. Sci. 2021, 22, 9582. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.J.; Huang, S.Y.; Kuo, C.H.; Wang, C.Y.; Wang, K.C.; Wu, C.C. Safety range of free valproic acid serum concentration in adult patients. PLoS ONE 2020, 15, e0238201. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B. An Updated Overview on Therapeutic Drug Monitoring of Recent Antiepileptic Drugs. Drugs R D 2016, 16, 303–316. [Google Scholar]
- He, N.; Su, S.; Ye, Z.; Du, G.; He, B.; Li, D.; Liu, Y.; Yang, K.; Zhang, X.; Zhang, Y.; et al. Evidence-based Guideline for Therapeutic Drug Monitoring of Vancomycin: 2020 Update by the Division of Therapeutic Drug Monitoring, Chinese Pharmacological Society. Clin. Infect. Dis. 2020, 71, S363–S371. [Google Scholar] [CrossRef] [PubMed]
- Dauphin-Ducharme, P.; Yang, K.; Arroyo-Curras, N.; Ploense, K.L.; Zhang, Y.; Gerson, J.; Kurnik, M.; Kippin, T.E.; Stojanovic, M.N.; Plaxco, K.W. Electrochemical Aptamer-Based Sensors for Improved Therapeutic Drug Monitoring and High-Precision, Feedback-Controlled Drug Delivery. ACS Sens. 2019, 4, 2832–2837. [Google Scholar] [CrossRef]
- Steffens, N.A.; Zimmermann, E.S.; Nichelle, S.M.; Brucker, N. Meropenem use and therapeutic drug monitoring in clinical practice: A literature review. J. Clin. Pharm. Ther. 2021, 46, 610–621. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.H.; Brady, K.; Cotta, M.O.; Roberts, J.A. Therapeutic Drug Monitoring of Antibiotics: Defining the Therapeutic Range. Ther. Drug Monit. 2022, 44, 19–31. [Google Scholar] [CrossRef]
- Minichmayr, I.K.; Schaeftlein, A.; Kuti, J.L.; Zeitlinger, M.; Kloft, C. Clinical Determinants of Target Non-Attainment of Linezolid in Plasma and Interstitial Space Fluid: A Pooled Population Pharmacokinetic Analysis with Focus on Critically Ill Patients. Clin. Pharmacokinet. 2017, 56, 617–633. [Google Scholar] [CrossRef] [PubMed]
- Reyhanoglu, G.; Reddivari, A.K.R. Tobramycin. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2023. [Google Scholar]
- Charfi, R.; Ben Sassi, M.; Gaies, E.; Jebabli, N.; Daghfous, R.; Trabelsi, S. Digoxin therapeutic drug monitoring: Age influence and adverse events. Tunis. Med. 2020, 98, 35–40. [Google Scholar]
- Klinger, E.; Steimer, J.L.; Jouvent, R.; Le Moël, G.; Mascart, J.Y.; Des Lauriers, A. Renal clearance technic for individualizing lithium dosage in routine hospital care. Encephale 1984, 10, 223–230. [Google Scholar]
- Kramer, C.; Tawney, M. A fatal overdose of transdermally administered fentanyl. J. Am. Osteopath. Assoc. 1998, 98, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Ait-m-bark, Z.; Schummer, C.; Lemmer, P.; Yegles, M.; Appenzeller, B.; Wennig, R. Determination of Fentanyl in Sweat and Hair of a Patient using Transdermal Patches. J. Anal. Toxicol. 2008, 32, 260–264. [Google Scholar] [CrossRef] [PubMed]
- Badhan, R.K.S.; Gittins, R.; Al Zabit, D. The optimization of methadone dosing whilst treating with rifampicin: A pharmacokinetic modeling study. Drug Alcohol. Depend. 2019, 200, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Fucci, N.; De Giovanni, N. Methadone in hair and sweat from patients in long-term maintenance therapy. Ther. Drug Monit. 2007, 29, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Self, T.H.; Heilker, G.M.; Alloway, R.R.; Kelso, T.M.; Abou-Shala, N. Reassessing the therapeutic range for theophylline on laboratory report forms: The importance of 5–15 micrograms/mL. Pharmacotherapy 1993, 13, 590–594. [Google Scholar]
- Urban, A.E.; Cubała, W.J. Therapeutic drug monitoring of atypical antipsychotics. Psychiatr. Pol. 2017, 51, 1059–1077. [Google Scholar] [CrossRef]
- Dahl, S.G. Plasma level monitoring of antipsychotic drugs. Clinical utility. Clin. Pharmacokinet. 1986, 11, 36–61. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2015, 30, 1600–1611. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Yang, Y.; Zhang, L.; Zhang, Q.; Balbuena, L.; Ungvari, G.S.; Zang, Y.-F.; Xiang, Y.-T. Quality of life in Parkinson’s disease: A systematic review and meta-analysis of comparative studies. CNS Neurosci. Ther. 2021, 27, 270–279. [Google Scholar] [CrossRef]
- Liu, J.-P.; Li, J.; Lu, Y.; Wang, L.; Chen, G. Impulse control disorder, lysosomal malfunction and ATP13A2 insufficiency in Parkinsonism. Clin. Exp. Pharmacol. Physiol. 2017, 44, 172–179. [Google Scholar] [CrossRef]
- Freitas, M.E.; Ruiz-Lopez, M.; Fox, S.H. Novel levodopa formulations for Parkinson’s disease. CNS Drugs 2016, 30, 1079–1095. [Google Scholar] [CrossRef]
- Deleu, D.; Jacob, P.; Chand, P.; Sarre, S.; Colwell, A. Effects of caffeine on levodopa pharmacokinetics and pharmacodynamics in Parkinson disease. Neurology 2006, 67, 897–899. [Google Scholar] [CrossRef]
- Senek, M.; Aquilonius, S.-M.; Askmark, H.; Bergquist, F.; Constantinescu, R.; Ericsson, A.; Lycke, S.; Medvedev, A.; Memedi, M.; Ohlsson, F. Levodopa/carbidopa microtablets in Parkinson’s disease: A study of pharmacokinetics and blinded motor assessment. Eur. J. Clin. Pharmacol. 2017, 73, 563–571. [Google Scholar] [CrossRef]
- Tsunoda, M.; Hirayama, M.; Tsuda, T.; Ohno, K. Noninvasive monitoring of plasma l-dopa concentrations using sweat samples in Parkinson’s disease. Clin. Chim. Acta 2015, 442, 52–55. [Google Scholar] [CrossRef]
