Recent Progress in Plasmonic based Electrochemiluminescence Biosensors: A Review
Abstract
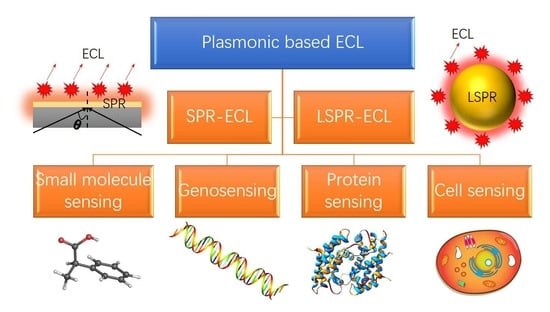
1. Introduction
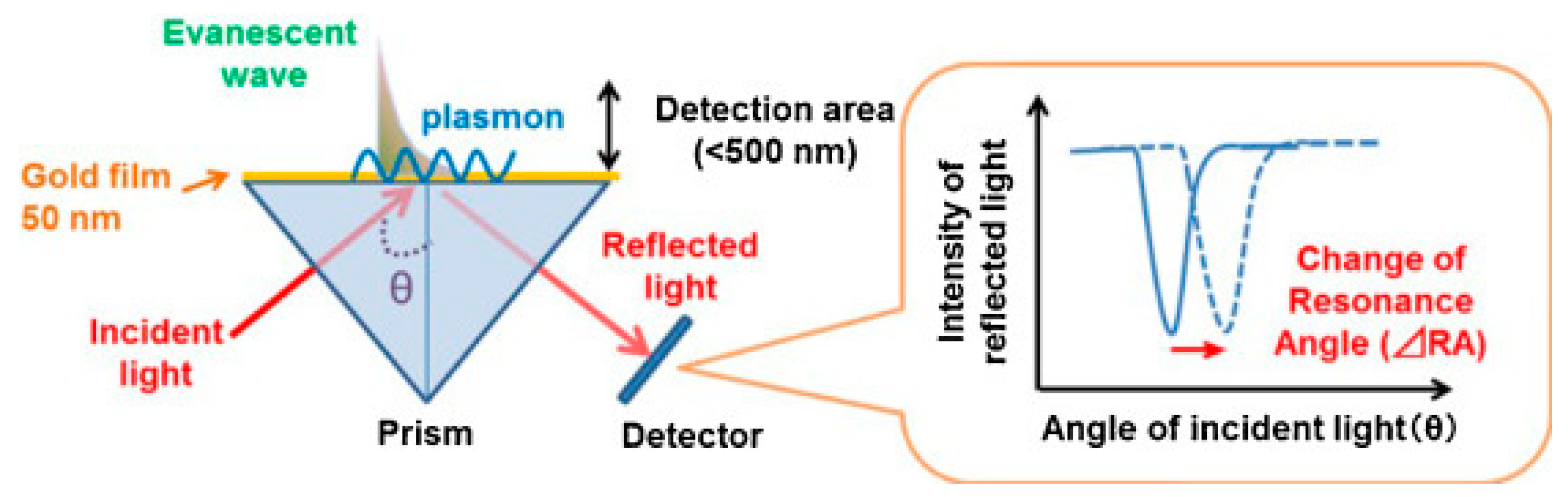
2. Fundamentals of Plasmon-Coupled ECL
2.1. SPR-Coupled ECL
2.2. LSPR-coupled ECL
3. Plasmonic-Based ECL Biosensors
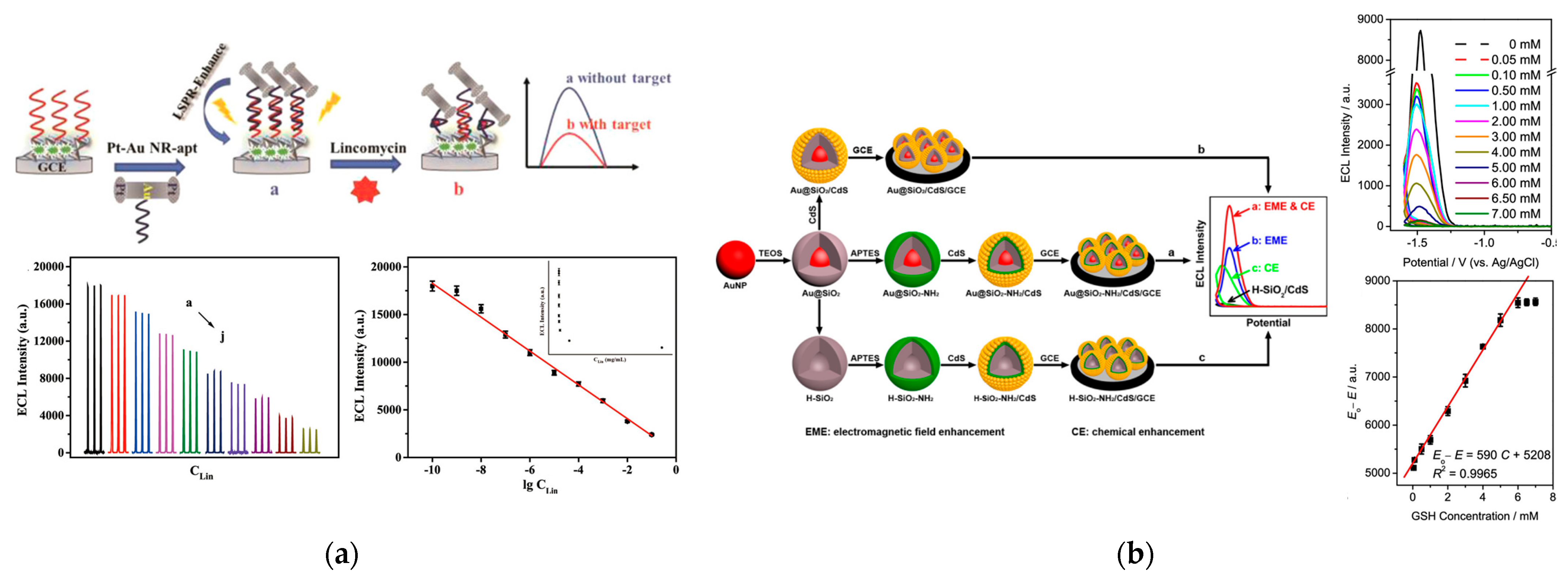
3.1. Small Molecule Sensing
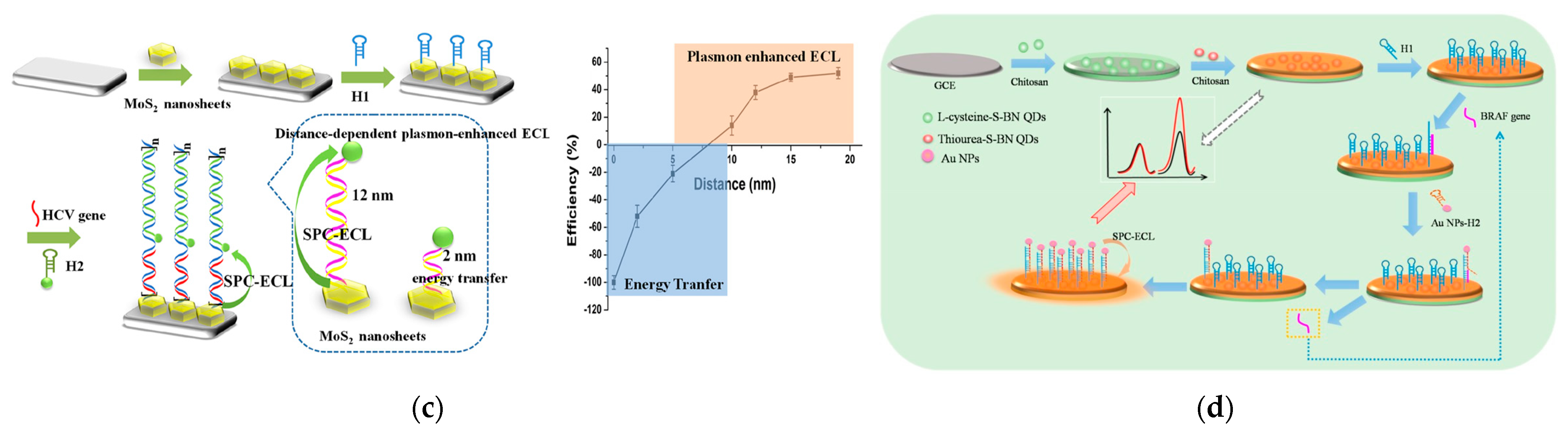
3.2. Genosensing
3.3. Protein Sensing
3.4. Cells Sensing and Microscopy
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, C.; Cao, Y.; Gou, X.; Zhu, J.J. Recent Progress in Electrochemiluminescence Sensing and Imaging. Anal. Chem. 2020, 92, 431–454. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Lin, X.; Ding, D.; Diao, G. Graphitic-phase carbon nitride-based electrochemiluminescence sensing analyses: Recent advances and perspectives. RSC Adv. 2018, 8, 19369–19380. [Google Scholar] [CrossRef] [PubMed]
- Valenti, G.; Rampazzo, E.; Kesarkar, S.; Genovese, D.; Fiorani, A.; Zanut, A.; Palomba, F.; Marcaccio, M.; Paolucci, F.; Prodi, L. Electrogenerated chemiluminescence from metal complexes-based nanoparticles for highly sensitive sensors applications. Coord. Chem. Rev. 2018, 367, 65–81. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y. Recent Progress of Novel Electrochemiluminescence Nanoprobes and Their Analytical Applications. Front. Chem. 2020, 8, 626243. [Google Scholar] [CrossRef]
- Zanut, A.; Fiorani, A.; Rebeccani, S.; Kesarkar, S.; Valenti, G. Electrochemiluminescence as emerging microscopy techniques. Anal. Bioanal. Chem. 2019, 411, 4375–4382. [Google Scholar] [CrossRef]
- Ding, H.; Su, B.; Jiang, D. Recent Advances in Single Cell Analysis by Electrochemiluminescence. ChemistryOpen 2022, 2022, e202200113. [Google Scholar] [CrossRef]
- Knezevic, S.; Bouffier, L.; Liu, B.; Jiang, D.; Sojic, N. Electrochemiluminescence microscopy: From single objects to living cells. Curr. Opin. Electrochem. 2022, 35, 101096. [Google Scholar] [CrossRef]
- Rebeccani, S.; Zanut, A.; Santo, C.I.; Valenti, G.; Paolucci, F. A Guide Inside Electrochemiluminescent Microscopy Mechanisms for Analytical Performance Improvement. Anal. Chem. 2022, 94, 336–348. [Google Scholar] [CrossRef]
- Zhou, J.H.; Zhang, S.Y.; Liu, Y. Electrochemiluminescence Single-cell Analysis on Nanostructured Interface. Electroanal 2022, 34, 937–946. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, C.; Xu, Q.; Zhu, J.-J. Recent progress in electrochemiluminescence microscopy analysis of single cells. Analyst 2022, 147, 2884–2894. [Google Scholar] [CrossRef]
- Fereja, T.H.; Du, F.; Wang, C.; Snizhko, D.; Guan, Y.; Xu, G. Electrochemiluminescence Imaging Techniques for Analysis and Visualizing. J. Anal. Test. 2020, 4, 76–91. [Google Scholar] [CrossRef]
- Lv, X.; Li, Y.; Cui, B.; Fang, Y.; Wang, L. Electrochemiluminescent sensor based on an aggregation-induced emission probe for bioanalytical detection. Analyst 2022, 147, 2338–2354. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, M.J.; Yan, H.; Lu, C.; Xu, J.J. Recent Advances in Aggregation-Induced Electrochemiluminescence. Chem. A Eur. J. 2019, 25, 12671–12683. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Ma, C.; Zhu, J.-J. Carbon-based dots for electrochemiluminescence sensing. Mater. Chem. Front. 2020, 4, 369–385. [Google Scholar] [CrossRef]
- Ma, C.; Wu, W.; Peng, Y.; Wang, M.X.; Chen, G.; Chen, Z.; Zhu, J.J. A Spectral Shift-Based Electrochemiluminescence Sensor for Hydrogen Sulphide. Anal. Chem. 2018, 90, 1334–1339. [Google Scholar] [CrossRef]
