Machine Learning-Based 30-Day Hospital Readmission Predictions for COPD Patients Using Physical Activity Data of Daily Living with Accelerometer-Based Device
Abstract
:1. Introduction
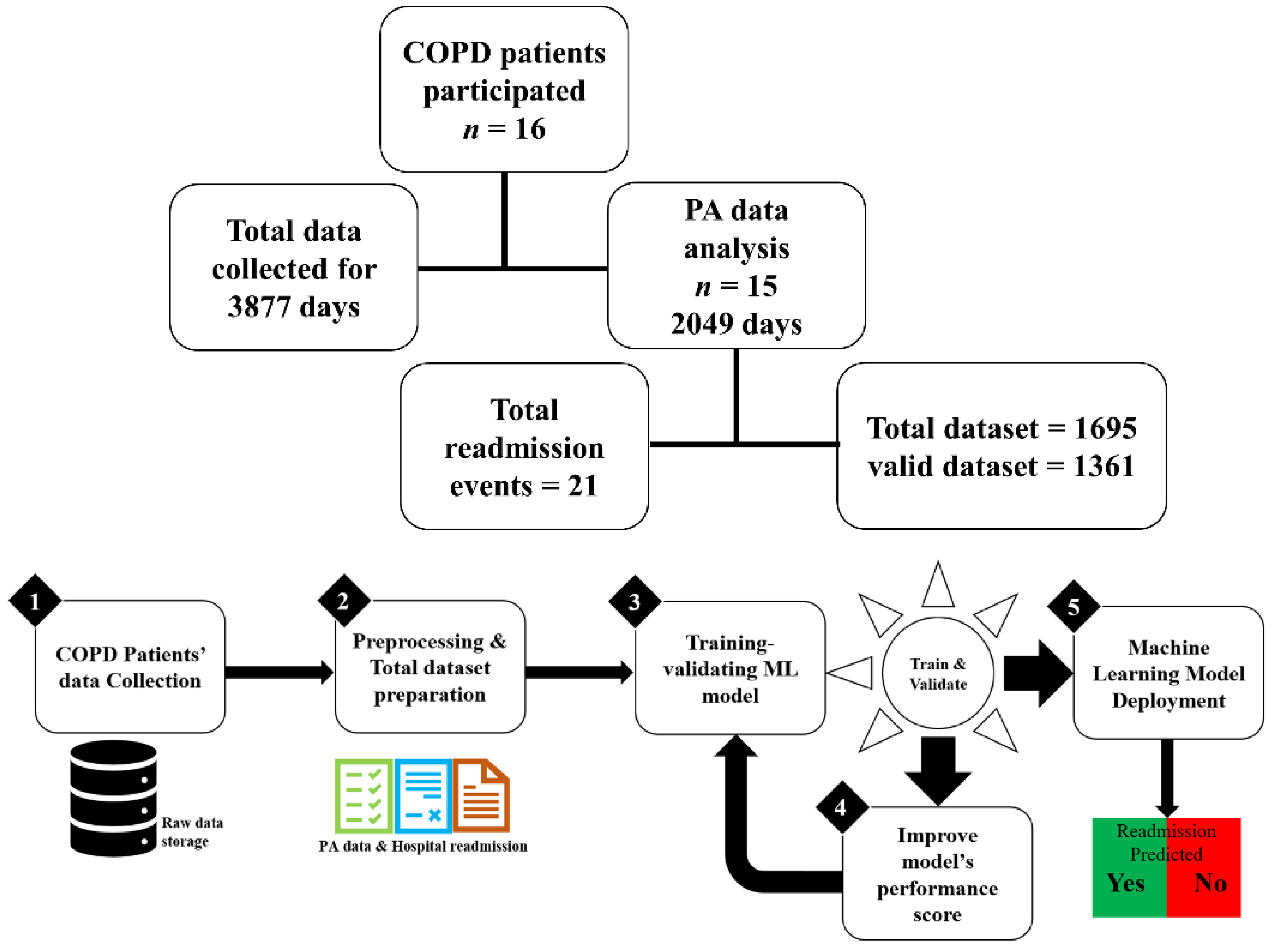
2. Materials and Methods
2.1. Study Design and Setting
2.2. Participants
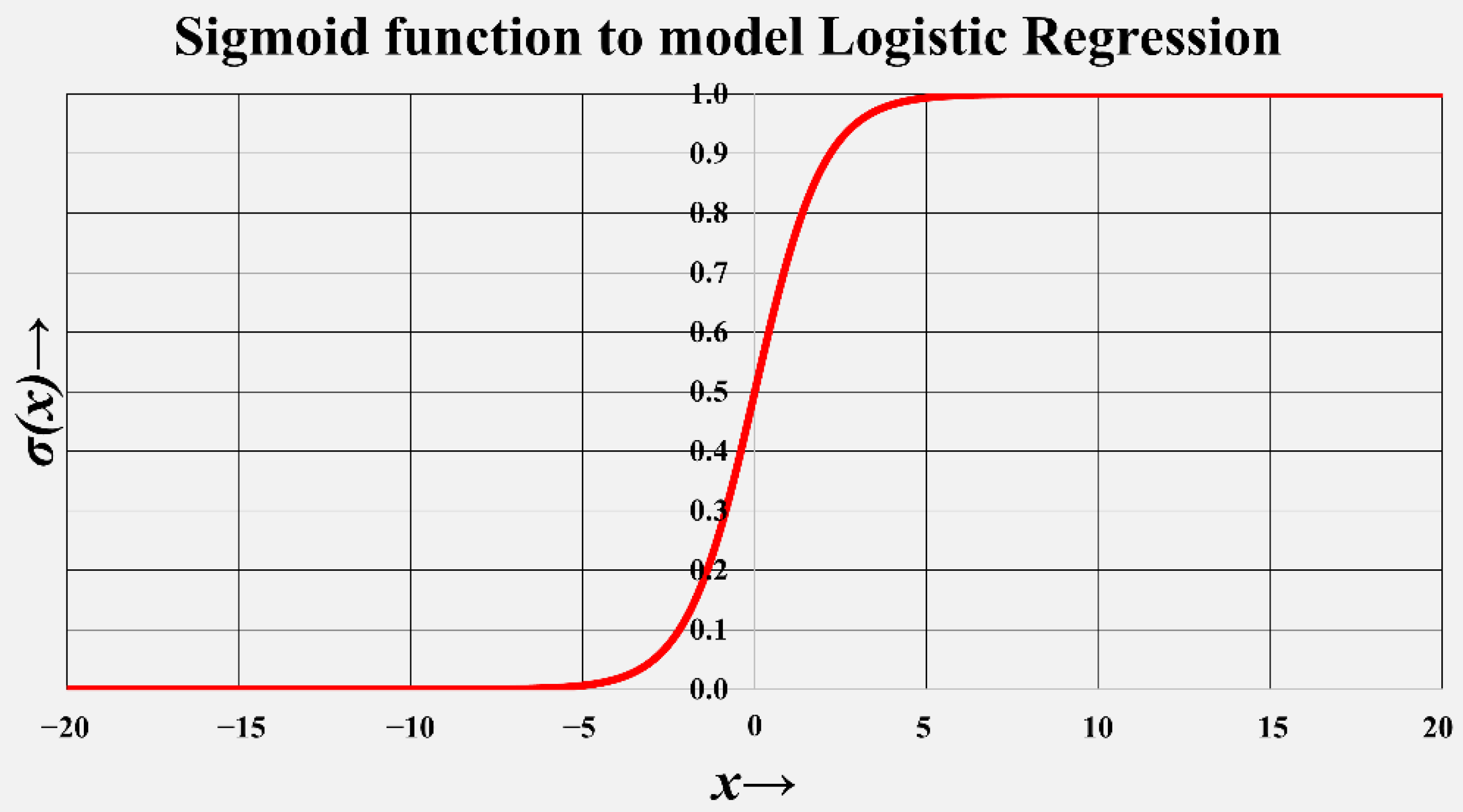
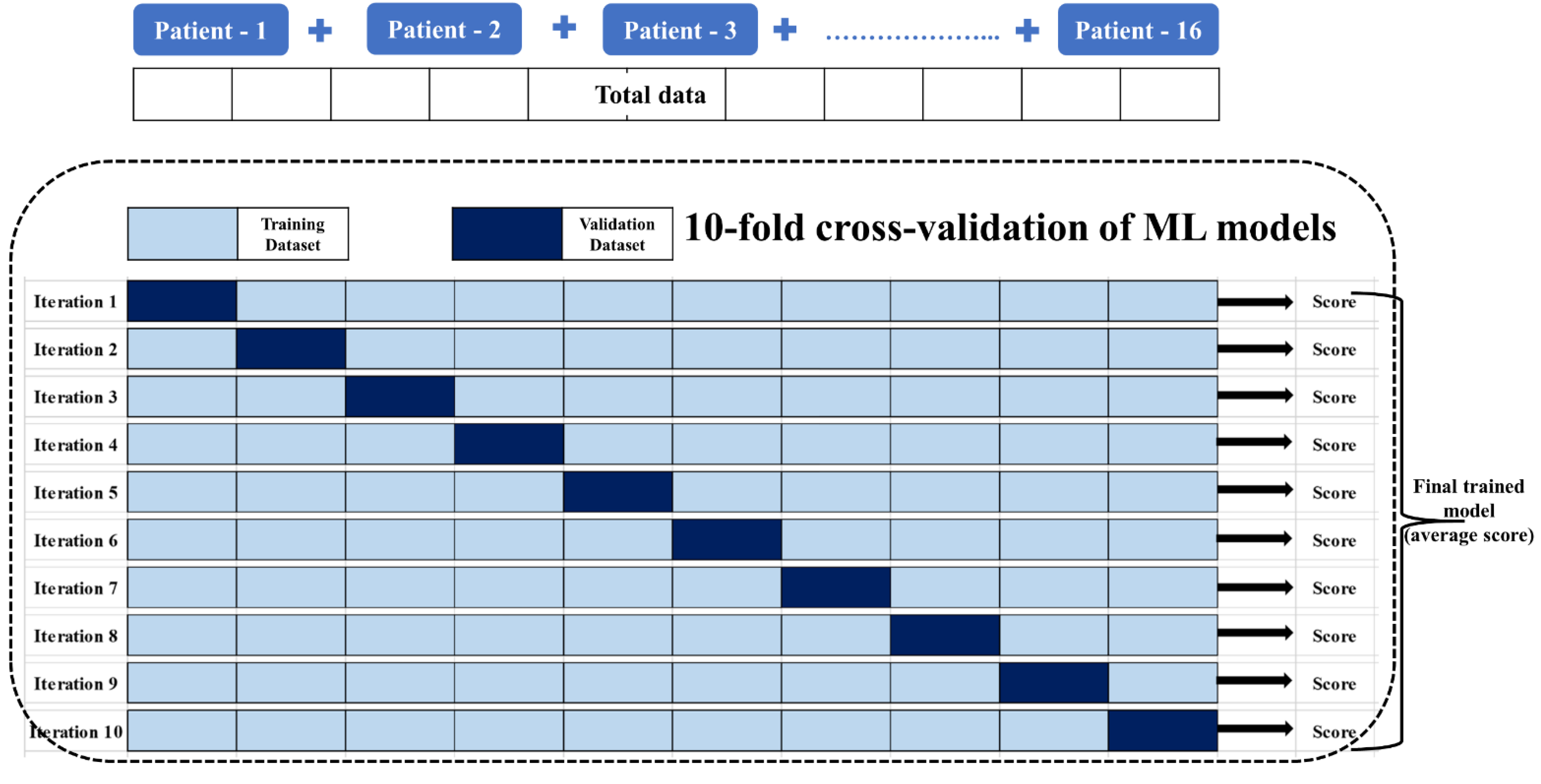
2.3. Data Preprocessing, Statistical Analysis, and Data Reduction
2.4. Dataset
2.4.1. Valid Dataset
2.4.2. Positive Dataset
2.4.3. Negative Dataset
3. Results
3.1. Prediction-Based Performance
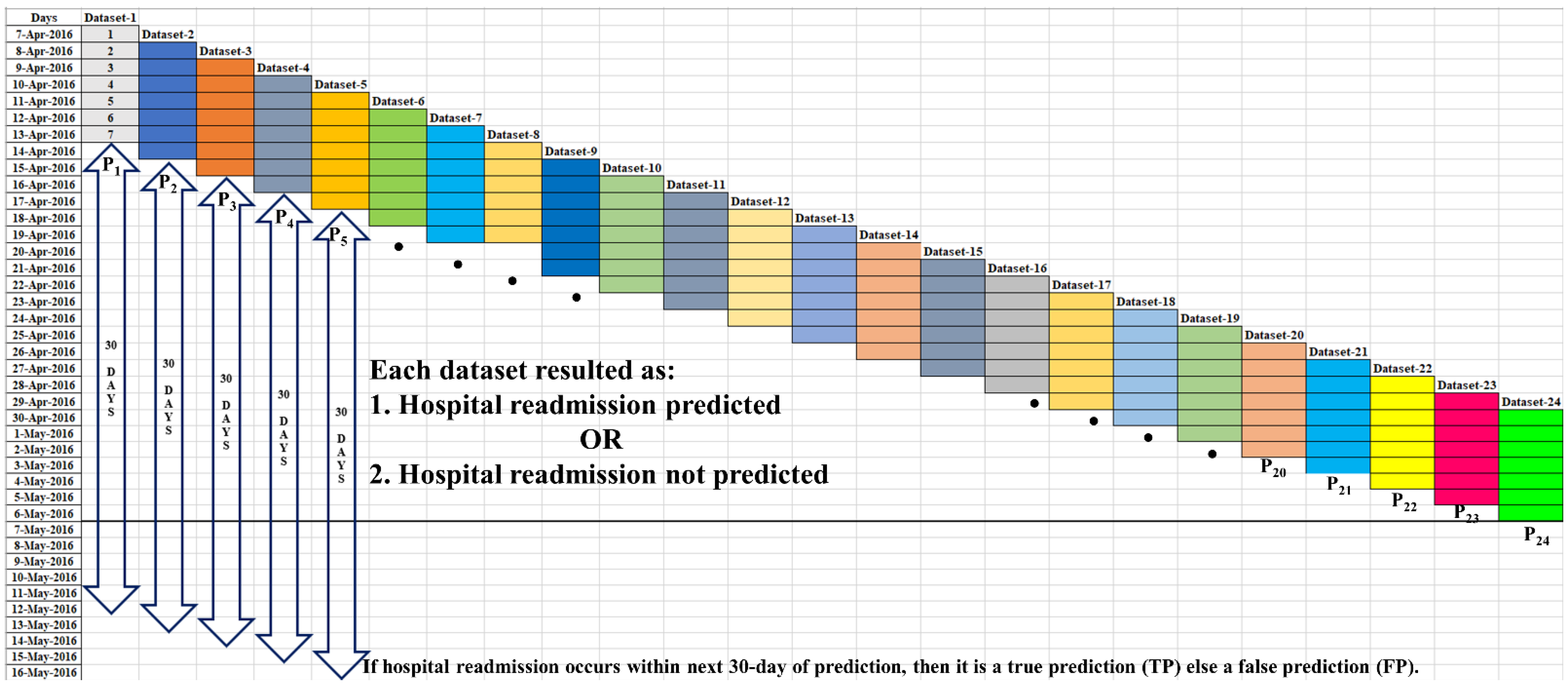
3.1.1. True Prediction ()
3.1.2. False Prediction ()
3.2. Event-Based Performance
3.2.1. Truly Predicted Event ()
3.2.2. Mispredicted Event ()
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Available online: https://goldcopd.org/2022-gold-reports-2/ (accessed on 21 April 2022).
- Nguyen, H.Q.; Chu, L.; Liu, I.-L.A.; See, J.S.; Suh, D.; Korotzer, B.; Yuen, G.; Desai, S.; Coleman, K.J.; Xiang, A.H.; et al. Associations between physical activity and 30-day readmission risk in chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2014, 11, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Hakim, M.A.; Garden, F.L.; Jennings, M.D.; Dobler, C.C. Performance of the LACE index to predict 30-day hospital readmissions in patients with chronic obstructive pulmonary disease. Clin. Epidemiol. 2018, 10, 51–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, T.; Press, V.G.; Huisingh-Scheetz, M.; White, S.R. COPD Readmissions: Addressing COPD in the era of value-based health care. Chest 2016, 150, 916–926. [Google Scholar] [CrossRef] [Green Version]
- Amalakuhan, B.; Kiljanek, L.; Parvathaneni, A.; Hester, M.; Cheriyath, P.; Fischman, D. A prediction model for COPD readmissions: Catching up, catching our breath, and improving a national problem. J. Community Hosp. Intern. Med. Perspect. 2012, 2, 9915. [Google Scholar] [CrossRef]
