Applications of Microfluidics and Organ-on-a-Chip in Cancer Research
Abstract
:1. Introduction and Overview
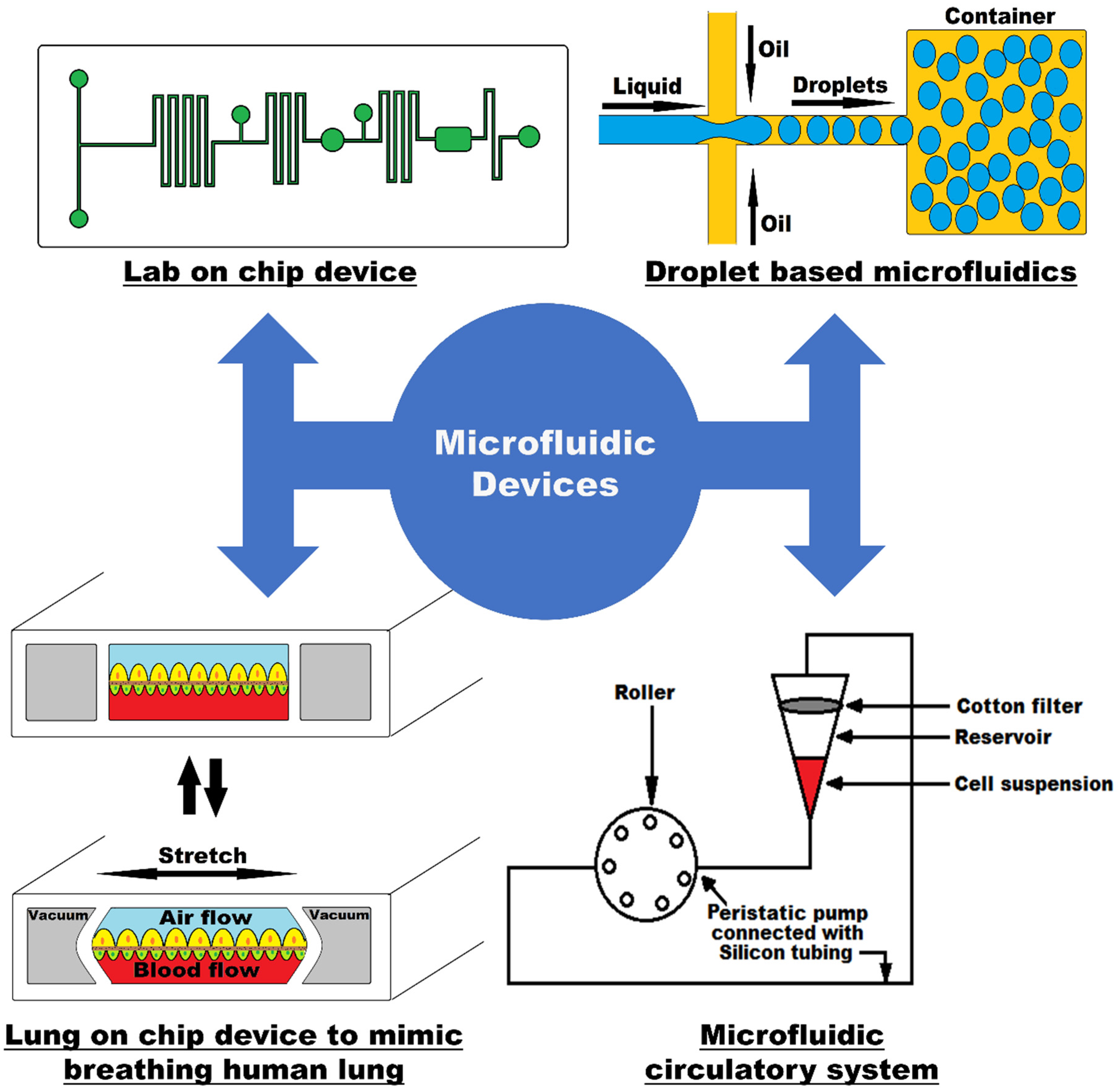
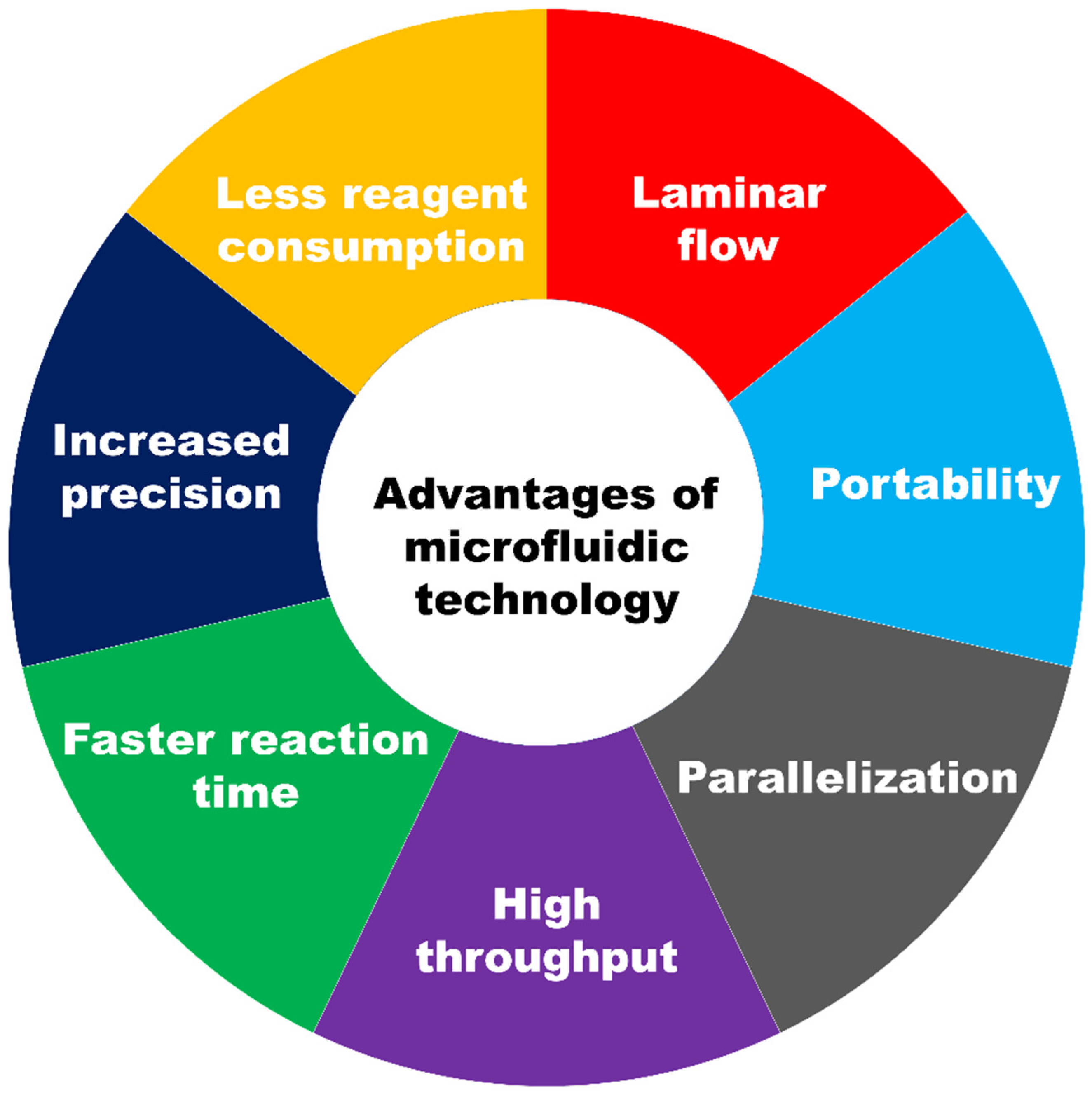
1.1. Introduction to Microfluidic Technology
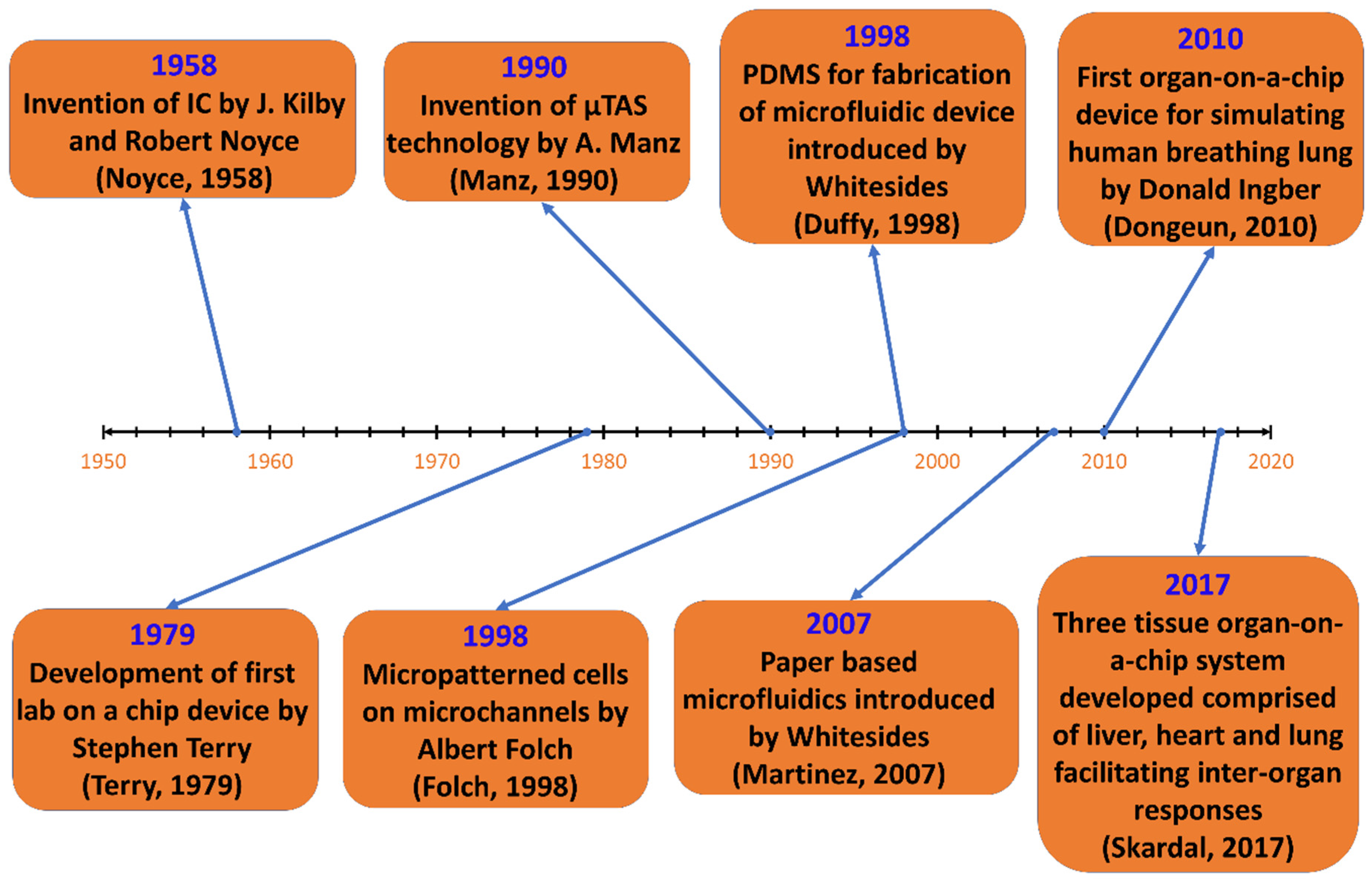
1.2. Historical Developments of Microfluidics
1.3. How Microfluidic Devices Work
1.3.1. Reynold’s Number
1.3.2. Peclet’s Number
1.3.3. Diffusion
1.3.4. Fluidic Resistance
1.3.5. Viscous Drag Force
1.3.6. Inertial Focusing
1.3.7. Surface Area to Volume Ratio
1.3.8. Surface Tension
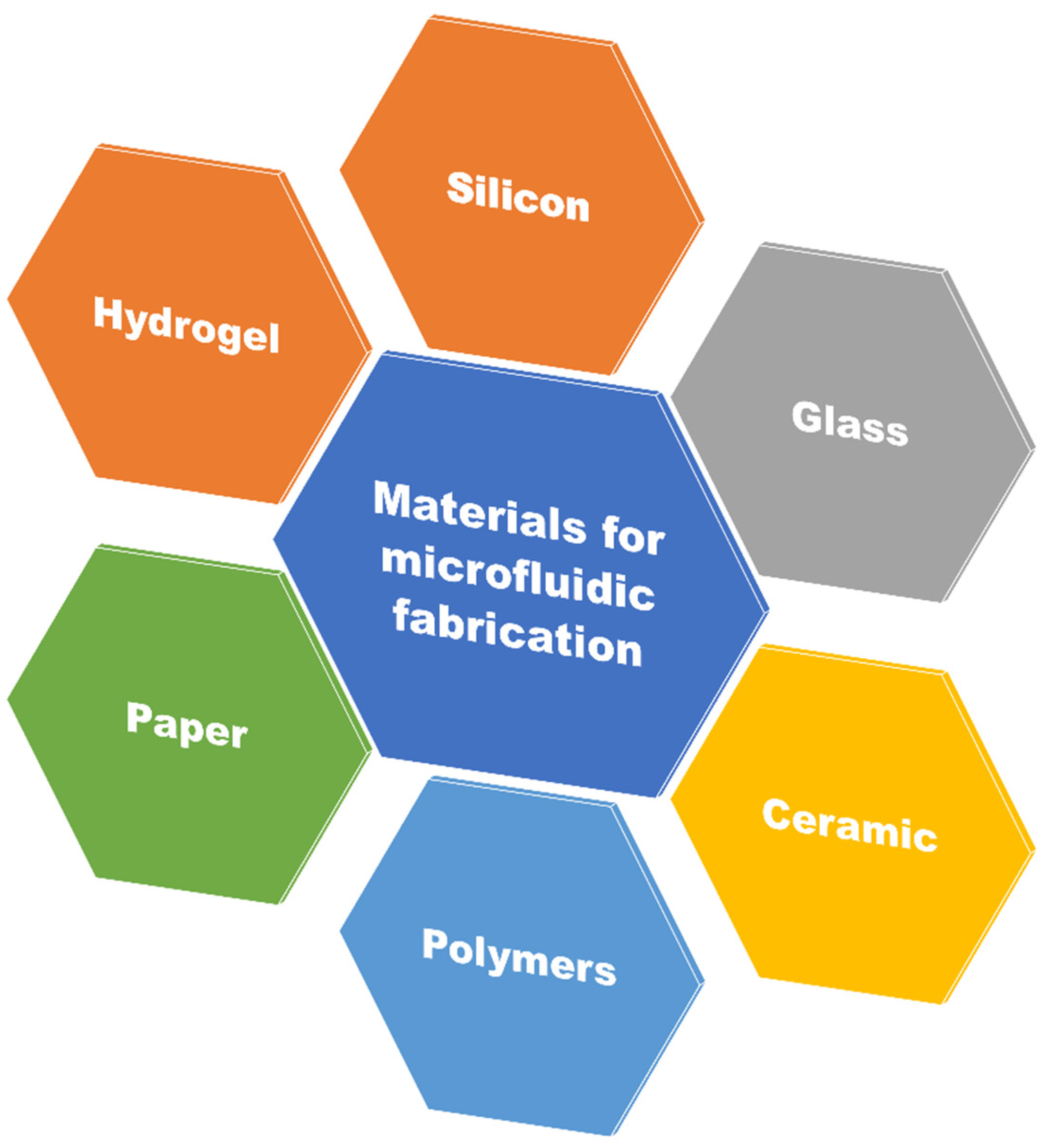
1.4. Designing Materials for Microfluidics
1.4.1. Polydimethylsiloxane (PDMS)
1.4.2. Silicon
1.4.3. Glass
1.4.4. Paper
2. Microfluidic Devices in Cancer Research
2.1. Fundamentals of Cancer Metastasis
2.2. Microfluidics in Cancer Research
| Application of Microfluidic Technology in Oncology | Description | References |
|---|---|---|
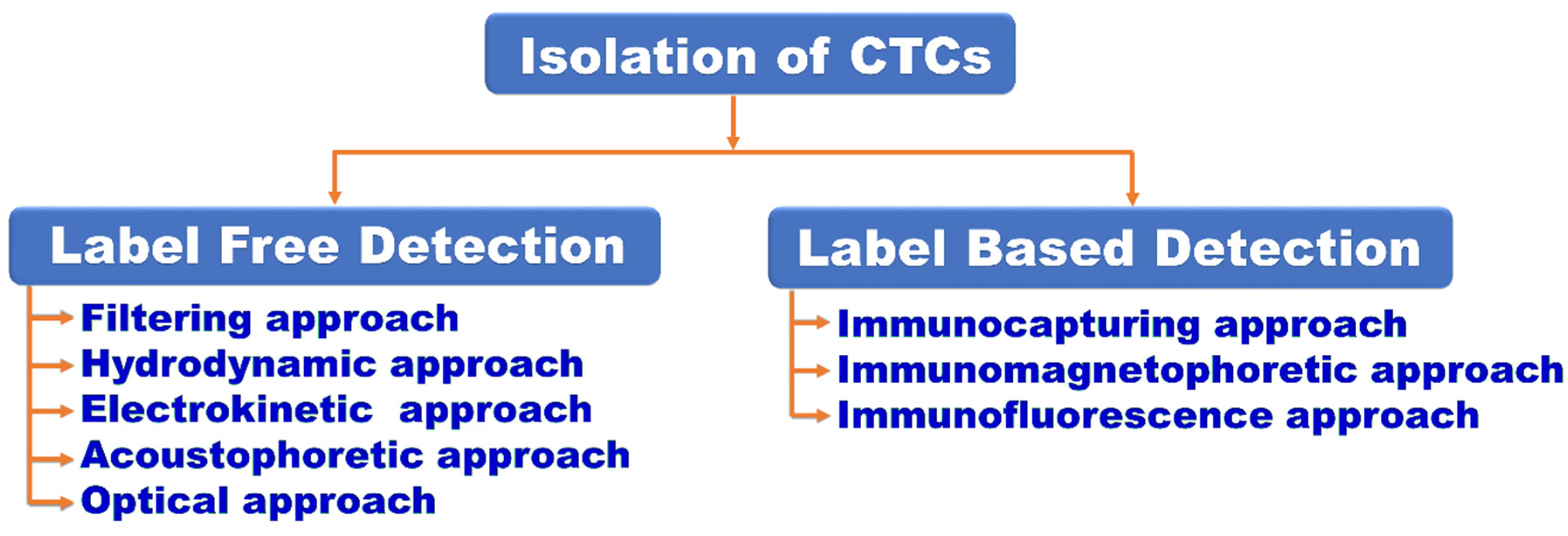
| Isolation of CTCs | Performing label free and label-based methods for separation of cancer cells from background blood cells | [32,33,34,35,36,37,38,39,40] |
| Studying cancer cell phenotype | For studying the mechanical qualities that influence the migration of cancer cells and metastatic pattern | [41,42,43,44,45] |
| Studying shear stress | For characterizing the biophysical response of tumor cells due to shear stress in circulation | [46,47,48,49,50] |
| Studying metastasis | For studying the metastatic cascade by developing microfluidic tools able to reproduce biophysical, biomechanical and biochemical environment | [51,52,53,54,55,56] |
| Anti-cancer drug screening using droplet microfluidics | For allowing programmable drug absorption, confinement and controlled release | [57,58,59,60] |
| Replication of tumor microenvironment (TME) on chip | For recapitulating the key features of tumor microenvironment including tumor-stromal interaction, extracellular matrix (ECM) components, biophysical and metabolic factors | [61,62,63] |
| Studying angiogenesis and developing vascularized tumor on chip | For recreating prominent features of TME for oxygen and nutrient delivery to tumor cells | [64,65,66] |
| Organ-on-a-chip | For replicating the physiological aspects of an organ for replicating the structural, mechanical and biological factors for understanding cancer biology and advancing drug development process | [67,68,69,70] |
2.2.1. Microfluidic Device for Isolation of CTC
2.2.2. Microfluidic Devices for Studying Cancer Cell Phenotype
2.2.3. Microfluidic Devices for Studying Shear Stress
2.2.4. Microfluidic Device for Studying Metastasis
3. Microfluidic Device in Anti-Cancer Drug Screening
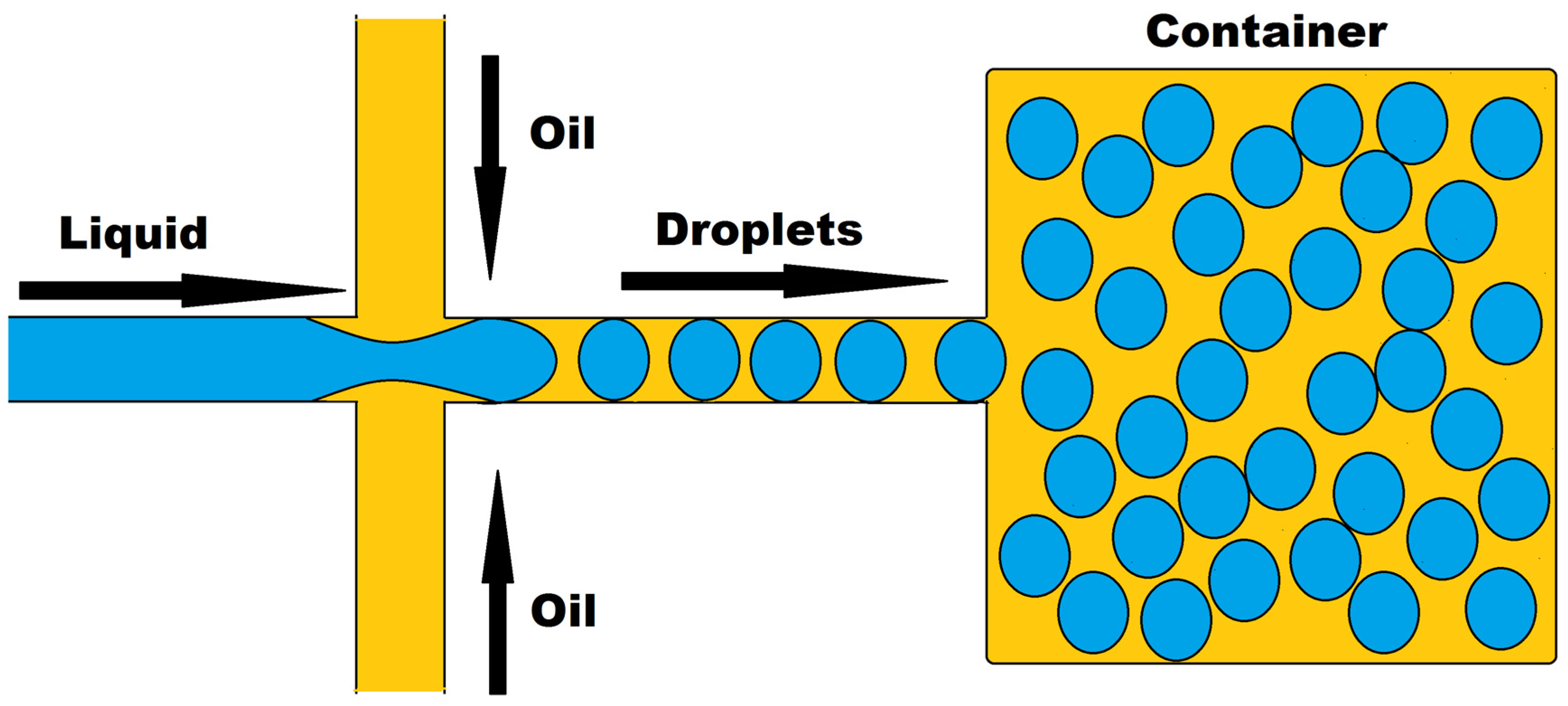
3.1. Drug Response Studies Using Droplet Microfluidics
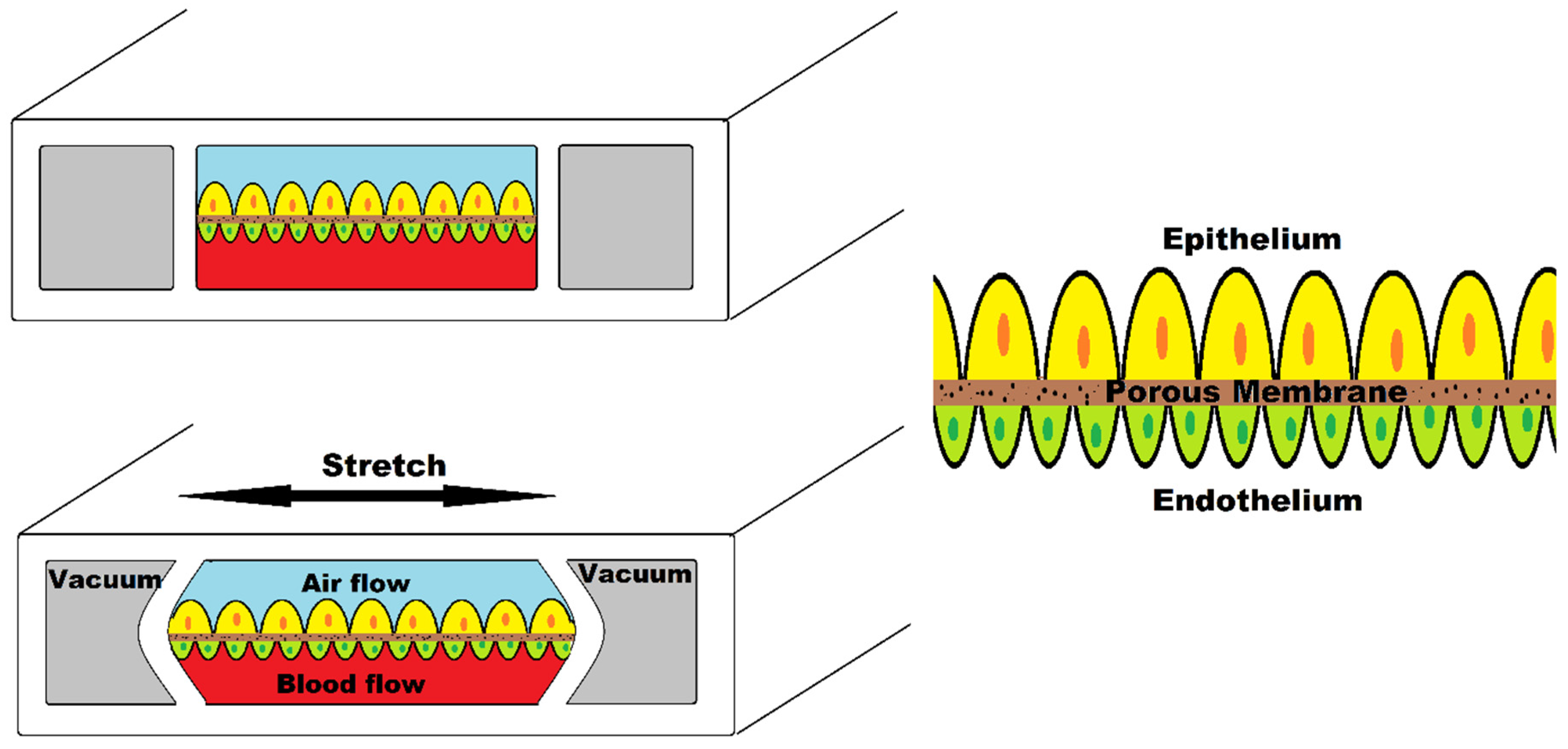
3.2. Organ-on-a-Chip Platform
3.3. Organ-on-a-Chip in Cancer Research
3.4. Replication of Tumor Microenvironment on Chip
3.5. Modelling Angiogenesis
3.6. Modelling Vascularized Tumor Models
3.7. Metastatic Cascade in Organ-on-a-Chip
4. Concluding Remarks
4.1. Significance of Microfluidic and Organ-on-a-Chip Device in Cancer Research
4.2. Limitations of Microfluidic and Organ-on-a-Chip Device in Cancer Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Cottet, J.; Renaud, P. Introduction to microfluidics. In Drug Delivery Devices and Therapeutic Systems; Elsevier: Amsterdam, The Netherlands, 2021; pp. 3–17. [Google Scholar]
- Tabeling, P. Introduction to Microfluidics; OUP Oxford: Oxford, UK, 2005. [Google Scholar]
