AIEgen-Peptide Bioprobes for the Imaging of Organelles
Abstract
:1. Introduction
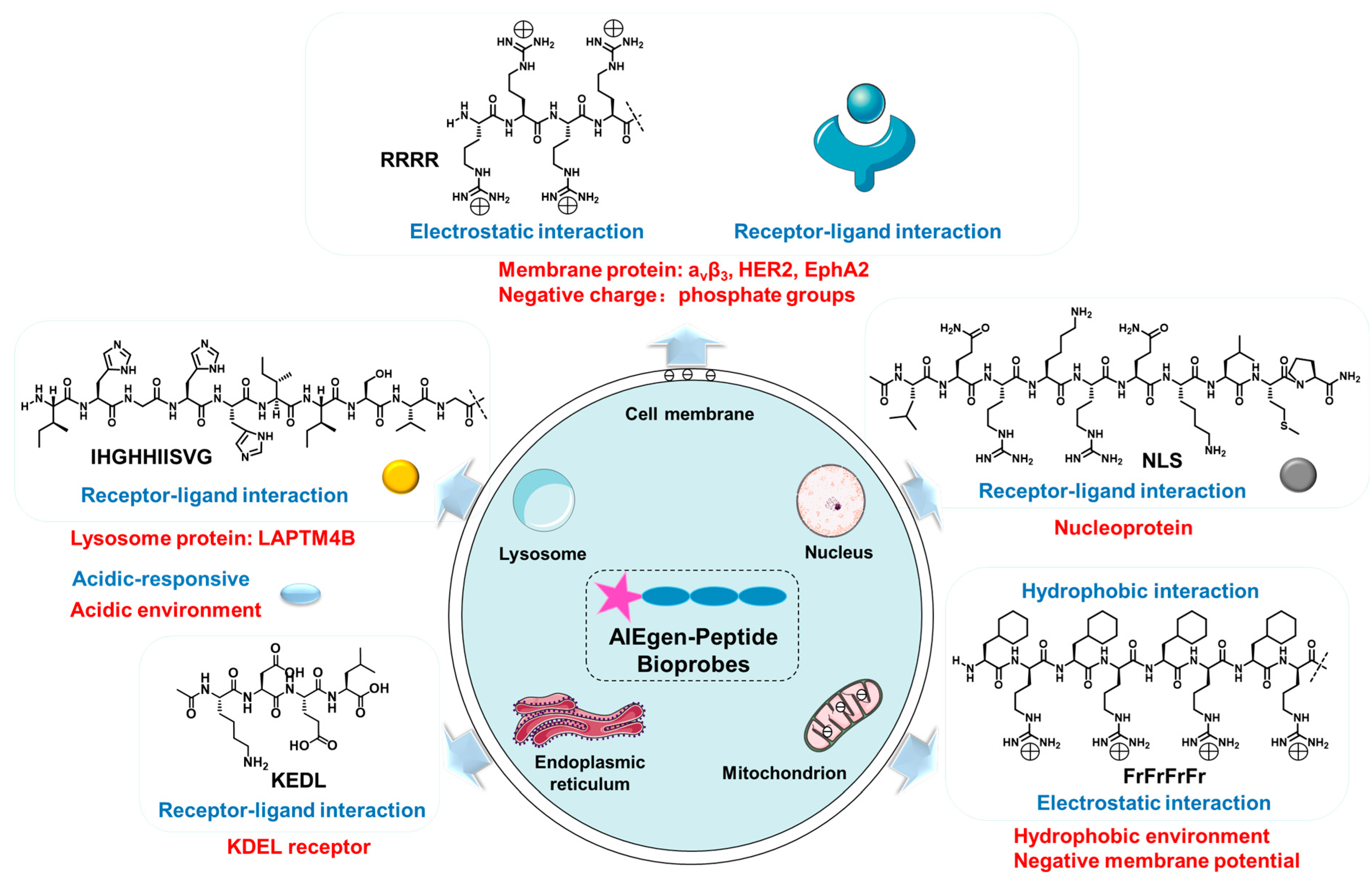
2. Design Strategies and Recent Examples
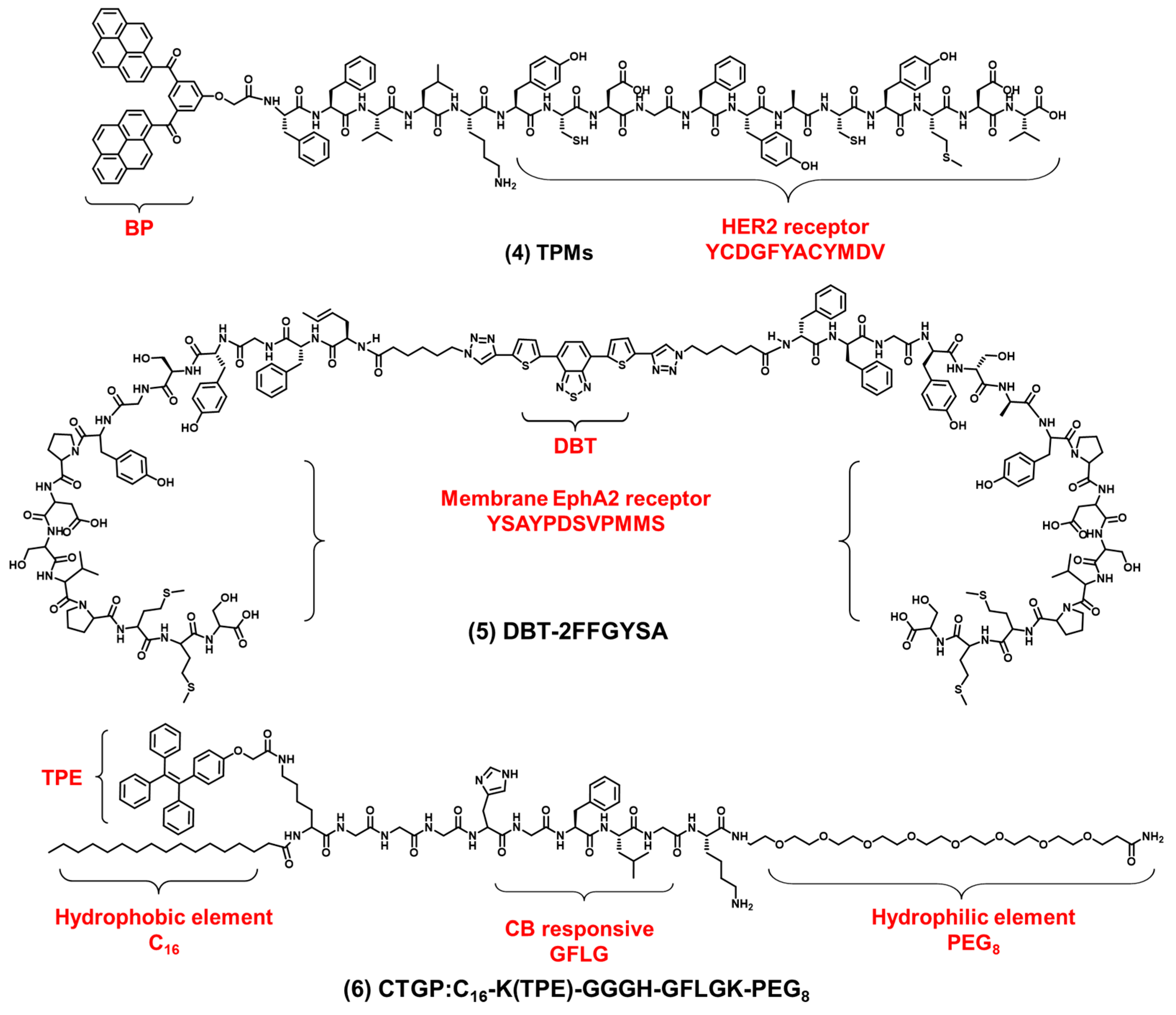
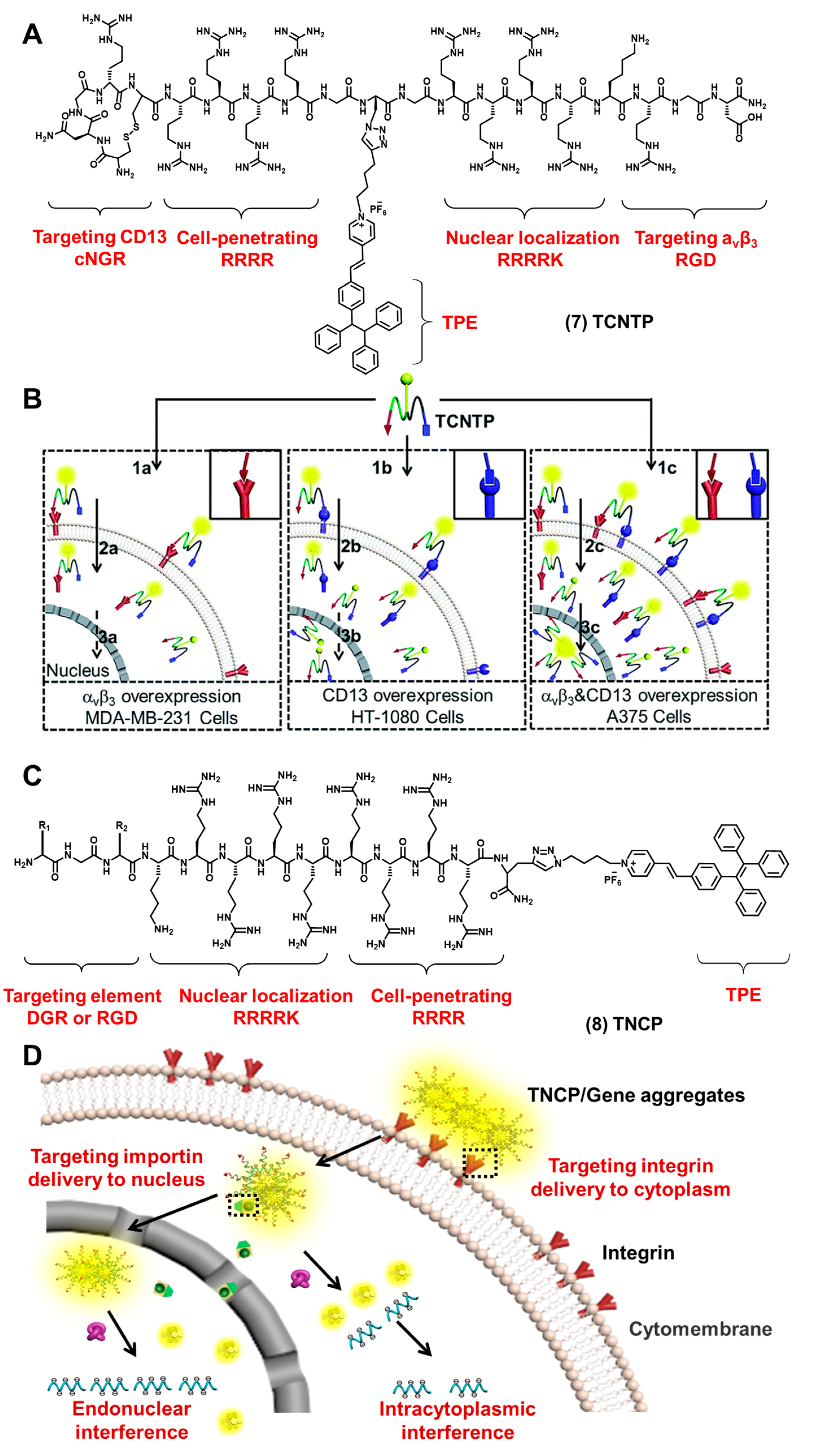
2.1. Cell Membrane-Targeted Bioprobes
2.2. Nucleus-Targeted Bioprobes
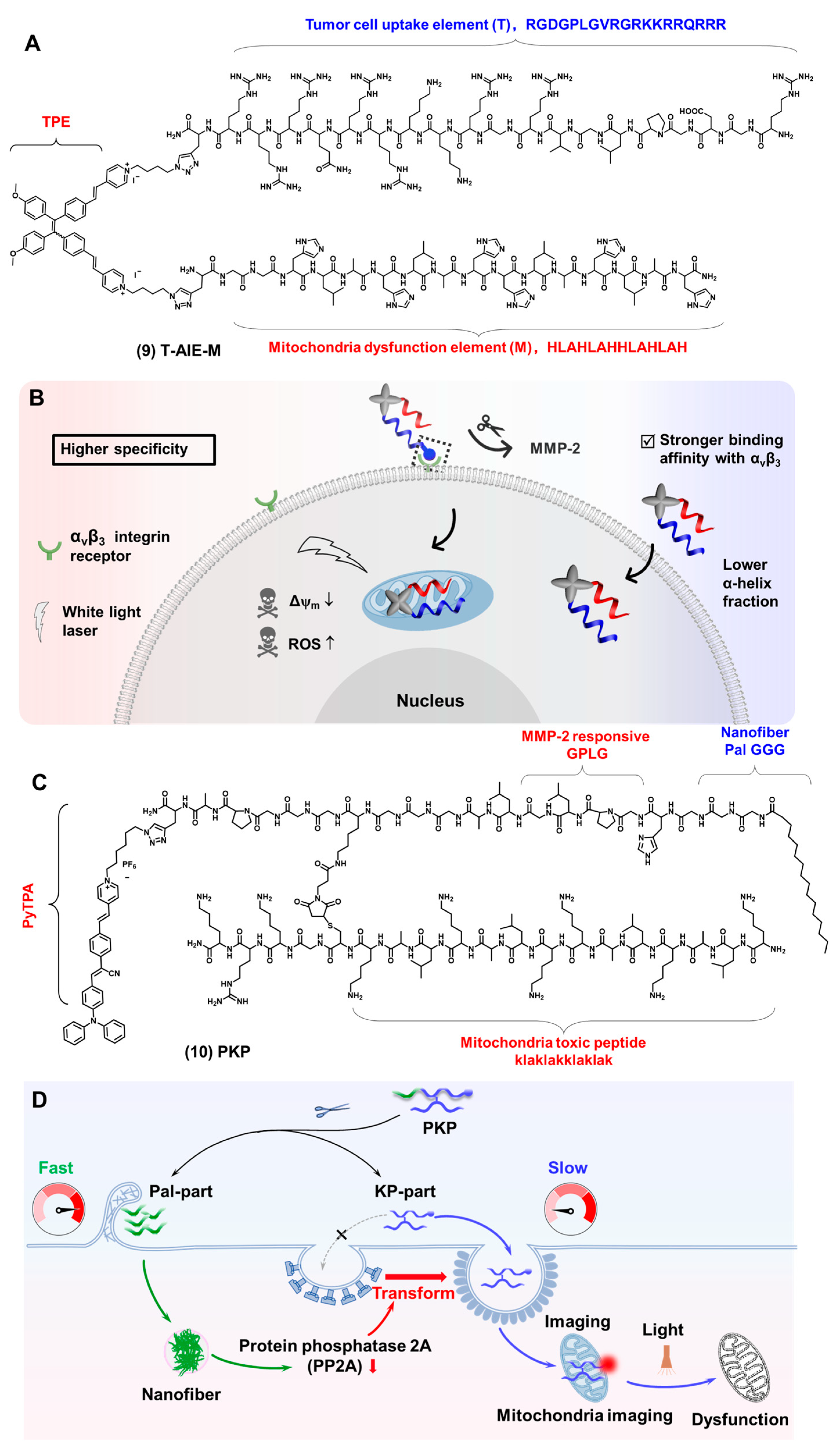
2.3. Mitochondria-Targeted Bioprobes
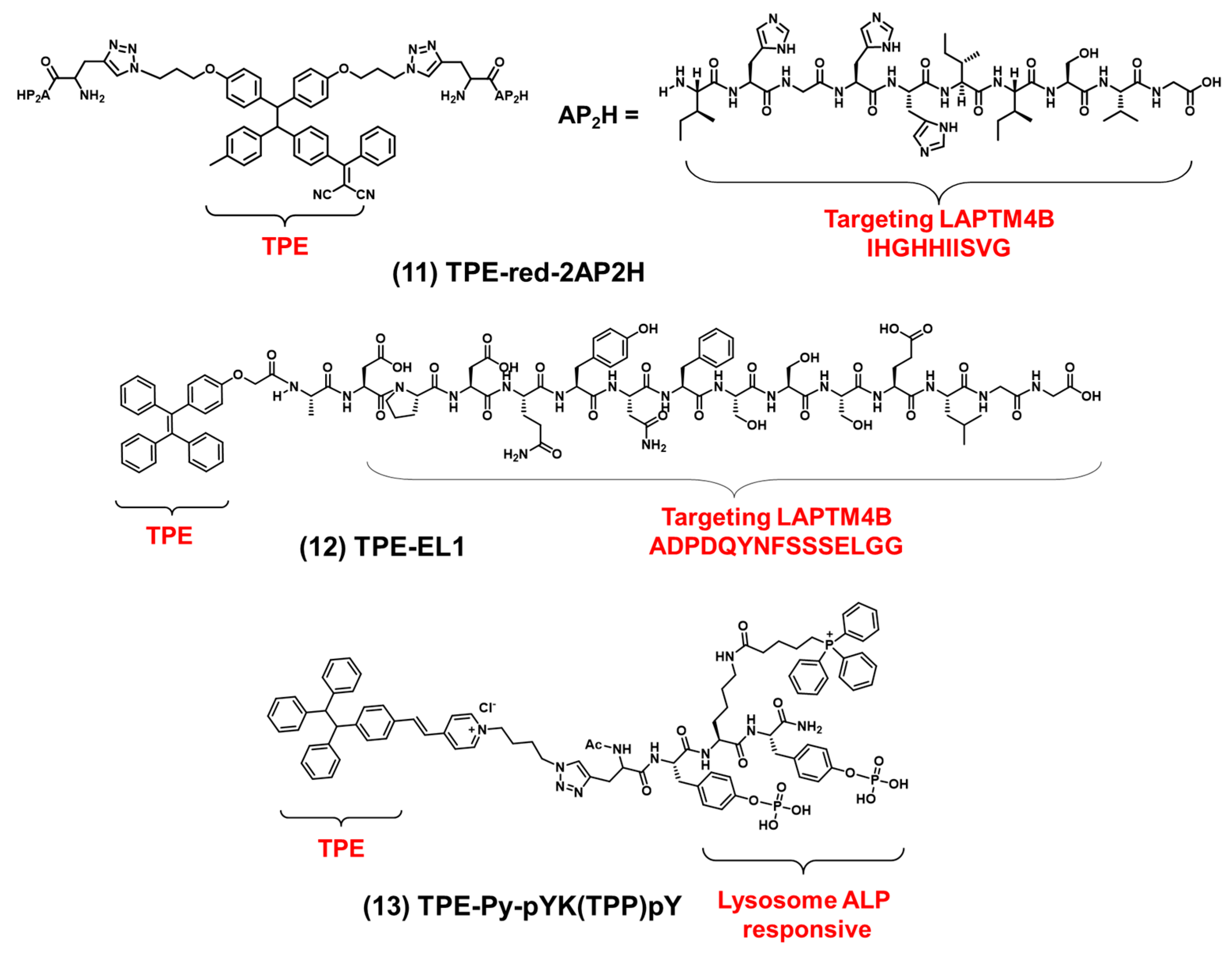
2.4. Lysosome-Targeted Bioprobes
2.5. Endoplasmic Reticulum-Targeted Bioprobes
3. Conclusions and Future Prospects
| Target | Peptide | AIEgen | Responsive Site | λex/λem (nm) | Cytotoxicity | References |
|---|---|---|---|---|---|---|
| Cell membrane | cRGD | TPS | αvβ3 | 356/480 | no cytotoxicity | [53] |
| RRRR | TPE | 330/466 | no cytotoxicity | [54] | ||
| RGD-Pal-RRRR | T-MY | 330/500 | no cytotoxicity | [55] | ||
| C16-K(TPE)-GGGH-GFLGK-PEG8 | TPE | 370/470 | IC50 = 0.1 mg/mL | [56] | ||
| YCDGFYACYMDV | BP | HER2 | 380/520 | no cytotoxicity | [57] | |
| YSAYPDSVPMMS | DBT | EphA2 | 490/642 | IC50 = 38.3 × 10−6 M | [58] | |
| Nucleus | NLS | PyTPE | 405/570 | no cytotoxicity | [65] | |
| NLS | PyTPE | 405/580 | no cytotoxicity | [66] | ||
| Mitochondria | HLAHLAHHLAHLAH | TPE | 420/720 | no cytotoxicity | [76] | |
| klaklakklaklak | PyTPA | 450/620 | High cytotoxicity | [77] | ||
| Lysosome | GFLG | PyTPA | CB | 450/650 | High cytotoxicity | [79] |
| IHGHHIISVG | TPE | LAPTM4B | 445/620 | EC50 = 3.1 μM | [80] | |
| ADPDQYNFSSSELGG | TPE | LAPTM4B | 330/470 | no cytotoxicity | [81] | |
| pYK(TPP)pY | TPE | ALP | 400/595 | IC50 = 9.7 μM | [82] | |
| Endoplasmic reticulum | KDEL | TPE | 430/620 | no cytotoxicity | [86] | |
| TPA | 480/653 | no cytotoxicity | [87] |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, M.; Zhan, J.; Lin, W. Single fluorescent probes enabling simultaneous visualization of duple organelles: Design principles, mechanisms, and applications. Coord. Chem. Rev. 2022, 451, 214–266. [Google Scholar] [CrossRef]
- Saminathan, A.; Zajac, M.; Anees, P.; Krishnan, Y. Organelle-level precision with next-generation targeting technologies. Nat. Rev. Mater. 2021, 7, 355–371. [Google Scholar] [CrossRef]
- Yu, H.; Guo, Y.; Zhu, W.; Havener, K.; Zheng, X. Recent advances in 1,8-naphthalimide-based small-molecule fluorescent probes for organelles imaging and tracking in living cells. Coord. Chem. Rev. 2021, 444, 214019. [Google Scholar] [CrossRef]
- Friedman, J.R.; Nunnari, J. Mitochondrial form and function. Nature 2014, 505, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Cho, H.; Cho, Y.Y.; Shim, M.S.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondria-targeted drug delivery in cancers. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165808. [Google Scholar] [CrossRef]