- Goto, T.; Faridi, M.K.; Gibo, K.; Toh, S.; Hanania, N.A.; Camargo, A., Jr.; Hasegawa, K. Trends in 30-day readmission rates after COPD hospitalization, 2006–2012. Respir. Med. 2017, 130, 92–97. [Google Scholar] [CrossRef] [Green Version]
- Thorpe, O.; Kumar, S.; Johnston, K. Barriers to and enablers of physical activity in patients with COPD following a hospital admission: A qualitative study. Int. J. COPD 2014, 9, 115–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awais, M.; Chiari, L.; Ihlen, E.A.F.; Helbostad, J.L.; Palmerini, L. Physical activity classification for elderly people in free-living conditions. IEEE J. Biomed. Health Inform. 2019, 23, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Mesanza, A.B.; Lucas, S.; Zubizarreta, A.; Cabanes, I.; Portillo, E.; Rodriguez-Larrad, A. A machine learning approach to perform physical activity classification using a sensorized crutch tip. IEEE Access 2020, 8, 210023–210034. [Google Scholar] [CrossRef]
- Fridriksdottir, E.; Bonomi, A.G. Accelerometer-based human activity recognition for patient monitoring using a deep neural network. Sensors 2020, 20, 6424. [Google Scholar] [CrossRef]
- Waschki, B.; Kirsten, A.; Holz, O.; Müller KC, H.; Magnussen, H. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: A prospective cohort study. Chest 2011, 140, 331–342. [Google Scholar] [CrossRef]
- Spruit, M.A.; Pitta, F.; McAuley, E.; ZuWallack, R.L.; Nici, L. Pulmonary rehabilitation and physical activity in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015, 192, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Watz, H.; Calverley, P.; Chanez, P.; Dahl; Decramer, M.; Disse, B.; Finnigan, H.; Kirsten, A.M.; Rodriguez-Roisin, R.; Tetzlaff, K.; et al. An official European respiratory society statement on physical activity in COPD. Eur. Respir. J. 2014, 44, 1521–1537. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Centurion, V.; Casaburi, R.; Coultas, D.B.; Kansai, M.M.; Kitsiou, S.; Luo, J.J.; Ma, J.; Rand, C.S.; Tan, A.