Bimetallic Biogenic Pt-Ag Nanoparticle and Their Application for Electrochemical Dopamine Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of Pt-Ag Nanoparticle
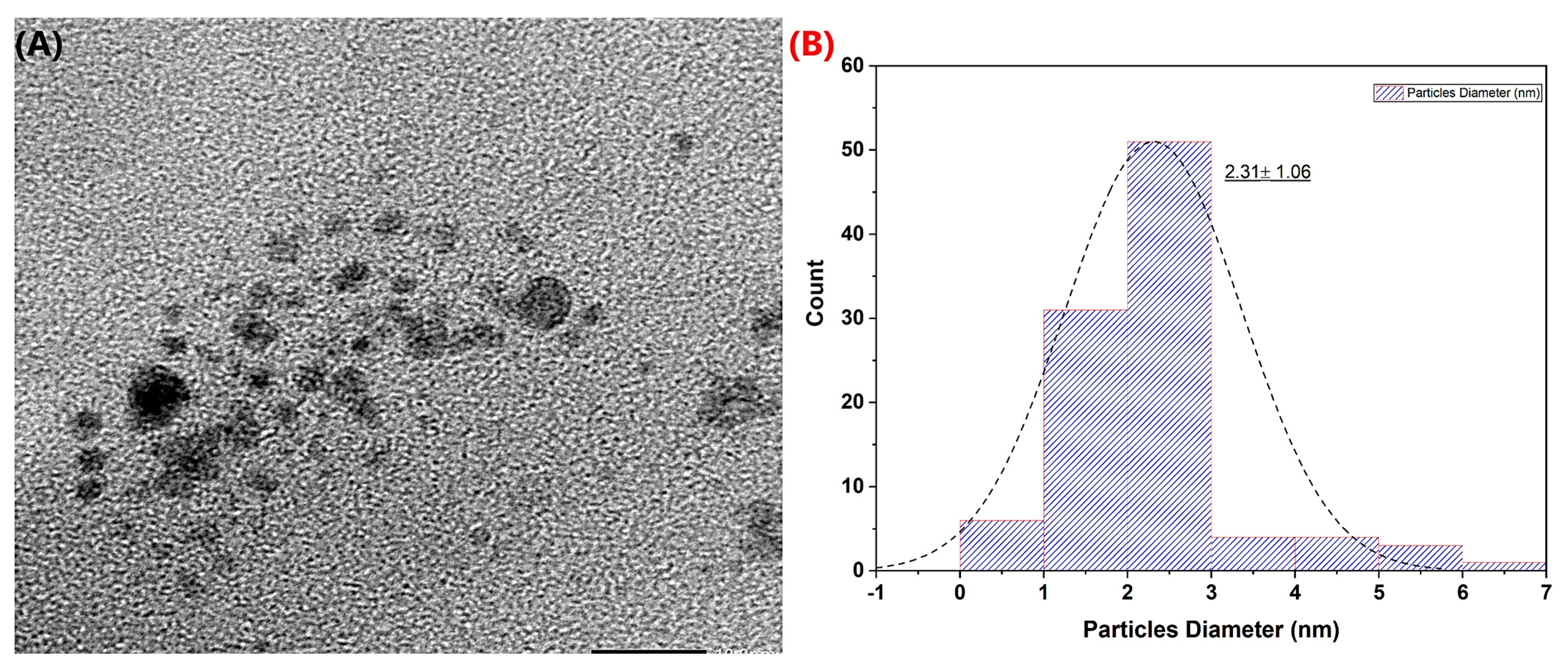
2.3. Characterization of Pt-Ag Nanoparticles
2.4. Electrochemical Characterization
2.5. Antibacterial Characterization
3. Conclusion and Discussion
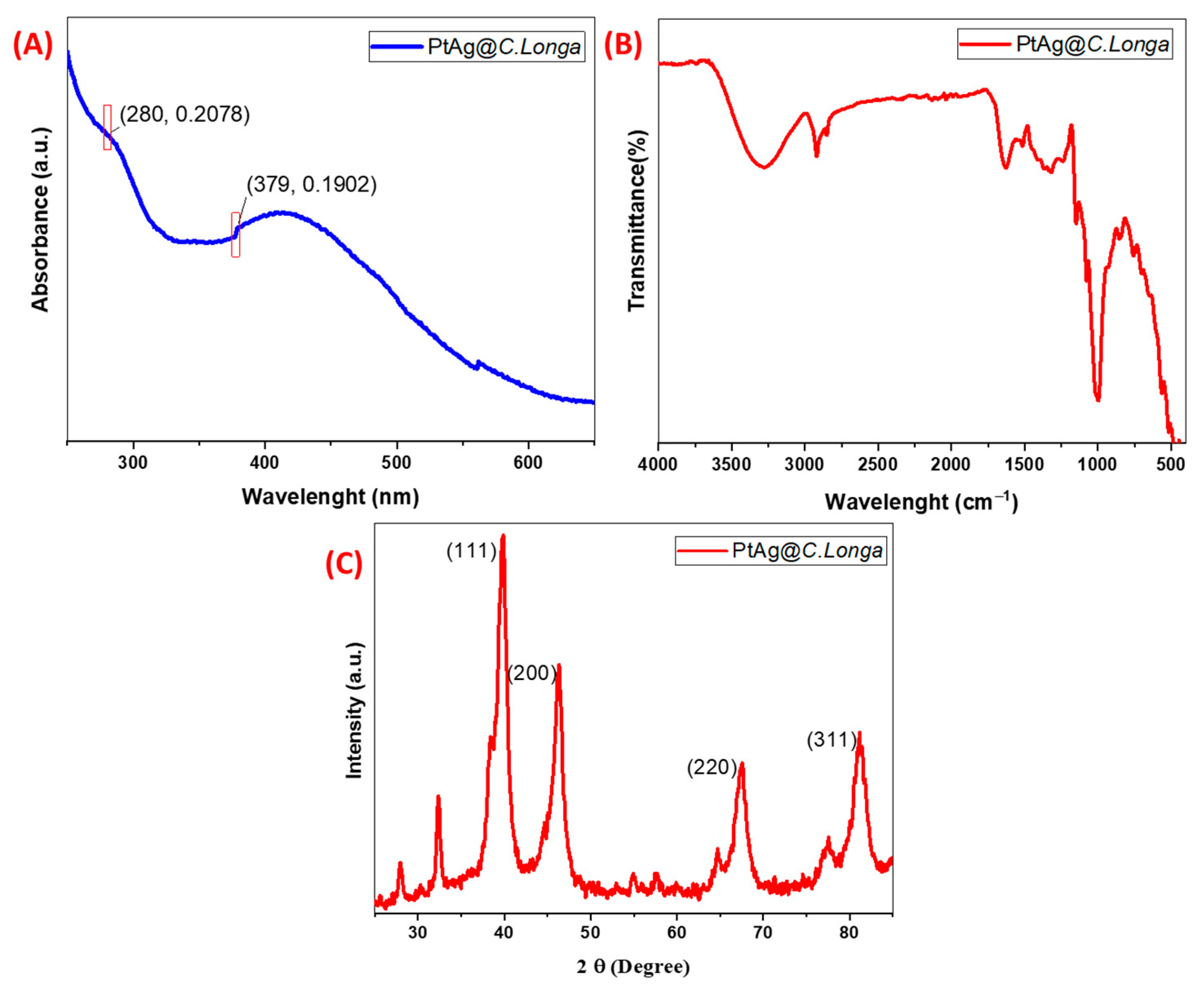
Ultraviolet-Visible (UV-VIS) Spectroscopy, FTIR, and XRD Characterization
4. Electrochemical Characterization
4.1. Use of Pt-Ag Bimetallic Modified Electrode
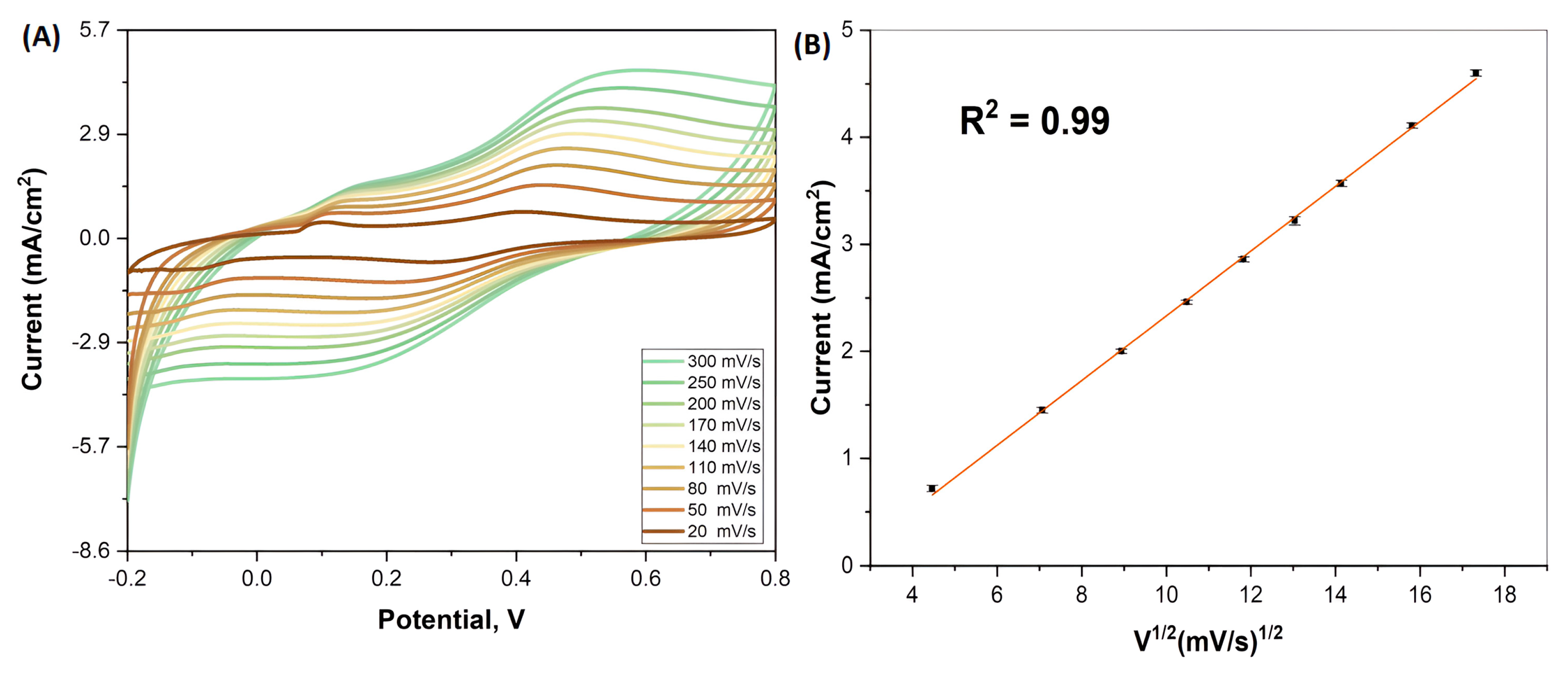
4.2. Effect of Scan Rate
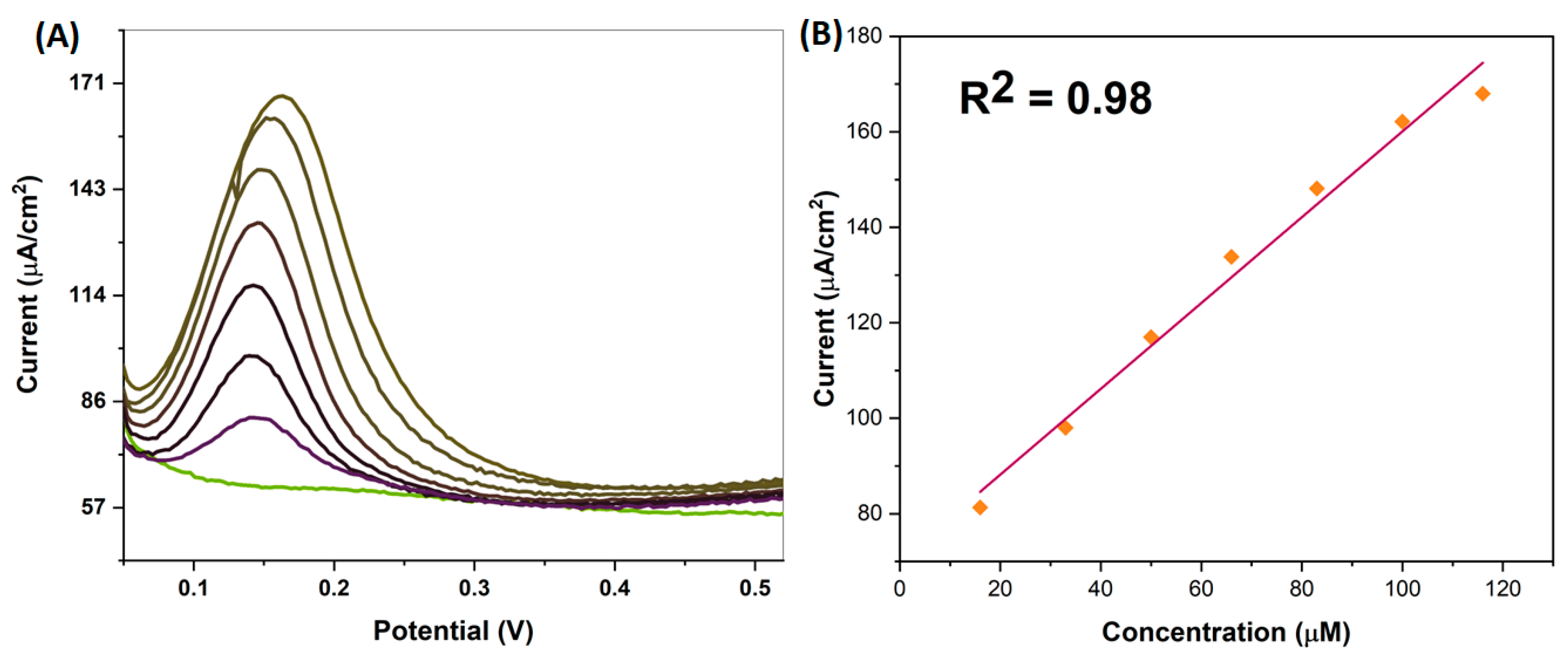
4.3. Studying Differential Pulse Voltammetry (DPV)
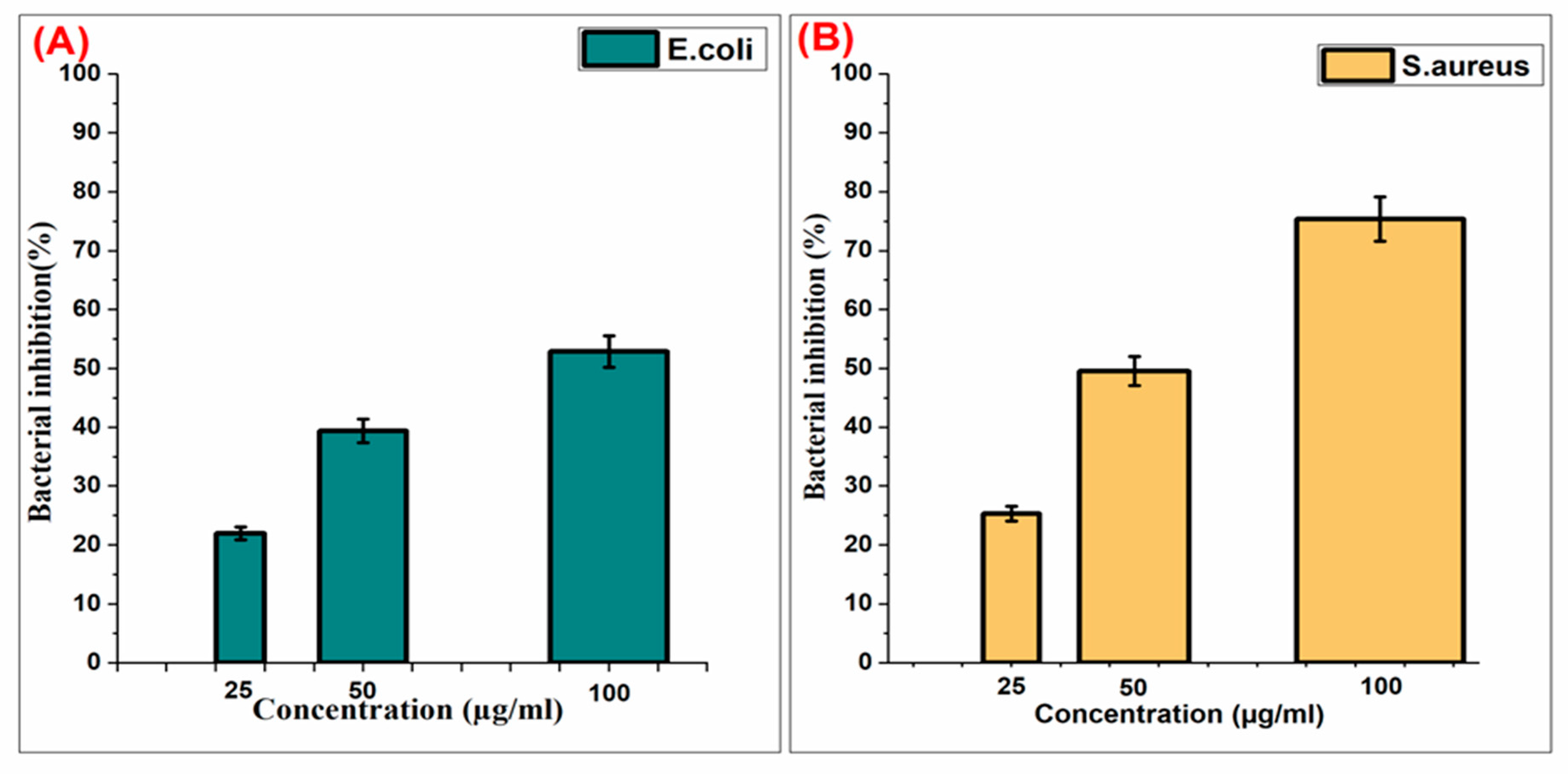
5. Bacterial Inhibition Rate of Pt-Ag Nps
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, A.; Kamal, T.; Saad, M.; Ameen, F.; Bhat, S.; Khan, M.A.; Rahman, F. Synthesis and antibacterial activity of nanoenhanced conjugate of Ag-doped ZnO nanorods with graphene oxide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122296. [Google Scholar] [CrossRef]
- Ameen, F.; Aygun, A.; Seyrankaya, A.; Tiri, R.N.E.; Gulbagca, F.; Kaynak, İ.; Majrashi, N.; Orfali, R.; Dragoi, E.N.; Sen, F. Photocatalytic investigation of textile dyes and E. coli bacteria from wastewater using Fe3O4@ MnO2 heterojunction and investigation for hydrogen generation on NaBH4 hydrolysis. Environ. Res. 2023, 220, 115231. [Google Scholar] [CrossRef] [PubMed]
- Raya, I.; Kzar, H.H.; Mahmoud, Z.H.; Al Ayub Ahmed, A.; Ibatova, A.Z.; Kianfar, E. A review of gas sensors based on carbon nanomaterial. Carbon Lett. 2021, 32, 339–364. [Google Scholar] [CrossRef]
- Prabhu, S.A.; Kavithayeni, V.; Suganthy, R.; Geetha, K. Graphene quantum dots synthesis and energy application: A review. Carbon Lett. 2021, 31, 1–12. [Google Scholar] [CrossRef]