- Jeena, M.T.; Kim, S.; Jin, S.; Ryu, J.H. Recent progress in mitochondria-targeted drug and drug-free agents for cancer therapy. Cancers 2019, 12, 4. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, P.C.; Bartlett, J.J.; Pulinilkunnil, T. Lysosomal biology and function: Modern view of cellular debris bin. Cells 2020, 9, 1131. [Google Scholar] [CrossRef]
- Perera, R.M.; Zoncu, R. Lysosome as a regulatory hub. Annu. Rev. Cell Dev. Biol. 2016, 32, 223–253. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, D.S.; Blower, M.D. Endoplasmic reticulum: Structure, function and response to cellular signaling. Cell. Mol. Life Sci. 2016, 73, 79–94. [Google Scholar] [CrossRef] [Green Version]
- King, A.P.; Wilson, J.J. Endoplasmic reticulum stress: An arising target for metal-based anticancer agents. Chem. Soc. Rev. 2020, 49, 8113–8136. [Google Scholar] [CrossRef]
- Liu, J.; Zhai, Z.; Niu, H.; Zhang, Y.; Song, X.; Zhang, P.; Ye, Y. Endoplasmic reticulum-targetable fluorescent probe for visualizing HClO in EC1 cells. Tetrahedron Lett. 2020, 61, 152301. [Google Scholar] [CrossRef]
- Klinge, S.; Woolford, J.L., Jr. Ribosome assembly coming into focus. Nat. Rev. Mol. Cell Biol. 2019, 20, 116–131. [Google Scholar] [CrossRef] [PubMed]
- Qiu, K.; Chen, Y.; Rees, T.W.; Ji, L.; Chao, H. Organelle-targeting metal complexes: From molecular design to bio-applications. Coord. Chem. Rev. 2019, 378, 66–86. [Google Scholar] [CrossRef]
- Huang, S.; Wang, Y. Golgi structure formation, function, and post-translational modifications in mammalian cells. F1000Research 2017, 6, 2050. [Google Scholar] [CrossRef] [PubMed]
- Garrido, C.; Galluzzi, L.; Brunet, M.; Puig, P.E.; Didelot, C.; Kroemer, G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006, 13, 1423–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sathasivam, S.; Ince, P.G.; Shaw, P.J. Apoptosis in amyotrophic lateral sclerosis: A review of the evidence. Neuropathology 2010, 27, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 90, 487–498. [Google Scholar] [CrossRef]
- Tian, M.; Ma, Y.; Lin, W. Fluorescent probes for the visualization of cell viability. Acc. Chem. Res. 2019, 52, 2147–2157. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.D.; Min, X.; Lou, X.D.; Xia, F. One-dimensional and two-dimensional nanomaterials for the detection of multiple biomolecules. Chin. Chem. Lett. 2019, 30, 1557–1564. [Google Scholar]
- Gao, P.; Pan, W.; Li, N.; Tang, B.Z. Fluorescent probes for organelle-targeted bioactive species imaging. Chem. Sci. 2019, 10, 6035–6071. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Leung, N.L.; Kwok, R.T.; Lam, J.W.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, L.H.; Kwok, R.T.K.; He, X.; Lam, J.W.Y.; Tang, B.Z. AIE bioconjugates for biomedical applications. Adv. Opt. Mater. 2020, 8, 2000162. [Google Scholar] [CrossRef]
