Metal Coordination-Driven Supramolecular Nanozyme as an Effective Colorimetric Biosensor for Neurotransmitters and Organophosphorus Pesticides
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Fabrication of SupraZyme
2.3. Sensing of Acetylcholine Esterase and Neurotransmitter Surrogate Acetylthiocholine
2.4. Sensing of Neurotransmitter Epinephrine and Norepinephrine
2.5. Sensing of Organophosphorus Pesticides
2.6. Sensing of Organophosphorus Pesticides in Spiked Samples
3. Results and Discussion
3.1. SupraZyme Preparation and Characterization
3.2. Inhibition of Oxidase-like Nanozyme Activity by Thiol Groups
3.3. Oxidase-like Nanozyme Activity to Generate Colorimetric Response for Biosensing
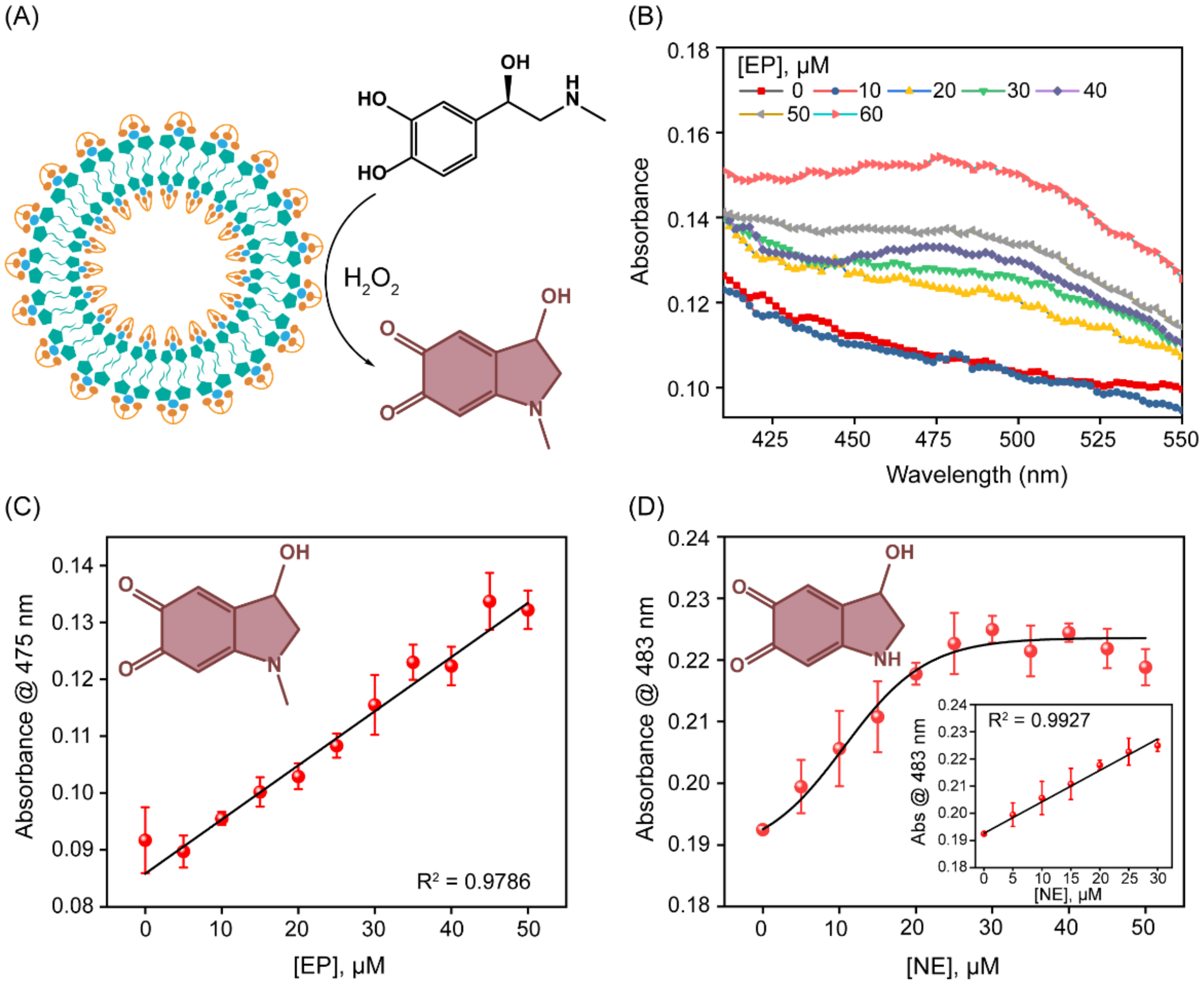
3.4. Detection of Epinephrine and Norepinephrine
3.5. Detection of Organophosphorus (OP) Pesticides
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, J.-X.; Xia, J.-J.; Deng, F.-L.; Liang, W.-W.; Wu, J.; Yin, B.-M.; Dong, M.-X.; Chen, J.-J.; Ye, F.; Wang, H.-Y.; et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: A targeted metabolomics study. Transl. Psychiatry 2018, 8, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Nagatsu, T. The catecholamine system in health and disease -Relation to tyrosine 3-monooxygenase and other catecholamine-synthesizing enzymes. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2007, 82, 388–415. [Google Scholar] [CrossRef] [PubMed]
- Elugoke, S.E.; Adekunle, A.S.; Fayemi, O.E.; Mamba, B.B.; Nkambule, T.T.I.; Sherif, E.-S.M.; Ebenso, E.E. Progress in electrochemical detection of neurotransmitters using carbon nanotubes/nanocomposite based materials: A chronological review. Nano Select 2020, 1, 561–611. [Google Scholar] [CrossRef]
- Herholz, K. Acetylcholine esterase activity in mild cognitive impairment and Alzheimer’s disease. Eur. J. Nucl. Med. Mol. Imaging 2008, 35 (Suppl. 1), S25–S29. [Google Scholar] [CrossRef]
- Kiso, Y.; Li, H.; Shigetoh, K.; Kitao, T.; Jinno, K. Pesticide analysis by high-performance liquid chromatography using the direct injection method. J. Chromatogr. A 1996, 733, 259–265. [Google Scholar] [CrossRef]
- Ashrafi Tafreshi, F.; Fatahi, Z.; Ghasemi, S.F.; Taherian, A.; Esfandiari, N. Ultrasensitive fluorescent detection of pesticides in real sample by using green carbon dots. PLoS ONE 2020, 15, e0230646. [Google Scholar] [CrossRef]
- An, J.; Chen, M.; Hu, N.; Hu, Y.; Chen, R.; Lyu, Y.; Guo, W.; Li, L.; Liu, Y. Carbon dots-based dual-emission ratiometric fluorescence sensor for dopamine detection. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 243, 118804. [Google Scholar] [CrossRef]
