Graphene-Based Metamaterial Sensor for Pesticide Trace Detection
Abstract
1. Introduction
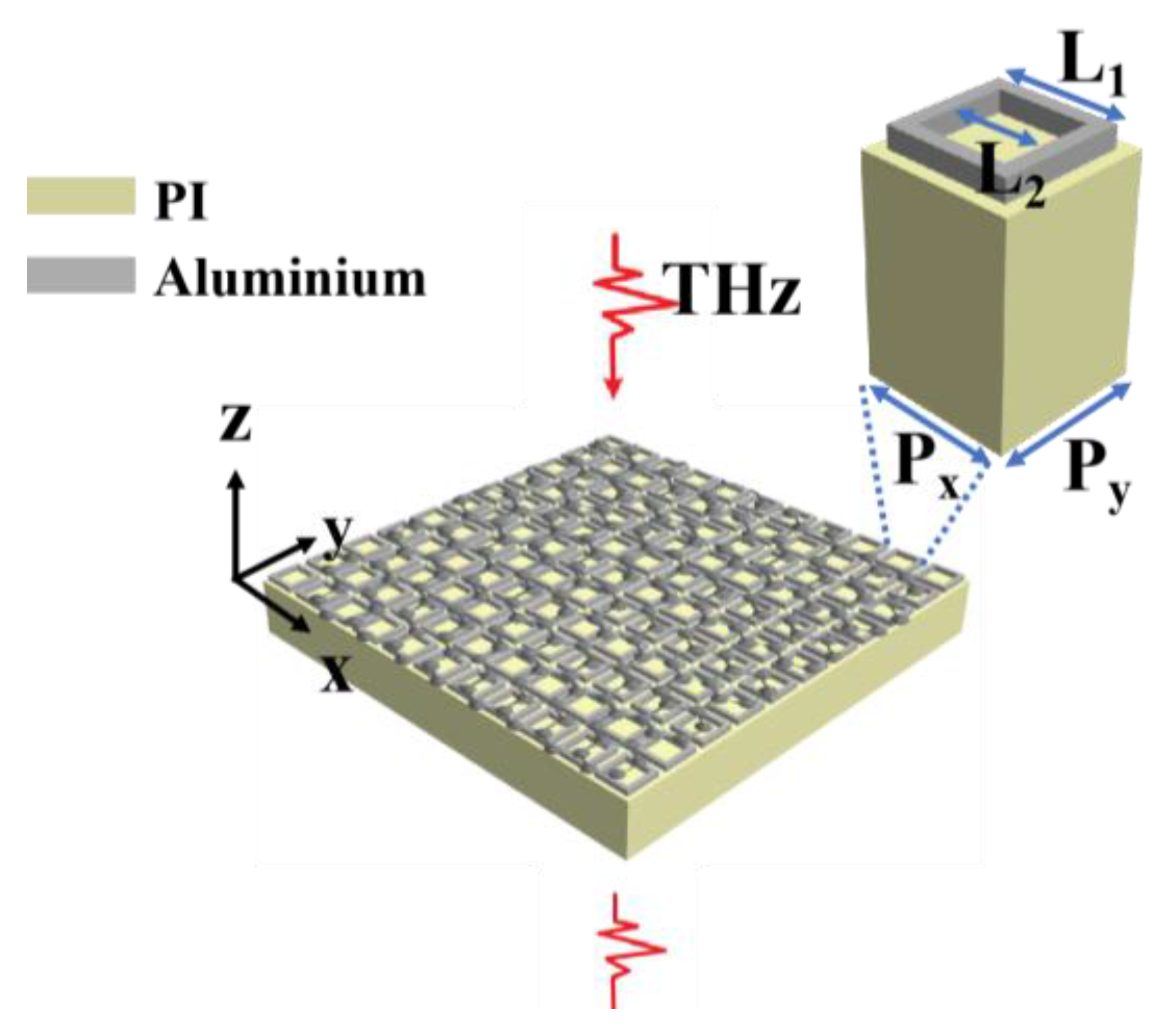
2. Structural Design
3. Simulation Results
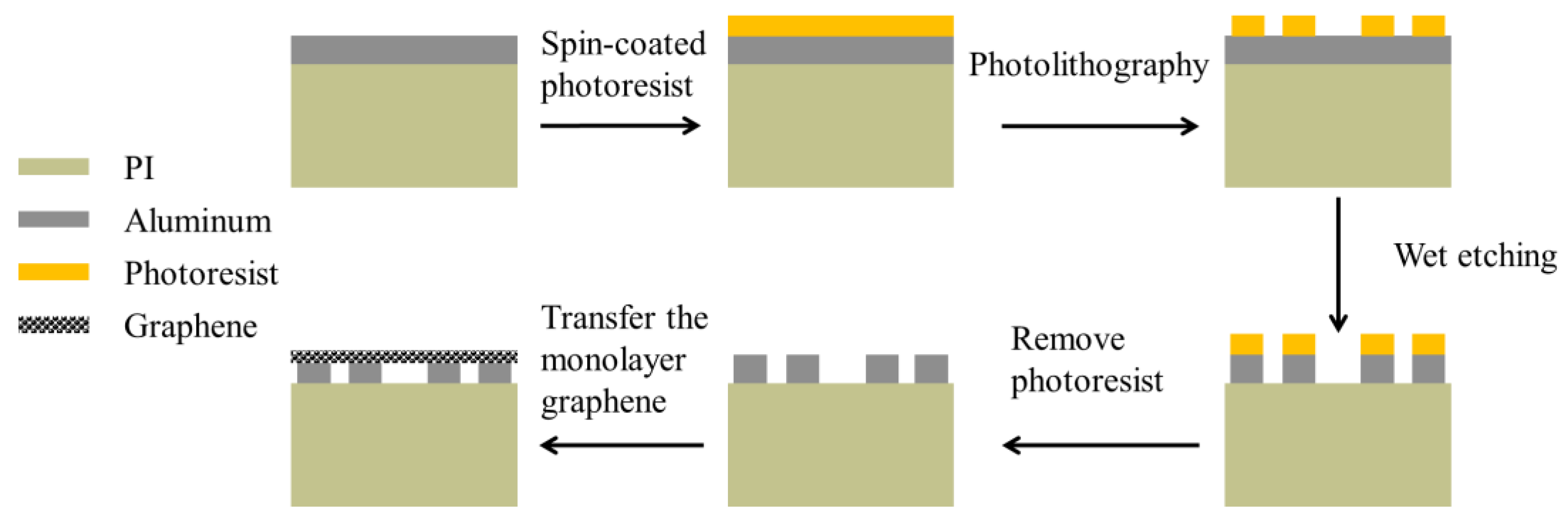
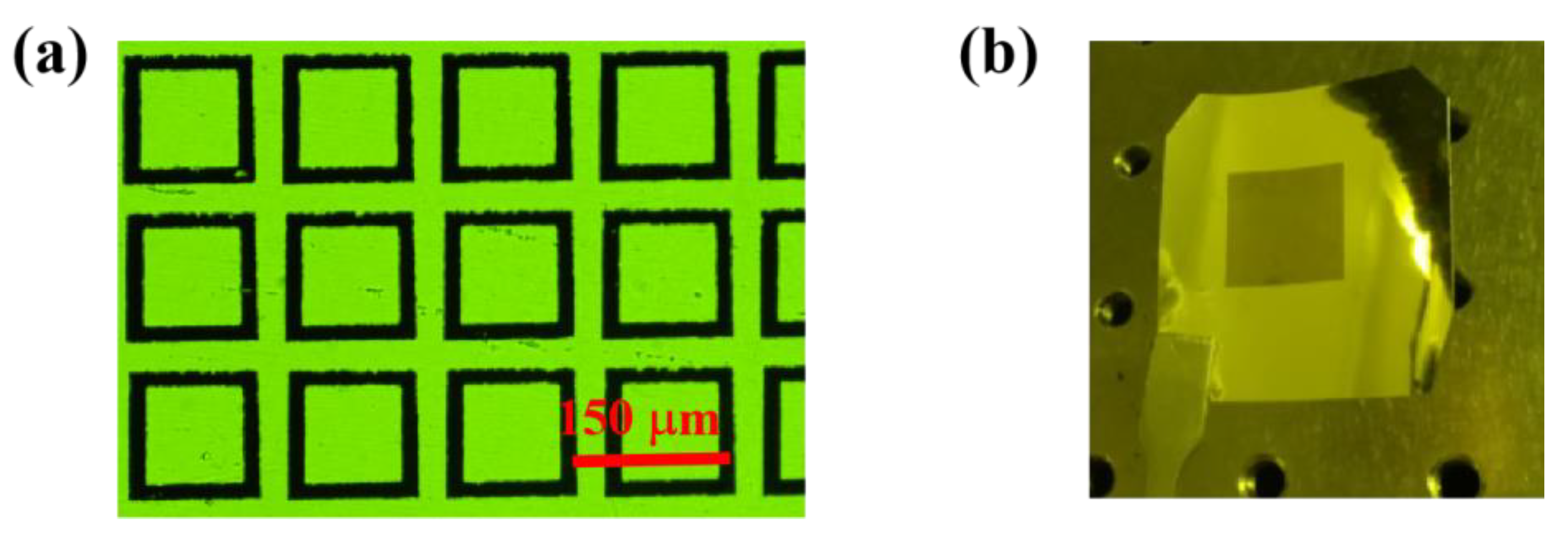
4. Fabrication
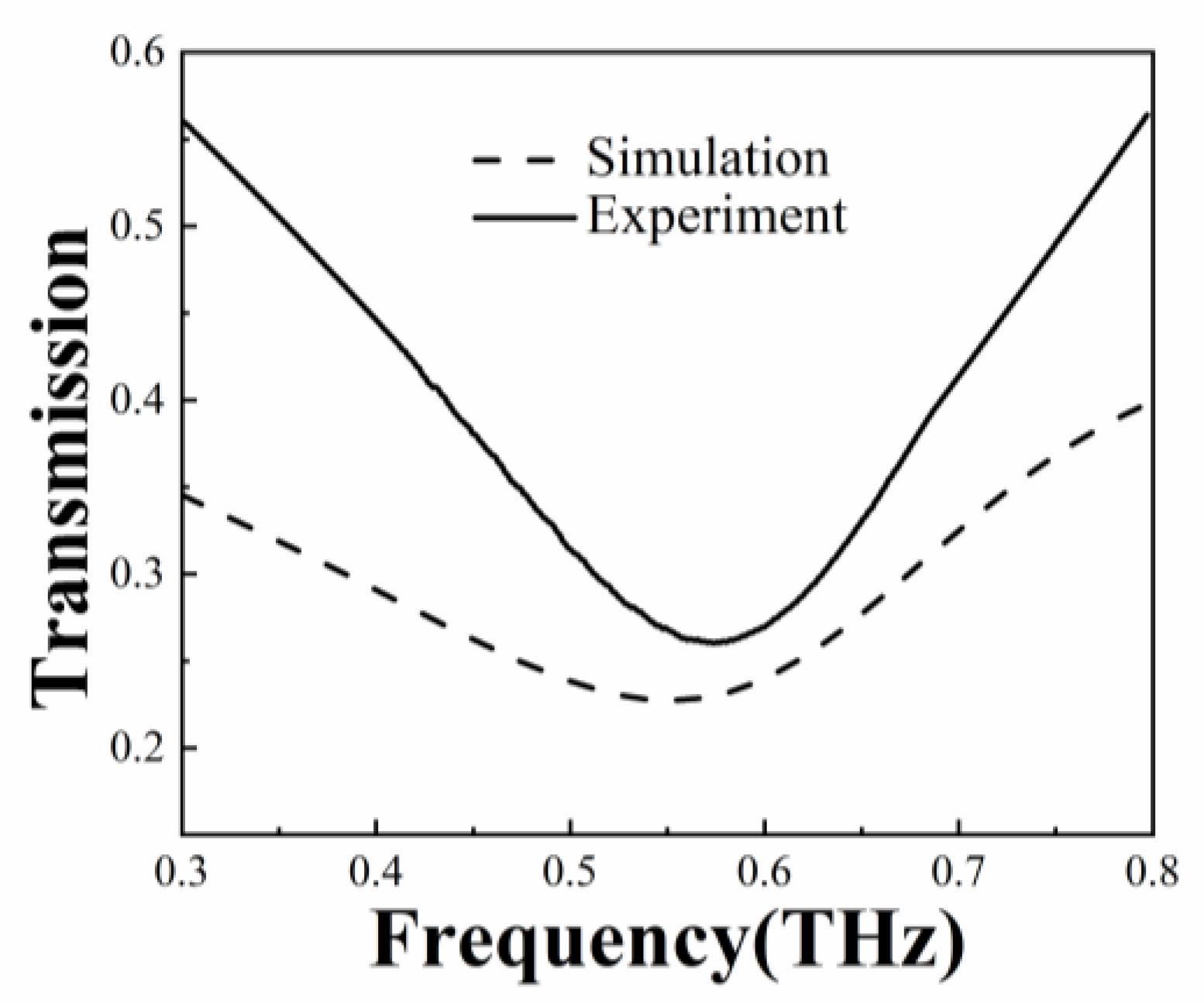
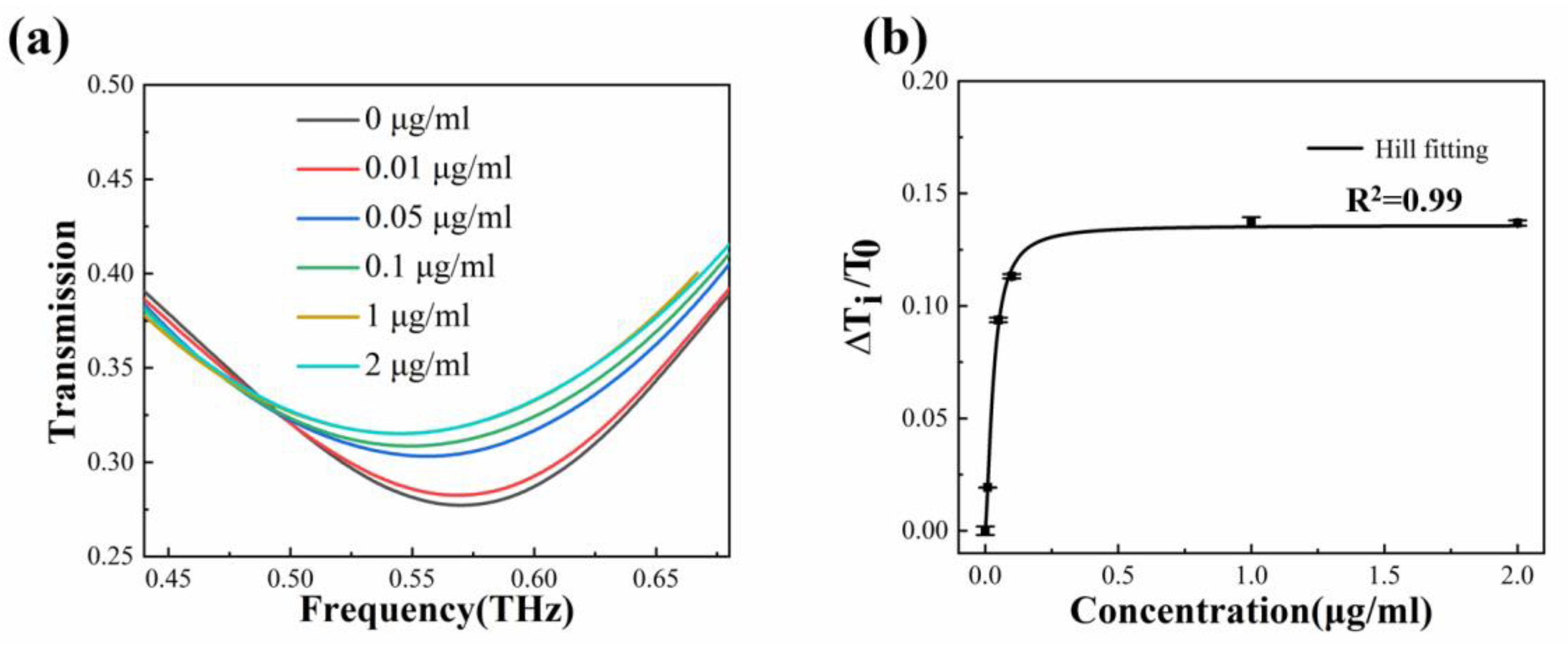
5. Experimental Results
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, P.; Wan, Y.; Ali, A.; Deng, S.; Su, Y.; Fan, C.; Yang, S. Aptamer-wrapped gold nanoparticles for the colorimetric detection of omethoate. Sci. China Chem. 2016, 59, 237–242. [Google Scholar] [CrossRef]
- Liu, M.; Khan, A.; Wang, Z.; Liu, Y.; Yang, G.; Deng, Y.; He, N. Aptasensors for pesticide detection. Biosens. Bioelectron. 2019, 130, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, E.; Barroso, M.; Margalho, C.; Cruz, A.; Vieira, D.N.; López-Rivadulla, M. Determination of quinalphos in blood and urine by direct solid-phase microextraction combined with gas chromatography–mass spectrometry. J. Chromatogr. A 2006, 832, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Martínez Salvador, I.; Garrido Frenich, A.; Egea González, F.J.; Martínez Vidal, J.L. Determination of organophosphorus pesticides in vegetables by GC with pulsed flame-photometric detection, and confirmation by MS. Chromatographia 2006, 64, 667–672. [Google Scholar] [CrossRef]
- Berijani, S.; Assadi, Y.; Anbia, M.; Hosseini, M.R.M.; Aghaee, E. Dispersive liquid–liquid microextraction combined with gas chromatography-flame photometric detection: Very simple, rapid and sensitive method for the determination of organophosphorus pesticides in water. J. Chromatogr. A. 2006, 1123, 1–9. [Google Scholar] [CrossRef]