- Xu, W.; Lee, M.M.S.; Zhang, Z.; Sung, H.H.Y.; Williams, I.D.; Kwok, R.T.K.; Lam, J.W.Y.; Wang, D.; Tang, B.Z. Facile synthesis of AIEgens with wide color tunability for cellular imaging and therapy. Chem. Sci. 2019, 10, 3494–3501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, J.; Wu, X.; Ding, S.; Lou, X.; Xia, F.; Wang, S.; Hong, Y. Aggregation-induced emission photosensitizers: From molecular design to photodynamic therapy. J. Med. Chem. 2020, 63, 1996–2012. [Google Scholar] [CrossRef]
- Cai, X.; Liu, B. Aggregation-induced emission: Recent advances in materials and biomedical applications. Angew. Chem. Int. Ed. 2020, 59, 9868–9886. [Google Scholar] [CrossRef]
- Xu, S.; Duan, Y.; Liu, B. Precise molecular design for high-performance luminogens with aggregation-induced emission. Adv. Mater. 2020, 32, e1903530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, J.; Huang, Y.; Tian, H. Progress and trends in AIE-based bioprobes: A brief overview. ACS Appl. Mater. Interfaces 2018, 10, 12217–12261. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tang, R.; Liu, X.; Gong, J.; Zhao, Z.; Sheng, Z.; Zhang, J.; Li, X.; Niu, G.; Kwok, R.T.K.; et al. Bright aggregation-induced emission nanoparticles for two-photon imaging and localized compound therapy of cancers. ACS Nano 2020, 14, 16840–16853. [Google Scholar] [CrossRef]
- Wu, W.; Yang, Y.Q.; Yang, Y.; Yang, Y.M.; Wang, H.; Zhang, K.Y.; Guo, L.; Ge, H.F.; Liu, J.; Feng, H. An organic NIR-II nanofluorophore with aggregation-induced emission characteristics for in vivo fluorescence imaging. Int. J. Nanomed. 2019, 14, 3571–3582. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, Y.; Wang, J.; Liang, X.J. Aggregation-induced emission (AIE) fluorophores as imaging tools to trace the biological fate of nano-based drug delivery systems. Adv. Drug Deliv. Rev. 2019, 143, 161–176. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, B.; Yuan, Y. AIEgen based drug delivery systems for cancer therapy. J. Control. Release 2018, 290, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yao, Q.; Xu, F.; Li, Y.; Kim, D.; Chung, J.; Baek, G.; Wu, X.; Hillman, P.F.; Lee, E.Y.; et al. An activatable AIEgen probe for high-fidelity monitoring of overexpressed tumor enzyme activity and its application to surgical tumor excision. Angew. Chem. Int. Ed. 2020, 59, 10186–10195. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ni, X.; Tian, H.W.; Liu, Q.; Guo, D.S.; Ding, D. Calixarene-based supramolecular AIE dots with highly inhibited nonradiative decay and intersystem crossing for ultrasensitive fluorescence image-guided cancer surgery. Angew. Chem. Int. Ed. 2020, 59, 10008–10012. