Development of a Wearable Ultrasound Transducer for Sensing Muscle Activities in Assistive Robotics Applications
Abstract
1. Introduction
- The design and fabrication procedure of a flexible US transducer incorporating PZT-5A elements into PDMS substrate was demonstrated. Consequently, the proposed US transducer exhibits high flexibility and wearability.
- Sizes of PDMS substrates and transducer arrangements can be easily customized to fit the location and shape of the target muscles. As a result of the customization of the US transducer, it is possible to measure multiple muscle groups simultaneously and individually.
- A proof-of-concept study has established that the wearable device can accurately monitor muscle movements both regionally and at different depths.
2. Materials and Methods
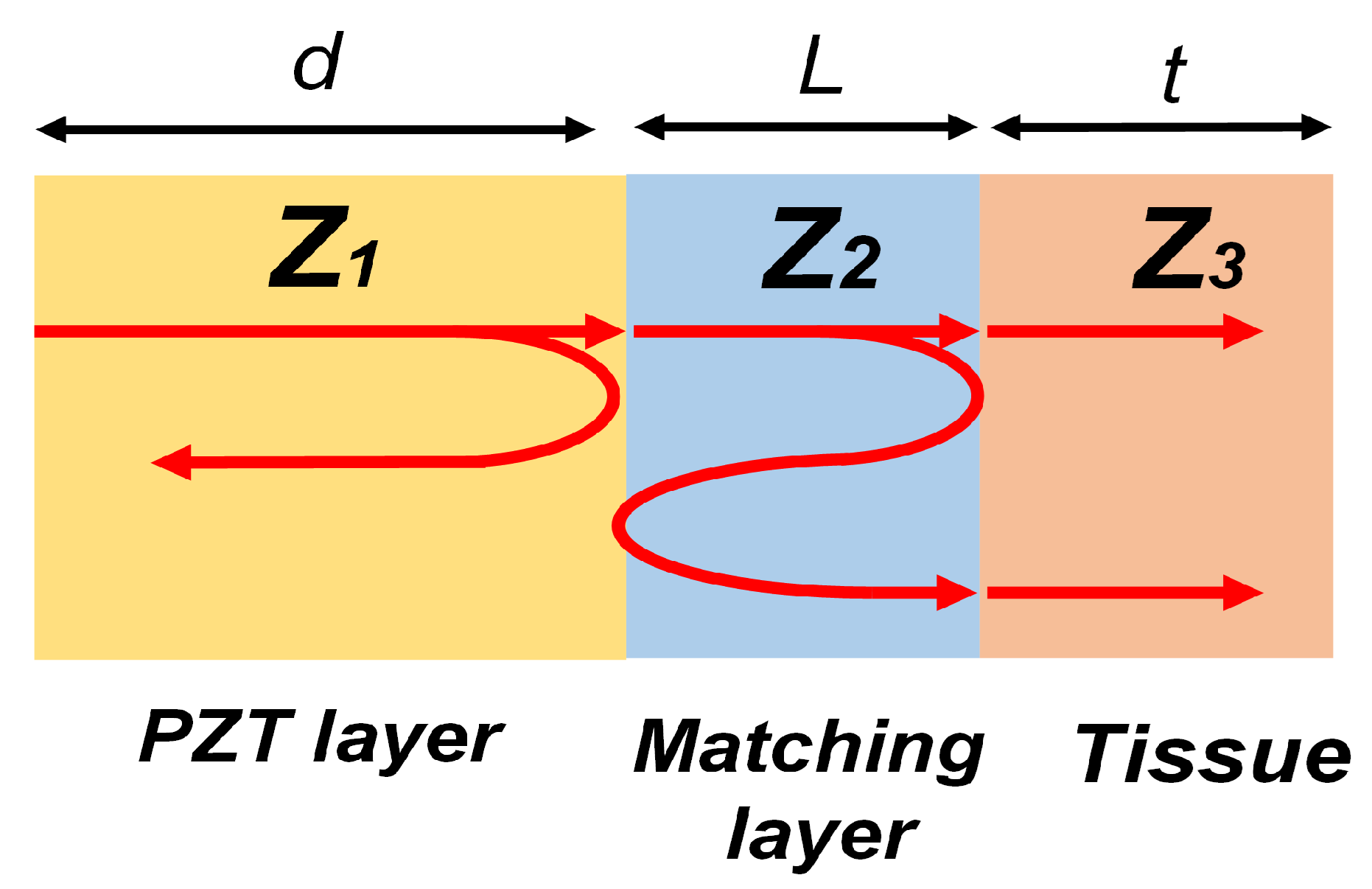
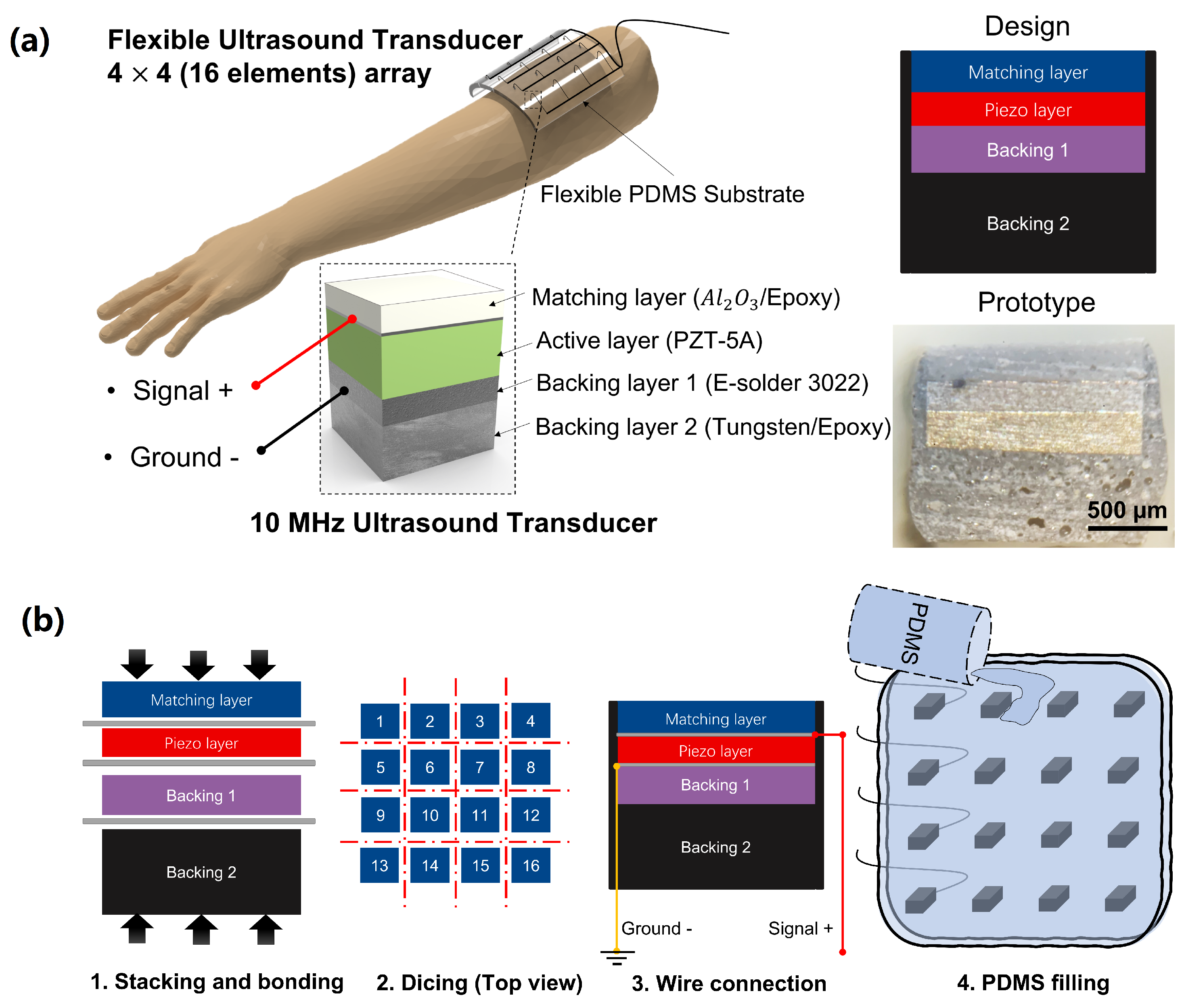
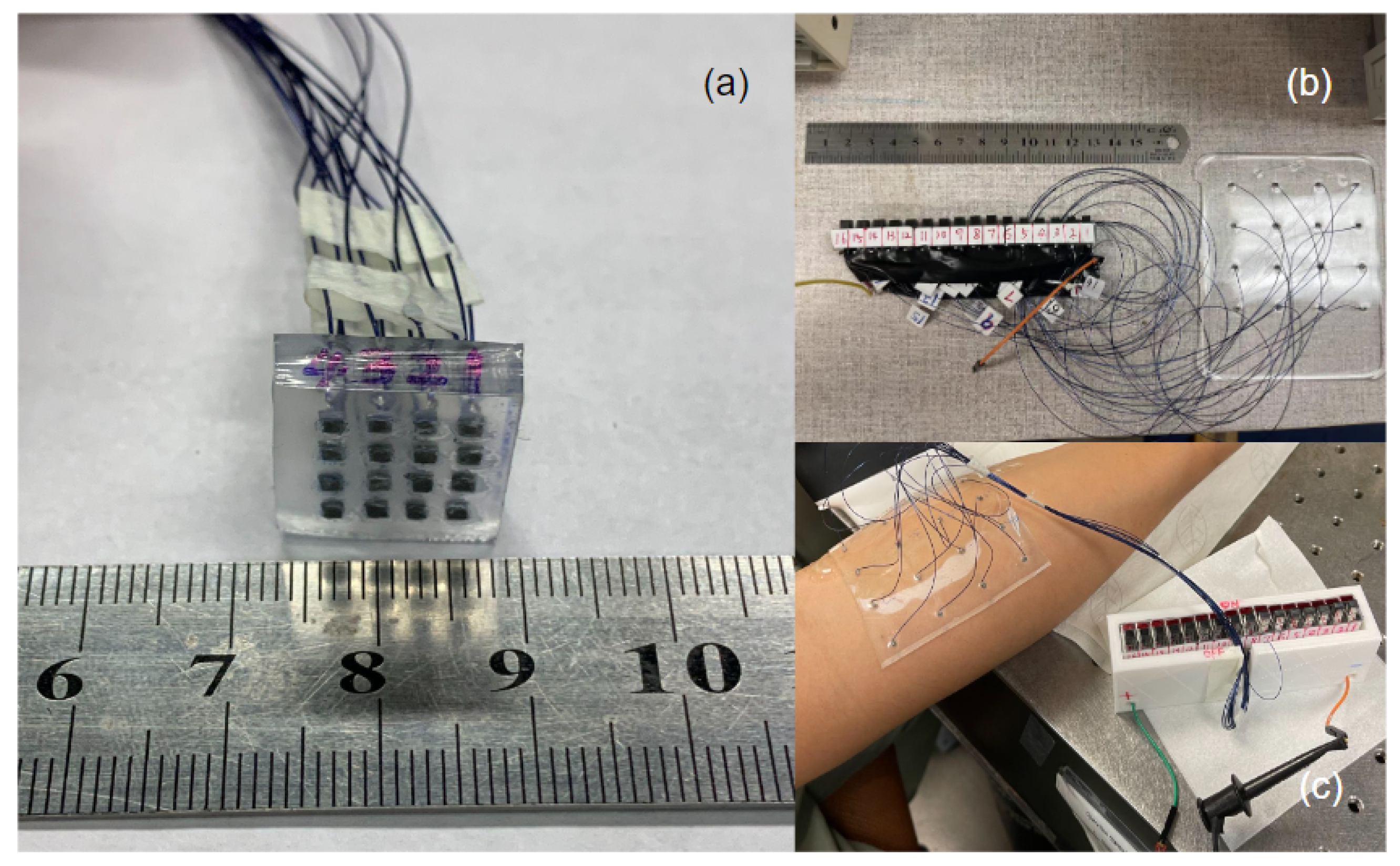
2.1. Transducer Design and Fabrication
2.2. Transducer Characterizations
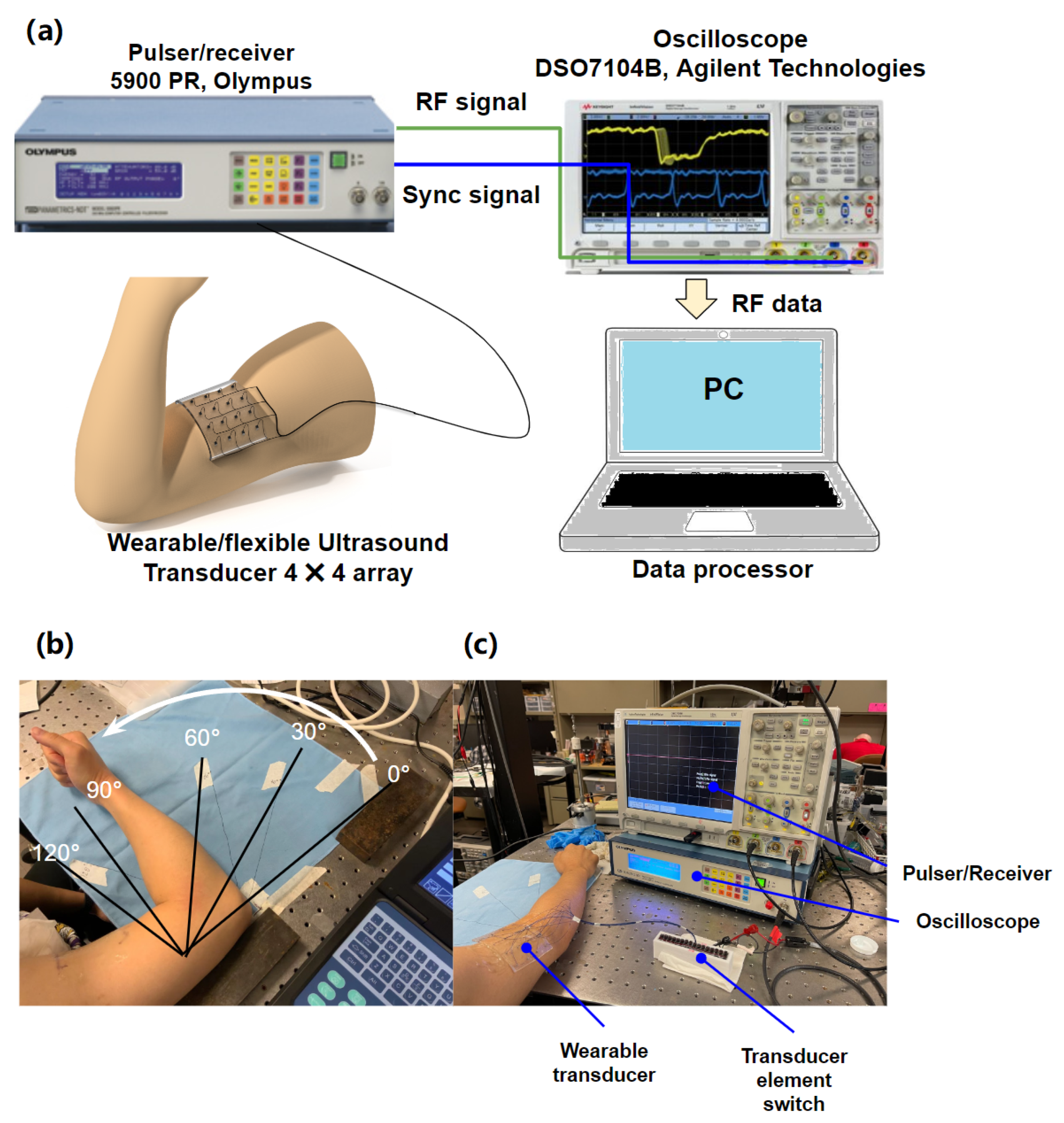
2.3. In Vitro Experimental Setup
2.4. Preliminary In Vivo Experimental Setup
2.5. RF Data Processing Procedure
3. Results
3.1. Transducer Characterizations
3.2. In Vitro Results
3.3. Preliminary In Vivo Results
4. Discussion and Future Works
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.P. Assistive robotics: An overview. In Assistive Technology and Artificial Intelligence; Springer: Berlin/Heidelberg, Germany, 1998; pp. 126–136. [Google Scholar]
- Össur PowerKnee. 2022. Available online: https://www.ossur.com/en-us/prosthetics/knees/power-knee (accessed on 1 January 2023).
- Esposito, D.; Andreozzi, E.; Fratini, A.; Gargiulo, G.D.; Savino, S.; Niola, V.; Bifulco, P. A piezoresistive sensor to measure muscle contraction and mechanomyography. Sensors 2018, 18, 2553. [Google Scholar] [CrossRef]
- Jayaraman, C.; Hoppe-Ludwig, S.; Deems-Dluhy, S.; McGuire, M.; Mummidisetty, C.; Siegal, R.; Naef, A.; Lawson, B.E.; Goldfarb, M.; Gordon, K.E.; et al. Impact of powered knee-ankle prosthesis on low back muscle mechanics in transfemoral amputees: A case series. Front. Neurosci. 2018, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Akhtar, A.; Liu, Y.; Chen, H.; Yeo, W.H.; Park, S.I.; Boyce, B.; Kim, H.; Yu, J.; Lai, H.Y.; et al. An epidermal stimulation and sensing platform for sensorimotor prosthetic control, management of lower back exertion, and electrical muscle activation. Adv. Mater. 2016, 28, 4462–4471. [Google Scholar] [CrossRef] [PubMed]
