Odorant Binding Causes Cytoskeletal Rearrangement, Leading to Detectable Changes in Endothelial and Epithelial Barrier Function and Micromotion
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells
2.2. Odorants
2.3. Electric-Cell Substrate Impedance Sensing (ECIS)
2.4. Micromotion
2.5. cAMP Activity
2.6. Actin and Tubulin Staining
2.7. Fluorescence Quantification
2.8. LDH Release
2.9. Statistics
3. Results and Discussion
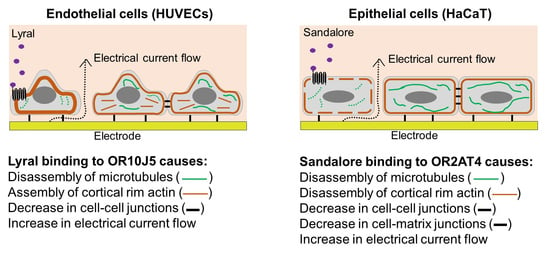
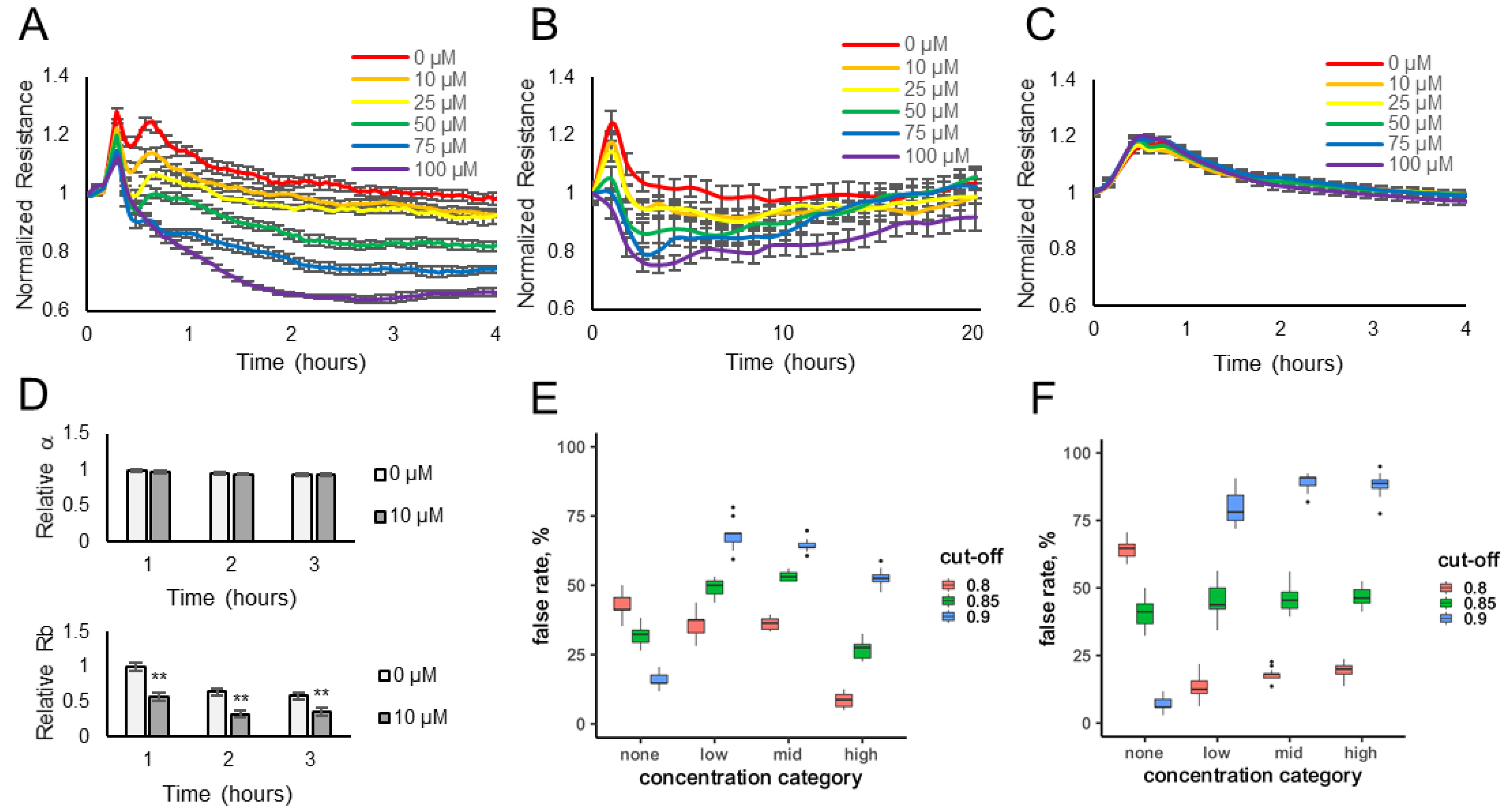
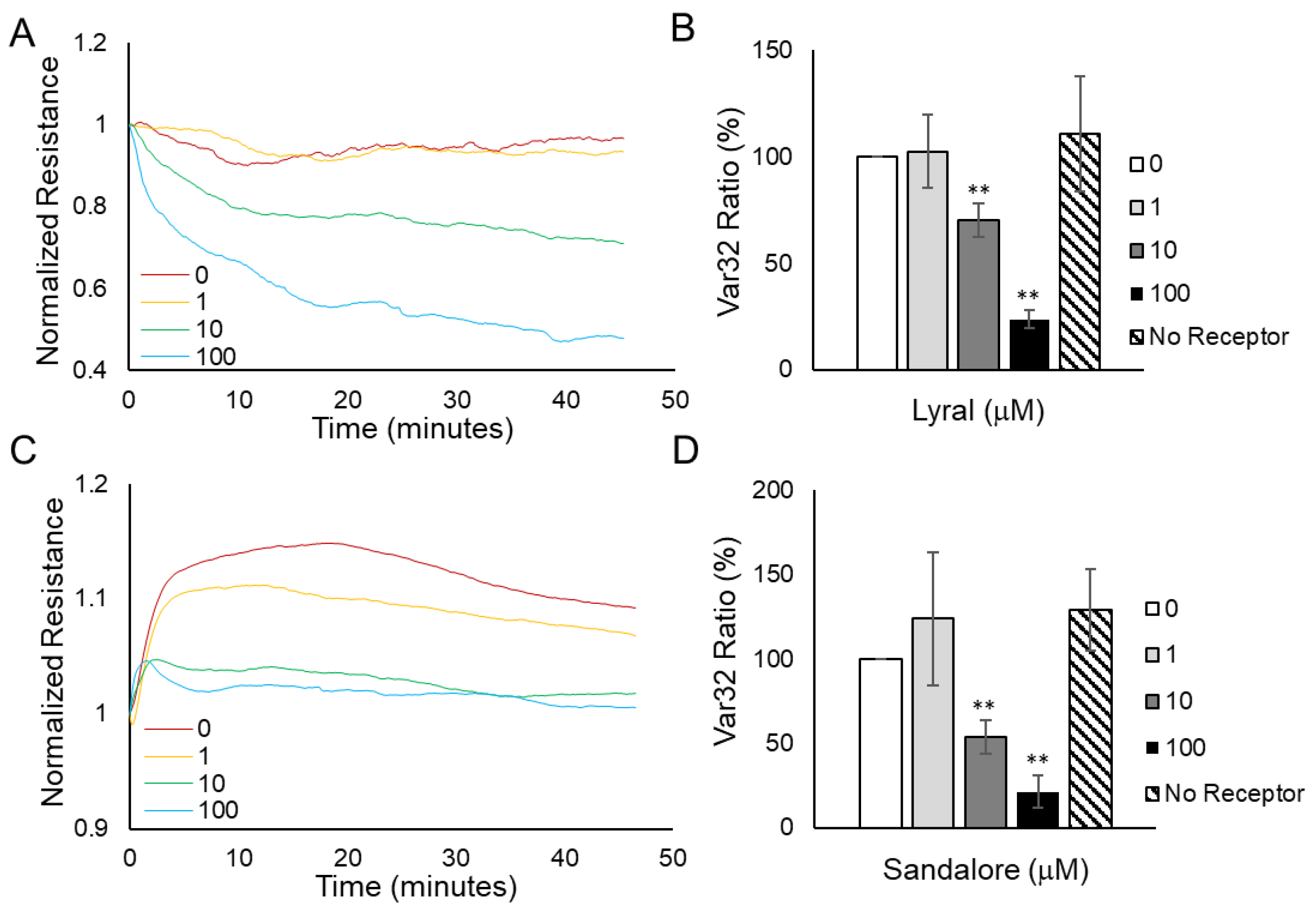
3.1. Detection of Odorants Using Cellular Resistance
3.2. Determination of cAMP Activity after Odorant Exposure
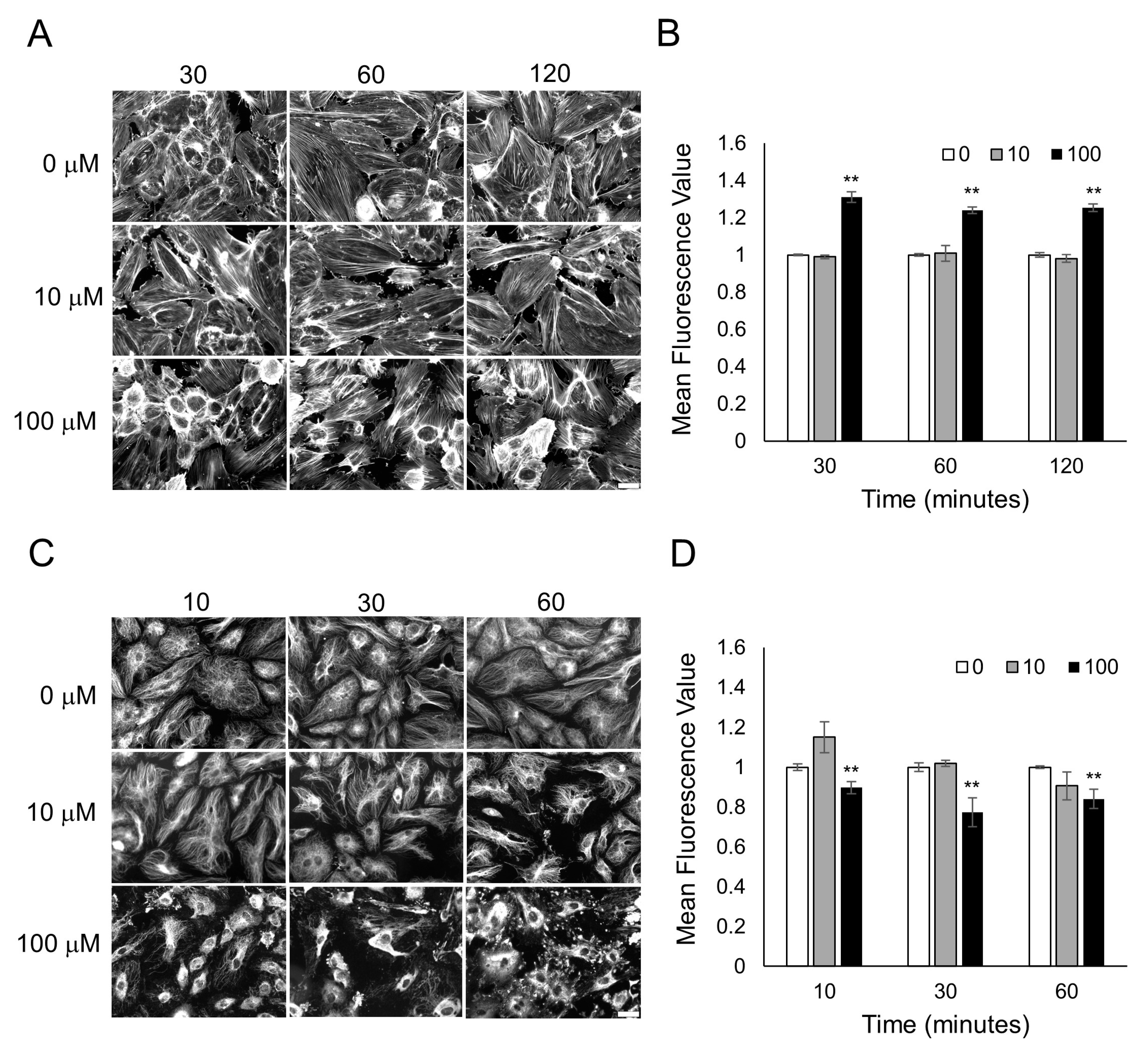
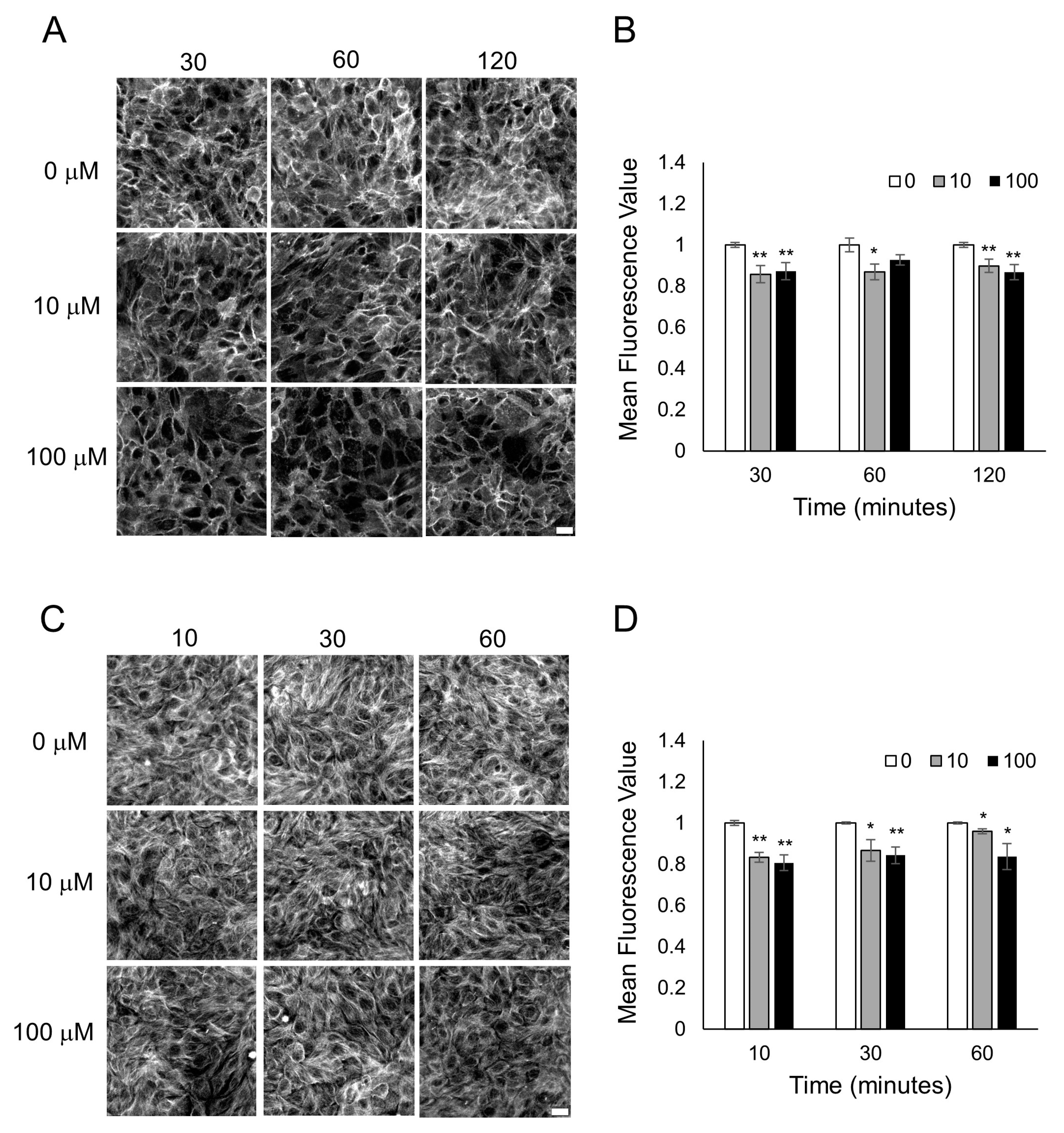
3.3. Characterization of Cytoskeletal Organization after Odorant Exposure
3.4. Detection of Odorants Using Cellular Micromotion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bohbot, J.D.; Vernick, S. The Emergence of Insect Odorant Receptor-Based Biosensors. Biosensors 2020, 10, 26. [Google Scholar] [CrossRef]
- Gao, K.; Gao, F.; Du, L.; He, C.; Wan, H.; Wang, P. Integrated Olfaction, Gustation and Toxicity Detection by a Versatile Bioengineered Cell-Based Biomimetic Sensor. Bioelectrochemistry 2019, 128, 1–8. [Google Scholar] [CrossRef]
- Lee, S.H.; Jun, S.B.; Ko, H.J.; Kim, S.J.; Park, T.H. Cell-Based Olfactory Biosensor Using Microfabricated Planar Electrode. Biosens. Bioelectron. 2009, 24, 2659–2664. [Google Scholar] [CrossRef]
- Wu, C.; Lillehoj, P.B.; Wang, P. Bioanalytical and Chemical Sensors Using Living Taste, Olfactory, and Neural Cells and Tissues: A Short Review. Analyst 2015, 140, 7048–7061. [Google Scholar] [CrossRef]