- Unger, M.A.; Chou, H.P.; Thorsen, T.; Scherer, A.; Quake, S.R. Monolithic microfabricated valves and pumps by multilayer soft lithography. Science 2000, 288, 113–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Fohlerova, Z.; Pekarek, J.; Basova, E.; Neuzil, P. Recent advances in lab-on-a-chip technologies for viral diagnosis. Biosens. Bioelectron. 2020, 153, 112041. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.J.; Ingber, D.E.; den Toonder, J. Organs on Chips 2013. Lab Chip 2013, 13, 3447–3448. [Google Scholar] [CrossRef] [PubMed]
- Brock, D.; Laws, D. The early history of microcircuitry: An overview. IEEE Ann. Hist. Comput. 2011, 34, 7–19. [Google Scholar] [CrossRef]
- Lathrop, J.W. The Diamond Ordnance Fuze Laboratory’s Photolithographic Approach to Microcircuits. IEEE Ann. Hist. Comput. 2011, 35, 48–55. [Google Scholar] [CrossRef]
- Terry, S.C.; Jerman, J.H.; Angell, J.B. A gas chromatographic air analyzer fabricated on a silicon wafer. IEEE Trans. Electron Devices 1979, 26, 1880–1886. [Google Scholar] [CrossRef]
- Thomas, N.R. Frederic Stanley Kipping—Pioneer in silicon chemistry: His life & legacy. Silicon 2010, 2, 187–193. [Google Scholar]
- Hum, P.W. Exploration of Large Scale Manufacturing of Polydimethylsiloxane (pdms) Microfluidic Devices. Ph.D. Thesis, Massachusetts Institute of Technology, Cambridge, MA, USA, 2006. [Google Scholar]
- Wang, M.; Duan, B. Materials and their biomedical applications. In Encyclopedia of Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–152. [Google Scholar]
- Folch, A.; Toner, M. Cellular micropatterns on biocompatible materials. Biotechnol. Prog. 1998, 14, 388–392. [Google Scholar] [CrossRef]
- Squires, T.M.; Quake, S.R. Microfluidics: Fluid physics at the nanoliter scale. Rev. Mod. Phys. 2005, 77, 977. [Google Scholar] [CrossRef] [Green Version]
- Beebe, D.J.; Mensing, G.A.; Walker, G.M. Physics and applications of microfluidics in biology. Annu. Rev. Biomed. Eng. 2002, 4, 261–286. [Google Scholar] [CrossRef] [PubMed]
- Passioura, J.B. Hydraulic resistance of plants. I. Constant or variable? Aust. J. Plant Physiol. 1984, 11, 333–339. [Google Scholar] [CrossRef]
- Loudet, J.; Hanusse, P.; Poulin, P. Stokes drag on a sphere in a nematic liquid crystal. Science 2004, 306, 1525. [Google Scholar] [CrossRef] [PubMed]
- Mashhadian, A.; Shamloo, A. Inertial microfluidics: A method for fast prediction of focusing pattern of particles in the cross section of the channel. Anal. Chim. Acta 2019, 1083, 137–149. [Google Scholar] [CrossRef]
- Abdulla, A.; Liu, W.; Gholamipour-Shirazi, A.; Sun, J.; Ding, X. High-throughput isolation of circulating tumor cells using cascaded inertial focusing microfluidic channel. Anal. Chem. 2018, 90, 4397–4405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yuan, D.; Zhang, J.; Li, W. A Review of Secondary Flow in Inertial Microfluidics. Micromachines 2020, 11, 461. [Google Scholar] [CrossRef]
- Pennathur, S.; Meinhart, C.D.; Soh, H.T. How to exploit the features of microfluidics technology. Lab Chip 2008, 8, 20–22. [Google Scholar]
- Ren, K.; Zhou, J.; Wu, H. Materials for microfluidic chip fabrication. Acc. Chem. Res. 2013, 46, 2396–2406. [Google Scholar] [CrossRef]
- Roy, E.; Pallandre, A.; Zribi, B.; Horny, M.C.; Delapierre, F.D.; Cattoni, A.; Gamby, J.; Haghiri-Gosnet, A.-M. Overview of materials for microfluidic applications. In Advances in Microfluidics—New Applications in Biology, Energy, and Materials Sciences; BoD–Books on Demand: Norderstedt, Germany, 2016. [Google Scholar]
- Borok, A.; Laboda, K.; Bonyar, A. PDMS Bonding Technologies for Microfluidic Applications: A Review. Biosensors 2021, 11, 292. [Google Scholar] [CrossRef]
- Lamberti, A.; Marasso, S.; Cocuzza, M.J.R.A. PDMS membranes with tunable gas permeability for microfluidic applications. RSC Adv. 2014, 4, 61415–61419. [Google Scholar] [CrossRef]
