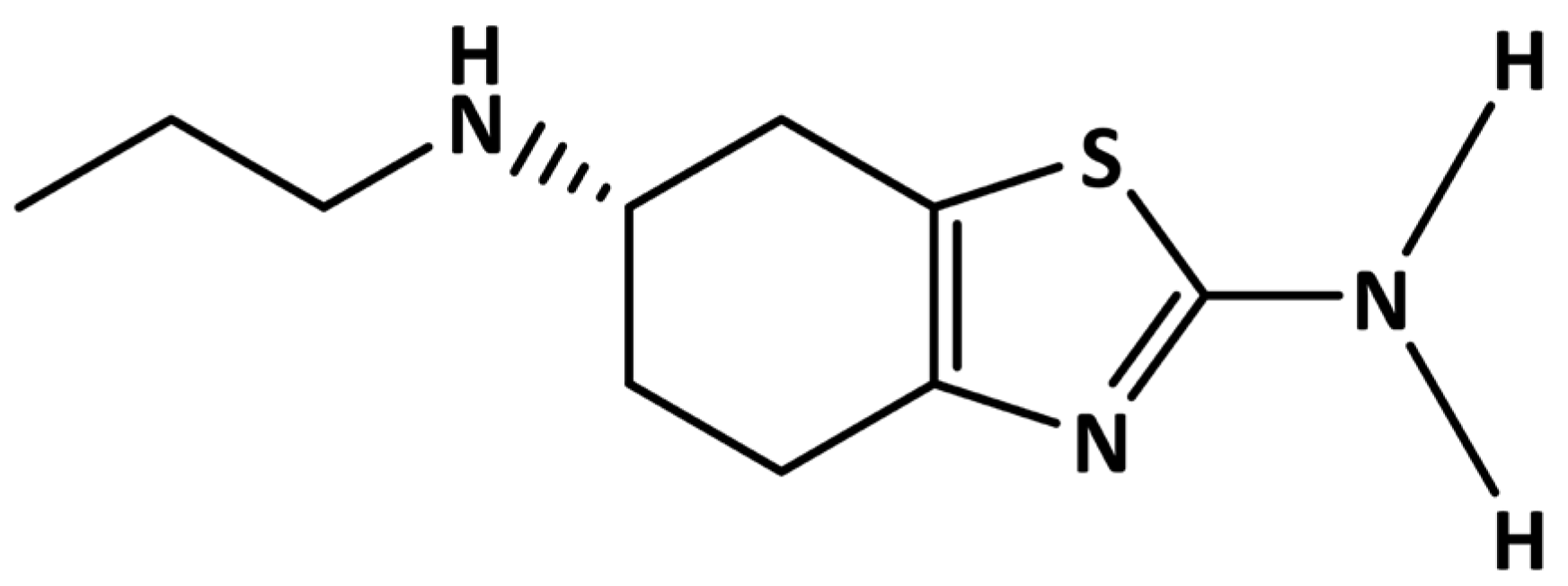
Direct and Sensitive Electrochemical Evaluation of Pramipexole Using Graphitic Carbon Nitride (gCN) Sensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Used Chemicals and Reagents
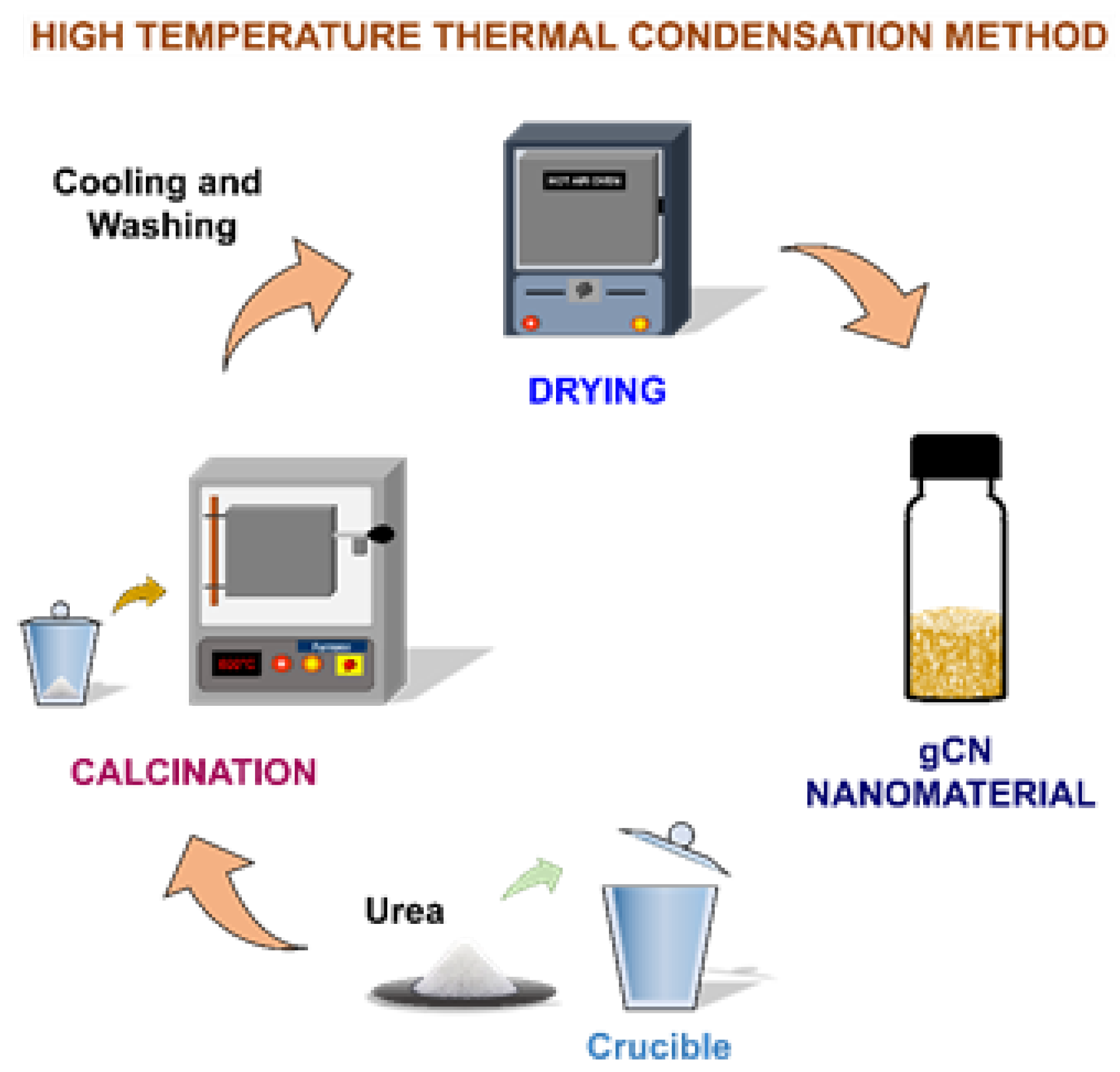
2.2. Synthesis of gCN
2.3. Equipment and Instruments Used
2.4. Construction of Working Electrode
2.5. Method of Analysis
2.6. Formulation of Drug Sample
2.7. Interference Study
3. Results and Discussions
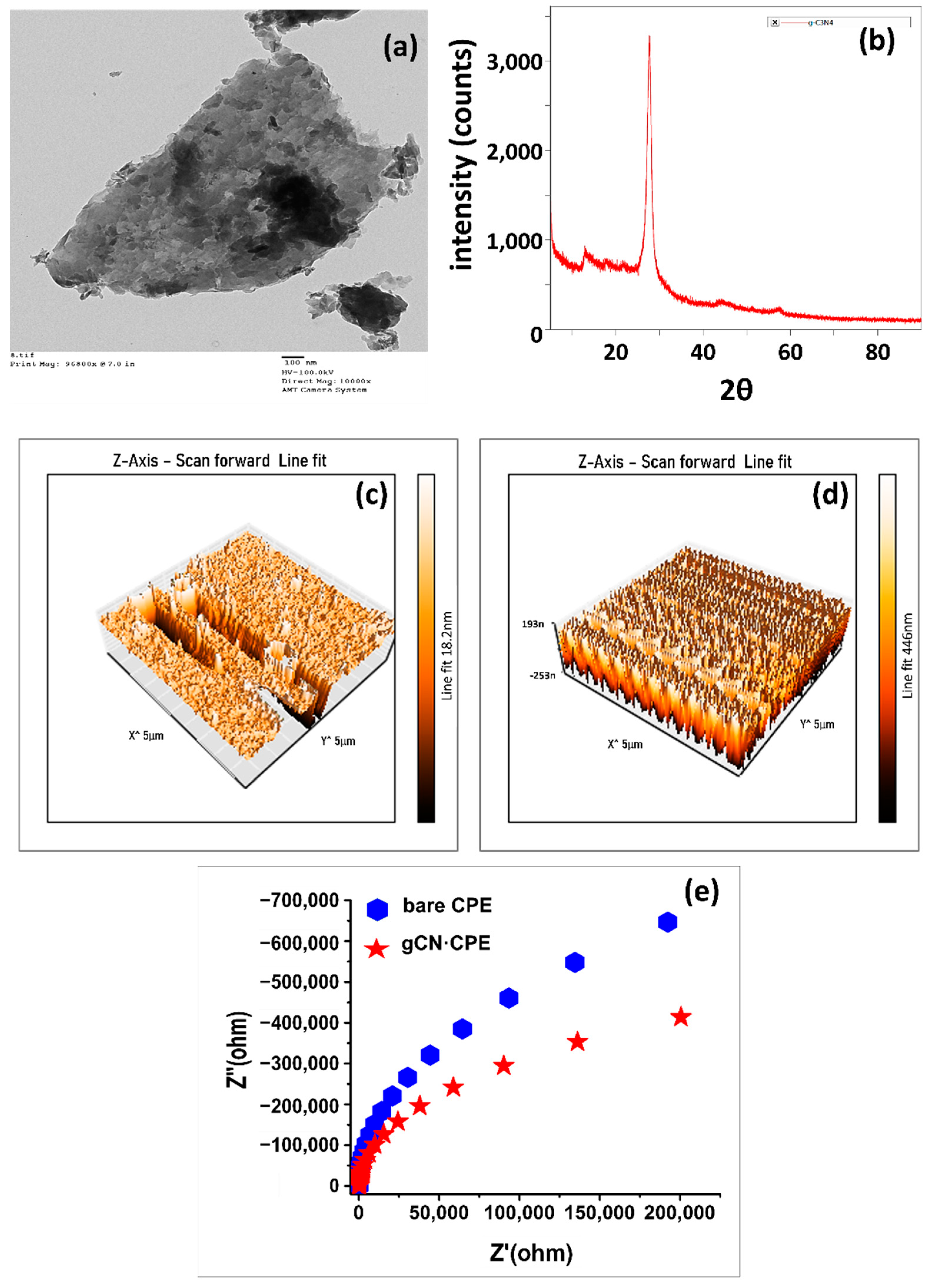
3.1. Characterization of the Electrode Material
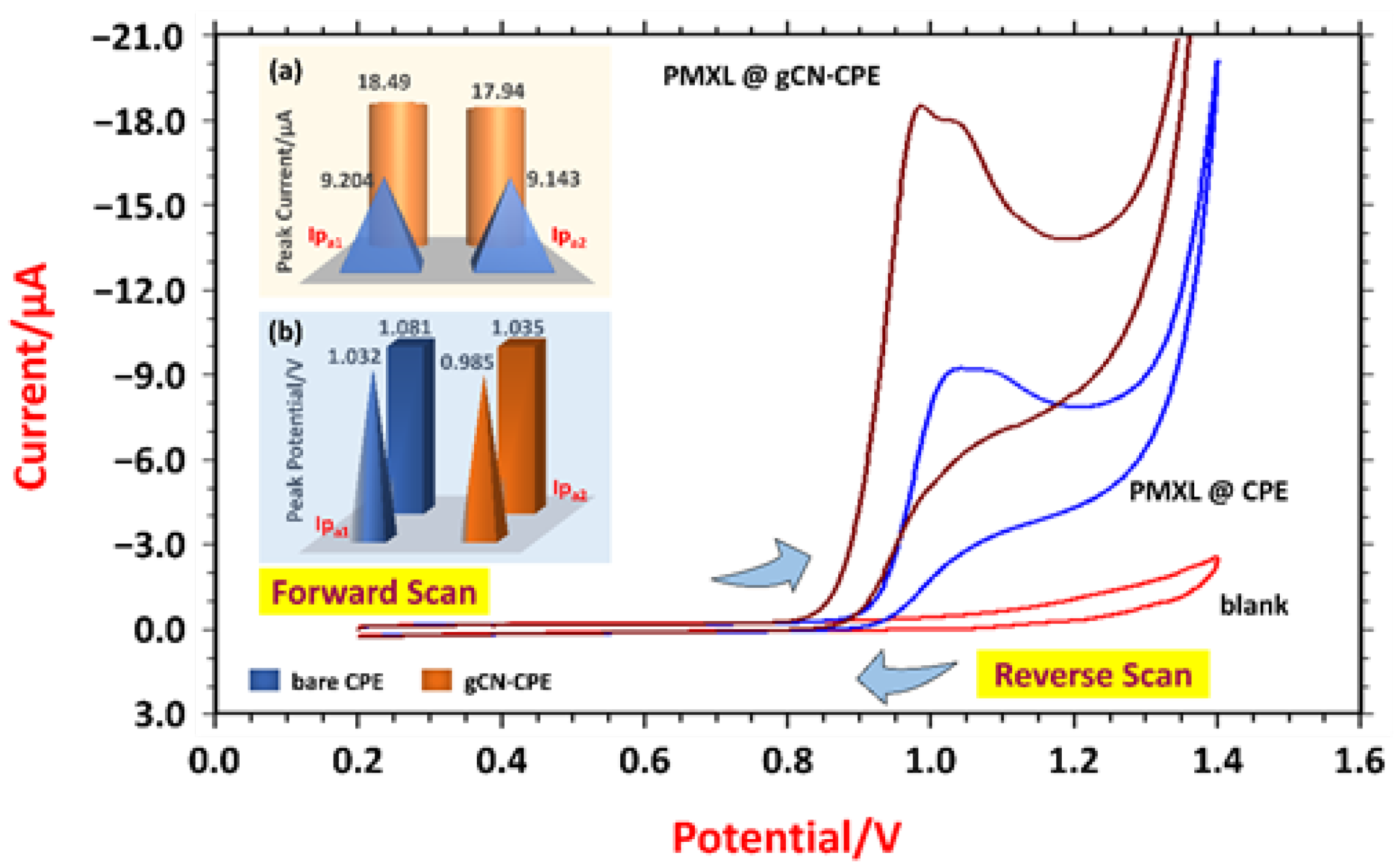
3.2. Voltammetric Response
3.3. Optimization Study
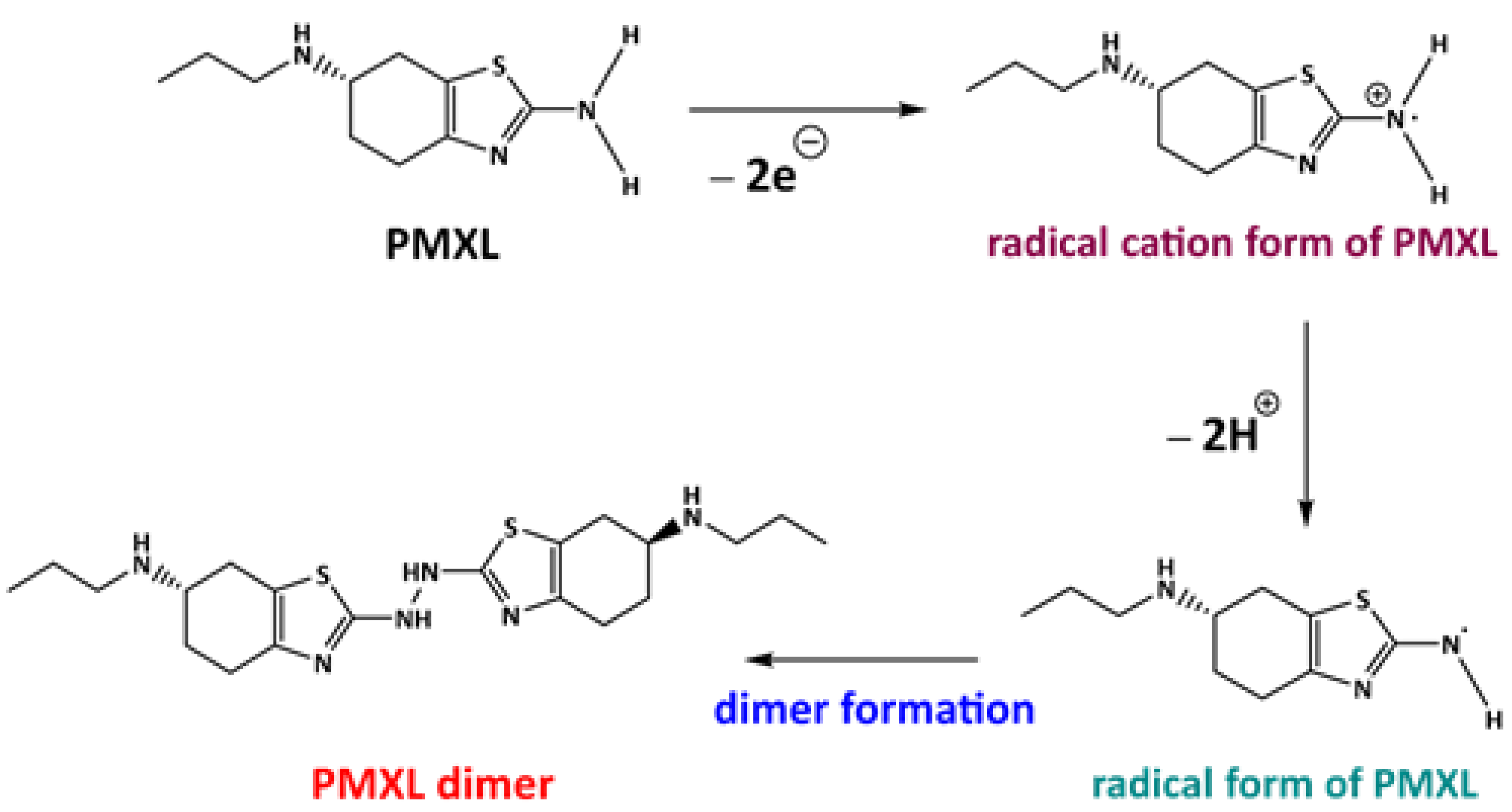
3.4. Probable Electrochemical Sensing Mechanism of PMXL at gCN·CPE
3.5. Feasible Reaction Mechanism of PMXL at gCN·CPE
3.6. Impact of Scan Rates on PXML
3.7. Quantification of PMXL Using gCN·CPE
3.8. Diagnostic Analysis
3.9. Interference Study
3.10. Stability of the Electrode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wiench, P.; González, Z.; Menéndez, R.; Grzyb, B.; Gryglewicz, G. Beneficial impact of oxygen on the electrochemical perfor-mance of dopamine sensors based on N-doped reduced graphene oxides. Sens. Actuators B Chem. 2018, 257, 143–153. [Google Scholar] [CrossRef]
- Jin, H.; Zhao, C.; Gui, R.; Gao, X.; Wang, Z. Reduced graphene oxide/nile blue/gold nanoparticles complex-modified glassy carbon electrode used as a sensitive and label-free aptasensor for ratiometric electrochemical sensing of dopamine. Anal. Chim. Acta 2018, 1025, 154–162. [Google Scholar] [CrossRef]
- Aparna, T.; Sivasubramanian, R.; Dar, M.A. One-pot synthesis of Au-Cu2O/rGO nanocomposite based electrochemical sensor for selective and simultaneous detection of dopamine and uric acid. J. Alloy. Compd. 2018, 741, 1130–1141. [Google Scholar] [CrossRef]
- Bennett, J.P., Jr.; Piercey, M.F. Pramipexole—A new dopamine agonist for the treatment of Parkinson’s disease. J. Neurol. Sci. 1999, 163, 25–31. [Google Scholar] [CrossRef]
