A Peculiar Binding Characterization of DNA (RNA) Nucleobases at MoOS-Based Janus Biosensor: Dissimilar Facets Role on Selectivity and Sensitivity
Abstract
:1. Introduction
2. Methodology
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hu, Q.; Li, H.; Wang, L.; Gu, H.; Fan, C. DNA Nanotechnology-Enabled Drug Delivery Systems. Chem. Rev. 2019, 119, 6459–6506. [Google Scholar] [CrossRef] [PubMed]
- Storhoff, J.J.; Lazarides, A.A.; Mucic, R.C.; Mirkin, C.A.; Letsinger, R.L.; Schatz, G.C. What Controls the Optical Properties of DNA-Linked Gold Nanoparticle Assemblies? J. Am. Chem. Soc. 2000, 122, 4640–4650. [Google Scholar] [CrossRef]
- Lermusiaux, L.; Sereda, A.; Portier, B.; Larquet, E.; Bidault, S. Reversible Switching of the Interparticle Distance in DNA—Templated Gold Nanoparticle Dimers. ACS Nano 2012, 6, 10992–10998. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Liu, P.; Zhou, W.; Ding, J.; Liu, J. Bioorthogonal DNA Adsorption on Polydopamine Nanoparticles Mediated by Metal Coordination for Highly Robust Sensing in Serum and Living Cells. ACS Nano 2018, 12, 9070–9080. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. A Colorimetric Lead Biosensor Using DNAzyme—Directed Assembly of Gold Nanoparticles. J. Am. Chem. Soc. 2003, 125, 6642–6643. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Non—Base Pairing DNA Provides a New Dimension for Controlling Aptamer—Linked Nanoparticles and Sensors. J. Am. Chem. Soc. 2007, 129, 8634–8643. [Google Scholar] [CrossRef]
- Vovusha, H.; Sanyal, B. Adsorption of nucleobases on 2D transition—Metal dichalcogenides and graphene sheet: A first principles density functional theory study. RSC Adv. 2015, 5, 67427–67434. [Google Scholar] [CrossRef]
