Determination Methods of the Risk Factors in Food Based on Nanozymes: A Review
Abstract
1. Introduction
2. Pathogenic Microorganism
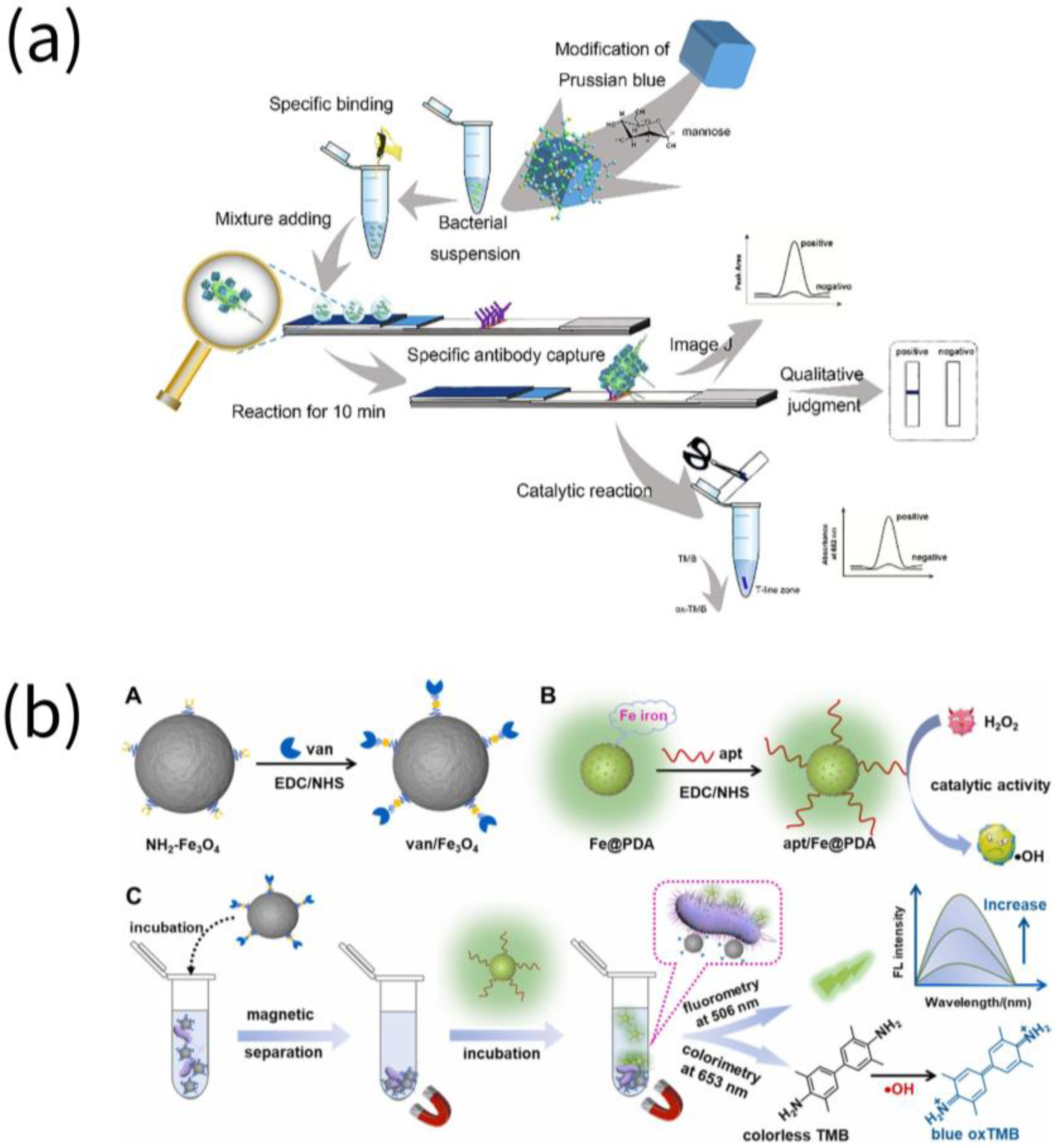
3. Toxins
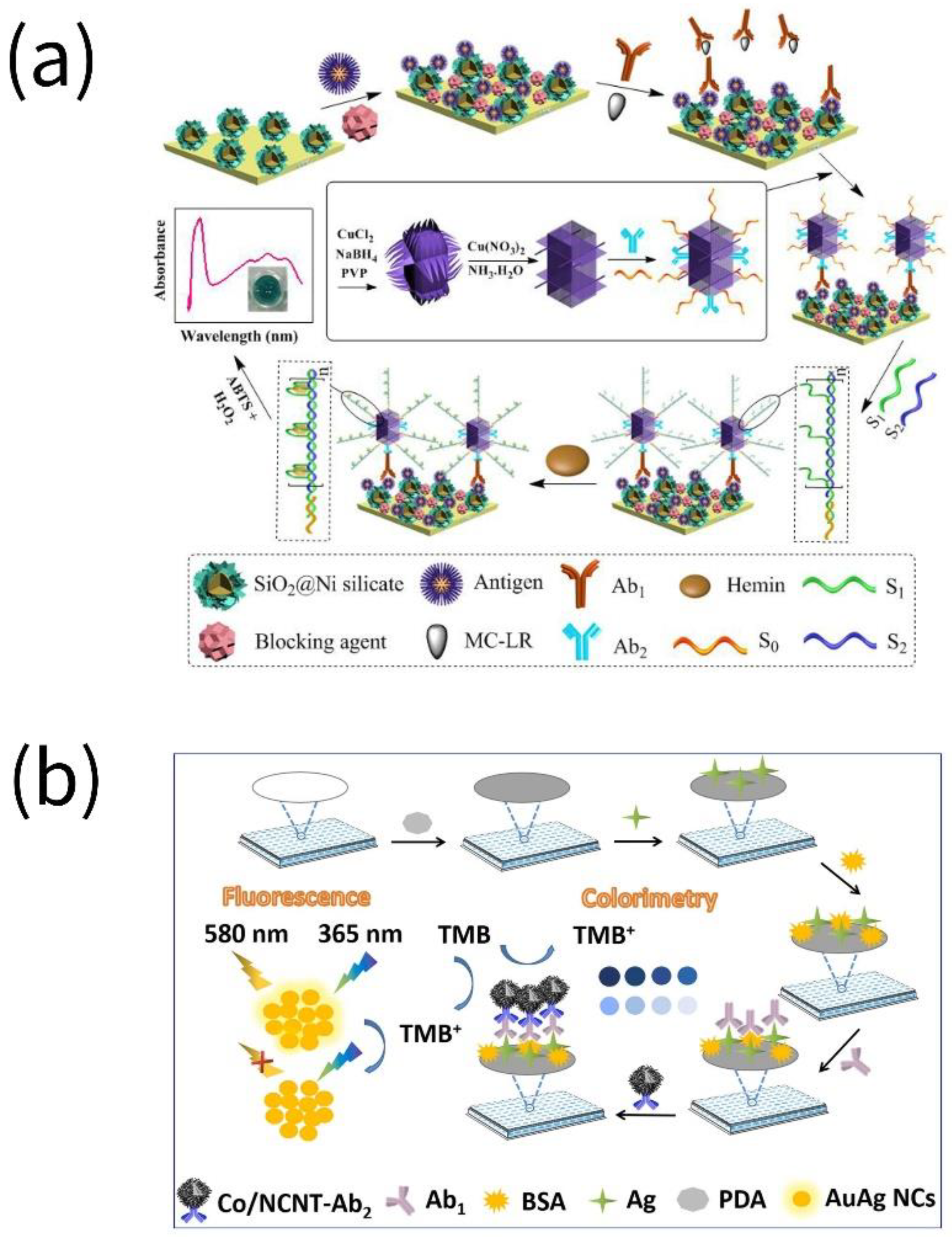
4. Pesticide Residues

5. Veterinary Drugs

6. Heavy Metals
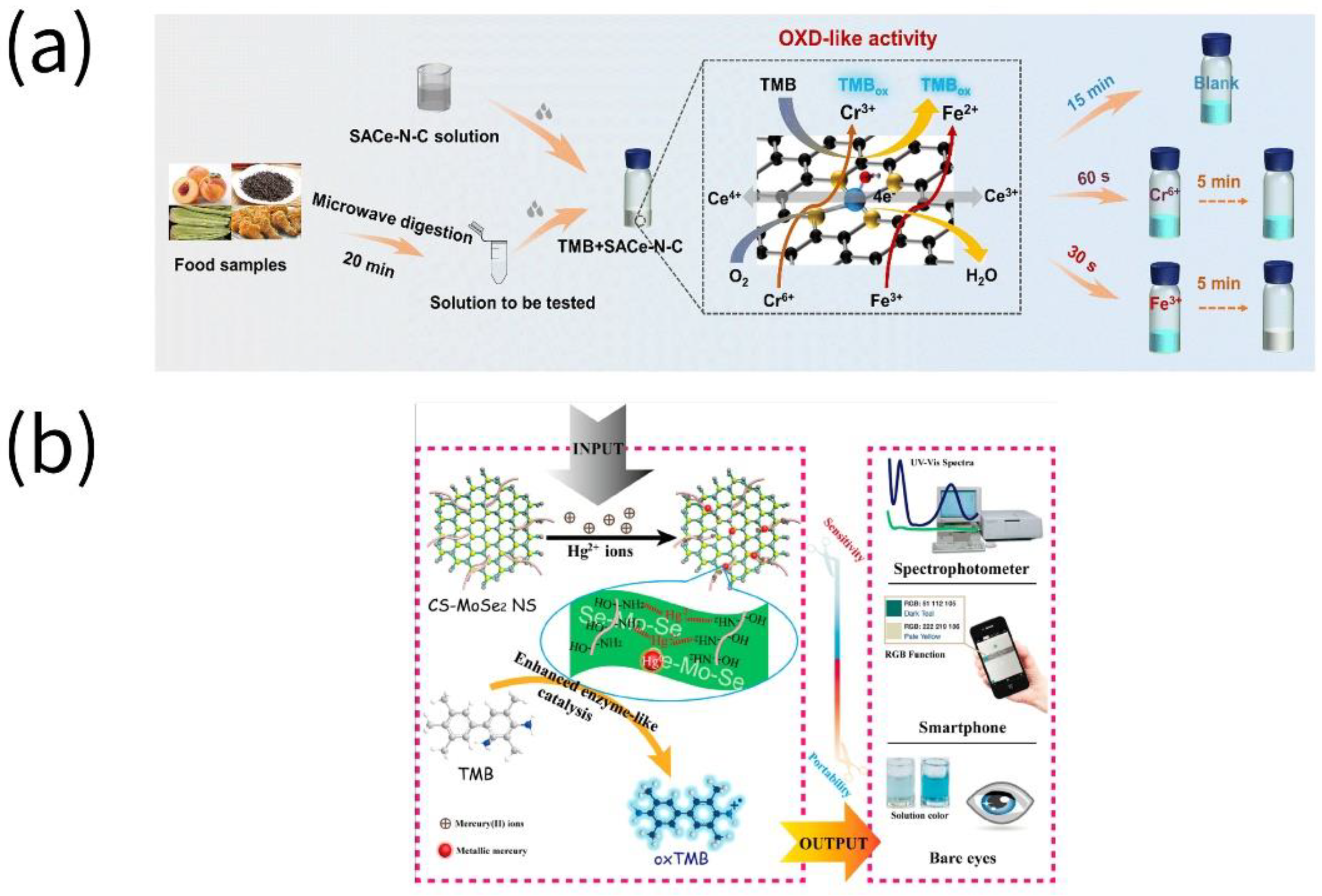
7. Others

8. Conclusions and Perspectives
| Analytes | Biosensors | Nanozymes | LODs | Food matrix | Ref. |
|---|---|---|---|---|---|
| Pathogenic microorganism | |||||
| Escherichia coli O157:H7 (E. coli O157:H7) | Colorimetric | Platinum-coated magnetic nanoparticle clusters (Pt/MNCs) | 10 CFU/mL | milk | [86] |
| E. coli O157:H7 | Colorimetric | Hemin-concanavalin A hybrid nanoflowers (HCH nanoflowers) | 4.1 CFU/mL | milk | [87] |
| Salmonella Enteritidis | Colorimetric | Fe-MOF nanoparticles | 34 CFU/mL | milk | [88] |
| Salmonella typhimurium | Colorimetric | Prussian blue nanoparticles (PBNPs) | 6 × 103 CFU/mL | powdered milk | [89] |
| Salmonella enterica serovar typhimurium | Colorimetric | ZnFe2O4-reduced graphene oxide nanostructures | 11 CFU/mL | milk | [90] |
| Listeria monocytogenes (L. monocytogenes) | Colorimetric | AgNCs | 10 CFU/mL | pork | [91] |
| Toxins | |||||
| Aflatoxin B1 (AFB1) | Colorimetric | Mesoporous SiO2/Au-Pt (m-SAP) | 0.005 ng/mL | peanut | [92] |
| AFB1 | Colorimetric | Porphyrin NanoMOFs (NanoPCN-223(Fe)) | 0.003 ng/mL | milk | [93] |
| AFB1 and Salmonella Enteritidis | Colorimetric/Fluorescent | Pt@PCN-224-HRP-initiator DNA (PP-HRP-iDNA) | 6.5 × 10−4 ng/mL and 4 CFU/mL for AFB1 and Salmonella Enteritidis respectively | rice and milk | [94] |
| Ochratoxin A (OTA) | Colorimetric | Co(OH)2 nanocages | 2.6 × 10−4 ng/mL | corn | [95] |
| Saxitoxin (STX) | Colorimetric | AuNPs | 4.246 × 10−4 nM | shellfish | [96] |
| Pesticide residues | |||||
| Diazinon | Fluorescent | Fe3O4 nanoparticles@ZIF-8 (Fe3O4 NPs@ZIF-8) | 0.2 nM | water and fruit juices | [97] |
| Acetamiprid | Colorimetric | Gold nanoparticles (GNPs) | 0.1 ng/mL | - | [98] |
| Methyl-paraoxon | Colorimetric/Fluorescent | nanoceria | 420 nM | Semen nelumbinis, Semen Armeniacae Amarum, Rhizoma Dioscoreae | [99] |
| Paraoxon | Fluorescent | Carbon quantum dots (CQDs) | 0.05 ng/mL | tap and river water | [100] |
| Paraoxon | Fluorescent | MnO2 Nanosheet-Carbon Dots | 0.015 ng/mL | tap water, river water, rice, and cabbage | [101] |
| Paraoxon, Parathion, Fenitrothion and Diazinon | Colorimetric | AuNPs | 0.13 ng/mL, 0.37 ng/mL, 0.42 ng/mL and 0.20 ng/mL for paraoxon, parathion, fenitrothion and diazinon, respectively | water | [102] |
| Glyphosate | Colorimetric/Fluorescence/Photothermal | N-CDs/FMOF-Zr | 13.1 ng/mL, 1.5 ng/mL and 11.5 ng/mL for colorimetric, fluorescence and photothermal respectively | rice, millet, and soybeans | [103] |
| Veterinary drugs | |||||
| Tetracycline (TC) | Colorimetric | AuNCs | 46 nM | drugs and milk | [104] |
| Kanamycin | Colorimetric | Gold nanoparticles (GNPs) | 1.49 nM | - | [105] |
| Enrofloxacin | Chemiluminescence | Co(OH)2 nanosheets | 4.1 × 10−5 ng/mL | shrimp, chicken, and duck meat | [106] |
| Norfloxacin (NOR) | Colorimetric | 1-methyl-D-tryptophan-capped gold nanoclusters (1-Me-D-Trp@AuNCs) | 200 nM | drugs | [107] |
| Sulfaquinoxaline (SQX) | Chemiluminescence | Cu(II)-anchored unzipped covalent triazine framework (UnZ-CCTF) | 7.6 × 10−4 nM | milk | [108] |
| Chloramphenicol (CAP) | Electrochemiluminescence | Ultrathin PtNi | 2.6 × 10−7 nM | pig urine, river water, and milk | [109] |
| Heavy metals | |||||
| Hg2+ | Colorimetric | Pt NP | 16.9 nM, 26 nM and 47.3 nM for MilliQ water, tap water and ground waters, respectively | MilliQ water, tap water, and ground waters | [110] |
| Hg2+ and MeHg | Fluorescent | Copper oxide-based nanocomposites | 3.0 nM and 3.3 nM for Hg2+ and MeHg, respectively | tap water, river water, seawater, and dogfish muscle | [111] |
| Ag2+ | Colorimetric | Chitosan-PtNPs (Ch-PtNPs) | 4 nM | tap and lake water | [112] |
| Ag2+ | Colorimetric | Pt nanoparticles | 7.8 × 10−3 nM | river water | [113] |
| Pb2+ | Colorimetric | Tungsten disulfide (WS2) nanosheets | 4 ng/mL | tap water, soil, wheat, and fish serum | [114] |
| Pb2+ | Colorimetric | Au@Pt nanoparticles | 3.0 nM | lake water | [115] |
| Pb2+ and Hg2+ | Fluorescent | Metal-deposited bismuth oxyiodide (BiOI) nanonetworks | nanomolar quantities | tap water, river water, lake water, and sea water | [116] |