- Mierau, J.; Schingnitz, G. Biochemical and pharmacological studies on pramipexole, a potent and selective dopamine D2 receptor agonist. Eur. J. Pharmacol. 1992, 215, 161–170. [Google Scholar] [CrossRef]
- Svensson, K.; Carlsson, A.; Huff, R.M.; Kling-Petersen, T.; Waters, N. Behavioral and neurochemical data suggest functional differences between dopamine D2 and D3 receptors. Eur. J. Pharmacol. 1994, 263, 235–243. [Google Scholar] [CrossRef]
- Häselbarth, V.; Justus-Obenauer, H.; Peil, H. Pharmacokinectics and Bioavailability of Pramipexole: Comparison of Plasma Levels after Intravenous and Oral Administration in Healthy Volunteers (M/2730/0029). Upjohn Technical Report; 7215–94–016. 1994. Available online: https://www.ema.europa.eu/en/documents/scientific-discussion/sifrol-epar-scientific-discussion_en.pdf (accessed on 29 June 2022).
- Wright, C.E.; Sisson, T.L.; Ichhpurani, A.K.; Peters, G.R. Steady-state pharmacokinetic properties of pramipexole in healthy volunteers. J. Clin. Pharmacol. 1997, 37, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, M.; Demir, E.; Aydogdu, N.; Zare, N.; Karimi, F.; Kandomal, S.M.; Rokni, H.; Ghasemi, Y. Recent advantages in elec-trochemical monitoring for the analysis of amaranth and carminic acid food colors. Food Chem. Toxicol. 2022, 163, 112929. [Google Scholar] [CrossRef]
- Bansod, B.; Kumar, T.; Thakur, R.; Rana, S.; Singh, I. A review on various electrochemical techniques for heavy metal ions de-tection with different sensing platforms. Biosens. Bioelectron. 2017, 94, 443–455. [Google Scholar] [CrossRef]
- Killedar, L.; Ilager, D.; Malode, S.J.; Shetti, N.P. Fast and facile electrochemical detection and determination of fungicide car-bendazim at titanium dioxide designed carbon-based sensor. Mater. Chem. Phys. 2022, 285, 126131. [Google Scholar] [CrossRef]
- Krishnan, R.G.; Saraswathyamma, B. Disposable electrochemical sensor for coumarin induced milk toxicity in raw milk samples. Measurement 2020, 170, 108709. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nano-materials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef] [PubMed]