- Curtis, T.M.; Widder, M.W.; Brennan, L.M.; Schwager, S.J.; van der Schalie, W.H.; Fey, J.; Salazar, N. A Portable Cell-Based Impedance Sensor for Toxicity Testing of Drinking Water. Lab Chip 2009, 9, 2176–2183. [Google Scholar] [CrossRef]
- Curtis, T.M.; Collins, A.M.; Gerlach, B.D.; Brennan, L.M.; Widder, M.W.; van der Schalie, W.H.; Vo, N.T.K.; Bols, N.C. Suitability of Invertebrate and Vertebrate Cells in a Portable Impedance-Based Toxicity Sensor: Temperature Mediated Impacts on Long-Term Survival. Toxicol. Vitr. 2013, 27, 2061–2066. [Google Scholar] [CrossRef]
- Brennan, L.M.; Widder, M.W.; Lee, L.E.J.; van der Schalie, W.H. Long-Term Storage and Impedance-Based Water Toxicity Testing Capabilities of Fluidic Biochips Seeded with RTgill-W1 Cells. Toxicol. Vitr. 2012, 26, 736–745. [Google Scholar] [CrossRef]
- Drew, L. Olfactory Receptors Are Not Unique to the Nose. Nature 2022, 606, S14–S17. [Google Scholar] [CrossRef] [PubMed]
- Antunes, G.; de Souza, F.M.S. Olfactory Receptor Signaling. In Methods in Cell Biology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 132, pp. 127–145. ISBN 0091-679X. [Google Scholar]
- Kang, W.; Son, B.; Park, S.; Choi, D.; Park, T. UV-Irradiation- and Inflammation-Induced Skin Barrier Dysfunction Is Associated with the Expression of Olfactory Receptor Genes in Human Keratinocytes. Int. J. Mol. Sci. 2021, 22, 2799. [Google Scholar] [CrossRef] [PubMed]
- Tong, T.; Wang, Y.; Kang, S.G.; Huang, K. Ectopic Odorant Receptor Responding to Flavor Compounds: Versatile Roles in Health and Disease. Pharmaceutics 2021, 13, 1314. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Saxena, R.; Chattopadhyay, A. Reorganization of the Actin Cytoskeleton upon G-Protein Coupled Receptor Signaling. Biochim. Biophys. Acta Biomembr. 2011, 1808, 1921–1929. [Google Scholar] [CrossRef] [PubMed]