| Others | |||||
| Sulfide | Colorimetric | GMP-Cu nanozyme with laccase activity | 670 nM | baking soda, rock sugar, konjac flour, and xylitol | [117] |
| Nitrite | Colorimetric/Electrochemical | Histidine(His)@AuNCs/rGO | 2 nM and 700 nM for Colorimetric and Electrochemical respectively | sausage | [118] |
| Nitrite | Colorimetric | Hollow MnFeO particles | 200 nM | sausage, pickles, and salted eggs | [119] |
| Salbutamol | Colorimetric | AgNPs | 2.614 × 10−4 ng/mL | tap water and artificial urine | [120] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shenashen, M.A.; Emran, M.Y.; El Sabagh, A.; Selim, M.M.; Elmarakbi, A.; El-Safty, S.A. Progress in Sensory Devices of Pesticides, Pathogens, Coronavirus, and Chemical Additives and Hazards in Food Assessment: Food Safety Concerns. Prog. Mater. Sci. 2022, 124, 100866. [Google Scholar] [CrossRef]
- van Asselt, E.D.; van der Fels-Klerx, H.J.; Marvin, H.J.P.; van Bokhorst-van de Veen, H.; Groot, M.N. Overview of Food Safety Hazards in the European Dairy Supply Chain: Food Safety Hazards in the Dairy Chain. Compr. Rev. Food Sci. Food Saf. 2017, 16, 59–75. [Google Scholar] [CrossRef] [PubMed]
- Proietti, I.; Frazzoli, C.; Mantovani, A. Identification and Management of Toxicological Hazards of Street Foods in Developing Countries. Food Chem. Toxicol. 2014, 63, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tan, C.-P.; Liu, Y.-F.; Xu, Y.-J. Interactions between Food Hazards and Intestinal Barrier: Impact on Foodborne Diseases. J. Agric. Food Chem. 2020, 68, 14728–14738. [Google Scholar] [CrossRef]
- Piatkowska, M.; Jedziniak, P.; Zmudzki, J. Multiresidue Method for the Simultaneous Determination of Veterinary Medicinal Products, Feed Additives and Illegal Dyes in Eggs Using Liquid Chromatography-Tandem Mass Spectrometry. Food Chem. 2016, 197, 571–580. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, C.; Lu, X.; Xu, G. Nontargeted Screening of Chemical Contaminants and Illegal Additives in Food Based on Liquid Chromatography-High Resolution Mass Spectrometry. TrAC-Trend Anal. Chem. 2017, 96, 89–98. [Google Scholar] [CrossRef]
- Li, G.; Xu, L.; Wu, W.; Wang, D.; Jiang, J.; Chen, X.; Zhang, W.; Poapolathep, S.; Poapolathep, A.; Zhang, Z.; et al. On-Site Ultrasensitive Detection Paper for Multiclass Chemical Contaminants via Universal Bridge-Antibody Labeling: Mycotoxin and Illegal Additives in Milk as an Example. Anal. Chem. 2019, 91, 1968–1973. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Zhou, X.; Yu, Y.; Liu, J.; Hu, N.; Wang, H.; Li, G.; Wu, Y. Recent Progress in the Construction of Nanozyme-Based Biosensors and Their Applications to Food Safety Assay. TrAC-Trend Anal. Chem. 2019, 121, 115668. [Google Scholar] [CrossRef]
- Gao, L.; Yan, X. Nanozymes: An Emerging Field Bridging Nanotechnology and Biology. Sci. China Life Sci. 2016, 59, 400–402. [Google Scholar] [CrossRef]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-Nanoparticle-Based Transphosphorylation Catalysts. Angew. Chem. Int. Edit. 2004, 116, 6291–6295. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wu, L.; Yao, H.; Zhao, L. Catalase-Like Nanozymes: Classification, Catalytic Mechanisms, and Their Applications. Small 2022, 18, 2203400. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Nath, I.; Chakraborty, J.; Verpoort, F. Metal Organic Frameworks Mimicking Natural Enzymes: A Structural and Functional Analogy. Chem. Soc. Rev. 2016, 45, 4127–4170. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhou, Y.; Ren, J.; Qu, X. Carbon Nanozymes: Enzymatic Properties, Catalytic Mechanism, and Applications. Angew. Chem. Int. Edit. 2018, 57, 9224–9237. [Google Scholar] [CrossRef]
- Huang, L.; Sun, D.; Pu, H.; Wei, Q. Development of Nanozymes for Food Quality and Safety Detection: Principles and Recent Applications. Compr. Rev. Food Sci. F 2019, 18, 1496–1513. [Google Scholar] [CrossRef]
- Rohani Bastami, T.; Bayat, M.; Paolesse, R. Naked-Eye Detection of Morphine by Au@Ag Nanoparticles-Based Colorimetric Chemosensors. Sensors 2022, 22, 2072. [Google Scholar] [CrossRef]
- Jiang, D.; Ni, D.; Rosenkrans, Z.T.; Huang, P.; Yan, X.; Cai, W. Nanozyme: New Horizons for Responsive Biomedical Applications. Chem. Soc. Rev. 2019, 48, 3683–3704. [Google Scholar] [CrossRef]
