A Metasurface Plasmonic Analysis Platform Combined with Gold Nanoparticles for Ultrasensitive Quantitative Detection of Small Molecules
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Fabrication of the MetaSPR Chip and Biosensor
2.3. Synthesis of AuNP and Labeled Antibodies
2.4. Functionalizing the Surface of the MetaSPR Chip
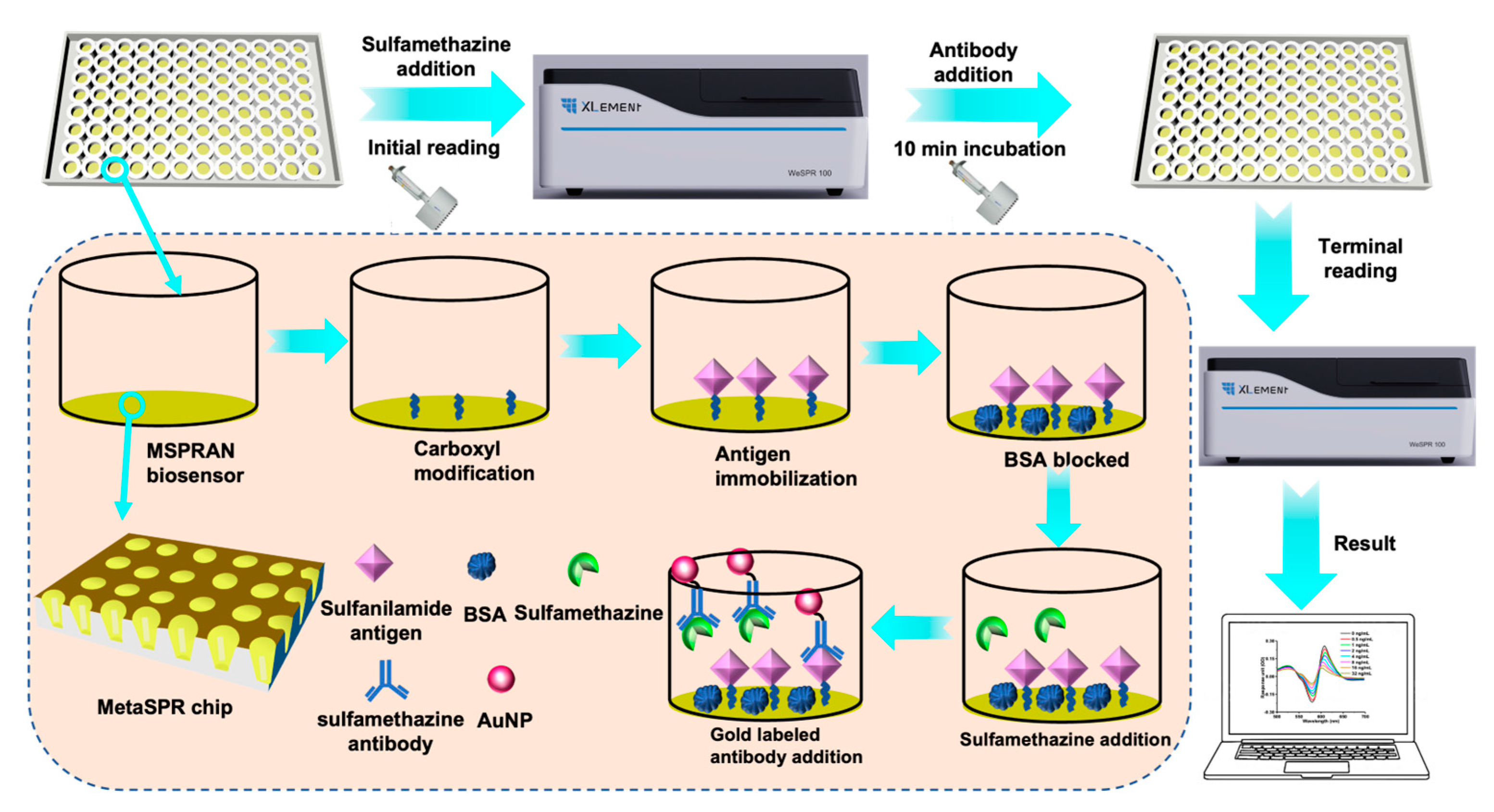
2.5. Measurement of Small Molecules Using the MSPRAN Biosensor
2.6. Small-Molecule Detection in Whole Egg
3. Results and Discussion
3.1. Principle of Small-Molecule Detection Using the MSPRAN Biosensor
3.2. Characterization of MetaSPR Chip and AuNPs
3.3. Assessing Antibody Immobilization and Optimization of Testing Conditions
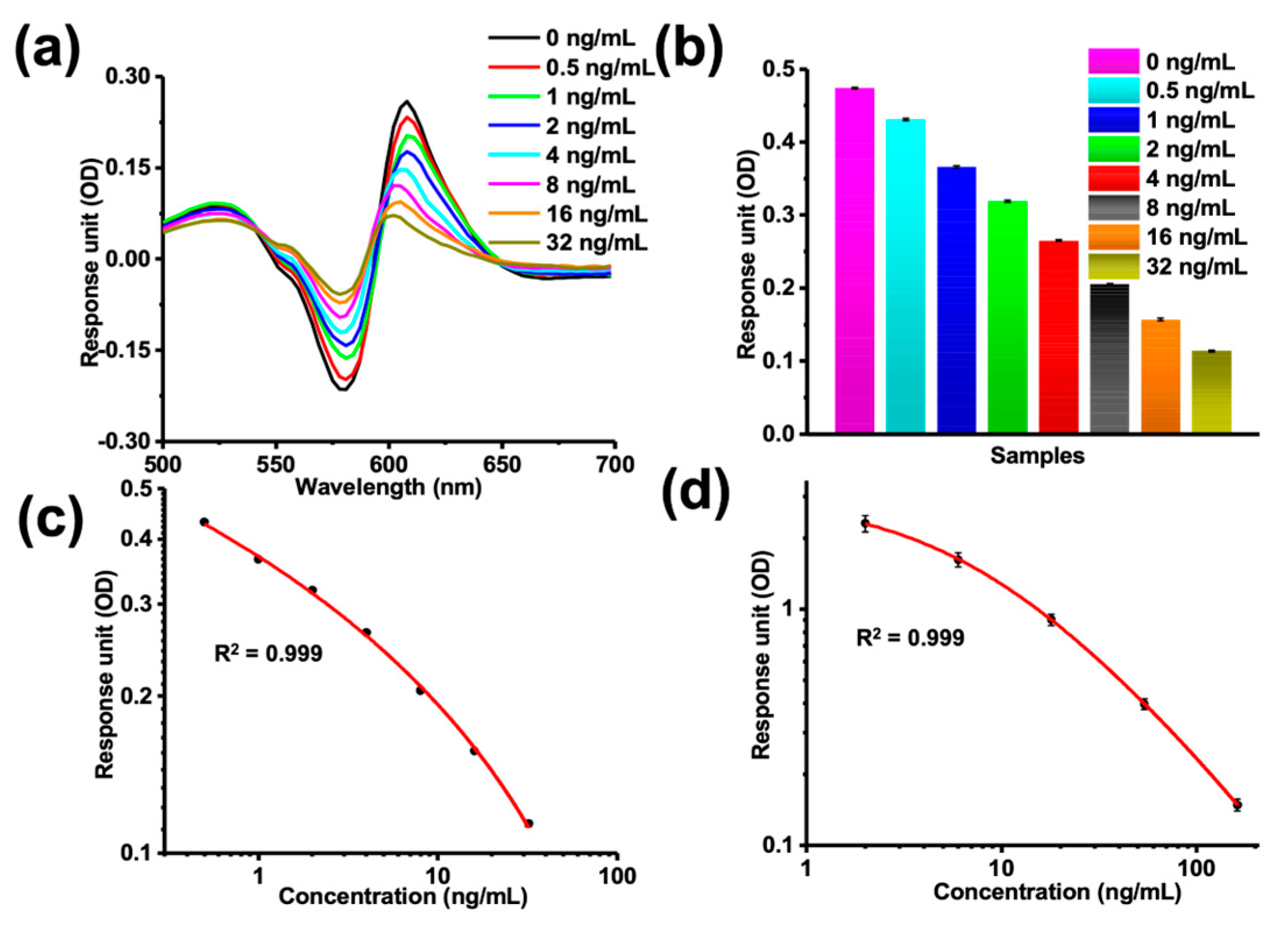
3.4. Establishment of a Standard Curve for Small-Molecule Quantitative Analysis
3.5. MSPRAN Performance Evaluation for Sulfamethazine Detection
3.6. Sulfamethazine Analysis in Whole Eggs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Campbell, K.; McGrath, T.; Sjölander, S.; Hanson, T.; Tidare, M.; Jansson, Ö.; Moberg, A.; Mooney, M.; Elliott, C.; Buijs, J. Use of a novel micro-fluidic device to create arrays for multiplex analysis of large and small molecular weight compounds by surface plasmon resonance. Biosens. Bioelectron. 2011, 26, 3029–3036. [Google Scholar] [CrossRef]
- Guo, Z.; Bai, L.; Gong, S. Government regulations and voluntary certifications in food safety in China: A review. Trends Food Sci. Technol. 2019, 90, 160–165. [Google Scholar] [CrossRef]
- Gavage, M.; Delahaut, P.; Gillard, N. Suitability of high-resolution mass spectrometry for routine analysis of small molecules in food, feed and water for safety and authenticity purposes: A review. Foods 2021, 10, 601. [Google Scholar] [CrossRef]
- Medina, D.A.V.; Borsatto, J.V.B.; Maciel, E.V.S.; Lancas, F.M. Current role of modern chromatography and mass spectrometry in the analysis of mycotoxins in food. TrAC Trends Anal. Chem. 2021, 135, 116156. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, C.X.; Li, Z.Y.; Zheng, Z.Y.; Xiang, Y.; Liu, Y.; Zhao, R.F.; Fang, J. Detection of fluoroquinolone and sulfamethazine residues in poultry eggs in Kunming city, southwest China. Poult. Sci. 2022, 101, 101892. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef]