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yang, Y.; Guo, Y.; Lu, S.; Du, Y.; Li, J.J.; Zhang, X.; Leung, N.L.C.; Zhao, Z.; Niu, G.; et al. Phage-guided targeting, discriminative imaging, and synergistic killing of bacteria by AIE bioconjugates. J. Am. Chem. Soc. 2020, 142, 3959–3969. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Li, Y.; Long, Z.; Jiang, R.; Zhuang, Z.; Wang, Z.; Zhao, Z.; Lou, X.; Xia, F.; Tang, B.Z. Efficient near-infrared photosensitizer with aggregation-induced emission for imaging-guided photodynamic therapy in multiple xenograft tumor models. ACS Nano 2020, 14, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Hu, J.J.; Dai, J.; Lou, X.; Zhao, Z.; Xia, F.; Tang, B.Z. Self-guiding polymeric prodrug micelles with two aggregation-induced emission photosensitizers for enhanced chemo-photodynamic therapy. ACS Nano 2021, 15, 3026–3037. [Google Scholar] [CrossRef]
- Jiang, R.; Dai, J.; Dong, X.; Wang, Q.; Meng, Z.; Guo, J.; Yu, Y.; Wang, S.; Xia, F.; Zhao, Z.; et al. Improving image-guided surgical and immunological tumor treatment efficacy by photothermal and photodynamic therapies based on a multifunctional NIR AIEgen. Adv. Mater. 2021, 33, e2101158. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, L.; Wang, Y.; Wang, C.; Mu, Q.; Liu, X.; Yu, M.; Wang, K.N.; Yao, G.; Yu, Z. Dynamic adjust of non-radiative and radiative attenuation of AIE molecules reinforces NIR-II imaging mediated photothermal therapy and immunotherapy. Adv. Sci. 2022, 9, e2104793. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Morsch, M.; Lu, Y.; Shangguan, P.; Han, L.; Wang, Z.; Chen, X.; Song, C.; Liu, S.; et al. Brain-targeted aggregation-induced-emission nanoparticles with near-infrared imaging at 1550 nm boosts orthotopic glioblastoma theranostics. Adv. Mater. 2022, 34, e2106082. [Google Scholar] [CrossRef]
- Yan, D.; Wang, M.; Wu, Q.; Niu, N.; Li, M.; Song, R.; Rao, J.; Kang, M.; Zhang, Z.; Zhou, F.; et al. Multimodal imaging-guided photothermal immunotherapy based on a versatile NIR-II aggregation-induced emission luminogen. Angew. Chem. Int. Ed. 2022, 61, e202202614. [Google Scholar] [CrossRef]
- Jia, H.; Ding, D.; Hu, J.; Dai, J.; Yang, J.; Li, G.; Lou, X.; Xia, F. AIEgen-based lifetime-probes for precise furin quantification and identification of cell subtypes. Adv. Mater. 2021, 33, e2104615. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Hu, J.J.; Dong, X.; Chen, B.; Dong, X.; Liu, R.; Xia, F.; Lou, X. Deep downregulation of PD-L1 by caged peptide-conjugated AIEgen/miR-140 nanoparticles for enhanced immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202117798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, W.; Li, R.Q.; Qiu, W.X.; Zhuang, Z.N.; Cheng, S.X.; Zhang, X.Z. Peptide-based multifunctional nanomaterials for tumor imaging and therapy. Adv. Funct. Mater. 2018, 28, 1804492. [Google Scholar] [CrossRef]