- Huang, J.; Chu, J.; Wang, Z.; Zhang, J.; Yang, A.; Li, X.; Gao, C.; Huang, H.; Wang, X.; Cheng, Y. Chemisorption of NO2 to MoS2 Nanostructures and its Effects for MoS2 Sensors. Chem. Nano Mat. 2019, 5, 1123–1130. [Google Scholar]
- Wang, D.; Yang, A.; Lan, T.; Fan, C.; Pan, J.; Liu, Z.; Chu, J.; Yuan, H.; Wang, X.; Rong, M.; et al. Tellurene based chemical sensor. J. Mater. Chem. A 2019, 7, 26326–26333. [Google Scholar] [CrossRef]
- Urban, F.; Martucciello, N.; Peters, L.; McEvoy, N.; di Bartolomeo, A. Environmental Effects on the Electrical Characteristics of Back-Gated WSe2 Field-Effect Transistors. Nanomaterials 2018, 8, 901. [Google Scholar] [CrossRef] [Green Version]
- di Bartolomeo, A.; Pelella, A.; Urban, F.; Grillo, A.; Iemmo, L.; Passacantando, M.; Liu, X.; Giubileo, F. Field Emission in Ultrathin PdSe2 Back-Gated Transistors. Adv. Electron. Mater. 2020, 6, 2000094. [Google Scholar] [CrossRef]
- Yang, A.; Wang, D.; Wang, X.; Zhang, D.; Koratkar, N.; Rong, M. Recent advances in phosphorene as a sensing material. Nano Today 2018, 20, 13–32. [Google Scholar] [CrossRef]
- Zhang, L.; Khan, K.; Zou, J.; Zhang, H.; Li, Y. Recent Advances in Emerging 2D Material-Based Gas Sensors: Potential in Disease Diagnosis. Adv. Mater. Interfaces 2019, 6, 1901329. [Google Scholar] [CrossRef]
- Laref, S.; Wang, B.; Laleg-Kirati, T.; Gao, X.; Gojobori, T. Quantum Chemical Investigations of Biorelevant Molecules on Black—Phosphorus Nanocarrier: Implications for Antiviral Drugs Therapy in the Early Disease Circumstances. 