An Integrated and Multi-Target Nucleic Acid Isothermal Analysis System for Rapid Diagnosis of Vulvovaginal Candidiasis
Abstract
1. Introduction
2. Materials and Methods
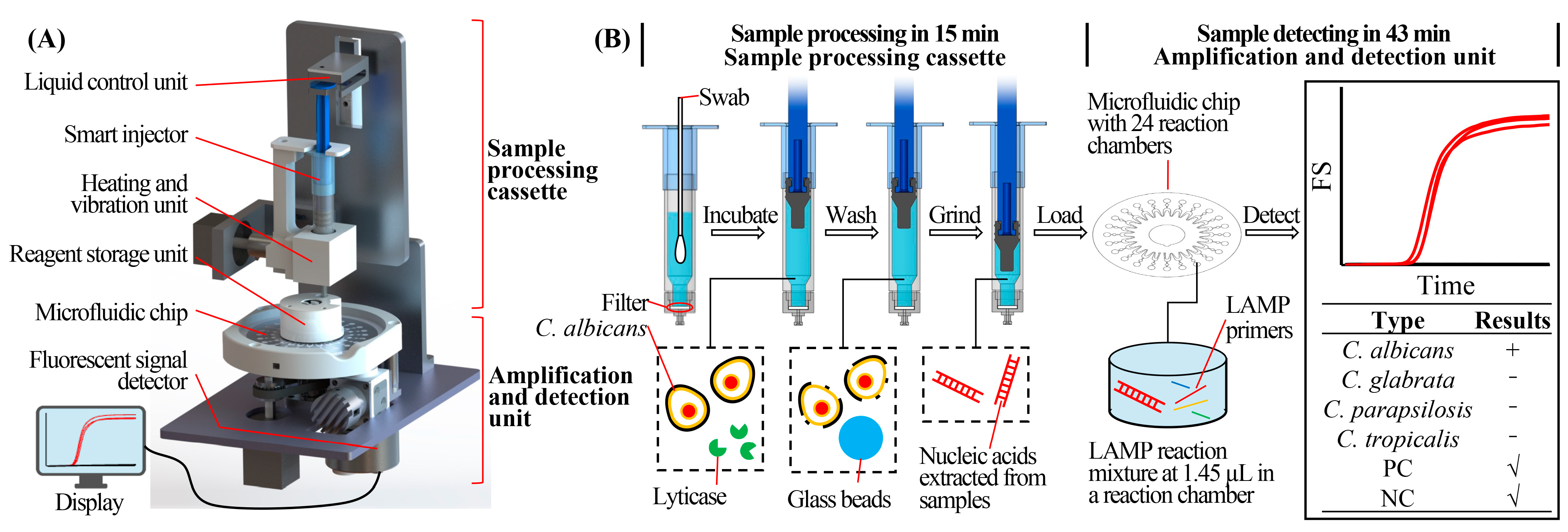
2.1. Structure and Working Principle of the RPT System
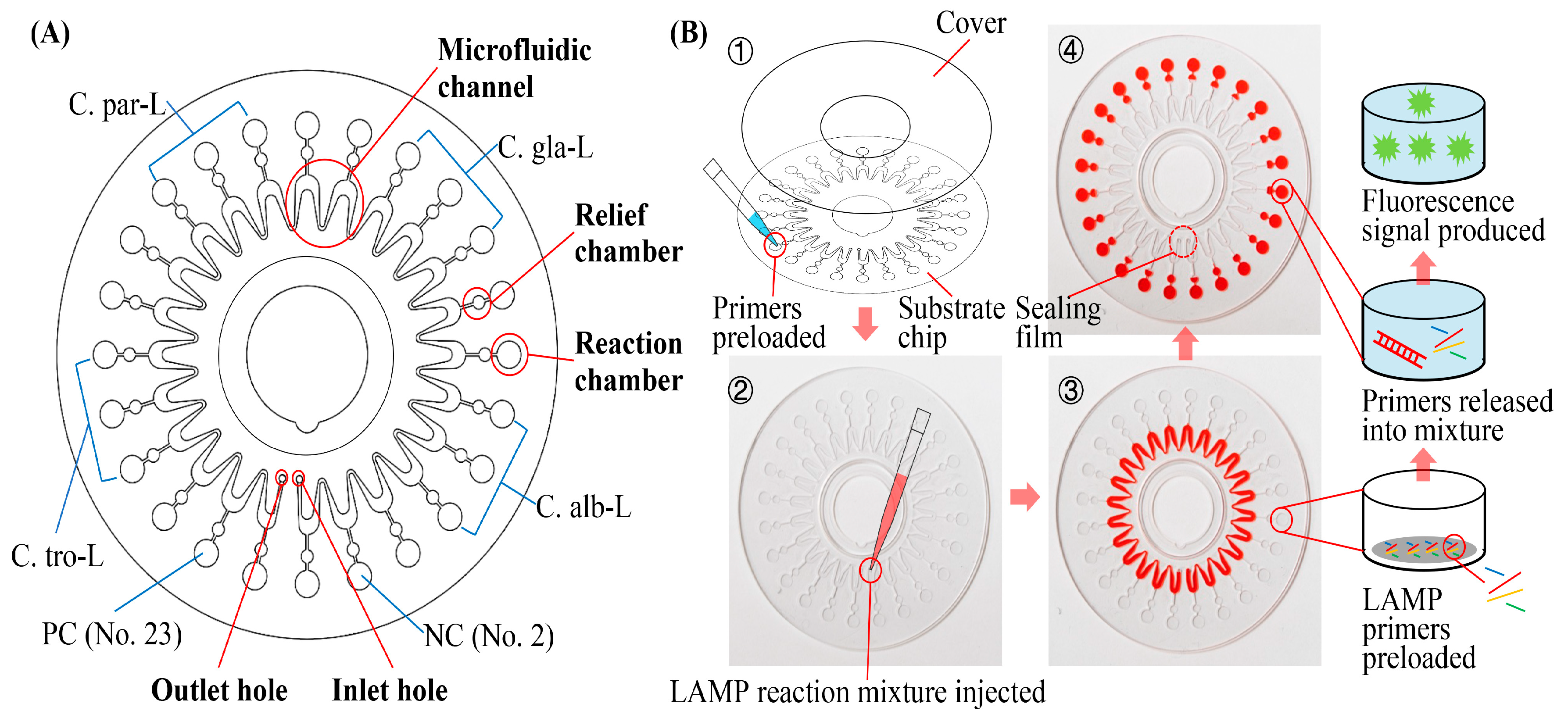
2.2. Structure and Working Principle of the Microfluidic Chip
2.3. Preparation of Mycological Typing LAMP Primers
2.4. Preparation of Nucleic Acids and Clinical Samples
2.5. LAMP and PCR Assays
3. Results and Discussion
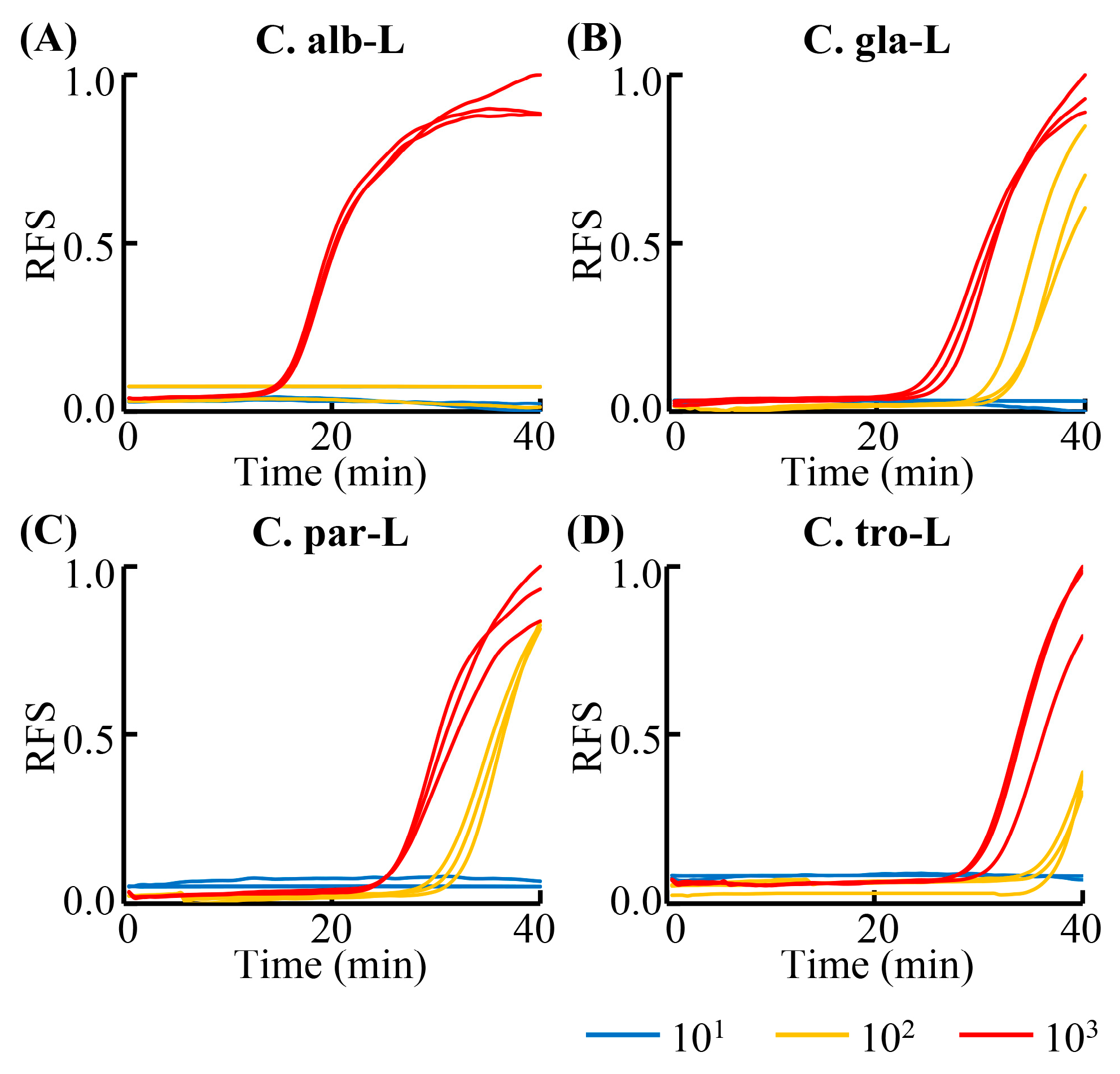
3.1. Sensitivity of the LAMP Primers
3.2. Performance of the Sample Processing Cassette
3.3. Detection Sensitivity and Specificity of the RPT System
3.4. Validation of the RPT System Assay with Clinical Samples
3.5. Period of Validity of the Reagents and Chips Used in the RPT System
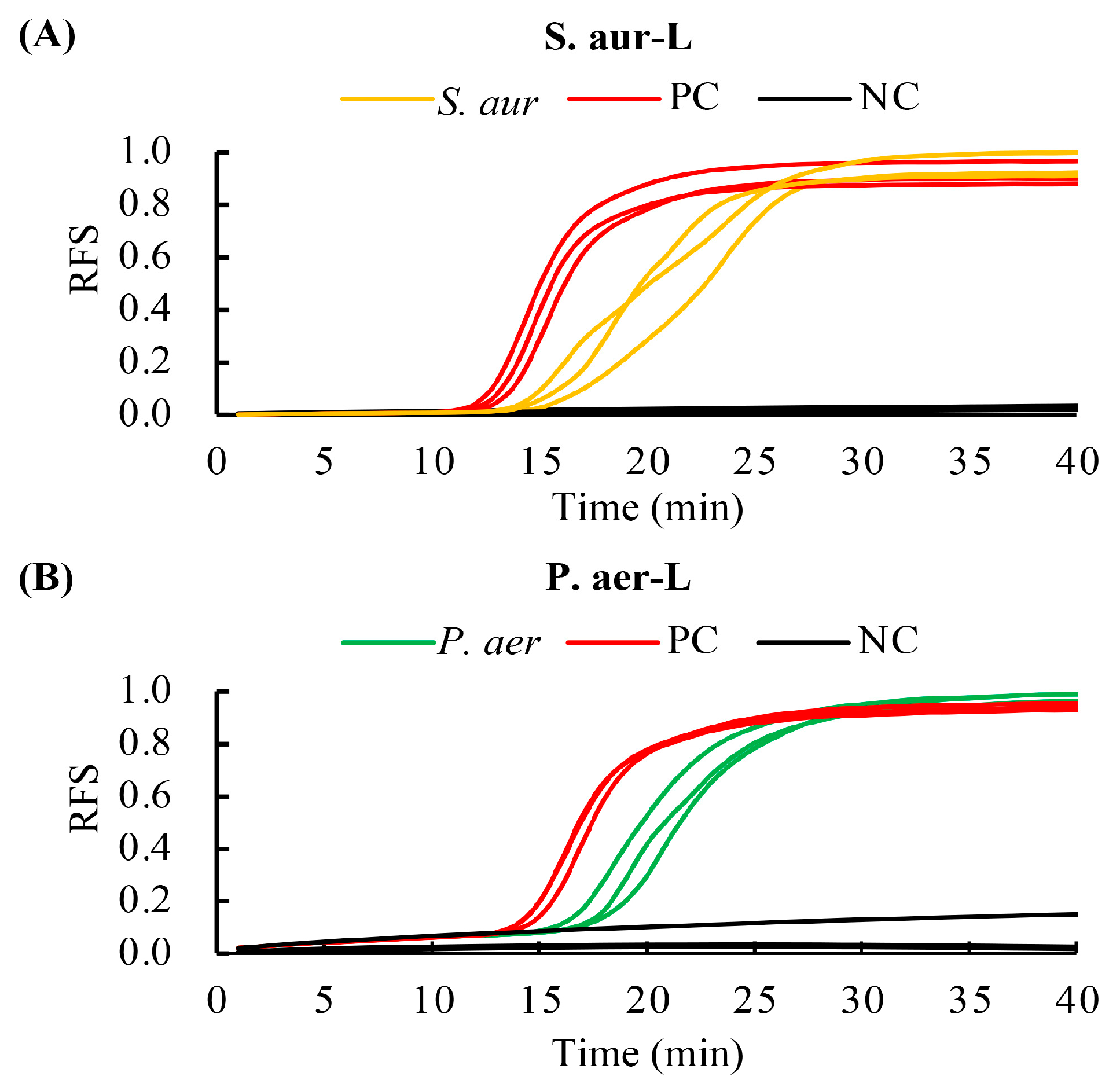
3.6. Processing and Detecting Ability of Bacteria Using the RPT System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goncalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef] [PubMed]
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi. 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Workowski, K.A.; Bolan, G.A.; Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm. Rep. 2015, 64, 1–137. [Google Scholar] [PubMed]
- Sobel, J.D. Vaginitis. N. Engl. J. Med. 1997, 337, 1896–1903. [Google Scholar] [CrossRef]
- Ilkit, M.; Guzel, A.B. The epidemiology, pathogenesis, and diagnosis of vulvovaginal candidosis: A mycological perspective. Crit. Rev. Microbiol. 2011, 37, 250–261. [Google Scholar] [CrossRef]
- Sobel, J.D.; Faro, S.; Force, R.W.; Foxman, B.; Ledger, W.J.; Nyirjesy, P.R.; Reed, B.D.; Summers, P.R. Vulvovaginal candidiasis: Epidemiologic, diagnostic, and therapeutic considerations. Am. J. Obstet. Gynecol. 1998, 178, 203–211. [Google Scholar] [CrossRef]
- Eckert, L.O.; Hawes, S.E.; Stevens, C.E.; Koutsky, L.A.; Eschenbach, D.A.; Holmes, K.K. Vulvovaginal candidiasis: Clinical manifestations, risk factors, management algorithm. Obstet. Gynecol. 1998, 92, 757–765. [Google Scholar] [CrossRef]
