A Novel Full-length IgG Recombinant Antibody Highly Specific to Clothianidin and Its Application in Immunochromatographic Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Hybridoma Screening
2.3. Sequencing and Analysis of Antibody VR Genes
2.4. Cloning Antibody Genes into Full-Length RAbs
2.4.1. Construction of Expression Vectors
2.4.2. Production of Full-Length RAbs
2.5. Functional Analyses of Full-Length RAbs
2.5.1. Heterologous ic-ELISAs
2.5.2. Non-Competitive SPR
2.6. Development of Full-Length RAb-Based GICA for Clothianidin Detection
2.6.1. Selectivity and Sensitivity Experiment
2.6.2. Sample Matrix Effect Analysis
2.6.3. Analysis of the Spiked Samples
3. Results and Discussion
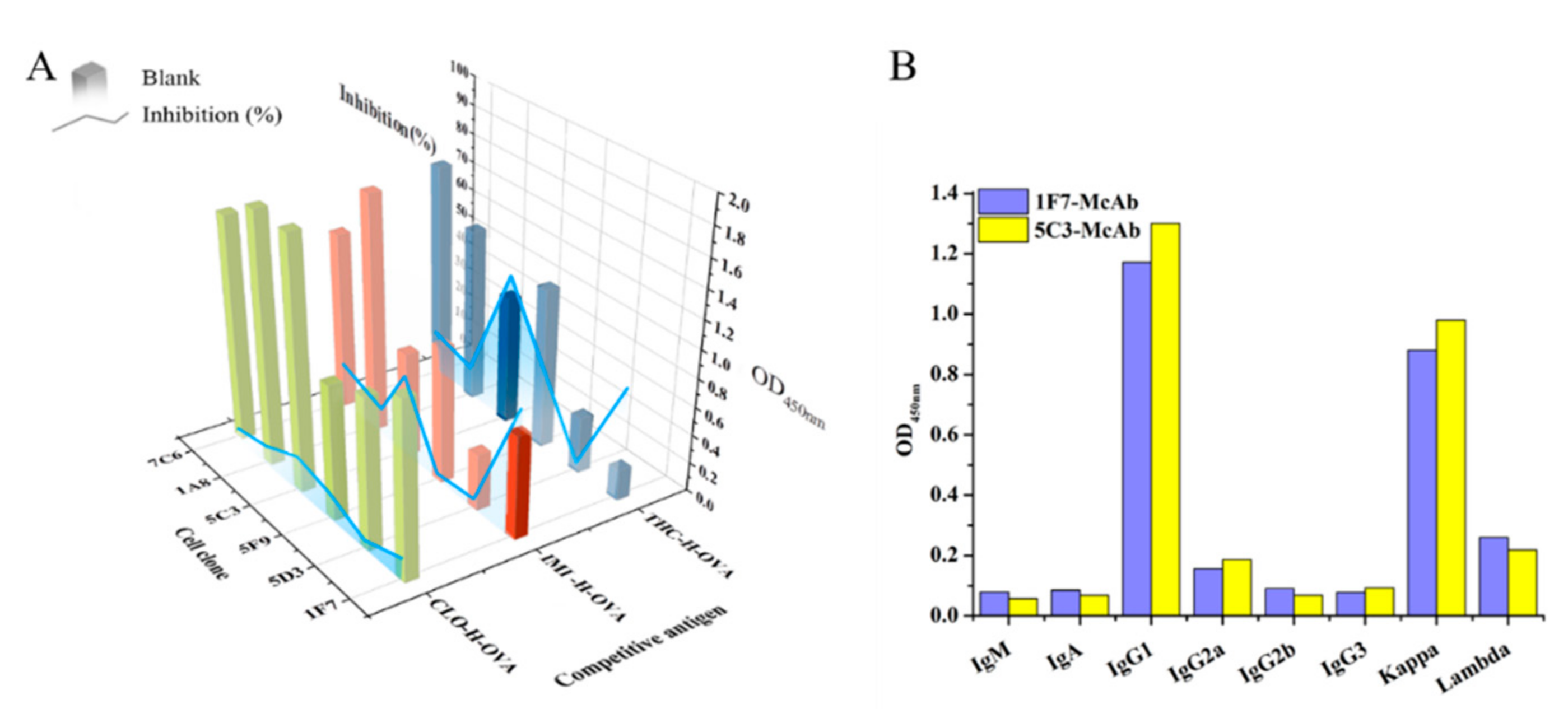
3.1. Hybridoma Screening
3.2. Sequencing of VR Genes and Expression of Full-Length IG RAbs
3.3. Heterologous ic-ELISAs for Functional Characterization of Full-Length RAbs
3.4. Non-Competitive SPR Assay for Functional Characterization of Full-Length RAbs
3.5. Application of a Highly Specific RAb in a GICA for Clothianidin Detection
3.5.1. Specificity and Sensitivity of GICA
3.5.2. Matrix Effects
3.5.3. Application of the GICA in Spiked Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fei, X.U.; Kang, R.E.N.; Yang, Y.Z.; Guo, J.P.; Liu, Y.M.; Lu, Y.Q.; Li, X.B. Immunoassay of chemical contaminants in milk: A review. J. Integr. Agric. 2015, 14, 2282–2295. [Google Scholar]
- Zhang, F.; Liu, B.; Wang, S. Review of Immunoassay Methods for the Detection of Sulfonamides. Curr. Org. Chem. 2017, 21, 2662–2674. [Google Scholar] [CrossRef]
- Bradbury, A.R.; Trinklein, N.D.; Thie, H.; Wilkinson, I.C.; Tandon, A.K.; Anderson, S.; Bladen, C.L.; Jones, B.; Aldred, S.F.; Bestagno, M.; et al. When monoclonal antibodies are not monospecific: Hybridomas frequently express additional functional variable regions. MAbs 2018, 10, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiao, S.S.; Chang, Y.Y.; Lu, X.Y.; Liu, P.Y.; Zhao, Y.; Zha, C.C.; Shen, L.R.; Guo, Y.R.; Zhu, G.N. High-affinity recombinant full-length antibody-based immunochromatographic strip assay for rapid and reliable detection of pyrazoxystrobin residues in food samples. Food Agric. Immunol. 2020, 31, 985–1003. [Google Scholar] [CrossRef]
- Baker, M. Antibody Anarchy: A Call to Order. Nature 2015, 527, 545–551. [Google Scholar] [CrossRef]
- Xu, C.; He, D.; Zu, Y.; Hong, S.; Li, J. Microcystin-LR heterologous genetically engineered antibody recombinant and its binding activity improvement and application in immunoassay. J. Hazard Mater. 2021, 406, 124596. [Google Scholar] [CrossRef]
- Plana, E.; Moreno, M.; Montoya, A.; Manclus, J.J. Development and application of recombinant antibody-based immunoassays to tetraconazole residue analysis in fruit juices. Food Chem. 2014, 143, 205–213. [Google Scholar] [CrossRef]