- Zou, R.; Teng, X.; Lin, Y.; Lu, C. Graphitic carbon nitride-based nanocomposites electrochemiluminescence systems and their applications in biosensors. TrAC Trends Anal. Chem. 2020, 132, 116054. [Google Scholar] [CrossRef]
- Xue, J.; Zhang, Z.; Zheng, F.; Xu, Q.; Xu, J.; Zou, G.; Li, L.; Zhu, J.-J. Efficient Solid-State Electrochemiluminescence from High-Quality Perovskite Quantum Dot Films. Anal. Chem. 2017, 89, 8212–8216. [Google Scholar] [CrossRef]
- Cao, Y.; Zhu, W.; Wei, H.; Ma, C.; Lin, Y.; Zhu, J.-J. Stable and Monochromatic All-Inorganic Halide Perovskite Assisted by Hollow Carbon Nitride Nanosphere for Ratiometric Electrochemiluminescence Bioanalysis. Anal. Chem. 2020, 92, 4123–4130. [Google Scholar] [CrossRef]
- Han, T.; Cao, Y.; Wang, J.; Jiao, J.; Song, Y.; Wang, L.; Ma, C.; Chen, H.-Y.; Zhu, J.-J. Crystallization-Induced Enhanced Electrochemiluminescence from a New Tris(bipyridine)ruthenium(II) Derivative. Adv. Funct. Mater. 2023, 2023, 2212394. [Google Scholar] [CrossRef]
- Cai, W.-R.; Zeng, H.-B.; Xue, H.-G.; Marks, R.S.; Cosnier, S.; Zhang, X.-J.; Shan, D. Enhanced Electrochemiluminescence of Porphyrin-Based Metal–Organic Frameworks Controlled via Coordination Modulation. Anal. Chem. 2020, 92, 1916–1924. [Google Scholar] [CrossRef]
- Bezuneh, T.T.; Fereja, T.H.; Kitte, S.A.; Li, H.; Jin, Y. Gold nanoparticle-based signal amplified electrochemiluminescence for biosensing applications. Talanta 2022, 248, 123611. [Google Scholar] [CrossRef]
- Chu, Y.; Han, T.; Deng, A.; Li, L.; Zhu, J.-J. Resonance energy transfer in electrochemiluminescent and photoelectrochemical bioanalysis. Trac-Trends Anal. Chem. 2020, 123, 115745. [Google Scholar] [CrossRef]
- Li, L.; Chen, Y.; Zhu, J.J. Recent Advances in Electrochemiluminescence Analysis. Anal. Chem. 2017, 89, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Ma, C.; Zhu, J.-J. DNA Technology-assisted Signal Amplification Strategies in Electrochemiluminescence Bioanalysis. J. Anal. Test. 2021, 5, 95–111. [Google Scholar] [CrossRef]
- Ma, C.; Wu, S.; Zhou, Y.; Wei, H.-F.; Zhang, J.; Chen, Z.; Zhu, J.-J.; Lin, Y.; Zhu, W. Bio-Coreactant-Enhanced Electrochemiluminescence Microscopy of Intracellular Structure and Transport. Angew. Chem. 2021, 60, 4907–4914. [Google Scholar] [CrossRef]
- Guo, W.; Zhou, P.; Sun, L.; Ding, H.; Su, B. Microtube Electrodes for Imaging the Electrochemiluminescence Layer and Deciphering the Reaction Mechanism. Angew. Chem. 2021, 60, 2089–2093. [Google Scholar] [CrossRef]
- Wang, Q.; Ren, Z.H.; Zhao, W.M.; Wang, L.; Yan, X.; Zhu, A.S.; Qiu, F.M.; Zhang, K.K. Research advances on surface plasmon resonance biosensors. Nanoscale 2022, 14, 564–591. [Google Scholar] [CrossRef]
- Pan, S.; Li, X.; Yadav, J. Single-nanoparticle spectroelectrochemistry studies enabled by localized surface plasmon resonance. Phys. Chem. Chem. Phys. 2021, 23, 19120–19129. [Google Scholar] [CrossRef]
- Mouloua, D.; Kotbi, A.; Deokar, G.; Kaja, K.; El Marssi, M.; El Khakani, M.A.; Jouiad, M. Recent Progress in the Synthesis of MoS(2) Thin Films for Sensing, Photovoltaic and Plasmonic Applications: A Review. Materials 2021, 14, 3283. [Google Scholar] [CrossRef]
- Gong, L.; Feng, L.; Zheng, Y.; Luo, Y.; Zhu, D.; Chao, J.; Su, S.; Wang, L. Molybdenum Disulfide-Based Nanoprobes: Preparation and Sensing Application. Biosensors 2022, 12, 87. [Google Scholar] [CrossRef]
- Dao, T.D.; Han, G.; Arai, N.; Nabatame, T.; Wada, Y.; Hoang, C.V.; Aono, M.; Nagao, T. Plasmon-mediated photocatalytic activity of wet-chemically prepared ZnO nanowire arrays. Phys. Chem. Chem. Phys. 2015, 17, 7395–7403. [Google Scholar] [CrossRef]
