Abstract
This review summarizes and compares the available surface treatment and bonding techniques (e.g., corona triggered surface activation, oxygen plasma surface activation, chemical gluing, and mixed techniques) and quality/bond-strength testing methods (e.g., pulling test, shear test, peel test, leakage test) for bonding PDMS (polydimethylsiloxane) with other materials, such as PDMS, glass, silicon, PET (polyethylene terephthalate), PI (polyimide), PMMA (poly(methyl methacrylate)), PVC (polyvinyl chloride), PC (polycarbonate), COC (cyclic olefin copolymer), PS (polystyrene) and PEN (polyethylene naphthalate). The optimized process parameters for the best achievable bond strengths are collected for each substrate, and the advantages and disadvantages of each method are discussed in detail.
1. Introduction
Polydimethylsiloxane (PDMS), a silicon-based elastomer, is considered a fundamental building block in microfluidics due to its many desirable material properties (e.g., elasticity, optical transparency, biocompatibility, low autofluorescence, excellent thermal and chemical stability, etc.) [1,2,3,4]. Additionally, due to its convenient patterning technologies, such as replica molding or soft lithography, traditional fabrication methods centering on glass or silicon etching could be replaced with faster, less-expensive solutions [5,6,7]. Today, for a functional microfluidic device, microchannels are usually created in PDMS, then a chip is formed by closing them with a solid or flexible substrate (e.g., glass, silicon, or various polymers) [8,9,10,11]. This closing method is referred to as bonding technology since irreversible chemical bonds are formed between the activated or functionalized PDMS and substrate surfaces. Silicon-based materials (e.g., glass or silicon) can be relatively easily bonded to PDMS via simple surface activation, and thus these are the most widespread ways of sealing the microchannels.
However, in several cases, another type of substrate is required for a given application area. A common requirement could be the flexibility of the substrate. With the spreading of flexible electronics created by additive manufacturing [12,13] (e.g., electrodes on flexible polymer substrates, such as polyimide, created by 2D or 3D printing of nanomaterials [14]), functional elements such as sensors, biosensors, or microelectrode arrays can be integrated onto the surface of the polymer substrate [15,16]. Besides microfluidics, PDMS is also often used for the encapsulation of surface integrated electronics, or as protective coatings [17,18]. For complex Lab-on-a-Chip (LoC) or micro total analysis systems (Micro-TAS), PDMS is also often required to be bonded to other polymer-based materials, such as PET (polyethylene terephthalate), PI (polyimide), PMMA (poly(methyl methacrylate)), PVC (polyvinyl chloride), PC (polycarbonate), COC (cyclic olefin copolymer), PS (polystyrene) or PEN (polyethylene naphthalate) [19,20,21]. PDMS–polymer bonding is usually more complicated and requires the chemical functionalization of at least one bonded surface. These methods are called chemical gluing since chemical bonds between the applied molecular monolayers and surface functional groups provide a strong bond between the attached surfaces. In the past ten years, many techniques have been developed based on the combinations of surface activation, chemical gluing, and adhesive-based methods to bond PDMS with various substrates [22,23,24]. Since for most microfluidic applications a good quality bond with high strength is critical (especially where high-pressures or flow rates are applied), it is imperative to select the most appropriate bonding method for our target application. Additionally, the optimization of the technological process parameters is essential since, as demonstrated later, the quality of the bond greatly relies on it.
Our paper provides a review of these various technologies to bond PDMS with other surfaces. It pivots around three comprehensive tables, and the sections are structured accordingly. Table 1 and Section 2 compare the testing methods which are commonly used to quantify bond strengths. The differences between these methods should be understood in order to be able to evaluate and compare our bond quality with others. Table 2 and Table 3 compare the bonding methods for various substrates, which are discussed in detail in Section 3 and Section 4. While Table 2 focuses on the achievable bond strengths for a given substrate (based on the test method), Table 3 provides the optimized process parameters to reach these results. It is important to emphasize that both tables contain only the optimized process parameters. For bond quantification in a wider parameter range, please see the references. Our review is also content with focusing only on these aspects of PDMS-based microfluidic fabrication. Recent comprehensive reviews on the general application of PDMS for microfluidics [2], biomedicine [25], or fabrication technologies [5,6,7,26] can be recommended for those interested in other aspects.

Table 1.
The most frequently used bonding strength measurement methods and test.

Table 2.
Bond strengths measured with different test methods on PDMS–substrate bonds, categorized by the substrate type. In the table only the test results corresponding to the optimized process parameters are given.

Table 3.
Optimized process parameters for bonding PDMS with different substrates. For the bonding strength test results see Table 2. Abbreviations: APTES, 3-aminopropyl)triethoxysilane; CNC, Computer Numerical Control; COC, Cyclic Olefin Copolymer; DMPMS, Dimethyl-MethylPhenylMethoxy Siloxane; DRIE, Deep Reactive Ion Etching; GPTES, 3-glycidyloxypropyl)triethoxysilane; GPTMS, 3-glycidyloxypropyl)trimethoxysilane; KOH, Potassium hydroxide; MPTMS, 3-mercaptopropyl)trimethoxysilane; NOA74, Norland Optical Adhesive; PC, Polycarbonate; PDMS, Polydimethylsiloxane; PEN, Polyethylene Naphthalate; PET, Polyethylene Terephthalate; PI, Polyimide; PMMA, Poly(methyl methacrylate); PS, Polystyrene; PVC, Polyvinyl Chloride; RT, Room Temperature; TESPSA, 3-triethoxysilyl)propylsuccinic anhydride; UV, Ultraviolet; Physical quantities: R–PDMS curing agent/base polymer ratio, t—time, P—RF plasma power, p—pressure, f—gas flow rate. Conc.—concentration. The label in the bracket refers to the used medium as a—aqueous, e—ethanol, m—methanol.
2. Bond-Strength Testing Methods
Quality check of the prepared bond and the quantification of bond strength are important for all applications. Many different testing methods are used for this purpose; as shown in Table 1, 12 different functionally different techniques could be distinguished. Although a high bond strength—quantified by either tensile strength or shear strength—is a general requirement, many applications have special needs, e.g., long-term channel integrity [27,28], high working pressures inside the channel [29,30], or high inlet velocity [31,32,33]. Thus, understanding the differences between these testing methods and their provided information is essential for picking the right test for a given application. Comprehension of the testing methods is also crucial for interpreting the different bond strengths that characterize the bonding technologies.
2.1. Manual Peeling/Delamination Test
In this simple qualitative test, the bonded PDMS piece is forcibly removed from the substrate by hand or with the help of some basic tools like a scalpel. If the PDMS cannot be easily removed, or upon removal, the PDMS block tears/breaks (cohesive failure) instead of detaching cleanly from the substrate (adhesive failure), the formation of chemical bonds can be assumed. This test is the cheapest and easiest to implement: no special equipment or sample preparation is needed. On the other hand, the test does not yield any quantitative data, the results are quite subjective and have poor reproducibility. Usually, the main purpose of this test is to verify bond formation and/or filter out faulty workpieces before quantitative bond-strength testing with another method [19,20,21,34,35,36,37].
2.2. Tensile Strength Measurements
The quality of the prepared bond is best characterized by its tensile strength; thus, this is one of the most commonly used quantitative testing methods. Several different approaches exist to measure this property.
With a standard tensile testing machine, grips can be used to pull the bonded materials apart from each other and measure the force required to detach the PDMS from the substrate. The general advantage of this test is that the experimental conditions are well defined, only normal forces act on the materials, and thus the results can be easily compared with other similarly executed measurements. The three approaches in Figure 1a–d differ in how the samples are fixed to the pulling grips. Although using adhesives to fix the upper (non-bonded) site of the PDMS block to the grip (or a specifically machined stump) might seem straightforward, this might cause inconvenience for some equipment. Additionally, the adhesive strength between the PDMS and the grip head (stump) must be higher than the characterized PDMS–substrate bond, otherwise, it will break prematurely (Figure 1a). Sunkara et al. used a silicone sealant (LC909N, Henkel, Germany) to bond the upper part of the PDMS to an aluminum jig, which held up to 490 kPa [38].