- Bastami, T.R.; Dabirifar, Z. AuNPs@PMo12 Nanozyme: Highly Oxidase Mimetic Activity for Sensitive and Specific Colorimetric Detection of Acetaminophen. RSC. Adv. 2020, 10, 35949–35956. [Google Scholar] [CrossRef]
- Ruan, X.; Liu, D.; Niu, X.; Wang, Y.; Simpson, C.D.; Cheng, N.; Du, D.; Lin, Y. 2D Graphene Oxide/Fe-MOF Nanozyme Nest with Superior Peroxidase-Like Activity and Its Application for Detection of Woodsmoke Exposure Biomarker. Anal. Chem. 2019, 91, 13847–13854. [Google Scholar] [CrossRef] [PubMed]
- Sayad, A.A.; Ibrahim, F.; Uddin, S.M.; Pei, K.X.; Mohktar, M.S.; Madou, M.; Thong, K.L. A Microfluidic Lab-on-a-Disc Integrated Loop Mediated Isothermal Amplification for Foodborne Pathogen Detection. Sens. Actuat. B-Chem. 2016, 227, 600–609. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, L.; Li, Y. Biosensors for Rapid Detection of Salmonella in Food: A Review. Compr. Rev. Food Sci. 2021, 20, 149–197. [Google Scholar] [CrossRef] [PubMed]
- Alahi, M.E.E.; Mukhopadhyay, S.C. Detection Methodologies for Pathogen and Toxins: A Review. Sensors 2017, 17, 1885. [Google Scholar] [CrossRef]
- Abdalhai, M.H.; Fernandes, A.M.; Xia, X.; Musa, A.; Ji, J.; Sun, X. Electrochemical Genosensor to Detect Pathogenic Bacteria (Escherichia coli O157:H7) As Applied in Real Food Samples (Fresh Beef) To Improve Food Safety and Quality Control. J. Agric. Food Chem. 2015, 63, 5017–5025. [Google Scholar] [CrossRef] [PubMed]
- Primiceri, E.; Chiriacò, M.S.; de Feo, F.; Santovito, E.; Fusco, V.; Maruccio, G. A Multipurpose Biochip for Food Pathogen Detection. Anal. Methods 2016, 8, 3055–3060. [Google Scholar] [CrossRef]
- Hu, J.; Tang, F.; Wang, L.; Tang, M.; Jiang, Y.-Z.; Liu, C. Nanozyme Sensor Based-on Platinum-Decorated Polymer Nanosphere for Rapid and Sensitive Detection of Salmonella typhimurium with the Naked Eye. Sens. Actuat. B-Chem. 2021, 346, 130560. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Han, L.; Cai, Y.; Ren, H.; Ma, T.; Li, X.; Petrenko, V.A.; Liu, A. Colorimetric Assay of Bacterial Pathogens Based on Co3O4 Magnetic Nanozymes Conjugated with Specific Fusion Phage Proteins and Magnetophoretic Chromatography. ACS Appl. Mater. Interfaces 2020, 12, 9090–9097. [Google Scholar] [CrossRef]
- Zheng, L.; Cai, G.; Qi, W.; Wang, S.; Wang, M.; Lin, J. Optical Biosensor for Rapid Detection of Salmonella typhimurium Based on Porous Gold@Platinum Nanocatalysts and a 3D Fluidic Chip. ACS Sens. 2020, 5, 65–72. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, X.; Zhang, Y.; Wang, R.; Ji, Y.; Sun, J.; Zhang, D.; Wang, J. Functional Nanozyme Mediated Multi-Readout and Label-Free Lateral Flow Immunoassay for Rapid Detection of Escherichia coli O157:H7. Food Chem. 2020, 329, 127224. [Google Scholar] [CrossRef]
- Cheng, N.; Song, Y.; Zeinhom, M.M.A.; Chang, Y.-C.; Sheng, L.; Li, H.; Du, D.; Li, L.; Zhu, M.-J.; Luo, Y.; et al. Nanozyme-Mediated Dual Immunoassay Integrated with Smartphone for Use in Simultaneous Detection of Pathogens. ACS Appl. Mater. Interfaces 2017, 9, 40671–40680. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Gao, X.; Zhang, Y.; Chen, H.; Ye, Y.; Wu, Y. Polydopamine-Based Nanozyme with Dual-Recognition Strategy-Driven Fluorescence-Colorimetric Dual-Mode Platform for Listeria monocytogenes Detection. J. Hazard. Mater. 2022, 439, 129582. [Google Scholar] [CrossRef] [PubMed]
- Stephen Inbaraj, B.; Chen, B.H. Nanomaterial-Based Sensors for Detection of Foodborne Bacterial Pathogens and Toxins as Well as Pork Adulteration in Meat Products. J. Food Drug Anal. 2016, 24, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Reiner-Rozman, C.; Hageneder, S.; Dubiak-Szepietowska, M.; Dostálek, J.; Feller, K.-H. Fast and Sensitive Detection of Ochratoxin A in Red Wine by Nanoparticle-Enhanced SPR. Anal. Chim. Acta 2016, 937, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Nishimwe, K.; Wanjuki, I.; Karangwa, C.; Darnell, R.; Harvey, J. An Initial Characterization of Aflatoxin B1 Contamination of Maize Sold in the Principal Retail Markets of Kigali, Rwanda. Food Control 2017, 73, 574–580. [Google Scholar] [CrossRef]
- Martinović, T.; Andjelković, U.; Gajdošik, M.Š.; Rešetar, D.; Josić, D. Foodborne Pathogens and Their Toxins. J. Proteomics 2016, 147, 226–235. [Google Scholar] [CrossRef]
- Xu, Z.; Long, L.; Chen, Y.; Chen, M.-L.; Cheng, Y.-H. A Nanozyme-Linked Immunosorbent Assay Based on Metal-Organic Frameworks (MOFs) for Sensitive Detection of Aflatoxin B1. Food Chem. 2021, 338, 128039. [Google Scholar] [CrossRef]