- Liu, Z.; Liang, G.; Zhan, W. In situ activatable peptide-based nanoprobes for tumor imaging. Chem. Res. Chin. 2021, 37, 889–899. [Google Scholar] [CrossRef]
- Qi, G.B.; Gao, Y.J.; Wang, L.; Wang, H. Self-assembled peptide-based nanomaterials for biomedical imaging and therapy. Adv. Mater. 2018, 30, e1703444. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Wu, X.; Duan, Z.; Huang, Y.; Lou, X.; Xia, F. Biomacromolecule-functionalized AIEgens for advanced biomedical studies. Small 2019, 15, e1804839. [Google Scholar] [CrossRef] [PubMed]
- Daniel, L.; Kai, S. Lipid rafts as a membrane-organizing principle. Science 2010, 327, 46–50. [Google Scholar]
- Desale, K.; Kuche, K.; Jain, S. Cell-penetrating peptides (CPPs): An overview of applications for improving the potential of nanotherapeutics. Biomater. Sci. 2021, 9, 1153–1188. [Google Scholar] [CrossRef]
- Shi, L.; Liu, Y.H.; Li, K.; Sharma, A.; Yu, K.K.; Ji, M.S.; Li, L.L.; Zhou, Q.; Zhang, H.; Kim, J.S.; et al. An AIE-based probe for rapid and ultrasensitive imaging of plasma membranes in biosystems. Angew. Chem. Int. Ed. 2020, 59, 9962–9966. [Google Scholar] [CrossRef]
- Chen, S.; Xu, Z.; Li, S.; Liang, H.; Zhang, C.; Wang, Z.; Li, J.; Li, J.; Yang, H. Systematic interrogation of cellular signaling in live cells using a membrane-anchored DNA multitasking processor. Angew. Chem. Int. Ed. 2022, 61, e202113795. [Google Scholar]
- Wang, M.D.; Lv, G.T.; An, H.W.; Zhang, N.Y.; Wang, H. In situ self-assembly of bispecific peptide for cancer immunotherapy. Angew. Chem. Int. Ed. 2022, 61, e202113649. [Google Scholar]
- Wu, L.; Wang, Y.; Xu, X.; Liu, Y.; Lin, B.; Zhang, M.; Zhang, J.; Wan, S.; Yang, C.; Tan, W. Aptamer-based detection of circulating targets for precision medicine. Chem. Rev. 2021, 121, 12035–12105. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Liu, J.; Geng, J.; Tang, B.Z.; Liu, B. Specific detection of integrin αvβ3 by light-up bioprobe with aggregation-induced emission characteristics. J. Am. Chem. Soc. 2012, 134, 9569–9572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jin, S.; Yang, K.; Xue, X.; Li, Z.; Jiang, Y.; Chen, W.Q.; Dai, L.; Zou, G.; Liang, X.J. Cell membrane tracker based on restriction of intramolecular rotation. ACS Appl. Mater. Interfaces 2014, 6, 8971–8975. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hu, J.-J.; Wei, J.; Dai, J.; Liu, R.; Xia, F.; Lou, X. Peptide-conjugated aggregation-induced emission fluorogen: Precise and firm cell membrane labeling by multiple weak interactions. CCS Chem. 2022, 4, 464–475. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, D.; Jiang, N.; Rojalin, T.; Baehr, C.M.; Zhang, D.; Xiao, W.; Wu, Y.; Cong, Z.; Li, J.J.; et al. Transformable peptide nanoparticles arrest HER2 signalling and cause cancer cell death in vivo. Nat. Nanotechnol. 2020, 15, 145–153. [Google Scholar] [CrossRef]