- Moon, J.-M.; Teymourian, H.; De la Paz, E.; Sempionatto, J.R.; Mahato, K.; Sonsa-ard, T.; Huang, N.; Longardner, K.; Litvan, I.; Wang, J. Non-Invasive Sweat-Based Tracking of L-Dopa Pharmacokinetic Profiles Following an Oral Tablet Administration. Angew. Chem.-Int. Ed. 2021, 60, 19074–19078. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Wang, Y.; Li, J.; Da, P.; Geng, J.; Zheng, G. Sensitive enzymatic glucose detection by TiO2 nanowire photoelectrochemical biosensors. J. Mater. Chem. A 2014, 2, 6153–6157. [Google Scholar] [CrossRef]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Fan, C.; Xu, T.; Su, L.; Zhang, X. An electrochemical wearable sensor for levodopa quantification in sweat based on a metal–Organic framework/graphene oxide composite with integrated enzymes. Sens. Actuators B Chem. 2022, 359, 131586. [Google Scholar] [CrossRef]
- Goud, K.Y.; Moonla, C.; Mishra, R.K.; Yu, C.; Narayan, R.; Litvan, I.; Wang, J. Wearable electrochemical microneedle sensor for continuous monitoring of levodopa: Toward Parkinson management. ACS Sens. 2019, 4, 2196–2204. [Google Scholar] [CrossRef]
- Gowers, S.A.N.; Freeman, D.M.E.; Rawson, T.M.; Rogers, M.L.; Wilson, R.C.; Holmes, A.H.; Cass, A.E.; O’Hare, D. Development of a Minimally Invasive Microneedle-Based Sensor for Continuous Monitoring of β-Lactam Antibiotic Concentrations in Vivo. ACS Sens. 2019, 4, 1072–1080. [Google Scholar] [CrossRef]
- Levine, D.P. Vancomycin: A history. Clin. Infect. Dis. 2006, 42, S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Chien, J.-C.; Baker, S.W.; Soh, H.T.; Arbabian, A. Design and analysis of a sample-and-hold CMOS electrochemical sensor for aptamer-based therapeutic drug monitoring. IEEE J. Solid-State Circuits 2020, 55, 2914–2929. [Google Scholar] [CrossRef]
- Ferreira, A.; Martins, H.; Oliveira, J.C.; Lapa, R.; Vale, N. PBPK Modeling and Simulation of Antibiotics Amikacin, Gentamicin, Tobramycin, and Vancomycin Used in Hospital Practice. Life 2021, 11, 1130. [Google Scholar] [CrossRef]
- Wu, Y.; Tehrani, F.; Teymourian, H.; Mack, J.; Shaver, A.; Reynoso, M.; Kavner, J.; Huang, N.; Furmidge, A.; Duvvuri, A. Microneedle aptamer-based sensors for continuous, real-time therapeutic drug monitoring. Anal. Chem. 2022, 94, 8335–8345. [Google Scholar] [CrossRef] [PubMed]
- Kaefer, K.; Krüger, K.; Schlapp, F.; Uzun, H.; Celiksoy, S.; Flietel, B.; Heimann, A.; Schroeder, T.; Kempski, O.; Sonnichsen, C. Implantable sensors based on gold nanoparticles for continuous long-term concentration monitoring in the body. Nano Lett. 2021, 21, 3325–3330. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Gowers, S.A.; Freeman, D.M.; Wilson, R.C.; Sharma, S.; Gilchrist, M.; MacGowan, A.; Lovering, A.; Bayliss, M.; Kyriakides, M. Microneedle biosensors for real-time, minimally invasive drug monitoring of phenoxymethylpenicillin: A first-in-human evaluation in healthy volunteers. Lancet Digit. Health 2019, 1, e335–e343. [Google Scholar] [CrossRef] [PubMed]
- Saleem, M.; Deters, B.; de la Bastide, A.; Korzen, M. Antibiotics overuse and bacterial resistance. Ann. Microbiol. Res. 2019, 3, 93. [Google Scholar]
- Mishra, R.K.; Goud, K.Y.; Li, Z.; Moonla, C.; Mohamed, M.A.; Tehrani, F.; Teymourian, H.; Wang, J. Continuous opioid monitoring along with nerve agents on a wearable microneedle sensor array. J. Am. Chem. Soc. 2020, 142, 5991–5995. [Google Scholar] [CrossRef] [PubMed]
- Raymundo-Pereira, P.A.; Gomes, N.O.; Machado, S.A.S.; Oliveira, O.N. Wearable glove-embedded sensors for therapeutic drug monitoring in sweat for personalized medicine. Chem. Eng. J. 2022, 435, 135047. [Google Scholar] [CrossRef]
- Ameer, B.; Greenblatt, D.J. Acetaminophen. Ann. Intern. Med. 1977, 87, 202–209. [Google Scholar] [CrossRef]
- Khandelwal, N.; James, L.P.; Sanders, C.; Larson, A.M.; Lee, W.M.; The Acute Liver Failure Study Group. Unrecognized acetaminophen toxicity as a cause of indeterminate acute liver failure. Hepatology 2011, 53, 567–576. [Google Scholar] [CrossRef]
- Bunchorntavakul, C.; Reddy, K.R. Acetaminophen (APAP or N-acetyl-p-aminophenol) and acute liver failure. Clin. Liver Dis. 2018, 22, 325–346. [Google Scholar] [CrossRef]
- Heard, K.; Green, J.L.; Anderson, V.; Bucher-Bartelson, B.; Dart, R.C. Paracetamol (acetaminophen) protein adduct concentrations during therapeutic dosing. Br. J. Clin. Pharmacol. 2016, 81, 562–568. [Google Scholar] [CrossRef]
- Lin, S.; Yu, W.; Wang, B.; Zhao, Y.; En, K.; Zhu, J.; Cheng, X.; Zhou, C.; Lin, H.; Wang, Z. Noninvasive wearable electroactive pharmaceutical monitoring for personalized therapeutics. Proc. Natl. Acad. Sci. USA 2020, 117, 19017–19025. [Google Scholar] [CrossRef]
- Tsong, J.L.; Robert, R.; Khor, S.M. Emerging trends in wearable glove-based sensors: A review. Anal. Chim. Acta 2023, 1262, 341277. [Google Scholar] [CrossRef] [PubMed]
- Comer, S.D.; Cahill, C.M. Fentanyl: Receptor pharmacology, abuse potential, and implications for treatment. Neurosci. Biobehav. Rev. 2019, 106, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Kelly, E.; Sutcliffe, K.; Cavallo, D.; Ramos-Gonzalez, N.; Alhosan, N.; Henderson, G. The anomalous pharmacology of fentanyl. Br. J. Pharmacol. 2023, 180, 797–812. [Google Scholar] [CrossRef] [PubMed]
- Voskanyan, A. Chemical warfare agent terrorist attacks in Latin America and the Caribbean region (CWA-LAC). Prehosp. Disaster Med. 2019, 34, s13. [Google Scholar]
- Thompson, J.G.; Baker, A.M.; Bracey, A.H.; Seningen, J.; Kloss, J.S.; Strobl, A.Q.; Apple, F.S. Fentanyl concentrations in 23 postmortem cases from the hennepin county medical examiner’s office. J. Forensic Sci. 2007, 52, 978–981. [Google Scholar] [CrossRef] [PubMed]