- Viswanathan, S.; Radecka, H.; Radecki, J. Electrochemical biosensor for pesticides based on acetylcholinesterase immobilized on polyaniline deposited on vertically assembled carbon nanotubes wrapped with ssDNA. Biosens. Bioelectron. 2009, 24, 2772–2777. [Google Scholar] [CrossRef]
- Ratnam, K.V.; Manjunatha, H.; Janardan, S.; Babu Naidu, K.C.; Ramesh, S. Nonenzymatic electrochemical sensor based on metal oxide, MO (M = Cu, Ni, Zn, and Fe) nanomaterials for neurotransmitters: An abridged review. Sens. Int. 2020, 1, 100047. [Google Scholar] [CrossRef]
- Land, K.J.; Boeras, D.I.; Chen, X.-S.; Ramsay, A.R.; Peeling, R.W. REASSURED diagnostics to inform disease control strategies, strengthen health systems and improve patient outcomes. Nat. Microbiol. 2019, 4, 46–54. [Google Scholar] [CrossRef]
- De, M.; Rana, S.; Akpinar, H. Sensing of proteins in human serum using conjugates of nanoparticles and green fluorescent protein. Nat. Chem. 2009, 1, 461–465. [Google Scholar] [CrossRef]
- Agasti, S.S.; Rana, S.; Park, M.-H.; Kim, C.K.; You, C.-C.; Rotello, V.M. Nanoparticles for detection and diagnosis. Adv. Drug Deliv. Rev. 2010, 62, 316–328. [Google Scholar] [CrossRef]
- Rana, S.; Le, N.D.B.; Mout, R.; Duncan, B.; Elci, S.G.; Saha, K.; Rotello, V.M. A Multichannel Biosensor for Rapid Determination of Cell Surface Glycomic Signatures. ACS Cent. Sci. 2015, 1, 191–197. [Google Scholar] [CrossRef]
- Broto, M.; Kaminski, M.M.; Adrianus, C.; Kim, N.; Greensmith, R.; Dissanayake-Perera, S.; Schubert, A.J.; Tan, X.; Kim, H.; Dighe, A.S.; et al. Nanozyme-catalysed CRISPR assay for preamplification-free detection of non-coding RNAs. Nat. Nanotechnol. 2022, 17, 1120–1126. [Google Scholar] [CrossRef]
- Wang, J.; Huang, R.; Qi, W.; Su, R.; He, Z. Preparation of amorphous MOF based biomimetic nanozyme with high laccase- and catecholase-like activity for the degradation and detection of phenolic compounds. Chem. Eng. J. 2022, 434, 134677. [Google Scholar] [CrossRef]
- Ouyang, Y.; Fadeev, M.; Zhang, P.; Carmieli, R.; Sohn, Y.S.; Karmi, O.; Qin, Y.; Chen, X.; Nechushtai, R.; Willner, I. Aptamer-Functionalized Ce4+-Ion-Modified C-Dots: Peroxidase Mimicking Aptananozymes for the Oxidation of Dopamine and Cytotoxic Effects toward Cancer Cells. ACS Appl. Mater. Interfaces 2022, 14, 55365–55375. [Google Scholar] [CrossRef]
- Fedeli, S.; Im, J.; Gopalakrishnan, S.; Elia, J.L.; Gupta, A.; Kim, D.; Rotello, V.M. Nanomaterial-based bioorthogonal nanozymes for biological applications. Chem. Soc. Rev. 2021, 50, 13467–13480. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Kim, M.I. Nanomaterial-mediated paper-based biosensors for colorimetric pathogen detection. TrAC Trends Anal. Chem. 2020, 132, 116038–116050. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Kim, M.I. Using Nanomaterials in Colorimetric Toxin Detection. BioChip J. 2021, 15, 123–134. [Google Scholar] [CrossRef]