- Padmanaban, A.; Murugadoss, G.; Venkatesh, N.; Hazra, S.; Kumar, M.R.; Tamilselvi, R.; Sakthivel, P. Electrochemical deter-mination of harmful catechol and rapid decolorization of textile dyes using ceria and tin doped ZnO nanoparticles. J. Environ. Chem. Eng. 2021, 9, 105976. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Shetti, N.P.; Kalanur, S.S.; Pollet, B.G.; Nadagouda, M.N.; Aminabhavi, T.M. Hafnium doped tungsten oxide intercalated carbon matrix for electrochemical detection of perfluorooctanoic acid. Chem. Eng. J. 2022, 434, 134700. [Google Scholar] [CrossRef]
- Tümay, S.O.; Şenocak, A.; Sarı, E.; Şanko, V.; Durmuş, M.; Demirbas, E. A new perspective for electrochemical determination of parathion and chlorantraniliprole pesticides via carbon nanotube-based thiophene-ferrocene appended hybrid nanosensor. Sens. Actuators B Chem. 2021, 345, 130344. [Google Scholar] [CrossRef]
- Shetti, N.P.; Sampangi, L.V.; Hegde, R.N.; Nandibewoor, S.T. Electrochemical oxidation of loop diuretic furosemide at gold electrode and its analytical applications. Int. J. Electrochem. Sci. 2009, 4, 104–121. [Google Scholar]
- Ostojić, J.; Herenda, S.; Bešić, Z.; Miloš, M.; Galić, B. Advantages of an electrochemical method compared to the spectrophoto-metric kinetic study of peroxidase inhibition by boroxine derivative. Molecules 2017, 22, 1120. [Google Scholar] [CrossRef]
- PVernekar, R.; Shanbhag, M.M.; Shetti, N.P.; Mascarenhas, R.J. Silica-gel incorporated carbon paste sensor for the electrocat-alytic oxidation of famotidine and its application in biological sample analysis. Electrochem. Sci. Adv. 2021, e2100093. [Google Scholar] [CrossRef]
- Manasa, G.; Mascarenhas, R.J.; Bhakta, A.K.; Mekhalif, Z. Nano-graphene-platelet/Brilliant-green composite coated carbon paste electrode interface for electrocatalytic oxidation of flavanone Hesperidin. Microchem. J. 2020, 160, 105768. [Google Scholar] [CrossRef]