- Golge, O.; Kabak, B. Evaluation of QuEChERS sample preparation and liquid chromatography–triple-quadrupole mass spectrometry method for the determination of 109 pesticide residues in tomatoes. Food Chem. 2015, 176, 319–332. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Q.; Hu, B.; Shen, Q.; Yang, G.; Liang, X.; Sun, X.; Liu, F. Development of a sensitive ELISA for the analysis of the organophosphorous insecticide fenthion in fruit samples. Food Chem. 2008, 106, 1278–1284. [Google Scholar] [CrossRef]
- Dzudzevic Cancar, H.; Soylemez, S.; Akpinar, Y.; Kesik, M.; Göker, S.; Gunbas, G.; Volkan, M.; Toppare, L. A novel acetylcholinesterase biosensor: Core–shell magnetic nanoparticles incorporating a conjugated polymer for the detection of organophosphorus pesticides. ACS Appl. Mater. Interface 2016, 8, 8058–8067. [Google Scholar] [CrossRef]
- Zhao, H.; Ji, X.; Wang, B.; Wang, N.; Li, X.; Ni, R.; Ren, J. An ultra-sensitive acetylcholinesterase biosensor based on reduced graphene oxide-Au nanoparticles-β-cyclodextrin/Prussian blue-chitosan nanocomposites for organophosphorus pesticides detection. Biosens. Bioelectron. 2015, 65, 23–30. [Google Scholar] [CrossRef]
- Wang, G.; Zhu, F.; Lang, T.; Liu, J.; Hong, Z.; Qin, J. All-metal terahertz metamaterial biosensor for protein detection. Nanoscale Res. Lett. 2021, 16, 1–10. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Y.J.; Yang, J.; Su, X.; Deng, J.; Chum, C.C.; Hong, M.; Teng, J. High sensitivity molecule detection by plasmonic nanoantennas with selective binding at electromagnetic hotspots. Nanoscale 2014, 6, 1416–1422. [Google Scholar] [CrossRef]