- Lobo-Prat, J.; Kooren, P.N.; Stienen, A.H.; Herder, J.L.; Koopman, B.F.; Veltink, P.H. Non-invasive control interfaces for intention detection in active movement-assistive devices. J. Neuroeng. Rehabil. 2014, 11, 168. [Google Scholar] [CrossRef]
- Vesa, E.; Ilie, B. Equipment for SEMG signals acquisition and processing. In Proceedings of the International Conference on Advancements of Medicine and Health Care through Technology, Cluj-Napoca, Romania, 5–7 June 2014; pp. 187–192. [Google Scholar]
- Bi, L.; Feleke, A.G.; Guan, C. A review on EMG-based motor intention prediction of continuous human upper limb motion for human-robot collaboration. Biomed. Signal Process. Control 2019, 51, 113–127. [Google Scholar] [CrossRef]
- Li, K.; Zhang, J.; Wang, L.; Zhang, M.; Li, J.; Bao, S. A review of the key technologies for sEMG-based human-robot interaction systems. Biomed. Signal Process. Control 2020, 62, 102074. [Google Scholar] [CrossRef]
- Talib, I.; Sundaraj, K.; Lam, C.K.; Hussain, J.; Ali, M. A review on crosstalk in myographic signals. Eur. J. Appl. Physiol. 2019, 119, 9–28. [Google Scholar] [CrossRef]
- Waasdorp, R.; Mugge, W.; Vos, H.J.; De Groot, J.H.; Verweij, M.D.; De Jong, N.; Schouten, A.C.; Daeichin, V. Combining ultrafast ultrasound and high-density EMG to assess local electromechanical muscle dynamics: A feasibility study. IEEE Access 2021, 9, 45277–45288. [Google Scholar] [CrossRef]
- Becerra-Fajardo, L.; Ivorra, A. First steps towards an implantable electromyography (EMG) sensor powered and controlled by galvanic coupling. In World Congress on Medical Physics and Biomedical Engineering 2018; Springer: Singapore, 2019; pp. 19–22. [Google Scholar]
- Zealear, D.; Li, Y.; Huang, S. An implantable system for chronic in vivo electromyography. JoVE (J. Vis. Exp.) 2020, 158, e60345. [Google Scholar] [CrossRef]