- Kumar, S.; Bukkitgar, S.; Singh, S.; Pratibha; Singh, V.; Reddy, K.R.; Shetti, N.P.; Reddy, C.V.; Sadhu, V.; Naveen, S. Electrochemical Sensors and Biosensors Based on Graphene Functionalized with Metal Oxide Nanostructures for Healthcare Applications. ChemistrySelect 2019, 4, 5322–5337. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Ilager, D.; Mahapatra, S.; Shetti, N.P.; Chandra, P. Amberlite XAD-4 based electrochemical sensor for diclofenac detection in urine and commercial tablets. Mater. Chem. Phys. 2021, 273, 125044. [Google Scholar] [CrossRef]
- Yan, D.; Lou, Y.; Yang, Y.; Chen, Z.; Cai, Y.; Guo, Z.; Zhan, H.; Chen, B. Dye-Modified Metal–Organic Framework as a Recyclable Luminescent Sensor for Nicotine Determination in Urine Solution and Living Cell. ACS Appl. Mater. Interfaces 2019, 11, 47253–47258. [Google Scholar] [CrossRef] [PubMed]
- Malode, S.J.; Keerthi, P.K.; Shetti, N.P.; Kulkarni, R.M. Electroanalysis of carbendazim using MWCNT/Ca-ZnO modified elec-trode. Electroanalysis 2020, 32, 1590–1599. [Google Scholar] [CrossRef]
- Liu, A.Y.; Cohen, M.L. Prediction of New Low Compressibility Solids. Science 1989, 245, 841–842. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.L. Calculation of bulk moduli of diamond and zinc-blende solids. Phys. Rev. B 1985, 32, 7988–7991. [Google Scholar] [CrossRef]
- Liebig, J. Ueber die Constitution des Aethers und seiner Verbindungen. Eur. J. Org. Chem. 1834, 9, 1–39. [Google Scholar] [CrossRef]
- Khan, M.A.; Mutahir, S.; Wang, F.; Lei, W.; Xia, M.; Zhu, S. Facile one-step economical methodology of metal free g-C3N4 syn-thesis with remarkable photocatalytic performance under visible light to degrade trans-resveratrol. J. Hazard. Mater. 2019, 367, 293–303. [Google Scholar] [CrossRef]
- Ilager, D.; Shetti, N.P.; Reddy, K.R.; Tuwar, S.M.; Aminabhavi, T.M. Nanostructured graphitic carbon nitride (g-C3N4)-CTAB modified electrode for the highly sensitive detection of amino-triazole and linuron herbicides. Environ. Res. 2021, 204, 111856. [Google Scholar] [CrossRef]