- Desouky, M.; Anisur, M.R.; Alba, M.; Raman, R.S.; Swillam, M.A.; Voelcker, N.H.; Kasry, A. Near-Field Mapping of Localized Plasmon Resonances in Metal-Free, Nanomembrane Graphene for Mid-Infrared Sensing Applications. ACS Appl. Nano Mater. 2018, 1, 6454–6462. [Google Scholar] [CrossRef]
- Lou, J.; Liang, J.; Yu, Y.; Ma, H.; Yang, R.; Fan, Y.; Wang, G.; Cai, T. Silicon-based terahertz meta-devices for electrical modulation of Fano resonance and transmission amplitude. Adv. Opt. Mater. 2020, 8, 2000449. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, Z.; Liang, L.; Yan, X.; Wei, D.; Song, X.; Wang, M.; Yao, J. Sensitive detection of the concentrations for normal epithelial cells based on Fano resonance metamaterial biosensors in terahertz range. Appl. Opt. 2019, 58, 6268–6273. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lang, T.; Shen, T.; Shen, C.; Hong, Z.; Lu, C. Numerical study of an ultra-broadband all-silicon terahertz absorber. Appl. Sci. 2020, 10, 436. [Google Scholar] [CrossRef]
- Wang, J.; Lang, T.; Hong, Z.; Xiao, M.; Yu, J. Design and fabrication of a triple-band terahertz metamaterial absorber. Nanomaterials 2021, 11, 1110. [Google Scholar] [CrossRef]
- Yan, R.; Arezoomandan, S.; Sensale-Rodriguez, B.; Xing, H.G. Exceptional terahertz wave modulation in graphene enhanced by frequency selective surfaces. ACS Photonics 2016, 3, 315–323. [Google Scholar] [CrossRef]