- Liu, W.; Gan, C.; Chang, W.; Qileng, A.; Lei, H.; Liu, Y. Double-Integrated Mimic Enzymes for the Visual Screening of Microcystin-LR: Copper Hydroxide Nanozyme and G-Quadruplex/Hemin DNAzyme. Anal. Chim. Acta 2019, 1054, 128–136. [Google Scholar] [CrossRef]
- Lu, D.; Jiang, H.; Zhang, G.; Luo, Q.; Zhao, Q.; Shi, X. An In Situ Generated Prussian Blue Nanoparticle-Mediated Multimode Nanozyme-Linked Immunosorbent Assay for the Detection of Aflatoxin B1. ACS Appl. Mater. Interfaces 2021, 13, 25738–25747. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Z.; Guan, Y.; Chen, Y.; Liu, W.; Liu, Y. Zeolitic Imidazolate Frameworks-Derived Hollow Co/N-Doped CNTs as Oxidase-Mimic for Colorimetric-Fluorescence Immunoassay of Ochratoxin A. Sens. Actuat. B-Chem. 2022, 359, 131609. [Google Scholar] [CrossRef]
- Zhu, H.; Cai, Y.; Qileng, A.; Quan, Z.; Zeng, W.; He, K.; Liu, Y. Template-Assisted Cu2O@Fe(OH)3 Yolk-Shell Nanocages as Biomimetic Peroxidase: A Multi-Colorimetry and Ratiometric Fluorescence Separated-Type Immunosensor for the Detection of Ochratoxin A. J. Hazard. Mater. 2021, 411, 125090. [Google Scholar] [CrossRef] [PubMed]
- Tomer, V.; Sangha, J.K.; Ramya, H.G. Pesticide: An Appraisal on Human Health Implications. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015, 85, 451–463. [Google Scholar] [CrossRef]
- Singh, B.K. Organophosphorus-Degrading Bacteria: Ecology and Industrial Applications. Nat. Rev. Microbiol. 2009, 7, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Shi, T.; Luo, X.; Xiong, H.; Min, F.; Chen, Y.; Nie, S.; Xie, M. Determination of Multi-Pesticide Residues in Green Tea with a Modified QuEChERS Protocol Coupled to HPLC-MS/MS. Food Chem. 2019, 275, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhou, R.; Zhang, H.; Cai, D.; Lin, X.; Lang, Y.; Qiu, Y.; Shentu, X.; Ye, Z.; Yu, X. A Smartphone Colorimetric Sensor Based on Pt@Au Nanozyme for Visual and Quantitative Detection of Omethoate. Foods 2022, 11, 2900. [Google Scholar] [CrossRef]
- Naveen Prasad, S.; Bansal, V.; Ramanathan, R. Detection of Pesticides Using Nanozymes: Trends, Challenges and Outlook. TrAC-Trend Anal. Chem. 2021, 144, 116429. [Google Scholar] [CrossRef]
- Ge, J.; Yang, L.; Li, Z.; Wan, Y.; Mao, D.; Deng, R.; Zhou, Q.; Yang, Y.; Tan, W. A Colorimetric Smartphone-Based Platform for Pesticides Detection Using Fe-N/C Single-Atom Nanozyme as Oxidase Mimetics. J. Hazard. Mater. 2022, 436, 129199. [Google Scholar] [CrossRef]
- He, L.; Jiang, Z.W.; Li, W.; Li, C.M.; Huang, C.Z.; Li, Y.F. In Situ Synthesis of Gold Nanoparticles/Metal-Organic Gels Hybrids with Excellent Peroxidase-Like Activity for Sensitive Chemiluminescence Detection of Organophosphorus Pesticides. ACS Appl. Mater. Interfaces 2018, 10, 28868–28876. [Google Scholar] [CrossRef]
- Liang, X.; Han, L. White Peroxidase Mimicking Nanozymes: Colorimetric Pesticide Assay without Interferences of O2 and Color. Adv. Funct. Mater. 2020, 30, 2001933. [Google Scholar] [CrossRef]
- Liang, B.; Han, L. Displaying of Acetylcholinesterase Mutants on Surface of Yeast for Ultra-Trace Fluorescence Detection of Organophosphate Pesticides with Gold Nanoclusters. Biosens. Bioelectron. 2020, 148, 111825. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Han, L.; Wang, X.; Li, W.; Guo, H.; Wei, H. Nanozyme Sensor Arrays Based on Heteroatom-Doped Graphene for Detecting Pesticides. Anal. Chem. 2020, 92, 7444–7452. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Du, D.; Lin, Y. Recent Progress on Nanomaterial-Based Biosensors for Veterinary Drug Residues in Animal-Derived Food. TrAC-Trend Anal. Chem. 2016, 83, 95–101. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, S.; Wang, G.; Yun, Y.; Liu, G.; Zhang, W. Nanozyme Applications: A Glimpse of Insight in Food Safety. Front. Bioeng. Biotechnol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xie, K.; Lee, K. Veterinary Drug Residues in Animal-Derived Foods: Sample Preparation and Analytical Methods. Foods 2021, 10, 555. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, S.; Yin, X.; Wang, S.; Tian, Y.; Shu, R.; Jia, C.; Chen, Y.; Sun, J.; Zhang, D.; et al. Nature-Inspired Nanozymes as Signal Markers for in-Situ Signal Amplification Strategy: A Portable Dual-Colorimetric Immunochromatographic Analysis Based on Smartphone. Biosens. Bioelectron. 2022, 210, 114289. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Pang, C.; Wang, M.; Yin, G.; Xu, Z.; Li, J.; Luo, J. Novel Chloramphenicol Sensor Based on Aggregation-Induced Electrochemiluminescence and Nanozyme Amplification. Biosens. Bioelectron. 2021, 176, 112944. [Google Scholar] [CrossRef]