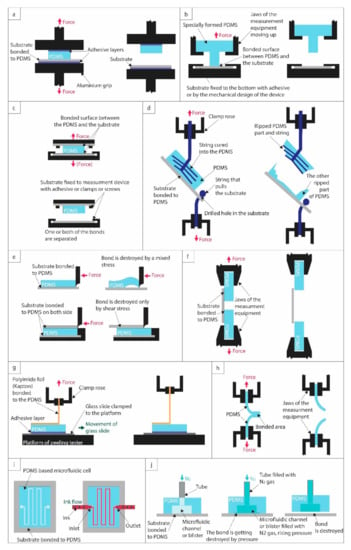
Figure 1.
Illustrations of different bond-strength testing methods. (a) Tensile strength measurement with adhesive. (b) Cylinder-based tensile strength measurement with a specially designed PDMS piece. (c) Double-substrate bonding-based tensile strength measurement. (d) String-based tensile strength measurement. (e) Pushing-based shear strength measurement. (f) Pulling-based shear strength measurement. (g) Peeling test. (h) T-peeling test. (i) Leakage test. (j) Burst test.
To avoid using adhesives, it is possible to create special jaws/grips to hold the PDMS and the substrate in the heads of the tensile testing machine. For this, the PDMS part should be explicitly molded for the purpose, e.g., in a cylinder shape (illustrated in Figure 1b, referred to as cylinder-based tensile strength measurement in Table 1). The protruding part of the cylinder is bonded to the substrate, while the flat part of the PDMS can be easily clamped to the equipment [39,40]. The substrate is similarly clamped to the bottom jaw. A disadvantage of this technique is that the used PDMS shape (which requires a separate molding form) is quite special and most possibly significantly differs from the design of the functional element, which cannot be directly used for this test. By contrast, the adhesive-assisted tensile testing could be performed even on the functional elements, e.g., bonded microfluidic cells, and thus combined with other testing methods (leakage test, burst test). The cylinder’s geometry should also be optimized: a too-long cylinder or a cylinder with a too-small base diameter can be torn down from the PDMS base. Too thin or fragile substrates (e.g., glass, silicon) are also vulnerable to breaking during the pulling.
The third solution is illustrated in Figure 1c. In this case, two layers of the substrate are bonded to both sides of the PDMS block. The substrate is fixed to the tensile strength testing machine with either clamps, screws, or adhesives [41,42]. The substrates should be thick enough to avoid breaking them during pulling. Additionally, if screws are used, holes are needed to be drilled into the substrates. The areas of the two bonds should be controlled precisely and taken into account upon the calculation of the tensile strength.
An alternative way for testing tensile strength is shown in Figure 1d. Here two twines are used to pull the PDMS/substrate assembly apart. One fixes the substrate to a pulling head via a drilled hole. A second twine is inserted into the PDMS pre-polymer and permanently cured into it. After bonding the PDMS with the substrates, the assembly is pulled apart. Although this pull test is performed in several works (e.g., by the team of Nae Yoon Lee [43,44,45,46,47]), it might have some drawbacks. Although cohesive failures can be easily distinguished from adhesive failures, the tensile strength cannot be adequately calculated in the latter case. Due to the nature of the assembly and the twine distribution inside the PDMS block, the pulling force cannot be considered perfectly normal and homogenously distributed along the bonded area. The bond between the twine and the PDMS is also crucial. The twine might be torn out from the block without detaching the PDMS from the substrate.
2.3. Shear Strength Measurement
Although shear forces might be a bit less relevant in microfluidic applications, in some instances, shear strength measurements are also performed on the bonded structures. In a classical shear testing machine, a force parallel to the bonding plane is applied with a given shear rate, as illustrated in Figure 1e. The shear force and displacement are measured, which can be used to calculate shear strength (failing load divided by the bond area) and shear strain [48].
A general problem with the shear test in Figure 1e is that the PDMS detaches gradually from the substrates, which requires monitoring the propagation of peeling to calculate the delamination area, as demonstrated in [49]. Pushing a partially peeled PDMS can also modify the direction of the acting forces, distorting the results.
To tackle this issue, Wang et al. bonded two rigid substrates to either side of the PDMS, and their shear tester pushed the upper substrate until the joint pair failed in one step. In this way, the shear strength and shear strain of the bonds could be characterized more conveniently and precisely [48].
An alternative approach could be using a tensile testing machine in shear mode, as illustrated in Figure 1f. Although in some works the results of these tests are referred to as tensile strength [50], due to the parallel forces, it is more appropriate to refer to them as lap shear strength, which is defined as the failure load divided by the bond area [51]. There are two ways to perform this test: either two PDMS blocks can be bonded to one substrate (as in [50]), or two substrates can be bonded to one PDMS block to avoid the difficulty of clamping PDMS. The bond areas should be precisely controlled and considered in lap shear strength calculation.
2.4. Peel Test
Peel tests are also frequently used to assess the bond quality of adhesive joints exposed to peel forces. Peel testing requires at least one flexible component, which refers to the ability of the adherend to bend through 90° without breaking or cracking [52]. For example, Hoang et al. used a Kapton foil bent at 90° angle, as illustrated in Figure 1g [53]. As the upper clamp roses and pulls the Kapton with a constant speed, at the same time, the lower platform shifts to maintain the 90°. Samples were glued onto glass slides using a silicone adhesive to prevent the PDMS from lifting during peeling. The resulting peel strength is calculated as the constant load per the bonded area’s width required to continue peeling the joint after initiation, determined from the flat portion of the force–extension curve [52,53].
Another method for peel strength measurement is the so-called T-peel test, illustrated in Figure 1h, which is also adopted by standard bodies (e.g., ISO 11339 or ASTM D 1876) [52]. A drawback of this method is that it requires both parts of the bonded structure to be flexible. It is most commonly used to detach two bonded PDMS blocks, as in [54] and [55], but a PDMS block bonded with a flexible foil could also be investigated this way. Overall, peel tests are harder to be performed correctly and could be done on a limited number of substrate types. The resulting peel strength is also a bit less relevant than the more widely used tensile strength.
2.5. Leakage Test
The most practical way to test the functionality of a microfluidic chip is leakage testing. In this family of methods (illustrated in Figure 1i), different approaches are used to test different qualities. In the simplest static leakage test (sometimes also referred to as durability test), a fluid is injected into the microchannels and kept there for a given amount of time, from the shortest test durations of minutes–hours [40,42] to even as long as months [20], and channel integrity is tested afterward. For these tests, no flow is applied on the fluid after injection (static conditions), and for extended tests, the ports are hermetically sealed. Besides colored water, other solutions are sometimes also used to test the long-term durability and swelling of PDMS (e.g., acids, i.e., 1M HCl [36], bases, i.e., 1 M NaOH [36], or organic solvents, i.e., tetrahydrofuran (THF) [43]).
By contrast, in the case of dynamic leakage tests, a constant flow is applied to evaluate the hydrodynamic stability of the device. Usually, the flow rate is gradually increased to a point where channel integrity fails. For the sake of better comparability between different structures/geometries, besides the absolute maximum flow rates (expressed in mL/min), the ratio of the liquid volume that flows through the channel within 1 min and the channel volume is also frequently given (expressed as flow-volume/channel-volume/min). Without a doubt, this is one of the most widespread methods for testing microchannel functionality [19,35,36,38,43,44,45,46,47,56,57].
2.6. Burst Test
Although technically this method could be considered to be a special type of leakage test, it is commonly referred to as burst pressure test (illustrated if Figure 1j). In this case, fluids or gases are introduced into either the microchannels or a blister specifically designed for this test. The pressure is slowly increased until the integrity of the investigated structure is compromised, either by delamination, leakage, or burst. This test requires appropriate instrumentation, including pressure control (pump and pressure sensor). Although usually the burst pressure level can be easily identified as a rapid drop in the measured pressure, sometimes the continuous optical monitoring of the structures might be needed to detect finer damage (e.g., slight leakage might happen before the burst of the cell). It has to be noted that—depending on the bonding protocol—there might be a huge difference between the burst pressures measured with air/nitrogen and water. For example, Pečar et al. measured a 5-fold difference between the burst pressures obtained with air and water, respectively [37], explained by the hydrolysis of covalent bonds [58]. (For this reason, in Table 2, the medium used for the test is also indicated.) Burst testing is probably the most used functional method to quantify the strength of the prepared bonds [21,36,37,38,39,40,41,46,47,48,57,59,60,61,62].