2021; Unpublished. [Google Scholar]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-Dimensional Nanocrystals Produced by Exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Peng, J.; Chen, X.; Ong, W.J.; Zhao, X.; Li, N. Surface and Heterointerface Engineering of 2D MXenes and Their Nanocomposites: Insights into Electro—and Photocatalysis. Chem 2019, 5, 18–50. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Vovusha, H.; Kaewmaraya, T.; Amornkitbamrung, V.; Ahuja, R. Adsorption characteristics of DNA nucleobases, aromatic amino acids and heterocyclic molecules on silicene and germanene monolayers. Sens. Actuators B Chem. 2018, 255, 2713–2720. [Google Scholar] [CrossRef] [Green Version]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Lu, S.Z.; Pan, J.; Qin, Z.; Wang, Y.Q.; Wang, Y.; Cao, C.Y.; Du, S.; Gao, H.J. Buckled Germanene Formation on Pt(111). Adv. Mater. 2014, 26, 4820–4824. [Google Scholar] [CrossRef]
- Sihag, A.; Mallajosyula, S.S. Adsorption of DNA Bases on Two-Dimensional Boron Sheets. ChemistrySelect 2019, 4, 3308–3314. [Google Scholar] [CrossRef]
- Novoselov, S.K.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Lu, A.Y.; Zhu, H.; Xiao, J.; Chuu, C.P.; Han, Y.; Chiu, M.H.; Cheng, C.C.; Yang, C.W.; Wei, K.H.; Yang, Y.; et al. Janus monolayers of transition metal dichalcogenides. Nat. Nanotechnol. 2017, 12, 744–749. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Jia, S.; Kholmanov, I.; Dong, L.; Er, D.; Chen, W.; Guo, H.; Jin, Z.; Shenoy, V.B.; Shi, L.; et al. Janus Monolayer Transition—Metal Dichalcogenides. ACS Nano 2017, 11, 8192–8198. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.C.; Zhu, Z.Y.; Tahir, M.; Schwingenschlögl, U. Spin-orbit-induced spin splittings in polar transition metal dichalcogenide monolayers. Europhys. Lett. 2013, 102, 57001. [Google Scholar] [CrossRef]