- Zeng, R.; Luo, Z.; Zhang, L.; Tang, D. Platinum Nanozyme-Catalyzed Gas Generation for Pressure-Based Bioassay Using Polyaniline Nanowires-Functionalized Graphene Oxide Framework. Anal. Chem. 2018, 90, 12299–12306. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Zhang, Y.; Wang, L.; Geng, Q.; Liu, D.; Duan, L.; Wang, Y.; Cui, J. Ratiometric Dual Signal-Enhancing-Based Electrochemical Biosensor for Ultrasensitive Kanamycin Detection. ACS Appl. Mater. Interfaces 2020, 12, 52713–52720. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy Metal Pollution in the Environment and Their Toxicological Effects on Humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Liu, L.; Lin, H. Paper-Based Colorimetric Array Test Strip for Selective and Semiquantitative Multi-Ion Analysis: Simultaneous Detection of Hg2+, Ag+, and Cu2+. Anal. Chem. 2014, 86, 8829–8834. [Google Scholar] [CrossRef]
- Fang, Y.; Pan, Y.; Li, P.; Xue, M.; Pei, F.; Yang, W.; Ma, N.; Hu, Q. Simultaneous Determination of Arsenic and Mercury Species in Rice by Ion-Pairing Reversed Phase Chromatography with Inductively Coupled Plasma Mass Spectrometry. Food Chem. 2016, 213, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi-Najafabadi, H.; Pasdaran, A.; Rezaei Bezenjani, R.; Bozorgzadeh, E. Determination of Toxic Heavy Metals in Rice Samples Using Ultrasound Assisted Emulsification Microextraction Combined with Inductively Coupled Plasma Optical Emission Spectroscopy. Food Chem. 2019, 289, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Zhang, Y.; Cao, L.; Yang, J.; Hu, M.; Pang, Z.; He, J. Portable Hg2+ Nanosensor with Ppt Level Sensitivity Using Nanozyme as the Recognition Unit, Enrichment Carrier, and Signal Amplifier. ACS Appl. Mater. Interfaces 2020, 12, 11761–11768. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Gao, S.; Yao, L.; Wang, L.; Qu, H.; Wu, Y.; Chen, Y.; Zheng, L. Single-Atom Nanozyme Enabled Fast and Highly Sensitive Colorimetric Detection of Cr(VI). J. Hazard. Mater. 2021, 408, 124898. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Zhang, C.; Jiang, W.; Liang, R.; Wen, S.; Peng, D.; Qiu, J. Covalent Organic Framework Nanosheet-Based Ultrasensitive and Selective Colorimetric Sensor for Trace Hg2+ Detection. ACS Sustain. Chem. Eng. 2019, 7, 9408–9415. [Google Scholar] [CrossRef]
- Zou, W.; Tang, Y.; Zeng, H.; Wang, C.; Wu, Y. Porous Co3O4 Nanodisks as Robust Peroxidase Mimetics in an Ultrasensitive Colorimetric Sensor for the Rapid Detection of Multiple Heavy Metal Residues in Environmental Water Samples. J. Hazard. Mater. 2021, 417, 125994. [Google Scholar] [CrossRef]
- Song, G.; Zhang, Q.; Liang, S.; Yao, Y.; Feng, M.; Majid, Z.; He, X.; Huang, K.; Li, J.-C.; Cheng, N. Oxidation Activity Modulation of a Single Atom Ce-N-C Nanozyme Enabling a Time-Resolved Sensor to Detect Fe3+ and Cr6+. J. Mater. Chem. C 2022. [Google Scholar] [CrossRef]
- Huang, L.; Zhu, Q.; Zhu, J.; Luo, L.; Pu, S.; Zhang, W.; Zhu, W.; Sun, J.; Wang, J. Portable Colorimetric Detection of Mercury(II) Based on a Non-Noble Metal Nanozyme with Tunable Activity. Inorg. Chem. 2019, 58, 1638–1646. [Google Scholar] [CrossRef]
- Carocho, M.; Barreiro, M.F.; Morales, P.; Ferreira, I.C.F.R. Adding Molecules to Food, Pros and Cons: A Review on Synthetic and Natural Food Additives. Compr. Rev. Food Sci. Food Saf. 2014, 13, 377–399. [Google Scholar] [CrossRef]
- Gagic, M.; Jamroz, E.; Krizkova, S.; Milosavljevic, V.; Kopel, P.; Adam, V. Current Trends in Detection of Histamine in Food and Beverages. J. Agric. Food Chem. 2019, 67, 773–783. [Google Scholar] [CrossRef]