3. Bonding Methods
In this section, the different strategies used to bond PDMS with various substrates are introduced in a general way. The specific protocols will be discussed in greater detail concerning the different substrate materials in Section 4. The general bonding strategies are illustrated in Figure 2. Silicon-based materials (e.g., glass or silicon) can be relatively easily bonded to PDMS. The process only requires surface activation (Figure 2a), which can be achieved either using corona discharge treatment [63,64] or oxygen plasma treatment on the surfaces [65,66,67]. For other substrate materials (e.g., thermoplastics), the bondable functional groups need to be created via surface functionalization. This method is often referred to as chemical gluing (Figure 2b,c). Sometimes these surface functionalization protocols are mixed with microscopic amounts of adhesives, as in Figure 2d.
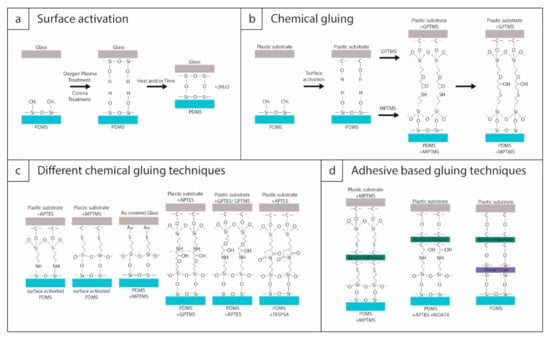
Figure 2.
Illustrations of the process steps of (a) surface activation with oxygen plasma or corona treatment, (b) chemical gluing. (c) The most often used chemical gluing methods. (d) Adhesive-based or combined gluing methods.
3.1. Surface Activation by Oxygen Plasma Treatment
The general aim of surface activation is to remove contaminants and generate reactive chemical groups for covalent bonding. The convenient bonding of silicon-based materials through silanol groups (–Si–OH) is well-known in MEMS technologies (e.g., silicon wafer-level bonding) [68]. Through surface activation, the terminal methyl groups (–CH3) in PDMS (generally comprised of repeating units of –O–Si(CH3)2–) can be replaced by silanol groups. These can form covalent siloxane bonds (Si-O-Si) with a similar silanol group on another activated surface after the loss of a water molecule (Figure 2a) [59]. The processes taking place upon plasma/corona exposure can be quite complex since the material is simultaneously subjected to a mixture of energetic particles and radiation, e.g., electrons, ions, UV, and ozone, which can cause at least 12 different interactions (for details see [69]). The surface oxidation of PDMS and the increased concentration of hydroxyl groups can also lead to the formation of strong intermolecular bonds [69,70]. These reactions may change the surface properties significantly [71], either by the formation of a thin SiOx layer, by increased cross-linking, or on the contrary, by the degradation of the network structure—all depending on the experimental conditions (e.g., plasma power, exposure time, etc.). Most of the proposed interactions upon plasma/corona exposure make the surface hydrophilic [69,72], and thus the quality of surface activation is generally monitored by water drop tests (contact-angle measurements). Exposed to normal ambient conditions, the treated surfaces quickly regain their hydrophobicity, mostly due to the migration of low molar mass chains from the bulk to the surface, the reorientation of polar groups on the surface into the bulk, or simply due to contamination [69,73,74]. Mechanical deformation (even compressive strains of less than 1%) were confirmed to speed up the recovery process [73], and thus treated PDMS samples should be handled with great care during alignment. Overtreated PDMS was also found to regain hydrophobicity faster, emphasizing the optimization of experimental conditions [73].
Plasma treatment is by far the most commonly used method for PDMS surface modification [74]. A large variety of gases can be used (e.g., oxygen, argon, nitrogen, hydrogen, fluorinate, etc.) from medium vacuum to atmospheric pressure for different purposes [75,76]. To create a silanol rich surface for PDMS bonding, oxygen plasma is used with pressures in the low–middle vacuum range. Besides gas content and pressure, the two other main process parameters are the plasma power and exposure time, which need to be optimized for successful bonding [20,49,54,55]. A significant advantage of plasma treatment is that with a common plasma chamber (reactor), these conditions can be fine-tuned and controlled precisely, yielding reproducible results and overall better bond quality. Low-pressure plasma is also less harmful to functional elements (e.g., thin-film metallic sensors) that might be integrated/embedded onto the treated surface, compared to corona treatment. Oxygen plasma surface activation is also frequently used as the first step in chemical gluing procedures [41,46].
3.2. Surface Activation by Corona Treatment
A corona treater is a device that generates a high voltage across an electrode at the tip of the unit. This handheld device is usually supplied with electrodes in different shapes for various applications [77,78]. The high potential of the electrode ionizes the surrounding air, creating a localized plasma called corona discharge. Corona treatment is a simple, cheap, and fast method which does not require expensive equipment or a special environment (the discharge can be generated at room temperature and atmospheric pressure, without the need for a vacuum system); it is portable, safe, and easy to be used [47,60,79]. A disadvantage of the technique is that it is usually performed manually. Although the discharge can be expanded to cover larger areas (with appropriate electrodes), the sample-electrode distance, movement speed, treatment time are all factors that need to be precisely controlled [19]. It has to be noted that the discharge could be harmful to metallic structures, e.g., it has been reported to have damaged a 35 nm thick plasmonic gold thin film on glass beyond applicability [50]. Corona surface activation can also be used combined with chemical gluing [43,47].
3.3. Surface Activation by UV/Ozone Treatment
The third possibility for enriching the PDMS surface with silanol groups is UV/ozone treatment [80,81]. Although this technique is significantly slower compared to both plasma and corona treatment, due to the greater penetration of photons, deeper surface modification (i.e., several 10 µm) could be achieved without any surface damage [74,82]. The process parameters (UV line, lamp power, exposure time) should be precisely selected to control the surface properties and hydrophobicity recovery [83,84]. The formation of a glassy, brittle SiOx layer [85] might not be desirable for some applications that rely on the flexibility of PDMS and a matching substrate. UV/ozone is also used for surface activation in chemical gluing [50].
3.4. Chemical Gluing
As discussed before, PDMS can be conveniently bonded to glass or other silicon-based materials through siloxane bonds. To bond PDMS with other materials, e.g., thermoplastics, functional groups should be created either only on the target substrate or on both materials. These techniques are often referred to as chemical gluing, where molecular monolayers (acting as coupling agents) are anchored on the surfaces with specific terminal functional groups [36,45,86,87,88]. The most commonly used molecules are all organosilanes, namely APTES ((3-aminopropyl)triethoxysilane), MPTMS ((3-mercaptopropyl)trimethoxysilane), GPTES ((3-glycidyloxypropyl)triethoxysilane), GPTMS ((3-glycidyloxypropyl)trimethoxysilane), and TESPSA ((3-triethoxysilyl)propylsuccinic anhydride). Figure 2c illustrates the different bonding schemes between PDMS and a general polymer substrate by using these functional molecular layers. As can be seen, the silanol groups of the PDMS can be directly used to form bonds with the amino groups (–NH2) of APTES or the thiol groups (–SH) of MPTMS functionalized polymers. APTES can also be used paired with GPTES, GPTMS, or TESPSA, depending on the substrate, while MPTMS is usually paired with GPTMS.
During surface activation, the carbon backbones of thermoplastic substrates (e.g., PMMA or PC) are broken with corona or oxygen plasma treatment. These free carbons react with inorganic silicons of the silane coupling agent, which is used to functionalize substrate surfaces to form Si–O–C bonds [89]. It is important to emphasize that the process parameters of chemical gluing (e.g., concentration and temperature of the solution, thorough rinsing, drying, and subsequent thermal treatment to stabilize the self-assembled layers before bonding) need to be controlled for optimal results, similarly to the surface activation [41]. The hydrolytic stability of Si–O–C bonds and various chemical gluing protocols were also investigated in detail [89]. The exact protocols and achievable bonding strengths are given in Table 2 and Table 3, and discussed in the next section.
3.5. Adhesive-Based Gluing
Adhesive-based gluing—when a microscopic amount of adhesive materials is applied between the two surfaces instead of molecular layers—is usually not considered bonding. Depending on the target substrate, applying epoxy or silicone-based adhesives might prove significantly weaker than chemical gluing methods [89]. However, since adhesives are sometimes used in combination with chemical gluing methods to improve bond quality, these hybrid techniques are worth mentioning. A few examples are given in Figure 2d, using different types of epoxy adhesives (e.g., Norland Optical Adhesive (NOA74) [61], LePage Gel epoxy adhesive [53], Biocompatible Epoxy 301-2 [57]). MPTMS [53] and APTES [61] can both be used to covalently bind the epoxy adhesive layer (with thickness in a few µm range) with the PDMS. In a different technique, a silicone-based adhesive (PrimeCoat, ~1 µm) and an epoxy adhesive (3–4 µm) were spin-coated on top of the substrate [57].