- Volkow, N.D. The epidemic of fentanyl misuse and overdoses: Challenges and strategies. World Psychiatry 2021, 20, 195–196. [Google Scholar] [CrossRef] [PubMed]
- Joshi, P.; Riley, P.R.; Mishra, R.; Azizi Machekposhti, S.; Narayan, R. Transdermal polymeric microneedle sensing platform for fentanyl detection in biofluid. Biosensors 2022, 12, 198. [Google Scholar] [CrossRef]
- Volkow, N.D.; Frieden, T.R.; Hyde, P.S.; Cha, S.S. Medication-assisted therapies—Tackling the opioid-overdose epidemic. N. Engl. J. Med. 2014, 370, 2063–2066. [Google Scholar] [CrossRef]
- Volkow, N.D.; Collins, F.S. The role of science in addressing the opioid crisis. N. Engl. J. Med. 2017, 377, 391–394. [Google Scholar] [CrossRef]
- Chen, X.; Deng, X.; Zhang, Y.; Wu, Y.; Yang, K.; Li, Q.; Wang, J.; Yao, W.; Tong, J.; Xie, T.; et al. Computational Design and Crystal Structure of a Highly Efficient Benzoylecgonine Hydrolase. Angew. Chem. Int. Ed. 2021, 60, 21959–21965. [Google Scholar] [CrossRef]
- Decosterd, I.; Beggah, A.T.; Durrer, A.; Buchser, E. Spinal opioids: Mechanisms of action and chronic pain management. Rev. Medicale Suisse 2006, 2, 1636–1640. [Google Scholar]
- Duan, G.; Guo, S.; Zhan, H.; Qi, D.; Zhang, Y.; Zhang, X. A new real-time method for detecting the effect of fentanyl using the preoperative pressure pain threshold and Narcotrend index: A randomized study in female surgery patients. Medicine 2015, 94, e316. [Google Scholar] [CrossRef]
- Volkow, N.D.; Jones, E.B.; Einstein, E.B.; Wargo, E.M. Prevention and treatment of opioid misuse and addiction: A review. JAMA Psychiatry 2019, 76, 208–216. [Google Scholar] [CrossRef]
- Campana, C.; Griffin, P.L.; Simon, E.L. Caffeine overdose resulting in severe rhabdomyolysis and acute renal failure. Am. J. Emerg. Med. 2013, 32, 111.e3–111.e4. [Google Scholar] [CrossRef]
- Riesselmann, B.; Rosenbaum, F.; Roscher, S.; Schneider, V. Fatal caffeine intoxication. Forensic Sci. Int. 1999, 103, S49–S52. [Google Scholar] [CrossRef]
- Tai, L.C.; Gao, W.; Chao, M.; Bariya, M.; Ngo, Q.P.; Shahpar, Z.; Nyein, H.Y.; Park, H.; Sun, J.; Jung, Y. Methylxanthine drug monitoring with wearable sweat sensors. Adv. Mater. 2018, 30, 1707442. [Google Scholar] [CrossRef]
- Graham, T.E.; Spriet, L.L. Metabolic, catecholamine, and exercise performance responses to various doses of caffeine. J. Appl. Physiol. 1995, 78, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Bell, D.G.; McLellan, T.M. Exercise endurance 1, 3, and 6 h after caffeine ingestion in caffeine users and nonusers. J. Appl. Physiol. 2002, 93, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Collomp, K.; Anselme, F.; Audran, M.; Gay, J.; Chanal, J.; Prefaut, C. Effects of moderate exercise on the pharmacokinetics of caffeine. Eur. J. Clin. Pharmacol. 1991, 40, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Noda, S.; Kitagawa, S.; Morishita, T. Proposal of sampling process for collecting human sweat and determination of caffeine concentration in it by using GC/MS. Biomed. Chromatogr. 2000, 14, 505–510. [Google Scholar] [CrossRef]
- Capriola, M. Synthetic cathinone abuse. Clin. Pharmacol. Adv. Appl. 2013, 5, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.L.; Nelson, K.H.; To, P.; López-Arnau, R.; Xu, P.; Wang, D.; Wang, Y.; Shen, H.-w.; Kuhn, D.M.; Angoa-Perez, M. Abuse potential and toxicity of the synthetic cathinones (i.e., “Bath salts”). Neurosci. Biobehav. Rev. 2020, 110, 150–173. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, Y.; Wu, H.; Wang, Y.; Niu, L.; Li, F. Integrated Aptasensor Array for Sweat Drug Analysis. Anal. Chem. 2022, 94, 7936–7943. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, C.C.; Dyer, K.R. A review of the clinical pharmacology of methamphetamine. Addiction 2009, 104, 1085–1099. [Google Scholar] [CrossRef] [PubMed]
- Kevil, C.G.; Goeders, N.E.; Woolard, M.D.; Bhuiyan, M.S.; Dominic, P.; Kolluru, G.K.; Arnold, C.L.; Traylor, J.G.; Orr, A.W. Methamphetamine use and cardiovascular disease: In search of answers. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1739–1746. [Google Scholar] [CrossRef]
- Han, B.; Compton, W.M.; Jones, C.M.; Einstein, E.B.; Volkow, N.D. Methamphetamine use, methamphetamine use disorder, and associated overdose deaths among US adults. JAMA Psychiatry 2021, 78, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.H.; Lee, W.-C.; Choi, Y.-J.; Moon, J.-I.; Jang, J.; Park, S.-G.; Choo, J.; Kim, D.-H.; Jung, H.S. A wearable surface-enhanced Raman scattering sensor for label-free molecular detection. ACS Appl. Mater. Interfaces 2021, 13, 3024–3032. [Google Scholar] [CrossRef]
- Criscuolo, F.; Hanitra, I.N.; Aiassa, S.; Taurino, I.; Oliva, N.; Carrara, S.; De Micheli, G. Wearable multifunctional sweat-sensing system for efficient healthcare monitoring. Sens. Actuators B Chem. 2021, 328, 129017. [Google Scholar] [CrossRef]
- Bonato, P. Advances in wearable technology and applications in physical medicine and rehabilitation. J. Neuroeng. Rehabil. 2005, 2, 2. [Google Scholar] [CrossRef]
- Lombardi, J.R.; Birke, R.L. A Unified View of Surface-Enhanced Raman Scattering. Acc. Chem. Res. 2009, 42, 734–742. [Google Scholar] [CrossRef]
- Schatz, G.C.; Young, M.A.; Van Duyne, R.P. Electromagnetic Mechanism of SERS. In Surface-Enhanced Raman Scattering: Physics and Applications; Kneipp, K., Moskovits, M., Kneipp, H., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 19–45. [Google Scholar]