- Li, J.; Fang, Y.; Zhang, Y.; Wang, H.; Yang, Z.; Ding, D. Supramolecular self-assembly-facilitated aggregation of tumor-specific transmembrane receptors for signaling activation and converting immunologically cold to hot tumors. Adv. Mater. 2021, 33, e2008518. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, L.H.; Qiu, W.X.; Zhang, Y.H.; Song, W.; Zhang, L.; Wang, S.B.; Zhang, X.Z. A transformable chimeric peptide for cell encapsulation to overcome multidrug resistance. Small 2018, 14, e1703321. [Google Scholar] [CrossRef]
- Li, Q.; Hao, X.; Wang, H.; Guo, J.; Ren, X.K.; Xia, S.; Zhang, W.; Feng, Y. Multifunctional REDV-G-TAT-G-NLS-Cys peptide sequence conjugated gene carriers to enhance gene transfection efficiency in endothelial cells. Colloids Surf. B Biointerfaces 2019, 184, 110510. [Google Scholar] [CrossRef]
- Kalderon, D.; Roberts, B.L.; Richardson, W.D.; Kalderon, D.; Roberts, B.L.; Richardson, W.D.; Smith, A.E. A short amino acid sequence able to specify nuclear location. Cell 1985, 39, 499–509. [Google Scholar] [CrossRef]
- Escriou, V.; Carrière, M.; Scherman, D.; Wils, P. NLS bioconjugates for targeting therapeutic genes to nucleus. Adv. Drug Deliv. Rev. 2003, 55, 295–306. [Google Scholar] [CrossRef]
- Brandén, L.J.; Mohamed, A.J.; Smith, C.I.E. A peptide nucleic acid-nuclear localization signal fusion that mediates nuclear transport of DNA. Nat. Biotechnol. 1999, 17, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Liu, J.; Shi, J. Cancer cell nucleus-targeting nanocomposites for advanced tumor therapeutics. Chem. Soc. Rev. 2018, 47, 6930–6946. [Google Scholar] [CrossRef] [PubMed]
- Tammam, S.N.; Azzazy, H.M.E.; Lamprecht, A. How successful is nuclear targeting by nanocarriers? J. Control. Release 2016, 229, 140–153. [Google Scholar] [CrossRef]
- Cheng, Y.; Sun, C.; Ou, X.W.; Liu, B.; Lou, X.D.; Xia, F. Dual-targeted peptide-conjugated multifunctional fluorescent probe with AIEgen for efficient nucleus-specific imaging and long-term tracing of cancer cells. Chem. Sci. 2017, 8, 4571–4578. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Sun, C.; Liu, R.; Yang, J.; Dai, J.; Zhai, T.; Lou, X.; Xia, F. A multifunctional peptide-conjugated AIEgen for efficient and sequential targeted gene delivery into nucleus. Angew. Chem. Int. Ed. 2019, 58, 5049–5053. [Google Scholar] [CrossRef]
- Liang, J.; Feng, G.; Kwok, R.T.K.; Ding, D.; Tang, B.; Liu, B. AIEgen based light-up probes for live cell imaging. Sci. China Chem. 2015, 59, 53–61. [Google Scholar] [CrossRef]