- Kurnaz Yetim, N.; Kurşun Baysak, F.; Koç, M.M.; Nartop, D. Synthesis and characterization of Au and Bi2O3 decorated Fe3O4@ PAMAM dendrimer nanocomposites for medical applications. J. Nanostruct. Chem. 2021, 11, 589–599. [Google Scholar] [CrossRef]
- Whatmore, R.W. Nanotechnology—What is it? Should we be worried? Occup. Med. 2006, 56, 295–299. [Google Scholar] [CrossRef]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The history of nanoscience and nanotechnology: From chemical–physical applications to nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Mahmood, K.; Amara, U.; Siddique, S.; Usman, M.; Peng, Q.; Khalid, M.; Hussain, A.; Ajmal, M.; Ahmad, A.; Sumrra, S.H. Green synthesis of Ag@ CdO nanocomposite and their application towards brilliant green dye degradation from wastewater. J. Nanostruct. Chem. 2022, 12, 329–341. [Google Scholar] [CrossRef]
- Zhang, Z.; Karimi-Maleh, H. Label-free electrochemical aptasensor based on gold nanoparticles/titanium carbide MXene for lead detection with its reduction peak as index signal. Adv. Compos. Hybrid Mater. 2023, 6, 68. [Google Scholar] [CrossRef]
- Zhang, Z.; Karimi-Maleh, H. In situ synthesis of label-free electrochemical aptasensor-based sandwich-like AuNPs/PPy/Ti3C2Tx for ultrasensitive detection of lead ions as hazardous pollutants in environmental fluids. Chemosphere 2023, 324, 138302. [Google Scholar] [CrossRef]
- Jones, W.; Gibb, A.; Goodier, C.; Bust, P.; Song, M.; Jin, J. Nanomaterials in construction—What is being used, and where? Proc. Inst. Civ. Eng. Constr. Mater. 2019, 172, 49–62. [Google Scholar] [CrossRef]
- Yassin, M.T.; Elgorban, A.M.; Al-Askar, A.A.; Sholkamy, E.N.; Ameen, F.; Maniah, K. Synergistic Anticandidal Activities of Greenly Synthesized ZnO Nanomaterials with Commercial Antifungal Agents against Candidal Infections. Micromachines 2023, 14, 209. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I. Nanomaterial and nanoparticle: Origin and activity. Nanosci. Plant–Soil Syst. 2017, 48, 71–112. [Google Scholar]