-Y.M.; Krishan, J.A. Daily physical activity in patients with COPD after hospital discharge in a minority population. Chronic Obstr. Pulm. Dis. 2019, 6, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Rio, F.; Lores, V.; Mediano, O.; Rojo, B.; Hernanz, A.; López-Collazo, E.; Alvarez-Sala, R. Daily physical activity in patients with chronic obstructive pulmonary disease is mainly associated with dynamic hyperinflation. Am. J. Respir. Crit. Care Med. 2009, 180, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Watz, H.; Waschki, B.; Boehme, C.; Claussen, M.; Meyer, T.; Magnussen, H. Extrapulmonary effects of chronic obstructive pulmonary disease on physical activity: A cross-sectional study. Am. J. Respir. Crit. Care Med. 2008, 177, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aymerich, J.; Lange, P.; Benet, M.; Schnohr, P.; Antó, J.M. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: A population based cohort study. Thorax 2006, 61, 772–778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.; Archer, K.R.; Choi, L. Statistical and machine learning models for classification of human wear and delivery days in accelerometry data. Sensors 2021, 21, 2726. [Google Scholar] [CrossRef]
- Patel, S.A.; Benzo, R.P.; Slivka, W.A.; Sciurba, F.C. Activity monitoring and energy expenditure in COPD patients: A validation study. COPD J. Chronic Obstr. Pulm. Dis. 2007, 4, 107–112. [Google Scholar] [CrossRef] [Green Version]
- Watz, H.; Waschki, B.; Meyer, T.; Magnussen, H. Physical activity in patients with COPD. Eur. Respir. J. 2009, 33, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Demeyer, H.; Burtin, C.; Van Remoortel, H.; Hornikx, M.; Langer, D.; Dcramer, M.; Gosselink, R.; Janssens, W.; Troosters, T. Standardizing the analysis of physical activity in patients with COPD following a pulmonary rehabilitation program. Chest 2014, 146, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.Y.; Verma, V.K.; Lee, M.Y.; Lai, C.S. Activity monitoring with a