- Li, F.; He, K.; Tang, T.; Mao, Y.; Wang, R.; Li, C.; Shen, J. The terahertz metamaterials for sensitive biosensors in the detection of ethanol solutions. Opt. Commun. 2020, 475, 126287. [Google Scholar] [CrossRef]
- Yang, M.; Liang, L.; Zhang, Z.; Xin, Y.; Wei, D.; Song, X.; Zhang, H.; Lu, Y.; Wang, M.; Zhang, M.; et al. Electromagnetically induced transparency-like metamaterials for detection of lung cancer cells. Opt. Express 2019, 27, 19520–19529. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Chen, X.; Li, T.; Li, Y.; Tian, Z.; Wang, M. Highly sensitive terahertz metamaterial biosensor for bovine serum albumin (BSA) detection. Opt. Mater. Express 2021, 11, 2268–2277. [Google Scholar] [CrossRef]
- Hu, S.; Liu, D.; Yang, H.; Wang, H.; Wang, Y. Staggered H-shaped metamaterial based on electromagnetically induced transparency effect and its refractive index sensing performance. Opt. Commun. 2019, 450, 202–207. [Google Scholar] [CrossRef]
- Wang, R.; Xu, W.; Chen, D.; Zhou, R.; Ying, Y. Ultrahigh-sensitivity molecular sensing with carbon nanotube terahertz metamaterials. ACS Appl. Mater. Interfaces 2020, 12, 40629–40634. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, Z.; Zhang, X.; Zhang, X.; Zhu, Y.; Chen, S.; Hu, H. Excitation of Surface Plasmon Resonance on Multiwalled Carbon Nanotube Metasurfaces for Pesticide Sensors. ACS Appl. Mater. Interfaces 2020, 12, 52082–52088. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, X.; Zhang, X.; Zhou, T.; Cui, Z.; Zhang, K. A novel terahertz metasurface based on a single-walled carbon nanotube film for sensing application. J. Mater. Chem. A 2022, 10, 1780–1787. [Google Scholar] [CrossRef]