- Hewavitharana, T.; Wedegaertner, P.B. Non-Canonical Signaling and Localizations of Heterotrimeric G Proteins. Cell. Signal. 2012, 24, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Victorio, G.; González-Espinosa, C.; Espinosa-Riquer, Z.P.; Macías-Silva, M. GPCRs and Actin-Cytoskeleton Dynamics. Methods Cell Biol. 2016, 132, 165–188. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C.R. A Morphological Biosensor for Mammalian Cells. Nature 1993, 366, 591–592. [Google Scholar] [CrossRef]
- Scott, C.W.; Peters, M.F. Label-Free Whole-Cell Assays: Expanding the Scope of GPCR Screening. Drug Discov. Today 2010, 15, 704–716. [Google Scholar] [CrossRef]
- Stolwijk, J.A.; Matrougui, K.; Renken, C.W.; Trebak, M. Impedance Analysis of GPCR-Mediated Changes in Endothelial Barrier Function: Overview and Fundamental Considerations for Stable and Reproducible Measurements. Pflug. Arch. 2015, 467, 2193–2218. [Google Scholar] [CrossRef]
- Kim, S.H.; Yoon, Y.C.; Lee, A.S.; Kang, N.; Koo, J.; Rhyu, M.R.; Park, J.H. Expression of Human Olfactory Receptor 10J5 in Heart Aorta, Coronary Artery, and Endothelial Cells and Its Functional Role in Angiogenesis. Biochem. Biophys. Res. Commun. 2015, 460, 404–408. [Google Scholar] [CrossRef]
- Busse, D.; Kudella, P.; Grüning, N.M.; Gisselmann, G.; Ständer, S.; Luger, T.; Jacobsen, F.; Steinsträßer, L.; Paus, R.; Gkogkolou, P.; et al. A Synthetic Sandalwood Odorant Induces Wound-Healing Processes in Human Keratinocytes via the Olfactory Receptor OR2AT4. J. Investig. Dermatol. 2014, 134, 2823–2832. [Google Scholar] [CrossRef]