- Dong, L.; Lou, J.; Shenoy, V.B. Large In-Plane and Vertical Piezoelectricity in Janus Transition Metal Dichalcogenides. ACS Nano 2017, 11, 8242–8248. [Google Scholar] [CrossRef]
- Haldar, S.; Vovusha, H.; Yadav, M.K.; Eriksson, O.; Sanyal, B. Systematic study of structural, electronic, and optical properties of atomic-scale defects in the two-dimensional transition metal dichalcogenides MX2. Phys. Rev. B 2015, 92, 235408. [Google Scholar] [CrossRef] [Green Version]
- Do, T.N.; Nguyen, C.V.; Tan, L.V.; Idrees, M.; Amin, B.; Hieu, N.V.; Hoai, N.Y.X.; Nieu, N.N.; Phuc, H.V. Effects of La and Ce doping on electronic structure and optical properties of janus MoSSe monolayer. Superlattices Microstruct. 2021, 151, 106841. [Google Scholar] [CrossRef]
- Guan, Z.; Ni, S.; Hu, S. Tunable Electronic and Optical Properties of Monolayer and Multilayer Janus MoSSe as a Photocatalyst for Solar Water Splitting: A First–Principles Stu. J. Phys. Chem. C 2018, 122, 6209–6216. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, X.; Huang, Y.; Zhou, Y.; Chen, J.; Xia, H.; Wang, Y.; Xie, H.; Yu, J.; Lei, D.; et al. A library of atomically thin metal chalcogenides. Nature 2018, 556, 355–359. [Google Scholar] [CrossRef]
- Senthilkumar, V.; Tam, L.C.; Kim, Y.S.; Sim, Y.; Seong, M.J.; Jang, J.I. Direct vapor phase growth process and robust photoluminescence properties of large area MoS2 layers. Nano Res. 2014, 7, 1759–1768. [Google Scholar] [CrossRef]
- Wang, L.; Chen, L.; Wong, L.S.; Huang, X.; Liao, W.; Zhu, C.; Ang, W.K. 2D Electronics: Electronic Devices and Circuits Based on Wafer—Scale Polycrystalline Monolayer MoS2 by Chemical Vapor Deposition. Adv. Electron. Mat. 2019, 8, 1900393. [Google Scholar] [CrossRef]