- Guo, L.; Yin, H.; Xu, M.; Zheng, Z.; Fang, X.; Chong, R.; Zhou, Y.; Xu, L.; Xu, Q.; Li, J.; et al. In Situ Generated Plasmonic Silver Nanoparticle-Sensitized Amorphous Titanium Dioxide for Ultrasensitive Photoelectrochemical Sensing of Formaldehyde. ACS Sens. 2019, 4, 2724–2729. [Google Scholar] [CrossRef]
- Zheng, Z.; Murakami, N.; Liu, J.; Teng, Z.; Zhang, Q.; Cao, Y.; Cheng, H.; Ohno, T. Development of Plasmonic Photocatalyst by Site-selective Loading of Bimetallic Nanoparticles of Au and Ag on Titanium(IV) Oxide. ChemCatChem 2020, 12, 3783–3792. [Google Scholar] [CrossRef]
- Li, M.; Singh, R.; Soares, M.S.; Marques, C.; Zhang, B.; Kumar, S. Convex fiber-tapered seven core fiber-convex fiber (CTC) structure-based biosensor for creatinine detection in aquaculture. Opt. Express 2022, 30, 13898–13914. [Google Scholar] [CrossRef]
- Wang, Y.; Singh, R.; Chaudhary, S.; Zhang, B.; Kumar, S. 2-D Nanomaterials Assisted LSPR MPM Optical Fiber Sensor Probe for Cardiac Troponin I Detection. IEEE Trans. Instrum. Meas. 2022, 71, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Singh, R.; Marques, C.; Jha, R.; Zhang, B.; Kumar, S. Taper-in-taper fiber structure-based LSPR sensor for alanine aminotransferase detection. Opt. Express 2021, 29, 43793–43810. [Google Scholar] [CrossRef]
- Wang, Z.; Singh, R.; Zhang, B.; Kumar, S. SMF tapered fiber/AuNPs/ZnO based sensor for detection of acetylcholine. In Optics in Health Care and Biomedical Optics XI; Luo, Q., Li, X., Gu, Y., Zhu, D., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2021; Volune 11900, p. 119003D. [Google Scholar]
- Kumar, S.; Singh, R.; Kaushik, B.K.; Chen, N.-k.; Yang, Q.S.; Zhang, X. LSPR-Based Cholesterol Biosensor Using Hollow Core Fiber Structure. IEEE Sens. J. 2019, 19, 7399–7406. [Google Scholar] [CrossRef]
- Singh, L.; Singh, R.; Zhang, B.; Cheng, S.; Kumar Kaushik, B.; Kumar, S. LSPR based uric acid sensor using graphene oxide and gold nanoparticles functionalized tapered fiber. Opt. Fiber Technol. 2019, 53, 102043. [Google Scholar] [CrossRef]
- Kumar, S.; Wang, Y.; Li, M.Y.; Wang, Q.L.; Malathi, S.; Marques, C.; Singh, R.; Zhang, B.Y. Plasmon-Based Tapered-in-Tapered Fiber Structure for p-Cresol Detection: From Human Healthcare to Aquaculture Application. Ieee Sens. J. 2022, 22, 18493–18500. [Google Scholar] [CrossRef]
- Su, Y.; Xu, S.; Zhang, J.; Chen, X.; Jiang, L.-P.; Zheng, T.; Zhu, J.-J. Plasmon Near-Field Coupling of Bimetallic Nanostars and a Hierarchical Bimetallic SERS “Hot Field”: Toward Ultrasensitive Simultaneous Detection of Multiple Cardiorenal Syndrome Biomarkers. Anal. Chem. 2019, 91, 864–872. [Google Scholar] [CrossRef]
- Wen, S.; Su, Y.; Dai, C.; Jia, J.; Fan, G.-C.; Jiang, L.-P.; Song, R.-B.; Zhu, J.-J. Plasmon Coupling-Enhanced Raman Sensing Platform Integrated with Exonuclease-Assisted Target Recycling Amplification for Ultrasensitive and Selective Detection of microRNA-21. Anal. Chem. 2019, 91, 12298–12306. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xue, J.; Bi, C.; Xin, H.; Wang, Y.; Cao, X. Quantitative and specific detection of cancer-related microRNAs in living cells using surface-enhanced Raman scattering imaging based on hairpin DNA-functionalized gold nanocages. Analyst 2019, 144, 7250–7262. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Xia, J.; Deng, Z.; Cao, X. Detection of squamous cell carcinoma antigen in cervical cancer by surface-enhanced Raman scattering-based immunoassay. Anal. Methods 2019, 11, 2809–2818. [Google Scholar] [CrossRef]