- Eckert, L.O. Clinical practice. Acute vulvovaginitis. N. Engl. J. Med. 2006, 355, 1244–1252. [Google Scholar] [CrossRef]
- Sobel, J.D. Vulvovaginal candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef]
- Horowitz, B.J.; Giaquinta, D.; Ito, S. Evolving pathogens in vulvovaginal candidiasis: Implications for patient care. J. Clin. Pharmacol. 1992, 32, 248–255. [Google Scholar] [CrossRef]
- Vermitsky, J.P.; Self, M.J.; Chadwick, S.G.; Trama, J.P.; Adelson, M.E.; Mordechai, E.; Gygax, S.E. Survey of vaginal-flora Candida species isolates from women of different age groups by use of species-specific PCR detection. J. Clin. Microbiol. 2008, 46, 1501–1503. [Google Scholar] [CrossRef]
- Achkar, J.M.; Fries, B.C. Candida infections of the genitourinary tract. Clin. Microbiol. Rev. 2010, 23, 253–273. [Google Scholar] [CrossRef]
- Richter, S.S.; Galask, R.P.; Messer, S.A.; Hollis, R.J.; Diekema, D.J.; Pfaller, M.A. Antifungal susceptibilities of Candida species causing vulvovaginitis and epidemiology of recurrent cases. J. Clin. Microbiol. 2005, 43, 2155–2162. [Google Scholar] [CrossRef] [PubMed]
- Vaginitis in Nonpregnant Patients: ACOG Practice Bulletin, Number 215. Obstet. Gynecol. 2020, 135, e1–e17. [CrossRef]
- National guideline for the management of vulvovaginal candidiasis. Clinical Effectiveness Group (Association of Genitourinary Medicine and the Medical Society for the Study of Venereal Diseases). Sex Transm. Infect. 1999, 75 (Suppl. 1), S19–S20. [Google Scholar]
- Lowe, N.K.; Neal, J.L.; Ryan-Wenger, N.A. Accuracy of the clinical diagnosis of vaginitis compared with a DNA probe laboratory standard. Obstet. Gynecol. 2009, 113, 89–95. [Google Scholar] [CrossRef]
- Bergman, J.J.; Berg, A.O.; Schneeweiss, R.; Heidrich, F.E. Clinical comparison of microscopic and culture techniques in the diagnosis of Candida vaginitis. J. Fam. Pract. 1984, 18, 549–552. [Google Scholar] [PubMed]
