Abstract
We explored the feasibility of developing immunoassay technology with a linear carrier, to develop a simpler and cheaper rapid immunoassay technology. We selected aflatoxins as an example for research, as they are a group of highly toxic and carcinogenic compounds representing a worldwide threat to human health and life. With a non-competitive immunoassay, we detected and evaluated the effect of 28 different linear materials on antibody immobilization. Mercerized cotton and Dyneema line were chosen from the linear materials for further comparison using a competitive immunoassay, because both showed high-signal values and relatively low background noise. The results showed the sensitive IC50 of mercerized cotton as the reaction carrier was 0.33 ng/mL, and the linear range was 0.16~3.25 ng/mL. The sensitivity using Dyneema line as the reaction carrier was 1.16 ng/mL. The competitive curves of four sample matrices were established to evaluate the stability of the detection system; these were basically consistent with those without sample matrices. In conclusion, both mercerized cotton and Dyneema, will be suggested for the novel development of linear immobilization carrier-based immunoassays for other analytes, and especially to construct inexpensive and easy-to-obtain biological and environmental analytical technologies and biosensors.
1. Introduction
A point of care test (POCT) usually refers to the rapid detection that can be completed without professional technicians and equipment. In recent years, with the development of microfluidic analysis devices with a low cost and low consumption of costly material, researchers strive to find materials with a low cost and small volume for highly sensitive and rapid detection. Paper and textile have become two very important carriers of chip laboratory and sensing applications because of their low cost, wide availability and easy deformation. Recently, the proposed paper electrode and microfluidic paper-based analysis devices can be used for a high throughput, fast, stable point of care test, which makes the design of new paper-based sensors possible [1,2,3]. By constructing ordinary fibers into linear electrochemical transistor devices, it provides a way for multifunctional fabric systems [4,5,6]. A lateral flow immunochromatographic assay is a low-cost, simple, and rapid detection method, so threads have recently been proposed to transport and mix liquids in branch immunochromatographic assays [7,8,9,10,11,12].
It is difficult to prevent mycotoxins because fungi exist widely in nature. Mycotoxins are toxic low-molecular compounds, which seriously threaten the health of humans and animals. Mycotoxins are amongst the main pollutants in grain. The Food and Agriculture Organization of the United Nations reports that 25% of food production around the world is seriously contaminated with mycotoxins. Mycotoxins can destroy the growth of agricultural products, change metabolism, tissue integrity, and transcriptome response [13]. Food safety also affects international trade in food commodities. Thousands of tons of food are rejected every year because the contaminant exceeds the limit standards [14]. These toxins place a huge burden on countries that must deal with the consequences of contamination. The responses include increased public health attention, increased medical and health expenditure, and other economic tolls. According to the World Health Organization estimates for the year 2010, more than 0.4 million people died and more than 600 million became ill due to the 31 most common causes of foodborne diseases [15]. Aflatoxin (AF) is a secondary metabolite of Aspergillus flavus and Aspergillus parasiticus [16,17]. AFB1 mainly affects the development of rural agriculture in developing countries [18]. AFB1 is the most toxic and common aflatoxin [19,20], and can result in serious health problems including carcinogenesis, mutagenesis, and growth retardation [21]. AFB1 has genotoxicity [22] and can cause inhibited lipid utilization, defective intestinal development, and inflammation [23], and seriously threaten the health and life of human beings worldwide.
To monitor a variety of mycotoxins produced by A. flavus, highly sensitive and high-throughput detection methods have been developed. Chromatography is the most common commercial method for routine mycotoxin analysis in official laboratories, including thin-layer chromatography (TLC) [24], high-performance liquid chromatography (HPLC) [25,26], and liquid chromatography-tandem mass spectrometry (LC-MS) [27,28], which have made outstanding contributions in multiple mycotoxin detections. However, chromatography is limited by the need for expensive instruments, experienced operators, and cumbersome pretreatments. As a result, various portable detection technologies have been developed, and work to further develop sensitive, portable, simple, and inexpensive rapid diagnostic components is necessary.
In this project we aimed to find a microfluidic analysis device with a low cost and low consumption of costly materials. Cheap materials have been used to build sensitive, portable, simple analytical devices for the rapid detection of environmental pollution. We selected aflatoxins for this research project as they are a group of highly toxic and carcinogenic compounds that threaten human health and life worldwide, and restrict the development of national economies. We selected 28 types of polymer synthetic fiber and cotton fiber materials from the market. These shared the common morphological characteristic of being linear materials. The effects of these 28 different linear materials on antibody immobilization were detected and evaluated, and the two materials with the best immobilization effect were selected. Both materials can be used as antigen-antibody reaction carriers to complete ELISA experiments with a high sensitivity. This experiment will provide suggestions for the novel development of linear immobilization carrier-based immunoassays for other analytes, and especially to construct inexpensive and easy-to-obtain biological and environmental analytical technologies and biosensors.