- De Girolamo, A.; Lippolis, V.; Pascale, M. Overview of Recent Liquid Chromatography Mass Spectrometry-Based Methods for Natural Toxins Detection in Food Products. Toxins 2022, 14, 328. [Google Scholar] [CrossRef]
- Sang, P.; Lu, G.; Yu, D.; Song, X.; Guo, Y.; Xie, Y.; Yao, W.; Qian, H.; Hu, Z. Simultaneous Determination of Antibiotics, Mycotoxins, and Hormones in Milk by an 8–17 DNAzyme-Based Enzyme-Linked Immunosorbent Assay. J. Agric. Food Chem. 2022, 70, 12681–12688. [Google Scholar] [CrossRef]
- Yang, F.; Wang, C.; Yu, H.; Guo, Y.; Cheng, Y.; Yao, W.; Xie, Y. Establishment of the thin-layer chromatography-surface-enhanced Raman spectroscopy and chemometrics method for simultaneous identification of eleven illegal drugs in anti-rheumatic health food. Food Biosci. 2022, 49, 101842. [Google Scholar] [CrossRef]
- Majer-Baranyi, K.; Adányi, N.; Székács, A. Biosensors for Deoxynivalenol and Zearalenone Determination in Feed Quality Control. Toxins 2021, 13, 499. [Google Scholar] [CrossRef]
- Jia, M.; Liao, X.; Fang, L.; Jia, B.; Liu, M.; Li, D.; Zhou, L.; Kong, W. Recent advances on immunosensors for mycotoxins in foods and other commodities. TrAC Trends Anal. Chem. 2021, 136, 116193. [Google Scholar] [CrossRef]
- Huang, L.; Ding, L.; Zhou, J.; Chen, S.; Chen, F.; Zhao, C.; Xu, J.; Hu, W.; Ji, J.; Xu, H. One-step rapid quantification of SARS-CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point-of-care device. Biosens. Bioelectron. 2021, 171, 112685. [Google Scholar] [CrossRef]
- An, N.; Li, K.; Zhang, Y.; Wen, T.; Jin, W. A multiplex and regenerable surface plasmon resonance (MR-SPR) biosensor for DNA detection of genetically modified organisms. Talanta 2021, 231, 122361. [Google Scholar] [CrossRef]
- Arsenin, A.V.; Stebunov, Y.V.; Volkov, V.S. Graphene oxide linking layers for highly sensitive SPR biosensing of small molecules. Mater. Today Proc. 2018, 5, 17437–17441. [Google Scholar] [CrossRef]
- Huang, H.; Li, J.; Pan, S.; Wang, H.; Liang, A.; Jiang, Z. A novel small molecular liquid crystal catalytic amplification-nanogold SPR aptamer absorption assay for trace oxytetracycline. Talanta 2021, 233, 122528. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Huang, L.; Li, R.; Chen, M.; Huang, J.; Xu, H.; Hu, W.; Liu, G.L. A Nanoplasmonic Portable Molecular Interaction Platform for High-Throughput Drug Screening. Adv. Funct. Mater. 2022, 2203635. [Google Scholar] [CrossRef]