- Arshad, F.; Mohd-Naim, N.F.; Chandrawati, R.; Cozzolino, D.; Ahmed, M.U. Nanozyme-based sensors for detection of food biomarkers: A review. RSC Adv. 2022, 12, 26160–26175. [Google Scholar] [CrossRef]
- Maity, T.; Jain, S.; Solra, M.; Barman, S.; Rana, S. Robust and Reusable Laccase Mimetic Copper Oxide Nanozyme for Phenolic Oxidation and Biosensing. ACS Sustain. Chem. Eng. 2022, 10, 1398–1407. [Google Scholar] [CrossRef]
- Das, R.; Hardie, J.; Joshi, B.P.; Zhang, X.; Gupta, A.; Luther, D.C.; Fedeli, S.; Farkas, M.E.; Rotello, V.M. Macrophage-Encapsulated Bioorthogonal Nanozymes for Targeting Cancer Cells. JACS Au. 2022, 2, 1679–1685. [Google Scholar] [CrossRef]
- Mohamad, A.; Keasberry, N.A.; Ahmed, M.U. Enzyme-free Gold-silver Core-shell Nanozyme Immunosensor for the Detection of Haptoglobin. Anal. Sci. 2018, 34, 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Khataee, A.; Hassanzadeh, J.; Habibi, B. Sensitive biosensing of organophosphate pesticides using enzyme mimics of magnetic ZIF-8. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 209, 118–125. [Google Scholar] [CrossRef]
- Lin, L.; Ma, H.; Yang, C.; Chen, W.; Zeng, S.; Hu, Y. A colorimetric sensing platform based on self-assembled 3D porous CeGONR nanozymes for label-free visual detection of organophosphate pesticides. Mater. Adv. 2020, 1, 2789–2796. [Google Scholar] [CrossRef]
- Bagheri, N.; Khataee, A.; Habibi, B.; Hassanzadeh, J. Mimetic Ag nanoparticle/Zn-based MOF nanocomposite (AgNPs@ZnMOF) capped with molecularly imprinted polymer for the selective detection of patulin. Talanta 2018, 179, 710–718. [Google Scholar] [CrossRef]
- Dietrich, B.; Lehn, J.; Sauvage, J. Diaza-polyoxa-macrocycles et macrobicycles. Tetrahedron Lett. 1969, 10, 2885–2888. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1967, 89, 2495–2496. [Google Scholar] [CrossRef]
- Della Sala, F.; Neri, S.; Maiti, S.; Chen, J.L.Y.; Prins, L.J. Transient self-assembly of molecular nanostructures driven by chemical fuels. Curr. Opin. Biotechnol. 2017, 46, 27–33. [Google Scholar] [CrossRef]
- Maiti, S.; Fortunati, I.; Ferrante, C.; Scrimin, P.; Prins, L.J. Dissipative self-assembly of vesicular nanoreactors. Nat. Chem. 2016, 8, 725–731. [Google Scholar] [CrossRef]
- Khalily, M.A.; Gulseren, G.; Tekinay, A.B.; Guler, M.O. Biocompatible Supramolecular Catalytic One-Dimensional Nanofibers for Efficient Labeling of Live Cells. Bioconjugate Chem. 2015, 26, 2371–2375. [Google Scholar] [CrossRef]