4. Bonding Strategies for Various Substrates
In this section, the exact protocols used to bond PDMS with various substrates will be discussed in detail. Table 2 collects the applicable protocols for each substrate type and presents the achievable bonding strength, systematically ordered along with the testing methods of Table 1. Table 3 gives each protocol’s optimized process parameters to achieve the best results for a given substrate type.
4.1. PDMS
One of the most straightforward approaches to close a PDMS microchannel with a substrate is PDMS–PDMS bonding since a simple surface activation is sufficient, which can be performed in many ways. The most comprehensive studies on the optimization of oxygen plasma and corona treatment process parameters for the best achievable bonding strengths were performed by Bhattacharya et al. [59,60]. They mapped a wide range of RIE (Reacting Ion Etching) power (5–150 W), chamber pressure (20–1000 mTorr), and time of exposure (5–60 s) for both an inductively coupled high-density (ICP) plasma system, and a plasma-enhanced chemical vapor deposition (PECVD) system. The optimal parameters were found to be 700 mTorr, 20 W, and 30 s exposure, with an ICP system, which resulted in 400 kPa (58 psi) burst pressure [59]. In a newer work, they managed to reproduce these results with the same experimental conditions, also demonstrating huge variation (average burst pressure: 300 kPa, range: 180–715 kPa), and obtaining slightly smaller values with corona treated surfaces (average: 290 kPa, range: 227–380 kPa) [60]. The better reproducibility of corona treatment (handheld discharge unit, 15 kV output voltage for 30 s) is admittedly against expectations. It has to be noted that these burst pressures, obtained with optimized process parameters, are 2–3× better compared to other similar available data, e.g., Yousuff et al., who measured 105–180 kPa after oxygen plasma, and 87–95 kPa for UV/ozone treatment, respectively [62]. However, as also shown in Table 3, in many of these cases, the exact process parameters were not elaborated in the papers [45,62]. The parameters of atmospheric RF glow discharge plasma were optimized by Jung et al. [55], and similarly good results—in terms of peel test—were demonstrated with low-pressure RF air plasma as well [54].
As for chemical gluing, Lee et al. demonstrated a 2× increase in tensile strength (from 91 to 184 kPa) when using APTES/GPTMS gluing compared to normal oxygen plasma activation—although the plasma parameters are not given and presumably were not optimized either [45].
Another important technique that needs to be noted here is pre-polymer gluing (also referred to as ‘uncured PDMS adhesive’ or ‘partially cured adhesive’). In this approach, uncured/partially cured PDMS is placed between two fully cured substrates, or one of the two bonded pieces is only partially cured before making contact with the other substrate. Here, the pre-polymer adhesive or partially cured piece is cured during/after the bonding process. As an example, for partial cure bonding, Bhattacharya et al. pre-cured PDMS at 60 °C for 35 min before bonding and then allowed the piece to fully cure overnight following the bonding [60]. For both groups who tested this approach, the measured maximum burst pressures were at least 2× higher, compared to the same structures bonded with only either oxygen plasma or corona treatment (e.g., an average of 671 kPa), making it superior compared to other techniques [60,62]. Similarly good results were reported by Cao et al. with DMPMS-based (dimethyl-methylphenylmethoxy siloxane) pre-polymer gluing [35].
4.2. Glass (Silicon)
PDMS–glass and PDMS–silicon are also regularly used pairs to close PDMS microchannels. In the latter case, PDMS is bonded to the native oxide of silicon, so although glass is more frequently used, the protocols for bonding PDMS to silicon can be considered to be the same.
All of the approaches listed in Table 2 are based on surface activation similar to PDMS–PDMS bonding. The optimized oxygen plasma parameters with an ICP equipment were found to be 1000 mTorr chamber pressure, 20 W plasma power, and 30 sec exposure time [59]. The resulting 510 kPa (74 psi) burst pressure is more than 25% higher compared to PDMS–PDMS bonds also prepared with optimized oxygen plasma parameters [59]. For identical process parameters, others also reported significantly higher bonds between PDMS–glass than PDMS–PDMS, and the difference was even higher for UV/ozone treatment (95 to 314 kPa, respectively) [62]. Prepolymer gluing was also successfully used with glass substrates [35,62].
In Table 2, two application-specific examples are also given. Bonding PDMS to gold-coated glass [90] could be important for several sensor applications (either electrochemical or optical). For this purpose, surface activation + MPTMS functionalization of the PDMS could be used, relying on the terminal thiol groups to bond covalently to the gold surface. Bakouche et al. successfully demonstrated this method with an SPR (surface plasmon resonance) chip and even obtained higher lap shear strength values than for PDMS–glass bonding with simple corona surface activation [50]. Parylene, an organic polymer with a para-xylylene backbone, has many favorable properties considering microfabrication or microfluidics [91]. To irreversibly bond parylene with PDMS, Rezai et al. tried both pre-polymer gluing and plasma treatment with success [39]. By optimizing the process parameters of the latter (using a mixture of SF6 and N2), they obtained a very high bond tensile strength of 1.4 MPa and 145 kPa burst pressure (tested with water) [39].
4.3. PMMA
Similar to PDMS, poly(methylmethacrylate) (PMMA) is also a popular material for micro-TAS or LoC fabrication [92]. PMMA is an optically transparent thermoplastic (one of the least hydrophobic materials used in microfluidics), and its low price and compatibility with several microfabrication methods (e.g., injection molding, milling, hot embossing, etc.) makes it an ideal choice for disposable microfluidic devices. Since its direct bonding with PDMS is not possible [93] with an adequate bonding strength (e.g., with pre-polymer gluing, only 15 kPa tensile strength could be obtained [42]), a chemical gluing method has to be selected. There are numerous possibilities: nearly all of the illustrated approaches presented in Figure 2b,c can be used for PDMS–PMMA bonding, combined with either oxygen plasma or corona surface activation. Most of the protocols listed in Table 2 uses only one molecular adhesive. In these cases, the PMMA is functionalized with either APTES [37,38,41] or MPTMS [43] then bonded to a surface-activated PDMS. Others functionalize both surfaces with APTES/GPTMS [44] or APTES/TESPSA [46] pairs. By comparing the resulting tensile strength and burst pressure values, no direct improvement can be observed in favor of the double functionalization. With optimized plasma treatment and APTES coating parameters, high tensile strength (1.6 MPa [41]) and high burst pressures (>500 kPa, measured in air [37]) were obtained by coating only the PMMA piece.
An interesting approach is presented by Zhang et al., who used APTES functionalization on PMMA, followed by the addition of monoglycidyl ether terminated, low-molecular-weight PDMS [47]. In this way, PDMS-like terminals were placed onto the substrate (via the amine–epoxy bond), which can be subsequently bonded with bulk PDMS through classical siloxane bond formation. The aminosilane + monoglycidyl ether terminated PDMS combined functionalization thus leads back to PDMS–PDMS bonding, which can be used with a variety of substrates (e.g., PS, PC), with good results (580–620 kPa burst pressures) [47].
4.4. PC
Similar to PMMA, PC is another optically transparent thermoplastic with a high glass transition temperature (145 °C), low moisture absorption, and durability that makes it ideal for high-temperature applications. All of the chemical gluing protocols previously discussed for PMMA-PDMS bonding can be used for polycarbonate (PC) as well, including surface activation and APTES [37,38,58] or MTPMS [43] treatment, using APTES+ monoglycidyl ether terminated, low-molecular-weight PDMS functionalization [47], or using APTES/TESPSA [46] or APTES/ GPTMS [44] linker pairs. A notable difference is that with the identical bonding techniques, the resulting bond tensile strength or burst pressure is 10–50% higher for PDMS–PC than PDMS–PMMA bonds, which makes PC a good choice for high-pressure or high flow-rate applications.
4.5. PS
Polystyrene (PS) is another optically transparent material that is frequently used material for cell cultures and biosensor applications, thanks to its good biocompatibility [57,94,95]. For PDMS–PS bonding, three previously discussed techniques were also successfully applied, namely, surface activation combined with either APTES [38,40], APTES/TESPSA [46], or APTES + low molecular weight PDMS linker [47] treatment.