- Su, Y.-w.; Wang, W. Surface plasmon resonance sensing: From purified biomolecules to intact cells. Anal. Bioanal. Chem. 2018, 410, 3943–3951. [Google Scholar] [CrossRef]
- Li, J.; Qi, H.; Wang, H.; Yang, Z.; Zhu, P.; Diao, G. Fluorescence energy transfer-based multiplexed hybridization assay using gold nanoparticles and quantum dot conjugates on photonic crystal beads. Microchim. Acta 2014, 181, 1109–1115. [Google Scholar] [CrossRef]
- Cui, C.; Jin, R.; Jiang, D.; Zhang, J.; Zhu, J. Visualization of an Accelerated Electrochemical Reaction under an Enhanced Electric Field. Research 2021, 2021, 1742919. [Google Scholar] [CrossRef]
- Chen, M.M.; Xu, C.H.; Zhao, W.; Chen, H.Y.; Xu, J.J. Super-Resolution Electrogenerated Chemiluminescence Microscopy for Single-Nanocatalyst Imaging. J. Am. Chem. Soc. 2021, 143, 18511–18518. [Google Scholar] [CrossRef]
- Chen, M.M.; Zhao, W.; Zhu, M.J.; Li, X.L.; Xu, C.H.; Chen, H.Y.; Xu, J.J. Spatiotemporal imaging of electrocatalytic activity on single 2D gold nanoplates via electrogenerated chemiluminescence microscopy. Chem. Sci. 2019, 10, 4141–4147. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, J.; Wang, Z.; Liang, Z.; Nie, Y.; Xu, S.; Ma, Q. Polarization-Resolved Electrochemiluminescence Sensor Based on the Surface Plasmon Coupling Effect of a Au Nanotriangle-Patterned Structure. Anal. Chem. 2021, 93, 15785–15793. [Google Scholar] [CrossRef]
- Lu, H.-J.; Xu, J.-J.; Zhou, H.; Chen, H.-Y. Recent advances in electrochemiluminescence resonance energy transfer for bioanalysis: Fundamentals and applications. TrAC Trends Anal. Chem. 2020, 122, 115746. [Google Scholar] [CrossRef]
- Xia, J.; Zhou, J.; Zhang, R.; Jiang, D.; Jiang, D. Gold-coated polydimethylsiloxane microwells for high-throughput electrochemiluminescence analysis of intracellular glucose at single cells. Anal. Bioanal. Chem. 2018, 410, 4787–4792. [Google Scholar] [CrossRef]
- Villani, E.; Valenti, G.; Marcaccio, M.; Mattarozzi, L.; Barison, S.; Garoli, D.; Cattarin, S.; Paolucci, F. Coreactant electrochemiluminescence at nanoporous gold electrodes. Electrochim. Acta 2018, 277, 168–175. [Google Scholar] [CrossRef]
- Yanase, Y.; Hiragun, T.; Ishii, K.; Kawaguchi, T.; Yanase, T.; Kawai, M.; Sakamoto, K.; Hide, M. Surface Plasmon Resonance for Cell-Based Clinical Diagnosis. Sensors 2014, 14, 4948–4959. [Google Scholar] [CrossRef]
- Dinel, M.P.; Tartaggia, S.; Wallace, G.Q.; Boudreau, D.; Masson, J.F.; Polo, F. The Fundamentals of Real-Time Surface Plasmon Resonance/Electrogenerated Chemiluminescence. Angew. Chem. Int. Ed. 2019, 58, 18202–18206. [Google Scholar] [CrossRef]
- Yu, J.; Jia, P.; Wang, S.; Ebendorff-Heidepriem, H.; Abell, A.D. Electrochemical plasmonic optical fiber probe for real-time insight into coreactant electrochemiluminescence. Sens. Actuators B Chem. 2020, 321, 128469. [Google Scholar] [CrossRef]
- Perez-Tejeda, P.; Grueso, E.; Marin-Gordillo, A.; Torres-Marquez, C.; Giraldez-Perez, R.M. Aqueous Gold Nanoparticle Solutions for Improved Efficiency in Electrogenerated Chemiluminescent Reactions. Acs. Appl. Nano. Mater. 2018, 1, 5307–5315. [Google Scholar] [CrossRef]
- Pan, S.; Liu, J.; Hill, C.M. Observation of Local Redox Events at Individual Au Nanoparticles Using Electrogenerated Chemiluminescence Microscopy. J. Phys. Chem. C 2015, 119, 27095–27103. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Pan, J.; Chen, T.; Xing, X.; Zhang, W.; Lu, Z. Plasmon-Enhanced Electrochemiluminescence at the Single-Nanoparticle Level. Angew. Chem. 2022, 2022, e202214103. [Google Scholar] [CrossRef]