- Munaro, A.P.; da Cunha, G.P.; Filgueiras, J.G.; Pinto, J.M.; Munaro, M.; de Azevedo, E.R.; Akcelrud, L.C. Ageing and structural changes in PDMS rubber investigated by time domain NMR. Polym. Degrad. Stab. 2019, 166, 300–306. [Google Scholar] [CrossRef]
- Martin, A.; Teychené, S.; Camy, S.; Aubin, J. Fast and inexpensive method for the fabrication of transparent pressure-resistant microfluidic chips. Microfluid. Nanofluid. 2016, 20, 92. [Google Scholar] [CrossRef] [Green Version]
- Boobphahom, S.; Ly, M.N.; Soum, V.; Pyun, N.; Kwon, O.S.; Rodthongkum, N.; Shin, K. Recent Advances in Microfluidic Paper-Based Analytical Devices toward High-Throughput Screening. Molecules 2020, 25, 2970. [Google Scholar] [CrossRef] [PubMed]
- Hapach, L.A.; Mosier, J.A.; Wang, W.; Reinhart-King, C.A. Engineered models to parse apart the metastatic cascade. NPJ Precis. Oncol. 2019, 3, 20. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Cubero, M.J.; Lorente, J.A.; Robles-Fernandez, I.; Rodriguez-Martinez, A.; Puche, J.L.; Serrano, M.J. Circulating Tumor Cells: Markers and Methodologies for Enrichment and Detection. Methods Mol. Biol. 2017, 1634, 283–303. [Google Scholar]
- Zhang, Z.; Nagrath, S. Microfluidics and cancer: Are we there yet? Biomed. Microdevices 2013, 15, 595–609. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.S.; Hu, M.; Huang, M.C.; Cheong, W.C.; Gan, A.T.L.; Looi, X.L.; Leong, S.M.; Siew-Chang Koay, E.; Li, M.-H. Microsieve lab-chip device for rapid enumeration and fluorescence in situ hybridization of circulating tumor cells. Lab Chip 2012, 12, 4388–4396. [Google Scholar] [CrossRef]
- Wang, S.; Wan, Y.; Liu, Y. Effects of nanopillar array diameter and spacing on cancer cell capture and cell behaviors. Nanoscale 2014, 6, 12482–12489. [Google Scholar] [CrossRef] [Green Version]
- Loutherback, K.; D’Silva, J.; Liu, L.; Wu, A.; Austin, R.H.; Sturm, J.C. Deterministic separation of cancer cells from blood at 10 mL/min. AIP Adv. 2012, 2, 042107. [Google Scholar] [CrossRef] [Green Version]
- Wang, R. Hydrodynamic trapping of particles in an expansion-contraction microfluidic device. In Abstract and Applied Analysis; Hindawi: London, UK, 2013. [Google Scholar]
- Bhagat, A.A.S.; Hou, H.W.; Li, L.D.; Lim, C.T.; Han, J. Dean flow fractionation (DFF) isolation of circulating tumor cells (CTCs) from blood. In Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Seattle, WA, USA, 2–6 October 2011. [Google Scholar]
- Pødenphant, M.; Ashley, N.; Koprowska, K.; Mir, K.U.; Zalkovskij, M.; Bilenberg, B.; Bodmer, W.; Kristensen, A.; Marie, R. Separation of cancer cells from white blood cells by pinched flow fractionation. Lab Chip 2015, 15, 4598–4606. [Google Scholar] [CrossRef] [Green Version]
- Alshareef, M.; Metrakos, N.; Juarez Perez, E.; Azer, F.; Yang, F.; Yang, X.; Wang, G. Separation of tumor cells with dielectrophoresis-based microfluidic chip. Biomicrofluidics 2013, 7, 011803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, H.-S.; Kwon, K.; Kim, S.I.; Han, H.; Sohn, J.; Lee, S.; Jung, H.I. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP). Lab Chip 2011, 11, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, K.; Huang, Y.Y.; Lane, N.; Huebschman, M.; Uhr, J.W.; Frenkel, E.P.; Zhang, X. Microchip-based immunomagnetic detection of circulating tumor cells. Lab Chip 2011, 11, 3449–3457. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.W.; Li, Q.S.; Lee, G.Y.H.; Kumar, A.P.; Ong, C.N.; Lim, C.T. Deformability study of breast cancer cells using microfluidics. Biomed. Microdevices 2009, 11, 557–564. [Google Scholar] [CrossRef]