2. Materials and Methods
2.1. Materials and Reagents
Aflatoxin B1 standards, antigens of AFB1, and 3,3′,5,5′-tetramethylbenzidine (TMB) were purchased from Sigma (St. Louis, MO, USA). Goat anti-mouse monoclonal antibody conjugated to horseradish peroxidase (HRP) was purchased from Solarbio (Beijing, China). The monoclonal antibody (mAb) against AFB1 was produced in our laboratory. Scissors, tweezers, rulers, and milk powder were purchased at a supermarket (Wuhan, China). Phosphate-buffered saline (PBS, 0.01 M, pH 7.4) was prepared by adding 8 g of NaCl, 2.9 g of Na2HPO4·12H2O, 0.2 g of KH2PO4, and 0.2 g of KCl to 1000 mL of deionized water. Phosphate-buffered saline with Tween-20 (PBST) was prepared by dissolving the Tween-20 with PBS buffer. Carbonate buffer solution (0.05 M, pH 9.6) was obtained by adding 2.93 g of NaHCO3 and 1.59 g of Na2CO3 to deionized water to make a volume of 1 L. TMB substrate chromogenic solution consists of 9.5 mL citrate buffer solution, 0.5 mL TMB solution, and 32 µL urea hydrogen peroxide solution. All other inorganic chemicals and organic solvents were of analytical reagent grade, unless stated otherwise.
Water was obtained from a Milli-Q purification system (Millipore, Danvers, MA, USA). The Coster 96-well EIA/RIA plate and 1.5 mL microtubes were purchased from Corning Incorporated (Corning, NY, USA). The absorbance at 450 nm was detected using a SpectraMax i3x Microplate Reader from Molecular Devices (Sunnyvale, CA, USA). Microplate washer was purchased form Wuhan Bai Leizhen Biotechnology Co., Ltd. (Wuhan, China).
2.2. Linear Material
The experiment used a variety of linear materials (Table 1). These materials were 2.5 mm ± 0.5 mm in diameter and 2 cm ± 0.5 mm in length. Monofilaments were purchased from Nantong Cintiq Monofilament Technology Co., Ltd. (Nantong, China). Other linear materials were purchased in online stores.

Table 1.
Linear materials used in the experiment.
2.3. Development of Anti-AFB1 Monoclonal Antibody (mAb)
Cell line 1C11 screened by ELISA in our laboratory was used for cell culture to obtain monoclonal antibodies [29]. The antibody secreted by this cell line was highly sensitive to aflatoxin, so this antibody was used for the experiment.
2.4. Preparation of Linear Materials
Antibody proteins can bind tightly to linear materials through electrostatic, hydrophilic, and hydrophobic interactions. We used three schemes to pretreat the linear materials to improve antibody fixation. The first scheme was to maintain the original properties of these materials, rinsing them three times with ddH2O and drying them at 37 °C for standby. The second scheme was to use citric acid to modify the fiber materials. These materials were immersed in a mixture of 10% citric acid and 5% NaH2PO4 by fractional weight, then soaked for 5 min. After removing excessive moisture, they were pre-dried at 80 °C for 5 min, and then heated to 140 °C for 1.5 min to obtain the modified fiber materials. The third scheme was to precook these materials in boiling water containing 2 mM NaCl solution for 30 min, and then soak them in 0.01% H2O2 and 0.01 mM HCl for 5 min, in turn. The materials were fully washed with ddH2O and dried in an oven at 80 °C for 3 h. The best treatment method of these linear materials for antibody protein immobilization was determined by comparing these three treatment schemes.
2.5. Screening of Immobilizable Materials for Antibody Protein
Through the specific reaction of mAB and the enzyme-conjugated secondary antibody, the linear material that can be fixed by antibodies was screened out according to the signal changes caused by the reaction of chromogenic liquid catalyzed by enzyme-conjugated secondary antibodies, and the optimal immobilized slow-release solution of antibody protein was determined. The screening diagram of immobilized antibody materials is shown in Figure 1. The specific experimental scheme was as follows: The mAB against AFB1 were diluted to 1 µg/mL with PBS (pH 7.4), sodium bicarbonate (pH 8.5), and carbonate solution (pH 9.6), respectively. The linear materials were placed in an EP tube and 500 µL antibody solution was added to cover overnight at 4 °C, and the control group was established. After washing three times with the same amount of PBST solution, the unbound sites on the material were blocked with the same volume of 4% skim milk-PBST solution at 37 °C for 1 h. The washing step was then repeated. The goat anti-mouse IgG labeled with HRP diluted 1:5000 with PBST was added to the linear material and incubated at 37 °C for 1 h. After the material was washed five times with PBST, the linear material was transferred into the new EP tube by tweezers for the subsequent color reaction to exclude the influence of the original centrifuge tube on the coloration. The 200 µL TMB substrate chromogenic solution was added into the linear material and incubated at 37 °C for 10 min to make the reaction proceed. After the reaction, the color solution was fully mixed, 100 µL was removed and added to the enzyme label plate, and 50 µL concentrated sulfuric acid was added to terminate the reaction. Determination of absorbance at 450 nm by microplate reader. The optical density value reflected the immobilization of antibody proteins on the linear material, and the material with higher optical density value was selected as the best immobilization material.