- Giaever, I.; Keese, C.R. Micromotion of Mammalian Cells Measured Electrically. Proc. Natl. Acad. Sci. USA 1991, 88, 7896–7900. [Google Scholar] [CrossRef]
- Lo, C.M.; Keese, C.R.; Giaever, I. Impedance Analysis of MDCK Cells Measured by Electric Cell-Substrate Impedance Sensing. Biophys. J. 1995, 69, 2800–2807. [Google Scholar] [CrossRef]
- Lo, C.-M.; Keese, C.R.; Giaever, I. Cell–Substrate Contact: Another Factor May Influence Transepithelial Electrical Resistance of Cell Layers Cultured on Permeable Filters. Exp. Cell Res. 1999, 250, 576–580. [Google Scholar] [CrossRef]
- Chiu, S.P.; Lee, Y.W.; Wu, L.Y.; Tung, T.H.; Gomez, S.; Lo, C.M.; Wang, J.Y. Application of ECIS to Assess FCCP-Induced Changes of MSC Micromotion and Wound Healing Migration. Sensors 2019, 19, 3210. [Google Scholar] [CrossRef]
- Opp, D.; Wafula, B.; Lim, J.; Huang, E.; Lo, J.-C.; Lo, C.-M. Use of Electric Cell-Substrate Impedance Sensing to Assess in Vitro Cytotoxicity. Biosens. Bioelectron. 2009, 24, 2625–2629. [Google Scholar] [CrossRef]
- Aisenberg, W.H.; Huang, J.; Zhu, W.; Rajkumar, P.; Cruz, R.; Santhanam, L.; Natarajan, N.; Yong, H.M.; de Santiago, B.; Oh, J.J.; et al. Defining an Olfactory Receptor Function in Airway Smooth Muscle Cells. Sci. Rep. 2016, 6, 38231. [Google Scholar] [CrossRef] [PubMed]
- An, S.S.; Liggett, S.B. Taste and Smell GPCRs in the Lung: Evidence for a Previously Unrecognized Widespread Chemosensory System. Cell. Signal. 2018, 41, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Denda, M. Newly Discovered Olfactory Receptors in Epidermal Keratinocytes Are Associated with Proliferation, Migration, and Re-Epithelialization of Keratinocytes. J. Investig. Dermatol. 