- Li, X.L.; Li, Y.D. Formation of MoS2 Inorganic Fullerenes (IFs) by the Reaction of MoO3 Nanobelts and S. Chem. Eur. J. 2003, 9, 2726–2731. [Google Scholar] [CrossRef] [PubMed]
- Arnoldy, P.; van den Heijkant, J.A.M.; de Bok, G.D.; Moulijn, J.A. Temperature–programmed sulfiding of MoO3Al2O3 catalysts. J. Catal. 1985, 92, 35–55. [Google Scholar] [CrossRef]
- Okamoto, Y.; Kato, A.; Usman, R.N.; Fujikawa, T.; Koshika, H.; Hiromitsu, I.; Kubota, T. Effect of sulfidation temperature on the intrinsic activity of Co—MoS2 and Co—WS2 hydrodesulfurization catalysts. J. Catal. 2009, 265, 216–228. [Google Scholar] [CrossRef]
- Wu, Z.; Wang, D.; Sun, A. Preparation of MoS2 nanoflakes by a novel mechanical activation method. J. Cryst. Growth 2010, 312, 340–343. [Google Scholar] [CrossRef]
- Yagmurcukardes, M.; Peeters, F.M. Stable single layer of Janus MoSO: Strong out-of-plane piezoelectricity. Phys. Rev. B 2020, 101, 155205. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane—wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total–energy calculations using a plane–wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, S. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT—D) for the 94 elements H—Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comp. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Accurate description of van der Waals complexes by density functional theory including empirical corrections. J. Comput. Chem. 2004, 25, 1463–1473. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S. Semiempirical GGA–type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Yang, H.; Liu, Y.; Gao, C.; Meng, L.; Liu, Y.; Tang, X.; Ye, H. Adsorption Behavior of Nucleobases on Doped MoS2 Monolayer: A DFT Study. J. Phys. Chem. C 2019, 123, 30949–30957. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, M.; Jahanshahi, M.; Ghorbanzadeh, M.; Najafpour, G. Adsorption of DNA/RNA nucleobases onto single–layer MoS2 and Li-Doped MoS2: A dispersion—Corrected DFT study. Appl. Surf. Sci. 2018, 434, 176–187. [Google Scholar] [CrossRef]
- Mehdizadeh, A.; Zeynali, M.; Karimi, M. Engineering of MoS2 nanoribbons as high–performance materials for biosensing applications. Appl. Surf. Sci. 2021, 540, 148349. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, K.; Cui, H.; Yu, J.; Tao, L.-Q.; Li, X.; Liao, C.; Xie, L.; Chen, X. Tellurene based biosensor for detecting DNA/RNA nucleobases and amino acids: A theoretical insight. Appl. Surf. Sci. 2020, 532, 147451. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, Y.-K.; Kim, H.-J.; Scheicher, R.H.; Cho, J.-H. Physisorption of DNA Nucleobases on h—BN and Graphene: vdW—Corrected DFT Calculations. J. Phys. Chem. C 2013, 117, 13435–13441. [Google Scholar] [CrossRef] [Green Version]
- Cortés-Arriagada, D. Phosphorene as a Template Material for Physisorption of DNA/RNA Nucleobases and Resembling of Base Pairs: A Cluster DFT Study and Comparisons with Gra. J. Phys. Chem. C 2018, 122, 4870–4880. [Google Scholar] [CrossRef]
- Li, B.; Shao, Z. Adsorption of DNA/RNA nucleobases and base pairs on penta–graphene from first principles. Appl. Surf. Sci. 2020, 512, 145635. [Google Scholar] [CrossRef]
- Cortés-Arriagada, D.; Ortega, D.E. Effects on the aromatic character of DNA/RNA nucleobases due to its adsorption onto graphene. Int. J. Quant. Chem. 2018, 118, e25699. [Google Scholar] [CrossRef]