- Sobel, J.D. Recurrent vulvovaginal candidiasis. Am. J. Obstet. Gynecol. 2016, 214, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Neal, C.M.; Martens, M.G. Clinical challenges in diagnosis and treatment of recurrent vulvovaginal candidiasis. SAGE Open Med. 2022, 10, 20503121221115201. [Google Scholar] [CrossRef]
- Sobel, J.D.; Hay, P. Diagnostic techniques for bacterial vaginosis and vulvovaginal candidiasis—Requirement for a simple differential test. Expert. Opin. Med. Diagn. 2010, 4, 333–341. [Google Scholar] [CrossRef]
- Weissenbacher, T.; Witkin, S.S.; Ledger, W.J.; Tolbert, V.; Gingelmaier, A.; Scholz, C.; Weissenbacher, E.R.; Friese, K.; Mylonas, I. Relationship between clinical diagnosis of recurrent vulvovaginal candidiasis and detection of Candida species by culture and polymerase chain reaction. Arch. Gynecol. Obstet. 2009, 279, 125–129. [Google Scholar] [CrossRef]
- Mahmoudi Rad, M.; Zafarghandi, A.; Amel Zabihi, M.; Tavallaee, M.; Mirdamadi, Y. Identification of Candida species associated with vulvovaginal candidiasis by multiplex PCR. Infect. Dis. Obstet. Gynecol. 2012, 2012, 872169. [Google Scholar] [CrossRef]
- Yang, N.; Peng, J.; Wu, L.; Han, X.; Shaheen, N.; Zou, X. Hand-Held Zoom Micro-Imaging System Based on Microfluidic Chip for Point-of-Care Testing (POCT) of Vaginal Inflammation. IEEE J. Transl. Eng. Health Med. 2021, 9, 2800109. [Google Scholar] [CrossRef]
- Lai, W.; Shi, Y.; Zhong, J.; Zhou, X.; Yang, Y.; Chen, Z.; Zhang, C. A dry chemistry-based electrochemiluminescence device for point-of-care testing of alanine transaminase. Talanta 2023, 256, 124287. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, K.G.; Estrela, P.F.N.; Mendes, G.M.; Dos Santos, C.A.; Silveira-Lacerda, E.P.; Duarte, G.R.M. Rapid molecular diagnostics of COVID-19 by RT-LAMP in a centrifugal polystyrene-toner based microdevice with end-point visual detection. Analyst 2021, 146, 1178–1187. [Google Scholar] [CrossRef] [PubMed]