- Li, Q.; Ren, S.; Peng, Y.; Lv, Y.; Wang, W.; Wang, Z.; Gao, Z. A Colorimetric Strip for Rapid Detection and Real-Time Monitoring of Histamine in Fish Based on Self-Assembled Polydiacetylene Vesicles. Anal. Chem. 2020, 92, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Lehane, L.; Olley, J. Histamine Fish Poisoning Revisited. Int. J. Food Microbiol. 2000, 58, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Jia, B.; Wang, H.; Sun, Y.; Xu, X.; Wei, X.; Shen, Y.; Lei, H.; Xu, Z.; Luo, L. Prussian Blue Nanoparticles-Enabled Sensitive and Accurate Ratiometric Fluorescence Immunoassay for Histamine. Food Chem. 2022, 376, 131907. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wu, F.; Mu, F.; Dai, B. The Preparation of Fe-Based Peroxidase Mimetic Nanozymes and for the Electrochemical Detection of Histamine. Electroanal. Chem. 2022, 908, 116088. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Yu, R.; Wang, R.; Xu, Z. A Sensitive Biomimetic Enzyme-Linked Immunoassay Method Based on Au@Pt@Au Composite Nanozyme Label and Molecularly Imprinted Biomimetic Antibody for Histamine Detection. Food Agr. Immunol. 2021, 32, 592–605. [Google Scholar] [CrossRef]
- Yue, R.; Lu, Q.; Zhou, Y. A Novel Nitrite Biosensor Based on Single-Layer Graphene Nanoplatelet-Protein Composite Film. Biosens. Bioelectron. 2011, 26, 4436–4441. [Google Scholar] [CrossRef]
- Camargo, J.A.; Alonso, A.; Salamanca, A. Nitrate Toxicity to Aquatic Animals: A Review with New Data for Freshwater Invertebrates. Chemosphere 2005, 58, 1255–1267. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, H.; Liu, B.; Hu, P.; Pan, J.; Niu, X. Bifunctional Mn-Doped N-Rich Carbon Dots with Tunable Photoluminescence and Oxidase-Mimetic Activity Enabling Bimodal Ratiometric Colorimetric/Fluorometric Detection of Nitrite. ACS Appl. Mater. Interfaces 2022, 14, 44762–44771. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, M.; Liu, P.; Zhu, H.; Liu, B.; Hu, P.; Niu, X. Coupling Diazotization with Oxidase-Mimetic Catalysis to Realize Dual-Mode Double-Ratiometric Colorimetric and Electrochemical Sensing of Nitrite. Sens. Actuat. B-Chem. 2022, 355, 131308. [Google Scholar] [CrossRef]
- Adegoke, O.; Zolotovskaya, S.; Abdolvand, A.; Daeid, N.N. Rapid and Highly Selective Colorimetric Detection of Nitrite Based on the Catalytic-Enhanced Reaction of Mimetic Au Nanoparticle-CeO2 Nanoparticle-Graphene Oxide Hybrid Nanozyme. Talanta 2021, 224, 121875. [Google Scholar] [CrossRef]
- Payal, A.; Krishnamoorthy, S.; Elumalai, A.; Moses, J.A.; Anandharamakrishnan, C. A Review on Recent Developments and Applications of Nanozymes in Food Safety and Quality Analysis. Food Anal. Methods 2021, 14, 1537–1558. [Google Scholar] [CrossRef]
- Prabakaran, E.; Pandian, K. Amperometric Detection of Sudan I in Red Chili Powder Samples Using Ag Nanoparticles Decorated Graphene Oxide Modified Glassy Carbon Electrode. Food Chem. 2015, 166, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi-Moghaddam, H.; Tajik, S.; Beitollahi, H. Highly Sensitive Electrochemical Sensor Based on La3+-Doped Co3O4 Nanocubes for Determination of Sudan I Content in Food Samples. Food Chem. 2019, 286, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Chen, X.; Yang, J.; Wu, D.; Ma, J.; Kong, Y. Fabrication of CuO Nanoparticles-Decorated 3D N-Doped Porous Carbon as Electrochemical Sensing Platform for the Detection of Sudan I. Food Chem. 2019, 287, 375–381. [Google Scholar] [CrossRef]
- Yu, X.; Lee, J.K.; Liu, H.; Yang, H. Synthesis of Magnetic Nanoparticles to Detect Sudan Dye Adulteration in Chilli Powders. Food Chem. 2019, 299, 125144. [Google Scholar] [CrossRef]
- Kwon, D.; Lee, S.; Ahn, M.M.; Kang, I.S.; Park, K.-H.; Jeon, S. Colorimetric Detection of Pathogenic Bacteria Using Platinum-Coated Magnetic Nanoparticle Clusters and Magnetophoretic Chromatography. Anal. Chim. Acta 2015, 883, 61–66. [Google Scholar] [CrossRef]
- Wang, K.-Y.; Bu, S.-J.; Ju, C.-J.; Li, C.-T.; Li, Z.-Y.; Han, Y.; Ma, C.-Y.; Wang, C.-Y.; Hao, Z.; Liu, W.-S.; et al. Hemin-Incorporated Nanoflowers as Enzyme Mimics for Colorimetric Detection of Foodborne Pathogenic Bacteria. Bioorg. Med. Chem. Lett. 2018, 28, 3802–3807. [Google Scholar] [CrossRef]
- Cheng, N.; Zhu, C.; Wang, Y.; Du, D.; Zhu, M.-J.; Luo, Y.; Xu, W.; Lin, Y. Nanozyme Enhanced Colorimetric Immunoassay for Naked-Eye Detection of Salmonella enteritidis. J. Anal. Test. 2019, 3, 99–106. [Google Scholar] [CrossRef]