- Malhotra, B.D.; Ali, M.A. Nanomaterials in biosensors: Fundamentals and applications. Nanomater. Biosens. 2018, 1–74. [Google Scholar] [CrossRef]
- Ameen, F.; Hamidian, Y.; Mostafazadeh, R.; Darabi, R.; Erk, N.; Islam, M.A.; Orfali, R. A novel atropine electrochemical sensor based on silver nano particle-coated Spirulina platensis multicellular blue-green microalga. Chemosphere 2023, 324, 138180. [Google Scholar] [CrossRef] [PubMed]
- Ameen, F.; Mostafazadeh, R.; Hamidian, Y.; Erk, N.; Sanati, A.L.; Karaman, C.; Ayati, A. Modeling of adsorptive removal of azithromycin from aquatic media by CoFe2O4@ NiO anchored microalgae-derived nitrogen-doped porous activated carbon adsorbent and colorimetric quantifying of azithromycin in pharmaceutical products. Chemosphere 2023, 329, 138635. [Google Scholar] [CrossRef]
- Ghalkhani, M.; Zare, N.; Karimi, F.; Karaman, C.; Alizadeh, M.; Vasseghian, Y. Recent advances in Ponceau dyes monitoring as food colorant substances by electrochemical sensors and developed procedures for their removal from real samples. Food Chem. Toxicol. 2022, 161, 112830. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Maleh, H.; Karaman, C.; Karaman, O.; Karimi, F.; Vasseghian, Y.; Fu, L.; Baghayeri, M.; Rouhi, J.; Senthil Kumar, P.; Show, P.-L. Nanochemistry approach for the fabrication of Fe and N co-decorated biomass-derived activated carbon frameworks: A promising oxygen reduction reaction electrocatalyst in neutral media. J. Nanostruct. Chem. 2022, 12, 429–439. [Google Scholar] [CrossRef]
- Nimir, W.; Al-Othman, A.; Tawalbeh, M.; Al Makky, A.; Ali, A.; Karimi-Maleh, H.; Karimi, F.; Karaman, C. Approaches towards the development of heteropolyacid-based high temperature membranes for PEM fuel cells. Int. J. Hydrogen Energy 2021, 8, 6638–6656. [Google Scholar] [CrossRef]