- Heiderscheit, T.S.; Gallagher, M.J.; Baiyasi, R.; Collins, S.S.E.; Jebeli, S.A.H.; Scarabelli, L.; Al-Zubeidi, A.; Flatebo, C.; Chang, W.-S.; Landes, C.F.; et al. Nanoelectrode-emitter spectral overlap amplifies surface enhanced electrogenerated chemiluminescence. J. Chem. Phys. 2019, 151, 144712. [Google Scholar] [CrossRef]
- Heiderscheit, T.S.; Oikawa, S.; Sanders, S.; Minamimoto, H.; Searles, E.K.; Landes, C.F.; Murakoshi, K.; Manjavacas, A.; Link, S. Tuning Electrogenerated Chemiluminescence Intensity Enhancement Using Hexagonal Lattice Arrays of Gold Nanodisks. J. Phys. Chem. Lett. 2021, 12, 2516–2522. [Google Scholar] [CrossRef]
- Wilson, A.J.; Marchuk, K.; Willets, K.A. Imaging Electrogenerated Chemiluminescence at Single Gold Nanowire Electrodes. Nano Lett. 2015, 15, 6110–6115. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Xu, Y.; Zhang, Z.; Feng, J. Operando Imaging of Chemical Activity on Gold Plates with Single-Molecule Electrochemiluminescence Microscopy. Angew. Chem. 2022, 2022, e202200187. [Google Scholar] [CrossRef]
- Li, J.; Luo, M.; Jin, C.; Zhang, P.; Yang, H.; Cai, R.; Tan, W. Plasmon-Enhanced Electrochemiluminescence of PTP-Decorated Eu MOF-Based Pt-Tipped Au Bimetallic Nanorods for the Lincomycin Assay. ACS Appl. Mater. Interfaces 2022, 14, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Zeng, P.; Zhao, F.Q.; Zeng, B.Z. Au@SiO2@RuDS nanocomposite based plasmon-enhanced electrochemiluminescence sensor for the highly sensitive detection of glutathione. Talanta 2019, 204, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Y.; Chen, Y.; Wang, C.; Jiang, J.; Dong, J.; Yan, H.; Du, X. Dual Enhanced Electrochemiluminescence of Aminated Au@SiO2/CdS Quantum Dot Superstructures: Electromagnetic Field Enhancement and Chemical Enhancement. ACS Appl. Mater. Interfaces 2019, 11, 4488–4499. [Google Scholar] [CrossRef]
- Yuan, R.S.; Liu, Q.; Hong, H.H.; Ma, H.Y.; Xiao, L.T.; Li, Y.Q.; Jiang, D.; Hao, N.; Wang, K. Enhanced cathodic electrochemiluminescent microcystin-LR aptasensor based on surface plasmon resonance of Bi nanoparticles. J. Hazard. Mater. 2022, 434, 128877. [Google Scholar] [CrossRef]
- Li, X.Y.; Du, X.Z. Surface enhanced electrochemiluminescence of the Ru(bpy)(3)(2+)/tripropylamine system by Au@SiO2 nanoparticles for highly sensitive and selective detection of dopamine. Microchem. J. 2022, 176, 107837. [Google Scholar] [CrossRef]
- Li, J.X.; Shan, X.L.; Jiang, D.; Wang, W.C.; Xu, F.M.; Chen, Z.D. Au nanoparticle plasmon-enhanced electrochemiluminescence aptasensor based on the 1D/2D PTCA/CoP for diclofenac assay. Microchim. Acta 2021, 188, s00604-s021. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, Q.; Nie, Y.; Zhang, X.; Ma, Q. Polarized-Electrochemiluminescence Biosensor Based on Surface Plasmon Coupling Strategy and Fluorine-Doped BN Quantum Dots. Anal. Chem. 2020, 92, 9223–9229. [Google Scholar] [CrossRef]
- Wang, P.; Liang, Z.; Zhao, J.; Nie, Y.; Xu, S.; Ma, Q. A polarization-resolved ECL strategy based on the surface plasmon coupling effect of orientational Au nanobipyramids patterned structures. Chem. Eng. J. 2022, 448, 137630. [Google Scholar] [CrossRef]
- Li, J.X.; Cai, R.; Tan, W.H. A Novel ECL Sensing System for Ultrahigh Sensitivity miRNA-21 Detection Based on Catalytic Hairpin Assembly Cascade Nonmetallic SPR Effect. Anal. Chem. 2022, 94, 12280–12285. [Google Scholar] [CrossRef]