wrist-worn, accelerometer-based device. Micromachines 2018, 9, 450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chawla, H.; Bulathsinghala, C.; Tejada, J.P.; Wakefield, D.; ZuWallack, R. Physical activity as a predictor of thirty-day hospital readmission after a discharge for a clinical exacerbation of chronic obstructive pulmonary disease. Ann. Am. Thorac. Soc. 2014, 11, 1203–1209. [Google Scholar] [CrossRef] [PubMed]
- Osman, L.M.; Godden, D.J.; Friend, J.A.R.; Legge, J.S.; Douglas, J.G. Quality of life and hospital re-admission in patients with chronic obstructive pulmonary disease. Thorax 1997, 52, 67–71. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.Y.; Verma, V.K.; Lee, M.Y.; Lin, H.C.; Lai, C.S. Prediction of 30-day readmission for COPD patients using accelerometer-based activity monitoring. Sensors 2020, 20, 217. [Google Scholar] [CrossRef] [Green Version]
- Min, X.; Yu, B.; Wang, F. Predictive modeling of the hospital readmission risk from patients’ claims data using machine learning: A case study on COPD. Sci. Rep. 2019, 9, 2362. [Google Scholar] [CrossRef] [Green Version]
- Goto, T.; Jo, T.; Matsui, H.; Fushimi, K.; Hayashi, H.; Yasunaga, H. Machine learning-based prediction models for 30-day readmission after hospitalization for chronic obstructive pulmonary disease. COPD J. Chronic Obstr. Pulm. Dis. 2019, 16, 338–343. [Google Scholar] [CrossRef]
- Zhou, S.M.; Lyons, R.A.; Rahman, M.A.; Holborow, A.; Brophy, S. Predicting hospital readmission for campylobacteriosis from electronic health records: A machine learning and text mining perspective. J. Pers. Med. 2022, 12, 86. [Google Scholar] [CrossRef]
- Arnaud, E.; Elbattah, M.; Gignon, M.; Dequen, G. Deep learning to predict hospitalization at triage: Integration of structured data and unstructured text. In Proceedings of the 2020 IEEE International Conference on Big Data (Big Data), Atlanta, GA, USA, 10–13 December 2020; pp. 4836–4841. [Google Scholar] [CrossRef]