- Meenatchi, T.; Priyanka, V.; Subadevi, R.; Liu, W.-R.; Huang, C.-H.; Sivakumar, M. Probe on hard carbon electrode derived from orange peel for energy storage application. Carbon Lett. 2021, 31, 1033–1039. [Google Scholar] [CrossRef]
- Pandey, P.; Datta, M.; Malhotra, B. Prospects of nanomaterials in biosensors. Anal. Lett. 2008, 41, 159–209. [Google Scholar] [CrossRef]
- Altuner, E.E.; Akin, M.; Bayat, R.; Bekmezci, M.; Burhan, H.; Sen, F. Challenges in commercialization of carbon nanomaterial-based sensors. In Carbon Nanomaterials-Based Sensors; Elsevier: Kutahya, Turkey, 2022; pp. 381–392. [Google Scholar]
- Ermis, N.; Zare, N.; Darabi, R.; Alizadeh, M.; Karimi, F.; Singh, J.; Shahidi, S.-A.; Dragoi, E.N.; Camarada, M.B.; Baghayeri, M. Recent advantage in electrochemical monitoring of gallic acid and kojic acid: A new perspective in food science. J. Food Meas. Charact. 2023, 1–10. [Google Scholar] [CrossRef]
- Nasehi, P.; Moghaddam, M.S.; Rezaei-savadkouhi, N.; Alizadeh, M.; Yazdani, M.N.; Agheli, H. Monitoring of Bisphenol A in water and soft drink products using electrochemical sensor amplified with TiO2-SWCNTs and ionic liquid. J. Food Meas. Charact. 2022, 16, 2440–2445. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Darabi, R.; Karimi, F.; Karaman, C.; Shahidi, S.A.; Zare, N.; Baghayeri, M.; Fu, L.; Rostamnia, S.; Rouhi, J. State-of-art advances on removal, degradation and electrochemical monitoring of 4-aminophenol pollutants in real samples: A review. Environ. Res. 2023, 222, 115338. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, P.; Wang, A.; Yu, C.; Qian, T.; Wu, S.; Shen, J. Dopamine fluorescent sensors based on polypyrrole/graphene quantum dots core/shell hybrids. Biosens. Bioelectron. 2015, 64, 404–410. [Google Scholar] [CrossRef]
- Wise, R.A.; Robble, M.A. Dopamine and addiction. Annu. Rev. Psychol. 2020, 71, 79–106. [Google Scholar] [CrossRef] [PubMed]