- Jiang; Bosnick, K.; Maillard, M.; Brus, L. Single Molecule Raman Spectroscopy at the Junctions of Large Ag Nanocrystals. J. Phys. Chem. B 2003, 107, 9964–9972. [Google Scholar] [CrossRef]
- Gong, T.; Das, C.M.; Yin, M.-J.; Lv, T.-R.; Singh, N.M.; Soehartono, A.M.; Singh, G.; An, Q.-F.; Yong, K.-T. Development of SERS tags for human diseases screening and detection. Coord. Chem. Rev. 2022, 470, 214711. [Google Scholar] [CrossRef]
- Guerra Hernández, L.A.; Reynoso, A.A.; Fainstein, A. Does the chemical contribution have a secondary role in SERS? J. Opt. Soc. Am. B 2023, 40, C78–C85. [Google Scholar] [CrossRef]
- Zhao, Z.Q.; Li, Q.J.; Dong, Y.; Gong, J.X.; Li, Z.; Zhang, J.F. Core-shell structured gold nanorods on thread-embroidered fabric-based microfluidic device for Ex Situ detection of glucose and lactate in sweat. Sens. Actuators B-Chem. 2022, 353, 131154. [Google Scholar] [CrossRef]
- Jaworska, A.; Fornasaro, S.; Sergo, V.; Bonifacio, A. Potential of Surface Enhanced Raman Spectroscopy (SERS) in Therapeutic Drug Monitoring (TDM). A Critical Review. Biosensors 2016, 6, 47. [Google Scholar] [CrossRef]
- Sun, L.; Zhao, L.; Peng, R.-Y. Research progress in the effects of terahertz waves on biomacromolecules. Mil. Med. Res. 2021, 8, 28. [Google Scholar] [CrossRef]
- Baghelani, M.; Abbasi, Z.; Daneshmand, M.; Light, P.E. Non-Invasive Lactate Monitoring System Using Wearable Chipless Microwave Sensors With Enhanced Sensitivity and Zero Power Consumption. IEEE Trans. Biomed. Eng. 2022, 69, 3175–3182. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Bteich, M.; Tawk, Y.; Ramadan, A.H.; Dia, B.; Asadallah, F.A.; Eid, A.; Kanj, R.; Costantine, J.; Eid, A.A. Noninvasive, wearable, and tunable electromagnetic multisensing system for continuous glucose monitoring, mimicking vasculature anatomy. Sci. Adv. 2020, 6, eaba5320. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Malik, J.; Seo, J.M.; Cho, Y.M.; Bien, F. Subcutaneously implantable electromagnetic biosensor system for continuous glucose monitoring. Sci. Rep. 2022, 12, 17395. [Google Scholar] [CrossRef] [PubMed]
- Hanna, J.; Tawk, Y.; Azar, S.; Ramadan, A.H.; Dia, B.; Shamieh, E.; Zoghbi, S.; Kanj, R.; Costantine, J.; Eid, A.A. Wearable flexible body matched electromagnetic sensors for personalized non-invasive glucose monitoring. Sci. Rep. 2022, 12, 14885. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Wang, J.; Luo, Y.; Xu, T.; Zhang, X. Wearable Plasmonic Sweat Biosensor for Acetaminophen Drug Monitoring. ACS Sens. 2023, 8, 1766–1773. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, J.; Wang, F.; Kong, D. Stretchable and Superwettable Colorimetric Sensing Patch for Epidermal Collection and Analysis of Sweat. ACS Sens. 2021, 6, 2261–2269. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.Y.; Song, G.J.; Zhong, K.; Wang, Z.C.; Xia, X.; Tian, Y.Q. Wearable fluorescent contact lenses for monitoring glucose via a smartphone. Sens. Actuators B-Chem. 2022, 352, 131067. [Google Scholar] [CrossRef]
- Fan, R.; Andrew, T.L. Perspective—Challenges in Developing Wearable Electrochemical Sensors for Longitudinal Health Monitoring. J. Electrochem. Soc. 2020, 167, 037542. [Google Scholar] [CrossRef]
- Peng, B.; Zhao, F.; Ping, J.; Ying, Y. Recent Advances in Nanomaterial-Enabled Wearable Sensors: Material Synthesis, Sensor Design, and Personal Health Monitoring. Small 2020, 16, 2002681. [Google Scholar] [CrossRef]
- Sato, K. The physiology, pharmacology, and biochemistry of the eccrine sweat gland. Rev. Physiol. Biochem. Pharmacol. 1977, 79, 51–131. [Google Scholar]
- Cui, C.Y.; Schlessinger, D. Eccrine sweat gland development and sweat secretion. Exp. Dermatol. 2015, 24, 644–650. [Google Scholar] [CrossRef]
- Baker, L.B. Physiology of sweat gland function: The roles of sweating and sweat composition in human health. Temperature 2019, 6, 211–259. [Google Scholar] [CrossRef]
- Alvi, S.N.; Abuhdeeb, K.; Hammami, M.M. Sweat Cortisol and Cortisone Determination in Healthy Adults: UHPLC-MS/MS Assay Validation and Clinical Application. Adv. Pharmacol. Pharm. Sci. 2022, 2022, 3133640. [Google Scholar] [CrossRef]
- Basu, S.; Mitra, M.; Ghosh, A. Evaluation of sweat production by pilocarpine iontophoresis: A noninvasive screening tool for hypohidrosis in ectodermal dysplasia. Indian J. Clin. Biochem. IJCB 2013, 28, 433–435. [Google Scholar] [CrossRef]
- Simmers, P.; Li, S.K.; Kasting, G.; Heikenfeld, J. Prolonged and localized sweat stimulation by iontophoretic delivery of the slowly-metabolized cholinergic agent carbachol. J. Dermatol. Sci. 2018, 89, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Illigens, B.M.; Gibbons, C.H. Sweat testing to evaluate autonomic function. Clin. Auton. Res. 2009, 19, 79–87. [Google Scholar] [CrossRef]
- Allen, J.A.; Roddie, I. The role of circulating catecholamines in sweat production in man. J. Physiol. 1972, 227, 801–814. [Google Scholar] [CrossRef]
- Yang, P.; Wei, G.; Liu, A.; Huo, F.; Zhang, Z. A review of sampling, energy supply and intelligent monitoring for long-term sweat sensors. NPJ Flex. Electron. 2022, 6, 33. [Google Scholar] [CrossRef]
- Ravishankar, P.; Daily, A. Tears as the Next Diagnostic Biofluid: A Comparative Study between Ocular Fluid and Blood. Appl. Sci. 2022, 12, 2884. [Google Scholar] [CrossRef]