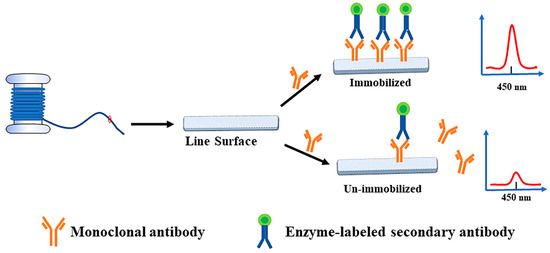
Figure 1.
Schematic diagram for screening antibody immobilization materials.
2.6. ELISA Experiment Based on Linear Materials
To verify the application effect of linear materials in the direction of aflatoxin immunoassay technology, we subjected the screened linear materials to ELISA. The experimental scheme was adjusted for the traditional ELISA. Development of a competitive ELISA based on linear materials was as follows: Linear materials were coated with 500 µL 1 µg/mL AFB1-BSA overnight at 4 °C. The most suitable sustained release solution was selected for the experiment. After removal of the coating solution, 500 µL PBST was used to wash the linear material three times, and then 4% skim milk was added to seal the residual site on the material and incubated at 37 °C for 1 h. The residual milk was washed with an equal amount of PBST. To determine the optimal working concentration of AFB1 mAB, 500 µL of gradient diluted AFB1 mAB was added to the material and specifically coupled with the fixed AFB1-BSA. Washing steps were then repeated. A blank control was also set up by only adding PBST solution. Goat anti-mouse HRP binding antibody diluted with PBST was added to the microtubules and incubated at 37 °C for 1 h. After washing 5 times with PBST, the linear material was transferred into the new EP tube by tweezers for subsequent color reaction, and the 200 µL TMB substrate color solution was added to the linear material, and the color reaction was carried out at 37 °C for 10 min. After full oscillation, 100 µL chromogenic liquid was removed and added to the enzyme label plate, and the color reaction was terminated with concentrated sulfuric acid. The optical density was measured immediately using a high-flux microplate reader. The concentration of monoclonal antibody with optical density value of 1 was determined as the optimal working concentration.
The coating and blocking processes of linear materials were the same as the indirect non-competitive ELISA. AFB1 diluted with 10% methanol/PBS was added to the linear material, and mABs diluted to the optimal working concentration in equal volume were added and heated to 37 °C for 1 h to allow the reaction. The addition of secondary antibody and the coloration procedure were the same as for the noncompetitive ELISA. After the termination of coloration, the optical density (450 nm) value was measured by a high-flux microplate reader, and the standard curve of ELISA was drawn by origin plot. The sensitivity (50% inhibitory concentration) of this program was determined.
2.7. Matrix Effect of Line-Load ELISA
To demonstrate the versatility of this method, we purchased four types of agricultural products from a local supermarket and measured the influence of matrix effect on its sensitivity. Samples included peanut, corn, rice, and wheat, which was ground to powder by a high-speed grinder. Methanol-PBS (70:30, v/v) was added to the sample powder, and the sample was extracted by shaking at 250 rpm and 10 °C for 1 h. The extract of each sample was concentrated by centrifugation at 6000 g for 15 min. The supernatant was filtered twice using a 0.45 µm organic phase filter, and the supernatant was collected for subsequent experiments. AFB1 standard solution was diluted serially with matrix solution. 250 µL of diluted AFB1 standard solution and an equal volume of mAB diluent were added to the coated and sealed linear materials, and other conditions were the same as for the blank standard curve of AFB1.
2.8. Evaluation of Line-Load ELISA Detection Technology
Three different concentrations of AFB1 (0.5, 1, 2 ng/mL) were added into the blank peanut matrix and detected by the established wire-load ELISA method, and repeated detection was set for intraday (n = 5) and daytime (n = 5). The stability, reproducibility and accuracy of the detection method established with linear materials as the carrier were evaluated.
2.9. 96-Well Microplate Sensitivity Determination
AFB1 was determined with a 96-well plate as the reaction carrier. The optimum working concentration of antibody was determined. AFB1-BSA was diluted to 1 µg/mL with carbonate solution, coated with 100 µL AFB1-BSA and overnight at 4 °C. They were washed three times with PBST, then sealed in PBST with 200 µL fat-free emulsion for 2 h at 37 °C. Monoclonal antibody 1C11 was diluted with a PBST gradient, added into the wells in turn, and incubated at 37 °C for 1 h. After washing, 100 µL goat anti-mouse HRP conjugated antibody was added to the hole for 1 h. It was finally washed three times and incubated with 100 µL TMB solution for 15 min. The reaction was terminated with 2 M H2SO4 (50 µL), and the absorbance was measured at 450 nm by an enzyme-labeled instrument.