- Alexander, J.H.; Jordan, S.W.; West, J.M.; Compston, A.; Fugitt, J.; Bowen, J.B.; Dumanian, G.A.; Pollock, R.; Mayerson, J.L.; Scharschmidt, T.J.; et al. Targeted muscle reinnervation in oncologic amputees: Early experience of a novel institutional protocol. J. Surg. Oncol. 2019, 120, 348–358. [Google Scholar] [CrossRef] [PubMed]
- Frantz, T.L.; Everhart, J.S.; West, J.M.; Ly, T.V.; Phieffer, L.S.; Valerio, I.L. Targeted muscle reinnervation at the time of major limb amputation in traumatic amputees: Early experience of an effective treatment strategy to improve pain. JBJS Open Access 2020, 5, e0067. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.W.; Shih, C.C.; Huang, C.C. Freehand three-dimensional ultrasound imaging of carotid artery using motion tracking technology. Ultrasonics 2017, 74, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chen, P.Y.; Huang, H.; Huang, C.C. In vivo visualization of vasculature in adult zebrafish by using high-frequency ultrafast ultrasound imaging. IEEE Trans. Biomed. Eng. 2018, 66, 1742–1751. [Google Scholar] [CrossRef]
- Tsui, P.H.; Wang, S.H.; Huang, C.C.; Chiu, C.Y. Quantitative analysis of noise influence on the detection of scatterer concentration by Nakagami parameter. J. Med Biol. Eng. 2005, 25, 45–51. [Google Scholar]
- Huang, C.C.; Tsui, P.H.; Wang, S.H. Detection of coagulating blood under steady flow by statistical analysis of backscattered signals. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2007, 54, 435–442. [Google Scholar] [CrossRef]
- Huang, C.C.; Su, T.H.; Shih, C.C. High-resolution tissue Doppler imaging of the zebrafish heart during its regeneration. Zebrafish 2015, 12, 48–57. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, P.Y.; Peng, P.H.; Lee, P.Y. 40 MHz high-frequency ultrafast ultrasound imaging. Med. Phys. 2017, 44, 2185–2195. [Google Scholar] [CrossRef]
- Huang, C.C.; Chen, R.; Tsui, P.H.; Zhou, Q.; Humayun, M.S.; Shung, K.K. Measurements of attenuation coefficient for evaluating the hardness of a cataract lens by a high-frequency ultrasonic needle transducer. Phys. Med. Biol. 2009, 54, 5981. [Google Scholar] [CrossRef]
- Ortenzi, V.; Tarantino, S.; Castellini, C.; Cipriani, C. Ultrasound imaging for hand prosthesis control: A comparative study of features and classification methods. In Proceedings of the 2015 IEEE International Conference on Rehabilitation Robotics (ICORR), Singapore, 11–14 August 2015; pp. 1–6. [Google Scholar]