- Pieragostino, D.; D’Alessandro, M.; di Ioia, M.; Di Ilio, C.; Sacchetta, P.; Del Boccio, P. Unraveling the molecular repertoire of tears as a source of biomarkers: Beyond ocular diseases. Proteom. Clin. Appl. 2015, 9, 169–186. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, S.Z.; Koh, S.K.; Chen, L.; Vaz, C.; Tanavde, V.; Li, X.R.; Beuerman, R.W. In-depth analysis of the human tear proteome. J. Proteom. 2012, 75, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Beuerman, R.W.; Foo, Y.; Liu, S.; Ang, L.P.; Tan, D.T. Characterisation of human tear proteins using high-resolution mass spectrometry. Ann.-Acad. Med. Singap. 2006, 35, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, S.P.; Williamson, R.T. A review of saliva: Normal composition, flow, and function. J. Prosthet. Dent. 2001, 85, 162–169. [Google Scholar] [CrossRef]
- Cawello, W.; Bökens, H.; Nickel, B.; Andreas, J.O.; Halabi, A. Tolerability, pharmacokinetics, and bioequivalence of the tablet and syrup formulations of lacosamide in plasma, saliva, and urine: Saliva as a surrogate of pharmacokinetics in the central compartment. Epilepsia 2013, 54, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Fatima, S.; Rehman, A.; Shah, K.; Kamran, M.; Mashal, S.; Rustam, S.; Sabir, M.; Nayab, A.; Muzammal, M. Composition and Function of Saliva: A Review. World J. Pharm. Pharm. Sci. 2020, 9, 1552–1567. [Google Scholar]
- Konstantinidi, E.M.; Lappas, A.S.; Tzortzi, A.S.; Behrakis, P.K. Exhaled breath condensate: Technical and diagnostic aspects. Sci. World J. 2015, 2015, 435160. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Dörfler, H.; Pagneux, Q.; Daniel, J.; Wadekar, S.; Woitrain, E.; Ladage, D.; Montaigne, D.; Boukherroub, R. Exhaled breath condensate as bioanalyte: From collection considerations to biomarker sensing. Anal. Bioanal. Chem. 2023, 415, 27–34. [Google Scholar] [CrossRef]
- Mutlu, G.M.; Garey, K.W.; Robbins, R.A.; Danziger, L.H.; Rubinstein, I. Collection and analysis of exhaled breath condensate in humans. Am. J. Respir. Crit. Care Med. 2001, 164, 731–737. [Google Scholar] [CrossRef]
- de Paiva, M.J.N.; Menezes, H.C.; de Lourdes Cardeal, Z. Sampling and analysis of metabolomes in biological fluids. Analyst 2014, 139, 3683–3694. [Google Scholar] [CrossRef]
- Ates, H.C.; Brunauer, A.; von Stetten, F.; Urban, G.A.; Güder, F.; Merkoçi, A.; Früh, S.M.; Dincer, C. Integrated Devices for Non-Invasive Diagnostics. Adv. Funct. Mater. 2021, 31, 2010388. [Google Scholar] [CrossRef]
- Guntner, A.T.; Abegg, S.; Konigstein, K.; Gerber, P.A.; Schmidt-Trucksass, A.; Pratsinis, S.E. Breath sensors for health monitoring. ACS Sens. 2019, 4, 268–280. [Google Scholar] [CrossRef]
- Marchei, E.; Farrè, M.; Pellegrini, M.; García-Algar, O.; Vall, O.; Pacifici, R.; Pichini, S. Pharmacokinetics of methylphenidate in oral fluid and sweat of a pediatric subject. Forensic Sci. Int. 2010, 196, 59–63. [Google Scholar] [CrossRef]
- Vasudev, A.; Tripathi, K.D.; Puri, V. Correlation of serum and salivary carbamazepine concentration in epileptic patients: Implications for therapeutic drug monitoring. Neurol. India 2002, 50, 60–62. [Google Scholar] [PubMed]
- Smink, B.E.; Hofman, B.J.; Dijkhuizen, A.; Lusthof, K.J.; De Gier, J.J.; Egberts, A.C.; Uges, D.R. The concentration of oxazepam and oxazepam glucuronide in oral fluid, blood and serum after controlled administration of 15 and 30 mg oxazepam. Br. J. Clin. Pharmacol. 2008, 66, 556–560. [Google Scholar] [CrossRef]
- Sankowski, B.; Michorowska, S.; Raćkowska, E.; Sikora, M.; Giebułtowicz, J. Saliva as Blood Alternative in Therapeutic Monitoring of Teriflunomide—Development and Validation of the Novel Analytical Method. Int. J. Mol. Sci. 2022, 23, 9544. [Google Scholar] [CrossRef]
- Yamada, E.; Takagi, R.; Moro, H.; Sudo, K.; Kato, S. Saliva as a potential matrix for evaluating pharmacologically active dolutegravir concentration in plasma. PLoS ONE 2021, 16, e0246994. [Google Scholar] [CrossRef]
- Dwivedi, R.; Gupta, Y.K.; Singh, M.; Joshi, R.; Tiwari, P.; Kaleekal, T.; Tripathi, M. Correlation of saliva and serum free valproic acid concentrations in persons with epilepsy. Seizure 2015, 25, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, Z.; Cao, Z.; Pan, L.; Shi, Y. Advanced Wearable Microfluidic Sensors for Healthcare Monitoring. Small 2020, 16, 1903822. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Y.; Su, L.; Zhao, D.; Zhao, L.; Zhang, X. Microfluidic Chip-Based Wearable Colorimetric Sensor for Simple and Facile Detection of Sweat Glucose. Anal. Chem. 2019, 91, 14803–14807. [Google Scholar] [CrossRef]
- Koh, A.; Kang, D.; Xue, Y.; Lee, S.; Pielak, R.M.; Kim, J.; Hwang, T.; Min, S.; Banks, A.; Bastien, P. A soft, wearable microfluidic device for the capture, storage, and colorimetric sensing of sweat. Sci. Transl. Med. 2016, 8, 366ra165. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, H.; Kim, S.B.; Wu, Y.; Ostojich, D.; Park, S.H.; Wang, X.; Weng, Z.; Li, R.; Bandodkar, A.J.; et al. Passive sweat collection and colorimetric analysis of biomarkers relevant to kidney disorders using a soft microfluidic system. Lab Chip 2019, 19, 1545–1555. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Jiao, L.; Xiao, X.; Nashalian, A.; Mathur, S.; Zhu, Z.J.; Wu, W.T.; Guo, W.W.; Zhai, Y.L.; Lu, X.Q.; et al. Flexible Prussian Blue-Au Fibers as Robust Peroxidase—Like Nanozymes for Wearable Hydrogen Peroxide and Uric Acid Monitoring. Electroanalysis 2022, 34, 1763–1771. [Google Scholar] [CrossRef]