Competitive ELISA was performed on the plate. AFB1 was diluted in a gradient from 50 ppb, and the 12th cell was set as a blank control. The concentration of antibody with optical density value of 1 was selected as the best working concentration. 50 µL of AFB1 and mAb 1C11 were added into the wells. After the color reaction was terminated, the IC50 value of the reaction was determined.
3. Results and Discussion
3.1. Determination of Linear Material Pretreatment Scheme
As detailed above, we selected three different pretreatment schemes to find a better method for fixing antibody protein to the linear materials. These schemes were ddH2O washing (Scheme One), dilute hydrochloric acid treatment (Scheme Two), and citric acid modification (Scheme Three). We randomly selected 10 kinds of materials to determine the optimal pretreatment method. The protein immobilization effect of citric acid on modified linear materials was poor (Figure 2). This may be due to the fact that the treatment results in the development of more carboxyl structures in the linear materials and a strong negative charge in the solution, so there is a strong repulsion between the antibody protein and the linear materials. Dilute hydrochloric acid treatment enhanced the nonspecific adsorption or amide bond formation between linear materials and antibodies; thus, the linear materials had a better ability to immobilize antibody proteins after dilute hydrochloric acid treatment. Treatment with dilute hydrochloric acid was also more effective than the use of citric acid, so the second pretreatment scheme was chosen for the linear materials.
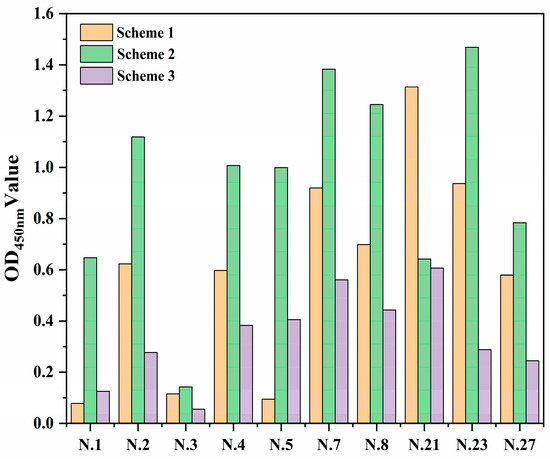
Figure 2.
Fixation effect of antibody protein on linear materials under three different pretreatments of ddH2O (Scheme 1), dilute acid treatment (Scheme 2), and citric acid modification (Scheme 3).
3.2. Material Screening to Demonstrate That mAb Can Be Immobilized
To verify the feasibility of mAb fixation in the linear materials, the intensities of the optical density value for different linear materials were studied under the same conditions. Twenty-eight different linear materials were selected from the market for this experiment. Three coated slow-release solutions (pH 7.4, 8.5, and 9.6) were set up to screen which of the linear materials had a better fixation of antibody protein and their optimal pH slow-release solutions. The mAb was immobilized on the linear material by non-specific adsorption and was specifically coupled with HRP-labeled signal antibody. The fixation effect of mAb was determined by measuring the optical density value (Figure 3). The results showed that each material had the ability to fix mAb, but that the fixation performance of each material was quite different. Mercerized cotton had advantages for the immobilization of mAb in carbonate solution (pH 9.6) and sodium bicarbonate buffer solution (pH 8.5), with optical density values of 1.329 and 1.240, respectively. That may have been because most of the components of mercerized cotton are cellulose, and there are high-density hydroxyl groups on the surface of cellulose. The immobilizing effect of Dyneema on mAb was second only to mercerized cotton, and showed the same performance as mercerized cotton in sodium bicarbonate solution (pH 8.5). Dyneema is a type of high-strength hydrophobic polyethylene fiber. The immobilization could be due to the salt bonds in the mAb, or that the side chains of some residues of the mAb participate in the formation of hydrogen bonds. Thus, the hydrophilicity of the residues is weakened, and the hydrophobic properties of some side chains are more prominent. Polypropylene fiber also has a good fixation effect in sodium bicarbonate solution due to this feature, but its fixation effect on antibody protein in other buffer solutions is significantly reduced. The immobilization effect of antibody protein in the alkaline solution showed advantages, which may be due to the alkaline buffer solution maintaining a higher activity of antibody protein.