2014, 134, 2677–2679. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Veitinger, S.; Peek, I.; Busse, D.; Eckardt, J.; Vladimirova, D.; Jovancevic, N.; Wojcik, S.; Gisselmann, G.; Altmüller, J.; et al. Two Olfactory Receptors—OR2A4/7 and OR51B5—Differentially Affect Epidermal Proliferation and Differentiation. Exp. Dermatol. 2017, 26, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Mashukova, A.; Spehr, M.; Hatt, H.; Neuhaus, E.M. Beta-Arrestin2-Mediated Internalization of Mammalian Odorant Receptors. J. Neurosci. 2006, 26, 9902–9912. [Google Scholar] [CrossRef]
- Zufall, F. The Cellular and Molecular Basis of Odor Adaptation. Chem. Senses 2000, 25, 473–481. [Google Scholar] [CrossRef]
- Fukuda, N.; Yomogida, K.; Okabe, M.; Touhara, K. Functional Characterization of a Mouse Testicular Olfactory Receptor and Its Role in Chemosensing and in Regulation of Sperm Motility. J. Cell Sci. 2004, 117, 5835–5845. [Google Scholar] [CrossRef]
- Griffin, C.A.; Kafadar, K.A.; Pavlath, G.K. MOR23 Promotes Muscle Regeneration and Regulates Cell Adhesion and Migration. Dev. Cell 2009, 17, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Pluznick, J.L.; Zou, D.-J.; Zhang, X.; Yan, Q.; Rodriguez-Gil, D.J.; Eisner, C.; Wells, E.; Greer, C.A.; Wang, T.; Firestein, S.; et al. Functional Expression of the Olfactory Signaling System in the Kidney. Proc. Natl. Acad. Sci. USA 2009, 106, 2059–2064. [Google Scholar] [CrossRef] [PubMed]
- Collinet, C.; Lecuit, T. Stability and Dynamics of Cell–Cell Junctions. Prog. Mol. Biol. Transl. Sci. 2013, 116, 25–47. [Google Scholar]