- Farka, Z.; Čunderlová, V.; Horáčková, V.; Pastucha, M.; Mikušová, Z.; Hlaváček, A.; Skládal, P. Prussian Blue Nanoparticles as a Catalytic Label in a Sandwich Nanozyme-Linked Immunosorbent Assay. Anal. Chem. 2018, 90, 2348–2354. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Qiu, Y.; Li, J.; Wang, Z. Colorimetric Aptasensor for the Detection of Salmonella Enterica Serovar Typhimurium Using ZnFe2O4-Reduced Graphene Oxide Nanostructures as an Effective Peroxidase Mimetics. Int. J. Food Microbiol. 2017, 261, 42–48. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Song, X.; Xu, K.; Chen, H.; Zhao, C.; Li, J. Colorimetric Immunoassay for Listeria Monocytogenes by Using Core Gold Nanoparticles, Silver Nanoclusters as Oxidase Mimetics, and Aptamer-Conjugated Magnetic Nanoparticles. Microchim. Acta 2018, 185, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Zhou, M.; Wang, Y.; Liu, J. Nanozyme and Aptamer- Based Immunosorbent Assay for Aflatoxin B1. J. Hazard. Mater. 2020, 399, 123154. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Li, K.; Wang, Y.; Li, L.; Cheng, Y.-H.; Xu, Z. Porphyrin NanoMOFs as a Catalytic Label in Nanozyme-Linked Immunosorbent Assay for Aflatoxin B1 Detection. Analytical Biochem. 2022, 655, 114829. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Hong, F.; Zeng, L.; Chen, Y. “Three-in-One” Zr-MOF Multifunctional Carrier-Mediated Fluorescent and Colorimetric Dual-Signal Readout Biosensing Platform to Enhance Analytical Performance. ACS Appl. Mater. Interfaces 2022, 14, 51234–51243. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Quan, Z.; Hou, H.; Cai, Y.; Liu, W.; Liu, Y. A Colorimetric Immunoassay Based on Cobalt Hydroxide Nanocages as Oxidase Mimics for Detection of Ochratoxin A. Anal. Chim. Acta 2020, 1132, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, L.; Ma, R.; Wang, L.; Yan, X.; Qi, X.; Wang, S.; Mao, X. A Competitive Colorimetric Aptasensor Transduced by Hybridization Chain Reaction-Facilitated Catalysis of AuNPs Nanozyme for Highly Sensitive Detection of Saxitoxin. Analy. Chim. Acta 2021, 1173, 338710. [Google Scholar] [CrossRef]
- Bagheri, N.; Khataee, A.; Hassanzadeh, J.; Habibi, B. Sensitive Biosensing of Organophosphate Pesticides Using Enzyme Mimics of Magnetic ZIF-8. Spectrochim. Acta A 2019, 209, 118–125. [Google Scholar] [CrossRef]
- Weerathunge, P.; Ramanathan, R.; Shukla, R.; Sharma, T.K.; Bansal, V. Aptamer-Controlled Reversible Inhibition of Gold Nanozyme Activity for Pesticide Sensing. Anal. Chem. 2014, 86, 11937–11941. [Google Scholar] [CrossRef]
- Wei, J.; Yang, L.; Luo, M.; Wang, Y.; Li, P. Nanozyme-Assisted Technique for Dual Mode Detection of Organophosphorus Pesticide. Ecotox. Environ. Safe. 2019, 179, 17–23. [Google Scholar] [CrossRef]
- Wu, X.; Song, Y.; Yan, X.; Zhu, C.; Ma, Y.; Du, D.; Lin, Y. Carbon Quantum Dots as Fluorescence Resonance Energy Transfer Sensors for Organophosphate Pesticides Determination. Biosens. Bioelectron. 2017, 94, 292–297. [Google Scholar] [CrossRef]
- Yan, X.; Song, Y.; Zhu, C.; Li, H.; Du, D.; Su, X.; Lin, Y. MnO2 Nanosheet-Carbon Dots Sensing Platform for Sensitive Detection of Organophosphorus Pesticides. Anal. Chem. 2018, 90, 2618–2624. [Google Scholar] [CrossRef] [PubMed]
- Satnami, M.L.; Korram, J.; Nagwanshi, R.; Vaishanav, S.K.; Karbhal, I.; Dewangan, H.K.; Ghosh, K.K. Gold Nanoprobe for Inhibition and Reactivation of Acetylcholinesterase: An Application to Detection of Organophosphorus Pesticides. Sens. Actuat. B-Chem. 2018, 267, 155–164. [Google Scholar] [CrossRef]
- Luo, X.; Huang, G.; Bai, C.; Wang, C.; Yu, Y.; Tan, Y.; Tang, C.; Kong, J.; Huang, J.; Li, Z. A Versatile Platform for Colorimetric, Fluorescence and Photothermal Multi-Mode Glyphosate Sensing by Carbon Dots Anchoring Ferrocene Metal-Organic Framework Nanosheet. J. Hazard. Mater. 2023, 443, 130277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Tian, Y.; Huang, P.; Wu, F.-Y. Using Target-Specific Aptamers to Enhance the Peroxidase-like Activity of Gold Nanoclusters for Colorimetric Detection of Tetracycline Antibiotics. Talanta 2020, 208, 120342. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.K.; Ramanathan, R.; Weerathunge, P.; Mohammadtaheri, M.; Daima, H.K.; Shukla, R.; Bansal, V. Aptamer-Mediated ‘Turn-off/Turn-on’ Nanozyme Activity of Gold Nanoparticles for Kanamycin Detection. Chem. Commun. 2014, 50, 15856–15859. [Google Scholar] [CrossRef] [PubMed]
- Pei, Y.; Zeng, L.; Wen, C.; Wu, K.; Deng, A.; Li, J. Detection of Enrofloxacin by Flow Injection Chemiluminescence Immunoassay Based on Cobalt Hydroxide Nanozyme. Microchim. Acta 2021, 188, 1–10. [Google Scholar] [CrossRef]
- Song, Y.; Qiao, J.; Liu, W.; Qi, L. Norfloxacin Detection Based on the Peroxidase-like Activity Enhancement of Gold Nanoclusters. Anal. Bioanal. Chem. 2021, 413, 979–985. [Google Scholar] [CrossRef]