- Solra, M.; Das, S.; Srivastava, A.; Sen, B.; Rana, S. Temporally Controlled Multienzyme Catalysis Using a Dissipative Supramolecular Nanozyme. ACS Appl. Mater. Interfaces 2022, 14, 45096–45109. [Google Scholar] [CrossRef]
- Kamra, A.; Das, S.; Bhatt, P.; Solra, M.; Maity, T.; Rana, S. A Dissipative Supramolecular Glue for Temporal Control of Amplified Enzyme Activity and Biocatalytic Cascades. ChemRxiv 2022, 1–23. [Google Scholar] [CrossRef]
- Pakiari, A.H.; Jamshidi, Z. Nature and Strength of M−S Bonds (M = Au, Ag, and Cu) in Binary Alloy Gold Clusters. J. Phys. Chem. A 2010, 114, 9212–9221. [Google Scholar] [CrossRef]
- Han, F.; Li, W.-C.; Li, D.; Lu, A.-H. In Situ Electrochemical Generation of Mesostructured Cu2S/C Composite for Enhanced Lithium Storage: Mechanism and Material Properties. ChemElectroChem 2014, 1, 733–740. [Google Scholar] [CrossRef]
- Sastry, K.S.R.; Karpova, Y.; Prokopovich, S.; Smith, A.J.; Essau, B.; Gersappe, A.; Carson, J.P.; Weber, M.J.; Register, T.C.; Chen, Y.Q.; et al. Epinephrine Protects Cancer Cells from Apoptosis via Activation of cAMP-dependent Protein Kinase and BAD Phosphorylation. J. Biol. Chem. 2007, 282, 14094–14100. [Google Scholar] [CrossRef]
- Pan, X.; Kaminga, A.C.; Jia, P.; Wen, S.W.; Acheampong, K.; Liu, A. Catecholamines in Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Front. Aging Neurosci. 2020, 12, 184. [Google Scholar] [CrossRef]
- Zuo, Z.; Kang, T.; Hu, S.; Su, W.; Gan, Y.; Miao, Z.; Zhao, H.; Feng, P.; Ke, B.; Li, M. A Bioluminescent Probe for Detecting Norepinephrine in Vivo. Anal. Chem. 2022, 94, 6441–6445. [Google Scholar] [CrossRef]
- Ivanov, A.N.; Younusov, R.R.; Evtugyn, G.A.; Arduini, F.; Moscone, D.; Palleschi, G. Acetylcholinesterase Biosensor based on Single-walled Carbon Nanotubes—Co phtalocyanine for Organophosphorus Pesticides Detection. Talanta 2011, 85, 216–221. [Google Scholar] [CrossRef]
- Dutta, R.R.; Puzari, P. Amperometric biosensing of Organophosphate and Organocarbamate Pesticides utilizing Polypyrrole entrapped Acetylcholinesterase Electrode. Biosen. Bioelectron. 2014, 52, 166–172. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Q.; Li, Q.; Li, H.; Li, F. Two-Dimensional MnO2 Nanozyme-Mediated Homogeneous Electrochemical Detection of Organophosphate Pesticides without the Interference of H2O2 and Color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wei, J.; Zou, J.; Cheng, Z.; Huang, Z.; Gu, L.; Zhong, Z.; Li, S.; Wang, Y.; Li, P. Electrochemical Detection of Methyl-paraoxon based on Bifunctional Cerium Oxide Nanozyme with Catalytic Activity and Signal Amplification Effect. J. Pharm. Anal. 2021, 11, 653–660. [Google Scholar] [CrossRef]