- Kansagara, D.; Englander, H.; Salanitro, A.; Kagen, D.; Theobald, C.; Freeman, M.; Kripalani, S. Risk Prediction Models for Hospital Readmission A Systematic Review. Available online: http://www.jama.com (accessed on 21 April 2022).
- Brindise, L.R.; Steele, R.J. Machine learning-based pre-discharge prediction of hospital readmission. In Proceedings of the 2018 International Conference on Computer, Information and Telecommunication Systems (CITS), Colmar, France, 11–13 July 2018. [Google Scholar] [CrossRef]
- Roberts, D.R.; Bahn, V.; Ciuti, S.; Boyce, M.S.; Elith, J.; Guillera-Arroita, G.; Hauenstein, S.; Lahoz-Monfort, J.J.; Schröder, B.; Thuiller, W.; et al. Cross-validation strategies for data with temporal, spatial, hierarchical, or phylogenetic structure. Ecography 2017, 40, 913–929. [Google Scholar] [CrossRef]
- Yagus, E.; Atnafu, S.W.; De Herrera, A.G.C.; Marzi, C.; Scheda, R.; Giannelli, M.; Tessa, C.; Citi, L.; Diciotti, S. Effect of data leakage in brain MRI classification using 2D convolutional neural networks. Sci. Rep. 2021, 11, 22544. [Google Scholar] [CrossRef] [PubMed]
- Shim, M.; Lee, S.H.; Hwang, H.J. Inflated prediction accuracy of neuropsychiatric biomarkers caused by data leakage in feature selection. Sci. Rep. 2021, 11, 7980. [Google Scholar] [CrossRef]

| Name | Statistical Expression | Description |
|---|---|---|
| Resultant acceleration, | , , and are accelerations along 3-orthogonal directions: the , , and axes at instance ”” | |
| Mean resultant acceleration, | The mean value is calculated over one epoch period (5 s) and values of | |
| Standard deviation (SD), | The SD value removes the constant gravity component included in the acceleration to represent actual quantified PA in an epoch period | |
| Activity index, | Summation of quantified PA for 12 epochs (total of 60 s for 5 s epoch) | |
| Regularity index, | On day “d”, the regularity of the hourly-PA () is defined as the correlation coefficient between the 24-h hourly-PA patterns of days “d − 1” and “d”. | |