- Badugu, R.; Jeng, B.H.; Reece, E.A.; Lakowicz, J.R. Contact lens to measure individual ion concentrations in tears and applications to dry eye disease. Anal. Biochem. 2018, 542, 84–94. [Google Scholar] [CrossRef]
- Xu, X.-Y.; Yan, B. A fluorescent wearable platform for sweat Cl− analysis and logic smart-device fabrication based on color adjustable lanthanide MOFs. J. Mater. Chem. C 2018, 6, 1863–1869. [Google Scholar] [CrossRef]
- Hu, B.; Kang, X.; Xu, S.; Zhu, J.; Yang, L.; Jiang, C. Multiplex Chroma Response Wearable Hydrogel Patch: Visual Monitoring of Urea in Body Fluids for Health Prognosis. Anal. Chem. 2023, 95, 3587–3595. [Google Scholar] [CrossRef]
- Bobacka, J. Electrochemical sensors for real-world applications. J. Solid State Electrochem. 2020, 24, 2039–2040. [Google Scholar] [CrossRef]
- Faridbod, F.; Gupta, V.K.; Zamani, H.A. Electrochemical Sensors and Biosensors. Int. J. Electrochem. 2011, 2011, 352546. [Google Scholar] [CrossRef]
- Mahato, K.; Wang, J. Electrochemical sensors: From the bench to the skin. Sens. Actuators B Chem. 2021, 344, 130178. [Google Scholar] [CrossRef]
- Sun, T.; Shen, L.; Jiang, Y.; Ma, J.; Lv, F.; Ma, H.; Chen, D.; Zhu, N. Wearable Textile Supercapacitors for Self-Powered Enzyme-Free Smartsensors. ACS Appl. Mater. Interfaces 2020, 12, 21779–21787. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Rinawati, M.; Zhan, J.-D.; Lin, K.-Y.; Huang, C.-J.; Chen, K.-J.; Mizuguchi, H.; Jiang, J.-C.; Hwang, B.-J.; Yeh, M.-H. Boron-Doped Graphene Quantum Dots Anchored to Carbon Nanotubes as Noble Metal-Free Electrocatalysts of Uric Acid for a Wearable Sweat Sensor. ACS Appl. Nano Mater. 2022, 5, 11100–11110. [Google Scholar] [CrossRef]
- Vivekanandan, A.K.; Muthukutty, B.; Chen, S.-M.; Sivakumar, M.; Chen, S.-H. Intermetallic Compound Cu2Sb Nanoparticles for Effective Electrocatalytic Oxidation of an Antibiotic Drug: Sulphadiazine. ACS Sustain. Chem. Eng. 2020, 8, 17718–17726. [Google Scholar] [CrossRef]
- Rajaji, U.; Raghu, M.S.; Kumar, K.Y.; Al-Kahtani, A.A.; Chen, C.-P.; Juang, R.-S.; Liu, T.-Y. Electrocatalytic oxidation and amperometric determination of sulfasalazine using bimetal oxide nanoparticles-decorated graphene oxide composite modified glassy carbon electrode at neutral pH. Microchim. Acta 2022, 189, 409. [Google Scholar] [CrossRef] [PubMed]
- Kesavan, G.; Vinothkumar, V.; Chen, S.-M. Sonochemical synthesis of copper vanadate nanoparticles for the highly selective voltammetric detection of antibiotic drug ornidazole. J. Alloys Compd. 2021, 867, 159019. [Google Scholar] [CrossRef]
- Kumar, V.; Suri, R.; Mittal, S. Review on new ionophore species for membrane ion selective electrodes. J. Iran. Chem. Soc. 2023, 20, 509–540. [Google Scholar] [CrossRef]
- Lyu, Y.; Gan, S.; Bao, Y.; Zhong, L.; Xu, J.; Wang, W.; Liu, Z.; Ma, Y.; Yang, G.; Niu, L. Solid-Contact Ion-Selective Electrodes: Response Mechanisms, Transducer Materials and Wearable Sensors. Membranes 2020, 10, 128. [Google Scholar] [CrossRef]
- Lim, H.-R.; Kim, Y.-S.; Kwon, S.; Mahmood, M.; Kwon, Y.-T.; Lee, Y.; Lee, S.M.; Yeo, W.-H. Wireless, Flexible, Ion-Selective Electrode System for Selective and Repeatable Detection of Sodium. Sensors 2020, 20, 3297. [Google Scholar] [CrossRef] [PubMed]
- Criscuolo, F.; Cantu, F.; Taurino, I.; Carrara, S.; De Micheli, G. A wearable electrochemical sensing system for non-invasive monitoring of lithium drug in bipolar disorder. IEEE Sens. J. 2020, 21, 9649–9656. [Google Scholar] [CrossRef]
- Kim, J.; Jeerapan, I.; Sempionatto, J.R.; Barfidokht, A.; Mishra, R.K.; Campbell, A.S.; Hubble, L.J.; Wang, J. Wearable Bioelectronics: Enzyme-Based Body-Worn Electronic Devices. Acc. Chem. Res. 2018, 51, 2820–2828. [Google Scholar] [CrossRef] [PubMed]
- Shiwaku, R.; Matsui, H.; Nagamine, K.; Uematsu, M.; Mano, T.; Maruyama, Y.; Nomura, A.; Tsuchiya, K.; Hayasaka, K.; Takeda, Y.; et al. A Printed Organic Circuit System for Wearable Amperometric Electrochemical Sensors. Sci. Rep. 2018, 8, 6368. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Zhang, X.R.; Liu, Y.Q.; Li, J.M.; Zhu, L.; Lu, Y.; Guo, X.S.; Chen, D.J. An enzyme-particle hybrid ink for one step screen-printing and long-term metabolism monitoring. Anal. Chim. Acta 2022, 1221, 340168. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhao, J.Q.; Wang, C.C.; Zhu, L.; Pan, X.Y.; Liu, Y.Q.; Li, J.M.; Guo, X.S.; Chen, D.J. Measurement of sucrose in beverages using a blood glucose meter with cascade-catalysis enzyme particle. Food Chem. 2023, 398, 133951. [Google Scholar] [CrossRef]
- Gill, H.S.; Denson, D.D.; Burris, B.A.; Prausnitz, M.R. Effect of microneedle design on pain in human volunteers. Clin. J. Pain. 2008, 24, 585–594. [Google Scholar] [CrossRef]
- Dhanjai; Lu, X.; Wu, L.; Chen, J.; Lu, Y. Robust Single-Molecule Enzyme Nanocapsules for Biosensing with Significantly Improved Biosensor Stability. Anal. Chem. 2020, 92, 5830–5837. [Google Scholar] [CrossRef] [PubMed]
- Hiraka, K.; Tsugawa, W.; Asano, R.; Yokus, M.A.; Ikebukuro, K.; Daniele, M.A.; Sode, K. Rational design of direct electron transfer type l-lactate dehydrogenase for the development of multiplexed biosensor. Biosens. Bioelectron. 2021, 176, 112933. [Google Scholar] [CrossRef]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Phypers, B.; Pierce, J.T. Lactate physiology in health and disease. Contin. Educ. Anaesth. Crit. Care Pain 2006, 6, 128–132. [Google Scholar] [CrossRef]