- Reddy, K.R.; Reddy, C.V.; Nadagouda, M.N.; Shetti, N.P.; Jaesool, S.; Aminabhavi, T.M. Polymeric graphitic carbon nitride (g-C3N4)-based semiconducting nanostructured materials: Synthesis methods, properties and photocatalytic applications. J. Environ. Manag. 2019, 238, 25–40. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Antonietti, M. Graphitic carbon nitride “reloaded”: Emerging applications beyond (photo) catalysis. Chem. Soc. Rev. 2016, 45, 2308–2326. [Google Scholar] [CrossRef]
- Sun, S.; Li, J.; Cui, J.; Gou, X.; Yang, Q.; Liang, S.; Yang, Z.; Zhang, J. Constructing oxygen-doped g-C3N4 nanosheets with an enlarged conductive band edge for enhanced visible-light-driven hydrogen evolution. Inorg. Chem. Front. 2018, 5, 1721–1727. [Google Scholar] [CrossRef]
- Liao, G.; He, F.; Li, Q.; Zhong, L.; Zhao, R.; Che, H.; Gao, H.; Fang, B. Emerging graphitic carbon nitride-based materials for biomedical applications. Prog. Mater. Sci. 2020, 112, 100666. [Google Scholar] [CrossRef]
- Nasir, M.S.; Yang, G.; Ayub, I.; Wang, S.; Wang, L.; Wang, X.; Yan, W.; Peng, S.; Ramakarishna, S. Recent development in graphitic carbon nitride based photocatalysis for hydrogen generation. Appl. Catal. B Environ. 2019, 257, 117855. [Google Scholar] [CrossRef]
- Mamba, G.; Mishra, A. Applied catalysis B: Environmental graphitic carbon nitride (gC3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Nirbhaya, V.; Chauhan, D.; Jain, R.; Chandra, R.; Kumar, S. Nanostructured graphitic carbon nitride based ultrasensing elec-trochemical biosensor for food toxin detection. Bioelectrochemistry 2021, 139, 107738. [Google Scholar] [CrossRef]
- Kesavan, G.; Vinothkumar, V.; Chen, S.-M.; Thangadurai, T.D. Construction of metal-free oxygen-doped graphitic carbon nitride as an electrochemical sensing platform for determination of antimicrobial drug metronidazole. Appl. Surf. Sci. 2021, 556, 149814. [Google Scholar] [CrossRef]
- Madhusudhana; Manasa, G.; Bhakta, A.K.; Mekhalif, Z.; Mascarenhas, R.J. Bismuth-nanoparticles decorated multi-wall-carbon-nanotubes cast-coated on carbon paste electrode; an electrochemical sensor for sensitive determination of Gallic Acid at neutral pH. Mater. Sci. Energy Technol. 2020, 3, 174–182. [Google Scholar] [CrossRef]