A one-step bonding technique, which stands out from the other chemical gluing methods, was also presented by Xu et al. [36]. They used air plasma (a mixture of oxygen and nitrogen) to activate the surface of both PS and PDMS substrates. Oxygen is known to create a large number of hydroxy (–OH) groups with a small portion of carbonyl (–C=O) groups on the surface of carbon-based plastics. The presence of nitrogen in the plasma enables the formation of imine groups ((R1, R2)C=NH) and their free radical forms, which is the essential contribution for irreversible PDMS–plastic bonding [36,96]. This effect of nitrogen was confirmed by both XPS performed on the treated surfaces and negative controls with oxygen/argon mixed plasma. Although this one-step bonding process was reproduced with COC and PP polymers, and they all performed well at dynamic leakage and burst tests (e.g., burst pressures above 500 kPa, measured with compressed air), this technique was still not tested for other mainstream polymers (PMMA, PC, PET, etc.).
Li et al. used an adhesive-based gluing technique to attach PDMS to PS by subsequently spin coating a silicone-based adhesive (PrimeCoat, ~1 µm) and an epoxy adhesive (3–4 µm) onto the substrates [57]. The resulting maximum burst pressure was comparable to other chemical gluing methods (see Table 2). The same adhesive-based technique was successfully implemented to bond PDMS with glass and PET as well [57].
4.6. PET
Poly(ethylene terephthalate) (PET) is optically transparent, strong, and lightweight plastic that is one of the favored substrates to choose when the planned application requires flexibility. Its chemical inertness, good gas permeability, and low cost can also be advantageous for several applications. Most previously mentioned chemical gluing technique can be selected for PDMS–PET bonding (namely, surface activation combined with either MPTMS [43], APTES/TESPSA [46], APTES/GPTMS [44,45], or APTES + low molecular weight PDMS linker [47] treatments. The resulting tensile strengths and burst pressures are a bit mixed (e.g., compared to PDMS–PMMA higher [43] and lower [47] tensile strengths are also reported with the same protocols), but all listed approaches can be successfully used to create reliable bonding with this flexible substrate.
Here, another technique that stands out has to be noted. Baraket et al. used KOH-based activation and hydrolyzed the surface of the tested polymers, which were then functionalized with MPTMS. Subsequently, oxygen plasma was made to substitute the propyl–thiol chain with hydroxyl groups by cleaving the terminal groups on the MPTMS treated polymers. These silanol groups form the siloxane bonds with the surface-activated PDMS [20]. Please note that this proposed approach is different from other chemical gluing methods which use MPTMS, e.g., proposed by Wu et al. [43], where the organosilane is bound to the corona treated surface of the PET substrate through thiol groups. The silane layer is in turn bonded with the activated PDMS, as illustrated in Figure 2d. Although Baraket et al. successfully applied this method to both PI and PEN besides PET [20], since no tensile or burst tests were performed, its comparison with the method of Wu et al. is not directly possible.
4.7. PI
Polyimides (including DuPont’s favored commercial product, ‘Kapton’) are popular substrate materials for flexible electronic devices [97,98]. One of their main advantages compared to the similarly flexible PET is their high glass transition temperature and stability over a wide temperature range (e.g., between −269 °C and 400 °C) [53]. Its high dielectric constant, good chemical resistance and compatibility with microelectronics packaging technologies also make it an excellent candidate to bridge the gap between microelectronics and microfluidics. The most detailed investigation of PDMS–PI bonding was performed by Hoang et al., who compared chemical gluing methods with adhesives (epoxy, silicon) and with hybrid MPTMS-epoxy adhesive methods, and tested the resulting bonds with peel tests [53]. Using adhesives only (e.g., LePage Gel epoxy adhesive), the resulting peel strengths were quite low (1.7–72 N/m). Out of the two chemical gluing methods, they only obtained a strong bond with the MPTMS/GPTMS pair (200 N/m), the APTES/GPTMS pair yielded a 100-times smaller 2.7 N/m peel strength. This result is somewhat surprising, since other groups successfully used the APTES/GPTMS pair with similar procedures (functionalizing the PDMS with APTES and the plastic with GPTMS) [21,44]. For PDMS–PI bonding, the best results were obtained by combining MPTMS surface functionalization with the epoxy adhesive (470 N/m). Both the MPTMS-epoxy and the MPTMS/GPTMS strategy yielded peel strengths that are comparable or even better than some values reported for PDMS–PDMS pairs [54,55], which indicates a strong and reliable bond.
4.8. Other Polymer Substrates
In Table 2, protocols that can be used for bonding PDMS with five more polymers are also given. These strategies were previously discussed in accordance with other substrates. In short, the air plasma surface activation used by Xu et al. was also successfully applied to bond PDMS with polypropylene (PP) and cyclic olefin copolymer (COC) [36]. For COC, Cortese et al. investigated other protocols from oxygen plasma treatment to oxygen plasma + APTES or GPTMS treatment, including oxygen plasma + APTES/GPTMS combined functionalization [21], which yielded the best results. For polyethylene naphthalate (PEN), the protocol of Baraket et al. was used with success, including KOH surface activation, MPTMS treatment, and oxygen plasma [20]. For polyvinyl chloride (PVC), a simple corona activation combined with MPTMS treatment yielded high bonding strengths [43], comparable to PDMS–PMMA or PDMS–PET with the same protocol. Finally, oxygen plasma combined with APTES was successfully used to bond PDMS with acrylonitrile butadiene styrene (ABS) as well.
4.9. Metals
Although metals cannot be considered as typical substrates in microfluidic fabrication, it has to be noted that by simple chemical gluing, PDMS can be conveniently bonded with various metals. The thiol–metal bonds, which were discussed in accordance with gold-coated glass [50], were successfully used to bond PDMS with iron, copper, aluminum, or brass by using a simple surface activation + MPTMS treatment protocol [43].
5. Conclusions
Strategies and methods to bond PDMS with various rigid and flexible substrate materials were collected and evaluated. As it was shown, with optimized process parameters, high-quality and high-strength bonds can be achieved. Commonly achievable strengths with optimized process parameters—quantitatively, based on the three most widely used testing methods, which are tensile strength tests, burst tests, and channel leakage tests—are >400 kPa tensile strength, >500 kPa burst pressure, or flow rates equal to several 1000× of the internal volume of the channels per minute. This could be achieved with simple surface activation methods for silicone-based substrates (e.g., glass, silicon, or PDMS). For polymers and metallic surfaces, chemical gluing is advised. In some exceptional cases, a combined adhesive + chemical gluing method could yield extra-strong bonds. It is hoped that the collected optimized process parameters in Table 3 will help others in this field to quickly tune their processes based on their substrates, available equipment, or application area.
Despite the growing trend and need for LoC and Micro-TAS devices, PDMS-based microfluidics remains an academic and small-scale R&D, rather than an industrial/commercial success, mostly owing to the scalability problems related to the micromachining and prototyping technologies considering large scale manufacture [99]. However, as demonstrated in the paper, high-quality bonds can now be formed between PDMS and many other plastic materials, some of which are compatible with either large-scale fabrication methods (e.g., PMMA), or electronics packaging technologies (e.g., PI). The combination of PDMS with such materials might open the door for its larger commercial/industrial success in the near future.
Author Contributions
A.B. (Alexandra Borók): literature survey, writing, visualization; K.L.: literature survey, writing; A.B. (Attila Bonyár): conceptualization, supervision, writing, review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The research reported in this paper and carried out at the Budapest University of Technology and Economics has been supported by the NRDI Fund (TKP2020 IES, Grant No. BME-IE-BIO) based on the charter of bolster issued by the NRDI Office under the auspices of the Ministry for Innovation and Technology. Prepared with the professional support of the Doctoral Student Scholarship Program of the Co-operative Doctoral Program of the Ministry for Innovation and Technology from the source of the National Research Development and Innovation Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The research reported in this paper and carried out at the Budapest University of Technology and Economics has been supported by the NRDI Fund (TKP2020 IES, Grant No. BME-IE-BIO) based on the charter of bolster issued by the NRDI Office under the auspices of the Ministry for Innovation and Technology. Prepared with the professional support of the Doctoral Student Scholarship Program of the Co-operative Doctoral Program of the Ministry for Innovation and Technology from the source of the National Research Development and Innovation Fund.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
APTES: 3-aminopropyl)triethoxysilane; CNC, Computer Numerical Control; COC, Cyclic Olefin Copolymer; DMPMS, Dimethyl-MethylPhenylMethoxy Siloxane; DRIE, Deep Reactive Ion Etching; GPTES, 3-glycidyloxypropyl)triethoxysilane; GPTMS, 3-glycidyloxypropyl)trimethoxysilane; ICP, Inductively Coupled High-Density Plasma System; KOH, Potassium hydroxide; LoC, Lab-on-a-Chip; MEMS, Micro Electro-Mechanical Systems; micro-TAS, micro Total Analysis Systems; MPTMS, 3-mercaptopropyl)trimethoxysilane; NOA74, Norland Optical Adhesive; PC, Polycarbonate; PDMS, Polydimethylsiloxane; PECVD, Plasma Enhanced Chemical Vapor Deposition; PEN, Polyethylene Naphthalate; PET, Polyethylene Terephthalate; PI, Polyimide; PMMA, Poly(methyl methacrylate); PS, Polystyrene; PVC, Polyvinyl Chloride; R&D, Research and Development; RIE, Reacting Ion Etching; RT, Room Temperature; SPR, Surface Plasmon Resonance; TESPSA, 3-triethoxysilyl)propylsuccinic anhydride; THF, Tetrahydrofuran; UV, Ultraviolet.