- Fiore, L.; Mazzaracchio, V.; Serani, A.; Fabiani, G.; Fabiani, L.; Volpe, G.; Moscone, D.; Bianco, G.M.; Occhiuzzi, C.; Marrocco, G.; et al. Microfluidic paper-based wearable electrochemical biosensor for reliable cortisol detection in sweat. Sens. Actuators B Chem. 2023, 379, 133258. [Google Scholar] [CrossRef]
- Huynh, V.L.; Trung, T.Q.; Meeseepong, M.; Lee, H.-B.; Nguyen, T.D.; Lee, N.-E. Hollow Microfibers of Elastomeric Nanocomposites for Fully Stretchable and Highly Sensitive Microfluidic Immunobiosensor Patch. Adv. Funct. Mater. 2020, 30, 2004684. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, Y.; Hu, X.; Zhao, F.; Zeng, B. Dual-Mode Electrochemical Competitive Immunosensor Based on Cd2+/Au/Polydopamine/Ti3C2 Composite and Copper-Based Metal–Organic Framework for 17β-Estradiol Detection. ACS Sens. 2022, 7, 3077–3084. [Google Scholar] [CrossRef] [PubMed]
- Madhu, S.; Ramasamy, S.; Magudeeswaran, V.; Manickam, P.; Nagamony, P.; Chinnuswamy, V. SnO2 nanoflakes deposited carbon yarn-based electrochemical immunosensor towards cortisol measurement. J. Nanostruct. Chem. 2023, 13, 115–127. [Google Scholar] [CrossRef]
- Martins, T.S.; Bott-Neto, J.L.; Machado, S.A.S.; Oliveira, O.N. Label-Free Electrochemical Immunosensor Made with Tree-like Gold Dendrites for Monitoring 25-Hydroxyvitamin D3 Metabolite. ACS Appl. Mater. Interfaces 2022, 14, 31455–31462. [Google Scholar] [CrossRef]
- Zhai, Q.; Yap, L.W.; Wang, R.; Gong, S.; Guo, Z.; Liu, Y.; Lyu, Q.; Wang, J.; Simon, G.P.; Cheng, W. Vertically Aligned Gold Nanowires as Stretchable and Wearable Epidermal Ion-Selective Electrode for Noninvasive Multiplexed Sweat Analysis. Anal. Chem. 2020, 92, 4647–4655. [Google Scholar] [CrossRef] [PubMed]
- Ribet, F.; Stemme, G.; Roxhed, N. Real-time intradermal continuous glucose monitoring using a minimally invasive microneedle-based system. Biomed. Microdevices 2018, 20, 101. [Google Scholar] [CrossRef]
- Li, S.; Lin, L.; Chang, X.; Si, Z.; Plaxco, K.W.; Khine, M.; Li, H.; Xia, F. A wrinkled structure of gold film greatly improves the signaling of electrochemical aptamer-based biosensors. RSC Adv. 2020, 11, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, Z.; Roushani, M.; Hosseini, H.; Choobin, H. Label-free electrochemical aptasensor for rapid detection of SARS-CoV-2 spike glycoprotein based on the composite of Cu(OH)(2) nanorods arrays as a high-performance surface substrate. Bioelectrochemistry 2022, 146, 108106. [Google Scholar] [CrossRef]
- Hou, Y.; Xu, Q.; Li, Y.; Long, N.; Li, P.; Wang, J.; Zhou, L.; Sheng, P.; Kong, W. Ultrasensitive electrochemical aptasensor with Nafion-stabilized f-MWCNTs as signal enhancers for OTA detection. Bioelectrochemistry 2023, 151, 108399. [Google Scholar] [CrossRef]
- Pellitero, M.A.; Arroyo-Curras, N. Study of surface modification strategies to create glassy carbon-supported, aptamer-based sensors for continuous molecular monitoring. Anal. Bioanal. Chem. 2022, 414, 5627–5641. [Google Scholar] [CrossRef]
- Nagata, M.; Lee, J.; Henley, S.; Ikebukuro, K.; Sode, K. An Amine-Reactive Phenazine Ethosulfate (arPES)—A Novel Redox Probe for Electrochemical Aptamer-Based Sensor. Sensors 2022, 22, 1760. [Google Scholar] [CrossRef]
- Ji, X.; Lin, X.; Rivnay, J. Organic electrochemical transistors as on-site signal amplifiers for electrochemical aptamer-based sensing. Nat. Commun. 2023, 14, 1665. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, C.; Figueroa-Miranda, G.; Offenhaeusser, A.; Mayer, D. Amplification of aptamer sensor signals by four orders of magnitude via interdigitated organic electrochemical transistors. Biosens. Bioelectron. 2019, 144, 111668. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Midinov, B.; White, R.J. Electrochemical Aptamer-Based Sensor for Real-Time Monitoring of Insulin. ACS Sens. 2019, 4, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Shaver, A.; Mahlum, J.D.; Scida, K.; Johnston, M.L.; Pellitero, M.A.; Wu, Y.; Carr, G.V.; Arroyo-Curras, N. Optimization of Vancomycin Aptamer Sequence Length Increases the Sensitivity of Electrochemical, Aptamer-Based Sensors In Vivo. ACS Sens. 2022, 7, 3895–3905. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, M.S.; Ngwa, W.; Chow, K.-F. Direct Electrochemical Aptamer-Based Detection of Digoxin. ChemistrySelect 2020, 5, 2408–2411. [Google Scholar] [CrossRef]
- Uehara, O.; Kusuhara, T.; Matsuzaki, K.; Yamamoto, Y.; Nakamura, T. Skin Electrical Impedance Model for Evaluation of the Thickness and Water Content of the Stratum Corneum. Adv. Biomed. Eng. 2022, 11, 98–108. [Google Scholar] [CrossRef]
- Scholten, K.; Meng, E. A review of implantable biosensors for closed-loop glucose control and other drug delivery applications. Int. J. Pharm. 2018, 544, 319–334. [Google Scholar] [CrossRef]
- Mei, R.; Wang, Y.; Shi, S.; Zhao, X.; Zhang, Z.; Wang, X.; Shen, D.; Kang, Q.; Chen, L. Highly Sensitive and Reliable Internal-Standard Surface-Enhanced Raman Scattering Microneedles for Determination of Bacterial Metabolites as Infection Biomarkers in Skin Interstitial Fluid. Anal. Chem. 2022, 94, 16069–16078. [Google Scholar] [CrossRef]
- Rojahn, T.B.; Vorstandlechner, V.; Krausgruber, T.; Bauer, W.M.; Alkon, N.; Bangert, C.; Thaler, F.M.; Sadeghyar, F.; Fortelny, N.; Gernedl, V.; et al. Single-cell transcriptomics combined with interstitial fluid proteomics defines cell type-specific immune regulation in atopic dermatitis. J. Allergy Clin. Immunol. 2020, 146, 1056–1069. [Google Scholar] [CrossRef]