- Hui, X.; Xuan, X.; Kim, J.; Park, J.Y. A highly flexible and selective dopamine sensor based on Pt-Au nanoparticle-modified laser-induced graphene. Electrochim. Acta 2019, 328, 135066. [Google Scholar] [CrossRef]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B.; Muthumary, J.; Srinivasan, K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 594–598. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal nanoparticles synthesis: An overview on methods of preparation, advantages and disadvantages, and applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Rana, A.; Yadav, K.; Jagadevan, S. A comprehensive review on green synthesis of nature-inspired metal nanoparticles: Mechanism, application and toxicity. J. Clean. Prod. 2020, 272, 122880. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, H.; Kumar, S.V.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles–An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Shumyantseva, V.V.; Suprun, E.V.; Bulko, T.V.; Archakov, A.I. Electrochemical methods for detection of post-translational modifications of proteins. Biosens. Bioelectron. 2014, 61, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Harandi, M.-H.; Shabani-Nooshabadi, M.; Darabi, R. Cu-BTC metal-organic frameworks as catalytic modifier for ultrasensitive electrochemical determination of methocarbamol in the presence of methadone. J. Electrochem. Soc. 2021, 168, 097507. [Google Scholar] [CrossRef]
- Darabi, R.; Shabani-Nooshabadi, M.; Khoobi, A. A potential strategy for simultaneous determination of deferoxamine and vitamin C using MCR-ALS with nanostructured electrochemical sensor in serum and urine of thalassemia and diabetic patients. J. Electrochem. Soc. 2021, 168, 046514. [Google Scholar] [CrossRef]
- Hussain, R.T.; Islam, A.S.; Khairuddean, M.; Suah, F.B.M. A polypyrrole/GO/ZnO nanocomposite modified pencil graphite electrode for the determination of andrographolide in aqueous samples. Alex. Eng. J. 2022, 61, 4209–4218. [Google Scholar] [CrossRef]