- Jiang, L.; Lan, R.; Huang, T.; Chan, C.F.; Li, H.; Lear, S.; Zong, J.; Wong, W.Y.; Muk Lan Lee, M.; Dow Chan, B.; et al. EBNA1-targeted probe for the imaging and growth inhibition of tumours associated with the Epstein Barr virus. Nat. Biomed. Eng. 2017, 1, 0042. [Google Scholar] [CrossRef] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Jean, S.R.; Ahmed, M.; Lei, E.K.; Wisnovsky, S.P.; Kelley, S.O. Peptide-mediated delivery of chemical probes and therapeutics to mitochondria. Acc. Chem. Res. 2016, 49, 1893–1902. [Google Scholar] [CrossRef]
- Pramanik, S.K.; Das, A. Fluorescent probes for imaging bioactive species in subcellular organelles. Chem. Commun. 2021, 57, 12058–12073. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Huo, F.; Yin, C. Organelle targetable fluorescent probes for hydrogen peroxide. Chin. Chem. Lett. 2019, 30, 1834–1842. [Google Scholar] [CrossRef]
- Rocha, M.; Hernandez, M.A.; Garcia, M.K.K.; Banuls, C.; Bellod, L. Mitochondria-targeted antioxidant peptides. Curr. Pharm. Des. 2010, 16, 3124–3131. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Byrne, A.; Burke, C.S.; Forster, R.J.; Keyes, T.E. Peptide-bridged dinuclear Ru(II) complex for mitochondrial targeted monitoring of dynamic changes to oxygen concentration and ROS generation in live mammalian cells. J. Am. Chem. Soc. 2014, 136, 15300–15309. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.P.; Kelley, S.O. Maximizing the therapeutic window of an antimicrobial drug by imparting mitochondrial sequestration in human cells. J. Am. Chem. Soc. 2011, 133, 3260–3263. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Hu, J.J.; Liu, R.; Dai, J.; Duan, M.; Yuan, L.; Xia, F.; Lou, X.D. Spatial order of functional modules enabling diverse intracellular performance of fluorescent probes. Angew. Chem. Int. Ed. 2021, 60, 18280–18288. [Google Scholar] [CrossRef]
- Yang, J.Y.; Hu, J.J.; Wei, J.M.; Dai, J.; Fang, H.; Xia, F.; Lou, X.D. Endocytosis pathway self-regulation for precise image-guided therapy through an enzyme-responsive modular peptide probe. Anal. Chem. 2022, 94, 7960–7969. [Google Scholar] [CrossRef]
- Choi, N.E.; Lee, J.Y.; Park, E.C.; Lee, J.H.; Lee, J. Recent advances in organelle-targeted fluorescent probes. Molecules 2021, 26, 217. [Google Scholar] [CrossRef]
- Yang, J.; Dai, J.; Wang, Q.; Cheng, Y.; Guo, J.; Zhao, Z.; Hong, Y.; Lou, X.; Xia, F. Tumor-triggered disassembly of a multiple-agent-therapy probe for efficient cellular internalization. Angew. Chem. Int. Ed. 2020, 59, 20405–20410. [Google Scholar] [CrossRef]
- Hu, F.; Huang, Y.; Zhang, G.; Zhao, R.; Yang, H.; Zhang, D. Targeted bioimaging and photodynamic therapy of cancer cells with an activatable red fluorescent bioprobe. Anal. Chem. 2014, 86, 7987–7995. [Google Scholar] [CrossRef]