- Li, X.; Cui, H.; Zeng, Z. A Simple Colorimetric and Fluorescent Sensor to Detect Organophosphate Pesticides Based on Adenosine Triphosphate-Modified Gold Nanoparticles. Sensors 2018, 18, 4302–4314. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.M.; Ren, W.; Irudayaraj, J.; Sajini, A.A.; Ali, M.I.; Ahmad, B. Colorimetric Detection of Organophosphate Pesticides Based on Acetylcholinesterase and Cysteamine Capped Gold Nanoparticles as Nanozyme. Sensors 2021, 21, 8050. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Fan, K.; Pan, Y.; Jiang, H.; Wang, F.; Yang, D.; Lu, D.; Feng, J.; Zhao, J.; Yang, L.; et al. Fe3O4 Magnetic Nanoparticle Peroxidase Mimetic-Based Colorimetric Assay for the Rapid Detection of Organophosphorus Pesticide and Nerve Agent. Anal. Chem. 2013, 85, 308–312. [Google Scholar] [CrossRef]
- Wei, J.; Yang, L.; Luo, M.; Wang, Y.; Li, P. Nanozyme-assisted technique for Dual Mode Detection of Organophosphorus Pesticide. Ecotoxicol. Environ. Saf. 2019, 179, 17–23. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, J.; Wu, Y. A Simple and Rapid Chemosensor for Colorimetric Detection of Dimethoate Pesticide based on the Peroxidase-mimicking Catalytic Activity of Gold Nanoparticles. Anal. Methods 2019, 11, 5337–5347. [Google Scholar] [CrossRef]
- Luo, D.; Huang, X.; Liu, B.; Zou, W.; Wu, Y. Facile Colorimetric Nanozyme Sheet for the Rapid Detection of Glyphosate in Agricultural Products Based on Inhibiting Peroxidase-Like Catalytic Activity of Porous Co3O4 Nanoplates. J. Agric. Food Chem. 2021, 69, 3537–3547. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Rosello, C.; Bélanger, R.; Ratti, C. Fate of Residual Pesticides in Fruit and Vegetable Waste (FVW) Processing. Foods 2020, 9, 1468. [Google Scholar] [CrossRef]
- Lv, J.; He, B.; Wang, N.; Li, M.; Lin, Y. A Gold Nanoparticle based Colorimetric and Fluorescent Dual-Channel Probe for Acetylcholinesterase Detection and Inhibitor Screening. RSC Adv. 2018, 8, 32893–32898. [Google Scholar] [CrossRef]
- Huang, L.; Sun, D.-W.; Pu, H.; Wei, Q.; Luo, L.; Wang, J. A Colorimetric Paper Sensor Based on the Domino Reaction of Acetylcholinesterase and Degradable γ-MnOOH Nanozyme for Sensitive Detection of Organophosphorus Pesticides. Sens. Actuators B Chem. 2019, 290, 573–580. [Google Scholar] [CrossRef]
- Qian, Z.; Chai, L.; Tang, C.; Huang, Y.; Chen, J.; Feng, H. A Fluorometric Assay for Acetylcholinesterase Activity and Inhibitor Screening with Carbon Quantum Dots. Sens. Actuators B Chem. 2016, 222, 879–886. [Google Scholar] [CrossRef]
- Wang, M.; Gu, X.; Zhang, G.; Zhang, D.; Zhu, D. Continuous Colorimetric Assay for Acetylcholinesterase and Inhibitor Screening with Gold Nanoparticles. Langmuir 2009, 25, 2504–2507. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, D.; Zhang, G.; Zhu, D. Colorimetric detection of Glucose and an Assay for Acetylcholinesterase with Amine-terminated Polydiacetylene Vesicles. Sci. Bull. 2011, 56, 1877–1883. [Google Scholar] [CrossRef]