- Shin, H.J.; Jang, H.W.; Ok, G. Highly sensitive detection of 4-Methylimidazole using a terahertz Metamaterial. Sensors 2018, 18, 4304. [Google Scholar] [CrossRef]
- Boltasseva, A.; Atwater, H.A. Low-loss plasmonic metamaterials. Science 2011, 331, 290–291. [Google Scholar] [CrossRef]
- Li, Q.; Tian, Z.; Zhang, X.; Xu, N.; Singh, R.; Gu, J.; Lu, P.; Luo, L.; Zhang, S.; Han, J.; et al. Dual control of active graphene–silicon hybrid metamaterial devices. Carbon 2015, 90, 146–153. [Google Scholar] [CrossRef]
- Davaji, B.; Cho, H.D.; Malakoutian, M.; Lee, J.K.; Panin, G.; Kang, T.W.; Lee, C.H. A patterned single layer graphene resistance temperature sensor. Sci. Rep. 2017, 7, 8811. [Google Scholar] [CrossRef]
- Yang, M.; Li, T.; Gao, J.; Yan, X.; Liang, L.; Yao, H.; Li, J.; Wei, D.; Wang, M.; Zhang, T.; et al. Graphene–polyimide-integrated metasurface for ultrasensitive modulation of higher-order terahertz Fano resonances at the Dirac point. Appl. Surf. Sci. 2021, 562, 150182. [Google Scholar] [CrossRef]
- Purkayastha, A.; Srivastava, T.; Jha, R. Ultrasensitive THz–Plasmonics gaseous sensor using doped graphene. Sens. Actuators B Chem. 2016, 227, 291–295. [Google Scholar] [CrossRef]
- Andryieuski, A.; Lavrinenko, A.V. Graphene metamaterials based tunable terahertz absorber: Effective surface conductivity approach. Opt. Express 2013, 21, 9144–9155. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lang, T.; Hong, Z.; Shen, T.; Wang, G. Tunable terahertz metamaterial absorber based on electricity and light modulation modes. Opt. Mater. Express 2020, 10, 2262–2273. [Google Scholar] [CrossRef]