References
- Bowen, J.; Cheneler, D.; Robinson, A.P.G. Direct e-beam lithography of PDMS. Microelectron. Eng. 2012, 97, 34–37. [Google Scholar] [CrossRef]
- Raj, M.K.; Chakraborty, S. PDMS microfluidics: A mini review. J. Appl. Polym. Sci. 2020, 137, 48958. [Google Scholar] [CrossRef]
- Becker, H.; Locascio, L.E. Polymer microfluidic devices. Talanta 2002, 56, 267–287. [Google Scholar] [CrossRef]
- Mata, A.; Fleischman, A.J.; Roy, S. Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 2005, 7, 281–293. [Google Scholar] [CrossRef]
- De Jong, J.; Lammertink, R.G.H.; Wessling, M. Membranes and microfluidics: A review. Lab Chip 2006, 6, 1125–1134. [Google Scholar] [CrossRef]
- Gale, B.K.; Jafek, A.R.; Lambert, C.J.; Goenner, B.L.; Moghimifam, H.; Nze, U.; Kamarapu, S.K. A Review of Current Methods in Microfluidic Device Fabrication and Future Commercialization Prospects. Inventions 2018, 3, 60. [Google Scholar] [CrossRef] [Green Version]
- McDonald, J.C.; Duffy, D.C.; Anderson, J.R.; Chiu, D.T.; Wu, H.K.; Schueller, O.J.A.; Whitesides, G.M. Fabrication of microfluidic systems in poly(dimethylsiloxane). Electrophoresis 2000, 21, 27–40. [Google Scholar] [CrossRef]
- Becker, H.; Gartner, C. Polymer microfabrication methods for microfluidic analytical applications. Electrophoresis 2000, 21, 12–26. [Google Scholar] [CrossRef]
- Sia, S.K.; Whitesides, G.M. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis 2003, 24, 3563–3576. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Koerner, T.; Horton, J.H.; Oleschuk, R.D. Fabrication and characterization of poly(methylmethacrylate) microfluidic devices bonded using surface modifications and solvents. Lab Chip 2006, 6, 66. [Google Scholar] [CrossRef] [PubMed]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Wang, R.D.; Yu, H.X.; Li, G.J.; Xu, K.X.; Tien, N.C.; Roberts, R.C.; Li, D.C. Inkjet-printed microelectrodes on PDMS as biosensors for functionalized microfluidic systems. Lab Chip 2015, 15, 690–695. [Google Scholar] [CrossRef]
- Alkhalaf, Q.; Pande, S.; Palkar, R. Review of Polydimethylsiloxane (PDMS) as a Material for Additive Manufacturing, Innovative Design, Analysis and Development Practices in Aerospace and Automotive Engineering. Lect. Notes Mech. Eng. Springer Singap. 2021, 7, 265–275. [Google Scholar]
- Magdassi, S.; Kamyshny, A. Nanomaterials for 2D and 3D Printing; Wiley: New York, NY, USA, 2017. [Google Scholar]
- Cheng, S.; Wu, Z.G. Microfluidic stretchable RF electronics. Lab Chip 2010, 10, 3227–3234. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.C.; Bruzewicz, D.A.; Weibel, D.B.; Whitesides, G.M. Microsolidics: Fabrication of three-dimensional metallic microstructures in poly(dimethylsiloxane). Adv. Mater. 2007, 19, 727–733. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Gupta, S.; Vilouras, A.; Dahiya, R. Polydimethylsiloxane as polymeric protective coating for fabrication of ultra-thin chips. Microelectron. Eng. 2020, 221, 7. [Google Scholar] [CrossRef]
- Yang, C.; Wang, W.; Li, Z. Optimization of Corona-triggered PDMS-PDMS Bonding Method. IEEE Int. Conf. Nano/Micro Eng. Mol. Syst. 2009, 5, 319–322. [Google Scholar]
- Baraket, A.; Zine, N.; Lee, M.; Bausells, J.; Jaffrezic-Renault, N.; Bessueille, F.; Yaakoubi, N.; Errachid, A. Development of a flexible microfluidic system based on a simple and reproducible sealing process between polymers and poly(dimethylsiloxane). Microelectron. Eng. 2013, 111, 332–338. [Google Scholar] [CrossRef]
- Cortesea, B.; Mowlemb, M.C.; Morgan, H. Characterisation of an irreversible bonding process for COC–COC and COC–PDMS–COC sandwich structures and application to microvalve. Sens. Actuators B Chem. 2011, 160, 1473–1480. [Google Scholar] [CrossRef]
- Thompson, C.S.; Abate, A.R. Adhesive-based bonding technique for PDMS microfluidic devices. Lab Chip 2013, 13, 632–635. [Google Scholar] [CrossRef]
- Tsao, C.W.; Syu, W.C. Bonding of thermoplastic microfluidics by using dry adhesive tape. RSC Adv. 2020, 10, 30289–30296. [Google Scholar] [CrossRef]
- Li, J.M.; Liang, C.; Zhang, H.; Liu, C. Reliable and high quality adhesive bonding for microfluidic devices. Micro Nano Lett. 2017, 12, 90–94. [Google Scholar] [CrossRef]
- Souza, A.; Ribeiro, J.; Araújo, F.S. Study of PDMS characterization and its applications in biomedicine: A review. J. Mech. Eng. Biomech. 2019, 4, 1–9. [Google Scholar]
- Hwang, Y.; Candler, R.N. Non-planar PDMS microfluidic channels and actuators: A review. Lab Chip 2017, 17, 3948–3959. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.F.; Wang, Z.; Zhang, W.; Jiang, X.Y. A simple PDMS-based microfluidic channel design that removes bubbles for long-term on-chip culture of mammalian cells. Lab Chip 2010, 10, 2906–2910. [Google Scholar] [CrossRef] [PubMed]
- Wengler, J.; Ognier, S.; Zhang, M.X.; Levernier, E.; Guyon, C.; Ollivier, C.; Fensterbank, L.; Tatoulian, M. Microfluidic chips for plasma flow chemistry: Application to controlled oxidative processes. React. Chem. Eng. 2018, 3, 930–941. [Google Scholar] [CrossRef]
- Sollier, E.; Murray, C.; Maoddi, P.; Di Carlo, D. Rapid prototyping polymers for microfluidic devices and high pressure injections. Lab Chip 2011, 11, 3752–3765. [Google Scholar] [CrossRef] [PubMed]
- Tiggelaar, R.M.; Benito-Lopez, F.; Hermes, D.C.; Rathgen, H.; Egberink, R.J.M.; Mugele, F.G.; Reinhoudt, D.N.; van den Berg, A.; Verboom, W.; Gardeniers, H.J.G.E. Fabrication, mechanical testing and application of high-pressure glass microreactor chips. Chem. Eng. J. 2007, 131, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.W.; Ran, T.; Gan, Y.; Zhou, J.J.; Zhang, Z.; Zhang, L.W.; Zhang, D.Y.; Jiang, L. Ultrafast water harvesting and transport in hierarchical microchannels. Nat. Mater. 2018, 17, 935. [Google Scholar] [CrossRef] [PubMed]
- Van Steijn, V.; Kreutzer, M.T.; Kleijn, C.R. Velocity fluctuations of segmented flow in microchannels. Chem. Eng. J. 2008, 135, S159–S165. [Google Scholar] [CrossRef]
- Abkarian, M.; Faivre, M.; Stone, H.A. High-speed microfluidic differential manometer for cellular-scale hydrodynamics. Proc. Natl. Acad. Sci. USA 2006, 103, 538–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofla, A.Y.N.; Martin, C. Study of the vapor-assisted method for bonding PDMS and glass: Effect of the vapor source. J. Micromechanics Microeng. 2010, 20, 125024. [Google Scholar] [CrossRef]
- Cao, H.H.; Dinh, T.H.N.; Hamdi, F.S.; Couty, M.; Martincic, E.; Woytasik, M.; Dufour-Gergam, E. Reversible bonding by dimethyl-methylphenylmethoxy siloxane based stamping technique for reusable poly(dimethyl)siloxane microfluidic chip. Micro Nano Lett. 2015, 10, 229–232. [Google Scholar] [CrossRef]