| Quality of physical activity, | Incorporating the effects of the different PAs and the regularity of PA on a day-to-day basis, ‘’ can be 1~1440 min in a 24-h day |
| Characteristics | Baseline Measure, n = 16 |
|---|---|
| Demographic | |
| Age (years), mean (SD) | 74.0 (±11.2) |
| Clinical | |
| Height (m), mean (SD) | 1.60 (±0.06) |
| Body weight (Kg.), mean (SD) | 55.39 (±9.01) |
| Body mass index (Kg/m2), median (IQR) | 21.96 (5.70 to 24.98) |
| mMRC, mean (SD) | 2.25 (±0.93) |
| 6MWD, mean (SD) | 282.56 (±98.10) |
| +FVC—Forced Vital Capacity Lung size (ltr), mean (SD) | 1.72 (±0.47) |
| FVC—Forced Vital Capacity FVC(%), mean (SD) | 59.56 (±16.05) |
| FEV—Forced expiratory volume FEV1(L), mean (SD) | 0.81 (±0.27) |
| FEV—Forced expiratory volume FEV1(%), mean (SD) | 38.25 (±15.68) |
| Tiffeneau-Pinelli index FEV1/FVC, mean (SD) | 48.25 (±15.03) |
| Study data statistics | |
| Actual number of hospital readmissions | 21 |
| Total testing days | 3877 |
| Total datatsets | 1695 |
| Total valid datatsets | 1361 |
| Datasets with predictions | 199 |
| Datasets with true prediction (TP) | 140 |
| Datasets with false prediction (FP) | 59 |
| Truly predicted event (TE) | 15 |
| Mispredicted event (ME) | 6 |
| Studies | Methods | Data | Prediction Model Performance |
|---|---|---|---|
| Current study | PA data with a logistic regression ML model | Continuous PA data, hospital medical records | Accuracy of predicted events: 71.43%Precision ( rate): 70.35% |
| Lin W.-Y. et al. [25] | PA data with statistical-mathematical model | Continuous PA data, hospital records | Accuracy of predicted events: 52.38%Precision ( rate): 37.78% |
| Amalakuhan B. et al. [5] | 55 feature variables for COPD exacerbations, random forest ML model | Demographic data, hospital medical records based on ICD-9 codes | Positive predictive value (accuracy in prediction): 70% (0.7) |