- Ren, W.; Xu, Y.; Huang, Z.; Li, Y.; Tu, Z.; Zou, L.; He, Q.; Fu, J.; Liu, S.; Hammock, B.D. Single-chain variable fragment antibody-based immunochromatographic strip for rapid detection of fumonisin B1 in maize samples. Food Chem. 2020, 319, 126546. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Mernaugh, R.L.; Zeng, X. Single chain fragment variable recombinant antibody functionalized gold nanoparticles for a highly sensitive colorimetric immunoassay. Biosens. Bioelectron. 2009, 24, 2853–2857. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; He, C.; Li, Z.; Huo, J.; Xue, Y.; Xu, X.; Qi, M.; Chen, L.; Hammock, B.D.; Zhang, J. Development of a Rapid Gold Nanoparticle Immunochromatographic Strip Based on the Nanobody for Detecting 2,4-DichloRophenoxyacetic Acid. Biosensors 2022, 12, 84. [Google Scholar] [CrossRef]
- Liu, P.; Guo, Y.; Jiao, S.; Chang, Y.; Liu, Y.; Zou, R.; Liu, Y.; Chen, M.; Guo, Y.; Zhu, G. Characterization of Variable Region Genes and Discovery of Key Recognition Sites in the Complementarity Determining Regions of the Anti-Thiacloprid Monoclonal Antibody. Int. J. Mol. Sci. 2020, 21, 6857. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhang, X.; Xu, J.; Guo, P.; Li, X.; Wang, H.; Xu, Z.; Lei, H.; Shen, X. Generation of recombinant antibodies by mammalian expression system for detecting S-metolachlor in environmental waters. J. Hazard. Mater. 2021, 418, 126305. [Google Scholar] [CrossRef] [PubMed]
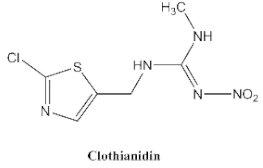
- Nauen, R.; Ebbinghaus-Kintscher, U.; Salgado, V.L.; Kaussmann, M. Thiamethoxam is a neonicotinoid precursor converted to clothianidin in insects and plants. Pestic. Biochem. Physiol. 2003, 76, 55–69. [Google Scholar] [CrossRef]
- Li, X.T.; Chen, J.H.; He, X.P.; Wang, Z.W.; Wu, D.N.; Zheng, X.L.; Zheng, L.; Wang, B.D. Simultaneous determination of neonicotinoids and fipronil and its metabolites in environmental water from coastal bay using disk-based solid-phase extraction and high-performance liquid chromatography–Tandem mass spectrometry. Chemosphere 2019, 234, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.H.; Zhang, C.; Liu, H.B.; Wu, R.R.; Tian, D.; Ruan, J.J.; Zhang, T.; Huang, M.Z.; Ying, G.G. Occurrence and distribution of neonicotinoid insecticides in surface water and sediment of the Guangzhou section of the Pearl River, South China. Environ. Pollut. 2019, 251, 892–900. [Google Scholar] [CrossRef]
- Bonmatin, J.; Noome, D.A.; Moreno, H.; Mitchell, E.A.D.; Glauser, G.; Soumana, O.S.; van Lexmond, M.B.; Sánchez-Bayo, F. A survey and risk assessment of neonicotinoids in water, soil and sediments of Belize. Environ. Pollut. 2019, 249, 949–958. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, J.; Wang, Z.; Zhang, B.; Sun, Z.; Yun, X.; Zhang, J. Levels and inhalation health risk of neonicotinoid insecticides in fine particulate matter (PM2.5) in urban and rural areas of China. Environ. Int. 2020, 142, 105822. [Google Scholar] [CrossRef]