- Miller, P.R.; Narayan, R.J.; Polsky, R. Microneedle-based sensors for medical diagnosis. J. Mater. Chem. B 2016, 4, 1379–1383. [Google Scholar] [CrossRef]
- Miller, P.R.; Taylor, R.M.; Tran, B.Q.; Boyd, G.; Glaros, T.; Chavez, V.H.; Krishnakumar, R.; Sinha, A.; Poorey, K.; Williams, K.P.; et al. Extraction and biomolecular analysis of dermal interstitial fluid collected with hollow microneedles. Commun. Biol. 2018, 1, 173. [Google Scholar] [CrossRef]
- Ita, K. Transdermal delivery of drugs with microneedles: Strategies and outcomes. J. Drug Deliv. Sci. Technol. 2015, 29, 16–23. [Google Scholar] [CrossRef]
- Parrilla, M.; Vanhooydonck, A.; Johns, M.; Watts, R.; De Wael, K. 3D-printed microneedle-based potentiometric sensor for pH monitoring in skin interstitial fluid. Sens. Actuators B-Chem. 2023, 378, 133159. [Google Scholar] [CrossRef]
- Lee, H.J.; Son, Y.; Kim, D.; Kim, Y.K.; Choi, N.; Yoon, E.-S.; Cho, I.-J. A new thin silicon microneedle with an embedded microchannel for deep brain drug infusion. Sens. Actuators B Chem. 2015, 209, 413–422. [Google Scholar] [CrossRef]
- Heifler, O.; Borberg, E.; Harpak, N.; Zverzhinetsky, M.; Krivitsky, V.; Gabriel, I.; Fourman, V.; Sherman, D.; Patolsky, F. Clinic-on-a-Needle Array toward Future Minimally Invasive Wearable Artificial Pancreas Applications. ACS Nano 2021, 15, 12019–12033. [Google Scholar] [CrossRef]
- Harvey, J.D.; Williams, R.M.; Tully, K.M.; Baker, H.A.; Shamay, Y.; Heller, D.A. An in Vivo Nanosensor Measures Compartmental Doxorubicin Exposure. Nano Lett. 2019, 19, 4343–4354. [Google Scholar] [CrossRef]
- Frost, M.C.; Meyerhoff, M.E. Implantable chemical sensors for real-time clinical monitoring: Progress and challenges. Curr. Opin. Chem. Biol. 2002, 6, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Barbosa, A.I.; Rebelo, R.; Kwon, I.K.; Reis, R.L.; Correlo, V.M. Skin-Integrated Wearable Systems and Implantable Biosensors: A Comprehensive Review. Biosensors 2020, 10, 79. [Google Scholar] [CrossRef]
- Moret, M.G.; Rodriguez, T.M. Ageism in the School: Do Stereotypes about Ageing Exist among Future Teachers? Rev. Educ. 2019, 43, 577–587. [Google Scholar]
- Chen, Z.; Chen, Y.; Hedenqvist, M.S.; Chen, C.; Cai, C.; Li, H.; Liu, H.; Fu, J. Multifunctional conductive hydrogels and their applications as smart wearable devices. J. Mater. Chem. B 2021, 9, 2561–2583. [Google Scholar] [CrossRef] [PubMed]
- Kougkolos, G.; Golzio, M.; Laudebat, L.; Valdez-Nava, Z.; Flahaut, E. Hydrogels with electrically conductive nanomaterials for biomedical applications. J. Mater. Chem. B 2023, 11, 2036–2062. [Google Scholar] [CrossRef] [PubMed]
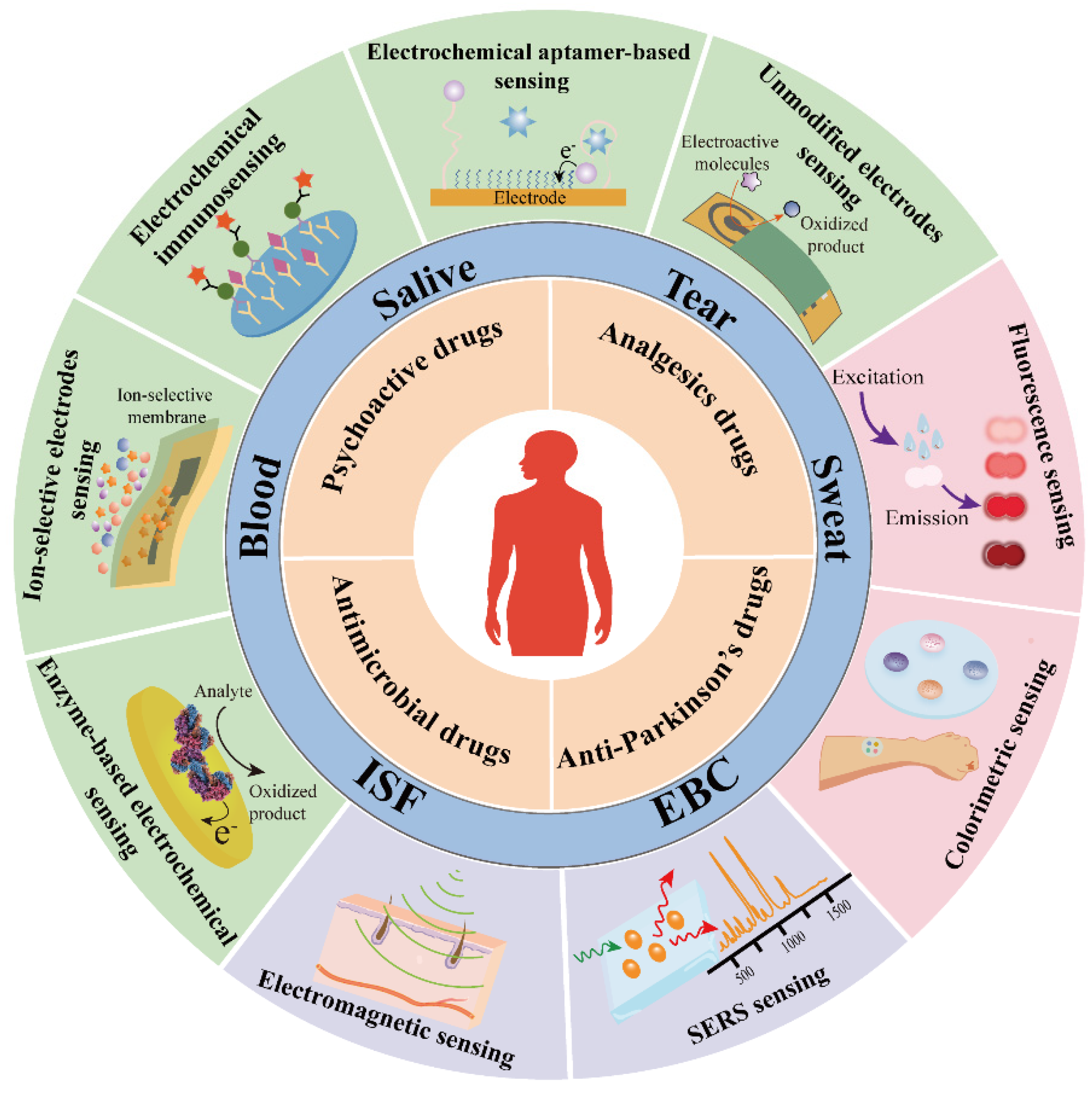
| Type of Drugs | Compound | Martrix | Biofluid Level | Ref |
|---|---|---|---|---|
| Immunosuppressants | Tacrolimus | Serum | 0.01–0.015 μg mL−1 | [20] |
| Cyclosporin | Serum | 80–1000 μg mL−1 | [21,22] | |
| Antiepileptic | Carbamazepine | Serum | 6000–8000 μg mL−1 | [23] |
| Phenytoin sodium | Serum | 10–20 μg mL−1 | [24,25] | |
| Phenobarbital | Serum | 10–40 μg mL−1 | [26] | |
| Valproic acid | Serum | 50–100 μg mL−1 | [27] | |
| Lamotrigine | Serum | 2.5–15 μg mL−1 | [28] | |
| Levetiracetam | Serum | 12–46 μg mL−1 | [28] | |
| Antimicrobial drugs | Vancomycin | Serum | 0.005–0.04 μg mL−1 | [29] |
| Sweat | 8.7–50.7 μg mL−1 | [30] | ||
| Meropenem | Serum | 8–32 μg mL−1 | [31] | |
| Linezolid | Serum | 2–7 μg mL−1 | [32] | |
| ISF | 0.101–1.2 μg mL−1 | [33] | ||
| Tobramycin | Serum | 4–6 μg mL−1 | [34] | |
| Voricnazole | Serum | 0.5–5 μg mL−1 | [32] | |
| Cardioactive drugs | Digoxin | Serum | 0.001–0.0025 μg mL−1 | [35] |
| Antidepressants | Lithium | Serum | 44.4–66.6 μg mL−1 | [36] |
| Analgesics drugs | Fentanyl | Serum | 1–3 μg mL−1 | [37] |
| Sweat | 0.17–1.02 μg mL−1 | [38] | ||
| Methadone | Serum | 0.08–0.7 μg mL−1 | [39] | |
| Sweat | 120–2160 ng patch−1 | [40] | ||
| Anti-asthmatic drugs | Theophylline | Serum | 5–15 μg mL−1 | [41] |
| Antipsychotic drugs | Clozapine | Serum | 0.35–0.5 μg mL−1 | [42] |
| Risperidone | Serum | 0.02–0.06 μg mL−1 | [42] | |
| Perphenazine | Plasma | 0.0012–0.0024 μg mL−1 | [43] | |
| Fluphenazine | Plasma | 0.0002–0.002 μg mL−1 | [43] | |
| Thiothixene | Plasma | 0.002–0.015 μg mL−1 | [43] | |
| Olanzapine | Serum | 0.002–0.004 μg mL−1 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Li, J.; Xiao, S.; Liu, Y.; Bai, M.; Gong, L.; Zhao, J.; Chen, D. Revolutionizing Precision Medicine: Exploring Wearable Sensors for Therapeutic Drug Monitoring and Personalized Therapy. Biosensors 2023, 13, 726. https://doi.org/10.3390/bios13070726
Liu Y, Li J, Xiao S, Liu Y, Bai M, Gong L, Zhao J, Chen D. Revolutionizing Precision Medicine: Exploring Wearable Sensors for Therapeutic Drug Monitoring and Personalized Therapy. Biosensors. 2023; 13(7):726. https://doi.org/10.3390/bios13070726
Chicago/Turabian StyleLiu, Yuqiao, Junmin Li, Shenghao Xiao, Yanhui Liu, Mingxia Bai, Lixiu Gong, Jiaqian Zhao, and Dajing Chen. 2023. "Revolutionizing Precision Medicine: Exploring Wearable Sensors for Therapeutic Drug Monitoring and Personalized Therapy" Biosensors 13, no. 7: 726. https://doi.org/10.3390/bios13070726
APA StyleLiu, Y., Li, J., Xiao, S., Liu, Y., Bai, M., Gong, L., Zhao, J., & Chen, D. (2023). Revolutionizing Precision Medicine: Exploring Wearable Sensors for Therapeutic Drug Monitoring and Personalized Therapy. Biosensors, 13(7), 726. https://doi.org/10.3390/bios13070726