- Adamo, A.; Sharei, A.; Adamo, L.; Lee, B.; Mao, S.; Jensen, K.F. Microfluidics-based assessment of cell deformability. Anal. Chem. 2012, 84, 6438–6443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pushkarsky, I.; Tseng, P.; Black, D.; France, B.; Warfe, L.; Koziol-White, C.J.; Jester, W.F., Jr.; Trinh, R.K.; Lin, J.; Scumpia, P.O.; et al. Elastomeric sensor surfaces for high-throughput single-cell force cytometry. Nat. Biomed. Eng. 2018, 2, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tian, Z.; Wang, Z.; Rufo, J.; Li, P.; Mai, J.; Xia, J.; Bachman, H.; Huang, P.-H.; Wu, M.; et al. Harmonic acoustics for dynamic and selective particle manipulation. Nat. Mater. 2022, 21, 540–546. [Google Scholar] [CrossRef]
- Augustsson, P.; Karlsen, J.T.; Su, H.-W.; Bruus, H.; Voldman, J. Iso-acoustic focusing of cells for size-insensitive acousto-mechanical phenotyping. Nat. Commun. 2016, 7, 11556. [Google Scholar] [CrossRef]
- Landwehr, G.M.; Kristof, A.J.; Rahman, S.M.; Pettigrew, J.H.; Coates, R.; Balhoff, J.B.; Triantafillu, U.L.; Kim, Y.; Melvin, A.T. Biophysical analysis of fluid shear stress induced cellular deformation in a microfluidic device. Biomicrofluidics 2018, 12, 054109. [Google Scholar] [CrossRef]
- Regmi, S.; Fu, A.; Luo, K.Q. High Shear Stresses under Exercise Condition Destroy Circulating Tumor Cells in a Microfluidic System. Sci. Rep. 2017, 7, 39975. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Mao, S.; Khan, M.; Zhang, Q.; Huang, Q.; Feng, S.; Lin, J.-M. Responses of Cellular Adhesion Strength and Stiffness to Fluid Shear Stress during Tumor Cell Rolling Motion. ACS Sens. 2019, 4, 1710–1715. [Google Scholar] [CrossRef]
- Regmi, S.; Fung, T.S.; Lim, S.; Luo, K.Q. Fluidic shear stress increases the anti-cancer effects of ROS-generating drugs in circulating tumor cells. Breast Cancer Res. Treat. 2018, 172, 297–312. [Google Scholar] [CrossRef]
- Marrella, A.; Fedi, A.; Varani, G.; Vaccari, I.; Fato, M.; Firpo, G.; Guida, P.; Aceto, N.; Scaglione, S. High blood flow shear stress values are associated with circulating tumor cells cluster disaggregation in a multi-channel microfluidic device. PLoS ONE 2021, 16, e0245536. [Google Scholar] [CrossRef]
- Kim, S.H.; Hwang, S.M.I.; Lee, J.M.; Kang, J.H.; Chung, I.Y.; Chung, B.G. Epithelial-to-mesenchymal transition of human lung alveolar epithelial cells in a microfluidic gradient device. Electrophoresis 2013, 34, 441–447. [Google Scholar] [CrossRef]
- Kuo, C.; Chiang, C.L.; Huang, R.Y.J.; Hsinyu, L.; Wo, A.M. Probing the traits of epithelial-mesenchymal transition in a microfluidic device. In Proceedings of the 15th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Seattle, WA, USA, 2–6 October 2011. [Google Scholar]
- Shin, M.K.; Kim, S.K.; Jung, H. Integration of intra-and extravasation in one cell-based microfluidic chip for the study of cancer metastasis. Lab Chip 2011, 11, 3880–3887. [Google Scholar] [CrossRef]
- Lee, H.; Park, W.; Ryu, H.; Jeon, N.L. A microfluidic platform for quantitative analysis of cancer angiogenesis and intravasation. Biomicrofluidics 2014, 8, 054102. [Google Scholar] [CrossRef] [Green Version]
- Nagaraju, S.; Truong, D.; Mouneimne, G.; Nikkhah, M. Microfluidic Tumor-Vascular Model to Study Breast Cancer Cell Invasion and Intravasation. Adv. Healthc. Mater. 2018, 7, e1701257. [Google Scholar] [CrossRef]
- Kuhlbach, C.; Luz, S.; Baganz, F.; Hass, V.C.; Mueller, M.M. A Microfluidic System for the Investigation of Tumor Cell Extravasation. Bioengineering 2018, 5, 40. [Google Scholar] [CrossRef] [Green Version]