- Nanayakkara, I.A.; White, I.M. Demonstration of a quantitative triplex LAMP assay with an improved probe-based readout for the detection of MRSA. Analyst 2019, 144, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- Owoicho, O.; Olwal, C.O.; Tettevi, E.J.; Atu, B.O.; Durugbo, E.U. Loop-mediated isothermal amplification for Candida species surveillance in under-resourced setting: A review of evidence. Expert. Rev. Mol. Diagn. 2022, 22, 643–653. [Google Scholar] [CrossRef]
- Lin, X.; Jin, X.; Du, W.; Shan, X.; Huang, Q.; Fu, R.; Lv, W.; Yang, H.; Su, Y.; Huang, G. Quantitative and specific detection of viable pathogens on a portable microfluidic chip system by combining improved propidium monoazide (PMAxx) and loop-mediated isothermal amplification (LAMP). Anal. Methods 2021, 13, 3569–3576. [Google Scholar] [CrossRef]
- Inacio, J.; Flores, O.; Spencer-Martins, I. Efficient identification of clinically relevant Candida yeast species by use of an assay combining panfungal loop-mediated isothermal DNA amplification with hybridization to species-specific oligonucleotide probes. J. Clin. Microbiol. 2008, 46, 713–720. [Google Scholar] [CrossRef]
- Kasahara, K.; Ishikawa, H.; Sato, S.; Shimakawa, Y.; Watanabe, K. Development of multiplex loop-mediated isothermal amplification assays to detect medically important yeasts in dairy products. FEMS Microbiol. Lett. 2014, 357, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Yamazaki, T.; Yo, A.; Tone, K.; Mahdi Alshahni, M.; Fujisaki, R.; Makimura, K. Detection of Fungi from an Indoor Environment using Loop-mediated Isothermal Amplification (LAMP) Method. Biocontrol. Sci. 2017, 22, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Ou, H.; Wang, Y.; Wang, Q.; Ma, Y.; Liu, C.; Jia, L.; Zhang, Q.; Li, M.; Feng, X.; Li, M.; et al. Rapid detection of multiple pathogens by the combined loop-mediated isothermal amplification technology and microfluidic chip technology. Ann. Palliat. Med. 2021, 10, 11053–11066. [Google Scholar] [CrossRef]