- Ma, X.; Li, S.; Pang, C.; Xiong, Y.; Li, J. A Cu(II)-Anchored Unzipped Covalent Triazine Framework with Peroxidase-Mimicking Properties for Molecular Imprinting-Based Electrochemiluminescent Detection of Sulfaquinoxaline. Microchim. Acta 2018, 185, 546. [Google Scholar] [CrossRef]
- Zhu, X.; Gao, L.; Tang, L.; Peng, B.; Huang, H.; Wang, J.; Yu, J.; Ouyang, X.; Tan, J. Ultrathin PtNi Nanozyme Based Self-Powered Photoelectrochemical Aptasensor for Ultrasensitive Chloramphenicol Detection. Biosens. Bioelectron. 2019, 146, 111756. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Peroxidase Activity of Biogenic Platinum Nanoparticles: A Colorimetric Probe towards Selective Detection of Mercuric Ions in Water Samples. Sens. Actuat. B-Chem. 2018, 254, 690–700. [Google Scholar] [CrossRef]
- Lien, C.; Yu, P.; Chang, H.; Hsu, P.; Wu, T.; Lin, Y.; Huang, C.; Lai, J. DNA Engineered Copper Oxide-Based Nanocomposites with Multiple Enzyme-like Activities for Specific Detection of Mercury Species in Environmental and Biological Samples. Anal. Chim. Acta 2019, 1084, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; He, S.; Lin, X.; Yang, L.; Lin, Z.; Chen, R.; Peng, H.; Chen, W. Target-Triggered Inhibiting Oxidase-Mimicking Activity of Platinum Nanoparticles for Ultrasensitive Colorimetric Detection of Silver Ion. Chinese Chem. Lett. 2019, 30, 1659–1662. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Wang, L.; Xu, H.; Tang, S.; Yang, H.; Zhang, L.; Song, H. A Simple Assay for Ultrasensitive Colorimetric Detection of Ag+ at Picomolar Levels Using Platinum Nanoparticles. Sensors 2017, 17, 2521. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Hu, Y.; Yang, Y.; Liu, B.; Wu, Y. A Facile Colorimetric Sensor for Ultrasensitive and Selective Detection of Lead(II) in Environmental and Biological Samples Based on Intrinsic Peroxidase-Mimic Activity of WS2 Nanosheets. Anal. Chim. Acta 2020, 1106, 115–125. [Google Scholar] [CrossRef]
- Xie, Z.; Shi, M.; Wang, L.; Peng, C.; Wei, X. Colorimetric Determination of Pb2+ Ions Based on Surface Leaching of Au@Pt Nanoparticles as Peroxidase Mimic. Microchim. Acta 2020, 187, 255. [Google Scholar] [CrossRef]
- Hsu, C.; Lien, C.; Harroun, S.; Ravindranath, R.; Chang, H.; Mao, J.; Huang, C. Metal-Deposited Bismuth Oxyiodide Nanonetworks with Tunable Enzyme-like Activity: Sensing of Mercury and Lead Ions. Mater. Chem. Front. 2017, 1, 893–899. [Google Scholar] [CrossRef]
- Huang, H.; Li, M.; Hao, M.; Yu, L. (Lucy); Li, Y. A Novel Selective Detection Method for Sulfide in Food Systems Based on the GMP-Cu Nanozyme with Laccase Activity. Talanta 2021, 235, 122775. [Google Scholar] [CrossRef]
- Liu, L.; Du, J.; Liu, W.; Guo, Y.; Wu, G.; Qi, W.; Lu, X. Enhanced His@AuNCs Oxidase-like Activity by Reduced Graphene Oxide and Its Application for Colorimetric and Electrochemical Detection of Nitrite. Anal. Bioanal. Chem. 2019, 411, 2189–2200. [Google Scholar] [CrossRef]
- Wang, M.; Liu, P.; Zhu, H.; Liu, B.; Niu, X. Ratiometric Colorimetric Detection of Nitrite Realized by Stringing Nanozyme Catalysis and Diazotization Together. Biosensors 2021, 11, 280. [Google Scholar] [CrossRef]
- He, H.; Sun, T.; Liu, W.; Xu, Z.; Han, Z.; Zhao, L.; Wu, X.; Ning, B.; Bai, J. Highly Sensitive Detection of Salbutamol by ALP-Mediated Plasmonic ELISA Based on Controlled Growth of AgNPs. Microchem. J. 2020, 156, 104804. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lang, Y.; Zhang, B.; Cai, D.; Tu, W.; Zhang, J.; Shentu, X.; Ye, Z.; Yu, X. Determination Methods of the Risk Factors in Food Based on Nanozymes: A Review. Biosensors 2023, 13, 69. https://doi.org/10.3390/bios13010069
Lang Y, Zhang B, Cai D, Tu W, Zhang J, Shentu X, Ye Z, Yu X. Determination Methods of the Risk Factors in Food Based on Nanozymes: A Review. Biosensors. 2023; 13(1):69. https://doi.org/10.3390/bios13010069
Chicago/Turabian StyleLang, Yihan, Biao Zhang, Danfeng Cai, Wanjun Tu, Jingyi Zhang, Xuping Shentu, Zihong Ye, and Xiaoping Yu. 2023. "Determination Methods of the Risk Factors in Food Based on Nanozymes: A Review" Biosensors 13, no. 1: 69. https://doi.org/10.3390/bios13010069
APA StyleLang, Y., Zhang, B., Cai, D., Tu, W., Zhang, J., Shentu, X., Ye, Z., & Yu, X. (2023). Determination Methods of the Risk Factors in Food Based on Nanozymes: A Review. Biosensors, 13(1), 69. https://doi.org/10.3390/bios13010069