- Kadioglu, Y.; Gorkan, T.; Aktürk, O.Ü.; Aktürk, E.; Ciraci, S. Enhanced Interactions of Amino Acids and Nucleic Acid Bases with Bare Black Phosphorene Monolayer Mediated by Coadsorbed Species. J. Phys. Chem. C 2019, 38, 23691–23704. [Google Scholar] [CrossRef] [Green Version]
- Mathew, K.; Sundararaman, R.; Letchworth-Weaver, K.; Arias, T.A.; Hennig, G.R. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 2014, 140, 084106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathew, K.; Kolluru, V.S.C.; Mula, S.; Steinmann, S.N.; Hennig, R.G. Implicit self-consistent electrolyte model in plane–wave density–functional theory. arXiv 2016, arXiv:1601.03346. [Google Scholar] [CrossRef] [Green Version]
- Heyd, J.; Scuseria, G.E. Hybrid functionals based on a screened Coulomb potential. J. Chem. Phys. 2003, 118, 8207. [Google Scholar] [CrossRef] [Green Version]
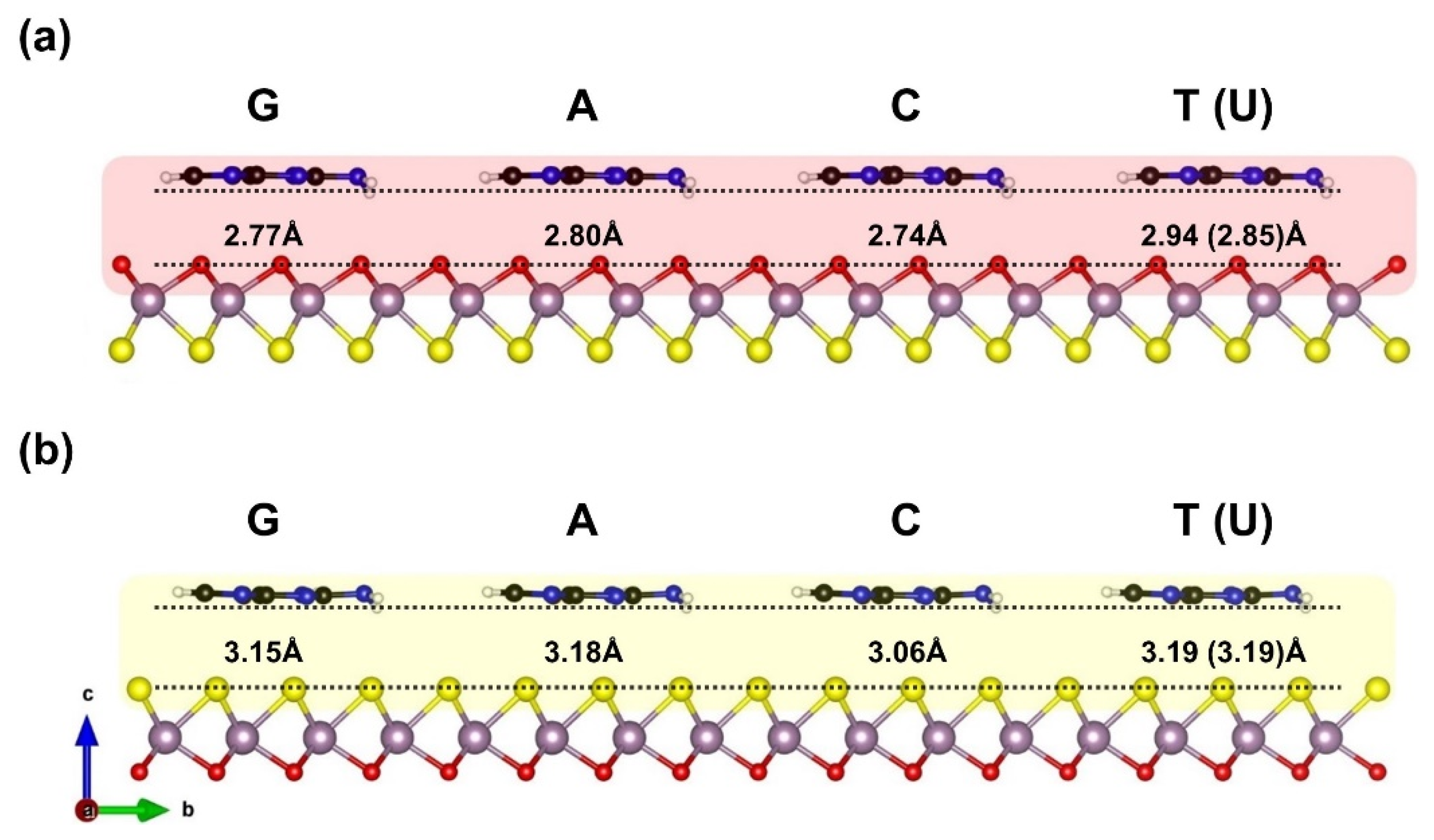

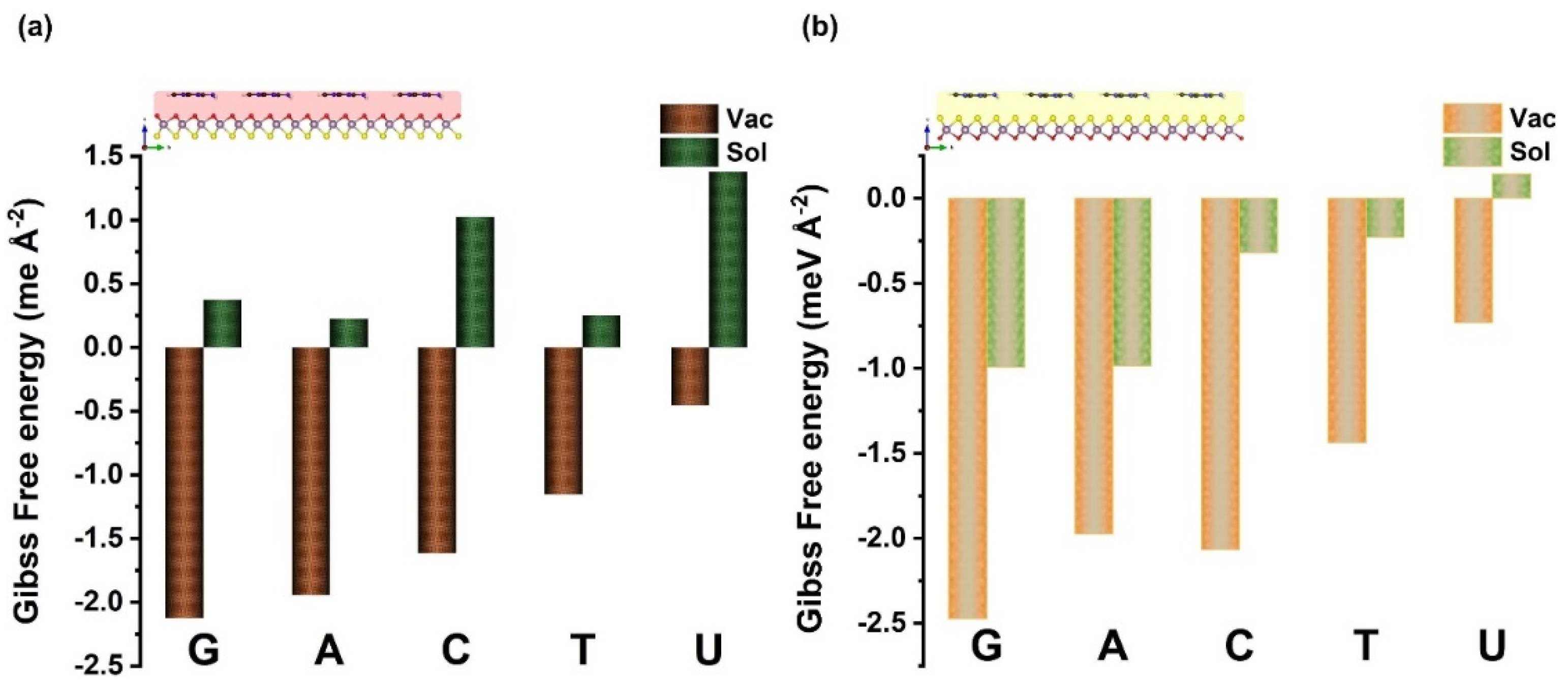
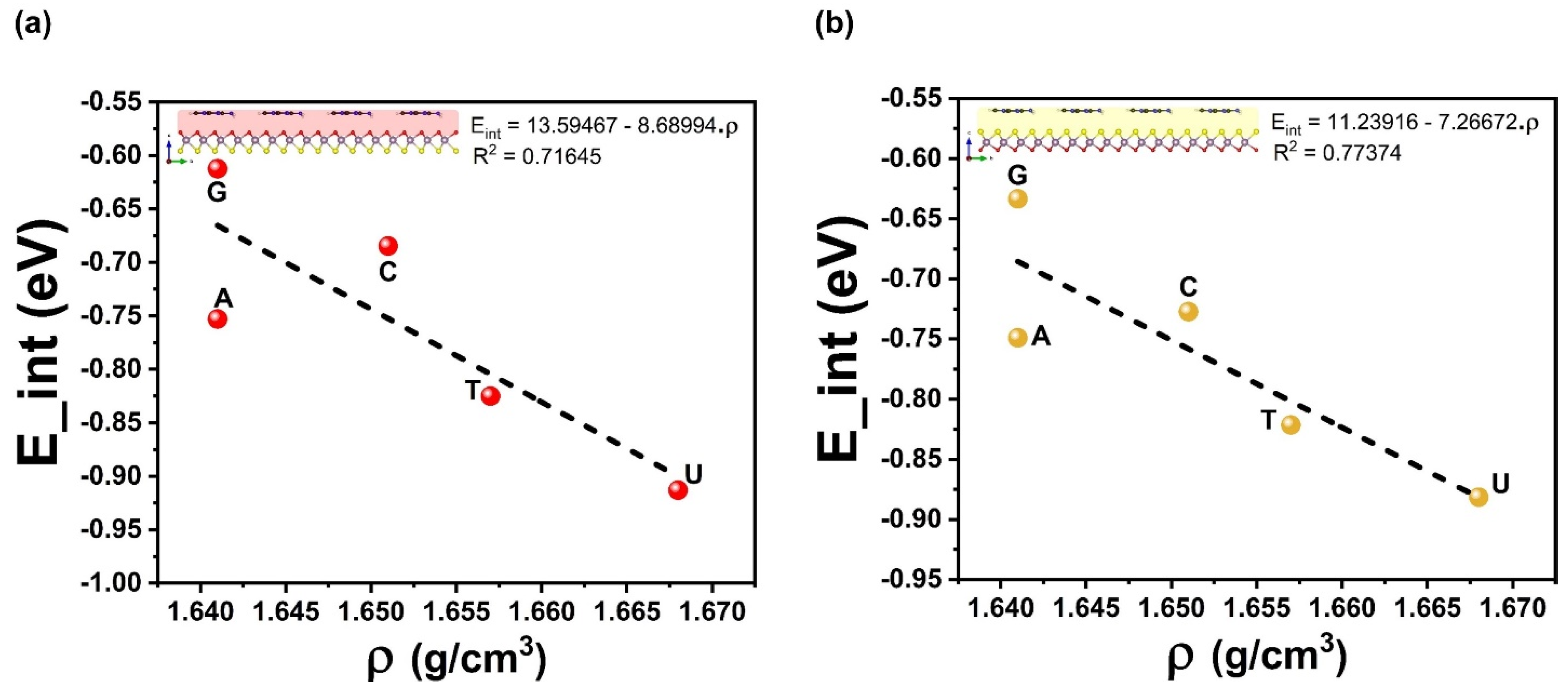

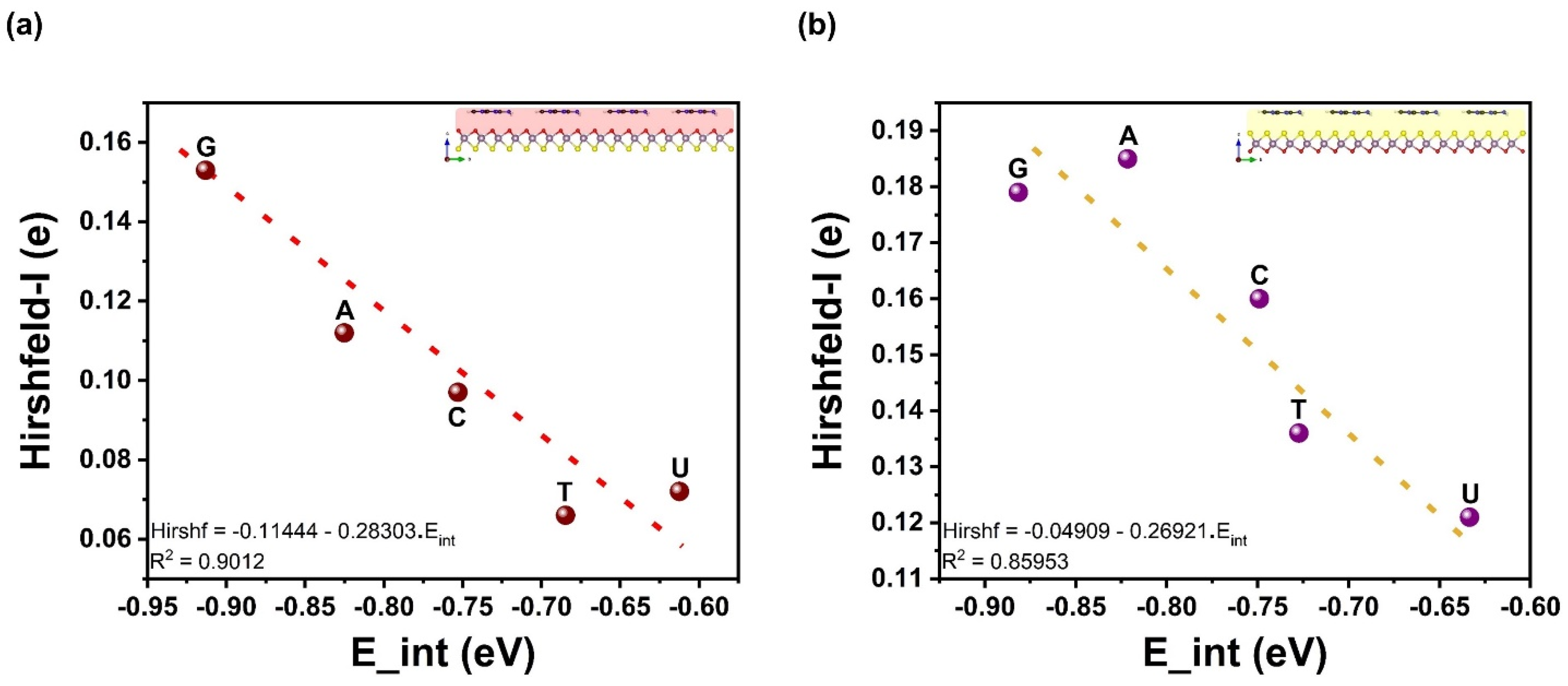
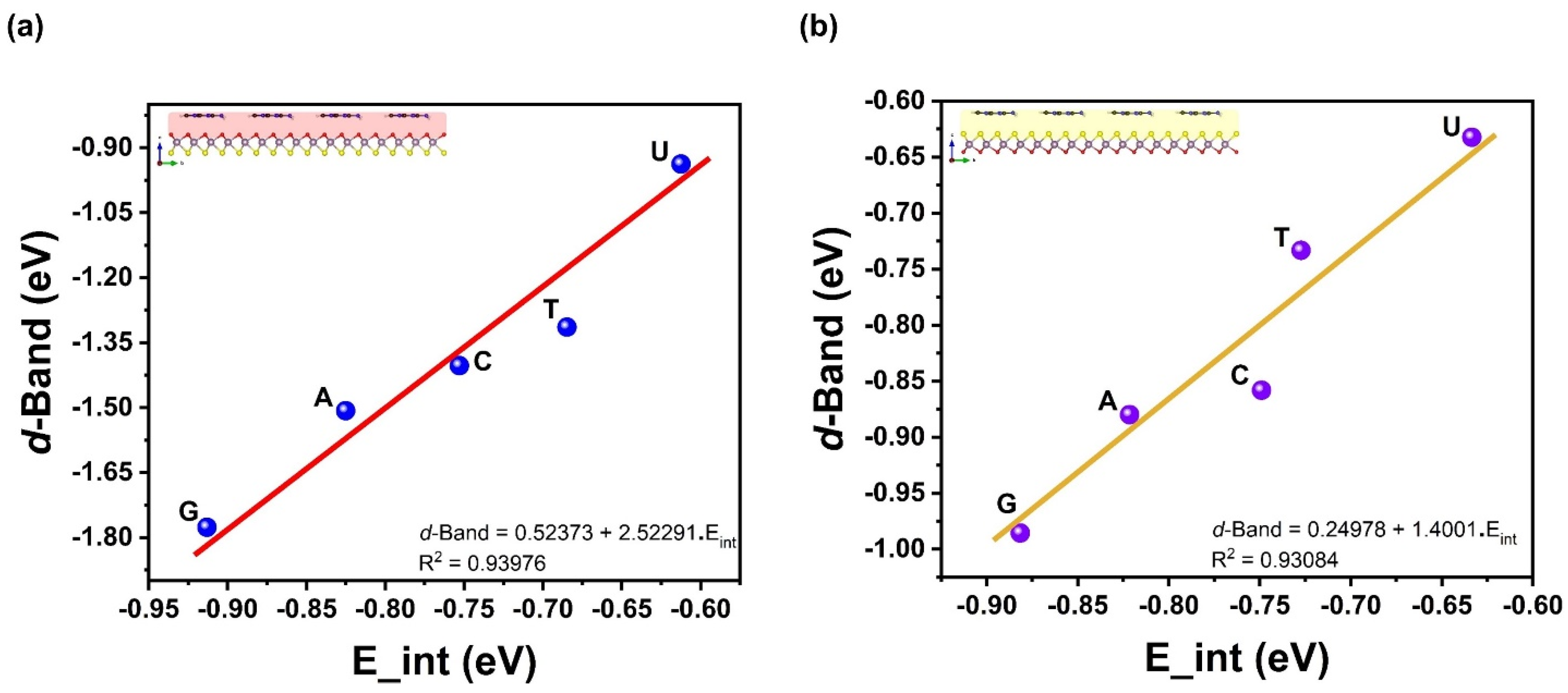
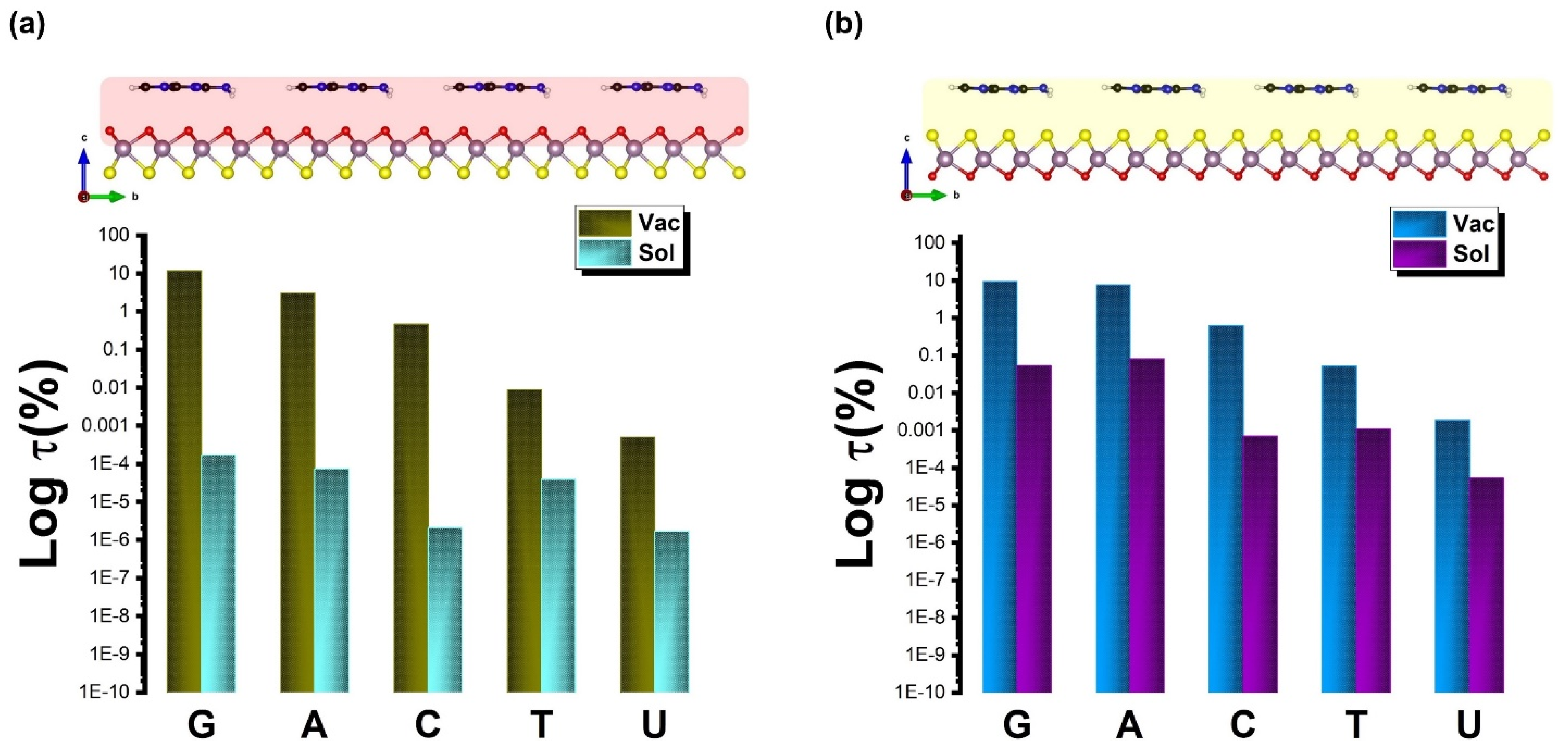
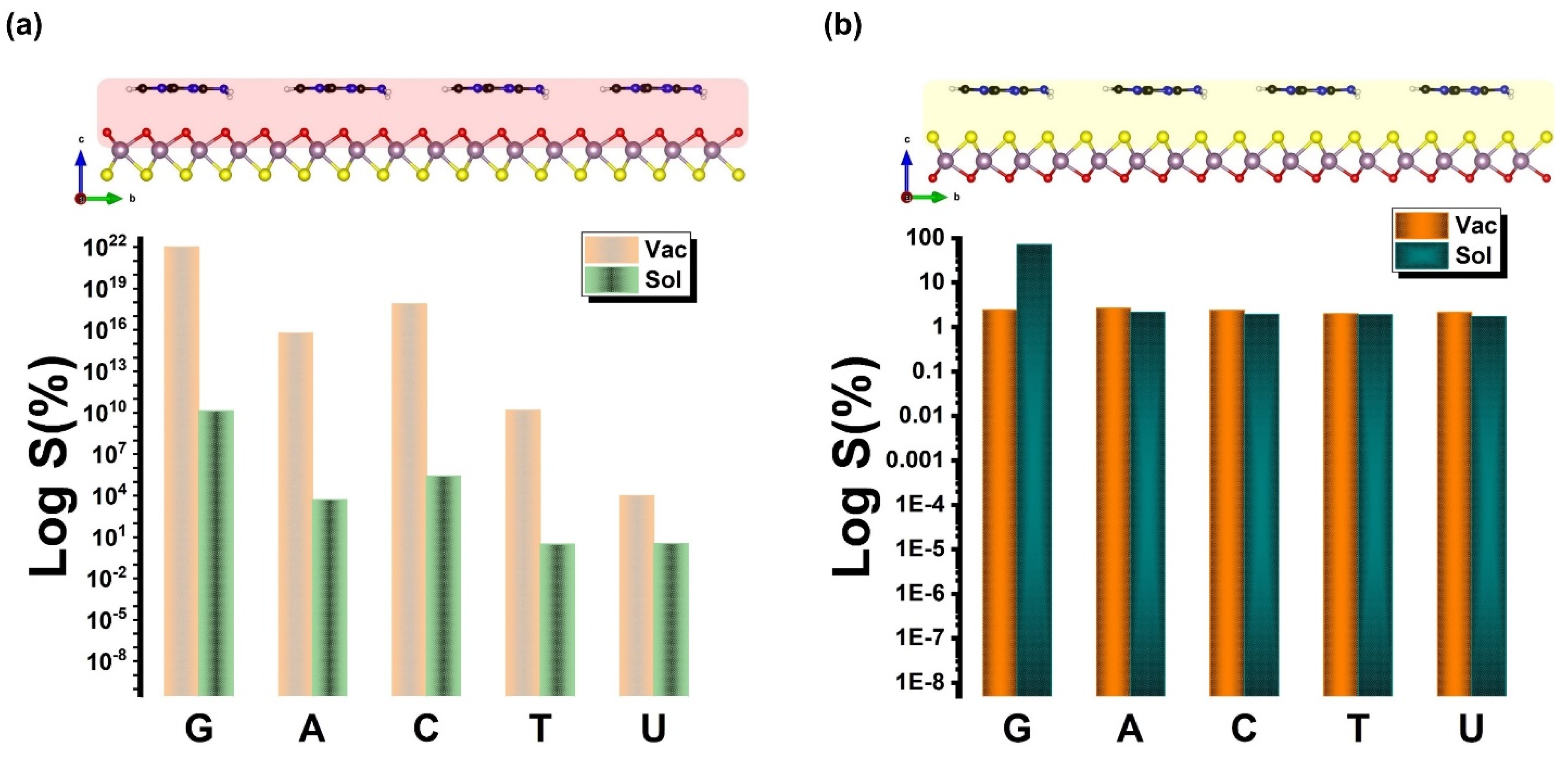
| Ads. State | DAds | EAds | EInt | EvdW | ECov |
|---|---|---|---|---|---|
| G–Oxy | 2.77 | −0.90 | −0.91 | −0.89 | −0.022 |
| A–Oxy | 2.80 | −0.86 | −0.82 | −0.82 | −0.004 |
| C–Oxy | 2.74 | −0.81 | −0.75 | −0.72 | −0.036 |
| T–Oxy | 2.94 | −0.71 | −0.68 | −0.71 | +0.027 |
| U–Oxy | 2.85 | −0.64 | −0.61 | −0.63 | +0.015 |
| G–Sul | 3.15 | −0.90 | −0.88 | −0.94 | +0.056 |
| A–Sul | 3.18 | −0.89 | −0.82 | −0.87 | +0.050 |
| C–Sul | 3.06 | −0.82 | −0.75 | −0.79 | +0.045 |
| T–Sul | 3.19 | −0.76 | −0.73 | −0.82 | +0.094 |
| U–Sul | 3.19 | −0.67 | −0.63 | −0.68 | +0.047 |
| Ads. State | EDef–Tot | EDef–Lyr | EDef–Mol | EDef–Cov |