- Xu, X.; Jia, Y.; Li, R.; Wen, Y.; Liang, Y.; Lao, G.; Liu, X.; Zhou, W.; Liu, H.; Xie, J.; et al. Rapid and simultaneous detection of multiple pathogens in the lower reproductive tract during pregnancy based on loop-mediated isothermal amplification-microfluidic chip. BMC Microbiol. 2022, 22, 260. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Alshahni, M.M.; Tamura, T.; Satoh, K.; Iguchi, S.; Kikuchi, K.; Mimaki, M.; Makimura, K. Rapid Detection of Candida auris Based on Loop-Mediated Isothermal Amplification (LAMP). J. Clin. Microbiol. 2018, 56, e00591-18. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.H.; Jee, H.; Moon, K.C.; Lim, C.S.; Jang, W.S. Development of a Simple DNA Extraction Method and Candida Pan Loop-Mediated Isothermal Amplification Assay for Diagnosis of Candidemia. Pathogens 2022, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Lipke, P.N.; Ovalle, R. Cell wall architecture in yeast: New structure and new challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [CrossRef]
- Huang, G.; Huang, Q.; Xie, L.; Xiang, G.; Wang, L.; Xu, H.; Ma, L.; Luo, X.; Xin, J.; Zhou, X.; et al. A rapid, low-cost, and microfluidic chip-based system for parallel identification of multiple pathogens related to clinical pneumonia. Sci. Rep. 2017, 7, 6441. [Google Scholar] [CrossRef]
- Schellenberg, J.J.; Ormond, M.; Keynan, Y. Extraction-free RT-LAMP to detect SARS-CoV-2 is less sensitive but highly specific compared to standard RT-PCR in 101 samples. J. Clin. Virol. 2021, 136, 104764. [Google Scholar] [CrossRef]
- Onderdonk, A.B.; Delaney, M.L.; Fichorova, R.N. The Human Microbiome during Bacterial Vaginosis. Clin. Microbiol. Rev. 2016, 29, 223–238. [Google Scholar] [CrossRef]