- Akhlaghi, N.; Baker, C.A.; Lahlou, M.; Zafar, H.; Murthy, K.G.; Rangwala, H.S.; Kosecka, J.; Joiner, W.M.; Pancrazio, J.J.; Sikdar, S. Real-time classification of hand motions using ultrasound imaging of forearm muscles. IEEE Trans. Biomed. Eng. 2015, 63, 1687–1698. [Google Scholar] [CrossRef]
- Sheng, Z.; Sharma, N.; Kim, K. Quantitative assessment of changes in muscle contractility due to fatigue during nmes: An ultrasound imaging approach. IEEE Trans. Biomed. Eng. 2019, 67, 832–841. [Google Scholar] [CrossRef]
- Zhang, Q.; Iyer, A.; Lambeth, K.; Kim, K.; Sharma, N. Ultrasound Echogenicity as an Indicator of Muscle Fatigue during Functional Electrical Stimulation. Sensors 2022, 22, 335. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Kim, K.; Sharma, N. Prediction of ankle dorsiflexion moment by combined ultrasound sonography and electromyography. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 28, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Iyer, A.; Kim, K.; Sharma, N. Evaluation of non-invasive ankle joint effort prediction methods for use in neurorehabilitation using electromyography and ultrasound imaging. IEEE Trans. Biomed. Eng. 2020, 68, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.Y.; Yang, T.H.; Huang, H.; Hsu, H.Y.; Kuo, L.C.; Su, F.C.; Huang, C.C. Evaluation of hand tendon movement by using high-frequency ultrasound vector Doppler imaging. IEEE Trans. Biomed. Eng. 2020, 67, 2945–2952. [Google Scholar] [CrossRef] [PubMed]
- La, T.G.; Le, L.H. Flexible and wearable ultrasound device for medical applications: A review on materials, structural designs, and current challenges. Adv. Mater. Technol. 2021, 7, 2100798. [Google Scholar] [CrossRef]
- Hamelmann, P.; Mischi, M.; Kolen, A.F.; Van Laar, J.O.; Vullings, R.; Bergmans, J.W. Fetal heart rate monitoring implemented by dynamic adaptation of transmission power of a flexible ultrasound transducer array. Sensors 2019, 19, 1195. [Google Scholar] [CrossRef]
- Wang, C.; Li, X.; Hu, H.; Zhang, L.; Huang, Z.; Lin, M.; Zhang, Z.; Yin, Z.; Huang, B.; Gong, H.; et al. Monitoring of the central blood pressure waveform via a conformal ultrasonic device. Nat. Biomed. Eng. 2018, 2, 687–695. [Google Scholar] [CrossRef]