- Liu, J.; Chen, P.; Hu, X.; Huang, L.; Geng, Z.; Xu, H.; Hu, W.; Wang, L.; Wu, P.; Liu, G.L. An ultra-sensitive and specific nanoplasmonic-enhanced isothermal amplification platform for the ultrafast point-of-care testing of SARS-CoV-2. Chem. Eng. J. 2023, 451, 138822. [Google Scholar] [CrossRef]
- Akgönüllü, S.; Yavuz, H.; Denizli, A. SPR nanosensor based on molecularly imprinted polymer film with gold nanoparticles for sensitive detection of aflatoxin B1. Talanta 2020, 219, 121219. [Google Scholar] [CrossRef]
- Vinci, G.; Rapa, M. Noble metal nanoparticles applications: Recent trends in food control. Bioengineering 2019, 6, 10. [Google Scholar] [CrossRef]
- Nejati, K.; Dadashpour, M.; Gharibi, T.; Mellatyar, H.; Akbarzadeh, A. Biomedical Applications of Functionalized Gold Nanoparticles: A Review. J. Clust. Sci. 2022, 33, 1–16. [Google Scholar] [CrossRef]
- Melaine, F.; Roupioz, Y.; Buhot, A. Gold Nanoparticles Surface Plasmon Resonance Enhanced Signal for the Detection of Small Molecules on Split-Aptamer Microarrays (Small Molecules Detection from Split-Aptamers). Microarrays 2015, 4, 41–52. [Google Scholar] [CrossRef]
- Hua, Z.; Yu, T.; Liu, D.; Xianyu, Y. Recent advances in gold nanoparticles-based biosensors for food safety detection. Biosens. Bioelectron. 2021, 179, 113076. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Griffith, B.; Bhomkar, P.; Wishart, D.S.; McDermott, M.T. Functionalized gold nanoparticle-enhanced competitive assay for sensitive small-molecule metabolite detection using surface plasmon resonance. Analyst 2018, 143, 289–296. [Google Scholar] [CrossRef]
- Dang, T.; Hu, W.; Zhang, W.; Song, Z.; Wang, Y.; Chen, M.; Xu, H.; Liu, G.L. Protein binding kinetics quantification via coupled plasmonic-photonic resonance nanosensors in generic microplate reader. Biosens. Bioelectron. 2019, 142, 111494. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Dang, T.; Li, Y.; Liang, J.; Xu, H.; Liu, G.L.; Hu, W. Digital plasmonic immunosorbent assay for dynamic imaging detection of protein binding. Sens. Actuators B Chem. 2021, 348, 130711. [Google Scholar] [CrossRef]