- Li, D.; Zhang, W.; Yu, X.; Wang, Z.; Su, Z.; Wei, G. When biomolecules meet graphene: From molecular level interactions to material design and applications. Nanoscale 2016, 8, 19491–19509. [Google Scholar] [CrossRef] [PubMed]
- Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Varga, B.; Juhasz, B.; Tosaki, A. The Hill equation and the origin of quantitative pharmacology. Arch. Hist. Exact. Sci. 2012, 66, 427–438. [Google Scholar] [CrossRef]
- Chen, Z.; Qu, F.; Wang, Y.; Nie, P. Terahertz dual-band metamaterial absorber for trace indole-3-acetic acid and tricyclazole molecular detection based on spectral response analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120222. [Google Scholar] [CrossRef]
- Liu, J. High-sensitivity detection method for organochlorine pesticide residues based on loop-shaped absorber. Mater. Chem. Phys. 2020, 242, 122542. [Google Scholar] [CrossRef]
- Yue, L.; Wang, Y.; Cui, Z.; Zhang, X.; Zhu, Y.; Zhang, X.; Chen, S.; Wang, X.; Zhang, K. Multi-band terahertz resonant absorption based on an all-dielectric grating metasurface for chlorpyrifos sensing. Opt. Express 2021, 29, 13563–13575. [Google Scholar] [CrossRef]
- Xu, W.; Xie, L.; Zhu, J.; Tang, L.; Singh, R.; Wang, C.; Ma, Y.; Chen, H.T.; Ying, Y. Terahertz biosensing with a graphene-metamaterial heterostructure platform. Carbon 2019, 141, 247–252. [Google Scholar] [CrossRef]