- Li, M.X.; Feng, Q.M.; Zhou, Z.; Zhao, W.; Xu, J.J.; Chen, H.Y. Plasmon-Enhanced Electrochemiluminescence for Nucleic Acid Detection Based on Gold Nanodendrites. Anal. Chem. 2018, 90, 1340–1347. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Wang, M.; Zhang, Q.; Ma, Q. Distance-dependent plasmon-enhanced electrochemiluminescence biosensor based on MoS2 nanosheets. Biosens. Bioelectron. 2020, 148, 111823. [Google Scholar] [CrossRef]
- Lu, H.-J.; Pan, J.-B.; Wang, Y.-Z.; Ji, S.-Y.; Zhao, W.; Luo, X.-L.; Xu, J.-J.; Chen, H.-Y. Electrochemiluminescence Energy Resonance Transfer System between RuSi Nanoparticles and Hollow Au Nanocages for Nucleic Acid Detection. Anal. Chem. 2018, 90, 10434–10441. [Google Scholar] [CrossRef]
- Kitte, S.A.; Bushira, F.A.; Xu, C.; Wang, Y.; Li, H.J.; Jin, Y.D. Plasmon-Enhanced Nitrogen Vacancy-Rich Carbon Nitride Electrochemiluminescence Aptasensor for Highly Sensitive Detection of miRNA. Anal. Chem. 2022, 94, 1406–1414. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Wang, M.; Ma, Q. A visual electrochemiluminescence resonance energy transfer/surface plasmon coupled electrochemiluminescence nanosensor for Shiga toxin-producing Escherichia coli detection. Green Chem. 2018, 20, 5520–5527. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, M.; Nie, Y.; Zhang, Q.; Ma, Q. Sulfur Regulated Boron Nitride Quantum Dots Electrochemiluminescence with Amplified Surface Plasmon Coupling Strategy for BRAF Gene Detection. Anal. Chem. 2019, 91, 6250–6258. [Google Scholar] [CrossRef]
- Liang, Z.H.; Nie, Y.X.; Zhang, X.; Wang, P.L.; Ma, Q. Multiplex Electrochemiluminescence Polarization Assay Based on the Surface Plasmon Coupling Effect of Au NPs and Ag@Au NPs. Anal. Chem. 2021, 93, 7491–7498. [Google Scholar] [CrossRef]
- Lu, H.J.; Xu, C.H.; Xu, J.J.; Chen, H.Y. Metallic Inverse Opals: An Electrochemiluminescence enhanced Substrate for Sensitive Bioanalysis. Anal. Chem. 2019, 91, 14757–14764. [Google Scholar] [CrossRef]
- Cumba, L.; Pellegrin, Y.; Melinato, F.; Forster, R.J. Enhanced Electrochemiluminescence from 3D Nanocavity Electrode Arrays. Sens. Actuators Rep. 2022, 4, 100082. [Google Scholar] [CrossRef]
- Chen, X.; Gui, W.; Ma, Q. Ultrasensitive detection of EGFR gene based on surface plasmon resonance enhanced electrochemiluminescence of CuZnInS quantum dots. Anal. Chim. Acta 2018, 1009, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Liu, Y.; Nie, Y.; Ma, Q. Magnetic-plasmonic yolk-shell nanostructure-based plasmon-enhanced electrochemiluminescence sensor. Sens. Actuators B Chem. 2020, 319, 128245. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.Y.; Nie, Y.X.; Liu, Y.; Ma, Q. Wavelength-Dependent Surface Plasmon Coupling Electrochemiluminescence Biosensor Based on Sulfur-Doped Carbon Nitride Quantum Dots for K-RAS Gene Detection. Anal. Chem. 2019, 91, 13780–13786. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.Y.; Han, T.; Xiong, Y.F.; Wang, S.C.; Dai, T.Y.; Chen, J.H.; Zhang, X.J.; Wang, G.F. Plasmon-Enhanced Electrochemiluminescence of Silver Nanoclusters for microRNA Detection. Acs Sens. 2019, 4, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.D.; Liao, M.Y.; Zhang, H.F.; Gong, J.B.; Yang, F.; Xu, M.Y.; Tremblay, P.L.; Zhang, T. An electrochemiluminescence resonance energy transfer biosensor for the detection of circulating tumor DNA from blood plasma. Iscience 2021, 24, 3543–3546. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Li, H.; Saqib, M.; Xu, C.; Jin, Y. Nanoengineered Metasurface Immunosensor with over 1000-Fold Electrochemiluminescence Enhancement for Ultra-sensitive Bioassay. iScience 2019, 17, 267–276. [Google Scholar] [CrossRef]