- Brouzes, E.; Medkova, M.; Savenelli, N.; Marran, D.; Twardowski, M.; Hutchison, J.B.; Rothberg, J.M.; Link, D.R.; Perrimon, N.; Samuels, M.L. Droplet microfluidic technology for single-cell high-throughput screening. Proc. Natl. Acad. Sci. USA 2009, 106, 14195–14200. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Chen, M.C.; Cheung, K.C. Droplet-based microfluidic system for multicellular tumor spheroid formation and anticancer drug testing. Lab Chip 2010, 10, 2424–2432. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J. Mixed hydrogel bead-based tumor spheroid formation and anticancer drug testing. Analyst 2014, 139, 2449–2458. [Google Scholar] [CrossRef] [PubMed]
- Sabhachandani, P.; Motwani, V.; Cohen, N.; Sarkar, S.; Torchilin, V.; Konry, T. Generation and functional assessment of 3D multicellular spheroids in droplet based microfluidics platform. Lab Chip 2016, 16, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Menon, N.V.; Chuah, Y.J.; Cao, B.; Lim, M.; Kang, Y. A microfluidic co-culture system to monitor tumor-stromal interactions on a chip. Biomicrofluidics 2014, 8, 064118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gioiella, F.; Urciuolo, F.; Imparato, G.; Brancato, V.; Netti, P.A. An Engineered Breast Cancer Model on a Chip to Replicate ECM-Activation In Vitro during Tumor Progression. Adv. Healthc. Mater. 2016, 5, 3074–3084. [Google Scholar] [CrossRef] [PubMed]
- Rijal, G.; Li, W. A versatile 3D tissue matrix scaffold system for tumor modeling and drug screening. Sci. Adv. 2017, 3, e1700764. [Google Scholar] [CrossRef] [Green Version]
- Nashimoto, Y.; Okada, R.; Hanada, S.; Arima, Y.; Nishiyama, K.; Miura, T.; Yokokawa, R. Vascularized cancer on a chip: The effect of perfusion on growth and drug delivery of tumor spheroid. Biomaterials 2020, 229, 119547. [Google Scholar] [CrossRef]
- Cao, X.; Ashfaq, R.; Cheng, F.; Maharjan, S.; Li, J.; Ying, G.; Hassan, S.; Xiao, H.; Yue, K.; Zhang, Y.S. A Tumor-on-a-Chip System with Bioprinted Blood and Lymphatic Vessel Pair. Adv. Funct. Mater. 2019, 29, 1807173. [Google Scholar] [CrossRef]
- Mannino, R.G.; Santiago-Miranda, A.N.; Pradhan, P.; Qiu, Y.; Mejias, J.C.; Neelapu, S.S.; Roy, K.; Lam, W.A. 3D microvascular model recapitulates the diffuse large B-cell lymphoma tumor microenvironment in vitro. Lab Chip 2017, 17, 407–414. [Google Scholar] [CrossRef]
- Skardal, A.; Devarasetty, M.; Forsyhte, S.; Atala, A.; Soker, S.A. A reductionist metastasis-on-a-chip platform for in vitro tumor progression modeling and drug screening. Biotechnol. Bioeng. 2016, 113, 2020–2032. [Google Scholar] [CrossRef] [Green Version]
- Zervantonakis, I.K.; Hughes-Alford, S.K.; Charest, J.L.; Condeelis, J.S.; Gertler, F.B.; Kamm, R.D. Three-dimensional microfluidic model for tumor cell intravasation and endothelial barrier function. Proc. Natl. Acad. Sci. USA 2012, 109, 13515–13520. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Li, E.; Guo, Z.; Yu, R.; Hao, H.; Xu, Y.; Sun, Z.; Li, X.; Lyu, J.; Wang, Q. Design and Construction of a Multi-Organ Microfluidic Chip Mimicking the in vivo Microenvironment of Lung Cancer Metastasis. ACS Appl. Mater. Interfaces 2016, 8, 25840–25847. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, D.; Wu, G.; Lu, S.; Lo, J.; He, Y.; Zhao, C.; Zhao, X.; Zhang, H.; Wang, S. Metastasis-on-a-chip mimicking the progression of kidney cancer in the liver for predicting treatment efficacy. Theranostics 2020, 10, 300–311. [Google Scholar] [CrossRef]
- Zou, D.; Cui, D. Advances in isolation and detection of circulating tumor cells based on microfluidics. Cancer Biol. Med. 2018, 15, 335–353. [Google Scholar] [PubMed] [Green Version]