- Peng, C.; Chen, M.; Sim, H.K.; Zhu, Y.; Jiang, X. Noninvasive and nonocclusive blood pressure monitoring via a flexible piezo-composite ultrasonic sensor. IEEE Sens. J. 2020, 21, 2642–2650. [Google Scholar] [CrossRef]
- Cueto, C.; Cudeiro, J.; Agudo, O.C.; Guasch, L.; Tang, M.X. Spatial response identification for flexible and accurate ultrasound transducer calibration and its application to brain imaging. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2020, 68, 143–153. [Google Scholar] [CrossRef]
- Zhuang, X.; Lin, D.S.; Oralkan, Ö.; Khuri-Yakub, B.T. Fabrication of flexible transducer arrays with through-wafer electrical interconnects based on trench refilling with PDMS. J. Microelectromech. Syst. 2008, 17, 446–452. [Google Scholar] [CrossRef]
- Pashaei, V.; Dehghanzadeh, P.; Enwia, G.; Bayat, M.; Majerus, S.J.; Mandal, S. Flexible body-conformal ultrasound patches for image-guided neuromodulation. IEEE Trans. Biomed. Circuits Syst. 2019, 14, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Cho, I.J.; Ko, K.; Yoon, E.S.; Park, H.H.; Kim, T.S. Flexible piezoelectric micromachined ultrasonic transducer (pMUT) for application in brain stimulation. Microsyst. Technol. 2017, 23, 2321–2328. [Google Scholar] [CrossRef]
- Xu, L.; Wang, P.; Xia, P.; Wu, P.; Chen, X.; Du, L.; Liu, J.; Xue, N.; Fang, Z. A Flexible Ultrasound Array for Local Pulse Wave Velocity Monitoring. Biosensors 2022, 12, 479. [Google Scholar] [CrossRef]
- Huang, C.C.; Lee, P.Y.; Chen, P.Y.; Liu, T.Y. Design and implementation of a smartphone-based portable ultrasound pulsed-wave Doppler device for blood flow measurement. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2012, 59, 182–188. [Google Scholar] [CrossRef]
- Jeong, J.W.; Yeo, W.H.; Akhtar, A.; Norton, J.J.; Kwack, Y.J.; Li, S.; Jung, S.Y.; Su, Y.; Lee, W.; Xia, J.; et al. Materials and optimized designs for human-machine interfaces via epidermal electronics. Adv. Mater. 2013, 25, 6839–6846. [Google Scholar] [CrossRef]