- Mège, R.-M.; Gavard, J.; Lambert, M. Regulation of Cell–Cell Junctions by the Cytoskeleton. Curr. Opin. Cell Biol. 2006, 18, 541–548. [Google Scholar] [CrossRef]
- Meng, W.; Mushika, Y.; Ichii, T.; Takeichi, M. Anchorage of Microtubule Minus Ends to Adherens Junctions Regulates Epithelial Cell-Cell Contacts. Cell 2008, 135, 948–959. [Google Scholar] [CrossRef] [PubMed]
- Prasain, N.; Stevens, T. The Actin Cytoskeleton in Endothelial Cell Phenotypes. Microvasc. Res. 2009, 77, 53–63. [Google Scholar] [CrossRef]
- Sluysmans, S.; Vasileva, E.; Spadaro, D.; Shah, J.; Rouaud, F.; Citi, S. The Role of Apical Cell-Cell Junctions and Associated Cytoskeleton in Mechanotransduction. Biol. Cell 2017, 109, 139–161. [Google Scholar] [CrossRef]
- Glotfelty, L.G.; Zahs, A.; Iancu, C.; Shen, L.; Hecht, G.A. Microtubules Are Required for Efficient Epithelial Tight Junction Homeostasis and Restoration. Am. J. Physiol. -Cell Physiol. 2014, 307, C245–C254. [Google Scholar] [CrossRef]
- Alieva, I.B.; Zemskov, E.A.; Smurova, K.M.; Kaverina, I.N.; Verin, A.D. The Leading Role of Microtubules in Endothelial Barrier Dysfunction: Disassembly of Peripheral Microtubules Leaves behind the Cytoskeletal Reorganization. J. Cell Biochem. 2013, 114, 2258–2272. [Google Scholar] [CrossRef]
- Ivanov, A.I.; Parkos, C.A.; Nusrat, A. Cytoskeletal Regulation of Epithelial Barrier Function During Inflammation. Am. J. Pathol. 2010, 177, 512–524. [Google Scholar] [CrossRef]
- Vouret-Craviari, V.; Boquet, P.; Pouysségur, J.; van Obberghen-Schilling, E. Regulation of the Actin Cytoskeleton by Thrombin in Human Endothelial Cells: Role of Rho Proteins in Endothelial Barrier Function. Mol. Biol. Cell 1998, 9, 2639–2653. [Google Scholar] [CrossRef] [PubMed]