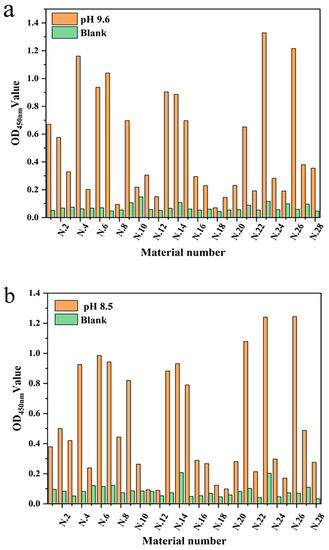
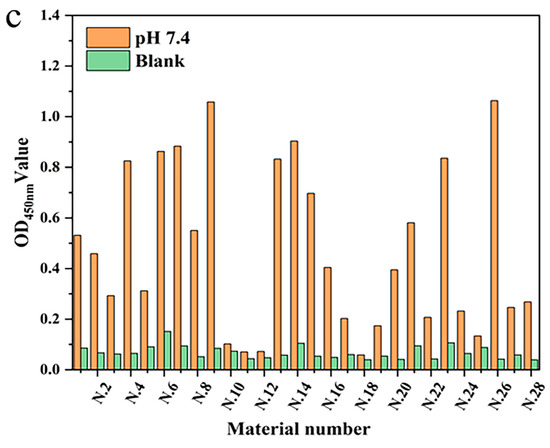
Figure 3.
The immobilization effect of antibody protein on linear materials under different buffer conditions: (a) Carbonate buffer (pH 9.6); (b) Sodium carbonate buffer (pH 8.5); and (c) PBS (pH 7.4).
3.3. Establishment of Standard Curves Based on Linear Material
To determine that mercerized cotton and Dyneema are superior carriers for the immune response, we established a standard curve based on linear materials. The AFB1 standard was diluted with 10% methanol-PBS. The competitive inhibition curve was made with the AFB1 standard concentration as the X axis and B/B0 as the Y axis. When mercerized cotton and Dyneema were used as immune response carriers, the IC50’s were 0.33 ng/mL and 1.16 ng/mL, respectively (Figure 4). The former had a higher sensitivity, and the equation of the inhibition curve was y = 0.01494 + 1.00532/(1 + (x/0.307)2.1956), R2 = 0.9993. This method had a linear detection range (IC20–IC80) of 0.16–3.25 ng/mL. The sensitivity and detection linear range of this immunoreaction program were close to those of 96-well plate method. The immune response can be carried out on the intact protein fixable material.
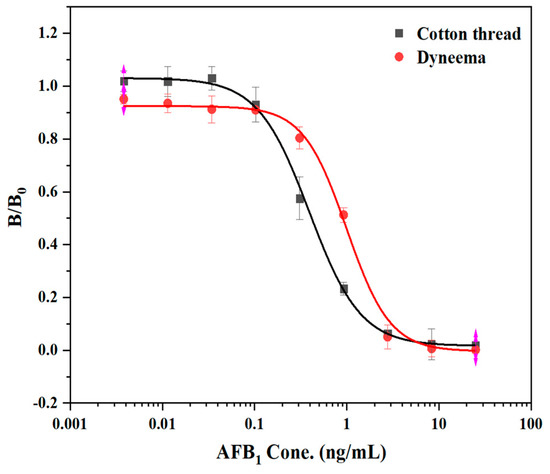
Figure 4.
Competitive standard curve of mAb against AFB1 on different materials.
3.4. Matrix Effect of Line-Load ELISA
The matrix effect is an important factor affecting the accuracy of ELISA. Impurities in sample extracts will cause false positive or false negative results [30]. It was found in the standard curve experiments that when mercerized cotton was used as the reaction carrier, the reaction procedure had a high sensitivity. Therefore, mercerized cotton was used to study the matrix effect on sensitivity. To explore the influence of sample matrix composition on the established method in the actual sample detection process, we purchased four agricultural products (peanut, corn, rice, and wheat) from the supermarket that were extracted with 70% methanol-PBS and prepared into a series of AFB1 standard solutions without dilution to establish the ELISA competition curve of each matrix extracts. The IC50 of peanut, corn, rice, and wheat were 0.37 ng/mL, 0.25 ng/L, 0.34 ng/mL, and 0.29 ng/mL, respectively. By comparing the IC50 values of the four sample matrices at a 450 nm wavelength (Figure 5), it was shown that the competition curves of the four sample matrices were not significantly different from those without a matrix. Thus, the materials used for protein immobilization can be applied to the laboratory immunoassay technology.
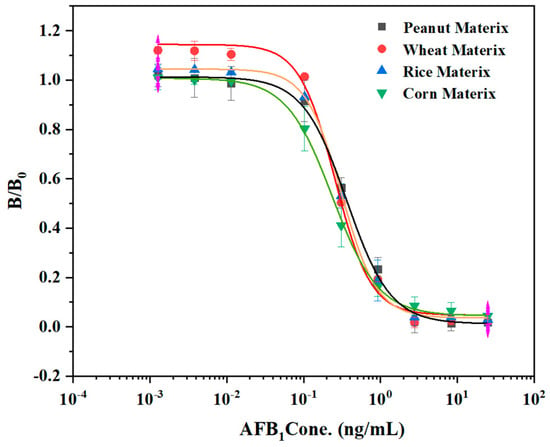
Figure 5.
Assay standard curve of mercerized cotton in peanut, wheat, rice, and corn matrix.