- BKorbi, H.; Tapsoba, I.; Benkhoud, M.; Boujlel, K. Electroxidation of ortho-substituted aromatic amines mechanistic investi-gation. J. Electroanal. Chem. 2004, 571, 241–246. [Google Scholar]
- Hassaninejad-Darzi, S.K.; Shajie, F. Simultaneous determination of acetaminophen, pramipexole and carbamazepine by ZSM-5 nanozeolite and TiO2 nanoparticles modified carbon paste electrode. Mater. Sci. Eng. C 2018, 91, 64–77. [Google Scholar] [CrossRef]
- Laviron, E. General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J. Electroanal. Chem. Interfacial Electrochem. 1979, 101, 19–28. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Shetti, N.P.; Kalanur, S.S.; Pollet, B.G.; Upadhyaya, K.P.; Ayachit, N.H.; Aminabhavi, T.M. Hf-Doped Tungsten Oxide Nanorods as Electrode Materials for Electrochemical Detection of Paracetamol and Salbutamol. ACS Appl. Nano Mater. 2021, 5, 1263–1275. [Google Scholar] [CrossRef]
- Bard, A.; Faulkner, L.; Leddy, J.; Zoski, C. Electrochemical Methods: Fundamentals and Applications; Wiley: New York, NY, USA, 1980; Volume 2, p. 231. [Google Scholar]
- Elgrishi, N.; Rountree, K.; McCarthy, B.D.; Rountree, E.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2017, 95, 197–206. [Google Scholar] [CrossRef]
- Dey, S.; Pradhan, P.K.; Upadhayay, U.; Desai, K.; Niranjani, D. Method development and validation of pramipexole by UV spectrophotometric method. J. Pharm. Res. 2012, 5, 5052–5054. [Google Scholar]
- Deng, X.; Hai, X.; Vervoort, R.; Pamperin, D.; Adams, E.; Van Schepdael, A. Development and validation of a chiral capillary electrophoresis method for assay and enantiomeric purity control of pramipexole. J. Sep. Sci. 2011, 34, 3070–3076. [Google Scholar] [CrossRef] [PubMed]
- Panditrao, V.M.; Sarkate, A.P.; Sangshetti, J.N.; Wakte, P.S.; Shinde, D.B. Stability-indicating HPLC determination of pramipexole dihydrochloride in bulk drug and pharmaceutical dosage form. J. Braz. Chem. Soc. 2011, 22, 1253–1258. [Google Scholar] [CrossRef][Green Version]