- Sayner, S.L.; Alexeyev, M.; Dessauer, C.W.; Stevens, T. Soluble Adenylyl Cyclase Reveals the Significance of CAMP Compartmentation on Pulmonary Microvascular Endothelial Cell Barrier. Circ. Res. 2006, 98, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Inda, C.; dos Santos Claro, P.A.; Bonfiglio, J.J.; Senin, S.A.; Maccarrone, G.; Turck, C.W.; Silberstein, S. Different CAMP Sources Are Critically Involved in G Protein-Coupled Receptor CRHR1 Signaling. J. Cell Biol. 2016, 214, 181–195. [Google Scholar] [CrossRef] [PubMed]
- Giaever, I.; Keese, C.R. Monitoring Fibroblast Behavior in Tissue Culture with an Applied Electric Field. Proc. Natl. Acad. Sci. USA 1984, 81, 3761–3764. [Google Scholar] [CrossRef]
- Lo, C.-M.; Keese, C.R.; Giaever, I. Monitoring Motion of Confluent Cells in Tissue Culture. Exp. Cell Res. 1993, 204, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Tarantola, M.; Sunnick, E.; Schneider, D.; Marel, A.-K.; Kunze, A.; Janshoff, A. Dynamic Changes of Acoustic Load and Complex Impedance as Reporters for the Cytotoxicity of Small Molecule Inhibitors. Chem. Res. Toxicol. 2011, 24, 1494–1506. [Google Scholar] [CrossRef]
- Reddy, L.; Wang, H.-S.; Keese, C.R.; Giaever, I.; Smith, T.J. Assessment of Rapid Morphological Changes Associated with Elevated CAMP Levels in Human Orbital Fibroblasts. Exp. Cell Res. 1998, 245, 360–367. [Google Scholar] [CrossRef]
- Peterlin, Z.; Firestein, S.; Rogers, M.E. The State of the Art of Odorant Receptor Deorphanization: A Report from the Orphanage. J. Gen. Physiol. 2014, 143, 527–542. [Google Scholar] [CrossRef]
- Jiang, Y.; Gong, N.N.; Hu, X.S.; Ni, M.J.; Pasi, R.; Matsunami, H. Molecular Profiling of Activated Olfactory Neurons Identifies Odorant Receptors for Odors in Vivo. Nat. Neurosci. 2015, 18, 1446–1454. [Google Scholar] [CrossRef]