3.5. Evaluation of Line-Load ELISA Detection Technology
To evaluate the stability and reproducibility of the detection method based on linear materials, three AFB1 concentrations (0.5, 1, and 2 ng/mL) were added to the peanut matrix. The determination results were shown in Table 2. The recovery rate of standard addition of the line-load ELISA was between 87.88–112.88%, the intra-day coefficient of variation was 8.10–10.12%, and the inter-day coefficient of variation was 7.27–12.87%, all within the acceptable range. Therefore, the detection method based on linear materials has a certain stability and reproducibility and can be used to develop other new detection technologies.

Table 2.
Evaluation of line-load ELISA detection technology.
3.6. Comparison of Traditional Detection Methods for AFB1
The 96-well microplate was used to study the antibody protein pair for the detection of AFB1. AFB1 was diluted with 10% methanol PBS to 25, 8.3, 2.8, 0.93, 0.30, 0.10, 0.03, 0.011, 0.0038, and 0.00127 ng/mL. The mAb ELISA method based on the plate reaction was established, and the standard curve is shown below (Figure 6). The IC50 of this reaction is 0.114 ng/mL. There was no significant difference in the sensitivity between the established linear-ELISA detection method and the traditional 96-well enzyme-plate detection method, indicating that the rapid detection technology immobilized linear materials as the carrier to establish a new model. The traditional AFB1 detection method and its detection carrier components are shown in Table 3. Developing sensitive, portable, simple and cheap rapid diagnostic equipment is a technical development trend. In this experiment, the detection technology is established based on low-cost, easily available antibody immobilized linear material, and the detection cost that is much lower than traditional detection methods. It also provides carrier materials for the development of other detection technologies, and provides ideas for the development of new sensors.
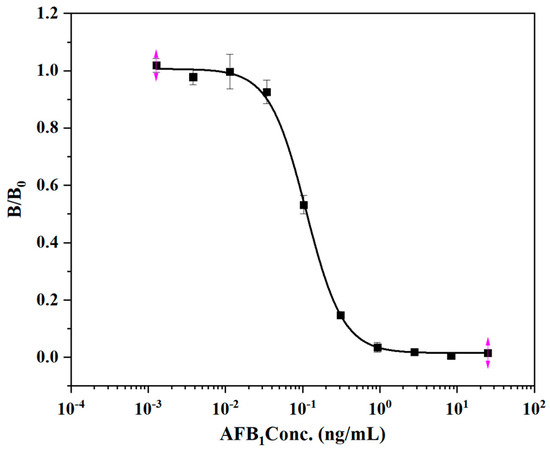
Figure 6.
Competitive standard curve of monoclonal antibody against AFB1 on enzyme label plate.

Table 3.
Carrier elements and characteristics of AFB1 detection method.
4. Conclusions
The development of immune rapid detection technology for small molecule substances such as mycotoxins continues to be a research focus, but the component cost for rapid detection technology is high. We explored the feasibility of developing immunoassay technology using linear carriers to develop a simpler and cheaper rapid immunoassay technology. Aflatoxins were selected for our research as they are a group of highly toxic and carcinogenic compounds that threaten human health and life worldwide. The effects of 28 different linear materials on antibody immobilization were evaluated by a non-competitive immunoassay. It was shown that mercerized cotton and Dyneema wire had a stable immobilization effect. Using ELISA it was found that the IC50 of anti-AFB1 mAB was 0.33 ng/mL using mercerized cotton as the immune response vector. With Dyneema as the reaction carrier, the IC50 was 1.16 ng/mL. Mercerized cotton had a high sensitivity as the immune response carrier, and the inhibition curve equation was y = 0.01494 + 1.00532/(1 + (x/0.307)2.1956), R2 = 0.9993. The linear detection range (IC20–IC80) of this method was 0.16~3.25 ng/mL. The competition curves of the four sample matrices (peanut, corn, rice, and wheat) were not significantly different from those without matrices. The mercerized cotton and Dyneema screened in this experiment will be recommended for the development of new linear immobilized carrier immunoassays for other analytes, and to promote the development of immunoassay equipment. The antibody protein can be stably immobilized on hydrophilic and hydrophobic materials. The materials used in this experiment are cheap, easy to obtain, and have broad application prospects in the field of rapid detection, especially in the construction of semi-quantitative and quantitative analysis techniques and biosensors in biological analysis and environmental detection.
Author Contributions
Methodology, H.Y., X.L., Y.Z. (Yating Zheng) and M.Z.; validation, H.Y.; formal analysis, H.Y. and Y.Z. (Yating Zheng); investigation, X.T. and Q.Z.; resources, Q.Z.; data curation, H.Y. and X.L.; writing—original draft preparation, H.Y.; writing—review and editing, Y.Z. (Yueju Zhao) and Q.Z.; visualization, X.T.; supervision, M.Z. and Y.Z. (Yueju Zhao); project administration, X.T.; funding acquisition, Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the key project of National Natural Sciences Foundation of China (32030085).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study can be obtained from the corresponding authors according to reasonable requirements.