- Tison, L.; Rößner, A.; Gerschewski, S.; Menzel, R. The neonicotinoid clothianidin impairs memory processing in honey bees. Ecotoxicol. Environ. Saf. 2019, 180, 139–145. [Google Scholar] [CrossRef]
- Li, H.; Wu, F.; Zhao, L.; Tan, J.; Jiang, H.; Hu, F. Neonicotinoid insecticide interact with honeybee odorant-binding protein: Implication for olfactory dysfunction. Int. J. Biol. Macromol. 2015, 81, 624–630. [Google Scholar] [CrossRef]
- Bandeira, F.O.; Alves, P.; Hennig, T.B.; Brancalione, J.; Matias, W.G. Chronic effects of clothianidin to non-target soil invertebrates: Ecological risk assessment using the species sensitivity distribution (SSD) approach. J. Hazard. Mater. 2021, 419, 126491. [Google Scholar] [CrossRef]
- Hladik, M.L.; Main, A.R.; Goulson, D. Environmental Risks and Challenges Associated with Neonicotinoid Insecticides. Environ. Sci. Technol. 2018, 52, 3329–3335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Sheng, E.Z.; Cong, L.J.; Wang, M.H. Development of Immunoassays for Detecting Clothianidin Residue in Agricultural Products. J. Agric. Food Chem. 2013, 61, 3619–3623. [Google Scholar] [CrossRef] [PubMed]
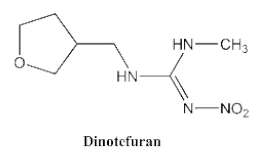
- Uchigashima, M.; Watanabe, E.; Ito, S.; Iwasa, S.; Miyake, S. Development of Immunoassay Based on Monoclonal Antibody Reacted with the Neonicotinoid Insecticides Clothianidin and Dinotefuran. Sensors 2012, 12, 15858–15872. [Google Scholar] [CrossRef] [PubMed]
- Sheng, E.; Shi, H.; Zhou, L.; Hua, X.; Feng, L.; Yu, T.; Wang, M. Dual-labeled time-resolved fluoroimmunoassay for simultaneous detection of clothianidin and diniconazole in agricultural samples. Food Chem. 2016, 192, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, X.; Hua, X.; Yin, W.; Fang, Q.; Wang, M. Fluorescence polarization immunoassay for highly efficient detection of clothianidin in agricultural samples. Anal. Methods 2014, 6, 6541–6547. [Google Scholar] [CrossRef]
- Yin, W.; Hua, X.; Liu, X.; Shi, H.; Gee, S.J.; Wang, M.; Hammock, B.D. Development of an enzyme-linked immunosorbent assay for thiacloprid in soil and agro-products with phage-displayed peptide. Anal. Biochem. 2015, 481, 27–32. [Google Scholar] [CrossRef]
- Guo, A.; Sheng, H.; Zhang, M.; Wu, R.; Xie, J. Development and Evaluation of a Colloidal Gold Immunochromatography Strip for Rapid Detection of Vibrio parahaemolyticus in Food. J. Food Qual. 2012, 35, 366–371. [Google Scholar] [CrossRef]
- Guo, Y.R.; Liu, R.; Liu, Y.; Xiang, D.D.; Liu, Y.H.; Gui, W.J.; Li, M.Y.; Zhu, G.N. A non-competitive surface plasmon resonance immunosensor for rapid detection of triazophos residue in environmental and agricultural samples. Sci. Total Environ. 2018, 613–614, 783–791. [Google Scholar] [CrossRef]
- Jiao, S.S.; Liu, P.Y.; Liu, Y.; Zou, R.B.; Zhao, Y.; Liu, Y.H.; Zhu, G.N.; Guo, Y.R. Binding properties of broad-specific monoclonal antibodies against three organophosphorus pesticides by a direct surface plasmon resonance immunosensor. Anal. Bioanal. Chem. 2018, 410, 7263–7273. [Google Scholar] [CrossRef]