- Ji, S.; Li, J.; Duan, X.; Zhang, J.; Zhang, Y.; Song, M.; Li, S.; Chen, H.; Ding, D. Targeted enrichment of enzyme-instructed assemblies in cancer cell lysosomes turns immunologically cold tumors hot. Angew. Chem. Int. Ed. 2021, 60, 26994–27004. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Gui, S.L.; Huang, Y.Y.; Hu, F.; Jin, Y.L.; Yu, Y.; Zhang, G.X.; Zhang, D.Q.; Zhao, R. Rapid, sensitive, and in-solution screening of peptide probes for targeted imaging of live cancer cells based on peptide recognition-induced emission. Chem. Commun. 2017, 53, 11091–11094. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Norton, A.S.; Pokharel, D.; Song, Y.; Hill, R.A. KDEL peptide gold nanoconstructs: Promising nanoplatforms for drug delivery. Nanomedicine 2013, 9, 366–374. [Google Scholar] [CrossRef]
- Xiao, H.; Wu, C.; Li, P.; Tang, B. Simultaneous fluorescence visualization of endoplasmic reticulum superoxide anion and polarity in myocardial cells and tissue. Anal. Chem. 2018, 90, 6081–6088. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Choi, S.H.; Hong, S.T.; Kim, M.S.; Ryu, S.S.; Yoon, Y.U.; Paik, K.C.; Han, M.S.; Sim, T.; Cho, B.R. Two-photon probes for endoplasmic reticulum: Its detection in a live tissue by two-photon microscopy. Chem. Commun. 2020, 56, 3657–3660. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, H.; Liu, R.; Chen, C.; Zeng, S.; Liu, Q.; Ding, D. Endoplasmic reticulum targeted AIE bioprobe as a highly efficient inducer of immunogenic cell death. Sci. China Chem. 2020, 63, 1428–1434. [Google Scholar] [CrossRef]
- Shi, L.; Gao, X.; Yuan, W.; Xu, L.; Deng, H.; Wu, C.; Yang, J.; Jin, X.; Zhang, C.; Zhu, X. Endoplasmic reticulum-targeted fluorescent nanodot with large stokes shift for vesicular transport monitoring and long-term bioimaging. Small 2018, 14, e1800223. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, B.; Yuan, H.; Zhang, W.; Hu, J.; Lou, X.; Xia, F. AIEgen-Peptide Bioprobes for the Imaging of Organelles. Biosensors 2022, 12, 667. https://doi.org/10.3390/bios12080667
Chen B, Yuan H, Zhang W, Hu J, Lou X, Xia F. AIEgen-Peptide Bioprobes for the Imaging of Organelles. Biosensors. 2022; 12(8):667. https://doi.org/10.3390/bios12080667
Chicago/Turabian StyleChen, Bochao, Haotong Yuan, Wei Zhang, Jingjing Hu, Xiaoding Lou, and Fan Xia. 2022. "AIEgen-Peptide Bioprobes for the Imaging of Organelles" Biosensors 12, no. 8: 667. https://doi.org/10.3390/bios12080667
APA StyleChen, B., Yuan, H., Zhang, W., Hu, J., Lou, X., & Xia, F. (2022). AIEgen-Peptide Bioprobes for the Imaging of Organelles. Biosensors, 12(8), 667. https://doi.org/10.3390/bios12080667