|---|---|---|---|---|
| G–Oxy | +0.016 | −0.002 | +0.017 | +0.021 |
| A–Oxy | −0.038 | −0.005 | −0.033 | −0.034 |
| C–Oxy | −0.061 | −0.017 | −0.047 | −0.058 |
| T–Oxy | −0.027 | −0.018 | −0.009 | −0.024 |
| U–Oxy | −0.025 | −0.013 | −0.013 | −0.024 |
| G–Sul | −0.011 | −0.014 | +0.004 | −0.005 |
| A–Sul | −0.065 | −0.018 | −0.047 | −0.061 |
| C–Sul | −0.073 | −0.016 | −0.056 | −0.070 |
| T–Sul | −0.030 | −0.021 | −0.009 | −0.027 |
| U–Sul | −0.038 | −0.023 | −0.015 | −0.036 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laref, S.; Wang, B.; Inal, S.; Al-Ghamdi, S.; Gao, X.; Gojobori, T. A Peculiar Binding Characterization of DNA (RNA) Nucleobases at MoOS-Based Janus Biosensor: Dissimilar Facets Role on Selectivity and Sensitivity. Biosensors 2022, 12, 442. https://doi.org/10.3390/bios12070442
Laref S, Wang B, Inal S, Al-Ghamdi S, Gao X, Gojobori T. A Peculiar Binding Characterization of DNA (RNA) Nucleobases at MoOS-Based Janus Biosensor: Dissimilar Facets Role on Selectivity and Sensitivity. Biosensors. 2022; 12(7):442. https://doi.org/10.3390/bios12070442
Chicago/Turabian StyleLaref, Slimane, Bin Wang, Sahika Inal, Salah Al-Ghamdi, Xin Gao, and Takashi Gojobori. 2022. "A Peculiar Binding Characterization of DNA (RNA) Nucleobases at MoOS-Based Janus Biosensor: Dissimilar Facets Role on Selectivity and Sensitivity" Biosensors 12, no. 7: 442. https://doi.org/10.3390/bios12070442
APA StyleLaref, S., Wang, B., Inal, S., Al-Ghamdi, S., Gao, X., & Gojobori, T. (2022). A Peculiar Binding Characterization of DNA (RNA) Nucleobases at MoOS-Based Janus Biosensor: Dissimilar Facets Role on Selectivity and Sensitivity. Biosensors, 12(7), 442. https://doi.org/10.3390/bios12070442