- von der Weid, B.; Rossier, D.; Lindup, M.; Tuberosa, J.; Widmer, A.; Col, J.D.; Kan, C.; Carleton, A.; Rodriguez, I. Large-Scale Transcriptional Profiling of Chemosensory Neurons Identifies Receptor-Ligand Pairs in Vivo. Nat. Neurosci. 2015, 18, 1455–1463. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Curtis, T.M.; Nilon, A.M.; Greenberg, A.J.; Besner, M.; Scibek, J.J.; Nichols, J.A.; Huie, J.L. Odorant Binding Causes Cytoskeletal Rearrangement, Leading to Detectable Changes in Endothelial and Epithelial Barrier Function and Micromotion. Biosensors 2023, 13, 329. https://doi.org/10.3390/bios13030329
Curtis TM, Nilon AM, Greenberg AJ, Besner M, Scibek JJ, Nichols JA, Huie JL. Odorant Binding Causes Cytoskeletal Rearrangement, Leading to Detectable Changes in Endothelial and Epithelial Barrier Function and Micromotion. Biosensors. 2023; 13(3):329. https://doi.org/10.3390/bios13030329
Chicago/Turabian StyleCurtis, Theresa M., Annabella M. Nilon, Anthony J. Greenberg, Matthew Besner, Jacob J. Scibek, Jennifer A. Nichols, and Janet L. Huie. 2023. "Odorant Binding Causes Cytoskeletal Rearrangement, Leading to Detectable Changes in Endothelial and Epithelial Barrier Function and Micromotion" Biosensors 13, no. 3: 329. https://doi.org/10.3390/bios13030329
APA StyleCurtis, T. M., Nilon, A. M., Greenberg, A. J., Besner, M., Scibek, J. J., Nichols, J. A., & Huie, J. L. (2023). Odorant Binding Causes Cytoskeletal Rearrangement, Leading to Detectable Changes in Endothelial and Epithelial Barrier Function and Micromotion. Biosensors, 13(3), 329. https://doi.org/10.3390/bios13030329