- Narayana, P.S.; Teradal, N.L.; Seetharamappa, J.; Satpati, A.K. A novel electrochemical sensor for non-ergoline dopamine ag-onist pramipexole based on electrochemically reduced graphene oxide nanoribbons. Anal. Methods 2015, 7, 3912–3919. [Google Scholar] [CrossRef]
- Bozal-Palabiyik, B.; Uslu, B. Comparative study for voltammetric investigation and trace determination of pramipexole at bare and carbon nanotube-modified glassy carbon electrodes. Ionics 2016, 22, 2519–2528. [Google Scholar] [CrossRef]
- Hassaninejad-Darzi, S.K.; Shajie, F. A sensitive voltammetric determination of Anti-Parkinson drug pramipexole using tita-nium dioxide nanoparticles modified carbon paste electrode. J. Braz. Chem. Soc. 2017, 28, 529–539. [Google Scholar]



| Comparative Study | ||||
|---|---|---|---|---|
| Methods/Sensors Utilized | Linearity Range (µM) | LD (µM) | Sensitivity (µA·µM−1·cm−2) | Reference |
| UV | 0.04 to 1.4 | 0.015 | - | [45] |
| Capillary electrophoresis | 0.2 to 3.5 | 0.04 | - | [46] |
| HPLC | 0.02 to 1.4 | 0.01 | - | [47] |
| ZSM-5 nanozeolite and TiO2 nanoparticles | 0.6 to 105.0 | 0.38 | 0.44 | [40] |
| Carbon nanotube–modified glassy carbon electrodes | 0.013 to 0.66 | 0.023 | - | [49] |
| Titanium dioxide nanoparticles–modified carbon paste electrode | 0.46 to 100 | 0.14 | 0.66 | [50] |
| Graphitic carbon nitride–modified carbon paste electrode | 0.05 to 500 | 0.012 | 7.44 | Present work |
| Drug Spiked | Tablet Samples | Spiked (10−6 M) | Detected * (10−6 M) | % Recovery | RSD | % RSD |
|---|---|---|---|---|---|---|
| PMXL | Aliquot-1 | 5.0 | 4.78 | 95.58 | 0.0092 | 0.91 |
| Aliquot-2 | 10.0 | 9.73 | 97.32 | 0.0090 | 0.89 | |
| Aliquot-3 | 15.0 | 14.44 | 96.24 | 0.0091 | 0.90 |
| Interferent | Detected Epa (V) | Standard Epa (V) | Change in % Epa |
|---|---|---|---|
| Excipients | |||
| Cellulose | 0.94 | 0.948 | −0.84 |
| Gum acacia | 0.944 | 0.948 | −0.42 |
| Mannitol | 0.944 | 0.948 | −0.42 |
| Starch | 0.936 | 0.948 | −1.26 |
| TiO2 | 0.96 | 0.948 | 1.26 |
| Metal Ions | |||