- Guo, Y.R.; Liu, S.; Gui, W.; Zhu, G. Gold immunochromatographic assay for simultaneous detection of carbofuran and triazophos in water samples. Anal. Biochem. 2009, 389, 32–39. [Google Scholar] [CrossRef]
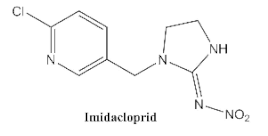
- Si, F.; Zou, R.; Jiao, S.; Qiao, X.; Guo, Y.; Zhu, G. Inner filter effect-based homogeneous immunoassay for rapid detection of imidacloprid residue in environmental and food samples. Ecotoxicol. Environ. Saf. 2018, 148, 862–868. [Google Scholar] [CrossRef]
- Zeng, L.; Xu, X.; Guo, L.; Wang, Z.; Ding, H.; Song, S.; Xu, L.; Kuang, H.; Liu, L.; Xu, C. An immunochromatographic sensor for ultrasensitive and direct detection of histamine in fish. J. Hazard. Mater. 2021, 419, 126533. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wu, H.; Xiao, Z.; Fu, H.; Shen, Y.; Luo, L.; Wang, H.; Lei, H.; Hongsibsong, S.; Xu, Z. Rational hapten design to produce high-quality antibodies against carbamate pesticides and development of immunochromatographic assays for simultaneous pesticide screening. J. Hazard. Mater. 2021, 412, 125241. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Liu, X.; Xiao, Z.; Fu, H.; Huang, Y.; Huang, S.; Shen, Y.; He, F.; Yang, X.; Hammock, B.; et al. Production of a specific monoclonal antibody for 1-naphthol based on novel hapten strategy and development of an easy-to-use ELISA in urine samples. Ecotoxicol. Environ. Saf. 2020, 196, 110533. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hua, X.D.; Ma, M.; Liu, J.S.; Zhou, L.L.; Wang, M.H. Detecting clothianidin residues in environmental and agricultural samples using rapid, sensitive enzyme-linked immunosorbent assay and gold immunochromatographic assay. Sci. Total Environ. 2014, 499, 1–6. [Google Scholar] [CrossRef]
- Yusakul, G.; Nuntawong, P.; Sakamoto, S.; Bhuket, P.R.N.; Kohno, T.; Kikkawa, N.; Rojsitthisak, P.; Shimizu, K.; Tanaka, H.; Morimoto, S. Bacterial Expression of a Single-Chain Variable Fragment (scFv) Antibody against Ganoderic Acid A: A Cost-Effective Approach for Quantitative Analysis Using the scFv-Based Enzyme-Linked Immunosorbent Assay. Biol. Pharm. Bull. 2017, 40, 1767–1774. [Google Scholar] [CrossRef] [Green Version]
- Liu, R.; Liang, X.; Xiang, D.; Guo, Y.; Liu, Y.; Zhu, G. Expression and Functional Properties of an Anti-Triazophos High-Affinity Single-Chain Variable Fragment Antibody with Specific Lambda Light Chain. Int. J. Mol. Sci. 2016, 17, 823. [Google Scholar] [CrossRef]
- Dong, J.; Li, Z.; Wang, Y.; Jin, M.; Shen, Y.; Xu, Z.; El-Aty, A.M.A.; Gee, S.J.; Hammock, B.D.; Sun, Y.; et al. Generation of functional single-chain fragment variable from hybridoma and development of chemiluminescence enzyme immunoassay for determination of total malachite green in tilapia fish. Food Chem. 2021, 337, 127780. [Google Scholar] [CrossRef]