Acknowledgments
The author thanked Beijing Mars Global Food Safety Center for its support and the National Natural Science Foundation of China (32030085). These grants have made significant contributions to the research described here.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wang, S.; Ge, L.; Song, X.; Yu, J.; Ge, S.; Huang, J.; Zeng, F. Paper-based chemiluminescence ELISA: Lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens. Bioelectron. 2012, 31, 212–218. [Google Scholar] [CrossRef]
- Wang, P.; Ge, L.; Yan, M.; Song, X.; Ge, S.; Yu, J. Paper-based three-dimensional electrochemical immunodevice based on multi-walled carbon nanotubes functionalized paper for sensitive point-of-care testing. Biosens. Bioelectron. 2012, 32, 238–243. [Google Scholar] [CrossRef]
- Lei, K.F.; Yang, S.I.; Tsai, S.W.; Hsu, H.T. Paper-based microfluidic sensing device for label-free immunoassay demonstrated by biotin-avidin binding interaction. Talanta 2015, 134, 264–270. [Google Scholar] [CrossRef]
- Shim, B.S.; Chen, W.; Doty, C.; Xu, C.; Kotov, N.A. Smart Electronic Yarns and Wearable Fabrics for Human Biomonitoring made by Carbon Nanotube Coating with Polyelectrolytes. Nano Lett. 2008, 8, 4151–4157. [Google Scholar] [CrossRef]
- Abouraddy, A.F.; Bayindir, M.; Benoit, G.; Hart, S.D.; Kuriki, K.; Orf, N.; Shapira, O.; Sorin, F.; Temelkuran, B.; Fink, Y.J.N.M. Towards multimaterial multifunctional fibres that see, hear, sense and communicate. Nat. Mater. 2007, 6, 336–347. [Google Scholar] [CrossRef]
- Hamedi, M.; Forchheimer, R.; Ingans, O.J.N.M. Towards woven logic from organic electronic fibres. Nat. Mater. 2007, 6, 357–362. [Google Scholar] [CrossRef]
- Zhou, G.; Mao, X.; Juncker, D. Immunochromatographic assay on thread. Anal. Chem. 2012, 84, 7736–7743. [Google Scholar] [CrossRef]
- Seth, M.; Mdetele, D.; Buza, J. Immunochromatographic thread-based test platform for diagnosis of infectious diseases. Microfluid. Nanofluidics 2018, 22, 45. [Google Scholar] [CrossRef]
- Meng, L.-L.; Song, T.-T.; Mao, X. Novel immunochromatographic assay on cotton thread based on carbon nanotubes reporter probe. Talanta 2017, 167, 379–384. [Google Scholar] [CrossRef]
- Mao, X.; Du, T.E.; Wang, Y.; Meng, L. Disposable dry-reagent cotton thread-based point-of-care diagnosis devices for protein and nucleic acid test. Biosens. Bioelectron. 2015, 65, 390–396. [Google Scholar] [CrossRef]
- Mao, X.; Du, T.-E.; Meng, L.; Song, T. Novel gold nanoparticle trimer reporter probe combined with dry-reagent cotton thread immunoassay device for rapid human ferritin test. Anal. Chim. Acta 2015, 889, 172–178. [Google Scholar] [CrossRef]
- Jia, X.; Song, T.; Liu, Y.; Meng, L.; Mao, X. An immunochromatographic assay for carcinoembryonic antigen on cotton thread using a composite of carbon nanotubes and gold nanoparticles as reporters. Anal. Chim. Acta 2017, 969, 57–62. [Google Scholar] [CrossRef]
- Barany, A.; Guilloto, M.; Cosano, J.; de Boevre, M.; Oliva, M.; de Saeger, S.; Fuentes, J.; Martínez-Rodriguez, G.; Mancera, J.M. Dietary aflatoxin B1 (AFB1) reduces growth performance, impacting growth axis, metabolism, and tissue integrity in juvenile gilthead sea bream (Sparus aurata). Aquaculture 2021, 533, 736189. [Google Scholar] [CrossRef]
- Akhtar, S.; Riaz, M.; Naeem, I.; Gong, Y.Y.; Ismail, A.; Hussain, M.; Akram, K. Risk assessment of aflatoxins and selected heavy metals through intake of branded and non-branded spices collected from the markets of Multan city of Pakistan. Food Control 2020, 112, 107132. [Google Scholar] [CrossRef]
- Pires, S.M.; Devleesschauwer, B. Estimates of global disease burden associated with foodborne pathogens. In Foodborne Infections and Intoxications; Academic Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Yu, L.; Ma, F.; Ding, X.; Wang, H.; Li, P. Silica/graphene oxide nanocomposites: Potential adsorbents for solid phase extraction of trace aflatoxins in cereal crops coupled with high performance liquid chromatography. Food Chem. 2018, 245, 1018–1024. [Google Scholar] [CrossRef]