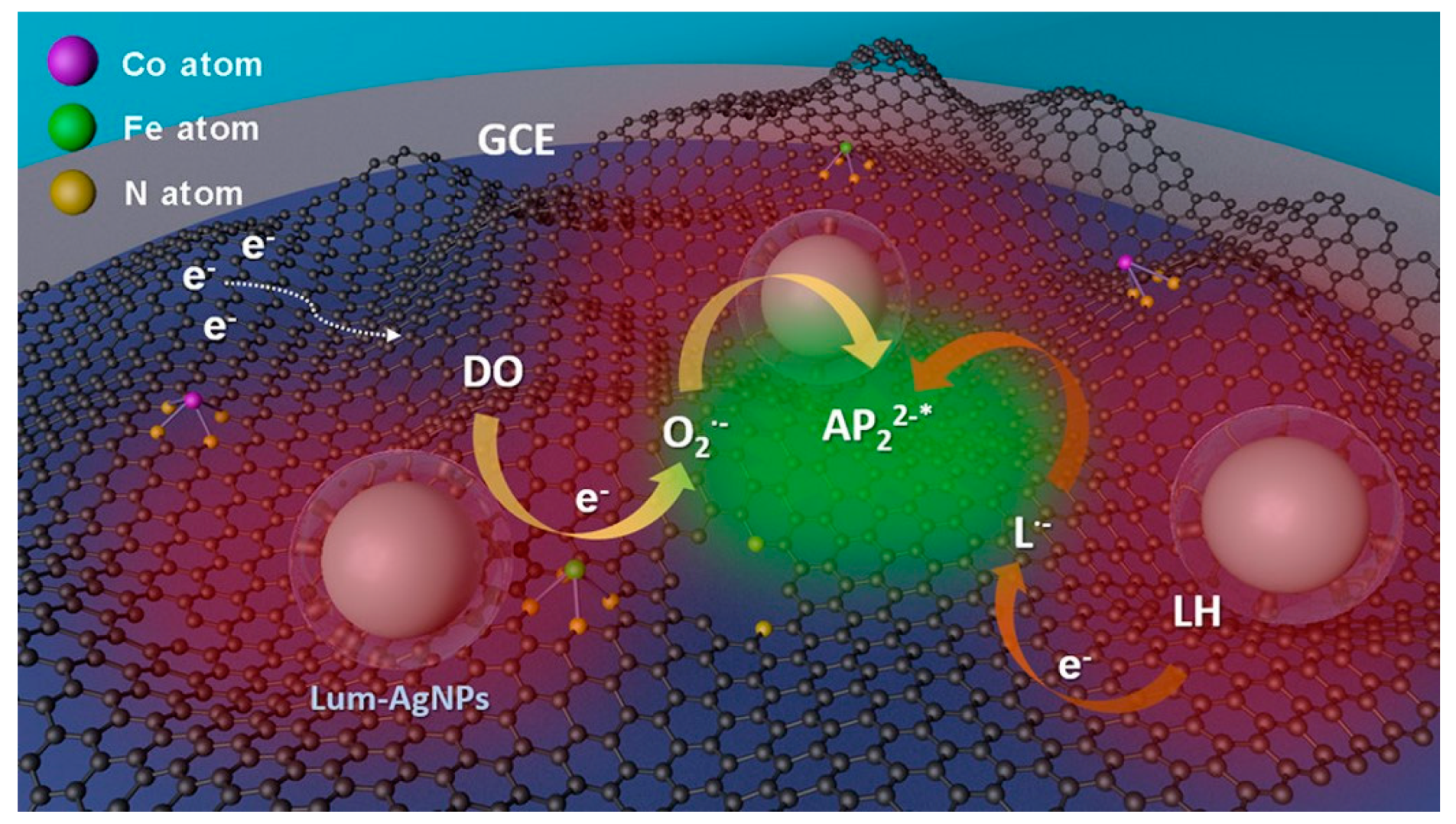
- Bushira, F.A.; Wang, P.; Wang, Y.; Hou, S.; Diao, X.; Li, H.; Zheng, L.; Jin, Y. Plasmon-Boosted Fe, Co Dual Single-Atom Catalysts for Ultrasensitive Luminol-Dissolved O-2 Electrochemiluminescence Detection of Prostate-Specific Antigen. Anal. Chem. 2022, 94, 9758–9765. [Google Scholar] [CrossRef]
- Liu, Z.M.; Wang, J.; Lu, Y.L.; Cui, C.; Zheng, L.M.; Hu, L.Q. Ultrasensitive prostate specific antigen monitoring based on electrochemiluminescent immunesystem with synergistic signal amplification effect of resonance energy transfer coupling with K2S2O8-H2O2 dual coreactants. J. Electroanal. Chem. 2021, 899, 115697. [Google Scholar] [CrossRef]
- Wang, D.F.; Zhou, J.; Guo, L.H.; Qiu, B.; Lin, Z.Y. A surface-enhanced electrochemiluminescence sensor based on Au-SiO2 core-shell nanocomposites doped with Ru(bpy)(3)(2+) for the ultrasensitive detection of prostate-specific antigen in human serum. Analyst 2020, 145, 132–138. [Google Scholar] [CrossRef]
- Isildak, I.; Navaeipour, F.; Afsharan, H.; Kanberoglu, G.S.; Agir, I.; Ozer, T.; Annabi, N.; Totu, E.E.; Khalilzadeh, B. Electrochemiluminescence methods using CdS quantum dots in aptamer-based thrombin biosensors: A comparative study. Mikrochim. Acta 2019, 187, 25. [Google Scholar] [CrossRef]
- Xiong, H.; Huang, Z.; Lin, Q.; Yang, B.; Yan, F.; Liu, B.; Chen, H.; Kong, J. Surface Plasmon Coupling Electrochemiluminescence Immunosensor Based on Polymer Dots and AuNPs for Ultrasensitive Detection of Pancreatic Cancer Exosomes. Anal. Chem. 2022, 94, 837–846. [Google Scholar] [CrossRef]
- Han, D.; Li, X.; Bian, X.; Wang, J.; Kong, L.; Ding, S.; Yan, Y. Localized surface plasmon-enhanced electrochemiluminescence biosensor for rapid, label-free, and single-step detection of broad-spectrum bacteria using urchin-like Au and Ag nanoparticles. Sens. Actuators B Chem. 2022, 355, 131120. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.; Sun, Y.; Su, J.; Jin, B.; Geng, L.; Song, Y.-Y.; Huang, X.; Yang, M. Signal-On Electrochemiluminescence of Self-Ordered Molybdenum Oxynitride Nanotube Arrays for Label-Free Cytosensing. Anal. Chem. 2018, 90, 10858–10864. [Google Scholar] [CrossRef]
- Zhou, H.; Ding, K.X.; Yu, Q.; Wang, H.Y.; Liu, J.; Wang, Z.H. Enhanced electrochemiluminescence ratiometric cytosensing based on surface plasmon resonance of Au nanoparticles and nanosucculent films. Biosens. Bioelectron. 2021, 189, 113367. [Google Scholar] [CrossRef]
- Cao, J.T.; Wang, Y.L.; Zhang, J.J.; Dong, Y.X.; Liu, F.R.; Ren, S.W.; Liu, Y.M. Immuno-Electrochemiluminescent Imaging of a Single Cell Based on Functional Nanoprobes of Heterogeneous Ru(bpy)32+@SiO2/Au Nanoparticles. Anal. Chem. 2018, 90, 10334–10339. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Li, B.; Liu, J.; Jiang, D.; Liu, B.; Sojic, N. Single Biomolecule Imaging by Electrochemiluminescence. J. Am. Chem. Soc. 2021, 143, 17910–17914. [Google Scholar] [CrossRef]
- Chen, Y.; Gou, X.; Ma, C.; Jiang, D.; Zhu, J.-J. A Synergistic Coreactant for Single-Cell Electrochemiluminescence Imaging: Guanine-Rich ssDNA-Loaded High-Index Faceted Gold Nanoflowers. Anal. Chem. 2021, 93, 7682–7689. [Google Scholar] [CrossRef]






| Targets | Plasmonic Materials | LOD | Linear Range | Ref | |
|---|---|---|---|---|---|
| Small molecule | Lincomycin | Au-Pt bimetallic nanorods | 0.026 ng/mL | 0.1 mg/mL–0.1 ng/mL | [64] |
| Glutathione | Au nanoparticles | 0.5 fM | 1.0 fM–1.0 nM | [65] | |