- Jang, K.I.; Han, S.Y.; Xu, S.; Mathewson, K.E.; Zhang, Y.; Jeong, J.W.; Kim, G.T.; Webb, R.C.; Lee, J.W.; Dawidczyk, T.J.; et al. Rugged and breathable forms of stretchable electronics with adherent composite substrates for transcutaneous monitoring. Nat. Commun. 2014, 5, 4779. [Google Scholar] [CrossRef]
- Wang, Z.; Xue, Q.T.; Chen, Y.Q.; Shu, Y.; Tian, H.; Yang, Y.; Xie, D.; Luo, J.W.; Ren, T.L. A flexible ultrasound transducer array with micro-machined bulk PZT. Sensors 2015, 15, 2538–2547. [Google Scholar] [CrossRef]
- Yang, Y.; Tian, H.; Yan, B.; Sun, H.; Wu, C.; Shu, Y.; Wang, L.G.; Ren, T.L. A flexible piezoelectric micromachined ultrasound transducer. Rsc Adv. 2013, 3, 24900–24905. [Google Scholar] [CrossRef]
- Kim, T.; Cui, Z.; Chang, W.Y.; Kim, H.; Zhu, Y.; Jiang, X. Flexible 1–3 composite ultrasound transducers with Silver-Nanowire-Based stretchable electrodes. IEEE Trans. Ind. Electron. 2019, 67, 6955–6962. [Google Scholar] [CrossRef]
- Kinsler, L.E.; Frey, A.R.; Coppens, A.B.; Sanders, J.V. Fundamentals of Acoustics; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Panda, P.; Sahoo, B. PZT to lead free piezo ceramics: A review. Ferroelectrics 2015, 474, 128–143. [Google Scholar] [CrossRef]
- Sherman, C.H.; Butler, J.L. Transducers and Arrays for Underwater Sound; Springer: New York, NY, USA, 2007; Volume 4. [Google Scholar]
- Seghir, R.; Arscott, S. Extended PDMS stiffness range for flexible systems. Sens. Actuators A Phys. 2015, 230, 33–39. [Google Scholar] [CrossRef]
- Lu, S.; Cai, W.; Cao, N.; Qian, H.j.; Lu, Z.y.; Cui, S. Understanding the Extraordinary Flexibility of Polydimethylsiloxane through Single-Molecule Mechanics. ACS Mater. Lett. 2022, 4, 329–335. [Google Scholar] [CrossRef]
- Barthwal, S.; Lim, S.H. Robust and chemically stable superhydrophobic aluminum-alloy surface with enhanced corrosion-resistance properties. Int. J. Precis. Eng. Manuf.-Green Technol. 2020, 7, 481–492. [Google Scholar] [CrossRef]
- Mol, C.R.; Breddels, P.A. Ultrasound velocity in muscle. J. Acoust. Soc. Am. 1982, 71, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Viola, F.; Walker, W.F. A comparison of the performance of time-delay estimators in medical ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2003, 50, 392–401. [Google Scholar] [CrossRef]