- Ren, L.; Li, H.; Liu, M.; Du, J. Light-accelerating Oxidase-mimicking Activity of Black Phosphorus Quantum Dots for Colorimetric detection of Acetylcholinesterase Activity and Inhibitor Screening. Analyst 2020, 145, 8022–8029. [Google Scholar] [CrossRef]
- Lu, L.; Hu, X.; Zeng, R.; Lin, Q.; Huang, X.; Li, M.; Tang, D. Dual-mode Colorimetric-Photothermal Sensing Platform of Acetylcholinesterase Activity based on the Peroxidase-like Activity of Fe–N–C Nanozyme. Anal. Chim. Acta 2022, 1229, 340383. [Google Scholar] [CrossRef]
- Han, T.; Wang, G. Peroxidase-like Activity of Acetylcholine-based Colorimetric detection of Acetylcholinesterase Activity and an Organophosphorus Inhibitor. J. Mater. Chem. B 2019, 7, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Huang, X.; Cai, J.; Zhang, A.; Ding, J.; Chen, S. An Amperometric Acetylthiocholine Sensor based on Immobilization of Acetylcholinesterase on a Multiwall Carbon Nanotube–Cross-linked Chitosan Composite. Anal. Bioanal. Chem. 2007, 387, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Duenas, L.; Gomez, F.A. Thread- and Capillary Tube-Based Electrodes for the Detection of Glucose and Acetylthiocholine. Micromachines 2020, 11, 920. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.; Wang, M.; Ma, R.; Liu, Q.; Sun, X.; Xiong, Y.; Chen, X. Single-atom Rh Nanozyme: An Efficient Catalyst for Highly Sensitive Colorimetric Detection of Acetylcholinesterase activity and Adrenaline. Sens. Actuators B Chem. 2023, 375, 132972. [Google Scholar] [CrossRef]
- Ying, M.; Yang, G.; Xu, Y.; Ye, H.; Lin, X.; Lu, Y.; Pan, H.; Bai, Y.; Du, M. Copper Fumarate with High-bifunctional Nanozyme activities at different pH values for Glucose and Epinephrine Colorimetric detection in Human Serum. Analyst 2022, 147, 40–47. [Google Scholar] [CrossRef]
- Guan, M.; Wang, M.; Qi, W.; Su, R.; He, Z. Biomineralization-inspired Copper-Cystine Nanoleaves Capable of Laccase-like Catalysis for the Colorimetric detection of Epinephrine. Front. Chem. Sci. Eng. 2021, 15, 310–318. [Google Scholar] [CrossRef]
- Alizadeh, N.; Ghasemi, S.; Salimi, A.; Sham, T.-K.; Hallaj, R. CuO nanorods as a Laccase mimicking enzyme for highly sensitive Colorimetric and Electrochemical Dual Biosensor: Application in living cell epinephrine analysis. Colloids Surf. B. 2020, 195, 111228. [Google Scholar] [CrossRef]
- Godoy-Reyes, T.M.; Costero, A.M.; Gaviña, P.; Martínez-Máñez, R.; Sancenón, F. A Colorimetric Probe for the Selective Detection of Norepinephrine Based on a Double Molecular Recognition with Functionalized Gold Nanoparticles. ACS Appl. Nano Mater. 2019, 2, 1367–1373. [Google Scholar] [CrossRef]