- Huang, L.; Li, Y.; Luo, C.; Chen, Y.; Touil, N.; Annaz, H.-E.; Zeng, S.; Dang, T.; Liang, J.; Hu, W. Novel nanostructure-coupled biosensor platform for one-step high-throughput quantification of serum neutralizing antibody after COVID-19 vaccination. Biosens. Bioelectron. 2022, 199, 113868. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Lu, X.-M.; Zhu, F.; Zhao, M. The preparation of gold nanoparticles and evaluation of their immunological function effects on rats. Bio-Med. Mater. Eng. 2014, 24, 885–892. [Google Scholar] [CrossRef]
- Philip, A.; Kumar, A.R. The performance enhancement of surface plasmon resonance optical sensors using nanomaterials: A review. Coord. Chem. Rev. 2022, 458, 214424. [Google Scholar] [CrossRef]
- Islam, M.A.; Jacob, M.V.; Antunes, E. A critical review on silver nanoparticles: From synthesis and applications to its mitigation through low-cost adsorption by biochar. J. Environ. Manag. 2021, 281, 111918. [Google Scholar] [CrossRef]
- Fukuma, M.; Noda, J. Optical properties of titanium-diffused LiNbO3 strip waveguides and their coupling-to-a-fiber characteristics. Appl. Opt. 1980, 19, 591–597. [Google Scholar] [CrossRef]
- Türkmen, D.; Bakhshpour, M.; Göktürk, I.; Aşır, S.; Yılmaz, F.; Denizli, A. Selective dopamine detection by SPR sensor signal amplification using gold nanoparticles. New J. Chem. 2021, 45, 18296–18306. [Google Scholar] [CrossRef]
- Shu, R.; Liu, S.; Huang, L.; Li, Y.; Sun, J.; Zhang, D.; Zhu, M.-Q.; Wang, J. Enzyme-Mimetic nano-immunosensors for amplified detection of food hazards: Recent advances and future trends. Biosens. Bioelectron. 2022, 217, 114577. [Google Scholar] [CrossRef] [PubMed]
- Masdor, N. Determination of the detection limit using the four-parameter logistic model for the double-antibody sandwich ELISA for the rapid detection of Bacillus cereus in food. J. Environ. Microbiol. Toxicol. 2017, 5, 12–13. [Google Scholar] [CrossRef]
- Li, S.; Zhang, C.; Tang, H.-X.; Gu, Y.; Guo, A.-J.; Wang, K.; Lian, K.-Q. Determination of 24 sulfamethazine antibiotics in instant pastries by modified QuEChERS coupled with ultra performance liquid chromatography-tandem mass spectrometry. J. Food Drug Anal. 2023, 31, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, Z.; Zhang, L.; Xu, Y.; Xiong, J.; Zhang, H.; He, Z.; Zheng, Y.; Jiang, H.; Shen, J. Adsorption and convenient ELISA detection of sulfamethazine in milk based on MOFs pretreatment. Food Chem. 2022, 374, 131712. [Google Scholar] [CrossRef] [PubMed]