- Amabebe, E.; Anumba, D.O.C. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Front. Med. 2018, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hu, Q.; Li, H.; Xie, Y.; Xiu, L.; Zhang, Y.; Guo, X.; Yin, K. Multiplex Detection of Infectious Diseases on Microfluidic Platforms. Biosensors 2023, 13, 410. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Guo, X.; Yang, Y.; Shi, H.; Hao, R.; Wang, S.; Li, Z.J.; Zhao, R.; Song, H. Microfluidic chip and isothermal amplification technologies for the detection of pathogenic nucleic acid. J. Biol. Eng. 2022, 16, 33. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Chaim, W. Treatment of Torulopsis glabrata vaginitis: Retrospective review of boric acid therapy. Clin. Infect. Dis. 1997, 24, 649–652. [Google Scholar] [CrossRef]
- Jafarzadeh, L.; Ranjbar, M.; Nazari, T.; Naeimi Eshkaleti, M.; Aghaei Gharehbolagh, S.; Sobel, J.D.; Mahmoudi, S. Vulvovaginal candidiasis: An overview of mycological, clinical, and immunological aspects. J. Obstet. Gynaecol. Res. 2022, 48, 1546–1560. [Google Scholar] [CrossRef]
- Esfahani, A.; Omran, A.N.; Salehi, Z.; Shams-Ghahfarokhi, M.; Ghane, M.; Eybpoosh, S.; Razzaghi-Abyaneh, M. Molecular epidemiology, antifungal susceptibility, and ERG11 gene mutation of Candida species isolated from vulvovaginal candidiasis: Comparison between recurrent and non-recurrent infections. Microb. Pathog. 2022, 170, 105696. [Google Scholar] [CrossRef]
- Posteraro, B.; Vella, A.; De Carolis, E.; Sanguinetti, M. Molecular Detection of Resistance to Echinocandins. Methods Mol. Biol. 2017, 1508, 413–421. [Google Scholar] [CrossRef]
- Wecke, J.; Lahav, M.; Ginsburg, I.; Giesbrecht, P. Cell wall degradation of Staphylococcus aureus by lysozyme. Arch. Microbiol. 1982, 131, 116–123. [Google Scholar] [CrossRef]



| Species | C. albicans | C. glabrata | |||
| Method | SPC | Kit | SPC | Kit | |
| LAMP | DS (CFU/mL) | 2.23 × 102 | 2.23 × 102 | 8.10 × 101 | 8.10 × 102 |
| R2 | 0.9795 | 0.9727 | 0.9754 | 0.9669 | |
| PCR | DS (CFU/mL) | 2.23 × 101 | 2.23 × 101 | 8.10 × 101 | 8.10 × 102 |
| R2 | 0.9894 | 0.9953 | 0.9569 | 0.9991 | |
| Species | C. parapsilosis | C. tropicalis | |||
| Method | SPC | Kit | SPC | Kit | |
| LAMP | DS (CFU/mL) | 3.53 × 101 | 3.53 × 102 | 2.80 × 101 | 2.80 × 101 |