| 1.0 nM–1.0 μM | |||||
| Au cores (ca. 55 nm) | 0.065 mM | 0.10–6.00 mM | [66] | ||
| Microcystins-LR | Bismuth nanoparticles | 0.003 pM | 0.01–5000 pM | [67] | |
| Dopamine | Au core (ca. 68 nm) | 0.004 μM | 0.01–600 μM | [68] | |
| Diclofenac | Au nanoparticles | 0.072 pM | 0.1 pM-10 μM | [69] | |
| Gene | K-ras gene | Au nanoparticles | 0.03 fM | 0.1 fM–10 nM | [70] |
| miRNA-21 | Au nanobipyramids | 3.3 fM | 0.01 pM–10 nM | [71] | |
| miRNA-205 | 1.7 fM | 5 fM–1 nM | |||
| miRNA-221 | Au nanotriangle | 0.71 fM | 1 fM–1 nM | [50] | |
| miRNA-21 | SnS2 nanoplates | 0.6 aM | n.r.a | [72] | |
| nucleic acid | Au nanodendrites | 30 aM | 1.0–500 fM | [73] | |
| Hepatitis C virus gene | MoS2 nanosheets | 0.17 pM | 0.5 pM–1 nM | [74] | |
| miRNA-141 | Au nanocages | 0.4 fM | 1.0 fM–10 pM | [75] | |
| miRNA-133a | Au nanoparticles | 0.87 aM | 1 aM–100 pM | [76] | |
| Shiga toxin-producing Escherichia coli gene | Au nanoparticles | 0.3 pM | 1 pM–5 nM | [77] | |
| BRAF gene | Au nanoparticles | 0.3 pM | 1 pM–1.5 nM | [78] | |
| Breast cancer-related genes (BRCA1, BRCA2) | Au nanoparticles, gold-coatedsilver nanoparticles | n.r.a | 100 aM–1 nM | [79] | |
| miRNA-21 | Gold inverse opals | 3.3 fM | 5.0 fM–5.0 pM | [80] | |
| MRSA DNA | 3D Au array | 1 μM | 10 nM–30 μM | [81] | |
| Epidermal growth factor receptor gene | Au nanoparticles | 0.0043 nM | 0.05 nM–1 nM | [82] | |
| K-RAS gene | gold shell | 0.3 fM | 1 fM–1 nM | [83] | |
| Au nanoparticles | 16 fM | 50 fM–1 nM | [84] | ||
| miRNA 21 | Au nanoparticles | 0.96 aM | 1 aM–104 fM | [85] | |
| Circulating tumor DNA | Au nanoparticles | 0.0023 fM | 0.01 fM–1 pM | [86] | |
| Protein | Prostate-specific antigen | Au nanoparticles | 3 fg/mL | 10 fg/mL–1 μg/mL | [87] |
| Ag nanoparticles | 0.98 fg/mL | 1 fg/mL–1 μg/mL | [88] | ||
| 0.0046 pg/mL | 1 × 10−5–500 ng/mL | [89] | |||
| Au nucleus | 7 × 10−7 ng/mL | n.r.a | [90] | ||
| Thrombin | Au nanoparticles | 92 pg/mL | 500–5000 pg/mL | [91] | |
| 6.5 pg/mL | 50–1000 pg/mL | ||||
| 500 fg/mL | 5–500 pg/mL | ||||
| Cell | Pancreatic cancer exosomes | Au nanoparticles | 400 particles/mL | 1.0 × 103–1.0 × 106 particles/mL | [92] |
| S. aurenus | Urchin-like Au and Ag nanoparticles | 52 CFU/mL | 2 × 102–2 × 108 CFU/mL | [93] | |
| HepG2 | Au nanoparticles | 47 cells/mL | 50–13800 cells/mL | [94] | |
| Ramos | Au nanoparticles | 20 cells/mL | 80–8 × 105 cells/mL | [95] | |
| PSA on LNCaP | Au nanoparticles | 3.0 pg/mL | 10 pg/mL–50 ng/mL | [96] | |
| 31 pg/mL | 0.05–50 ng/mL | ||||
| Cytokeratin 19 on MCF-7 | Au nanoparticles | 0.12 pg/mL | 0.01–10 ng/mL | [97] | |
| CEA on MCF-7 | High-index faceted gold nanoflower | n.r.a | n.r.a | [98] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, C.; Zhang, Z.; Tan, T.; Zhu, J.-J. Recent Progress in Plasmonic based Electrochemiluminescence Biosensors: A Review. Biosensors 2023, 13, 200. https://doi.org/10.3390/bios13020200
Ma C, Zhang Z, Tan T, Zhu J-J. Recent Progress in Plasmonic based Electrochemiluminescence Biosensors: A Review. Biosensors. 2023; 13(2):200. https://doi.org/10.3390/bios13020200
Chicago/Turabian StyleMa, Cheng, Zhichen Zhang, Tingting Tan, and Jun-Jie Zhu. 2023. "Recent Progress in Plasmonic based Electrochemiluminescence Biosensors: A Review" Biosensors 13, no. 2: 200. https://doi.org/10.3390/bios13020200
APA StyleMa, C., Zhang, Z., Tan, T., & Zhu, J.-J. (2023). Recent Progress in Plasmonic based Electrochemiluminescence Biosensors: A Review. Biosensors, 13(2), 200. https://doi.org/10.3390/bios13020200