- Perkampus, H.-H. UV-VIS Spectroscopy and Its Applications; Springer Science & Business Media: New York, NY, USA, 2013. [Google Scholar]
- Salem, M.A.; Bakr, E.A.; El-Attar, H.G. Pt@ Ag and Pd@ Ag core/shell nanoparticles for catalytic degradation of Congo red in aqueous solution. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 188, 155–163. [Google Scholar] [CrossRef]
- Govindan, S.; Nivethaa, E.; Saravanan, R.; Narayanan, V.; Stephen, A. Synthesis and characterization of chitosan–silver nanocomposite. Appl. Nanosci. 2012, 2, 299–303. [Google Scholar] [CrossRef]
- Thirumurugan, A.; Aswitha, P.; Kiruthika, C.; Nagarajan, S.; Christy, A.N. Green synthesis of platinum nanoparticles using Azadirachta indica–An eco-friendly approach. Mater. Lett. 2016, 170, 175–178. [Google Scholar] [CrossRef]
- Yang, M.; Lu, F.; Zhou, T.; Zhao, J.; Ding, C.; Fakhri, A.; Gupta, V.K. Biosynthesis of nano bimetallic Ag/Pt alloy from Crocus sativus L. extract: Biological efficacy and catalytic activity. J. Photochem. Photobiol. B Biol. 2020, 212, 112025. [Google Scholar] [CrossRef]
- Wang, R.; Feng, J.-J.; Xue, Y.; Wu, L.; Wang, A.-J. A label-free electrochemical immunosensor based on AgPt nanorings supported on reduced graphene oxide for ultrasensitive analysis of tumor marker. Sens. Actuators B Chem. 2018, 254, 1174–1181. [Google Scholar] [CrossRef]
- Shao, F.-Q.; Feng, J.-J.; Yang, Z.-Z.; Chen, S.-S.; Yuan, J.; Wang, A.-J. Cytosine assisted aqueous synthesis of AgPt hollow alloyed nanostructures as highly active electrocatalyst for ethylene glycol oxidation and hydrogen evolution. Int. J. Hydrogen Energy 2017, 42, 24767–24775. [Google Scholar] [CrossRef]
- Mehmandoust, M.; Erk, N.; Karaman, C.; Karaman, O. An electrochemical molecularly imprinted sensor based on CuBi2O4/rGO@ MoS2 nanocomposite and its utilization for highly selective and sensitive for linagliptin assay. Chemosphere 2022, 291, 132807. [Google Scholar] [CrossRef] [PubMed]