| R2 | 0.9405 | 0.9949 | 0.9690 | 0.9185 | |
| PCR | DS (CFU/mL) | 3.53 × 100 | 3.53 × 101 | 2.80 × 100 | 2.80 × 101 |
| R2 | 0.9869 | 0.9992 | 0.9909 | 0.9894 | |
| System/Method | Target | Sample | Sensitivity | Specificity | Cost | Efficiency | Ref |
|---|---|---|---|---|---|---|---|
| Membrane microarray hybridized with DIG-labeled LAMP amplicons | 7 Candida species and 2 other yeasts | Cultured sample | >6 cells/reaction | High when detecting another 8 nontarget yeasts | ~3.8 euros | ~5–6 h for one assay | [30] |
| LAMP in PCR tubes | Including 4 candida species | Plasmids, swabs from indoor environment | ≥10 plasmids/reaction | Universal primers for fungal species | N/A | ~2–2.5 h for one assay | [32] |
| LAMP in PCR tubes | Candida auris | Cultured sample, ear swab | 20 copies/reaction | High when detecting another 38 fungal species | 25 µL LAMP mix per reaction | ~2 h for one assay | [35] |
| PMAxx-LAMP with portable system | Candida albicans | Cultured sample | 103 CFU/mL | Distinguishing viable and dead cells | ~1 µL LAMP mix per reaction | ~2 h for one assay | [29] |
| Microfluidic chip and detector (iChip-400, Baicare) using LAMP | Candida albicans and 4 other species | Cultured sample, clinical sample | 463 pg/µL | 8 negative and 2 false positive results for another 10 species | ~7 µL LAMP mix per reaction | ~1.5 h or one assay | [33] |
| LAMP in PCR tubes, combined with rapid DNA extraction using Chelex-100 | Universal primers for 6 candida species | Cultured sample, blood | >104 cells/mL | High when detecting another 5 species | 25 µL LAMP mix per reaction | ~1 h for one assay | [36] |
| Microfluidic chip and detector (iChip-400, Baicare) using LAMP | Candida albicans and 4 other species | Cultured sample, clinical sample | 7.53 CFU/µL | High when detecting another 10 species | ~7 µL LAMP mix per reaction | ~1.5 h or one assay | [34] |
| The RPT system | 4 candida species | Cultured sample, Vaginal swab | <2 CFU/reaction | High when detecting corresponding another 10 species | 1.41 µL LAMP mix per reaction | <1 h for one assay | This work |