- Menon, S.; Jose, A.R.; Jesny, S.; Kumar, K.G. A Colorimetric and Fluorometric Sensor for the Determination of Norepinephrine. Anal. Methods. 2016, 8, 5801–5805. [Google Scholar] [CrossRef]
- Ally, N.; Hendricks, N.; Gumbi, B. A Colorimetric Detection of Noradrenaline in Wastewater Using Citrate-Capped Colloidal Gold Nanoparticles Probe. Colloids Inter. 2022, 6, 61. [Google Scholar] [CrossRef]
- Son, S.E.; Ko, E.; Tran, V.-K.; Hur, W.; Choi, H.; Lee, H.B.; Park, Y.; Seong, G.H. Highly Sensitive Electrochemical Determination of Norepinephrine Using Poly Acrylic Acid-Coated Nanoceria. ChemElectroChem. 2019, 6, 4666–4673. [Google Scholar] [CrossRef]





| Method | Material | Pesticide Detected | Detection Limit | Ref. |
|---|---|---|---|---|
| Electrochemical methods | AChE/SWCNT-Co phthalocyanine/GCE | Paraoxon | 2 ppb | [39] |
| PPy-AChE-Geltn-Glut/Pt | Paraoxon | 1.1 ppb | [40] | |
| manganese dioxide nanosheets (MnNS) | Paraoxon | 0.025 ppb | [41] | |
| CeO2 nanozyme | Paraoxon | 14.8 ppb | [42] | |
| Colorimetric methods | RB-AuNPs | Ethoprophos | 89 ppb | [43] |
| Cysteamine capped gold nanoparticles (C-AuNPs) | parathion ethyl | 5.8 ppb | [44] | |
| Fe3O4 magnetic nanoparticle | Paraoxon | 2.47 ppb | [45] | |
| Nanoceria | Paraoxon | 103.7 ppb | [46] | |
| AuNPs | Dimethoate | 4.7 ppb | [47] | |
| Co3O4 Nanoplates | Glyphosate | 175 ppb | [48] | |
| SupraZyme | Paraoxon | 0.48 ppb | This work | |
| Methamidophos | 15.8 ppb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatt, P.; Solra, M.; Chaudhury, S.I.; Rana, S. Metal Coordination-Driven Supramolecular Nanozyme as an Effective Colorimetric Biosensor for Neurotransmitters and Organophosphorus Pesticides. Biosensors 2023, 13, 277. https://doi.org/10.3390/bios13020277
Bhatt P, Solra M, Chaudhury SI, Rana S. Metal Coordination-Driven Supramolecular Nanozyme as an Effective Colorimetric Biosensor for Neurotransmitters and Organophosphorus Pesticides. Biosensors. 2023; 13(2):277. https://doi.org/10.3390/bios13020277
Chicago/Turabian StyleBhatt, Preeti, Manju Solra, Smarak Islam Chaudhury, and Subinoy Rana. 2023. "Metal Coordination-Driven Supramolecular Nanozyme as an Effective Colorimetric Biosensor for Neurotransmitters and Organophosphorus Pesticides" Biosensors 13, no. 2: 277. https://doi.org/10.3390/bios13020277
APA StyleBhatt, P., Solra, M., Chaudhury, S. I., & Rana, S. (2023). Metal Coordination-Driven Supramolecular Nanozyme as an Effective Colorimetric Biosensor for Neurotransmitters and Organophosphorus Pesticides. Biosensors, 13(2), 277. https://doi.org/10.3390/bios13020277