- Cima, I.; Yee, C.W.; Iliescu, F.S.; Phyo, W.M.; Lim, K.H.; Iliescu, C.; Tan, M.H. Label-free isolation of circulating tumor cells in microfluidic devices: Current research and perspectives. Biomicrofluidics 2013, 7, 11810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.; Cao, B.; Sun, B.; Lin, Y.-S. Highly-sensitive capture of circulating tumor cells using micro-ellipse filters. Sci. Rep. 2017, 7, 610. [Google Scholar] [CrossRef]
- Pohl, H.A. The motion and precipitation of suspensoids in divergent electric fields. J. Appl. Phys. 1951, 22, 869–871. [Google Scholar] [CrossRef]
- Li, P.; Mao, Z.; Peng, Z.; Zhou, L.; Chen, Y.; Huang, P.-H.; Truica, C.I.; Drabick, J.J.; El-Deiry, W.S.; Dao, M.; et al. Acoustic separation of circulating tumor cells. Proc. Natl. Acad. Sci. USA 2015, 112, 4970–4975. [Google Scholar] [CrossRef] [Green Version]
- Dalili, A.; Samiei, E.; Hoorfar, M. A review of sorting, separation and isolation of cells and microbeads for biomedical applications: Microfluidic approaches. Analyst 2018, 144, 87–113. [Google Scholar] [CrossRef]
- Went, P.T.; Lugli, A.; Meier, S.; Bundi, M.; Mirlacher, M.; Sauter, G.; Dirnhofer, S. Frequent EpCam protein expression in human carcinomas. Hum. Pathol. 2004, 35, 122–128. [Google Scholar] [CrossRef]
- Paterlini-Brechot, P.; Benali, N.L. Circulating tumor cells (CTC) detection: Clinical impact and future directions. Cancer Lett. 2007, 253, 180–204. [Google Scholar] [CrossRef]
- Nagrath, S.; Sequist, L.V.; Maheswaran, S.; Bell, D.W.; Irimia, D.; Ulkus, L.; Smith, M.R.; Kwak, E.L.; Digumarthy, S.; Muzikansky, A.; et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 2007, 450, 1235–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stroock, A.D.; Dertinger, S.K.W.; Ajdari, A.; Mezic, I.; Stone, H.A.; Whitesides, G.M. Chaotic mixer for microchannels. Science 2002, 295, 647–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, W.; Ogunwobi, O.O.; Chen, T.; Zhang, J.; George, T.J.; Liu, C.; Fan, Z.H. Capture, release and culture of circulating tumor cells from pancreatic cancer patients using an enhanced mixing chip. Lab Chip 2014, 14, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Thomas, A.; Lee, E.; Yang, S.; Cheng, X.; Liu, Y. Highly efficient and selective isolation of rare tumor cells using a microfluidic chip with wavy-herringbone micro-patterned surfaces. Analyst 2016, 141, 2228–2237. [Google Scholar] [CrossRef] [Green Version]
- Shaw Bagnall, J.; Byun, S.; Begum, S.; Miyamoto, D.T.; Hecht, V.C.; Maheswaran, S.; Stott, S.L.; Toner, M.; Hynes, R.O.; Manalis, S.R. Deformability of Tumor Cells versus Blood Cells. Sci. Rep. 2015, 5, 18542. [Google Scholar] [CrossRef] [Green Version]
- Hope, J.M.; Bersi, M.R.; Dombroski, J.A.; Clinch, A.B.; Pereles, R.S.; Merryman, W.D.; King, M.R. Circulating prostate cancer cells have differential resistance to fluid shear stress-induced cell death. J. Cell Sci. 2021, 134, jcs251470. [Google Scholar] [CrossRef]
- Zhu, C.; Yago, T.; Lou, J.; Zarnitsyna, V.I.; McEver, R.P. Mechanisms for flow-enhanced cell adhesion. Ann. Biomed. Eng. 2008, 36, 604–621. [Google Scholar] [CrossRef] [Green Version]
- Huang, Q.; Hu, X.; He, W.; Zhao, Y.; Hao, S.; Wu, Q.; Li, S.; Zhang, S.; Shi, M. Fluid shear stress and tumor metastasis. Am. J. Cancer Res. 2018, 8, 763–777. [Google Scholar]
- Fu, A.; Ma, S.; Wei, N.; Tan, B.X.X.; Tan, E.Y.; Luo, K.Q. High expression of MnSOD promotes survival of circulating breast cancer cells and increases their resistance to doxorubicin. Oncotarget 2016, 7, 50239. [Google Scholar] [CrossRef] [Green Version]
- Ma, S.; Fu, A.; Chiew, G.G.Y.; Luo, K.Q. Hemodynamic shear stress stimulates migration and extravasation of tumor cells by elevating cellular oxidative level. Cancer Lett. 2017, 388, 239–248. [Google Scholar] [CrossRef]