- Mehmandoust, M.; Erk, N.; Karaman, O.; Karimi, F.; Bijad, M.; Karaman, C. Three-dimensional porous reduced graphene oxide decorated with carbon quantum dots and platinum nanoparticles for highly selective determination of azo dye compound tartrazine. Food Chem. Toxicol. 2021, 158, 112698. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Zhang, B.; Saha, C.; Kumar, C.; Kaushik, B.K.; Kumar, S. Development of dopamine sensor using silver nanoparticles and PEG-functionalized tapered optical fiber structure. IEEE Trans. Biomed. Eng. 2019, 67, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Anuar, N.S.; Basirun, W.J.; Shalauddin, M.; Akhter, S. A dopamine electrochemical sensor based on a platinum–silver graphene nanocomposite modified electrode. RSC Adv. 2020, 10, 17336–17344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sharma, G.; Kumar, A.; Shekh, M.I.; Stadler, F.J. Fabrication and characterization of Ni/Ag/Zn trimetal oxide nanocomposites and its application in dopamine sensing. Mater. Today Commun. 2021, 29, 102726. [Google Scholar] [CrossRef]
- Li, Y.; Fan, C.; Zheng, J. A high-efficiency electrochemical sensor of dopamine based on WS2 nanosheets decorated with dandelion-like platinum–silver nanoparticles. J. Mater. Sci. Mater. Electron. 2022, 33, 5061–5072. [Google Scholar] [CrossRef]
- Karatas, E.; Ozden, D.S.; Yilmaz, M.; Yazan, Z.; Piskin, E. A sensitive nanocomposite design via polydopamine mediated Au and Ag nanoparticles: Voltammetric assay for dopamine in biological samples. Thin Solid Film. 2022, 756, 139354. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Kang, P.; Wang, S.-Q.; Liu, Z.-G.; Li, Y.-X.; Guo, Z. Ag nanoparticles anchored onto porous CuO nanobelts for the ultrasensitive electrochemical detection of dopamine in human serum. Sens. Actuators B Chem. 2021, 327, 128878. [Google Scholar] [CrossRef]