- Sobolev, V.; Arias, R.; Goodman, K.; Walk, T.; Orner, V.; Faustinelli, P.; Massa, A. Suppression of aflatoxin production in aspergillus species by selected peanut (Arachis hypogaea) stilbenoids. J. Agric. Food Chem. 2018, 66, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Aristil, J.; Venturini, G.; Maddalena, G.; Toffolatti, S.L.; Spada, A. Fungal contamination and aflatoxin content of maize, moringa and peanut foods from rural subsistence farms in South Haiti. J. Stored Prod. Res. 2020, 85, 101550. [Google Scholar] [CrossRef]
- Mary, V.S.; Valdehita, A.; Navas, J.M.; Rubinstein, H.R.; Fernandez-Cruz, M.L. Effects of aflatoxin B1, fumonisin B1 and their mixture on the aryl hydrocarbon receptor and cytochrome P450 1A induction. Food Chem. Toxicol. 2015, 75, 104–111. [Google Scholar] [CrossRef]
- Wang, L.; He, L.; Zeng, H.; Fu, W.; Wang, J.; Tan, Y.; Zheng, C.; Qiu, Z.; Luo, J.; Lv, C.; et al. Low-dose microcystin-LR antagonizes aflatoxin B1 induced hepatocarcinogenesis through decreasing cytochrome P450 1A2 expression and aflatoxin B1-DNA adduct generation. Chemosphere 2020, 248, 126036. [Google Scholar] [CrossRef]
- Dai, Y.; Huang, K.; Zhang, B.; Zhu, L.; Xu, W. Aflatoxin B1-induced epigenetic alterations: An overview. Food Chem. Toxicol. 2017, 109, 683–689. [Google Scholar] [CrossRef]
- Klvana, M.; Bren, U.J.M. Aflatoxin B1–Formamidopyrimidine DNA Adducts: Relationships between structures, free energies, and melting temperatures. Molecules 2019, 24, 150. [Google Scholar] [CrossRef] [Green Version]
- Ivanovics, B.; Gazsi, G.; Reining, M.; Berta, I.; Poliska, S.; Toth, M.; Domokos, A.; Nagy, B., Jr.; Staszny, A.; Cserhati, M.; et al. Embryonic exposure to low concentrations of aflatoxin B1 triggers global transcriptomic changes, defective yolk lipid mobilization, abnormal gastrointestinal tract development and inflammation in zebrafish. J. Hazard. Mater. 2021, 416, 125788. [Google Scholar] [CrossRef]
- Qu, L.-L.; Jia, Q.; Liu, C.; Wang, W.; Duan, L.; Yang, G.; Han, C.-Q.; Li, H. Thin layer chromatography combined with surface-enhanced raman spectroscopy for rapid sensing aflatoxins. J. Chromatogr. A 2018, 1579, 115–120. [Google Scholar] [CrossRef]
- Nouri, N.; Sereshti, H. Electrospun polymer composite nanofiber-based in-syringe solid phase extraction in tandem with dispersive liquid-liquid microextraction coupled with HPLC-FD for determination of aflatoxins in soybean. Food Chem. 2019, 289, 33–39. [Google Scholar] [CrossRef]
- Beltran, E.; Ibanez, M.; Portoles, T.; Ripolles, C.; Sancho, J.V.; Yusa, V.; Marin, S.; Hernandez, F. Development of sensitive and rapid analytical methodology for food analysis of 18 mycotoxins included in a total diet study. Anal. Chim. Acta 2013, 783, 39–48. [Google Scholar] [CrossRef]
- Chen, J.; Liu, F.; Li, Z.; Tan, L.; Zhang, M.; Xu, D. Solid phase extraction based microfluidic chip coupled with mass spectrometry for rapid determination of aflatoxins in peanut oil. Microchem. J. 2021, 167, 106298. [Google Scholar] [CrossRef]
- Xu, X.; Xu, X.; Han, M.; Qiu, S.; Hou, X. Development of a modified QuEChERS method based on magnetic multiwalled carbon nanotubes for the simultaneous determination of veterinary drugs, pesticides and mycotoxins in eggs by UPLC-MS/MS. Food Chem. 2019, 276, 419–426. [Google Scholar] [CrossRef]
- Zhang, D.; Li, P.; Zhang, Q.; Zhang, W.; Huang, Y.; Ding, X.; Jiang, J. Production of ultrasensitive generic monoclonal antibodies against major aflatoxins using a modified two-step screening procedure. Anal. Chim. Acta 2009, 636, 63–69. [Google Scholar] [CrossRef]
- Butler, J.E.; Heyermann, H.; Borca, M.; Bielecka, M.; Frenyo, L.V. The isotypic, allotypic and idiotypic heterogeneity of bovine IgG2. Vet. Immunol. Immunopathol. 1987, 17, 125–134. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).