- Ma, S.; Fu, A.; Lim, S.; Chiew, G.G.Y.; Luo, K.Q. MnSOD mediates shear stress-promoted tumor cell migration and adhesion. Free Radic. Biol. Med. 2018, 129, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Seyfried, T.N.; Huysentruyt, L.C. On the origin of cancer metastasis. Crit. Rev. Oncog. 2013, 18, 43–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.-H.V.; Middleton, K.; You, L.; Sun, S. A review of microfluidic approaches for investigating cancer extravasation during metastasis. Microsyst. Nanoeng. 2018, 4, 17104. [Google Scholar] [CrossRef] [Green Version]
- Laitala, A.; Erler, J.T. Hypoxic Signalling in Tumour Stroma. Front. Oncol. 2018, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Chiang, S.P.; Cabrera, R.M.; Segall, J.E. Tumor cell intravasation. Am. J. Physiol. Cell Physiol. 2016, 311, C1–C14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, H.; Xu, H.; Qin, J. Biomimetic tumor microenvironment on a microfluidic platform. Biomicrofluidics 2013, 7, 11501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boussommier-Calleja, A.; Li, R.; Chen, M.B.; Wong, S.C.; Kamm, R.D. Microfluidics: A new tool for modeling cancer-immune interactions. Trends Cancer 2016, 2, 6–19. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Dong, X.; Tu, Y.; Miao, G.; Zhang, Z.; Zhang, L.; Wei, Z.; Yu, D.; Qiu, X. Methods and platforms for analysis of nucleic acids from single-cell based on microfluidics. Microfluid. Nanofluid. 2021, 25, 87. [Google Scholar] [CrossRef]
- Tavakoli, H.; Li, X. Recent advances in microfluidic platforms for single-cell analysis in cancer biology, diagnosis and therapy. Trends Anal. Chem. 2019, 117, 13–26. [Google Scholar] [CrossRef]
- Sanchez Barea, J.; Lee, J.; Kang, D.K. Recent Advances in Droplet-based Microfluidic Technologies for Biochemistry and Molecular Biology. Micromachines 2019, 10, 412. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chen, Z.; Bian, F.; Shang, L.; Zhu, K.; Zhao, Y. Advances of droplet-based microfluidics in drug discovery. Expert Opin. Drug Discov. 2020, 15, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Dimitriou, P.; Li, J.; Tornillo, G.; McCloy, T.; Barrow, D. Droplet Microfluidics for Tumor Drug-Related Studies and Programmable Artificial Cells. Glob. Chall. 2021, 5, 2000123. [Google Scholar] [CrossRef] [PubMed]
- Dhiman, N.; Kingscott, P.; Sumer, H.; Sharma, C.S.; Rath, S.N. On-chip anticancer drug screening–Recent progress in microfluidic platforms to address challenges in chemotherapy. Biosens. Bioelectron. 2019, 137, 236–254. [Google Scholar] [CrossRef] [PubMed]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-chips: Into the next decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef]
- Kimura, H.; Sakai, Y.; Fujii, T. Organ/body-on-a-chip based on microfluidic technology for drug discovery. Drug Metab. Pharmacokinet. 2018, 33, 43–48. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Waring, M.J.; Arrowsmith, J.; Leach, A.R.; Leeson, P.D.; Mandrell, S.; Owen, R.M.; Pairaudeau, G.; Pennie, W.D.; Pickett, S.D.; Wang, J.; et al. An analysis of the attrition of drug candidates from four major pharmaceutical companies. Nat. Rev. Drug Discov. 2015, 14, 475–486. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, J.; Wang, X.; Feng, L.; Wu, J.; Zhu, X.; Wen, W.; Gong, X. Organ-on-a-chip: Recent breakthroughs and future prospects. Biomed. Eng. Online 2020, 19, 9. [Google Scholar] [CrossRef] [Green Version]
- Huh, D.; Matthews, B.D.; Mammoto, A.; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science 2010, 328, 1662–1668. [Google Scholar] [CrossRef] [Green Version]
- Hassell, B.A.; Goyal, G.; Lee, E.; Sontheimer-Phelps, A.; Levy, O.; Chen, C.S.; Ingber, D.E. Human Organ Chip Models Recapitulate Orthotopic Lung Cancer Growth, Therapeutic Responses, and Tumor Dormancy In Vitro. Cell Rep. 2017, 21, 508–516. [Google Scholar] [CrossRef] [Green Version]