- Hu, Z.; Li, H.; Liu, J.; Xue, S.; Gong, A.; Zhang, J.; Liao, Y. Production of a phage-displayed mouse ScFv antibody against fumonisin B1 and molecular docking analysis of their interactions. Biotechnol. Bioprocess Eng. 2016, 21, 134–143. [Google Scholar] [CrossRef]



| Neonicotinoid Analogues | PcAb [22] | McAb [23] | McAb [35] | 5C3-McAb in This Study | 5C3-RAb in This Study | 1F7-McAb in This Study | 1F7-RAb in This Study |
|---|---|---|---|---|---|---|---|
 | 100 a (46) b | 100 (4.4) | 100 (25.6) | 100 (13.16) | 100 (5.22) | 100 (5.38) | 100 (4.62) |
 | 11.8 c | 64 | 47.8 | 42.4 | 56 | <0.1 | <0.1 |
 | 0.8 | <0.1 | <0.03 | <0.1 | <0.1 | <0.1 | <0.1 |
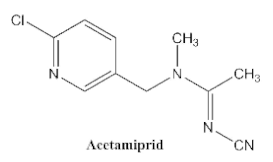 | <0.05 | <0.1 | <0.03 | <0.1 | <0.1 | <0.1 | <0.1 |
 | <0.05 | <0.1 | <0.03 | <0.1 | <0.1 | <0.1 | <0.1 |
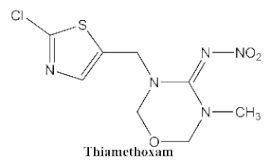 | <0.05 | <0.1 | <0.03 | <0.1 | <0.1 | <0.1 | <0.1 |
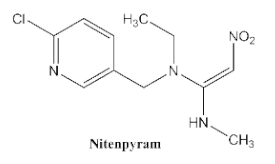 | <0.05 | <0.1 | <0.03 | <0.1 | <0.1 | <0.1 | <0.1 |
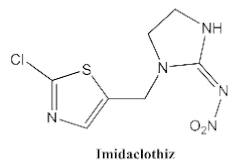 | <0.05 | <0.1 | <0.03 | <0.1 | <0.1 | <0.1 | <0.1 |
| Sample | Spiked Concentration (μg/L or ng/g) | Dilution Time d | Results (n = 3) | |
|---|---|---|---|---|
| Batch 1 e | Batch 2 | |||
| River water | 0 | 2 | −/−/− | −/−/− |
| 5 | ±/±/± | ±/±/± | ||
| 10 | +/+/+ | +/+/+ | ||
| 20 | +/+/+ | +/+/+ | ||
| Soil | 0 | 4 | −/−/− | −/−/− |
| 10 | ±/±/± | ±/±/± | ||
| 20 | +/+/+ | +/+/+ | ||
| 40 | +/+/+ | +/+/+ | ||
| Tomato | 0 | 6 | −/−/− | −/−/− |
| 15 | ±/±/± | ±/±/± | ||
| 30 | +/+/+ | +/+/+ | ||
| 60 | +/+/+ | +/+/+ | ||
| Orange | 0 | 6 | −/−/− | −/−/− |
| 15 | ±/±/± | ±/±/± | ||
| 30 | +/+/+ | +/+/+ | ||
| 60 | +/+/+ | +/+/+ | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Chen, Y.; Jiao, S.; Lu, X.; Fang, Y.; Liu, Y.; Zhao, Y.; Zhan, X.; Zhu, G.; Guo, Y. A Novel Full-length IgG Recombinant Antibody Highly Specific to Clothianidin and Its Application in Immunochromatographic Assay. Biosensors 2022, 12, 233. https://doi.org/10.3390/bios12040233
Chang Y, Chen Y, Jiao S, Lu X, Fang Y, Liu Y, Zhao Y, Zhan X, Zhu G, Guo Y. A Novel Full-length IgG Recombinant Antibody Highly Specific to Clothianidin and Its Application in Immunochromatographic Assay. Biosensors. 2022; 12(4):233. https://doi.org/10.3390/bios12040233
Chicago/Turabian StyleChang, Yunyun, Yang Chen, Shasha Jiao, Xinying Lu, Yihua Fang, Yihua Liu, Ying Zhao, Xiuping Zhan, Guonian Zhu, and Yirong Guo. 2022. "A Novel Full-length IgG Recombinant Antibody Highly Specific to Clothianidin and Its Application in Immunochromatographic Assay" Biosensors 12, no. 4: 233. https://doi.org/10.3390/bios12040233
APA StyleChang, Y., Chen, Y., Jiao, S., Lu, X., Fang, Y., Liu, Y., Zhao, Y., Zhan, X., Zhu, G., & Guo, Y. (2022). A Novel Full-length IgG Recombinant Antibody Highly Specific to Clothianidin and Its Application in Immunochromatographic Assay. Biosensors, 12(4), 233. https://doi.org/10.3390/bios12040233