- Xu, B.Y.; Yan, X.N.; Xu, J.J.; Chen, H.J. One step high quality poly(dimethylsiloxane)-hydrocarbon plastics bonding. Biomicrofluidics 2012, 6, 16507–165078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pečar, B.; Možek, M.; Vrtačnik, D. Thermoplastic—PDMS polymer covalent bonding for microfluidic applications. J. Microelectron. 2017, 47, 147–154. [Google Scholar]
- Sunkara, V.; Park, D.K.; Hwang, H.; Chantiwas, R.; Soper, S.A.; Cho, Y.K. Simple room temperature bonding of thermoplastics and poly(dimethylsiloxane). Lab Chip 2011, 11, 962–965. [Google Scholar] [CrossRef] [Green Version]
- Rezai, P.; Selvaganapathy, P.R.; Rwohl, G. Plasma enhanced bonding of polydimethylsiloxane with parylene and its optimization. J. Micromech. Microeng. 2011, 21, 065024. [Google Scholar] [CrossRef]
- Song, K.Y.; Zhang, H.B.; Zhang, W.J.; Teixeira, A. Enhancement of the surface free energy of PDMS for reversible and leakage-free bonding of PDMS-PS microfluidic cell-culture systems. Microfluid. Nanofluidics 2018, 22, 135. [Google Scholar] [CrossRef]
- Kim, K.; Park, S.W.; Yang, S.S. The optimization of PDMS-PMMA bonding process using silane primer. BioChip J. 2010, 4, 148–154. [Google Scholar] [CrossRef]
- Chow, W.W.Y.; Lei, K.F.; Shi, G.; Li, W.J.; Huang, Q. Micro Fluidic Channel Fabrication by PDMS-Interface Bonding. Smart Mater. Struct. 2005, 15, 141–148. [Google Scholar]
- Wu, W.; Wu, J.; Kima, J.H.; Lee, N.Y. Instantaneous room temperature bonding of a wide range of non-silicon substrates with poly(dimethylsiloxane) (PDMS) elastomer mediated by a mercaptosilane. Lab Chip 2015, 15, 2819–2825. [Google Scholar] [CrossRef]
- Tang, L.; Lee, N.Y. A facile route for irreversible bonding of plastic-PDMS hybrid microdevices at room temperature. Lab Chip 2010, 10, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.Y.; Chung, B.H. Novel Poly(dimethylsiloxane) Bonding Strategy via Room Temperature “Chemical Gluing”. Langmuir 2009, 25, 3861–3866. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Chemically robust succinimide group-assisted irreversible bonding of poly(dimethylsiloxane)-thermoplastic microfluidic devices at room temperature. Analyst 2020, 145, 6887–6894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.N.; Lee, N.Y. Non-silicon substrate bonding mediated by poly(dimethylsiloxane) interfacial coating. Appl. Surf. Sci. 2015, 327, 233–240. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Zhang, P.; Li, Y. Easy-Disassembly Bonding of PDMS Used for Leak-Tight Encapsulation of Microfluidic Devices. In Proceedings of the 18th International Conference on Electronic Packaging Technology (ICEPT), Harbin, China, 16–19 August 2017; pp. 1051–1055. [Google Scholar]
- Xionga, L.C.; Chena, P.; Zhoua, Q. Adhesion promotion between PDMS and glass by oxygen plasma pre-treatment, J. Adhes. Sci. Technol. 2014, 28, 1046–1154. [Google Scholar] [CrossRef]
- Bakouche, M.T.; Ganesan, S.; Guérin, S.; Hourlier, D.; Bouazaoui, M.; Vilcot, J.P.; Maricot, S. Leak-free integrated microfluidic channel fabrication for surface plasmon resonance applications. J. Micromech. Microeng. 2020, 30, 125003. [Google Scholar] [CrossRef]
- Campbell, F.C. Chapter 8—Adhesive Bonding and Integrally Cocured Structure: A Way to Reduce Assembly Costs through Parts Integration. In Manufacturing Processes for Advanced Composites; Elsevier Science: Amsterdam, The Neatherlands, 2004; pp. 241–301. [Google Scholar]
- Duncan, B. 14–Developments in testing adhesive joints. In Advances in Structural Adhesive Bonding; Woodhead Publishing: Sawston, UK, 2010; pp. 389–436. [Google Scholar]
- Hoang, M.V.; Chung, H.J.; Elias, A.L. Irreversible bonding of polyimide and polydimethylsiloxane (PDMS) based on a thiolepoxy click reaction. J. Micromech. Microeng. 2016, 26, 105019. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.F. Characterization of fracture energy and toughness of air plasma PDMS-PDMS bonding by T-peel testing. J. Adhes. Sci. Technol. 2017, 32, 1239–1252. [Google Scholar] [CrossRef]
- Jung, K.H.; Kim, D.G.; Jung, S.B. Adhesion of PDMS substrates assisted by Plasma Graft Polymerization. Surf. Interface Anal. 2016, 48, 597–600. [Google Scholar] [CrossRef]
- Abidin, U.; Daud, N.A.S.M.D.; Le Brun, V. Replication and leakage Test of Polydimethylsiloxane (PDMS) Microfluidics Channel. AIP Conf. Proc. 2019, 2062, 020064. [Google Scholar]
- Li, X.; Wu, N.; Rojanasakul, Y.; Liu, Y. Selective stamp bonding of PDMS microfluicdic devices to polymer substrates for biological applications. Sens. Actuators A Phys. 2013, 193, 186–192. [Google Scholar] [CrossRef]
- Aran, K.; Sasso, L.A.; Kamdar, N.; Zahn, J.D. Irreversible, direct bonding of nanoporous polymer membranes to PDMS or glass microdevices. Lab Chip 2010, 10, 548–552. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharya, S.; Datta, A.; Berg, J.M.; Gangopadhyay, S. Studies on Surface Wettability of Poly(Dimethyl) Siloxane (PDMS) and Glass Under Oxygen-Plasma Treatment and Correlation With Bond Strength. J. Microelectromechanical. Syst. 2005, 14, 590–597. [Google Scholar] [CrossRef]
- Eddings, M.A.; Johnson, M.A.; Gale, B.K. Determining the optimal PDMS–PDMS bonding technique for microfluidic devices. J. Micromech. Microeng. 2008, 18, 067001. [Google Scholar] [CrossRef]
- Agostini, M.; Greco, G.; Cecchini, M. Polydimethylsiloxane (PDMS) irreversible bonding to untreated plastics and metals for microfluidics applications. APL Mater. 2019, 7, 081108. [Google Scholar] [CrossRef] [Green Version]
- Yousuff, C.M.; Danish, M.; Ho, E.T.W.; Basha, I.H.K.; Hamid, N.H.B. Study on the Optimum Cutting Parameters of an Aluminum Mold for Effective Bonding Strength of a PDMS Microfluidic Device. Micromachines 2017, 8, 285. [Google Scholar] [CrossRef]
- Goldman, M.; Goldman, A.; Sigmond, R.S. The corona discharge, its properties and specific uses; Pure and Applied Chemistry. Pure Appl. Chem. 1985, 57, 1353–1362. [Google Scholar] [CrossRef] [Green Version]
- Hillborg, H.; Karlsson, S.; Gedde, U.W. Characterisation of low molar mass siloxanes extracted from crosslinked polydimethylsiloxanes exposed to corona discharges. Polymer 2001, 42, 8883–8889. [Google Scholar] [CrossRef]
- Foerch, R.; Izawa, J.; Spears, G. A comparative study of the effects of remote nitrogen plasma, remote oxygen plasma, and corona discharge treatments ont he surface properties of polyethylene. J. Adhes. Sci. Technol. 1991, 5, 549–564. [Google Scholar] [CrossRef]
- Grace, J.M.; Gerenser, L.J. Plasma Treatment of Polymers. J. Dispers. Sci. Technol. 2003, 24, 305–341. [Google Scholar] [CrossRef]