| Chawla H. et al. [23] | Vector magnitude units (VMU), i.e., summed movements in three planes over each minute, logistic regression ML model | PA data recorded with GT3X+ accelerometer, derived indices, hospital medical records | 31.58% of patients had all-cause hospital readmissions, patients with lower PA are 6.7 times more likely to be readmitted |
| Min X. et al. [26] | Traditional and deep learning ML models: logistic regression, support vector machine, random forest, and multilayer perceptron | Knowledge-driven: hospital Score, LACE index, handcrafted features; Data-driven: reshaped data grouped into categories | Prediction performance with data-driven features: 65% Combined (knowledge-driven and data-driven): 65.30% |
| Goto T. et al. [27] | Recorded PA data used with logistic regression and Lasso regression ML models | Self-reported, manually assessed, static PA data | 7% of patients had 30-day readmissions. Prediction classification ability (precision): 61.00% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verma, V.K.; Lin, W.-Y. Machine Learning-Based 30-Day Hospital Readmission Predictions for COPD Patients Using Physical Activity Data of Daily Living with Accelerometer-Based Device. Biosensors 2022, 12, 605. https://doi.org/10.3390/bios12080605
Verma VK, Lin W-Y. Machine Learning-Based 30-Day Hospital Readmission Predictions for COPD Patients Using Physical Activity Data of Daily Living with Accelerometer-Based Device. Biosensors. 2022; 12(8):605. https://doi.org/10.3390/bios12080605
Chicago/Turabian StyleVerma, Vijay Kumar, and Wen-Yen Lin. 2022. "Machine Learning-Based 30-Day Hospital Readmission Predictions for COPD Patients Using Physical Activity Data of Daily Living with Accelerometer-Based Device" Biosensors 12, no. 8: 605. https://doi.org/10.3390/bios12080605
APA StyleVerma, V. K., & Lin, W.-Y. (2022). Machine Learning-Based 30-Day Hospital Readmission Predictions for COPD Patients Using Physical Activity Data of Daily Living with Accelerometer-Based Device. Biosensors, 12(8), 605. https://doi.org/10.3390/bios12080605