- Wu, H.; Goel, L.D.; Kim, H.; Zhang, B.; Kim, J.; Dayton, P.A.; Xu, Z.; Jiang, X. Dual-Frequency Intravascular Sonothrombolysis: An In Vitro Study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2021, 68, 3599–3607. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, B.; Huang, C.C.; Peng, C.; Zhou, Q.; Jiang, X. Ultrasound-guided Intravascular Sonothrombolysis with a Dual Mode Ultrasound Catheter: In-vitro study. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2022, 69, 1917–1925. [Google Scholar] [CrossRef]
- Daft, C.M. Conformable transducers for large-volume, operator-independent imaging. In Proceedings of the 2010 IEEE International Ultrasonics Symposium, San Diego, CA, USA, 11–14 October 2010; pp. 798–808. [Google Scholar]
- de Oliveira, T.F.; Pai, C.N.; Matuda, M.Y.; Adamowski, J.C.; Buiochi, F. Development of a 2.25 MHz flexible array ultrasonic transducer. Res. Biomed. Eng. 2019, 35, 27–37. [Google Scholar] [CrossRef]
- Lin, M.; Hu, H.; Zhou, S.; Xu, S. Soft wearable devices for deep-tissue sensing. Nat. Rev. Mater. 2022, 7, 850–869. [Google Scholar] [CrossRef]
- Charaya, H.; La, T.G.; Rieger, J.; Chung, H.J. Thermochromic and piezocapacitive flexible sensor array by combining composite elastomer dielectrics and transparent ionic hydrogel electrodes. Adv. Mater. Technol. 2019, 4, 1900327. [Google Scholar] [CrossRef]
- Castellini, C.; Passig, G.; Zarka, E. Using ultrasound images of the forearm to predict finger positions. IEEE Trans. Neural Syst. Rehabil. Eng. 2012, 20, 788–797. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Chen, X.; Wang, L.; Makihata, M.; Liu, H.C.; Zhou, T.; Zhao, X. Bioadhesive ultrasound for long-term continuous imaging of diverse organs. Science 2022, 377, 517–523. [Google Scholar] [CrossRef] [PubMed]





| Property | Pulse-Echo Response Test | Electrical Impedance Test | |||||
|---|---|---|---|---|---|---|---|
| Central Frequency (MHz) | Fractional Bandwidth (−6 dB) % | Loop Sensitivity (dB) | Capacitance (@ 1 kHz, pF) | Loss (@ 1 kHz, mU) | Impedance in Air () | Impedance in Water () | |
| Element #1 | 10.04 | 37.05 | −39.28 | 199.00 | 9.80 | 79.06 | 78.30 |
| Element #2 | 10.71 | 30.44 | −49.82 | 186.50 | 9.90 | 80.53 | 81.35 |
| Element #3 | 10.69 | 21.89 | −39.05 | 199.80 | 9.77 | 76.09 | 72.50 |