- Vesel, A.; Junkar, I.; Cvelbar, U.; Kovac, J.; Mozetic, M. Surface modification of polyester by oxygen- and nitrogen-plasma treatment. Surf. Interface Anal. 2008, 40, 1444–1453. [Google Scholar] [CrossRef]
- Najafi, K.; Harpster, T.J.; Kim, H.; Mitchell, J.S.; Welch, W.C. 1.09–Wafer Bonding. In Comprehensive Microsystems; Elsevier: Amsterdam, The Netherlands, 2008; pp. 235–270. [Google Scholar]
- Hillborg, H.; Gedde, U.W. Hydrophobicity changes in silicone rubbers. IEEE Trans. Dielect. Elect. Insul. 1999, 6, 703–717. [Google Scholar] [CrossRef]
- Garbassi, F.; Morra, M.; Barino, L.; Occhiello, E. Polymer Surfaces. In From Physics to Technology; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Waters, L.J.; Finch, C.V.; Bhuiyan, A.K.M.H.; Hemming, K.; Mitchell, J.C. Effect of plasma surface treatment of poly(dimethylsiloxane) on the permeation of ppharmaceutical compounds. J. Pharm. Anal. 2017, 7, 338–342. [Google Scholar] [CrossRef]
- Zou, J.; Wong, P.Y. Thermal Effects in Plasma Treatment of Patterned PDMS for Bonding Stacked Channels. MRS Online Proc. Libr. 2003, 782, A5.5. [Google Scholar] [CrossRef]
- Hillborg, H.; Gedde, U.W. Hydrophobicity recovery of polydimethyl siloxane after exposure to corona discharges. Polymer 1998, 39, 1991–1998. [Google Scholar] [CrossRef]
- Zhou, J.; Ellis, A.V.; Voelcker, N.H. Recent developments in PDMS surface modification for microfluidic devices. Electrophoresis 2010, 31, 2–16. [Google Scholar] [CrossRef]
- Lopera, S.; Mansano, R.D. Plasma-Based Surface Modification of Polydimethylsiloxane for PDMS-PDMS Molding. Int. Sch. Res. Not. (ISRN) Polym. Sci. 2012, 5, 767151. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.T.; Jeong, O.C. PDMS surface modification using atmospheric pressure plasma. Microelectron. Eng. 2011, 88, 2281–2285. [Google Scholar] [CrossRef]
- Sugawara, M. Generation of a highly uniform and large area corona discharge source adaptable to surface treatment. Surf. Coat. Technol. 2001, 142, 290–292. [Google Scholar] [CrossRef]
- Adamiak, K.; Inculet, I.I.; Castle, G.S.P. The Control of Corona Current Distribution Using Shaped Electrodes. J. Electrost. 1993, 30, 381–392. [Google Scholar] [CrossRef]
- Haubert, K.; Drier, T.; Beebe, D. PDMS bonding by means of a portable, low-cost corona system. Lab Chip 2006, 6, 1548–1549. [Google Scholar] [CrossRef]
- Özçam, A.E.; Efimenko, K.; Genzer, J. Effect of ultraviolet/ozone treatment on the surface and bulk properties of poly(dimethyl siloxane) and poly(vinylmethyl siloxane) networks. Polymer 2014, 55, 3107–3119. [Google Scholar] [CrossRef]
- Kim, J.; Chaudhury, M.K.; Owen, M.J. Hydrophobic recovery of polydimethylsiloxane elastomer exposed to partial electrical discharge. J. Colloid. Interface Sci. 2000, 226, 231–236. [Google Scholar] [CrossRef] [Green Version]
- Berdichevsky, Y.; Khandurina, J.; Guttman, A.; Lo, Y.H. UV/ozone modification of poly(dimethylsiloxane) microfluidic channels. Sens. Actuators B Chem. 2004, 97, 402–408. [Google Scholar] [CrossRef]
- Fu, Y.J.; Qui, H.Z.; Liao, K.S.; Lue, S.J.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Effect of UV-Ozone Treatment on Poly(dimethylsiloxane) Membranes: Surface Characterization and Gas Separation Performance. Langmuir 2009, 26, 4392–4399. [Google Scholar] [CrossRef] [PubMed]
- Efimenko, K.; Wallace, W.E.; Genzer, J. Surface Modification of Sylgard-184 Poly(dimethylsiloxane) Networks by Ultraviolet and Ultraviolet/Ozone Treatment; J. Colloid. Interface Sci. 2002, 254, 306–315. [Google Scholar] [CrossRef]
- Schnyder, B.; Lippert, T.; Kötz, R.; Wokaun, A.; Graubner, V.M.; Nuyken, O. UV-irradiation induced modification of PDMS films investigated by XPS and spectroscopic ellipsometry. Surf. Sci. 2003, 532, 1067–1071. [Google Scholar] [CrossRef]
- Li, C.H.; Wilkes, G.L. The mechanism for 3-aminopropyltriethoxysilane to strengthen the interface of polycarbonate substrates with organic-inorganic sol-gel coatings. J. Inorg. Organomet. Polym. 1998, 8, 33–45. [Google Scholar] [CrossRef]
- Vlachopoulou, M.E.; Tserepi, A.; Pavli, P.; Argitis, P.; Sanopoulou, M.; Misiakos, K. A low temperature surface modification assisted method for bonding plastic substrates. J. Micromech. Microeng. 2009, 19, 015007. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lee, N.Y. Microfluidic device fabrication mediated by surface chemical bonding. Analyst 2020, 145, 4096–4110. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Ram, R.J. Plastic-PDMS bonding for high pressure hydrolytically stable active microfluidics. Lab Chip 2009, 9, 1618–1624. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Moreno, J.; To, J.; Yang, C.W.T.; Turner, R.F.B.; Bizzotto, D.; Cheung, K.C. Fabricating devices with improved adhesion between PDMS and gold-patterned glass. Sens. Actuators B Chem. 2017, 246, 904–909. [Google Scholar] [CrossRef]
- Shin, Y.S.; Cho, K.; Lim, S.H.; Chung, S.; Park, S.J.; Chung, C.; Han, D.C.; Chang, J.K. PDMS-based micro PCR chip with Parylene coating; J. Micromech. Microeng. 2003, 13, 768–774. [Google Scholar] [CrossRef]
- Henry, A.C.; Tutt, T.J.; Galloway, M.; Davidson, Y.Y.; McWhorter, C.S.; Soper, S.A.; McCarley, R.L. Surface Modification of Poly(methyl methacrylate) Used in the Fabrication of Microanalytical Devices. Anal. Chem. 2000, 72, 5331–5337. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.A.; Whitesides, G.M. Rapid protyping of microfluidic systems in poly (dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, X.; Shen, J.; Zhu, J.J.; Hou, W. Fabrication of a Novel Glucose Biosensor Based on a Highly Electroactive Polystyrene/Polyaniline/Au Nanocomposite. J. Phys. Chem. B 2008, 112, 9237–9242. [Google Scholar] [CrossRef]
- Qian, W.P.; Yao, D.; Yu, F.; Xu, B.; Zhou, R.; Bao, X.; Lu, Z. Immobilization of Antibodies on Ultraflat Polystyrene Surfaces. Clin. Chem. 2000, 46, 1456–1463. [Google Scholar] [CrossRef] [Green Version]
- Fridman, A.A. Plasma Chemistry, 1st ed.; Cambridge University Press: New York, NY, USA, 2008. [Google Scholar]
- Kaltenbrunner, M.; Sekitani, T.; Reeder, J.; Yokota, T.; Kuribara, K.; Tokuhara, T.; Drack, M.; Schwödiauer, R.; Graz, I.; Bauer-Gogonea, S.; et al. An ultra-lightweight design for imperceptible plastic electronics. Nature 2013, 499, 458. [Google Scholar] [CrossRef]
- Weber, J.; Potje-Kamloth, K.; Haase, F.; Detemple, P.; Volklein, F.; Doll, T. Coin-size coiled-up polymer foil thermoelectric power generator for wearable electronics. Sens. Actuators A Phys. 2006, 132, 325–330. [Google Scholar] [CrossRef]
- Convery, N.; Gadegaard, N. 30 years of microfluidics. Micro Nano Eng. 2019, 2, 76–91. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).