| Clinical Samples | Species | LAMP | PCR | |
|---|---|---|---|---|
| VVC | 18 | C. albicans | 9 | 9 |
| C. glabrata | 5 | 5 | ||
| C. parapsilosis | 1 | 1 | ||
| C. tropicalis | 3 | 3 | ||
| non-VVC | 7 | No Candida species were detected. | ||
| Normal | 7 | |||
| Methods | Microscopic Examination | Sensitivity | Specificity | Positive Predictive Value | Negative Predictive Value | Kappa Value | |
|---|---|---|---|---|---|---|---|
| Positive | Negative | ||||||
| LAMP | 100% | 100% | 100% | 100% | 1 | ||
| Positive | 18 | 0 | |||||
| Negative | 0 | 14 | |||||
| PCR | 100% | 100% | 100% | 100% | 1 | ||
| Positive | 18 | 0 | |||||
| Negative | 0 | 14 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, X.; Li, M.; Mao, Z.; Deng, A.; Lv, W.; Huang, L.; Zhong, H.; Yang, H.; Zhang, L.; Liao, Q.; et al. An Integrated and Multi-Target Nucleic Acid Isothermal Analysis System for Rapid Diagnosis of Vulvovaginal Candidiasis. Biosensors 2023, 13, 559. https://doi.org/10.3390/bios13050559
Jin X, Li M, Mao Z, Deng A, Lv W, Huang L, Zhong H, Yang H, Zhang L, Liao Q, et al. An Integrated and Multi-Target Nucleic Acid Isothermal Analysis System for Rapid Diagnosis of Vulvovaginal Candidiasis. Biosensors. 2023; 13(5):559. https://doi.org/10.3390/bios13050559
Chicago/Turabian StyleJin, Xiangyu, Meng Li, Zeyin Mao, Anni Deng, Wenqi Lv, Leyang Huang, Hao Zhong, Han Yang, Lei Zhang, Qinping Liao, and et al. 2023. "An Integrated and Multi-Target Nucleic Acid Isothermal Analysis System for Rapid Diagnosis of Vulvovaginal Candidiasis" Biosensors 13, no. 5: 559. https://doi.org/10.3390/bios13050559
APA StyleJin, X., Li, M., Mao, Z., Deng, A., Lv, W., Huang, L., Zhong, H., Yang, H., Zhang, L., Liao, Q., & Huang, G. (2023). An Integrated and Multi-Target Nucleic Acid Isothermal Analysis System for Rapid Diagnosis of Vulvovaginal Candidiasis. Biosensors, 13(5), 559. https://doi.org/10.3390/bios13050559