- Nie, P.; Zhu, D.; Cui, Z.; Qu, F.; Lin, L.; Wang, Y. Sensitive detection of chlorpyrifos pesticide using an all-dielectric broadband terahertz metamaterial absorber. Sens. Actuators B Chem. 2020, 307, 127642. [Google Scholar] [CrossRef]



| Type of Sensor | Pesticide Types | Band (THz) | LOD | Reference |
|---|---|---|---|---|
| Metamaterial absorber based on a split ring resonator structure | Indole-3-acetic acid and tricyclazole | 0–3.5 | 10 ng/L | [35] |
| Loop-shaped absorber | Toxaphene | 0.1–2 | 0.213 mg/L | [36] |
| All-dielectric grating metasurface | Chlorpyrifos | 0.2–2.5 | / | [37] |
| Graphene metamaterial sensor | Chlorpyrifos methyl | 0.8–1.1 | 0.2 ng | [38] |
| All-dielectric broadband terahertz absorber | Chlorpyrifos | 0.2–2 | 0.1 mg/L | [39] |
| Graphene-based metamaterial biosensor | Phosalone | 0.3–0.8 | 0.01 mg/L | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, T.; Xiao, M.; Cen, W. Graphene-Based Metamaterial Sensor for Pesticide Trace Detection. Biosensors 2023, 13, 560. https://doi.org/10.3390/bios13050560
Lang T, Xiao M, Cen W. Graphene-Based Metamaterial Sensor for Pesticide Trace Detection. Biosensors. 2023; 13(5):560. https://doi.org/10.3390/bios13050560
Chicago/Turabian StyleLang, Tingting, Meiyu Xiao, and Wenyang Cen. 2023. "Graphene-Based Metamaterial Sensor for Pesticide Trace Detection" Biosensors 13, no. 5: 560. https://doi.org/10.3390/bios13050560
APA StyleLang, T., Xiao, M., & Cen, W. (2023). Graphene-Based Metamaterial Sensor for Pesticide Trace Detection. Biosensors, 13(5), 560. https://doi.org/10.3390/bios13050560