- Tiri, R.N.E.; Gulbagca, F.; Aygun, A.; Cherif, A.; Sen, F. Biosynthesis of Ag–Pt bimetallic nanoparticles using propolis extract: Antibacterial effects and catalytic activity on NaBH4 hydrolysis. Environ. Res. 2022, 206, 112622. [Google Scholar] [CrossRef]

| Material | Detection Method | Linear Range | LOD | References |
|---|---|---|---|---|
| AgNPs | LSPR | 10 nM–1 µM | 0.058 µM | [47] |
| Pt–Ag/Gr | DPV | 0.1–60 µM | 0.012 μM | [48] |
| Ni/Ag/Zn | DPV | 1–25 μM | 0.3 μM | [49] |
| PtAg/WS2/GCE | Amperometry | 0.6–1000 μM | 0.2 μM | [50] |
| Cit-AuNP@PDA@PGE | DPV | 0.5–7.0 μM | 0.96 µM | [51] |
| Ag/CuO/ITO | CV | 0.04–10 μM | 7 nM | [52] |
| Biogenic Pt-Ag | DPV | 16 μM–0.11 mM | 0.03 µM | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekmezci, M.; Ozturk, H.; Akin, M.; Bayat, R.; Sen, F.; Darabi, R.; Karimi-Maleh, H. Bimetallic Biogenic Pt-Ag Nanoparticle and Their Application for Electrochemical Dopamine Sensor. Biosensors 2023, 13, 531. https://doi.org/10.3390/bios13050531
Bekmezci M, Ozturk H, Akin M, Bayat R, Sen F, Darabi R, Karimi-Maleh H. Bimetallic Biogenic Pt-Ag Nanoparticle and Their Application for Electrochemical Dopamine Sensor. Biosensors. 2023; 13(5):531. https://doi.org/10.3390/bios13050531
Chicago/Turabian StyleBekmezci, Muhammed, Hudanur Ozturk, Merve Akin, Ramazan Bayat, Fatih Sen, Rozhin Darabi, and Hassan Karimi-Maleh. 2023. "Bimetallic Biogenic Pt-Ag Nanoparticle and Their Application for Electrochemical Dopamine Sensor" Biosensors 13, no. 5: 531. https://doi.org/10.3390/bios13050531
APA StyleBekmezci, M., Ozturk, H., Akin, M., Bayat, R., Sen, F., Darabi, R., & Karimi-Maleh, H. (2023). Bimetallic Biogenic Pt-Ag Nanoparticle and Their Application for Electrochemical Dopamine Sensor. Biosensors, 13(5), 531. https://doi.org/10.3390/bios13050531