| Ca(NO3)2 | 0.936 | 0.948 | −1.26 |
| CuSO4 | 0.94 | 0.948 | −0.84 |
| FeSO4 | 0.956 | 0.948 | 0.84 |
| KCl | 0.944 | 0.948 | −0.42 |
| MnSO4 | 0.944 | 0.948 | −0.42 |
| NaCl | 0.952 | 0.948 | 0.42 |
| Reproducibility | |||||
|---|---|---|---|---|---|
| CV Responses | Detected Response | Original Response | Response Retention % | RSD | % RSD |
| At 1st day | 22.57 | 22.57 | 100.00 | 0.031 | 3.14 |
| At 4th day | 22.57 | 21.86 | 96.85 | 0.032 | 3.24 |
| At 8th day | 22.57 | 21.29 | 94.33 | 0.033 | 3.33 |
| At 12th day | 22.57 | 20.95 | 92.82 | 0.033 | 3.38 |
| % Retention: 92.82–96.85; Average % retention: 96.01; % RSD: 3.14 | |||||
| Repeatability | |||||
| At 0 h | 22.57 | 22.57 | 100.00 | 0.021 | 2.18 |
| After 12 h | 22.57 | 21.76 | 96.41 | 0.022 | 2.26 |
| After 24 h | 22.57 | 21.68 | 96.06 | 0.022 | 2.27 |
| % Retention: 96.06–100.0; Average % retention: 97.5; % RSD: 2.24 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanbhag, Y.M.; Shanbhag, M.M.; Malode, S.J.; Dhanalakshmi, S.; Mondal, K.; Shetti, N.P. Direct and Sensitive Electrochemical Evaluation of Pramipexole Using Graphitic Carbon Nitride (gCN) Sensor. Biosensors 2022, 12, 552. https://doi.org/10.3390/bios12080552
Shanbhag YM, Shanbhag MM, Malode SJ, Dhanalakshmi S, Mondal K, Shetti NP. Direct and Sensitive Electrochemical Evaluation of Pramipexole Using Graphitic Carbon Nitride (gCN) Sensor. Biosensors. 2022; 12(8):552. https://doi.org/10.3390/bios12080552
Chicago/Turabian StyleShanbhag, Yogesh M., Mahesh M. Shanbhag, Shweta J. Malode, S. Dhanalakshmi, Kunal Mondal, and Nagaraj P. Shetti. 2022. "Direct and Sensitive Electrochemical Evaluation of Pramipexole Using Graphitic Carbon Nitride (gCN) Sensor" Biosensors 12, no. 8: 552. https://doi.org/10.3390/bios12080552
APA StyleShanbhag, Y. M., Shanbhag, M. M., Malode, S. J., Dhanalakshmi, S., Mondal, K., & Shetti, N. P. (2022). Direct and Sensitive Electrochemical Evaluation of Pramipexole Using Graphitic Carbon Nitride (gCN) Sensor. Biosensors, 12(8), 552. https://doi.org/10.3390/bios12080552