- Strelez, C.; Chilakala, S.; Ghaffarian, K.; Lau, R.; Spiller, E.; Ung, N.; Hixon, D.; Yoon, A.Y.; Sun, R.X.; Lenz, H.-J.; et al. Human colorectal cancer-on-chip model to study the microenvironmental influence on early metastatic spread. iScience 2021, 24, 102509. [Google Scholar] [CrossRef] [PubMed]
- Miri, A.K.; Mostafavi, E.; Khorsandi, D.; Hu, S.-K.; Malpica, M.; Khademhosseini, A. Bioprinters for organs-on-chips. Biofabrication 2019, 11, 042002. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jang, J.; Kang, H.-W. 3D Bioprinting and its application to organ-on-a-chip. Microelectron. Eng. 2018, 200, 1–11. [Google Scholar] [CrossRef]
- Mason, J.; Visintini, S.; Quay, T. An Overview of Clinical Applications of 3-D Printing and Bioprinting. In CADTH Issues in Emerging Health Technologies; CADTH: Ottawa, ON, Canada, 2016; pp. 1–19. [Google Scholar]
- Ma, C.; Peng, Y.; Li, H.; Chen, W. Organ-on-a-Chip: A New Paradigm for Drug Development. Trends Pharmacol. Sci. 2021, 42, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Shang, M.; Soon, R.H.; Lim, C.T.; Khoo, B.L.; Han, J. Microfluidic modelling of the tumor microenvironment for anti-cancer drug development. Lab Chip 2019, 19, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Calar, K.; Evans, C.; Petrasko, M.; De la Puente, P. Bioengineering the Oxygen-Deprived Tumor Microenvironment within a Three-Dimensional Platform for Studying Tumor-Immune Interactions. Front. Bioeng. Biotechnol. 2020, 8, 1040. [Google Scholar] [CrossRef]
- Berzina, S.; Harrison, A.; Taly, V.; Xiao, W. Technological Advances in Tumor-On-Chip Technology: From Bench to Bedside. Cancers 2021, 13, 4192. [Google Scholar] [CrossRef]
- Trujillo-de Santiago, G.; Flores-Garza, B.G.; Tavares-Negrete, J.A.; Lara-Mayorga, I.M.; Gonzalez-Gamboa, I.; Zhang, Y.S.; Rojas-Martinez, A.; Ortiz-Lopez, R.; Alvarez, M.M. The Tumor-on-Chip: Recent Advances in the Development of Microfluidic Systems to Recapitulate the Physiology of Solid Tumors. Materials 2019, 12, 2945. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Ching, H.; Yoon, J.-K.; Jeon, N.L.; Kim, Y. Microvascularized tumor organoids-on-chips: Advancing preclinical drug screening with pathophysiological relevance. Nano Converg. 2021, 8, 12. [Google Scholar] [CrossRef]
- Hachey, S.J.; Hughes, C.C.W. Applications of tumor chip technology. Lab Chip 2018, 18, 2893–2912. [Google Scholar] [CrossRef]
- Cho, H.; Kim, J.; Song, H.; Sohn, K.Y.; Jeon, M.; Han, K.-H. Microfluidic technologies for circulating tumor cell isolation. Analyst 2018, 143, 2936–2970. [Google Scholar] [CrossRef] [PubMed]
- Papapetrou, E.P. Patient-derived induced pluripotent stem cells in cancer research and precision oncology. Nat. Med. 2016, 22, 1392–1401. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Regmi, S.; Poudel, C.; Adhikari, R.; Luo, K.Q. Applications of Microfluidics and Organ-on-a-Chip in Cancer Research. Biosensors 2022, 12, 459. https://doi.org/10.3390/bios12070459
Regmi S, Poudel C, Adhikari R, Luo KQ. Applications of Microfluidics and Organ-on-a-Chip in Cancer Research. Biosensors. 2022; 12(7):459. https://doi.org/10.3390/bios12070459
Chicago/Turabian StyleRegmi, Sagar, Chetan Poudel, Rameshwar Adhikari, and Kathy Qian Luo. 2022. "Applications of Microfluidics and Organ-on-a-Chip in Cancer Research" Biosensors 12, no. 7: 459. https://doi.org/10.3390/bios12070459
APA StyleRegmi, S., Poudel, C., Adhikari, R., & Luo, K. Q. (2022). Applications of Microfluidics and Organ-on-a-Chip in Cancer Research. Biosensors, 12(7), 459. https://doi.org/10.3390/bios12070459