| Element #4 | 9.97 | 59.18 | −50.27 | 189.70 | 9.37 | 79.64 | 80.83 |
| Element #5 | 10.61 | 32.61 | −38.87 | 194.68 | 10.55 | 79.17 | 79.04 |
| Element #6 | 10.82 | 29.57 | −37.42 | 196.27 | 9.70 | 75.07 | 76.23 |
| Element #7 | 10.37 | 45.71 | −45.96 | 193.89 | 10.90 | 81.04 | 86.28 |
| Element #8 | 10.85 | 32.26 | −40.74 | 191.54 | 9.70 | 76.50 | 75.46 |
| Element #9 | 10.85 | 36.31 | −35.09 | 199.46 | 10.00 | 76.19 | 73.00 |
| Element #10 | 10.61 | 48.82 | −38.02 | 176.98 | 9.37 | 77.94 | 75.27 |
| Element #11 | 10.18 | 40.47 | −37.27 | 194.72 | 10.64 | 74.85 | 75.70 |
| Element #12 | 10.97 | 30.81 | −40.47 | 199.50 | 10.20 | 76.92 | 77.75 |
| Element #13 | 10.83 | 27.52 | −35.83 | 191.45 | 10.10 | 79.60 | 80.00 |
| Element #14 | 10.81 | 32.75 | −39.42 | 184.60 | 9.90 | 85.88 | 80.43 |
| Element #15 | 10.93 | 33.12 | −37.18 | 184.80 | 10.30 | 85.69 | 79.65 |
| Element #16 | 10.16 | 64.57 | −39.42 | 180.10 | 9.10 | 83.36 | 82.81 |
| Average | 10.59 | 37.69 | −40.26 | 191.44 | 9.96 | 79.22 | 78.41 |
| Standard Deviation | 0.33 | 11.55 | 4.53 | 7.13 | 0.48 | 3.45 | 3.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, X.; Zhang, B.; Moon, S.; Xu, G.-X.; Huang, C.-C.; Sharma, N.; Jiang, X. Development of a Wearable Ultrasound Transducer for Sensing Muscle Activities in Assistive Robotics Applications. Biosensors 2023, 13, 134. https://doi.org/10.3390/bios13010134
Xue X, Zhang B, Moon S, Xu G-X, Huang C-C, Sharma N, Jiang X. Development of a Wearable Ultrasound Transducer for Sensing Muscle Activities in Assistive Robotics Applications. Biosensors. 2023; 13(1):134. https://doi.org/10.3390/bios13010134
Chicago/Turabian StyleXue, Xiangming, Bohua Zhang, Sunho Moon, Guo-Xuan Xu, Chih-Chung Huang, Nitin Sharma, and Xiaoning Jiang. 2023. "Development of a Wearable Ultrasound Transducer for Sensing Muscle Activities in Assistive Robotics Applications" Biosensors 13, no. 1: 134. https://doi.org/10.3390/bios13010134
APA StyleXue, X., Zhang, B., Moon, S., Xu, G.-X., Huang, C.-C., Sharma, N., & Jiang, X. (2023). Development of a Wearable Ultrasound Transducer for Sensing Muscle Activities in Assistive Robotics Applications. Biosensors, 13(1), 134. https://doi.org/10.3390/bios13010134