- Premarathne, J.M.K.J.K.; Satharasinghe, D.A.; Gunasena, A.R.C.; Wanigasekara, A.; Munasinghe, D.M.S.; Abeynayake, P. Thin-layer Chromatographic Method for Quantification of Sulfamethazines in Chicken Meat. Food Anal. Methods 2018, 11, 2666–2672. [Google Scholar] [CrossRef]



| Sample | Detection Method | Limit of Detection | Reference |
|---|---|---|---|
| Sulfamethazine | UPLC–MS/MS | 0.14 μg/kg | [34] |
| ELISA | 0.19 ng/mL | [35] | |
| TLC | 40 ng/g | [36] | |
| MSPRAN biosensor | 73 pg/mL | This work |
| Concentration (ppb) | ELISA | MSPRAN | ||
|---|---|---|---|---|
| Average (ppb) | Recovery (%) | Average (ppb) | Recovery (%) | |
| 2 | 1.92 ± 0.03 | 96.20 | 1.75 ± 0.16 | 87.29 |
| 4 | 4.51 ± 0.07 | 112.60 | 3.92 ± 0.12 | 98.00 |
| 16 | 17.4 ± 006 | 109.30 | 19.1 ± 0.18 | 119.30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Ji, W.; Fan, H.; Zhang, L.; Wan, X.; Fan, Z.; Liu, G.L.; Peng, Q.; Huang, L. A Metasurface Plasmonic Analysis Platform Combined with Gold Nanoparticles for Ultrasensitive Quantitative Detection of Small Molecules. Biosensors 2023, 13, 681. https://doi.org/10.3390/bios13070681
Zhou T, Ji W, Fan H, Zhang L, Wan X, Fan Z, Liu GL, Peng Q, Huang L. A Metasurface Plasmonic Analysis Platform Combined with Gold Nanoparticles for Ultrasensitive Quantitative Detection of Small Molecules. Biosensors. 2023; 13(7):681. https://doi.org/10.3390/bios13070681
Chicago/Turabian StyleZhou, Taohong, Weihao Ji, Hongli Fan, Li Zhang, Xugang Wan, Zhiyong Fan, Gang Logan Liu, Qingzhi Peng, and Liping Huang. 2023. "A Metasurface Plasmonic Analysis Platform Combined with Gold Nanoparticles for Ultrasensitive Quantitative Detection of Small Molecules" Biosensors 13, no. 7: 681. https://doi.org/10.3390/bios13070681
APA StyleZhou, T., Ji, W., Fan, H., Zhang, L., Wan, X., Fan, Z., Liu, G. L., Peng, Q., & Huang, L. (2023). A Metasurface Plasmonic Analysis Platform Combined with Gold Nanoparticles for Ultrasensitive Quantitative Detection of Small Molecules. Biosensors, 13(7), 681. https://doi.org/10.3390/bios13070681






